Abstract
Chemotherapy and radiotherapy have drastically improved cancer survival, but they can result in significant short- and long-term cardiovascular complications, most commonly heart failure from chemotherapy, whilst radiotherapy increases the risk of premature coronary artery disease (CAD), valve, and pericardial diseases. Cardiac computed tomography (CT) with calcium scoring has a role in screening asymptomatic patients for premature CAD, cardiac CT angiography (CTCA) allows the identification of significant CAD, also in the acute settings where concerns exist towards invasive angiography. CTCA integrates the diagnostic work-up and guides surgical/percutaneous management of valvular heart diseases and allows the assessment of pericardial conditions, including detection of effusion and pericardial calcification. It is a widely available and fast imaging modality that allows a one-step evaluation of CAD, myocardial, valvular, and pericardial disease. This review aims to provide an update on its current use and accompanying evidence-base for cardiac CT in the management of cardio-oncology patients.
Keywords: cardio-oncology, cardiac CT, coronary artery disease, valvular disease, pericardial disease
Introduction
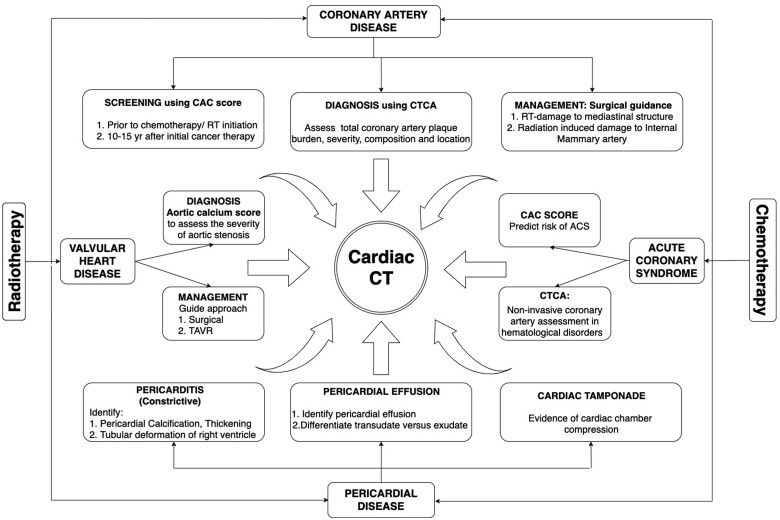
Recent and continued successes in cancer treatment research have improved many cancer patients’ survival and increased the rates of complete remission and cure from disease. However, cancer treatment’s success is often tempered by debilitating and, at times, life-threatening early and late cardiovascular complications, the incidence of which continues to rise in tandem with increasing cancer survivorship.1 Cardiac computed tomography (CT) is a widely available and fast technique to assess coronary artery disease (CAD) and myocardial, valvular, and pericardial disease2 (Figure 1). Recent technical developments allow the performance of cardiac CT at very low radiation doses,3 making it an attractive imaging modality and an essential adjunct to echocardiography, cardiac magnetic resonance, and nuclear imaging in the assessment of cardio-oncology patients.4 Compared to nuclear testing for ischaemia assessment and invasive angiography, on average cardiac CT delivers a lower effective radiation dose of 2–5 vs. 6–21 mSv for single-photon emission CT and 2–20 mSv for invasive angiography.5
Figure 1.
Different roles of Cardiac CT in screening, diagnosis, and management in cancer patients.
Cancer treatment-related cardiotoxicity
The spectrum of cancer treatment-related cardiotoxicity is broad and can be due to both systemic treatments and radiotherapy (Table 1). Systemic cancer therapy can cause several cardiac complications through different pathophysiological mechanisms, ranging from a direct vasospastic effect to endothelial injury and acute arterial thrombosis, or long-term changes in lipid metabolism and consequent premature arteriosclerosis.6 Radiation therapy (RT) is an integral part of cancer treatment in several oncologic conditions, including Hodgkin and non-Hodgkin lymphomas, breast, and lung cancers, as well as oesophageal and gastric neoplasia. Radiotherapy that includes the mediastinal area is associated with an increased risk of premature coronary artery disease (CAD), valve disease, and pericardial disease.7
Table 1.
Cardiac complications of chemotherapy and radiotherapy and their proposed mechanism.
| Chemotherapeutic drug | Mechanism of injury | Cardiotoxic effects |
|---|---|---|
| Anthracycline | Direct myocardial and endothelial injury | Heart failure |
| Trastuzumab | Disrupts HER signaling and activates autophagy; affects cardiomyocyte’s ability to recycle toxic cellular substrates resulting in cardiotoxicity | Heart failure |
| Vascular endothelial growth factor signaling pathway (VEGF) inhibitors |
Inhibit the downstream kinase involved in VEGF receptor signaling removes the positive, stimulating effects on endothelial cell proliferation, which maintains endothelial viability and vascular integrity VEGF inhibition-related impairment in the production of nitric oxide and prostacyclin →leads to vasoconstriction, increased peripheral vascular resistance Increased haematocrit and blood viscosity secondary to overproduction of erythropoietin |
Thromboembolism Hypertension Arterial thrombosis |
| Antimetabolites such as Capecitabine and 5-fluorouracil | Direct toxic effect on the coronary endothelium leading to micro-thrombotic occlusion and coronary vasospasm |
Acute coronary syndromes Sudden cardiac death |
| Angiogenesis inhibitors | Transient elevation in factor VIII and vWF and reduction in soluble thrombomodulin | Thromboembolism |
| Antimicrotubule agents | Chronotropic effect either indirectly through histamine release or directly on the Purkinje system |
Arrhythmia Heart block |
| Small molecule tyrosine kinase | Interaction with myocardial hERG K+channels; impeded electrical flow and delayed impulse conduction |
Conduction abnormalities QTc prolongation |
| Cytarabine | Immune-mediated | Acute pericarditis |
| Radiotherapy | Direct radiation to the mediastinal area |
Premature coronary artery disease (CAD) Valve disease Pericardial disease |
CAD, coronary artery disease; vWF, von Willebrand Factor.
Coronary artery disease
Accelerated atherosclerosis is one of the significant manifestations of radiation-induced cardiotoxicity. The risk of subsequent coronary events is proportional to the radiation dose. A recent study of more than 2500 Hodgkin lymphoma survivors showed a 7.4% increase in the risk of developing CAD per Gray of mean heart irradiation dose.8 RT-induced CAD results from endothelial cell damage, inflammation, and eventually fibrosis,9 with preferential involvement of the ostia and proximal segments of the coronary arteries.10
Coronary calcification is part of atherosclerosis development, and non-contrast CT allows for the quantification of coronary calcium; a calcific lesion is defined by a computed tomographic density of 130 Hounsfield units having an area ≥1 mm.11 The identification of coronary artery calcium (CAC) was initially established with electron beam CT,12 but multidetector CT has now become the modality of choice for CAC evaluation.13 CAC can be quantified by the Agatston score. A higher score corresponds to a higher risk of acute coronary events,14 whilst a negative calcium score confers a very low probability of obstructive CAD and carries a high negative predictive value in asymptomatic patients.15
Whilst gated CT allows the most accurate detection of CAC, current generation multidetector CT scanners equipped with larger numbers of detectors and faster gantry speeds mean that non-gated studies are now able to provide a qualitative CAC assessment that has been shown to correlate well with gated CT studies and cardiovascular outcomes.16 CAC is the best predictor of future events, and screening oncologic patients undergoing scans for cancer staging is beneficial.17 Moreover, assessment of CAC on non-gated CT chest performed for cancer screening staging has been used to identify the risk of developing CAD in relation to radiation exposure.18 Several research studies have also been performed assessing the utility of the CAC score to predict coronary events in patients undergoing RT (Table 2).
Table 2.
Studies investigating the role of CAC and CT coronary angiography in the assessment of CAD in cancer patients.
| Author | No. of patients | Type of cancer | Type of therapy | Mean dose/radiation dose | Risk of developing CAC/CAD |
|---|---|---|---|---|---|
| Coronary artery calcification (CAC) | |||||
| Whitlock et al., J Am Heart Assoc 2015 | 3122 | Different types of cancer vs. no cancer | No therapy | Not applicable | Higher incidence of new CAC in both men and women, even after accounting for CV risk factors |
| Andersen et al., Am J Cardiol 2010 | 47 | Hodgkin lymphoma (HL) | Radiotherapy | 40.6 Gy | Post-irradiation CAC may be a suitable and straightforward method to screen for CAD |
| Chang et al., Front Oncol 2013 | 20 | Breast cancer (left) | Radiotherapy | 4500–5040 cGy | No significant CAC of the coronaries and aorta at 5 or more years following RT |
| Brann et al., Cardio-Oncology 2016 | 83 | All types of cancers | Radiotherapy and/or chemotherapy | Not available | Less prevalent/severe CAC burden after controlling for significant CV risk factors |
| Takx et al., Int J Cardiovasc Imaging 2017 | 333 | Breast cancer | Radiotherapy | 60 Gy | Patients receiving RT had significantly lower the risk of a positive CAC score |
| CT coronary angiography (CTCA) | |||||
| Kupeli et al., J Clin Oncol 2010 | 119 | HL | Radiotherapy and/or chemotherapy | ≥20 Gy | 6.8 times higher in patients receiving mediastinal radiotherapy |
| Girinsky et al., Int J Radiat Oncol Biol Phys 2014 | 179 | HL | Radiotherapy and/or chemotherapy |
Doxorubicin: 330 mg RT: 36 Gy |
CTCA abnormalities were detected in 15% and 34% of patients within the first 5-10 years. |
| Mulrooney et al., Cancer 2014 | 31 | HL | Radiotherapy or multimodality therapy |
RT alone: ≥30 Gy In the Multimodal group RT: 20–29 Gy. Anthracycline: 191 mg/m2 |
CCTA identified CAD in a substantial portion of HL survivors; possible effective screening modality |
| Van Rosendael et al., Radiother Oncol 2017 | 79 | HL and non-HL | Radiotherapy with or without chemotherapy | 36 Gy | Cancer survivors showed a higher presence, greater severity, larger extent, and more proximally located CAD. |
CV, cardiovascular; HL, Hodgkin lymphoma; RT, radiotherapy.
The evidence around the impact of RT on the development of CAC remains heterogeneous. The Multi-Ethnic Study of Atherosclerosis (MESA) study highlighted that a diagnosis of cancer and chemotherapy alone or in conjunction with RT was associated with an increased incidence of developing new CAC compared with the general population, even after accounting for atherosclerotic risk factors.19 Similarly, in a cohort of 47 Hodgkin lymphoma survivors who received a mean cardiac dose of 40.6 Gy, CAC imaging demonstrated a strong association between the severity of CAC and the presence of coronary artery disease verified by angiography.20 On the other hand, Chang et al.21 demonstrated that patients referred to a cardio-oncology clinic had similar or less CAC on a chest CT, even though about a quarter of them received left-sided or whole chest radiation, compared to patients without a prior cancer diagnosis (and hence no RT). Another recent study22 on breast cancer survivors compared subjects with chest CT ≥6 months after RT’s start to subjects who had a CT scan either before or without undergoing RT, demonstrating a significantly lower risk of a positive CAC score in radiotherapy patients.
CT coronary angiography (CTCA) can be used to screen patients undergoing RT as it allows for the identification of CAD and has a prognostic utility in identifying subjects at increased risk for all-cause death,23 while a negative CTCA portends an extremely low risk of cardiac death.24
In patients treated with mediastinal RT and/or cardiotoxic chemotherapy for childhood Hodgkin lymphoma, 16% of patients had an abnormal CTCA result at a mean interval of 8 years after completion of cancer therapy, with an almost seven-fold higher risk in patients receiving mediastinal radiation doses ≥20 Gy compared to patients who did not receive mediastinal RT.25 In asymptomatic Hodgkin lymphoma survivors treated with mediastinal irradiation, CTCA abnormalities were detected in nearly 15% of the patients within the first 5 years after treatment, and up to 34% at 10 years after treatment. Correspondingly, obstructive CAD was confirmed by invasive angiography in 10% of patients, and 6% went on to receive revascularization.26 Van Rosendael et al.27 studied a population of Hodgkin and non-Hodgkin lymphoma survivors treated with mediastinal irradiation with or without chemotherapy with non-irradiated controls matched for age, gender, and risk factors. CTCA demonstrated a higher prevalence, greater severity, and a more considerable extent and more proximally located CAD in irradiated patients than non-irradiated controls. Significantly, more RT-treated patients had two-vessel CAD, or three-vessel/left main CAD, with more severe stenosis and more coronary artery plaques in proximal segments. However, this historic patient population was treated 15–35 years ago, and contemporary RT now utilizes several measures to minimize the mean heart dose.28
The risk of an acute coronary syndrome (ACS) is increased following RT by ∼16% per Gray of mean heart dose. A significant dose–effect relationship was demonstrated between RT volume–dose distribution and coronary events, highlighting the importance of reducing heart exposure to radiation.29 CT has also been used to predict the risk of ACS in relation to the baseline CAC in a study that evaluated the baseline CAC detected after RT in staging non-gated CT chest scans in patients with breast cancer.30 A high CAC score (>100 HU) was associated with acute coronary events, even after correction for confounding factors, such as age, history of ischaemic heart disease, diabetes, BMI ≥30, mean heart dose, hypercholesterolaemia, and hypertension. All these studies showed that the absolute excess risk induced by RT strongly depends on baseline cardiovascular risk factors, and therefore, the optimization of cardiovascular risk factors remains essential.
Current guidelines suggest screening for CAD 5–10 years after chest irradiation as part of surveillance for late cardiovascular toxicity.31 These patients may be minimally symptomatic or even asymptomatic, despite the presence of inducible ischaemia, possibly due to concomitant central and autonomic nervous system damage from radiotherapy.32 Approaches for early detection of CAD include anatomical and/or functional assessment. CTCA allows direct visualization of coronary artery atherosclerosis and is particularly suitable for detecting CAD at an early stage. It has the unique ability to assess the total coronary artery plaque burden, severity, composition, and location of lesions, which have clinical and prognostic relevance.24
The assessment and diagnosis of ACS in cancer patients can be complicated by haematological abnormalities, such as anaemia, thrombocytopenia, and coagulation disorders, which portent a higher risk of invasive angiography complications. Cardiac CT may, therefore, represent a valid alternative for the diagnosis of significant coronary artery disease, even in the settings of ACS.33 Moreover, in the acute settings, when clinical features are overlapping and of difficult interpretation, cardiac CT can be considered for a triple rule-out strategy.34
Where surgical revascularization is warranted, cardiac CT provides a further assessment of potential RT-induced damage to mediastinal structures, such as the pericardium, and atherosclerotic disease to the internal mammary arteries,35 which may complicate the surgery.
Case example
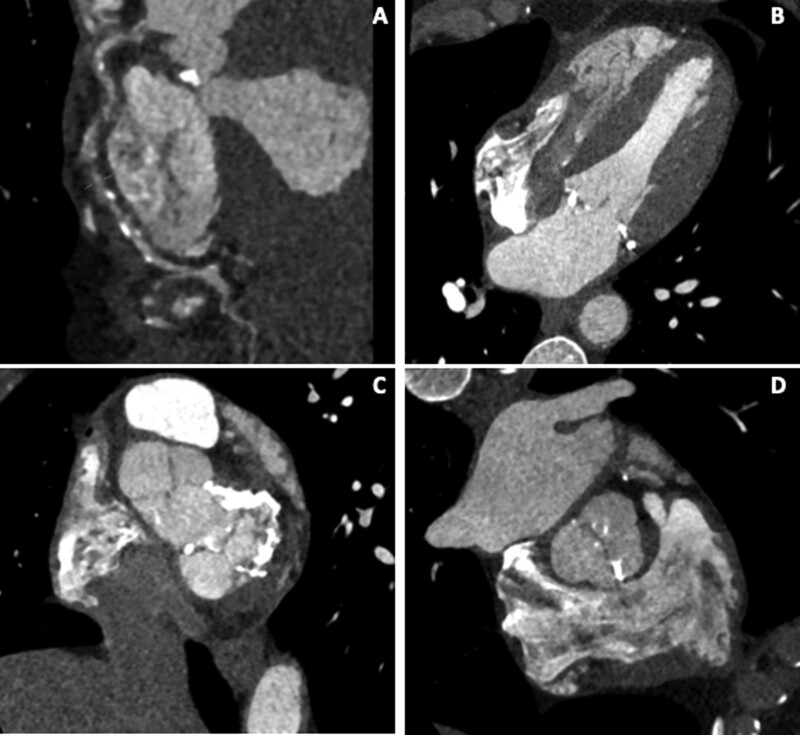
A 49-year-old man with previous Hodgkin lymphoma treated with chemo and radiotherapy aged 26 was investigated due to worsening shortness of breath on exertion on the background of the previous stenting to the left circumflex artery 2 years prior for angina. The transthoracic echocardiogram showed moderate to severe aortic stenosis, moderate mitral stenosis, and septal motion consistent with ventricular interdependence. Cardiac CT showed severe right coronary artery disease (Figure 2A), mild to moderate disease in the left anterior descending artery, and a thickened but non-calcified pericardium around the right ventricular free wall (0.7 cm) (Figure 2B). Significant calcification of the mitral valve and annulus and mild calcification of the aortic valve were also noted (Figure 2C and D). The patient underwent successful mechanical aortic and mitral valve replacement with a 21-mm St. Jude aortic valve and a 27-mm St. Jude mitral valve and bypass grafting.
Figure 2.
Coronary, valvular, and pericardial disease in Hodgkin lymphoma survivor treated with chemo and radiotherapy.
Valvular disease
Valvular heart disease (VHD) can be a complication of chest irradiation, and the risk of VHD is related to the radiation dose.36 Radiation-induced valvular changes have been described in ∼6–15% of patients treated with radiotherapy and usually occur up to 10–20 years after treatment.37 Heidenreich et al.38 found a 34-fold increased risk of developing a valvular disease in patients receiving mediastinal irradiation compared to the Framingham population. The mechanism appears to be direct damage to the valve, its apparatus, and surrounding myocardium, causing fibrotic thickening with valvular retraction leading to stenosis or regurgitation, with the anteriorly positioned mitral and aortic valves being most commonly implicated.39
Multislice cardiac CT is increasingly used as an adjunct for the diagnosis and evaluation of VHD, particularly in the setting of aortic stenosis (AS). Aortic calcium quantification has become a vital component of the diagnosis of low-flow, low-gradient AS with preserved ejection fraction,40 where the aortic calcium score has been shown to correlate with the degree of severity.
CT is also crucial to surgical planning as RT is frequently associated with mediastinal fibrosis and a porcelain aorta, impacting the suitability for aortic cross-clamping and cannulation; conventional surgical treatment of valvular disease is challenging and not always feasible.41 New interventional techniques, such as transcatheter aortic valve implantation (TAVI) may represent a more favourable treatment option, and CT also plays a significant role in the work-up and pre-procedural planning of TAVI procedures.42 Dijos et al.43 have shown TAVI’s efficacy in radiation-induced AS, which appears to have acceptable risk, low mortality, and high clinical effectiveness at mid-term follow-up. Schechter et al.44 corroborated this, who found that patients with cancer and severe AS who underwent aortic valve replacement, predominantly with TAVI, experienced better survival than those who had no valve replacement regardless of cancer type or cancer treatment and supports the use of TAVI in this population.
Fewer data are available instead of the treatment of radiation-induced mitral valve disease with a transcatheter approach45 and, although transcatheter interventions appear a reasonable alternative to surgery, the experience in mitral valve disease is limited and requires further validation.
Case example
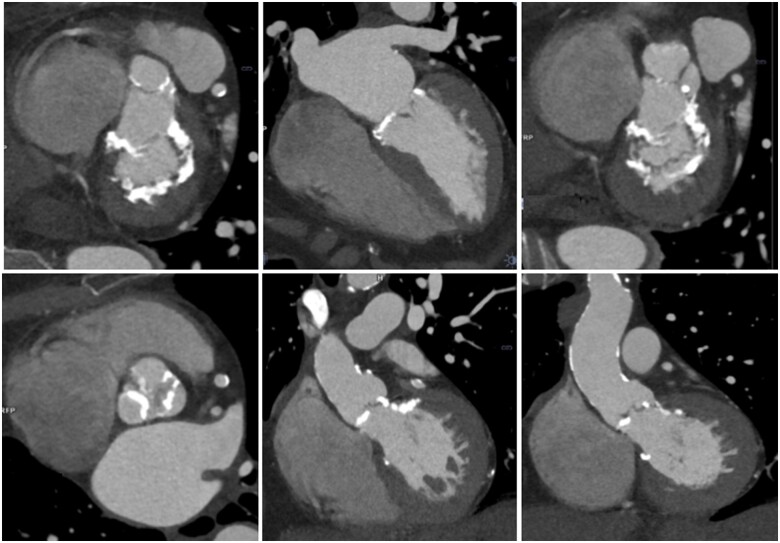
A 70-year-old woman had previously undergone mediastinal surgery and radiotherapy for Hodgkin’s lymphoma at 17, on a more recent background of hypertension and diabetes mellitus. Transthoracic echocardiography showed moderate mitral stenosis with a mean gradient 7 mmHg, severe aortic stenosis with peak velocity 4.4 m/s, mean gradient of 45 mmHg, and aortic valve area by continuity equation of 0.5 cm2. Cardiac CT showed significant calcification of the mitral valve and annulus (Figure 3, top row), aortic valve, and ascending aorta (Figure 3, bottom row). The patient underwent stress echocardiography with cardiopulmonary monitoring achieving an adequate level of exercise with no symptoms and evidence of good capacity on gas exchange. A follow-up at 6 months was therefore planned.
Figure 3.
Radiation-induced valvular heart disease.
Pericardial disease
Mediastinal irradiation can be complicated by acute and chronic radiation pericarditis.46 Its incidence is related to the cumulative radiation dose, and despite contemporary low-dose protocols, the incidence of chronic pericarditis increases by a factor of 1.6 in patients undergoing RT for left breast cancer compared to those receiving RT for right-sided breast cancer.47 More recently, with lower radiation doses and shielding methods (like subcarinal blocking), the incidence of radiation-induced pericarditis has significantly decreased from 20% to 2.5%.48,49
Frequently, in cases of RT-associated pericarditis, especially in the acute setting, there is an associated pericardial effusion, usually an exudate characterized by high protein count. With time, fibrous adhesions may occur with the development of effusive-constrictive pericarditis in cancer survivors treated with RT.50
The pericardium can be easily assessed on cardiac CT images; average pericardium thickness measures 0.7–2 mm.51 CT is superior to other imaging modalities for the identification of pericardial calcification.52 Furthermore, it allows the identification and differentiation of transudates and exudates. A CT attenuation value of 4.7 Hounsfield unit (HU) or higher provides an 80% sensitivity and 87.7% specificity for the prediction of an exudative pericardial effusion,53 and therefore high CT attenuation values of a pericardial effusion of unknown cause may prompt diagnostic drainage to exclude recurrent or new underlying malignancy. Cardiac tamponade may be suspected where considerable fluid accumulation causes compression of the cardiac chambers and right-sided venous congestion. In the same study, a cut-off value of 6.5 HU provided 71.4% sensitivity and 72.3% specificity for identifying cardiac tamponade.53
Constrictive pericarditis can be associated with pericardial calcification, pericardial thickening, narrowing, or tubular deformation of the right ventricle, as well as manifestations of venous congestion on CT.54
Case example
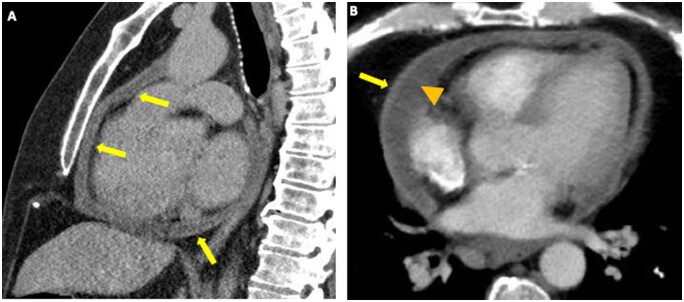
A 59-year-old man with systemic AL amyloidosis received treatment with Lenalidomide, Bortezomib, and Dexamethasone. In the absence of symptoms, routine echocardiography revealed a new moderate-sized pericardial effusion developed over 6 months. Worsening dyspnoea and lower limb oedema developed, prompting hospital admission. A cardiac CT showed a small to moderate pericardial effusion along with thickened pericardium (Figure 4A and B, arrows) with a localized pericardial effusion (Figure 4B, arrowhead). A transthoracic echocardiogram confirmed constrictive physiology, demonstrating ventricular interdependence, respirophasic mitral flow variations, and plethoric and non-compliant inferior vena cava in keeping with the clinical suspicion of haemodynamic compromise. A subsequent right-heart catheterization also confirmed constrictive physiology. A non-steroidal anti-inflammatory agent and Colchicine were initiated with only a limited response, and he subsequently went on to a successful pericardiectomy.
Figure 4.
Chemotherapy-induced pericardial disease.
Future perspectives and conclusion
Improvements in cancer survival in recent decades have resulted in a large and growing population of long-term cancer survivors. Ultimately, a comprehensive assessment of cancer treatment-related cardiac disease in cardio-oncology requires complementary cardiac CT, CMR, and echocardiography; each modality has particular strengths and weaknesses. In this regard, cardiac CT has seen an expanding role in the assessment of coronary artery disease and provides essential information for the management of other complications of cancer therapies, such as valvular and pericardial disease.
Cardiac CT is associated with radiation and decreased temporal resolution compared to cardiac magnetic resonance (CMR) and echocardiography, which do not utilize ionizing radiation.
On the other hand, cardiac CT has a superior spatial resolution.55 In addition to providing an excellent negative predictive value and prognostic information for the assessment of coronary artery disease, retrospectively gated cardiac CT is considered an accurate and reproducible alternative to echocardiography and CMR for the evaluation of biventricular volumes and function when this information cannot be achieved due to suboptimal acoustic windows, claustrophobia, significant artifact from metallic implants limiting the utility of echocardiography and CMR.56 Cardiac CT-derived ventricular volumes and EF have been shown to correlate well with CMR as a gold standard and may be superior to both 2D and 3D echocardiography.57 It is a quicker scan, requires shorter and fewer breath holds and is often better tolerated than CMR. Furthermore, recent development in scanners and acquisition techniques allows achieving high-quality images with lower radiation doses, making the technique more attractive in this population.58
New techniques in cardiac CT continue to be developed. This includes the use of vasodilator stress agents that will enable cardiac CT to assess differences in the myocardial distribution of iodinated contrast, providing information about myocardial perfusion, identification of epicardial and mid-myocardial scar patterns, and estimation of extracellular volume similar to the tissue characterization provided by CMR.59,60 Furthermore, strain imaging to detect pre-clinical cancer treatment-related cardiac disease can also be calculated with cardiac CT via velocity gradients between two points in the myocardium, similar to the information provided by strain imaging on echocardiography.61 Whilst these techniques remain in the pre-clinical phase, validation of the data will eventually seek to broaden the clinical applications of cardiac CT.
Cardiac CT is already an essential adjunct to other cardiac imaging modalities in diagnosing and screening cancer treatment-related cardiac disease; the continued development of other applications promises a diagnostic tool that will offer more outstanding quantitative and qualitative cardiac assessments.
Conflict of interest: none declared.
References
- 1.Chung R, Ghosh AK, Banerjee A. Cardiotoxicity: precision medicine with imprecise definitions. Open Heart 2018;5:e000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celutkiene J, Pudil R, Lopez-Fernandez T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:1504–24. [DOI] [PubMed] [Google Scholar]
- 3.Achenbach S, Marwan M, Ropers D, Schepis T, Pflederer T, Anders K et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340–6. [DOI] [PubMed] [Google Scholar]
- 4.Biersmith MA, Tong MS, Guha A, Simonetti OP, Addison D. Multimodality cardiac imaging in the era of emerging cancer therapies. J Am Heart Assoc 2020;9:e013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MC, Stewart C, Weir NW, Newby DE. Using radiation safely in cardiology: what imagers need to know. Heart 2019;105:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das D, Asher A, Ghosh AK. Cancer and coronary artery disease: common associations, diagnosis and management challenges. Curr Treat Options Oncol 2019;20:46. [DOI] [PubMed] [Google Scholar]
- 7.Rygiel K. Cardiotoxic effects of radiotherapy and strategies to reduce them in patients with breast cancer: an overview. J Can Res Ther 2017;13:186–92. [DOI] [PubMed] [Google Scholar]
- 8.van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CP, Krol AD, Hauptmann M et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol 2016;34:235–43. [DOI] [PubMed] [Google Scholar]
- 9.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-induced heart disease: pathologic abnormalities and putative mechanisms. Front Oncol 2015;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinnasami BR, Schwartz RC, Pink SB, Skotnicki RA. Isolated left main coronary stenosis and mediastinal irradiation. Clin Cardiol 1992;15:459–61. [DOI] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 2006;114:1761–91. [DOI] [PubMed] [Google Scholar]
- 13.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126–33. [DOI] [PubMed] [Google Scholar]
- 14.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med 2004;164:1285–92. [DOI] [PubMed] [Google Scholar]
- 15.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017;11:157–68. [DOI] [PubMed] [Google Scholar]
- 16.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, Narula J et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging 2017;32:W54–66. [DOI] [PubMed] [Google Scholar]
- 18.Ravenel JG, Nance JW. Coronary artery calcification in lung cancer screening. Transl Lung Cancer Res 2018;7:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitlock MC, Yeboah J, Burke GL, Chen H, Klepin HD, Hundley WG. Cancer and its association with the development of coronary artery calcification: an assessment from the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2015;4:e002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen R, Wethal T, Gunther A, Fossa A, Edvardsen T, Fossa SD et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin's lymphoma. Am J Cardiol 2010;105:149–52. [DOI] [PubMed] [Google Scholar]
- 21.Chang M, Suh J, Kirtani V, Dobrescu A, Haas J, Zeldis S et al. Coronary calcium scanning in patients after adjuvant radiation for early breast cancer and ductal carcinoma in situ. Front Oncol 2013;3:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takx RAP, Vliegenthart R, Schoepf UJ, Pilz LR, Schoenberg SO, Morris PB et al. Coronary artery calcium in breast cancer survivors after radiation therapy. Int J Cardiovasc Imaging 2017;33:1425–31. [DOI] [PubMed] [Google Scholar]
- 23.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–70. [DOI] [PubMed] [Google Scholar]
- 24.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 25.Kupeli S, Hazirolan T, Varan A, Akata D, Alehan D, Hayran M et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin's lymphoma. J Clin Oncol 2010;28:1025–30. [DOI] [PubMed] [Google Scholar]
- 26.Girinsky T, M’Kacher R, Lessard N, Koscielny S, Elfassy E, Raoux F et al. Prospective coronary heart disease screening in asymptomatic Hodgkin lymphoma patients using coronary computed tomography angiography: results and risk factor analysis. Int J Radiat Oncol Biol Phys 2014;89:59–66. [DOI] [PubMed] [Google Scholar]
- 27.van Rosendael AR, Daniels LA, Dimitriu-Leen AC, Smit JM, van Rosendael PJ, Schalij MJ et al. Different manifestation of irradiation induced coronary artery disease detected with coronary computed tomography compared with matched non-irradiated controls. Radiother Oncol 2017;125:55–61. [DOI] [PubMed] [Google Scholar]
- 28.Fuller SA, Haybittle JL, Smith RE, Dobbs HJ. Cardiac doses in post-operative breast irradiation. Radiother Oncol 1992;25:19–24. [DOI] [PubMed] [Google Scholar]
- 29.van den Bogaard VA, Ta BD, van der Schaaf A, Bouma AB, Middag AM, Bantema-Joppe EJ et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol 2017;35:1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos CTG, van den Bogaard VAB, Greuter MJW, Vliegenthart R, Schuit E, Langendijk JA et al. Is the coronary artery calcium score associated with acute coronary events in breast cancer patients treated with radiotherapy? Radiother Oncol 2018;126:170–6. [DOI] [PubMed] [Google Scholar]
- 31.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L et al. ; In oration with the European Society of Cardiology Working Groups on Nuclear Cardiology and Cardiac Computed Tomography and Cardiovascular Magnetic Resonance and the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:721–40. [DOI] [PubMed] [Google Scholar]
- 32.van Leeuwen-Segarceanu EM, Bos WJ, Dorresteijn LD, Rensing BJ, der Heyden JA, Vogels OJ et al. Screening Hodgkin lymphoma survivors for radiotherapy induced cardiovascular disease. Cancer Treat Rev 2011;37:391–403. [DOI] [PubMed] [Google Scholar]
- 33.Bittner DO, Mayrhofer T, Puchner SB, Lu MT, Maurovich-Horvat P, Ghemigian K et al. Coronary computed tomography angiography-specific definitions of high-risk plaque features improve detection of acute coronary syndrome. Circ Cardiovasc Imaging 2018;11:e007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burris AC, Boura JA, Raff GL, Chinnaiyan KM. Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC Consortium. JACC Cardiovasc Imaging 2015;8:817–25. [DOI] [PubMed] [Google Scholar]
- 35.Fender EA, Chandrashekar P, Liang JJ, Dhar PR, Sio TT, Stulak JM et al. Coronary artery bypass grafting in patients treated with thoracic radiation: a case-control study. Open Heart 2018;5:e000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol AD et al. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107:djv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wethal T, Lund MB, Edvardsen T, Fossa SD, Pripp AH, Holte H et al. Valvular dysfunction and left ventricular changes in Hodgkin's lymphoma survivors. A longitudinal study. Br J Cancer 2009;101:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol 2003;42:743–9. [DOI] [PubMed] [Google Scholar]
- 39.Carlson RG, Mayfield WR, Normann S, Alexander JA, Radiation-associated valvular disease. Chest 1991;99:538–45. [DOI] [PubMed] [Google Scholar]
- 40.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al. ; ESC Scientific Document Group. ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. 2017; [DOI] [PubMed] [Google Scholar]
- 41.Kamdar AR, Meadows TA, Roselli EE, Gorodeski EZ, Curtin RJ, Sabik JF et al. Multidetector computed tomographic angiography in planning of reoperative cardiothoracic surgery. Ann Thorac Surg 2008;85:1239–45. [DOI] [PubMed] [Google Scholar]
- 42.Hamm CW, Mollmann H, Holzhey D, Beckmann A, Veit C, Figulla HR et al. ; for the GARY-Executive Board. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J 2014;35:1588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dijos M, Reynaud A, Leroux L, Reant P, Cornolle C, Roudaut R et al. Efficacy and follow-up of transcatheter aortic valve implantation in patients with radiation-induced aortic stenosis. Open Heart 2015;2:e000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schechter M, Balanescu DV, Donisan T, Dayah TJ, Kar B, Gregoric I et al. An update on the management and outcomes of cancer patients with severe aortic stenosis. Catheter Cardiovasc Interv 2019;94:438–45. [DOI] [PubMed] [Google Scholar]
- 45.Paven E, Cimadevilla C, Urena M, Dilly MP, Nataf P, Raffoul R et al. Management of radiation-induced valvular heart disease due to Hodgkin's Lymphoma in the modern era. Eurointervention 2018;13:e1771–3. [DOI] [PubMed] [Google Scholar]
- 46.Ghosh AK, Crake T, Manisty C, Westwood M. Pericardial disease in cancer patients. Curr Treat Options Cardiovasc Med 2018;20:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167–75. [DOI] [PubMed] [Google Scholar]
- 48.Carmel RJ, Kaplan HS. Mantle irradiation in Hodgkin's disease. An analysis of technique, tumor eradication, and complications. Cancer 1976;37:2813–25. [DOI] [PubMed] [Google Scholar]
- 49.Mauch PM, Weinstein H, Botnick L, Belli J, Cassady JR. An evaluation of long-term survival and treatment complications in children with Hodgkin's disease. Cancer 1983;51:925–32. [DOI] [PubMed] [Google Scholar]
- 50.Sagrista-Sauleda J, Angel J, Sanchez A, Permanyer-Miralda G, Soler-Soler J. Effusive-constrictive pericarditis. N Engl J Med 2004;350:469–75. [DOI] [PubMed] [Google Scholar]
- 51.O'Leary SM, Williams PL, Williams MP, Edwards AJ, Roobottom CA, Morgan-Hughes GJ et al. Imaging the pericardium: appearances on ECG-gated 64-detector row cardiac computed tomography. Br J Radiol 2010;83:194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogaert J, Francone M. Pericardial disease: value of CT and MR imaging. Radiology 2013;267:340–56. [DOI] [PubMed] [Google Scholar]
- 53.Cetin MS, Ozcan Cetin EH, Ozdemir M, Topaloglu S, Aras D, Temizhan A et al. Effectiveness of computed tomography attenuation values in characterization of pericardial effusion. Anatol J Cardiol 2017;17:322–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alter P, Figiel JH, Rupp TP, Bachmann GF, Maisch B, Rominger MB. MR, CT, and PET imaging in pericardial disease. Heart Fail Rev 2013;18:289–306. [DOI] [PubMed] [Google Scholar]
- 55.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the Prospective Multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) Trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 56.Aziz W, Claridge S, Ntalas I, Gould J, de Vecchi A, Razeghi O et al. Emerging role of cardiac computed tomography in heart failure. ESC Heart Fail 2019;6:909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greupner J, Zimmermann E, Grohmann A, Dübel H-P, Althoff T, Borges AC et al. Head-to-head comparison of left ventricular function assessment with 64-row computed tomography, biplane left cineventriculography, and both 2- and 3-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging as the reference standard. J Am Coll Cardiol 2012;59:1897–907. [DOI] [PubMed] [Google Scholar]
- 58.Ramsey BC, Fentanes E, Choi AD, Branch KR, Thomas DM. Myocardial assessment with cardiac CT: ischemic heart disease and beyond. Curr Cardiovasc Imaging Rep 2018;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nacif MS, Kawel N, Lee JJ, Chen X, Yao J, Zavodni A et al. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology 2012;264:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H-J, Im DJ, Youn J-C, Chang S, Suh YJ, Hong YJ et al. Myocardial extracellular volume fraction with dual-energy equilibrium contrast-enhanced cardiac CT in nonischemic cardiomyopathy: a prospective comparison with cardiac MR imaging. Radiology 2016;280:49–57. [DOI] [PubMed] [Google Scholar]
- 61.Buss SJ, Schulz F, Mereles D, Hosch W, Galuschky C, Schummers G et al. Quantitative analysis of left ventricular strain using cardiac computed tomography. Eur J Radiol 2014;83:e123–30. [DOI] [PubMed] [Google Scholar]