Abstract
The use of probiotics in gastrointestinal ailments has shown therapeutic effects. The imbalance of the microbiota caused by antibiotic treatment or others has been shown to be restored to normal with probiotic treatment. In this study, a genomically and phenotypically safe probiotic Alkalihalobacillus clausii 088AE has been evaluated for ameliorating antibiotic-associated diarrhea (AAD) in pediatrics (PE, n = 60, 2–10 years), adolescent and adults (AA, n = 60, 11–65 years) through a randomized controlled clinical trial. A. clausii 088AE was administered for seven days (PE, 4 and AA, 6 billion/day) and primary and secondary endpoints were evaluated on different visits. Compared to the respective placebo arms, A. clausii 088AE improved the diarrheal (time to last unformed stool and diarrheal frequency) conditions in children, adolescents and adults. A. clausii 088AE treatment decreased AAD-severity score on visit 5 in both pediatric (0.12 ± 0.33, 12.39 folds), adult and adolescent (0.54 ± 0.36, 2.34 folds) groups compared to those respective placebo arm (p < 0.05). A. clausii 088AE was well tolerated, did not cause significant changes in vital and clinical safety parameters and subjects reported no adverse effects or serious adverse reactions. A. clausii 088AE is safe and therapeutically effective against AAD, reducing onset of diarrhea and related severity symptoms including abdominal discomfort and pain, bloating and flatulence. A. clausii 088AE may be recommended as a live bio-therapeutic agent for improving clinical pathophysiology of gastrointestinal ailments, in particular antibiotic-associated diarrhea and related symptoms.
Keywords: Alkalihalobacillus clausii, Antibiotic-associated diarrhea, Time to last informed stool, Bristol stool scale, Adverse effects, Efficacy
Alkalihalobacillus clausii, antibiotic-associated diarrhea, time to last informed stool, Bristol stool scale, adverse effects, efficacy.
1. Introduction
Treatment with common and new antibiotics is becoming progressively ineffective and complicated due to rapid emergence of antimicrobial resistance. Antibiotic therapy has most prevalent adverse reactions such as antibiotic-associated diarrhea and other gastrointestinal, physiological and psychological illnesses [1, 2]. Commonly associated symptoms of antibiotic-associated diarrhea are dehydration, fever, nausea, vomiting, abdominal cramps, watery diarrhea, bloody and foul-smelling diarrhea range from mild and self-limiting to severe. Clinically significant antibiotic-associated diarrhea may appear mushy (loose) or watery stools at least three episodes a day right after few hours up to two months of antibiotics intake [3].
The use of antibiotics systematically inducts a multimodal disturbances of the microbial ecology through the longitudinal decrease in the distribution, diversity and richness of autochthonous microorganisms in the gastrointestinal tract [4, 5]. The aminoglycoside streptomycin can perturb the balance of microbiota and increases the abundance of phyla Bacteroidetes and Firmicutes [6]. Ampicillin and ciprofloxacin augmented genus Acinetobacter and Lactobacillus, respectively [7]. Certain antibiotics can cause the proliferation of Fusobacteria, Clostridia and Eubacteria while demolishing beneficial groups like Bacteroides and Bifidobacteria [8]. Such imbalance (dysbiosis) affects the microbial synergisms by depriving microbial network, nutrients, metabolites of microbial and host origin, promotes the horizontal transfer of antibiotic resistant genes (ARGs) and mobile genetic elements (MGEs) to sensitive microorganisms. The collapsed resident microbiota often fails to resist the overgrowth of endogenous opportunistic microorganisms and inhibit the pathogenic invasion. Re-establishment of normal gut microbiota happens over time after cessation of antibiotic therapy [9]. The microbiota recovered after antibiotic treatment can be unstable and therefore patients can be susceptible to secondary infections and other diseases. Therefore, antibiotic-associated diarrhea can lead to prolonged hospitalization with persistent illness and increased cost of medical care [10].
Therefore, the restoration of normal gut microbiota is a fundamental therapeutic treatment paradigm, in which probiotics can provide promising healthcare solutions for antibiotic-associated diarrhea [11]. Probiotics are potential live bio-therapeutics, which maintain intestinal microecology during or after antibiotic treatment through competition for epithelial receptors and nutrients, inhibition of epithelial and mucosal adhesion of pathogens, producing short chain fatty acids (SCFAs), balancing non-favorable intestinal pH (low), regulating immunity and synthesizing antimicrobial peptides and metabolites. With these mechanisms, probiotics provide a promising route to treat many gastrointestinal ailments including antibiotic-associated diarrhea without a risk of spreading antibiotic resistance among other microorganisms [12, 13].
The therapeutic effects of probiotics in the treatment of infectious and antibiotic-associated diarrhea have been evaluated through randomized controlled trials (RCTs) and meta-analysis [14]. The probiotics intervention adopts either a monoculture or mixed culture formula and consists of a variety of microbial species, including Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Bacillus coagulans, Bacillus clausii (current nomenclature Alkalihalobacillus clausii or A. clausii) and Saccharomyces boulardii [15]. Among them, probiotic Alkalihalobacillus clausii (basionym Bacillus clausii) has been studied for variety of acute and infectious diarrheal diseases, such as acute and chronic diarrhea, acute community-acquired diarrhea (ACAD), antibiotic associated diarrhea (AAD), Clostridium difficile-induced diarrhea (CDID) across different geographical locations. The adjunctive treatment with A. clausii strains has been reported safe among children, adult and elderly population [16]. It is currently available as an over the counter (OTC) medical product and used as a complementary treatment [17].
A. clausii (average genome size 4197324–4598557 bp and 42.8–44.75% GC content), a sporogenous probiotic bacteria belongs to genus Alkalihalobacillus and is widely studied for the production of high alkaline proteases, antimicrobials like type-A lantibiotic clausin (Mw 2107.94–5,158.11 Da) and other lantibiotics [18, 19]. A. clausii maintains gut health by improving digestive microenvironment, reprogramming intestinal microbiota and regulating host's immunity [20, 21, 22]. The naturally encapsulated coating of bacterial spores provides protection against various drought conditions such as high temperature, desiccation, osmotic pressure, etc. This highly resilient allochthonous probiotic can survive and proliferate in harsh gastrointestinal conditions comprising gastric acid, pepsin, pancreatin, ions, digestive enzymes, biles and mucins [23]. The efficacy of probiotic A. clausii varies among strains; differs as per dosages used in the finished formulations and severity of clinical conditions in order to exhibit the intended health benefits [12, 20, 24].
A. clausii strains have long been consumed safely by the general human population [24]. A. clausii is considered as the Qualified Presumption of Safety (QPS) listed bacteria by the European Food Safety Authority (EFSA) [25]. A. clausii strains show resistance to one or multiple antibiotics; however, the relevant genes are intrinsically present in chromosomal DNA rendering them highly stable, nontransferable and nonfunctional [26, 27]. In addition, the absence of extrachromosomal genome makes A. clausii a risk-free and safe probiotic for human and animal applications [28]. The intrinsic antibiotic(s) resistance in probiotics can be advantageous as these probiotics can be used in combination with antibiotics to restore microbiota in various gastrointestinal ailments including antibiotic-associated diarrhea [24, 29].
The comparative evaluation on A. clausii strains showed heterogeneity and variable clinical benefit in different diarrheal conditions. Such effects happen primarily due to differential ability of the strain to survive and germinate under harsh gastrointestinal conditions, the adhesion and colonization on epithelial cells, the modulation immune system, varying compatibility to complex treatment conditions and host adaptability [30]. A. clausii strain with fulfillment of these attributes can provide a consistent therapeutic outcome in gastrointestinal illnesses, thus this study is originated. The current study evaluated the clinical efficacy and safety of the probiotic A. clausii 088AE [MCC 0538] in improving antibiotic-associated diarrhea and related symptoms in pediatric, adolescent and adult population. A. clausii 088AE (genome size 4598557 bp, GC content 44.74%, NCBI Reference Sequence No. CP031128 & MH532550), a genomically stable and phenotypically safe probiotic bacterium is used in this clinical trial. The frequency of diarrhea, severity of related symptoms and stool consistency in the intervened group and control group were assessed as outcome variables.
2. Methods
2.1. Investigational product (IP) and method of analysis
The investigational product (IP), spore preparation of A. clausii 088AE (2 billion colony-forming unit per gram, CFU/g) with excipient (maltodextrin) was used in powder form (one g per sachet). The placebo contained only excipient, maltodextrin (1.00 g). Both test and placebo products were supplied by Advanced Enzyme Technologies Ltd., Thane, India and complied with the standard specifications (Method of Analysis; Supplement File__088AE-AAD_2021). Physical appearance and sensory profile, packaging and labelling were same and only the coded batch numbers could differentiate two products.
2.2. Ethics and informed consent
The trial was prospectively registered in the Clinical Trial Registry, India [CTRI/2020/03/023936 (13/03/2020)] before patient enrollment began. The written ethical approval obtained from Rajalakshmi Hospital, Karnataka (India) and Sai hospital, Maharashtra (India). Approved study protocol was in accordance with the Declaration of Helsinki [31], the ICH-harmonized tripartite guideline for good clinical practice [32] and the Indian Council of Medical Research Guidelines for Biomedical Research on Human subjects [33]; and the same was followed with no further changes or amendments during trial [WHO Format Research Protocol_088AE-AAD(v01) and CONSORT(2010)_Checklist__088AE-AAD_2021]. Written and oral information was provided to all patients in understandable language. Every subject had given written informed consent to investigator after understating the objective of this trial, including possible risks and benefits.
2.3. Trial design and selection of subjects
The prospective interventional trial was randomized, double-blinded, parallel, placebo controlled and had a total of 5 scheduled visits to the clinical site by the study subjects. Subject selection was based on the defined inclusion and exclusion criteria.
2.3.1. Inclusion criteria
Male and female subjects (2–65 years) were included on following criteria such as – acute infectious diarrhea (three or more loose stools in last 24 h) and treated with physician's prescribed broad spectrum antibiotics (e.g., β-lactam, lincomycin, cephalosporin and macrolide class) for last five days; a positive stool microbial culture test and clinical evaluation by the principal investigator; and willing to participate with written informed consent/assent following applicable guideline.
2.3.2. Exclusion criteria
Patients excluded from study - with bloody or purulent stool, with pus or mucus and severe dehydration needing hospitalization. Subjects with symptoms of septicemia, faster heart rate and breathing, shortness of breath, sweaty or clammy skin were excluded. Subjects were excluded if they were on other bacteriotherapy prior to this study or during study other than interventional product. Subjects with history of gastric and duodenal ulcer, upper gastrointestinal bleeding, autoimmune gastritis and gastroesophageal reflux disease (GERD) were excluded (Details of Exclusion Criteria; Supplement File__088AE-AAD_2021). In addition, subjects having adverse effects or serious adverse effects, pregnancies, disease emergencies and study protocol violation are removed from the trial (Details of Withdrawal Criteria; Supplement File__088AE-AAD_2021).
2.4. Randomization and treatment procedures
A total of 120 subjects comprising sixty paediatric subjects (PS, 2–10 years) and sixty from adolescent and adults (AA, 11–65 years) were enrolled. Each age group was allocated with 30 randomized subjects in each of the test and the placebo group. Both the test groups, i.e., paediatric (Test-PS, n = 30) and adolescent and adults (Test-AA, n = 30) were on A. clausii 088AE with two billion colony forming unit activity (Method of Analysis; Supplement File__088AE-AAD_2021) powder (carrier maltodextrin) respectively for twice (bid) and thrice (tid) a day. A similar dosing schedule (on maltodextrin) were given to the respective control [Placebo-PS (n = 30) and Placebo-AA (n = 30)] groups. All subjects were on the prescribed concomitant (ongoing antibiotic therapy) therapy along with IP treatment during the treatment duration. Total study and treatment duration were 17 and 7 days, respectively. The subject randomization (followed by SAS random number generation method), treatment allocation and procedures are represented in a schematic diagram (Figure 1). Study investigators remained blind until the completion of the study whereas un-blinding was done only at the end of clinical phase completion. Supportive treatments were recommended to the subjects along with IP, if required, but without changing concomitant (antibiotics) treatment as deemed necessary by the investigator. No changes or amendments were made to the approved protocol after the trial commenced and no interim data analysis was done during the study period.
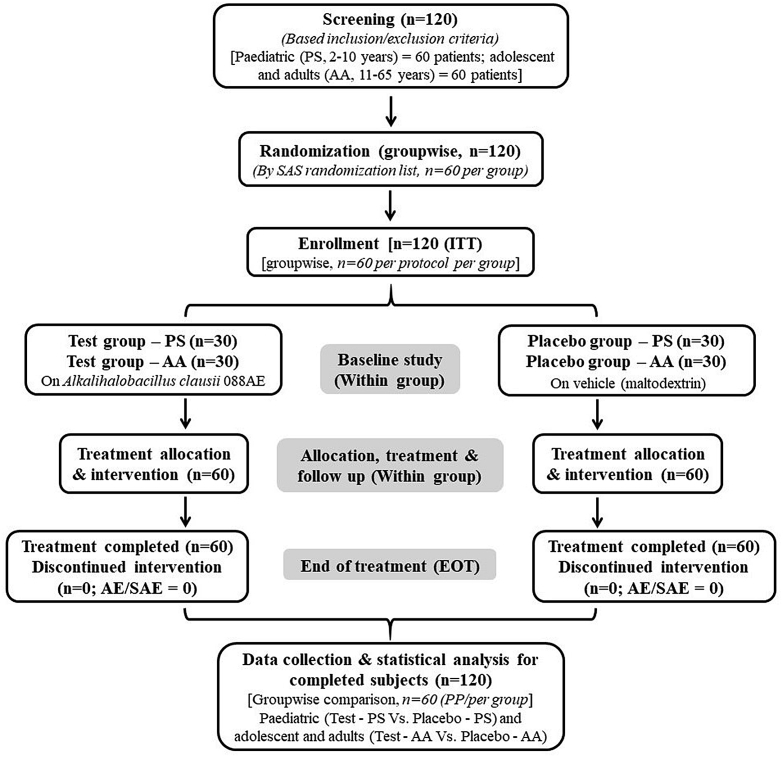
Figure 1.
Schematic diagram of the clinical study on evaluation of the efficacy and safety of Alkalihalobacillus clausii 088AE in the treatment of antibiotic-associated diarrhea. The study enrolled total 120 intension to treat (ITT) subjects (each 60 subjects in pediatric group and adolescent and adult group) through the inclusion and exclusion criteria screening. Each age group was allocated with 60 subjects (each 30 subjects in test and placebo arm) following the SAS random number generation method. With no adverse effects (AEs) or serious adverse events (SAEs) report and no discontinuation of subjects, completed 120 per protocol (PP) subjects were considered for endpoints evaluation. Data comparison between test and placebo, and statistical analysis were performed within age group. Study compliance was checked at every follow up visits by a team of physician-investigator and officers.
2.5. Endpoints analysis: efficacy and safety variables
Primary and secondary endpoints evaluated different efficacy and safety variables of A. clausii 088AE used for treating antibiotic-associated diarrhea (AAD). This study was conducted on two population groups, i.e., pediatric group and adolescent adult group. Different endpoint results of test arm were compared with the respective placebo arm within specific population group. In primary endpoints, the efficacy of A. clausii 088AE was evaluated from time to last unformed stool (TTLUS), total number of unformed stools, time to complete resolution of functional gastrointestinal discomforts and percentage (%) of responders with complete remission of diarrhea at different treatment durations (24–168 h).
Secondary endpoints evaluated changes of severity of AAD related symptoms, visual analog scale (VAS) and tolerance to investigational product at baseline to EOT. Severity of AAD was assessed by physician's investigation for bloating, distension, flatulence, odorous flatulence, difficult gas evacuation, stomach rumbling, belching, bad breath, abdominal movement and excessive gas evacuation. The mean severity score was calculated from a four-point scale on symptoms (0 = none, 1 = mild, 2 = moderate and 3 = Severe). AAD-associated symptoms like abdominal pain, bloating and flatulence, vomiting and nausea, perception of mental wellbeing and influence on daily life were assessed through visual analogue scale (VAS, 0–10). Safety of A. clausii 088AE was assessed by examining general physical health, systemic biomarkers and adverse effect reports. The clinical safety was assessed through systemic biomarkers like hematological (complete blood count), hepatic [aspartate aminotransferase (SGOT) and alanine aminotransferase (SGPT)], renal function test (serum concentration of creatinine, albumin, sodium, potassium and blood urea nitrogen), random blood sugar (RBS) and total cholesterol at baseline and EOT. Biomarkers analysis were performed following the standard medical test protocols. The adverse effect (AE) is defined as any medically untoward event detected in a clinical study subject after use of the study agents, whether or not caused by the use of the agents. Whereas, serious adverse event (SAE) is defined as any untoward medical incidence which is life-threatening and results into death or hospitalization, disability or incapacity and congenital anomaly.
2.6. Sample power and statistical analysis
Following the maximum sample size among primary endpoints, each group with sixty subjects conferred 90% sample power (α = 0.050 when MD = 1.00). Randomization was done using SAS® software, version 9.1.3. Safety and efficacy analysis was performed on per-protocol (PP) population within group. Separate analyses were performed for primary and secondary endpoints. The entire statistical analysis was performed as per the statistical analysis plan (SAP). Significance of treatment effects were determined either by the null-hypothesis (H0 = μs-μe ≥ d) or the alternative hypothesis (H1 = μs − μe ≤ d). Where, μe is the mean change in the test arm and μs is mean change in the placebo arm. Results were analyzed with 5% significance level (confidence interval 95%) and the differences within the groups were assessed using t-test. Differences between two groups were measured using Kruskal-Wallis one-way ANOVA test.
3. Results
From March 2020 to October 2020, a total of 60 pediatric and 60 adolescent and adult patients with antibiotic-associated diarrhea (AAD) were enrolled with no exclusion to the study in two hospitals in India. All intention to treat (ITT) patients completed the study without dropout, hence, per protocol’ (PP) population. Pediatric (PS) group enrolled patients within age range of 2–10 years, whereas, adolescent and adults (AA) group enrolled patients within 10–65 years of age. Each age group had two arms with 30 patients per arm and received two different treatments. The test arm of pediatric (Test-PS) and adolescent and adult (Test-AA) groups received Alkalihalobacillus clausii 088AE (2 billion per sachet) respectively two and three times in a day. The control arms [pediatric (Placebo-PS) and adolescent and adult (Placebo-AA)] of both groups received maltodextrin with corresponding dosing schedule. Out of 60 patients in pediatric group, Test-PS had total 12 male (M, 40.00%) and 18 females (F, 60.00%) and Placebo-PS had 8M (26.67%):22F (73.33%). In adolescent and adult group, Test-AA had 23M (76.67%) and 7F (23.33%), and Placebo-AA had 22M (73.33%) and 8F (26.67%). Demographic parameters (age, height, body weight and body mass index) of all treatment arms compared within age group were not significantly different (Table 1). Principal investigator and clinical trial team assessed study regulations at each visit along with all the safety and efficacy assays as per the schedule of events (Table 2). Subjects reported an acute or persistent mild to severe form of antibiotic-associated diarrhea mainly due β-lactam, lincomycin, cephalosporin and macrolide class antibiotics before enrollment began. Enrolled patients were treated with A. clausii 088AE along with ongoing concomitant antibiotics. IP treatment was started on visit 1 (baseline, day 1) and end of treatment was on visit 5 (EOT, day 8). All patients completed the study, reported no drop out, and missed dosage or discontinuation of investigational product. The safety and efficacy analyses were evaluated on PP population, i.e., full analysis set (FAS, n = 60) within the age group. The clinical trial was concluded after the telephonic follow up [on visit 06 (day 17 ± 3)] of the last enrolled patient and completion of target sample size according to the study protocol.
Table 1.
The demographic details of pediatric (PS) and adolescent and adult (AA) subjects of four treatment arms (Test-PS and Placebo-PS; Test-AA and Placebo-AA) participated in the current clinical trial and their descriptive statistics.
| Parameters | Pediatric group (PS) |
P value [95% CI] (p < 0.05) | Adolescent and adult group (AA) |
P value [95% CI] (p < 0.05) | ||
|---|---|---|---|---|---|---|
| Test-PS | Placebo-PS | Test-AA | Placebo-AA | |||
| Subjects number (n) |
30 |
30 |
30 |
30 |
||
| Gender [n (%)] | ||||||
| Male | 12 (40.00%) | 8 (26.67%) | 23 (76.67%) | 22 (73.33%) | ||
| Female | 18 (60.00%) | 22 (73.33%) | 7 (23.33%) | 8 (26.67%) | ||
| Age (years) [min/max] | 4.89 ± 1.65 [2.10/7.30] | 5.28 ± 1.08 [3.00/7.50] | 0.283 [-0.331, 1.111] | 26.67 ± 16.03 [12.00/62.00] | 27.07 ± 17.41 [12.00/62.00] | 0.926 [-8.249, 9.049] |
| Height (cm) [min/max] | 99.95 ± 12.87 [63.50/121.00] | 106.48 ± 14.97 [80.00/125.00] | 0.075 [-0.689, 3.745] | 159.53 ± 11.23 [139.00/176.00] | 159.10 ± 11.10 [136.00/174.00] | 0.882 [-6.201, 5.341] |
| Weight (kg) [min/max] | 18.54 ± 5.31 [10.70/29.80] | 19.25 ± 3.98 [11.00/27.20] | 0.560 [-1.715, 3.135] | 61.43 ± 15.24 [32.00/86.00] | 60.80 ± 17.38 [34.00/97.00] | 0.882 [-9.078, 7.818] |
| Body Mass Index (kg m−2) [min/max] | 18.61 ± 4.47 [10.70/27.50] | 17.02 ± 3.22 [12.00/24.90] | 0.119 [-3.603, 0.423] | 23.53 ± 3.13 [14.60/28.07] | 23.21 ± 3.87 [15.40/32.00] | 0.726 [-2.139, 1.499] |
Values expressed as mean ± SD and the difference between two mean is reported with 95% Confidence Interval (CI).
Table 2.
Schematic schedule of the clinical trial to evaluate the safety and efficacy of Alkalihalobacillus clausii 088AE on antibiotic-associated diarrhea (AAD) patients.
| Visits | Visit 01 (Day 1)a | Visit 02 (Day 2)b | Visit 03 (Day 3)c | Visit 04 (Day 5)d | Visit 05 (Day 8)e | Visit 06 (Day 17 ± 3)f | Visit 07g |
|---|---|---|---|---|---|---|---|
| Informed consent/assent | √ | ||||||
| Demography | √ | ||||||
| Medical/surgical history | √ | ||||||
| Inclusion/exclusion criteria | √ | ||||||
| IP Dispensing | √ | √ | √ | √ | |||
| Issue diary card | √ | √ | √ | √ | |||
| Physical examination | √ | √ | √ | √ | √ | ||
| Vital signs | √ | √ | √ | √ | √ | √ | |
| Investigator assessments for severity of diarrhea | √ | √ | √ | √ | √ | ||
| Laboratory tests | √ | √ | |||||
| Compliance check | √ | √ | √ | √ | |||
| Primary endpoints | √ | √ | √ | √ | |||
| Secondary endpoints | √ | √ | √ | √ | |||
| Concomitant medications | √ | √ | √ | √ | √ | √ | |
| AE/SAE assessment | √ | √ | √ | √ | √ | √ |
Visit 01 was for screening, randomization and initiation of treatment (baseline).
Visit 02 was second day of treatment.
Visit 03 was third day of treatment.
Visit 04 was fifth day of treatment.
Visit 05 was eighth day of treatment, i.e., end of treatment (EOT).
Visit 06 was on 17 ± 3 day and for telephonic follow up.
Visit 07 was unscheduled visit.
3.1. Primary endpoint: efficacy evaluation
3.1.1. Total number of unformed stools
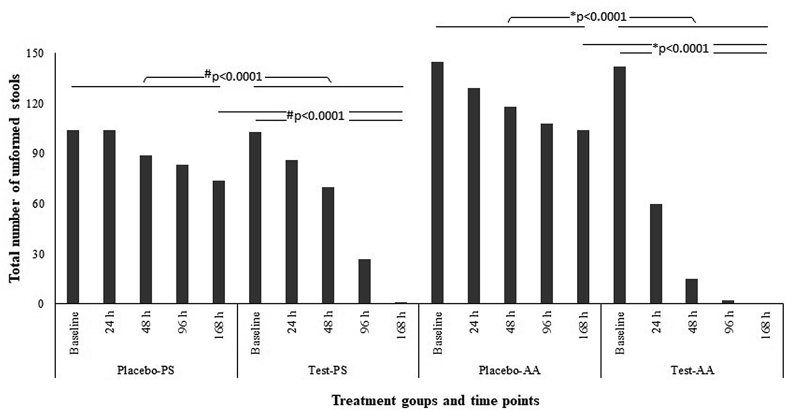
Treatment of AAD with Alkalihalobacillus clausii 088AE decreased the total number of unformed stools in pediatrics as well as in adolescent and adult groups. In pediatric group, treatment of A. clausii 088AE reduced 16.91% of total number of unformed stools by 24 h compared to placebo where no reduction was recorded from the baseline (average total 103.5 diarrheal episodes at baseline for both arms). Importantly, A. clausii 088AE (Test-PS) improved diarrheal condition in pediatric group by 99.03% at the end of treatment (EOT, 168 h), whereas, 28.50% in placebo (Placebo-PS) arm (p < 0.0001) (Figure 2). A 57.74% drop in total number of unformed stools was recorded after 24 h of A. clausii 088AE treatment in adolescent and adult groups compared to placebo (11.03%). After 96 h of treatment, A. clausii 088AE (Test-AA) arm reported a few (1.41%) incidence of unformed stools compared to placebo (Placebo-AA, 71.72%) (Figure 2).
Figure 2.
Total number of unformed stools (induced by antibiotic-associated diarrhea) recorded in pediatric# (Placebo-PS and Test-PS) and adolescent and adult∗ (Placebo-AA and Test-AA) groups from the baseline and first day of Alkalihalobacillus clausii 088AE administration to the end of treatment (EOT) (#/∗p < 0.0001).
3.1.2. Time to last unformed stool (TTLUS)
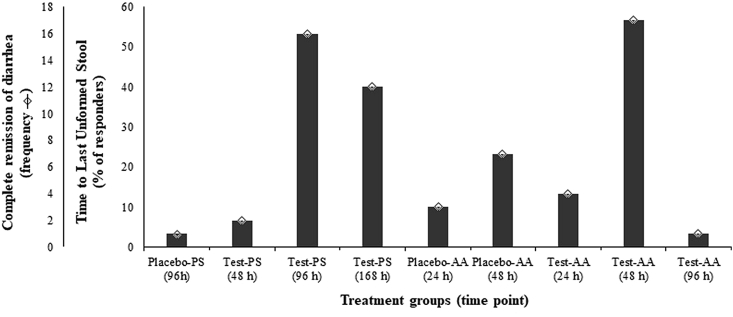
Efficacy of Alkalihalobacillus clausii 088AE in improving antibiotic-associated diarrhea was determined from frequency of subjects reporting time to last unformed stools following Bristol stool form scale. Incidence of ‘normal’ Bristol stool (type 3 or 4) for the first time was captured by subjects frequency to express TTLUS. In pediatric group, A. clausii 088AE improved the diarrheal conditions by reporting higher frequency normal stools [n2 at 72 h (6.67%), n16 at 96 h (53.33%) and n12 at 168 h (40.00%)] within shorter duration compared to placebo group [n1 at 96 h (3.33%). Similarly, diarrheal condition was improved by A. clausii 088AE in adolescent and adult groups by higher TTLUS [n4 at 48 (26.67%), n17 at 72 h (56.67%) and n1 at 96 h (3.33%)] compared to placebo group [n3 at 48 h (10%) and n7 at 72 h (23.33%)] (Figure 3).
Figure 3.
The complete remission of diarrhea and relative percentage (%) of responders on time to last unformed stool (TTLUS) at different time points from both pediatric (Placebo-PS and Test-PS) and adolescent and adult (Placebo-AA and Test-AA) groups after the administration of Alkalihalobacillus clausii 088AE to the end of treatment (EOT).
3.1.3. Complete remission of diarrhea [by Bristol stool scale]
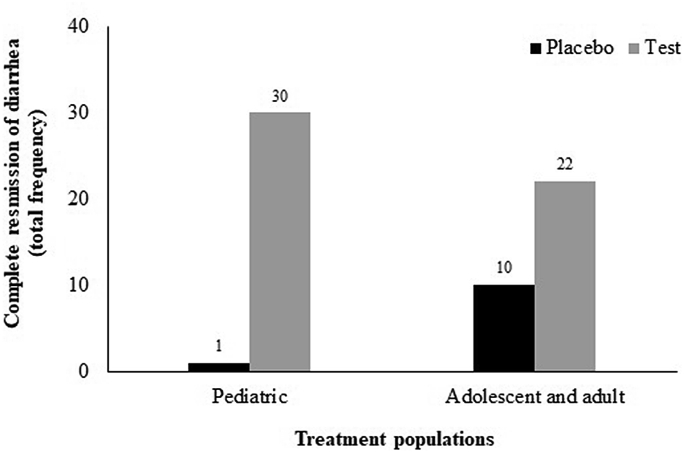
Based on Bristol Stool Form Scale (BSFC) questionnaire, percentage responders as defined by the number of patients who reported the ‘normal’ stool form [type 3 (like a sausage but with cracks on its surface) or type 4 (like a sausage or snake, smooth and soft] at different visits were considered to calculate the complete remission of diarrhea. The Alkalihalobacillus clausii 088AE-mediated improvement was reflected by percentage responders with complete remission of diarrhea at different visits both in pediatric and adult and adolescents compared placebo. In pediatric group, normal stool appearance was reported by 30 patients at different time points (16 patients in 96 h, 2 patients in 72 h and 12 patients in 168 h) in A. clausii 088AE treatment arm (Test-PS) compared to Placebo-PS (1 patient in 48 h) [p < 0.0001; RR = 0.03 (95%CI, 0.01–0.23); ARR = 0.97 (95%CI, 0.79–0.99)]. In adolescent and adult group, total 22 patients (04 patients in 48 h, 17 patients in 72 h and 1 patient in 96 h) reported complete remission of diarrhea from A. clausii 088AE treatment arm (Test-AA), whereas, 10 patients reported from Placebo-AA arm (3 patients in 48 h and 7 patients in 72 h) [p < 0.0001; RR = 2.20 (95%CI, 1.27–3.81); ARR = -0.40 (95%CI, -0.58–0.15); OR = 5.50 (95%CI, 1.81–16.68)] (Figure 4).
Figure 4.
Total frequency of complete remission from antibiotic-associated diarrhea (AAD) treated with Alkalihalobacillus clausii 088AE compared to respective placebo arms in two treatment groups, i.e., pediatric, adolescent and adult groups.
3.2. Secondary endpoints: safety evaluation
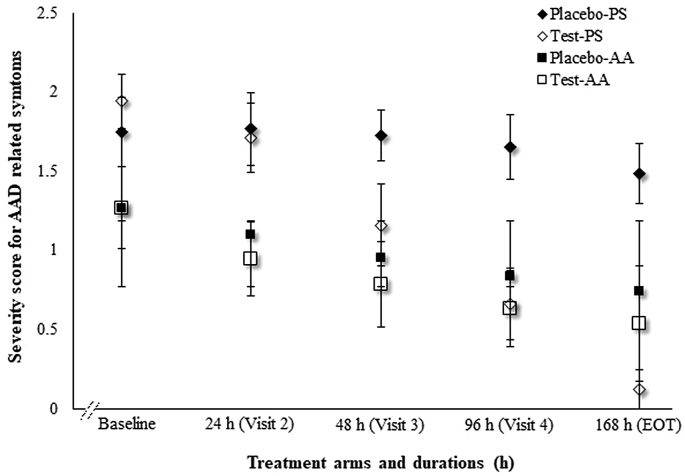
3.2.1. Change in severity of AAD related symptoms
Severity of AAD was determined on a four-point scale for symptoms like bloating, distension, flatulence, odorous flatulence, difficult gas evacuation, stomach rumbling, belching, bad breath, abdominal movement and excessive gas evacuation. In current study, treatment with A. clausii 088AE (test arms) significantly reduced the severity score of AAD in pediatric, adolescent and adult groups compared to respective placebo arms. At baseline, severity scores were not significantly different between test and placebo arm in pediatric group [p = 0.1231 (95%CI, -0.05–0.45)] and adolescent and adult group [p = 0.9554 (95%CI, -0.15–0.14)]. In pediatric group, A. clausii 088AE reduced the severity of AAD gradually on each visit, eventually 12.39 folds reduction on EOT compared to placebo arm [mean difference (MD) = 1.36; p < 0.0001 (95%CI, 1.20–1.52)]. The placebo arm did not show any improvement in AAD severity (Figure 5). In adolescent and adult group, A. clausii 088AE reduced AAD severity over time and 2.34 folds on EOT than its baseline, and was significantly different compared to placebo arm [MD = 0.20; p = 0.0237 (95%CI, 0.03–0.37)] (Figure 5).
Figure 5.
Changes in investigators assessed severity score of AAD related symptoms at different time points both in pediatric [Placebo-PS (♦) and Test-PS (◊)] and adolescent and adult [Placebo-AA (■;) and Test-AA (□)] groups.
3.2.2. VAS assessment of AAD symptoms
Alkalihalobacillus clausii 088AE treatment reduced other associated symptoms like abdominal pain, bloating and flatulence, vomiting and nausea in both pediatric, adult and adolescent groups compared to placebo (p < 0.0001). These were assessed through visual analogue scale (VAS, 0–10) assessment. Trial data showed that pediatric subjects from Test-PS arm (A. clausii 088AE) were relived from abdominal pain [MD -6.67 ± 0.19; 95%CI, -7.06-6.28] than Placebo-PS [MD -0.60 ± 0.26; 95%CI, -1.12–0.09]. In adolescent and adult group, Test-AA arm reported reduced abdominal pain [MD -3.40 ± 0.31; 95%CI,-4.02-2.77] compared to Placebo-AA [MD -2.20 ± 0.26; 95%CI, -2.73–1.67] (Table 3). The bowel movement (diarrhea) was improved remarkably both in pediatric, adolescent and adult group (p < 0.0001). In pediatric group, the mean VAS score difference for bowel movement (diarrhea) was -6.20 ± 0.23 (95%CI, -6.66–5.73) in A. clausii 088AE treated (Test-PS) arm compared to an insignificant mean difference in Placebo-PS [MD -0.60 ± 0.29; 95%CI, -1.18–0.01]. The adolescent and adult group reported a reduced bowel movement (diarrhea) after A. clausii 088AE treatment [Test-AA, MD 5.04 ± 0.49 (95%CI, -6.02-4.05)] than its Placebo-AA arm [MD -3.56 ± 0.43; 95%CI, -4.43–2.68] (Table 3).
Table 3.
Comparative visual analogue scale (VAS) scores evaluated in antibiotic-associated diarrhea (AAD) at baseline and EOT in pediatric (Test-PS and Placebo-PS) and adolescent and adult group (Test-AA and Placebo-AA) (±SD). In both groups, test arms received Alkalihalobacillus clausii 088AE. Symptoms considered were abdominal pain, diarrhea, bloating and flatulence, nausea and vomiting, perception of mental well-being and influence daily life. Intragroup [MD = baseline-EOT] and intergroup mean differences [in pediatric (MD = Placebo-PS – Test-PS) and adolescent and adult group (MD = Placebo-AA – Test-AA)] for all the symptoms was analyzed through ANOVA and 95% confidence interval (CI) estimation at EOT and mean.
| Parameters | Arms | Baseline | EOT | Mean change | p (<0.05) | 95% CI |
|---|---|---|---|---|---|---|
| Abdominal pain | Placebo-PS | 6.53 ± 0.51 | 5.93 ± 1.31 | -0.60 ± 0.26 | <0.0001 | -1.12,-0.09 |
| Test-PS | 7.40 ± 0.67 | 0.73 ± 0.83 | -6.67 ± 0.19 | -7.06,-6.28 | ||
| Between groups (Placebo-PS – Test-PS) | -6.07 ± 0.05 | -5.76,-4.63 (at EOT) 5.95, 6.18 (at mean) | ||||
| Placebo-AA | 4.50 ± 1.33 | 2.30 ± 0.58 | -2.20 ± 0.26 | <0.0001 | -2.73,-1.67 | |
| Test-AA | 4.67 ± 1.62 | 1.27 ± 0.51 | -3.40 ± 0.31 | -4.02,-2.77 | ||
| Between groups (Placebo-AA – Test-AA) | -1.20 ± 0.07 | -1.31, -0.75 (at EOT) -1.34,-1.05 (at mean) | ||||
| Diarrhea (bowel movement) | Placebo-PS | 6.40 ± 0.62 | 5.80 ± 1.49 | -0.60 ± 0.29 | <0.0001 | -1.18,-0.01 |
| Test-PS | 6.96 ± 0.72 | 0.76 ± 1.04 | -6.20 ± 0.23 | -6.66,-5.73 | ||
| Between groups (Placebo-PS – Test-PS) | -5.60 ± 0.06 | -5.70,-4.37 (at EOT) 5.46, 5.73 (at mean) | ||||
| Placebo-AA | 6.76 ± 0.93 | 3.20 ± 2.21 | -3.56 ± 0.43 | <0.0001 | -4.43,-2.68 | |
| Test-AA | 6.80 ± 1.49 | 1.76 ± 2.25 | -5.04 ± 0.49 | -6.02,-4.05 | ||
| Between groups (Placebo-AA – Test-AA) | -1.48 ± 0.12 | -2.59,-0.28 (at EOT) 1.24,1.72 (at mean) | ||||
| Bloating and flatulence | Placebo-PS | 6.23 ± 0.62 | 5.63 ± 1.24 | -0.60 ± 0.25 | <0.0001 | -1.10,-0.09 |
| Test-PS | 6.50 ± 0.63 | 0.40 ± 0.62 | -6.10 ± 0.16 | -6.42,-5.77 | ||
| Between groups (Placebo-PS – Test-PS) | -5.50 ± 0.05 | -5.73,-4.72 (at EOT) -5.61,-5.39 (at mean) | ||||
| Placebo-AA | 3.23 ± 1.16 | 1.43 ± 1.04 | -1.80 ± 0.28 | <0.0001 | -2.36,-1.23 | |
| Test-AA | 3.03 ± 0.92 | 0.90 ± 0.80 | -2.13 ± 0.22 | -2.57,-1.68 | ||
| Between groups (Placebo-AA – Test-AA) | -0.33 ± 0.06 | -1.01,-0.05 (at EOT) -0.46,-0.19 (at mean) | ||||
| Nausea and vomiting | Placebo-PS | 6.16 ± 0.64 | 5.40 ± 1.54 | -0.76 ± 0.30 | <0.0001 | -1.36,-0.15 |
| Test-PS | 6.30 ± 0.65 | 0.03 ± 0.18 | -6.27 ± 0.12 | -6.51,-6.02 | ||
| Between groups (Placebo-PS – Test-PS) | -5.51 ± 0.05 | -5.93,-4.80 (at EOT) -5.63,-5.39 (at mean) | ||||
| Placebo-AA | 2.56 ± 1.30 | 1.16 ± 0.98 | -1.40 ± 0.29 | <0.0001 | -1.99,-0.80 | |
| Test-AA | 2.56 ± 1.50 | 0.63 ± 0.89 | -1.93 ± 0.31 | -2.56,-1.29 | ||
| Between groups (Placebo-AA – Test-AA) | -0.53 ± 0.07 | -1.01,-0.04 (at EOT) -0.68,-0.37 (at mean) | ||||
| Perception of mental well-being | Placebo-PS | 6.06 ± 0.78 | 5.33 ± 1.34 | -0.73 ± 0.28 | <0.0001 | -1.29,-0.16 |
| Test-PS | 6.60 ± 1.89 | 0.06 ± 0.25 | -6.54 ± 0.35 | -7.23,-5.84 | ||
| Between groups (Placebo-PS – Test-PS) | -5.81 ± 0.08 | -5.76,-4.77 (at EOT) -5.97,-5.64 (at mean) | ||||
| Placebo-AA | 3.20 ± 0.92 | 1.43 ± 1.16 | -1.77 ± 0.27 | <0.0001 | -2.31,-1.22 | |
| Test-AA | 3.10 ± 1.06 | 0.83 ± 0.95 | -2.27 ± 0.26 | -2.79,-1.74 | ||
| Between groups (Placebo-AA – Test-AA) | -0.50 ± 0.06 | -1.14,-0.05 (at EOT) -0.63,-0.36 (at mean) | ||||
| Influence daily life | Placebo-PS | 6.30 ± 0.87 | 5.13 ± 1.27 | -1.17 ± 0.28 | <0.0001 | -1.73,-0.60 |
| Test-PS | 6.67 ± 0.92 | 0.30 ± 0.46 | -6.37 ± 0.18 | -6.74,-5.99 | ||
| Between groups (Placebo-PS – Test-PS) | -5.20 ± 0.06 | -5.32,-4.33 (at EOT) -5.32,-5.07 (at mean) | ||||
| Placebo-AA | 5.50 ± 1.16 | 3.53 ± 2.40 | -1.97 ± 0.48 | <0.0001 | -2.94,-0.99 | |
| Test-AA | 5.56 ± 1.30 | 2.10 ± 2.38 | -3.46 ± 0.49 | -4.45,-2.46 | ||
| Between groups (Placebo-AA – Test-AA) | -1.49 ± 0.12 | -2.66,-0.19 (at EOT) -1.74,-1.23 (at mean) |
Similarly, pediatric group showed a remarkable improvement in bloating and flatulence symptoms after A. clausii 088AE treatment [Test-PS, MD -6.10 ± 0.16 (95%CI, -6.42–5.77)] compared to Placebo-PS arm [MD 0.60 ± 0.25; 95%CI, -1.10–0.09] (p < 0.0001). The mean difference between Test-AA and Placebo-AA arm was however small in adolescent and adult group [MD -0.33 ± 0.06; 95%CI, -0.46,-0.19] (p < 0.0001). Simultaneously, Test-PS arm of pediatric group reported a sharp reduction of nausea and vomiting symptoms after A. clausii 088AE treatment [MD -6.27 ± 0.12; 95%CI, -6.51–6.02] than Placebo-PS arm [MD -0.76 ± 0.30; 95%CI, -1.36–0.15] (p < 0.0001). Whereas, adolescent and adult group reported a marginal improvement in nausea and vomiting in Test-AA arm [MD -1.93 ± 0.31; 95%CI, -2.56–1.29] than Placebo-AA [MD -1.40 ± 0.29; 95%CI, -1.99–0.80] (p < 0.0001) (Table 3).
The perception of mental wellbeing and influence on daily life were improved in subjects receiving Alkalihalobacillus clausii 088AE. The perception of mental wellbeing of pediatric group was ‘severe’ to ‘very sever’ in both Test-PS and Placebo-PS at baseline [p = 0.1534 (95%CI, -0.21–1.28)]. However, the mental wellbeing was improved over time after A. clausii 088AE intervention (Test-PS) and significantly normalized at EOT [MD -6.54 ± 0.35; 95%CI, -7.23–5.84] (p < 0.0001). In comparison to Test-PS, mental wellbeing was not improved from baseline to EOT in Placebo-PS [MD -0.73 ± 0.28; 95%CI, -1.29–0.16]. The perception of mental wellbeing was ‘mild’ to ‘moderate’ in adolescent and adult group and it was improved from baseline to EOT in Test-AA arm compared to Placebo-AA arm [MD -0.50 ± 0.06; 95%CI, -0.63–0.36] (Table 3). Similarly, intervention of A. clausii 088AE improved influence on daily life was found improved both in pediatric [Test-PS, MD -5.20 ± 0.06 (95%CI, -5.32–5.07)] and adolescent and adult [Test-AA, MD -1.49 ± 0.12 (95%CI, -1.74–1.23)] group compared to their respective placebo arms (p < 0.0001) (Table 3).
3.2.3. Assessment of IP tolerance, adverse events (AEs) and serious adverse effects (SAEs) and systematic biomarkers
The investigational product (IP), A. clausii 088AE was tolerated well by the study participants. They reported no adverse events (AEs), serious adverse effects (SAEs) or adverse drug reactions (ADRs) during study period.
All vital signs like pulse, respiratory rate, systolic blood pressure, diastolic blood pressure and body temperature were assessed for adolescent and adult group at each visit (Table S1). All vital parameters, except, systolic and diastolic blood pressure were measured in pediatric subjects at each visit (Table S2). No significant differences (p < 0.05) were observed in vital parameters in Test-AA [pmin = 0.075 and pmax = 0.916] and Test-PS [pmin = 0.143 and pmax = 0.917] compared to their respective Placebo arms among all visits and results remained within the normal range.
The complete blood count (hemoglobin, total RBC and leukocyte count, neutrophils, lymphocytes, eosinophils, monocytes, basophils, hematocrit, platelet counts, erythrocyte sedimentation rate) in hematology, liver [serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT)] and renal (creatinine, blood urea nitrogen, albumin, serum sodium and potassium level) function test and serum biochemistry (total cholesterol and random blood sugar) produced no statistically significant difference (p < 0.05) between baseline (visit 1) and EOT (visit 5) in adolescent and adult [Test-AA and Placebo-AA; pmin = 0.063 (95%CI, -0.987–0.027) and pmax = 0.945 (95% CI, -1.411–1.510)] (Table S3) and pediatric [Test-PS and Placebo-PS; pmin = 0.068 (95% CI, -3.304–0.124) and pmax = 1.000 (95% CI, -0.189–0.189)] (Table S4) groups. These clinical safety parameter results were within standard range of reference values.
4. Discussion
Gut microbial dysbiosis is a known cause for many gastrointestinal ailments including antibiotic-associated diarrhea (AAD) [34, 35, 36]. Probiotics offer therapeutic solution to such disorders within gut microbiota. Probiotic strains of Alkalihalobacillus clausii lessen AAD-related clinical symptoms by reprogramming the microbial balance of gut; restore healthy and complex host-microbiota interactions. However, heterogeneous study designs often demonstrated variable probiotic efficacies in various gastrointestinal ailments including AAD. These are majorly due to the variability in host-specific clinical circumstances and strain-specific probiotic attributes.
In this CONSORT-compliant randomized placebo-controlled trial (RCT), A. clausii 088AE is evaluated for its therapeutic effectiveness in antibiotic-associated diarrhea (AAD) and related symptoms in pediatric, adolescent and adult population [CONSORT(2010)_Checklist__088AE-AAD_2021]. A. clausii 088AE, MCC 0538 (genome size 4598557 bp, GC content 44.74%, NCBI Reference Sequence No. CP031128 & MH532550) is a genomically stable and phenotypically safe probiotic bacterium.
The intervention of A. clausii 088AE unveiled significant improvement in total number of unformed stools and time to last unformed stool (TTLUS) in pediatrics (Test-PS) as well as in adolescent and adult (Test-AA) compared to their respective placebo groups (Placebo-PS and Placebo-AA). Children are extremely vulnerable to many diarrheal conditions like, acute, infectious, viral and antibiotic-associated diarrhea [16]. In this study, pediatric group (children below 10 years of age) reported a significant reduction of total number of unformed stools by 16.91% after 24 h and 99.03% after 168 h (EOT) of A. clausii 088AE (4 billion CFU per day) intervention compared to placebo arm (p < 0.0001) (Figure 2). Several other reports mentioned about A. clausii-intervened decrease in number and frequency of stools in children suffered from acute diarrhea. Maugo [37] reported a significant decrease in mean number of diarrhoeal motions on third and fourth day of A. clausii treatment (4 billion CFU per day) in children (<5 y, n = 136) with acute diarrhea with severe dehydration. In another study, a same strength and dosage of A. clausii reduced the frequency and duration of diarrhea in children (<12 y, n = 131) after 24 and 60 h of treatment [38]. A. clausii 088AE (Test-PS) reported maximum frequency of cessation of diarrhea and complete recovery to normal stools (100%) within average shorter duration of 5.04 day. The shorter duration of therapeutic effect was observed within 48 h of A. clausii 088AE treatment. In contrary, 96.7% pediatric patients reported no cessation of diarrhea from Placebo-PS arm even after EOT (168 h). The mean frequency of loose stools (diarrhea) in Test-PS and Placebo-PS was 3.43 ± 0.07 and 3.47 ± 0.11 at baseline [p = 0.0983 (95%CI, -0.01–0.09)]; which was changed to 0.03 ± 0.01 and 2.47 ± 0.04 at EOT [p < 0.0001 (95%CI, 2.42–2.45)], respectively. These significant results clearly indicated that A. clausii 088AE was effective against AAD by decreasing diarrheal frequency and duration and improving consistency of stools among children. Sudha et al. [39] reported about drop of stools frequency and increase in stool consistency among children (<5 y, n = 119) receiving B. clausii suspension after 96 h of treatment (4th day). In an open-label study reported by De Castro et al. [40], B. clausii (2–4 billion CFU per day) resolved diarrhea (52.6 %) with reduced stools frequency within first 3 days of treatment in Filipino children (<6 y, n = 3178) suffered with acute community-acquired diarrhea of viral origin or antibiotic administration. However, the IP, compounded with four different strains of B. clausii, e.g., O/C, SIN, N/R, and T observed low rate of adverse events among study subjects. Shah et al. [41], in an interesting study (n = 225), determined the comparative therapeutic efficacy between B. clausii and Lactic Acid Bacillus (LAB) in children (<5 y). The study showed that a 4 billion CFU/day of B. clausii along with ORS + Zinc supplement showed better efficacy than LAB in terms of faster recovery and achieving normal stool frequency. In fact, other spore-forming Bacillus like B. subtilis, B. coagulans, B. licheniformis, Clostridium butyricum and Lactobacillus spp., Pediococcus spp., Saccharomyces boulardii have clinically evaluated to exert therapeutic efficacies against various pediatric diarrhea including antibiotic-associated diarrhea [2, 13, 42].
Similar to improvement in diarrheal conditions in children, A. clausii 088AE (6 billion CFU per day) treatment reduced total number of unformed stool in adolescent and adult group by 57.74% after 24 h compared to placebo arm (p < 0.0001) (Figure 2). At baseline, both Placebo-AA and Test-AA had mean diarrheal frequency of 4.83 ± 0.29 and 4.73 ± 0.17 [p = 0.1087 (95%CI, -0.22–0.02)]; which was changed to 3.47 ± 0.12 and 0.00 ± 0.00 [p < 0.0001 (95%CI, -3.51–3.42)], respectively. Treatment with A. clausii 088AE reported higher time to last unformed stools, viz., 26.67, 56.67 and 3.33% respectively after 48, 72 and 96h (Figure 3). Results of clinical effectiveness of A. clausii 088AE are in agreement with other strains reported [43]. Earlier, Sudha et al. [43] reported on B. clausii strain UBBC07 which reduced the mean duration and frequency of acute diarrhoea, with improvement in stool consistency in a second phase clinical trial (n = 27). In another study, Plomer et al. [44] reported about therapeutic effectiveness of a commercially available medicinal product Enterogermina®, contains four different strains of poly-antibiotic resistant B. clausii (O/C, SIN, N/R and T) that decreased the incidence (39%) and duration of diarrhea in patients (n = 130) receiving H. pylori eradication therapy. Several other pathogens like C. perfringens, S. aureus, K. oxytoca, Candida spp., Pseudomonas spp. and Salmonella spp. infrequently distort the intestinal microbial ecology. β-lactam, lincomycin, cephalosporin and macrolide class of antibiotics are routinely applied to control such pathogenic overgrowth that often induce adverse effects like infectious or antibiotic-associated diarrhea [45]. Certainly, the compatibility of A. clausii 088AE with antibiotics is a key attribute behind its therapeutic effectiveness against gastrointestinal ailments including antibiotic induced infectious diarrhea.
The antibiotic-associated diarrheal severity is associated with symptoms like bloating, fever, distension, flatulence, odorous flatulence, difficult gas evacuation, stomach rumbling, belching, bad breath, abdominal movement and excessive gas evacuation. In this study, A. clausii 088AE showed significant reduction in severity scores on EOT in pediatric [p < 0.0001 (95%CI, -1.52–1.21)] as well as adolescent and adult [p = 0.0237 (95%CI, 0.03–0.37)] groups compared to their respective placebo arms. Few other studies also evaluated severity parameters during AAD. With a triple-therapy RCT study [rabeprazole 20 mg-bid, clarithromycin 500 mg-bid, amoxicillin 1 g-bid and B. clausii 2 billion CFU-tid (n = 130)] on H. pylori-positive patients, Plomer et al. [44] reported that B. clauii reduced (29%) incidence of diarrhea in first week and remained lower in second week. In secondary efficacy outcomes, B. clausii with triple-therapy reduced the patient-reported frequency/intensity of associated symptoms like vomiting, taste disturbance, loss of appetite, nausea, epigastric pain, flatulence, constipation, or skin rash from first to second week. In a similar triple-therapy, Nista et al. [46] reported on lower incidence of nausea, diarrhea and epigastric pain in Helicobacter pylori-positive patients (n = 120) after 14 days of B. clausii treatment. On a similar note, this study evaluated AAD-associated symptoms on patients receiving broad-spectrum antibiotics treatment and recorded the degree of improvement using VAS assessment scale. Results indicated that A. clausii 088AE was efficacious in reducing symptoms like abdominal pain, bloating and flatulence, vomiting and nausea, perception of mental wellbeing and influence on daily life in both pediatric, adult and adolescent groups compared to placebo (p < 0.05). The improvement [change between two arm means (Test vs. Placebo) on EOT and mean change between baseline to EOT means of two arms] was time-dependent and measured statistically significant on EOT (p < 0.0001) compared to respective placebo. Interestingly, a comparative analysis implied on A. clausii 088AE that might exert better clinical efficacies than other commercially available poly-antibiotic resistant A. clausii strains. This can be due to distinguished gastrointestinal stability characteristics, antipathogenic and immunomodulatory profile of the current strain A. clausii 088AE. Urdaci et al. [47] particularly demonstrated the inhibitory activity of B. clausii OC against S. aureus CIP35053156, E. faecium LMBA27323 and Clostridium difficile 514. A. clausii supports the restoration of intestinal microbiota, aids in improving digestion and absorption of nutrients, antibiotic-induced vitamin deficiency and may facilitate systematic detoxification of residual antibiotics [48, 49]. Diarrheal severity varies on grade of inflammation of mucosal lining. A. clausii may modulate various inflammasome markers like nuclear factor κβ (NF-kβ), mitogen-activated protein kinases (MAPK), nitric oxide synthase (NOS), γ-interferon (IFN-γ) and tumor necrosis factor (TNF) [47, 50]. Increasing evidences support that A. clausii can modulate epithelial barrier and resume gastrointestinal homeostasis with the differential expression of genes related to immune response and inflammation (IL-8, IL-1β and IL-1 receptor ligands, CD79β, FCGR2β, β-defensin, IFN-β), apoptosis and cell growth (caspase 5, BCL2-antagonist), cell differentiation (IL-13), cell-cell signaling (IL6R), cell adhesion (protocadherin 12, vitronectin, type 2-cadherin 6), signal transcription and transduction (MAX dimerization protein 1, NF-κβ binding protein), tight junction proteins (mucin 5AC, occludin and zonula occludens-1) [51, 52, 53].
Safety risk assessment unveiled that the investigational spore preparation of A. clausii 088AE (2 billion CFU) was well tolerated by pediatric (bid) and adolescent and adult (tid) group when administered with antibiotics. A. clausii 088AE was safe in both pediatric (Test-PS and Placebo-PS) and adolescent and adult (Test-AA and Placebo-AA) groups as no statistically significant difference was estimated between baseline (visit 1) and EOT (visit 5) for vital signs, hematology, liver and renal function test and serum biochemistry parameters (p < 0.05). Each safety parameter result was within standard range of reference values. Simultaneously, no study drug related adverse events (AEs), serious adverse effects (SAEs) or adverse drug reactions (ADRs) were noted during the trial, which conclude that investigational product is safe to use. In contrary to the safety of A. clausii 088AE, other strains of A. clausii have been reported for adverse effects of mild to moderate severity in a very small group of study participants [24, 44]. In many clinical studies, spore preparation of A. clausii is administered orally with a dosage of 1–4 × 109 CFU/day to neonates, infants and children and 2–8×109 CFU/day to adults [16, 24, 38, 43, 50, 53, 54, 55].
In summary, 2 × 109 CFU of probiotic Alkalihalobacillus clausii 088AE (basionym Bacillus clausii 088AE) was widely accepted by children (bid) and adolescents and adults (tid) with no treatment-related adverse reactions. Co-administration of A. clausii 088AE with antibiotics can significantly reduce diarrhea and alleviate the symptoms of diarrhea induced by antibiotics, such as abdominal pain, diarrhea (defecation), bloating and flatulence, nausea and vomiting, perception of mental well-being and influence daily life, compared to those placebo arms. Treatment with A. clausii 088AE significantly reduced stool frequency and shortened the duration of unformed stools with "normal" stool consistency, reducing the severity of AAD. No serious adverse reactions further confirmed the treatment safety and therapeutic effects of A. clausii 088AE. A. clausii 088AE can be used as an effective adjunct bacterial therapy for the pathophysiology of diarrhea, especially AAD and acute infectious diarrhea in children, adolescents, and adults.
Declarations
Author contribution statement
Chiranjit Maity: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Anil Kumar Gupta: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by Advanced Enzyme Technologies Ltd., Thane, India.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are grateful and would like to acknowledge to Mr. V.L. Rathi and Mr. M. Kabra at Advanced Enzyme Technologies Ltd. for supporting this study.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bartlett J.G. Antibiotic-associated diarrhea. N. Engl. J. Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.McFarland L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 2006;101:812–822. doi: 10.1111/j.1572-0241.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 3.Hogenauer C. Mechanisms and management of antibiotic-associated diarrhea. Clin. Infect. Dis. 1998;27:702–710. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 4.Lange K. Effects of antibiotics on gut microbiota. Dig. Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 5.Ianiro G. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65:1906–1915. doi: 10.1136/gutjnl-2016-312297. [DOI] [PubMed] [Google Scholar]
- 6.Ng K.M. Recovery of the gut microbiota after antibiotics depends on host diet, community context, and environmental reservoirs. Cell Host Microbe. 2019;26:650–665. doi: 10.1016/j.chom.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu L. The effect of antibiotics on the gut microbiome: a metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genom. 2020;21:263. doi: 10.1186/s12864-020-6665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickson M. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Therap. Adv. Gastroenterol. 2011;4:185–197. doi: 10.1177/1756283X11399115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willing B.P. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011;9:233–243. doi: 10.1038/nrmicro2536. [DOI] [PubMed] [Google Scholar]
- 10.Elvers K.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wischmeyer P.E. Role of the microbiome, probiotics, and 'dysbiosis therapy' in critical illness. Curr. Opin. Crit. Care. 2016;22:347–353. doi: 10.1097/MCC.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutting S.M. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Maity C., Gupta A.K. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur. J. Clin. Pharmacol. 2019;75:21–31. doi: 10.1007/s00228-018-2562-x. [DOI] [PubMed] [Google Scholar]
- 14.Videlock E.J., Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2012;35:1355–1369. doi: 10.1111/j.1365-2036.2012.05104.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins T., Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am. Fam. Physician. 2017;96:170–178. [PubMed] [Google Scholar]
- 16.Ianiro G. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10:1074. doi: 10.3390/nu10081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency (EMA) 2017. List of Nationally Authorised Medicinal Products.https://www.ema.europa.eu/en/medicines/ema_group_types/ema_document-psusa?search_api_views_fulltext=b+clausii [Google Scholar]
- 18.Senesi S. Molecular characterization and identification of Bacillus clausii Strains marketed for use in oral bacteriotherapy. Appl. Environ. Microbiol. 2001;67:834–839. doi: 10.1128/AEM.67.2.834-839.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouhss A. Specific interactions of clausin, a new lantibiotic, with lipid precursors of the bacterial cell wall. Biophys. J. 2009;97:1390–1397. doi: 10.1016/j.bpj.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ripert G. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016;60:3445–3454. doi: 10.1128/AAC.02815-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatri I. Composite genome sequence of Bacillus clausii, a probiotic commercially available as Enterogermina®, and insights into its probiotic properties. BMC Microbiol. 2019;19:307. doi: 10.1186/s12866-019-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paparo L. Protective action of Bacillus clausii probiotic strains in an in vitro model of Rotavirus infection. Sci. Rep. 2020;10:12636. doi: 10.1038/s41598-020-69533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cenci G. Tolerance to challenges miming gastrointestinal transit by spores and vegetative cells of Bacillus clausii. J. Appl. Microbiol. 2006;101:1208–1215. doi: 10.1111/j.1365-2672.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- 24.De Castro J.A. Recommendations for the adjuvant use of the poly-antibiotic–resistant probiotic Bacillus clausii (O/C, SIN, N/R, T) in acute, chronic, and antibiotic-associated diarrhea in children: consensus from Asian experts. Trop. Dis. Travel Med. Vaccines. 2020;6:21. doi: 10.1186/s40794-020-00120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) Koutsoumanis K. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 14: suitability of taxonomic units notified to EFSA until March 2021. EFSA J. 2021;19:6689. doi: 10.2903/j.efsa.2021.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozdogan B. Characterization of a new erm-related macrolide resistance gene present in probiotic strains of Bacillus clausii. Appl. Environ. Microbiol. 2004;70:280–284. doi: 10.1128/AEM.70.1.280-284.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galopin S. A chromosomal chloramphenicol acetyltransferase determinant from a probiotic strain of Bacillus clausii. FEMS Microbiol. Lett. 2009;296:185–189. doi: 10.1111/j.1574-6968.2009.01633.x. [DOI] [PubMed] [Google Scholar]
- 28.Lakshmi S.G. Safety assesment of Bacillus clausii UBBC07, a spore forming probiotic. Toxicol. Rep. 2017;4:62–71. doi: 10.1016/j.toxrep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbrescia A. Antibiotic sensitivity of Bacillus clausii strains in commercial preparation. Curr. Med. Chem. 2014;1:102–110. [Google Scholar]
- 30.Ghelardi E. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J. Appl. Microbiol. 2015;119:552–559. doi: 10.1111/jam.12848. [DOI] [PubMed] [Google Scholar]
- 31.World Medical Association (WMA) 52nd WMA general assembly; Scotland, Edinburgh: 2000. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. [Google Scholar]
- 32.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice [E6(R1)] 1996. [Google Scholar]
- 33.Indian Council of Medical Research (ICMR) 2006. Ethical Guidelines for Biomedical Research on Human Participants. New Delhi.http://www.cns.iisc.ac.in/wordpress/wpcontent/uploads/2017/01/ethical_guidelines.pdf [Google Scholar]
- 34.Young V.B., Schmidt T.M. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J. Clin. Microbiol. 2004;42:1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon M.Y., Yoon S.S. Disruption of the gut ecosystem by antibiotics. Yonsei Med. J. 2018;59:4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao H. Gut microbiota characteristics in mice with antibiotic-associated diarrhea. BMC Microbiol. 2020;20:313. doi: 10.1186/s12866-020-01999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maugo B.M. University of Nairobi; Kenya: 2012. Effectiveness of Bacillus Clausii in Reducing Duration of Illness in Acute Diarrhoea in Children 6-59 Months of Age Admitted with Severe Dehydration.http://erepository.uonbi.ac.ke:8080/xmlui/handle/123456789/8325 Doctoral dissertation. [Google Scholar]
- 38.Keya L. Bacillus clausii as an adjuvant therapy in acute childhood diarrhoea. IOSR J. Dent. Med. Sci. 2015;14:74–76. [Google Scholar]
- 39.Sudha M.R. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: a double blind placebo controlled study. Benef. Microbes. 2019;10:149–154. doi: 10.3920/BM2018.0094. [DOI] [PubMed] [Google Scholar]
- 40.De Castro J.A. Bacillus clausii as adjunctive treatment for acute community-acquired diarrhea among Filipino children: a large-scale, multicenter, open-label study (CODDLE) Trop. Dis. Travel Med. Vaccines. 2019;5:14. doi: 10.1186/s40794-019-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah A.C. Clinical effectiveness of Bacillus clausii and Lactic acid bacillus in acute diarrhea. Indian J. Child Health. 2020;7:109–112. [Google Scholar]
- 42.Horosheva T.V. Efficacy of Bacillus probiotics in prevention of antibiotic-associated diarrhoea: a randomized, double-blind, placebo-controlled clinical trial. JMM Case Rep. 2014;1:1–6. [Google Scholar]
- 43.Sudha M.R. Efficacy of Bacillus clausii strain UBBC-07 in the treatment of patients suffering from acute diarrhoea. Benef. Microbes. 2013;4:211–216. doi: 10.3920/BM2012.0034. [DOI] [PubMed] [Google Scholar]
- 44.Plomer M. Effect of Bacillus clausii capsules in reducing adverse effects associated with Helicobacter pylori eradication therapy: a randomized, double-blind, controlled trial. Infect. Dis. Ther. 2020;9:867–878. doi: 10.1007/s40121-020-00333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Q. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst. Rev. 2019;4 doi: 10.1002/14651858.CD004827.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nista E.C. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment. Pharmacol. Ther. 2004;20:1181–1188. doi: 10.1111/j.1365-2036.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- 47.Urdaci M.C. Bacillus clausii probiotic strains: antimicrobial and immunomodulatory activities. J. Clin. Gastroenterol. 2004;38:S86–S90. doi: 10.1097/01.mcg.0000128925.06662.69. [DOI] [PubMed] [Google Scholar]
- 48.Tramonti A. Molecular mechanism of PdxR – a transcriptional activator involved in the regulation of vitamin B6 biosynthesis in the probiotic bacterium Bacillus clausii. FEBS J. 2015;282:2966–2984. doi: 10.1111/febs.13338. [DOI] [PubMed] [Google Scholar]
- 49.Kong X.X. The biodegradation of cefuroxime, cefotaxime and cefpirome by the synthetic consortium with probiotic Bacillus clausii and investigation of their potential biodegradation pathways. Sci. Total Environ. 2019;651:271–280. doi: 10.1016/j.scitotenv.2018.09.187. [DOI] [PubMed] [Google Scholar]
- 50.Ciprandi G. Cytokines evaluation in nasal lavage of allergic children after Bacillus clausii administration: a pilot study. Pediatr. Allergy Immunol. 2004;15:148–151. doi: 10.1046/j.1399-3038.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 51.Di Caro S. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur. J. Gastroenterol. Hepatol. 2005;17:951–960. doi: 10.1097/00042737-200509000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Lopetuso L.R. Bacillus clausii and gut homeostasis: state of the art and future perspectives. Expet Rev. Gastroenterol. Hepatol. 2016;10:943–948. doi: 10.1080/17474124.2016.1200465. [DOI] [PubMed] [Google Scholar]
- 53.Prato, T. Solid composition containing bacillus-type non-pathogenic bacterial spores. US Patent No. US8039006B2 (active), Sanofi Aventis SpA. Available at https://patents.google.com/patent/US8039006B2/en (2011).
- 54.Tewari V.V. Bacillus clausii for prevention of late-onset sepsis in preterm infants: a randomized controlled trial. J. Trop. Pediatr. 2015;61:377–385. doi: 10.1093/tropej/fmv050. [DOI] [PubMed] [Google Scholar]
- 55.Jayanthi N., Sudha M.R. Bacillus clausii - the probiotic of choice in the treatment of diarrhoea. J. Yoga Phys. Ther. 2015;5:211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.