Abstract
Objective
Low back pain is one of the most common reasons for which people visit their doctor. Between 12% and 15% of the US population seek care for spine pain each year, with associated costs exceeding $200 billion. Up to 80% of adults will experience acute low back pain at some point in their lives. This staggering prevalence supports the need for increased research to support tailored clinical care of low back pain. This work proposes a multidimensional conceptual taxonomy.
Methods
A multidisciplinary task force of the ACTTION-APS-AAPM Pain Taxonomy (AAAPT) with clinical and research expertise performed a focused review and analysis, applying the AAAPT five-dimensional framework to acute low back pain.
Results
Application of the AAAPT framework yielded the following: 1) Core Criteria: location, timing, and severity of acute low back pain were defined; 2) Common Features: character and expected trajectories were established in relevant subgroups, and common pain assessment tools were identified; 3) Modulating Factors: biological, psychological, and social factors that modulate interindividual variability were delineated; 4) Impact/Functional Consequences: domains of impact were outlined and defined; 5) Neurobiological Mechanisms: putative mechanisms were specified including nerve injury, inflammation, peripheral and central sensitization, and affective and social processing of acute low back pain.
Conclusions
The goal of applying the AAAPT taxonomy to acute low back pain is to improve its assessment through a defined evidence and consensus-driven structure. The criteria proposed will enable more rigorous meta-analyses and promote more generalizable studies of interindividual variation in acute low back pain and its potential underlying mechanisms.
Keywords: Acute Pain, Low Back Pain, Physical Function, Psychological Function, Coping Responses, Taxonomy
Introduction
Acute low back pain is one of the most common reasons for adults to seek medical care. Between 12% and 15% of the US population seek care for acute low back pain each year, with associated costs exceeding $200 billion [1–3]. Activity-limiting acute low back pain can lead to increased work absence and high health care utilization, resulting in a vast economic burden on individuals, families, communities, governments, and industries [4–6]. Activity-limiting acute low back pain has a worldwide lifetime prevalence of about 39% and an annual prevalence of 38% [7]. Although many patients recover spontaneously, proper evaluation can guide treatment and improve outcomes [8–12]. A majority of those who experience acute low back pain develop recurrence of these symptoms [13].
Since 2000, low back pain has been listed on the World Health Organization’s leading causes of worldwide disability, rising in the ranking from 18th to 13th [14]. In the United States, low back pain has been reported as the leading cause of disability [15]. Despite increasing prevalence, low back pain remains poorly understood from mechanistic, diagnostic, and treatment perspectives. While the National Institutes of Health (NIH) has developed research standards for chronic low back pain, acute low back pain with or without lower extremity pain does not have a defined classification system in research and/or clinical care. The absence of such a framework limits the assessment of acute low back pain and a better understanding of treatment responsive phenotypes.
The focus of this working group was developing multifaceted criteria that move beyond simple diagnostic coding to better characterize acute low back ± lower extremity pain clinical presentation and enhance research approaches. The NIH Task Force on Research Standards for Chronic Low Back Pain concluded that a major challenge for improved understanding of chronic low back pain is the ambiguity of the taxonomy for this diagnosis within many research studies, limiting the creation of clinical or research standards [16]. This challenge is reflected in the acute low back pain domain as well. The goal of this project is to curate the existing literature and enhance understanding of the acute low back pain ± lower extremity pain dimensional taxonomy for clinical and research applications moving forward.
The goal of applying the ACTTION-APS-AAPM Pain Taxonomy (AAAPT) acute pain taxonomy to acute low back pain is to improve its assessment through a defined evidence and consensus–driven structure [17]. The spectrum of causes of low back pain is far-reaching and includes potential pathology from almost every organ system. In an effort to remain consistent with a recently published ACTTION-APS Pain Taxonomy (AAPT) manuscript on spine-related chronic low back pain [18], our interdisciplinary and multidisciplinary working group of clinician scientists developed a consensus to define the broad concept of low back pain into four separate acute lumbosacral spine–related pain subtypes. A narrative review and synthesis were performed to support the distinct categorizations of these subtype conditions and to develop a working taxonomy for clinical and research use. While there are several overlapping domains across these conditions, the proposed taxonomy was developed by the working group to best subclassify them within the general AAAPT taxonomical structure. The criteria proposed will enable more rigorous meta-analyses and promote more generalizable studies of interindividual variation in acute low back pain and its potential underlying mechanisms. This emerging classification system will also enhance current efforts toward data standardization while advancing understanding of acute lumbosacral spine–related pain through enhanced generalizability of research efforts.
Methods
A collaborative effort between the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION), the American Pain Society (APS), and the American Academy of Pain Medicine (AAPM) was initiated and formed the ACTTION-APS-AAPM Pain Taxonomy (AAAPT) [17]. This effort was intended to develop a common language and taxonomy for a broad group of acute pain conditions. As a part of the AAAPT, a working group (WG) of six physicians and nursing professionals with expertise in research, education, and clinical management of acute lumbosacral spine–related pain convened in Washington, DC, in November 2017. Two additional individuals (SM, DE) were unable to attend and participated following the meeting. The goal of the WG was to specifically and methodically address issues around the assessment, study, and treatment of acute low back pain and related lower extremity pain. Before the in-person meeting, WG members were provided with taxonomy templates and background articles [19–21]. Each member of the WG conducted her or his focused narrative literature review, with special attention to their particular area of expertise (i.e., preclinical or clinical research, pharmacologic/interventional lumbosacral spine care, surgical lumbosacral spine care, biopsychosocial modulators of pain) and based on taxonomy dimension. Before the in-person meeting, the WG discussed topical areas of importance through conference calls and e-mail exchanges, and a consensus was reached. Based on extensive discussions among the WG members, the topic of the narrative review focused upon the broad criterion of classifying acute low back pain. A secondary goal in creating this research taxonomy was to maintain clinical relevance, expanding the impact of the classification.
At the in-person meeting, WG members summarized data and discussed their application with respect to the previously published five AAAPT dimensions for acute pain: 1) core criteria, 2) common features, 3) modulating factors, 4) impact/functional consequences, and 5) neurobiological mechanisms [17]. The literature review performed by the WG members before the meeting revealed a broad group of definitions and identified highly common conditions. Given the spectrum of diagnoses related to acute low back pain, the WG adapted the AAAPT dimensions into the following subtypes: 1) acute lumbosacral pain (axial low back pain), 2), lumbosacral radiculopathy, 3) lumbosacral radicular pain, and 4) lumbar neurogenic claudication. Pediatric populations (age <18), cancer pain, fracture, infection, trauma, lumbosacral spine surgery, heterotopic ossification, myelopathy, and spinal cord injury or compression were excluded from the classification system. Differential diagnoses for these acute pain conditions included piriformis syndrome, vascular claudication, meralgia paresthetica, widespread pain, cluneal neuralgia, and other myofascial pain syndromes.
Dimensional criteria for the larger AAAPT framework were evaluated based upon subcriteria relevant to acute low back–related pain in the literature. Dimension 1, focusing on diagnoses, had several important criteria. Key differential domains included inciting event and causation, timing of symptoms, anatomical location, and involved tissue type. Dimension 2 evaluated based on signs, radiological findings, neurological exam findings, provocation test exam findings, neurophysiological testing, and symptoms. Dimension 3 included demographics, low back pain–specific defined comorbidities, other pain conditions, treatments, and measures of physical, psychological, and social functioning, as outlined in the NIH chronic low back pain standards [16]. The working group felt it was important to align with existing research standards to allow broader interoperability of the criteria. Reflecting key pain-related NIH PROMIS measures [22], this dimension included biopsychosocial factors, sociodemographic variables, and treatments as modulating factors. Dimension 4 included similar variables to Dimension 3 for evaluation including demographics, comorbidities, treatments, and functional status, but expanded to evaluate pain interference. Dimension 5 was evaluated for tissue-based mechanisms, pain processing, and genetic components.
An initial presentation of findings was made to other acute pain WGs during the in-person AAAPT meeting to align definitions and categories among the dimensions and across other acute pain types. Consensus agreement of the AAAPT participants was reached for these conditions and definitions, as has been done with other AAAPT WGs [23,24]. Knowledge gaps were identified, and strategies to translate findings into research and clinical practice were formulated.
Results
Definition of Acute Low Back Pain
The Acute Low Back Pain WG chose to use the definition for chronic low back pain developed by the NIH Task Force on Research Standards [16] to guide the definition of acute low back pain. We defined low back pain as pain involving or derived from structures in the lumbosacral region between the lower posterior margin of the rib cage and the horizontal gluteal fold. We recognized that the NIH Task Force defined chronic low back pain as pain that is present on half the days or more in six months. Therefore, a history for acute low back pain had to include pain that is present on less than half the days of six months. This definition allows for intermittent/recurring or constant pain.
Acute Lumbosacral Pain
Dimension 1: Core Diagnostic Criteria
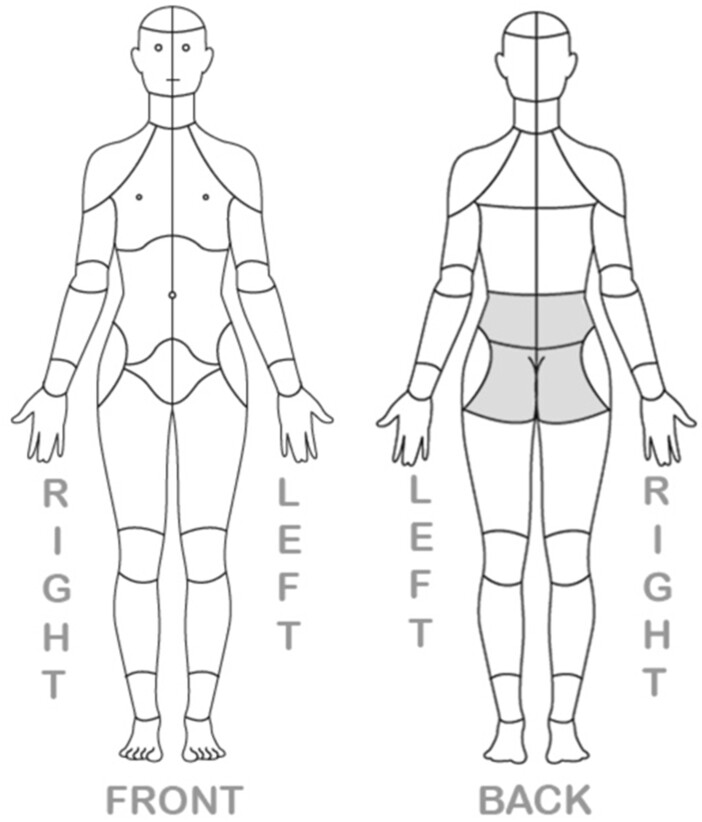
The history of acute lumbosacral pain, also known as acute axial low back pain, must include pain in the area between the lower posterior margin of the rib cage and the horizontal gluteal fold (Figure 1) [16]. Lower extremity pain may be present in patients with lumbosacral pain but is not necessary for the diagnosis and may represent a distinct pathologic process with a separate etiology. In addition to the appropriate time course and location listed above, for the diagnosis of acute lumbosacral pain to be met, the inciting tissues may include pathology related to the lumbar facet joints, sacroiliac joints, intervertebral discs, lumbar spinal nerves, muscles, ligaments, tendons, and other bony structures of the lumbosacral spine. Neurophysiologic testing such as electromyography (EMG), nerve conduction studies (NCS), and skin/nerve biopsy may have no abnormal findings. The WG definition does not require neurologic deficits related to their lumbosacral spine condition. Finally, a key exclusion criterion for this definition of acute lumbosacral pain was lumbosacral spine surgery within the preceding three-month period. The AAAPT core diagnostic criteria for acute lumbosacral pain are summarized in Table 1.
Figure 1.
Low back or lumbosacral region (highlighted) between the lower posterior margin of therib cage and the horizontal gluteal fold.
Table 1.
Diagnostic criteria for acute lumbosacral pain
|
EMG = electromyography; NCS = nerve conduction study.
While not required to meet Core Diagnostic Criteria, this may be used to support classification.
Dimension 2: Common Features
The key feature of acute lumbosacral pain is patient-reported pain of any descriptive quality that can be either nociceptive and/or nociplastic per International Association for the Study of Pain (IASP) definitions [25]. Acute lumbosacral pain is generally described as dull, throbbing, aching, stabbing, sharp, gnawing, nagging, and annoying [26]. Clinical examination of the low back region can include hyperalgesia and/or allodynia, with the potential for decreased range of motion (ROM) of the lumbar spine and pain with ROM testing. Provocation maneuvers may elicit pain in the low back region that correlates with the anatomic structure of interest. Acute lumbosacral pain may or may not have radiographic evidence of pathology. Pain may be spontaneous (at rest) or evoked (with changes in activity or position).
Dimension 3: Modulating Factors
For Dimension 3, the focus is on the “preexisting phenomena” that may serve as modulators for acute lumbosacral spine pain conditions. The WG identified the following subgroups of potential modulating factors across all included conditions: demographics, medical comorbidities, treatments, functional status (including physical and psychological parameters), and a history of other pain diagnoses (Table 2). The NIH Research Standards for Chronic Low Back Pain criteria were incorporated after WG discussion into the AAAPT criteria for acute lumbosacral pain to improve alignment of future acute low back pain and chronic low back pain data sets [16].
Table 2.
Modulating factors
| Demographics | Comorbidities | Treatments | Functional Status | Other Pain Dx: |
|---|---|---|---|---|
|
|
|
|
|
ADLs = activities of daily living; BMI = body mass index; ETOH = alcohol; Dx = diagnoses; NSAID = nonsteroidal anti-inflammatory drugs; PT = physical therapy; WC = workers’ compensation.
Demographic Factors
Increasing age and female sex have ben associated with a higher incidence of acute lumbosacral pain in most published literature [27–32]. Racial and ethnic differences have been shown to influence the development of acute lumbosacral pain, with white and black subjects having a higher incidence than Hispanic, Native American, and Asian/Pacific Islanders [27,30]. Very few studies have investigated the impact of marital status as a risk factor. A single study suggested that there was an increased prevalence in people who were married as opposed to single individuals [27]. Lower educational achievement and socioeconomic status were identified in a few publications as risk factors for the development of acute lumbosacral pain [2,29,33,34]. No studies were identified regarding the influence of employment status and litigation as risk factors for the development of acute lumbosacral pain, although they may play a role in chronicity of painful conditions [35]. Occupational factors such as work–family imbalance, physically or psychologically strenuous work, sedentary work, whole-body vibration, low social support in the workplace, job dissatisfaction, and workers’ compensation status have all been associated with development of acute lumbosacral pain [2,29,31–33,36].
Medical Comorbidities
Increased body mass index and the classification of obesity are reported as a strong predictor and risk factor for the development of acute lumbosacral pain [29,37]. No studies have investigated alcohol use as a modulator, but strong evidence implicates tobacco use in its development [29,31,37]. The chronic use of opioid medications, illicit drug use, and nonsteroidal anti-inflammatory drugs (NSAIDs) have not been reported as risk factors for the development of acute lumbosacral pain [38]. Anxiety and depression have been closely linked to the risk of developing acute lumbosacral pain [39–42]. Genetic predictors of recovery from acute lumbosacral pain have also been explored and include polymorphisms and allele-based associations with OPRM1 rs1799971 and MMP9 rs17576 [43]. Alterations in pain-related gene expression have also been discovered in patients with acute lumbosacral pain compared with healthy controls [44].
In addition to influencing the development of acute lumbosacral pain, individual patient factors can impact outcomes. Smoking status, anxiety, and depression have been shown to negatively alter recovery trajectories [37,45,46]. Clinical implications include optimization of these comorbidities at presentation to improve pain outcomes and prevent pain chronification [39,46,47].
Psychosocial Status
Increased life stress is a risk factor for the development of acute lumbosacral pain [29]. Pain catastrophizing, fear avoidance, and history of lifetime traumatic events can influence the development of acute pain states, playing a key role in the recovery trajectory of acute lumbosacral pain. This condition can have a profound influence on a person’s physical, psychological, and social well-being. For example, anxiety, depression, anger, fatigue, kinesiophobia, and fear of chronic pain are common responses to acute lumbosacral pain and can impact a patient’s pain experience and recovery. While less frequently reported, a variety of aspects of social functioning appear to amplify the broader experience of pain. Several studies suggest that a more restricted social attachment approach is associated with greater distress [48], greater pain catastrophizing [49], lower self-efficacy to decrease pain [50], more disability due to pain [51], and greater pain sensitivity [52]. Conversely, protective factors like resilience, high self-efficacy, and positive affect may decrease the risk or severity of acute lumbosacral pain [53].
Other Pain Diagnoses
Inclusion of other pain diagnoses such as myofascial pain in the classification aligns with the minimal data set of the NIH research standards for chronic low back pain [16]. While there may be additional pain conditions that are relevant for acute lumbosacral pain characterization (such as diffuse amplified musculoskeletal pain syndrome and fibromyalgia), the current focus of this taxonomy does not strongly support their inclusion as specific comorbid lumbosacral pain conditions at this time.
Treatments
Treatments (both physical, educational, and psychological) have the opportunity to positively impact the pain trajectory and recovery in patients with acute lumbosacral pain conditions. Identifying at-risk patients (e.g., anxious or depressed patients, patients with high catastrophizing and low self-efficacy) in order to tailor treatments and provide personalized analgesia can provide the opportunity to improve outcomes. For many patients, early recognition and educational intervention improve pain outcomes [54,55].
Dimension 4: Impact and Functional Consequences
Dimension 4 evaluates the downstream impact of acute lumbosacral pain on functioning in the physical, social, psychological, and vocational domains. Leveraging PROMIS outcomes as a reference guide, the WG identified subgroups of potential factors to consider, including demographics and functional status (including physical and psychological parameters) and pain interference outcomes.
Demographics
Acute lumbosacral pain negatively impacts a patient’s likelihood of returning to work; however, the trajectory of recovery and return to work can be improved by early intervention, education, multidisciplinary care, and by addressing psychological and affective disturbances (such as catastrophizing, fear avoidance, anxiety, and depression) [4,29,46,54,56,57].
Functional Status/Pain Interference Outcomes
Increased life stress, pain catastrophizing, anxiety, depression, and fear avoidance can influence the development of acute pain states, as discussed in Dimension 3. However, these can be a consequence of the acute lumbosacral pain experience and may influence recovery and disease prognosis. The presence of these negative constructs and affective states can decrease the likelihood of a positive and meaningful recovery from acute lumbosacral pain and can play a role in the transition from acute to chronic pain [40,41,46,47,54,58–63]. Finally, pain interference may negatively influence functional status, including the domains of sleep, walking, activities of daily living, or sexual activity.
Dimension 5: Putative Pain Mechanisms
Understanding the mechanistic pathways that contribute to acute lumbosacral pain may help guide treating clinicians in a logical and multimodal approach to analgesia. It is important to note that acute lumbosacral pain with no previous lumbosacral pain episodes can occur in many patients; however, it is also very possible that patients with a history of chronic lumbosacral pain can experience acute exacerbations of this pain and that the putative pain mechanisms listed in the following paragraphs are likely true for both patient scenarios [64]. Identifying sources of potential nociceptive sensitization (e.g., both peripheral and central) may provide a “blueprint” of treatment options. Focusing on dampening or reducing these areas of sensitization to improve the trajectory of recovery from the acute pain episode could potentially prevent the development of chronic pain [65]. Acute lumbosacral pain frequently results from a variety of pathophysiological or anatomic alterations of numerous tissue types, including lumbar vertebral bodies, intervertebral discs, lumbar spinal nerves, lumbar facet (zygapophyseal) joints, lumbar ligamentous or vascular structures, lumbar musculature, or sacroiliac joints. Furthermore, alterations in pain processing, both peripherally and centrally, affect the ultimate perception of acute lumbosacral pain (Table 3).
Table 3.
Putative neurobiological mechanisms
| Tissue-Based Mechanisms | Pain Processing | Genetic Components |
|---|---|---|
| Collagen vascular disordersInflammatory mechanisms | Central mechanismsPeripheral mechanisms | Polymorphisms and allele-based associations |
Different subsets or combinations of these tissue alterations may be involved depending on the pathogenesis, including but not limited to acute disc herniation, acute annular tear, acute spondylolysis or pars interarticularis stress reaction, zygapophyseal (facet) joint extension stress, Schmorl’s node end plate disruption, and muscle contusions. Acute processes involving these structures may occur; however, nociceptive activation of preexisting previously nonpainful age-related degenerative changes may lead to acute lumbosacral pain as well. True nociceptive mechanisms are typically tissue-based, including congenital or induced anatomic alterations of non-nerve tissue spinal anatomic structures. Underlying collagen-vascular disorders can contribute to the development of acute lumbosacral pain [28,29,66,67].
Pain processing alterations in the peripheral and central nervous system are associated with acute pain conditions. Mechanical or structural pathology (e.g., disc protrusion or herniation, facet joint or pars interarticularis stress reaction, annular tear) triggers the development of edema and local cellular abnormalities [68]. With these local changes, there is increased production of pro-inflammatory cytokines in the spinal cord, which leads to the expression of multiple algesic mediators and heightened nociceptive activity. Peripheral T cells and macrophages cross the blood–brain barrier into the parenchyma of the spinal cord, causing further increased neuroimmune activation and peripheral sensitization [68]. Peripheral sensitization results from the local action of inflammatory mediators on the peripheral terminals of high-threshold nociceptive sensory neurons [69]. The desensitization and hyperpolarization of low-threshold mechanoreceptors in combination with sensitization of high-threshold mechanoreceptors leads to behavioral sensitization [70]. These actions activate intracellular signaling pathways that lead to phosphorylation of ion channels and receptors on the nociceptive terminal membrane, which reduces threshold and increases excitability [71,72]. This hypersensitivity reduces the intensity of the peripheral stimulus needed to activate nociceptors at the site of inflammation (primary hyperalgesia). Once the source of the inflammation subsides, the peripheral sensitization process should de-escalate. It has been well described that these peripheral sensitization processes contribute to central nervous system inflammatory and signaling events. These events contribute to the development of central sensitization and maintenance of pain in the days to weeks after an acute lumbosacral pain episode [73].
Central sensitization is a form of central nervous system neuroplasticity that amplifies pain signaling [74]. Initially, the plasticity is produced first by the intense firing of noxious peripheral stimuli and the consequent synaptic activity generated therein in the dorsal horn neurons [75]. Alterations in preexisting proteins in the dorsal horn neurons are produced, increasing the trafficking of ion channels and receptors to the membrane and changing their function. Some hours after the onset of acute pain and tissue injury, there is altered gene transcription in sensory neurons and in the spinal cord that augments the release and action of excitatory neurotransmitters and reduces inhibitory neurotransmitters [75,76]. Central sensitization is an abnormal persistent response to a normal sensory input and may result in a transition to chronic pain in addition to the spread of sensitivity beyond the original site of injury [77]. After induction of central sensitization, the responsiveness of neurons increases sufficiently in that normally ineffective synaptic inputs, including those elicited by non-noxious stimuli or high-threshold mechano-sensitivity, activates pain transmission neurons [70,78].
Psychological modifiers (e.g., depression, anxiety) likely interact with functional connectivity in pain-relevant higher brain centers that could magnify incoming nociceptive signals [79]. In addition, stress-related hormones or neurotrophic factors may increase and impact inflammatory processes in the periphery [80,81].
Acute Painful Lumbosacral Radiculopathy
Dimension 1: Core Diagnostic Criteria
Acute painful lumbosacral radiculopathy includes unilateral or bilateral lower extremity pain accompanied by numbness, weakness, and/or abnormal reflexes in the lower extremity. Lumbosacral pain (axial low back pain) may or may not be present along with the radiculopathy. Lumbosacral radiculopathy is related to axonal conduction block of the spinal nerve(s) or roots (e.g., most commonly compression of the lumbar spinal nerve(s) due to one or more of a variety of lumbosacral spine pathologic conditions and less commonly due to vascular infarction or autoimmune damage), resulting in objective physical examination findings. Weakness results from blockade of motor fibers and numbness from blockade of sensory fibers. Diminishment of deep tendon reflexes results from either motor or sensory block [82]. Although pain is not required to establish the diagnosis of lumbosacral radiculopathy, the WG included radicular pain and termed the condition painful lumbosacral radiculopathy. The AAAPT core diagnostic criteria for this condition are summarized in Table 4.
Table 4.
Diagnostic criteria for acute painful lumbosacral radiculopathy
|
CT = computed tomography; EMG = electromyography; MRI = magnetic resonance imaging; NCS = nerve conduction study.
While not required to meet Core Diagnostic Criteria, this may be used to support classification.
Dimension 2: Common Features
The key feature of acute painful lumbosacral radiculopathy is patient-reported pain of any descriptive quality that can be characterized as neuropathic and/or nociceptive by the International Association for the Study of Pain (IASP) [25]. Painful lumbosacral radiculopathy can be bilateral or unilateral. Typical descriptors that are used for acute painful lumbosacral radiculopathy include burning, numb, shooting, and stabbing [83]. Pain may be spontaneous (at rest) or evoked (with changes in activity or position). Clinical examination over the previously defined low back region may include hyperalgesia to noxious stimuli and allodynia to non-noxious stimuli, with the potential for decreased range of motion (ROM) of the lumbosacral spine and pain with ROM testing. Patients with acute painful lumbosacral radiculopathy will have neurologic deficits including diminished sensation (proprioception, fine touch, and temperature), motor strength, and reflexes in areas served by the nerves of the lumbosacral spine. Provocation maneuvers such as the Lasegue test (straight leg test) may elicit pain in the lower extremity that suggests lower lumbosacral nerve involvement [67,84]. The Ely test (reverse straight leg raise) may elicit pain in the lower extremity, suggesting upper lumbar nerve involvement [67,84]. Acute painful lumbosacral radiculopathy may have radiographic evidence of nerve compression or inflammation consistent with the findings from the physical exam. Neurophysiologic testing such as EMG and NCS will likely show abnormal findings; however, if the pain and neurologic deficits have been present for less than six weeks, EMG/NCS may be normal [85].
Dimension 3: Modulating Factors
Obesity is both a common comorbid condition and a risk factor for the development of acute painful lumbosacral radiculopathy [86,87]. There is no clear association between this condition and other medical comorbidities; however, the most prevalent health issues in this acute pain condition likely overlap with the common comorbidities seen in a chronic low back pain and middle-aged population including osteoarthritis and depression (Table 2) [88,89]. As in acute lumbosacral pain, it may be true for acute painful lumbosacral radiculopathy that early recognition and intervention improve pain outcomes [54,55]; however, no studies have been performed to identify this in acute painful lumbosacral radiculopathy. There are some data that suggest that epidural steroid injections can be beneficial in the short term [90]. Genetics may play a role in modulation, but current practice does not reflect widespread genetic testing [43,91].
Dimension 4: Impact and Functional Consequences
Few studies have been performed looking at the impact and consequences of acute painful lumbosacral radiculopathy; however, limitations in daily activities, reduced quality of life, sleep disturbances, and anxiety and mood disorders are consequences of chronic painful radiculopathy [92,93]. Frequent consequences of chronic painful lumbosacral radiculopathy include sleep disruption, lack of energy, difficulty concentrating, drowsiness, and an inability to work, engage in hobbies, or interact socially [18]. A major limitation in the present body of knowledge is a lack of data on the impact and consequences of the acute painful lumbosacral radiculopathy experience.
Dimension 5: Putative Pain Mechanisms
Acute painful lumbosacral radiculopathy may arise following an inciting event that causes mechanical or inflammatory injury or from entrapment related to disc herniation or foraminal stenosis [94]. Disc herniation is the most common cause of nerve root impingement when a structural correlate is identified (80–89%). Other causes may include synovial cyst formation, facet joint overgrowth, and spondylolisthesis with segmental instability [95,96]. Additionally, nerve tissue alterations can lead to both nociceptive and neuropathic pain. In acute painful lumbosacral radiculopathy, mechanical nerve and vascular compression can lead to neuropathic pain symptoms [29,66,67,97–100]. Release of calcitonin gene-related peptide (CGRP) and Substance P after nerve injury leads to neurogenic inflammation. Neurogenic inflammation occurs by acting to increase the epithelial permeability of local capillaries, allowing movement of immune cells into the area of tissue injury [101–103]. As with acute lumbosacral pain, pain-processing alterations in the peripheral and central nervous system are associated with acute painful conditions and likely play a role in acute painful lumbosacral radiculopathy (Table 3).
Acute Lumbosacral Radicular Pain
Dimension 1: Core Diagnostic Criteria
The history for acute lumbosacral radicular pain includes unilateral or bilateral lower extremity pain. Low back pain may or may not be present along with the radicular pain. Importantly, radicular pain differs from acute lumbosacral radiculopathy in that it is not accompanied by neurologic deficits. For the diagnosis of acute lumbosacral radicular pain, the inciting tissues may include pathology related to the lumbar facet joints, sacroiliac joints, intervertebral discs, muscles in the lumbosacral region, and lumbar spinal nerve(s). The AAAPT core diagnostic criteria for acute lumbosacral radicular pain are summarized in Table 5.
Table 5.
Diagnostic criteria for acute lumbosacral radicular pain
|
EMG = electromyography; NCS = nerve conduction study.
While not required to meet Core Diagnostic Criteria, this may be used to support classification.
Dimension 2: Common Features
The key feature of acute lumbosacral radicular pain is patient-reported pain of any descriptive quality characterized by the International Association for the Study of Pain (IASP) as nociceptive, neuropathic, and/or nociplastic [25]. This pain exists in either the unilateral or bilateral lower extremities and/or the low back region as defined by the NIH Task Force [16]. Typical pain descriptors that are used for lumbosacral radicular pain include burning, shooting, and stabbing. Pain may be spontaneous (at rest) or evoked (with changes in activity or position). Clinical examination over the previously defined low back region can but does not have to include hyperalgesia to noxious stimuli or allodynia to non-noxious stimuli, with the potential for decreased ROM of the lumbar spine and pain with ROM testing. Acute lumbosacral radicular pain may or may not have radiographic evidence of pathology that correlates with the findings from the history and physical exam. Neurophysiologic testing such as EMG and NCS will be without abnormalities.
Dimension 3: Modulating Factors
Obesity is both a common comorbid condition and risk factor for the development of acute lumbosacral radicular pain [86,87]. There is no clear association between acute lumbosacral radicular pain and other medical comorbidities. However, similar to acute painful lumbosacral radiculopathy, the most prevalent health issues in this acute pain condition likely overlap with the common comorbidities seen in a chronic low back pain and middle-aged population including osteoarthritis and depression (Table 2) [88,89]. As in acute lumbosacral pain and painful radiculopathy, it may be true that early recognition and educational intervention about pain and neuroscience improve pain outcomes [54,55]; however, no studies have been performed to identify this in acute lumbosacral radicular pain. Few studies have been performed to determine if interventional pain procedures for the treatment of acute lumbosacral radicular pain alter the trajectory of patients with acute pain or if pain relief by these measures affects the acute-to-chronic pain trajectory. There are some data that suggest that epidural steroid injections can be beneficial in the short term for acute lumbosacral radicular pain [90].
Dimension 4: Impact and Functional Consequences
Few studies have been performed looking at the impact and consequences of acute lumbosacral radicular pain; however, limitations in daily activities, reduced quality of life, sleep disturbances, and anxiety and mood disorders are associated with chronic lumbosacral radicular pain [92,93]. As with painful lumbosacral radiculopathy, sleep disruption, lack of energy, difficulty concentrating, drowsiness, and an inability to work, engage in hobbies, or interact socially are commonly correlated with acute lumbosacral radicular pain [18]. A major limitation in the present body of knowledge is a lack of data on the impact and consequences of the acute lumbosacral radicular pain experience.
Dimension 5: Putative Pain Mechanisms
Acute lumbosacral radicular pain may arise following an inciting event that causes mechanical or inflammatory injury or from entrapment related to disc herniation or foraminal stenosis but does not result in conduction blockade, as with lumbosacral radiculopathy [94]. As with acute lumbosacral radiculopathy, disc herniation is the most common cause of nerve root impingement when a structural correlate is identified (80–89%), and other causes may include synovial cyst formation, facet joint overgrowth [95], spondylolisthesis with segmental instability [96], and chemical irritation from intradiscal inflammatory mediators [82]. In acute lumbosacral radicular pain, the underlying mechanism has features of both an inflammatory and neuropathic response. Nerve root injury or compression is associated with release of inflammatory mediators that sensitize peripheral nociceptors [104]. Mechanical causes can exist in the form of herniated disc fragment, foraminal stenosis, or root traction that can then lead to structural changes that cause edema and local cellular abnormalities [68]. As with acute lumbosacral pain and acute painful lumbosacral radiculopathy, pain-processing alterations in the peripheral and central nervous system are associated with acute painful conditions and likely play a role in acute lumbosacral radicular pain. Increased production of proinflammatory cytokines in the central nervous system induces increased expression of multiple pronociceptive mediators, and augmented afferent input from the sensitized peripheral nociceptors may lead to heightened responsiveness of neurons in the central nervous system, which is a proposed mechanism for central sensitization in radicular conditions [69,94]. Additionally, peripheral sensitization mechanisms lead to enhanced perception of both spontaneous and evoked radicular symptoms (Table 5) [105].
Acute Lumbosacral Neurogenic Claudication
Dimension 1: Core Diagnostic Criteria
The history for acute lumbosacral neurogenic claudication includes bilateral lower extremity pain that may or may not include low back pain and may be accompanied by subjective or objective findings of numbness, weakness, or abnormal reflexes. No published duration of time exists to define acute vs chronic lumbar neurogenic claudication. For the diagnosis of acute lumbosacral neurogenic claudication, the inciting tissue will include pathology related to the lumbar and/or sacral spinal nerve(s) (e.g., compression of lumbar spinal nerve[s] due one or more of a variety of lumbar spine conditions, usually central spinal canal stenosis at any of the lumbar levels). The AAAPT core diagnostic criteria for acute lumbosacral neurogenic claudication are summarized in Table 6.
Table 6.
Diagnostic criteria for acute lumbosacral neurogenic claudication
|
CT = computed tomography; EMG = electromyography; MRI = magnetic resonance imaging; NCS = nerve conduction study.
While not required to meet Core Diagnostic Criteria, this may be used to support classification.
Dimension 2: Common Features
The key feature of acute lumbosacral neurogenic claudication is patient-reported pain of any descriptive quality that can be characterized by the International Association for the Study of Pain (IASP) as neuropathic and/or nociceptive [25]. This pain exists in the bilateral lower extremities and/or the low back region, as defined by the NIH Task Force [16]. Typical word descriptors that are used for acute lumbosacral neurogenic claudication include cramping, burning, aching, numbness, weakness, and heaviness [106]. Clinical examination over the previously defined low back region can but does not have to include hyperalgesia to noxious stimuli and allodynia to non-noxious stimuli, with the potential for decreased ROM of the lumbar spine and pain with ROM testing. A hallmark of acute lumbosacral neurogenic claudication is that pain and/or neurologic deficits are evoked with walking or standing and are relieved with sitting or position change, including flexion of the lumbar spine. Acute lumbosacral neurogenic claudication will have radiographic evidence of spine canal stenosis consistent with the patient’s history and physical exam [107]. Neurophysiologic tests such as EMG and NCS are unlikely to be abnormal.
Dimension 3: Modulating Factors
Increased body mass index and the classification of obesity are reported as a strong predictor and risk factor for the development of acute lumbosacral neurogenic claudication due to lumbosacral spinal stenosis [108]. Tobacco use has been reported as a risk factor for the development of acute neurogenic claudication [108]. Increasing age has been associated with lumbosacral spinal stenosis, and the average age of clinical presentation of patients with neurogenic claudication is 60–65 years [108–111]. Approximately 32% to 50% of older patients with chronic neurogenic claudication have coexisting diseases, including hypertension, gastrointestinal issues, other joint disorders, heart disease, diabetes, and depression [110,111]. Occupational factors such repetitive spinal stress have been associated with acute neurogenic claudication due to lumbar spinal stenosis [108]. Other factors such as congenital or other musculoskeletal conditions (e.g., congenital spinal stenosis, spondyloarthropathies) are likely risk factors for the development of acute lumbosacral neurogenic claudication (Table 2) [112].
Dimension 4: Impact and Functional Consequences
The reduced independent mobility experienced by patients with acute lumbosacral neurogenic claudication due to spinal stenosis can significantly impair their ability to provide care to themselves. As the primary difficulty of the condition is elicited by ambulation, this leads to disruption of regular activities around the household and recreational activities, such as hobbies, sports, or social interactions [113–115]. The downstream effects of these physical limitations, loss of independence, and reduction in participation in social events and hobbies can lead to depression, social isolation, and feelings of sadness in patients with acute lumbosacral neurogenic claudication [113–115].
Dimension 5: Putative Pain Mechanisms
There is a combination of degenerative, age-related findings that leads to lumbosacral spinal stenosis including alterations of the intervertebral discs, vertebral body osteophytes, ligamentum flavum, and facet joints that collectively lead to a narrowing of the space around the neurovascular structures within the central spinal canal [116]. These degenerative changes may eventually lead to cauda equina compression and dysfunction, which is associated with increased intraspinal compartment pressures [117]. Localized inflammation due to compression leads to sensitization of the nerve roots and cauda equina, reduced arterial blood flow and venous congestion may result in reversible ischemic changes, and dilation of venous structures can contribute to nerve root compression and additional perfusion deficits [107]. Collectively, these mechanisms will eventually lead to structural damage to the cauda equina lumbar and sacral nerve fibers (Table 4) [118].
Discussion
One of the challenges of treating acute low back pain is the lack of agreement in terminology and classification of the conditions within the nonspecific terms of “acute” and “low back pain.” We have therefore defined acute as pain that is intermittent or constant for less than half the days in a six-month period. We defined low back pain as pain involving or derived from structures in the lumbosacral region between the lower posterior margin of the rib cage and the horizontal gluteal fold. This work proposes an organizational classification taxonomy aligned with existing research standards to meaningfully move our understanding of acute low back pain forward. Significant differences in modulating factors, impact, and neurobiological mechanisms may exist, but our current literature does not rigorously classify them in the biological, social, or psychological domains. This work proposes the next iterative step forward to provide a framework for study design and clinical data collection. Possible avenues for use of this taxonomy may be the development of an acute low back pain data registry or testing new treatments in defined subgroups presented here.
Adoption of this systematic approach to classifying acute low back pain will enhance our ability to design research studies and provide clinical care that connect to a larger collection of data. By establishing standardized classification, identification of individual factors and patterns impacting pain outcome will become feasible. Creating the framework for analyzing results across the translational spectrum provides scaffolding for larger analysis of pooled data. The classification reflects the ongoing shift in pain research from diagnosis-based classification to a system reflecting the complex biopsychosocial factors impacting acute pain. Mapping painful conditions related to the lumbosacral spine to the AAAPT dimensions creates an opportunity to design and support interventions that will help further refine classification and research. Although our WG mirrored the paradigm provided in the AAAPT taxonomy, we also strove to provide an overarching set of diagnostic criteria for acute low back pain for broader application and secondary data analysis. Limitations associated with the taxonomy are the known gaps in literature to fully understand mechanisms and modulating factors associated with acute low back pain. Future work is needed to validate this taxonomy. However, the focus of this work is to create a classification that will address this limitation and lay the foundation for future investigations with data that are able to be combined and compared with other studies.
Advances in translational acute low back pain are evaluating intersections of pain and other predictive factors. Future work incorporating individual biopsychosocial, economic, and geographic factors may leverage this AAAPT dimensionality as we further evaluate acute spine pain. The evidence at this point for acute low back pain shows benefit for multidisciplinary pain treatment, but not necessarily comparative superiority due to lack of granularity [119]. For larger-scale acute spine-related pain research, the Quebec Low Back Pain Cohort is proposing to build on work of existing international cohorts to expand data collection to include biomechanical, epigenetic, genetic, and neuroanatomical characteristics, which would address many of the Dimension 3, 4, and 5 gaps in knowledge [120]. At the environmental level, occupational factors influencing acute low back pain may include work–family imbalance, exposure to a hostile work environment, and job insecurity [36]. At the mechanistic level, the COMT rs4680 genotype and independent COMT expression have been demonstrated to have an association with the transition from acute to chronic low back pain, although the time frame for wider genetic testing may not support regular use [121]. Acute low back pain patients and controls demonstrated lower fatty acid amide hydrolase and transient receptor potential cation channel subfamily V member 1 (TRPV1) mRNA expression compared with chronic low back pain patients [122]. The relationship between acute phase inflammatory response and recovery outcomes is in the early stages, but there is a potential connection between high inflammation (measured by increased levels of C-reactive protein and interleukin) and good recovery, compared with high tumor necrosis factor paired with depressive symptoms, which is associated with poor recovery [123]. The majority of these studies align with the anatomical and chronological framework defined by this WG and support future directions for research and dimensional study of acute lumbosacral spine–related pain.
Conclusions
It deserves mention that the taxonomy presented here should be considered a “living taxonomy,” in that it will likely undergo further testing and refinement. We anticipate that further validation, refinement, and adaptation of this taxonomy will require the time and effort of many within and outside of the AAAPT Low Back Pain WG. Nonetheless, the WG believes that this first iteration of the acute lumbosacral spine–related pain taxonomy and the ones that will follow will help resolve the heterogeneity in the classification and research of acute low back pain.
Acknowledgments
The AAAPT Low Back Pain WG gratefully acknowledges the vision of Robert Dworkin and Dennis Turk in establishing this initiative to improve the communication among clinicians and scientists. The leadership of Michael Kent and Patrick Tighe in coordinating the larger AAAPT endeavor was invaluable to the completion of this work. We also thank Drs. Stephen Bruehl, Robert Dworkin, and Dennis Turk for their valuable insights and helpful editorial advice.
Funding sources: This work was supported by the AAAPT (Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks [ACTTION]–American Academy of Pain Medicine [AAPM]–American Pain Society [APS] Pain Taxonomy), National Institutes of Health (NIH) K23 GM123320 (ALN), and NIH K08 EB022631 (MBCA).
Disclosures and conflicts of interest: Dr. Nicol holds funding through NIH K23 GM123320. Dr. Adams holds funding through NIH K08 EB022631. Dr. Hurley holds funding through NIH R21 DA046085, R33 DA046085, Agency for HealthCare Research and Quality R01 HS023306, the Robert Wood Johnson Foundation (Clinical Scholar) and Medtronic, Inc. (Informatics Consultancy). Dr. Mackey holds funding through NIH K24 DA029262, R01 DA 045027, R01 AT008561. Dr. Edwards holds funding through NIH R01 DA045052, R01 DA037891, R01 AT009680; Centers for Disease Control NU90TP921981, and an educational grant to American Academy of Pain Medicine sponsored by Mallinckrodt (2017–2019). All other authors have no conflicts of interest to disclose.
References
- 1.Health Care Utilization and Economic Cost of Musculoskeletal Diseases. United States Bone and Joint Initiative: The Burden of Musculoskeletal Diseases in the United States, Second Edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: Socioeconomic factors and consequences. J Bone Jt Surg Ame 2006;88(Suppl 2):21–4. [DOI] [PubMed] [Google Scholar]
- 3.Davis MA, Onega T, Weeks WB, Lurie JD. Where the United States spends its spine dollars: Expenditures on different ambulatory services for the management of back and neck conditions. Spine 2012;37(19):1693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steenstra IA, Verbeek JH, Heymans MW, Bongers PM. Prognostic factors for duration of sick leave in patients sick listed with acute low back pain: A systematic review of the literature. Occup Environ Med 2005;62(12):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thelin A, Holmberg S, Thelin N. Functioning in neck and low back pain from a 12-year perspective: A prospective population-based study. J Rehabil Med 2008;40(7):555–61. [DOI] [PubMed] [Google Scholar]
- 6.Kent PM, Keating JL. The epidemiology of low back pain in primary care. Chiropr Osteopat 2005;13(1):13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012;64(6):2028–37. [DOI] [PubMed] [Google Scholar]
- 8.Hebert JJ, Koppenhaver SL, Walker BF. Subgrouping patients with low back pain: A treatment-based approach to classification. Sports Health 2011;3(6):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute “nonspecific” low back pain: Results of a randomized clinical trial. Spine 2006;31(6):623–31. [DOI] [PubMed] [Google Scholar]
- 10.Fritz JM, Brennan GP, Clifford SN, Hunter SJ, Thackeray A. An examination of the reliability of a classification algorithm for subgrouping patients with low back pain. Spine 2006;31(1):77–82. [DOI] [PubMed] [Google Scholar]
- 11.Fritz JM, Delitto A, Erhard RE. Comparison of classification-based physical therapy with therapy based on clinical practice guidelines for patients with acute low back pain: A randomized clinical trial. Spine 2003;28(13):1363–71, discussion 72. [DOI] [PubMed] [Google Scholar]
- 12.Delitto A, Erhard RE, Bowling RW. A treatment-based classification approach to low back syndrome: Identifying and staging patients for conservative treatment. Phys Ther 1995;75(6):470–85; discussion 485–9. [DOI] [PubMed] [Google Scholar]
- 13.Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol 2010;24(6):769–81. [DOI] [PubMed] [Google Scholar]
- 14.Briggs AM, Woolf AD, Dreinhofer K, et al. Reducing the global burden of musculoskeletal conditions. Bull World Health Organ 2018;96(5):366–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokdad AH, Ballestros K, Echko M, et al. State of US health, 1990-2016. Burden of diseases, injuries, and risk factors among US states. JAMA 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. J Pain 2014;15(6):569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent ML, Tighe PJ, Belfer I, et al. The ACTTION-APS-AAPM Pain Taxonomy (AAAPT) multidimensional approach to classifying acute pain conditions. Pain Med 2017;18(5):947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markman JD, Czerniecka-Foxx K, Khalsa PS, et al. AAPT diagnostic criteria for chronic low back pain. J Pain 2020;S1526-5900(20)30009-2 (doi: 10.1016/j.jpain.2020.01.008). [DOI] [PubMed] [Google Scholar]
- 19.Widerstrom-Noga E, Loeser JD, Jensen TS, Finnerup NB. AAPT diagnostic criteria for central neuropathic pain. J Pain 2017;18(12):1417–26. [DOI] [PubMed] [Google Scholar]
- 20.Paice JA, Mulvey M, Bennett M, et al. Diagnostic criteria for chronic cancer pain conditions. J Pain 2017;18(3):233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain 2017;18(5):490–8. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(Suppl 1):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Field JJ, Ballas SK, Campbell CM, et al. AAAPT diagnostic criteria for acute sickle cell disease pain. J Pain 2019;20(7):746–59. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber KL, Belfer I, Miaskowski C, Schumacher M, Stacey BR, Van De Ven T. AAAPT diagnostic criteria for acute pain following breast surgery. J Pain 2019;S1526-5900(19)30790-4 (doi: 10.1016/j.jpain.2019.08.008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholz J, Finnerup NB, Attal N, et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019;160(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Main CJ. Pain assessment in context: A state of the science review of the McGill Pain Questionnaire 40 years on. Pain 2016;157(7):1387–99. [DOI] [PubMed] [Google Scholar]
- 27.Ernat J, Knox J, Orchowski J, Owens B. Incidence and risk factors for acute low back pain in active duty infantry. Mil Med 2012;177(11):1348–51. [DOI] [PubMed] [Google Scholar]
- 28.Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am 2016;100(1):169–81. [DOI] [PubMed] [Google Scholar]
- 29.Golob AL, Wipf JE. Low back pain. Med Clin North Am 2014;98(3):405–28. [DOI] [PubMed] [Google Scholar]
- 30.Knox JB, Orchowski JR, Owens B. Racial differences in the incidence of acute low back pain in United States military service members. Spine 2012;37(19):1688–92. [DOI] [PubMed] [Google Scholar]
- 31.Ochsmann E, Rüger H, Kraus T, Drexler H, Letzel S, Münster E. Gender-specific risk factors for acute low back pain: Starting points for target-group-specific prevention [in German]. Schmerz 2009;23(4):377–84. [DOI] [PubMed] [Google Scholar]
- 32.Downie AS, Hancock MJ, Rzewuska M, Williams CM, Lin CW, Maher CG. Trajectories of acute low back pain: A latent class growth analysis. Pain 2016;157(1):225–34. [DOI] [PubMed] [Google Scholar]
- 33.Cassidy JD, Carroll LJ, Cote P. The Saskatchewan Health and Back Pain Survey. The prevalence of low back pain and related disability in Saskatchewan adults. Spine 1998;23(17):1860–6, discussion 1867. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda T, Sugiyama K, Aida J, et al. Socioeconomic inequalities in low back pain among older people: The JAGES cross-sectional study. Int J Equity Health 2019;18(1):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giummarra MJ, Ioannou L, Ponsford J, et al. Chronic pain following motor vehicle collision: A systematic review of outcomes associated with seeking or receiving compensation. Clin J Pain 2016;32(9):817–27. [DOI] [PubMed] [Google Scholar]
- 36.Yang H, Haldeman S, Lu ML, Baker D. Low back pain prevalence and related workplace psychosocial risk factors: A study using data from the 2010 National Health Interview Survey. J Manipulative Physiol Ther 2016;39(7):459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiri R, Falah-Hassani K, Heliovaara M, et al. Risk factors for low back pain: A population-based longitudinal study. Arthritis Care Res (Hoboken) 2019;71(2):290–26. [DOI] [PubMed] [Google Scholar]
- 38.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med 2007;147(7):478–91. [DOI] [PubMed] [Google Scholar]
- 39.Edwards D, Gatchel R, Adams L, Stowell AW. Emotional distress and medication use in two acute pain populations: Jaw and low back. Pain Pract 2006;6(4):242–53. [DOI] [PubMed] [Google Scholar]
- 40.Shaw WS, Hartvigsen J, Woiszwillo MJ, Linton SJ, Reme SE. Psychological distress in acute low back pain: A review of measurement scales and levels of distress reported in the first 2 months after pain onset. Arch Phys Med Rehabil 2016;97(9):1573–87. [DOI] [PubMed] [Google Scholar]
- 41.Wand BM, Chiffelle LA, O’Connell NE, McAuley JH, Desouza LH. Self-reported assessment of disability and performance-based assessment of disability are influenced by different patient characteristics in acute low back pain. Eur Spine J 2010;19(4):633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elfering A, Kaser A, Melloh M. Relationship between depressive symptoms and acute low back pain at first medical consultation, three and six weeks of primary care. Psychol Health Med 2014;19(2):235–46. [DOI] [PubMed] [Google Scholar]
- 43.Bjorland S, Roe C, Moen A, Schistad E, Mahmood A, Gjerstad J. Genetic predictors of recovery in low back and lumbar radicular pain. Pain 2017;158(8):1456–60. [DOI] [PubMed] [Google Scholar]
- 44.Starkweather AR, Ramesh D, Lyon DE, et al. Acute low back pain: Differential somatosensory function and gene expression compared with healthy no-pain controls. Clin J Pain 2016;32(11):933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grotle M, Brox JI, Veierød MB, Glomsrød B, Lønn JH, Vøllestad NK. Clinical course and prognostic factors in acute low back pain: Patients consulting primary care for the first time. Spine 2005;30(8):976–82. [DOI] [PubMed] [Google Scholar]
- 46.Lanier DC, Stockton P. Clinical predictors of outcome of acute episodes of low back pain. J Fam Pract 1988;27(5):483–9. [PubMed] [Google Scholar]
- 47.Wand BM, Bird C, McAuley JH, Doré CJ, MacDowell M, De Souza LH. Early intervention for the management of acute low back pain: A single-blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine 2004;29(21):2350–6. [DOI] [PubMed] [Google Scholar]
- 48.Ciechanowski P, Sullivan M, Jensen M, Romano J, Summers H. The relationship of attachment style to depression, catastrophizing and health care utilization in patients with chronic pain. Pain 2003;104(3):627–37. [12927635] [CVOTICKCVO] [DOI] [PubMed] [Google Scholar]
- 49.Kratz AL, Davis MC, Zautra AJ. Attachment predicts daily catastrophizing and social coping in women with pain. Health psychology: Official journal of the Division of Health Psychology. Am Psychol Assoc 2012;31(3):278–85. [DOI] [PubMed] [Google Scholar]
- 50.Meredith P, Strong J, Feeney JA. Adult attachment, anxiety, and pain self-efficacy as predictors of pain intensity and disability. Pain 2006;123(1–2):146–54. [DOI] [PubMed] [Google Scholar]
- 51.Davies KA, Macfarlane GJ, McBeth J, Morriss R, Dickens C. Insecure attachment style is associated with chronic widespread pain. Pain 2009;143(3):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McWilliams LA, Asmundson GJ. The relationship of adult attachment dimensions to pain-related fear, hypervigilance, and catastrophizing. Pain 2007;127(1–2):27–34. [DOI] [PubMed] [Google Scholar]
- 53.Esteve R, Bendayan R, Lopez-Martinez AE, Ramirez-Maestre C. Resilience and vulnerability factors when pain is acute as predictors of disability: Findings from a two-year longitudinal study. Pain Med 2017;18(11):2116–25. [DOI] [PubMed] [Google Scholar]
- 54.Zimney K, Louw A, Puentedura EJ. Use of therapeutic neuroscience education to address psychosocial factors associated with acute low back pain: A case report. Physiother Theory Pract 2014;30(3):202–9. [DOI] [PubMed] [Google Scholar]
- 55.Traeger AC, Hubscher M, Henschke N, Moseley GL, Lee H, McAuley JH. Effect of primary care-based education on reassurance in patients with acute low back pain: Systematic review and meta-analysis. JAMA Intern Med 2015;175(5):733–43. [DOI] [PubMed] [Google Scholar]
- 56.Fischer CA, Neubauer E, Adams HS, Schiltenwolf M, Wang H. Effects of multidisciplinary pain treatment can be predicted without elaborate questionnaires. Int Orthop 2014;38(3):617–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallegraeff JM, Krijnen WP, van der Schans CP, de Greef MH. Expectations about recovery from acute non-specific low back pain predict absence from usual work due to chronic low back pain: A systematic review. J Physiother 2012;58(3):165–72. [DOI] [PubMed] [Google Scholar]
- 58.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J Behav Med 2007;30(1):77–94. [DOI] [PubMed] [Google Scholar]
- 59.Stisen DB, Tegner H, Bendix T, Esbensen BA. The experience of patients with fear-avoidance belief hospitalised for low back pain - a qualitative study. Disabil Rehabil 2016;38(4):307–14. [DOI] [PubMed] [Google Scholar]
- 60.Swinkels-Meewisse IE, Roelofs J, Oostendorp RA, Verbeek AL, Vlaeyen JW. Acute low back pain: Pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain 2006;120(1–2):36–43. [DOI] [PubMed] [Google Scholar]
- 61.Thomas JS, France CR. Pain-related fear is associated with avoidance of spinal motion during recovery from low back pain. Spine 2007;32(16):E460–6. [DOI] [PubMed] [Google Scholar]
- 62.Grotle M, Brox JI, Glomsrod B, Lonn JH, Vollestad NK. Prognostic factors in first-time care seekers due to acute low back pain. Eur J Pain 2007;11(3):290–8. [DOI] [PubMed] [Google Scholar]
- 63.Traeger AC, Hubscher M, Henschke N, et al. Emotional distress drives health services overuse in patients with acute low back pain: A longitudinal observational study. Eur Spine J 2016;25(9):2767–73. [DOI] [PubMed] [Google Scholar]
- 64.Devereaux M. Low back pain. Med Clin North Am 2009;93(2):477–501. [DOI] [PubMed] [Google Scholar]
- 65.Carroll I, Hah J, Mackey S, et al. Perioperative interventions to reduce chronic postsurgical pain. J Reconstr Microsurg 2013;29(04):213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Semin Arthritis Rheum 1998;28(1):60–71. [DOI] [PubMed] [Google Scholar]
- 67.Urits I, Burshtein A, Sharma M, et al. Low back pain, a comprehensive review: Pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep 2019;23(3):23. [DOI] [PubMed] [Google Scholar]
- 68.DeLeo JA, Winkelstein BA. Physiology of chronic spinal pain syndromes: From animal models to biomechanics. Spine 2002;27(22):2526–37. [DOI] [PubMed] [Google Scholar]
- 69.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science 2000;288(5472):1765–9. [DOI] [PubMed] [Google Scholar]
- 70.Boada MD, Gutierrez S, Eisenach JC. Peripheral oxytocin restores light touch and nociceptor sensory afferents towards normal after nerve injury. Pain 2019;160(5):1146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhave G, Gereau RW. Posttranslational mechanisms of peripheral sensitization. J Neurobiol 2004;61(1):88–106. [DOI] [PubMed] [Google Scholar]
- 72.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001;413(6852):203–10. [DOI] [PubMed] [Google Scholar]
- 73.Inoue K, Tsuda M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 2018;19(3):138–52. [DOI] [PubMed] [Google Scholar]
- 74.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: Do pain and memory share similar mechanisms? Trends Neurosci 2003;26(12):696–705. [DOI] [PubMed] [Google Scholar]
- 75.Samad TA, Moore KA, Sapirstein A, et al. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 2001;410(6827):471–5. CrossRef][10.1038/35068566] [DOI] [PubMed] [Google Scholar]
- 76.Harvey RJ, Depner UB, Wassle H, et al. GlyR alpha3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004;304(5672):884–7. [DOI] [PubMed] [Google Scholar]
- 77.Woolf CJ; American College of Physicians, American Physiological Society. Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 2004;140(6):441–51. [DOI] [PubMed] [Google Scholar]
- 78.Woolf CJ, King AE. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J Neurosci 1990;10(8):2717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang SS, Wu W, Yang JM, Wang CH. Abnormal spontaneous brain activity in acute low-back pain revealed by resting-state functional MRI. Am J Phys Med Rehabil 2017;96(4):253–9. [DOI] [PubMed] [Google Scholar]
- 80.Sudhaus S, Fricke B, Stachon A, et al. Salivary cortisol and psychological mechanisms in patients with acute versus chronic low back pain. Psychoneuroendocrinology 2009;34(4):513–26. [DOI] [PubMed] [Google Scholar]
- 81.Diz JBM, de Souza Moreira B, Felicio DC, et al. Brain-derived neurotrophic factor plasma levels are increased in older women after an acute episode of low back pain. Arch Gerontol Geriatr 2017;71:75–82. [DOI] [PubMed] [Google Scholar]
- 82.Bogduk N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain 2009;147(1–3):17–9. [19762151] [CVOTICKCVO] [DOI] [PubMed] [Google Scholar]
- 83.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain 2004;5(9):491–7. [DOI] [PubMed] [Google Scholar]
- 84.Alexander CE, Varacallo M. Lumbosacral Radiculopathy. Treasure Island, FL: StatPearls; 2019. [PubMed] [Google Scholar]
- 85.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res Int 2014;2014:698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cook CE, Taylor J, Wright A, Milosavljevic S, Goode A, Whitford M. Risk factors for first time incidence sciatica: A systematic review. Physiother Res Int 2014;19(2):65–78. [DOI] [PubMed] [Google Scholar]
- 87.Shiri R, Lallukka T, Karppinen J, Viikari-Juntura E. Obesity as a risk factor for sciatica: A meta-analysis. Am J Epidemiol 2014;179(8):929–37. [DOI] [PubMed] [Google Scholar]
- 88.Iversen T, Solberg TK, Wilsgaard T, Waterloo K, Brox JI, Ingebrigtsen T. Outcome prediction in chronic unilateral lumbar radiculopathy: Prospective cohort study. BMC Musculoskelet Disord 2015;16(1):4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: Four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33(25):2789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen SP, Bicket MC, Jamison D, Wilkinson I, Rathmell JP. Epidural steroids: A comprehensive, evidence-based review. Reg Anesth Pain Med 2013;38(3):175–200. [DOI] [PubMed] [Google Scholar]
- 91.Hasvik E, Iordanova Schistad E, Grøvle L, Julsrud Haugen A, Røe C, Gjerstad J. Subjective health complaints in patients with lumbar radicular pain and disc herniation are associated with a sex - OPRM1 A118G polymorphism interaction: A prospective 1-year observational study. BMC Musculoskelet Disord 2014;15(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hider SL, Whitehurst DG, Thomas E, Foster NE. Pain location matters: The impact of leg pain on health care use, work disability and quality of life in patients with low back pain. Eur Spine J 2015;24(3):444–51. [DOI] [PubMed] [Google Scholar]
- 93.Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: A novel treatment approach. Clin J Pain 2007;23(1):15–22. [DOI] [PubMed] [Google Scholar]
- 94.Hunt JL, Winkelstein BA, Rutkowski MD, Weinstein JN, DeLeo JA. Repeated injury to the lumbar nerve roots produces enhanced mechanical allodynia and persistent spinal neuroinflammation. Spine 2001;26(19):2073–9. [DOI] [PubMed] [Google Scholar]
- 95.Suri P, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine 2011;36(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamada K, Aota Y, Higashi T, et al. Roentgenographic and computed tomographic findings in symptomatic lumbar foraminal stenosis. Eur Spine J 2015;24(2):333–8. [DOI] [PubMed] [Google Scholar]
- 97.Kato K, Sekiguchi M, Kikuchi S, Konno S. The effect of a 5-HT2A receptor antagonist on pain-related behavior, endogenous 5-hydroxytryptamine production, and the expression 5-HT2A receptors in dorsal root ganglia in a rat lumbar disc herniation model. Spine 2015;40(6):357–62. [DOI] [PubMed] [Google Scholar]
- 98.Sekiguchi M, Konno S, Kikuchi S. Effects of 5-HT2A receptor antagonist on blood flow in chronically compressed nerve roots. J Peripher Nerv Syst 2004;9(4):263–9. [DOI] [PubMed] [Google Scholar]
- 99.Kato K, Kikuchi S, Konno S, Sekiguchi M. Participation of 5-hydroxytryptamine in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine 2008;33(12):1330–6. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi H, Kikuchi S, Konno S, Kato K, Sekiguchi M. Interaction of 5-hydroxytryptamine and tumor necrosis factor-alpha to pain-related behavior by nucleus pulposus applied on the nerve root in rats. Spine 2011;36(3):210–8. [DOI] [PubMed] [Google Scholar]
- 101.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med 2010;16(11):1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hucho T, Levine JD. Signaling pathways in sensitization: Toward a nociceptor cell biology. Neuron 2007;55(3):365–76. [DOI] [PubMed] [Google Scholar]
- 103.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014;13(7):533–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scholz J, Woolf CJ. The neuropathic pain triad: Neurons, immune cells and glia. Nat Neurosci 2007;10(11):1361–8. [DOI] [PubMed] [Google Scholar]
- 105.Shiri R, Falah-Hassani K, Viikari-Juntura E, Coggon D. Leisure-time physical activity and sciatica: A systematic review and meta-analysis. Eur J Pain 2016;20(10):1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fritz JM, Delitto A, Welch WC, Erhard RE. Lumbar spinal stenosis: A review of current concepts in evaluation, management, and outcome measurements. Arch Phys Med Rehabil 1998;79(6):700–8. [DOI] [PubMed] [Google Scholar]
- 107.Siebert E, Prüss H, Klingebiel R, Failli V, Einhäupl KM, Schwab JM. Lumbar spinal stenosis: Syndrome, diagnostics and treatment. Nat Rev Neurol 2009;5(7):392–403. [DOI] [PubMed] [Google Scholar]
- 108.Bagley C, MacAllister M, Dosselman L, Moreno J, Aoun SG, El Ahmadieh TY. Current concepts and recent advances in understanding and managing lumbar spine stenosis. F1000Res 2019;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine 1996;21(15):1787–94, discussion 94–5. [DOI] [PubMed] [Google Scholar]
- 110.Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine 2007;32(1):1–8. [DOI] [PubMed] [Google Scholar]
- 111.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine 2010;35(14):1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Singh K, Samartzis D, Vaccaro AR, et al. Congenital lumbar spinal stenosis: A prospective, control-matched, cohort radiographic analysis. Spine J 2005;5(6):615–22. [DOI] [PubMed] [Google Scholar]
- 113.Ammendolia C, Schneider M, Williams K, et al. The physical and psychological impact of neurogenic claudication: The patients’ perspectives. J Can Chiropr Assoc 2017;61(1):18–31. [PMC free article] [PubMed] [Google Scholar]
- 114.Edwards TC, Lavallee DC, Bauer Z, et al. Problem areas identified as important to older adults with lumbar spinal stenosis. Spine J 2015;15(7):1636–44. [DOI] [PubMed] [Google Scholar]
- 115.Lyle S, Williamson E, Darton F, Griffiths F, Lamb SE. A qualitative study of older people’s experience of living with neurogenic claudication to inform the development of a physiotherapy intervention. Disabil Rehabil 2017;39(10):1009–17. [DOI] [PubMed] [Google Scholar]
- 116.Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine 1995;20(24):2746–9. [DOI] [PubMed] [Google Scholar]
- 118.Rydevik B, Brown MD, Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine 1984;9(1):7–15. [DOI] [PubMed] [Google Scholar]
- 119.Marin TJ, Van Eerd D, Irvin E, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low back pain. Cochrane Database Syst Rev 2017;6:CD002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Page GM, Lacasse A, Quebec Back Pain Consortium. The Quebec Low Back Pain Study: A protocol for an innovative 2-tier provincial cohort. Pain Rep 2020;5(1):e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Baumbauer KM, Perry RD, M, Carney KB, et al. Contribution of COMT and BDNF genotype and expression to the risk of transition from acute to chronic low back pain. Clin J Pain 2020;36:430–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramesh D, D'Agata A, Starkweather AR, Young EE. Contribution of endocannabinoid gene expression and genotype on low back pain susceptibility and chronicity. Clin J Pain 2018;34(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klyne DM, Barbe MF, van den Hoorn W, Hodges PW. ISSLS PRIZE IN CLINICAL SCIENCE 2018: Longitudinal analysis of inflammatory, psychological, and sleep-related factors following an acute low back pain episode-the good, the bad, and the ugly. Eur Spine J 2018;27(4):763–77. [DOI] [PubMed] [Google Scholar]