Abstract
Aims
Out‐of‐hospital cardiac arrest (OHCA) mostly results from ventricular tachycardia/ventricular fibrillation (VT/VF), often triggered by acute myocardial infarction (AMI). Sulfonylurea (SU) antidiabetics can block myocardial ATP‐regulated K+ channels (KATP channels), activated during AMI, thereby modulating action potential duration (APD). We studied whether SU drugs impact on OHCA risk, and whether these effects are related to APD changes.
Methods
We conducted a population‐based case–control study in 219 VT/VF‐documented OHCA cases with diabetes and 697 non‐OHCA controls with diabetes. We studied the association of SU drugs (alone or in combination with metformin) with OHCA risk compared to metformin monotherapy, and of individual SU drugs compared to glimepiride, using multivariable logistic regression analysis. We studied the effects of these drugs on APD during simulated ischaemia using patch‐clamp studies in human induced pluripotent stem cell‐derived cardiomyocytes.
Results
Compared to metformin, use of SU drugs alone or in combination with metformin was associated with reduced OHCA risk (ORSUdrugs‐alone 0.6 [95% CI 0.4–0.9], ORSUdrugs + metformin 0.6 [95% CI 0.4–0.9]). We found no differences in OHCA risk between SU drug users who suffered OHCA inside or outside the context of AMI. Reduction of OHCA risk compared to glimepiride was found with gliclazide (ORadj 0.5 [95% CI 0.3–0.9]), but not glibenclamide (ORadj 1.3 [95% CI 0.6–2.7]); for tolbutamide, the association with reduced OHCA risk just failed to reach statistical significance (ORadj 0.6 [95% CI 0.3–1.002]). Glibenclamide attenuated simulated ischaemia‐induced APD shortening, while the other SU drugs had no effect.
Conclusions
SU drugs were associated with reduced OHCA risk compared to metformin monotherapy, with gliclazide having a lower risk than glimepiride. The differential effects of SU drugs are not explained by differential effects on APD.
Keywords: ESCAPE‐NET, KATP channelssulfonylurea, sudden cardiac arrest
What is already known about this subject
An increase in the incidence of out‐of‐hospital cardiac arrest (OHCA) among individuals with diabetes mellitus (DM) represents a global public health problem.
There is strong interest in minimizing OHCA risk in individuals with DM.
A few experimental and observational studies suggest that some sulfonylurea drugs (SU drugs) may reduce OHCA risk, but the evidence from clinical studies is inconclusive because these studies were small and had other important limitations in their design.
What this study adds
The effects of SU drugs on OHCA risk are here studied in a large cohort specifically designed to study OHCA (total 219 patients with ECG‐documented OHCA).
Use of SU drugs (alone or in combination with metformin) is associated with reduced OHCA risk compared to metformin monotherapy.
Gliclazide, but not glibenclamide, is associated with decreased risk of OHCA compared to glimepiride; tolbutamide is also associated with reduced OHCA risk, but this association just failed to reach statistical significance.
1. INTRODUCTION
Out‐of‐hospital cardiac arrest (OHCA) is a leading cause of death in industrialized societies. OHCA is predominantly caused by ventricular tachycardia/ventricular fibrillation (VT/VF) that arises from disruptions in cardiac electrophysiology.1 Diabetes mellitus is an important risk factor for OHCA.2 Multiple pathophysiologic changes in diabetes may result in VT/VF, in particular, development of ischaemic heart disease.2 Myocardial ischaemia may lead to VT/VF by inducing various electrophysiological changes. One key mechanism is change in the duration of the action potential (AP) of ventricular cardiomyocytes. AP‐shortening, following in large part from opening of myocardial ATP‐regulated K+ channels (KATP channels) during ischaemia and acute myocardial infarction (AMI),3 may facilitate re‐entrant excitation and VT/VF.3 Conversely, AP‐shortening during ischaemia and AMI may be a cardioprotective mechanism, and lack thereof may result in intracellular Ca2+ overload and delayed afterdepolarizations3 and/or impaired cell‐to‐cell transmission of the electrical wavefront;4 these changes may also culminate in VT/VF. Sulfonylurea (SU) drugs, used commonly to achieve glycaemic control in diabetes, exert their therapeutic action by blocking pancreatic KATP channels, thereby inducing release of insulin.5 Importantly, SU drugs may also block myocardial KATP channels,5 thereby potentially impacting on AP duration of ventricular cardiomyocytes. We therefore hypothesized that SU drugs impact on the risk of OHCA, especially during AMI. To test our hypothesis, we studied whether the use of SU drugs (alone or in combination with metformin) is associated with reduced OHCA risk compared with metformin (both designed for the treatment of type 2 diabetes), in a population‐based case–control study based on data from an emergency medical services (EMS) attended OHCA registry, and stratified our analysis according to the immediate cause of OHCA (presence or absence of AMI), expecting that use of SU drugs is more strongly associated with lower OHCA risk in subgroups of OHCA cases who suffered OHCA in the presence of AMI than in the absence of AMI. Moreover, we studied the association between individual SU drugs (glibenclamide, gliclazide, tolbutamide) compared to glimepiride. In addition, we explored the underlying cellular electrophysiological mechanisms by performing patch‐clamp studies in human‐induced pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs).
2. METHODS
2.1. Study design and setting
We conducted a population‐based case–control study. Cases were individuals who suffered OHCA from presumed cardiac causes with ECG‐documented VT/VF, drawn from the Amsterdam Resuscitation Studies (ARREST,6) registry in the study period 2005–2011. We excluded cases without complete drug‐dispensing record 1year before index date (OHCA date), those who suffered OHCA from obvious non‐cardiac causes (e.g., trauma, drowning), and those who suffered their second OHCA episode. Each case was matched with up to five non‐OHCA controls who were alive on the index date (OHCA date) using risk set matching based on age, sex and index date. From this original case–control data set, we included all persons who had used metformin in monotherapy or any SU drug within 90 days before index date, and excluded all persons who used insulin (proxy for advanced stage of diabetes) or other oral glucose‐lowering drugs (e.g., thiazolidinediones), thereby increasing comparability with respect to underlying diabetes severity. By subselecting all individuals with drug‐dispensing for metformin or SU drugs 90 days before the index date from the original case–control data set, the original matching was lost. This study was conducted according to the principles expressed in the Declaration of Helsinki and was approved by the Medical Ethics Committee of Academic Medical Center.
2.2. Data sources
Details of the ARREST registry were reported previously.6 In short, ARREST is an ongoing population‐based registry of all EMS‐attended OHCAs in one contiguous study region of the Netherlands, representative for the community at large (~2.6 million inhabitants, urban and rural areas, capture rate >90%). The ARREST study centre is notified by all dispatch centres in the study region of every EMS‐attended OHCA. ECGs are collected from the manual defibrillator used by EMS personnel and/or the automated external defibrillator used by first responders or citizen‐responders. Information from these ECGs with additional information from the dispatch centres and EMS personnel are used to verify the presence of VT/VF. The immediate cause of VT/VF was retrieved from hospital records and was classified as AMI, no AMI (any other cardiac cause) or unknown, as diagnosed by the treating cardiologist.6 These data were obtained for those individuals who survived to hospital admission. Drug‐dispensing records in the year before OHCA was retrieved from the patients' pharmacist using standardized protocols. Controls were derived from the PHARMO Database Network, which contains drug‐dispensing records from community pharmacies.7 We also obtained complete drug‐dispensing records in the year before index date from controls. As virtually all individuals in the Netherlands are registered at a single pharmacy, drug‐dispensing records are considered complete.
2.3. Exposure of interest
We studied use of the most commonly prescribed SU drugs in the Netherlands (glibenclamide, glimepiride, gliclazide, tolbutamide) by using the Anatomical Therapeutic Chemical classification (ATC) system (see Table S1 in the Supporting Information for the ATC codes). Use of metformin and SU drugs was defined as having a drug‐dispensing record within 90 days before the index date, since, in the Netherlands, the average repeat prescription length for drugs used for chronic diseases is 90 days. We classified users of antidiabetics into one of the following mutually exclusive categories: (1) use of metformin alone and (2) use of SU drugs (alone or in combination with metformin).
2.4. Covariates
As covariates, we assessed the following known risk factors for OHCA: cardiovascular disease, use of non‐antiarrhythmic QT‐prolonging drugs, and use of Vaughan‐Williams class 1 or 3 antiarrhythmic drugs. Presence of cardiovascular disease was based on drug proxies and was defined as use of any of the following drugs within 6 months before the index date: beta blockers, calcium channel blockers, renin‐angiotensin system inhibitors, diuretics, nitrates, antithrombotics and statins. Non‐antiarrhythmic QT‐prolonging drugs were defined as advised by the CredibleMeds list (www.CredibleMeds.org). Use of Vaughan‐Williams antiarrhythmic drugs class 1 or 3 and/or non‐cardiac QT‐prolonging drugs was defined as having a drug‐dispensing record within 90 days prior to index date. We used a period of 90 days before index date for Vaughan‐Williams antiarrhythmic drugs class 1 or 3 and/or non‐cardiac QT‐prolonging drugs in order to adjust for the direct cardiac electrophysiological effects of these drugs. The cardiovascular drug groups were included in the analysis as proxies for cardiovascular disease, and not because of their direct effects on cardiac electrophysiology. Therefore, their exposure window was set at 6 months.
2.5. Cellular electrophysiological studies
The effects of the SU drugs on AP‐shortening during simulated ischaemia (SI) were measured in hiPSC‐CMs, a well‐established human cell model for cardiac disease and drug screening studies.8 APs of hiPSC‐CMs were recorded at 36 ± 0.2°C using the perforated patch‐clamp technique. APs were elicited at 1 Hz and AP duration at 90% of repolarization (APD90) was analysed. The dynamic clamp technique with injection of an in silico inward rectifier K+ current (IK1) was used to achieve a close‐to‐physiological resting membrane potential. The effects of SU drugs on AP‐shortening were studied during SI, induced by omission of extracellular glucose in combination with metabolic inhibition achieved using 2 mmol/L sodium cyanide (NaCn) and 1 mmol/L iodoacetate. An extended methods section is provided in the Supporting Information. The SU drug concentrations studied (glibenclamide 10 nM, glimepiride 10 nM, gliclazide 10 μM, tolbutamide 10 μM) were used because they are reported to cause 50% block of KATP current (IK,ATP) in cardiac and skeletal muscle.9, 10, 11
2.6. Statistical analyses
We used multivariable logistic regression analysis to estimate the strength of the association between SU drugs and OHCA risk by calculating the odds ratio (OR) and 95% confidence interval (CI), employing two models. By subselecting all individuals with drug‐dispensing for metformin or SU drugs 90 days before the index date from the original case–control data set, the original matching was lost. Therefore, we adjusted for age and sex (Model 1). Estimates were additionally adjusted for all covariates listed in Table 1 (Model 2). Analyses were conducted for the overall use of SU drugs (reference: metformin alone), and separately for use of SU drugs alone or in combination with metformin (reference: metformin alone). Subgroup analyses were conducted in the subgroups of OHCA cases who suffered OHCA in the presence or absence of AMI. We studied the association between individual SU drugs and OHCA risk in which the reference category consisted of glimepiride. We chose glimepiride as reference category because a previous study found that glimepiride showed no association with sudden cardiac arrest/ventricular arrhythmia.12 Estimates for individual SU drugs were additionally adjusted for diabetes severity that was defined according to medication prescriptions (Model 3): (stage 1) metformin or SU drugs only; (stage 2) metformin and SU drugs. Finally, we examined whether patient characteristics or concomitant drug use was different between users of individual SU drugs.
TABLE 1.
Baseline characteristics of cases and controls
| Cases | Controls | P‐value | |
|---|---|---|---|
| (n = 219) | (n = 697) | ||
| Mean age, years (SD) | 71.4 (10.3) | 71.9 (10.0) | .581 |
| Male sex | 168 (76.7) | 556 (79.8) | .332 |
| Concomitant drug use | |||
| Beta blockers | 110 (50.2) | 261 (37.4) | .001 |
| Calcium channel blockers | 46 (21.0) | 155 (22.2) | .700 |
| Antithrombotics | 95 (43.4) | 296 (42.5) | .812 |
| Diuretics | 131 (59.8) | 308 (44.2) | <.001 |
| Renin‐angiotensin system inhibitors | 148 (67.6) | 422 (60.5) | .061 |
| Nitrates | 54 (24.7) | 78 (11.2) | <.001 |
| Statins | 129 (58.9) | 450 (64.6) | .130 |
| Vaughan‐Williams class 1 or 3 antiarrhythmic drugs | 14 (6.4) | 10 (1.4) | <.001 |
| Non‐cardiac QT‐prolonging drugs | 13 (5.9) | 33 (4.7) | .478 |
Numbers are number (%) unless indicated otherwise. P‐values are calculated using the Student's t‐test or χ2 statistics.
Use of beta blockers, calcium channel blockers, antithrombotics, diuretics, renin‐angiotensin system inhibitors, nitrates and/or statins was defined as use within 6 months before the index date. Use of Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and/or non‐cardiac QT‐prolonging drugs was defined as use within 90 days before the index date.
Comparisons for continuous variables were made with Student's t‐test or analysis of variance (ANOVA). The χ2 analyses were used when discrete variables were compared across groups. Statistical tests were two‐tailed, with P‐value of <.05 considered as statistically significant.
For the cellular electrophysiological studies, statistical analysis was carried out with SigmaStat 3.5 software and data are presented as mean ± SEM. Normality and equal variance assumptions were tested with the Kolmogorov–Smirnov and the Levene median test, respectively. Groups were compared using ANOVA based on ranks test (Kruskal‐Wallis test) followed by Dunn's test. P < .05 was defined as statistical significance.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20.13
3. RESULTS
3.1. Subject characteristics
We identified 2503 OHCA cases from cardiac causes with ECG documented VT/VF and complete drug‐dispensing records; among these cases, 219 used metformin in monotherapy, SU drugs alone or SU drugs in combination with metformin (mean age 71.4 years, 76.7% male, Table 1, Figure 1). Among 10 543 non‐OHCA controls with complete medication records, 697 used metformin in monotherapy, SU drugs alone or SU drugs in combination with metformin (mean age 71.9 years, 79.5% male, Figure 1).
FIGURE 1.
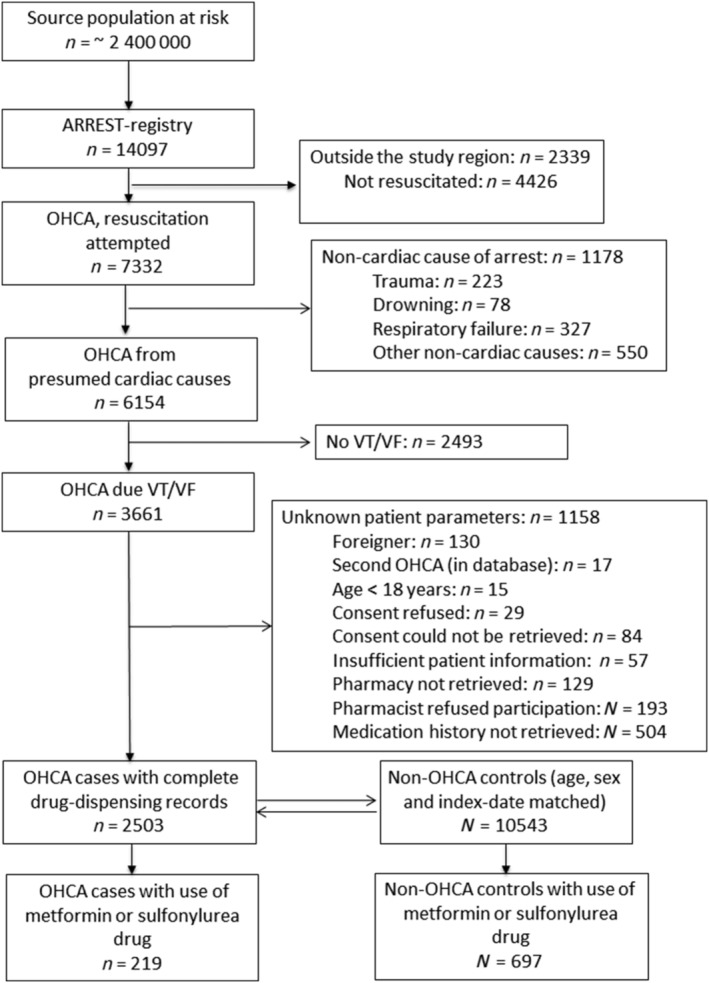
Flow chart of inclusion of out‐of‐hospital cardiac arrest (OHCA) casesOHCA, out‐of‐hospital cardiac arrest; VT/VF, ventricular tachycardia/ventricular fibrillation
3.2. Association between SU drugs and OHCA risk
Compared to use of metformin alone (cases: 50.2%; controls: 39.5%), the overall use of SU drugs (cases: 49.8%; controls: 60.5%) was associated with reduced risk of OHCA (ORSUdrugs‐overall 0.6 [95% CI 0.5–0.9]). Use of SU drugs alone (cases: 19.6%; controls: 24.7%) or in combination with metformin (cases: 30.1%; controls: 35.9%) was associated with reduced OHCA risk (ORSUdrugs‐alone 0.6 [95% CI 0.4–0.9], ORSUdrugs + metformin 0.6 [95% CI 0.4–0.9], Table 2). We found higher prevalence of statin use among metformin users, but found no further evidence that users of SU drugs had less cardiovascular drug use (Table 3). When we stratified according to the immediate cause of VF (AMI vs. no AMI), we found no differences in OHCA risk between SU drug users who suffered OHCA inside or outside the context of AMI (ORAMI‐SUdrugs 0.6 [95% CI 0.4–1.1]; ORnonAMI‐SUdrugs 0.7 [95% CI 0.7–1.2], Table 4). Next, we examined OHCA risk of the individual SU drugs compared to glimepiride, and found that OHCA risk was reduced in individuals who used gliclazide (ORadj 0.5 [95% CI 0.3–0.9]), but not glibenclamide (ORadj 1.3 [95% CI 0.6–2.7]), Table 5). Use of tolbutamide also appeared to be associated with reduced OHCA risk, but this association just failed to reach statistical significance (ORadj 0.6 [95% CI: 0.3–1.002]). When we compared concomitant drug use between patients who used each of the studied SU drugs, we found no significant differences in cardiovascular drug use between individual SU drugs (Table 6).
TABLE 2.
Use of sulfonylurea and the risk for out‐of‐hospital cardiac arrest compared to use of metformin in monotherapy
| Cases | Controls | Crude OR | Adjusted OR | |
|---|---|---|---|---|
| (n = 219) | (n = 697) | (model 1) | (model 2) | |
| Metformin alone | 110 (50.2) | 275 (39.5) | 1.0 (reference) | 1.0 (reference) |
| Sulfonylurea drugs | 109 (49.8) | 422 (60.5) | 0.7 (0.5–0.9) | 0.6 (0.5–0.9) |
| Sulfonylurea drugs alone | 43 (19.6) | 172 (24.7) | 0.6 (0.4–0.9) | 0.6 (0.4–0.9) |
| Sulfonylurea drugs + metformin | 66 (30.1) | 250 (35.9) | 0.7 (0.5–1.0) | 0.6 (0.4–0.9) |
Use of metformin and/or sulfonylurea drugs was defined as use within 90 days before the index date.
Model 1: OR adjusted for age and sex.
Model 2: OR adjusted for age, sex, use of cardiovascular drug use, Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and non‐cardiac QT‐prolonging drugs.
TABLE 3.
Characteristics of users of metformin alone, sulfonylurea drugs alone or sulfonylurea drugs + metformin
| Metformin alone | Sulfonylurea drugs alone | Sulfonylurea drugs + metformin | P‐value | |
|---|---|---|---|---|
| N | 385 | 215 | 316 | |
| Age (years, standard deviation) | 69.6 (10.1) | 75.2 (9.7) | 72.1 (9.5) | <.001 |
| Sex male | 302 (78.4) | 162 (75.3) | 260 (82.3) | .146 |
| Concomitant drug use | ||||
| Beta blockers | 150 (39.0) | 88 (40.9) | 133 (42.1) | .696 |
| Calcium channel blockers | 80 (20.8) | 53 (24.7) | 68 (21.5) | .533 |
| Antithrombotics | 165 (42.9) | 87 (40.5) | 139 (44.0) | .720 |
| Diuretics | 185 (48.1) | 101 (47.0) | 153 (48.4) | .946 |
| Renin‐angiotensin system inhibitors | 234 (60.8) | 128 (59.5) | 208 (65.8) | .254 |
| Nitrates | 52 (13.5) | 37 (17.2) | 43 (13.6) | .409 |
| Statins | 260 (67.5) | 122 (56.7) | 197 (62.3) | .029 |
| Vaughan‐Williams class 1 or 3 antiarrhythmic drugs | 8 (2.1) | 7 (3.3) | 9 (2.8) | .654 |
| Non‐cardiac QT‐prolonging drugs | 24 (6.2) | 10 (4.7) | 12 (3.8) | .326 |
Numbers are number (%) unless indicated otherwise. P‐values are calculated using ANOVA or χ2 statistics.
Use of metformin and/or sulfonylurea drugs was defined as use within 90 days before the index date. Use of beta blockers, calcium channel blockers, antithrombotics, diuretics, renin‐angiotensin system inhibitors, nitrates and/or statins was defined as use within 6 months before the index date. Use of Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and/or non‐cardiac QT‐prolonging drugs was defined as use within 90 days before the index date.
TABLE 4.
Use of sulfonylurea drugs and out‐of‐hospital cardiac arrest (OHCA) risk in patients within or outside the context of acute myocardial infarction (AMI)
| Cases | Controls | Crude OR | Adjusted OR | |
|---|---|---|---|---|
| (model 1) | (model 2) | |||
| OHCA‐AMI | 63 | 697 | ||
| Metformin alone | 32 (50.8) | 275 (39.5) | 1.0 (reference) | 1.0 (reference) |
| Sulfonylurea drugs | 31 (49.2) | 422 (60.5) | 0.6 (0.4–1.1) | 0.6 (0.4–1.1) |
| Sulfonylurea drugs alone | 12 (19.0) | 172 (24.7) | 0.6 (0.30–1.2) | 0.6 (0.3–1.2) |
| Sulfonylurea drugs + metformin | 19 (30.2) | 250 (35.9) | 0.6 (0.36–1.2) | 0.7 (0.4–1.2) |
| OHCA‐no AMI | 56 | 697 | ||
| Metformin alone | 26 (46.4) | 275 (39.5) | 1.0 (reference) | 1.0 (reference) |
| Sulfonylurea drugs | 30 (53.6) | 422 (60.5) | 0.7 (0.4–1.3) | 0.7 (0.4–1.2) |
| Sulfonylurea drugs alone | 11 (19.6) | 172 (24.7) | 0.6 (0.3–1.3) | 0.6 (0.3–1.3) |
| Sulfonylurea drugs + metformin | 19 (33.9) | 250 (35.9) | 0.8 (0.4–1.5) | 0.7 (0.4–1.4) |
The immediate cause of OHCA could only obtained for those individuals who survived to hospital admission. Use of metformin and/or sulfonylurea drugs was defined as use within 90 days before the index‐date.
Model 1: OR adjusted for age and sex.
Model 2: OR adjusted for age, sex, use of cardiovascular drugs, Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and non‐cardiac QT‐prolonging drugs.
TABLE 5.
Use of individual sulfonylurea drugs and risk of out‐of‐hospital cardiac arrest compared to use of glimepiride
| Cases | Controls | Crude OR | Adjusted OR | Adjusted OR | |
|---|---|---|---|---|---|
| (n = 219) | (n = 697) | (model 1) | (model 2) | (model 3) | |
| Glimepiride | 58 (26.5) | 164 (23.5) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Glibenclamide | 12 (5.5) | 29 (4.2) | 1.2 (0.6–2.5) | 1.3 (0.6–2.7) | 1.3 (0.6–2.7) |
| Gliclazide | 16 (7.3) | 101 (14.5) | 0.5 (0.3–0.8) | 0.5 (0.3–0.9) | 0.5 (0.3–0.9) |
| Tolbutamide | 23 (10.5) | 125 (17.9) | 0.5 (0.3–0.9) | 0.6 (0.3–1.002) | 0.6 (0.3–1.002) |
Not included in the table: Three controls that used multiple sulfonylurea drugs concomitantly and no users of SU drugs. Use of individual sulfonylurea drugs was defined as use within 90 days before the index‐date.
Model 1: OR adjusted for age and sex.
Model 2: OR adjusted for age, sex, use of cardiovascular drugs, Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and non‐cardiac QT‐prolonging drugs.
Model 3: OR adjusted for age, sex, use of cardiovascular drugs, Vaughan‐Williams class 1 or 3 antiarrhythmic drugs, non‐cardiac QT‐prolonging drugs and diabetes severity.
TABLE 6.
Characteristics of individual sulfonylurea drug users
| Glimepiride | Glibenclamide | Gliclazide | Tolbutamide | P‐value | |
|---|---|---|---|---|---|
| N | 222 | 41 | 117 | 148 | |
| Age, years, mean (SD) | 72.0 (9.6) | 73.6 (9.5) | 73.8 (9.5) | 75.0 (10.0) | .034 |
| Male, n (%) | 167 (75.2) | 33 (80.5) | 97 (82.9) | 122 (82.4) | .251 |
| Concomitant drug use, n (%) | |||||
| Beta blockers | 95 (42.8) | 14 (34.1) | 53 (45.3) | 58 (39.2) | .556 |
| Calcium channel blockers | 50 (22.5) | 11 (26.8) | 32 (27.4) | 28 (18.9) | .389 |
| Antithrombotics | 89 (40.1) | 21 (51.2) | 54 (46.2) | 61 (41.2) | .469 |
| Diuretics | 114 (51.4) | 21 (51.2) | 57 (48.7) | 62 (41.9) | .334 |
| Renin‐angiotensin system inhibitors | 144 (64.9) | 25 (61.0) | 80 (68.4) | 87 (58.8) | .405 |
| Nitrates | 34 (15.3) | 5 (12.2) | 16 (13.7) | 25 (16.9) | .842 |
| Statins | 140 (63.1) | 17 (41.5) | 72 (61.5) | 89 (60.1) | .077 |
| Vaughan‐Williams class 1 or 3 antiarrhythmic drugs | 10 (4.5) | 1 (2.4) | 2 (1.7) | 3 (2.0) | .409 |
| Non‐cardiac QT‐prolonging drugs | 10 (4.5) | 1 (2.4) | 4 (3.4) | 7 (4.7) | .885 |
Numbers are number (%) unless indicated otherwise. P‐values are calculated using ANOVA or χ2 statistics.
Use of individual sulfonylurea drugs was defined as use within 90 days before the index date. Use of beta blockers, calcium channel blockers, antithrombotics, diuretics, renin‐angiotensin system inhibitors, nitrates and/or statins was defined as use within 6 months before the index date. Use of Vaughan‐Williams class 1 or 3 antiarrhythmic drugs and/or non‐cardiac QT‐prolonging drugs was defined as use within 90 days before the index date.
3.3. Cellular electrophysiological studies
Figure 2A, left panels, shows typical SI‐induced APD90 changes in time in absence (top panels) and presence (bottom panels) of 10 nM glibenclamide. In both conditions, SI resulted in an initial APD90 prolongation. Subsequently, APD90 shortened (Figure 2A), which is importantly due to activation of IK,ATP. In presence of glibenclamide, the APD90 shortening was less pronounced (Figure 2A). Figure 2B, left panel, summarizes the average SI‐induced APD90 changes in time without or in the presence of the four SU drugs. Only glibenclamide resulted in significantly less APD90 shortening after 15 minutes, while the other SU drugs had no significant effects (Figure 2B, right panel). Similar effects were found in a different set of experiments where we used 10 μM of all drugs (Supplemental Figure S1).
FIGURE 2.
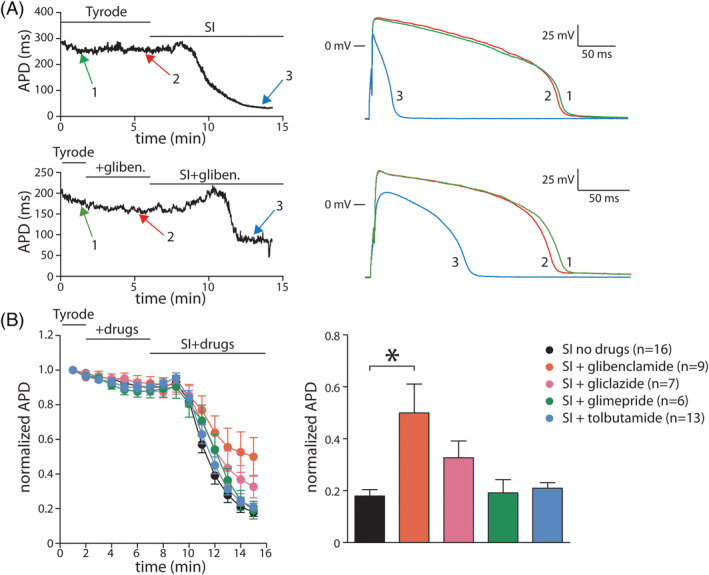
Effects of sulfonylurea drugs on action potentials (APs) during simulated ischemia (SI)A, Typical AP duration (APD) changes in time in response to SI in absence (top panels) or presence (bottom panels) of 10 nM glibenclamide. SI‐induced APD shortening is less pronounced in presence of glibenclamide. B, Average SI‐induced APD changes in time (left panel) and average APD shortening at 15 min in absence or presence of glibenclamide (10 nM), gliclazide (10 μM), glimepride (10 nM), and tolbutamide (10 μM). * indicates P < .05
4. DISCUSSION
In this observational study using real‐world population‐based data, we found that use of SU drugs (alone or in combination with metformin) was associated with decreased OHCA risk compared to use of metformin alone. Although users of metformin were younger and the prevalence of statin use was higher among metformin users, we found no further evidence that differences in patient profiles between SU drug users and metformin users accounted for the OHCA risk‐reducing effects of SU drugs. There was no difference in OHCA risk reduction between patients with or without AMI. Finally, we found that gliclazide, but not glibenclamide, was associated with decreased OHCA risk compared to glimepiride; tolbutamide also appeared to be associated with reduced OHCA risk, but this association just failed to reach statistical significance. The differences between the SU drugs were not explained by differences in patient characteristics or by different effects on APD90 changes during simulated ischaemia in cellular electrophysiologic studies in hiPSc‐CMs.
To find the explanation for the decreased risk of OHCA upon use of SU drugs, we first investigated whether our finding was explained by differences in patient profiles, in particular, presence of factors that increase OHCA risk (i.e., cardiovascular comorbidity). Although the prevalence of statin use was higher among users of metformin, we found no further evidence that users of SU drugs had fewer prescriptions for cardiovascular drugs than users of metformin. Second, since diabetes itself is a known risk factor for OHCA, confounding by indication must be considered.2 Such confounding by indication may arise when comparing treatments at different stages of the disease process (time‐lag bias). In our study, we therefore compared use of SU drugs as a whole and separately as monotherapy and in combination with metformin, to use of metformin in monotherapy. Even when we compared use of SU drugs in combination with metformin (a second‐ to third‐line treatment strategy) to metformin monotherapy (a first‐line treatment strategy), use of SU drugs was associated with decreased OHCA risk. Furthermore, patients using SU drugs (with or without metformin) might have more diabetes severity than patients using metformin only (our reference category). Nonetheless, despite probably having more advanced stage of diabetes than users of metformin only, users of SU drugs still had lower OHCA risk; this provides additional support for the notion that SU drugs reduce OHCA risk.
Since SU drugs are usually prescribed in addition to metformin as a second‐ to third‐line treatment strategy, individuals had to survive to receive SU drugs. Thus, individuals who only used metformin in the 90 days prior to index date and an OHCA occurred were classified in the metformin group. However, individuals who subsequently received SU drugs in the 90 days prior to index date would have been classified in the SU drugs + metformin group. This may result in immortal bias, because the period during which individuals were on metformin and survived (immortal) was not counted (in the metformin group) in the calculation of the effect size.
We found no stronger OHCA risk reduction associated with SU drugs in OHCA patients with proven AMI than in OHCA patients without proven AMI. This does not rule out that the OHCA risk‐reducing effects of SU drugs occur in the presence of myocardial ischaemia, because myocardial ischaemia may have been present, but may not have led to AMI, e.g., because of spontaneous reperfusion by thrombus resolution and/or relaxation of the culprit vessel.14 This is of particular relevance, since AMI status could only be established in OHCA victims who survived to hospital admission and received cardiologic workup (post‐mortem analysis after OHCA is not mandatory in the Netherlands and very seldom performed in OHCA victims who die before hospital admission).
We next studied the effects of each individual SU drug separately considering that SU drugs differ substantially between each other in several important pharmacodynamic and pharmacokinetic properties.5 Our findings indicate that the OHCA risk‐reducing effects of SU drugs are drug‐specific, rather than a class effect. We investigated whether they were explained by differences in patient profiles, but found no relevant differences in concomitant drug use between users of the different SU drugs. In addition, we studied whether the SU drugs have distinct potencies to inhibit KATP channels during SI. We found that this was the case, with glibenclamide preventing AP‐shortening during SI more than the other SU drugs studied, including glimepiride. However, these findings were not consistent with our epidemiologic finding that the SU drugs were grouped into two groups: drugs that reduced OHCA risk (gliclazide, tolbutamide, although not statistically significant for tolbutamide) and drugs that did not (glibenclamide, glimepiride). Thus, our cellular electrophysiologic studies provided no evidence to support the possibility that our observed effects of SU drugs were explained by their effects on APD shortening during ischaemia.
Although we found an association between SU drugs and reduced OHCA risk, it is important to note that some guidelines have already lowered the recommendation for the use of SU drugs15 due to their association with increased risk of hypoglycaemia16 and the increasing concerns regarding their cardiovascular safety.17, 18 Compared to newer oral antidiabetic drugs, SU drugs are associated with higher risk of hypoglycaemia. A recent study reported that hypoglycaemic adverse events occurred in 10.6% of linagliptin users, but in 37.7% of glimepiride users.19 Still, given the low cost and demonstrated ability to reduce microvascular complications, SU drugs remain important in the treatment of diabetes mellitus type 2.20 SU drugs may differ with respect to hypoglycaemia risk with glibenclamide carrying the highest and gliclazide the lowest risk of hypoglycaemia among second‐generation SU drugs, which may also influence OHCA risk, since hypoglycaemia has been associated with QT‐prolongation.12 This may have influenced our outcome, since we could not control for hypoglycaemia in our study. This may have masked a possible association between glibenclamide and reduced OHCA risk, while we expect that the association between gliclazide and OHCA may have been less affected.
A previous study showed an increased mortality and cardiovascular risk with most first‐ and second‐generation SU drugs compared with metformin in patients with or without previous AMI.17 However, results were not statistically different from metformin in both patients with AMI and those without AMI for gliclazide,17 indicating that gliclazide has a more favourable cardiovascular risk profile over other SU drugs. These findings were supported by another retrospective cohort study, where an increased total and cardiovascular mortality associated with glibenclamide compared with gliclazide was found.18 Our finding that gliclazide was associated with lower OHCA risk adds to the accumulating evidence of a favourable cardiovascular risk profile for gliclazide over other SU drugs. Regardless of the underlying mechanisms of our epidemiologic findings, our results are of clinical importance given the sharp rise in the prevalence of diabetes, and the fact that diabetes is associated with increased OHCA risk.2 Therefore, a potential relation between gliclazide and lower OHCA risk and the mechanisms involved warrants future replication studies in other settings.
Animal studies on the effect of KATP channel modulators on cardiac arrhythmias produced seemingly contradictory findings. Glibenclamide was associated with decreased incidence of sustained VT and VF during ischaemia–reperfusion,21 but another study found increased occurrence of VT.22 Conversely, use of KATP channel openers was associated with increased incidence of VT/VF during ischaemia–reperfusion in one study,21 but with decreased incidence of arrhythmias in another study.23 Moreover, opposite effects in the propensity for VF of KATP channel openers were demonstrated in different species.23 While these drugs inhibited VF in anesthetized dogs, they promoted VF in rat hearts. These discrepancies indicate that the effects of KATP channel modulators in relation to cardiac arrhythmias depend upon the experimental model.19 The effects of SU drugs on VT/VF on a population level are less known, and previous clinical studies on the association between SU drugs and VT/VF had important limitations.12, 24, 25, 26 A retrospective study by Davis et al. showed that glibenclamide, but not gliclazide, was associated with lower VF incidence compared to insulin, but not to gliclazide.24 However, important facts were not reported, e.g., the time of VF occurrence, the temporal relationship between VF and SU drug use, and the number of VF cases. Also, the diagnosis of diabetes partly relied on self‐reported data, and VF ascertainment was not systematic, e.g., fewer diabetes patients received rhythm monitoring.24 Lomuscio et al. found lower VF incidence in the first 36 hours after AMI among individuals with diabetes who used glibenclamide compared with individuals with diabetes who used no glibenclamide.25 That study, however, was small (VF occurred in two individuals with diabetes who used glibenclamide, and six individuals with diabetes who did not use glibenclamide), while VT/VF episodes occurred at an unspecified time during the 36 hour period (and may not have been related to IK‐ATP activity), and it was not specified whether all patients used glibenclamide at the time of those episodes. Cacciapuoti et al. reported that premature ventricular complexes and nonsustained ventricular tachycardias following presumed ischaemic episodes occurred less often during glibenclamide use than metformin use in a crossover study.26 However, the study was small (19 individuals with diabetes), and ascertainment of ischaemia was uncertain, relying solely on ST segment changes on Holter recordings with an unspecified number of leads.26 Leonard et al. investigated in a large cohort (519 272 patients) whether SU drugs are associated with risk of sudden cardiac arrest and ventricular arrhythmia, and found reduced risk among users of glibenclamide, but not glimepiride, compared with glipizide (tolbutamide and gliclazide were not examined).12 We could not compare our findings with that study, because glipizide is not marketed in the Netherlands, while gliclazide is not marketed in the United States and in all European countries. Moreover, the study populations are distinct, with far larger proportions of women and non‐Caucasians among study participants in that study than in ours. In addition, the study by Leonard et al. had important limitations. First, that study relied solely on emergency department and in‐hospital diagnosis, and omitted patients who died before hospital admission. This causes important inclusion bias, particularly since diabetes is associated with reduced pre‐hospital survival rates after OHCA.27 Moreover, ascertainment of VF was not certain because it was based on claims rather than actual ECG recordings. Our study resolved these limitations. Our ARREST registry was specifically designed to study OHCA and, thanks to participation of all EMS departments in the study region, enrolled both patients who survived to hospital admission and those who died pre‐hospital. Moreover, all cases in the ARREST registry had ECG documentation to ascertain the presence or absence of VT/VF.
4.1. Strengths and limitations
A major strength of the ARREST registry is the presence of ECG documentation of VT/VF. Given the highly unpredictable way in which OHCA occurs and the low survival rates after OHCA, it is very difficult to study OHCA. Such studies require a dedicated study design, in particular, to ascertain that OHCA resulted from cardiac causes and to reduce misclassification by inclusion of OHCAs from non‐cardiac causes. ECG documentation of VT/VF may be the best method to achieve this. Moreover, the population‐based real‐world design of our ARREST registry minimizes selection bias by ensuring that virtually all OHCAs in our study region are prospectively captured. This is particularly relevant in the present study, since patients with diabetes have reduced pre‐hospital survival rates after OHCA.23 Finally, information about drug use in cases and controls was based on drug‐dispensing records, which is already one step closer to ascertainment of use than analysis of drug prescription.
Our study has also some limitations, e.g., data regarding the comorbidities could not be included in this study. To deal with this, we used concomitant drug use as proxy. Although we have no direct evidence that this method captures the most important cardiovascular diseases sufficiently accurately, we derive assurance that it did from our previous study in which we found similar results when we used this method or direct information on comorbidities.28 Moreover, possible misclassification arising from this was probably similarly distributed between cases and controls. Furthermore, possible confounding by indication might play a role in our study since diabetes itself is a known risk factor for OHCA.2 An approach could be to match case and control subjects on duration of diabetes. Here, duration of antidiabetic treatment might be considered as a proxy for diabetes duration. However, the fact that we only had information on medication use 1 year before index year prevented us from identifying disease duration in all patients. To minimize this potential bias, we selected only patients with diabetes, aiming to make the cases and controls comparable with respect to underlying condition and excluded patients using second‐line antidiabetic drugs or insulin to create a study population with a similar disease severity. Moreover, we compared users of SU drugs to users of metformin only. Still, (unmeasured) residual confounders could not be ruled out since data on several important risk factors such as physical activity, body mass index, left ventricular ejection fraction and blood glucose regulation were not available. Similarly, we had no information about smoking and alcohol use, although we cannot rule out that these confounders were unequally distributed between cases and controls, thereby leading to different baseline risk of cardiovascular disease between both groups. Also, we had no information about renal function and were not able to adjust for it. Yet, the risk of OHCA increases as kidney function declines.29 Socioeconomic position is another risk factor for which we could not adjust because of lack of data, but it is of possible relevance, as it is likely associated with OHCA incidence.30 Finally, misclassification in the use of metformin and SU drugs may have occurred, as we defined drug use as presence of drug‐dispensing records ≤90 days prior to index date. We considered an exposure window of 90 days, since, in the Netherlands, the average repeat prescription length for drugs used for chronic diseases is 90 days. Lengthening of this exposure window might lead to inclusion of drugs that were not used at index date, confounding the analysis of direct drug effects on the primary endpoint of OHCA, as we planned in the present study. Lastly, patients without a proven immediate cause of OHCA were excluded from the analysis that was stratified according to AMI status. Yet, exposure and AMI status are unrelated to missingness, and therefore we do not expect that the exclusion of cases with missing AMI status has impacted our results. Our subgroup analysis was based on small sample sizes, which may have resulted in possibly low statistical power. Thus, findings regarding our subgroup analyses should be interpreted with caution.
5. CONCLUSION
SU drugs (alone or in combination with metformin) were associated with reduced OHCA risk compared to metformin monotherapy. Gliclazide was associated with lower risk than glimepiride. These effects were not solely explained by effects on AP‐shortening during ischaemia.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
T.E.E. and H.L.T. conceived the study idea. T.E.E., M.T.B., A.O.V., P.C.S., A.d.B., H.L.T. designed the research. T.E.E. performed the statistical analyses and wrote the manuscript; A.O.V. and L.J. conducted the patch‐clamp experiments. P.C.S. worked up the original data to a data matrix ready for statistical analyses. All authors critically revised and approved the manuscript.
Supporting information
FIGURE S1 Effects of 10 μM sulfonylurea drugs on action potentials (APs) during simulated ischemia (SI). Left panel, Average changes over time in AP duration (APD) induced by SI in absence or presence of 10 μM sulfonylurea drugs. Right panel, Average APD shortening at 15 min after start of SI in absence or presence of 10 μM sulfonylureas. * indicates P < 0.05
TABLE S1 ATC codes of sulfonylurea drugs
ACKNOWLEDGEMENTS
The authors greatly appreciate the contributions of Paulien Homma, Remy Stieglis and Sandra de Haas for data management of the ARREST registry, and are greatly indebted to all participating EMS dispatch centres (Amsterdam, Haarlem and Alkmaar), regional ambulance services (Ambulance Amsterdam, GGD Kennemerland, Witte Kruis and Veiligheidsregio Noord‐Holland Noord Ambulancezorg), fire brigades, and police departments in the study region for their contribution and support. The authors would also like to thank Leontien Bosch for providing the hiPSC‐CMs, and all the healthcare providers contributing information to the PHARMO Database Network. The authors would also like to thank Stichting Farmaceutische Kerngetallen and the pharmacists for their participation in this study.
This work was supported by the European Union's Horizon 2020 research and innovation programme under the acronym ESCAPE‐NET, registered under grant agreement No. 733381 (T.E.E., M.T.B., H.L.T.), and the COST Action PARQ (grant agreement No. CA19137) supported by COST (European Cooperation in Science and Technology), and the Netherlands CardioVascular Research Initiative (Dutch Heart Foundation, Dutch Federation of University Medical Centers, Netherlands Organization for Health Research and Development, and Royal Netherlands Academy of Sciences) grants CVON‐2017‐15 RESCUED (H.L.T.) and CVON‐2018‐30 Predict‐2 (M.T.B., H.L.T.). The ARREST registry is supported by an unconditional grant from Physio‐Control Inc., part of Stryker, Redmond, WA, USA. The funders were not involved in designing the study, collecting and analysing the data, preparing the manuscript, or the decision to publish.
Eroglu TE, Jia L, Blom MT, et al. Sulfonylurea antidiabetics are associated with lower risk of out‐of‐hospital cardiac arrest: Real‐world data from a population‐based study. Brit Jnl Clinical Pharma. 2021;87(9):3588–3598. 10.1111/bcp.14774
Talip E. Eroglu and Lixia Jia contributed equally to this study.
The authors confirm that the PI for this paper is Hanno L. Tan.
Funding information COST Action PARQ, Grant/Award Number: CA19137; European Union's Horizon 2020, Grant/Award Number: 733381; Netherlands CardioVascular Research Initiative, Grant/Award Numbers: CVON‐2017‐15 RESCUED, CVON‐2018‐30 Predict‐2; Physio‐Control Inc., part of Stryker
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.
REFERENCES
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473‐1482. [DOI] [PubMed] [Google Scholar]
- 2.Siscovick DS, Sotoodehnia N, Rea TD, Raghunathan TE, Jouven X, Lemaitre RN. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev Endocr Metab Disord. 2010;11(1):53‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilde AA, Janse MJ. Electrophysiological effects of ATP sensitive potassium channel modulation: implications for arrhythmogenesis. Cardiovasc Res. 1994;28(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 4.Tan HL, Mazón P, Verberne HJ, et al. Ischaemic preconditioning delays ischaemia induced cellular electrical uncoupling in rabbit myocardium by activation of ATP sensitive potassium channels. Cardiovasc Res. 1993;27(4):644‐651. [DOI] [PubMed] [Google Scholar]
- 5.Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65(3):385‐411. [DOI] [PubMed] [Google Scholar]
- 6.Blom MT, van Hoeijen DA, Bardai A, et al. Genetic, clinical and pharmacological determinants of out‐of‐hospital cardiac arrest: rationale and outline of the AmsteRdam Resuscitation Studies (ARREST) registry. Open Heart. 2014;1(1):e000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herings R, Pedersen L. Pharmacy‐based medical record linkage systems. In: Strom B, Kimmel S, eds. Pharmacoepidemiology. 5th ed.New York: John Wiley & Sons; 2012:270‐286. [Google Scholar]
- 8.Navarrete EG, Liang P, Lan F, et al. Screening drug‐induced arrhythmia [corrected] using human induced pluripotent stem cell‐derived cardiomyocytes and low‐impedance microelectrode arrays. Circulation. 2013;128(11_suppl_1):S3‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrence CL, Proks P, Rodrigo GC, et al. Glicazide produces high‐affinity block of KATP channels in mouse isolated pancreatic beta cells but not rat heart or arterial smooth muscle cells. Diabetologia. 2001;44(8):1019‐1025. [DOI] [PubMed] [Google Scholar]
- 10.Barrett‐Jolley R, McPherson GA. Characterization of KATP channels in intact mammalian skeletal muscle fibres. Br J Pharmacol. 1998;123(6):1103‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song DK, Ashcroft FM. Glimepiride block of cloned β‐cell, cardiac and smooth muscle KATP channels. Br J Pharmacol. 2001;133(1):193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard CE, Brensinger CM, Aquilante CL, et al. Comparative safety of sulfonylureas and the risk of sudden cardiac arrest and ventricular arrhythmia. Diabetes Care. 2018;41(4):713‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander SPH, Mathie A, Peters JA, et al. The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. Br J Pharmacol. 2019;176(Supp 1):S142‐S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114(12):1852‐1866. [DOI] [PubMed] [Google Scholar]
- 15.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23(2):207‐238. [DOI] [PubMed] [Google Scholar]
- 16.Leonard CE, Bilker WB, Brensinger CM, et al. Severe hypoglycemia in users of sulfonylurea antidiabetic agents and antihyperlipidemics. Clinical Pharmacology & Therapeutics. 2016;99(5):538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900‐1908. [DOI] [PubMed] [Google Scholar]
- 18.Khalangot M, Tronko M, Kravchenko V, Kovtun V. Glibenclamide‐related excess in total and cardiovascular mortality risks: data from large Ukrainian observational cohort study. Diabetes Res Clin Pract. 2009;86(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonard CE, Hennessy S, Han X, Siscovick DS, Flory JH, Deo R. Pro‐ and antiarrhythmic actions of sulfonylureas: mechanistic and clinical evidence. Trends Endocrinol Metabol. 2017;28(8):561‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolleben CD, Sanguinetti MC, Siegl PK. Influence of ATP‐sensitive potassium channel modulators on ischemia‐induced fibrillation in isolated rat hearts. J Mol Cell Cardiol. 1989;21(8):783‐788. [DOI] [PubMed] [Google Scholar]
- 22.Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M. Pharmacological evidence for the persistent activation of ATP‐sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation. 1995;92(8):2266‐2275. [DOI] [PubMed] [Google Scholar]
- 23.Grover GJ, Sleph PG, Dzwonczyk S. Pharmacologic profile of cromakalim in the treatment of myocardial ischemia in isolated rat hearts and anesthetized dogs. J Cardiovasc Pharmacol. 1990;16(6):853‐864. [DOI] [PubMed] [Google Scholar]
- 24.Davis TM, Parsons RW, Broadhurst RJ, Hobbs MS, Jamrozik K. Arrhythmias and mortality after myocardial infarction in diabetic patients. Relationship to diabetes treatment. Diabetes Care. 1998;21(4):637‐640. [DOI] [PubMed] [Google Scholar]
- 25.Lomuscio A, Vergani D, Marano L, Castagnone M, Fiorentini C. Effects of glibenclamide on ventricular fibrillation in non‐insulin‐dependent diabetics with acute myocardial infarction. Coron Artery Dis. 1994;5(9):767‐771. [PubMed] [Google Scholar]
- 26.Cacciapuoti F, Spiezia R, Bianchi U, Lama D, D'Avino M, Varricchio M. Effectiveness of glibenclamide on myocardial ischemic ventricular arrhythmias in non‐insulin‐dependent diabetes mellitus. Am J Cardiol. 1991;67(9):843‐847. [DOI] [PubMed] [Google Scholar]
- 27.Van Hoeijen DA, Blom MT, Bardai A, Souverein PC, De Boer A, Tan HL. Reduced pre‐hospital and in‐hospital survival rates after out‐of‐hospital cardiac arrest of patients with type‐2 diabetes mellitus: an observational prospective community‐based study. Europace. 2015;17(5):753‐760. [DOI] [PubMed] [Google Scholar]
- 28.Eroglu TE, Mohr GH, Blom MT, et al. Differential effects on out‐of‐hospital cardiac arrest of dihydropyridines: real‐world data from population‐based cohorts across two European countries. Eur Heart J Cardiovasc Pharmacother. 2020;6(6):347‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pun PH. The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis. 2014;21(6):480‐488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Nieuwenhuizen BP, Oving I, Kunst AE, et al. Socio‐economic differences in incidence, bystander cardiopulmonary resuscitation and survival from out‐of‐hospital cardiac arrest: a systematic review. Resuscitation. 2019;141:44‐62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Effects of 10 μM sulfonylurea drugs on action potentials (APs) during simulated ischemia (SI). Left panel, Average changes over time in AP duration (APD) induced by SI in absence or presence of 10 μM sulfonylurea drugs. Right panel, Average APD shortening at 15 min after start of SI in absence or presence of 10 μM sulfonylureas. * indicates P < 0.05
TABLE S1 ATC codes of sulfonylurea drugs
Data Availability Statement
The data underlying this article cannot be shared publicly due to ethical/privacy reasons.


