Abstract
Diabetic retinopathy (DR) is a global health burden. Screening for sight-threatening DR (STDR) is the first cost-effective step to decrease this burden. We analyzed the similarities and variations between the recent country-specific and the International Council of Ophthalmology (ICO) DR guideline to identify gaps and suggest possible solutions for future universal screening. We selected six representative national DR guidelines, one from each World Health Organization region, including Canada (North America), England (Europe), India (South- East Asia), Kenya (Africa), New Zealand (Western Pacific), and American Academy of Ophthalmology Preferred Practice Pattern (used in Latin America and East Mediterranean). We weighed the newer camera and artificial intelligence (AI) technology against the traditional screening methodologies. All guidelines agree that screening for DR and STDR in people with diabetes is currently led by an ophthalmologist; few engage non-ophthalmologists. Significant variations exist in the screening location and referral timelines. Screening with digital fundus photography has largely replaced traditional slit-lamp examination and ophthalmoscopy. The use of mydriatic digital 2-or 4-field fundus photography is the current norm; there is increasing interest in using non-mydriatic fundus cameras. The use of automated DR grading and tele-screening is currently sparse. Country-specific guidelines are necessary to align with national priorities and human resources. International guidelines such as the ICO DR guidelines remain useful in countries where no guidelines exist. Validation studies on AI and tele-screening call for urgent policy decisions to integrate DR screening into universal health coverage to reduce this global public health burden.
Subject terms: Public health, Diseases
摘要
糖尿病视网膜病变(DR)是全球性的健康负担。筛查威胁视力的DR (STDR)是降低这一负担的第一个具有成本效益的步骤。我们分析了不同国家特有的指南和国际眼科理事会(ICO)指南之间的共同点和差异点, 以确定差距, 并为未来的普遍筛查提出可能的解决方案。我们选择了六个具有代表性国家的DR指南, 每个世界卫生组织区域各一个, 包括加拿大 (北美) 、英国 (欧洲) 、印度 (东南亚) 、肯尼亚 (非洲) 、新西兰 (西太平洋) 和美国眼科学会首选的临床模式 (拉丁美洲和东地中海) 。我们考量了较新的照相技术、人工智能 (AI) 技术与传统筛查方法之间的差别。所有指南都认为糖尿病患者的DR和STDR筛查应该由眼科医生主导进行, 仅少量主张非眼科医生参与。但筛选地点和转诊时间不同指南之间存在显著差异。数字眼底照相筛检在很大程度上取代了传统的裂隙灯检查和检眼镜。散瞳后的数字眼底照相视野2个或4个视野范围是目前的标准, 但免散瞳的眼底照相也逐渐兴起。目前很少使用自动进行DR分级和远程筛查。根据各国的重点政策和人力资源情况制定相应的具体指南必不可少。但国际指南如ICO-DR指南在没有具体指南的国家仍然可以使用。为了减轻这一全球公共卫生负担, 有关AI和远程筛查的有效性研究需要立刻进行政策决策, 以将DR筛查纳入全民健康的覆盖范围。
Introduction
The vision loss expert group (VLEG) reported that diabetic retinopathy (DR) accounted for 1.07% of blindness and 1.25% of moderate to severe visual impairment (MSVI) in 2015 [1]. Despite global efforts to reducing visual impairment, the prevalence of DR is increasing (Crude prevalence 1990: 0.03%; 2015: 0.04%) while the prevalence of all other causes of visual impairment is decreasing consequent to concerted global efforts [1]. This disparity is explained by the estimated global demographic changes between 2019 and 2030–24.8% increase in people with diabetes (International Diabetes Federation, IDF, 2019 estimation: 463 million; 2030 projection: 578 million) [2], 10.7% increase in population (2019 estimate: 7.71 billion; 2030 projection: 8.54 billion) [3], 13.1% longer longevity of people (life expectancy: 2019 estimation: 64.2 years; 2030 projection: 72.6 years) [3], and the increasing aging population of 65+ years (2019 estimate: 9.1%; 2030 projection: 11.7%) [3]. Table 1 lists the key elements of diabetes care in the world [2].
Table 1.
Key elements in global status of diabetes [2].
| Components | AFR 47 countries | EUR 57 countries | MENA 21 countries | NAC 24 countries | SACA 19 countries | SEA 7 countries | WP 36 countries | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2030 | 2019 | 2030 | 2019 | 2030 | 2019 | 2030 | 2019 | 2030 | 2019 | 2030 | 2019 | 2030 | |
| Adult population (20–79 years) | 501.3m | 703.9m | 665.4m | 673.8m | 426.3m | 533.8m | 357.1m | 393.5m | 335.1m | 381.0m | 997.4m | 1.2b | 1.7b | 1.8b |
| DM regional prevalence | 3.9% | 4.1% | 8.9% | 9.8% | 12.8% | 14.2% | 13.3% | 14.2% | 9.4% | 10.6% | 8.8% | 9.7% | 9.6% | 11.0% |
| DM age-adjusted prevalence | 4.7% | 5.1% | 6.3% | 7.3% | 12.2% | 13.3% | 11.1% | 12.3% | 8.5% | 9.5% | 11.3% | 12.2% | 11.4% | 12.4% |
| DM people | 19.4m | 28.6m | 59.3m | 66.0m | 54.8m | 76.0m | 47.6m | 56.0m | 31.6m | 40.2m | 87.6m | 115.1m | 162.6m | 196.5m |
| DM undiagnosed prevalence | 59.7% | - | 40.7% | - | 44.7% | - | 37.8% | - | 41.9% | - | 56.7% | - | 55.8% | - |
| DM undiagnosed people | 11.6m | - | 24.2m | - | 24.5m | - | 18.0m | - | 13.3m | - | 49.6m | - | 90.8m | - |
| T1 DM people | 25,800 | - | 296,500 | - | 149,400 | - | 224,900 | - | 127,200 | - | 184,100 | - | 102,200 | - |
| DM deaths | 366,200 | - | 465,900 | - | 418,900 | - | 301,700 | - | 243,200 | - | 1.15m | - | 1.26m | - |
| DM health expense USDa | 9.7b | 12.7b | 161.4b | 168.5b | 24.9b | 32.5b | 324.5b | 338.8b | 69.7b | 80.4b | 8.1b | 10.1b | 162.2b | 181.8b |
| IGT. Regional prevalence | 9.0% | 9.5% | 5.5% | 5.9% | 8.3% | 8.9% | 15.5% | 16.3% | 10.1% | 10.8% | 3.1% | 3.3% | 8.0% | 8.7% |
| IGT. Age-adjusted prevalence | 10.1% | 10.5% | 4.4% | 4.9% | 9.2% | 9.7% | 12.3% | 13.2% | 9.7% | 10.3% | 7.7% | 7.9% | 10.4% | 11.0% |
| IGT. People | 46.3m | 66.8m | 36.6 m | 39.7 m | 35.5 m | 47.3 m | 55.5 m | 64.0 m | 33.9 m | 41.0 m | 30.6 m | 39.1 m | 136.5 m | 155.9 m |
AFR Africa, EUR Europe, DM diabetes mellitus, MENA middle east and north Africa, NAC North America & Caribbean, SACA South And Central America, SEA South East Asia, T1DM Type 1 DM, WP Western Pacific, USD US dollars, IGT impaired glucose tolerance, b Billion, m million.
aDirect cost.
There is a concurrent rise in the worldwide cost of diabetes care; in 2030, the direct cost could be USD 825 billion (2019 estimate: USD 760 billion) [2] and total cost, both direct and indirect, could be USD 2.1–2.5 trillion (2015 cost: USD 1.3 trillion) [4]. However, there is gross inequity on planned expenditure on diabetes and its complications globally (the region with higher prevalence and the low-middle income countries, LMIC, are spending less). As one of the six building blocks of the WHO health system, health finance is an important consideration. In less developed countries, the consideration of the cost of DR care assumes more significance for three reasons: (a) higher resource allocation for cataract surgery and uncorrected refractive error; (b) a significant amount of out-of-pocket spending, and occasionally, catastrophic health expenditure; (c) late presentation of patients with advanced diabetic retinopathy and vision loss.
Progression of DR follows a particular pattern from non-proliferative DR (NPDR) to proliferative DR (PDR). Typically, it takes years for NPDR to convert to sight-threatening diabetic retinopathy (STDR). The STDR includes macular edema and proliferative diabetic retinopathy (PDR). Using retinal photography, a recent review estimated the global burden of DR in people with diabetes mellitus (DM) could be as high as 27% [5]. The estimated quality-adjusted life years (QALY) loss due to visual impairment is also high (−74.93 years per 100,000 person-years in a study from Korea) [6].
The key to reducing visual impairment and blindness for a chronic disease like DR is early detection and treatment of STDR. Screening is a useful tool, and DR meets nearly all criteria laid down by Wilson and Jungner [7]. Screening for DR has also added benefit to identifying people at risk of other diabetes-related complications such as neuropathy, nephropathy, cardiovascular disease (CVD), stroke, and peripheral vascular disease [8].
The regional and national perspectives have been evolving globally to incorporate newer advances and innovations in DR screening. However, there are variations as per the existing public health requirements and available resources. The objective of this review was to identify potential areas for improvement to enable global coverage with DR screening. We reviewed the currently available and recently updated DR screening guidelines across the globe, one from each region of the World Health Organization (WHO)/International Agency for the Prevention of Blindness (IAPB). We compared them with the International Council of Ophthalmology (ICO) guidelines for evaluating the similarities and variations in screening for STDR.
Methods
A search of available electronic databases, including the WHO, ICO, IAPB, VISION 2020 Right to Sight, American Academy of Ophthalmology (AAO) sources, was completed to identify the existing country or professional ophthalmological society- approved DR guidelines for people with type 1 (T1) DM and type 2 (T2) DM. The reference terms were “diabetic retinopathy,” “screening,” “guidelines,” and “practice pattern.” We reviewed 12 guidelines available in the English-language published or updated in the last 5 years and selected one from each region of the IAPB. The publication period was chosen as the previous 5 years to include only those guidelines that have possibly incorporated the recent DR screening updates (Table 2a, b).
Table 2.
Literature review of DR and DM screening.
| (A) Diabetic retinopathy [9–15] | |||
| Region | Country | Commissioning authority | Publication year |
| North America | Canada | Clinical Practice Guidelines Expert Com | 2018 |
| Latin America | LA | AAO PPP | 2019 |
| East Mediterranean | EMR | AAO PPP | 2019 |
| Africa | Kenya | Division on NCD, Kenya | 2017 |
| South-East Asia | India | Indian institute of public health | 2019 |
| Europe | England | NHS DR screening program | 2017 |
| West Pacific | New Zealand | Ministry of health, New Zealand | 2016 |
| International Council of Ophthalmology (ICO) | 2017 | ||
| (B) Diabetes mellitus [26–32] | |||
| Region | Country | Commissioning authority | Publication year |
| North America | USA | ADA | 2019 |
| South America | LA | AAO PPP | 2019 |
| East Mediterranean | Pakistan | WHO CC, Pakistan | 2019 |
| Africa | South Africa | SEMDSA | 2017 |
| South East Asia | India | ICMR | 2018 |
| Europe | - | EASD | 2017 |
| West Pacific | Australia | Diabetes Australia | 2014 |
| Japan | Japan Diabetes Society | 2016 | |
AAO American Academy of Ophthalmology, ADA American Diabetes Association, DR Diabetic retinopathy, EASD European Association for the study of diabetes, ICMR Indian Council of Medical Research, LA Latin America, PPP preferred practice pattern, NCD non-communicable disease, NHS National Health Service, SEMDSA Society of Endocrinology, Metabolism, and Diabetes of South Africa; WHO CC World Health Organization Collaborating Center.
The final section of the guideline and reason for such selection is as follows: Canada (2018; North America-Canada practices systematic tele-screening), England (2017; Europe-England practices national screening), India (2019; South-East Asia-India is home to the second largest population of DM), Kenya (2017; Africa- Kenya has developed a detailed DM/DR protocol), New Zealand (2016; Western Pacific- New Zealand practices systematic national DR screening), and AAO Preferred Practice Pattern (PPP) (2019; guidelines used in Latin America and Eastern Mediterranean countries) [9–14]. All these guidelines were compared between them and against the ICO (2017) guidelines (Table 2a, b) [15].
Questions
The ICO guidelines on diabetic eye care provide a framework for developing regionally applicable DR screening guidelines. We reviewed the ICO guidelines and identified questions relevant to DR screening. The answers to these questions are central components for any DR screening program.
Classification of DR: Which classification is easy to use and reliable that could be applied with optimal training of human resources?
Systemic factors in DR: What are the target parameters associated with DM care that impacts DR outcome?
Screening and referral for DR: Why, Who, How, and What?
Results
The following answers to the questions derived from the review of the guidelines are included in this communication.
Definition and classification of DR: what is easy to use and yet reliable?
The earliest classification of DR is the Airlie House classification [16]. The proposed classification, with little modification, was, used in the Diabetic Retinopathy Study (DRS) and, for the first time, the standards of photo-documentation using stereo photographs of 7 standard fields (around the optic disc and macula) were laid [17]. Later, this classification was further modified and used in the Early Treatment of Diabetic Retinopathy Study (ETDRS) [18]. The ETDRS introduced a new term- the clinically significant macular edema (CSMO) [18]. The ETDRS also measured the DR severity scale into 13 levels.
The ETDRS classification became the new gold standard of the DR severity scale; it was suitable for research but suffered from its complexity. In 2003 the Clinical Disease Severity Scale for DR proposed a new classification, the International Classification of Diabetic Retinopathy (ICDR) [19]. There are five categories in DR; diabetic macular edema (DMO), when present, was classified into three categories. (Table 3). Using the optical coherence tomography heatmap, the DMO is also classified into “center involving” and “non-center involving” DMO [20]. All examined/selected guidelines currently follow the ICDR classification, and color fundus photography is the recommended standard for DR screening.
Table 3.
International classification of diabetic retinopathy (ICDR) classification [19].
| Disease severity | Findings observable upon dilated ophthalmoscopy |
| Diabetic retinopathy (DR) | |
| No apparent DR | No abnormalities |
| Mild NPDR | Microaneurysms only |
| Moderate NPDR | More than just microaneurysms but less than Severe NPDR, (microaneurysms with other signs like intraretinal hemorrhages, hard exudates, cotton wool spots) |
| Severe NPDR |
Any of the following: (4:2:1) 1 More than 20 intraretinal hemorrhages in each of 4 quadrants 2 Definite venous beading in 2+ quadrants 3 Prominent intraretinal microvascular abnormalities IRMA in 1+ quadrant (And no signs of PDR) |
| PDR |
One or more of the following: 1. Neovascularization 2. Vitreous/preretinal hemorrhage |
| Diabetic macular edema (DME) by clinical appearance | |
| No apparent DME | No retinal thickening or hard exudates at macula |
| Mild DME | Some retinal thickening or hard exudates in posterior pole but distant from the center of the macula |
| Moderate DME | Retinal thickening or hard exudates approaching the center of the macula but not involving the center |
| Severe DME | Retinal thickening or hard exudates involving the center of the macula |
| DME classification by Center of macula involvement using Optical Coherence Tomography (OCT) | |
| Non-central involving DME | Retinal thickening in the macula that does not involve central subfield zone in OCT (1 mm diameter) |
| Center involving DME | Retina thickening in the macula that involves the central subfield zone in OCT (1 mm diameter) |
DR diabetic retinopathy, NPDR non-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy, DME diabetic macular edema.
Systemic factors in DR: what are the target parameters?
Control of diabetes mellitus and many associated co-morbidities is necessary for maximum treatment benefit to people with STDR [21–25]. This consists of a variable combination of retinal laser and intravitreal anti-vascular endothelial growth factor (VEGF) injection and vitreoretinal surgery [21].
There are several modifiable systemic factors of DM, but the two risk factors with the most convincing evidence and affordable treatment are hyperglycemia and hypertension. The ACCORD and its follow-up studies provided recent evidence that intensive glycaemic control remains beneficial for reducing DR progression. The legacy effect is evident in people with type 2 DM [22, 24]. The Diabetes Control and Complications Trial (DCCT) evaluated intensive control of hyperglycemia in Type 1 DM, and long term results showed definite benefits in risk reduction of DR [25]. The United Kingdom Prospective Diabetes Study (UKPDS) has shown a decreased incidence of DR with tight control of blood pressure and glucose in patients with Type 2 DM [23]. In the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), diastolic blood pressure was a significant predictor for DR progression to PDR over 14-year follow-up in people with T1DM [26]. The DM guidelines from different countries of the region [27–33] have set targets for diabetes, blood pressure, and cholesterol control (Table 4). Other important systemic factors are kidney disease (greater association with T1DM) [34], microalbuminuria, anemia (for retinopathy progression and DMO) [35], and obesity (strong relationship with insulin resistance) [36]. With the recent surge of novel anti-diabetes strategies, the initial worsening of DR should be monitored before the retina begins to stabilize, as observed with insulin therapy initiation [37]. A multi-disciplinary approach and close interaction between the diabetologist and ophthalmologist helps, and housing them together is beneficial.
Table 4.
| Region/ country | HbA1C% (in IFCC units) | FPG (mg/dl) | BP (mm Hg) | HDL (mg/dl) | LDL (mg/dl) |
|---|---|---|---|---|---|
| USA | <7.0% (<53.0) | 80–130 | <140/90 if not high risk, else <130/80 | >39 | <70 if high riska |
| Europe | <7.0% (<53.0) | <126 | 120–130 /70–80 120–139/70–80 (≥65 years) | NS | <70 if high risk |
| Australia | <7.0% (<53.0) | 106–145 | <130/80 | >39 | <77 |
| Japan | <7.0% (<53.0) | <130 | <130/80 | >40 | <100 |
| MENA | 6.5–7.0% (47.5–53.0) | 80–120 | <140/90 | NS | NS |
| South Africa | <7.0% (<53.0) | 72–126 | <140/90 | >39 for men | <70 |
| India | <7.0% (<53.0) | 80–110 | <130/80 | >40 for men | <70 for high risk |
MENA Middle East and North Africa, NS not specified, IFCC International Federation of Clinical Chemistry (mmol/mol).
aHigh risk refers to patients at high risk for macrovascular complications as defined by regional organizations, e.g., established cerebral vascular disease (CVD), smoking, obesity, hypertension, family history of premature CVD and evidence of other vascular disease [32].
Screening and referral for DR: Why Who, How, and When
Why should DR screening be established?
The vast majority of patients who develop DR have no symptoms until the late stages due to DMO and PDR complications, such as vitreous hemorrhage or tractional retinal detachment. The late presentation of people with an advanced disease state is a worldwide phenomenon related to a lack of public knowledge, awareness, and social deprivation [38–41]. Direct medical costs for DR care are substantial, so also the indirect costs of visual impairment with respect to loss of productivity, increasing hospital admissions, and decreased quality of life [42]. From a public health perspective, blindness, and its treatment cost results in poverty at the individual level and retards economic development at the national level [43]. Good health and wellbeing (Sustainable Development Goal, SDG 3) is intimately connected with SDG 1 and 2 (No poverty and Zero hunger) [44]. All guidelines agree to screen for diabetic retinopathy, and DR screening fits all chronic disease screening criteria [45].
Who should perform DR screening?
In the past, countries have relied on ophthalmologists and physicians to screen all people with diabetes. With the shift from ophthalmoscopy to digital retinal photography, technically trained and certified screeners such as optometrists and allied ophthalmic personnel, including trained retinal photographers, are more cost-effective [46]. The reliability of screening by optometrists and/or retinal photographers has reached 91% sensitivity, and 78% specificity [47] against the National Institute for Clinical Excellence (NICE, UK) recommended acceptance level for DR screening at 80% sensitivity, 95% specificity (and clinical failure rate <5%) [48]. In many countries, the existing law does not allow optometrists to dilate pupils without ophthalmologist supervision. This is a barrier, but reading and grading fundus photos obtained in a non-mydriatic camera could overcome this barrier with the current technology.
How often and where should DR screening be done
Most guidelines suggest that people with T2DM are screened first at the time of diagnosis, and people with T1 DM are screened first at puberty or five years after the diagnosis.
The follow-up care depends on the disease severity; it could vary from 1 to 2 years when there is no retinopathy to half-yearly review once the retinopathy is stabilized after adequate treatment. Longer intervals of follow-up have also been suggested after cohort studies on T2 DM [49]. There are different opinions on the time of retinal examination during pregnancy (Table 5). Examining all people with DM at fixed facilities (eye hospital/ophthalmology services) may not always be feasible in many low-middle income countries (LMICs) and in countries/regions that do not have enough workforce and technology resources. In most countries, there is no standardized, systematic nation-wide screening for DR. In the studied guidelines, only England and New Zealand practice a national DR screening policy. Maintaining a national registry of patients with DM will help direct patients with DM for periodic screening efficiently. Annual eye evaluation may not be cost-effective for low-risk patients [50].
Table 5.
| Question | Condition | Strategy | County guidelines | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Canada | Latin Am | England | India | Kenya | NZ | AAO PPP | |||
| Who | All DM | Trained personnel | + | - | + | + | + | + | + |
| Ophthalmologist | + | + | + | + | + | + | + | ||
| Teleophthalmology | + | - | + | + | - | + | + | ||
| How | All DM | Fundus photo | + | + | + | + | + | + | + |
| Ophthalmoscopy | + | + | + | + | + | + | + | ||
| National screening | - | - | + | - | - | + | |||
| When | T2DM | At diagnosis | + | + | + | + | + | + | |
| T1DM | On basis of puberty | - | + | + | |||||
| Review on basis of age | + (>15 years) | + (>15 years) | 12 years and above | + (>10 years) | + (>10 years) | ||||
| Review on basis of duration of DM(5- year interval) | + | + | + | + | + | + | |||
| Pregnancy | Pre-existing DM only | + | + | + | + | ||||
| All gestational DM | + | + | + | + | |||||
| Basic tests | ALL DM | Visual acuity | + | + | + | + | + | + | + |
| Fundus\camera preference | Non-mydriatic | Mydriatic | Non-mydriatic | Non-mydriatic | Mydriatic | Non mydriatc | Color | ||
| Fundus field | 7-field/UWF | NS | 2 × 450 | Variable | NS | 4–750 | Not specified | ||
| Mydraisis prefered | + | + | + | + | + | + | + | ||
| Referral | Disease severity | Non STDR | Annual | Annual | R0–R3a | Annual | Annual | R0–R5a M0-M6a | Annual |
| STDR | Urgent | Urgent | Urgent | Urgent | Urgent | ||||
| ICO | Referral by disease and county economics | Referral criteria by VA, disease, infrastructure | |||||||
| No/mild NPDR. Referral | LMIC: 1–2 years | HIC-6–12 months | Vision | <20/40; VA reduction symptoms | |||||
| Mod- Severe NPDR & worse | LMIC-Urgent | HIC-Urgent | Disease status | Mod/severe NPDR & worse | |||||
| Pregnancy. Pre-existing DM | LMIC-28 weeks | HIC- 16–20 weeks | Infrastructure | VA, retinal exam not obtained | |||||
DM diabetes mellitus, NS not specified, NZ New Zealand, UWF Ultra-widefield.
aBased on disease severity of disease.
Studies from India, Malawi, and the USA have shown that screening closer to people and/or coupled with the application of laser to eyes with STDR detected in screening improves compliance and is cost-effective, including the gain in quality-adjusted life years (QALY) [51–53]. Based on the current resources, we suspect reaching people with mobile services or opportunistic screening in mass medical congregations would continue for quite some time in all LMICs.
What should be included in DR screening?
The ICO screening guideline suggests a record of disease and treatment history (duration, status, and medication). The basic eye examinations include presenting (and spectacles corrected, if any) vision and fundus photography [15]. A comprehensive eye examination would also include slit-lamp examination, intraocular pressure measurement (gonioscopy when intraocular pressure is high), and dilated eye examination. However, essentially, a screening examination should be short enough for people to adopt it and yet informative enough for intelligent referrals.
What are the follow-up and referral criteria
The key indicators of DR screening’s success are the robustness of the referral system and compliance with these referrals. Three outcomes could emerge from a DR screening episode (a) a routine re-examination when there is no or mild retinopathy, (b) non-urgent referral for moderate non-sight-threatening DR and (c) urgent referral for sight-threatening DR. Most guidelines agree that annual eye check-up is necessary when retinopathy is not detected (some countries recommend two years) and this interval is reduced depending on the degree of retinopathy. The ICO has suggested different guidelines for LMIC- it is twice longer for mild retinopathy (1–2 years in LMIC and 6–12 months in high-income countries, HIC). England and New Zealand guidelines have a systematic referral pattern: England (R0–R3) and New Zealand (R0–R5), depending on the disease severity. All guidelines are unanimous on the referral of all people with STDR and those with reduced visual symptoms. However, crucial to the right referral is the quality of the retinal image and grading of these images. All guidelines suggest referrals of all people with ungradable fundus photographs. Table 5 lists the DR screening strategy [8–16].
Discussion
All the included guidelines uphold the fundamental aim of DR screening to identify STDR. There are many similarities and a few variations in the screening guidelines (Table 5). It appears that two regions, the Eastern Mediterranean and Latin America region, follow the AAO PPP. We also believe the ICO guideline is used in many LMICs that have not yet developed their DR guidelines. Important areas of dissimilarity are the technical details, screening personnel, the need for mydriasis, and the choice of retinal camera.
Screening personnel
A 2015 ICO survey estimated 232,866 ophthalmologists globally, and they were unequally distributed, much less in low-income countries (3.7/million population) than in high-income countries (76.2/million population), and within the LMICs, they were located more in urban than in rural areas [54]. In the same year, 2015, there were 415 million people with diabetes globally, and 75% of them lived in LMICs [55]. Given that the global annual growth of the number of ophthalmologists is 2.9% and the yearly global increase in people with diabetes is 4.47% [2], it would be impossible for ophthalmologists alone to screen all people with diabetes all the time. Therefore, we advocate that non-ophthalmologists be trained to capture retinal images and use computer-aided grading of DR images, where available.
Need for mydriasis
Mydriatic examination generally allows screening of a greater retinal area compared to an undilated fundus view. Pupil dilatation enables the ophthalmologists to have a good view of the retina and a photographer to obtain artifact-free retinal photographs. However, dilatation for all patients and a routine mydriatic screening test that makes the person wait for at least 30 min and incapacitates the individual for near work for the next couple of hours may not always be necessary. With newer technology of non-mydriatic devices (such as non-mydriatic retinal camera and optical coherence tomography angiography), one could reserve pupillary dilatation to people where a readable/gradable image could not be obtained and obviously for those who need treatment. To achieve universal coverage in countries with steep increases in numbers of people with diabetes, innovative approaches in using non-mydriatic cameras should be encouraged. Policies that prevent mydriasis by non-clinician or non-clinical environment should be reviewed, risk-assessed, and situation-specific strategies tailored to facilitate mydriasis.
Creating referral system
One of the prime motivating factors of a screening program for DR is the evidence-based standardized care for all levels of STDR that includes laser, intravitreal anti-VEGF injection, and vitreoretinal surgery [56–59]. The detailed referral system practiced in England and New Zealand national screening is not practically possible everywhere, certainly not in LMICs. Ideally, countries must develop/revise their country-specific DR screening and treatment guidelines based on the local resources and trained human resources for health. The global benchmark, such as the ICO guideline that has taken different resources into account, is a good option as a national guideline or a template to develop/revise national policies.
Future of DR screening
Borne out of technological advances and policy planning, there are emerging game-changers. These should be considered in new or revised DR screening guidelines. These include camera technology, artificial intelligence, e-health, and universal health coverage.
Camera technology
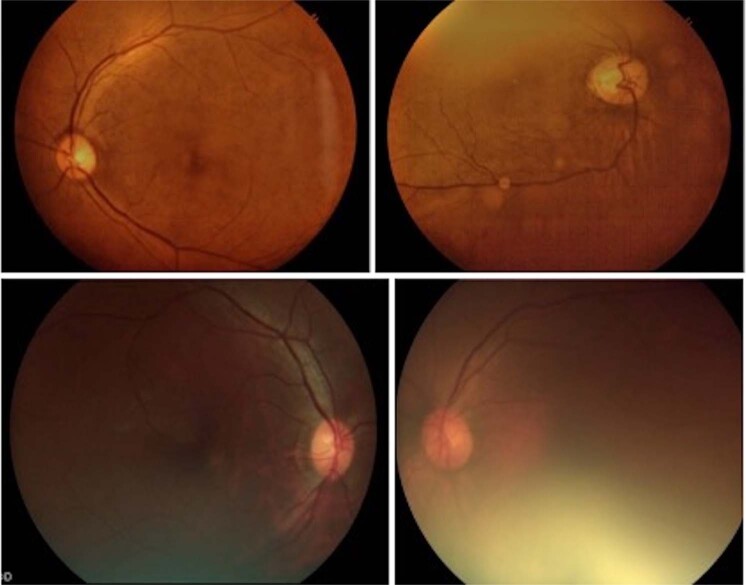
The concept of 7-field fundus photography (300 views) originated with the DRS in the early 1970s. The 7-field fundus photos were arranged around the optic disc in a particular sequence [17, 18]. Since then, camera technology and techniques have undergone sweeping changes. In brief, these changes are black and white to color photography, film-based to digital photography, narrow (300) field to wide (450–500, and ultra-wide (2000) field imaging. Recent studies have demonstrated that digital photography is as good as film-based photography [60]; monoscopic digital fundus photographs also match the rigor of stereo photos [61]; and a mosaic of four- or five- 450 fields could create a field of view reaching the area covered by classic 7-field fundus photography [62]. Single ultra-widefield color fundus photo is also reported good for DR grading [63]. One drawback of digital retinal imaging is its inability to identify and grade macular edema accurately. Over the years, the camera hardware technology has advanced to produce less bulky and hand-held cameras, smartphone-based cameras, and non-mydriatic fundus cameras. This has immensely increased the opportunity to reach people closer to their home and transmit their images to a remotely located reading site for grading and management planning. In the last two decades, the quality of handheld cameras has considerably improved with better prediction capability [64, 65]. A good-quality fundus image is one where both disc and macula are captured in the same frame with good focus and illumination; a poor-quality fundus image captures either disc or macula and has poor illumination (Fig. 1).
Fig. 1. Example of fundus photo obtained in DR screening using non-mydriatic camera.
Upper panel: 3 Nethra™ (Forus, India)- good (left) and poor (right) quality fundus image. Lower panel: Visuscout™ (Zeiss, Germany)- good (right) and poor (left) quality fundus image.
A technical comparison of the currently available non-mydriatic cameras is listed in Table 6. Technology has improved over the years; the questions at present are (1) what is the minimum field required for DR screening in most instances; (2) what are financial resources, as the camera cost steeply increases with an increase in the field of view; (3) what is the choice between mydriatic and non-mydriatic camera for retinal photography and its applicability in DR screening. A systematic review on the use of non-mydriatic cameras reported that two-field retinal photographs were predictable (sensitivity 91%, specificity 94%). It matched the predictability of images obtained by the mydriatic fundus camera once the ungradable images (18.4%) are excluded [66].
Table 6.
Technical comparison of commercially available non-mydriatic cameras.
| Make and modela | Model | Type | Minimum pupil size (mm) | Field of view (degrees) | Autofocus | Imagingc | Other features |
|---|---|---|---|---|---|---|---|
| Zeiss | Visuscout 100 | HH | 3.5 | 40 | + | C, RF, IR | Wi fi enable AI integration |
| Bosch | Fundus | HH | NS | 40 | + | C, RF, IR | - |
| Topcon | TRC-NW400 | TT | >4 | 45 | + | C | Image share with internet |
| Topcon | TRC-NW8F | TT | 3.3 | 45 | + | CC, RF, FFA, FAF | - |
| Optovue | Vivocon | TT | 4 | ≥45 | + | C | Wi fi enable |
| Welch Allyn | RetinaVue RetinaVue100 | HH | NS | 45 | + | C | - |
| RetinaVue 700 | HH | 2.5b | 60 | + | C | EMR integratable | |
| CenterVue DRS | TT | 2.7b | 45 | + | C | EMR integratable | |
| Forus | 3nethra Royal | TT | NS | 45 | NS | C | EMR integratable AI integration |
| Canon | CR-2 | TT | 4 | 45 | + | C, RF | Internal fixation |
| Kowa | VX-20 | TT | 4 | 45 | + | C | Internal fixation |
| Nonmyd DIII | TT | 3.5 | 45/30 | NS | C | Internal fixation | |
| Nidek | AFC-330 | TT | 3.3 | 45 | + | C | EMR integratable |
| Verscam | HH | NS | 45 | + | C | slit-lamp attachment Internal fixation | |
| Centervue | DRS camera | TT | 4 | 45 | + | C | Wi fi enable |
| Eidon | TT | 2.5 | 60 | + | C, RF, IR | Confocal true color | |
| Coburn | SK-650A | TT | 3.3 | 45 | No | C, RF, anterior | Internal fixation |
| Volk | Pictor plus | HH | 2.7 | 40 | + | C, anterior | Wi fi enable |
| EasyScan | Retinal imaging | TT | 1.5 | 45 | + | C, IR | Confocal SLO |
| Optosd | California | TT | NS | 200 | No | C, RF, FFA, FAF | Confocal SLO, EMR integratable |
| Optomed | Aurora | HH | 3.1 | 50 | + | C, RF, IR | EMR integratable Wi fi enable |
| Remedio | Fundus on phone camera | Hand held | 3.3 | 45 | No | C, RF, IR | Smart phone based, SL mount, tele med capable |
The information presented in table has been compiled from the specifications mentioned at the websites of the respective manufacturers.
C color, FAF fundus auto fluorescence, FFA fundus fluorescein angiography, HH hand held, IR infra red, NS not specified, RF red free, SL slit-lamp, TT table top.
aExcluding small pupil modes and multi modal imaging systems.
bMay need little chemical dilation.
cAs available in the specifications.
dTechnically not a non-mydriatic camera, but has been extensively used as the same.
Artificial intelligence
Artificial intelligence (AI) in health care uses complex algorithms to emulate human cognition to analyze complex medical data without direct human input. It provides a well-defined output to the end-user. In several large studies, deep learning (one of the tools of AI) in DR has shown good sensitivity (87% to 100%), specificity (87% to 98%), and receiver operating characteristic curve (AUC; 0.93 to 0.99) for referable DR and/or STDR [67–69]. The current technology cannot capture macular edema (would improve once OCT images are included). AI has not addressed the issue of images captured in different cameras, the image quality, and the field of view. Despite technological advances, deep learning is still not tried in a real-world screening of DR. The final barriers are the health policy of different countries and the trust of both clinicians and patients in the machine verdicts [70].
E-healthcare-ophthalmology
E-health refers to the intersection of medical informatics, public health, and business, referring to health services and information delivered or enhanced through the Internet and related technologies [71]. The World Health Assembly (WHA) 2018 acknowledged digital technologies’ potential to play a major role in improving public health worldwide (WHA 71.7) [72]. The most integral part of e-health and telemedicine is robust information and communication technology (ICT). Broadly, telemedicine applications are divided into (1) “asynchronous” (Store-and-forward) that involves an exchange of pre-recorded data between two or more individuals; and (2) “synchronous” (Real-time) that involves the simultaneous presence of individuals for an immediate exchange of information. Both are possible, though; the asynchronous method is more suitable for DR screening. The patient’s management information is shared at the soonest possible time, and appropriate care is offered after that.
In India, remote screening has been used in DR successfully using a low-cost portable retinal camera [73, 74]. Realizing the importance of tele-screening, the Canadian Retina Research Network (CR2N) has recently laid guidelines to standardize tele-screening methodology in DR. The important elements of these guidelines are DR severity classification and recommendation for retinal photography (two-450 field fundus photo, one centered on the disc and the other centered on the macula; 600 horizontal and 450 vertical), and optical coherence tomography (imaging macula) standards [75]. Some of the important barriers of e-health that applies equally to tele-screening of DR include the availability of reliable ICTs in regions where it matters the most (such as in LMICs), the language of communication across different parts of the world, human, culture, and behavior of shifting from face-to-face encounters to virtual ones and the local legal requirements.
Universal health care
Universal health coverage (UHC) (equitable access, quality care, no catastrophic financial hardship) is the world’s aspiration [76], and the continuum of health intervention by integrated people-centered eye care (IPCEC) is WHO’s recommendation [77]. The IPCEC addresses the full spectrum of eye conditions, according to people’s needs and throughout their life course [77]. It has four layers of care from community to tertiary care. Adhering to both the principles of UHC and IPCEC, different components of DR screening could be conveniently distributed from community level to tertiary level care [78] (Table 7).
Table 7.
DR screening activities at different levels of health care [78] along the WHO guidelines of integrated people-centered eye care.
| Activity | DM center | Community | Primary | Secondary | Tertiary |
|---|---|---|---|---|---|
| DM/DR history | + | + | + | + | + |
| Blood test | + | − | + | + | + |
| VA measurement | + | + | + | + | + |
| Dilated eye exam | +/− | − | + | + | + |
| Fundus photo | +/− | +/− | +/− | + | + |
| Referral to next level | + | + | + | + | + |
| Advocacy | + | + | + | + | + |
With permission from Indian Journal of Ophthalmology.
Systemic care
Monitoring for systemic factors forms an essential aspect of the management of DM and DR. The targets for systemic care are generally uniform across the geographic territories, barring minor differences (Table 4). There is consensus that hyperglycemia and hypertension could be managed as per the available resources at primary through tertiary centers (Table 7). This would result in a decrease in microvascular (including DR) and macrovascular complications of DM. In line with the UHC, the closer this intervention is taken to the community, the higher likelihood of compliance.
Task sharing
Screening of DR in a cost-effective way is important and necessary when there is a huge burden of DM and a shortage of human resources, particularly in regions with a high prevalence of diabetic eye disease [79]. Effective and efficient use of the available trained human non-ophthalmologist workforce would free the ophthalmologists to perform a technically more difficult task in DR care, such as delivering retinal laser, performing intravitreal injections/vitreoretinal surgery, and follow up care of the treated patients.
Screening is not without limitations. These include: (a) screening can reduce the risk of developing a disease or its complications but cannot offer a guarantee of protection; (b) there is a minimum risk of false-positive and false-negative results; (c) false-positive results could lead to distress and possibly unnecessary treatment; (d) false-negative results could lead to false reassurance to patients and doctors. Screening is effective only when it is combined with proper referral and timely treatment. Therefore, it is imperative that screening is not established without creating suitable referral pathways, appropriate treatment, and follow-up care.
Weakness and strengths
The main weakness of this analysis is the limited number of guidelines examined and confined to the English language only. Incidentally, more countries have guidelines for DM (including one from the IDF for T2DM) than DR. The only global guideline for DR is the one recommended by the ICO. Also, we selected English-language DR guidelines, one from each IAPB region and the ones recently published.
The strength of this analysis was the evaluation of the most recent (2016–2019) published DR guidelines. Given the technological advances of devices used in DR detection, increased advocacy, and friendly eye health policy, the inclusion of the DR guidelines formulated in the recent five years was important. This report also compares the current commercially available non-mydriatic fundus camera, which is soon likely to be the standard of DR screening. The analysis also provided evidence on gaps in guidelines, and recommendations are made based on potential solutions from practices in different health systems.
In conclusion, a uniform protocol for DR screening in each country would help improve case detection. National guidelines on timely and evidence-based treatments should be put in place to complement a good screening program. Using newer technology of the camera, e-health, artificial intelligence, and the use of available health care personnel beyond ophthalmologists such as the allied eye health personnel will improve universal coverage of screening. International and national policies need to prioritize DR screening and treatment to align with universal health coverage to improve the efficiency of the screening programs in diabetic retinopathy.
Acknowledgements
Hyderabad Eye Research Foundation for research support of TD, BT, and PKR.
Funding
SS, TD, PKR: GCRF UKRI (MR/P207881/1). TD/BT/PKR: general funding support by HERF. TD, BT, PKR: Hyderabad Eye Research Foundation, Hyderabad, India (2020). SS, TD, PKR: GCRF UKRI (MR/P207881/1).
Author contributions
Concepts: TD, BT, PDN. Design: TD, BT, SS, HT, JN, RK. Definition of intellectual content: TD, BT, SS, TT, ST, PW, JN, PDN, PKR, RK. Literature search: TD, BT, SS, TT, PW, JN, PDN, PKR, RK. Data acquisition: TD, BT, ST, JN. Data analysis: TD, BT. Statistical analysis: TD, BT. Manuscript preparation: TD, BT, SS, RK. Manuscript editing: TD, BT, SS, TT, HT, PW, JN, PDN, PKR, RK. Manuscript review: TD, BT, SS, TT, HT, PW, JN, PDN, PKR, RK. Guarantor: TD.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bourne RRA, Flaxman SR, Braithwaite T, Cicineli M, Das A, Jonas JB, on behalf of the Vision Loss Expert Group. et al. Magnitude, temporal trends and projection of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–97. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 2.IDF Atlas 2019. 9th edn. www.daibetesatlas.org [accessed 01042020].
- 3.World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). United Nations: Department of Economic and Social Affairs, Population Division; 2019
- 4.Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun A, Barnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care. 2018;41:963–70. doi: 10.2337/dc17-1962. [DOI] [PubMed] [Google Scholar]
- 5.Thomas RL, Halim S, Gurudas S, Sivaprasad S, Owens DR. IDF diabetes atlas: a review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pr. 2019;157:107840. doi: 10.1016/j.diabres.2019.107840. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Ahn S, Park KH. Burden of visual impairment and chronic diseases. JAMA Ophthalmol. 2016;134:778–84. doi: 10.1001/jamaophthalmol.2016.1158. [DOI] [PubMed] [Google Scholar]
- 7.Wilson JM, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. https://apps.who.int [last accessed 06 April 2020]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–8. [PubMed] [Google Scholar]
- 9.Diabetes Canada Clinical Practice Guidelines Expert Committee, Altomare F, Kherani A, Lovshin J. Retinopathy. Can J Diabetes. 2018;42 Suppl 1:S210–6. [DOI] [PubMed]
- 10.Scanlon PH. The English national screening programme for diabetic retinopathy 2003–2016. Acta Diabetologica. 2017;54:515–25. doi: 10.1007/s00592-017-0974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indian Institute of Public Health. Guidelines for the prevention and management of diabetic retinopathy and diabetic eye disease in India. Hyderabad: Indian Institute of Public Health; 2019. http://www.phfi.org›wp-content›uploads›2019/09>
- 12.Ministry of Health. Guidelines for the screening and management of Diabetic Retinopathy in Kenya. Nairobi, Kenya: Ministry of Health; 2017. http://www.health.go.ke/wp-content/uploads/2017/11/Guidelines-for-Screening-and-Management-of-Diabetic-Retinopathy-in-Kenya.pdf
- 13.Ministry of Health. Diabetic retinal screening, grading, monitoring and referral guidance. Wellington: Ministry of Health; 2016. https://www.health.govt.nz/system/files/documents/publications/diabetic-retinal-screening-grading-monitoring-referral-guidance-mar16.pdf
- 14.AO diabetic retinopathy PPP 2019. www.aao.org [accessed 03 Apr 2020]
- 15.International council of Ophthalmology. Guidelines for diabetic eye care. International council of Ophthalmology; 2017. http://www.icoph.org/downloads/ICOGuidelinesforDiabeticEyeCare.pdf
- 16.Goldberg MF, Jampol LM. Knowledge of diabetic retinopathy before and 18 years after the Airlie House symposium on treatment of diabetic retinopathy. Ophthalmology. 1987;94:741–6. doi: 10.1016/S0161-6420(87)33524-9. [DOI] [PubMed] [Google Scholar]
- 17.Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the diabetic retinopathy. Invest Ophthalmol Vis Sci. 1981;21:1–226. [PubMed] [Google Scholar]
- 18.Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806. doi: 10.1016/S0161-6420(13)38012-9. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 20.Trichonas G, Kaiser PK. Optical coherence tomography imaging of macular oedema. Br J Ophthalmol. 2014;98:24–9. doi: 10.1136/bjophthalmol-2014-305305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, et al. for the American Diabetes Assocation. Retinopathy in diabetes. Diabetes Care. 2004;S1:s 84–7. doi: 10.2337/diacare.27.2007.S84. [DOI] [PubMed] [Google Scholar]
- 22.Action to Control Cardiovascular Risk in Diabetes Follow-On Eye Study G, the Action to Control Cardiovascular Risk in Diabetes Follow-On Study G. Persistent effects of intensive glycemic control on retinopathy in type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-on study. Diabetes Care. 2016;39:1089–1100. doi: 10.2337/dc16-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2. Diabetes (UKPDS 38) Br Med J. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 24.ACCORD Study Group and ACCORD Eye Study Group. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Klein BEK, Moss SE, Cruickshanks RJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association. Standards of medical care in diabetes. Abridged for primary care providers. American Diabetes Association; 2019. www.clinical.diabtetesjournals.org [DOI] [PMC free article] [PubMed]
- 28.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, The Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Association for the Study of Diabetes (EASD) et al. 2019 ESC Guidelines on diabetes, pre-diabetes,and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 29.The Royal Australia College of General Practioners and Diabetes Australia (RACGP). General practice management of type 2 diabetes 2014-15. The Royal Australia College of General Practioners and Diabetes Australia (RACGP). www.diabetesaustralia.com.au [accessed 5 April 2020]
- 30.Shera AS, Basit A, Fawwad A. Middle East and North Africa region guidelines for the management of type 2 diabetes. J Diabetol. 2019;10:134–9. doi: 10.4103/jod.jod_43_18. [DOI] [Google Scholar]
- 31.SEMDSA 2017 Guidelines for the Management of Type 2 Diabetes Mellitus. J Endocrinolol Meta Diab South Africa. 2017; 22: S1-96
- 32.Haneda M, Noda M, Origasa H, Noto H, Yabes D, Fujita Y, et al. Japanese clinical practice guideline for diabetes 2016. J Diabetes Invetig. 2018 doi: 10.1111/jdi.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Indian Council of Medical Research. ICMR guidelines for management of type 2 diabetes mellitus 2018. Indian Council of Medical Research; 2018. www.icmr.nic.inguidelines [accessed 5 April 2020]
- 34.Pearce I, Simo R, Lovestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–78. doi: 10.1111/dom.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ajoy Mohan VK, Nithyanandam S, Idiculla J. Microalbuminuria and low hemoglobin as risk factorsfor the occurrence and increasing severity of diabetic retinopathy. Indian J Ophthalmol. 2011;59:207–10. doi: 10.4103/0301-4738.81029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Goblan AS, Al-Alfi M, Khan MZ. Mecahnism linking diabetes and obesity. Diabetes Metab Syndr Obes. 2014;7:587–91. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bain SC, Klufas MA, Ho A, Mathewa DR. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metab. 2019;21:454–66. doi: 10.1111/dom.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukla R, Gudlavalleti MV, Bandoyopadhyay S, Anchala R, Gudlavalleti ASV, Jotheeswaran AT, et al. Perception of care and barriers to treatment in individuals with diabetic retinopathy in India: 11‑city 9‑state study. Indian J Endocrinol Metab. 2016;20:S33–41. doi: 10.4103/2230-8210.179772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane M, Mathewson PA, Shrama HE, Palmer H, Shah P, Nightingale P, et al. Social deprivation as a risk for late presentation of proliferative diabetic retinopathy. Clin Ophthalmol. 2015;9:347–52. doi: 10.2147/OPTH.S73272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piyasena MMPN, Murthy GVS, Yip JLY, Gilbert C, Zuurmond M, Peto T, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS ONE. 2019;14:e0198979. doi: 10.1371/journal.pone.0198979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rani PK, Nangia V, Murthy KR, Khanna RC, Das T. Community care for diabetic retinopathy and glaucoma in India: a panel discussion. Indian J Ophthalmol. 2018;66:916–20. doi: 10.4103/ijo.IJO_910_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coney JM. Addressing unmet needs in diabetic retinopathy. Am J Manag Care. 2019;25(16 Suppl):S311–16. [PubMed] [Google Scholar]
- 43.Poverty and blindness: a survey of literature. http://www.icoph.org>downloads>povertyandblindness>downloads>poverty and blindness> [accessed 13 April 2020]
- 44.Sustainable Development Goals. www.sustainabledevelopment.un.org [accessed 13 April 2020]
- 45.Das T, Raman R, Ramasamy K, Rani PK. Telemedicine in diabetic retinopathy: current status and future directions. Middle East Afr J Ophthalmol. 2015;22:174–78. doi: 10.4103/0974-9233.154391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avidor D, Loewenstein A, Waisbourd M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: a systematic review. Cost Eff Resour Alloc. 2020;18:16. doi: 10.1186/s12962-020-00211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan S, Shetty S, Natarajan V, Sharma T, Raman R. Development and validation of a diabetic retinopathy referral algorithm based on single-field fundus photography. PLoS ONE. 2016;11:e0163108. doi: 10.1371/journal.pone.0163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.National Institute for Clinical Excellence. Management of type-2 diabetes: retinopathy-screening and early management. London, UK: Inherited Clinical Guidelines; 2002.
- 49.van der Heijden AA, Rauh SP, Dekker JM, Beulens JW, Elders P, ‘t Hart LM, et al. The Hoorn Diabetes Care System (DCS) cohort. A prospective cohort of persons with type 2 diabetes treated in primary care in the Netherlands. BMJ Open. 2017;7:e015599. doi: 10.1136/bmjopen-2016-015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rein DB, Wittenborn JS, Zhang X, Allaire BA, Song MS, Klein R, et al. The cost-effectiveness of three screening alternatives for people with diabetes with no or early diabetic retinopathy. Health Serv Res. 2011;46:1534–61. doi: 10.1111/j.1475-6773.2011.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh S, Shukla AK, Sheikh A, Gupta G, More A. Effect of health education and screening location on compliance with diabetic retinopathy screening in a rural population in Maharashtra. Indian J Ophthalmol. 2020;68:S47–51. doi: 10.4103/ijo.IJO_1976_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vetrini D, Kiire CA, Burgess PI, Harding SP, Kayange PC, Kalua K, et al. Incremental cost-effectiveness of screening and laser treatment for diabetic retinopathy and macular edema in Malawi. PLoS ONE. 2018;13:e0190742. doi: 10.1371/journal.pone.0190742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garoon RB, Lin WV, Young AK, Yeh AG, Chu YI, Weng CY. Cost savings analysis for a diabetic retoinopathy teleretinal screening program using an activity-based costing approach. Ophthalmol Retin. 2018;2:906–13. doi: 10.1016/j.oret.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Resnikoff S, Lansingh VC, Washburn L, Felch W, Marie- Gauthier T, Taylor HR, et al. Estimated number of ophthalmologists worldwide (International Council of Ophthalmology update): will we meet the needs? Br J Ophthalmol. 2020;104:588–92. doi: 10.1136/bjophthalmol-2019-314336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pr. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 56.Evans JR, Michelssi M, Virgili G. Laser photocoagualtion for proliferative diabetic retiniapthy. Cochrane Database Syst Rev. 2014; CD011234. [DOI] [PMC free article] [PubMed]
- 57.Cheung N, Wong IY, Wong TY. Ocular anti VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. 2014;37:900–15. doi: 10.2337/dc13-1990. [DOI] [PubMed] [Google Scholar]
- 58.Das T, Aurora A, Chhablani J, Giridhar A, Kumar A, Raman R, et al. Evidence based review of diabetic macular edema management: consensus statement on Indian treatment guidelines. Indian J Ophthalmol. 2016;64:14–25. doi: 10.4103/0301-4738.178142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Maria M, Panchal B, Coassin M. Update on indications for diabetic vitrectomy and management of complications. Ann Eye Sci. 2018;3:51. doi: 10.21037/aes.2018.09.04. [DOI] [Google Scholar]
- 60.Gangaputra S, Almukhtar T, Glassman AR, Aiello LP, Bressler N, Bressler SB, et al. Diabetic retinopathy clinical research network. Comparison of film and digital fundus photographs in eyes of individuals with diabetes mellitus. Invest Ophthalmol Vis Sci. 2011;52:6168–73. doi: 10.1167/iovs.11-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li HK, Hubbard LD, Danis RP, Esquivel A, Florez-Arango J, Krupinski EA. Monoscopic versus stereoscopic retinal photography for grading diabetic retinopathy severity. Invest Ophthalmol Vis Sci. 2010;51:3184–92. doi: 10.1167/iovs.09-4886. [DOI] [PubMed] [Google Scholar]
- 62.Srihatrai P, Hlowchitsieng T. The diagnostic accuracy of single- and five-field fundus photography in diabetic retinopathy screening by primary care physicians. Indian J Ophthalmol. 2018;66:94–7. doi: 10.4103/ijo.IJO_657_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aiello LP, Odia I, Glassman AR, Melia M, Jampol LM, Bressler NM, et al. Diabetic retinopathy clinical research network. Comparison of Early Treatment Diabetic Retinopathy Study standard 7-field imaging with ultrawide-field imaging for determining severity of diabetic retinopathy. JAMA Ophthalmol. 2019;137:65–73. doi: 10.1001/jamaophthalmol.2018.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piyasena MMPN, Yip JLY, D MacLeod, Kim M, Gudlavelleti VSM. Diagnostic test accuracy of diabetic retinopathy screening by physician graders using a hand-held non-mydriatic retinal camera at a tertiary level medical clinic. BMC Ophthalmol. 2019;19:89. doi: 10.1186/s12886-019-1092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pratibha V, Rajalakshmi R, Arulmalar S, Usha M, Subhalakshmi R, Gilbert CE, et al. Accuracy of the smartphone-based non-mydrriatic retinal camera in the detection of sighi-threatening diabetic retinopathy. Indian J Ophthalmol. 2020;68:S42–6. doi: 10.4103/ijo.IJO_1937_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piyasena M, Murthy GVS, Yip JLY, Gilbert C, Peto T, Gordon I, et al. Systematic review and meta-analysis of diagnostic accuracy of detection of any level of diabetic retinopathy using digital retinal imaging. Syst Rev. 2018;7:182. doi: 10.1186/s13643-018-0846-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abramoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Niemeijer M. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci. 2016;57:5200–06. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- 68.Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanswamy A. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316:2402–10. doi: 10.1001/jama.2016.17216. [DOI] [PubMed] [Google Scholar]
- 69.Ting DSW, Cheung CY, Tan GSW, Quang ND, Hamzah H, Garcia- Franco R, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiple population with diabetes. JAMA. 2017;318:2211–23. doi: 10.1001/jama.2017.18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong TY, Bressler NM. Artifical intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA. 2016;316:2366–67. doi: 10.1001/jama.2016.17563. [DOI] [PubMed] [Google Scholar]
- 71.Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):220. doi: 10.2196/jmir.3.2.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.eHealth-World health Organization. World health Organization; 2020. http://www.who.intehealth. [accessed 22 April 2020]
- 73.Rajalakshmi R, Arulmalar S, Usha M, Pratibha V, Kareemuddin KS, Anjana RM, et al. Validation of smartphone-based retinal photography for diabetic retinopathy screening. PLoS ONE. 2015;10:e0138285. doi: 10.1371/journal.pone.0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Natarajan S, Jain A, Krishnan R, Rogya A, Sivaprasad S. Diagnostic accuracy of community-based diabetic retinopathy screening with an offline artificial intelligence system on a smartphone. JAMA Ophthalmol. 2019;137:1182–88. doi: 10.1001/jamaophthalmol.2019.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boucher MC, Qian Jn Brent MH, Wong T, Sheidow T, Duval R, Kherani A, et al. Evidence based Canadian guidelines for tele-retina screening for diabetic retinopathy: recommendations from the Canadian Retina Research Network (CR2N) Tele-Retina steering Committee. Can J Ophthalmol. 2020;55:14–24. doi: 10.1016/j.jcjo.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 76.WHO. What is universal coverage? WHO; 2020. http://www.who.intuniversal_coverage_definition [accessed 08 April 2020]
- 77.World Report on Vision. WHO, 2020. https://www.who.int. Accessed 08 April 2020.
- 78.Murthy GVS, Sundar G, Gilbert C, Shukla R, on behalf of the IIPH DR Project Implementation Core Team. Operational guidelines for diabetic retinopathy in India: summary. India J Ophthalmol. 2020;68:S59–62. doi: 10.4103/ijo.IJO_1966_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Das T, Keeffe J, Sivaprasad S, Rao GN. Capacity building for universal eye health coverage in South East Asia beyond 2020. Eye. 2020 doi: 10.1038/s41433-020-0801-8. [DOI] [PMC free article] [PubMed] [Google Scholar]