Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP, Adcyap1) activation of PAC1 receptors (Adcyap1r1) can significantly increase the excitability of diverse neurons through differential mechanisms. For guinea pig cardiac neurons, the modulation of excitability can be mediated in part by PAC1 receptor plasma membrane G protein-dependent activation of adenylyl cyclase and downstream signaling cascades. By contrast, PAC1 receptor-mediated excitability of hippocampal dentate gyrus granule cells appears independent of membrane delimited AC/cAMP/PKA and PLC/PKC signaling. For both neuronal types, there is mechanistic convergence demonstrating that endosomal PAC1 receptor signaling has prominent roles. In these models, neuronal exposure to Pitstop2 to inhibit β-arrestin/clathrin-mediated PAC1 receptor internalization eliminates PACAP modulation of excitability. β-arrestin is a scaffold for a number of effectors especially MEK/ERK and notably, paradigms that inhibit PAC1 receptor endosome formation and ERK signaling also blunt the PACAP-induced increase in excitability. Detailed PAC1 receptor internalization and endosomal ERK signaling mechanisms have been confirmed in HEK PAC1R-EGFP cells and shown to be long lasting which appear to recapitulate the sustained electrophysiological responses. Thus, PAC1 receptor internalization/endosomal recruitment efficiently and efficaciously activates MEK/ERK signaling and appears to represent a singular and critical common denominator in regulating neuronal excitability by PACAP.
Keywords: Parasympathetic cardiac neuron, hippocampal dentate gyrus cell, PACAP, PAC1 receptor internalization/endosomal signalling, MAPK modulation of sodium channels
INTRODUCTION
Pituitary adenylate cyclase activating polypeptides (PACAP, Adcyap1) are evolutionarily well-preserved trophic and intercellular signaling molecules that are widely distributed within neural and endocrine tissues (Arimura, 1998; Hannibal, 2002; Jaworski and Proctor, 2000; Vaudrey et al., 2009). PACAP peptides are members of the vasoactive intestinal peptide (VIP)-secretin-glucagon family of bioactive peptides and were first isolated from ovine hypothalami based on their abilities to stimulate adenylyl cyclase (AC) activity in anterior pituitary cells (Kimura et al., 1990; Miyata et al., 1989). Alternative posttranslational processing of the precursor molecule gives rise to two α-amidated forms of the peptide, PACAP38 and PACAP27. PACAP38 has 38 amino acid residues [rat pro-PACAP(131-168)], while PACAP27 corresponds to the amino terminal segment of PACAP38 [proPACAP(131-157)]. The levels of PACAP38 predominate in most tissues, although PACAP38 : PACAP27 ratio appears tissue-specific (Arimura et al., 1991). PACAP27 exhibits 68% amino acid identity with the 28-amino acid peptide VIP (Kimura et al., 1990; Miyata et al., 1989).
The actions of PACAP are mediated through several heptahelical G-protein-coupled receptor (GPCR) subtypes including the PACAP-selective PAC1 receptor (Adcyap1r1) and PACAP/VIP VPAC receptors (Vipr1 and Vipr2; also VPAC1 and VPAC2, respectively) (Harmar and Lutz 1994; Arimura, 1998; Braas and May, 1999; Vaudry et al., 2009). There are multiple PAC1 receptor isoforms from alternative splicing depending on the absence or presence of Hip and/or Hop cassette inserts into the third cytoplasmic loop that may serve to fine-tune the second messenger responses. The PAC1Null (neither Hip nor Hop insert) and PAC1Hop receptor variants predominate in the CNS and PNS (Spengler et al., 1993; Braas and May, 1999). There is duality in canonical membrane delimited PAC1 receptor signaling through the recruitment of Gαs and Gαq/11 leading to adenylyl cyclase (AC) and phospholipase C (PLC) activation, respectively (Deutsch and Sun, 1992; Spengler et al., 1993; Pisegna and Wank, 1996; Braas and May, 1999). From these pathways, other intracellular signaling cascades, such as mitogen-activated protein kinase (MAPK) and Akt (also protein kinase B) (Pisegna and Wank, 1996; Barrie et al., 1997; Bouschet et al., 2003; May et al., 2010), may be engaged to diversify cellular responses. In addition to these well studied plasma membrane initiated signaling mechanisms, the PAC1 receptors, as in other GPCR systems, can internalize and initiate long-term ERK activation via endosomal signaling (May et al., 2010; May et al., 2014). These endosomal mechanisms are still not well understood but can be functionally critical in potentially delivering second messengers to intracellular sites with high spatial and temporal resolution. The ability for neurons to deliver the right signals to the right place and at the right time provides the mechanistic means to initiate and modulate cellular responses with high precision (Calebiro et al., 2010; Scita and Di Fiore, 2010; McMahon and Boucrot, 2011; Irannejad et al., 2013).
Among its many physiological effects, PACAP can play key roles in sensory and autonomic functioning, learning and memory, and responses to injury and stress through actions on peripheral and central nervous systems neurons (Braas et al., 1998; Cho et al., 2012; Hammack et al., 2009; Hammack and May, 2014; Hill et al., 2011; Legradi et al., 2007; Stroth et al., 2012; Tompkins et al., 2007; Vaudry et al., 2009). As peripheral and central neuronal models, we have investigated and compared, using guinea pig intracardiac parasympathetic postganglionic neurons and rat hippocampal dentate gyrus granule cells, the importance of PACAP-induced PAC1 receptor internalization and endosomal MEK/ERK signaling in modulating neuronal excitability. Both neuron types express PAC1 receptors and are innervated by PACAP containing fibers.
PACAP increases cardiac neuron excitability through multiple signaling pathways
PACAP is present in parasympathetic cholinergic preganglionic nerve terminals innervating guinea pig cardiac ganglia neurons (Braas et al.,1998; Calupca et al., 2000). The effects of PACAP on cardiac neurons were studied using intracellular recordings from neurons in whole mount preparations of the guinea pig intracardiac ganglia (Braas et al., 1998; Merriam et al., 2013); both neurally-released and exogenous PACAP application depolarized and increased excitability of the cardiac neurons via selective PAC1 receptor activation (Braas et al.. 1998; Hoover et al., 2009; Tompkins et al., 2007). The primary focus of our past studies involved elucidating mechanisms contributing to the peptide-induced increase in excitability, which was evident from the shift in excitability curves, as determined by plotting the number of action potentials generated by 1 second depolarizing current steps of increasing magnitude before and in the presence of PACAP (Braas et al., 1998; Merriam et al., 2013; May and Parsons, 2016; Parsons and May, 2019). In intracellular recordings of control unstimulated whole mount preparations, the majority of the cardiac neurons exhibited a phasic or accommodating firing pattern in response to long suprathesthold depolarizing current steps; approximately 10% of cardiac neurons exhibited tonic firing patterns. The application of PACAP to these preparations markedly enhanced excitability in all cardiac neurons regardless of control response phenotype.
Modulation of multiple ionic conductances contributed to the PACAP-induced increase in cardiac neurons excitability (Parsons et al., 2016; May and Parsons, 2016; Parsons and May, 2019; Tompkins et al., 2006; Tompkins et al., 2016), which appeared to reflect concerted plasma membrane delimited and endosomal signaling mechanisms. While activated PAC1 receptor recruitment of Gαq/11 for PLC-mediated signaling did not appear to play any role in PACAP-induced increase in cardiac neuron excitability (Parsons et al., 2008; Parsons et al., 2016), plasma membrane PAC1 receptor Gαs/adenylyl cyclase activation and cAMP-induced enhancement of the hyperpolarization-induced nonselective cationic current Ih contributed to the PACAP-stimulated increase in excitability in some cardiac neurons. The PACAP responses were suppressed following treatments with cesium to block Ih (Merriam et al., 2004; Tompkins et al., 2009); further, the PACAP effect was reduced by nickel treatment, indicating that PACAP activation of the low voltage-activated calcium current It also participated in the PACAP-induced increase in excitability (Tompkins et al., 2015). In coherence, protein kinase A (PKA) phosphorylation of T-type channel α subunits has been shown to enhance IT (Talavera and Nilius, 2006; Chemin et al., 2007; Iftinca and Zamponi, 2008; Simms and Zamboni, 2014). Hence in sum, cardiac neuron membrane PACAP/PAC1 receptor activation of the canonical downstream AC/cAMP/PKA pathway has abilities to enhance dual Ih and IT, contributing synergistically to PACAP regulation of cardiac neuron excitability.
Importantly however, in more detailed surveys to probe underlying mechanisms using pathway inhibitors, cardiac neuron pretreatments with the MEK inhibitor PD98059 also significantly suppressed PACAP enhancement of excitability. Cardiac neuron PAC1 receptor activation of MEK/ERK signaling can be generated through both cAMP/PKA and endosomal signaling (Clason et al., 2016; see schematic) and the ERK-induced enhancement of an inward current appeared to be generated through the voltage-dependent sodium channel Nav1.7 (Tompkins et al., 2016). To delineate which mechanistic route to ERK activation was more prominent, the conditions that blunt clathrin-mediated receptor endocytosis, including treatment with the clathrin inhibitor Pitstop2 or the dynamin inhibitor dynasore, as well as decreasing ambient temperature, strikingly eliminated the PACAP effect on cardiac neuron excitability (Merriam et al., 2013; May et al., 2014;Tompkins et al., 2018; Parsons and May, 2019). From these observations, PAC1 receptor internalization and recruitment of endosomal signaling appeared to be a critical mechanism underlying the PACAP effect on cardiac neuron excitability. Although plasma membrane AC/cAMP production and endosomal ERK signaling may act synergistically to modulate the currents necessary to enhance excitability, recent studies have shown that a significant fraction of total cellular AC activity and cAMP levels can be produced by neuronal endosomes following receptor endocytosis (Calebiro et al., 2009; Ferrandon et al., 2009; Calebiro et al., 2010, Irannejad et al., 2013; Vilardaga et al., 2014; Di Fiore and von Zastrow, 2016). While the concept of a unifying mechanism remains to be tested, these results may implicate endosomal signaling platforms as the primary means of sustained cAMP and activated ERK generation underlying PAC1 receptor-mediated neuronal excitability.
PACAP increases granule cell excitability through endosomal ERK activation.
High levels of PAC1 receptor transcripts in the granule cell layer of the dentate gyrus in the hippocampus were initially determined by in situ hybridization studies (Jaworski and Proctor, 2000; Hannibal, 2002) and hippocampal PACAP hilar mossy neurons were shown to send fiber projections to the DG inner molecular layer (IML) (Condro et al., 2016; Johnson et al., 2020b); hence, there are short PACAPergic synaptic circuits between PACAP-expressing hilar mossy cells and the proximal dendrites on PAC1 receptor-expressing DG granule cells. Accordingly, PACAP application to hippocampal dentate gyrus slice preparations increased the excitability of granule cells (Johnson et al., 2020a; Johnson et al., 2020b). In whole-cell recordings in current clamp mode, the granule cells fired tonically in response to suprathreshold 1 second depolarizing current injections; the action potential frequency increased with increasing current intensity (Johnson et al., 2020a; Johnson et al., 2020b). PACAP enhanced the excitability of DG granule cells assessed by determining the shift in excitability curves determined by plotting action potential frequency before and in PACAP with increasing current steps. The increase in excitability was associated with a negative shift in the threshold for action potential generation and appeared to be independent of changes in input resistance and changes in resting membrane potential (Johnson et al., 2020a). Some hippocampal interneurons express VIP, but as VIP did not mimic the actions of PACAP, PACAP increased DG granule cells excitability through PAC1 receptor signaling.
In contrast to cardiac neurons, neither AC/cAMP/PKA nor PLC/DAG/IP3 signaling appeared to be required for the PACAP modulation of DG cell excitability. But to a greater extent than cardiac neurons, treatments with the MEK inhibitor PD98059, virtually eliminated PACAP-enhanced induced excitability of DG granule cells (Johnson et al., 2020a). The effect of PACAP on DG granule cells was also blunted markedly by treatment with the cell-permeable clathrin-mediated endocytosis inhibitor Pitstop2; hence the effects of PD98059 or Pitstop2 to block the PACAP-mediated responses appeared comparable. Thus for hippocampal dentate granule cells, PAC1 receptor internalization and endosomal recruitment of MEK/ERK signaling appeared to represent the primary if not the sole second messenger mechanism contributing to the PACAP modulation of neuronal excitability in DG granule cells (Johnson et al., 2020a; see schematic). The PACAP effect remained in the presence of a number of well-established blockers of voltage-dependent potassium and calcium currents, suggesting modulations by these conductances were not essential (Johnson et al., 2020a). However, PACAP failed to increase excitability in DG cells pretreated with the persistent sodium channel blocker riluzole, indicating that the PACAP effect required this component of the inward sodium current (Johnson et al., 2020a).
PACAP induces PAC1 receptor internalization in HEK cell cultures
Whole mount and ex vivo tissue preparations are not always amenable to detailed mechanistic and visualization studies, and accordingly, the electrophysiological studies were coupled with PACAP/PAC1 receptor internalization and activated MEK/ERK assessments using HEK cells stably expressing a human PAC1 receptor C-terminally tethered to EGFP (HEK PAC1R-EGFP receptor cells) (Merriam et al., 2013; May et al., 2014). From Western blots, PACAP-mediated ERK activation was BimI-sensitive, which unlike the neurons above, implicated roles for PLC/PKC signaling in HEK cells. But notably, Pistop2 and dynasore blocked PACAP/PAC1 receptor internalization/endosomal signaling that contributed to pERK generation (May et al., 2014). These biochemical measures were correlated with PACAP induced PAC1 internalization determined using confocal microscopy (Merriam et al., 2013; May et al., 2014). Under control conditions at 37°C, the receptor remained primarily in the cell membrane, whereas within a few minutes of exposure to nanomolar PACAP concentrations, there was extensive PAC1R-EGFP endocytosis into intracellular vesicles with a concomitant reduction in cell surface fluorescence. In contrast, PACAP exposures of similar duration at 22 - 24°C resulted in scant receptor translocation into intracellular vesicles and no loss of membrane fluorescence, indicating that receptor internalization was suppressed at room temperature. Similar to the reduction in activated PAC1 receptor trafficking at ambient temperature, pretreatment of the cells at 37 C with Pitstop2, a clathrin inhibitor (von Kleist et al., 2011), or dynasore, a dynamin I/II inhibitor (Maca et al., 2006) also blunted markedly PACAP-stimulated PAC1R-EGFP internalization resulting in receptor retention on the cell surface.
From these observations, the HEK PAC1R-EGFP internalization studies aligned with the neuronal results and allowed more detailed analyses of the endosome signaling process. In treating the HEK PAC1R-EGFP with PACAP and signaling inhibitors, the characteristics of PAC1 receptor internalization and endosomal ERK signaling were determined. A brief 5 minute pulse exposure to 25 nM PACAP alone produced a sustained elevation of pERK that peaked at 10 −15 minutes and returned to baseline levels in about 60 minutes (Figure 1), demonstrating a long lasting generation of pERK by PACAP. Additional experiments tested whether either membrane delimited PLC/PKC or internalization/endosomal signaling was most prominent. Across treatment times and PACAP concentrations, Pitstop 2 blocked PACAP-stimulated ERK activation more than 70% whereas BimI attenuated activation approximately 40 – 60% (Figure 2). By these measures, the endosomal signaling pathway appeared more prevalent. Even with low PACAP concentrations (1 nM), a 15 minute exposure significantly increased pERK levels that was sensitive to Pitstop 2 and not by BimI (Figure 2, right panels), further implicating receptor internalization and endosomal signaling as predominant mechanisms for PAC1 receptor long-term MEK/ERK signaling.
Figure 1.
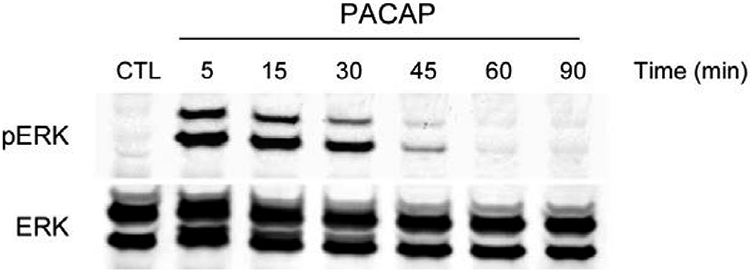
Brief PAC1 receptor activation results in long term ERK activation duration. Stable HEK PAC1 receptor-EGFP cell lines were acutely pulse treated with 25 nM PACAP27 for 5 min followed by washout, incubation in control medium without peptide and harvest at the times shown (5 – 90 min). Even after brief PACAP exposure, the duration of ERK activation (pERK) was extended markedly over 30 min and attenuated to baseline levels at 60 min. The same Western blot was reprobed with a pan ERK antibody to illustrate equal sample loading.
Figure 2.
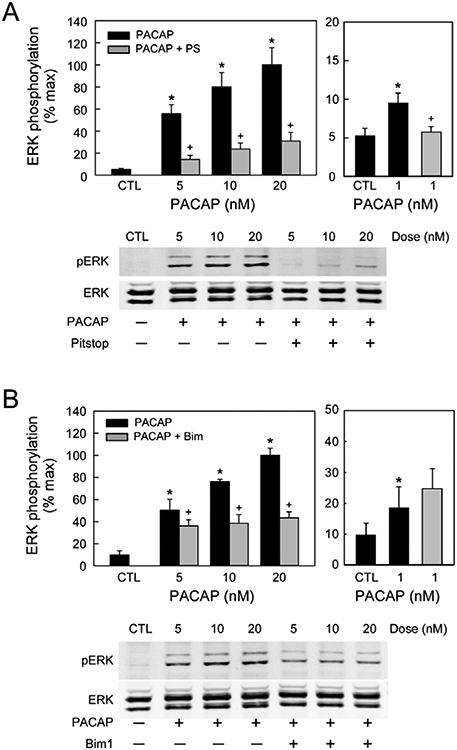
Pitstop2 and BimI blocked PACAP-mediated ERK activation at varying PACAP concentrations. HEK PAC1 receptor-EGFP cells were pretreated with 20 μM Pitstop 2 (A) or 15 μM BimI (B) for 15 min before the addition of PACAP (5 - 20 nM) for 15 min. Pitstop and Bim I inhibited ERK activation at all PACAP peptide concentrations tested, suggesting that receptor internalization/endosomal signaling occurred even at the low peptide concentrations. Even at 1 nM PACAP, ERK activation was more sensitive to receptor internalization/endosome-mediated mechanisms than PKC signaling. Representative Western blots for each treatment shown in replicate. *, significantly different from untreated control (CTL); +, different from sample at same PACAP concentration. Data represent mean ± SEM, n = 3.
Conclusion
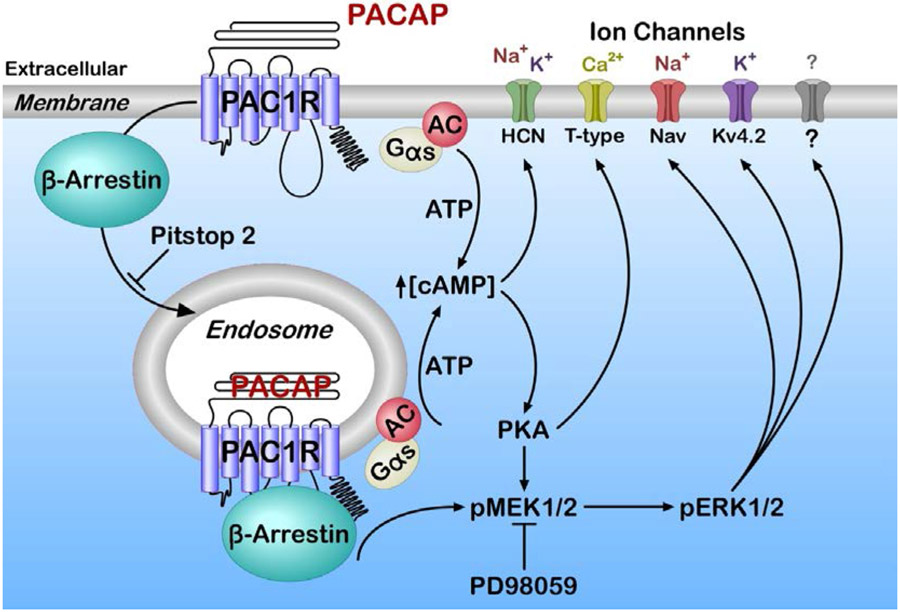
In addition to membrane delimited G protein-dependent signaling, GPCRs undergo a sequence of steps leading to receptor internalization and endosomal signaling (Calebiro et al., 2009; Ferrandon et al., 2009; Calebiro et al., 2010, Irannejad et al., 2013; Vilardaga et al., 2014). GPCR endocytosis had initially been associated with receptor desensitization and recycling pathways, but is now recognized to represent a mechanism supporting sustained second messenger generation via signaling endosomes (Calebiro et al., 2009; Ferrandon et al., 2009; Calebiro et al., 2010, Irannejad et al., 2013; Vilardaga et al., 2014). A significant component of pERK generation by PACAP is dependent on PAC1 receptor internalization and endosomal signaling (Figure 3, schematic). Further, the MEK/ERK signaling cascade has critical roles in synaptic plasticity and regulation of neuronal excitability (Sweatt, 2004). We propose that PAC1 receptor internalization/endosomal recruitment of MEK/ERK signaling is a critical mechanism supporting the PACAP modulation of neuronal excitability.
Figure 3.
Schematic of PAC1 receptor plasma membrane and endosomal signaling. PACAP/PAC1 receptor activation can generate plasma membrane delimited and endosomal signals following β-arrestin-mediated receptor internalization. PACAP/PAC1 receptor-stimulated AC/cAMP production is generated largely from the plasma membrane although a smaller fraction can also be derived from endosomes; both can contribute to the gating of hyperpolarization-activated cyclic nucleotide (HCN) channel and Ih. Cyclic AMP pathways can lead to ERK signaling, although sustained ERK activation may result from effectors such as MEK, docked to endosomal receptor/β-arrestin signaling platforms to regulate T-type, sodium, potassium and/or yet uncharacterized channels. Pitstop 2 blocks receptor/β-arrestin internalization and endosomal signaling. MEK inhibitor PD98059 blocks ERK signaling and channel activation.
Acknowledgements:
This work was supported in part by National Institutes of Health (NIH) grant National Institute of General Medical Sciences (NIGMS) P30 GM103498 / National Center for Research Resources (NCRR) P30 RR032135 (RLP) and National Institute of Mental Health (NIMH) MH097988 (SEH and VM).
We thank Thomas Buttolph for excellent technical assistance.
Footnotes
Competing interests: The authors declare no competing interests.
Availability of data and materials: All original supporting data in the text are within the manuscript.
The authors declare that they have no conflict of interest.
References
- Arimura A (1998) Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jap J Physiol. 48:301–331. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvári-Vigh A, Miyata A, Mizuno K, Coy DH, Kitada C. (1991) Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129:2787–2789. [DOI] [PubMed] [Google Scholar]
- Barrie AP, Clohessy AM, Buensuceso CS, Rogers MV, Allen JM (1997) Pituitary adenylyl cyclase-activating peptide stimulates extracellular signal-regulated kinase 1 or 2 (ERK1/2) activity in a Ras-independent, mitogen-activated protein Kinase/ERK kinase 1 or 2-dependent manner in PC12 cells. J Biol Chem. 272: 19666–19671. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Perez V, Fernandez C, Bockaert J, Eychene A, Journot L (2003) Stimulation of the ERK pathway by GTP-loaded Rap1 requires the concomitant activation of Ras, protein kinase C, and protein kinase A in neuronal cells. J Biol Chem. 278: 4778–4785. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V (1999) Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC1 receptor isoform activation of specific intracellular signaling pathways. J Biol Chem. 274: 27702–27710. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL (1998) Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci. 18: 9766–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, Persani L, Lohse M J (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 7: e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ (2010) Signaling by internalized G-protein-coupled receptors. Trends Pharmacol Sci 31: 221–228. [DOI] [PubMed] [Google Scholar]
- Calupca MA, Vizzard MA, Parsons RL (2000) Origin of pituitary adenylate cyclase-activating polypeptide (PACAP)-immunoreactive fibers innervating guinea pig parasympathetic cardiac ganglia. J Comp Neurol. 423: 26–39. [PubMed] [Google Scholar]
- Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, Barrère C, Nargeot J, Lory P (2007). Temperature-dependent modulation of CaV3 T-type calcium channels by protein kinases C and A in mammalian cells. J Biol Chem. 282: 32710–32718. [DOI] [PubMed] [Google Scholar]
- Cho J-H, Zushida K, Shumyatsky GP, Carlezon WA, Meloni EG, Bolshakov VY (2012). Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci 32: 14165–141177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clason TA, Girard BM, May V, Parsons RL (2016). Activation of MEK/ERK signaling by PACAP in guinea pig cardiac neurons. J Mol Neurosci 59: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Van C, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek JA. (2016) High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol. 524:3827–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch PJ, Sun Y (1992) The 38-amino acid form of pituitary adenylate cyclase-activating polypeptide stimulates dual signaling cascades in PC12 cells and promotes neurite outgrowth. J Biol Chem. 267: 5108–5113. [PubMed] [Google Scholar]
- Di Fiore PP, von Zastrow M (2016). Endocytosis, signaling and beyond. Cold Spring Harb Perspect Biol 6: a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP (2009). Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 5: 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Chung J, Rhoades KM, Schutz KC, Falls WA, Braas KM, May V (2009) Chronic stress increases pituitary adenylate cyclase-activating polypeptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST); roles for PACAP in anxiety-like behavior. Psychoneuroendrocrinology 34: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, May V (2014) Pituitary adenylate cyclase activating polypeptide in stress-related disorders: Data convergence from animal and human studies. Biological Psychiatry 78(3):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. (2002) Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 453:389–417. [DOI] [PubMed] [Google Scholar]
- Harmar T, Lutz E (1994) Multiple receptors for PACAP and VIP. Trends Pharmacol. Sci 15:97–99. [DOI] [PubMed] [Google Scholar]
- Hill J, Chan S-A, Kuri B, Smith C (2011) Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286: 42459–42469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover DB, Tompkins JD, Parsons RL (2009) Differential activation of guinea pig intrinsic cardiac neurons by the PAC1 agonists maxadilan and pituitary adenylate cyclase-activating polypeptide 27 (PACAP27). J Pharmacol Exp Ther 331: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftinca MC, Zamponi GW (2008) Regulation of neuronal T-type calcium channels. Trends in Pharmacol Sci. 30:32–40. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, von Zastrow M (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski DM, Proctor MD. (2000) Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res. 120:27–39, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson GC, Parsons RL, May V, and Hammack SE (2020a). PACAP-induced PAC1 receptor internalization and recruitment of MEK/ERK signaling enhances excitability of dentate gyrus granule cells. Am J Physiol. 318: C870–C878, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GC, Parsons RL, May V, and Hammack SE (2020b). The role of pituity adenylate cyclase-activating polypeptide (PACAP) signaling in the hippocampal dendate gyrus Frontiers in Cellular Neuroscience doi: 10.3389/fncel.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura C, Ohkubo S, Ogi K, Hosoya M, Itoh Y, Onda H, Miyata A, Jiang L, Dahl RR, Stibbs HH, Arimura A, Fujino M. (1990) A novel peptide which stimulates adenylate cyclase: Molecular cloning and characterization of bovine and human cDNAs. Biochem Biophys Res Commun. 166: 81–89. [DOI] [PubMed] [Google Scholar]
- Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM (2007). Microinjection of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdale of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast. 2007: 79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maca E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10: 839–850. [DOI] [PubMed] [Google Scholar]
- May V, Buttolph TR, Girard BM, Clason TA, Parsons RL (2014) PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol. 306: C1068–C1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Lutz E, MacKenzie C, Schutz KC, Dozark K, Braas KM (2010) Pituitary adenylate cyclase-activating polypeptide (PACAP)/PACAP1HOP1receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J Biol Chem 285: 9749–9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, Parsons RL (2016) G Protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: Signaling options and lessons from the PAC1 receptor. J Cell Physiol 232:698–706. [DOI] [PubMed] [Google Scholar]
- McMahon HT and Boucrot E (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 12: 517–533. [DOI] [PubMed] [Google Scholar]
- Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL (2013) Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci. 33: 4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam LA, Barstow KL, Parsons RL (2004) Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regul Pept 123: 123–133. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574, 1989. [DOI] [PubMed] [Google Scholar]
- Parsons RL and May V. (2019) PACAP-induced PAC1 receptor internalization and recruitment of endosomal signaling regulate cardiac neuron excitability. J Mol Neurosci. 68:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RL, Tompkins JD, Hardwick JC, Merriam LA, Girard BM, May V (2016) Multiple mechanisms contribute to the PAC1 modulation of parasympathetic cardiac neuron excitability. In: Pituitary Adenylate Cyclase Activating Polypeptide – PACAP. (Reglodi D, Tamas A, ed) New York, Springer Nature, 205–225. [Google Scholar]
- Parsons RL, Tompkins JD, Merriam LA (2008). Source and action of pituitary adenylate cyclase-activating polypeptide in guinea pig intrinsic cardiac ganglia. Tzu Chi Med J 20: 11–18 [Google Scholar]
- Pisegna JR, Wank SA (1996) Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem 271: 17267–17274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463: 464–473. [DOI] [PubMed] [Google Scholar]
- Simms BA and Zamponi GW (2014) Neuronal voltage-gated calcium channels: Structure, function and dysfunction. Neuron 82: 24–45. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170–175. [DOI] [PubMed] [Google Scholar]
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE (2012) PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiolo 14:311–317. [DOI] [PubMed] [Google Scholar]
- Talavera K, Nilius B (2006) Biophysics and structure-function relationships of T-type Ca2+ channels. Cell Calcium 40: 97–114. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Ardell JL, Hoover DB, Parsons RL (2007) Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Clason TA, Buttolph TR, Girard BM, Linden AK, Hardwick JC, Merriam LA, May V, Parsons RL (2018) Src family inhibitors blunt the PACAP-induced PAC1 receptor endocytosis, phosphorylation of ERK and increase in cardiac neuron excitability. Am J Physiol Cell Physiol. 314: C233–C241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL (2016) Activation of MEK/ERK signalling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 311: C643–C651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Hardwick JC, Locknar SA, Merriam LA, Parsons RL (2006) Ca2+ influx, but not Ca2+ release from internal stores, is required for the PACAP-induced increase in excitability in guinea pig intracardiac neurons. J Neurophysiol 95: 2134–2142. [DOI] [PubMed] [Google Scholar]
- Tompkins JD, Lawrence YT, Parsons RL (2009) Enhancement of Ih, but not inhibition of IM, is a key mechanism underlying the PACAP-induced increase in excitability of guinea pig intrinsic cardiac neurons. Am J Physiol Regul Integr Comp Physiol 297: R52–R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL (2015) Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol. 308: C857–C866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357. [DOI] [PubMed] [Google Scholar]
- Vilardaga J-P, Jean-Alphonse FG, Gardella T (2014) Endosomal generation of cAMP in GPCR signalling. Nat Chem Biol. 10: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146: 471–484. [DOI] [PubMed] [Google Scholar]