Abstract
Sea turtles are exposed to trace elements through water, sediment, and food. Exposure to these elements has been shown to decrease immune function, impair growth, and decrease reproductive output in wildlife. This study compares trace element concentrations in green turtles in captivity at Sea Life Park Hawaii (n = 6) to wild green turtles in Kapoho Bay, HI (n = 5 to 7). Blood and scute samples were collected and analyzed for eleven elements via inductively coupled plasma-mass spectrometry (ICP-MS). Selenium was significantly greater (p < 0.05) in the blood of captive turtles compared to wild turtles, while V, Ni, and Pb were significantly greater in the blood of wild turtles. In scute, V, Cu, Se, and Cr were significantly greater in captive turtles, while As was significantly greater in wild turtles. Pelleted food fed to the captive turtles and representative samples of the wild turtle diet were analyzed via ICP-MS to calculate trophic transfer factors and daily intake values. Wild turtles had greater estimated daily intake than captive turtles for all elements except Cu and Se. Because captive turtles are fed a diet very different from their wild counterparts, captive turtles do not represent control or reference samples for chemical exposure studies in wild turtles. No toxic thresholds are known for sea turtles, but rehabilitation and managed care facilities should monitor sea turtle elemental concentrations to ensure the animals’ health.
Keywords: Marine turtle, reptile, aquarium, Hawaii, captive, heavy metals
Graphical Abstract
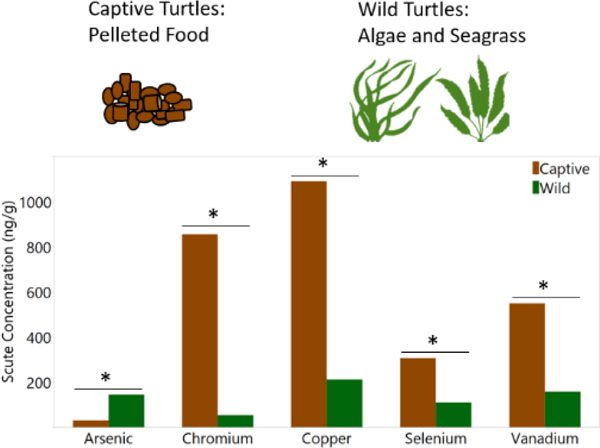
The differences in elemental concentrations between captive and wild turtles are primarily due to their food source. Captive turtles are given a pelleted food that is a mixture of animal and plant protein products with additives, while wild turtles have a primarily herbivorous diet.
INTRODUCTION
Trace elements have been found in marine vertebrates around the world including cetaceans, seabirds, fishes, and sea turtles (Gochfeld et al. 1999; Gardner et al. 2006; Araújo and Cedeño-Macias 2016; Hansen et al. 2016). Some elements, including K, Na, Mg, Ca, Mn, Fe, Co, Ni, Cu, Zn, Mo, and Se, are essential for enzymatic activity, cell structure, immune response, and other bodily functions. Essential elements can become toxic at high concentrations, while nonessential elements, e.g., As, Hg, and Pb, can be toxic at very low concentrations (Gadd 1992; Liu et al. 2008; Cortes-Gomez et al. 2017). These nonessential elements can gain access to cells by mimicking essential elements, potentially disrupting cellular processes (Liu et al. 2008; Perrault et al. 2017). This can adversely affect the health of organs, the central nervous system, and the immune system through acute or chronic exposure (Liang et al. 2004).
Hawaiian green turtles (Chelonia mydas) face anthropogenic threats such as pollution, fisheries bycatch, and habitat loss. C. mydas are classified as threatened under the United States Endangered Species Act and listed in Appendix I of the Convention of International Trade in Endangered Species (IUCN 2019; Sinaei and Bolouki 2017). Age classes are defined based on straight carapace length (SCL) measured from nuchal notch to the tip of the posterior-most marginal scute. Juvenile sea turtles have a SCL < 75 cm, subadult turtles range between 75 cm to 80 cm SCL and adult turtles have a SCL > 80 cm. Hawaiian green turtles recruit to nearshore habitats at around 35 cm SCL. Once in nearshore habitats, subadult and adult turtles exhibit high site fidelity to a particular location. Green turtles are bioindicators of the environment due to their long lives, high site fidelity, and accumulation of contaminants from food, water, and sediment inadvertently ingested while eating (Aguirre and Lutz 2004; Lam et al. 2007; Andreani et al. 2008; Duncan et al. 2019).
Natural and anthropogenic contaminants, including metals, are one of the hazards potentially contributing to the decline of green turtles worldwide (Villa et al. 2015). Wild green sea turtles have a lifespan of 75 years, potentially bioaccumulating metals; though trace element concentrations in tissues are generally one to two orders of magnitude lower in sea turtles than seabirds or marine mammals (Lutcavage et al. 1997; Milton and Lutz 2003; Mayne et al. 2020).
Contaminant monitoring in sea turtles can help elucidate the risk of trace elements to the species as well as to the ecosystems in which they inhabit (Cortes-Gomez et al. 2017). Blood samples can be used to estimate elemental contamination in liver, muscle, and kidney tissue of green turtles (Van de Merwe et al. 2010). Whole blood allows measurement of elements in both intracellular and extracellular compartments and represents recent (weeks to months) exposure to contaminants (Takeuchi et al. 2016; Villa et al. 2017). Conversely, scutes are hard, keratinized plates that make up the shell of a sea turtle. Many trace elements bind to the keratin of the scutes, incorporating and storing contaminants over time, giving a history of exposure (Sakai et al. 2000; Day et al. 2005; Innis et al. 2008; Van de Merwe 2008; Komoroske et al. 2011; Bezerra et al. 2013; Bryan 2013; Perrault et al. 2017). Once incorporated into scute, elements become metabolically inactive and are unavailable for remobilization (Day et al. 2010). All species of sea turtle are threatened or endangered and protected by the Endangered Species Act, making non-lethal samples a requirement. Blood and scute, along with food samples, can be used to monitor elemental contaminant exposure in live turtles. Trophic transfer factor (TTF) is defined as the ratio between the concentration of an element in the animals’ tissue to the concentration of the element in its food (DeForest et al. 2007). A TTF value > 1 indicates elements may biomagnify, while a TTF <1 means biomagnification is unlikely to occur (Mathews and Fisher 2008).
Contaminant monitoring in captive turtles may help answer questions about wild turtles. Sea turtles have been raised and rehabilitated in captivity for decades (Owens and Blainvillain 2013). In addition to rehabilitation, sea turtles in captivity can provide valuable insight into disease pathways and physiology (Falk et al. 2007). Captive turtles are fed a prescribed diet and offer a unique opportunity to study the trophic transfer of trace elements, and may potentially serve as a baseline of elemental concentrations in sea turtles.
The present study quantified the mass fractions (hereinafter called concentrations) of the inorganic elements: As, Cd, Co, Cr, Cu, Ni, Pb, Sb, Se, Sr, and V in the blood and scutes of adult captive and wild Hawaiian green turtles. Studies have previously been conducted on sea turtles in captivity, quantifying trace elements in headstarted (captive reared) Kemp’s Ridley turtles (Lepidochelys kempii); a green turtle in rehabilitation, and captive hawksbill (Eretmochelys imbricata), green, and loggerhead (Caretta caretta) turtles (Bezerra et al. 2014; Orvik 1997; Suzuki et al. 2012a,2012b). To our knowledge this is the first study to measure elemental concentrations in captive turtle food and to calculate TTF and dietary intake values. Past studies have incorrectly suggested that captive turtles may serve as control or reference samples for health assessments, contaminant exposure, or toxicology studies because they might be exposed to lesser contaminants than wild turtles (Arthur et al. 2008; Casal et al. 2009; Basile et al. 2012; Suzuki et al. 2012a, Suzuki et al. 2012b). Since sea turtles receive most of their contaminant load from diet, captive turtles are still exposed to chemicals through provided food, whether wild caught or formulated, but likely at different concentrations than wild turtles (Keller 2013).
MATERIALS AND METHODS
Sampling
Samples were collected according to the Biological and Environmental Monitoring and Archival of Sea Turtle Tissues protocols (Keller et al. 2014). Blood, scute, algae, pelleted food, and water samples were stored in liquid nitrogen vapor freezers (LN2, −150 °C) until shipped frozen to Texas Tech University for analysis. Health status and morphometrics of all turtles can be found in Table S1. Wild green turtles were captured in November 2011 and April 2015 by hand or scoop net in Kapoho Bay on the eastern side of Hawaii Island (19.496291 N −154.820698 W, Figure 1), which is now covered by new lava from the 2018 eruption of Kilauea. After capture, turtles were brought ashore and blood samples taken. Briefly, double-ended stainless-steel needles were used to draw blood into glass sodium heparin Vacutainer blood collection tubes within 15 min of capture. An average of 16.4 mL of blood was taken from each turtle (range: 13 mL – 19 mL). Blood was kept on ice until aliquoted and frozen.
Figure 1:
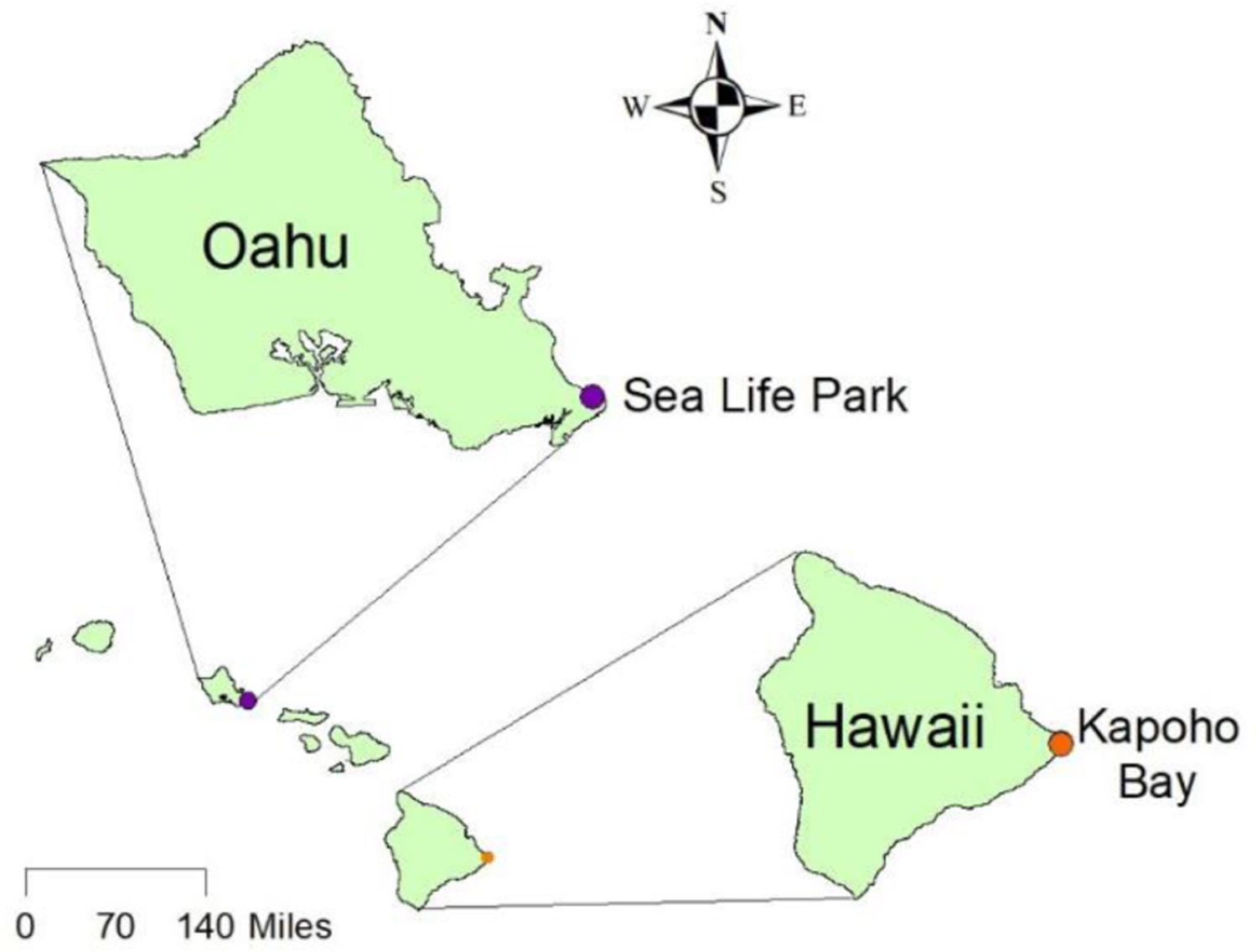
Location of Sea Life Park Hawaii on the island of Oahu and Kapoho Bay on the island of Hawaii.
Scute shavings were collected from the fifth central scute. The scute was cleaned of epiphytic/epibiotic organisms and sloughing keratin removed with a wet plastic scrubbing pad. The scute and knife were cleaned with 70% isopropanol and Millipore high purity 18 M Ω cm−1 water (hereinafter referred to as high purity deionized water), and dried with a cleanroom wiper. The top layer of scute that is penetrated with algae was shaved off and discarded. The knife and scute were cleaned again with 70% isopropanol and high purity deionized water and dried. The knife was used to shave keratin layers off the entire surface of the 5th central scute, being careful to avoid scute seams or shaving too deep. Scute shavings were collected in a Teflon bag and sealed with a cable tie. An average of 0.86 g of scute was collected from each individual (range: 0.69 g – 1.04 g). Scutes were homogenized by mortar and pestle (pre-cleaned by soapy water sonication, high purity deionized water and acid rinsing) prior to analysis. Samples of known algae prey items (Gracilaria salicornia and Amancia spp.) were collected from Kapoho Bay in December 2013 while snorkeling to represent the wild green turtle diet (Arthur and Balazs 2008). Dive gloves were worn while collecting algae samples, which were placed in centrifuge tubes, and stored in LN2 until analysis.
Green turtles were brought into Sea Life Park Hawaii (SLPH) from the wild in the mid-to-late 1960s and are one of the few captive breeding colonies in the world. They are fed a commercially available pelleted floating compound (35% protein turtle finisher, Melick Aquafeed, Catawissa, PA 17820 USA) designed to meet the nutritional requirements of green turtles. The 14 adult green turtles (9 females, 5 males) living in the pond are fed 2.3 kg of pelleted food per day directly into the exhibit without turtle separation. Their diet is supplemented with fresh lettuce. Blood and scute were sampled from six adult captive turtles (three male and three female, without additional selection criteria) in August 2012 following the same protocol as the wild turtles. Seawater samples were collected in October 2017 in 50 mL centrifuge tubes (Corning Inc., Corning, NY 14831 USA) from three points in the pond (the water inlet, the center of the pond and near the nesting beach). The Kapoho Bay turtles (three males, two females, and two of unknown sex) without externally visible fibropapillomatosis tumors were selected based on a similar SCL (nuchal notch to tip) as the captive turtles. All sea turtles in this study were adult or sub-adult sea turtles, with a SCL of at least 76 cm. A Welch’s two sample t-test (p = 0.43, t = 0.83) showed no difference in mean SCL between the two groups, reducing the variability in elemental concentrations due to life stage.
Sample digestion and ICP-MS analysis
A small mass digestion method was used (French et al. 2017). Trace metal grade nitric acid (HNO3; 0.2 mL, 5.53 mol/L, Fisher Scientific, Fair Lawn, New Jersey 07410, USA), trace metals grade hydrochloric acid (HCl; 0.1 mL, 0.99 mol/L, Fisher Scientific), and high purity deionized water (0.1 mL) were added to approximately 0.1 g scute in 15 mL centrifuge tubes. Tubes were heated in a reciprocal shaking hot water bath (Model 66800, Precision Scientific, Chennai, Tamil Nadu, India 600086) at 90 °C (± 5 (SD) °C) for 1 h. After cooling, 0.1 mL high purity 30% hydrogen peroxide (H2O2, Fisher Scientific) was added and samples were heated again for 30 min. Samples were subsequently diluted to 10 mL with high purity deionized water and filtered through 0.45 µm polytetrafluoroethylene (PTFE) filters (Whatman 0.45 µm GMF-150, Maidstone, ME England). Captive turtle food (0.1 g) was digested in the same manner as the scute samples. For blood samples, 0.4 mL HNO3, 0.1 mL HCl, and 0.1 mL high purity deionized water were added to 0.2 mL blood in 15 mL centrifuge tubes; vortexed; sonicated for 10 min; and heated in a hot water bath at 95 °C ± 5 (SD) °C for 1 h. After cooling, 0.2 mL H2O2 was added to the blood samples, vortexed, sonicated, and heated for 30 min. This process was repeated two more times until the blood samples were completely digested. Blood samples were diluted to 10 mL with high purity deionized water and filtered through 0.45 µm PTFE filters.
Algae samples were dried to complete dry mass overnight at 55 °C. Moisture content was determined by subtracting the dry weight from the wet weight and dividing that value by the wet weight. Moisture content of both samples was 46%. A subsample of the algae (0.2 g) was combined with 0.2 mL HNO3, 0.1 mL HCl, and 3 mL high purity deionized water in a 15 mL centrifuge tube. The samples were placed in a hot water bath at 95 °C (± 5 (SD) °C) for 1 h. After cooling, 0.2 mL H2O2 was added and the samples were heated for 30 min. Samples were filtered through 0.45 µm PTFE filters and diluted to 10 mL for analysis. Seawater samples collected from SLPH (0.5 mL) were acidified with 1.4 mL HNO3 and subsequently diluted with high purity deionized water in a 1:100 ratio. An in-house matrix control material was created using a seawater and algae sample. The seawater and algae samples were spiked with a custom multi-element standard containing Ag, Al, As, Cd, Co, Cr, Cu, Hg, Ni, Pb, Sb, Se, Sn, Sr, V, and Z (TTUNIV-1, Inorganic Ventures, Christianburg, VA USA) at 100 ng/g. The trace element concentrations in the natural samples were subtracted from the spiked samples to account for the natural trace element mass fractions in the sample. The in house matrix spike control material matched the spiked concentration by ± 20% (Eastern-Research-Group 2012, Table S2).
Elemental analyses were performed in helium collision mode using an Agilent Technologies 7900 inductively coupled plasma-mass spectrometer (ICP-MS) equipped with an Agilent Technologies ASX-500 Series autosampler (Agilent, Santa Clara, CA 95051 USA). A custom multi-element calibration standard (TTUNIV-1, Inorganic Ventures) was used to build a seven-point external calibration curve with concentrations ranging from 0.1 ng/g to 1000 ng/g. The calibration solutions were analyzed at the beginning and end of every run; and a check standard solution of 10 ng/g or 50 ng/g run every ten samples. Internal standards Bismuth (Bi), Germanium (Ge), Indium (In), Lutetium (Lu), Rhodium (Rh), Scandium (Sc), and Terbium (Tb; Agilent Technologies) were added online and samples were reanalyzed if the recovery was outside the acceptable recovery range of 80% to 120%. Quality assurance was conducted using method blanks, field blanks produced from the same lot numbers of blood collection supplies, and certified reference materials (CRMs; DOLT-5: Dogfish Liver from National Research Council Canada, Ottawa, Ontario, and Seronorm™ Trace Elements Whole Blood L-3 [REF 210313, LOT 1509408] from Sero AS, Billingstad, Norway). Measured values of As, Cd, Co, Cr, Cu, Pb, Se, and V are in agreement with the certified values in the Seronorm CRM and Ni and Sb overlap with the certified value (Table S2). Measured values of Co, Cr, Cu, Ni, and Pb are in agreement with the certified values in the Dolt 5 CRM (Table S2). The remaining elements (As, Cd, Se, Sr, and V) were within 20% of the certified values. The instrument detection limit (IDL) was determined by analyzing seven replicates of the 0.1 ng/g multi-element standard (Inorganic Ventures) and multiplying the standard deviation of these replicates by the Student’s t-test value (3.143), giving an IDL ranging from 0.02 ng/g to 0.39 ng/g (Table S2; Creed et al. 1994). Limit of quantification (LOQ) was calculated by multiplying the lowest concentration of the calibration curve by the dilution factor. The LOQ (ng/g) were 10, 0.29, 2, and 0.83 for seawater, algae, scute/food pellets, and blood, respectively. Both field and method blanks were subtracted from the samples. All blood values are in ng/g wet mass (wm); scute and captive turtle food in ng/g dry mass as received (not dried in an oven; dm) and algae in ng/g dry mass (oven dried).
Statistical analysis and data handling
All statistical analyses were performed using the program R (version 3.2.3, The R foundation for Statistical Computing, Vienna, Austria) and the Nondetects and Data Analysis for Environmental Data (NADA) package, recommended for left censored data (Helsel 2005), with a p ≤ 0.05 considered significant. Mean, median, and standard deviations were calculated using Kaplan-Meier (K-M) or regression on order statistical (ROS) models. Shapiro-Wilk and Bartlett tests were used to test normality and homoscedasticity of data. Differences in elemental concentrations between captive and wild turtles were determined by parametric (using the NADA function cenmle) or nonparametric (using cendiff) tests. A Kendall’s tau correlation was run (using cenken) to determine the relationships between SCL and elements in the blood or scute as well as to determine the relationship between blood and scute elemental concentrations.
Trophic transfer factor was calculated as the ratio between the concentration of an element in the tissue (blood or scute) to the concentration in the diet (pelleted food or algae; DeForest et al. 2007). For blood or scute concentrations below the LOQ, half the LOQ was used. Estimated daily intake (EDI) was calculated to estimate the daily exposure of captive and wild turtles to elemental contaminants (Perrault 2014). Concentrations of elements in algae was measured using the dry mass of the algae. Algae concentrations were converted from ng/g dm to ng/g wm using the moisture content of the algae (46%). The fourteen captive turtles are fed approximately 2.3 kg of pelleted food each day, giving a consumption rate of 164 g pelleted food per turtle per day. The consumption rate was multiplied by the measured elemental concentration of the pelleted food, giving a daily intake rate in µg per turtle per day. Captive turtles are fed additional, undocumented amounts of vegetables (lettuce) per day, but because of the amount uncertainties these were not included in the EDI. Wild turtle EDI was calculated using a daily food intake of 127 g (dm) per turtle per day as determined earlier (Williams 1988). G. salicornia made up approximately 40.9% of the green turtles’ diet at Kapoho and Amansia spp. approximately 30% (Russell and Balazs 2009). The remaining 29.1% was estimated using a 50:50 combination of the Amansia and G. salicornia concentrations.
RESULTS
Individual measurements of element concentrations in scute and blood samples are provided in Tables S3 and S4. Eleven elements were above LOQ in at least one blood or scute sample of captive and wild turtles (Table 1). Essential element Sr was found at the greatest concentration in the blood and scute of all sea turtles in this study. Lead, a toxic heavy metal, was found in the blood of all wild and captive turtles. Turtle E, the largest of the captive turtles, had the greatest scute concentrations of all elements amongst the captive turtles.
Table 1.
Elemental concentrations in the scute (ng/g dm) and blood (ng/g wm) of captive turtles at Sea Life Park Hawaii and wild turtles at Kapoho Bay, HI.
| SCUTE |
||||||
|---|---|---|---|---|---|---|
| Captive |
Wild |
|||||
| Element | Median | Mean (SD) | % detected | Median | Mean (SD) | % detected |
|
|
|
|||||
| As*a | 9.2 | 30.3 (55.8) | 50 | 138.0 | 144 (22.8) | 100 |
| Cdb | 9.72 | 15.5 (11.6) | 66.7 | 14.7 | 14.4 (3.70) | 80 |
| Cob | - | - | 16.7 | - | - | 0 |
| Cr*b | 119 | 855 (1825) | 100 | 55.5 | 53.8 (37.2) | 100 |
| Cu*a | 1030 | 1090 (372) | 100 | 221.0 | 212 (29.0) | 100 |
| Nib | 180 | 270 (241) | 100 | 111.0 | 134 (74.1) | 100 |
| Pba | 14.6 | 20.8 (16.8) | 33.3 | 26.8 | 32.9 (12.0) | 60 |
| Sb | - | - | 0 | - | - | 0 |
| Se*a | 350 | 306 (90.7) | 100 | 126 | 110 (33.9) | 100 |
| Sra | 7370 | 9150 (4660) | 100 | 5420 | 5920 (2160) | 100 |
| V*b | 450 | 549 (310) | 100 | 140 | 158 (80.9) | 100 |
| BLOOD |
||||||
| Captive |
Wild |
|||||
| Element | Median | Mean (SD) | % detected | Median | Mean (SD) | % detected |
|
|
|
|||||
| Asa | 22.8 | 28.8 (17.3) | 100 | 28.0 | 35.6 (24.2) | 100 |
| Cd | - | - | 0 | - | - | 14.3 |
| Co | - | - | 0 | 4.23 | 4.61 (1.47) | 42.9 |
| Crb | - | - | 0 | - | - | 14.3 |
| Cua | 611 | 609 (87.5) | 100 | 645 | 628 (75.3) | 100 |
| Ni*b | - | - | 16.7 | 38.0 | 40.4 (19.3) | 100 |
| Pb*a | 25.1 | 24.6 (12.7) | 100 | 55.3 | 69.3 (30.5) | 100 |
| Sb | - | - | 0 | - | - | 0 |
| Se*a | 440 | 439 (103) | 100 | 74.2 | 102 (61.4) | 100 |
| Sra | 717 | 789 (427) | 100 | 631 | 694 (137) | 100 |
| V*b | - | - | 0 | 11.6 | 12.7 (4.07) | 71.4 |
difference between captive and wild was determined by a parametric function (R NADA cenmle)
difference between captive and wild was determined by a nonparametric function (R NADA cendiff)
significant difference (p < 0.05) between captive and wild turtles
Ten elements were measured in the pelleted food at SLPH as well as representative samples of algae (Amansia spp. and G. salicornia) from Kapoho Bay (Table 2). Seawater samples from SLPH contained four elements > LOQ; Cr, Cu, Se, and Sr (Table 3). Seawater samples from Kapoho Bay were not analyzed in this study, however Cr, Cu, and Se concentrations published previously for Kapoho Tidepool water (Bienfang et al. 2009) were less than those in SLPH (Table 3).
Table 2.
Mean elemental concentrations in pelleted food (n = 3) and algae (n = 1) in ng/g dm.
| Element | Pelleted Food | Amansia spp. * | Gracilaria salicornia * |
|---|---|---|---|
|
|
|
||
| As | 259 ± 5.8 | 1,520 | 4,270 |
| Cd | 53.5 ± 1.9 | 518 | 196 |
| Co | 95.5 ± 6.1 | 281 | 39.3 |
| Cr | 523 ± 105 | 1,630 | 663 |
| Cu | 10,400 ± 2,460 | 3,610 | 985 |
| Ni | 2,490 ± 118 | 8,970 | 532 |
| Pb | 97.5 ± 16.8 | 1,030 | 388 |
| Sb | 11.3 ± 0.5 | 14.9 | 14.1 |
| Se | 767 ± 54.3 | 237 | 77.6 |
| Sr | 8,730 ± 593 | 79,000 | 31,100 |
| V | 208 ± 24.7 | 2,020 | 3,370 |
Moisture content of Amansia spp. was 46.2% and G. salicornia was 45.1%.
Table 3.
Elemental concentrations (ng/mL) at the water inlet, middle of the pond, and near the nesting beach in the sea turtle habitat at SLPH compared to seawater from Kapoho Tidepools published previously (Bienfang et al. 2009).
| Element | Water Inlet | Middle | Nesting Beach | Kapoho Tide Pool |
|---|---|---|---|---|
|
|
|
|||
| As | < LOQ | < LOQ | < LOQ | 1.48 |
| Cd | < LOQ | < LOQ | < LOQ | - |
| Co | < LOQ | < LOQ | < LOQ | 0.01 |
| Cr | 15.8 | 14.1 | 15 | 0.14 |
| Cu | 280 | < LOQ | < LOQ | 0.19 |
| Ni | < LOQ | < LOQ | < LOQ | 0.24 |
| Pb | < LOQ | < LOQ | < LOQ | 0.01 |
| Sb | < LOQ | < LOQ | < LOQ | - |
| Se | 32.8 | < LOQ | 23.9 | 0.02 |
| Sr | 4,450 | 4,490 | 4,200 | - |
| V | < LOQ | < LOQ | < LOQ | 1.52 |
<LOQ = less than the limit of quantitation
Significant differences (p < 0.05) were observed in blood and scute elemental concentrations between captive and wild turtles (Table 1). Captive turtles had significantly greater blood Se, an essential element, than wild turtles (Table 1). Three toxic elements, Cd, Ni, and Pb, were significantly greater in the blood of wild turtles (Table 1). Captive turtles had significantly greater scute Cr, Cu, Se, and V, while As was greater in the scute of wild turtles (Table 1).
A Kendall’s rank correlation showed three metals in the captive turtles scute that were significantly, positively correlated with SCL; V (τ = 0.87, p = 0.02), Cd (τ = 0.80, p = 0.02) and Pb (τ = 0.80, p = 0.04, Figure S1). Selenium was the only element in the blood of wild turtles to be significantly correlated with SCL (τ = 0.70, p = 0.03, Figure S1). No correlations were found between SCL and scute in wild turtles or between SCL and blood in captive turtles.
Three metals in captive turtle scutes, Cr, Sr, and V, had a TTF > 1 (Table 4). No TTF was > 1 in the blood of captive turtles. No elements in Amansia spp. had a TTF > 1 in scute or blood of wild turtles. Selenium in G. salicornia showed the potential for bioaccumulation in scutes and blood of wild turtles (Table 4). Only one element, Cu, was negatively correlated between blood and scute tissue in wild turtles (τ = −1.0, p = 0.027; Figure S2). No elements were correlated between blood and scute in captive turtles.
Table 4.
Trophic transfer values for blood and scute in captive and wild turtles. Estimated daily intake (µg/day) is calculated for captive turtles at SLPH using only pelleted food and wild turtles using a combination of Amansia spp. and G. salicornia. Bold values highlight a TTF > 1, indicating potential toxicological risk for that element.
| Captive | Wild | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Pelleted Food | Amansia spp. | G. salicornia | ||||||
|
|
||||||||
| Trophic Transfer Scute | Trophic Transfer Blood | Daily Intake | Trophic Transfer Scute | Trophic Transfer Blood | Trophic Transfer Scute | Trophic Transfer Blood | Daily Intake | |
| As | 0.12 | 0.11 | 42 | 0.09 | 0.04 | 0.03 | 0.02 | 387 |
| Cd | 0.29 | < 0.01 | 9 | 0.03 | < 0.01 | 0.07 | < 0.01 | 43 |
| Co | 0.01 | < 0.01 | 16 | < 0.01 | 0.03 | 0.03 | 0.21 | 19 |
| Cr | 1.63 | < 0.01 | 86 | 0.03 | 0.03 | 0.08 | 0.07 | 139 |
| Cu | 0.10 | 0.06 | 1700 | 0.06 | 0.02 | 0.22 | 0.07 | 273 |
| Ni | 0.11 | < 0.01 | 408 | 0.01 | 0.01 | 0.25 | 0.14 | 544 |
| Pb | 0.21 | 0.25 | 16 | 0.03 | 0.13 | 0.11 | 0.33 | 85 |
| Sb | 0.01 | < 0.01 | 2 | 0.07 | 0.05 | 0.07 | 0.05 | 2 |
| Se | 0.40 | 0.57 | 126 | 0.47 | 0.80 | 1.42 | 2.39 | 19 |
| Sr | 1.05 | 0.09 | 1430 | 0.07 | 0.02 | 0.19 | 0.04 | 6660 |
| V | 2.64 | < 0.01 | 34 | 0.08 | 0.01 | 0.05 | 0.01 | 352 |
Sex influenced differences in some element concentrations in captive turtles. Scute Pb was significantly greater in females than males. Blood Se was greater in males, and blood Sr was greater in females (Figure S3).
DISCUSSION
Elemental concentrations in sea turtles are affected by diet, species, sex, age, health status, type of tissue, and location, including captive vs wild. The differences in elemental concentrations between captive and wild turtles are primarily due to their food source. Captive turtles are given a pelleted food that is a mixture of animal and plant protein products, with sodium selenite added as a dietary supplement. Consequently, the concentration of Se in the pelleted food was about 3 to 9 times greater than the samples of algae from Kapoho Bay. Captive turtles in this study had significantly greater concentrations of Se in their blood and scute than wild turtles. This difference in Se concentrations was also seen in loggerhead turtles in rehabilitation whereby blood selenium concentration increased through rehabilitation (Camacho et al. 2014b). Selenium concentrations in the present study are likely not of concern when compared to established avian toxicity reference values. Adequate Se concentrations in the whole blood of avian species ranges from 130 ng/g to 200 ng/g, sublethal effects such as weight loss are seen at 900 ng/g, and concentrations of 12,000 ng/g to 16,000 ng/g cause death (O’Toole and Raisbeck 1997; Stout et al. 2010). Furthermore, yellow-bellied sliders (Trachemys scripta scripta) were exposed to Se at doses of 15,000 ng/g and 30,000 ng/g for 5 weeks. Sliders in the 30,000 ng/g treatment group had a blood Se concentration of 14,000 ± 900 ng/g and exhibited anemia. Mortality was only seen in the 30,000 ng/g treatment group and not in the control or 15,000 ng/g groups (Haskins et al. 2017). However, leatherback sea turtles have been documented with blood Se concentrations up to 10,300 ng/g, indicating leatherback turtles may tolerate high Se concentrations (Innis et al. 2010; Perrault et al. 2013). Selenium may represent a greater hazard to eggs and hatchlings than adult sea turtles. A study of metals in green sea turtle eggs determined that the Se concentrations (464 ± 26 ng/g wm) were likely to affect hatching success (van de Merwe et al. 2009). Most sea turtle populations have adequate blood Se concentrations, with few populations including green turtles in San Diego Bay and Boyne River Estuary at or above sublethal concentrations in birds (Figure 2; Komoroske et al. 2011; Gaus et al. 2012). Chelonian species are likely more resilient than avian species from Se toxicity, as seen from high blood Se concentrations in green sea turtles from Boyne River Estuary, Australia, leatherbacks from the US Virgin Islands, and effects observed in laboratory-exposed yellow-bellied sliders (Gaus et al. 2012; Perrault et al. 2013; Haskins et al. 2017).
Figure 2:
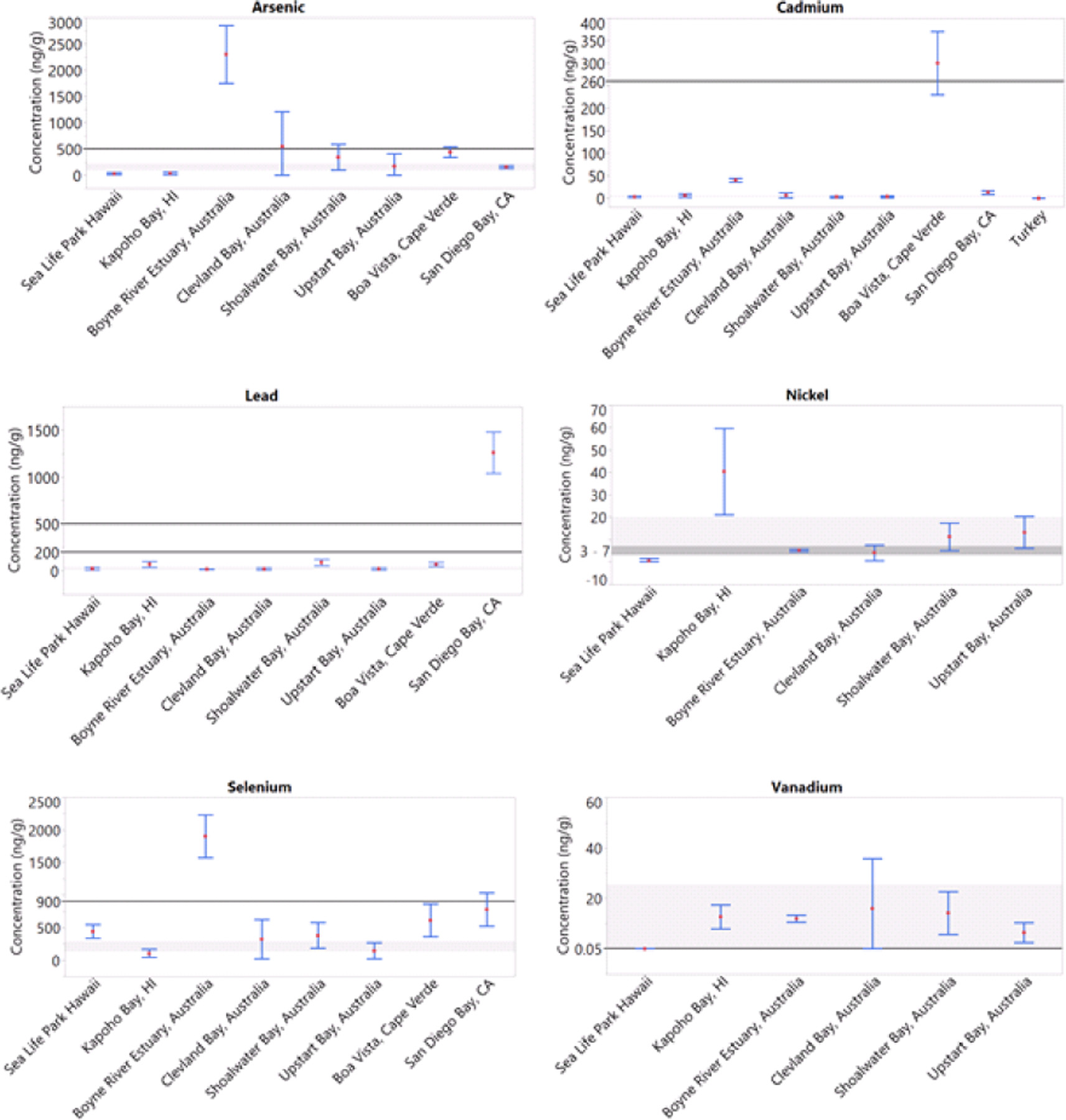
Elemental concentrations (mean ± SD, ng/g wm) in the whole blood of green turtles worldwide. Data taken from Komoroske et al. (2011), Gaus et al. (2012); Camacho et al. (2014a), and Villa et al. 2017). Horizontal lines indicate thresholds as defined as: As toxicity in humans (500 ng/g, Saha et al. 1999); Cd toxicity in avian species (260 ng/g, Wayland and Scheuhammer 2011); Pb 200 ng/g is background levels in avian species and 500 ng/g is clinical poisoning in avian species (Stout et al. 2010; Franson and Pain 2011); Ni 3 ng/g to 7 ng/g is background concentration in humans (Gaus et al. 2012); Se weight loss in avian species (900 ng/g, O’Toole and Raisbeck 1997; Stout et al. 2010); and V 0.05 ng/g is the background concentration in humans (Gaus et al. 2012). Grey shaded areas are concentrations documented in green sea turtles from the Howick Islands, a relatively undisturbed region in Australia used as a reference population (Villa et al. 2017).
Another essential element, Cu, was 11 times and 3 times greater in pelleted food then G. salicornia and Amansia spp., respectively. Due to the high concentrations of Cu in the pelleted food, scute Cu concentration was significantly greater in the captive turtles than the wild turtles. However, blood Cu concentrations were similar between the two groups, because Cu is known to be tightly regulated in the blood through excretion (Liu et al. 2008). The similar blood Cu concentrations between the two groups, despite their major differences in exposure, indicate that sea turtles can regulate blood Cu concentrations. High concentrations of Cu in pelleted food led to increased sequestration of Cu in the scute as measured in the captive turtles. A negative relationship was seen in Cu between scute and blood tissues of wild turtles (Figure S2). This relationship is difficult to explain, but may have to do with (1) blood representing recent exposure to Cu that has not yet been incorporated in the scute tissue, (2) Cu is tightly regulated in the blood through rapid elimination pathways that do not involve scute growth, and/or (3) turtles with higher Cu exposure have induced metallothioneins or other elimination pathways that eliminate the Cu before it is sequestered into scute (Storelli et al. 2008). A decrease in the efficiency of copper excretion leading to copper toxicosis has not been shown in reptiles.
Vanadium, As, Cd, and Pb were all at greater concentrations in both of the algae samples than the pelleted food and Ni was greatest in the Amansia spp. followed by the pelleted food. Some elements are naturally high in marine algae species including V, As, and Cd. Marine algae may be high in V because V is an essential element for some species while As is high because some marine algae can accumulate As. Cadmium is highly mobile between the environment and algae and will bioaccumulate in seaweed (Wever and Kustin 1990; Dawczynski et al. 2007; Ardiyansyah et al. 2019). Similarly, these elements were all at greater concentrations in the blood of wild turtles than captive turtles.
The TTF > 1 seen in V, Cr, and Sr in captive turtle scutes indicates these elements may biomagnify in captive turtles. Turtle E had much greater concentrations of all elements in its scute tissue, and may have influenced the TTF calculations. When Turtle E was removed, only V had a TTF > 1. A larger sample size of captive turtles is needed to conclusively determine which elements may biomagnify in green turtles.
Turtle blood concentrations (ng/g wm) of V, Cd, Pb, As, Se, and Ni in this study were compared to green turtles around the world, with associated toxicity reference values for related species, as toxicity reference values do not exist for green turtles (Figure 2). All sea turtle populations shown in Figure 2 have blood V concentrations greater than background levels in humans (0.05 ng/g), but show concentrations less than the maximum concentration measured in osprey blood from the Chesapeake Bay area (54 ng/g wm) with known pollution from metal-working and petroleum refinery activities (Rattner et al. 2008; Gaus et al. 2012). A population of sea turtles at an uncontaminated site in the Howick Islands, Australia had blood V concentrations ranging from 1.23 ng/g – 94.3 ng/g (Villa et al. 2017). All green sea turtle populations considered in Figure 2 are within this range.
Cadmium concentrations in almost all green sea turtle populations are very similar, well below the value associated with toxic effects in birds (260 ng/g wm) and similar to concentrations seen in Howick Island sea turtles (Wayland and Scheuhammer 2011; Villa et al. 2017). Leatherback turtles have been shown to have higher Cd concentrations than green turtles, from 14 ng/g – 182 ng/g (Harris et al. 2011; Innis et al. 2010).
Blood Pb concentrations of ≤ 200 ng/g are considered background levels in three orders of birds (Anseriformes, Falconifomes, and Columbiformes), and 500 ng/g is considered clinical poisoning in Anseriformes and Falconiformes (Stout et al. 2010; Franson and Pain 2011). However, crocodiles (Crocodylus porosus) with sustained blood Pb concentrations of 3,420 ng/g for several months showed no adverse effects (Hammerton et al. 2003). All green sea turtle populations are well below these levels.
Arsenic blood concentrations were greatest in turtles from Boyne River Estuary in Australia, above ≈ 500 ng/g, which is potentially toxic in humans (Saha et al. 1999; Gaus et al. 2012). Sea turtles in the Howick Islands had blood As concentrations of 160 ± 66 ng/g. Kapoho Bay and SLPH turtles are below these values, indicating As is not a concern for these two populations.
Nickel blood concentrations of turtles in this study were above background levels in humans (3 ng/g to 7 ng/g; Gaus et al. 2012). The worst case hazard quotient for Ni in green sea turtle eggs from Hong Kong was established to be 26.4 ng/g (Lam et al. 2007). Kapoho Bay turtles in this study had mean blood Ni concentrations greater than 26.4 ng/g (40.4 ± 19.3 ng/g), but toxicity reference values are unknown for adult sea turtle blood. However, adult sea turtles are likely less sensitive to Ni than embryos and hatchlings.
Reference values for one species may not accurately extrapolate to another. In the case of Pb, As, and Se, sea turtles may be more tolerant to these elements than birds or humans because the populations with greater concentrations of these elements (Figure 2) are considered healthy.
Diet played the largest role in differences between captive and wild turtles, but sex is often a confounding factor. Female captive turtles had greater concentrations of scute lead than males. When females lay eggs, calcium (Ca) is mobilized from the bone for eggshell formation. Lead mimics Ca and is stored in and mobilized from bone following the same kinetics as Ca (Assi et al. 2016). For example, blood Pb concentrations in leatherbacks have been shown to increase as the nesting season progresses (Guirlet et al. 2008). In the wild, female turtles are capital breeders and in general do not feed during the nesting season, though opportunistic feeding can occur. Instead their energy is supplied from fat stores (Bjorndal 1985; Hays et al. 2002; Tucker and Read 2017; Page-Karjian et al. 2020). One hypothesis to explain the greater scute lead concentrations in these captive female turtles is that as Ca, and hence Pb as a Ca mimic, was mobilized from bones during egg vitellogenesis/egg production, blood Pb concentrations increased. Unlike wild turtles, the captive turtles would continue to eat during the nesting season, and continue to be exposed to Pb and Ca. While some of those nutrients would be offloaded into developing eggs, excess Pb could be sequestered in scute.
Three elements in captive turtle scutes, V, Cd, and Pb, and one element in the wild turtle blood, Se, were positively correlated with SCL (Figure S1). Larger, potentially older, turtles have greater concentrations of these elements, as seen with largest captive Turtle E. This suggests accumulation through age of V, Cd or Pb in their scutes. Accumulation through age is also suspected for Se in blood, based on these correlations. The biomagnifying potential, as seen by a TTF>1 for Se in blood of wild turtles, supports this suggestion. In addition, the bioaccumulation of Se has been documented in leatherbacks from the northwestern Atlantic Ocean (Perrault 2012).
This study only found one element, Cu in wild turtles, to be significantly correlated between blood and scute tissues. In previous studies, total mercury concentrations were correlated between scutes and blood in loggerhead sea turtles, scute manganese, zinc, and Hg concentrations were correlated with whole body burdens of these elements in loggerhead and green turtles, and Se concentrations in the blood of green turtles were correlated with concentrations in the liver, kidney, and muscle (Day et al. 2005; Sakai et al. 2000; van de Merwe 2008). The sample size in this study was small and may be the reason why more elements were not correlated between blood and scute. Additional studies should be done for green turtles to determine relationships between blood and scute element concentrations to prevent unnecessary sampling and stress to these threatened and endangered species.
One limitation of the current study was the lack of analysis of elements in the lettuce that captive turtles are fed at SLPH. This contribution could not be included in the daily intake or TTF calculations for the captive turtles, so the EDI and TTFs are conservatively underestimated. However, this study remains the first to analyze the food source of captive turtles and increases the knowledge of the impacts of pelleted food on captive turtles.
CONCLUSION
All metals found in the captive and wild Hawaiian green turtles in this study are similar to or less than concentrations found in green sea turtles elsewhere (Figure 2). It is important to establish baseline concentrations of elements in sea turtles to understand the toxicity threat of elements if and when they are found to be elevated and to follow contaminant exposure over time. Captive sea turtles do not represent wild turtles’ exposure to trace elements. Sea Life Park Hawaii and other aquaria or rehabilitation centers feed captive sea turtles a diet very different than their natural prey. In the case of SLPH, the pelleted food they consume has greater concentrations of some elements than the wild turtles’ food source, and thus their tissues have greater concentrations. Reference concentrations for some elements may be better established in healthy, wild populations living in relatively unpolluted areas.
Supplementary Material
Acknowledgment—
We gratefully acknowledge Department of Environmental Toxicology at Texas Tech University for providing the facilities to perform the chemical analysis. We thank F. Nilsen, A. Fong, J. Jacob, T. Schock, and H. Lynch for sample collection and processing assistance, and C. Bryan Sallee and J. Cañas-Carell for manuscript reviews.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
Disclaimer—The authors declare no competing financial interest. Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Data availability statement—
Data, associated metadata, and calculation tools are available from the corresponding author (katherine.shaw@nist.gov).
REFERENCES
- Aguirre AA, Lutz PL. 2004. Marine turtles as Ssentinels of ecosystem health: Is fibropapillomatosis an indicator? Ecohealth 1:275–283. 10.1007/s10393-004-0097-3. [DOI] [Google Scholar]
- Andreani G, Santoro M, Cottignoli S, Fabbri M, Carpenè E, Isani G. 2008. Metal distribution and metallothionein in loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. Sci Total Environ 390:287–294. 10.1016/j.scitotenv.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Araújo CVM, Cedeño-Macias LA. 2016. Heavy metals in yellowfin tuna (Thunnus albacares) and common dolphinfish (Coryphaena hippurus) landed on the Ecuadorian coast. Sci Total Environ 541:149–154. 10.1016/j.scitotenv.2015.09.090. [DOI] [PubMed] [Google Scholar]
- Ardiyansyah O, Sudarno Rosmanida. 2019. Bioaccumulation of cadmium (Cd) heavy metal on seaweed (Gracilaria sp.) in traditional fishpond of Jabon Subdistrict, Sidoarjo District. IOP Conf Ser Earth Environ Sci 236:1–6. 10.1088/17551315/236/1/012059. [DOI] [Google Scholar]
- Arthur KE, Balazs GH. 2008. A comparison of immature green turtles (Chelonia mydas) diets among seven sites in the main Hawaiian Islands. Pacific Sci 62:205–217. 10.2984/1534-6188(2008)62[205:acoigt]2.0.co;2. [DOI] [Google Scholar]
- Assi MA, Hezmee MNM, Haron AW, Sabri MYM, Rajion MA. 2016. The detrimental effects of lead on human and animal health. Vet World 9:660–671. 10.14202/vetworld.2016.660-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile F, Di Santi A, Ferretti L, Bentivegna F, Pica A. 2012. Hematology of the Mediterranean population of sea turtle (Caretta caretta): comparison of blood values in wild and captive, juvenile and adult animals. Comp Clin Pathol 21:1401–1406. 10.1007/s00580-011-1306-4 [DOI] [Google Scholar]
- Bezerra MF, Lacerda LD, Lima EHSM, Melo MTD. 2013. Monitoring mercury in green sea turtles using keratinized carapace fragments (scutes). Mar Pollut Bull 77:424–427. 10.1016/j.marpolbul.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Bienfang P, De Carlo EH, Christopher S, DeFelice S, Moeller P. 2009. Trace element concentrations in Coastal Hawaiian waters. Mar Chem 113:164–171. 10.1016/j.marchem.2009.01.007. [DOI] [Google Scholar]
- Bjorndal KA. 1985. Nutritional ecology of sea turtles. Copeia. 3:736–751. [Google Scholar]
- Bryan JM. 2013. Concentrations of heavy metals in scute samples from nesting female olive Ridley, Lepidochelys olivacea, and Eastern Pacific green, Chelonia mydas agassizii, sea turtles in Costa Rica. Master’s Thesis. Purdue University, Fort Wayne, IN, USA. [Google Scholar]
- Camacho M, Boada LD, Orós J, López P, Zumbado M, Almeida-González M, Luzardo OP. 2014a. Monitoring organic and inorganic pollutants in juvenile live sea turtles: Results from a study of Chelonia mydas and Eretmochelys imbricata in Cape Verde. Sci Total Environ 481:303–310. 10.1016/j.scitotenv.2014.02.051. [DOI] [PubMed] [Google Scholar]
- Camacho M, Orós J, Henríquez-hernández LA, Valerón PF, Boada LD, Zaccaroni A, Zumbado M, Luzardo OP. 2014b. Influence of the rehabilitation of injured loggerhead turtles (Caretta caretta) on their blood levels of environmental organic pollutants and elements. Sci Total Environ 487:436–442. 10.1016/j.scitotenv.2014.04.062. [DOI] [PubMed] [Google Scholar]
- Casal AB, Camacho M, Lopez-Jurado LF, Juste C, Oros J. 2009. Comparative study of hematologic and plasma biochemical variablesin Eastern Atlantic juvenile and adlt nesting loggerhead sea turtles (Caretta caretta). Vet Clin Pathol 38:213–218. 10.1111/j.1939-165X.2008.00106.x [DOI] [PubMed] [Google Scholar]
- Cortes-Gomez AA, Romero D, Girondot M. 2017. The current situation of inorganic elements in marine turtles: A general review and meta-analysis. Environ Pollut 229:567–585. 10.1016/j.envpol.2017.06.077. [DOI] [PubMed] [Google Scholar]
- Creed JT, Brockhoff CA, Martin TD. 1994. Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma – Mass Spectrometry. Report no. R-94–111, Method 200.8, Revision 5.4. U.S. Environmental Protection Agency, Cincinnati, OH, USA. [Google Scholar]
- Dawczynski C, Schäfer U, Leiterer M, Jahreis G. 2007. Nutritional and toxicological importance of macro, trace, and ultra-trace elements in algae food products. J Agric Food Chem 55:10470–10475. 10.1021/jf0721500. [DOI] [PubMed] [Google Scholar]
- Day RD, Christopher SJ, Becker PR, Whitaker DW. 2005. Monitoring mercury in the loggerhead sea turtle, Caretta caretta. Environ Sci Technol 39:437–446. 10.1021/es049628q. [DOI] [PubMed] [Google Scholar]
- Day RD, Keller JM, Harms CA, Segars AL, Cluse WM, Godfrey MH, Lee AM, Peden-Adams M, Thorvalson K, Dodd M, Norton, T. 2010. Comparison of mercury burdens in chronically debilitated and healthy loggerhead sea turtles (Caretta caretta). J Wildl Dis 46:111–117. 10.7589/0090-3558-46.1.111. [DOI] [PubMed] [Google Scholar]
- DeForest DK, Brix KV, Adams WJ. 2007. Assessing metal bioaccumulation in aquatic environments: The inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat Toxicol 84:236–246. 10.1016/j.aquatox.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Duncan EM, Broderick AC, Fuller WJ, Galloway TS, Godfrey MH, Hamann M, Limpus CJ, Lindeque PK, Mayes AG, Omeyer LCM, Santillo D, Snape RTE, Godley BJ. 2019. Microplastic ingestion ubiquitous in marine turtles. Glob Chang Biol 25:744–752. 10.1111/gcb.14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastern-Research-Group. 2012. Standard Operating Procedure for Determination of Lead in TSP by Inductively Coupled Plasma Mass Spectrometry ( ICP-MS) with Hot Block Dilute Acid and Hydrogen Peroxide Filter Extraction. Morrisville, NC. (USEPA Generic ICPMS FEM SOP Pb TSP, EQL-0512–201). [Google Scholar]
- Falk JH, Reinhard EM, Vernon CL, Bronnenkant K, Heimlich JE, Deans NL. 2007. Why zoos & aquariums matter: Assessing the impact of a visit to a zoo or aquarium institute for learning innovation principal investigator. Association of Zoos & Aquariums, Silver Springs, MD. [Google Scholar]
- Franson CJ, Pain DJ. 2011. Lead in birds. In Beyer WN, Meador JP, eds, Environmental Contaminants in Biota: Interpreting Tissue Concentrations, 2nd ed. CRC Press, Boca Raton, FL, USA, pp 563–593. [Google Scholar]
- French AD, Ashbaugh HM, Steinmetz G, Barnes M, Conway WC, Klein DM. 2017. The S.M.A.R.T (small mass, affordable, rapid, transfer-less) digestion method for heavy metal determinations. Int J Environ Anal Chem 97:499–507. 10.1080/03067319.2017.1328060. [DOI] [Google Scholar]
- Gadd GM. 1992. Metals and microorganisms: A problem of definition. FEMS Microbiol Lett 100:197–203. 10.1111/j.1574-6968.1992.tb14040.x/abstract. [DOI] [PubMed] [Google Scholar]
- Gardner SC, Fitzgerald SL, Vargas BA, Rodríguez LM. 2006. Heavy metal accumulation in four species of sea turtles from the Baja California peninsula, Mexico. BioMetals 19:91–99. 10.1007/s10534-005-8660-0. [DOI] [PubMed] [Google Scholar]
- Gaus C, Grant S, Jin NL, Goot K, Villa A, Neugebauer F, Qi L, Limpus C. 2012. Investigation of Contaminant Levels in Green Turtles from Gladstone. Final/Technical Report. National Research Centre for Environmental Toxicology. Queensland, Australia. [Google Scholar]
- Gochfeld M, Gochfeld DJ, Minton D, Murray BG, Pyle P, Seto N, Smith D, Burger J. 1999. Metals in feathers of Bonin petrel, Christmas shearwater, wedge-tailed shearwater, and red-tailed tropicbird in the Hawaian Islands, northern Pacific. Environ Monit Assess 59:343–358. 10.1023/A:1006134125236. [DOI] [Google Scholar]
- Guirlet E, Das K, Girondot M. 2008. Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat Toxicol 88:267–276. 10.1016/j.aquatox.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Hammerton KM, Jayasinghe N, Jeffree RA, Lim RP. 2003. Experimental study of blood lead kinetics in estuarine crocodiles (Crocodylus porosus) exposed to ingested lead shot. Arch Environ Contam Toxicol 45:390–398. 10.1007/s00244-003-0234-y [DOI] [PubMed] [Google Scholar]
- Hansen AM, Bryan CE, West K, Jensen BA. 2016. Trace element concentrations in liver of 16 species of cetaceans stranded on Pacific Islands from 1997 through 2013. Arch Environ Contam Toxicol 70:75–95. 10.1007/s00244-015-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Heather S, Scott R, Kirsten V, Robert H, Thierry M. 2011. Comparative health assessment of western pacific leatherback turtles (Dermochelys coriacea) foraging off the coast of California, 2005 – 2007. J Wildl Di 47:321–337. 10.7589/0090-3558-47.2.321 [DOI] [PubMed] [Google Scholar]
- Haskins DL, Hamilton MT, Stacy NI, Finger JW, Tuberville TD. 2017. Effects of selenium exposure on the hematology, innate immunity, and metabolic rate of yellow-bellied sliders (Trachemys scripta scripta). Ecotoxicology 26:1134–1146. 10.1007/s10646-017-1839-7. [DOI] [PubMed] [Google Scholar]
- Hays GC, Broderick AC, Glen F, Godley BJ. 2002. Change in body mass associated with long-term fasting in a marine reptile: the case of green turtles (Chelonia mydas) at Ascension Island. Can. J. Zool 80:1299–1302. 10.1139/z02-110 [DOI] [Google Scholar]
- Helsel DR. 2005. Nondetects And Data Analysis: Statistics for Censored Environmental Data. John Wiley & Sons, Inc., Hoboken, NJ, USA. [Google Scholar]
- Innis C, Tlusty M, Perkins C, Holladay S, Merigo C, Weber ES. 2008. Trace metal and organochlorine pesticide concentrations in cold-stunned juvenile Kemp’s ridley turtles (Lepidochelys kempii) from Cape Cod, Massachusetts. Chelonian Conserv Biol 7:30–239. 10.2744/ccb-0707.1. [DOI] [Google Scholar]
- Innis CHI, Merigo CO, Dodge K, Tlusty M, Dodge M, Sharp B, Myers A, McIntosh A, Wunn D, Perkins C, Herdt TH, Norton T, Lutcavage M. 2010. Health evaluation of leatherback turtles (Dermochelys coriacea) in the Northwestern Atlantic during direct capture and fisheries gear disentanglement. Chelonian Conserv Biol 9:205–222. 10.2744/CCB-0838.1. [DOI] [Google Scholar]
- International Union for Conservation of Nature (IUCN). IUCN red list of threatened species. [cited2019 May 1]. Available from: http://www.redlist.org.
- Keller JM. 2013. Exposure to and effects of persistant organic pollutants. In Wyneken J, Lohman K, Musick J, eds, The Biology of Sea Turtles Volume III. Boca Raton, FL, USA, pp 285–328. [Google Scholar]
- Keller JM, Pugh RS, Becker PR. 2014. Biological and Environmental Monitoring and Archival of Sea Turtle Tissues (BEMAST): Rationale, Protocols, and Initial Collections of Banked Sea Turtle Tissues. NIST, Gaithersburg, MD. [Google Scholar]
- Komoroske LM, Lewison RL, Seminoff JA, Deheyn DD, Dutton PH. 2011. Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere 84:544–552. 10.1016/j.chemosphere.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Lam JCW, Tanabe S, Chan SKF, Lam MHW, Martin M, Lam PKS. 2007. Levels of trace elements in green turtle eggs collected from Hong Kong: Evidence of risks due to selenium and nickel. Enviornmental Pollut 144:790–801. 10.1016/j.envpol.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Liang L, Hu J, Chen D, Zhou Q, He B, Jiang G. 2004. Primary investigation of heavy metal contamination status in molluscs collected from Chinese coastal sites. Bull Environ Contam Toxicol 72:937–944. 10.1007/s00128-004-0334-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Goyer RA, Waalkes MP. 2008. Chapter 23: Toxic effects of metals. In Klaassen CD, ed, Casarett & Doull’s Toxicology: The Basic Science of Poisons, 7th ed. McGraw-Hill Education, New York, NY, USA. [Google Scholar]
- Lutcavage ME, Plotkin P, Witherington B, Lutz PL. 1997. Human impacts on sea turtle survival. In Lutz PL, Musick JA, eds, The Biology of Sea Turtles. CRC Press, Boca Raton, FL, USA, pp 387–409. [Google Scholar]
- Mathews T, Fisher NS. 2008. Trophic transfer of seven trace metals in a four-step marine food chain. Mar Ecol Prog Ser 367:23–33. 10.3354/meps07536. [DOI] [Google Scholar]
- Milton SL, Lutz PL. 2003. Physiological and genetic responses to environmental stress. In Lutz PL, Musick JA, Wyneken J, eds, The Biology of Sea Turtles Volume II. CRC Press, Boca Raton, FL, USA, pp 163–197. [Google Scholar]
- O’Toole D, Raisbeck MF. 1997. Experimentally induced selenosis of adult mallard ducks: clinical signs, lesions, and toxicology. Vet Pathol. 34:330–340. [DOI] [PubMed] [Google Scholar]
- Orvik LM. 1997. Trace Metal Concentrations in Blood of the Kemp’s Ridley Sea Turtle (Lepidochelys kempii). Master’s Thesis. Texas A&M University, College Station, TX, USA. [Google Scholar]
- Owens DW, Blainvillain G. 2013. Captive reproduction of sea turtles: an important success story. Proceedings, International Symposium on Reproduction of Marine Life: Birth of New Life, Okinawa, Japan, February 21–22, 2009, 23–40. [Google Scholar]
- Page-Karjian AP, Chabot R, Stacy NI, Morgan AS, Valverde RA, Stewart S, Coppenrath CM, Manire CA, Herbst LH, Gregory CR, Ritchie BW, Perrault JR. 2020. Comprehensive health assessment of green turtles Chelonia mydas nesting in southeastern Florida, USA. Endang Species Res 42:21–35. 10.3354/esr01036 [DOI] [Google Scholar]
- Perrault JR. 2012. Assessment of mercury and selenium concentrations in tissues of stranded leatherback sea turtles (Dermochelys coriacea). J Herpetol Med Surg 22:76–85. 10.5818/1529-9651-22.3.76 [DOI] [Google Scholar]
- Perrault JR, Miller DL, Garner J, Wyneken J. 2013. Mercury and selenium concentrations in leatherback sea turtles (Dermochelys coriacea): Population comparisons, implications for reproductive success, hazard quotients and directions for future research. Sci Total Environ 463:61–71. 10.1016/j.scitotenv.2013.05.067. [DOI] [PubMed] [Google Scholar]
- Perrault JR. 2014. Mercury and selenium ingestion rates of Atlantic leatherback sea turtles (Dermochelys coriacea): A cause for concern in this species? Mar Environ Res 99:160–169. 10.1016/j.marenvres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Perrault JR, Stacy NI, Lehner AF, Poor SK, Buchweitz JP, Walsh CJ. 2017. Toxic elements and associations with hematology, plasma biochemistry, and protein electrophoresis in nesting loggerhead sea turtles (Caretta caretta) from Casey Key, Florida. Environ Pollut 231:1398–1411. 10.1016/j.envpol.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Rattner BA, Golden NH, Toschik PC, McGowan PC, Custer TW. 2008. Concentrations of metals in blood and feathers of nestling ospreys (Pandion haliaetus) in Chesapeake and Delaware Bays. Arch Environ Contam Toxicol 54:114–122. 10.1007/s00244-007-9004-6. [DOI] [PubMed] [Google Scholar]
- Russell DJ, Balazs GH. 2009. Dietary shifts by green turtles (Chelonia mydas) in the Kāne’ohe Bay region of the Hawaiian islands: A 28-year study. Pacific Sci 63:181–192. 10.2984/049.063.0202. [DOI] [Google Scholar]
- Saha JC, Dikshit AK, Bandyopadhyay M, Saha KC. 1999. A review of arsenic poisoning and its effects on human health. Crit Rev Env Sci Technol 29:281–313. 10.1080/10643389991259227 [DOI] [Google Scholar]
- Sakai H, Saeki K, Ichihashi H, Suganuma H, Tanabe S, Tatsukawa R. 2000. Species-specific distribution of heavy metals in tissues and organs of loggerhead turtle (Caretta caretta) and green turtle (Chelonia mydas) from Japanese coastal waters. Mar Pollut Bull 40:701–709. 10.1016/S0025-326X(00)00008-4. [DOI] [Google Scholar]
- Sinaei M, Bolouki M. 2017. Metals in blood and eggs of green sea turtles (Chelonia mydas) from nesting colonies of the northern coast of the Sea of Oman. Arch Environ Contam Toxicol 73:552–561. 10.1007/s00244-017-0421-x. [DOI] [PubMed] [Google Scholar]
- Storelli MM, Barone G, Storelli A, Marcotrigiano GO. 2008. Total and subcellular distribution of trace elements (Cd, Cu and Zn) in the liver and kidney of green turtles (Chelonia mydas) from the Mediterranean Sea. Chemosphere 70:908–913. 10.1016/j.chemosphere.2007.06.069 [DOI] [PubMed] [Google Scholar]
- Stout JD, Brinker DF, Driscoll CP, Davison S, Murphy LA. 2010. Serum biochemistry values, plasma mineral levels, and whole blood heavy metal measurements in wild northern goshawks (Accipiter gentilis). J Zoo Wildl Med 41:649–655. 10.1638/2009-0258.1. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Noda J, Yanagisawa M. 2012a. Particle-induced x-ray emission analysis of elements in plasma from wild and captive sea turtles (Eretmochelys imbricata, Chelonia mydas, and Caretta caretta) in Okinawa, Japan. Biol Trace Elem Res 148:302–308. 10.1007/s12011-012-9375-z. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Noda J, Yanagisawa M, Kawazu I, Sera K, Fukui D. 2012b. Relationships between carapace sizes and plasma major and trace element status in captive hawksbill sea turtles (Eretmochelys imbricata). J Vet Med Sci 74:1677–1680. 10.1292/jvms.12-0099. [DOI] [PubMed] [Google Scholar]
- Takeuchi NY, Walsh MT, Bonde RK, Powell JA, Bass DA, Gaspard JC III, Barber DS. 2016. Baseline reference range for trace metal concentrations in whole blood of wild and managed west indian manatees (Trichechus manatus) in Florida and Belize. Aquat Mamm 42:440–453. 10.1578/AM.42.4.2016.440. [DOI] [Google Scholar]
- Tucker AD, Read M. 2017. Frequency of foraging by gravid green turtles (Chelonia mydas) at Raine Island, Great Barrier Reef. J. Herpetol 35:500–503. 10.2307/1565970 [DOI] [Google Scholar]
- Van de Merwe JP. 2008. Persistent organic pollutants and heavy metals in the green sea turtle, Chelonia mydas. Master’s Thesis. Griffith University, Queensland, Australia. [Google Scholar]
- Van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Ibrahim K, Lee SY. 2009. Chemical contamination of green turtle (Chelonia mydas) eggs in Peninsular Malaysia: Implications for conservation and public health. Environ Health Perspect 117:1397–1401. 10.1289/ehp.0900813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Lee SY. 2010. Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar Pollut Bull 60:579–588. 10.1016/j.marpolbul.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Villa CA, Finlayson S, Limpus C, Gaus C. 2015. A multi-element screening method to identify metal targets for blood biomonitoring in green sea turtles (Chelonia mydas). Sci Total Environ. 10.1016/j.scitotenv.2014.11.100. [DOI] [PubMed]
- Villa CA, Flint M, Bell I, Hof C, Limpus CJ, Gaus C. 2017. Trace element reference intervals in the blood of healthy green sea turtles to evaluate exposure of coastal populations. Environ Pollut 220:1465–1476. 10.1016/j.envpol.2016.10.085. [DOI] [PubMed] [Google Scholar]
- Wayland M, Scheuhammer AM. 2011. Cadmium in birds. In Beyer WN, Meador JP, eds, Environmental Contaminants in Biota: Interpreting Tissues Concentrations. 2nd ed, CRC Press, Boca Raton, FL, USA, pp 645–668. [Google Scholar]
- Wever R, Kustin K. 1990. Vanadium: A biologically relevant element. In Skyes AG, ed, Advances in Inorganic Chemistry, Vol 35. Academic Press, Cambridge, MA, USA, pp 81–115. [Google Scholar]
- Williams SL. 1988. Thalassia testudinum productivity and grazing by green turtles in a highly disturbed seagrass bed. Mar Biol 98:447–455. 10.1007/BF00391121. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, associated metadata, and calculation tools are available from the corresponding author (katherine.shaw@nist.gov).


