Key Points
Question
Which hospital-led interventions are associated with reducing length of stay (LOS) for high-risk populations?
Findings
In this systematic review including 19 systematic reviews, 8 strategies for reducing LOS in high-risk populations were identified: discharge planning, geriatric assessment, medication management, clinical pathways, interdisciplinary or multidisciplinary care, case management, hospitalist services, and telehealth. Interventions were most frequently designed for older patients or patients with heart failure and were often associated with inconsistent outcomes in LOS, readmissions, and mortality across populations.
Meaning
This systematic review found that across all high-risk populations, there are inconsistent results on the effectiveness associated with interventions to reduce LOS, such as discharge planning, which are often widely used by health systems.
This systematic review identifies and analyzes evidence regarding potential systems-led strategies to reduce hospital length of stay for high-risk populations.
Abstract
Importance
Many strategies to reduce hospital length of stay (LOS) have been implemented, but few studies have evaluated hospital-led interventions focused on high-risk populations. The Agency for Healthcare Research and Quality (AHRQ) Learning Health System panel commissioned this study to further evaluate system-level interventions for LOS reduction.
Objective
To identify and synthesize evidence regarding potential systems-level strategies to reduce LOS for patients at high risk for prolonged LOS.
Evidence Review
Multiple databases, including MEDLINE and Embase, were searched for English-language systematic reviews from January 1, 2010, through September 30, 2020, with updated searches through January 19, 2021. The scope of the protocol was determined with input from AHRQ Key Informants. Systematic reviews were included if they reported on hospital-led interventions intended to decrease LOS for high-risk populations, defined as those with high-risk medical conditions or socioeconomically vulnerable populations (eg, patients with high levels of socioeconomic risk, who are medically uninsured or underinsured, with limited English proficiency, or who are hospitalized at a safety-net, tertiary, or quaternary care institution). Exclusion criteria included interventions that were conducted outside of the hospital setting, including community health programs. Data extraction was conducted independently, with extraction of strength of evidence (SOE) ratings provided by systematic reviews; if unavailable, SOE was assessed using the AHRQ Evidence-Based Practice Center methods guide.
Findings
Our searches yielded 4432 potential studies. We included 19 systematic reviews reported in 20 articles. The reviews described 8 strategies for reducing LOS in high-risk populations: discharge planning, geriatric assessment, medication management, clinical pathways, interdisciplinary or multidisciplinary care, case management, hospitalist services, and telehealth. Interventions were most frequently designed for older patients, often those who were frail (9 studies), or patients with heart failure. There were notable evidence gaps, as there were no systematic reviews studying interventions for patients with socioeconomic risk. For patients with medically complex conditions, discharge planning, medication management, and interdisciplinary care teams were associated with inconsistent outcomes (LOS, readmissions, mortality) across populations. For patients with heart failure, clinical pathways and case management were associated with reduced length of stay (clinical pathways: mean difference reduction, 1.89 [95% CI, 1.33 to 2.44] days; case management: mean difference reduction, 1.28 [95% CI, 0.52 to 2.04] days).
Conclusions and Relevance
This systematic review found inconsistent results across all high-risk populations on the effectiveness associated with interventions, such as discharge planning, that are often widely used by health systems. This systematic review highlights important evidence gaps, such as the lack of existing systematic reviews focused on patients with socioeconomic risk factors, and the need for further research.
Introduction
Hospital length of stay (LOS) is a quality metric health systems use as a proxy of efficient hospital management. Reduction in LOS improves bed turnover, allowing hospitals to match demand with capacity for elective and emergent admissions, intensive care unit (ICU) care, and interhospital transfers.1,2 When demand exceeds capacity, emergency department crowding, ICU strain, and ward strain occur, all of which are associated with worse outcomes, including mortality.3,4,5,6,7,8,9 Classifying patient hospital stays into diagnosis-related groups with fixed reimbursements further incentivizes hospitals to improve LOS to maintain operating margins.10 However, important potential trade-offs between LOS reduction and postdischarge adverse outcomes (eg, readmissions, mortality) exist. Furthermore, prolonged LOS may be associated with negative patient and staff experience, as well as increased inpatient complications (eg, hospital acquired infections, falls), many of which may be preventable.11 Therefore, many hospitals aim to implement systems-level approaches to provide optimal care and a safe discharge while avoiding prolonged hospital stays.
Many strategies to reduce hospital LOS have been developed, including some targeting different aspects of patient management, such as clinical care (eg, enhanced recovery programs and early mobility programs12,13,14,15), and others focusing on staffing models16,17 and logistics of care coordination.18,19,20,21,22 Although evidence is limited, some interventions, such as enhanced recovery after surgery programs, have focused on elective admissions and have been reported as consistently associated with improved LOS for planned, scheduled surgeries.23 In contrast to elective admissions, much less is known about the effectiveness of interventions in unplanned hospitalizations, especially among populations at-risk for poor outcomes. This includes patients who are medically complex, such as older adults, patients with heart failure, or patients with other chronic comorbidities who are at increased risk for prolonged LOS. Similarly, such interventions may be less generalizable to patients with socioeconomic risk factors more likely to be affected by health care disparities and at increased risk for adverse events related to hospitalization24 and unnecessary delays in discharge.25,26,27 Furthermore, many hospitals within Learning Health Systems, including safety-net hospitals, serve populations at disproportionately high risk for prolonged LOS and often struggle to maintain operating margins as a result. Long-term financial viability of these hospitals is crucial to ensuring access to care for underserved populations.5 Therefore, the Agency for Healthcare Research and Quality (AHRQ) Learning Health System Panel identified a need to identify broad system-level interventions to reduce LOS among patients with high risk of prolonged LOS.28 To address this need, we performed an overview of systematic reviews to identify interventions intended to reduce LOS for high-risk populations and identify evidence gaps. In this systematic review, we summarize existing evidence, with a specific focus on hospitalized older adults and patients with heart failure.
Methods
This systematic review is based on a technical brief performed by the ECRI-Penn Medicine Evidence-based Practice Center for the AHRQ. The protocol and final report are available elsewhere.29 We interviewed 7 key informants with expertise in health systems, health care delivery processes, high-risk populations, care model transformation, and hospital quality and safety and incorporated input to refine scope and aims. This report follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for systematic reviews.
Data Sources and Search Strategy
Medical librarians searched MEDLINE, PubMed, Embase, CINAHL, the Cochrane Library, and gray literature sources, including from government agencies and relevant stakeholder organizations, from January 1, 2010, through September 30, 2020, with updated searches through January 19, 2021. Search terms are provided in eTable 1 in the Supplement. The full search strategy is available in the full report, published elsewhere.29
Study Selection
Abstracts and full-text articles were screened in duplicate by a methodologist and a clinician using DistillerSR (Evidence Partners) using predetermined criteria (Table 1). Based on input from key informants, we focused on 2 populations at high-risk for prolonged LOS: patients with socioeconomic risk factors (eg, underinsured or uninsured) and patients who are medically complex (eg, with frailty or multimorbidity) (Table 1). Systematic reviews were included if they examined acute unplanned hospitalizations in the United States, evaluated hospital-initiated interventions (such as case management or multidisciplinary team models), aimed to reduce hospital LOS, reported on LOS as a primary outcome, and were published in English. We also required systematic reviews to provide explicit search criteria and inclusion and exclusion criteria and assess risk of bias (ROB) for included studies. We excluded systematic reviews focused on admission for nonemergent elective procedures, utilizing interventions not initiated within hospital settings (eg, community-based programs or ambulatory care visits after discharge), or including more than 50% of studies from outside of the United States.
Table 1. Populations, Interventions, Comparators, Outcomes, Timing, Settings, and Inclusion and Exclusion Criteria.
| Category | Criteria |
|---|---|
| Population |
|
| Interventions |
|
| Comparators | Include: Usual care, any comparison, or other active intervention |
| Outcomes |
|
| Timing | Include: All |
| Setting |
|
Abbreviation: LOS, length of stay.
Data Extraction and Quality Assessment
A standardized data extraction form was used to collect patient population, hospital and intervention characteristics, comparators, and outcomes assessed. Included systematic reviews were required to meet several thresholds for quality, including explicit search strategies, inclusion and exclusion criteria, and ROB assessment; therefore, independent quality assessment of included reviews was not performed. LOS was extracted as the primary outcome, with associated balancing measures as secondary outcomes, including readmission and mortality rates. For each outcome, when available, we extracted strength of evidence (SOE) ratings provided by systematic reviews. If not provided, we assessed SOE using AHRQ Evidence-based Practice Center guidance.30
Data Synthesis
We narratively summarized evidence for heart failure and older populations and developed an evidence map to summarize the direction of association, volume, and quality of existing data for interventions across key outcomes (ie, LOS, readmission, and mortality). We also highlighted important knowledge gaps in the evidence base.
Results
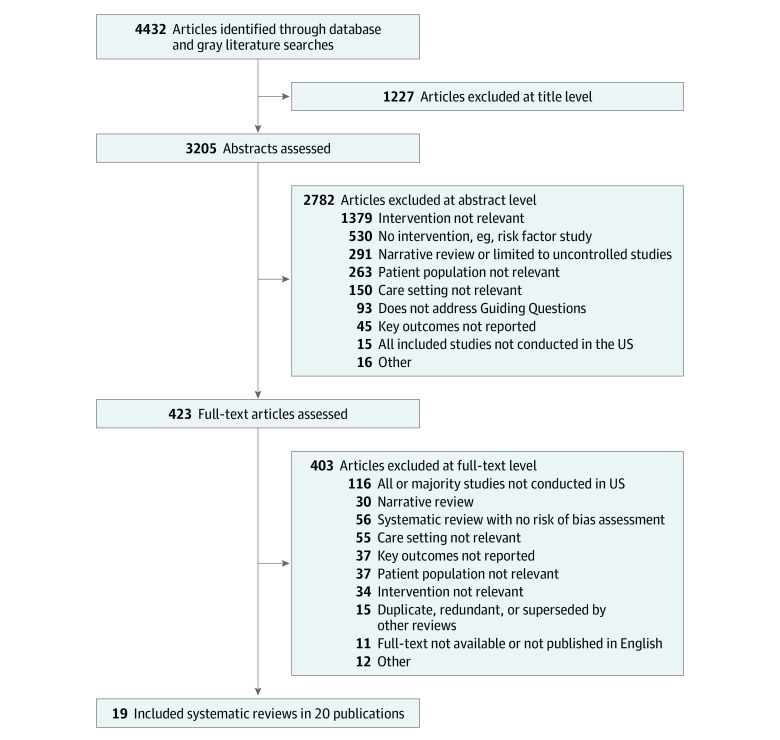
Of 4432 potentially relevant studies, we included 19 systematic reviews in 20 publications, 1 of which was identified in our gray literature search.20,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 The most common reason for exclusion was that the systematic review did not describe a hospital-led intervention. Study selection is shown in the Figure. Characteristics of included systematic reviews are shown in eTable 2 in the Supplement.
Figure. Diagram of Included Systematic Reviews.
Of 19 systematic reviews analyzed, 10 included a mix of study designs (eg, randomized clinical trials [RCTs], observational cohort studies),31,32,33,34,35,36,37,38,39,40,41 8 included only RCTs,20,42,43,44,45,46,47,48 and 1 included only retrospective cohort studies.49
Included systematic reviews addressed 8 types of systems-level hospital or health system interventions: discharge planning,20,37,38,43,46 geriatric assessment or consultation,33,39,44,47,49 medication management,31,34,45 clinical pathways,36,42 interdisciplinary or multidisciplinary care,40,48 case management,35 hospitalist services,41 and telehealth.32 There was an overlap of 10 individual studies across 8 systematic reviews evaluating discharge planning, geriatric assessment, or multidisciplinary care.20,37,38,39,42,43,44,46,49 The description and distribution of these categories is shown in Table 2, and individual study overlap is presented in eTable 2 in the Supplement.
Table 2. Description of Interventions to Reduce Length of Stay.
| Intervention | Included systematic reviews | Description of intervention |
|---|---|---|
| Geriatric assessment | Bakker et al,33 2011 | Multidisciplinary team with geriatrics consultation at various stages of patient care, including comanagement with surgical teams for preoperative optimization |
| Patel et al,39 2020 | ||
| Ellis et al,44 2017 | ||
| Van Craen et al,47 2010 | ||
| Eagles et al,49 2020 | ||
| Discharge planning | Zhu et al,20 2015 | Included an assessment of suitability for discharge, planning, implementation, and/or post-discharge follow-up. Follow-up care involved a phone call within 24 h of discharge, scheduling outpatient visits, home visits, and/or on-call services. |
| Mabire et al,37 2018 | ||
| Mabire et al,38 2016 | ||
| Bryant-Lukosius et al,43 2015 | ||
| Goncalves-Bradley et al,46 2010 | ||
| Medication management | Austin et al,31 2020 | Often targeted for high-risk medications, such as anticoagulants or antibiotics, with known adverse effects. Interventions included computerized entry systems, clinical decision support tools, dashboard utilization, pharmacist-led anticoagulation consultation services, and systematic education and feedback programs for patients. |
| Gillaizeau et al,34 2013 | ||
| Frazer et al,45 2019 | ||
| Clinical pathways | Kul et al,36 2012 | Included studies on multicomponent interventions, such as quality-improvement initiatives, including inpatient clinical pathway for heart failure management, standardized admission orders, education for staff and patients, or telephone surveillance after discharge |
| Agarwal et al,42 2019 | ||
| Multidisciplinary care | Pannick et al,40 2015 | Team coordination with inclusion of specialists during rounds, communication strategies, and task delegation for implementation of consultative recommendations |
| Zhang et al,48 2013 | ||
| Case management | Huntley et al,35 2016 | Directed by nurse case managers and included various strategies, such as medication review, family conferencing, education, home environment assessment, or referral to other services |
| Hospitalist service | White et al,41 2011 | Utilization of hospitalist physician staffing evaluated based on assessments of physician performance on quality of care |
| Telehealth | Baratloo et al,32 2018 | Hospital-based telehealth services for patients with stroke, linking hospital-based clinicians to outside clinical care teams |
The clinical conditions reported included patients with heart failure and other patient groups, such as patients with acute stroke, pregnant women at high risk, and infants. Some systematic reviews synthesized data quantitatively for the LOS outcome, while others presented either a narrative synthesis or data from individual studies on reported LOS. Included reviews evaluated older adults and those with chronic conditions and quantitatively synthesized LOS and other outcomes (Table 2). Results from included systematic reviews are shown in eTable 1 in the Supplement, and those performing meta-analysis for LOS are presented in eTable 3 in the Supplement.
Systematic reviews reported limited information on the setting of included studies. There were 13 reviews that described interventions conducted in multiple types of hospitals, including academic medical centers, community hospitals, and, less frequently, Department of Veterans Affairs hospitals. Only 1 systematic review focused on trauma centers, and 6 reviews did not report hospital type. Only 5 reviews reported whether all included studies were conducted in urban, suburban, or rural settings: 3 included both urban and rural hospitals, 1 was limited to urban settings, and 1 included only rural hospitals. Few reviews reported hospital bed size or affiliation with a health system.
Evidence Map
Table 3 summarizes quantitative findings from systematic reviews for the outcomes of LOS, readmissions, and mortality in an evidence map. The map provides an overview of direction of association, SOE, size and study designs contributing to the evidence base, and patient populations addressed. For most interventions, evidence for LOS reduction was inconsistent.
Table 3. Evidence Map of Systematic Reviews With Quantitative Synthesisa.
| Patient population | Intervention | Systematic review(s) | Study designs (No. of included patients)b | Outcome (strength of evidence)c | ||
|---|---|---|---|---|---|---|
| LOS | Readmissions | Mortality | ||||
| Older adults | Discharge planning | Mabire et al,37 2018 | 4 RCTs, 1 pre-post study, 1 cohort (2370) | ↑ (L) | ↓ (M) | NR |
| Mabire et al,38 2016 | ||||||
| Goncalves-Bradley et al,46 2016 | 12 RCTs (2193) | ↓ (M) | ↓ (M) | NR | ||
| Bryant-Lukosius et al,43 2015 | 3 RCTs (396) | ↔ (L) | NR | ↔ (L) | ||
| Geriatric assessment | Eagles et al,49 2020 | 2 retrospective cohort studies (5414) | ↓ (M) | NR | ↓ (M) | |
| Van Craen et al,47 2010 | 7 RCTs (4759) | ↔ (H) | ↔ (M) | ↔ (H) | ||
| Ellis et al,44 2017 | 11 RCTs (4346) | NR | NR | ↔ (H) | ||
| Patients with heart failure | Discharge planning | Bryant-Lukosius et al,43 2015 | 2 RCTs (495) | NR | ↔ (L) | ↔ (L) |
| Clinical pathways | Kul et al,36 2012 | 1 RCT and 4 observational studies (2095) | ↓ (L) | ↓ (M) | ↓ (M) | |
| Case management | Huntley et al,35 2016 | 8 RCTs and 1 observational study (1765) | ↓ (M) | ↓ (M) | NR | |
| Patients with chronic conditions | Discharge planning | Zhu et al,20 2015 | 5 RCTs (1912) | ↔ (M) | ↓ (M) | ↓ (H) |
| Medication management | Gillaizeau et al,34 2013 | 8 RCTs and 1 observational study (n = 18 507) | ↔ (L) | NR | NR | |
| Interdisciplinary care | Pannick et al,40 2015 | 2 RCTs, 2 non-RCT cluster studies, 2 before/after studies (NR) | ↔ (M) | ↑ (L) | ↔ (M) | |
| Infants | Discharge planning | Bryant-Lukosius et al,43 2015 | 2 RCTs (495) | NR | ↔ (L) | NR |
| Pregnant women | Discharge planning | Bryant-Lukosius et al,43 2015 | 2 RCTs (15) | ↓ (M) | NR | NR |
| Patients with stroke | Telehealth | Baratloo et al,32 2018 | 1 RCT, 2 prospective controlled studies, 6 retrospective controlled studies (2850) | ↓ (M) | NR | ↔ (M) |
Abbreviations: L, low or very low; H, high; LOS, length of stay; M, moderate; NR, not reported; RCT, randomized clinical trial.
All reviews reported LOS data. NR in the LOS column indicates that the authors reported a narrative synthesis or results from individual trials instead of a quantitative synthesis. Narrative syntheses are not included here.
Number of patients included in quantitative synthesis for LOS. If LOS was not quantitatively synthesized, the number of patients for the outcome depicted with quantitative synthesis is reported.
Direction of association is indicated by arrows, with ↑ indicating increase; ↓, decrease; and ↔, inconclusive.
Evidence Gaps
We found notable evidence gaps for high-risk populations. No systematic reviews focused on patients with socioeconomic risk factors (eg, housing instability, social isolation, no medical insurance or underinsuance, social mobility, lack of social network, lack of social support, limited access to health care services or social services, rural settings), limited English proficiency, or hospitalization at a safety-net or tertiary or quaternary care hospital. Furthermore, although systematic reviews did address certain populations of patients who were medically complex (including patients with chronic diseases, infants with high risk, and pregnant women with high risk), no systematic reviews assessed interventions for other key medically complex populations, such as patients with substance use disorder, comorbid psychiatric or behavioral health problems, or common chronic diseases, such as chronic kidney disease and chronic obstructive pulmonary disease.
Older Patients
There were 9 systematic reviews (in 10 publications) that included older patients,33,37,38,39,43,44,46,47,48,49 with 4 systematic reviews focused on elderly patients who were frail. Of these, 5 systematic reviews performed quantitative synthesis of LOS and assessed discharge planning interventions (3 systematic reviews) and geriatric assessment or consultation (2 systematic reviews).
Discharge planning interventions (eg, early assessment of discharge suitability, establishing discharge plan and follow-up) had mixed results among older patients (Table 2). One systematic review by Mabire et al37,38 included 4 RCTs, 1 pre-post study, and 1 cohort study (including 2370 patients) assessing nursing-led discharge planning interventions, and found the intervention was associated with increased hospital LOS (weighted mean difference, 0.29 [95% CI, 0.24 to 0.35] days; low SOE). Although LOS increased, discharge planning was associated with improved readmission rates (odds ratio [OR], 0.57 [95% CI, 0.40 to 0.81]; P = .01), although a subanalysis specific to transitional care did not find an association with improvement (OR, 0.70 [95% CI, 0.38 to 1.27]). Another review by Goncalves-Bradley et al46 examined transitional care programs run by clinical nurse specialists across 12 RCTs (including 2193 patients) and found a decrease in LOS (mean difference, −0.73 [95% CI, −1.33 to −0.12] days; moderate SOE). A subgroup analysis focused on older surgical patients, including 2 RCTs (including 184 patients), found no change in LOS (mean difference, −0.06 [95% CI, −1.23 to 1.11] days). The third review by Bryant-Lukosius et al43 also evaluated a nurse-led intervention in 3 RCTs (including 396 patients) and found no difference in LOS (mean difference, −0.69 [95% CI, −1.95 to 0.56] days; low SOE).
There were 2 systematic reviews that quantitatively assessed outcomes associated with geriatric assessment or consultation interventions and also found mixed LOS outcomes. Eagles et al49 evaluated geriatric assessment performed by a geriatrician for reducing LOS for patients aged 65 years or older admitted to a trauma center. Based on 2 retrospective cohort studies (including 5414 patients), Eagles et al49 found that compared with standard care, geriatric assessment was associated with reduced LOS (mean difference, −1.11 [95% CI, −1.43 to −0.79] days; moderate SOE).49 Pre-post studies (including 7408 patients) from the systematic review by Eagles et al49 did not show improvement in mortality (unadjusted OR, 0.91 [95% CI, 0.70 to 1.18]), but cohort data comparing geriatric trauma consultation with standard of care (including 482 patients) did show improvement associated with consultation in inpatient mortality (unadjusted OR, 0.24 [95% CI, 0.12 to 0.52]; I2 = 0%; moderate SOE). In contrast, another review by Van Craen et al47 included 7 RCTs (including 4759 patients) and found no difference in LOS (mean reduction, 0.07 [95% CI, −0.11 to 0.26] days) or mortality at 12 months (relative risk [RR], 0.97 [95% CI, 0.88 to 1.08]; high SOE). Notably, the review by Van Craen et al47 focused on elderly patients who were frail, which may have represented a higher-risk population than other studies. Van Craen et al47 noted that the components and implementation of the intervention (geriatric evaluation and treatment unit) across included studies was heterogeneous.
Heart Failure
We found 4 systematic reviews assessing 3 interventions (ie, discharge planning, clinical pathways, and case management) in patients with heart failure. However, only 2 of 4 systematic reviews meta-analyzed outcomes for LOS. One systematic review did not provide quantitative synthesis for LOS but found no difference in readmission (defined as unplanned readmission at 90 days) or mortality, although the intervention was associated with improved patient satisfaction.43
One systematic review performed a quantitative assessment of LOS for clinical pathway interventions. Kul et al36 included 1 RCT and 4 observational studies (including 2095 patients) and found that clinical pathway utilization was associated with reduced LOS (mean difference reduction, 1.89 [95% CI, 1.33 to 2.44] days; I2 = 42%; low SOE). There was also a reduction in readmission rate (RR, 0.81 [95% CI, 0.66 to 0.99]; I2 = 16%; moderate SOE) and inpatient mortality (RR, 0.45 [95% CI, 0.21 to 0.94]; I2 = 73%; low SOE).
A single systematic review by Huntley et al35 examined case management interventions. Huntley et al35 included 8 RCTs and 1 observational study (including 1765 patients) and found an association with decreased LOS (mean difference reduction, 1.28 [95% CI, 0.52 to 2.04] days; I2 = 63%; moderate SOE). Additionally, a sensitivity analysis excluding studies at high ROB found a mean reduction of 1.76 (95% CI, 1.23-2.29) days (I2 = 14%). Regarding readmission, Huntley et al35 found that case management interventions studied in 12 RCTs and 1 observational study (including 3346 patients) were associated with reduced readmission rates (RR, 0.74 [95% CI, 0.60 to 0.92]; moderate SOE) with increased heterogeneity (I2 = 69%), with similar results when high ROB studies were excluded (RR, 0.77 [95% CI, 0.61 to 0.96]; I2 = 68%).
A review by Bryant-Lukosius et al43 evaluated discharge planning interventions across multiple patient populations aged in their early to mid-70s, including patients with heart failure (2 RCTs with 495 patients). Although the systematic review did not perform quantitative synthesis of LOS for patients with heart failure, Bryant-Lukosius et al43 found no difference in readmission rates, defined as unplanned readmission at 90 days (RR, 0.81 [95% CI, 0.57 to 1.13]), and no difference in mortality (including 345 patients; RR, 0.76 [95% CI, 0.41 to 1.42]). However, the intervention was associated with improved patient satisfaction (including 403 patients; mean difference, 6.09 [95% CI, 3.55 to 8.63]; P < .001; moderate SOE).
Discussion
This systematic review identified evidence for 8 hospital-based interventions targeting high-risk patient populations: discharge planning,20,37,38,43,46 geriatric assessment or consultation,33,39,44,47,49 medication management,31,34,45 clinical pathways,36,42 interdisciplinary or multidisciplinary care,40,48 case management,35 hospitalist services,41 and telehealth.32 As shown Table 3, aside from interventions for patients with heart failure, interventions were not consistently associated with reduced hospital LOS for medically complex populations. It is important to note that patients who are medically complex do not exist in silos, as older patients may also have chronic conditions, such as heart failure; therefore, identifying interventions that could reduce LOS across populations is important.
For patients with heart failure, clinical pathways and case management interventions were both associated with reduced LOS, readmission rates, and mortality.36 These findings are notable, since prior research has shown that interventions may improve LOS but worsen readmission rates or mortality.50 However, it is important to recognize the significant heterogeneity across studies in the systematic review by Kul et al36; similarly, another included narrative review on clinical pathways did not perform meta-analysis owing to increased heterogeneity.42 This suggests local context and resources (eg, team dynamics, hospital priorities, processes and staffing resources, and administrative support) are likely important factors in how successful interventions, such as clinical pathways or case management, are in streamlining care.36,51,52
Hospitalized older patients often have increased costs, complications, worse outcomes,53,54 and longer LOS compared with younger patients. For example, a study by Freeman et al53 estimated that compared with younger patients (aged 18-44 years), older adults (aged 65-84 years) had hospital stays that were a mean of 1.4 days longer. However, no intervention was consistently associated with reduced LOS for older patients. While 1 review found discharge planning was associated with a 0.73-day reduction in LOS in older patients,46 others found no association with reduced LOS, or even found an association with increased LOS.37,38,43 Inpatient geriatric assessment interventions also yielded inconsistent results. The largest review on this topic was unable to meta-analyze results owing to heterogeneity in study designs and settings across 11 RCTs.44 Studies’ settings included academic medical centers as well as community hospitals, and intervention components varied across studies. Thus, the variable associations with LOS across studies may reflect organizational differences, as LOS may vary depending on hospital factors, such as patient transfers, bed capacity, and demand. Future studies are needed to identify if interventions would provide more consistent benefits for particular types of hospitals or wards.
Limitations
This systematic review has several important limitations. First, we used systematic reviews instead of primary studies to characterize interventions. Using systematic reviews facilitated searching for evidence across a broad range of populations and interventions and supported identification of evidence gaps. However, systematic reviews rarely reported key aspects of local environments (eg, demographic data on patient volume, bed size, payer mix, or other organizational capacity), which individual studies may have reported and would provide valuable context for health systems gauging feasibility and likelihood of success. Future systematic reviews assessing hospital LOS should certainly highlight these factors. Second, as systematic reviews inherently lag behind primary research, our evidence base may have missed interventions not yet captured in a published review. However, a targeted search of RCTs through January 19, 2021, identified no additional relevant studies pertinent to older or heart failure populations. Third, we did not formally assess systematic review quality; however, because systematic reviews were required to meet certain methodological standards for inclusion (ie, explicit search strategies, inclusion and exclusion criteria, and ROB assessment), all included systematic reviews met this threshold for quality.
Conclusions
In this systematic review, we found 19 systematic reviews that identified 8 strategies for reducing LOS in high-risk populations: discharge planning, geriatric assessment; medication management; clinical pathways; inter- or multidisciplinary care; case management; hospitalist services; and telehealth. Identifying hospital-initiated interventions to reduce LOS without increasing readmissions or mortality is of interest to most hospitals and health systems. While many hospitals are searching for a one-size-fits-all solution to streamline care, our systematic review found that no single intervention was consistently associated with reduced LOS across all high-risk populations. A 2011 study by Hansen et al55 found that no single intervention was associated with reduced readmissions across broad populations. Since then, national initiatives have resulted in development of multifaceted approaches that include a range or combination of interventions that can be adapted for local context.56 Our findings suggest that efforts to reduce LOS, particularly for high-risk populations, could benefit from a similar approach. Future research assessing interventions for LOS reduction in high-risk populations or subpopulations should also consider implementation science measures to inform local adaption.
eTable 1. Embase and MEDLINE Search Terms in Embase Syntax
eTable 2. Characteristics of Systematic Reviews on Reducing Length of Hospital Stay
eTable 3. Summary of Findings for Length of Stay Meta-analyses
References
- 1.Bueno H, Ross JS, Wang Y, et al. Trends in length of stay and short-term outcomes among Medicare patients hospitalized for heart failure, 1993-2006. JAMA. 2010;303(21):2141-2147. doi: 10.1001/jama.2010.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005–2014. HCUP Statistical Brief #225. Agency for Healthcare Research and Quality; 2017:18. [Google Scholar]
- 3.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011;17(6):648-657. doi: 10.1097/MCC.0b013e32834c7a53 [DOI] [PubMed] [Google Scholar]
- 4.Gabler NB, Ratcliffe SJ, Wagner J, et al. Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med. 2013;188(7):800-806. doi: 10.1164/rccm.201304-0622OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilman M, Adams EK, Hockenberry JM, Milstein AS, Wilson IB, Becker ER. Safety-net hospitals more likely than other hospitals to fare poorly under Medicare’s value-based purchasing. Health Aff (Millwood). 2015;34(3):398-405. doi: 10.1377/hlthaff.2014.1059 [DOI] [PubMed] [Google Scholar]
- 6.Kohn R, Harhay MO, Weissman GE, et al. Ward capacity strain: a novel predictor of delays in intensive care unit survivor throughput. Ann Am Thorac Soc. 2019;16(3):387-390. doi: 10.1513/AnnalsATS.201809-621RL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohn R, Harhay MO, Bayes B, et al. Ward capacity strain: a novel predictor of 30-day hospital readmissions. J Gen Intern Med. 2018;33(11):1851-1853. doi: 10.1007/s11606-018-4564-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605-611.e6. doi: 10.1016/j.annemergmed.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer AJ, Thode HC Jr, Viccellio P, Pines JM. The association between length of emergency department boarding and mortality. Acad Emerg Med. 2011;18(12):1324-1329. doi: 10.1111/j.1553-2712.2011.01236.x [DOI] [PubMed] [Google Scholar]
- 10.Centers for Medicare & Medicaid Services . MS-DRG classifications and software. Accessed August 18, 2021. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/MS-DRG-Classifications-and-Software
- 11.Rojas-García A, Turner S, Pizzo E, Hudson E, Thomas J, Raine R. Impact and experiences of delayed discharge: a mixed-studies systematic review. Health Expect. 2018;21(1):41-56. doi: 10.1111/hex.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer NL, Gunnar WP, Dahm P, et al. Enhanced recovery protocols for adults undergoing colorectal surgery: a systematic review and meta-analysis. Dis Colon Rectum. 2018;61(9):1108-1118. doi: 10.1097/DCR.0000000000001160 [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Yu J, Doumouras AG, Li J, Hong D. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: a meta-analysis of randomized controlled trials. Surg Oncol. 2020;32:75-87. doi: 10.1016/j.suronc.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 14.Dietz N, Sharma M, Adams S, et al. Enhanced recovery after surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. 2019;130:415-426. doi: 10.1016/j.wneu.2019.06.181 [DOI] [PubMed] [Google Scholar]
- 15.Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: a quality-improvement project. J Hosp Med. 2016;11(5):341-347. doi: 10.1002/jhm.2546 [DOI] [PubMed] [Google Scholar]
- 16.Mercedes A, Fairman P, Hogan L, Thomas R, Slyer JT. Effectiveness of structured multidisciplinary rounding in acute care units on length of stay and satisfaction of patients and staff: a quantitative systematic review. JBI Database System Rev Implement Rep. 2016;14(7):131-168. doi: 10.11124/JBISRIR-2016-003014 [DOI] [PubMed] [Google Scholar]
- 17.Butler M, Schultz TJ, Halligan P, et al. Hospital nurse-staffing models and patient- and staff-related outcomes. Cochrane Database Syst Rev. 2019;4(4):CD007019. doi: 10.1002/14651858.CD007019.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridwan ES, Hadi H, Wu YL, Tsai PS. Effects of transitional care on hospital readmission and mortality rate in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2019;64(9):1146-1156. doi: 10.4187/respcare.06959 [DOI] [PubMed] [Google Scholar]
- 19.Van Spall HGC, Rahman T, Mytton O, et al. Comparative effectiveness of transitional care services in patients discharged from the hospital with heart failure: a systematic review and network meta-analysis. Eur J Heart Fail. 2017;19(11):1427-1443. doi: 10.1002/ejhf.765 [DOI] [PubMed] [Google Scholar]
- 20.Zhu QM, Liu J, Hu HY, Wang S. Effectiveness of nurse-led early discharge planning programmes for hospital inpatients with chronic disease or rehabilitation needs: a systematic review and meta-analysis. J Clin Nurs. 2015;24(19-20):2993-3005. doi: 10.1111/jocn.12895 [DOI] [PubMed] [Google Scholar]
- 21.Grover CA, Sughair J, Stoopes S, et al. Case management reduces length of stay, charges, and testing in emergency department frequent users. West J Emerg Med. 2018;19(2):238-244. doi: 10.5811/westjem.2017.9.34710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okere AN, Renier CM, Frye A. Predictors of hospital length of stay and readmissions in ischemic stroke patients and the impact of inpatient medication management. J Stroke Cerebrovasc Dis. 2016;25(8):1939-1951. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 23.Paton F, Chambers D, Wilson P, et al. Effectiveness and implementation of enhanced recovery after surgery programmes: a rapid evidence synthesis. BMJ Open. 2014;4(7):e005015. doi: 10.1136/bmjopen-2014-005015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naessens JM, Campbell CR, Shah N, et al. Effect of illness severity and comorbidity on patient safety and adverse events. Am J Med Qual. 2012;27(1):48-57. doi: 10.1177/1062860611413456 [DOI] [PubMed] [Google Scholar]
- 25.Gruneir A, Bronskill SE, Maxwell CJ, et al. The association between multimorbidity and hospitalization is modified by individual demographics and physician continuity of care: a retrospective cohort study. BMC Health Serv Res. 2016;16:154. doi: 10.1186/s12913-016-1415-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore L, Cisse B, Batomen Kuimi BL, et al. Impact of socio-economic status on hospital length of stay following injury: a multicenter cohort study. BMC Health Serv Res. 2015;15:285. doi: 10.1186/s12913-015-0949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadhera RK, Choi E, Shen C, Yeh RW, Joynt Maddox KE. Trends, causes, and outcomes of hospitalizations for homeless individuals: a retrospective cohort study. Med Care. 2019;57(1):21-27. doi: 10.1097/MLR.0000000000001015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality . Learning Health Systems Panel: series overview. Accessed August 17, 2021. https://effectivehealthcare.ahrq.gov/products/learning-health-systems-panel/overview
- 29.Agency for Healthcare Research and Quality . Interventions to decrease hospital length of stay: technical brief. Accessed August 19, 2021. https://effectivehealthcare.ahrq.gov/products/hospital-length-of-stay/research [PubMed]
- 30.Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; 2013:44. [PubMed] [Google Scholar]
- 31.Austin J, Barras M, Sullivan C. Interventions designed to improve the safety and quality of therapeutic anticoagulation in an inpatient electronic medical record. Int J Med Inform. 2020;135:104066. doi: 10.1016/j.ijmedinf.2019.104066 [DOI] [PubMed] [Google Scholar]
- 32.Baratloo A, Rahimpour L, Abushouk AI, Safari S, Lee CW, Abdalvand A. Effects of telestroke on thrombolysis times and outcomes: a meta-analysis. Prehosp Emerg Care. 2018;22(4):472-484. doi: 10.1080/10903127.2017.1408728 [DOI] [PubMed] [Google Scholar]
- 33.Bakker FC, Robben SHM, Olde Rikkert MG. Effects of hospital-wide interventions to improve care for frail older inpatients: a systematic review. BMJ Qual Saf. 2011;20(8):680-691. Published online February 25, 2011. doi: 10.1136/bmjqs.2010.047183 [DOI] [PubMed] [Google Scholar]
- 34.Gillaizeau F, Chan E, Trinquart L, et al. Computerized advice on drug dosage to improve prescribing practice. Cochrane Database Syst Rev. 2013;(11):CD002894. doi: 10.1002/14651858.CD002894.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huntley AL, Johnson R, King A, Morris RW, Purdy S. Does case management for patients with heart failure based in the community reduce unplanned hospital admissions: a systematic review and meta-analysis. BMJ Open. 2016;6(5):e010933. doi: 10.1136/bmjopen-2015-010933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kul S, Barbieri A, Milan E, Montag I, Vanhaecht K, Panella M. Effects of care pathways on the in-hospital treatment of heart failure: a systematic review. BMC Cardiovasc Disord. 2012;12:81. doi: 10.1186/1471-2261-12-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mabire C, Dwyer A, Garnier A, Pellet J. Meta-analysis of the effectiveness of nursing discharge planning interventions for older inpatients discharged home. J Adv Nurs. 2018;74(4):788-799. doi: 10.1111/jan.13475 [DOI] [PubMed] [Google Scholar]
- 38.Mabire C, Dwyer A, Garnier A, Pellet J. Effectiveness of nursing discharge planning interventions on health-related outcomes in discharged elderly inpatients: a systematic review. JBI Database System Rev Implement Rep. 2016;14(9):217-260. doi: 10.11124/JBISRIR-2016-003085 [DOI] [PubMed] [Google Scholar]
- 39.Patel JN, Klein DS, Sreekumar S, Liporace FA, Yoon RS. Outcomes in multidisciplinary team-based approach in geriatric hip fracture care: a systematic review. J Am Acad Orthop Surg. 2020;28(3):128-133. doi: 10.5435/JAAOS-D-18-00425 [DOI] [PubMed] [Google Scholar]
- 40.Pannick S, Davis R, Ashrafian H, et al. Effects of interdisciplinary team care interventions on general medical wards: a systematic review. JAMA Intern Med. 2015;175(8):1288-1298. doi: 10.1001/jamainternmed.2015.2421 [DOI] [PubMed] [Google Scholar]
- 41.White HL, Glazier RH. Do hospitalist physicians improve the quality of inpatient care delivery: a systematic review of process, efficiency and outcome measures. BMC Med. 2011;9:58. doi: 10.1186/1741-7015-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal A, Bahiru E, Yoo SGK, et al. Hospital-based quality improvement interventions for patients with heart failure: a systematic review. Heart. 2019;105(6):431-438. doi: 10.1136/heartjnl-2018-314129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryant-Lukosius D, Carter N, Reid K, et al. The clinical effectiveness and cost-effectiveness of clinical nurse specialist-led hospital to home transitional care: a systematic review. J Eval Clin Pract. 2015;21(5):763-781. doi: 10.1111/jep.12401 [DOI] [PubMed] [Google Scholar]
- 44.Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9(9):CD006211. doi: 10.1002/14651858.CD006211.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frazer A, Rowland J, Mudge A, Barras M, Martin J, Donovan P. Systematic review of interventions to improve safety and quality of anticoagulant prescribing for therapeutic indications for hospital inpatients. Eur J Clin Pharmacol. 2019;75(12):1645-1657. doi: 10.1007/s00228-019-02752-8 [DOI] [PubMed] [Google Scholar]
- 46.Gonçalves-Bradley DC, Lannin NA, Clemson LM, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;(1):CD000313. doi: 10.1002/14651858.CD000313.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Craen K, Braes T, Wellens N, et al. The effectiveness of inpatient geriatric evaluation and management units: a systematic review and meta-analysis. J Am Geriatr Soc. 2010;58(1):83-92. doi: 10.1111/j.1532-5415.2009.02621.x [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Lu Y, Liu M, et al. Strategies for prevention of postoperative delirium: a systematic review and meta-analysis of randomized trials. Crit Care. 2013;17(2):R47. doi: 10.1186/cc12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eagles D, Godwin B, Cheng W, et al. A systematic review and meta-analysis evaluating geriatric consultation on older trauma patients. J Trauma Acute Care Surg. 2020;88(3):446-453. doi: 10.1097/TA.0000000000002571 [DOI] [PubMed] [Google Scholar]
- 50.Kaboli PJ, Go JT, Hockenberry J, et al. Associations between reduced hospital length of stay and 30-day readmission rate and mortality: 14-year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837-845. doi: 10.7326/0003-4819-157-12-201212180-00003 [DOI] [PubMed] [Google Scholar]
- 51.Jabbour M, Newton AS, Johnson D, Curran JA. Defining barriers and enablers for clinical pathway implementation in complex clinical settings. Implement Sci. 2018;13(1):139. doi: 10.1186/s13012-018-0832-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores EJ, Mull NK, Lavenberg JG, et al. Using a 10-step framework to support the implementation of an evidence-based clinical pathways programme. BMJ Qual Saf. 2019;28(6):476-485. doi: 10.1136/bmjqs-2018-008454 [DOI] [PubMed] [Google Scholar]
- 53.Freeman WJ, Weiss AJ, Heslin KC. Overview of U.S. Hospital Stays in 2016: Variation by Geographic Region. HCUP Statistical Brief #246. Agency for Healthcare Research and Quality; 2018. [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention . Number, percent distribution, rate, days of care with average length of stay, and standard error of discharges from short-stay hospitals, by sex and age: United States, 2010. Accessed August 17, 2021. https://www.cdc.gov/nchs/data/nhds/2average/2010ave2_ratesexage.pdf
- 55.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. doi: 10.7326/0003-4819-155-8-201110180-00008 [DOI] [PubMed] [Google Scholar]
- 56.Agency for Healthcare Research and Quality . Re-Engineered Discharge (RED) toolkit. Accessed August 17, 2021. https://www.ahrq.gov/patient-safety/settings/hospital/red/toolkit/redtool1.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Embase and MEDLINE Search Terms in Embase Syntax
eTable 2. Characteristics of Systematic Reviews on Reducing Length of Hospital Stay
eTable 3. Summary of Findings for Length of Stay Meta-analyses