Abstract
Background
In preclinical studies trifluridine/tipiracil (FTD/TPI) plus oxaliplatin (Industriestrasse, Holzkirchen, Germany) sensitised microsatellite stable (MSS) metastatic colorectal cancer (mCRC) to anti-programmed cell death protein-1; the addition of oxaliplatin or bevacizumab (F Hoffmann- la ROCHE AG, Kaiseraugst, Switzerland) enhanced the antitumour effects of FTD/TPI. This study aimed to investigate the safety and efficacy of FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab (Uxbridge business Park, Uxbridge, United Kingdom) in patients with mCRC who had progressed after at least one prior line of treatment.
Patients and methods
In 14-day cycles, patients received FTD/TPI 35 mg/m2 (twice daily, days 1-5) plus oxaliplatin 85 mg/m2 (day 1), and, on day 1, either bevacizumab 5 mg/kg (cohort A) or nivolumab 3 mg/kg (cohort B). Patients in Cohort B had confirmed MSS status.
Results
In total, 54 patients were enrolled: 37 in cohort A and 17 in cohort B. Recruitment in cohort B was stopped early due to the low response rate (RR) observed at interim analyses of efficacy. The most common adverse events (AEs) in cohort A were neutropenia/decreased neutrophils (75.7%), nausea (59.5%), vomiting (40.5%), diarrhoea (37.8%), peripheral sensory neuropathy (37.8%), fatigue (35.1%) and decreased appetite (35.1%). In cohort B, the most common AEs were neutropenia/decreased neutrophils (70.6%), diarrhoea (58.8%), nausea (47.1%), vomiting (47.1%), fatigue (47.1%), asthenia (41.2%), paraesthesia (41.2%), thrombocytopenia/decreased platelets (35.3%) and decreased appetite (35.3%). Confirmed objective RR was 17.1% in cohort A and 7.1% in cohort B; the corresponding values for median progression-free survival in the two cohorts were 6.3 and 6.0 months.
Conclusion
FTD/TPI plus oxaliplatin and bevacizumab or nivolumab had an acceptable safety profile and demonstrated antitumour activity in previously treated patients with mCRC.
Key words: trifluridine/tipiracil, oxaliplatin, metastatic colorectal cancer, fluoropyrimidines
Highlights
-
•
This study evaluated the safety and efficacy of FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab in mCRC patients.
-
•
FTD/TPI plus oxaliplatin in combination with bevacizumab or nivolumab had an acceptable and manageable safety profile.
-
•
Antitumour activity was observed following treatment with FTD/TPI plus oxaliplatin and bevacizumab.
-
•
Despite a modest RR with the addition of nivolumab, survival data were promising in these poor-prognosis patients.
Introduction
Despite progress in the treatments for metastatic colorectal cancer (mCRC), acquired resistance to systemic therapy continues to be a major challenge.1 Fluoropyrimidine-based irinotecan or oxaliplatin (Industriestrasse, Holzkirchen, Germany) chemotherapy is widely used in mCRC as either first- or second-line therapy, and the addition of a biological agent such as bevacizumab (F Hoffmann- la ROCHE AG, Kaiseraugst, Switzerland) or cetuximab significantly improves patient outcomes.2 However, the overall 5-year survival rate for mCRC patients remains low, mainly due to acquired drug resistance.1 In addition, the use of immune checkpoint inhibitors is restricted to a minority of patients with mismatch repair-deficient tumours with high levels of microsatellite instability (5% of mCRC tumours).3 Thus, further research is needed to identify new treatment strategies to overcome drug resistance.
Trifluridine/tipiracil (FTD/TPI, also known as TAS-102) is an oral antitumour drug4 approved for previously treated patients with mCRC. In preclinical studies, FTD/TPI plus oxaliplatin sensitised microsatellite stable (MSS) mCRC to anti-programmed cell death protein (PD)-1 immune checkpoint inhibitors,5, 6, 7, 8 suggesting that FTD/TPI plus oxaliplatin may improve responses to immunotherapy. Furthermore, the antitumour effects of FTD/TPI were enhanced when it was combined with oxaliplatin or bevacizumab.9,10 Therefore, this phase I study evaluated the combination of FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab (Uxbridge business Park, Uxbridge, United Kingdom) in patients with mCRC who had received at least one line of standard chemotherapy. The study included a dose-escalation part, during which the recommended dose of FTD/TPI plus oxaliplatin combination was established.11 Here, we report the results of the expansion part, evaluating the safety and efficacy of FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab.
Methods
Design and patients
This was an open-label, multicohort, phase I study conducted at 25 sites in France, Spain, Italy, Germany, Austria, Hungary and the UK (ClinicalTrials.gov number: NCT02848443). During dose escalation, the recommended dose was determined to be FTD/TPI 35 mg/m2 twice daily on days 1-5, and oxaliplatin 85 mg/m2 intravenous infusion on day 1. A 14-day treatment cycle was used instead of the standard 28-day FTD/TPI cycle to reduce the additive toxicity of the chemotherapy combination.
In addition to the recommended dose of FTD/TPI plus oxaliplatin, patients received on day 1 either intravenous bevacizumab 5 mg/kg (cohort A) or intravenous nivolumab 3 mg/kg (cohort B). Treatment was discontinued upon disease progression, unacceptable toxicity or patient withdrawal. In cohort B, treatment could be continued after a first disease progression if the patient could derive a clinical benefit.
Eligible patients were aged ≥18 years with histologically confirmed CRC who had previously received one or more lines of standard chemotherapy excluding oxaliplatin (previous adjuvant chemotherapy was allowed), had an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, adequate bone marrow, liver and kidney function and measurable disease at baseline. In addition, patients in cohort B were required to have a confirmed MSS status.
The expansion part of the study used a Bayesian three-stage design, which allowed early cohort termination based on interim analyses of efficacy (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100270). Patients were recruited simultaneously and inclusion in either cohort was at the discretion of the investigator; it was not possible to switch to the other cohort. Recruitment of up to 35 patients per cohort was planned. Stage 1 started with a safety run-in phase; the first six patients were monitored during their first two cycles before allowing the recruitment of nine additional patients. The study progressed to stage 2, and later to stage 3, and enrolled an additional 10 patients only if the response rate (RR) at the previous stage was >10%; otherwise the cohort was terminated for futility. At the end of stage 2, recruitment could be terminated for early evidence of efficacy if an RR of >30% was observed.
Assessments
Evaluation of antitumour activity was made according to RECIST, version 1.1, based on radiological assessments conducted at baseline, every four treatment cycles and at the end of treatment. All toxicities were assessed according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Peripheral sensory neuropathy was assessed using Levi grading.12 To investigate biomarkers and gene expression signatures related to immune function, image-guided biopsies were carried out at baseline and cycle 4 for all patients in cohort B.
Outcomes
The primary endpoints were safety and tolerability. Secondary endpoints included the objective RR (ORR; the proportion of patients achieving a complete or partial response), the disease control rate (DCR; the proportion of patients with a complete or partial response or stable disease) and progression-free survival (PFS; time from the date of inclusion to the date of progression or death whichever occurred first).
Biomarker assessment (cohort B)
Evaluation of CD8+ T cells and programmed cell death ligand 1 (PD-L1)-positive tumour cells was performed using Halioseek, a dual-staining immunohistochemistry and digital quantification assay (HalioDx, France) from formalin-fixed paraffin-embedded (FFPE) samples. The immune cells infiltration was evaluated using Immunoscore (HalioDx) based on densities of CD8+ and CD3+ T cells both in the core of the tumour and in the invasive margin from FFPE samples. Immune-related gene signatures were analysed on RNA isolated from FFPE samples using NanoString nCounter assay (NanoString Technology Inc., USA). Scores for gene expression were calculated by averaging z-score across a panel of 770 major immune-related genes (PanCancer Pathways Panel from NanoString).
Statistics
The sample size was selected to allow assessment of safety and antitumour activity. In the Bayesian framework, a beta-binomial model with a minimally informative prior beta distribution with parameters equal to 2.6 and 9.6 was chosen for the RR. Using this approach, the probability of promising activity was ≥71% when the observed RR was 31.4%.
Safety was assessed in all patients who received at least one dose of the study drugs. Efficacy was assessed in patients who received at least one dose of the study drugs and who had at least one evaluable postbaseline tumour assessment. The statistical analyses were mainly descriptive. Wilson's 95% confidence intervals (CIs) were calculated for the ORR and DCR. Time-to-event endpoints and 95% CIs were estimated using the Kaplan–Meier method.
Ethics
The study protocol, participant information and consent form were reviewed and approved by the ethics committees of participating institutions. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and all patients provided written informed consent prior to entering the study.
Results
Between 23 November 2017 and 1 February 2019, a total of 54 patients were recruited for the expansion part of the study: 37 patients in cohort A and 17 patients in cohort B.
For this analysis, the data cut-off date was 1 August 2019, at which time 32 patients (86.5%) in cohort A and 16 (94.1%) in cohort B had discontinued study treatments, with disease progression being the most common reason (59.5% of patients in cohort A and 64.7% in cohort B).
Baseline characteristics of patients are summarised in Table 1. Almost all patients had previously received irinotecan (94.4%) and/or 5-fluorouracil (5-FU) [98.1%] and the majority (55.6%) had previously received bevacizumab. Of the patients who had previously received oxaliplatin [20 patients (37.0%)], the majority received it in the perioperative setting (19 patients). Of these, its use represented a protocol deviation in two patients (one in each cohort) who had progressed during or within 6 months of oxaliplatin treatment. In addition, one patient in cohort A underwent hyperthermic intraperitoneal chemotherapy in the metastatic setting.
Table 1.
Baseline characteristics of patients (N = 54)
| Characteristic | Cohort A (n = 37) | Cohort B (n = 17) |
|---|---|---|
| Age, years | ||
| Median | 64.0 | 64.0 |
| Range | 43.0-83.0 | 33.0-76.0 |
| Sex, n (%) | ||
| Female | 17 (46.0) | 5 (29.4) |
| Male | 20 (54.1) | 12 (70.6) |
| ECOG performance status, n (%) | ||
| 0 | 24 (64.9) | 9 (52.9) |
| 1 | 13 (35.1) | 8 (47.1) |
| Primary tumour site, n (%) | ||
| Left colon | 16 (43.2) | 6 (35.3) |
| Right colon | 10 (27.0) | 3 (17.7) |
| Transverse colon | 1 (2.7) | 0 (0) |
| Othera | 1 (2.7) | 0 (0) |
| Rectum | 9 (24.3) | 8 (47.1) |
| Disease duration, years | ||
| Median | 2.2 | 1.8 |
| Range | 0.4-11.5 | 0.5-6.5 |
| Time from diagnosis to first metastasis, months | ||
| Median | 14.1 | 10.0 |
| Range | 1.3-107.8 | 0.7-67.3 |
| Prior number of regimens for advanced disease | ||
| Mean ± standard deviation | 1.78 ± 1.11 | 2.35 ± 1.54 |
| Prior systemic anticancer agent, n (%)b | ||
| Fluorouracil | 36 (97.3) | 17 (100) |
| Capecitabine | 10 (27.0) | 8 (47.1) |
| Irinotecan | 35 (94.6) | 16 (94.1) |
| Bevacizumab | 21 (56.8) | 9 (52.9) |
| Cetuximab | 8 (21.6) | 7 (41.2) |
| Panitumumab | 3 (8.1) | 3 (17.6) |
| Oxaliplatin | 14 (37.8) | 6 (35.3) |
ECOG, Eastern Cooperative Oncology Group.
In one patient the primary tumour site was the caecum.
Agents used in >2% of all patients are listed.
In cohort A, patients received a median of 12 treatment cycles (range 1-37 cycles) with a median duration of exposure to FTD/TPI and bevacizumab of 6.2 and 6.1 months, respectively, and to oxaliplatin of 4.8 months. In cohort B, patients received a median of eight treatment cycles (range 1-43) with a median duration of exposure to FTD/TPI, nivolumab and oxaliplatin of 4.1 months.
Safety
All patients reported at least one adverse event (AE) of any grade. Frequently reported AEs (occurring in ≥10% of patients) in both cohorts were neutropenia and/or decreased neutrophil count, thrombocytopenia and/or decreased platelet count, nausea, vomiting, diarrhoea, fatigue, asthenia and decreased appetite; additionally, peripheral sensory neuropathy and paraesthesia were commonly reported (Table 2).
Table 2.
Treatment-emergent adverse events that occurred in ≥10% of patients in either cohort (N = 54)
| Adverse eventa | Cohort A (n = 37) |
Cohort B (n = 17) |
||
|---|---|---|---|---|
| Any, n (%) | Grade ≥3, n (%) | Any, n (%) | Grade ≥3, n (%) | |
| All | 37 (100.0) | 29 (78.4) | 17 (100.0) | 14 (82.4) |
| Nausea | 22 (59.5) | 1 (2.7) | 8 (47.1) | 0 (0) |
| Diarrhoea | 14 (37.8) | 1 (2.7) | 10 (58.8) | 2 (11.8) |
| Vomiting | 15 (40.5) | 2 (5.4) | 8 (47.1) | 1 (5.9) |
| Stomatitis | 9 (24.3) | 1 (2.7) | 2 (11.8) | 0 (0) |
| Abdominal pain | 7 (18.9) | 0 (0) | 3 (17.6) | 0 (0) |
| Constipation | 6 (16.2) | 0 (0) | 2 (11.8) | 0 (0) |
| Paraesthesia | 9 (24.3) | 0 (0) | 7 (41.2) | 1 (5.9) |
| Peripheral sensory neuropathy | 14 (37.8) | 1 (2.7) | 4 (23.5) | 0 (0) |
| Neuropathy peripheral | 4 (10.8) | 0 (0) | 4 (23.5) | 1 (5.9) |
| Neurotoxicity | 0 (0) | 0 (0) | 2 (11.8) | 0 (0) |
| Headache | 5 (13.5) | 0 (0) | 1 (5.9) | 0 (0) |
| Dizziness | 4 (10.8) | 0 (0) | 1 (5.9) | 0 (0) |
| Dysgeusia | 3 (8.1) | 0 (0) | 2 (11.8) | 0 (0) |
| Fatigue | 13 (35.1) | 3 (8.1) | 8 (47.1) | 4 (23.5) |
| Asthenia | 12 (32.4) | 2 (5.4) | 7 (41.2) | 0 (0) |
| Pyrexia | 8 (21.6) | 0 | 3 (17.6) | 0 (0) |
| Neutropenia/decreased neutrophil count | 28 (75.7) | 14 (37.8) | 12 (70.6) | 8 (47.1) |
| Thrombocytopenia/decreased platelet count | 12 (32.4) | 1 (2.7) | 6 (35.3) | 2 (11.8) |
| Anaemia/decreased haemoglobin | 10 (27.0) | 3 (8.1) | 5 (29.4) | 3 (17.6) |
| Weight decreased | 5 (13.5) | 1 (2.7) | 3 (17.6) | 0 (0) |
| Back pain | 4 (10.8) | 0 (0) | 2 (11.8) | 0 (0) |
| Arthralgia | 4 (10.8) | 0 (0) | 0 (0) | 0 (0) |
| Decreased appetite | 13 (35.1) | 1 (2.7) | 6 (35.3) | 0 (0) |
| Epistaxis | 4 (10.8) | 0 (0) | 0 (0) | 0 (0) |
| Hiccups | 1 (2.7) | 0 (0) | 2 (11.8) | 0 (0) |
| Pulmonary embolism | 0 (0) | 0 (0) | 2 (11.8) | 1 (5.9) |
| Hypertension | 7 (18.9) | 6 (16.2) | 0 (0) | 0 (0) |
| Malignant neoplasm progression | 4 (10.8) | 4 (10.8) | 3 (17.6) | 1 (5.9) |
| Insomnia | 4 (10.8) | 0 (0) | 0 (0) | 0 (0) |
Adverse events were coded using MedDRA 21.0 (MedDRA is registered by IFPMA on behalf of ICH [International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use]).
Grade ≥3 AEs occurred in 29 patients (78.4%) in cohort A and in 14 patients (82.4%) in cohort B. These events were considered treatment related in 27 patients (73%) in cohort A; most commonly neutropenia and/or decreased neutrophil count (38%, n = 14) and hypertension (8.1%, n = 3) were noted. In cohort B, grade ≥3 treatment-related AEs were reported in 11 patients (64.7%); most commonly neutropenia and/or decreased neutrophil count (47%, n = 8), fatigue (23.5%, n = 4), anaemia (11.8%, n = 2) and thrombocytopenia (11.8%, n = 2) were noted.
Serious AEs occurred in 12 patients (32.4%) in cohort A [treatment related in 9 (24.3%)] and 9 (52.9%) in cohort B [treatment-related in 5 (29.4%); Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100270].
Treatment-related AEs led to permanent treatment discontinuation in six cohort A patients (16.2%; neutropenia in three patients, and one instance each of thrombocytopenia, abdominal abscess and renal failure) and one cohort B patient (5.9%; pancreatitis and muscular weakness).
Four patients died during the study; three died because of disease progression (two in cohort A and one in cohort B) and one in cohort A died because of pneumonitis which was deemed to be related to oxaliplatin.
Efficacy
Treatment response data were available for 35 cohort A patients and 14 cohort B patients (Table 3). Before the first tumour evaluation, two patients in cohort A discontinued because of AEs, one in cohort B withdrew consent and another in cohort B died from disease progression; one in cohort B had a microsatellite-high tumour and was excluded from the analysis.
Table 3.
Objective response rate and disease control rate in patients with evaluable treatment response data (N = 49)
| Cohort A (n = 35) | Cohort B (n = 14) | |
|---|---|---|
| Best overall response, n (%) | ||
| Complete response | 1 (2.9) | — |
| Partial response | 5 (14.3) | 1 (7.1) |
| Stable disease | 25 (71.4) | 9 (64.3) |
| Progressive disease | 4 (11.4) | 4 (28.6) |
| Objective response ratea, n (%) | 6 (17.1) | 1 (7.1) |
| 95% CIb | 8.1-32.7 | 1.3-31.5 |
| Disease control ratec, n (%) | 31 (88.6) | 10 (71.4) |
| 95% CIb | 74.1-95.5 | 45.4-88.3 |
| Progression-free survival, months | ||
| Median | 6.3 | 6.0 |
| 95% CId | 5.5-15.6 | 2.0-8.0 |
| Overall survival, months | ||
| Median | 15.1 | NR |
| 95% CId | 10.7-NR | 6.5-NR |
| Survival probability at 6 months | 0.886 | 0.857 |
CI, confidence interval; CR, complete response; NR, not yet reached; PR, partial response; SD, stable disease.
Objective response rate = best overall response (CR or PR).
95% confidence interval calculated using Wilson's method.
Disease control rate = best overall response (CR, PR or SD).
95% confidence interval calculated using the Kaplan–Meier method.
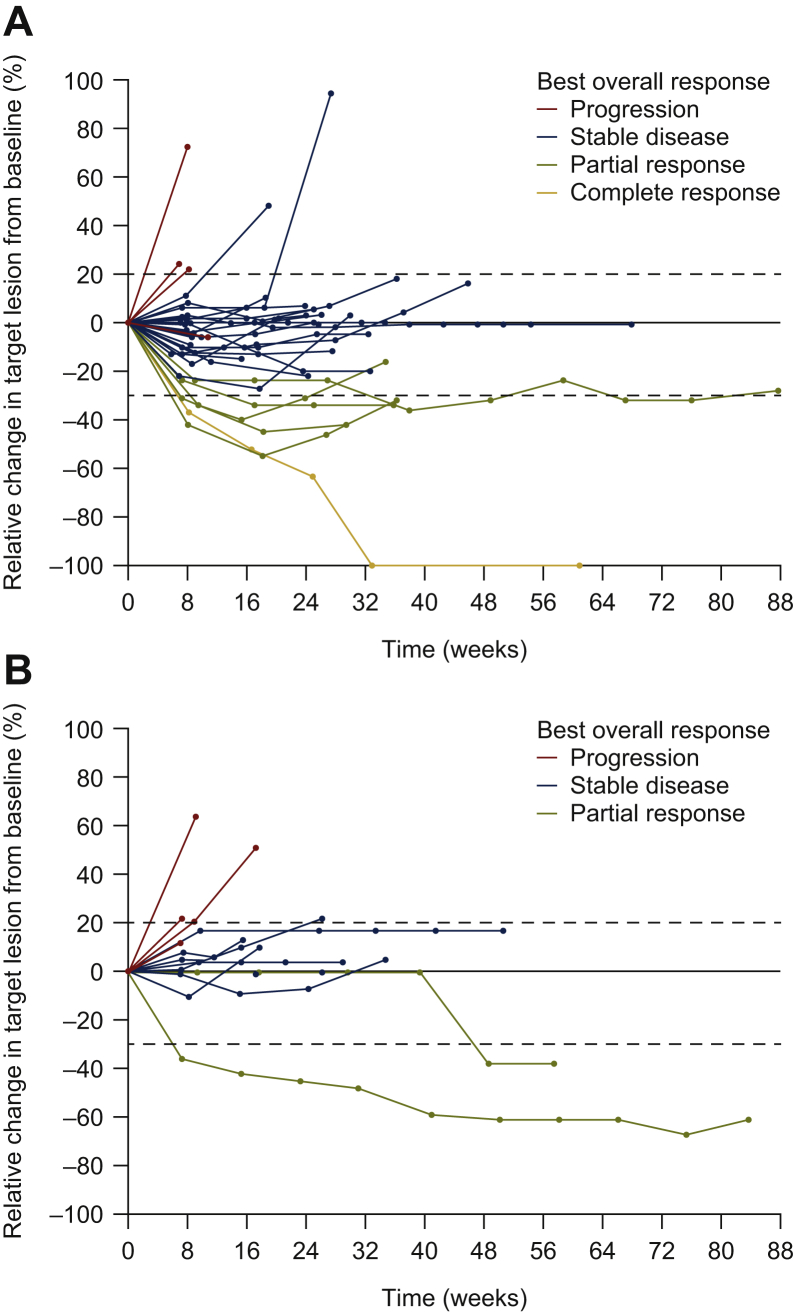
The confirmed ORR was 17.1% (n = 6; 95% CI 8.1%-32.7%) in cohort A and 7.1% (n = 1; 95% CI 1.3%-31.5%) in cohort B (Table 3, Figure 1); therefore, the probability of promising activity was 12% in cohort A and 6% in cohort B.
Figure 1.
Relative change from baseline in the target lesion size according to best overall response for evaluable patients in (A) cohort A and (B) cohort B. Cohort B includes one patient who was not included in the final efficacy analysis (this patient had high levels of microsatellite instability).
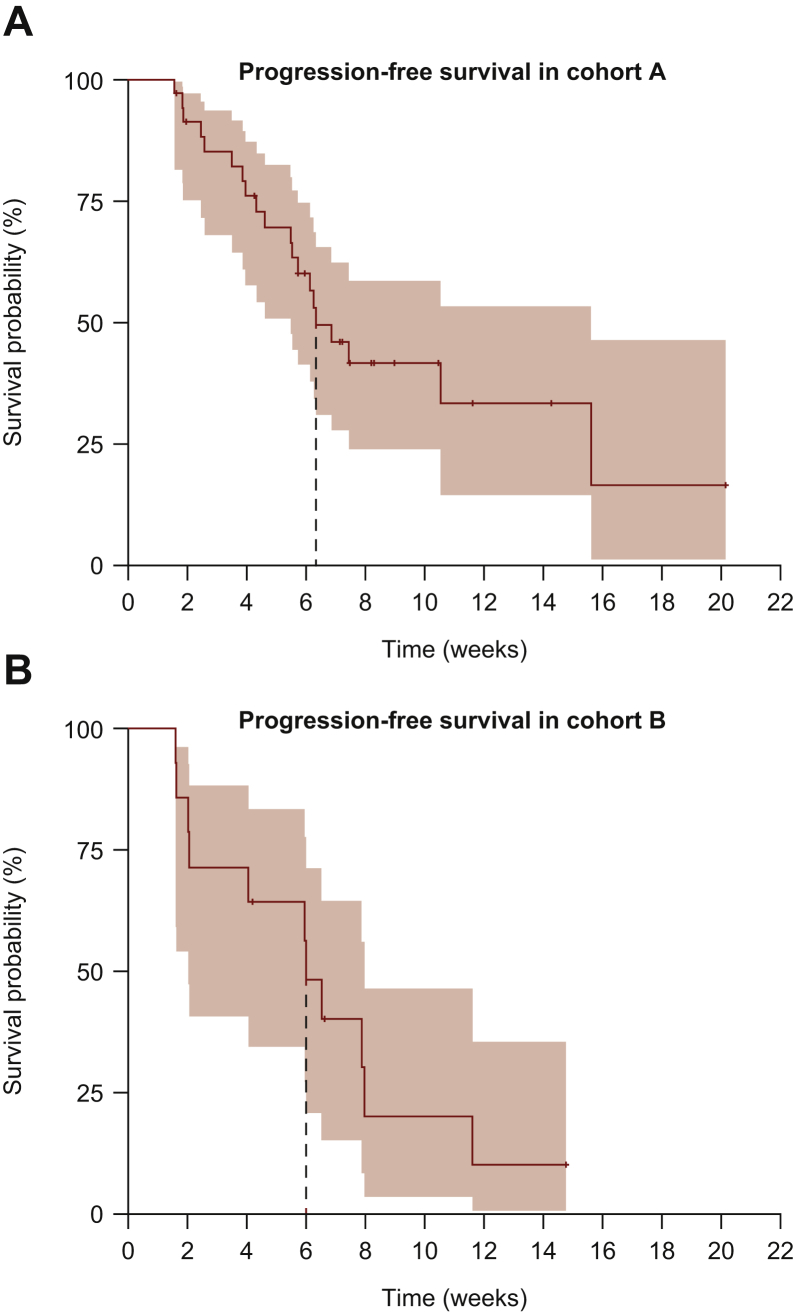
In cohort A, the DCR was 88.6% (n = 31; 95% CI 74.0%-95.5%), and in cohort B it was 71.4% (n = 10; 95% CI 45.4%-88.3%). The median PFS was 6.3 (95% CI 5.5-15.6) months in cohort A and 6.0 (95% CI 2.0-8.0) months in cohort B (Figure 2).
Figure 2.
Kaplan–Meier plots of progression-free survival for (A) cohort A and (B) cohort B.
Biomarker assessment (cohort B)
All patients from cohort B had a biopsy at baseline, and 10 had a biopsy at cycle 4, but samples from only eight and five of these, respectively, were of sufficient quality and/or quantity for analysis.
At baseline, all evaluable patients had PD-L1-negative tumours (defined as <5% of tumour cells); intratumoural CD8+ T-cell density was low in six patients (2-31 cells/mm2) and high in two patients (57-132 cells/mm2; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100270). On treatment, one patient showed a positive conversion of PD-L1 expression to above the 5% threshold, which was associated with a clinical benefit (i.e. disease stabilisation for >8 months). PD-L1 conversion was also associated with enhanced infiltration of CD8+ T cells (from 14 to 80 cells/mm2), upregulation of genes related to inflammatory response, T-cell activation and enriched interferon-gamma and tumour inflammation signature (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100270). Detailed heat maps are presented in Supplementary Figures S3-S6, available at https://doi.org/10.1016/j.esmoop.2021.100270.
Discussion
The results of this study show that the FTD/TPI plus oxaliplatin in combination with bevacizumab or nivolumab had a manageable safety profile in patients with mCRC previously treated with at least one line of standard chemotherapy, excluding oxaliplatin.
These safety results were in line with those from the dose-escalation part of this study,11 where grade ≥3 AEs were primarily haematological and manageable with basic supportive care and treatment delays, reduction or interruptions. Two other phase I, dose-escalation studies evaluated the re-introduction of oxaliplatin, given in combination with FTD/TPI, in patients with refractory mCRC.13,14 In each study, 12 patients were administered FTD/TPI on the same schedule and at the same dose as in this study, and neutropenia was the most common grade ≥3 AE.
The addition of bevacizumab or nivolumab did not appear to markedly increase the toxicity of combination therapy. The type and incidence of AEs observed with the FTD/TPI plus oxaliplatin and bevacizumab or nivolumab combination were consistent with those of the individual drugs reported in similar patient populations.15, 16, 17 In both cohorts, common AEs included neutropenia and/or decreased neutrophil count, thrombocytopenia and/or decreased platelet count, nausea, vomiting, diarrhoea, fatigue, asthenia and decreased appetite. Additional common AEs were peripheral sensory neuropathy in cohort A, and paraesthesia in cohort B. Of note, one patient in cohort A had grade 3 prolonged activated partial thromboplastin time, considered to be related to FTD/TPI, suggesting that bleeding parameters should be carefully monitored in patients also receiving bevacizumab.
In this study, antitumour activity was observed following treatment with FTD/TPI plus oxaliplatin and bevacizumab (ORR 17.1%). Treatment was terminated early in the cohort receiving FTD/TPI plus oxaliplatin and nivolumab due to low RR (<10%). However, the median PFS was similar in the two cohorts (6.3 months in cohort A and 6.0 months in cohort B).
In a randomised, phase III study conducted in 829 patients with mCRC who had been previously treated with a fluoropyrimidine and irinotecan, the combination of FOLFOX plus bevacizumab was associated with a PFS of 7.3 months and an ORR of 22.7%.18 However, in that study, patients received bevacizumab 10 mg/kg and all patients were bevacizumab treatment naïve at baseline. In cohort A of our study, patients received bevacizumab 5 mg/kg, and >50% had previously received bevacizumab treatment, while 37.8% had previously received oxaliplatin in the adjuvant/neoadjuvant setting. Thus it is difficult to compare the results of these two studies. In another randomised, phase III study that was conducted in 185 patients with mCRC who had received first-line therapy with a fluoropyrimidine and bevacizumab, FOLFOX or FOLFIRI plus bevacizumab were associated with a median PFS of 6.8 months and an RR of 21%.19 There was no significant difference between FOLFOX and FOLFIRI in terms of PFS (P = 0.470).19 In a further study in which 409 patients with mCRC received bevacizumab plus 5-FU or capecitabine and irinotecan or oxaliplatin after progressing on bevacizumab-based first-line therapy, median PFS was 5.7 months and the RR was <6%.20
The RR in cohort B of the following study was modest with only one response observed among the 14 evaluable patients treated in stage 1 of the Bayesian three-stage design and the recruitment was halted in the cohort. These results were in line with those of previous studies conducted in pretreated patients with MSS mCRC, in which immune checkpoint inhibitors as monotherapy or in combination21,22 failed to demonstrate efficacy with low RR and poor PFS. A phase II study of FTD/TPI plus nivolumab recently published23 failed also to demonstrate clinical benefit in patients with refractory MSS mCRC. However, it should be noted that the patients in cohort B of the current study were more heavily pretreated than those in cohort A. The mean number of prior regimens for advanced disease was 1.78 ± 1.11 for cohort A and 2.35 ± 1.54 for cohort B (Table 1). As the PFS was similar in both cohorts, it is possible that adding nivolumab to standard chemotherapy may be of benefit in earlier stages of treatment. Our available biomarker data were not sufficient to analyse a correlation between immunosuppressive pathways within the tumour microenvironment and clinical response. In one patient, PD-L1 conversion was observed upon treatment; although this was associated with stable disease for 8 months, it was not sufficient to induce a clinical response. As reported in multiple immuno-oncology trials with pembrolizumab, the presence of an inflamed tumour and an adaptive immune response is not sufficient for clinical benefit from PD-1 blockade, likely due to the presence of multiple immunosuppressive pathways.24
This study had a number of limitations. Evaluation of antitumour activity was based on the investigator's assessment, rather than by centralised review. Furthermore, as would be expected for a phase I study, the number of patients evaluated was small.
In conclusion, this study showed that FTD/TPI plus oxaliplatin and either bevacizumab or nivolumab had an acceptable safety profile in previously treated patients with mCRC. In addition, the FTD/TPI plus oxaliplatin plus bevacizumab combination demonstrated encouraging antitumour activity. Although the RR with FTD/TPI, oxaliplatin and nivolumab was modest, the survival data were promising in these patients with poor prognosis.
Acknowledgements
The authors thank the study team for conducting the study, and the patients and their families for their participation. Editorial support was provided by Georgii Filatov and Toni Dando of Springer Healthcare Communications and funded by Institut de Recherches Internationales Servier, Suresnes, France.
Funding
The study was funded jointly by Servier, France and Taiho Pharmaceutical, Japan.
Disclosure
RB has received honoraria from Bayer, AstraZeneca, Sanofi, Novartis, Amgen, Hoffmann La Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb and Merck. AC has received consulting/advisory honoraria from Lilly, Amgen, Servier, BMS and Sanofi, and travel accommodation from Amgen, Servier and Merck. AH reports personal fees and nonfinancial support from Servier, during the conduct of the study; grants from Incyte; personal fees from Amgen, Lilly, Debiopharm, Incyte, Bayer, EISAI; grants and nonfinancial support from AstraZeneca; grants from Boehringer Ingelheim, Janssen-Cilag, Merck, Novartis, Pfizer, BMS and Sanofi. GR received honoraria from Novartis, Pfizer, Lilly, Amgen, Roche, SWIXX and Merck. MPS reports personal fees from Servier, Amgen and Merck. GP reports advisory role and speakers honorarium from Servier. AS reports grants and/or personal fees or consulting or advisory role, speakers bureau, research funding, travel/accommodations/expenses from Merck KGaA, Bristol-Myers Squibb, Amgen, Roche, MSD, Servier, Sanofi, AstraZeneca, Bayer, Lilly and Celgene. TA has served in a consulting/advisory role and/or received honoraria for Amgen, Bristol-Myers Squibb, Chugai, Clovis, Grindstone, GSK, HalioDx, MSD Oncology, Pierre Fabre, Roche/Ventana, Sanofi, Servier and Tesaro, and has received travel, accommodation and other expenses from Roche/Genentech/Ventana, MSD Oncology and Bristol-Myers Squibb. GA received honoraria for consulting/advisory roles from Amgen, Bristol-Myers Squibb, Merck Serono, Roche, Bayer, Servier and Sanofi; travel and accommodation expenses from Amgen, Roche, Servier, Bayer and Sanofi and has had an advisory role without compensation for Treos Bio Limited. JE has received honoraria from Servier, Eisai, MSD, BTG, BMS, Ipsen, Bayer and Roche. JT reports personal financial interest in the form of a scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, BeiGene, Biocartis, Boehringer Ingelheim, Chugai, F. Hoffmann-La Roche Ltd., Foundation Medicine, Genentech Inc., Genmab A/S, HalioDx SAS, Halozyme, Imugene Ltd., Inflection Biosciences Ltd., Ipsen, Kura Oncology, Lilly, MSD, Menarini, Merck Serono, Merrimack, Merus, Molecular Partners, Novartis, Peptomyc, Pfizer, Pharmacyclics, ProteoDesign SL, Rafael Pharmaceuticals, Roche Diagnostics, Sanofi, Seagen, Seattle Genetics, Servier, Symphogen, Taiho and VCN Biosciences. CL, NA, VC and RF are employees of Servier. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Hammond W.A., Swaika A., Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol. 2016;8(1):57–84. doi: 10.1177/1758834015614530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E., Cervantes A., Adam R. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 3.Tintelnot J., Stein A. Immunotherapy in colorectal cancer: available clinical evidence, challenges and novel approaches. World J Gastroenterol. 2019;25(29):3920–3928. doi: 10.3748/wjg.v25.i29.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz H.J., Stintzing S., Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777–783. doi: 10.1016/j.ctrv.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limagne E., Thibaudin M., Nuttin L. Trifluridine/tipiracil plus oxaliplatin improves PD-1 blockade in colorectal cancer by inducing immunogenic cell death and depleting macrophages. Cancer Immunol Res. 2019;7(12):1958–1969. doi: 10.1158/2326-6066.CIR-19-0228. [DOI] [PubMed] [Google Scholar]
- 6.Nukatsuka M., Nakagawa F., Takechi T. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, with oxaliplatin on human colorectal and gastric cancer xenografts. Anticancer Res. 2015;35(9):4605–4615. [PubMed] [Google Scholar]
- 7.Limagne E., Nuttin L., Spill A. P-256 Trifluridine/Tipiracil combined to oxaliplatin sensitizes microsatellite stable colorectal cancer to anti-PD-1 blockade. Ann Oncol. 2017;28(suppl 3):iii90. [Google Scholar]
- 8.Ghiringhelli F., Limagne E., Thibaudin M. 558P The combination of trifluridine/tipiracil and oxaliplatin induces immunogenic cell death in microsatellite stable colorectal cancer. Ann Oncol. 2018;29(suppl 8):viii186. [Google Scholar]
- 9.Temmink O.H., Hoebe E.K., van der Born K., Ackland S.P., Fukushima M., Peters G.J. Mechanism of trifluorothymidine potentiation of oxaliplatin-induced cytotoxicity to colorectal cancer cells. Br J Cancer. 2007;96(2):231–240. doi: 10.1038/sj.bjc.6603549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukihara H., Nakagawa F., Sakamoto K. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep. 2015;33(5):2135–2142. doi: 10.3892/or.2015.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argiles G., Andre T., Hollebecque A. Phase I dose-escalation of trifluridine/tipiracil in combination with oxaliplatin in patients with metastatic colorectal cancer. Eur J Cancer. 2019;112:12–19. doi: 10.1016/j.ejca.2019.01.101. [DOI] [PubMed] [Google Scholar]
- 12.Kautio A.L., Haanpaa M., Kautiainen H., Leminen A., Kalso E., Saarto T. Oxaliplatin scale and National Cancer Institute-Common Toxicity Criteria in the assessment of chemotherapy-induced peripheral neuropathy. Anticancer Res. 2011;31(10):3493–3496. [PubMed] [Google Scholar]
- 13.Suenaga M., Wakatsuki T., Mashima T. A phase I study to determine the maximum tolerated dose of trifluridine/tipiracil and oxaliplatin in patients with refractory metastatic colorectal cancer: LUPIN study. Invest New Drugs. 2020;38:111–119. doi: 10.1007/s10637-019-00749-9. [DOI] [PubMed] [Google Scholar]
- 14.Cecchini M., Kortmansky J.S., Lacy J. A phase I study of TAS-102 in combination with oxaliplatin (TAS-OX) for refractory metastatic colorectal cancer (mCRC) Am Soc Clin Oncol. 2019;37:630. [Google Scholar]
- 15.European Medicines Agency (EMA) Avastin: European Public Assessment Report (EPAR) – Product information. EMA. 2009. https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf Available at.
- 16.European Medicines Agency (EMA) Opdivo: European Public Assessment Report (EPAR) – Product information. EMA. 2015. https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf Available at.
- 17.European Medicines Agency (EMA) Lonsurf: European Public Assessment Report (EPAR) – Product information. EMA. 2016. https://www.ema.europa.eu/en/documents/product-information/lonsurf-epar-product-information_en.pdf Available at.
- 18.Giantonio B.J., Catalano P.J., Meropol N.J. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 19.Masi G., Salvatore L., Boni L. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol. 2015;26(4):724–730. doi: 10.1093/annonc/mdv012. [DOI] [PubMed] [Google Scholar]
- 20.Bennouna J., Sastre J., Arnold D. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 21.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng C., Kim T.W., Bendell J. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 23.Patel M.R., Falchook G.S., Hamada K., Makris L., Bendell J.C. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med. 2021;10:1183–1190. doi: 10.1002/cam4.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayers M., Lunceford J., Nebozhyn M. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.