Abstract
Rationale:
Drug use during adolescence results in a life-long risk to develop substance-use disorders. Adolescent rats are less reactive to cocaine-associated cues compared to adults; however, the contribution of adolescent-formed context-drug-associations to elicit relapse-like behavior is underexplored. Although it is known that social isolation can impact drug-seeking behavior, the effects of housing conditions on context-induced cocaine-seeking during adolescence vs adulthood is unknown.
Objectives:
The present study compared the effect of adolescent vs adult-formed context-drug associations under differential housing conditions (pair vs single) on cocaine-seeking behavior during adolescence or adulthood. This objective was accomplished using operant cocaine self-administration (Coc-SA) under a standard, non-abbreviated (Non-ABRV) or modified, abbreviated (ABRV) paradigm.
Methods:
In experiment 1, adolescent and adult rats received Non-ABRV Coc-SA in a distinct context (2 hr, 1x/day, 10 days), extinction training (EXT) in a second context (1 hr, 1x/day, 8 days) with reinstatement test (TEST) during adulthood in the cocaine-paired context. In experiments 2–3, rats received all behavioral phases during adolescence or adulthood: ABRV Coc-SA (2 hr, 2x/day, 5 days), EXT (1 hr, 4x/day, 2 days) with TEST in a cocaine-paired or novel, unpaired context. All experiments included pair and single-housing conditions.
Results & Conclusions:
Age at cocaine exposure did not influence behavior in Non-ABRV or ABRV paradigms. Under Non-ABRV conditions, adolescent and adult single-housed rats had higher seeking behavior than pair housed. These data suggest that social isolation influences context-induced cocaine-seeking regardless of age at drug exposure and provides a condensed, ABRV paradigm to investigate context-induced, cocaine-seeking behavior during adolescence.
Keywords: Adolescence, Context, Self-Administration, Cocaine Seeking, Reinstatement, Isolation Housing, Pair Housing, Extinction
INTRODUCTION
Adolescence is a developmental time period characterized by increased sensation seeking, impulsivity and initiation of drug use (Spear and Silveri 2016; Steinberg 2016; Walker et al. 2017; Dahl et al. 2018). Adolescent initiation of drug use may lead to vulnerability associated with faster progression to drug dependence and a life-long risk of relapse (Anthony and Petronis 1995; Clark et al. 1998; Doremus-Fitzwater and Spear 2016; Johnston et al. 2018). Cocaine-use disorders are characterized by cycles of relapse to drug-seeking and taking behavior despite extended periods of abstinence (American Psychiatric Association 2013; Saunders 2017). The successful treatment of repeated relapse is challenging given that drug-associated stimuli, such as explicit cues or environmental contexts, can precipitate craving and subsequent relapse behaviors (Ehrman et al. 1992; Foltin and Haney 2000). In line with the idea that early vulnerability to drug use can elicit negative outcomes later in life, there is evidence that adolescents display greater resistance to behavioral interventions targeted to prevent relapse compared to adults (Ramo and Brown 2008; Ramo et al. 2012; Winters et al. 2014; Zbukvic et al. 2016). Therefore, it is essential to understand how adolescents and adults may be differentially sensitive to specific types of drug-associated stimuli during relapse.
Rodent models of operant cocaine self-administration (Coc-SA) allow for a methodical approach to study voluntary drug taking and subsequent relapse-like behavior. Exposure to a number of drug-associated stimuli (explicit cue, environmental context or drug itself) can elicit relapse-like behavior which is measured as an increase in responses on a previous drug-associated lever (de Wit and Stewart 1981; Shaham et al. 2003; Fuchs et al. 2008; Venniro et al. 2016). While operant models are used extensively to study relapse in adults, less is known about this process during adolescence. Previous literature has demonstrated that adolescent rodents self-administer more cocaine (Li and Frantz 2009; Zbukvic et al. 2016; Madsen et al. 2017) and display increased cocaine-primed and stress-induced reinstatement during adulthood, when compared to their adult-exposed counterparts (Anker and Carroll 2010; Wong et al. 2013; Wong and Marinelli 2016). Rats with adolescent-onset of cocaine use show either similar or reduced cue-induced cocaine-seeking as adults, when compared to adult-onset controls (Anker and Carroll 2010; Zbukvic et al. 2016; Wong and Marinelli 2016). In regards to contextual drug associations, rats trained in cocaine conditioned place preference (CPP) during adolescence show increased preference for a cocaine-paired (Coc-Pair) context when tested as adults (Badanich et al. 2006; Brenhouse and Andersen 2008). Furthermore, mice with a history of oral sucrose-cocaine display increased habit-like, context-induced reinstatement, compared to adults (DePoy et al. 2016). Taken together, these data suggest that adolescent responsiveness to drug-related stimuli critically depends on the modality or type of cue. While there is ample literature examining the role of drug-context-associations in precipitating relapse-like behavior in adult rats, it is unclear how relapse is altered when operant contextual self-administration occurs during adolescence (Crombag and Shaham 2002; Fuchs et al. 2005; Lasseter et al. 2011; Arguello et al. 2017). Therefore, the first objective of the present study was to examine and compare the influence of adolescent vs adult-formed, context-drug-associations on cocaine-seeking behavior during adulthood by utilizing a standard, contextual Coc-SA model of relapse (Non-Abbreviated; Non-ABRV) (Fuchs et al. 2008; Perry et al. 2014; Venniro et al. 2016; Khoo et al. 2017).
Despite adolescent-specific vulnerabilities to drug use, it is a challenge to examine both drug-taking and seeking behaviors during the narrow time window of adolescence (PND 35–63), due to the extended time needed to conduct Coc-SA procedures (Spear 2000; McCutcheon and Marinelli 2009; Dahl et al. 2018). To address this gap in knowledge, we modified and abbreviated (ABRV) the standard, contextual reinstatement model to conduct all behavioral phases- Coc-SA, extinction training (EXT) and reinstatement test (TEST) during adolescence. The use of an ABRV paradigm allowed us to address the second objective of the present study, which was to evaluate the influence of context-drug-associations formed during adolescence on reinstatement of drug-seeking during adolescence, compared to all phases occurring during adulthood. In addition, to test whether potential increases in cocaine seeking were specific to the Coc-Pair context, we included a group in which TEST occurred in a novel, unpaired context (Nov-Unpair).
Lastly, it is known that adolescent initiation of drug use is highly influenced by peer and social factors (Neisewander et al. 2012; Trezza et al. 2014; Walker et al. 2019). There is evidence that socially-isolated adolescent rats self-administer more cocaine at low, but not high, doses compared to pair-housed controls (Boyle et al. 1991; Ding et al. 2005; Gipson et al. 2011; Whitaker et al. 2013). In addition, adolescent rodents that are isolated or exposed to social stress display increased escalation of Coc-SA as well as increased cue-induced reinstatement (Burke and Miczek 2015; Fosnocht et al. 2019). Given that rearing conditions during adolescence can impact subsequent drug-taking behavior, a third objective of the study was to determine whether different housing conditions (pair vs single) during adolescence vs adulthood would affect acquisition of Coc-SA, EXT or TEST behaviors in both the Non-ABRV and ABRV paradigms.
MATERIALS & METHODS
Animals:
Male Sprague Dawley rats (Envigo Inc, Haslett, MI) arrived at post-natal day 25, P25 (60–80 gm, n=51), or P56 (260–280 gm, n=32) and were pair housed upon arrival with ad libitum access to food and water. Humidity and temperature were regulated within the vivarium and rats were maintained on reverse lighting conditions (7 am off, 7 pm on) with all behavioral conditions occurring during the rats’ active cycle. Rats underwent habituation and handling for 7–8 days prior to surgery. Following surgical procedures, rodents were separated into two housing groups (pair vs single) and maintained on 15 gm (adolescent) or 18 gm (adult) of chow, which were adjusted to allow for normal growth (Carroll ME 1985; DePoy et al. 2016; Cho et al. 2018). Female rats were not included in the current study to focus on Age x Context × Housing factors. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan State University (MSU) and followed the National Research Council’s Guide for the Care and Use of Laboratory Rats.
Surgery:
Rats were fully anesthetized using a mixture of ketamine + xylazine (80–100 mg/kg + 5–10 mg/kg, respectively; Henry Schein) via intraperitoneal (IP) injection. Intravenous (IV) catheters were constructed in house and consisted of: 22 gauge, 11 mm length guide cannula with a screw type connector (Plastics One, Roanoke, VA), 210/125 μm polypropylene mesh (Sefar Propyltex, Buffalo, NY), 0.64 mm I.D, 1.19 mm O.D. silastic tubing (Dow Corning Corporation, Midland, MI), dental cement and liquid acrylic (Lang Dental Manufacturing; Wheeling, IL) and clear silicone sealant (Henkel Corp., Rocky Hill, CT). Catheter length for adolescents was adjusted based on previous reports, with silastic tubing length of 9.7 cm (adult=10.0 cm), with a silicone ball located 0.3 cm from the end that would be inserted into the vein (Wong et al. 2013; Zbukvic et al. 2016). Catheters implanted into the right jugular vein ran subcutaneously and exited at the back near the shoulder blade (Fuchs et al. 2005; Bal et al. 2019). Rats were administered pre and post-surgical oral meloxicam analgesia (Ceva Animal Health, Lenexa, KS). During post-surgery recovery (5 days), catheters were flushed with 0.1 mL cefazolin (100 mg/mL; WG Critical Care, Paramus, NJ) dissolved in heparinized saline (Hep-Sal; 70 U/mL; Fresenius Kabi, Lake Zurich, IL), followed by 0.1 mL of 10 U/mL Hep-Sal to maintain catheter patency. Catheters were flushed with 0.1 mL of 70 U/mL Hep-Sal prior to each Coc-SA session. After the final Coc-SA session of the day, catheters were flushed with 0.1 mL cefazolin and 0.1 mL 10 U/mL Hep-Sal, respectively. If the patency of catheters was uncertain, rats were given 0.1 mL Propofol IV (10 mg/mL; Hospira Inc, Lake Forest, IL) after the final Coc-SA session and observed for brief loss of movement.
General Coc-SA Training:
Rats were randomly assigned to begin Coc-SA in operant boxes (29.5 × 24 × 28 cm; Med Associates Inc., St. Albans, NY) configured to one of two distinct contexts. Context A consisted of a continuous red house light (0.4 fc brightness), intermittent pure tone (80 dB, 1 kHz; 2 sec on, 2 sec off), pine-scented air freshener (Car Freshener Corp., Watertown, NY), and wire mesh flooring (26 cm × 27 cm). Context B consisted of an intermittent white stimulus light over the inactive lever (1.2 fc brightness; 2 sec on, 2 sec off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener (Car Freshener Corp., Watertown, NY), and a slanted black acrylic panel bisecting the bar flooring (19 cm × 27 cm). Rats were counterbalanced to start Coc-SA in either Context A or B.
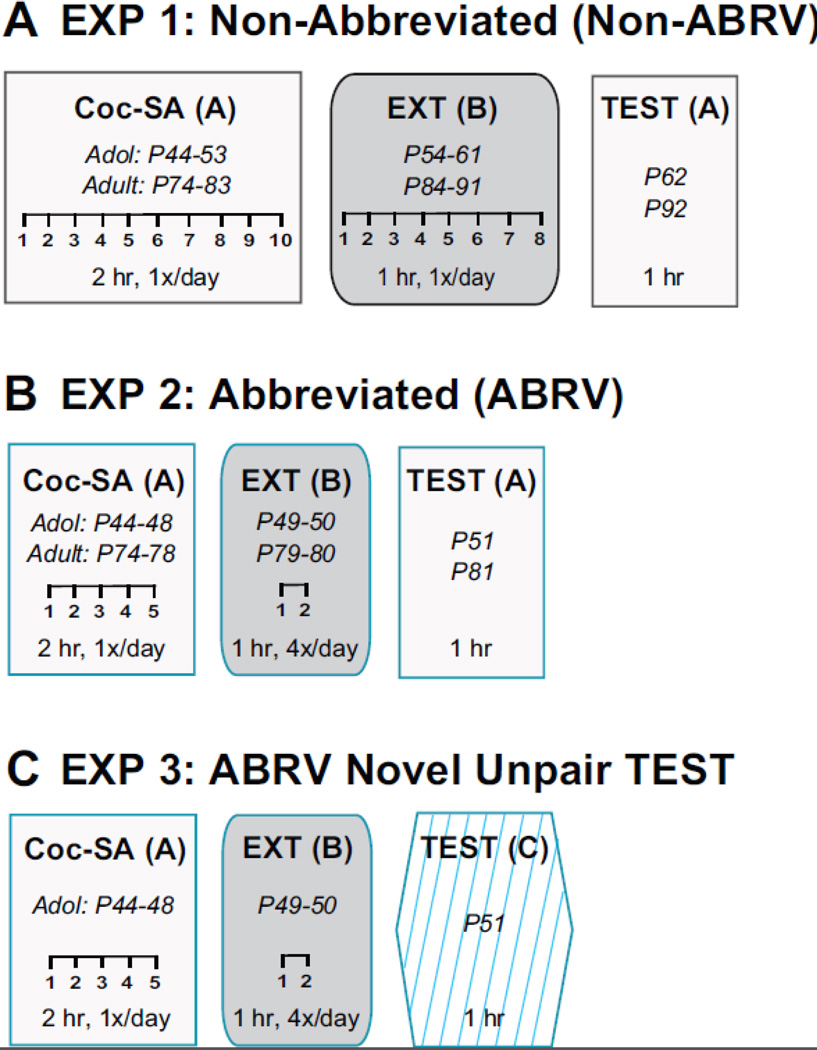
Cocaine hydrochloride (Cocaine-HCl; NIDA Drug Supply System, Research Triangle Park, NC) was dissolved in sterile saline (Hospira Inc, Lake Forest, IL). Responses on the active lever resulted in an IV infusion of 0.05 ml of 1.5 mg/mL Cocaine-HCl (0.5 mg/kg per each infusion) on a Fixed-Ratio 1 (FR1) schedule of reinforcement, whereas responses on the inactive lever did not result in infusions. Drug delivery was controlled by an infusion pump (Med Associates Inc; Model PHM-107) with each infusion lasting for 2 sec with a 20 sec time-out period, during which active lever responses resulted in no consequences. All lever responses and infusion data were recorded using Med-PC IV. Weight was recorded daily and cocaine concentration was adjusted to account for every 50 gm increase. Cocaine concentration was adjusted to avoid changes in rates or length of infusion which can impact reinstatement behavior as previously reported (Roberts et al. 1989; Fredriksson and Li 2019). Two Coc-SA and EXT paradigms were used throughout the study (Fig 1) and are outlined below.
Figure 1: Schematic of behavioral experiments.
All experiments (EXP) included cocaine self-administration training (Coc-SA), extinction training (EXT) and a cocaine-seeking test (TEST). (A) EXP 1: Both adolescent (P44) and adult (P74) rats underwent Non-Abbreviated (Non-ABRV) Coc-SA in Context A (2 hr sessions, 1x/day over 10 days, minimum of 10 sessions) followed by EXT in Context B (1 hr sessions, 1x/day over 8 days, minimum of 8 sessions). The 1 hr TEST occurred in the previous cocaine-paired context (Coc-Pair) when both groups were in adulthood. (B) EXP 2: Both adolescent and adult rats were used for Abbreviated (ABRV) Coc-SA (2 hr sessions, 2x/day over 5 days, minimum of 10 sessions) and EXT (1 hr sessions, 4x/day over 2 days, minimum of 8 sessions). The 1 hr TEST occurred in the Coc-Pair context during either late adolescence (P51) or adulthood (P81). (C) For EXP 3, adolescent rats went through all phases of the ABRV paradigm, similar to EXP2, with the exception that groups received 1 hr TEST in either the Coc-Pair or a novel unpaired (Nov-Unpair) Context C.
EXP 1: Adolescent vs adult-onset Coc-SA and cocaine-seeking behavior in adulthood
Non-ABRV Behavioral Phases:
Adolescent rats started behavior at P40 and began each phase on average: Coc-SA (P44), EXT (P54) and TEST (P62). Adult rats started behavior at P70 and each phase on average: Coc-SA (P74), EXT (P84) and TEST (P92). The age range at which Coc-SA training began during adolescence was based on previous studies which utilized a timepoint when hormonal fluctuation stabilized (McCutcheon and Marinelli 2009; Wong et al. 2013; Sisk 2015; Dahl et al. 2018). Coc-SA consisted of 2 hr sessions, 1x/day over 10 days, for a minimum of 10 sessions until a criterion of ≥ 10 drug infusions/session was reached. Rats then received EXT training which consisted of a 1 hr session, 1x/day over 8 days for a minimum of 8 sessions until two consecutive sessions of ≤ 25 active responses were reached. Both active and inactive lever responses were recorded but resulted in no programmed consequences. After completing EXT, rats were returned to the original Coc-Pair context for a 1 hr TEST session during which active and inactive lever responses resulted in no programmed consequences. Due to the absence of cocaine reinforcement, active lever responses during TEST served as a measure of drug-seeking behavior.
EXP 2–3: Adolescent-onset Coc-SA and cocaine-seeking behavior in adolescence, compared to adult controls.
ABRV Behavioral Phases:
Adolescent rats started behavior at P40 and began each phase on average: Coc-SA (P44), EXT (P49) and TEST (P51). Adult rats started behavior at P70 and began each phase on average: Coc-SA (P74), EXT (P79) and TEST (P81). To allow for both Coc-SA and drug-seeking TEST to occur during adolescence, a second ABRV Coc-SA paradigm was utilized. While a previous study used ABRV Coc-SA, it was preceded by standard, Non-ABRV training (Anker and Carroll, 2010). The current ABRV Coc-SA consisted of 2 hr sessions, 2x/day over 5 days, for a minimum of 10 sessions, followed by EXT which consisted of 1 hr sessions, 4x/day over 2 days, for a minimum of 8 sessions with responses on both levers resulting in no programmed consequences. The two training sessions/day were separated by a 2 hr period during which rats were returned to their home-cage environments (Grimm et al. 2001; Li and Frantz 2009, 2017). The 1 hr TEST of cocaine-seeking behavior occurred in the Coc-Pair context.
For EXP 3, the 1 hr drug-seeking TEST occurred in either the Coc-Pair or Nov-Unpair context during adolescence. The Nov-Unpair context consisted of an alternating tone (80 dB, cycles of 1–1.5–2.5 kHz), constant light (1.2 fc brightness) above both the active and inactive levers, lemon-scented air freshener (Car Freshener Corp., Watertown, NY), and black acrylic flooring (26 cm × 27 cm). Both single and pair-housed conditions were combined since no differences were observed with the ABRV paradigm.
Statistical Analyses:
Separate analyses of variance (ANOVAs) or independent t-tests were conducted to examine for possible pre-existing differences between housing groups for: cocaine infusions, lever responses during Coc-SA training (Mean ± SEM, final 3 sessions), EXT (final 1 hr session) and number of sessions to reach acquisition criteria for Coc-SA and EXT. Rats that did not acquire Coc-SA or failed catheter patency tests were excluded from analysis (n=4). Lever responses during the non-reinforced reinstatement TEST were analyzed with mixed factorial ANOVAs. For EXP 1–2, 2×2×2 ANOVAs were analyzed with Age (adolescent vs adult), Context (EXT S8 vs Coc-Pair TEST) and Housing (pair vs single) as factors. For all behavioral phases (Coc-SA, EXT and TEST), no interaction effects were found (see Supplementary Table 1) and therefore subsequent mixed factorial ANOVAs were analyzed with Context as the within-subjects factor (EXT S8 vs Coc-Pair TEST) and Housing as the between-subjects factor (pair vs single). For EXP 3, ANOVAs were analyzed with Context (EXT S8 vs Coc-Pair TEST) as the within-subjects factor and TEST Group (Coc-Pair vs Nov-Unpair) as the between-subjects factor. Significant effects, when appropriate, were followed by analysis by Tukey’s HSD post-hoc tests, with alpha set at 0.05.
RESULTS
For all Non-ABRV and ABRV behavioral phases (Coc-SA, EXT and TEST), no Age × Context × Housing interaction effects were found (see Supplementary Table 1), therefore Context × Housing effects were explored separately for adolescents and adults.
Do housing conditions affect adolescent, vs adult, Coc-SA and subsequent reinstatement of cocaine-seeking behavior during adulthood (EXP 1)?
Non-ABRV Adolescent Behavior:
Adolescent pair and single-housed rats displayed stable responding on both levers during the last three sessions of Coc-SA training. There were no pre-existing differences between housing groups in the number of sessions to acquire Coc-SA, cocaine infusions or lever responses during the last three sessions of Coc-SA (Fig 2A). Adolescent pair and single-housed rats did not differ in active or inactive lever responses during EXT training (Fig 2B). 2×8 ANOVAs of lever responses revealed a significant EXT session main effect (active: F7,18=14.063, p<0.0001; inactive: F7,18=10.755, p<0.0001), with no EXT session × Housing interaction or Housing main effects. Therefore, responding on both levers decreased in both housing groups by the final EXT session (active and inactive: EXT S1>S8, p<0.01).
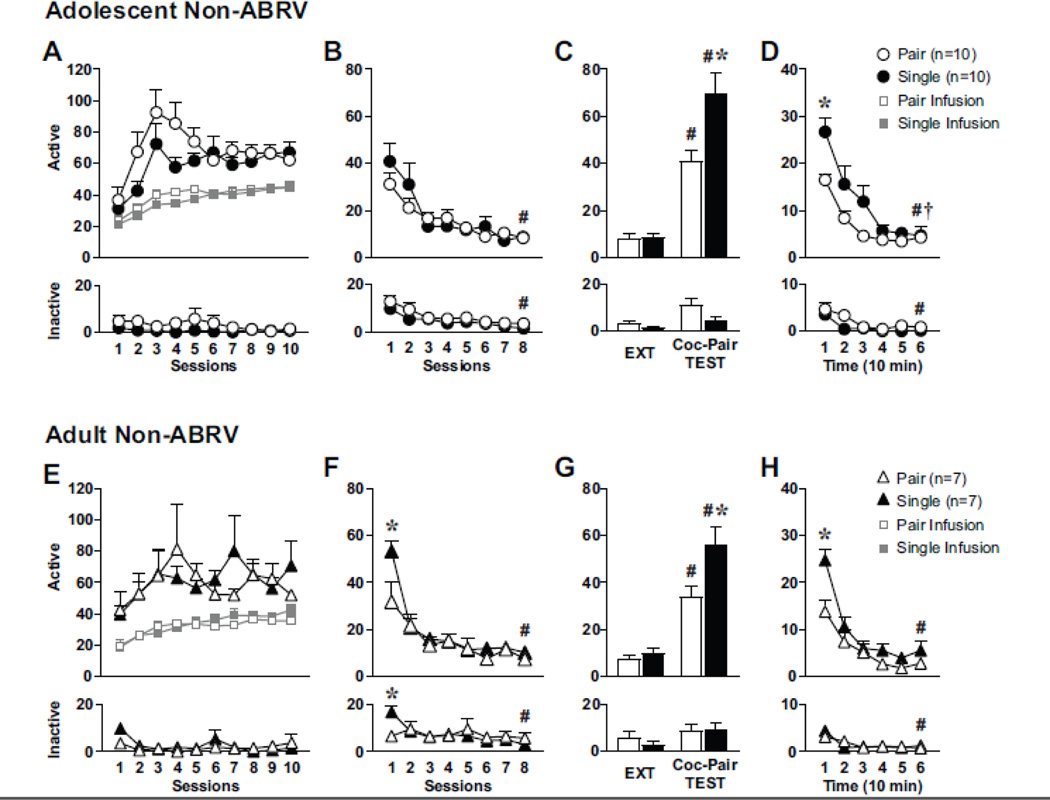
Figure 2: Effect of housing conditions in adolescent vs adult-onset Coc-SA and cocaine-seeking behavior during adulthood.
Behavior during Non-ABRV Coc-SA, EXT and Coc-Pair TEST for (A-D) adolescent and (E-H) adult rats. Mean (±SEM) of active and inactive lever responses during (A, E) Coc-SA for adolescent and adult rats and number of infusions, (B, F) 1 hr EXT sessions, (C, G) the last 1 hr EXT session and 1 hr Coc-Pair TEST, (D, H) during 10 min Bins of the 1 hr Coc-Pair TEST. Symbols indicate significant within-subject (#, †) or between-subject (*) differences revealed by Tukey’s test, p<0.05. (B, F) # EXT S1>8, (C, G) # last session of EXT>Coc-Pair TEST, (D, H) * Time Bin 1>6, † Time Bin 2>6, * Housing differences (F) EXT S1, (C, G) Coc-Pair TEST, (H) during first 10 min of TEST. Circles indicate adolescent group; triangles indicate adult group.
Adolescent Coc-SA pair and single-housed rats displayed increased cocaine-seeking behavior upon exposure to the Coc-Pair context during adulthood (Fig 2C). A 2×2 ANOVA of active lever responses during the final EXT vs TEST session revealed a significant Context × Housing interaction (F1,18=6.422, p=0.021), Context main (F1,18=70.400, p<0.0001) and Housing main (F1,18=9.760, p=0.006) effects. Therefore, both housing groups exhibited greater active lever responses during the TEST within the Coc-Pair context than on the final EXT session (TEST>EXT S8, #p<0.01). In addition, single-housed rats demonstrated higher drug-seeking behavior than pair-housed during the TEST (Single>Pair, *p<0.01). A 2×2 ANOVA of inactive lever responses during the final EXT vs TEST session revealed a significant Context main (F1,18=12.252, p=0.003) and Housing main (F1,18=5.872, p=0.026) effects with no significant Context × Housing interaction effect (F1,18=2.111, p=0.163).
A time-course analysis of lever responses during 10 min Bins of the 1 hr TEST revealed significant Bin main (active: F5,18=25.728, p<0.0001; inactive: F5,18=11.225, p<0.0001 ), active lever Housing main (F1,18=8.294, p=0.010), trend for inactive Housing main (F1,18=4.345, p=0.052) and no Bin × Housing interaction effects (Fig 2D). Thus, active responses for pair-housed rats declined after the initial 10 min (Bin 1>6, #p<0.01); whereas responses for single housed declined after 20 min (Bin 1–2>6, #p<0.05). For both housing groups, inactive responses declined after the initial 10 min (Bin 1>6, #p<0.05).
Non-ABRV Adult Behavior:
Adult pair and single-housed rats displayed stable responding on both levers during the last three sessions of Coc-SA training. There were no pre-existing differences between housing groups in the number of sessions to acquire Coc-SA, cocaine infusions or lever responses during the last three sessions of Coc-SA (Fig 2E). Adult pair and single-housed rats did not differ in active lever responses during EXT training (Fig 2F). 2×8 ANOVAs of lever responses revealed a significant EXT session × Housing interaction (active: F7,12=3.485, p=0.003; inactive: F7,12=4.303, p<0.0001) and EXT session main (active: F7,12=30.968, p<0.0001; inactive: F7,12=5.318, p<0.0001) effects, with no Housing main effects. Therefore, responding on the active lever decreased in both housing groups by the final EXT session (EXT S1>S8, #p<0.01). In addition, single-housed rats demonstrated higher responses on the first EXT session compared to pair housed (active and inactive, EXT S1: Single>Pair, *p<0.01).
Adult Coc-SA pair and single-housed rats displayed increased cocaine-seeking behavior upon exposure to the Coc-Pair context during adulthood (Fig 2G). A 2×2 ANOVA of active lever responses during the final EXT vs TEST session revealed a significant Context × Housing interaction (F1,12=5.081, p=0.044), Context main (F1,12=67.579, p<0.0001) and Housing main (F1,12=8.120, p=0.015) effects. Therefore, both housing groups exhibited greater active lever responses during the TEST within the Coc-Pair context than on the final EXT session (TEST>EXT S8, #p<0.01). In addition, single-housed rats demonstrated higher drug-seeking behavior than pair housed during the TEST (Single>Pair, *p<0.05) A 2×2 ANOVA of inactive lever responses during the final EXT vs TEST session revealed a significant Context main effect (F1,12=5.718, p=0.034) with no Housing main or Context × Housing interaction effects.
A time-course analysis of active lever responses during 10 min Bins of the 1 hr TEST revealed significant Bin × Housing interaction (F5,12=2.598, p=0.034), Bin main (F5,12=30.645, p<0.0001) and Housing main (F1,12=7.028, p=0.021) effects (Fig 2H). Therefore, in both housing groups, active lever responses declined after the initial 10 min (Bin 1>6, #p<0.01). In addition, single-housed rats responded higher during the initial 10 min of TEST than pair housed (Bin 1: Single>Pair, #p<0.01). For inactive lever responses there was a Bin main effect (F5,12=6.932, p<0.0001), with no Housing main or Bin × Housing interaction effects. For the single-housed group, inactive responses declined after the initial 10 min (Bin 1>6, #p<0.01).
Do housing conditions affect adolescent Coc-SA and subsequent reinstatement of cocaine-seeking behavior during adolescence, compared to adults (EXP 2)?
ABRV Adolescent Behavior:
Adolescent pair and single-housed rats displayed stable responding on both levers during the last three sessions of Coc-SA training. There were no pre-existing differences between housing groups in the number of sessions to acquire Coc-SA, cocaine infusions or lever responses during the last three sessions of Coc-SA (Fig 3A). Adolescent pair and single-housed rats did not differ in lever responses during EXT training (Fig 3B). 2×8 ANOVAs of lever responses revealed a significant EXT session main effect (active: F7,13=5.693, p<0.0001; inactive: F7,13=9.871, p<0.0001), with no EXT session × Housing interaction or Housing main effects. Therefore, responding on both levers decreased in both housing groups by the final EXT session (active and inactive: EXT S1>S8, #p<0.05).
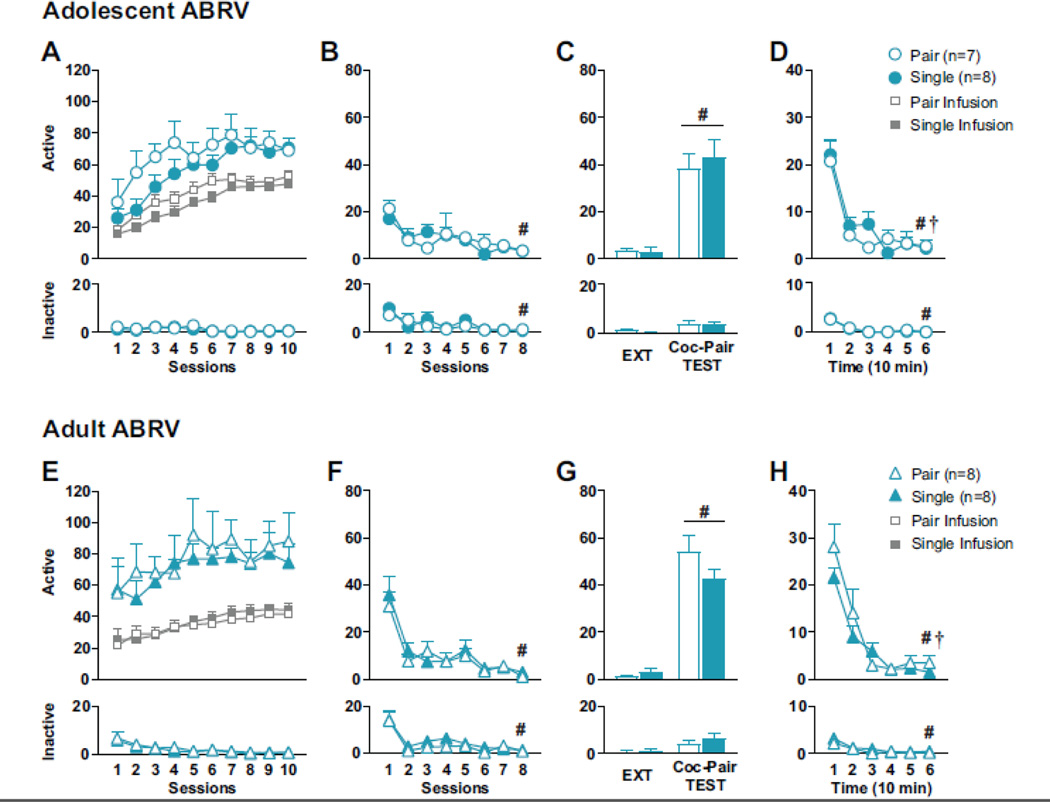
Figure 3: Effect of housing conditions in adolescent-onset Coc-SA and cocaine-seeking behavior during adolescence, compared to adult controls.
Behavior during ABRV Coc-SA, EXT and Coc-Pair TEST for (A-D) adolescent and (E-H) adult rats. Mean (±SEM) of active and inactive lever responses during (A, E) Coc-SA for adolescent and adult rats and the number of infusions (B, F) 1 hr EXT sessions, (C, G) the last EXT session and 1 hr Coc-Pair TEST, (D, H) 10 min Bins of the 1 hr Coc-Pair TEST. Symbols indicate significant within-subject (#, †) differences revealed by Tukey’s test, p<0.05: (B, F) # EXT S1>8, (C, G) # last session of EXT>Coc-Pair TEST, (D, H) # Time Bin 1>6, † Time Bin 2>6. Circles indicate adolescent group; triangles indicate adult group.
Adolescent Coc-SA pair and single-housed rats displayed increased cocaine-seeking behavior upon exposure to the Coc-Pair context during adolescence (Fig 3C). A 2×2 ANOVA of active and inactive lever responses during the final EXT vs TEST session revealed a significant Context main (active: F1,13=58.184, p<0.0001; inactive: F1,13=11.750, p=0.004) with no Housing main or Context × Housing interaction effects. Therefore, both housing groups exhibited greater active lever responses during the TEST within the Coc-Pair context than on the final EXT session. A time-course analysis of lever responses during 10 min Bins of the 1 hr TEST revealed significant Bin main (active: F5,13=27.629, p<0.0001; inactive: F5,13=9.217, p<0.0001), with no Housing main or Bin × Housing interaction effects (Fig 3D). Therefore, responses on both levers declined in both housing groups after the initial 10 min (active and inactive, Bin 1>6, #p<0.01).
ABRV Adult Behavior:
Adult rats displayed stable responding on both levers during the last three sessions of Coc-SA training. There were no pre-existing differences between housing groups in the number of sessions to acquire Coc-SA, cocaine infusions or lever responses during the last three sessions of Coc-SA (Fig 3E). Adult pair and single-housed rats did not differ in lever responses during EXT training (Fig 3F). 2×8 ANOVAs of lever responses revealed a significant EXT session main effect (active: F7,14=18.301, p<0.0001; inactive: F7,14=16.596, p<0.0001), with no Housing main or EXT session × Housing interaction effects. Therefore, lever responding decreased in both housing groups by the final EXT session (active and inactive: EXT S1>S8, #p<0.01).
Adult Coc-SA pair and single-housed rats displayed increased cocaine-seeking behavior upon exposure to the Coc-Pair context during adulthood (Fig 3G). A 2×2 ANOVA of lever responses during the final EXT vs TEST session revealed a significant Context main (active: F1,14=136.259, p<0.0001; inactive: F1,14=17.330, p<0.0001) with no Housing main or Context × Housing interaction effects. Therefore, both housing groups exhibited greater responses on both levers during the TEST within the Coc-Pair context than on the final EXT session. A time-course analysis of lever responses during the 10 min Bins of the 1 hr TEST revealed significant Bin main (active: F5,14=26.023, p<0.0001; inactive: F5,14=7.390, p<0.0001), with no Housing main or Bin × Housing interaction effects (Fig 3H). Therefore, lever responses declined in both housing groups after the initial 10 min (active and inactive, Bin 1>6, #p<0.05).
Does exposure to a novel context elicit reinstatement of cocaine-seeking behaviors during adolescence (EXP 3)?
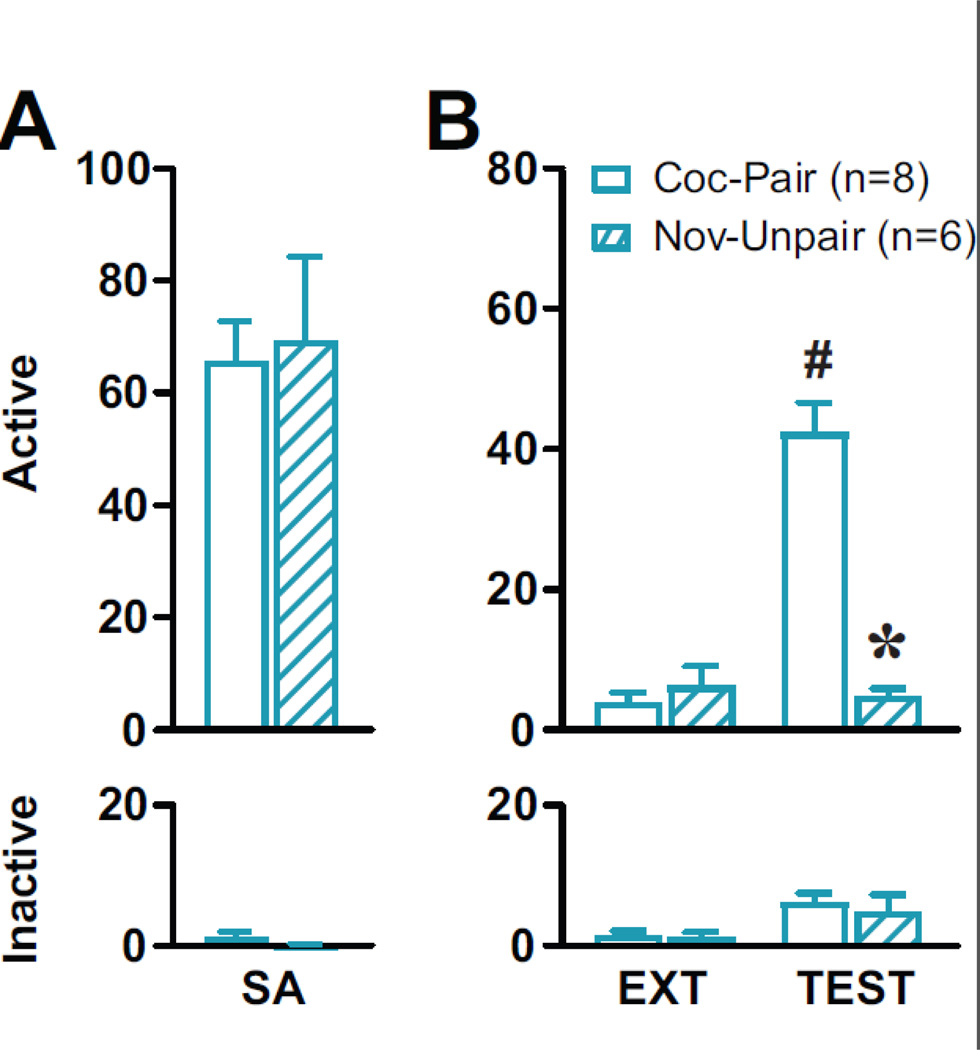
For EXP 3, groups that would be tested in the Coc-Pair vs Nov-Unpair context displayed stable responding on both levers during the last three sessions of Coc-SA. There were no pre-existing differences between TEST groups in the number of sessions to acquire Coc-SA, cocaine infusions and active or inactive lever responses during the last three sessions of Coc-SA or on the last EXT session (Fig 4A). Exposure to the Coc-Pair, but not Nov-Unpair, context resulted in increased cocaine-seeking behavior during adolescence (Fig 4B). 2×2 ANOVAs of active lever responses during the final EXT vs TEST session revealed a significant Context main (F1,12=35.887, p<0.0001), Housing main (F1,12=41.093, p<0.0001) and Context × TEST group interaction (F1,12=41.942, p<0.0001) effects. Therefore, the Coc-Pair group displayed increased drug seeking in the Coc-Pair context compared to the last EXT session (Coc-Pair TEST>EXT S8, #p<0.01). In contrast, the Nov-Unpair group did not increase drug seeking during TEST compared to the last EXT session. Furthermore, the Nov-Unpair group displayed significantly lower reinstatement behavior compared to the Coc-Pair group during the TEST session (Coc-Pair>Nov-Unpair, *p<0.01). A 2×2 ANOVA of inactive lever responses revealed a significant Context main effect (F1,12=12.203, p=0.004), with no Housing main or Context × Housing interaction effects.
Figure 4: Adolescent-onset Coc-SA and cocaine-seeking behavior in Coc-Pair vs Nov-Unpair context during adolescence.
Adolescent rats went through all phases of the ABRV paradigm similar to EXP 2. The 1 hr TEST occurred in either the Coc-Pair or Nov-Unpair contexts. Mean (±SEM) of active and inactive lever responses on (A) the last 3 sessions of Coc-SA, (B) the last 1 hr EXT session and 1 hr TEST in either the Coc-Pair (open bar) or Nov-Unpair Context (striped bar). Symbols represent significant differences revealed by Tukey’s test, p<0.05: # within-subjects effect, * between-subjects effect.
DISCUSSION
The present study determined that adolescent and adult-formed, context-cocaine-associations elicited similar levels of relapse-like behavior during adulthood with a standard, Non-ABRV paradigm (Fig 2). Implementation of a condensed, ABRV paradigm in which all behavioral phases (Coc-SA, EXT and TEST) occurred during adolescence resulted in robust context-induced drug-seeking during adolescence. However, no differences were observed in any behavioral phase between adolescent and adult-exposed rats (Fig 3). While the age of drug initiation did not result in differences in cocaine-taking or seeking behavior, differences in housing condition were observed in the Non-ABRV paradigm, with both adolescent and adult single-housed rats displaying elevated cocaine seeking compared to their pair-housed counterparts. The present study also found that adolescent drug-seeking was specific to the previous Coc-Pair context, given that exposure to a non-drug associated, Nov-Unpair context at TEST, did not reinstate cocaine-seeking behaviors (Fig 4). To our knowledge, the present study is the first to examine the role of adolescent-formed, cocaine-context-associations to precipitate relapse-like behavior during adolescence using operant self-administration procedures (Crombag and Shaham 2002; Fuchs et al. 2005; Hamlin et al. 2008). Furthermore, we provide a condensed, ABRV self-administration paradigm that can be used to investigate the underlying mechanisms that contribute to adolescent, context-induced relapse-like behaviors.
We observed no differences in Coc-SA behavior between adolescent and adult rats trained with a Non-ABRV or ABRV paradigm. Adolescent rats trained under Non-ABRV conditions acquired Coc-SA and were able to discriminate between the active and inactive levers in a diffuse, environmental context in which explicit cues were not paired with cocaine, as has been observed in adults (Crombag and Shaham 2002; Fuchs et al. 2005; Hamlin et al. 2008). When trained under ABRV conditions, both adolescent and adult rats displayed similar patterns of cocaine-taking behavior. Existing literature which examined adolescent drug taking are complex: at moderate doses (0.3–0.6 mg/kg), adolescent rats take more cocaine than adults (Kantak et al. 2007; Kerstetter and Kantak 2007; Anker and Carroll 2010; Schramm-Sapyta et al. 2011; Wong et al. 2013; Wong and Marinelli 2016), but there is also data supporting no difference in cocaine intake based on age (Li and Frantz 2009; Zbukvic et al. 2016; Madsen et al. 2017). In the current study, we also observed no differences in cocaine intake based on age (at 0.5 mg/kg, within the moderate range) under both Non-ABRV and ABRV conditions (Fig 2, 3).
Several methodological factors could contribute to the varying observations between adolescent and adult cocaine intake: use of different rat strains, age at start of Coc-SA, reinforcement schedules and short vs long-access conditions. While post-pubertal rats self-administer more cocaine than adults, similar or greater adolescent intake has been observed with both short vs long-access and varying reinforcement schedules (McCutcheon and Marinelli 2009); however, there is little evidence that adolescents self-administer less cocaine than adults. Our current data add to the field by showing that adolescent and adults have similar drug-taking behavior in a diffuse environmental context. While all adolescent rats started Coc-SA post-pubertally, we found no differences in taking behavior between rats exposed to cocaine for extended or brief periods of adolescence (Non-ABRV vs ABRV). Furthermore, we find that housing conditions (pair vs single) do not change cocaine intake dependent on age of cocaine exposure.
In the current study, similar EXT learning was observed between adolescent and adult cocaine-exposed rats, regardless of whether they were trained on a Non-ABRV or ABRV paradigm (Fig 2, 3). Despite age differences on the first day of EXT, all groups displayed comparable lever responses and time necessary to reach EXT criteria. As with Coc-SA, previous reports show that adolescent and adult rats display similar behavioral responses during EXT (Zbukvic et al. 2016; Madsen et al. 2017), but also that increased time is needed for adolescent rats to extinguish lever responding or cocaine CPP, compared to adults (Brenhouse and Andersen 2008; Anker and Carroll 2010). Although no Age × Context × Housing interactions were observed in any of our experiments, an Age × EXT session interaction was present with ABRV training conditions (Fig 3B, F). However, Tukey’s post-hoc comparisons did not reveal significant effects of Age on the first EXT session. Interestingly, a significant effect of Housing was observed in adult rats with the Non-ABRV paradigm. Specifically, single-housed adults exhibited increased lever responses on the first non-reinforced EXT session in a new context, compared to pair-housed rats (Fig 2F). While these data contrast with findings that adolescents are generally more resistant to EXT, they are in agreement with studies showing that isolation elevates cocaine seeking under extinction conditions (Brenhouse and Andersen 2008; Fosnocht et al. 2019).
In all our experiments, adolescent and adult cocaine-exposed rats had similar levels of context-elicited, relapse-like behavior when tested during adolescence or adulthood. While no differences were observed in drug-seeking due to Age, significant Housing effects were observed in both age groups in the Non-ABRV paradigm (Fig 2, 3). Previous studies determined that socially-isolated adolescent rats self-administer more cocaine at low, but not moderate, doses (Boyle et al. 1991; Howes et al. 2000; Ding et al. 2005; Neisewander et al. 2012; Fosnocht et al. 2019). In addition, isolation shortly after weaning can result in increased stress and anxiety-related behaviors (McCutcheon and Marinelli 2009; Zakharova et al. 2009; Gipson et al. 2011; Whitaker et al. 2013; Walker et al. 2019). In our experimental design, single housing began at P37 or P67 for adolescents and adults. Although housing conditions did not affect taking behavior, increased responding during the cocaine-seeking test was observed in single vs pair-housed groups under Non-ABRV conditions. Furthermore, the largest difference in seeking was observed in the initial 10–20 min of the reinstatement test (Fig 2G, H). Given that the effect of Housing was observed only for Non-ABRV conditions, we hypothesize this may be due to a difference in the total time of single housing between the Non-ABRV and ABRV group (21 vs 10 days) before the TEST of drug seeking. Therefore, the ABRV paradigm may not be sensitive to detect housing effects on behavior but will be useful for understanding the underlying mechanisms of drug seeking during adolescence.
Lastly, the present study addressed whether increased cocaine-seeking behavior during adolescence was specific to the Coc-Pair context, rather than an effect of removal from the EXT context (Bouton 2002, 2019). Our data show that exposure to a Nov-Unpair context during TEST did not elicit drug-seeking behaviors (Fig 4) with minimal responses on both the active and inactive levers, suggesting that adolescent rats did not display an increase in exploratory behaviors in a novel context. The Nov-Unpair data is consistent with previous studies that indicate reinstatement is specific to the context in which cocaine was previously administered, however, these processes may differ for natural reinforcers such as food or sucrose (Crombag and Shaham 2002; Fuchs et al. 2005; Bouton et al. 2011; Trask et al. 2017). In regards to contextual reinstatement, the current study is the first to our knowledge that explicitly examines the role of context-drug-associations formed during adolescence and their effects on reinstatement in both adolescence and adulthood.
In general, it seems that adolescent rats are more responsive to stress-elicited and cocaine-primed reinstatement but less responsive to cocaine-paired cues, compared to adults (Li and Frantz 2009; Anker and Carroll 2010; Wong and Marinelli 2016). Specifically, in a rodent model of cue-exposure therapy, adult rats that receive passive presentation of conditioned, drug-associated cues show diminished levels of seeking behavior compared to adolescents. Our study highlights that adolescent rats exposed to cocaine display equal reactivity to cocaine-paired contexts as adults. While this is in contrast with studies in which adolescents display heightened CPP and habit-like, context-seeking (oral sucrose) behavior, this could be attributed to procedural differences in conditioning paradigms. Finally, our findings of increased context-induced seeking under isolation add to growing evidence that external vs internal social factors can differentially affect relapse-like behavior. For example, while social interaction within a drug-taking context can enhance Coc-SA, external social interaction can diminish drug-taking behavior, a phenomenon that adolescent rats may be particularly sensitive to (Boyle et al. 1991; Neisewander et al. 2012; El Rawas and Saria 2016).
Contrary to our hypothesis, adolescent rats did not appear more vulnerable than adults to early drug exposure or isolated housing conditions. What could account for this invulnerability? Given that the mesocorticolimbic drug-reward circuit may not be fully developed during this time period, perhaps adolescent rats were protected from the potential negative effects of cocaine exposure (Walker et al. 2017; Hoops and Flores 2017). Relevant to our findings, increased cue-induced drug-seeking elicited by isolated conditions recruits several brain nuclei (Fosnocht et al. 2019). While we observed similar behavior in adolescent and adult cocaine-exposed rats, perhaps distinct brain circuits may be engaged in this process. While some brain regions support both cue and contextually-induced reinstatement (e.g. orbitofrontal cortex, basolateral amygdala, dorsomedial prefrontal cortex), other regions (dorsal and ventral hippocampus) selectively contribute to contextual relapse (Bossert et al. 2013). Future studies can begin to examine which of these regions are activated upon contextual relapse during adolescence and whether they are influenced by housing conditions.
Supplementary Material
Acknowledgements:
This work was supported by NIDA grant R00 DA037271.
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders DMS V. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition. 10.1176/appi.books.9780890425596.744053 [DOI] [Google Scholar]
- Anker JJ, Carroll ME (2010) Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 208:211–222. 10.1007/s00213-009-1721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Petronis KR (1995) Early-onset drug use and risk of later drug problems. Drug Alcohol Depend 40:9–15. 10.1016/0376-8716(95)01194-3 [DOI] [PubMed] [Google Scholar]
- Arguello AA, Wang R, Lyons CM, et al. (2017) Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology (Berl) 234:2431–2441. 10.1007/s00213-017-4632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL (2006) Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol 550:95–106. 10.1016/j.ejphar.2006.08.034 [DOI] [PubMed] [Google Scholar]
- Bal A, Gerena J, Olekanma DI, Arguello AA (2019) Neuronal Activation in Orbitofrontal Cortex Subregions: Cfos Expression Following Cue-Induced Reinstatement of Cocaine-Seeking Behavior. Behav Neurosci 133:489–495. 10.1037/bne0000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y (2013) The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229:453–476. 10.1007/s00213-013-3120-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME (2002) Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol. Psychiatry 52:976–986. 10.1016/s0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- Bouton ME (2019) Extinction of instrumental (operant) learning: interference, varieties of context, and mechanisms of contextual control. Psychopharmacology (Berl) 236:7–19. 10.1007/s00213-018-5076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, Winterbauer NE (2011) Renewal after the extinction of free operant behavior. Learn Behav 39:57–67. 10.3758/s13420-011-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AE, Gill K, Smith BR, Amit Z (1991) Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav 39:269–274. 10.1016/0091-3057(91)90178-5 [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL (2008) Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci 122:460–465. 10.1037/0735-7044.122.2.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AR, Miczel KA (2015) Escalation of cocaine self-administration in adulthood after social defeat of adolescent rats: role of social experience and adaptive coping behavior. Psychopharmacology (Berl) 232:3067–3079. 10.1007/s00213-015-3947-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME (1985) The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug Alcohol Depend 16:95–109. 10.1016/0376-8716(85)90109-7 [DOI] [PubMed] [Google Scholar]
- Cho BC, Kwak MJ, Kim WY, Kim JH (2018) Impulsive action and impulsive choice are differentially expressed in rats depending on the age at exposure to a gambling task. Front Psychiatry 9:503. 10.3389/fpsyt.2018.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Kirisci L, Tarter RE (1998) Adolescent versus adult onset and the development of substance use disorders in males. Drug Alcohol Depend 49:115–121. 10.1016/S0376-8716(97)00154-3 [DOI] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y (2002) Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116:169–173. 10.1037/0735-7044.116.1.169 [DOI] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, Suleiman AB (2018) Importance of investing in adolescence from a developmental science perspective. Nature 554:441–450. 10.1038/nature25770 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 75:134–143. 10.1007/BF00432175 [DOI] [PubMed] [Google Scholar]
- DePoy LM, Allen AG, Gourley SL (2016) Adolescent cocaine self-administration induces habit behavior in adulthood: sex differences and structural consequences. Transl Psychiatry 6:e875. 10.1038/tp.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Kang L, Li B, Ma L (2005) Enhanced cocaine self-administration in adult rats with adolescent isolation experience. Pharmacol Biochem Behav 82:673–677. 10.1016/j.pbb.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP (2016) Reward-centricity and attenuated aversions: An adolescent phenotype emerging from studies in laboratory animals. Neurosci Biobehav Rev 70:121–134. 10.1016/j.neubiorev.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP (1992) Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 107:523–529. 10.1007/BF02245266 [DOI] [PubMed] [Google Scholar]
- El Rawas R, Saria A (2016) The Two Faces of Social Interaction Reward in Animal Models of Drug Dependence. Neurochem Res 41:492–499. 10.1007/s11064-015-1637-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Haney M (2000) Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 149:24–33. 10.1007/s002139900340 [DOI] [PubMed] [Google Scholar]
- Fosnocht AQ, Lucerne KE, Ellis AS, et al. (2019) Adolescent social isolation increases cocaine seeking in male and female mice. Behav Brain Res 359:589–596. 10.1016/j.bbr.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson I, Li X (2019) Rate matters: rapid cocaine delivery promotes incubation of cocaine craving. Neuropsychopharmacology 44:1009–1010. 10.1038/s41386-018-0275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, et al. (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30:296–309. 10.1038/sj.npp.1300579 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X (2008) Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov. Today Dis. Model 5:251–258. 10.1016/j.ddmod.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, et al. (2011) Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 214:557–566. 10.1007/s00213-010-2060-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Incubation of cocaine craving after withdrawal. Nature 412:141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neuroscience 151:659–670. 10.1016/j.neuroscience.2007.11.018 [DOI] [PubMed] [Google Scholar]
- Hoops D, Flores C (2017) Making Dopamine Connections in Adolescence. Trends Neurosci. 40:709–719. 10.1016/j.tins.2017.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes SR, Dalley JW, Morrison CH, et al. (2000) Leftward shift in the acquisition of cocaine self-administration in isolation-reared rats: relationship to extracellular levels of dopamine, serotonin and glutamate in the nucleus accumbens and amygdala-striatal FOS expression. Psychopharmacology (Berl) 151:55–63. 10.1007/s002130000451 [DOI] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, et al. (2019) Monitoring the Future national survey results on drug use 1975–2018: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan. 10.3998/2027.42/148123 [DOI] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V (2007) Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus). Exp Clin Psychopharmacol 15:37–47. 10.1037/1064-1297.15.1.37 [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM (2007) Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 194:403–11. 10.1007/s00213-007-0852-6 [DOI] [PubMed] [Google Scholar]
- Khoo SYS, Gibson GD, Prasad AA, McNally GP (2017) How contexts promote and prevent relapse to drug seeking. Genes, Brain Behav. 16:185–204. 10.1111/gbb.12328 [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Wells AM, Xie X, Fuchs RA (2011) Interaction of the basolateral amygdala and orbitofrontal cortex is critical for drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 36:711–720. 10.1038/npp.2010.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ (2009) Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacology (Berl) 204:725–733. 10.1007/s00213-009-1502-y [DOI] [PubMed] [Google Scholar]
- Li C, Frantz KJ (2017) Abstinence environment contributes to age differences in reinstatement of cocaine seeking between adolescent and adult male rats. Pharmacol Biochem Behav 158:49–56. 10.1016/j.pbb.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Madsen HB, Zbukvic IC, Luikinga SJ, et al. (2017) Extinction of conditioned cues attenuates incubation of cocaine craving in adolescent and adult rats. Neurobiol Learn Mem 143:88–93. 10.1016/j.nlm.2016.09.002 [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M (2009) Age matters. Eur J Neurosci 29:997–1014. 10.1111/j.1460-9568.2009.06648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS (2012) Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology (Berl). 224:33–56. 10.1007/s00213-012-2853-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Zbukvic I, Kim JH, Lawrence AJ (2014) Role of cues and contexts on drug-seeking behaviour. British J of Pharmacology 171: 4636–4672. 10.1111/bph.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Brown SA (2008) Classes of Substance Abuse Relapse Situations: A Comparison of Adolescents and Adults. Psychol Addict Behav 22:372–379. 10.1037/0893-164X.22.3.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramo DE, Prince MA, Roesch SC, Brown SA (2012) Variation in substance use relapse episodes among adolescents: A longitudinal investigation. J Subst Abuse Treat 43:44–52. 10.1016/j.jsat.2011.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G (1989) Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 97:535–538. 10.1007/bf00439560 [DOI] [PubMed] [Google Scholar]
- Saunders JB (2017) Substance use and addictive disorders in DSM-5 and ICD 10 and the draft ICD 11. Curr. Opin. Psychiatry 30:227–237. 10.1097/YCO.0000000000000332 [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cauley MC, Stangl DK, et al. (2011) Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berl) 215:493–504. 10.1007/s00213-011-2216-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, et al. (2003) The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology (Berl). 168:3–20. 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Sisk CL (2015) Gonadal hormones organize the adolescent brain and behavior. Res Perspect Endocr Interact 13:15–27. 10.1007/978-3-319-09168-6_2 [DOI] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Spear LP, Silveri MM (2016) Special Issue on the Adolescent Brain. Neurosci. Biobehav. Rev 70:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2016) Commentary on Special Issue on the Adolescent Brain: Redefining Adolescence. Neurosci. Biobehav. Rev 70:343–346. 10.1016/j.neubiorev.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Trask S, Shipman ML, Green JT, Bouton ME (2017) Inactivation of the prelimbic cortex attenuates context-dependent operant responding. J Neurosci 37:2317–2324. 10.1523/JNEUROSCI.3361-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ (2014) On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacology (Berl). 231:1715–1729. 10.1007/s00213-014-3471-z [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Shaham Y (2016) Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Progress in Brain Research. 224: 25–52. 10.1016/bs.pbr.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, et al. (2017) Adolescence and reward: Making sense of neural and behavioral changes amid the chaos. J. Neurosci 37:10855–10866. 10.1523/JNEUROSCI.1834-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Cunningham AM, Gregory JK, Nestler EJ (2019) Long-term behavioral effects of post-weaning social isolation in males and females. Front Behav Neurosci 13:1–20. 10.3389/fnbeh.2019.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, Morikawa H (2013) Social Deprivation Enhances VTA Synaptic Plasticity and Drug-Induced Contextual Learning. Neuron 77:335–345. 10.1016/j.neuron.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Tanner-Smith EE, Bresani E, Meyers K (2014) Current advances in the treatment of adolescent drug use. Adolesc. Health. Med. Ther 5:199–209. 10.2147/AHMT.S48053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Ford KA, Pagels NE, et al. (2013) Adolescents are more vulnerable to cocaine addiction: Behavioral and electrophysiological evidence. J Neurosci 33:4913–4922. 10.1523/JNEUROSCI.1371-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Marinelli M (2016) Adolescent-onset of cocaine use is associated with heightened stress-induced reinstatement of cocaine seeking. Addict Biol 21:634–645. 10.1111/adb.12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, et al. (2009) Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience 163:890–897. 10.1016/j.neuroscience.2009.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbukvic IC, Ganella DE, Perry CJ, et al. (2016) Role of Dopamine 2 Receptor in Impaired Drug-Cue Extinction in Adolescent Rats. Cereb cortex 26:2895–904. 10.1093/cercor/bhw051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.