Abstract
E2F transcription factors play a critical role in cell cycle progression through the regulation of genes required for G1/S transition. They are also thought to be important for growth arrest; however, their potential role in the cell differentiation process has not been previously examined. Here, we demonstrate that E2F4 is highly upregulated following the neuronal differentiation of PC12 cells with nerve growth factor (NGF), while E2F1, E2F3, and E2F5 are downregulated. Immunoprecipitation and subcellular fractionation studies demonstrated that both the nuclear localization of E2F4 and its association with the Rb family member p130 increased following neuronal differentiation. The forced expression of E2F4 markedly enhanced the rate of PC12 cell differentiation induced by NGF and also greatly lowered the rate at which cells lost their neuronal phenotype following NGF removal. Importantly, this effect occurred in the absence of any significant change in the growth regulation of PC12 cells by NGF. Further, the downregulation of E2F4 expression with antisense oligodeoxynucleotides inhibited NGF-induced neurite outgrowth, indicating an important role for this factor during PC12 cell differentiation. Finally, E2F4 expression was found to increase dramatically in the developing rat cerebral cortex and cerebellum, as neuroblasts became postmitotic and initiated terminal differentiation. These findings demonstrate that, in addition to its effects on cell proliferation, E2F4 actively promotes the neuronal differentiation of PC12 cells as well as the retention of this state. Further, this effect is independent of alterations in cell growth and may involve interactions between E2F4 and the neuronal differentiation program itself. E2F4 may be an important participant in the terminal differentiation of neuroblasts.
While cell differentiation and growth arrest are distinct processes that can occur independently, they are generally intimately linked, with cell cycle exit being a prerequisite for terminal differentiation. For example, proliferating neuroblasts already exhibit several neuronal features, but the onset of terminal differentiation does not occur until the final cell division (14, 45). Clearly, mechanisms operating at the interface between the growth arrest and differentiation programs play critical roles in establishing the differentiated states of neurons and other cell types. Such mechanisms are also likely to be important in maintaining the irreversibility of terminal differentiation, and their disruption plays an important role in cell immortalization and tumorigenesis. Several cell cycle-associated proteins have been directly implicated in the terminal differentiation process, including the retinoblastoma protein (pRb) and the cyclin-dependent kinase inhibitor p21/WAF1 (17, 19). Importantly, recent studies have shown that these proteins have dual regulatory effects on cell cycle progression and cell differentiation that occur by distinct mechanisms (12, 47).
Members of the E2F family of transcriptional regulators have a central role in the cell cycle-dependent expression of genes required for DNA synthesis and S-phase entry, and functional studies have provided direct evidence for their involvement in the G1/S transition as well as apoptosis (for a review, see references 22 and 40). Six E2F family members have been characterized (E2F1 through E2F6), and they exhibit various degrees of structural relatedness (6, 13, 22, 51). E2Fs exist as heteromeric complexes consisting of an E2F and a DP family member (the so-called “free” E2F) and, with the exception of E2F6, also associate with a retinoblastoma (Rb) family member under specific conditions. Dimerization with a DP family member is necessary for optimal E2F transcriptional activity, while complex formation with Rb family members converts E2Fs into repressors of E2F-regulated genes. The ectopic expression of E2F1, E2F2, or E2F3 is sufficient to induce S-phase entry, while E2F4 or E2F5 by itself cannot (33). This difference apparently reflects the fact that nuclear import of E2F4 or E2F5 is cell cycle dependent (32, 35, 54).
While E2Fs are clearly important for cell cycle progression, little is currently known about their potential functions during cell differentiation. Differential changes in the expression or activities of E2Fs following cell cycle exit and differentiation have been demonstrated for several cell types, with E2F4 often being the predominant species (42, 48, 53, 56). In certain differentiated tissues, E2F5 appears to be selectively induced (11). These observations suggest that, in addition to contributing to cell proliferation, E2F4 and E2F5 may be important transcriptional regulators of growth arrest and/or differentiation. However, the direct demonstration of the ability of these transcriptional regulators to promote cell differentiation has been lacking. In the present study, we show that E2F4 enhances both the initiation and maintenance of nerve growth factor (NGF)-induced differentiation of PC12 cells into sympathetic-like neurons and that these effects do not involve changes in the regulation of cell growth. We also demonstrate that inhibition of E2F4 protein levels interferes with the differentiation process.
MATERIALS AND METHODS
Cell cultures.
PC12 cells were grown on collagen-coated plates in Dulbecco’s modified Eagle medium (DMEM) with or without 10% horse serum–5% fetal bovine serum and incubated at 37°C in 10% CO2. To induce neuronal differentiation, cells were treated with 50 ng of NGF (Harlan Bioproducts) per ml for various times. In washing experiments, NGF-treated cells were rinsed three times with DMEM with or without serum and cultured for up to four additional days.
Plasmids and stable transfections.
The plasmid pUHD15-1neo, containing the tetracycline resistance repressor (tTA) fused in frame with the activation domain of virion protein 16 of the herpes simplex virus, and the expression vector pTETHAE2F4, containing the human E2F4 sequence inserted into pUHD10-3, have been described (33). PC12 cells were transfected with equimolar amounts of pTETHAE2F4 and pUHD15-1neo with Lipofectamine (GIBCO/BRL). Cells were allowed to recover for 72 h prior to selection with G418 (200 μg/ml) and tetracycline (2 mg/ml). Multiple colonies of PC12 cell derivatives (E2F4-tet cells) were isolated, and the E2F4 induction and differentiation responses of two of these colonies were fully characterized. The colonies behaved very similarly to each other and to the original population of pooled clones.
RNA isolation and analysis.
Total RNA was isolated from PC12 cells and rat cerebral cortex by the guanidinium isothiocyanate-CsCl method, and Northern blot analyses were performed as previously described (27). The various probes used for Northern analysis were generated by restriction digestion with the following: (i) for E2F1, a StyI-XhoI fragment from pSp72-RBAP1 (26); (ii) for E2F3, a BamHI-BamHI fragment from pCMV-E2F3 (38); (iii) for E2F5, an XbaI-HincII fragment from pCMV-E2F5 (23); and (iv) for cyclin A, an EcoRI-SphI fragment from pCycA (55). The amount of RNA in each lane was normalized with a human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) cDNA probe generated by PstI digestion of pHcGAP (52).
Protein extracts and Western blotting.
Whole-cell extracts from PC12 cells and rat cortex and cerebellum were prepared as previously described (2). Briefly, cells were homogenized in whole-cell extraction buffer containing 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 10 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25% glycerol, 2 mg of pepstatin A per ml, 5 mg of leupeptin per ml, 5 mg of ubenimex (Bestatin) per ml, and 5 mg of aprotinin per ml and centrifuged for 10 min at 15,000 × g. Supernatants were used for analysis. Extracts were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels, blotted onto polyvinylidene difluoride membranes, and reacted with rabbit antibodies against E2F1, E2F4, E2F5, pRb, p107, p130, cyclin A, Sp1 (Santa Cruz Biotech), and β-tubulin type III (Sigma). Incubations were carried out in Tris-buffered saline containing 0.1% Tween 20 and 10% nonfat dry milk for 1 h at room temperature. Protein bands were visualized by enhanced chemiluminescence (kit from DuPont/NEN, Boston, Mass.).
Cellular analyses.
The extent of neurite outgrowth in E2F4-tet PC12 cell derivatives was determined by the ratio of neurite length to cell body diameter (9) (at least 300 to 400 cells were counted for each data point). The number of viable cells was determined by cell counting by trypan blue exclusion. The rate of DNA synthesis in E2F4-tet cells was determined by labelling 0.5 × 106 cells for 2 h with 1 μCi of [3H]thymidine. Washed cells were extracted at various times with 2 N NaOH. Significance was examined by a one-way analysis of variance, followed by Bonferroni multiple-comparison testing.
Isolation of nuclear and cytoplasmic fractions.
For nuclear and cytoplasmic extracts, cells were homogenized in 10 mM HEPES (pH 7.2) containing 150 mM NaCl, 1.5 mM MgCl2, and protease inhibitors. Complete cellular disruption was verified by trypan blue exclusion, and nuclear and cytoplasmic fractions were generated by centrifugation at 800 × g for 10 min. The nuclear pellet was washed once with hypotonic buffer and then lysed with radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 8.1], 150 mM NaCl, 0.2% SDS, 1% sodium deoxycholate, 1% Nonidet P-40, 5 mM EDTA).
Immunoprecipitations.
Immunoprecipitations were performed on 200 μg of whole-cell extracts in a final volume of 0.2 ml at 4°C for 1 h with 10 μg of p130 antibody (sc-317; Santa Cruz Biotech). Protein A/G Plus agarose (Santa Cruz Biotech) was then added, and incubations were continued for an additional 16 h at 4°C. Pellets were collected by centrifugation, washed four times with whole-cell extraction buffer, and dissolved in electrophoresis sample buffer. Immunoprecipitates were resolved on SDS–10% polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and probed with E2F4 antiserum (sc-866; Santa Cruz Biotech).
Antisense ODN treatment.
Synthetic phosphorothioate oligodeoxynucleotides (ODNs) were obtained from Oligos Etc. (Redding Center, Conn.). E2F4 antisense ODNs were based on conserved mouse and human E2F4 sequences (3, 46) and were as follows: AS1, 5′-GCCTCCGCCATCGC-3′ (residues +14 to −3 in mouse E2F4 cDNA); AS2, 5′-CCTGTGGCCCGGCCTCCG-3′ (residues +22 to +5); and AS3, 5′-TCGTGCCGGCTTGGAGTC-3′ (residues +56 to +89). Control ODNs contained either the sense sequences complementary to AS1 and AS3 (S1 and S3) or the unrelated sequences 5′-AGCCTCCGCCAGCAAGGCTGC-3′ (CS1) and 5′-GTTTTGAGCAGCCATGGTGGA-3′ (CS2). Treatment of PC12 cells with ODNs (5 μM) was performed with or without NGF in the presence of Transfast reagent (Promega, Madison, Wis.) as recommended by the manufacturer. Fresh ODNs were added every 48 h for up to 6 days during NGF treatment.
RESULTS
Regulation of E2F and Rb family members during neuronal differentiation of PC12 cells.
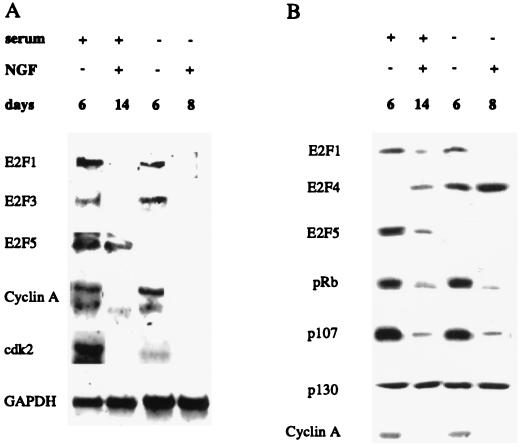
We utilized the PC12 cell model to explore the role of E2Fs during neuronal differentiation. These pheochromocytoma cells undergo growth arrest and differentiation into postmitotic sympathetic-like neurons in the presence of NGF and faithfully reproduce many important features of sympathetic differentiation, including neurite outgrowth, regulation of cell cycle-associated genes, and NGF-dependent survival (7, 37, 49, 59). Exposure of PC12 cells to 50 ng of NGF per ml for 8 days in the absence of serum or for 14 days in the presence of serum markedly downregulated the levels of protein and/or mRNA for E2F1, E2F3, and E2F5 (Fig. 1). In contrast, E2F4 protein levels were markedly increased in differentiated PC12 neurons (Fig. 1B). The increase in E2F4 protein levels was even greater in neuronal derivatives generated in the absence of serum, due to the upregulation of E2F4 by serum removal alone (Fig. 1B), while the expression of E2F5 protein and mRNA was inhibited under serum-free conditions (Fig. 1). Thus, E2F4 protein levels increase markedly following PC12 cell differentiation, while the levels of other E2F proteins are greatly diminished or extremely low.
FIG. 1.
Expression of E2F and Rb family members following NGF treatment of PC12 cells. (A) Northern analysis. GAPDH mRNA was used to monitor the relative loading of total RNA (30 μg/lane). (B) Western analysis (30 μg of protein/lane). Cells were cultured in the presence or absence of serum and with or without NGF for 6, 8, or 14 days, as indicated. Cells were treated with NGF for a longer time in the presence of serum, since the differentiation response is slower under these conditions (15).
Analysis of Rb family members revealed that pRb and p107 proteins also were suppressed in neuronal derivatives, while p130 was unchanged (Fig. 1B). We also examined the regulation of cyclin A and cdk2, both of which are important for cell cycle regulation and one of which (cyclin A) is regulated by E2Fs in PC12 cells (22). In both cases, expression was markedly suppressed following NGF-induced differentiation (Fig. 1).
E2F4 binds p130 and localizes to the nucleus following PC12 cell differentiation.
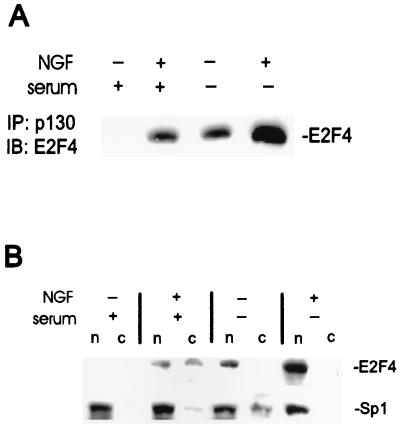
E2F4 interactions with Rb family proteins have a major impact on E2F4 function, including its conversion from a trans-activator into a repressor of E2F-regulated cell cycle genes (22, 39). In differentiated and growth-arrested cells, E2F4 is often associated with the Rb family member p130 (8, 42, 53, 57), and the fact that p130 was unchanged by the NGF-induced differentiation of PC12 cells, while pRb and p107 were downregulated, suggests that E2F4-p130 complexes may exist in these neuronal derivatives. p130-containing complexes were immunoprecipitated and examined for the presence of E2F4. E2F4 protein was not detectable in p130 immunoprecipitates derived from proliferating PC12 cells but was clearly evident in complexes from cells treated with NGF (Fig. 2A). The number of E2F4-p130 complexes was higher in PC12 cells treated with NGF in the absence of serum than in cells treated in the presence of serum, which was consistent with the increased concentrations of total E2F4 protein in these cells. Also, E2F4 was readily detected in p130 immunoprecipitates derived from PC12 cells cultured in serum-free medium alone. The negative control protein Sp1 was undetectable on immunoblots of the same p130 precipitates (data not shown). Thus, E2F4 associates with p130 in PC12 cells following growth arrest and neuronal differentiation.
FIG. 2.
E2F4 associates with p130 and localizes to the nucleus in neuronal derivatives of PC12 cells. Wild-type cells were cultured with or without serum and with or without NGF for 8 days. (A) p130 complexes were immunoprecipitated (IP) with p130 antibodies from 200 μg of whole-cell extracts and subjected to Western blot (immunoblot [IB]) analysis with E2F4 antiserum. (B) Western blot analysis of nuclear (n) and cytosolic (c) protein fractions from wild-type PC12 cells. A total of 50 μg of protein was loaded per lane. Transcription factor Sp1 was used as a nuclear marker.
An additional effect of the interaction of E2F4 with Rb family proteins is increased nuclear localization and, therefore, enhanced transcriptional regulation (32, 35, 54). This was examined by the subcellular fractionation of PC12 cells with the transcription factor Sp1 as a marker for the nuclear fraction (25). Expression of Sp1 in PC12 cells is unaffected by NGF treatment (58). The ratio of E2F4 within the nuclear and cytosolic fractions increased considerably following NGF treatment for 8 days (Fig. 2B). This was particularly evident in cells which differentiated in serum-free medium (the presence of significant amounts of cytosolic E2F4 in cells which differentiated in the presence of serum likely reflects the fact that differentiation proceeds more slowly under these conditions [15] and is incomplete after 8 days of NGF treatment). The majority of E2F4 in growth-arrested, serum-deprived PC12 cells was also associated with the nuclear fraction (Fig. 2B). These findings indicate that E2F4 is mainly or exclusively localized in the nucleus following neuronal differentiation.
Development of PC12 cell derivatives expressing E2F4 under the control of a tetracycline-regulated promoter.
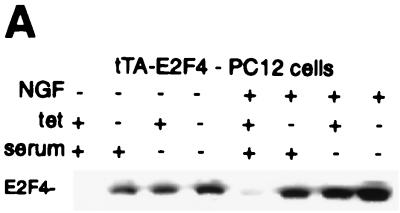
The above results suggested that E2F4 has a primary role in neuronal differentiation relative to other E2Fs. To directly investigate this, we developed PC12 cell derivatives stably transfected with a tetracycline-regulated expression plasmid for this factor, together with a constitutively active vector encoding the tetracycline transactivator tTA. With this system, E2F4 expression is repressed in the presence of tetracycline and induced following its removal. Multiple cell lines (E2F4-tet cells) were generated and examined for the expression of E2F4 in the presence and absence of tetracycline (Fig. 3A). No E2F4 protein was detected in noninduced, proliferating E2F4-tet cells, indicating tight regulation of the expression vector by tetracycline. In contrast, E2F4 was readily detected following tetracycline removal. The levels of induced E2F4 protein were, in fact, similar to those obtained following growth arrest and differentiation of uninduced PC12 cells (Fig. 3A) and thus were quite physiological. Under serum-free, growth-arrested conditions in which endogenous E2F4 is upregulated, the removal of tetracycline resulted in a further, apparently additive increase in E2F4 levels. Similarly, tetracycline removal resulted in a marked increase in E2F4 levels in cells treated with NGF for 6 days in either the presence or absence of serum (Fig. 3A). Thus, E2F4-tet cells exhibit substantial induction of E2F4 expression in proliferating, quiescent, and differentiated PC12 cells.
FIG. 3.
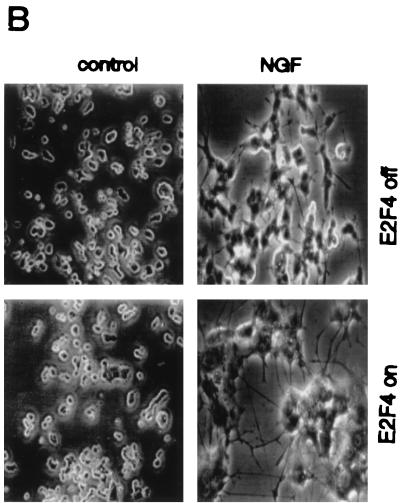
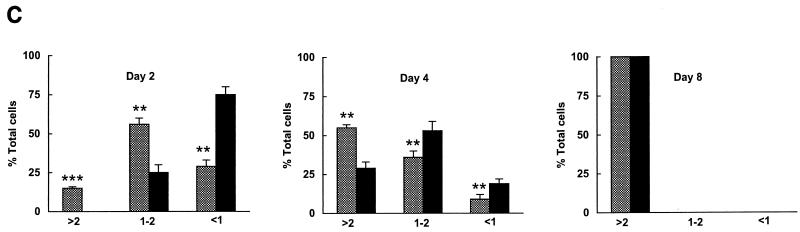
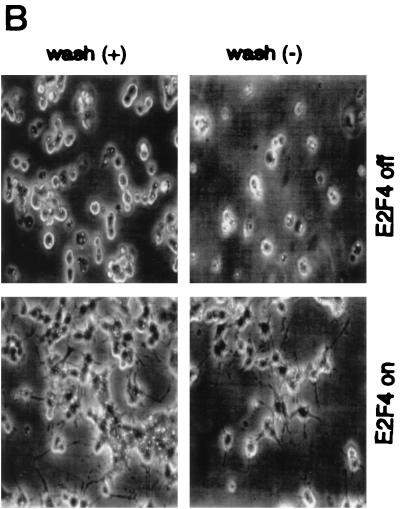
E2F4 induction accelerates the differentiation response of PC12 cells. (A) Western analysis of E2F4 protein expression in E2F4-tet cells. Cells were cultured for 6 days in the presence or absence of NGF and tetracycline (tet) in serum-free or serum-containing medium. Ectopic E2F4 expression was induced following the removal of tetracycline. (B) Effect of E2F4 induction on the morphological differentiation of PC12 cells by NGF. Phase-contrast microscopy of E2F4-tet PC12 cells cultured with or without NGF for 4 days in the presence of serum was done. Tetracycline was either present in (E2F4 off) or absent from (E2F4 on) the culture medium. (C) Quantitation of neurite outgrowth in E2F4-tet cells following NGF treatment. The extent of outgrowth was determined by the ratio of neurite length to cell body diameter (9). Results are presented as the percentage of total cells counted and represent means + standard errors of three to four independent experiments. The x axis shows cell body diameters. Cells were cultured in either the presence (black bars, not induced) or absence (shaded bars, induced) of tetracycline and were treated with NGF in serum-containing DMEM for 2 to 8 days. One-way analysis of variance was used for statistical analysis. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (D) Western analysis of β-tubulin expression in E2F4-tet cells. Cell extracts were analyzed at various days following NGF treatment in the presence of serum, with and without tetracycline (tet) in the culture medium. The same membrane was reprobed with antibodies against transcription factor Sp1.
Effects of E2F4 induction on neuronal differentiation of PC12 cells.
To examine its effects on PC12 cell differentiation, E2F4 was induced prior to the initiation of NGF treatment. Control studies verified that tetracycline treatment had no effect on either the differentiation of wild-type PC12 cells or their response to NGF withdrawal (data not shown). E2F4-tet cells were treated with 50 ng of NGF per ml for up to 8 days in serum-containing medium in the presence and absence of tetracycline. Major differences in the rate of morphological differentiation that were most dramatic after 2 to 3 days of NGF treatment were observed (Fig. 3B). E2F4-induced cells had a much more mature neuronal appearance at this time, including a flattened morphology and the prevalence of markedly longer neurites. In contrast, E2F4 induction did not alter the morphology of proliferating PC12 cells (data not shown).
The effects of E2F4 preinduction on PC12 cell differentiation were quantified by determining the ratio of neurite length to cell body diameter. Cells were classified according to this ratio (<1, 1 to 2, and >2 cell body diameters), with more-differentiated cells possessing higher values. On day 2 of NGF treatment, >70% of E2F4-induced cells were significantly differentiated (bearing neurites 1 cell diameter or greater in length), while only 25% of uninduced cells possessed neurite lengths of 1 to 2 cell diameters; none had neurites longer than 2 cell diameters (Fig. 3C). By day 8 of NGF treatment, 100% of uninduced cells exhibited a differentiated phenotype, and thus E2F4 enhancement of differentiation could no longer be detected.
The neuronal marker β-tubulin type III (18) was used to monitor the effects of E2F4 preinduction on the biochemical differentiation of PC12 cells by NGF. β-Tubulin protein levels increased following the NGF treatment of E2F4-tet cells, and the rate of this increase was substantially higher when E2F4 was preinduced (Fig. 3D) on days 2 and 4 of NGF treatment. Thus, the ectopic expression of E2F4 markedly enhances the onset of neuronal differentiation in PC12 cells.
E2F4 and maintenance of the neuronal phenotype.
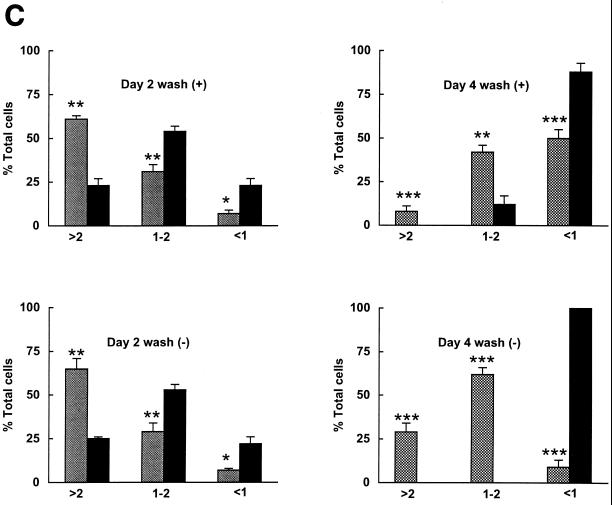
A characteristic feature of NGF-treated PC12 cells is their ability to undergo reversible differentiation and renewed proliferation upon NGF removal in the presence of serum (16). Western analysis demonstrated that E2F4 protein decreased to undetectable levels after the removal of NGF from differentiated PC12 cells and further culturing for 4 days in the presence of serum, while the expression of both E2F1 and E2F5 increased (Fig. 4A). We therefore examined whether ectopic expression of E2F4 would promote retention of the differentiated state in neuronal derivatives undergoing dedifferentiation. E2F4-tet cells were differentiated with NGF in the presence of serum for 8 days with and without tetracycline and then washed and cultured in serum-containing medium alone. Cells in which E2F4 was induced (without tetracycline) lost neurites at a considerably lower rate than uninduced E2F4-tet cells after NGF removal (Fig. 4B). The number of cells possessing neurites of 1 to 2 cell body diameters or more was 4.3-fold higher on the fourth day of washing (Fig. 4C). Further, the expression of β-tubulin type III decreased at a lower rate following NGF removal from induced E2F4-tet cells (Fig. 4D).
FIG. 4.
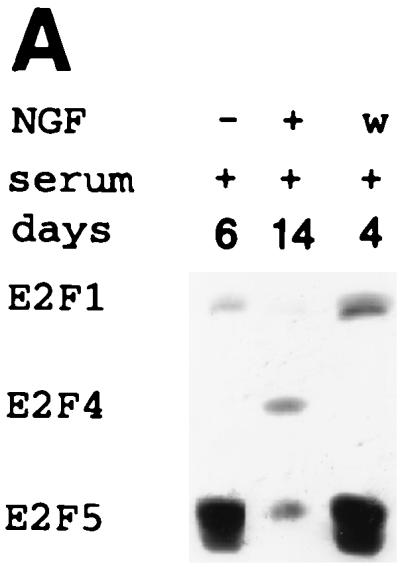
Effect of E2F4 induction on the response of differentiated PC12 cells to NGF withdrawal. (A) Western analysis of E2F protein expression following removal of NGF from wild-type PC12 cells. PC12 cells were treated with NGF in serum-containing medium for 14 days and then washed (w) and cultured with serum for up to 4 days. (B) Phase-contrast microscopy of E2F4-tet cells treated with NGF in the presence of serum for 8 days, then washed three times to remove NGF, and incubated in either the presence [wash (+)] or absence [wash (−)] of serum for 2 days, as indicated. Tetracycline was either present in (E2F4 off) or absent from (E2F4 on) the culture medium. (C) Neurite outgrowth in E2F4-tet derivatives following the washing of NGF-treated cells. Cells were treated with NGF in serum-containing DMEM for 8 days and then were washed in medium with or without serum for 2 or 4 days. Data were analyzed as for Fig. 3. (D) Western analysis of β-tubulin expression. E2F4-tet cells were treated with NGF in serum-containing medium for 8 days with or without tetracycline (tet) and then either harvested (8 days) or washed (w) and cultured in the absence of NGF for 2 or 4 days. The same membrane was probed for Sp1 protein as an internal control.
Neuronal derivatives of PC12 cells lose their neurites and subsequently die via an apoptotic mechanism if cultured in serum-free medium without NGF (37). When differentiated E2F4-tet cells were washed and cultured in medium lacking both serum and NGF, the induced cells retained their differentiated morphology to a much greater degree than did the uninduced cells (Fig. 4B). This effect was particularly dramatic on the fourth day following NGF removal, when >90% of induced E2F4-tet cells contained neurites of intermediate length or greater while no uninduced cells had this phenotype (Fig. 4C). Thus, in addition to accelerating the onset of neuronal differentiation, E2F4 also promotes the maintenance of the differentiated state of PC12 cell-derived neurons.
Growth regulation in E2F4-induced PC12 cells during differentiation.
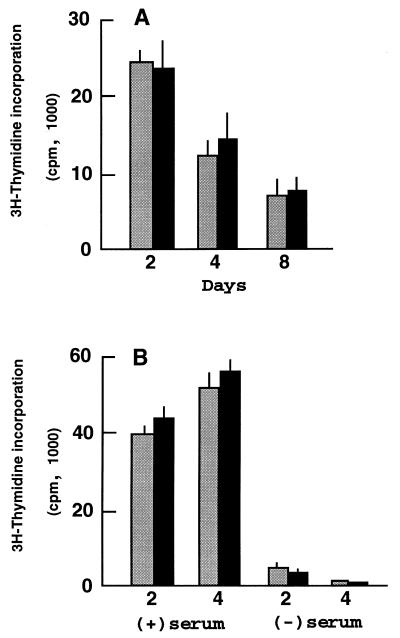
In contrast to its impact on cell differentiation, E2F4 induction had no significant effect on DNA synthesis or cell number in PC12 cells undergoing growth arrest and differentiation in response to NGF (Fig. 5A and data not shown). Similarly, these parameters were unaltered during the dedifferentiation and growth renewal of PC12 cell-derived neurons following NGF withdrawal in the presence of serum (Fig. 5B and data not shown). The ectopic expression of E2F4 also had no major effect on the survival rate of NGF-deprived neuronal derivatives in serum-free medium, although there was a trend toward decreased cell loss (data not shown). Thus, E2F4 enhancement of both the onset and retention of PC12 cell differentiation is independent of any marked alteration of growth arrest or cell proliferation.
FIG. 5.
Induction of E2F4 does not alter DNA synthesis in NGF-treated PC12 cells. (A) [3H]thymidine incorporation was determined during the differentiation response to NGF. Cells were treated with NGF in serum-containing medium for 2, 4, or 8 days in either the presence (black bars, uninduced) or absence (shaded bars, induced) of tetracycline. (B) DNA synthesis was determined following washing of differentiated E2F4-tet cells. Cells were treated with NGF in the presence of serum for 8 days and then washed and cultured with (+) or without (−) serum in either the presence or absence of tetracycline, as for panel A. Data are expressed as the means ± standard errors of three to four independent experiments.
Role of endogenous E2F4 in neuronal differentiation of PC12 cells.
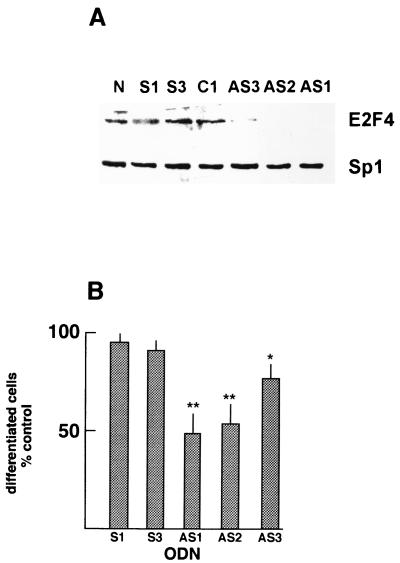
To determine whether E2F4 was normally involved in PC12 cell differentiation, antisense ODNs (AS1 to AS3) were designed for sequences spanning or adjacent to the translation initiation region which are unrelated to sequences within the other E2Fs. All three antisense ODNs markedly reduced the expression of E2F4 in NGF-treated PC12 cells, with AS1 and AS2 being more effective than AS3 (Fig. 6A). When tested for their effects on NGF-dependent neurite outgrowth from PC12 cells, the antisense ODNs inhibited this process with the same order of efficacy, while sense or unrelated control ODNs had negligible effects on both E2F4 protein levels and neurite outgrowth (Fig. 6 and data not shown). Thus, E2F4 has an important regulatory role in the NGF-induced differentiation of PC12 cells.
FIG. 6.
Analyses with antisense ODNs. (A) Analysis of E2F4 and Sp1 proteins in extracts following ODN treatment of PC12 cells. PC12 cells were treated with NGF for 6 days in the presence or absence of ODNs. (B) Neurite outgrowth in PC12 cells following antisense-ODN treatment for 5 days. The percentage of total cells bearing neurites more than 2 cell body diameters in length were determined. Results are expressed relative to those for cells treated with NGF in the absence of ODNs and are the averages of five independent experiments. N, cells treated with NGF without ODN; C1, control unrelated ODN.
Regulation of E2F4 expression during development of the rat central nervous system.
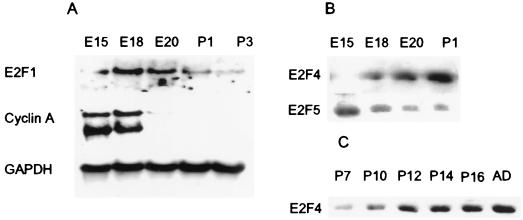
We also examined whether the pattern of E2F expression in developing neural tissue was similar to that observed in differentiating PC12 cells. During neurogenesis of the rat cerebral cortex, proliferating neuroblasts within the subventricular zone become postmitotic and begin their migration to upper cortical layers in a wave occurring in the late embryonic and early postnatal period (36). An analysis of the rat cerebral cortex revealed that the expression of both E2F1 and E2F5 was downregulated as cortical neurons became postmitotic between E18 and P1 to P3 (Fig. 7A and B). Cyclin A expression was similarly downregulated in the cerebral cortex, as previously reported (21). In contrast, E2F4 protein was substantially upregulated during this period of terminal growth arrest and neuronal differentiation (Fig. 7B).
FIG. 7.
Developmental regulation of E2F gene expression in rat central nervous system. Rat cortex (A and B) and cerebellum (C) were examined. (A) Northern analysis was performed as for Fig. 1. (B and C) Western analyses. Thirty micrograms each of total RNA and protein extracts was loaded per lane.
Similar analyses were performed with developing rat cerebellum. During postnatal weeks 2 and 3, granule cell neuroblasts within the extragranular layer terminally exit the cell cycle and migrate inward (1). Western analysis of rat cerebellar extracts during this period revealed a gradual but large increase in E2F4 expression (Fig. 7C). In contrast, E2F5 protein was essentially undetectable during this developmental period in the rat cerebellum (data not shown). Thus, E2F4 is upregulated in the rat central nervous system as neuroblasts become postmitotic and differentiate, as occurs in PC12 cell-derived neurons.
DISCUSSION
E2FA facilitates the differentiation process.
E2F4 is one of a family of factors known to be critical for normal progression through the G1 and S phases of the cell cycle. The present studies have demonstrated an additional activity for this factor, specifically in the differentiated state. The expression of E2F4 increases markedly in neuronal derivatives of PC12 cells, and its ectopic expression enhances both the initiation of neuronal differentiation and its maintenance when examined under reversal conditions. Further, experiments with antisense ODNs have shown that E2F4 is an important regulator of NGF-induced differentiation. To our knowledge, this is the first demonstration of a direct role for mammalian E2F factors in promoting the differentiation of any cell lineage. Mutation studies of the Drosophila melanogaster E2F gene have previously suggested a role for this factor in postmitotic cells (5), and a nonproliferative function in secretion by epithelial cells of the choroid plexus was recently demonstrated for mouse E2F5 in gene knockout studies (31). The present findings suggest that E2F4 may have therapeutic potential in inhibiting the reversal of differentiation that often follows the treatment of certain tumors with differentiating agents (20).
While important for the neuronal differentiation of PC12 cells, E2F4 induction by itself was not sufficient to initiate this process. This is not surprising in light of the dual roles of E2F4 in cell cycle progression and differentiation. To promote differentiation, E2F4 presumably must form complexes with factors which promote its nuclear localization and appropriately regulate its transcriptional activity. That is, its nuclear entry depends on its association with either a DP or Rb partner (32, 35, 54), and the nature of the facilitating partner(s) is an important determinant of its transcriptional activity: e.g., DP partners enhance transactivation, while Rb proteins convert the E2F complex into a transrepressor. Association with p130 is, therefore, presumably important for the nuclear localization and function of E2F4 during the neuronal differentiation of PC12 cells. In contrast to the present results, the ectopic expression of E2F4 induces cell cycle reentry in quiescent muscle cells when overexpressed with DP3Δ (43). This apparently reflects limiting levels of Rb family members under the latter conditions, since E2F4 complexes formed with p130 and p107 are associated with growth arrest in these cells. Thus, whether E2F4 promotes cell proliferation or differentiation is determined to a great degree by the nature of its associated partners.
E2Fs and terminal differentiation of neuroblasts.
The terminal differentiation of postmitotic neurons is not initiated until permanent exit from the cell cycle (36), and this event represents a critical juncture in nervous system development. Loss of either pRb or p130 leads to the uncontrolled proliferation and subsequent apoptosis of central and peripheral neuroblasts, as well as the incomplete differentiation of surviving cells (28, 29). Studies employing adenovirus E1A proteins have also implicated Rb family members in the neuronal differentiation of PC12 cells by NGF (4). A central mechanism by which Rb members presumably regulate the arrest and differentiation of neurons is via interactions with E2Fs. Consistent with this, the presence of free E2F complexes (i.e., lacking Rb family members) as well as the increased expression of an E2F target, the cyclin E gene, is associated with the altered proliferation of central nervous system neurons in Rb-deficient mice (34).
The present studies suggest that E2F4 is an important transcriptional regulator of the terminal differentiation of neuroblasts. Expression of E2F4 protein markedly increases in the rat cerebral cortex and cerebellum as neurons become postmitotic and is consistent with the pattern of E2F4 mRNA expression observed during neurogenesis in the mouse (10). It has been found that E2F1, E2F2, and E2F5 mRNAs are downregulated while E2F4 transcripts are readily detected in postmitotic neurons of the mouse (10, 50). E2F4 is also upregulated following the differentiation of human neuroblastoma cells (44). The demonstration of E2F4-p130 complexes following the neuronal differentiation of PC12 cells, together with the required role for p130 in neuronal development (28), further implicates E2F4 in this process.
Mechanisms of E2F4 action during differentiation.
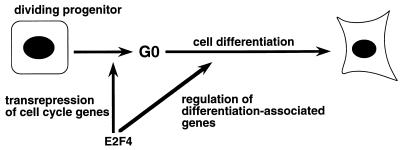
The specific mechanisms by which E2F4 enhances differentiation of PC12 cell-derived neurons and possibly other cell types remain to be determined. E2F complexes containing p130 predominate in the G0 state of many differentiated cells and tissues (although complexes with other Rb family members are also sometimes observed), and E2F4 is often the major E2F species detected (8, 38, 42, 57). E2F-p130 complexes are thought to function by repressing the expression of genes that are required for G1/S-phase transition and DNA synthesis (24, 30). Thus, one mechanism by which E2F4 may promote neuronal differentiation is by the repression of G1/S-associated genes, which, in turn, would facilitate cell cycle exit (Fig. 8). The downregulation of E2F target genes, i.e., the cyclin A, p107, and pRb genes as well as the gene for E2F1 itself, in differentiated PC12 cells and neurons, as described here and in other studies (10, 21, 41), is consistent with this mechanism. However, E2F4 induction had no effect on the growth regulation of PC12 cells by NGF, indicating that additional, cell cycle-independent mechanisms are operative. In particular, these results suggest that E2F4 may directly interact with the differentiation program itself, either as complexes with p130 or in some other form (Fig. 8). Both pRb and p21/WAF1 can influence cell differentiation independent of their effects on the cell cycle (12, 47), and E2F4 may also have dual yet distinct roles in regulating cell growth and differentiation. The present study defines a system in which these and other important aspects of E2F4 function during cell differentiation can now be addressed.
FIG. 8.
Mechanisms by which E2F4 may facilitate the differentiation process. E2F4 may act indirectly by downregulating the expression of genes involved in cell cycle progression, thus promoting growth arrest and subsequent differentiation, or by direct participation in the differentiation process itself. In PC12 cells, its effects appear to be largely independent of the cell cycle.
ACKNOWLEDGMENTS
We thank René Bernards and Hermann Bujard for providing us with the E2F4 and tTA expression vectors, respectively, and Nicholas La Thangue for E2F5 antiserum. We also thank Cathy Warren for her assistance in preparing the manuscript.
This publication was made possible by PHS grant DK36468 as well as an Annual Research Fund Award from the Worcester Foundation for Biomedical Research to D.L.K.
REFERENCES
- 1.Altman J. Postnatal development of the cerebellar cortex in the rat. J Comp Neurol. 1972;145:465–514. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- 2.Beijersbergen R L, Carlée L, Kerkhoven R M, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes Dev. 1995;9:1340–1353. doi: 10.1101/gad.9.11.1340. [DOI] [PubMed] [Google Scholar]
- 3.Beijersbergen R L, Kerkhoven R M, Zhu L, Carlée L, Voorhoeve P M, Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994;8:2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- 4.Boulukos K E, Ziff E B. Adenovirus 5 E1A proteins disrupt the neuronal phenotype and growth factor responsiveness of PC12 cells by a conserved region 1-dependent mechanism. Oncogene. 1993;8:237–248. [PubMed] [Google Scholar]
- 5.Brook A, Xie J E, Du W, Dyson N. Requirements for dE2F function in proliferating cells and in post-mitotic differentiating cells. EMBO J. 1996;15:3676–3683. [PMC free article] [PubMed] [Google Scholar]
- 6.Cartwright P, Muller H, Wagener C, Holm K, Helin K. E2F-6: a novel member of the E2F family is an inhibitor of E2F-dependent transcription. Oncogene. 1998;17:611–623. doi: 10.1038/sj.onc.1201975. [DOI] [PubMed] [Google Scholar]
- 7.Chong J A, Tapia-Ramirez J, Kim S, Toledo-Aral J J, Zheng Y, Boutros M C, Altshuller Y M, Frohman M A, Kraner S D, Mandel G. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell. 1995;80:949–957. doi: 10.1016/0092-8674(95)90298-8. [DOI] [PubMed] [Google Scholar]
- 8.Corbeil H B, Branton P E. Characterization of an E2F-p130 complex formed during growth arrest. Oncogene. 1997;15:657–668. doi: 10.1038/sj.onc.1201224. [DOI] [PubMed] [Google Scholar]
- 9.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Dagnino L, Fry C J, Bartley S M, Farnham P, Gallie B L, Phillips R A. Expression patterns of the E2F family of transcription factors during mouse nervous system development. Mech Dev. 1997;66:13–25. doi: 10.1016/s0925-4773(97)00083-x. [DOI] [PubMed] [Google Scholar]
- 11.Dagnino L, Fry C J, Bartley S M, Farnham P, Gallie B L, Phillips R A. Expression patterns of the E2F family of transcription factors during murine epithelial development. Cell Growth Differ. 1997;8:553–563. [PubMed] [Google Scholar]
- 12.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Inhibitory function of p21Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 13.Gaubatz S, Wood J G, Livingston D M. Unusual proliferation arrest and transcriptional control properties of a newly discovered E2F family member, E2F-6. Proc Natl Acad Sci USA. 1998;95:9190–9195. doi: 10.1073/pnas.95.16.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Sanchez A, Bader D. In vitro analysis of cardiac progenitor cell differentiation. Dev Biol. 1990;139:197–209. doi: 10.1016/0012-1606(90)90288-t. [DOI] [PubMed] [Google Scholar]
- 15.Greene L A. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene L A, Tischler A S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu W, Schneider J W, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- 18.Halegoua S, Armstrong R C, Kremer N E. Dissecting the mode of action of a neuronal growth factor. Curr Top Microbiol Immunol. 1991;165:119–170. doi: 10.1007/978-3-642-75747-1_7. [DOI] [PubMed] [Google Scholar]
- 19.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by myoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 20.Hass R, Gunji H, Datta R, Kharbanda S, Hartmann A, Weichselbaum R, Kufe D. Differentiation and retrodifferentiation of human myeloid leukemia cells is associated with reversible induction of cell cycle-regulatory genes. Cancer Res. 1992;52:1445–1450. [PubMed] [Google Scholar]
- 21.Hayes T E, Valtz N L M, McKay R D G. Downregulation of CDC2 upon terminal differentiation of neurons. New Biol. 1991;3:259–269. [PubMed] [Google Scholar]
- 22.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 23.Hijmans E M, Voorhoeve P M, Beijersbergen R L, van ’t Veer L J, Bernards R. E2F-5, a new E2F family member that interacts with p130 in vivo. Mol Cell Biol. 1995;15:3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurford R K, Jr, Cobrinik D, Lee M-H, Dyson N. pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 1997;11:1447–1463. doi: 10.1101/gad.11.11.1447. [DOI] [PubMed] [Google Scholar]
- 25.Kadonaga J T, Carner K R, Masiarz F R, Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987;51:1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin W G, Jr, Krek W, Sellers W R, DeCaprio J A, Ajchenbaum F, Fuchs C S, Chittenden T, Yue L, Farnham P J, Blanar M A, Livingston D M, Flemington E K. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992;70:351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- 27.Kew D, Kilpatrick D L. Widespread organ expression of the rat proenkephalin gene during early postnatal development. Mol Endocrinol. 1990;4:337–340. doi: 10.1210/mend-4-2-337. [DOI] [PubMed] [Google Scholar]
- 28.LeCouter J E, Kablar B, Whyte P F, Ying C, Rudnicki M A. Strain-dependent embryonic lethality in mice lacking the retinoblastoma-related p130 gene. Development. 1998;125:4669–4679. doi: 10.1242/dev.125.23.4669. [DOI] [PubMed] [Google Scholar]
- 29.Lee E Y-H P, Hu N, Yuan S-S F, Cox L A, Bradley A, Lee W-H, Herrup K. Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev. 1994;8:2008–2021. doi: 10.1101/gad.8.17.2008. [DOI] [PubMed] [Google Scholar]
- 30.Li J-M, Hu P P-C, Shen X, Yu Y, Wang X-F. E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor β through E2F binding sites. Proc Natl Acad Sci USA. 1997;94:4948–4953. doi: 10.1073/pnas.94.10.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindeman G J, Dagnino L, Gaubatz S, Xu Y, Bronson R T, Warren H B, Livingston D M. A specific, nonproliferative role for E2F-5 in choroid plexus function revealed by gene targeting. Genes Dev. 1998;12:1092–1098. doi: 10.1101/gad.12.8.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindeman G J, Gaubatz S, Livingston D M, Ginsberg D. The subcellular localization of E2F-4 is cell-cycle dependent. Proc Natl Acad Sci USA. 1997;94:5095–5100. doi: 10.1073/pnas.94.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukas J, Petersen B O, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996;16:1047–1057. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macleod K F, Hu Y, Jacks T. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 1996;15:6178–6188. [PMC free article] [PubMed] [Google Scholar]
- 35.Magae J, Wu C-L, Illenye S, Harlow E, Heintz N H. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J Cell Sci. 1996;109:1717–1726. doi: 10.1242/jcs.109.7.1717. [DOI] [PubMed] [Google Scholar]
- 36.McConnell S K. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Mesner P W, Winters T R, Green S H. Nerve growth factor withdrawal-induced cell death in neuronal PC12 cells resembles that in sympathetic neurons. J Cell Biol. 1992;119:1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moberg K, Starz M A, Lees J A. E2F-4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol Cell Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller R. Transcriptional regulation during the mammalian cell cycle. Trends Genet. 1995;11:173–178. doi: 10.1016/S0168-9525(00)89039-3. [DOI] [PubMed] [Google Scholar]
- 40.Nevins J R. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 1998;9:585–593. [PubMed] [Google Scholar]
- 41.Okano H J, Pfaff D W, Gibbis R B. RB and Cdc2 expression in brain: correlations with 3H-thymidine incorporation and neurogenesis. J Neurosci. 1993;13:2930–2938. doi: 10.1523/JNEUROSCI.13-07-02930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puri P L, Balsano C, Burgio V L, Chirillo P, Natoli G, Ricci L, Mattei E, Graessmann A, Levrero M. MyoD prevents cyclinA/cdk2 containing E2F complexes formation in terminally differentiated myocytes. Oncogene. 1997;14:1171–1184. doi: 10.1038/sj.onc.1200941. [DOI] [PubMed] [Google Scholar]
- 43.Puri P L, Cimino L, Fulco M, Zimmerman C, La Thangue N B, Giordano A, Graessmann A, Levrero M. Regulation of E2F4 mitogenic activity during terminal differentiation by its heterodimerization partners for nuclear translocation. Cancer Res. 1998;58:1325–1331. [PubMed] [Google Scholar]
- 44.Raschella G, Tanno B, Bonetto F, Amendola R, Battista T, De Luca A, Giordano A, Paggi M G. Retinoblastoma-related protein pRb2/p130 and its binding to the B-myb promoter increase during human neuroblastoma differentiation. J Cell Biochem. 1997;67:297–303. [PubMed] [Google Scholar]
- 45.Ross M E. Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 1996;19:62–68. doi: 10.1016/0166-2236(96)89622-6. [DOI] [PubMed] [Google Scholar]
- 46.Sardet C, Vidal M, Cobrinik D, Geng Y, Onufryk C, Chen A, Weinberg R A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci USA. 1995;92:2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sellers W R, Novitch B G, Miyake S, Heith A, Otterson G A, Kaye F J, Lassar A B, Kaelin W G., Jr Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith E J, Leone G, DeGregori J, Jakoi L, Nevins J R. The accumulation of an E2F-p130 transcriptional repressor distinguishes a G0 cell state from a G1 cell state. Mol Cell Biol. 1996;16:6965–6976. doi: 10.1128/mcb.16.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strom A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes Dev. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tevosian S G, Paulson K E, Bronson R, Yee A S. Expression of the E2F-1/DP-1 transcription factor in murine development. Cell Growth Differ. 1996;7:43–52. [PubMed] [Google Scholar]
- 51.Trimarchi J M, Fairchild B, Verona R, Moberg K, Andon N, Lees J A. E2F-6, a member of the E2F family that can behave as a transcriptional repressor. Proc Natl Acad Sci USA. 1998;95:2850–2855. doi: 10.1073/pnas.95.6.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tso J Y, Sun X H, Kao T H, Reece K S, Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vairo G, Livingston D M, Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995;9:869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- 54.Verona R, Moberg K, Estes S, Starz M, Vernon J P, Lees J A. E2F activity is regulated by cell cycle-dependent changes in subcellular localization. Mol Cell Biol. 1997;17:7268–7282. doi: 10.1128/mcb.17.12.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 56.Williams C D, Linch D C, Sorensen T S, La Thangue N B, Thomas N S B. The predominant E2F complex in human primary haemopoietic cells and in AML blasts contains E2F-4, DP-1 and p130. Br J Haematol. 1997;96:686–696. doi: 10.1046/j.1365-2141.1997.d01-2086.x. [DOI] [PubMed] [Google Scholar]
- 57.Williams C D, Linch D C, Watts M J, Thomas N S. Characterization of cell cycle status and E2F complexes in mobilized CD34+ cells before and after cytokine stimulation. Blood. 1997;90:194–203. [PubMed] [Google Scholar]
- 58.Yan G Z, Ziff E B. Nerve growth factor induces transcription of the p21 WAF1/CIP1 and cyclin D1 genes in PC12 cells by activating the Sp1 transcription factor. J Neurosci. 1997;17:6122–6132. doi: 10.1523/JNEUROSCI.17-16-06122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan G-Z, Ziff E B. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]