Abstract
Posttraumatic stress disorder (PTSD) is a highly prevalent, debilitating mental health condition. A better understanding of contributory neurobiological mechanisms will lead to effective treatments, improving quality of life for patients. Given that not all trauma-exposed individuals develop PTSD, identification of pre-trauma susceptibility factors that can modulate posttraumatic outcomes is important. Recent clinical evidence supports a strong link between inflammatory conditions and PTSD. A particularly strong association has been reported between asthma and PTSD prevalence and severity. Unlike many other PTSD-comorbid inflammatory conditions, asthma often develops in children, sensitizing them to subsequent posttraumatic pathology throughout their lifetime. Currently, there is a significant need to understand the neurobiology, shared mechanisms, and inflammatory mediators that may contribute to comorbid asthma and PTSD. Here, we provide a translational perspective of asthma and PTSD risk and comorbidity, focusing on clinical associations, relevant rodent paradigms and potential mechanisms that may translate asthma-associated inflammation to PTSD development.
Introduction
Posttraumatic stress disorder (PTSD) is a trauma-induced psychiatric condition with a lifetime prevalence of approximately 4–5 % in civilians and 14–20% in combat veterans (Kessler et al., 2005; Richardson et al., 2010; Koenen et al., 2017). Individuals with PTSD experience chronic disability, comorbidity, as well as compromised quality of life (Watson, 2019). PTSD symptomology is characterized under the Diagnostic Statistical Manual of Mental Disorders-5 (DSM-5) by re-experiencing and intrusive memories of trauma, avoidance of trauma reminders, hyperarousal and negative alterations in cognition and mood lasting more than one month. Approved pharmacological treatments for PTSD such as selective serotonin reuptake blockers have limited efficacy (~60% treatment response) (Berger et al 2009), necessitating the need to identify underlying mechanisms and risk factors associated with PTSD development.
Although exposure to intense psychological trauma is unfortunately common (Benjet et al., 2016), for most trauma-exposed individuals, the re-experiencing of traumatic memories and arousal symptoms resolve over time. However, in 10%−20% of cases symptoms persist, resulting in PTSD (Kilpatrick et al 2013). It is unclear what factors promote PTSD development; it is likely a combination of the intensity of trauma, genetics, early life experiences and pre-existing disease. As a more complete understanding of risk factors will facilitate the development of a more effective screening and treatment regimen for individuals at elevated risk for PTSD, identification of pre-trauma factors that increase susceptibility to PTSD development, and dissection of mechanisms modulating posttraumatic outcomes is an area of high priority.
Chronic inflammation predisposes to PTSD
Mounting evidence in the past decade supports a role for inflammation in the pathophysiology of PTSD (Pace and Heim, 2011; Passos et al., 2015; Deslauriers et al., 2017a; Kim et al., 2020). Although substantial literature suggests that inflammatory responses are exacerbated by PTSD, the alternative association - that inflammatory signals promote PTSD development after trauma - has also been proposed (reviewed in (Sumner et al., 2020). Numerous longitudinal studies (particularly military cohorts) investigated whether pre-trauma inflammatory markers predicted the development of PTSD (Eraly et al, 2014, Breen et al 2015, Glatt et al 2013, van Zuiden et al 2012). These studies supported an association between markers of increased immune activation and increased likelihood of PTSD symptoms. Two studies employing gene expression measures at pre-deployment demonstrated upregulation of immune networks in men who developed PTSD versus those that did not (Glatt et al., 2013; Breen et al., 2015). In addition, increased rates of comorbidity are observed between PTSD and somatic disorders of immune dysfunction such as chronic idiopathic urticaria, rheumatoid arthritis, and asthma (Devoto et al., 2016; Hunkin & Quarterly, 2012; Maloley et al., 2019). Despite the rising awareness of inflammation-PTSD links, there is a poor understanding of potential underlying mechanisms.
Asthma and PTSD Link: Evidence from human literature
i). Epidemiological Studies
Accumulating evidence suggests that among physical illnesses, there is a strong link between PTSD and asthma. An epidemiological study in Australian Vietnam Veterans (O’Toole and Catts, 2008), studied the association of combat exposure and PTSD with self-reported measures of physical health conditions including asthma, eczema, arthritis, back and other musculoskeletal disorders, and hypertension. Interestingly, the strongest association between PTSD and poor physical health (odds ratio (OR) - 5.38) concerned asthma. Furthermore, the OR was even higher (8.26) after controlling for other psychiatric conditions and comorbidities such as depression. Given the lack of association of PTSD with hay fever in this study, the authors concluded that the link between asthma and PTSD was not due to allergic immune responses (i.e. Th2 cytokine production), but rather with other non-Th2 associated immune responses active during allergic asthma, although other studies reported an association of seasonal allergies with PTSD (OR 1.32) (Kelly and Poole, 2019). Other studies reported elevated OR for PTSD in patients with asthma-related symptoms such as airflow limitation (OR 3.2–8.8) (Kean et al., 2006; Spitzer et al., 2011). Increased asthma prevalence has also been reported in individuals with PTSD following the 9/11 terrorist attack (Shiratori and Samuelson, 2012). A large sample size, longitudinal study in Taiwanese population also corroborates increased asthma prevalence in PTSD patients (Hung et al., 2019). Collectively, these reports suggest a potential relationship between PTSD and asthma, which is likely to play in a bidirectional manner.
ii). Genetic studies
Although epidemiological studies support an association between asthma and PTSD, little is known about the shared genetics underlying the causality of this association. Genetic studies investigating asthma or PTSD suggest that both are moderately heritable conditions that are additionally influenced by gene-environment interactions. In a recent meta-analysis study, a pairwise genetic correlation found a high association between PTSD and asthma, the strongest among somatic conditions (Nievergelt et al., 2019). Intriguingly, no correlations of asthma were observed with other psychiatric conditions including major depression, schizophrenia, bipolar disorder and ADHD. The Vietnam Era Twin Registry was used to dissociate genetic versus environmental factors in asthma-PTSD risk in mono-and dizygotic twins (Goodwin et al., 2007). PTSD symptoms were significantly associated with asthma regardless of zygosity, suggesting contributions of both familial/genetic and environmental in the asthma-PTSD link. Traumatic stress exposure may play an environmental role in PTSD and asthma association. Accumulating evidence from GWAS studies (reviewed in Rosenberg et al., 2014) suggest that stress and trauma may modulate susceptibility conferred by inherited genetic variants. As an example, a pituitary adenylate cyclase-activating peptide (PACAP) receptor ADCYAP1R1 gene variant associated with PTSD (Ressler et al., 2011) was linked with an increased risk for asthma after interaction with exposure to violence in children (Chen et al., 2013). Collectively, this evidence suggests that a potential gene x environment interaction may underlie asthma-PTSD risk and comorbidity although directionality has not been clearly investigated.
iii). PTSD Risk with asthma severity
In several studies, PTSD has been associated with severe asthma. For example, a study of children with asthma in the Boston community demonstrated that PTSD symptoms were found to be significantly elevated in children with severe asthma (Vanderbilt et al., 2008). In a sample of adolescents, higher posttraumatic symptoms and risk for PTSD was reported for individuals with severe asthma as compared to mild or no asthma (Kean et al., 2006). A recent study reported higher prevalence of PTSD (50% of sample) in individuals with moderate to severe asthma compared to other psychiatric conditions including depression and anxiety disorders (Paquet et al., 2019). In another investigation, individuals with asthma prior to PTSD developed more aggravated asthma symptoms after the development of PTSD, suggesting overlapping mediators and shared mechanisms (Underner et al., 2019). Further prospective and longitudinal studies are needed to assess whether severe asthma and associated immune dysregulation serve as a predisposition factor for the development of PTSD.
Immune pathways in Asthma
More than 300 million people suffer from allergic asthma worldwide (Vos et al., 2017). Allergic asthma is a chronic inflammatory lung disease characterized by transient periods of shortness of breath, impaired breathing, wheezing, and coughing. These symptoms are caused by aberrant immune responses to otherwise innocuous environmental proteins. This results in airway inflammation due to eosinophilic and lymphocytic recruitment to the lung, mucus hypersecretion, smooth muscle hypertrophy, airway remodeling, and production of allergen-specific immunoglobulins, specifically IgE. Importantly, asthma is considered a disease of childhood as ~ 11% of US children between the ages of 15 and 19 have been diagnosed with asthma, making it the most common chronic inflammatory disease in children (Asher and Pearce, 2014). Thus, unlike many other panic and PTSD-comorbid conditions, childhood asthma can have a long-term effect, further enhancing its capacity to sensitize and increase risk of PTSD development (Stowman et al., 2015).
Innate immune pathways in asthma
Asthma is associated with a well-defined, progressive activation of both innate and adaptive immune responses to normally innocuous environmental antigens (allergens) (see Figure 1). Excellent reviews are available on the mechanisms underlying allergen-driven activation of the immune system (Lambrecht et al., 2019; Hellings and Steelant, 2020). To briefly summarize these reviews, activation of the innate immune system is driven by allergen-associated pathogen associated molecular patterns (PAMPs) inhaled with allergens like house dust mite (HDM). PAMP recognition by pattern recognition receptors (PRRs) on epithelial cells lining the airways is key to immune responses to allergen. Activation of PRRs (eg, Toll-like or C-type lectin receptors) leads to the production of inflammatory mediators (GM-CSF, CCL20, TNFα, IL-1β) important in the recruitment and activation of phagocytic antigen presenting cells like dendritic cells (DCs). In the airways, DCs encounter allergen, become activated through associated TLRs activation, and migrate to draining lymph nodes where they can activate naïve T cells. Importantly, DC exposure to epithelial cell-derived alarmins such as thymic cytokine thymic stromal lymphopoietin (TSLP), IL-25, IL-33, and IL-1α, in the airways facilitates the differentiation of naïve T cells interacting with the DCs to T helper-2 (Th2) effector cells.
Figure 1: Immune mechanisms active during allergic asthma response.
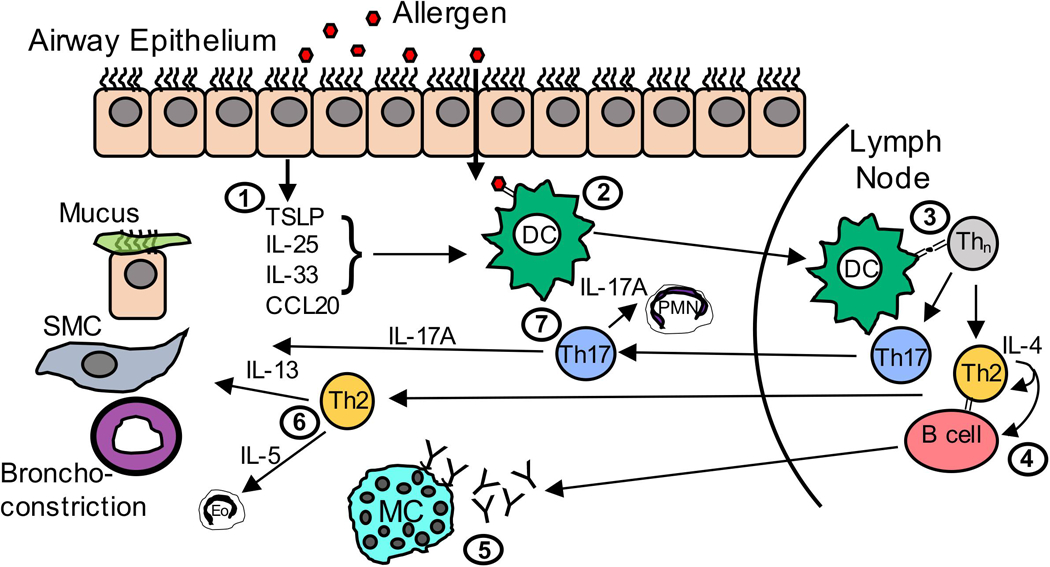
(1) Pathogen Associated Molecular Patterns (PAMPs) on inhaled allergens are recognized by Pattern Recognition Receptors (PRRs) expressed by airway epithelial cells, which produce cytokines and chemokines upon activation. (2) Chemokines, such as CCL20, recruit lung Dendritic cells (DCs) to the epithelial cell barrier where they encounter allergen and become activated. (3) Activated DCs migrate to the draining lymph node (LN) where they present allergen in the context of Major Histocompatability Class II molecules (MHCII) to activate naïve CD4+ T cells and induce differentiation into cytokine-producing effector T helper 2 (Th2) cells. (4) These Th2 cells produce IL-4, which induces B cells to class switch to IgE. (5) IgE produced by B cells binds to Fc𝜀RI on the surface of lung-resident mast cells. Once mast cell-bound IgE is crosslinked by allergen, mast cells can degranulate to release mediators, such as histamine, that induce inflammation and contribute to many of the hallmark airway symptoms of allergic asthma. (6) Activated, allergen-specific Th2 cells also migrate from the lymph node to the lung where they proliferate and produce IL-13 that acts upon epithelial cells to induce goblet cell hyperplasia and mucus production, and smooth muscle cells (SMC) to induce proliferation and mediate airway hyperresponsiveness (AHR). Th2 cells also produce IL-5, which recruits and prolongs the survival of eosinophils. (7) In severe asthma, Th17 cells also differentiate in the LN and migrate to the airways where they produce IL-17A, which both enhance the effects of IL-13, and recruit polymorphonuclear (PMN) cells to the airways.
Adaptive immune pathways in asthma
Once allergen-specific Th2 cells develop, PRR-induced production of inflammatory mediators then triggers recruitment of Th2 cells into the lungs. Cytokine produced by Th2 cells are central to asthma pathology. These include IL-4 (which induces IgE production by B cells), IL-13 (which drives mucus production, AHR, and smooth muscle hypertrophy), and IL-5 (which recruits and regulates survival of eosinophils). Indeed, there are many ongoing trials to test the efficacy of Th2 cytokine-blocking reagents in the treatment of allergic asthma.
Although Th2 cytokines are thought to be important drivers of asthma symptomology, there is a growing sentiment that more severe forms of asthma may involve additional, non-Th2 derived cytokines. As PTSD is more pronounced in the context of severe allergic asthma it is therefore important to distinguish factors driving mild versus severe asthma. Recently, Th17 cells (and production of Th17-associated cytokines like IL-17A or IL-17F) have been shown to promote more severe asthma when present with Th2 cells. Murine asthma models eliciting mixed Th2/Th17 responses are associated with more severe airway hyperresponsiveness and inflammation than purely Th2 responses (Wakashin et al., 2008; Lajoie et al., 2010). This is in line with recent reports that suggest more severe asthma in humans is also associated with a mixed Th2/Th17 immune response. In human asthmatics, IL-17A levels in lung biopsies, sputum, and serum correlated with asthma severity (Molet et al., 2001; Al-Ramli et al., 2009). Furthermore, asthmatics with mixed Th2/Th17 cells in the bronchoalveolar lavage fluid exhibited the greatest airway obstruction, hyperreactivity, and steroid use compared to Th2 or Th2/Th17-low subgroups (Irvin et al., 2014). However, the importance of cytokines associated with general asthma (Th2 cytokines like IL-4, IL-5, and IL-13) versus those associated severe asthma (Th17 cytokines like IL-17A) in PTSD physiology is unclear.
Asthma-relevant immune mediators in PTSD: Clinical studies
Several excellent reviews have been published that summarize studies reporting altered cytokines, chemokines and T cells in PTSD subjects (Wang et al., 2016, 2017b; Michopoulos et al., 2017; Kim et al., 2020). However, in this section we will primarily discuss immune mediators relevant to severe asthma physiology as described above, including Th2 and Th17 cells and related cytokines. PTSD patients were reported to have increased PBMC counts, and a linear correlation between PBMC counts and anxiety symptoms (Zhou et al., 2014). PTSD patients demonstrated a decreased frequency of circulating regulatory T cells and an increased frequency of Th1 cells, while the frequency of Th2 cells was unaltered. In another study, IL-5 (a Th2-associated cytokine) was associated with hostility scores in military personnel although trauma and PTSD were not investigated in this group (Mommersteeg et al., 2008). These observations, coupled with evidence showing weaker associations between PTSD and other Th2-associated allergic disorders (hay fever) (O’Toole and Catts, 2008), and the particularly strong association between PTSD and severe asthma (itself more associated with other immune responses) suggest that Th2 responses may not play a defining role in the asthma PTSD-link.
As stated above, Th17 cells are particularly associated with more severe forms of asthma. Although Th17 cells were not different in PTSD patients, there was a significant linear correlation of peripheral blood mononuclear Th17 cell counts and PTSD score, suggesting a potential association between Th17 cells and PTSD symptomology (Zhou et al., 2014). Consistent with a Th17/IL-17A-PTSD association, concentration of serum IL-17A was significantly higher in PTSD subjects (Zhou et al., 2014). Furthermore, the same study investigated microRNA (miRs) expression coupled with Ingenuity Systems Pathway Analysis and revealed a upregulation of IL-23 expression heavily involved in Th17 induction. In subjects with rheumatoid arthritis, higher IL-17A levels were observed in patients with PTSD compared to those without (Maloley et al., 2019). In another group of combat veterans tightly controlled for trauma type, higher levels of IL-17A, as well as IL-2 and IFNγ) were reported in the plasma and saliva of PTSD subjects compared to the trauma-exposed no-PTSD group (Wang et al., 2016). Importantly, compared to Th17 cells producing IL-17A alone, Th17 cells producing a mixture of IL-17A, IL-2 and IFNγ have proven to be more pro-inflammatory (Ghoreschi et al., 2010). Collectively these studies support that immune mediators elevated in the context of severe asthma, particularly Th17/IL17A may contribute to PTSD/asthma association.
Mechanistic Insights on the PTSD/severe asthma link: Pre-clinical models
Preclinical paradigms can provide valuable mechanistic insights that complement clinical observations. Several rodent paradigms have been developed to simulate PTSD relevant behavioral, or asthma-relevant physiological/immunological responses. Given the availability of several PTSD and asthma models (see Tables 1 & 2), assessment of the relationship and mechanistic interaction between the two is plausible, yet, surprisingly, rigorous studies to assess this link are currently lacking. Here, we describe rodent studies where animals were exposed to either traumatic stress (relevant to PTSD) or allergens (relevant to asthma). We highlight studies where asthma-relevant inflammatory mediators were assessed in PTSD paradigms or behavioral measures relevant to psychiatric outcomes were investigated following allergen exposure.
Table 1:
Animal models of PTSD-relevant behavior pertinent to asthma-related inflammatory mediators
| Model | Behavior | Immune Response | Citations |
|---|---|---|---|
| Chronic Social Defeat Stress (SDS) | ↑Social avoidance ↑Cued/context fear ↑Anxiety |
↑Mature Th2 ↑Pro-inflammatory profile (i.e. IL-17A, IL-4) ↑Dendritic priming |
Munshi et al., 2020
Moshfegh et al., 2019 Powell et al., 2011 Bailey et al., 2009 Ambrée et al., 2019 |
| Predator Stress Paradigm (PSP) | ↑ Anxiety ↑ Startle |
T-cell mediated T-cell entrance via BBB |
Wilson et al., 2013 Vargas-Caraveo, 2015 Cohen et al., 2006 Lewitus et al., 2008 |
| Inescapable Footshock Stress (IFS) | ↑Learned helplessness ↑Anhedonia |
↑Th17 differentiation in susceptible ↑CNS Th17 |
Beurel et al., 2013, 2018 Beurel & Lowell, 2018 Madina-Rodriguez et al., 2018 |
| Single Prolonged Stress (SPS) | ↑ Anxiety ↓ Cognition ↓ Memory |
↑General inflammation Peripheral: IL-1β, TNFα Central: IL-1 β, IFNγ |
Wang et al., 2018
Lee et al., 2016 Lee et al., 2018 |
Table 2:
Animal models of asthma pertinent to PTSD-relevant behaviors
| Model | Behavior | Immune Response | Citations |
|---|---|---|---|
|
Ovalbumin
(non-CNS, non-self antigen) |
↑ Anxiety ↓ Spatial learning ↑Fear Response |
Antibodies: IgE IgG1 T-cell: Th2 cyokines, IL-17, Treg TNFα |
Costa-Pinto et al., 2005 Bei et al., 2013 Tonelli et al., 2009 Dehdar et al., 2019 Zuo et al., 2014 |
| House Dust Mite | ↑ Anxiety ↑ Anhedonia ↑ Extinction Freezing |
Cortisol Th2 cytokines: IL-4, IL-5, IL-13 |
Caulfield et al., 2017
Caulfield et al., 2018 Lewkowich et al., 2020 |
PTSD-relevant preclinical models.
The interaction of stress with asthma induction and severity is well established (see (Chen and Miller, 2007; Bailey et al., 2009; Haczku and Panettieri, 2010; Barnthouse and Jones, 2019; Lopes et al., 2020)). As clinical evidence in PTSD patients strongly supports the contribution of immune mediators, many pre-clinical studies have assessed traumatic stress-evoked global changes in pro- and anti-inflammatory molecules. In this section, we selectively focus on paradigms validated for PTSD-relevant behavior and physiology where alterations in adaptive immune mediators relevant to asthma were assessed regardless of whether the behavior / physiology outcomes were found to alter asthma pathology, or vice versa (see Table 1). Although not directly examining the asthma-PTSD link, these studies report a temporal association of altered inflammatory mediators with PTSD-relevant behavior, suggesting their potential use for assessment of asthma physiology.
(i). Chronic Social Defeat Paradigm
The social defeat stress (SDS) model has been used extensively to simulate depression and anxiety associated behaviors, however recent studies suggest relevance to posttraumatic pathophysiology (Deslauriers et al., 2018). Mice exposed to repeated SDS demonstrated increased anxiety-like behavior, microglial alterations and increased neuronal activity within the amygdala, and these changes were associated with increased blood levels of Th2 cells and pro-inflammatory cytokines (Munshi et al., 2020). Mechanistically, SDS can induce the priming of dendritic cells (Powell et al., 2011) that are important in shaping the primary adaptive immune response in asthma (see Fig 1). Recent studies suggest a contribution of norepinephrine (NE), a sympathetic hormone upregulated in PTSD (Southwick et al., 1999; Strawn and Geracioti et al., 2008), in regulation of T cell activation in isolated splenocytes (Case et al., 2016). In SDS exposed mice increased T cell activation was accompanied by elevated levels of tyrosine hydroxylase a catecholaminergic biosynthetic enzyme (Moshfegh et al., 2019). Interestingly, SDS increased circulating IL17A levels and splenic IL-17A expression was positively correlated with increased expression of tyrosine hydroxylase (Table 1). Consistent with this hypothesis, one study investigated the effects of chronic SDS exposure in a murine model of allergic asthma (Bailey et al., 2009). Psychosocial stress exposure exacerbated the airway hyperactivity response to allergen exposure and increased anxiety-like behavior and significantly elevated levels of Th2-associated inflammatory cytokines, such as IL-4, IL-5, and IL-13 observed in lung tissue. Another study reported SDS-evoked alterations in the adaptive immune response that was associated with stress susceptibility and resilience (Ambrée et al., 2019). Significantly higher numbers of splenic IL-17A producing CD4+ and CD8+ T cells were observed only in susceptible animals compared to control mice. Collectively, these studies support the utility of the SDS model for studying T cell alterations that are relevant to the development of allergic asthma.
(ii). Predator Stress Paradigm
Predator-stress consists of a single exposure to a predator, either unprotected, with a physical barrier, or exposure to a predator odor. These manipulations result in enduring behavioral and physiological alterations including general avoidance, exaggerated fear response, and hyperarousal (Deslauriers et al., 2018). Some studies have explored immune involvement and T cell activity/trafficking following predator stress (Table 2). Immunodeficient mice exposed to predator odor demonstrate higher anxiety and startle response compared to immunosufficient mice suggesting that adaptation to predator odor stress requires a controlled adaptive immune response (Cohen et al., 2006). Furthermore, T cell trafficking into BBB-devoid areas such as the choroid plexus was associated with resilience in predator stress. Direct induction of T cell recruitment into the brain (via immunization with a peptide derived from myelin, pMOG35–55) also induced this resilient phenotype, suggesting that increasing T cell numbers in the brain was associated with resilience.
(iii). Inescapable Footshock Stress paradigm
Repeated exposure to inescapable footshocks (IFS) has been used for simulating learned helplessness characteristic of depression. However, given that footshocks are highly aversive and fear inducing, they constitute an intense traumatic stressor and thus have also been used in rodent PTSD paradigms (Deslauriers et al., 2018; Conoscenti and Fanselow, 2019). In several studies (Beurel et al., 2013, 2018; Beurel and Lowell, 2018; Medina-Rodriguez et al., 2018), activation of Th17 T cells was observed in the hippocampus of mice susceptible to learned helplessness, but not in resilient mice (Beurel et al., 2013). Furthermore, Th17 cell transfer promoted learned helplessness while impairing novelty suppressed feeding and social interaction behaviors. Pharmacological inhibition of Rorγt (a transcription factor necessary for Th17 cell differentiation) prevented these behaviors, supporting a central role and sufficiency of Th17 cells in IFS-evoked behavioral deficiencies. In a follow up study (Beurel et al., 2018), tagged donor Th17 cells were localized to the hippocampus and prefrontal cortex of host mice suggesting potential infiltration of peripheral Th17 cells into these sites were driving behavioral alterations.
(iv). Single Prolonged Stress (SPS) Paradigm
The rodent SPS paradigm is widely used to simulate intense traumatic stress experience that is relevant to PTSD. SPS involves successive exposure to three stressors (restraint, swim and ether inhalation) (Yamamoto et al., 2009). SPS exposure results in behavioral/physiological changes that are relevant to PTSD, particularly impaired fear extinction, neuroendocrine dysfunction, increased startle, and sleep disturbances (reviewed in (Deslauriers et al., 2017b; Souza et al., 2017; Lisieski et al., 2018). Few studies have explored immune cell alterations following SPS exposure. SPS-exposed rats displayed increased plasma and brain concentrations of IL-1β, IFN-γ, and TNF-α concurrent with deficits in fear extinction (Wang et al., 2018). Increased expression of brain TNF-α, IL-1β and IL-6 following SPS was reported in rats that exhibit impairments in cognitive memory and increased anxiety associated behaviors (Lee et al., 2016, 2018). Currently, SPS effects on the adaptive immune system are not known.
Across models, studies support that post trauma behaviors are influenced by adaptive and innate immune responses in the periphery. Thus, it is conceivable that chronically altered homeostatic balance of T cell populations observed in asthma may impact posttraumatic outcomes. However, these relationships have not been investigated.
Asthma-relevant preclinical models
Although current rodent models of asthma simulate inflammatory, structural and physiological features associated with asthma in humans (reviewed in Nials and Uddin, 2008; Chapman et al., 2014; Martin et al., 2014; Kianmehr et al., 2016), studies expanding these models to include behavioral measurements are limited. In this section, we discuss studies where an allergen-induced lung dysfunction was coupled with behavioral measurements that may be relevant to posttraumatic pathophysiology. The most commonly used asthma protocols in rodents - Ovalbumin (OVA) and house dust mite (HDM) extract-based protocols - and their effects on behaviors is discussed in this section (see Table 2).
i). Ovalbumin Model
In OVA-based models, animals are systemically sensitized by OVA injection (i.e. intraperitoneally) often in the presence of an adjuvant such as aluminum salts or the gram-negative bacterial cell wall component, lipopolysaccharide (LPS). To induce localized pulmonary inflammatory responses, sensitized mice are given ovalbumin either as an aerosol or through intranasal/intratracheal challenge. This protocol promotes robust inflammatory responses relevant to asthma, including local production of Th2 cytokines (IL-4, IL-5, IL-13), airway eosinophilia, excessive mucus production, elevated IgE levels and airway hyperreactivity (AHR). The effects of OVA-induced asthma on conditioned passive avoidance behavior using a light dark box have been reported. For example, OVA sensitized mice showed significant avoidance of the dark compartment (usually preferable to mice) after this area was associated with OVA treatment, suggesting a conditioned place avoidance associated with the aeroallergen (Costa-Pinto et al., 2005). Additionally, OVA administration induced significant Fos activation in stress and emotion regulatory areas of the brain: the central nucleus of the amygdala and the hypothalamus. In another study, sensitization and challenge with OVA increased anxiety-associated behaviors in the social interaction and open field tests while simultaneously increasing expression of Th2 cytokines (IL-4, IL-5, IL-13) in the prefrontal cortex (Tonelli et al., 2009). Furthermore, increased immobility behaviors in the forced swim and tail suspension tests representing depression-like behaviors were reported in OVA-challenged mice (Zuo et al., 2014). OVA-sensitized mice also display potentiated stress-evoked anxiety-like behaviors and increased Th2-associated cytokines (Bailey et al., 2009) suggesting a potential role of Th2-associated responses in modulation of behavior.
Importantly, alterations in important fear regulatory regions have been noted in asthma. Increased prefrontal activity and anxiety is reported in asthma patients (Rosenkranz et al., 2012), and OVA-challenged rats showed increased anxiety behavior coupled with elevated mPFC activity, amygdala delta-gamma coupling, and functional connectivity within mPFC-amygdala circuits (Dehdar et al., 2019). Interestingly, a central role of mPFC-amygdala dysfunction has also been reported in PTSD (Gilboa et al., 2004; Milad et al., 2009) suggesting that asthma can target brain regions relevant to PTSD pathophysiology. Collectively, OVA sensitization/challenge models support behavioral effects and peripheral-central inflammation in forebrain areas regulating stress and emotion and could be further refined for PTSD-relevant behaviors and physiology.
ii). House Dust Mite extract (HDM) treatment paradigm
There is an increasing shift towards natural allergens for experimental models of asthma. One widely utilized allergen is and extract of house dust mite (HDM). This allergen offers several benefits over OVA models including translational relevance (~85% of asthmatics demonstrate sensitivity to HDM) (Nelson et al., 1996; Gregory and Lloyd, 2011) and that asthma phenotypes can be induced following mucosal allergen exposures (i.e. through the lung) without prior systemic sensitization. This mirrors the development of asthma in humans, where allergen exposure typically occurs via the inhaled route. HDM contains multiple PAMPs that can fully activate and damage respiratory epithelial cells and allow for robust activation of both innate and adaptive immune responses (see Fig 1).
The impact of HDM-induced asthma on behaviors relevant to psychiatric conditions is limited, however available data suggest a potentiating effect of HDM-induced asthma on behavioral changes. Anxiety-related behaviors in animals with airway inflammation induced by early life HDM exposure (starting postnatal day 7) versus labored breathing (induced by methacholine starting postnatal day 22) (Caulfield et al., 2017) has been assessed. Only animals with labored breathing, but not airway inflammation, had reduced open arm exploration in the elevated plus maze. Importantly, AI appeared to reverse the effects of LB breathing on anxiety or depression associated behaviors suggesting that airway dysfunction was an important driver of the observed behavioral changes. More recently this same group explored the impact of peri-adolescent exposures on behaviors in adult animals (Caulfield et al., 2018). Interestingly, the effects of peri-adolescent asthma models could be dichotomized based on the frequency of neonatal (postnatal day 3–5) ultrasonic vocalizations (USV). LB-exposed neonates with high USV demonstrated increased anxiety like behaviors, whereas those with low USV demonstrated decreases in such behaviors. As high calling mice were found to have lower IL-5 expression, this further supports the hypothesis that Th2 cytokines responses may limit anxiety like behaviors in this model. However, early airway inflammation reduced adult latency to immobility in the forced swim test with no dichotomization by USV. This may suggest developmental effects on depression-associated behaviors related to inflammation (Caulfield et al., 2018).
Recently, using an HDM-induced model of allergic asthma in A/J mice, a strain which develops a mixed Th2/Th17 response (Lewkowich et al., 2008; Lajoie et al., 2010) and steroid refractory features observed in severely asthmatic humans (Serra et al., 2018), we observed effects of intra-tracheal HDM treatment on fear extinction behavior, with no significant differences in anxiety, depression or panic associated behaviors (Lewkowich et al., 2020). Importantly, impaired fear extinction is a hallmark of PTSD (Milad et al., 2009), and contributes to the persistence of trauma memories (Jovanovic et al., 2010; Maddox et al., 2019). Interestingly, although pulmonary inflammation induced by HDM was associated with increased recruitment of both IL-13 and IL-17A-producing cells to the lungs, only IL-17A producing cells were elevated in the brains of HDM-exposed A/J animals. The increased frequency of IL-17A-producing cells in the brain was accompanied by microglial alterations in specialized blood-brain-barrier (BBB) compromised circumventricular area, subfornical organ, suggesting that local IL-17 production may be important in regulating microglial, and neural circuit function. Furthermore, post extinction measurements revealed increased ΔFosB staining within areas regulating fear extinction; the medial prefrontal cortex and basolateral amygdala in HDM treated mice. Collectively, these data show modulation of brain immune mechanisms and fear circuits by peripheral airway inflammation. HDM-fear conditioning models may be useful for understanding mechanisms underlying the risk and comorbidity of asthma-PTSD.
The asthma-PTSD link - Potential peripheral-CNS associations and contributory mechanisms
The brain has historically been considered immune privileged as compared to other organs, based on protection against the peripheral milieu and the apparent absence of a lymphatic system, a view that has been challenged by recent studies (Louveau et al., 2018). Complex tight junctions between adjacent endothelial cells in the cerebral vasculature make up a ‘physical barrier’ called the blood brain barrier (BBB). The BBB facilitates the entry of required nutrients into the brain, and excludes larger molecules, and immune cells, such as T cells, from entering the CNS. However, mounting evidence suggest that multiple mechanisms enabling communication between peripheral and CNS compartments may operate in the absence of pathological BBB breakdown. Excellent reviews have been published on communication pathways and neuroimmune links between the periphery and brain (Ransohoff et al., 2003; Quan and Banks, 2007; Shechter et al., 2013; Dantzer, 2018)). In this section we briefly discuss potential routes by which asthma-associated, T-cell-mediated immune perturbations in bronchopulmonary and humoral compartments may regulate brain areas and circuits relevant to PTSD (See Fig 2).
Figure 2. llustration of potential routes by which asthma-associated airway inflammation may impact brain physiology and behavior.
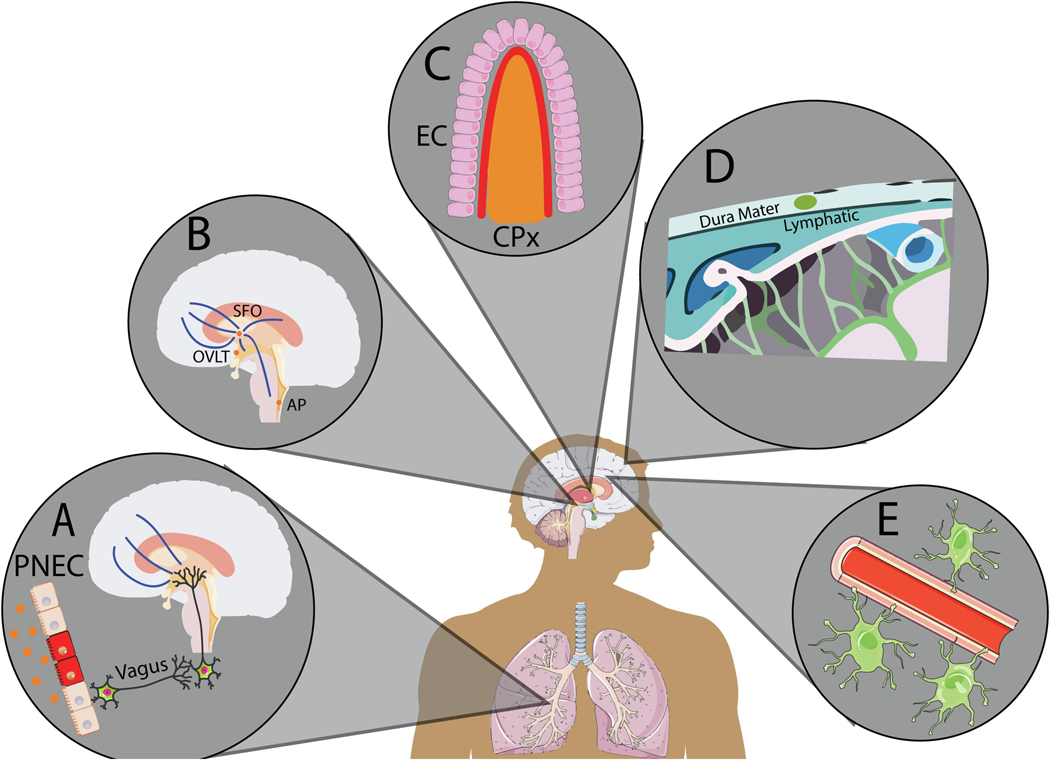
(A) Pulmonary vagal-brain stem pathway: Afferent nerve fibers from sensory ganglia innervate pulmonary neuroendocrine cells. Intratracheal allergens and related inflammatory signals can activate vagal nerve afferents innervating brainstem nuclei that project to forebrain areas regulating emotional behavior, neuroendocrine and autonomic responses. (B) Sensory circumventricular organs: Fenestrated, “leaky”, blood vessels and peri-ventricular location allow CVOs to sense blood and CSF milieu and permit diffusion of cytokines and T cell extravasation. Presence of neuronal cell bodies projecting to forebrain-hindbrain centers controlling cardiovascular, neurovascular and behavioral responses makes CVOs particularly attractive in body-to-brain signaling. (C) Choroid plexus-blood-cerebrospinal fluid interphase. Specialized monolayer of epithelial cells (EC) facilitates the entry of adaptive immune mediators, particularly T cells from blood into the CSF compartment and interstitial spaces thereby modulating brain homeostasis and function. (D) Meningeal lymphatic system: Meningeal interphase provides a site for T cell diapedesis, as well as, T cell drainage from the CSF into cervical lymph nodes, providing an opportunity for generation and trafficking of reactive T cells into the parenchyma for modulating brain physiology and behavior.(E) Blood-brain barrier interphase: A protective barrier under homeostatic conditions, BBB integrity can be compromised by chronic exposure to pollutants, nanoparticles and stress. Elevated cytokine levels can increase BBB permeability by modifying tight junction proteins and endothelial cells allowing extravasation of immune mediators into the brain.
I). Pulmonary Vagal- Brain Stem pathway
The vagus nerve provides a primary pathway for immune-brain communication (reviewed in Mazzone and Undem, 2016), and vagal sensory mechanisms may play an important role in conditions such as asthma. Neural tracing studies have shown vagal afferent fibers from the jugular and nodose ganglia innervating lower airways (Berthoud and Neuhuber, 2000). Afferent neurons communicate with the diffuse neuroendocrine system in lungs comprising of neuroepithelial cell bodies that serve as airway sensors for irritant stimuli (Hale et al., 2012). It is likely that intratracheal allergens in asthma and related inflammatory cytokines activate vagal nerve afferents. Signals from the nodose ganglion have been reported to communicate with the brain stem via C fibers (Hale et al., 2012), targeting the vagal nerve nuclei, primarily nucleus of solitary tract (NTS), which in turn innervates cortical and subcortical areas such as the hypothalamus to regulate sympathetic/cardiovascular and neuroendocrine responses (Ulrich-Lai and JP, 2009).
Of relevance to asthma, previous work has shown that pulmonary neuroepithelial cells (PNECs) are required for the Th2 immune response in asthma, as Ascl1-mutant mice that have no PNECs demonstrate a suppressed asthma response (Sui et al., 2018). OVA sensitization resulted in activation of the dorsolateral part of the NTS in the absence of activation of the area postrema, a CVO, supporting that localized inflammation in the lung can signal to the brain via vagal pathways, in the absence of circulating proinflammatory mediators (Hale et al., 2012). The pulmonary-vagal-brain stem communication pathway thus provides a direct mechanism by which asthma-relevant immune mediators and lung airway distension can generate appropriate autonomic, endocrine, and behavioral responses via central pathways. Sensitized sympathetic tone and abnormal neuroendocrine hypothalamic pituitary adrenal response are observed in PTSD patients (Pitman et al., 2012). Furthermore, NTS projections to fear regulatory areas such as the hippocampus, amygdala, and prefrontal cortex may be relevant to dysregulated fear memory reported in PTSD patients. Interestingly, in rats exposed to SPS, a PTSD-relevant paradigm, vagal nerve stimulation improved extinction of fear and reduced acoustic startle and anxiety-like behavior (Souza et al., 2017) supporting the relevance of vagal inputs in regulation of PTSD-relevant behaviors.
II). Sensory Circumventricular Organs
Circumventricular organs (CVOs), are specialized areas located in proximity to brain ventricles and characterized by a compromised BBB with fenestrated capillaries (McKinley et al., 2003; Miyata, 2015). Sensory CVOs comprising of the subfornical organ (SFO) vascular organ of lamina terminalis (OVLT) in the forebrain and area postrema (AP) in the hindbrain contain neuronal cell bodies in close apposition to blood capillaries and ventricular CSF. Lymphopenic Rag2(−/−) mice reconstituted with GFP-expressing CD4(+) and CD8(+) T cells display an abundance of lymphoid cells within the circumventricular organs similar to the choroid plexus and meninges (Song et al., 2016), suggesting that the CVOs represent potential homing sites whereby T cell populations can impact brain function. Due to these features and their efferent projections to several forebrain and hind brain areas, CVO-resident neurons have the unique capacity to detect alterations in the blood-CSF milieu and impact physiological and behavioral responses (McKinley et al., 1998; Johnson et al., 2013). In addition, CVOs such as the AP receive direct innervation from the vagus (Ferguson, 1991) enabling further communication with lung-nodose ganglion pathway (discussed above). SFO neurons have been reported to modulate neuroendocrine responses and behavior via projections to the hypothalamus, BNST and prefrontal cortex (McKinley et al., 2003; Krause et al., 2011). Studies from our group have shown regulation of panic and PTSD relevant fear behaviors via microglial chemosensory mechanisms and angiotensin receptor type 1 (AT1R) within the SFO (Vollmer et al., 2016; Winter et al., 2019). Interestingly, renin angiotensin system (RAS) has been implicated in allergic asthma (Kim and Im, 2019), as well as PTSD (Khoury et al., 2012). Recently, using a murine HDM model of severe allergic asthma, we reported alterations in microglia within the SFO that temporally coincided with increased Th17 cells in the brain and preceded the development of deficits in fear extinction (Lewkowich et al., 2020). This suggests a potential role of the CVOs, particularly, the SFO in translating asthma-relevant inflammation into behaviors relevant to PTSD.
III). The choroid plexus blood–cerebrospinal fluid interphase
The choroid plexus (CPx), a secretory CVO, gates the blood CSF barrier and serves as an immunological interface allowing the exchange of signals between the periphery and brain (Ransohoff and Engelhardt, 2012). Accumulating evidence supports the CPx, composed of vascular and connective tissue and surrounded by specialized epithelial monolayer, as a site through which adaptive immune cells, particularly T cells, can regulate brain function (reviewed in (Baruch and Schwartz, 2013). Interestingly, mice lacking integrin β4 on pulmonary epithelial cells display increased sensitivity to asthma development, and increased bipolar-like behaviors that were associated with increased airway inflammation and interleukin 4 receptor α expression in the CPx (Han et al., 2018) suggesting that elevated Th2-like responses may contribute to altered behavior. However, the CPx also serves as an important site for Th17 entry into the brain (reviewed in Cipollini et al., 2019). Previous studies have reported that the CPx regulates the trafficking of homeostatic T cells into the non-inflamed CNS for surveillance and pathogenic Th17 cells in early stages of EAE (Ransohoff et al., 2003). Importantly, IL-17A upregulates CCL20 expression on CPx epithelial cells enabling the entry of CCR6+ Th17 cells into the brain (Reboldi et al., 2009). After CPx-gated entry into the CSF, Th17 cells can distribute into the perivascular space as well as activate neurons within the sensory CVOs. It has been proposed that following traumatic stress, immune surveillance function of the CPx may be altered promoting maladaptive signaling and behavioral manifestations (Kertser et al., 2019). Collectively, this supports a key role of the CPx in adaptive immune modulation of brain function under physiological as well as pathological states such as severe asthma where chronic elevations in Th2 and Th17 cells are observed.
IV). Meningeal Lymphatic System
Recent work (Louveau et al., 2018; Brioschi and Colonna, 2019) has identified entry points for immune cells and cytokines through distinct anatomical routes in the meninges, a three-layered membrane comprising of the dura, subarachnoid and pia mater that covers the brain parenchyma. Fluorescently labelled T cells injected intravenously can be detected in the leptomeningeal spaces (Carrithers et al., 2000), suggesting T cell diapedesis and bypassing of the BBB. Furthermore, meningeal lymphatic vessels allow drainage of T cells from CSF to cervical lymph nodes, providing an opportunity for generation and trafficking of reactive T cells into the parenchyma. Thus, meningeal mechanisms provide another interface for peripheral immune system interaction with the CNS. Under physiological conditions, T cells within the subarachnoid space have been reported to regulate learning and memory, as RAG deficient mice, which are devoid of adaptive immune cells, show cognitive impairments (Derecki et al., 2010; Kipnis et al., 2012). In EAE, Th17 cells can enter the meningeal subarachnoid space after crossing the blood-CSF barrier via the choroid plexus, and eventually extravasate into brain parenchyma after reactivation mechanisms at the meningeal-cervical lymph node site (Zepp et al., 2011; Shechter et al., 2013; Brioschi and Colonna, 2019). Of relevance to the central role of Th17/IL-17A in asthma and cortical dysfunction evident in PTSD, recent studies report a homeostatic role of meningeal γδ17 T cells and physiological release of IL-17A by these cells in regulating anxiety-like behavior in mice via IL-17A receptor in cortical glutamatergic neurons (Alves de Lima et al., 2020). It is currently unclear whether chronic T cell inflammation in asthma could alter neuroimmune homeostasis within leptomeningeal spaces or impact γδ17 T cell/IL-17A-mediated regulation of cortical neurons. The capacity of meningeal T cells to modulate cognitive and anxiety-like behaviors makes this interphase an important candidate for future investigations in asthma paradigms and traumatic stress associated models of relevance to PTSD.
V). Blood-Brain Barrier (BBB) Interphase
T cell extravasation through the BBB is reported in conditions such as multiple sclerosis and encephalomyelitis (Kebir et al., 2007; Balasa et al., 2020). Similarly, exposure to inhaled, asthma relevant exposures such diesel exhaust, disrupts the BBB (Hartz et al., 2008; Heidari Nejad et al., 2015). Further, postmortem studies have shown an accumulation of particulate matter in cerebral capillaries and frontal lobes of individuals from areas of high air pollution (Wang et al., 2017a) and evidence suggests that nanoparticles can compromise the tight-junction proteins constituting the BBB (Wang et al., 2017a). Such conditions may facilitate the passage of peripheral T cells that are chronically elevated in severe asthmatics into brain parenchyma. Beyond inhaled toxicants, pro-inflammatory cytokines like IL-17A are relatively small molecules, and have been demonstrated to cross the BBB through both passive, and active transport mechanisms (Banks and Erickson, 2010; Huppert et al., 2010).
In particular, IL-17A-BBB interactions have been extensively studied in the context of multiple sclerosis and its rodent model counterpart, experimental autoimmune encephalomyelitis (EAE) (Komiyama et al., 2006; Rothhammer et al., 2011; Du et al., 2016). IL-17A can rapidly increase BBB permeability by modifying the localization of tight junction proteins and endothelial changes (Rahman et al., 2018). The endothelial cells of the BBB express functional IL-17A receptors and upon IL-17A binding, decrease expression of claudin-5, a protein that maintains the tight junctions necessary for the integrity of the BBB (Cipollini et al., 2019). With the breakdown, other cytokines and T helper cells are now able to penetrate the brain in a pathological context. It is promising that IL-17A facilitates this breakdown, as it indicates its ability to act in not already compromised BBBs. It is conceivable that similar IL-17A-mediated disruption of the BBB may occur in severe asthma associated with a mixed Th2/Th17 response.
Conclusions and Future Directions
As described in preceding sections, evidence from clinical and epidemiological studies, as well as limited preclinical observations supports a strong association between asthma and PTSD, although and mechanistic links have yet to be explored. Based on existing knowledge, several questions need to be addressed. The directionality between asthma and PTSD needs to be investigated in longitudinal studies. Furthermore, as current cross sectional studies have mostly focused on combat exposure or events like 9/11 (Goodwin et al., 2007; O’Toole and Catts, 2008; Shiratori and Samuelson, 2012; Underner et al., 2019), an important consideration would be to dissociate the contribution of psychological versus environmental triggers in promoting PTSD or asthma symptomology. Given the prevalence of asthma in younger population, studies in pediatric cohorts with an emphasis on psychiatric assessments and long-term outcomes is necessary. There is growing evidence that events occurring early in life, both before and after birth, are significantly associated with the risk of asthma (reviewed in (Renz and Skevaki, 2021), and pre-gestational inflammatory insults can promote later development and persistence of asthma. In this regard neurodevelopmental maternal immune activation models, especially those involving Th17/IL-17A mediated behavioral deficits and cortical dysfunction may be relevant (Choi et al., 2016; Reed et al., 2020). It would also be important to assess contribution of stress, genetics and other inflammatory conditions in modulating asthma-PTSD physiology. Perhaps most crucial is the need to develop suitable preclinical paradigms for mechanistic understanding of asthma-PTSD, focusing on assessment of asthma and PTSD-relevant physiological and behavioral outcomes as well as tissue analysis of immune mediators in the brain and periphery. These models will also be relevant for identification and testing of novel targets and therapeutic agents. Although preliminary evidence from our group and others (Beurel et al., 2013; Caulfield et al., 2017, 2018; Alves de Lima et al., 2020; Lewkowich et al., 2020) suggests a role for severe-asthma related Th17/IL-17A in PTSD-relevant behaviors and comorbidities, future studies are required to determine how T cell mediators impact brain function at a cell-circuit level. Moreover, as IL-17A is produced in excess in many other chronic inflammatory conditions, elucidating the mechanisms through which IL-17A may mediate these effects may have wide reaching implications for the co-morbid PTSD that accompanies many other pro-inflammatory conditions. As outlined by us, several periphery-brain communication pathways exist that can translate asthma-related inflammation into the CNS. In conclusion, we highlight asthma and PTSD association, an emerging and relevant area of research investigation that will not only provide information on body-brain communication pathways but further our understanding on the risk, comorbidity and treatment of these highly prevalent conditions.
Highlights.
Genetic and epidemiological evidence supports a severe asthma-PTSD association
Asthma, particularly severe forms, involve mixed T helper Th2 and Th17 immune cells
Links between Th2/Th17, and behavior are seen in rodent models of asthma and PTSD
Peripheral immune-brain pathways may translate asthma inflammation to PTSD
Acknowledgements
The authors would like to acknowledge support from NIH grant MH117483–01 (RS and IL). Financial support was also provided by the UC pilot translation research project (PTRP) grant (RS) and CCHMC Mind Brain and Behavior Research Innovation Program (IL). EJ would like to acknowledge support from a T32 pre-doctoral training program in Neuroscience grant (T32NS 007453)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q (2009) T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 123:1185–1187. [DOI] [PubMed] [Google Scholar]
- Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J (2020) Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol:1–9 [DOI] [PMC free article] [PubMed]
- Ambrée O, Ruland C, Zwanzger P, Klotz L, Baune BT, Arolt V, Scheu S, Alferink J (2019) Social defeat modulates T helper cell percentages in stress susceptible and resilient mice. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher I, Pearce N (2014) Global burden of asthma among children. Int J Tuberc Lung Dis 18:1269–1278 [DOI] [PubMed] [Google Scholar]
- Bailey MT, Kierstein S, Sharma S, Spaits M, Kinsey SG, Tliba O, Sheridan JF, Panettieri RA, Haczku A (2009) Social Stress Enhances Allergen-Induced Airway Inflammation in Mice and Inhibits Corticosteroid Responsiveness of Cytokine Production. J Immunol 182:7888–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasa R, Barcutean L, Balasa A, Motataianu A, Roman-Filip C, Manu D (2020) The action of TH17 cells on blood brain barrier in multiple sclerosis and experimental autoimmune encephalomyelitis. Hum Immunol 81:237–243. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA (2010) The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 37:26–32 [DOI] [PubMed] [Google Scholar]
- Barnthouse M, Jones BL (2019) The Impact of Environmental Chronic and Toxic Stress on Asthma. Clin Rev Allergy Immunol 57:427–438 [DOI] [PubMed] [Google Scholar]
- Baruch K, Schwartz M (2013) CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun 34:11–16. [DOI] [PubMed] [Google Scholar]
- Benjet C et al. (2016) The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med 46:327–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Mendlowicz MV, Marques-Portella C, Kinrys G, Fontenelle LF, Marmar CR, Figueira I. (2009) Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 33:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. In: Autonomic Neuroscience: Basic and Clinical, pp 1–17. Auton Neurosci. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS (2013) Inflammatory T Helper 17 Cells Promote Depression-like Behavior in Mice. Biol Psychiatry 73:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA (2018) Th17 cells in depression. Brain Behav Immun 69:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Lowell JA, Jope RS (2018) Distinct characteristics of hippocampal pathogenic TH17 cells in a mouse model of depression. Brain Behav Immun 73:180–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, Risbrough VB, Baker DG, O’Connor DT, Nievergelt CM, Woelk CH (2015) Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry 20:1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brioschi S, Colonna M (2019) The CNS Immune-Privilege Goes Down the Drain(age). Trends Pharmacol Sci 40:1–3. [DOI] [PubMed] [Google Scholar]
- Carrithers MD, Visintin I, Kang SJ, Janeway CA (2000) Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123:1092–1101. [DOI] [PubMed] [Google Scholar]
- Case AJ, Roessner CT, Tian J, Zimmerman MC (2016) Mitochondrial superoxide signaling contributes to norepinephrine-mediated T-lymphocytecytokine profiles. PLoS One 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield JI, Caruso MJ, Bourne RA, Chirichella NR, Klein LC, Craig T, Bonneau RH, August A, Cavigelli SA (2018) Asthma Induction During Development and Adult Lung Function, Behavior and Brain Gene Expression. Front Behav Neurosci 12:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield JI, Caruso MJ, Michael KC, Bourne RA, Chirichella NR, Klein LC, Craig T, Bonneau RH, August A, Cavigelli SA (2017) Peri-adolescent asthma symptoms cause adult anxiety-related behavior and neurobiological processes in mice. Behav Brain Res 326:244–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DG, Tully JE, Nolin JD, Janssen-Heininger YM, Irvin CG (2014) Animal models of allergic airways disease: Where are we and where to next? J Cell Biochem 115:2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE (2007) Stress and inflammation in exacerbations of asthma. Brain Behav Immun 21:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, Acosta-Pérez E, Alvarez M, Colón-Semidey A, Baccarelli AA, Weeks DE, Kolls JK, Canino G, Celedón JC (2013) ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med 187:584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim S V., Hoeffer CA, Littman DR, Huh JR (2016) The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science (80- ) 351:933–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipollini V, Anrather J, Orzi F, Iadecola C (2019) Th17 and Cognitive Impairment: Possible Mechanisms of Action. Front Neuroanat 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J (2006) Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J Neurobiol 66:552–563 [DOI] [PubMed] [Google Scholar]
- Conoscenti MA, Fanselow MS (2019) Dissociation in effective treatment and behavioral phenotype between stress-enhanced fear learning and learned helplessness. Front Behav Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pinto FA, Basso AS, Britto LRG, Malucelli BE, Russo M (2005) Avoidance behavior and neural correlates of allergen exposure in a murine model of asthma. Brain Behav Immun 19:52–60 Available at: http://www.ncbi.nlm.nih.gov/pubmed/15581738 [Accessed March 31, 2020]. [DOI] [PubMed] [Google Scholar]
- Dantzer R (2018) Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol Rev 98:477–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehdar K, Mahdidoust S, Salimi M, Gholami-Mahtaj L, Nazari M, Mohammadi S, Dehghan S, Jamaati H, Khosrowabadi R, Nasiraei-Moghaddam A, Barkley V, Javan M, Mirnajafi-Zadeh J, Sumiyoshi A, Raoufy MR (2019) Allergen-induced anxiety-like behavior is associated with disruption of medial prefrontal cortex - amygdala circuit. Sci Rep 9:19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J (2010) Regulation of learning and memory by meningeal immunity: A key role for IL-4. J Exp Med 207:1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Powell SB, Risbrough VB (2017a) Immune signaling mechanisms of PTSD risk and symptom development: insights from animal models. Curr Opin Behav Sci 14:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Powell SB, Risbrough VB (2017b) Immune signaling mechanisms of PTSD risk and symptom development: insights from animal models. Curr Opin Behav Sci 14:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, Risbrough VB (2018) Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol Psychiatry 83:895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Garg AV., Kosar K, Majumder S, Kugler DG, Mir GH, Maggio M, Henkel M, Lacy-Hulbert A, McGeachy MJ (2016) Inflammatory Th17 Cells Express Integrin αvβ3 for Pathogenic Function. Cell Rep 16:1339–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV (1991) The area postrema: A cardiovascular control centre at the blood-brain interface? In: Canadian Journal of Physiology and Pharmacology, pp 1026–1034. Can J Physiol Pharmacol. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K et al. (2010) Generation of pathogenic TH 17 cells in the absence of TGF-β 2 signalling. Nature. [DOI] [PMC free article] [PubMed]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O (2004) Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 55:263–272 [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH, Baker DG, Lohr JB, Kremen WS, Litz BT, Tsuang MT (2013) Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: A pilot study. Am J Med Genet Part B Neuropsychiatr Genet 162:313–326. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Fischer ME, Goldberg J (2007) A twin study of post-traumatic stress disorder symptoms and asthma. Am J Respir Crit Care Med 176:983–987 [DOI] [PubMed] [Google Scholar]
- Gregory LG, Lloyd CM (2011) Orchestrating house dust mite-associated allergy in the lung. Trends Immunol 32:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haczku A, Panettieri RA (2010) Social stress and asthma: The role of corticosteroid insensitivity. J Allergy Clin Immunol 125:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Rook GAW, Lowry CA (2012) Pathways Underlying Afferent Signaling of Bronchopulmonary Immune Activation to the Central Nervous System. In: Chemical immunology and allergy, pp 118–141. Chem Immunol Allergy. [DOI] [PubMed]
- Han L, Wang L, Tang S, Yuan L, Wu S, Du X, Xiang Y, Qu X, Liu H, Luo H, Qin X, Liu C (2018) ITGB4 deficiency in bronchial epithelial cells directs airway inflammation and bipolar disorder-related behavior. J Neuroinflammation 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AMS, Bauer B, Block ML, Hong J-S, Miller DS (2008) Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J 22:2723–2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidari Nejad S, Takechi R, Mullins BJ, Giles C, Larcombe AN, Bertolatti D, Rumchev K, Dhaliwal S, Mamo J (2015) The effect of diesel exhaust exposure on blood-brain barrier integrity and function in a murine model. J Appl Toxicol 35:41–47 [DOI] [PubMed] [Google Scholar]
- Hellings PW, Steelant B (2020) Epithelial barriers in allergy and asthma. J Allergy Clin Immunol 145:1499–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YH, Cheng CM, Lin WC, Bai YM, Su TP, Li CT, Tsai SJ, Pan TL, Chen TJ, Chen MH (2019) Post-traumatic stress disorder and asthma risk: A nationwide longitudinal study. Psychiatry Res 276:25–30. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CRW (2010) Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J 24:1023–1034 [DOI] [PubMed] [Google Scholar]
- Irvin C, Zafar I, Good J, Rollins D, Christianson C, Gorska MM, Martin RJ, Alam R (2014) Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 134:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Sajdyk TJ, Fitz SD, Hale MW, Lowry CA, Hay-Schmidt A, Shekhar A (2013) Angiotensin II’s role in sodium lactate-induced panic-like responses in rats with repeated urocortin 1 injections into the basolateral amygdala. Prog Neuro-Psychopharmacology Biol Psychiatry 44:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, SD N, NQ B, Davis M, Duncan E, Bradley B, KJ R (2010) Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 27:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean EM, Kelsay K, Wamboldt F, Wamboldt MZ (2006) Posttraumatic stress in adolescents with asthma and their parents. J Am Acad Child Adolesc Psychiatry 45:78–86 [DOI] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A (2007) Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med 13:1173–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ, Poole JA (2019) Pollutants in the workplace: Effect on occupational asthma. J Allergy Clin Immunol 143:2014–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertser A, Baruch K, Deczkowska A, Weiner A, Croese T, Kenigsbuch M, Cooper I, Tsoory M, Ben-Hamo S, Amit I, Schwartz M (2019) Corticosteroid signaling at the brain-immune interface impedes coping with severe psychological stress. Sci Adv 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. ArchGenPsychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ (2012) The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73:849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianmehr M, Ghorani V, Boskabady MH (2016) Animal model of asthma, various methods and measured parameters, a methodological review. Iran J Allergy, Asthma Immunol 15:445–465. [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ (2013) National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 26:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Im DS (2019) Suppressive effects of type I angiotensin receptor antagonists, candesartan and irbesartan on allergic asthma. Eur J Pharmacol 852:25–33. [DOI] [PubMed] [Google Scholar]
- Kim TD, Lee S, Yoon S (2020) Inflammation in Post-Traumatic Stress Disorder (PTSD): A Review of Potential Correlates of PTSD with a Neurological Perspective. Antioxidants (Basel, Switzerland) 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC (2012) Pro-cognitive properties of T cells. Nat Rev Immunol 12:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC et al. (2017) Posttraumatic stress disorder in the World Mental Health Surveys. Psychol Med 47:2260–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y (2006) IL-17 Plays an Important Role in the Development of Experimental Autoimmune Encephalomyelitis. J Immunol 177:566–573 [DOI] [PubMed] [Google Scholar]
- Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR (2011) Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 31:15009–15015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie S, Lewkowich IP, Suzuki Y, Clark JR, Sproles AA, Dienger K, Budelsky AL, Wills-Karp M (2010) Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 11:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H, Fahy J V (2019) The Cytokines of Asthma. Immunity 50:975–991 [DOI] [PubMed] [Google Scholar]
- Lee B, Shim I, Lee H, Hahm DH (2018) Melatonin ameliorates cognitive memory by regulation of cAMP-response element-binding protein expression and the anti-inflammatory response in a rat model of post-traumatic stress disorder. BMC Neurosci 19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH (2016) Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol 20:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich I, Ahlbrand R, Johnson E, McAlees J, Nawreen N, Raman R, Lingel I, Hargis J, Hoover C, Sah R (2020) Modulation of fear behavior and neuroimmune alterations in house dust mite exposed A/J mice, a model of severe asthma. Brain Behav Immun. 88:688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M (2008) Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One 3:e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisieski MJ, Eagle AL, Conti AC, Liberzon I, Perrine SA (2018) Single-prolonged stress: A review of two decades of progress in a rodent model of post-traumatic stress disorder. Front Psychiatry 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes S, Hallak JEC, Machado de Sousa JP, Osório F de L (2020) Adverse childhood experiences and chronic lung diseases in adulthood: a systematic review and meta-analysis. Eur J Psychotraumatol 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A et al. (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21:1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Hartmann J, Ross RA, Ressler KJ (2019) Deconstructing the Gestalt: Mechanisms of Fear, Threat, and Trauma Memory Encoding. Neuron 102:60–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloley PM, England BR, Sayles H, Thiele GM, Michaud K, Sokolove J, Cannon GW, Reimold AM, Kerr GS, Baker JF, Caplan L, Case AJ, Mikuls TR (2019) Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis✰. Semin Arthritis Rheum 49:229–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RA, Hodgkins SR, Dixon AE, Poynter ME (2014) Aligning mouse models of asthma to human endotypes of disease. Respirology 19:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Undem BJ (2016) Vagal afferent innervation of the airways in health and disease. Physiol Rev 96:975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ (1998) Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl 25:S61–7 [DOI] [PubMed] [Google Scholar]
- McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ (2003) The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172:III–XII, 1–122, back cover. [DOI] [PubMed] [Google Scholar]
- Medina-Rodriguez EM, Lowell JA, Worthen RJ, Syed SA, Beurel E (2018) Involvement of innate and adaptive immune systems alterations in the pathophysiology and treatment of depression. Front Neurosci 12:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T (2017) Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42:254–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL (2009) Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biol Psychiatry 66:1075–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S (2015) New aspects in fenestrated capillary and tissue dynamics in the sensory circumventricular organs of adult brains. Front Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J (2001) IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 108:430–438 [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Vermetten E, Kavelaars A, Geuze E, Heijnen CJ (2008) Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology 33:1041–1050 [DOI] [PubMed] [Google Scholar]
- Moshfegh CM, Elkhatib SK, Collins CW, Kohl AJ, Case AJ (2019) Autonomic and redox imbalance correlates with T-Lymphocyte inflammation in a model of chronic social defeat stress. Front Behav Neurosci 13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi S, Loh MK, Ferrara N, DeJoseph MR, Ritger A, Padival M, Record MJ, Urban JH, Rosenkranz JA (2020) Repeated stress induces a pro-inflammatory state, increases amygdala neuronal and microglial activation, and causes anxiety in adult male rats. Brain Behav Immun 84:180–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RP, DiNicolo R, Fernández-Caldas E, Seleznick MJ, Lockey RF, Good RA (1996) Allergen-specific IgE levels and mite allergen exposure in children with acute asthma first seen in an emergency department and in nonasthmatic control subjects. J Allergy Clin Immunol 98:258–263. [DOI] [PubMed] [Google Scholar]
- Nials AT, Uddin S (2008) Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech 1:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM et al. (2019) International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole BI, Catts SV. (2008) Trauma, PTSD, and physical health: An epidemiological study of Australian Vietnam veterans. J Psychosom Res 64:33–40 [DOI] [PubMed] [Google Scholar]
- Pace TWW, Heim CM (2011) A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav Immun 25:6–13 [DOI] [PubMed] [Google Scholar]
- Paquet J, Mah D, Saad E, Beach J, Vethanayagam D (2019) Psychiatric co-morbidity and asthma: A pilot study utilizing a free use tool to improve asthma care. Clin Investig Med 42:E22–E27 [DOI] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhães PV, Kapczinski F, Kauer-Sant’Anna M (2015) Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. The Lancet Psychiatry 2:1002–1012. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13:769–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Mays JW, Bailey MT, Hanke ML, Sheridan JF (2011) Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behav Immun 25:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA (2007) Brain-immune communication pathways. Brain Behav Immun 21:727–735 [DOI] [PubMed] [Google Scholar]
- Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, Marchi N, van Boxel-Dezaire AHH (2018) IFN-γ IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun 507:274–279. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisäkk P, Kidd G (2003) Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3:569–581. [DOI] [PubMed] [Google Scholar]
- Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F (2009) C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol 10:514–523. [DOI] [PubMed] [Google Scholar]
- Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, King HO, Waisman A, Halassa MM, Huh JR, Choi GB (2020) IL-17a promotes sociability in mouse models of neurodevelopmental disorders.Nature 577:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Skevaki C (2021) Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol 21:177–191 [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V (2011) Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LK, Frueh BC, Acierno R (2010) Prevalence estimates of combat-related post-traumatic stress disorder: critical review. Aust N Z J Psychiatry 44:4–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SL, Miller GE, Brehm JM, Celedón JC (2014) Stress and asthma: Novel insights on genetic, epigenetic, and immunologic mechanisms. J Allergy Clin Immunol 134:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz MA, Busse WW, Sheridan JF, Crisafi GM, Davidson RJ (2012) Are there neurophenotypes for asthma? functional brain imaging of the interaction between emotion and inflammation in asthma. PLoS One 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, Srivastava R, Claussen MC, Hemmer B, Korn T (2011) Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J Exp Med 208:2465–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MF, Cotias AC, Pão CRR, Daleprane JB, Jurgilas PB, Couto GC, Anjos-Valotta EA, Cordeiro RSB, Carvalho VF, Silva PMR, Martins MA (2018) Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability. J Immunol 201:851–860. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Schwartz M (2013) Orchestrated leukocyte recruitment to immune-privileged sites: Absolute barriers versus educational gates. Nat Rev Immunol 13:206–218. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, Samuelson KW (2012) Relationship between posttraumatic stress disorder and asthma among New York area residents exposed to the World Trade Center disaster. J Psychosom Res 73:122–125. [DOI] [PubMed] [Google Scholar]
- Song C, Nicholson JD, Clark SM, Li X, Keegan AD, Tonelli LH (2016) Expansion of brain T cells in homeostatic conditions in lymphopenic Rag2−/− mice. Brain Behav Immun 57:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick S, Bremner J, Rasmusson A, III MCA, Arnstein A, DS C (1999) Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46:1192–1204. [DOI] [PubMed] [Google Scholar]
- Souza RR, Noble LJ, McIntyre CK (2017) Using the single prolonged stress model to examine the pathophysiology of PTSD. Front Pharmacol 8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Koch B, Grabe HJ, Ewert R, Barnow S, Felix SB, Ittermann T, Obst A, Volzke H, Glaser S, Schaper C (2011) Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J 37:1068–1075 [DOI] [PubMed] [Google Scholar]
- Stowman S, Kearney CA, Daphtary K (2015) Mediators of Initial Acute and Later Posttraumatic Stress in Youth in a PICU*. Pediatr Crit Care Med 16:e113–e118 [DOI] [PubMed] [Google Scholar]
- Strawn JR and Geracioti TD (2008) Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety 25(3):260–71 [DOI] [PubMed] [Google Scholar]
- Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, Lashua A, Yu C, Klein BS, Locksley RM, Deutsch G, Sun X (2018) Pulmonary neuroendocrine cells amplify allergic asthma responses. Science (80- ) 360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD (2020) Posttraumatic Stress Disorder and Inflammation: Untangling Issues of Bidirectionality. Biol Psychiatry 87(10):885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Katz M, Kovacsics CE, Gould TD, Joppy B, Hoshino A, Hoffman G, Komarow H, Postolache TT (2009) Allergic rhinitis induces anxiety-like behavior and altered social interaction in rodents. Brain Behav Immun 23:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai Y, JP H (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underner M, Goutaudier N, Peiffer G, Perriot J, Harika-Germaneau G, Jaafari N (2019) Influence of post-traumatic stress disorder on asthma. Press Medicale 48:488–502 [DOI] [PubMed] [Google Scholar]
- Vanderbilt D, Young R, MacDonald HZ, Grant-Knight W, Saxe G, Zuckerman B (2008) Asthma severity and PTSD symptoms among inner city children: a pilot study. J Trauma Dissociation 9:191–207 [DOI] [PubMed] [Google Scholar]
- Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li K-Y, Santin JM, Ratliff-Rang CA, Patrone LGA, Rush J, Lewkowich IP, Herman JP, Putnam RW, Sah R (2016) Microglial Acid Sensing Regulates Carbon Dioxide-Evoked Fear. Biol Psychiatry 80:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T et al. (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 390(10100):1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakashin H, Hirose K, Maezawa Y, Kagami SI, Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, Puccetti P, Iwamoto I, Nakajima H (2008) IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic airway inflammation in mice. Am J Respir Crit Care Med 178:1023–1032. [DOI] [PubMed] [Google Scholar]
- Wang SC, Lin CC, Chen CC, Tzeng NS, Liu YP (2018) Effects of oxytocin on fear memory and neuroinflammation in a rodent model of posttraumatic stress disorder. Int J Mol Sci 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiong L, Tang M (2017a) Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol 37:644–667 [DOI] [PubMed] [Google Scholar]
- Wang Z, Caughron B, Young MRI (2017b) Posttraumatic stress disorder: An immunological disorder? Front Psychiatry 8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Mandel H, Levingston CA, Young MRI (2016) An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of Veterans with combat-related PTSD. Hum Immunol 77:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P (2019) PTSD as a Public Mental Health Priority. Curr Psychiatry Rep 21(7):61. [DOI] [PubMed] [Google Scholar]
- Winter A, Ahlbrand R, Sah R (2019) Recruitment of central angiotensin II type 1 receptor associated neurocircuits in carbon dioxide associated fear. Prog Neuro-Psychopharmacology Biol Psychiatry 92:378–386 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I (2009) Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety 26:1110–1117 [DOI] [PubMed] [Google Scholar]
- Zepp J, Wu L, Li X (2011) IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol 32:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, Nagarkatti M (2014) Dysregulation in microRNA Expression Is Associated with Alterations in Immune Functions in Combat Veterans with Post-Traumatic Stress Disorder Gonzalez P, ed. PLoS One 9:e94075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo HX, Li JQ, Han B, Ke CJ, Liu XD, Zhang YC, Li L, Yang X (2014) Di-(n-butyl)-phthalate-induced oxidative stress and depression-like behavior in mice with or without ovalbumin immunization. Biomed Environ Sci 27:268–280 [DOI] [PubMed] [Google Scholar]


