Abstract
Objectives
To develop a consensus statement on the use of lung ultrasound (LUS) in the assessment of symptomatic general medical inpatients with known or suspected coronavirus disease 2019 (COVID‐19).
Methods
Our LUS expert panel consisted of 14 multidisciplinary international experts. Experts voted in 3 rounds on the strength of 26 recommendations as “strong,” “weak,” or “do not recommend.” For recommendations that reached consensus for do not recommend, a fourth round was conducted to determine the strength of those recommendations, with 2 additional recommendations considered.
Results
Of the 26 recommendations, experts reached consensus on 6 in the first round, 13 in the second, and 7 in the third. Four recommendations were removed because of redundancy. In the fourth round, experts considered 4 recommendations that reached consensus for do not recommend and 2 additional scenarios; consensus was reached for 4 of these. Our final recommendations consist of 24 consensus statements; for 2 of these, the strength of the recommendations did not reach consensus.
Conclusions
In symptomatic medical inpatients with known or suspected COVID‐19, we recommend the use of LUS to: (1) support the diagnosis of pneumonitis but not diagnose COVID‐19, (2) rule out concerning ultrasound features, (3) monitor patients with a change in the clinical status, and (4) avoid unnecessary additional imaging for patients whose pretest probability of an alternative or superimposed diagnosis is low. We do not recommend the use of LUS to guide admission and discharge decisions. We do not recommend routine serial LUS in patients without a change in their clinical condition.
Keywords: consensus, coronavirus disease 2019, internal medicine, lung, point‐of‐care ultrasound
Abbreviations
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- LUS
lung ultrasound
- POCUS
point‐of‐care ultrasound
- RT‐PCR
reverse transcription polymerase chain reaction
- US
ultrasound
As of October 18, 2020, according to the World Health Organization Weekly Epidemiological Update, there were more than 40 million confirmed cases of coronavirus disease 2019 (COVID‐19) infection worldwide, with 1.1 million deaths reported.1 In response to this disease, multiple health care processes had to be adapted.2 The scope of these adaptions has been extensive. For example, physical distancing measures had to be incorporated into the work flow. Health care processes had to be modified on the basis of the testing indications,3 availability of personal protective equipment, and other diagnostic capability, all of which may change depending on the stage of the COVID‐19 surge.4, 5
The use of lung ultrasound (LUS) in the evaluation of patients with known or suspected COVID‐19 has shown promise.6 Coronavirus disease 2019 predominantly affects the periphery of the lung.7, 8 Therefore, from a theoretical perspective, LUS should be able to readily identify these pleural‐based abnormalities. Indeed, published reports to date suggest that LUS findings in COVID‐19 appear to correspond directly to the peripheral lesions found on computed tomography (CT).9, 10, 11, 12 An LUS examination is performed at the bedside by the treating clinician. Thus, from a practical standpoint, LUS does not expose additional personnel, transport staff, or imaging departments to infectious risks. In addition, point‐of‐care ultrasound (POCUS) devices tend to be smaller than equipment used for chest radiography and CT and thus are easier to clean and disinfect, and when used appropriately, the use of LUS in COVID‐19 may reduce unnecessary use of other imaging modalities, thereby improving care efficiency.13, 14
However, the use of LUS is not risk free. Although LUS examinations can be quickly performed by those who are proficient in the use of LUS, often in less than 5 minutes,13 its use does impose additional exposure time for the practitioner in close proximity to the patient. Furthermore, the device must be appropriately disinfected after each use, requiring disinfectant and wipes, which may already be in low supply. Thus, the routine use of LUS without regard for its indications, expected clinical utility, and implications on health care resource use cannot be recommended.15 There is a clear need for guidance on the appropriate use of LUS in the assessment of medical inpatients with known or suspected COVID‐19. To address this gap, we developed a consensus statement on the use of LUS in the assessment of symptomatic general medical inpatients in Canada, based on expert opinions from an international multidisciplinary panel.
Materials and Methods
We did not seek ethics approval for this study based on a pRoject Ethics Community Consensus Initiative Ethics Screening tool score indicating minimal risk (https://albertainnovates.ca/programs/arecci/). All experts consented to participate in this consensus statement work. Our LUS expert panel consisted of 3 existing Canadian Internal Medicine Ultrasound (CIMUS) group members (2 general internists [I.W.Y.M. and S.A.] and 1 critical care physician [B.B.]). To ensure an appropriate breadth of specialist involvement and clinical experience with the various stages of the surge of COVID‐19, using a nonprobability sampling strategy, we invited additional experts on the basis of their known clinical expertise, specialized POCUS training, or international reputation. Additional panel members included 4 Canadian pulmonary specialists (D.J.M., C.A.H., B.W., and E.D.), 1 American pulmonary specialist (A.C.), 3 American emergency physicians (V.N., A.S.L., and R.B.L.), 3 American physicians specializing in hospital medicine (G.M., T.W., and M.W.), and 1 internist from Brazil (A.C.T.). In addition, we had 1 external consulting member (A.H., an American intensivist/anesthesiologist working overseas and president of an ultrasound [US] society).
The chair of the group (I.W.Y.M.) created the draft guideline elements and did not vote; the external consulting member also did not vote. The remaining 14 experts participated in voting. A total of 26 recommendations were considered. Recommendations were drafted on the basis of scenarios relevant to the inpatient practice of internal medicine and a literature review, performed in PubMed in April 2020 using the search terms COVID, COVID‐19, coronavirus*; point‐of‐care ultrasound, ultrasound, lung ultrasound, sonography, ultrasonography, imaging; and pneumon*, lung, resulting in 554 articles, of which 57 were deemed highly relevant (available at https://tinyurl.com/y38cpr67). For each recommendation, experts were asked to rate the strength of the recommendation based on the Grading of Recommendations Assessment, Development, and Evaluation system, as either “strong,” “weak,” or “do not recommend”; rating was based on the individual expert‐based estimated quality of the supporting evidence and the balance between benefits and anticipated harm.16, 17 We determined a priori that no more than 3 rounds of online surveys would be conducted. These were conducted between April 30 and June 2, 2020. Surveys were administered on an online platform (Qualtrics, Provo, UT). Consensus was defined as agreement by at least 70% of the experts.18 Experts voted anonymously. Feedback to experts on results from the preceding rounds consisted of percentages of participants who chose each option as well as key relevant comments. Recommendations that did not reach consensus were considered in subsequent rounds, with or without modifications based on expert comments. For recommendations that reached consensus for do not recommend, an additional fourth round was conducted in August 2020 to determine the strength of these recommendations. Two additional questions were also included in this final round.
Results
All 14 experts participated in all 4 rounds. Of the 26 recommendations considered, 10 related to recommendations on the general use of LUS (Table 1), 11 on its use on patients with moderate or severe symptoms, and 5 on its use on patients with minimal symptoms. Of the 26 recommendations, experts reached consensus on 6 of these in the first round; consensus was reached on 11 for inclusion but not on strength, and no consensus was achieved on 9 recommendations. Of the 20 recommendations considered in the second round, experts reached consensus on 13 of these for strength, 3 on inclusion, but not on strength, and no consensus was attained on 4 recommendations. Of the 7 recommendations considered in the third round, experts reached consensus on all. Four recommendations were removed because of redundancy, resulting in 22 recommendations. An additional fourth round was conducted to ascertain consensus on the strength of 4 recommendations that reached consensus for do not recommend and 2 additional scenarios that were not considered in the initial rounds (patients with moderate or severe symptoms and negative chest CT findings and patients with minimal symptoms and positive chest radiographic findings). For these, experts reached consensus on 4 of these. There was no consensus on the strength of 2 recommendations, with 50% of the panel voting for strong and 50% for weak in both cases (Table 1).
Table 1.
Recommendations on the Use of Lung POCUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19
| Recommendation | Strengtha |
|---|---|
| General | |
| 1. In addition to the usual lung scan regions, posterior lung zones of the patient should be scanned wherever possible. | Strong2 |
| 2. Pneumonitis on LUS imaging is supported by the following findings: pleural irregularity; patchy B‐lines that may be focal, multifocal, or confluent; subpleural consolidation; and localized/focal pleural effusion. These findings may be present in any combination and are not diagnostic of COVID‐19. | Strong1 |
| 3. In patients with preexisting pleural or interstitial lung disease, we recommend that LUS findings be interpreted with caution. | Strong1 |
| 4. Presence of any of the following on LUS imaging should prompt an additional workup, as these are considered less likely for COVID‐19: large consolidation and large or complex pleural effusion. | Strong1 |
| 5. In a patient with suspected COVID‐19, while performing the LUS examination, we recommend evaluating concurrently for the presence/absence of pneumothorax. | Strong2 |
| 6. We do not recommend that admission decisions be made on the basis of the severity of LUS findings. | Weak3 |
| 7. We do not recommend that discharge decisions be made on the basis of LUS findings. | Weak3 |
| 8. For admitted patients, we do not recommend that serial LUS examinations be performed routinely without a change in the clinical condition. | Weak2 |
| 9. For admitted patients, serial LUS examinations should be performed in the presence of a change in the clinical status. | Strong2 |
| Assessment of patients with moderate or severe symptoms | |
| 10. LUS should be the initial imaging of choice. | Strong3 |
| 11. Positive LUS findings for pneumonitis would not diagnose COVID‐19 but would support the diagnosis of pneumonitis. | Strong1 |
| 12. Positive LUS findings for pneumonitis may preclude the need to pursue additional imaging if the pretest probability of an alternative or superimposed diagnosis is low. | Strong2 |
| 13. Entirely negative LUS findings for pneumonitis would make COVID‐19 less likely. An additional workup for other causes of pulmonary symptoms is recommended. | Strong2 |
| If chest radiography has already been performed | |
| 14. If the chest radiographic findings are is negative, an LUS examination should be performed next. | Strong2 |
| 15. If the chest radiographic findings are negative, positive LUS findings for pneumonitis may preclude the need to pursue additional imaging if the pretest probability of an alternative or secondary diagnosis is low. | Strong2 |
| 16. If the chest radiographic findings are negative, negative LUS findings for pneumonitis should prompt an additional workup. | Strong1 |
| 17. If the chest radiographic findings are positive for pneumonitis, and the pretest probability of an alternative or secondary diagnosis is low, an LUS examination should be performed. | Weak3 |
| If a chest CT was already performed | |
| 18. If the chest CT findings are positive for pneumonitis without additional concerning features, and the pretest probability of an alternative or secondary diagnosis is low, we do not recommend performing an LUS examination. | Weak3 |
| 19. If the chest CT findings are negative for pneumonitis or other concerning features such as pulmonary embolism, and the pretest probability of an alternative or secondary diagnosis is low, we do not recommend performing an LUS examination. | Weak4 |
| Assessment of patients with minimal symptoms | |
| 20. LUS should be the initial imaging of choice. | Strong2 |
| If chest radiography has already been performed | |
| 21. Negative chest radiographic findings alone are insufficient for ruling out pneumonitis; additional chest imaging such as LUS is recommended. | Strong2 |
| 22. Negative chest radiographic findings and positive LUS findings would support the diagnosis of pneumonitis. | Strong1 |
| 23. Negative chest radiographic findings and negative LUS findings are sufficient for ruling out pneumonitis; we do not recommend additional imaging if the pretest probability of an alternative or secondary diagnosis is low. | Strong3 |
| 24. Positive chest radiographic findings for pneumonitis without additional concerning features would support the diagnosis of pneumonitis. Additional imaging such as LUS can still be considered. | Weak4 |
Superscript number indicates the round in which consensus was achieved.
Scope of Recommendations
All recommendations were considered in the context of the following conditions: (1) a POCUS device is readily available; (2) a trained POCUS practitioner is available and skilled at image acquisition, interpretation, and clinical integration; (3) POCUS images/clips are archivable for later review; (4) POCUS devices are appropriately cleaned and disinfected after each use; and (5) the health care capacity is such that other imaging modalities can be available. Last, these recommendations are intended for the Canadian internal medicine inpatient setting. They do not apply to patients in the outpatient or critical care/intubated setting.
Final Consensus‐Based Recommendations
General Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19
1. In addition to the usual lung scan regions, posterior lung zones of the patient should be scanned wherever possible (strong recommendation).
Before COVID‐19, a number of standardized lung‐scanning protocols were published,19, 20, 21, 22, 23 which varied in terms of the number and locations of lung zones recommended to be scanned. Existing radiologic data on COVID‐19 suggest that the patchy bilateral, multifocal ground‐glass opacity and abnormalities typically found on CT were predominantly identified in the lower24, 25, 26, 27, 28, 29, 30, 31 and posterior lung zones.25, 28, 29, 30, 32 These areas were similarly involved according to studies on LUS findings.33, 34 Although a number of LUS‐scanning protocols have been proposed for COVID‐19,35, 36, 37, 38, 39, 40 there currently are no data to support the use of one protocol over another. Moreover, although the 8‐zone method was commonly used before COVID,19 variability in practice exists. Thus, rather than adopting a prescriptive approach, we recommend that, based on the known distribution of lung findings in patients with COVID‐19, posterior lung zones be scanned wherever possible, in addition to the usual scan areas.
2. Pneumonitis on LUS imaging is supported by the following findings: pleural irregularity; patchy B‐lines that may be focal, multifocal, or confluent; subpleural consolidation; and localized/focal pleural effusion. These findings may be present in any combination and are not diagnostic of COVID‐19 (strong recommendation).
A number of LUS findings have been described in patients with COVID‐19 without preexisting lung disease, including pleural irregularity, subpleural consolidation, localized/focal pleural effusion, and B‐lines.10, 11, 41, 42, 43 Furthermore, B‐lines may be focal, multifocal, or confluent.41, 42 In addition, they have been described by some to take on a “light beam” configuration, where a large beam of light appears and disappears during respiration.44 Although these findings are considered typical of COVID‐19 pneumonitis on LUS imaging and may be present in any combination, radiologic findings are not considered diagnostic of COVID‐19, as any pneumonitis can present with these same findings. Nucleic acid amplification testing remains the reference standard for the diagnosis of COVID‐19.45
3. In patients with preexisting pleural or interstitial lung disease, we recommend that LUS findings be interpreted with caution (strong recommendation).
As the presence of B‐lines and pleural irregularities are also hallmarks of interstitial lung disease,46, 47, 48, 49 LUS findings in these patients with known or suspected COVID‐19 must be interpreted with caution. Comparisons of LUS findings with the patient's baseline, where available, may be helpful.
4. The presence of any of the following on LUS imaging should prompt an additional workup, as these are considered less likely for COVID‐19: large consolidation and large or complex pleural effusion (strong recommendation).
Based on studies on imaging, a number of findings were noted to be uncommon in COVID‐19: isolated lobar consolidation,50 lymphadenopathy,24, 31, 51, 52, 53 and pleural effusions (seen in <10% of cases).8, 24, 31, 32, 41, 53, 54, 55, 56, 57 It is important to note, however, that although large lobar consolidation and pleural effusions were rare, subpleural consolidation and localized or focal pleural effusions were commonly noted on LUS imaging.10, 11, 41, 42 Thus, consistent with a number of published recommendations, the presence of large consolidation or large or complex pleural effusions should suggest alternative diagnoses and prompt an additional workup.44, 58, 59, 60, 61
5. In a patient with suspected COVID‐19, while performing an LUS examination, we recommend evaluating concurrently for the presence/absence of pneumothorax (strong recommendation).
Although the presence of pneumothorax is rare in COVID‐19, reported only in 1% to 2% of cases,62, 63 during general lung scanning, the presence of pneumothorax may be easily ruled out,64 especially at sites where B‐lines are observed.65 Definitively ruling in pneumothorax, however, may be a bit more challenging unless a lung point is observed,66 since lung sliding may be absent in cases of severe disease.67 Currently, although an LUS assessment for the presence or absence of pneumothorax is not considered a core competency for the Canadian internal medicine POCUS curriculum,68 only minimal training may be required.69 In addition, evaluating for the presence or absence of pneumothorax is unlikely to add substantial additional time or result in undue harm. Therefore, we recommend that while performing an LUS examination, the practitioner should evaluate concurrently for the presence or absence of pneumothorax.
6. We do not recommend that admission decisions be made based on the severity of LUS findings (weak recommendation).
Imaging abnormalities have been shown to be present even in asymptomatic individuals with COVID‐19.70 Currently, decisions for admission are recommended to be made on the basis of illness and symptom severity, rather than on imaging findings alone.71, 72 Unnecessary hospitalization increases avoidable nosocomial transmission risks. To date, there are limited data to support the severity of LUS findings in determining the need for hospitalization. Thus, currently, we do not recommend that admission decisions be made solely on the basis of LUS findings.
7. We do not recommend that discharge decisions be made on the basis of LUS findings (weak recommendation).
With respect to decisions regarding discharge from a facility, the current recommendations from the World Health Organization and the Centers for Disease Control and Prevention recommend that discharge decisions be made on the basis of clinical indications.73, 74 Although discharge recommendations/guidelines differ in the parameters outlined, most are based on clinical indications, with or without polymerase chain reaction tests.75 Of the 3 guidelines that consider the improvement or resolution of pulmonary imaging findings as clinical discharge criteria,72, 76, 77 in an updated version, the European Centre for Disease Prevention and Control no longer lists imaging findings as criteria.78 Although radiologic abnormalities improve over time,79 ground glass opacities can persist beyond 26 days.27 In one study, only 5.7% of patients at the time of discharge had resolution of CT lung abnormalities.80 Thus, the use of LUS findings for discharge decisions cannot be recommended at this time.
8. For admitted patients, we do not recommend that serial LUS examinations be performed routinely without a change in the clinical condition (weak recommendation).
Although serial LUS may be useful for the longitudinal monitoring of patients,42, 81 its use may come at a cost of unnecessary infection transmission risks. During scanning, LUS places the provider at close proximity to the patient, exposing the provider to infectious risks.15, 82 The US device itself may also potentially serve as a vector if improperly disinfected.82, 83 Although appropriate cleaning and disinfecting protocols for US devices are available,82, 84 using LUS without a defined clinical indication or expected change in management cannot be recommended at this time.68
9. For admitted patients, serial LUS examinations should be performed in the presence of a change in the clinical status (strong recommendation).
In the presence of clinical status deterioration, an argument could be made for performing an LUS examination, especially if its findings can change management.68, 85 Thus, if serial LUS examinations were to be performed, we recommend that they be done in the presence of a clinical status change.
Recommendations on the Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19 and Moderate or Severe Symptoms
10. Lung US should be the initial imaging modality of choice (strong recommendation).
Unlike CT scans and chest radiographs, LUS imaging does not expose the patient to radiation, does not expose the imaging department and transport personnel to infectious risks, and can be performed quickly, typically within 5 minutes.13 Furthermore, if an LUS examination is performed by the same clinician who needs to assess the patient at the bedside, it would not require additional personal protective equipment use and carries minimal additional infectious risks to the practitioner. Thus, in settings in which a POCUS device is readily available and the practitioner is skilled at LUS, we recommend that LUS be considered the initial imaging modality of choice (Figure 1).
Figure 1.
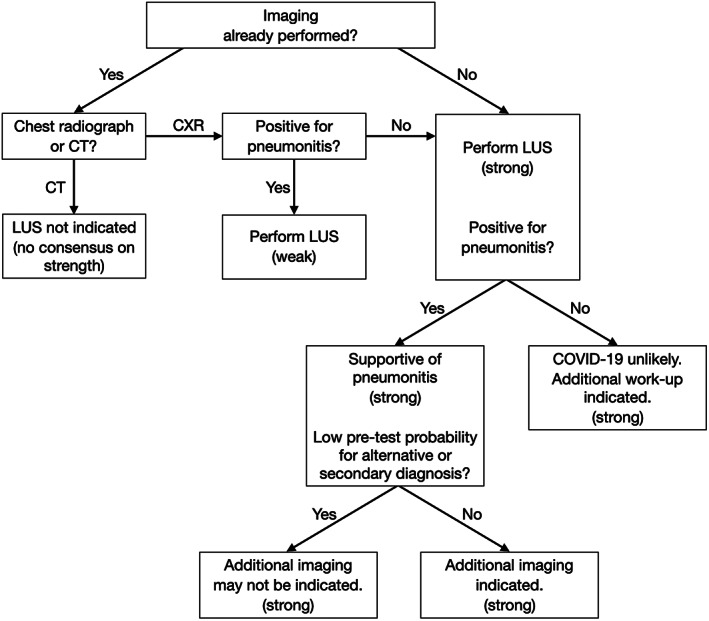
Recommended algorithm on LUS in the assessment of medical inpatients with known or suspected COVID‐19 with moderate or severe symptoms, with the strength of the recommendation indicated in parentheses. CXR indicates chest radiography.
11. Positive LUS findings for pneumonitis would not diagnose COVID‐19 but would support the diagnosis of pneumonitis (strong recommendation).
It is important to note that the constellation of LUS findings in COVID‐19 (pleural irregularity, subpleural consolidation, localized/focal pleural effusion, and B‐lines) are nonspecific. The presence of these findings supports the diagnosis of pneumonitis, but the definitive diagnosis of COVID‐19 requires the use of acid amplification testing.45
12. Positive LUS findings for pneumonitis may preclude the need to pursue additional imaging if the pretest probability of an alternative or secondary diagnosis is low (strong recommendation).
The clinical context must be taken into account when deciding whether to pursue additional imaging in a patient suspected of having COVID‐19 who has LUS findings suggestive of pneumonitis and no additional concerning features, such as large consolidation and large or complex pleural effusions.44 If the pretest probability of an alternative or secondary process is low, additional imaging may not be necessary. However, a number of complications and secondary processes are known in COVID‐19. For example, in non–intensive care unit medical patients, the prevalence of venous thromboembolism in patients with COVID‐19, even with thromboprophylaxis, may be as high as 22.5%.86, 87, 88 Thus, factors that affect pretest probability (such as hemoptysis, preexisting malignancy, a history of venous thromboembolism, and findings of deep venous thrombosis on the physical examination, among others) must be taken into account in the decision to pursue or not pursue additional imaging. Furthermore, COVID‐19 may precipitate or worsen preexisting conditions, such as the exacerbation of chronic obstructive pulmonary disease or worsening heart failure. Thus, whether the presence of pneumonitis fully explains any given patient's presentation and decisions on the pursuit of additional imaging or workup must be individualized.
13. Entirely negative LUS findings for pneumonitis would make COVID‐19 less likely. An additional workup for other causes of pulmonary symptoms is recommended (strong recommendation).
In patients presenting with moderate or severe symptoms, the probability of entirely normal radiologic findings is low compared to patients with less severe symptoms. In one study of 167 patients with reverse transcription polymerase chain reaction (RT‐PCR) test results and chest CT, only 4% of patients with positive RT‐PCR results had initial negative CT findings.89 In another study of 41 admitted patients, 100% had abnormal CT findings.90 Even for chest radiography, known to have lower sensitivity than chest CT,91 in patients with severe disease, abnormalities are identified on imaging more than 75% of the time.92 Thus, entirely negative LUS findings in patients with moderate or severe symptoms should prompt an additional work‐up.
Recommendations on the Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19 and Moderate or Severe Symptoms if Chest Radiography Has Already Been Performed
14. If the chest radiographic findings are negative, an LUS examination should be performed next (strong recommendation).
As previously noted, for patients with moderate or severe symptoms, chest radiographic findings are expected to be abnormal most of the time.92 Lung US may have higher sensitivity, especially for patients with moderate‐to‐severe disease.33 Thus, in cases in which chest radiographic findings are negative, LUS may have a higher yield for findings that support the presence of pneumonitis (Figure 1).
15. If the chest radiographic findings are negative, positive LUS findings for pneumonitis may preclude the need to pursue additional imaging if the pretest probability of an alternative or secondary diagnosis is low (strong recommendation).
Similar to recommendation 12, in the setting of positive LUS findings for pneumonitis in which the pretest probability of an alternative or secondary diagnosis is low, additional imaging may not be necessary. The decision to pursue additional imaging must be individualized.
16. If the chest radiographic findings are negative, negative LUS findings for pneumonitis should prompt an additional workup (strong recommendation).
For patients with moderate or severe symptoms, negative findings from both chest radiography and LUS should prompt an additional workup or search for an alternative diagnosis. The use of additional imaging studies with higher sensitivities (such as CT) should be considered.93
17. If the chest radiographic findings are positive for pneumonitis, and the pretest probability of an alternative or secondary diagnosis is low, an LUS examination should be performed (weak recommendation).
For a patient with moderate or severe symptoms whose chest radiographic findings are positive for pneumonitis without additional concerning features such as large consolidation and large pleural effusion, and the pretest probability of an alternative or secondary diagnosis is low, an LUS examination should be performed. Although the results of LUS may not alter patient treatment in many of these instances, an argument could be made for its use in patient monitoring or to rule out concerning US features that may prompt an additional workup such as the presence of a complex pleural effusion.
Recommendations on the Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19 and Moderate or Severe Symptoms if chest CT Has Already Been Performed
18. If the chest CT findings are positive for pneumonitis without additional concerning features, and the pretest probability of an alternative or secondary diagnosis is low, we do not recommend performing an LUS examination (no consensus on the strength of the recommendation).
For a patient with moderate or severe symptoms whose chest CT findings are positive for pneumonitis without additional concerning features, and the pretest probability of an alternative or secondary diagnosis is low, LUS is not indicated, as the results are unlikely to alter patient treatment in these instances or assist substantially with monitoring (Figure 1).
19. If the chest CT findings are negative for pneumonitis or other concerning features such as pulmonary embolism, and the pretest probability of an alternative or secondary diagnosis is low, we do not recommend performing an LUS examination (no consensus on the strength of the recommendation).
Given the superior accuracy of chest CT over LUS,93 including its ability to image centrally based abnormalities and to diagnose pulmonary embolism, in the setting of negative chest CT findings, an additional role for LUS, although unlikely harmful, is likely limited and may not represent optimal use of available resources, as the results are unlikely to alter patient treatment. As such, we do not recommend performing an LUS examination.
Recommendations on the Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19 and Minimal Symptoms
20. Lung US should be the initial imaging modality of choice (strong recommendation).
For patients with COVID‐19 with minimal symptoms, chest radiographic findings may be negative in as high as 58% of cases,55 and the use of chest radiography in these patients is not currently recommended by either the Canadian Association of Thoracic Radiology/Canadian Association of Radiologists94 or the Fleischner Society.95 Lung US may potentially have higher sensitivity55 in addition to lower radiation risks. Thus, in settings in which a device is readily available and the practitioner is skilled at LUS, we recommend that LUS be considered the initial imaging modality of choice (Figure 2).
Figure 2.
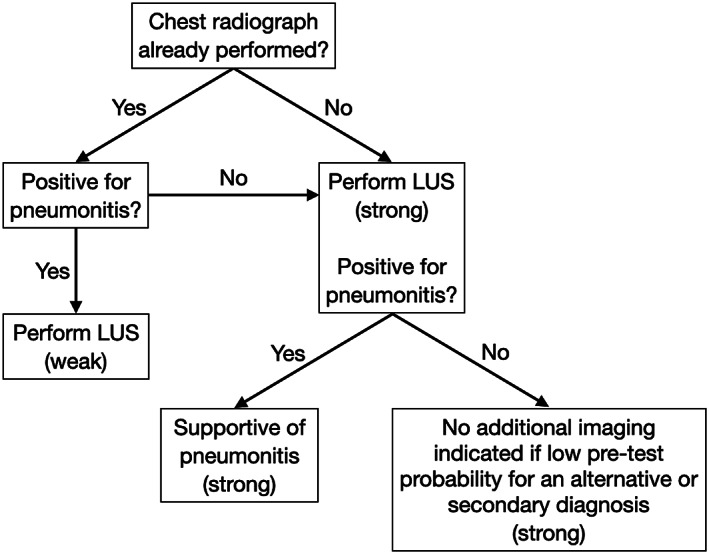
Recommended algorithm on LUS in the assessment of medical inpatients with known or suspected COVID‐19 with minimal symptoms, with the strength of the recommendation indicated in parentheses.
Recommendations on the Use of LUS in the Assessment of Medical Inpatients With Known or Suspected COVID‐19 and Minimal Symptoms if Chest Radiography Has Already Been Performed
21. Negative chest radiographic findings alone are insufficient for ruling out pneumonitis. Additional chest imaging such as LUS is recommended (strong recommendation).
Because of the low sensitivity of chest radiography for detecting pneumonitis in patients with minimal symptoms,55 additional chest imaging could be considered. Although CT has higher sensitivity,70 its use is not recommended in patients with mild symptoms.95 Thus, although LUS use in such settings is only supported by low‐certainty evidence,96 its use by trained practitioners is accompanied by minimal risks and thus should be considered (Figure 2).
22. Negative chest radiographic findings and positive LUS findings would support the diagnosis of pneumonitis (strong recommendation).
In a patient with mild symptoms, similar to recommendation 11, positive LUS findings for pneumonitis would be supportive of the diagnosis of pneumonitis but not diagnostic of COVID‐19. An additional workup such as RT‐PCR should still be performed in accordance with local protocols and policies.45
23. Negative chest radiographic findings and negative LUS findings are sufficient for ruling out pneumonitis. We do not recommend additional imaging if the pretest probability of an alternative or secondary diagnosis is low (strong recommendation).
In patients with mild symptoms, negative radiographic findings and negative LUS findings may preclude the need for additional thoracic imaging studies in patients with a low pretest probability of an alternative or secondary diagnosis. An additional workup such as RT‐PCR should still be performed in accordance with local protocols and policies.45
24. Positive chest radiographic findings for pneumonitis without additional concerning features would support the diagnosis of pneumonitis. Additional imaging such as LUS can still be considered (weak recommendation).
Although positive chest radiographic findings would support the diagnosis of pneumonitis, LUS may still be considered for monitoring purposes as well as for the higher sensitivity of LUS in identifying and visualizing pleural effusions.97, 98
Discussion
Our experts reached consensus on 24 recommendations on the use of LUS in the assessment of medical inpatients with known or suspected COVID‐19 in the Canadian internal medicine inpatient practice setting. Of these 24 recommendations, 9 are general ones, encompassing recommendations on technique, general interpretation, serial monitoring, and disposition decisions. Of the remaining 15 recommendations, 10 are directed toward the assessment of patients with moderate or severe symptoms and 5 toward those with minimal symptoms. We believe a symptom severity–based approach is necessary, as there is unlikely a setting whereby the use of LUS would be universally helpful or universally harmful. Context is a critical factor in clinical decision making. Our group strongly recommends that LUS be the initial imaging modality of choice in symptomatic patients with known or suspected COVID‐19. Although this recommendation may represent a substantial departure from the way internal medicine is usually practiced in Canada, there is increasingly robust evidence to support its use99 for centers with the capacity and capability for performing LUS examinations. Our recommendations were developed with a number of assumptions in mind. Specifically, we assumed that a POCUS device is readily available; a trained LUS practitioner is available and skilled at image acquisition, interpretation, and clinical integration; POCUS images/clips are archivable for later review; and the POCUS devices are appropriately cleaned and disinfected after each use. Last, we assumed that the health care capacity is such that other imaging modalities remain available. Should such time occur when the capacity for other clinically indicated imaging modalities is entirely overwhelmed, the role of POCUS and LUS may need to evolve accordingly to fill any necessary gap.
Our study has a number of limitations. First is the issue of generalizability. Our recommendations are focused on the Canadian medical inpatient setting, not critically ill patients in the intensive care unit or ambulatory outpatients. Given the input from a number of international experts on our panel, these recommendations will likely apply more broadly to medical inpatient settings outside Canada. However, adoption of these recommendations outside their intended setting should be mindful of contextual factors. We have also made a number of aforementioned assumptions, such that improperly trained practitioners using LUS, the use of improperly disinfected devices, or insufficient work flow processes to support its use would be associated with substantially higher risks of harm than that considered by our expert panel. Thus, our recommendations do not apply in those settings. Second, our recommendations only relate to LUS and not POCUS in other systems such as focused cardiac, deep venous thrombosis, and abdominal scans. Although there are likely benefits to the use of multisystem POCUS in a disease entity that involves multiple organ systems,100 the reality of the current internal medicine practice environment is such that only 5% of practitioners report using POCUS for clinical assessments.101 Thus, we restricted our focus to LUS, which is one of the easier applications to learn.102 Third, whereas we intended to conduct only 3 rounds of the survey, we ultimately needed to conduct a fourth round to determine the strength of recommendations for those that the experts reached consensus on do not recommend. With this additional round, we were unable to reach consensus on the strength of 2 such recommendations. Fourth, our literature review was not intended to be a systematic review: we used only a single database (PubMed) with abstract screening and review performed by only a single author (I.W.Y.M.). Fifth, our recommendations require that clinicians, in their decisions to pursue or not pursue additional investigations, consider the pretest probability of alternative or superimposed diagnoses. To do so will require the integration of multiple factors, which include but are not limited to age, sex, the presence or absence of comorbidities, presenting signs and symptoms, and investigation results. For example, the presence of pneumonitis on LUS imaging should not be used as evidence to forgo an additional workup in an elderly patient with a history of malignancy presenting with chest pain, hemoptysis, and a swollen leg. Clinical integration of LUS findings must be properly applied. Last but not least, the reality of COVID‐19 is that the evidence is rapidly evolving. For example, a systematic review and meta‐analysis is purportedly under way.103 Thus, new data and evidence will need to be incorporated as they emerge.
These limitations notwithstanding, our derived recommendations have some notable strengths. First, many of the members of the panel are well‐known POCUS experts, familiar not only with the technique of LUS and the literature but also with its inherent limitations. Second, our recommendations are pragmatic ones. For example, for patients with minimal symptoms, imaging is not recommended.94, 95 However, the reality is such that many of these patients referred to internal medicine will already have had imaging performed. Our recommendations give clinicians some guidance on how to incorporate existing imaging information with the use of LUS.
In summary, in symptomatic medical inpatients with known or suspected COVID‐19, we recommend the use of LUS, which can help support the diagnosis of pneumonitis (but not specifically diagnose COVID‐19), rule out concerning US features that may require an additional workup, and monitor patients with a change in their clinical status and may avoid unnecessary additional imaging for patients whose pretest probability of an alternative or superimposed diagnosis is low. We do not recommend the use of LUS findings to guide admission and discharge decisions. We do not recommend routine serial LUS examinations in patients without a change in their clinical condition.
Dr Ma is funded by the John A. Buchanan Chair in General Internal Medicine at the University of Calgary. The funder had no role in the design, execution, analyses, or interpretation of the data or the decision to submit this work. All of the other authors of this article have reported no disclosures.
References
- 1.World Health Organization . Coronavirus disease (COVID‐19) weekly epidemiological update. World Health Organization website. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed October 20, 2020.
- 2.Centers for Disease Control and Prevention . Preparedness tools for healthcare professionals and facilities responding to coronavirus (COVID‐19). Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/preparedness-checklists.html. Accessed October 20, 2020.
- 3.Centers for Disease Control and Prevention . Testing for COVID‐19. Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html. Accessed October 20, 2020.
- 4.American College of Radiology . ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID‐19 infection. American College of Radiology website. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed October 20, 2020.
- 5.Davenport MS, Bruno MA, Iyer RS, et al. ACR statement on safe resumption of routine radiology care during the coronavirus disease 2019 (COVID‐19) pandemic. J Am Coll Radiol 2020; 17:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargani L, Soliman‐Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID‐19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging 2020; 21:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng MY, Lee EY, Yang J, et al. Imaging profile of the COVID‐19 infection: radiologic findings and literature review. Radiol Cardiothorac Imaging 2020; 2:e200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID‐19 from viral pneumonia on chest CT. Radiology 2020; 296:E46–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Huang Y, Gao F, Yuan L, Wang Z. Lung ultrasonography versus chest CT in COVID‐19 pneumonia: a two‐centered retrospective comparison study from China. Intensive Care Med 2020; 46:1761–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19) [published online February 28, 2020]. SSRN. 10.2139/ssrn.3544750. [DOI]
- 11.Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology 2020; 295:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zieleskiewicz L, Markarian T, Lopez A, et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID‐19 pneumonia. Intensive Care Med 2020; 46:1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetrugno L, Bove T, Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow‐up of severity of lung involvement for management of patients with COVID‐19. Echocardiography 2020; 37:625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mongodi S, Orlando A, Arisi E, et al. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and health care provider exposure to COVID‐19. Ultrasound Med Biol 2020; 46:2090–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker K, Rippey J. Lung ultrasound in a COVID pandemic—choosing wisely. Australas J Ultrasound Med. 2020; 23(3):159–166. 10.1002/ajum.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines, 1: introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64:383–394. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humphrey‐Murto S, Varpio L, Gonsalves C, Wood TJ. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach 2017; 39:14–19. [DOI] [PubMed] [Google Scholar]
- 19.Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38:577–591. [DOI] [PubMed] [Google Scholar]
- 20.Jambrik Z, Monti S, Coppola V. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004; 93:1265–1270. [DOI] [PubMed] [Google Scholar]
- 21.Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound 2014; 12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B‐lines and N‐terminal pro‐brain‐type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009; 16:201–210. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID‐19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2020; 214:1287–1294. [DOI] [PubMed] [Google Scholar]
- 26.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology 2020; 295:202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID‐19). Radiology 2020; 295:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID‐19): analysis of nine patients treated in Korea. Korean J Radiol 2020; 21:494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID‐19 in Rome, Italy. Radiology 2020; 296:E79–E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang R, Li X, Liu H, et al. Chest CT severity score: an imaging tool for assessing severe COVID‐19. Radiol Cardiothorac Imaging 2020; 2:e200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 2020; 215:87–93. [DOI] [PubMed] [Google Scholar]
- 32.Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019‐nCoV) pneumonia. Radiology 2020; 295:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu W, Zhang S, Chen B, et al. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus sisease‐19 (COVID‐19) by bedside ultrasound. Ultraschall Med 2020; 41:300–307. [DOI] [PubMed] [Google Scholar]
- 34.Xing C, Li Q, Du H, Kang W, Lian J, Yuan L. Lung ultrasound findings in patients with COVID‐19 pneumonia. Crit Care 2020; 24:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID‐19: a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duggan NM, Shokoohi H, Liteplo AS, Huang C, Goldsmith AJ. Best practice recommendations for point‐of‐care lung ultrasound in patients with suspected COVID‐19. J Emerg Med 2020;59(4):515–520. 10.1016/j.jemermed.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.British Society of Thoracic Imaging . BSTI covid‐19 lung ultrasound guidance. British Society of Thoracic Imaging website. https://www.bsti.org.uk/standards-clinical-guidelines/clinical-guidelines/bsti-covid-19-lung-ultrasound-guidance/. Accessed October 20, 2020.
- 38.Kiamanesh O, Harper L, Wiskar K, et al. Lung ultrasound for cardiologists in the time of COVID‐19. Can J Cardiol 2020; 36:1144–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore S, Gardiner E. Point of care and intensive care lung ultrasound: a reference guide for practitioners during COVID‐19. Radiography 2020; 26:e297–e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassalou EE, Karantanas AH, Antoniou KM. Proposed lung ultrasound protocol during the COVID‐19 outbreak. J Ultrasound Med 2020. 10.1002/jum.15402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomoro P, Verde F, Zerboni F, et al. COVID‐19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single‐center study and comprehensive radiologic literature review. Eur J Radiol Open 2020; 7:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group . Findings of lung ultrasonography of novel coronavirus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020; 46:849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Xue H, Wang M, He N, Lv Z, Cui L. Lung ultrasound findings in patients with coronavirus disease (COVID‐19) [published online ahead of print July 22, 2020]. AJR Am J Roentgenol. https://www.ajronline.org/doi/10.2214/AJR.20.23513. [DOI] [PubMed] [Google Scholar]
- 44.Volpicelli G, Gargani L. Sonographic signs and patterns of COVID‐19 pneumonia. Ultrasound J 2020; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID‐19 [published online ahead of print June 16, 2020]. Clin Infect Dis. https://pubmed.ncbi.nlm.nih.gov/32556191/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gargani L, Doveri M, D'Errico L, et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology 2009; 48:1382–1387. [DOI] [PubMed] [Google Scholar]
- 47.Tardella M, Di Carlo M, Carotti M, Filippucci E, Grassi W, Salaffi F. Ultrasound B‐lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: cut‐off point definition for the presence of significant pulmonary fibrosis. Medicine (Baltimore) 2018; 97:e0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B‐lines in connective tissue disease‐associated interstitial lung disease: a literature review. Arthritis Res Ther 2017; 19:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinal‐Fernandez I, Pallisa‐Nuñez E, Selva‐O'Callaghan A, et al. Pleural irregularity, a new ultrasound sign for the study of interstitial lung disease in systemic sclerosis and antisynthetase syndrome. Clin Exp Rheumatol 2015; 33:S136–S141. [PMC free article] [PubMed] [Google Scholar]
- 50.Hani C, Trieu NH, Saab I, et al. COVID‐19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging 2020; 101:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID‐19) pneumonia. AJR Am J Roentgenol 2020; 215:338–343. [DOI] [PubMed] [Google Scholar]
- 52.Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology perspective of coronavirus disease 2019 (COVID‐19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol 2020; 214:1078–1082. [DOI] [PubMed] [Google Scholar]
- 53.Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect 2020; 80:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong HYF, Lam HYS, Fong AHT, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology 2020; 296:E72–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstock M, Echenique A, Russell J, et al. Chest x‐ray findings in 636 ambulatory patients with COVID‐19 presenting to an urgent care center: a normal chest x‐ray is no guarantee. J Urgent Care Med 2020; 14:13–18. [Google Scholar]
- 56.Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID‐19) outbreak: what the department of radiology should know. J Am Coll Radiol 2020; 17:447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID‐19). Eur Radiol 2020; 30:4007–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH. Essentials for radiologists on COVID‐19: an update—Radiology Scientific Expert Panel. Radiology 2020; 296:E113–E114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.British Society of Thoracic Imaging . Thoracic imaging in COVID‐19 infection: guidance for the reporting radiologist—updated version 2.0. British Society of Thoracic Imaging website. https://www.bsti.org.uk/standards-clinical-guidelines/clinical-guidelines/bsti-covid-19-guidance-for-the-reporting-radiologist/. Accessed October 20, 2020.
- 60.Hare SS, Rodrigues JCL, Jacob J, et al. A UK‐wide British Society of Thoracic Imaging COVID‐19 imaging repository and database: design, rationale and implications for education and research. Clin Radiol 2020; 75:326–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai WC, Zhang HW, Yu J, et al. CT imaging and differential diagnosis of COVID‐19. Can Assoc Radiol J 2020; 71:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med 2011; 37:224–232. [DOI] [PubMed] [Google Scholar]
- 65.Lichtenstein D, Mezière G, Biderman P, Gepner A. The comet‐tail artifact: an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999; 25:383–388. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein D, Mezière G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 2000; 26:1434–1440. [DOI] [PubMed] [Google Scholar]
- 67.Volpicelli G, Lamorte A, Villen T. What's new in lung ultrasound during the COVID‐19 pandemic. Intensive Care Med 2020; 47:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma I, Somayaji R, Rennert‐May E, et al. Canadian Internal Medicine Ultrasound (CIMUS) recommendations regarding internal medicine point‐of‐care ultrasound (POCUS) use during coronavirus (COVID‐19) pandemic. Can J Gen Intern Med 2020; 15:8–11. [Google Scholar]
- 69.Krishnan SMBBS, Kuhl TMD, Ahmed WMD, Togashi KMD, Ueda KMD. Efficacy of an online education program for ultrasound diagnosis of pneumothorax. Anesthesiology 2013; 118:715–721. [DOI] [PubMed] [Google Scholar]
- 70.Meng H, Xiong R, He R, et al. CT imaging and clinical course of asymptomatic cases with COVID‐19 pneumonia at admission in Wuhan, China. J Infect 2020; 81:e33–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Centers for Disease Control and Prevention . Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID‐19). Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed October 20, 2020.
- 72.Pediatric‐Adolescent Treatment Africa . International pulmonologist's consensus on COVID‐19. Pediatric‐Adolescent Treatment Africa website. http://teampata.org/wp-content/uploads/2020/03/INTERNATIONAL-PULMONOLOGIST%E2%80%99S-CONSENSUS-ON-COVID-19.pdf..pdf. Accessed October 20, 2020.
- 73.Centers for Disease Control and Prevention . Discontinuation of transmission‐based precautions and disposition of patients with COVID‐19 in healthcare settings (interim guidance). Centers for Disease Control and Prevention website. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html. Accessed October 20, 2020.
- 74.World Health Organization . Clinical management of COVID‐19: interim guidance. World Health Organization website. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed October 20, 2020.
- 75.Alberta Health Services COVID‐19 Scientific Advisory Group . Key research question: are there criteria or simple tools that can be used to determine which patients with suspected/confirmed COVID‐19 are stable and appropriate for safe discharge from hospital or an alternate care centre? What follow‐up is required? Alberta Health Services website. https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-criteria-for-safe-discharge-from-hospital-rapid-review.pdf. Accessed October 20, 2020.
- 76.National Health Commission of the People's Republic of China . Diagnosis and treatment protocol for COVID‐19 (trial version 7). National Health Commission of the People's Republic of China website. http://en.nhc.gov.cn/2020-03/29/c_78469.htm. Accessed October 20, 2020.
- 77.European Centre for Disease Prevention and Control. Technical Report. Novel coronavirus (SARS‐CoV‐2): discharge criteria for confirmed COVID‐19 cases—when is it safe to discharge COVID‐19 cases from the hospital or end home isolation? European Centre for Disease Prevention and Control website. https://www.ecdc.europa.eu/en/publications-data/novel-coronavirus-sars-cov-2-discharge-criteria-confirmed-covid-19-cases. Accessed October 20, 2020.
- 78.European Centre for Disease Prevention and Control . Technical Report. Guidance for discharge and ending isolation in the context of widespread community transmission of COVID‐19: first update. European Centre for Disease Prevention and Control website. https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation. Accessed October 20, 2020.
- 79.Zhou S, Zhu T, Wang Y, Xia L. Imaging features and evolution on CT in 100 COVID‐19 pneumonia patients in Wuhan, China. Eur Radiol 2020; 30:5446–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID‐19 pneumonia: a longitudinal study. Radiology 2020; 296:E55–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ji L, Li Y, Cao C, Lv Q, Xie M. Serial bedside lung ultrasonography in a critically ill COVID‐19 patient. QJM 2020; 113:491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Federation for Ultrasound in Medicine and Biology Safety Committee , Abramowicz JS, Basseal JM. World Federation for Ultrasound in Medicine and Biology position statement: how to perform a safe ultrasound examination and clean equipment in the context of COVID‐19. Ultrasound Med Biol 2020; 46:1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skowronek P, Wojciechowski A, Leszczyński P, et al. Can diagnostic ultrasound scanners be a potential vector of opportunistic bacterial infection? Med Ultrason 2016; 18:326–331. [DOI] [PubMed] [Google Scholar]
- 84.American Institute of Ultrasound in Medicine . Guidelines for cleaning and preparing external‐ and internal‐use ultrasound transducers and equipment between patients as well as safe handling and use of ultrasound coupling gel. American Institute of Ultrasound in Medicine website. https://www.aium.org/officialStatements/57. Accessed October 20, 2020. [DOI] [PubMed]
- 85.FUSIC . Committee on behalf of the Intensive Care Society and endorsed by the FAMUS working group on behalf of the Society for Acute Medicine. Decontamination guidelines: ultrasound transducer and equipment cleaning and disinfection. Intensive Care Society website. https://www.acutemedicine.org.uk/wp-content/uploads/2020/03/FUSIC_FAMUS-Decontamination-guidelines.pdf [Google Scholar]
- 86.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost 2020; 18:1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; 191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of d‐dimer as predictive factors. J Thromb Thrombolysis 2020; 50:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical coronavirus disease 2019 (COVID‐19) pneumonia: relationship to negative RT‐PCR testing. Radiology 2020; 296:E41–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adams SJ, Dennie C. Chest imaging in patients with suspected COVID‐19. CMAJ 2020; 192:E676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Besutti G, Giorgi Rossi P, Iotti V, et al. Accuracy of CT in a cohort of symptomatic patients with suspected COVID‐19 pneumonia during the outbreak peak in Italy. Eur Radiol 2020; 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dennie C, Hague C, Lim RS, et al. Canadian Society of Thoracic Radiology/Canadian Association of Radiologists consensus statement regarding chest imaging in suspected and confirmed COVID‐19. Can Assoc Radiol J 2020; 71:470–481. [DOI] [PubMed] [Google Scholar]
- 95.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner Society. Chest 2020; 158:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.World Health Organization . Use of chest imaging in COVID‐19: a rapid advice guide. World Health Organization website. https://www.who.int/publications/i/item/use-of-chest-imaging-in-covid-19. Accessed October 20, 2020.
- 97.Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med 2015; 10:811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yousefifard M, Baikpour M, Ghelichkhani P, et al. Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion: a meta‐analysis. Emerg (Tehran) 2016; 4:1–10. [PMC free article] [PubMed] [Google Scholar]
- 99.Emanuele P, Alberto G, Maria T, et al. Lung ultrasound for the diagnosis of SARS‐CoV‐2 pneumonia in the emergency department [published online ahead of print October 13, 2020]. Ann Emerg Med. . 10.1016/j.annemergmed.2020.10.008. [DOI] [Google Scholar]
- 100.Narasimhan M, Koenig SJ, Mayo PH. A whole‐body approach to point of care ultrasound. Chest 2016; 150:772–776. [DOI] [PubMed] [Google Scholar]
- 101.Wong J, Montague S, Wallace P, et al. Barriers to learning and using point‐of‐care ultrasound: a survey of practicing internists in six North American institutions. Ultrasound J 2020; 12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller A. Practical approach to lung ultrasound. BJA Educ 2015; 16:39–45. [Google Scholar]
- 103.Yang Y, Zhang D, Zhou C, Huang H, Wang R. Value of lung ultrasound for the diagnosis of COVID‐19 pneumonia: a protocol for a systematic review and meta‐analysis. BMJ Open 2020; 10:e039180. [DOI] [PMC free article] [PubMed] [Google Scholar]


