Abstract
Continuous venovenous hemofiltration (CVVH) is a life‐sustaining procedure in patients with severe burns and acute kidney injury. Physiologic changes from burn injury and use of CVVH may alter imipenem pharmacokinetics (PK). We aimed to compare imipenem clearance (CL) in burn patients with and without CVVH, determine the effect of burn on imipenem volume of distribution (CVVH, n = 12; no CVVH, n = 11), in combination with previously published models. Model qualification was performed with standard diagnostics and comparing predicted PK parameters/time‐concentration profiles with those in the existing literature. Monte Carlo simulations were conducted to evaluate the probability of target attainment. A 2‐compartment model best described the data. Utilizing albumin as a covariate on volume parameters and leveraging the clearance model from prior literature, our model predicted imipenem central volume and CL within a 10% margin of error across healthy, renally impaired, and burn populations. We provide direct comparison of imipenem CL in burn patients with and without CVVH. Notably, there was no significant difference. Large imipenem Vd in patients with severe burns is likely explained by increased capillary permeability, for which serum albumin may be a reasonable surrogate. Dosing 500 mg every 6 hours is adequate for burn patients on renally dosed CVVH; however, suspicion of augmented renal clearance or patients placed on CVVH without renal impairment may necessitate dosing of 1000 mg every 6 hours.
Keywords: antibiotic, burn, Monte Carlo simulations, pharmacokinetics
Severe burns, characterized by ≥20%‐30% total body surface area (TBSA), involve widespread skin and soft‐tissue damage leading to systemic inflammation, hypermetabolism, and multiorgan dysfunction.1, 2, 3, 4 Physiologic alterations associated with burns are complex and multiphasic, with increased capillary permeability,5 myocardial depression,6 and systemic hypotension7 observed immediately postinjury. Subsequent to the initial phase of injury, the second, or hypermetabolic, phase of severe burn is characterized by increased cardiac output, increased major organ blood flow, and accelerated catabolism3, 8 that can persist months after injury. Collectively, these multiphasic pathophysiologic changes contribute to the complexity in the management and care of burn victims.
In the modern burn care setting, individuals with severe burns are at high risk for significant morbidity and mortality, even in highly specialized centers.9 As such, the management of infection via pharmacotherapy is a frequent occurrence in burn care10; however, infection remains the leading cause of morbidity and death after injury.11, 12 The increased risk of developing a life‐threatening infection, leading to sepsis and multiple organ failure, necessitates immediate and adequate antimicrobial therapy to optimize chances of survival. Of particular importance, appropriate antibiotic dosing is challenging in critically ill patients (burn and nonburn) because of adaptations in physiology that impacts drug pharmacokinetics (PK), namely, for hydrophilic antimicrobials.13 Specifically, altered protein binding, increased volume of distribution (Vd), and augmented renal clearance are known permutations that contribute to substantial variability in antimicrobial PK.14, 15, 16 Achieving adequate antimicrobial concentrations can be further impeded by increased bacterial minimal inhibitory concentrations (MICs), which tend to be the predominant driver of failure to achieve adequate probability of target attainment (PTA).
Augmented renal clearance (ARC) is a phenomenon observed in burn victims,17, 18 with variable incidence (14%‐80%) in critically ill patients, potentially underlying suboptimal drug exposures, therapy failures,19, 20 and the development of bacterial drug resistance.16 Quantifying augmented renal clearance in critically ill patients is challenging, as it requires burdensome testing such as measuring 24‐hour creatinine clearance (CrCl). A recent study by Mulder et al used this approach to elucidate modifiable risk factors that contribute to augmented renal clearance, correlating 24‐hour CrCl with clinical estimates of glomerular filtration rate via the Cockcroft‐Gault equation. Interestingly, the major determinants of CrCl, body mass index (29 ± 6 vs 28 ± 6 kg/m2) and serum creatinine (0.97 ± 0.28 vs 1.09 ± 0.42 mg/dL) were similar in patients with and without augmented renal clearance, respectively.19 Moreover, the in‐depth analysis of risk factors and demographics associated with augmented renal clearance in trauma patients in this study highlighted the prevalence of augmented renal clearance and reduced mortality in young healthy males.
An important clinical consideration in burn patients is antibiotic clearance (CL) and the use of continuous renal replacement therapy (CRRT). The decision to incorporate CRRT in the care and management of critically ill patients, as well as the time to initiate and maintain therapy, has been the subject of extensive research.21, 22, 23 Traditionally, indications for the implementation of CRRT include the presence of severe acidosis, electrolyte disturbance, toxic ingestion, volume overload, or uremia.21, 22, 23 Continuous venovenous hemofiltration (CVVH), one of the most common modalities of CRRT, has been correlated with improved clinical incomes and associated with decreased mortality rates in burn and critically ill patients with acute kidney injury (AKI).24, 25, 26, 27, 28 As such, there is generally a low threshold to initiate CVVH in the critically ill burn population. CVVH allows for efficient metabolic solute CL and ultrafiltration of fluid; however, it imposes significant challenges on drug dosing, as factors beyond extracorporeal drug removal need to be accounted for, to include residual kidney function and changes in Vd and protein binding.29, 30 In addition, a recent observational study revealed that the average CVVH dose prescribed and delivered, to critically ill burn patients was relatively high compared with recommended doses based on high‐quality evidence.27 Early implementation and higher doses of CVVH, in addition to the high incidence of augmented renal clearance within this patient population, are important clinical considerations, as this combination may confound optimal dose selection and require higher dosing strategies.
Because of its broad‐spectrum antimicrobial activity, imipenem is often prescribed to critically ill patients treated with and without CVVH with high‐risk factors for multidrug resistance. To date, numerous studies have conducted imipenem population PK analyses and simulations in diverse patient populations that provide insight into appropriate dosing regimens to account for altered physiology.31, 32, 33, 34 Residual diuresis and burn injury are identified as important modifiers for endogenous imipenem CL, as the typical value in burn patients with CVVH was approximately 82% higher than that of CVVH patients without burn (11.12 and 6.11 L/h, respectively).33 In addition, recent evidence suggests that CVVH may contribute to greater clearance of imipenem than previously reported, with higher doses recommended to adequately treat and prevent resistance to pathogens with higher MICs (4‐8 µg/mL).35 Within these studies, there is wide variation in analytical techniques, software, and study sample size used.10, 36, 37 Consequently, this variability, in combination with the unpredictable physiology in burn patients, has resulted in model misspecification, with proposed models ranging from 1 to 3 compartments.33, 34, 35, 38 To our knowledge, no imipenem PK studies have quantified the effect of TBSA on imipenem Vd or directly compared burn patients treated with and without CVVH. Moreover, impaired and/or augmented renal function has not been assessed in burn populations to determine if dosing changes are necessary to achieve therapeutic levels of imipenem.
For this study, we sought to establish a comprehensive population PK model from our clinical data set and previously published literature to quantify the effects of burn on imipenem CL and Vd. Our findings presented here directly compare inherent CL of burn patients treated with and without CVVH and provide improved dosing recommendations in specific subpopulations, with the ability to extrapolate renal failure with CrCl and serum albumin as covariates.
Methods
Data
For the study, protocol and associated documents, to include informed consent forms, were reviewed and approved by the institutional review board at the United States Army Institute of Surgical Research Burn Center (San Antonio, Texas). There were a total of 23 patients, 11 who did not receive CVVH and 12 who received CVVH. For CVVH patients, the range of prescribed CVVH and delivered CVVH was 22.0‐54.5 and 20.5‐47.2 cc·h/kg, respectively. All but 2 patients received 500 mg imipenem every 6 hours infused over 30 minutes or 1 hour. The remaining 2 patients received either 1000 mg imipenem every 6 hours infused over 1 hour or 250 mg imipenem every 6 hours infused over 30 minutes. For each patient receiving concurrent CVVH, prefilter plasma (equivalent to a peripheral blood sample for non‐CVVH patients), postfilter plasma, and ultrafiltrate samples were collected at steady state. A baseline (trough) set of samples for each patient was drawn prior to the dose. The remaining samples were drawn from 0.5 to 8 hours postdose. There was a total of 81 prefilter plasma concentration observations. The mean number of prefilter postdose plasma samples per patient was 3.68 (range, 1‐4). One patient had no postdose plasma samples and was excluded from the PK analysis. One patient had missing CVVH data, but had postdose concentration observations available. Means for CVVH data including blood flow rate, ultrafiltrate rate, and prefilter fluid administration rate were imputed for this individual. Missing plasma concentration data from prefilter, postfilter, and ultrafiltrate samples were excluded from the analysis.
High‐Performance Liquid Chromatography
Plasma imipenem concentrations from patient samples were determined by high‐performance liquid chromatography (HPLC) using a method previously validated in our laboratory. A Dionex 3000 HPLC system (Dionex, Thermo‐Fisher Inc., Sunnyvale, California) with ultraviolet detection at 298 nm was used for analysis. Briefly, the mobile phases consisted of 0.2 borate buffer at pH 7.2 (mobile phase A) and 100% MeOH (mobile phase B). These were run at 0.6 mL/min isocratically (97:3) for 10 minutes, followed by a ramp of mobile phase B from 3% to 26% until 20 minutes. The stationary phase was a 150‐mm octadecyl column (Luna 5u C18 100A 150 × 4.6 mm; Phenomenex, Torrance, California). This resulted in retention times for imipenem and meropenem (internal standard [IS]) of approximately 7.5 and 20 minutes, respectively. Standard curves were constructed for imipenem by injecting reference solutions of known concentrations of analyte and IS. Peak areas of the eluted drugs were integrated, and concentrations were quantified using peak area ratios of analyte to IS. Linearity was confirmed from 0.50 to 25.0 µg/mL, with the mean ± SD between‐day calibration curve regression r 2 of 0.9992 ± 0.0008. Between‐day coefficients of variation at 0.5 and 25.0 µg/mL were 0.58% and 0.48%, respectively. The limit of detection for the assay was 0.02 µg/mL.
Samples were prepared for analysis by adding 15 µg of IS and 1 mL acetonitrile (MeCN). Following centrifugation (10 000g; 10 minutes), 900 µL of supernatant organic phase was decanted and evaporated to dryness using N2. The remaining residue was reconstituted in 200 µL mycophenolic acid, and 50‐μL aliquots were injected into the HPLC for analysis. The concentration of drug in each sample was determined by regression analysis of the peak area ratios.
Population Pharmacokinetic Modeling and Simulations
The general modeling approach was to first use standard statistical techniques to create a covariate model based on our data alone. Once this covariate model was finalized, it was compared with published imipenem population PK models, and strengths from these existing models were incorporated into our final model.
Population PK modeling and simulations were performed in Pumas (version 1.05),39 a PK/pharmacodynmaic estimation and simulation package in Julia.40 The first‐order conditional estimation method with interaction (FOCEI) was used to estimate population parameters. Data preparation, exploratory analysis, and graphs were performed in either Pumas or R (version 3.6.1). Data from all patients, both those positive and negative for CVVH were modeled simultaneously. The CL because of CVVH for each individual patient (CLCVVH) was calculated as the product of the ultrafiltrate flow rate (Qf ), the sieving coefficient (Sc ), and correction factor for prefilter fluid administration ( as follows:
| (1) |
where
| (2) |
and
| (3) |
where , , and denote the observed prefilter, postfilter, and ultrafiltrate concentrations, denotes the blood flow rate, and denotes the rate of prefilter replacement fluid.41, 42
Base Model
One‐, 2‐, and 3‐compartment models were explored. Between‐subject variability (BSV) was modeled using an exponential error model under the assumption that PK parameters are distributed log‐normally. Parameters generally took the form
| (4) |
where is the post hoc estimated parameter value for individual i, is the population mean parameter, and is the between‐subject random effects for individual i. However, as our approach was to simultaneously model data from CVVH and non‐CVVH patients, the base model equation for CL was
| (5) |
Selection of the base model was based on the likelihood ratio test with, plausibility and precision of parameter estimates, and diagnostic plots.
Covariate Model
Covariates were initially evaluated by plotting random effects of PK parameters from the base model against each covariate and observing the trends. Covariates evaluated were total body weight, lean body weight (LBW), CrCl, age, total burn surface area (TBSA), total second‐degree burn surface area, total third‐degree burn surface area, serum albumin, urine output, and use of CVVH. CrCl was calculated by the Cockcroft‐Gault equation,43 and LBW was calculated using Janmahasatian's formula.44 Continuous covariates were modeled as
| (6) |
where is the PK parameter in individual i, is the typical value of the PK parameter at the median value of the covariate (), is the covariate observed in individual i, and is the power estimate for the covariate.
Categorical covariates were modeled as
| (7) |
where is binary (coded as 0 or 1), represents the typical value of the PK parameter when , and represents the proportional change in when . Covariate modeling was initially performed with a forward addition process. A decrease of at least 3.84 units () in the objective function value (OFV) was considered statistically significant.
After determining which covariates were statistically significant in our data set, we compared our covariate model with established imipenem population PK models published in the literature. With a stepwise approach to ensure there were no major statistical liabilities, we included covariates in our model provided they were physiologically or clinically relevant.
Final Model Qualification
Final model qualification was based on both internal and external evaluations. The internal evaluation included examination of standard goodness‐of‐fit plots, precision of parameter estimates based on inference and bootstrap methods (n = 1000 runs), visual predictive checks (200 replicates, overall and stratified by CVVH), and normalized prediction distributed error analysis45 (performed in Pumas, 1000 replicates). External model evaluation was performed comparing pharmacokinetic PK estimates of our final model with those in the existing literature. In addition, the final model‐predicted time‐concentration data were graphically compared with time‐concentration data from the existing literature.
Monte Carlo Simulations
Monte Carlo simulations were performed with the final population PK model to evaluate surrogates for efficacy and safety in various burn subpopulations with current U.S. Food and Drug Administration (FDA)‐approved doses of imipenem. PTA was considered a surrogate for efficacy and defined as achieving free imipenem concentrations above minimum inhibitory concentrations greater than 40% of the time at steady state within the dosing interval (40% ). Simulations for renal function, namely, normal renal function (NRF) and augmented renal clearance (ARC) were performed by randomly selecting CrCl between 100 and 130 and between 150 and 250 mL/min, respectively. Median imipenem trough concentrations have been observed to range from 1 to 3.6 mg/L, depending on dose administered,46, 47 with patients with toxicity displaying higher trough levels than those without.48 As such, the probability of trough concentration > 5 mg/L was explored as a surrogate threshold for safety, which slightly exceeded the typical range of imipenem trough or minimum concentration (Cmin) observed clinically. Patients were simulated with a body weight of 70 kg. For each scenario, 1000 patient concentration‐time profiles were simulated assuming imipenem is 20% protein bound.49 The percentage of simulated patients who achieved 40% was calculated at MICs ranging from 0.5 to 16 mg/L, with PTA > 80% considered acceptable. Non‐FDA‐labeled dosing regimens were explored only if simulations with FDA‐labeled doses demonstrated PTA < 80% at a given target MIC.
Results
Patient Demographics
Patient demographics by CVVH status are summarized in Table 1. With the exception of weight and sex, the demographics reported in Table 1 were generally well balanced between the CVVH and no‐CVVH patient groups. The CVVH group had 5 women and 7 men, whereas the no‐CVVH group had only 1 woman and 10 men. The weight difference in the 2 patient groups corresponded to the sex imbalance, where mean weight in no‐CVVH patients was 105.06 ± 28.66 kg and mean weight in CVVH patients was 89.6 ± 22.38 kg. For CVVH patients, the average effluent (ultrafiltrate) flow rate was 30.13 ± 6.45 cc·h/kg. Median values and ranges for covariates used in the final population model were weight, 99.5 kg (57.9‐150.8 kg); albumin, 2.7 g/dL (1.5‐3.5 g/dL); and CrCl, 145.83 mL/min (88.08‐253.95 mL/min). The median CrCl was calculated only using data from the no‐CVVH population.
Table 1.
Patient Demographics (Data Presented as Mean and Standard Deviation)
| No CVVH | CVVH | |
|---|---|---|
| Age (years) | 51.09 (19.03) | 55 (19.99) |
| Sex | 1 woman and 10 men | 5 women and 7 men |
| Weight (kg) | 105.06 (28.66) | 89.6 (22.38) |
| Height (cm) | 176.16 (7.88) | 170.286 (7.03) |
| Total burn surface area (%) | 40.18 (20.88) | 45.31 (22.66) |
| Second‐degree burn (%) | 32.66 (18.3) | 19.47 (17.4) |
| Third‐degree burn (%) | 7.61 (10.65) | 25.92 (29.31) |
| Creatinine (mg/dL) (0.7‐1.4) | 0.86 (0.23) | 1.05 (0.52) |
| Albumin (g/dL) (3.4‐5.4) | 2.6 (0.55) | 2.92 (0.91) |
| Blood urea nitrogen (mg/dL) (14‐23) | 33.75 (18.35) | 34.66 (17.89) |
| Creatinine clearance (mL/min) | 151.92 (51.07) | 113.02 (58.92) |
| Urine output (mL) | 3144.46 (1660.99) | 948.83 (1255.43) |
| CLCVVH | – | 1.56 (0.7) |
| Effluent flow rate (cc/kg/h) | – | 30.13 (6.45) |
| Sieving coefficient | – | 0.67 (0.33) |
| Correction factor | – | 0.86 (0.04) |
Population Pharmacokinetic Models
Base Model
The model‐building process is summarized in Table S1 (supplementary material). Data from all patients (CVVH and no‐CVVH) were used to build 1 model. The data were best described by a 2‐compartment model with a combined additive/proportional error model. The 2‐compartment model compared with a 1‐compartment model led to a decrease in 27.2 units of the OFV. A 3‐compartment model led to a 20‐unit increase in the OFV compared with the 2‐compartment model. A 2‐compartment model with combined additive/proportional error led to a decrease in 7.28 units of the OFV compared with the same model with only proportional error. The 2‐compartment model was parameterized with terminal clearance CL, intracompartmental clearance Q, volume of the central compartment Vc, and volume of the peripheral compartment Vp.The BSV estimate for Q was approximately 0 and was therefore not estimated in any further model runs.
Internal Covariate Model
Covariate exploratory plots with random effects generated from the base model demonstrated strong trends between ηCL and CrCl and ηCL and weight. With regard to volume, exploratory plots demonstrated strong trends between and TBSA, ηVc and serum albumin, ηVp, and TBSA, and ηVp and serum albumin. Of note, serum albumin and TBSA were highly correlated, and the relationship of these covariates was adequately described by a linear model (Figure 1).
Figure 1.
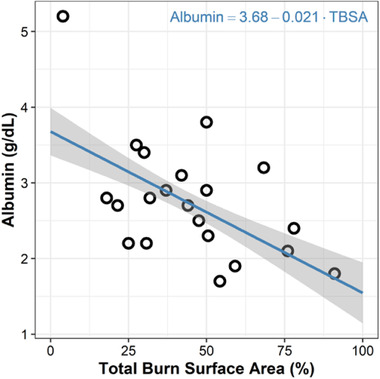
Albumin versus total burn surface area (TBSA). Linear model with intercept coefficient of 3.68 (P < 2 × 10−16) and slope coefficient of −0.021 (P = 8.33 × 10−9).
Parameter estimates of the internal covariate model are summarized in Table S2 (supplementary material). Based on our data alone (run 17, Table S1, supplementary material), we found that both weight and lean body weight were statistically significant covariates on terminal CL (P = .01 for both). The estimated exponent for weight as a covariate on terminal CL was 0.84 (95%CI, 0.17‐1.49). Weight was selected over ideal body weight (IBW) for further model runs given robust literature with which to directly compare our estimate. Of note, there was not a statistically significant difference in the inherent terminal CL of patients who received CVVH and those who did not.
Regarding covariates on volume, serum albumin was found to be a significant covariate on Vp (P = .043; power, −2.85; 95%CI, −5.95 to 0.28). However, the 1000 replicate bootstrap 95%CI for this estimate was −6.78 to 1.89. Neither weight nor IBW was a statistically significant covariates on Vc. There was large variability in Vp with BSV estimated to be 115 %CV as well as a low precision of estimates for Vp and, particularly noted by 1000 replicate bootstrap percent relative standard error (%RSE) of 62.99% and 249.58%, respectively. Other than estimates of and, PK parameters and random effects were similar to those reported for the final model (Table 2). Diagnostic plots were similar to those described below for the final model. Final equations for CL and Vp in this model were
| (8) |
and
| (9) |
respectively.
Table 2.
Pharmacokinetic Parameters for Final Model
| Parameter | Estimate (%RSE) | FOCEI 95%CI | Bootstrapd Estimate (%RSE) | Bootstrapd 95%CI |
|---|---|---|---|---|
| CL (L/h) | 15.31 (13) | 11.406‐19.21 | 15.31 (13.56) | 11.2‐19.92 |
| Vc (L) | 32.67 (27.18) | 15.29‐50.14 | 32.67 (29.6) | 14.45‐47.14 |
| Q (L/h) | 11 fixeda | – | – | – |
| Vp (L) | 41.23 fixedb | – | – | – |
| Covariates on CL | ||||
| CVVH (categorical) | −0.1 (130.86) | −0.36 to 0.16 | −0.1 (137.23) | −0.33 to 0.21 |
| CrCL (power) | 0.46 fixedc | – | – | – |
| Weight no CVVH (power) | 0.33 fixedc | – | – | – |
| Weight CVVH (power) | 0.75 fixed | – | – | – |
| Covariates on Vc | ||||
| Weight (power) | 0.74 fixedc | – | – | – |
| Albumin (power) | −1.17 (42.84) | −2.15 to −0.19 | −1.17 (81.61) | −3.78 to 0.5 |
| Covariates on Vp | ||||
| Albumin (power) | −3.68 (17) | −4.9 to −2.45 | −3.68 (37.69) | −5.98 to −0.39 |
| Random effects | ||||
| ω2 CL | 0.093 (28.18) | 0.035‐0.15 | 0.093 (28.77) | 0.016‐0.14 |
| ω2 Vc | 0.13 (36.45) | 0‐0.27 | 0.13 (38.1) | 0‐0.23 |
|
η‐shrinkage CL, 8.6%; η‐shrinkage Vc, 45.41%; Pearson's correlation between η‐Vc and η‐CL, 0.05 | ||||
| Residual unexplained variability | ||||
| Proportional error ϵ‐shrinkage, 13.21% | 0.3 (22) | 0.17‐0.43 | 0.3 (22.47) | 0.18‐0.44 |
aFixed from the literature (references 32 and 48‐50).
bFixed from the literature (references 32 and 48‐50).
cFixed from the literature (reference 48).
dBootstrap estimates based on 1000 samples.
Final Covariate Model
Final model parameter estimates are summarized in Table 2. Covariates for CL were parameterized, and fixed estimates for power for the CrCl and weight parameters were based on data taken from Bhagunde et al, a large FDA‐reviewed population PK model for imipenem/relebactam.50 These included CrCl (only used as a covariate for patients who did not undergo CVVH, power fixed at 0.46) and weight (power fixed at 0.33). For patients who underwent CVVH, weight was used as the sole covariate on CL (power fixed at 0.75). A non–statistically significant categorical covariate of CVVH status on CL was included, with rationale explained in the discussion. The final equation for terminal CL in patients without CVVH was
| (10) |
where the final equation for terminal CL in patients with CVVH is
| (11) |
Estimates for intercompartmental clearance and peripheral volume were fixed as a Q of 11 L/h and , respectively, based on previously published studies.32, 50, 51, 52 Albumin was included as a covariate on both Vc and Vp with rationale explained in the discussion. BSV on Vp was not estimated given low precision, as seen with bootstrap %RSE in the internal covariate model. Weight was used as a covariate on Vc, with power fixed to 0.74 based on Bhagunde et al.50 The final equations for Vc and Vp were
| (12) |
and
| (13) |
The condition number was (8 parameters), suggesting the model was not overparameterized.
Internal Validation of Final Model
Goodness‐of‐fit plots showed model predictions to be randomly scattered around the line of unity. There were no significant trend plots of conditional weighted residuals versus time or conditional weighted residuals versus predicted concentrations (Figure 2). Individual fit plots demonstrated both the population‐ and individual‐predicted concentrations fitted reasonably well to the observed data (Figure S1, supplementary material). Histograms of conditional weighted residuals and BSV random effects were consistent with normally distributed data centered at 0 (Figure S2, supplementary material). The normalized prediction distribution error (NPDE) analysis demonstrated NPDEs were distributed approximately normally centered at 0, with no significant trends in NPDEs over time or when plotted against predicted concentrations (Figure S3, supplementary material). Visual predictive checks demonstrated that the observed data and quantiles fell within the simulated 95%CIs (Figure S4, supplementary material). Plots of random effects versus covariates appropriately demonstrate eliminated or diminished trends on inclusion in the final model (Figure S5, supplementary material).
Figure 2.
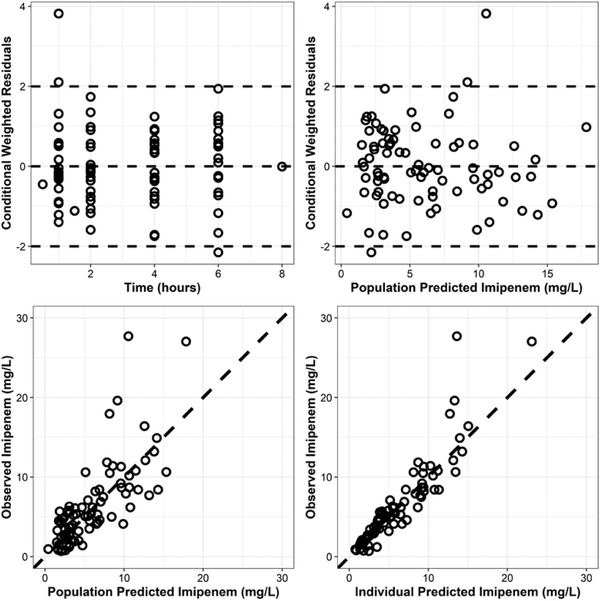
Goodness‐of‐fit plots. Top, conditional weighted residuals (CWRES) versus time and population model‐predicted concentration (mg/L). Bottom, observed imipenem concentration (mg/L) versus population and individual‐predicted concentrations (mg/L).
External Validation of Final Model
External validation demonstrated that mean simulated time‐concentration profiles are consistent with previously published literature31 (Figure 3). The final model could also predict typical values of CL and Vc within a 10% margin of error across burn, healthy, and renally impaired populations. The final model accurately predicted total volume of distribution in burn patients and predicted Vp with 30%‐60% margin of error in healthy and renally impaired populations (Table 3).
Figure 3.
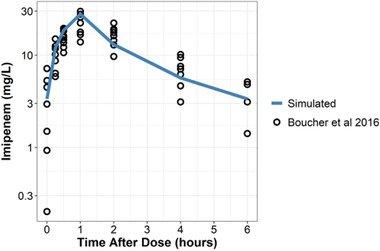
Visual comparison of mean simulations (blue line) with observed data (black circles) from Boucher et al (2016) after a 1‐hour infusion of 1000 mg imipenem every 6 hours at steady state. Mean demographics from Boucher et al used as covariates for the simulation were weight of 90 kg, total burn surface area of 23% (corresponding to albumin 3.2 g/dL), and CVVH CL of 3.27 L/h. Data from Boucher et al was recreated with WebPlotDigitzer 4.4 (https://apps.automeris.io/wpd/).
Table 3.
Comparison of Final Model‐Predicted Mean Pharmacokinetic Parameters With Literature‐Reported Mean Pharmacokinetic Parameters in Healthy, Renally Impaired, and Burn Populations
| Population | Conditions | Final Model‐Predicted Mean Parameters | Mean Parameters Observed in Literature | Citation |
|---|---|---|---|---|
| Healthy |
Albumin, 4 g/dL; weight, 76 kg; CrCl, 106 mL/min |
Vc, 16.89 L; Vp, 9.7 L; Cl, 12.1 L/h |
Vc, 9.37‐15.83 L; Vp, 5.84‐6.41 L; Cl, 11.5‐12.53 L/h |
Bhagunde et al, 2019; Coen van Hasselt et al, 2015 |
| Renal impairment |
Albumin, 4 g/dL; Weight, 58 kg; CrCl, 54.1 mL/min |
Vc, 13.14 L; Vp, 9.7 L; Cl, 8.19 L/h |
Vc, 11.4 L; Vp, 3.56 L Cl, 7.95 L/h |
Yoshizawa K et al, 2012 |
| Burn |
Albumin, 3 g/dL (corresponds to TBSA 32.26%); weight, 70.8 kg; CrCl, 126.3 mL/min |
Vtot, 50.43 L; Vc, 22.45 L; Vp, 27.98 L; Cl, 12.81 L/h |
Vtot, 15.69‐97.7 L; Vc, 28.28 L; Vp, 41.23 L; Cl, 11.1‐17.8 L/h |
Daily et al, 2003 (weight, 75.2 kg; TBSA, 27.6%; CrCl, 143 mL/min) Gomez et al, 2015 (weight, 68 kg; TBSA, 36.3%) Boucher et al, 1990 (weight, 71.3 kg; TBSA, 43.4%; CrCl, 109.6) Boucher et al, 2016 (weight, 90 kg; TBSA, 23%; all patients with CVVH) Machado et al, 2017 (weight, 67.5 kg; TBSA, 31%; CrCl not reported) Li et al, 2019 (burn data borrowed from Boucher et al, 2016) |
Monte Carlo Simulations
To evaluate the effects of renal function and burn severity on imipenem PTA, simulations were conducted with patients characterized by NRF or augmented renal clearance and varying degrees of burn injury (Figure 4). Simulations were performed assuming doses of either 500 mg every 6 hours or 1000 mg every 6 hours. Augmented renal clearance was simulated with a CrCl of 150‐250 mL/min, whereas NRF was simulated with a CrCl of 100‐130 mL/min. Serum albumin was varied and used as a surrogate for %TBSA based on the linear model in Figure 3 and further described in Figure 4. Results demonstrated that to satisfy a target MIC of 2 mg/L, 500 mg every 6 hours was adequate for patients with NRF and augmented renal clearance regardless of %TBSA. Of note, simulated patients with NRF and 70%‐100% burn had almost a 20% probability of imipenem trough > 5 mg/L. In addition, under the same simulated conditions, a dose of 1000 mg every 6 hours was adequate to achieve a target MIC of 4 mg/L (Figure S6, supplementary material); however, a high probability of imipenem trough > 5 mg/L (18%‐65%) was observed at this dose.
Figure 4.
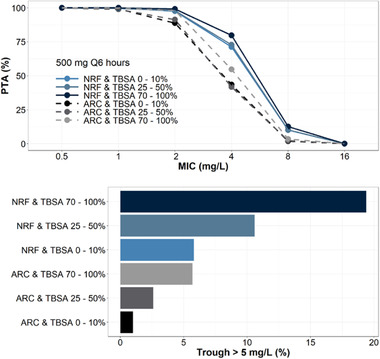
Probability of target attainment (top) and probability of imipenem trough > 5 mg/L (bottom) comparing normal renal function (NRF)—CrCl of 100‐130 mL/min—with augmented renal clearance (ARC)—CrCl of 150‐250 mL/min—in a 70‐kg critically ill patient with varying degrees of total burn surface area (TBSA). Each group is simulated with n = 1000 replicates with setting albumin of 3.45‐4 g/dL corresponding to TBSA of 0%‐10%, setting albumin of 2.6‐3.15 g/dL corresponding to a TBSA of 25%‐50%, and setting albumin of 1.5‐2.2 g/dL corresponding to a TBSA of 70%‐100%.
As a high incidence of augmented renal clearance has been observed among burn patients, who may be placed on CRRT as a part of therapy, PTA analyses were also conducted to evaluate simulated patients with NRF or augmented renal clearance and varying degrees of CL because of CVVH (Figure 5). Simulations were performed assuming doses of either 500 or 1000 mg every 6 hours. Serum albumin of 3.2 g/dL was used as a surrogate to simulate 30% TBSA (Figure 1). Results suggest that 500 mg every 6 hours would be an adequate dose to achieve an imipenem MIC of 2 mg/L (PTA 90%‐98%); however, the combination of augmented renal clearance and moderate‐ to high‐intensity CVVH would lead to possible treatment failure for an organism with an MIC of 2 mg/L (PTA 69%‐78%). Similarly, a dose of 1000 mg every 6 hours would be an adequate dose to satisfy a target MIC of 4 mg/L (PTA 89%‐98%), but again the combination of augmented renal clearance and moderate‐ to high‐intensity CVVH would lead to possible treatment failure (PTA 70%‐79%).
Figure 5.
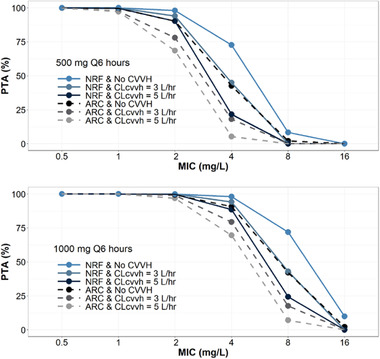
Probability of target attainment with either 500 mg every 6 hours (top) or 1000 mg every 6 hours (bottom) infused over 1 hour at steady state. Compares normal renal function (NRF)—CrCl of 100‐130 mL/min—with augmented renal clearance (ARC)—CrCl of 150‐250 mL/min—in a 70‐kg critically ill patient with a TBSA of 30% and varying intensity of CVVH. Each group is simulated with n = 1000 replicates with a setting albumin of 3 g/dL corresponding to a TBSA of 30%.
Additional simulations were performed assuming 30% TBSA (predicted by a serum albumin of 3.2 g/dL) and varying degrees of renal insufficiency (RI), with or without CVVH (3 or 5 L/h, respectively), to determine whether FDA‐labeled doses would be sufficient to achieve therapeutic imipenem levels (Figure S7). Doses and RI categories simulated were mild RI (CrCl 60‐89 mL/min, 400 mg every 6 hours), moderate RI (CrCl 30‐59 mL/min, 300 mg every 6 hours), or severe RI (CrCl 15‐29 mL/min, 200 mg every 6 hours). Results demonstrated that these FDA‐labeled doses would be adequate to satisfy a target MIC of 2 mg/L (PTA 86%‐99%) regardless of concurrent use of CVVH. Doubling the FDA‐labeled doses for respective RI categories would be appropriate to target an imipenem MIC of 4 mg/L (PTA 87%‐99%).
Discussion
In this study, we evaluated the PK of imipenem in 23 burn patients treated with or without CVVH and developed a population PK model using these data in combination with strengths of previously published models. To our knowledge, we provide the first direct comparison of imipenem CL in burn patients with and without CVVH. Notably, there was no significant difference (Table 2). Here we provide a population PK model in burn patients that allows for the extrapolation of effects of both CVVH and augmented renal clearance on imipenem CL. Importantly, to achieve a target MIC, neither CVVH nor augmented renal clearance alone had an impact on dosing requirements; however, the combination of augmented renal clearance and use of moderate‐ to high‐intensity (>35 cc·h/kg)53, 54 CVVH may result in treatment failure at standard doses of imipenem (Figure 5). In addition, we demonstrate that serum albumin was a reliable predictor of imipenem central and peripheral Vd, where decreased albumin was associated with higher Vd. This finding has some physiologic support where decreased albumin may be a reasonable surrogate marker for increased capillary permeability in patients with burn injury.
To date, numerous studies have evaluated imipenem PK in a variety of populations to include healthy,50, 52 renally impaired,55 and burn31, 32, 33, 34, 56, 57 patients. Taken together, these analyses have provided crucial understanding of physiological alterations and their impact on key imipenem PK parameters, namely, clearance and volume. Using our data set in combination with strengths of prior studies, our model consolidated these findings and generated parameter estimates and time‐concentration curves highly consistent with the published literature. There is strong evidence that renal CL is a significant covariate on imipenem CL. However, similar to our data set, previously published imipenem PK studies have relatively low patient numbers with variable renal clearance estimates, which are often confounded by the use of CVVH. Thus, we chose the approach to borrow a renal and weight covariate imipenem CL model from Bhagunde et al,50 a published FDA‐reviewed population PK analysis for imipenem/relebactam using data collected from phase 1‐3 clinical trials. The analysis included 815 patients with imipenem concentration observations and renal CL ranging from <15 to ≥250 mL/min. Although imipenem use was not specifically reported in burn patients, it is reasonable to assume that the effect of renal CL on imipenem is independent of disease state. Therefore, given the rigorous approach, large data set, and independent FDA review, data borrowed from Bhagunde et al provided an ideal renal clearance model to explore the effects of renal failure or augmented renal clearance in burn patients.
A limitation to utilizing the renal clearance model from Bhagunde et al is reliance on estimating CrCl with the Cockcroft‐Gault equation, which presents a unique challenge in the renal dosing of antibiotics in critically ill patients who may have unstable kidney function after AKI. Although there is evidence that the Jelliffe equation58 more accurately estimates CrCl in critically ill patients with unstable kidney function than the Cockcroft‐Gault equation, it has not been validated in the critically ill burn population and relies on assumptions of creatinine Vd derived from animal models or other noncomparable patient populations.59 In addition, methods for CrCl estimation are not interchangeable within population PK models, and the Cockcroft‐Gault and other steady equations have been shown to outperform the Jelliffe equation in terms of predictive performance for a variety of antibiotics.60, 61, 62 Further, Cockcroft‐Gault generally overestimates CrCl compared with the Jelliffe equation; however, the clinical consequence for unstable AKI antibiotic dosing would be supratherapeutic concentrations compared with the potential for subtherapeutic concentrations with Jelliffe estimation. Therefore, if the 2 methods provide discordant dosing recommendations, the clinical situation and risk benefit analysis should drive the choice. In the case of critically ill burn patients, as imipenem has a large therapeutic window and the consequence of underdosing may result in death, using the Cockcroft‐Gault estimate to select higher doses is sound clinical practice. For these reasons, in addition to the strength of the renal covariate model from Bhagunde et al and the current use of Cockcroft‐Gault for CrCl estimation in clinical practice, we opted for this method over the Jelliffe equation.
With regard to CVVH, we chose to model data from all patients together regardless of CVVH status. This approach allowed us to leverage the entire data set for better precision of estimates and more rigorously explore the difference in inherent total body CL of imipenem between these groups. On average, the CVVH group had a 10% decrease in inherent body CL compared with the no‐CVVH group. Even though this finding was not statistically significant, there likely is a true difference in inherent imipenem body CL between patients with and without CVVH. A significant portion of imipenem is renally cleared, and patients initiated on CVVH are more likely to have impaired renal function than those not on CVVH.27 This is consistent with our finding that patients in the CVVH group had 10% decreased inherent body CL compared with the no‐CVVH group. As a consequence of this clinical and physiologic context, CVVH was retained as a categorical covariate despite not achieving statistical significance. In addition, CVVH was retained to highlight this surprising finding and allow for better comparison of the typical value of imipenem CL in CVVH patients with the prior literature, in which data sets lacked both CVVH and no‐CVVH patients. The lack of statistical significance in inherent body imipenem CL in our data set is likely attributed to sample size, in conjunction with the significant mortality benefit and low threshold to implement CVVH in critically ill burn patients. It is possible that burn patients placed on CVVH have preserved renal function compared with other populations (ie, renally impaired) in which CVVH is commonly utilized. This is highlighted by an observational study in which as many as one‐third of severe burn patients had no AKI or stage 1 AKI at the time of RRT initiation.27 Furthermore, although there is a high incidence of augmented renal clearance in burn patients,16 serum creatinine is not a reliable predictor of augmented renal clearance. Thus, CVVH may be implemented in burn patients with augmented renal clearance based on traditional laboratory criteria for AKI.
In our final covariate model, we included albumin as a continuous covariate for both central and peripheral Vd (Table 2). Although there was a low level of statistical evidence to include albumin as a covariate on Vd, there was physiologic relevance and support from prior literature. Although direct comparisons are lacking, imipenem Vd is generally 2‐ to 5‐fold higher in severe burn patients compared with other patient populations.31, 32, 34, 50, 52, 55, 56, 57 Specifically, Gomez et al demonstrated that imipenem Vd nearly doubled in patients with >40% total burn surface area (TBSA), 0.97 L/kg, compared with those with <19% TBSA (0.56 L/kg).34 Therefore, we also sought to explore the effects of large changes in Vd related to severe burn injury on imipenem dosing. Our analyses revealed that central and peripheral Vd were highly negatively correlated with albumin and TBSA (Table 2). Furthermore, a strong negative correlation between albumin and TBSA was identified (Figure 1). The decision to select albumin over TBSA as a covariate in our model was 2‐fold. Albumin displayed internal statistical evidence (statistically significant covariate on Vp in the internal model, P = .044, α = 0.05), whereas TBSA was not a statistically significant covariate. In addition, albumin likely more closely represents the physiologic reason for increased Vd in burn patients, in whom lower levels of albumin suggest increased capillary permeability.63, 64 This decision was validated whereby using serum albumin as a covariate on Vc and Vp allowed for accurate extrapolation of typical values of Vc and Vp in healthy subjects (Table 3).
Regarding the physiologic relevance, hypoalbuminemia is a common clinical deficiency in burn patients and has been proposed as a marker of burn severity and indicator of mortality.65 Increased capillary permeability is a well‐established mechanism for hypoalbuminemia in the first 48 hours of severe burn injury. However, as imipenem is not highly protein bound, there are competing mechanisms of hypoalbuminemia in burn patients that would not explain increased imipenem Vd. These mechanisms include decreased liver production of albumin and loss of albumin through open wounds.66 Nevertheless, we demonstrated reasonable evidence that albumin is a significant covariate on imipenem Vc and Vp. Furthermore, including albumin as a covariate on Vc and Vp allowed for simulated trials of the effect of large changes inf Vd on dosing requirements. These simulations are highly valuable, as traditional clinical trials exploring Vd on dosing may not be feasible given the challenge to prospectively enroll enough critically ill burn patients to precisely characterize Vc and Vp.
Importantly, simulations performed in this study validated that large variations in Vc and Vp have minimal effect on PTA (Figure 4), with no recommended dosing adjustments required based on Vd. Interestingly, simulated patients with the highest Vd (70%‐100% TBSA) achieved the highest PTAs regardless of NRF or augmented renal clearance. This observation is likely explained by increases in half‐life of imipenem because of disproportionate increases in Vd compared with CL. Although there are no required imipenem dosing adjustments based on Vd, it is important to note that patients with the largest Vd have the highest probability of imipenem trough > 5 mg/L, which may be associated with a higher risk of adverse events.48 Therefore, in the most severe, critically ill burn patients there should be a high clinical suspicion for adverse events such as seizure and a low threshold to implement electroencephalogram testing.
As observed in prior literature, imipenem CL was the main determinant for dose adjustment.50 Importantly, neither the use of moderate‐ to high‐intensity CVVH or augmented renal clearance alone necessitated dose adjustment for specific MIC targets, but the combination of CVVH and augmented renal clearance together resulted in significant failure rates of achieving 40% (Figure 5). Given that diagnosis of augmented renal clearance requires a 24‐hour CrCl, along with a low threshold to implement CVVH and high incidence of augmented renal clearance, it may be a relatively frequent occurrence for critically ill burn patients to have augmented renal clearance and be treated with CVVH. Thus, to avoid burdensome testing and achieve a target MIC of 2 mg/L, it would be reasonable to use an empiric dosing regimen of 750 or 1000 mg every 6 hours intravenously infused over an hour in patients with preserved renal function at the time of CVVH initiation. Alternatively, drawing trough levels or obtaining a 24‐hour CrCl in the hypermetabolic phase of injury may allow for proper real‐time dose adjustments. Moreover, in patients with augmented renal clearance and CVVH, a dose of 1000 mg every 6 hours intravenously infused over 1 hour may not be adequate to achieve a PTA with a target MIC of 4 mg/L. Because the dosing regimen 1000 mg every 6 hours is the upper limit per the FDA label, if augmented renal clearance is suspected in a patient treated with CVVH and the goal is to empirically treat an infection with imipenem at a MIC of 4 mg/L, it may be advisable to use alternative antibiotics or trial off‐label extended infusion dosing protocols.67
In conclusion, we developed a comprehensive population PK model for burn patients using strengths from our own data and previously published literature. We explored the effects of CVVH, augmented renal clearance, and serum albumin/TBSA on PK parameters and subsequent requirements for dose adjustments. We found that serum albumin is likely a covariate for Vc and Vp, but large increases in volume parameters would not require a dose adjustment. Imipenem dosing of 500 mg every 6 hours was adequate for simulated burn patients on renal‐dosed CVVH; however, suspicion of augmented renal clearance and/or combination with CVVH may lead to high failure rates of imipenem therapy in burn victims, likely requiring dosing adjustments to 1000 mg every 6 hours.
Funding
Funding provided by a Uniformed Services University of the Health Sciences (USUHS)/Walter Reed Army Institute of Research (WRAIR) Clinical Pharmacology (P8) Fellowship.
Disclaimer
Material has been reviewed by the Walter Reed Army Institute of Research, the Uniformed Services University of the Health Sciences, and the United States Institute of Surgical Research. There is no objection to its presentation and/or publication. The views expressed in this article are those of the authors and do not reflect the official policy or position of the U.S. Army Medical Department, Department of the Army, Department of Defense, or the U.S. government. This study was conducted under a protocol reviewed and approved by the U.S. Army Medical Research and Development Command Institutional Review Board and in accordance with the approved protocol. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70‐25.
Supporting information
Supplementary information
Acknowledgments
We thank Ms. Zanete Wright for her support of the WRAIR/USUHS Clinical Pharmacology Fellowship. We also thank Dr. Vijay Gorantla and Dr. Joga Gobburu for their expertise and feedback on this study. This research would not have been possible without their assistance.
Data Availability Statement
Data presented in this article cannot be shared. For any other questions, please contact the corresponding author.
References
- 1.Farina JA Jr., Rosique MJ, Rosique RG. Curbing inflammation in burn patients. Int J Inflam. 2013;2013:715645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38(1):e469‐e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312‐319. [DOI] [PubMed] [Google Scholar]
- 4.Williams FN, Herndon DN, Jeschke MG. The hypermetabolic response to burn injury and interventions to modify this response. Clin Plast Surg. 2009;36(4):583‐+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganrot K, Jacobsson S, Rothman U. Transcapillary passage of plasma proteins in experimental burns. Acta Physiol Scand. 1974;91(4):497‐501. [DOI] [PubMed] [Google Scholar]
- 6.Raffa J, Trunkey DD. Myocardial depression in acute thermal injury. J Trauma. 1978;18(2):90‐93. [DOI] [PubMed] [Google Scholar]
- 7.Demling RH. The burn edema process: current concepts. J Burn Care Rehabil. 2005;26(3):207‐227. [PubMed] [Google Scholar]
- 8.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895‐1902. [DOI] [PubMed] [Google Scholar]
- 9.Jeschke MG, Pinto R, Kraft R, et al. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43(4):808‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele AN, Grimsrud KN, Sen S, Palmieri TL, Greenhalgh DG, Tran NK. Gap analysis of pharmacokinetics and pharmacodynamics in burn patients: a review. J Burn Care Res. 2015;36(3):e194‐211. [DOI] [PubMed] [Google Scholar]
- 11.Garrelts JC, Jost G, Kowalsky SF, Krol GJ, Lettieri JT. Ciprofloxacin pharmacokinetics in burn patients. Antimicrob Agents Chemother. 1996;40(5):1153‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenhalgh DG, Saffle JR, JHt Holmes, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28(6):776‐790. [DOI] [PubMed] [Google Scholar]
- 13.Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3‐11. [DOI] [PubMed] [Google Scholar]
- 14.Jaehde U, Sorgel F. Clinical pharmacokinetics in patients with burns. Clin Pharmacokinet. 1995;29(1):15‐28. [DOI] [PubMed] [Google Scholar]
- 15.Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37(3):840‐851; quiz 859. [DOI] [PubMed] [Google Scholar]
- 16.Udy AA, Roberts JA, Lipman J, Blot S. The effects of major burn related pathophysiological changes on the pharmacokinetics and pharmacodynamics of drug use: An appraisal utilizing antibiotics. Adv Drug Deliv Rev. 2018;123:65‐74. [DOI] [PubMed] [Google Scholar]
- 17.Conil JM, Georges B, Fourcade O, et al. Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol. 2007;63(5):583‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loirat P, Rohan J, Baillet A, Beaufils F, David R, Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299(17):915‐919. [DOI] [PubMed] [Google Scholar]
- 19.Mulder MB, Eidelson SA, Sussman MS, et al. Risk factors and clinical outcomes associated with augmented renal clearance in trauma patients. J Surg Res. 2019;244:477‐483. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson AJ Jr. Augmented renal clearance. Transl Clin Pharmacol. 2018;26(3):111‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta RL. Indications for dialysis in the ICU: renal replacement vs. renal support. Blood Purif. 2001;19(2):227‐232. [DOI] [PubMed] [Google Scholar]
- 22.Mehta R, Bouchard J, Macedo E. Renal replacement therapy in acute kidney injury: when, how and how much? Introduction. Semin Dial. 2011;24(2):123. [DOI] [PubMed] [Google Scholar]
- 23.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta‐analysis. Crit Care. 2011;15(1):R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KK, Lundy JB, Matson JR, et al. Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: a cohort study. Crit Care. 2009;13(3):R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carl DE, Grossman C, Behnke M, Sessler CN, Gehr TW. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial Int. 2010;14(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 26.Rhee H, Jang KS, Park JM, et al. Short‐ and long‐term mortality rates of elderly acute kidney injury patients who underwent continuous renal replacement therapy. PLoS One. 2016;11(11):e0167067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung KK, Coates EC, Hickerson WL, et al. Renal replacement therapy in severe burns: a multicenter observational study. J Burn Care Res. 2018;39(6):1017‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill DM, Rizzo JA, Aden JK, Hickerson WL, Chung KK, Investigators R. Continuous venovenous hemofiltration is associated with improved survival in burn patients with shock: a subset analysis of a multicenter observational study. Blood Purif. 2020:1‐8. doi: 10.1159/000512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pea F, Viale P, Pavan F, Furlanut M. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin Pharmacokinet. 2007;46(12):997‐1038. [DOI] [PubMed] [Google Scholar]
- 30.Mueller BA, Pasko DA, Sowinski KM. Higher renal replacement therapy dose delivery influences on drug therapy. Artif Organs. 2003;27(9):808‐814. [DOI] [PubMed] [Google Scholar]
- 31.Boucher BA, Hudson JQ, Hill DM, et al. Pharmacokinetics of imipenem/cilastatin burn intensive care unit patients undergoing high‐dose continuous venovenous hemofiltration. Pharmacotherapy. 2016;36(12):1229‐1237. [DOI] [PubMed] [Google Scholar]
- 32.Dailly E, Kergueris MF, Pannier M, Jolliet P, Bourin M. Population pharmacokinetics of imipenem in burn patients. Fundam Clin Pharmacol. 2003;17(6):645‐650. [DOI] [PubMed] [Google Scholar]
- 33.Li S, Xie F. Population pharmacokinetics and simulations of imipenem in critically ill patients undergoing continuous renal replacement therapy. Int J Antimicrob Agents. 2019;53(1):98‐105. [DOI] [PubMed] [Google Scholar]
- 34.Gomez DS, Sanches‐Giraud C, Silva CV Jr., et al. Imipenem in burn patients: pharmacokinetic profile and PK/PD target attainment. J Antibiot (Tokyo). 2015;68(3):143‐147. [DOI] [PubMed] [Google Scholar]
- 35.Fish DN, Teitelbaum I, Abraham E. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother. 2005;49(6):2421‐2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKindley DS, Boucher BA, Hess MM, Croce MA, Fabian TC. Pharmacokinetics of aztreonam and imipenem in critically ill patients with pneumonia. Pharmacotherapy. 1996;16(5):924‐931. [PubMed] [Google Scholar]
- 37.Tegeder I, Schmidtko A, Brautigam L, Kirschbaum A, Geisslinger G, Lotsch J. Tissue distribution of imipenem in critically ill patients. Clin Pharmacol Ther. 2002;71(5):325‐333. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Bai J, Wen A, Shen S, Duan M, Li X. Pharmacokinetic and pharmacodynamic analysis of critically ill patients undergoing continuous renal replacement therapy with imipenem. Clin Ther. 2020;42(8):1564‐1577 e1568. [DOI] [PubMed] [Google Scholar]
- 39.Rackacukas C, Nyberg, J, Ivaturi, V. PuMaS: a pharmaceutical modeling and simulation engine for drug development and clinical therapeutics in Julia. Abstracts for the Ninth American Conference on Pharmacometrics. J Pharmacokinet Pharmacodyn. 2018;45:3‐134.28884259 [Google Scholar]
- 40.Bezanson J, Edelman, A., Karpinski, S., Shah, V. Julia: a fresh approach to numerical computing. SIAM Rev. 2017;59(1):65‐98. [Google Scholar]
- 41.Choi G, Gomersall CD, Tian Q, Joynt GM, Li AM, Lipman J. Principles of antibacterial dosing in continuous renal replacement therapy. Blood Purif. 2010;30(3):195‐212. [DOI] [PubMed] [Google Scholar]
- 42.Hulko M, Haug U, Gauss J, Boschetti‐de‐Fierro A, Beck W, Krause B. Requirements and pitfalls of dialyzer sieving coefficients comparisons. Artif Organs. 2018;42(12):1164‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.FDA . FDA guidance for industry: pharmacokinetics in patients with impaired renal functionstudy design, data analysis, and impact on dosing and labeling; 2010. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/Pharmacokinetics-in-Patients-with-Impaired-Renal-Function-_-Study-Design-Data-Analysis-and-Impact-on-Dosing-and-Labeling.pdf. Accessed December 18, 2020.
- 44.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051‐1065. [DOI] [PubMed] [Google Scholar]
- 45.Brendel K, Comets E, Laffont C, Mentre F. Evaluation of different tests based on observations for external model evaluation of population analyses. J Pharmacokinet Pharmacodyn. 2010;37(1):49‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacGregor RR, Gibson GA, Bland JA. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high‐dose treatment for serious infections. Antimicrob Agents Chemother. 1986;29(2):188‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen A, Li Z, Yu J, et al. Clinical validation of therapeutic drug monitoring of imipenem in spent effluent in critically ill patients receiving continuous renal replacement therapy: a pilot study. PLoS One. 2016;11(4):e0153927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bricheux A, Lenggenhager L, Hughes S, Karmime A, Lescuyer P, Huttner A. Therapeutic drug monitoring of imipenem and the incidence of toxicity and failure in hospitalized patients: a retrospective cohort study. Clin Microbiol Infect. 2019;25(3):383 e381‐383 e384. [DOI] [PubMed] [Google Scholar]
- 49.Rodloff AC, Goldstein EJ, Torres A. Two decades of imipenem therapy. J Antimicrob Chemother. 2006;58(5):916‐929. [DOI] [PubMed] [Google Scholar]
- 50.Bhagunde P, Patel P, Lala M, et al. Population pharmacokinetic analysis for imipenem‐relebactam in healthy volunteers and patients with bacterial infections. CPT Pharmacometrics Syst Pharmacol. 2019;8(10):748‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couffignal C, Pajot O, Laouenan C, et al. Population pharmacokinetics of imipenem in critically ill patients with suspected ventilator‐associated pneumonia and evaluation of dosage regimens. Br J Clin Pharmacol. 2014;78(5):1022‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Hasselt JG, Rizk ML, Lala M, et al. Pooled population pharmacokinetic model of imipenem in plasma and the lung epithelial lining fluid. Br J Clin Pharmacol. 2016;81(6):1113‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fayad AI, Buamscha DG, Ciapponi A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst Rev. 2016;10(10):CD010613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Investigators RRTS, Bellomo R, Cass A, et al. Intensity of continuous renal‐replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627‐1638. [DOI] [PubMed] [Google Scholar]
- 55.Yoshizawa K, Ikawa K, Ikeda K, Kumon H, Ohge H, Morikawa N. Optimisation of imipenem regimens in patients with impaired renal function by pharmacokinetic‐pharmacodynamic target attainment analysis of plasma and urinary concentration data. Int J Antimicrob Agents. 2012;40(5):427‐433. [DOI] [PubMed] [Google Scholar]
- 56.Boucher BA, Hickerson WL, Kuhl DA, Bombassaro AM, Jaresko GS. Imipenem pharmacokinetics in patients with burns. Clin Pharmacol Ther. 1990;48(2):130‐137. [DOI] [PubMed] [Google Scholar]
- 57.Machado AS, Oliveira MS, Sanches C, et al. Clinical outcome and antimicrobial therapeutic drug monitoring for the treatment of infections in acute burn patients. Clin Ther. 2017;39(8):1649‐1657 e1643. [DOI] [PubMed] [Google Scholar]
- 58.Jelliffe RW. Letter: creatinine clearance: bedside estimate. Ann Intern Med. 1973;79(4):604‐605. [DOI] [PubMed] [Google Scholar]
- 59.Bouchard J, Macedo E, Soroko S, et al. Comparison of methods for estimating glomerular filtration rate in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2010;25(1):102‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charhon N, Neely MN, Bourguignon L, Maire P, Jelliffe RW, Goutelle S. Comparison of four renal function estimation equations for pharmacokinetic modeling of gentamicin in geriatric patients. Antimicrob Agents Chemother. 2012;56(4):1862‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Velde F, de Winter BCM, Neely MN, et al. Population pharmacokinetics of imipenem in critically ill patients: a parametric and nonparametric model converge on CKD‐EPI estimated glomerular filtration rate as an impactful covariate. Clin Pharmacokinet. 2020;59(7):885‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minichmayr IK, Roberts JA, Frey OR, Roehr AC, Kloft C, Brinkmann A. Development of a dosing nomogram for continuous‐infusion meropenem in critically ill patients based on a validated population pharmacokinetic model. J Antimicrob Chemother. 2018;73(5):1330‐1339. [DOI] [PubMed] [Google Scholar]
- 63.Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1(8432):781‐784. [DOI] [PubMed] [Google Scholar]
- 64.Soeters PB, Wolfe RR, Shenkin A.Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aguayo‐Becerra OA, Torres‐Garibay C, Macias‐Amezcua MD, et al. Serum albumin level as a risk factor for mortality in burn patients. Clinics (Sao Paulo). 2013;68(7):940‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melinyshyn A, Callum J, Jeschke MC, Cartotto R. Albumin supplementation for hypoalbuminemia following burns: unnecessary and costly! J Burn Care Res. 2013;34(1):8‐17. [DOI] [PubMed] [Google Scholar]
- 67.Ibrahim MM, Tammam TF, Ebaed MED, Sarhan HA, Gad GF, Hussein AK. Extended infusion versus intermittent infusion of imipenem in the treatment of ventilator‐associated pneumonia. Drug Des Devel Ther. 2017;11:2677‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Data Availability Statement
Data presented in this article cannot be shared. For any other questions, please contact the corresponding author.


