Abstract
Background
Infant anaesthesia causes acute brain cell apoptosis, and later in life cognitive deficits and behavioural alterations, in non-human primates (NHPs). Various brain injuries and neurodegenerative conditions are characterised by chronic astrocyte activation (astrogliosis). Glial fibrillary acidic protein (GFAP), an astrocyte-specific protein, increases during astrogliosis and remains elevated after an injury. Whether infant anaesthesia is associated with a sustained increase in GFAP is unknown. We hypothesised that GFAP is increased in specific brain areas of NHPs 2 yr after infant anaesthesia, consistent with prior injury.
Methods
Eight 6-day-old NHPs per group were exposed to 5 h isoflurane once (1×) or three times (3×), or to room air as a control (Ctr). Two years after exposure, their brains were assessed for GFAP density changes in the primary visual cortex (V1), perirhinal cortex (PRC), hippocampal subiculum, amygdala, and orbitofrontal cortex (OFC). We also assessed concomitant microglia activation and hippocampal neurogenesis.
Results
Compared with controls, GFAP densities in V1 were increased in exposed groups (Ctr: 0.208 [0.085–0.427], 1×: 0.313 [0.108–0.533], 3×: 0.389 [0.262–0.652]), whereas the density of activated microglia was unchanged. In addition, GFAP densities were increased in the 3× group in the PRC and the subiculum, and in both exposure groups in the amygdala, but there was no increase in the OFC. There were no differences in hippocampal neurogenesis among groups.
Conclusions
Two years after infant anaesthesia, NHPs show increased GFAP without concomitant microglia activation in specific brain areas. These long-lasting structural changes in the brain caused by infant anaesthesia exposure may be associated with functional alterations at this age.
Keywords: anaesthesia, apoptosis, astrogliosis, glial fibrillary protein, isoflurane, non-human primate
Editor's key points.
-
•
Infant anaesthesia causes acute brain cell apoptosis, cognitive deficits, and behavioural alterations later in life in non-human primates.
-
•
We hypothesised that infant anaesthesia causes chronic astrocyte activation (astrogliosis), consistent with prior injury, in specific brain areas 2 yr after exposure.
-
•
Two years after infant anaesthesia, non-human primates show increased astrogliosis without concomitant microglia activation in specific brain areas.
-
•
These novel long-term structural changes occur in brain regions that showed acute increased apoptosis after exposure and that are associated with functional alterations later in life
Anaesthesia exposure of young children has been associated with impaired neurobehavioral development.1,2 Most clinical studies have limitations that contribute to their ambiguous outcomes. According to a recent meta-analysis,3 three well-controlled studies in the field (Pediatric Anesthesia Neurodevelopment Assessment [PANDA], Mayo Anesthesia Safety in Kids [MASK], and General Anesthesia or Awake-regional Anesthesia in Infancy [GAS])4, 5, 6 share the finding that there is no measurable effect on primary outcome of intelligence quotient (IQ) after relatively short exposures to anaesthesia (1–2 h) during infancy. However, secondary outcomes of these studies suggest an association between anaesthesia exposure and alterations in executive function or behaviour in school-age children as reported in questionnaires by parents and caregivers. In contrast, a large body of animal studies provides robust evidence that anaesthesia exposure during infancy causes functional alterations in the developing brain. Initial findings in neonatal rats found that anaesthesia exposure induced long-term impairments in spatial learning and memory.7 Importantly, studies in infant non-human primates (NHPs) exposed to anaesthesia using similar physiological monitoring as for children, reported cognitive deficits8, 9, 10 and behavioural alterations11, 12, 13, 14 later in life.
Although the causes of the functional alterations are unknown, there is strong evidence that anaesthesia exposure of infant animals including NHPs causes an acute injury in the immature brain, initially evidenced by a widespread increase of neuronal apoptosis.7,15, 16, 17, 18, 19, 20 More recently, in vitro and in vivo studies have reported reduced neurogenesis and impaired dendritic arborisation after anaesthesia exposure.21, 22, 23, 24, 25, 26, 27 Although these neuronal changes may account for long-term functional impairments, glial cells play an essential role in neuronal function, and they are also affected by infant anaesthesia exposure. For example, in neonatal NHPs, oligodendrocytes were found to undergo acute apoptosis after anaesthesia exposure, similar to neurones.17,28, 29, 30 In addition, infant anaesthesia exposure resulted in myelination deficits in prefrontal brain areas of mice and NHPs,31 and in alterations in oligodendrocyte development and myelin formation in parts of the hippocampus that were associated with cognitive deficits in exposed mice.32
Growing evidence suggests that infant anaesthesia exposure also affects astrocyte structure and function,33, 34, 35, 36, 37 Anaesthesia-induced astrocytic dysfunction in neonatal mice is associated with behavioural alterations later in life.38 Chronic activation of astrocytes, called astrogliosis, is a common response to brain insults. Astrogliosis is found in various types of brain injuries, including hypoxic or traumatic injuries, and has also been associated with neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease, and with neurodevelopmental disorders, including autism.39, 40, 41, 42, 43, 44, 45, 46 Astrogliosis can be identified by increased levels of glial fibrillary acidic protein (GFAP),47 a cytoskeletal protein uniquely expressed in astrocytes that plays a critical role in their activation.24 In acute brain injury, GFAP expression increases and can remain elevated, representing a long-term structural change indicating a prior injury, called ‘glial scar’.48
Although acute structural changes in the developing brain caused by anaesthesia exposure such as apoptosis have been studied extensively, long-term alterations in brain structure at times when functional impairments become evident are not fully understood.49, 50, 51, 52 Furthermore, it is unknown whether such structural changes are present in brain areas involved in the functional alterations found after anaesthesia exposure of infants. We therefore investigated potential long-term structural changes in juvenile NHP brains 2 yr after exposure to isoflurane during infancy by assessing astrogliosis in selected brain areas. Initially, we examined the primary visual cortex (V1) because this region has shown robust increases in brain cell apoptosis in NHPs immediately after anaesthesia exposure in previous studies,15 and functional impairments connected to this brain area have been reported.8 Subsequently, we studied the perirhinal cortex (PRC), the subiculum in the hippocampus, the amygdala, and the orbitofrontal cortex (OFC) as these brain areas have also shown acute apoptosis.15,29 These areas represent major processing centres for visual recognition memory,53 social,54 and anxiety-related55,56 behaviours, which are impaired or altered in juvenile NHPs after infant anaesthesia exposure.8,11, 12, 13 We report that anaesthesia exposure during infancy is associated with increased astrogliosis in specific brain areas of juvenile NHPs indicated by increased GFAP expression. Our results provide novel evidence for long-term structural consequences 2 yr after infant anaesthesia exposure.
Methods
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Oregon National Primate Research Center (ONPRC) and was in compliance with all federal regulations, and the guidelines set forth in the Guide for the Care and Use of Laboratory Animals.57 The ONPRC is accredited by AAALAC International.
Conditions of animal housing and euthanasia
The housing conditions of the NHPs have been described.13,14 Briefly, 24 rhesus macaques (Macaca mulatta) with an equal number of females and males were born and housed at the ONPRC (Beaverton, OR, USA). After the first year of life, the NHPs were weaned from their dams, moved from outdoors into indoor home cages, and were housed with five to six juvenile peers. Twice a day, the animals were fed with commercial monkey chow (Lab Diet Monkey Diet, St. Louis, MO, USA) supplemented with grain or produce daily and had access to water ad libitum. There was a 12:12 h light/dark cycle, starting with lights on at 07:00. At the age of 2 yr, the NHPs were sedated with ketamine 20 mg kg−1 i.m. and transported to pathology, where basic morphometric measurements were taken. Pentobarbital 0.25 mg kg−1 i.v. was administered followed by exsanguination and in vivo transcardial perfusion–fixation with saline followed by 4% (w/v) paraformaldehyde to prepare the brain for histopathologic analysis.
Infant anaesthesia exposure
The conditions of infant anaesthesia exposure have been described.13,14 Briefly, 1 day before anaesthesia exposure, the postnatal day 5 (P5) NHP neonates were separated from the breeding group together with their dams. Each dam received a mild sedative (ketamine, i.m., 5 mg kg-1), and its neonate was transferred to the operating room. After taking baseline vital parameter measurements including heart rate, respiration, temperature, blood pressure, weight, blood gas analysis, and metabolic parameters, the neonate was carefully hand restrained and exposed to isoflurane via face mask and under spontaneous ventilation. This induction technique closely resembles the method used routinely to induce anaesthesia in young children. Subsequently, the trachea was intubated and the lungs mechanically ventilated, and a surgical plane of anaesthesia was maintained for 5 h at an end-tidal isoflurane of 0.7–1.5 vol% in 30 vol% oxygen. In our previous studies, dose–response experiments revealed that 5 h exposure to these isoflurane concentrations provided anaesthesia levels that allowed the NHPs to tolerate mild to moderate mechanical stimuli, and induced neurotoxicity in the developing NHP brain.15,28 Maintenance of this anaesthesia level was controlled by verifying the absence of movement and <10% increase in heart rate or blood pressure in response to a mechanical stimulus.
This management using full physiologic monitoring mimicked conditions in paediatric anaesthesia. Physiological parameters were maintained within narrow ranges throughout the exposure (Supplementary Table S1) using a similar approach as reported.58 After isoflurane exposure was stopped and the animal was awake, extubation was performed. Subsequently, animals were kept in an NHP incubator until fully recovered before they were transferred back to their dams. Animals were randomised to receive this procedure either one time (1×) on P6, three times on P6, P9, and P12 (3×), or to receive the same procedure on the same postnatal days but exposed to room air only. The NHPs in the 1× exposure group received room air on P9 and P12, after they had been exposed to isoflurane on P6. All three groups initially consisted of eight animals with equal sex distribution. One female NHP in the 3× group had to be removed from the study at about 1 yr of age because of therapy-resistant chronic diarrhoea.
Histopathology
Immediately after NHPs were euthanised, their brains were perfused with cold artificial cerebrospinal fluid by cannulation of the aorta. The left hemibrain was rapidly removed for electrophysiological studies and evaluation of dendritic morphology which required unfixed tissue. The right hemibrain was then perfused in situ with paraformaldehyde 4% and removed from the skull. Before further processing, the fixed hemibrains were coded to avoid bias by the investigators. The hemibrains were sliced at ~5 mm intervals, and paraffin blocks were prepared from frontal, occipital, and temporal regions, the latter including deep structures of the amygdala and the hippocampus. Several 6-μm-thick consecutive histological slices were cut from each paraffin block at three levels ~0.2 mm apart, aiming to include the brain regions of interest. Each slice was deparaffinised and stained with specific antibodies using standard immunohistochemical protocols that included antigen retrieval in citrate buffer pH 6.0. For each block, one slice was immunostained with the neuronal marker NeuN (Millipore #ABN78; Millipore, Burlington, VT, USA) to identify the brain regions of interest (i.e. V1, amygdala, hippocampus, and OFC) using the corresponding figures in a monkey brain atlas59 that illustrate the location of the brain regions. These regions were selected because previous studies found them to show robust apoptosis acutely15,29 or to be critically involved in long-term functional alterations associated with infant anaesthesia exposure.8,11, 12, 13 In addition, the subiculum was chosen as this hippocampal area is known to be highly vulnerable to brain injuries (e.g. hypoxia) early in life.60, 61, 62
The GFAP immunoreactivity was determined by incubating slices with a rabbit monoclonal anti-GFAP antibody (Abcam #ab68428; Abcam, Cambridge, UK) diluted 1:1000 in blocking solution (phosphate-buffered saline [PBS] with 1% bovine serum albumin [BSA] and 0.3% Triton, v/v) overnight at 4°C. Slices were washed in PBS and exposed to secondary antibody by incubation in Vector ImmPRESS™ (Vector Laboratories, Inc., Burlingame, CA, USA) reagent containing anti-rabbit IgG peroxidase conjugate for 30 min, and developed with diaminobenzidine (DAB). Iba-1 immunohistochemistry was performed using a protocol the primary antibody rabbit anti-Iba-1 (Wako #019–19741; Wako Chemicals, Richmond, VA, USA) diluted 1:3000 in blocking solution. For analysis of hippocampal neurogenesis 15-μm-thick sections were cut from temporal blocks and incubated with the ki67 primary antibody (Abcam #ab15580) diluted 1:2000 in blocking solution overnight at 4°C, followed by incubation in biotinylated goat anti-rabbit secondary antibody (Vector, #BA-1000; Vector Laboratories) diluted 1:200 in blocking solution, then incubated with avidin–biotin–peroxidase complex (Vectastain Elite kit; Vector Laboratories) and developed with DAB. All immunostained slices were lightly counterstained with Mayer's haematoxylin, dehydrated, cleared, and coverslipped with Permount (Fisher Scientific, Fair Lawn, NJ, USA). All histological slides were stored at room temperature for subsequent imaging analysis and quantification.
Quantification of GFAP area density and Iba-1 positive cells
Coded histological slides containing the brain regions of interest were scanned at high magnification using a 40× objective in an automatised slide scanner (Zeiss Axio Scan Z1 and ZEN software; Carl Zeiss, Oberkochen, Germany), and high-resolution images were stored in a hard drive for subsequent analysis. The ‘ZEN 2.3 lite’ software (free version) was used to create individual TIFF images containing the brain areas of interest. We measured astrogliosis and microglial activation using different units to be consistent with those used in previous studies that also reported these histopathological entities. Astrogliosis was measured as the ratio of GFAP-positive area to total area analysed, and expressed as GFAP density in percentage.63, 64, 65 This approach takes into consideration astrocyte hypertrophy and the accompanying increase in GFAP expression that characterises astrogliosis.40 Microglial activation was measured as number of positive cells divided by total area analysed and expressed as Iba-1 cell density in cells per mm.2,66,67 Quantification of GFAP positive area and Iba-1 positive cells in each brain region was performed using the ImageJ software.
To prevent bias introduced by selecting regions of interest within specific brain areas, quantification of GFAP and Iba-1 was performed on the entire grey matter of the brain region (i.e. V1, amygdala, or OFC) present on the histological slide. GFAP-positive area and Iba-1-positive cells were automatically recognised and counted using customised macros in the ImageJ that used the plugin IHC profiler (https://sourceforge.net/projects/ihcprofiler/). This plugin allows unbiased quantification of area positive for immunohistochemistry (brown areas) followed by an adjustable threshold to identify positive areas and exclude noise.68 The analysis outcome allows quantification of the number of positive counts (‘cells’) along with their area. These computer-assisted quantifications of GFAP and Iba-1 in specific brain regions were performed by an investigator blinded to animal group assignment and without access to the key to identify the coded histological slides. For quantification of hippocampal ki67-positive cells, coded slides were analysed by an experienced neuropathologist. Darkly stained (brown) nuclei were counted when located either in the dentate gyrus (DG) or within a band twice the thickness of the DG into the hilum, to ensure including all relevant cells.
Statistical analysis
All statistical analysis was performed in GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA). We tested for normality using the Wilk–Shapiro test. As GFAP area, Iba-1 cell density, and ki67 cell count data did not have normal distribution, descriptive statistical data for these variables are presented as median (25th–75th percentiles). Grubbs test was used to identify outliers, but no animals were excluded with this analysis. Comparison between medians was determined using Kruskal–Wallis test followed by Dunn's post hoc test. We used α=0.05 for all statistical tests.
Results
GFAP densities increased in V1 after isoflurane exposure
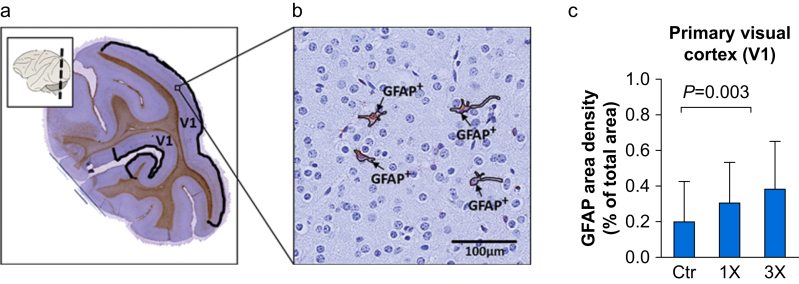
To evaluate whether isoflurane exposure during infancy results in long-term structural alterations, we assessed the density of GFAP expression in V1 in the brains of the 2-yr-old NHPs. This brain area was selected because it shows robust apoptosis acutely after infant anaesthesia exposure.15,28 Guided by the rhesus monkey brain atlas59 and strict cortical cytoarchitectural features, we identified the V1 region in our stained and scanned occipital brain slices (Fig. 1a) quantified GFAP-positive area (Fig. 1b). As shown in Fig. 1c, GFAP densities in V1 increased with exposure to isoflurane (Ctr: 0.21% [0.085–0.43], 1×: 0.31% [0.11–0.53], 3×: 0.39% [0.26–0.65], H(2)=10.24, P=0.006; Kruskal–Wallis test). Post hoc analysis with Dunn's multiple comparisons test showed that the differences among the control and exposed groups reached statistical significance for the 3× (P=0.003) but not for the 1× group (P=0.132).
Fig 1.
Glial fibrillary acidic protein (GFAP) expression in the visual cortex (V1) of juvenile non-human primates. (a) Representative image of a brain slice cut from the occipital region indicated in the inset, immunostained for GFAP (brown) and counterstained with haematoxylin (blue). The demarcated regions indicate the primary visual cortex (V1) contained in the slice. (b) High magnification image showing GFAP-positive areas (GFAP+) within V1. (c) GFAP area density (% of total area) in the visual cortex (V1) of juvenile non-human primates (NHPs) 2 yr after single (1×), three times (3×) or no exposure (Ctr) to isoflurane during infancy. Data are median (25th–75th percentiles), n (NHP)=Ctr: 8, 1×: 8, 3×: 7.
Iba-1 cell densities were unaffected after isoflurane exposure
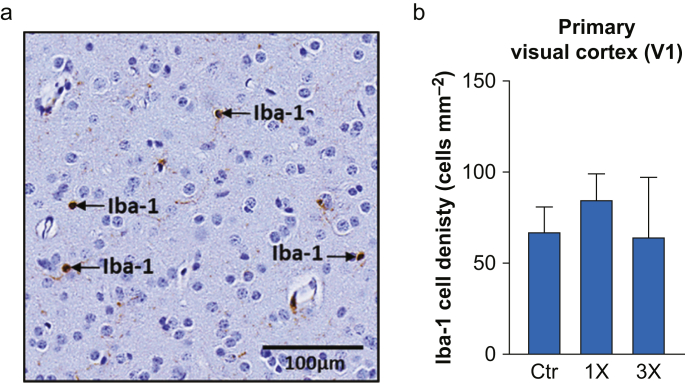
We analysed Iba-1 cell densities in V1 to test whether the increase in GFAP associated with isoflurane exposure during infancy was accompanied by activation of microglia in this region 2 yr after isoflurane exposure. Iba-1 positive cells (Fig. 2a) were divided by the V1 area to obtain Iba-1 cell density. In contrast to GFAP density, we did not find significant differences for Iba-1 cell densities in V1 among the groups (Fig. 2b; n.s., Kruskal–Wallis test).
Fig 2.
Iba-1 cell density in the visual cortex (V1) of juvenile non-human primates (NHPs). (a) Representative high magnification image showing Iba-1-positive cells (Iba-1) within V1. (b) Iba-1 cell density (cells mm−2) in the visual cortex (V1) of juvenile NHPs 2 yr after single (1×), three times (3×) or no exposure (Ctr) to isoflurane during infancy. Data are median (25th–75th percentiles), n (NHP)=Ctr: 8, 1×: 8, 3×: 7.
GFAP densities increased in the PRC, subiculum, and amygdala, but not in the OFC, after isoflurane exposure
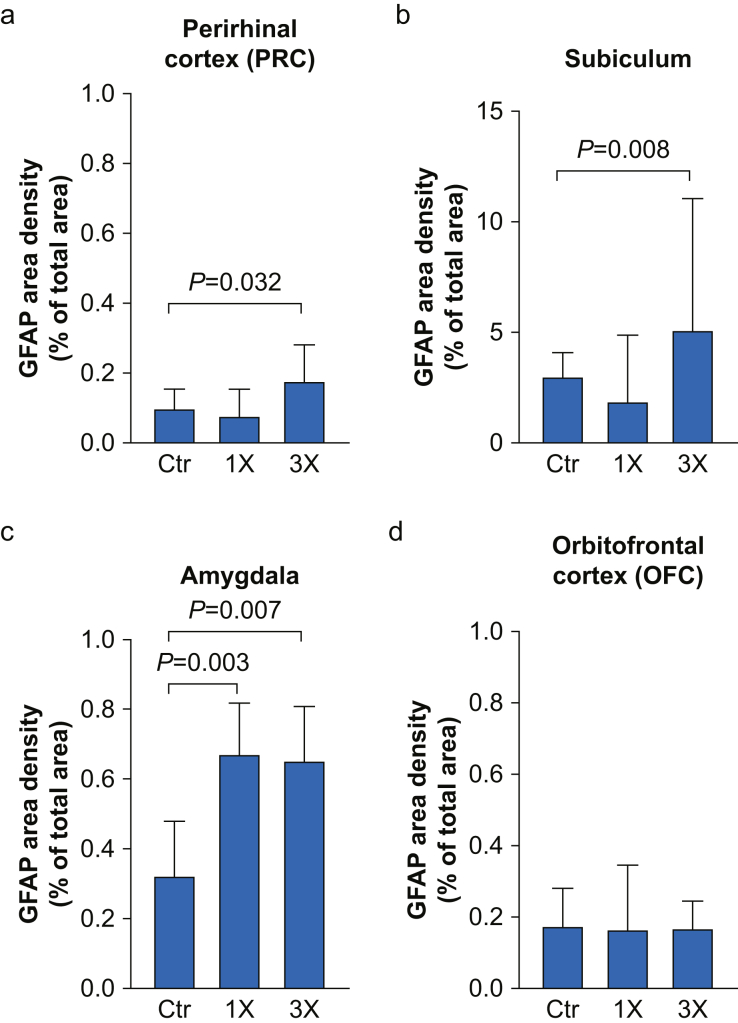
We extended our assessment of GFAP to other brain areas that participate in cognitive functions and behaviours that are altered after infant anaesthesia exposure. We assessed this marker in the PRC and subiculum because they are critically involved in memory function, and in the amygdala and the OFC, because they play major roles in anxiety and social behaviour. In the PRC of 2-yr-old NHPs, GFAP densities differed significantly between groups (Fig. 3a: Ctr: 0.10% [0.03–0.16], 1×: 0.082% [0.049–0.154], 3×: 0.18% [0.070–0.28], H(2)=6.96, P=0.031; Kruskal–Wallis test). Post hoc analysis with Dunn's multiple comparisons test showed that GFAP density was increased in the 3× group (P=0.032), but not in the 1× group (P>0.999) as compared with the control group. Similarly, the GFAP analysis in the subiculum revealed increased densities only for the 3× group when compared with control (Fig. 3b: Ctr: 3.07% [2.18–4.12], 1×: 1.93% [1.10–4.89], 3×: 5.16% [3.40–11.09], H(2)=13.52, P=0.001, Kruskal–Wallis test, post hoc Dunn test P=0.008). Analysis of the amygdala revealed increased GFAP densities in both exposed groups as compared with control (Fig. 3c: Ctr: 0.33% [0.19–0.48], 1×: 0.68% [0.46–0.82], 3×: 0.66% [0.434–0.81], H(2)=13.35, P=0.001; Kruskal–Wallis test). Dunn's multiple comparisons test revealed statistically significant differences for both exposure groups (1×: P=0.003, 3×: P=0.007). In contrast, in the OFC there were no significant differences in GFAP area densities between groups (Fig. 3d; n.s., Kruskal–Wallis test).
Fig 3.
GFAP expression in the perirhinal cortex, subiculum, amygdala, and orbitofrontal cortex of juvenile NHPs. GFAP area density (% of total area) in (a) the PRC, (b) subiculum, (c) amygdala, and (d) OFC of juvenile NHPs 2 yr after single (1×), three times (3×) or no exposure (Ctr) to isoflurane during infancy. Data are median (25th–75th percentiles); n (NHP)=PRC: Ctr: 8, 1×: 7, 3×: 7; subiculum: Ctr: 8, 1×: 8, 3× 7; amygdala: Ctr: 8, 1×: 7, 3×: 5; OFC: Ctr: 8, 1×: 8, 3×: 7. GFAP, glial fibrillary acidic protein; NHPs, non-human primates.
Hippocampal neurogenesis is not affected after isoflurane exposure
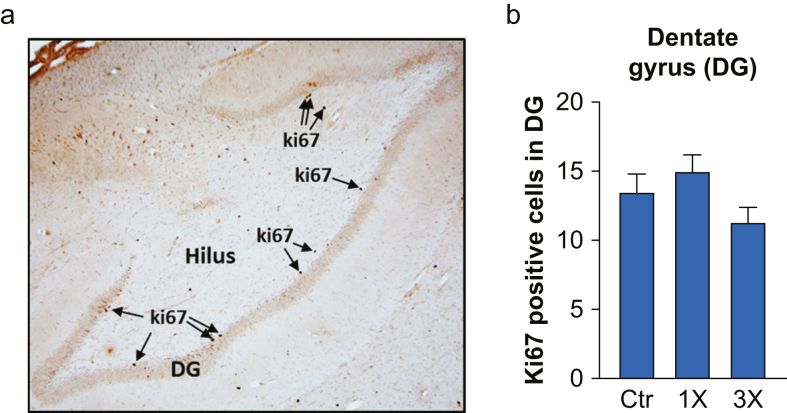
To explore whether exposure of infants to isoflurane affects neurogenesis in the DG of the hippocampus of juvenile NHPs, we evaluated the proliferative cell marker ki67. We found that there were no significant differences for ki67 cell counts in the indicated hippocampal area between the three groups (Fig. 4; n.s., Kruskal–Wallis test).
Fig 4.
ki67-positive cells in the dentate gyrus of juvenile non-human primates (NHPs). (a) Representative high magnification image (4×) showing ki67-positive cells (ki67) within the dentate gyrus (DG) of juvenile NHPs. (b) ki67-positive cells in DG in juvenile NHPs 2 yr after single (1×), three times (3×) or no exposure (Ctr) to isoflurane during infancy. Data are median (25th–75th percentiles); n (NHP)=Ctr: 8, 1×: 8, 3×: 7.
Discussion
We report increases in GFAP-positive area in specific brain regions of juvenile NHPs 2 yr after single or multiple 5 h isoflurane exposures during infancy. These results reveal novel long-term structural changes associated with anaesthesia exposure in the developing brain. Increased GFAP densities were found in V1, the PRC, and the subiculum, regions that participate in visual recognition memory, and in the amygdala, which plays a role in anxiety-related and social behaviours. These brain functions have been shown to be altered after infant anaesthesia exposure in NHPs.8,11, 12, 13 Such increases in GFAP area indicate chronic astrogliosis and resemble similar changes detectable after other types of brain injury including ischaemia/hypoxia,41,69,70 or those associated with neurodegenerative conditions such as Alzheimer's disease71 and developmental disorders such as autism.46 It is important to note that the long-term structural changes reported here are accompanied by alterations in behaviours detected at the same age.13
The increase in GFAP indicates activation of astrocytes, which is a common and non-specific response to CNS injury. It is likely that the increase in GFAP we found 2 yr after anaesthesia exposure was in response to the acute injury caused by isoflurane exposure during infancy that is characterised by a widespread increase in apoptosis of neurones and oligodendrocytes.28 Whereas mild to moderate astrogliosis is generally an acute and transient response, more severe and diffuse astrogliosis is chronic and can result in long-term tissue reorganisation. A localised increase in reactive astrocytes and GFAP density has been suggested to play a beneficial role as it represents a barrier separating the injured area from intact neuronal tissue thereby supporting restoration of tissue integrity and preventing further spread of cellular damage.72 However, such localised GFAP density increases also have been suggested to contribute to neuronal hyperexcitability and seizures.73 In contrast, a diffuse, chronic astrogliosis has been associated with detrimental effects and inhibition of tissue recovery including attenuation of axonal regrowth after tissue lesions.69,74,75 The astrogliosis we found in 2-yr-old NHPs after infant isoflurane exposure appears to be of the diffuse and chronic type, and likely occurred as a response to the acute widespread apoptotic injury.28 This type of gliosis can be compared with that associated with neonatal hypoxic injury, as both are chronic and diffuse in nature. However, we do not suspect that anaesthesia resulted in brain hypoxia as we were able to carefully monitor and control physiologic parameters during anaesthesia exposure, including oxygen saturation.
Astrocytes play important functions in maintaining extracellular homeostasis in the brain, modulating synaptic activity and shaping synaptogenesis (formation and pruning of synapses).40 Astrocyte dysfunction and astrogliosis have been associated with altered synaptic structure and function.69 Imbalances between excitatory and inhibitory synapses have been linked to behavioural alterations characteristic of autistic disorders, such as deficits in social interaction.76,77 Thus, it is possible that the astrogliosis we found may be associated with the reduced social behaviours that we found in the same NHPs at 2 yr of age after infant anaesthesia.13 In support of this hypothesis, chronic astrogliosis has been associated with altered behaviour in a murine traumatic brain injury model.78 However, further experiments in NHPs are needed to test this hypothesis.
Chronic activation of astrocytes might affect the development of critical brain areas and their connections, thereby impairing normal function.79,80 The astrogliosis we found in V1, the PRC, and the subiculum of NHPs 2 yr after exposure could be linked to the impaired visual recognition memory reported in similar aged NHPs.8 There is significant evidence that V1 could participate in visual recognition memory in rodents and NHPs. Early lesion experiments in rats indicated that the entire neocortex including V1 participates in memory storage,81 and more recent work suggests that V1 is involved in visual recognition and working memory.82, 83, 84 However, the critical areas for recognition memory processing are the PRC and the hippocampus, and damage to these structures was found to impair object recognition.53 Importantly, performance on the visual paired comparison task, the paradigm used in the study by Alvarado and colleagues,8 is dependent on PRC activity as reported in NHPs after neonatal perirhinal lesions.85 Therefore, the increase in astrogliosis in the PCR together with V1 found in the juvenile NHPs in our study may present the structural correlate for the impairments in visual recognition memory reported after infant anaesthesia exposure.8 In contrast, the hippocampus including the subicular cortex plays an essential role in recognition memory that involves spatial information,86,87 whereas neonatal perirhinal lesions in NHPs revealed that the PRC is dispensable for spatial location.88 We also found significant astrogliosis in the subiculum of juvenile NHPs in the 3× group, which may correlate with the lowest performance of the animals in this exposure group during the delayed response test that measures spatial working memory.13 Our findings of increased GFAP in temporal lobe structures (PRC and subiculum) in the brains of NHPs in the 3× group support clinical studies showing that multiple, but not single, infant anaesthesia exposures were associated with learning disabilities and attention deficit/hyperactivity disorder later in life.89,90
The astrogliosis we found in the amygdala may be associated with decreased close social behaviour and increased anxiety-related behaviours as reported in these NHPs,13 and in juvenile NHPs in other studies, after infant anaesthesia exposure.11,12,14 This component of the limbic system is substantially implicated in processing of social and anxiety-related behaviours.54,56,91,92 In addition, animals with ibotenic acid amygdala lesions during infancy exhibit decreased or altered social behaviour with familiar partners, and greater anxiety-related behaviours such as scratching and yawning later in life.93, 94, 95 Selective neurotoxic amygdala lesions in neonatal NHPs result in alterations in social behaviour, including loss of social status, decreased affiliative behaviours, and decreased social interactions.96, 97, 98, 99, 100 Hence, these alterations in social behaviours and increased anxiety-related behaviours after anaesthesia exposure during infancy could be associated with the astrogliosis that we found in the amygdala. Interestingly, we found astrogliosis in the 1× group only in the amygdala, suggesting that this area is more sensitive than the other areas analysed. Recent clinical studies report associations of early in life anaesthesia and alterations in behaviour that show some parallels with behavioural changes in animal studies as discussed extensively.3,13,101,102
Acute brain injury activates microglia, the primary immune cells of the CNS that respond by migrating to the site of injury where they release inflammatory factors, destroy pathogens, and remove damaged cells. Microglia-mediated inflammatory responses are also considered to be key in some neurodevelopmental diseases including autistic spectrum disorders.103,104 We detected astrogliosis, but we did not find evidence of ongoing microglia activation by Iba-1 stain 2 yr after infant anaesthesia exposure. Although we cannot disregard microglia activation early after infant anaesthesia exposure in these NHPs, previous studies in different species support our finding as they report acute, but not long-lasting, activation of microglia after infant anaesthesia exposure.67,105,106 For example in neonatal mice multiple exposures to sevoflurane induced an acute microglia activation in the hippocampus that was not evident 1 month after anaesthesia or later, suggesting that anaesthesia-induced microglia activation is acute and transient.67 Similarly, a subsequent study using in vivo micro-positron emission tomography (PET)/CT imaging in NHPs found that infant sevoflurane exposure resulted in acute activation of microglia that lasted a few days but was not present after 3 weeks.105 Although the molecular mechanisms of anaesthetic neurotoxicity are not completely understood, acute activation of microglia promoting inflammation and generation of reactive oxygen species (ROS) are among the proposed constituents of the underlying acute damage of the developing brain.107 The lack of microglia activation 2 yr after infant exposure suggests that there is no ongoing active injury, but instead that the astrogliosis found in the juvenile brains is a result of a prior injury. Thus, we propose that the increased levels of GFAP in specific brain regions represent a long-term structural marker of anaesthesia-induced brain injury in the developing brain.
Anaesthesia exposure during infancy impairs hippocampal neurogenesis.108 Several studies in neonatal rodents (P7) reported concurring findings that exposure to isoflurane, sevoflurane, propofol, or ketamine caused an acute decrease in progenitor proliferation in the DG that lasted for up to 10 days and was associated with long-term neurocognitive deficits.21,23, 24, 25 Another study in slightly older rodents (P14) reported persisting reductions of hippocampal neurogenesis for up to 4 weeks after exposure and deficits in memory function.22 We did not find differences in hippocampal neurogenesis 2 yr after infant anaesthesia exposure. However, we cannot discard the possibility of impaired neurogenesis at an earlier time, as this study or any other report did not investigate acute effects of anaesthesia exposure on hippocampal neurogenesis in the infant NHP.
Our study has several limitations. In the present study only a single method (GFAP staining) was used to detect astrogliosis. We chose GFAP labelling because detection of elevated GFAP levels in post mortem tissue is a traditional, well-established method to determine astrogliosis in various diseases.40,109,110 Because of its post mortem nature, our study can only provide information about astrogliosis at the time point of 2 yr after infant anaesthesia exposure, and further experiments are necessary to investigate the course of astrogliosis over time. The absence of astrogliosis in the OFC does not exclude its presence in other areas that have been implicated in social and anxiety-related behaviours such as the cingulate cortex, insula, other areas of the frontal cortex, or in white matter. We do not know whether our findings of astrogliosis 2 yr after infant exposure to isoflurane for 5 h (one or three times) can be generalised to other anaesthetics or to shorter exposure durations. Further studies will be needed to examine these issues.
Our study has several strengths. The NHP brain shares the highest structural similarities to the human brain, making this a preferred model for studies of chronic structural changes after infant anaesthesia exposure. In addition, this model allowed exposure of infants under physiologically controlled conditions to closely mimic paediatric clinical settings. The physiological parameters of neonatal NHPs were maintained within narrow ranges throughout the 5 h exposures and were comparable with those published earlier using a similar approach to monitor and control physiological parameters of neonatal NHPs during exposure to several anaesthetics including isoflurane.58 Our histopathological studies in the brains of juvenile NHPs revealed astrogliosis in several brain areas associated with anaesthesia exposure during infancy. This is the first report of this new type of structural alteration in the brain 2 yr after early-in-life anaesthesia exposure. This age in NHPs corresponds approximately to that of children aged 6–8 yr. The astrogliosis in the 2-yr-old NHPs was found concurrently with alterations in social and anxiety-related behaviours that we assessed at this age in the same animal cohort.13
Conclusions
We report chronic astrogliosis in juvenile non-human primates 2 yr after infant anaesthesia, providing the first neuroanatomical evidence for long-term structural consequences after exposure. The absence of microglia activation concurrently with the long-term astrogliosis suggests that it represents an indicator of past injury caused by infant anaesthesia exposure. The astrogliosis associated with infant anaesthesia exposure could be helpful as a long-term marker of anaesthesia-induced brain injury in future research. Preventing the sustained astrogliosis may represent a novel approach to prevent functional impairments associated with infant anaesthesia exposure.
Authors' contributions
Study design/planning: AMB, MG, GAD
Study conduct: AMB, MG, LDM
Data analysis: AMB, VN, JFPZ, MG
Writing of the paper: AMB, VN, JFPZ, MG
Manuscript revision: all authors
Acknowledgements
We thank Kristine Coleman at the ONRPC for all the care and handling of NHPs during their 2-yr life span; Xiao Jing Nie and Helen Liu for performing the histological preparations and staining; and Crystal Chaw at the Advanced Light Microscopy Core at OHSU for assistance with scanning and digitalising of the histological slides.
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: Long-term evidence of neonatal anaesthesia neurotoxicity linked to behavioural phenotypes in monkeys: where do we go from here? by Raper et al, Br J Anaesth 2021:127:343–345, doi: 10.1016/j.bja.2021.06.005
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.04.034.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Frontiers in Anaesthesia Research Award 2012 to AMB (awarded by the International Anaesthesia Research Society, San Francisco, CA, USA); US National Institutes of Health (Bethesda, MS, USA; grant P51OD011092) to the Oregon National Primate Research Center, Beaverton, OR, USA; the Board of Trustees Award of the International Anaesthesia Research Society (IARS) to AMB; and by departmental funds from the Departments of Anesthesiology, both at Oregon Health & Science University and at Columbia University.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Olsen E.A., Brambrink A.M. Anesthetic neurotoxicity in the newborn and infant. Curr Opin Anaesthesiol. 2013;26:535–542. doi: 10.1097/01.aco.0000433061.59939.b7. [DOI] [PubMed] [Google Scholar]
- 2.Andropoulos D.B. Effect of anesthesia on the developing brain: infant and fetus. Fetal Diagn Ther. 2018;43:1–11. doi: 10.1159/000475928. [DOI] [PubMed] [Google Scholar]
- 3.Ing C., Jackson W.M., Zaccariello M.J. Prospectively assessed neurodevelopmental outcomes in studies of anaesthetic neurotoxicity in children: a systematic review and meta-analysis. Br J Anaesth. 2021;126:433–444. doi: 10.1016/j.bja.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann M.E., de Graaff J.C., Dorris L. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. 2019;393:664–677. doi: 10.1016/S0140-6736(18)32485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L.S., Li G., Miller T.L. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warner D.O., Zaccariello M.J., Katusic S.K. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo Anesthesia Safety in Kids (MASK) study. Anesthesiology. 2018;129:89–105. doi: 10.1097/ALN.0000000000002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarado M.C., Murphy K.L., Baxter M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119:517–523. doi: 10.1093/bja/aew473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paule M.G., Li M., Allen R.R. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talpos J.C., Chelonis J.J., Li M., Hanig J.P., Paule M.G. Early life exposure to extended general anesthesia with isoflurane and nitrous oxide reduces responsivity on a cognitive test battery in the nonhuman primate. Neurotoxicology. 2019;70:80–90. doi: 10.1016/j.neuro.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raper J., De Biasio J.C., Murphy K.L., Alvarado M.C., Baxter M.G. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2018;120:761–767. doi: 10.1016/j.bja.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neudecker V., Perez-Zoghbi J.F., Coleman K. Infant isoflurane exposure affects social behaviours, but does not impair specific cognitive domains in juvenile non-human primates. Br J Anaesth. 2021;126:486–499. doi: 10.1016/j.bja.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman K., Robertson N.D., Dissen G.A. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brambrink A.M., Evers A.S., Avidan M.S. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brambrink A.M., Evers A.S., Avidan M.S. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creeley C., Dikranian K., Dissen G., Martin L., Olney J., Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth. 2013;110:29–38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noguchi K.K., Johnson S.A., Dissen G.A. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth. 2017;119:524–531. doi: 10.1093/bja/aex123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Zoghbi J.F., Zhu W., Neudecker V., Grafe M.R., Brambrink A.M. Neurotoxicity of sub-anesthetic doses of sevoflurane and dexmedetomidine co-administration in neonatal rats. Neurotoxicology. 2020;79:75–83. doi: 10.1016/j.neuro.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Slikker W., Jr., Zou X., Hotchkiss C.E. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 21.Stratmann G., Sall J.W., May L.D. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 22.Zhu C., Gao J., Karlsson N. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang F., Xue Z., Cang J. Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci Bull. 2012;28:499–508. doi: 10.1007/s12264-012-1260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J., Jing S., Chen X. Propofol administration during early postnatal life suppresses hippocampal neurogenesis. Mol Neurobiol. 2016;53:1031–1044. doi: 10.1007/s12035-014-9052-7. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y., Giri P.K., Lei S. Pretreatment with minocycline restores neurogenesis in the subventricular zone and subgranular zone of the hippocampus after ketamine exposure in neonatal rats. Neuroscience. 2017;352:144–154. doi: 10.1016/j.neuroscience.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 26.Briner A., De Roo M., Dayer A., Muller D., Habre W., Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 27.Kang E., Jiang D., Ryu Y.K. Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambrink A.M., Back S.A., Riddle A. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol. 2012;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creeley C.E., Dikranian K.T., Dissen G.A., Back S.A., Olney J.W., Brambrink A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenning K.J., Noguchi K.K., Martin L.D. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Xue Z., Liu Q. Disrupted folate metabolism with anesthesia leads to myelination deficits mediated by epigenetic regulation of ERMN. EBioMedicine. 2019;43:473–486. doi: 10.1016/j.ebiom.2019.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Q., Mathena R.P., Xu J., Eregha O.N., Wen J., Mintz C.D. Early postnatal exposure to isoflurane disrupts oligodendrocyte development and myelin formation in the mouse hippocampus. Anesthesiology. 2019;131:1077–1091. doi: 10.1097/ALN.0000000000002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culley D.J., Cotran E.K., Karlsson E. Isoflurane affects the cytoskeleton but not survival, proliferation, or synaptogenic properties of rat astrocytes in vitro. Br J Anaesth. 2013;110:19–28. doi: 10.1093/bja/aet169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lunardi N., Hucklenbruch C., Latham J.R., Scarpa J., Jevtovic-Todorovic V. Isoflurane impairs immature astroglia development in vitro: the role of actin cytoskeleton. J Neuropathol Exp Neurol. 2011;70:281–291. doi: 10.1097/NEN.0b013e31821284e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Yan Y., Inagaki Y., Logan S., Bosnjak Z.J., Bai X. Insufficient astrocyte-derived brain-derived neurotrophic factor contributes to propofol-Induced neuron death through Akt/glycogen synthase kinase 3beta/mitochondrial fission pathway. Anesth Analg. 2017;125:241–254. doi: 10.1213/ANE.0000000000002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Lu R., Feng D.Y., Zhang H. Sevoflurane inhibits glutamate-aspartate transporter and glial fibrillary acidic protein expression in hippocampal astrocytes of neonatal rats through the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. Anesth Analg. 2016;123:93–102. doi: 10.1213/ANE.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z., Xue H., Gao Q., Zhao P. Effects of early postnatal sevoflurane exposure on oligodendrocyte maturation and myelination in cerebral white matter of the rat. Biomed Pharmacother. 2020;131:110733. doi: 10.1016/j.biopha.2020.110733. [DOI] [PubMed] [Google Scholar]
- 38.Zhou B., Chen L., Liao P. Astroglial dysfunctions drive aberrant synaptogenesis and social behavioral deficits in mice with neonatal exposure to lengthy general anesthesia. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eddleston M., Mucke L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L., Wu Z.B., Zhuge Q. Glial scar formation occurs in the human brain after ischemic stroke. Int J Med Sci. 2014;11:344–348. doi: 10.7150/ijms.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verkhratsky A., Olabarria M., Noristani H.N., Yeh C.Y., Rodriguez J.J. Astrocytes in Alzheimer's disease. Neurotherapeutics. 2010;7:399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henstridge C.M., Tzioras M., Paolicelli R.C. Glial contribution to excitatory and inhibitory synapse loss in neurodegeneration. Front Cell Neurosci. 2019;13:63. doi: 10.3389/fncel.2019.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damier P., Hirsch E.C., Zhang P., Agid Y., Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 45.Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 46.Laurence J.A., Fatemi S.H. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- 47.Eng L.F., Ghirnikar R.S., Lee Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 48.Faulkner J.R., Herrmann J.E., Woo M.J., Tansey K.E., Doan N.B., Sofroniew M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lunardi N., Ori C., Erisir A., Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–188. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briner A., Nikonenko I., De Roo M., Dayer A., Muller D., Vutskits L. Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez V., Feinstein S.D., Lunardi N. General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology. 2011;115:992–1002. doi: 10.1097/ALN.0b013e3182303a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amrock L.G., Starner M.L., Murphy K.L., Baxter M.G. Long-term effects of single or multiple neonatal sevoflurane exposures on rat hippocampal ultrastructure. Anesthesiology. 2015;122:87–95. doi: 10.1097/ALN.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 53.Brown M.W., Aggleton J.P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 54.Machado C.J., Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 55.Kalin N.H., Shelton S.E., Davidson R.J. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalin N.H., Shelton S.E., Davidson R.J. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Institute of Laboratory Animal Resources . 8th edn. NRC; Washington, DC: 2011. Guide for the care and use of laboratory animals. [Google Scholar]
- 58.Martin L.D., Dissen G.A., McPike M.J., Brambrink A.M. Effects of anesthesia with isoflurane, ketamine, or propofol on physiologic parameters in neonatal rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2014;53:290–300. [PMC free article] [PubMed] [Google Scholar]
- 59.Paxinos G., Huang F.-X., Toga A.W. Academic Press; San Diego, USA: 2006. The rhesus monkey brain in stereotaxic coordinates. [Google Scholar]
- 60.Rossiter J.P., Anderson L.L., Yang F., Cole G.M. Caspase-3 activation and caspase-like proteolytic activity in human perinatal hypoxic–ischemic brain injury. Acta Neuropathol. 2002;103:66–73. doi: 10.1007/s004010100432. [DOI] [PubMed] [Google Scholar]
- 61.Stadelman C., Mews I., Srinivasan A., Deckwerth T.L., Lassmann H., Bruck W. Expression of cell death-associated proteins in neuronal apoptosis associated with pontosubicular neuron necrosis. Brain Pathol. 2001;11:273–281. doi: 10.1111/j.1750-3639.2001.tb00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friede R.L. Ponto-subicular lesions in perinatal anoxia. Arch Pathol. 1972;94:343–354. [PubMed] [Google Scholar]
- 63.van den Berge S.A., Kevenaar J.T., Sluijs J.A., Hol E.M. Dementia in Parkinson’s disease correlates with alpha-synuclein pathology but not with cortical astrogliosis. Parkinsons Dis. 2012;2012:420957. doi: 10.1155/2012/420957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allnoch L., Baumgartner W., Hansmann F. Impact of astrocyte depletion upon inflammation and demyelination in a murine animal model of multiple sclerosis. Int J Mol Sci. 2019;20:3922. doi: 10.3390/ijms20163922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wakasa S., Shiiya N., Tachibana T., Ooka T., Matsui Y. A semiquantitative analysis of reactive astrogliosis demonstrates its correlation with the number of intact motor neurons after transient spinal cord ischemia. J Thorac Cardiovasc Surg. 2009;137:983–990. doi: 10.1016/j.jtcvs.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Omri S., Behar-Cohen F., de Kozak Y. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. Am J Pathol. 2011;179:942–953. doi: 10.1016/j.ajpath.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen X., Dong Y., Xu Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varghese F., Bukhari A.B., Malhotra R., De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pekny M., Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta. 2016;1862:483–491. doi: 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Pekny M., Wilhelmsson U., Tatlisumak T., Pekna M. Astrocyte activation and reactive gliosis—a new target in stroke? Neurosci Lett. 2019;689:45–55. doi: 10.1016/j.neulet.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 71.Ross G.W., O'Callaghan J.P., Sharp D.S. Quantification of regional glial fibrillary acidic protein levels in Alzheimer's disease. Acta Neurol Scand. 2003;107:318–323. doi: 10.1034/j.1600-0404.2003.02098.x. [DOI] [PubMed] [Google Scholar]
- 72.Sofroniew M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robel S. Astroglial scarring and seizures: a cell biological perspective on epilepsy. Neuroscientist. 2017;23:152–168. doi: 10.1177/1073858416645498. [DOI] [PubMed] [Google Scholar]
- 74.Liuzzi F.J., Lasek R.J. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987;237:642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- 75.Anderson M.A., Burda J.E., Ren Y. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson S.B., Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–698. doi: 10.1016/j.neuron.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selimbeyoglu A., Kim C.K., Inoue M. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med. 2017;9:eaah6733. doi: 10.1126/scitranslmed.aah6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mannix R., Berglass J., Berkner J. Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J Neurosurg. 2014;121:1342–1350. doi: 10.3171/2014.7.JNS14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jo S., Yarishkin O., Hwang Y.J. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortinski P.I., Dong J., Mungenast A. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–591. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lashley K.S. Mass action in cerebral function. Science. 1931;73:245–254. doi: 10.1126/science.73.1888.245. [DOI] [PubMed] [Google Scholar]
- 82.Gavornik J.P., Bear M.F. Learned spatiotemporal sequence recognition and prediction in primary visual cortex. Nat Neurosci. 2014;17:732–737. doi: 10.1038/nn.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooke S.F., Komorowski R.W., Kaplan E.S., Gavornik J.P., Bear M.F. Visual recognition memory, manifested as long-term habituation, requires synaptic plasticity in V1. Nat Neurosci. 2015;18:262–271. doi: 10.1038/nn.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Super H., Spekreijse H., Lamme V.A. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293:120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- 85.Zeamer A., Richardson R.L., Weiss A.R., Bachevalier J. The development of object recognition memory in rhesus macaques with neonatal lesions of the perirhinal cortex. Dev Cogn Neurosci. 2015;11:31–41. doi: 10.1016/j.dcn.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nelson A.J.D., Kinnavane L., Amin E., O'Mara S.M., Aggleton J.P. Deconstructing the direct reciprocal hippocampal-anterior thalamic pathways for spatial learning. J Neurosci. 2020;40:6978–6990. doi: 10.1523/JNEUROSCI.0874-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bachevalier J. Nonhuman primate models of hippocampal development and dysfunction. Proc Natl Acad Sci U S A. 2019;116:26210–26216. doi: 10.1073/pnas.1902278116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weiss A.R., Bachevalier J. Object and spatial memory after neonatal perirhinal lesions in monkeys. Behav Brain Res. 2016;298:210–217. doi: 10.1016/j.bbr.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu D., Flick R.P., Zaccariello M.J. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127:227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sprung J., Flick R.P., Katusic S.K. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meunier M., Bachevalier J., Murray E.A., Malkova L., Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 92.Amaral D.G. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 93.Raper J., Stephens S.B., Sanchez M., Bachevalier J., Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev Psychobiol. 2014;56:1711–1722. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bauman M.D., Lavenex P., Mason W.A., Capitanio J.P., Amaral D.G. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. J Cogn Neurosci. 2004;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- 95.Moadab G., Bliss-Moreau E., Amaral D.G. Adult social behavior with familiar partners following neonatal amygdala or hippocampus damage. Behav Neurosci. 2015;129:339–350. doi: 10.1037/bne0000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bliss-Moreau E., Moadab G., Bauman M.D., Amaral D.G. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci. 2013;25:2124–2140. doi: 10.1162/jocn_a_00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bliss-Moreau E., Moadab G., Santistevan A., Amaral D.G. The effects of neonatal amygdala or hippocampus lesions on adult social behavior. Behav Brain Res. 2017;322:123–137. doi: 10.1016/j.bbr.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prather M.D., Lavenex P., Mauldin-Jourdain M.L. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- 99.Moadab G., Bliss-Moreau E., Bauman M.D., Amaral D.G. Early amygdala or hippocampus damage influences adolescent female social behavior during group formation. Behav Neurosci. 2017;131:68–82. doi: 10.1037/bne0000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Medina A., Torres J., Kazama A.M., Bachevalier J., Raper J. Emotional responses in monkeys differ depending on the stimulus type, sex, and neonatal amygdala lesion status. Behav Neurosci. 2020;134:153–165. doi: 10.1037/bne0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ing C., Brambrink A.M. Mayo Anesthesia Safety in Kids continued: two new studies and a potential redirection of the field. Br J Anaesth. 2019;122:716–719. doi: 10.1016/j.bja.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Neudecker V., Perez-Zhogbi J.F., Brambrink A.M. Recent advances in understanding cognitive and behavioral alterations after early-in-life anesthesia exposure and new mitigation/alternative strategies in preclinical studies. Curr Opin Anaesthesiol. 2021 doi: 10.1097/ACO.0000000000001016. Advance Access published on May 18. [DOI] [PubMed] [Google Scholar]
- 103.Stephenson J., Nutma E., van der Valk P., Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edmonson C.A., Ziats M.N., Rennert O.M. A Non-inflammatory role for microglia in autism spectrum disorders. Front Neurol. 2016;7:9. doi: 10.3389/fneur.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang X., Liu S., Newport G.D. In vivo monitoring of sevoflurane-induced adverse effects in neonatal nonhuman primates using small-animal positron emission tomography. Anesthesiology. 2016;125:133–146. doi: 10.1097/ALN.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 106.Broad K.D., Hassell J., Fleiss B. Isoflurane exposure induces cell death, microglial activation and modifies the expression of genes supporting neurodevelopment and cognitive function in the male newborn piglet brain. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Durga P. Neuronal apoptosis of the developing brain: influence of anesthetics. Indian J Cereb Palsy. 2016;2:71–78. [Google Scholar]
- 108.Kang E., Berg D.A., Furmanski O. Neurogenesis and developmental anesthetic neurotoxicity. Neurotoxicol Teratol. 2017;60:33–39. doi: 10.1016/j.ntt.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eng L.F., Ghirnikar R.S. GFAP and astrogliosis. Brain Pathol. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 110.Middeldorp J., Hol E.M. GFAP in health and disease. Prog Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.