Abstract
Chronic graft-versus-host disease (cGVHD) is the leading late complication after allogeneic hematopoietic stem cell transplantation (HSCT). Many patients receive multiple lines of systemic therapy until cGVHD resolves, but about 15% remain on systemic treatment for more than 7 years after cGVHD diagnosis. This study describes the clinical and biological factors of patients who present with cGVHD persisting for ≥7 years (persistent cGVHD). Patients with persistent cGVHD (n = 38) and those with cGVHD for <1 year (early cGVHD) (n = 83) were enrolled in a prospective cross-sectional natural history study. Patients in the persistent cGVHD group were a median of 10.2 years from cGVHD diagnosis (range 7–27 years). Fifty-eight percent of persistent cGVHD patients (22/38) were receiving systemic immunosuppression, compared to 88% (73/83) in the early cGVHD group. In multivariable analysis, bone marrow (BM) stem cell source, presence of ENA autoantibodies, higher NIH lung score, higher platelet counts, and higher IgA levels were significantly associated with persistent cGVHD. A high sensitivity panel of serum biomarkers including seven cytokines diagnostic for cGVHD was analyzed and showed significantly lower levels of BAFF and CXCL10 in patients with persistent cGVHD. In conclusion, standardly accepted clinical measures of disease severity may not accurately reflect disease activity in patients with persistent cGVHD. However, many patients with persistent cGVHD are still receiving systemic immunosuppression despite lacking evidence of disease activity. Development of reliable clinical biomarkers of cGVHD activity may help guide future systemic treatments.
1 |. INTRODUCTION
Chronic graft-versus-host disease (cGVHD) is the leading cause of late non-relapse morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT).1 cGVHD is a systemic immune disorder affecting multiple organs including skin, oral mucosa, eyes, genitalia, lungs, gastrointestinal tract, liver, joints and fascia.2 Due to its multi-organ nature, most treatments require systemic immunosuppression with corticosteroids or various other immunomodulators. Two-year cumulative incidence of cGVHD requiring systemic treatment is between 30% and 40%.3 The average duration of systemic immunosuppression for cGVHD is 2–3 years. However, approximately 15% of patients still receive systemic immunosuppression 7 years after diagnosis of cGVHD.4 The duration of immunosuppression with corticosteroids is of critical importance as its long term use is associated with debilitating side effects including increased susceptibility to infections, myopathy, cataracts, osteoporosis, steroid-induced diabetes, cardiovascular events, psychological changes, and weight changes.5 Even non-steroidal systemic therapies are not benign and have a wide range of toxicities.6,7 Thus, better understanding the natural history, biology, and course of cGVHD in patients requiring prolonged systemic therapy will enable development of appropriate treatments and ability to respond to individual patient needs.
Prior studies have identified some clinical factors that were associated with longer duration of systemic immunosuppression, including: peripheral blood HSCT graft source, female stem cell donor to male recipient, donor-recipient human leukocyte antigen (HLA) mismatch, serum bilirubin >2 mg/dL at diagnosis of cGVHD, and increased number of organ sites involved by cGVHD.4 However, there is paucity of information describing characteristics of patients with persistent cGVHD lasting for ≥7 years. The predictive factors and underlying pathogenesis driving persistent cGVHD are unknown. Symptoms in many of these patients, such as those related to eyes, salivary glands, lungs or joint contractures could also be a reflection of irreversible target organ damage and late-stage fibrosis, rather than a continued active immune inflammatory process.
A serious limitation in studying patients with persistent cGVHD is the absence of reliable diagnostic tools that can decipher symptoms and signs related to active disease vs cumulative target organ damage. The implication is that some patients might be exposed to prolonged and potentially unnecessary doses of systemic therapies despite less active cGVHD. Prior studies sought to identify potential serum biomarkers of cGVHD diagnosis, progression and response to immunosuppressive treatment.8 Cytokines including B cell activating factor (BAFF), CXCL9, and CXCL10 have been shown to be significantly increased in cGVHD patients compared to patients without cGVHD.9–12 However, such biomarkers of systemic inflammation have not been studied specifically in patient cohorts with persistent cGVHD. The aim of this study is to describe clinical and biological characteristics in clinically annotated patients referred with cGVHD persisting for more than 7 years after diagnosis.
2 |. METHODS
Patients were enrolled in a cross-sectional prospective study of the natural history of cGVHD at the National Institutes of Health (NIH) (NCT00092235). This study involves a multi-disciplinary team evaluation during a 1 week visit by specialists with expertise in cGVHD (dermatology, dentistry, rehabilitation medicine, occupational therapy, gynecology, pain and palliative care, hematology/oncology and ophthalmology). Patients referred by their primary transplant physician for cGVHD evaluation who were able to give written informed consent were eligible. Patients who enrolled on the study ≥7 years from the time of cGVHD diagnosis (defined as persistent cGVHD) were compared to those who enrolled <1 year from cGVHD diagnosis (defined as early cGVHD). Healthy volunteers without any evidence of cGVHD were also included as controls for the serum cytokine analyses.
Clinical variables collected included age, gender, Karnofsky Performance Status, underlying disease, donor relationship, type of stem cell source, conditioning regimen intensity, administration of total body irradiation, donor HLA mismatch, number of involved organs and cGVHD subclassification. Other clinical variables were type of cGVHD onset, NIH global severity, NIH individual and average organ scores, Lee cGVHD total symptom scale and subscales (skin, eyes and mouth, breathing, eating and digestion, muscles and joints, energy, mental and emotional). Additional variables were presence of moderate/high immunosuppression intensity (moderate intensity defined as prednisone ≥0.5 mg/kg/day and/or any single agent/modality; high intensity defined as two or more modalities/agents ± prednisone ≥0.5 mg/kg/day13), prednisone dose at the time of evaluation, Human Activity Profile (HAP) patient self-report, walk velocity, cGVHD clinician and patient assessed global severity and sclerotic and erythema body surface area involved. Patients were categorized based on their distance from the transplant center (0–25 miles, 26–100 miles, 101–300 miles and > 300 miles) to address if barriers to care may have an effect on the duration of cGVHD.
Patientsʼ cGVHD disease was also categorized based on status per the EBMT-NIH-CIBMTR consensus paper, into one of the four following categories: active, controlled, inactive or resolved.14 The “active” group had ongoing inflammatory or worsening manifestations irrespective of their treatment status or cGVHD sequelae. The “controlled” group did not have those manifestations and was on immunosuppressive therapy (IST) or off IST for <24 weeks. The rest of the patients with no active cGVHD and off IST >24 weeks were divided either into “inactive” or “resolved” groups, with the “inactive” group having cGVHD sequalae and the “resolved” group having no sequalae. The Lee cGHVD symptom scale was used to evaluate the symptom burden including skin, eyes and mouth, breathing, eating and digestion, muscle and joints, energy, mental and emotional, with a higher score indicating higher disease burden.15 HAP is a 94-item self-report measure of energy expenditure or physical fitness.16
Laboratory variables collected included serum bilirubin, C-reactive protein, erythrocyte sedimentation rate, C3 and C4 complement, albumin, ferritin, autoantibodies (CCP, ds-DNA, LKM-1, mitochondrial, ANA, ENA, anti-cardiolipin, RF), IgG, IgM, IgA and lymphocyte subsets CD3, CD4, CD8, CD19 and NK cells. IFN- γ, IL-6, IP-10, and MCP-1 were assayed using V-PLEX by from Meso-ScaleDiscovery (MSD). The BAFF, CXCL9, and ST2 high-sensitive assays were developed and customized for clinical testing using MSD electrochemiluminescence immunoassay technology with antibody pairs obtained from R&D Systems.
2.1 |. Selection of cytokines
The cytokines measured in this study included BAFF, CXCL9, IP-10 (CXCL10), IL-6, IFN-γ, ST2, and MCP-1. BAFF is a member of the TNF ligand family that functions to promote B-cell survival. BAFF is upregulated in patients with cGVHD and is also predictive of cGVHD development.11,17 Interferon- γ (IFN-γ) inducible pathways along with release of CXCL9 from myeloid tissues and local production of IL-6 may lead to initiation and persistence of cGHVD.18 In addition, CXCL9 levels were increased in newly diagnosed cGVHD and affected by disease activity.19 The IFN-γ inducible protein-10 (IP-10), also known as CXCL10, and ST2, a member of the IL-1 family, was also associated with active cGVHD.8–10,20 Monocyte chemoattractant protein-1 (MCP-1) is a known chemoattractant for monocytes and may similarly contribute to local inflammation seen in cGVHD.
2.2 |. Statistical analysis
Factors reported as a continuous parameter, or that could be essentially considered as if continuous, were compared between two groups using a Wilcoxon rank test, and among three groups using a Kruskal-Wallis test. Ordered categorical parameters were compared between the two groups using a Cochran- Armitage test for the trend. Dichotomous parameters were compared between the two groups using Fisherʼs exact test. Following an initial screening by the univariate methods described, univariate and multivariable logistic regression analysis was used to identify a set of factors that could jointly impact the persistent vs early cGVHD classification. All P-values reported are two-tailed and presented without any formal adjustment for multiple comparisons. In view of the number of the tests performed, P values for the univariate analyses such that P < .005 could be considered statistically important, while .005 < P < .05 would represent strong trends.
3 |. RESULTS
Between 2004 and 2015, 38 patients with persistent cGVHD and 83 patients with early cGVHD were prospectively enrolled as part of the cross-sectional natural history study. Fourteen patients were excluded due to patient declination to complete study (n = 2), lack of cGVHD (n = 9), and PI discretion (n = 3); patients (n = 138) who were 1 to 7 years from cGVHD diagnosis were also excluded. In patients with persistent cGVHD, the median time from diagnosis to enrollment was 10.2 years (range 7–27), (Figure S1). Patients with early cGVHD were slightly older (median age 48.5 years compared to 41.9 years in persistent cGVHD patients, P = .10), but both groups had similar performance status.
Univariate analysis revealed several clinical characteristics that were different between the groups (Tables 1 and 2). Factors that were associated with persistent cGVHD included: bone marrow (BM) stem cell source, myeloablative conditioning, higher breathing symptom burden on Lee cGVHD symptom scale, lower ferritin, lower doses of prednisone, positive ENA autoantibody, and higher platelets, CD19 cells, CD4 cells, IgG and IgA levels. Fifty-eight percent of patients (22/38) in the persistent cGVHD group were on systemic immunosuppression compared to 88% (73/83) in the early cGHVD group. Fewer patients with persistent cGVHD were on higher intensity immunosuppression than newly diagnosed patients (47% vs 80%, P < .001, Tables S1 and S2). In addition, lung involvement was more common among patients with persistent cGVHD (87% vs 65%, P = 0.002). This finding was supported by the fact that the persistent cGVHD group had lower FEV1 values than the early cGVHD group, consistent with long-standing lung damage. This finding is also consistent with earlier observations of lower lung scores in patients able to discontinue systemic immunosuppression21 (Table 1). Finally, there were no differences in factors associated with cGVHD disease characteristics, NIH global or organ severity, or distance from home to the transplant center.
TABLE 1.
Univariate analysis of clinical factors associated with persistent chronic GVHD
| Persistent cGVHD (>7 years from diagnosis) N = 38*** |
Early cGVHD (<1 year from cGVHD diagnosis) N = 83*** |
P value | |
|---|---|---|---|
| Baseline transplant characteristics | |||
| Patient age, median (range) | 41.9 (16–66) | 48.5 (7–72) | .10 |
| Karnofsky performance status % (median) | 80 | 80 | .81 |
| Donor/patient gender (F/M) | 15 (42%) | 14 (18%) | .01 |
| Underlying disease | |||
| • Lymphoid | 7 (18%) | 38 (46%) | .004 |
| • Myeloid/other | 31 (82%) | 44 (54%) | |
| Related donor | 28 (74%) | 45 (56%) | .07 |
| Bone marrow (BM) stem cell source | 22 (58%) | 9 (11%) | ≤.0001 |
| Myeloablative conditioning regimen | 32 (86%) | 37 (45%) | ≤.0001 |
| TBI in conditioning | 15 (41%) | 21 (26%) | .13 |
| HLA-mismatch | 6 (17%) | 8 (10%) | .36 |
| cGVHD characteristics | |||
| Number of organs involved (median) | 4 | 4 | .35 |
| Serum bilirubin at the time of evaluation in mg/dL (median) | 0.4 | 0.6 | .19 |
| Prednisone dose at the time of evaluation in mg/kg (median, range) | 0 (0–0.57) | 0.194 (0–3.37) | .001 |
| Progressive type of onset | 19 (51%) | 28 (34%) | .10 |
| cGVHD classification | |||
| • Classic | 34 (94%) | 61 (74%) | .01 |
| • Overlap | 2 (6%) | 21 (26%) | |
| NIH global severity | |||
| • Moderate | 10 (26%) | 35 (44%) | .10 |
| • Severe | 28 (74%) | 45 (56%) | |
| NIH average organ score (median) | 1 | 0.857 | 0.05 |
| NIH individual organ scores* | |||
| • Skin | 27 (71%) | 60 (73%) | .61 |
| • Mouth | 25 (66%) | 57 (70%) | .40 |
| • Eyes | 29 (76%) | 62 (77%) | .31 |
| • GI tract | 22 (58%) | 36 (44%) | .10 |
| • Liver | 16 (43%) | 45 (55%) | .28 |
| • Lungs | 33 (87%) | 53 (65%) | .002 |
| • Joints and fascia | 21 (55%) | 38 (46%) | .09 |
| • Genital | 10 (53%) | 23 (53%) | .98 |
| cGVHD by therapeutic intent | |||
| • Active | 17 (63%) | 24 (34%) | .01 |
| • Not active | 10 (37%) | 47 (66%) | |
| EBMT-NIH-CIBMTR classification | |||
| • Active | 22 (63%) | 39 (49%) | .007 |
| • Controlled | 9 (26%) | 40 (50%) | |
| • Inactive | 4 (11%) | 1 (1%) | |
| Moderate to high intensity of IS ** | 18 (47%) | 66 (80%) | <.001 |
| Lee symptom scale total (median) | 39.5 | 34 | 0.02 |
| Lee scale subscores (median) | |||
| • Skin | 6 | 5 | .83 |
| • Eyes and mouth | 8 | 6 | .09 |
| • Breathing | 5 | 2 | .001 |
| • Eating and digestion | 2 | 1 | .11 |
| • Muscles and joints | 7.5 | 5.5 | .05 |
| • Energy | 5.5 | 4 | .02 |
| • Mental and emotional | 4 | 3 | .31 |
| Sclerotic cGVHD (dermal) %BSA | 0.005 | 0 | .008 |
| Sclerotic cGVHD (deep) %BSA | 0 | 0 | .05 |
| Erythema, %BSA | 0 | 0.004 | .033 |
| Total BSA, percent (median) | 20 | 22 | .30 |
| Clinician severity, Form A**** (median) | 6 | 6 | .92 |
| Patient severity, Form B**** (median) | 6 | 5 | .55 |
| FEV1 (median) | 69 | 92.65 | <.001 |
| HAP, MAS***** (median) | 77 | 71 | .23 |
| HAP, AAS****** (median) | 65 | 58 | .35 |
| Two minutes walk test in feet (median) | 556 | 594 | .34 |
| Range of motion, percent predicted (median) | 40 | 55 | .16 |
| Socioeconomic characteristics | |||
| Distance from transplant center | |||
| • 0–25 miles | 9 (28%) | 17 (23%) | .78 |
| • 26–100 miles | 8 (25%) | 22 (29%) | |
| • 101–300 miles | 4 (13%) | 10 (13%) | |
| • >300 miles | 11 (34%) | 26 (35%) | |
Values in bold are the variables turned to be statistically important (P < .005) and their corresponding P values.
NIH organ scores are reported on a 0 to 3 scale indicating increasing category of involvement. The percentages shown are for scores of 1–3. P-values determined by Cochran-Armitage test for trend across all ordered categories.
Moderate: prednisone ≥0.5 mg/kg/day and/or any single agent/modality; high: two or more modalities/agents ±prednisone ≥0.5 mg/kg/day.
Percentages are based on numbers with complete information for a given parameter.
Form A and Form B are used to assess clinician assessed and patient reported cGVHD symptoms, respectively.
HAP, MAS: Human Activity Profile, Maximum Activity Score is the highest oxygen demanding activity still performed and is determined in comparison with peers of same age and gender.
HAP, AAS: Human Activity Profile, Adjusted Activity Score is the MAS minus the total number of stopped doing responses below MAS and represents the average level of activity.
TABLE 2.
Univariate analysis of laboratory factors associated with persistent chronic GVHD
| Persistent cGVHD (>7 years from diagnosis) N = 38 |
Early cGVHD (<1 year from diagnosis) N = 83 |
P value | |
|---|---|---|---|
| Platelet count, 109/L (median) | 277.5 | 193 | <.0001 |
| ESR, mm/hr (median) | 15 | 18.5 | .44 |
| CRP, mg/L (median) | 2.175 | 1.43 | .27 |
| C3, mg/dL (median) | 140.5 | 128 | .03 |
| C4, mg/dL (median) | 28.5 | 27 | .14 |
| Albumin, g/dL (median) | 4 | 3.5 | <.0001 |
| Ferritin, ng/mL (median) | 150 | 1240 | <.0001 |
| CD3, cells/μL (median) | 897.5 | 691 | .15 |
| CD4, cells, μL (median) | 533.5 | 327 | .002 |
| CD8, cells μL (median) | 320.5 | 310 | .88 |
| CD19, cells μL (median) | 265.5 | 87 | <.001 |
| NK, cells/μL (median) | 170.5 | 144.5 | .03 |
| IgG, mg/dL (median) | 756 | 516 | <.001 |
| IgM, mg/dL (median) | 70 | 49 | .007 |
| IgA, mg/dL (median) | 145 | 32 | <.0001 |
| Presence of autoantibodies (%) | |||
| Anti-CCP* | 5 (13%) | 4 (5%) | .151 |
| Anti-dsDNA* | 0 (0%) | 2 (3%) | 1.0000 |
| Anti-LKM-1* | 19 (50%) | 25 (32%) | .068 |
| Anti-mitochondrial | 3 (8%) | 10 (13%) | .545 |
| ANA* | 14 (37%) | 17 (21%) | .0789 |
| ENA * | 7 (18%) | 1 (1%) | .001 |
| Anti-cardiolipin IgM | 6 (16%) | 3 (4%) | .057 |
| Anti-cardiolipin IgG | 1 (3%) | 7 (9%) | .272 |
| Rheumatoid Factor | 5 (14%) | 5 (6%) | .284 |
CCP-1: Citric citrullinated peptide, dsDNA: double stranded DNA, LKM-1: Liver-kidney microsomal type 1, ANA: anti-nuclear antibody, ENA: Extractable nuclear antigen.
Approximately 30 parameters with P < .10 were identified in the univariate screening process, but only variables missing ≤15 data values were included in the multivariable logistic analysis. Factors that had P < .05 in a univariate logistic regression analysis were subsequently evaluated in the multivariable model by a backward selection process. In the multivariable model using this process, BM stem cell source, presence of ENA autoantibodies, higher NIH lung score, higher platelet count and higher IgA were identified as being significantly associated with persistent cGVHD (Table 3).
TABLE 3.
Multivariable analysis depicting factors associated with persistent cGVHD after adjusting for factors in the univariate analysis: bone marrow stem cell source, presence of ENA autoantibodies, higher NIH lung score, higher platelet count and higher IgA. Standard estimate stands for parameter estimate that is part of a model predicting patients with persistent cGVHD. The higher the value, the stronger the contribution of that variable to the model
| Parameter | DF | Estimate | Standard error | Wald Chi-square | P value |
|---|---|---|---|---|---|
| Intercept | 1 | −6.35 | 1.33 | 22.71 | <.0001 |
| Bone marrow (BM) stem cells | 1 | 1.85 | 0.67 | 7.71 | .006 |
| ENA autoantibody | 1 | 4.06 | 1.37 | 8.76 | .003 |
| NIH lung score | 1 | 1.02 | 0.36 | 7.96 | .005 |
| Platelets | 1 | 0.0077 | 0.0032 | 5.83 | .016 |
| IgA | 1 | 0.015 | 0.0045 | 11.97 | <.001 |
Estimates represent the difference in log(odds) or log(odds ratio). Estimates correspond to the following comparisons: patients who received bone marrow stem cells vs. patients who received peripheral blood or cord blood stem cells; patients with detectable extractable nuclear antigen (ENA) antibodies vs. patients with undetectable antibodies; patients who differ by one stage of NIH lung score; patients who differ by 109/L platelets; and patients who differ by 1mg/dL of IgA.
In the cytokine analysis, patients with persistent cGVHD had significantly lower levels of BAFF and CXCL10. Also, CXCL9, IFN-γ, MCP-1 and IL-6 showed a trend towards lower levels in the persistent cGVHD group. This suggests a lower level of inflammation in these patients (Figure 1). Additionally, IL-6, CXCL9, BAFF, CXCL10, MCP-1 and ST2 were all significantly higher in the early cGVHD group compared to healthy volunteers.
FIGURE 1.
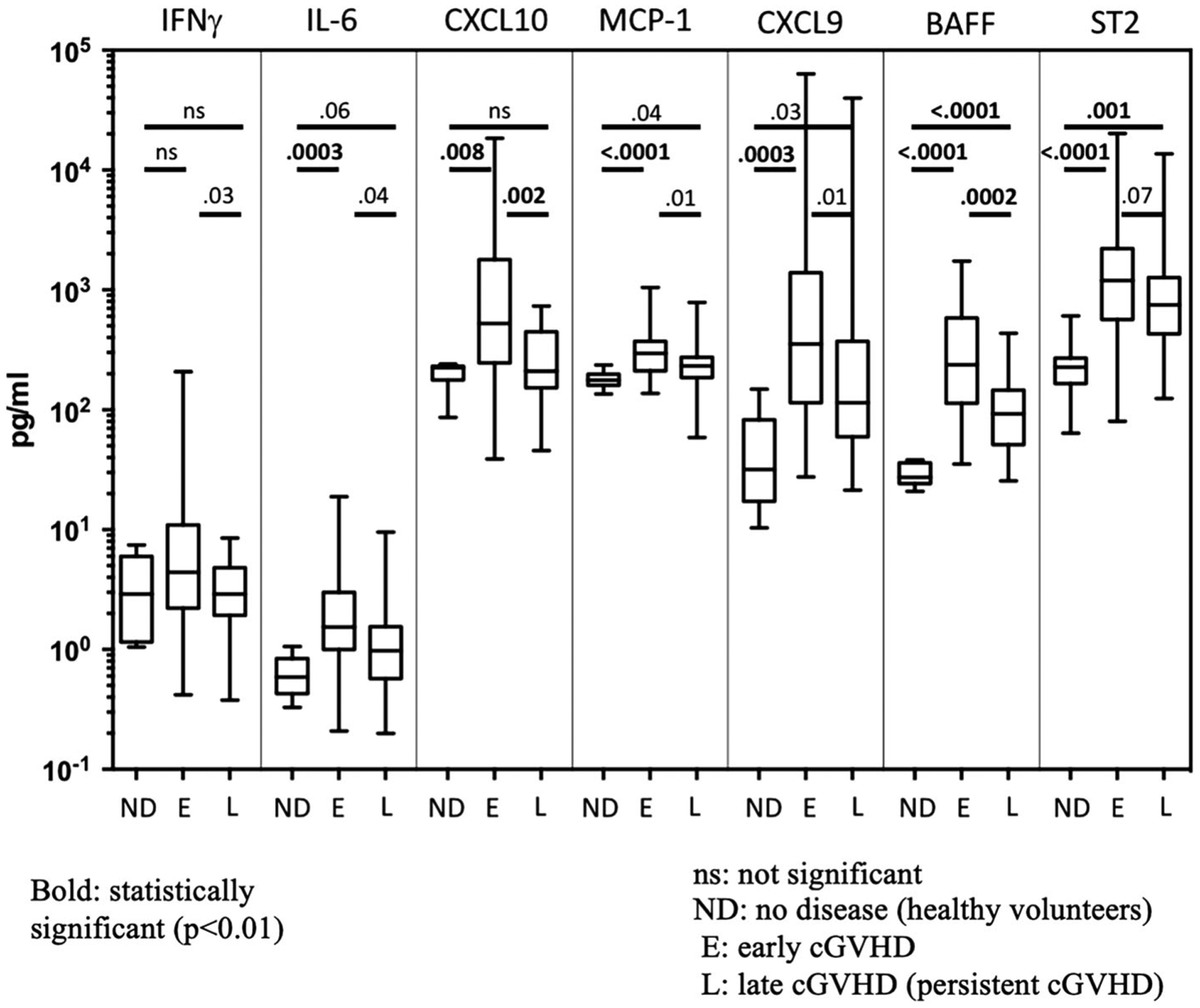
Cytokine analysis in persistent vs early chronic GVHD and healthy volunteer. Graph comparing levels of 7 selected cGVHD biomarkers (in pg/ml) among individuals with no disease (ND), early cGVHD (<1 year) (E) and persistent cGVHD >7 years (L) using Wilcoxon rank sum test. A total of 52 early and 26 persistent cGVHD patients were tested with the ELISA panels. Each bar represents the SD for the measurement within each group and solid line within the bar stands for the median value. Vertical lines represent the range of measurements. BAFF and CXCL10 levels were significantly higher in the early cGVHD group compared to persistent cGVHD group. IL-6, CXCL9, BAFF, CXCL10, MCP-1 and ST2 were all significantly higher in the early cGVHD group than in healthy volunteers
Per the EBMT-NIH-CIBMTR consensus GVHD categorizations, patients with persistent and early cGVHD were categorized into the “active,” “controlled”, and “inactive” groups. There were no patients fitting into the definition of “resolved” GVHD among our cohort. To see if worsening GVHD or treatment has any effect on the levels of biomarkers of cGVHD, the cytokine levels were compared among all the cGVHD status categories for patients in the persistent cGVHD group. The CXCL10 levels were higher in the active group (n = 16) compared to controlled (n = 5) and inactive (n = 2) groups (median 312.6 vs 144.5 and 167.6, respectively, P = .04, for overall comparison). However, there was no difference between controlled and inactive groups (P = .57) in CXCL10 levels, at least in part because of the limited number of patients in each group. BAFF and ST2 levels in persistent cGVHD did not demonstrate significant differences between active and controlled or inactive groups (data not shown).
4 |. DISCUSSION
This study aimed to describe clinical and biological characteristics of patients with persistent cGVHD ≥7 years after diagnosis. Patients with persistent cGVHD were more likely to have lung cGVHD and a higher Lee cGVHD breathing symptom burden, but lower levels of cytokines that are diagnostic and prognostic for cGVHD, including BAFF and CXCL10 compared to the patients with early cGVHD. Most (74%) of these patients with persistent cGVHD were determined to have severe cGVHD by NIH global severity and 58% of these patients remain on immunosuppression. Our findings suggest that patients with persistent cGVHD may have less active inflammatory processes and increased symptom burden, requiring a more tailored need for treatment due to accumulated irreversible damage, involving those focused on reversing sclerotic processes. Standard accepted clinical measures of disease severity may not be helpful in distinguishing active disease from accumulated late effects in target organs and tissues.
Interestingly, despite the severity of disease and high symptom burden scores, persistent cGVHD patients on this study showed fewer laboratory indicators of systemic inflammation, as reflected by significantly higher albumin and lower ferritin serum levels than in the early cGVHD group. In addition, plasma cytokine analysis revealed a significantly lower level of CXCL10 and BAFF in persistent cGVHD, compared to the <1 year early cGVHD group, and a trend towards lower levels for CXCL9, IFN-γ, MCP-1 and IL-6. In the persistent cGVHD group, patients were further categorized based on the EBMT-NIH-CIBMTR consensus disease status categories and patients with active cGVHD were found to have higher levels of CXCL10 compared to patients in the inactive and controlled cGVHD, despite the limited number of sample size in the latter two groups. Several groups have shown BAFF and CXCL10 to be diagnostic and prognostic for GVHD, and that BAFF levels are increased at the onset of and throughout pulmonary cGVHD.12,22,23 However, despite having increased lung scores and lower FEV1, patients in the persistent cGVHD group had significantly lower cytokine levels compared to those in the early cGVHD group, which cannot be explained by the lower median dose of steroids in this group (median 0 compared to 0.19 mg/kg prednisone equivalent). These findings suggest that clinically persistent cGVHD may not accurately reflect immunologically active disease. Symptoms in some of these patients might be driven by irreversible damage and end stage fibrosis rather than an active disease process. It is noteworthy that 63% of these patients with persistent cGVHD were determined after comprehensive multi-specialist clinical evaluation as having active cGVHD, and were recommended to consider further intensification or change of systemic therapy. As an alternate explanation, lower levels of BAFF in the persistent cGVHD group could possibly be explained by the higher number of B-cells compared to the early cGVHD group, as B-cells remove BAFF from the plasma.11,18 This finding is also supported by the higher IgA levels in the early cGVHD group. Regardless, the difference in the levels of BAFF between the two groups is conspicuous and is a finding that requires further research and understanding.
In addition to higher levels of B-cells and immunoglobulins, patients with persistent cGVHD also had higher levels of autoantibodies. In particular, the presence of extractable nuclear antigen (ENA) autoantibodies was predictive for persistent cGVHD in this analysis. The ENA is a set of antigens which include Ro, La, Sm and many other nuclear and ribonuclear antigens. Antibodies to these antigens are seen in a wide range of rheumatologic diseases, particularly Sjogrenʼs disease, systemic lupus erythematosus, and systemic sclerosis.24 These diseases have clinical manifestations including xerostomia, salivary gland destruction, oral sensitivity and keratoconjunctivitis sicca, which are similar to oral and eye cGVHD. A prior study showed that presence of various autoantibodies was common in oral cGVHD involvement.25 That study also found that patients with >1 year cGVHD duration had a higher incidence of autoantibodies compared to patients <1 year. Though it remains unclear if any of these autoantibodies have a pathogenic role in persistent cGVHD, their significance should be further investigated in this clinical setting.
Interestingly, variables shown in prior studies to predict longer time for discontinuation of systemic immunosuppression at time of cGVHD diagnosis, such as HLA mismatch, serum bilirubin levels, number of involved GVHD sites or peripheral blood stem cell source, did not show differences in frequencies between the early and persistent comparison groups here. This lack of association could be due to the study design. For example, BM stem cell source was predictive for persistent chronic GVHD in both univariate and multivariate analyses. This is contrary to the previous research stating that peripheral stem cell graft sources are associated with higher incidence of cGHVD and increased need for immunosuppression.26,27 This observation has a few possible explanations. As the persistent cGHVD group is followed ≥7 years and many of these patients underwent their transplant over a decade ago, that group had a higher usage of BM stem cell source (Table 1), as peripheral stem cells as a source in allogeneic HSCT became widespread only after the early-mid 2000s.26 The fact that 60% (23/38) of patients in the persistent cGVHD group were transplanted before the year 2000 and 83% (19/23) of those patients received BM stem cell source is in line with this explanation. In contrast, 100% of our early cGVHD group received their transplant after the year 2000. Alternatively, patients with peripheral stem cell source may have succumbed to their higher cGVHD burden earlier, thus leaving a higher percentage of patients with BM stem cell source in the persistent cGVHD group.28–30
This study had some limitations that should be taken into consideration. First, the patient population was selected from a cross sectional study, so it does not allow us to longitudinally analyze and associate clinical findings with potential laboratory markers of disease activity. Secondly, the cytokine panel used here does not include other cytokines of interest like MMP3 and ostepontin for example, that are putative biomarkers of cGVHD.20 A longitudinal study with a more comprehensive cytokine panel measured at scheduled and event-driven time points may provide more information about the biology in persistent cGVHD. Nevertheless, the results of the study presented here were obtained in a clinically annotated cohort of patients with long standing cGVHD, who have been determined by their primary clinicians as having active cGVHD and potentially requiring systemic therapy.
In conclusion, the findings presented here demonstrate that most patients with persistent cGVHD are still on substantial doses of systemic immunosuppression while our current measures including laboratory signs of systemic inflammation may not capture the degree of GVHD sequalae, such as fibrosis and sclerosis. Symptoms in these patients are commonly pulmonary and may be due to irreversible target organ damage or fibrosis rather than presence of active inflammation, and treatment approach should be tailored accordingly. The results presented here also support the practice to exert increased caution when making therapeutic decisions about systemic therapy in patients with persistent cGVHD, especially in patients with fibrotic changes. The development of clinically useful biomarkers of cGVHD activity is an imperative and high research priority.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the patients and their loved ones for their participation in the National Institutes of Health cGVHD natural history study. This study was supported by the Intramural Research Program of the National Institutes of Health, the Center for Cancer Research, and the National Cancer Institute. The views expressed in this work do not necessarily represent the views of the National Institutes of Health or the United States Government.
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest to report.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104: 3501–3506. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarantopoulos S, Cardones AR, Sullivan KM. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff D, Schleuning M, von Harsdorf S, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17: 1–17. [DOI] [PubMed] [Google Scholar]
- 8.Paczesny S Biomarkers for posttransplantation outcomes. Blood. 2018;131:2193–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paczesny S, Zaid MA. Transplantation: CXCL10: most consistent cGVHD biomarker? Blood. 2016;127:2950–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariminia A, Holtan SG, Ivison S, et al. Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood. 2016;127:3082–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeo-stasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff D, Greinix H, Lee S, et al. Biomarkers in chronic graft-versus-host disease: quo vadis? Bone Marrow Transplant. 2018;53: 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell S, Leidy NK, Mooney K, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant. 2010;45:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT− NIH− CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018; 53:1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–452. [DOI] [PubMed] [Google Scholar]
- 16.Davidson M, de Morton N. A systematic review of the Human Activity Profile. Clin Rehabil. 2007;21:151–162. [DOI] [PubMed] [Google Scholar]
- 17.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakim FT, Memon S, Jin P, et al. Upregulation of IFN-inducible and damage-response pathways in chronic graft-versus-host disease. J Immunol. 2016;197:3490–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitko CL, Levine JE, Storer BE, et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Storer BE, Kushekhar K, et al. Biomarker panel for chronic graft-versus-host disease. J Clin Oncol. 2016;34:2583–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis LM, Pirsl F, Steinberg SM, et al. Predictors for permanent discontinuation of systemic immunosuppression in severely affected chronic graft-versus-host disease patients. Biol Blood Marrow Transplant. 2017;23:1980–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzmina Z, Krenn K, Petkov V, et al. CD19+ CD21low B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121:1886–1895. [DOI] [PubMed] [Google Scholar]
- 23.Ren H-G, Adom D, Paczesny S. The search for drug-targetable diagnostic, prognostic and predictive biomarkers in chronic graft-versus-host disease. Expert Rev Clin Immunol. 2018;14:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan TG, Wong RC, Adelstein S. Autoantibodies to extractable nuclear antigens: making detection and interpretation more meaningful. Clin Diagn Lab Immunol. 2002;9:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuzmina Z, Gounden V, Curtis L, et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. 2015;90:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bensinger W, Weaver C, Appelbaum F, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1995;85:1655–1658. [PubMed] [Google Scholar]
- 27.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jim HS, Sutton SK, Jacobsen PB, et al. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer. 2016;122:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flowers ME, Parker PM, Johnston LJ, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415–419. [DOI] [PubMed] [Google Scholar]
- 30.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


