Abstract
Background:
The 2030 Sustainable Development Goals (SDGs) set an ambitious new benchmark for safely managed drinking water services (SMDWs), but many countries lack national data on the availability and quality of drinking water.
Objectives:
We quantified the availability and microbiological quality of drinking water, monitored SMDWs, and examined risk factors for Escherichia coli (E. coli) contamination in 27 low-and middle-income countries (LMICs).
Methods:
A new water quality module for household surveys was implemented in 27 Multiple Indicator Cluster Surveys. Teams used portable equipment to measure E. coli at the point of collection (PoC, ) and at the point of use (PoU, ) and asked respondents about the availability and accessibility of drinking water. Households were classified as having SMDW services if they used an improved water source that was free of E. coli contamination at PoC, accessible on premises, and available when needed. Compliance with individual SMDW criteria was also assessed. Modified Poisson regression was used to explore household and community risk factors for E. coli contamination.
Results:
E. coli was commonly detected at the PoC (range 16–90%) and was more likely at the PoU (range 19–99%). On average, 84% of households used an improved drinking water source, and 31% met all of the SMDW criteria. E. coli contamination was the primary reason SMDW criteria were not met (15 of 27 countries). The prevalence of E. coli in PoC samples was lower among households using improved water sources [; 95% confidence interval (CI): 0.64, 0.85] but not for households with water accessible on premises (; 95% CI: 0.94, 1.05) or available when needed (; 95% CI: 0.88, 1.02). E. coli contamination of PoU samples was less common for households in the richest vs. poorest wealth quintile (; 95% CI: 0.55, 0.88) and in communities with high () improved sanitation coverage (; 95% CI: 0.90, 0.97). Livestock ownership (; 95% CI: 1.04, 1.13), rural vs. urban residence (; 95% CI: 1.04, 1.16), and wet vs. dry season sampling (; 95% CI: 1.01, 1.15) were positively associated with contamination at the PoU.
Discussion:
Cross-sectional water quality data can be collected in household surveys and can be used to assess inequalities in service levels, to track the SDG indicator of SMDWs, and to examine risk factors for contamination. There is an urgent need for better risk management to reduce widespread exposure to fecal contamination through drinking water services in LMICs. https://doi.org/10.1289/EHP8459
Introduction
The Sustainable Development Goals (SDGs) set ambitious targets for universal access to safe drinking water, sanitation, and hygiene (WASH) by 2030. Expert consultations and negotiations between United Nations Member States led to the adoption of a new global indicator 6.1.1, the “use of safely managed drinking water services.” Safely managed drinking water services (SMDWs) are defined as improved sources of drinking water (piped water, boreholes/tube wells, protected dug wells and springs, rainwater collection, packaged or delivered water), that are accessible on premises and provide water that is available when needed and free from fecal and priority chemical contamination (WHO/UNICEF 2017a).
The World Health Organization (WHO) and United Nations Children’s Fund (UNICEF), through the Joint Monitoring Program (JMP) for Water Supply, Sanitation and Hygiene, are mandated to monitor the SDG global indicators for WASH and released baseline estimates in July 2017 (WHO/UNICEF 2017a) and updated estimates in 2019 (WHO/UNICEF 2019) and 2021 (WHO/UNICEF 2021). The JMP reports confirm earlier studies suggesting widespread exposure to contamination through drinking water in low- and middle-income countries (LMICs) (Bain et al. 2014) and estimating that people drink water from sources that contain fecal indicator bacteria (Onda et al. 2012). Although the importance of water quality is increasingly recognized by policymakers, the 2019 JMP report found that only 98 of the 193 UN Member States had sufficient data at the national level to report on water quality for Target 6.1 (WHO/UNICEF 2019). There is an urgent need to expand the monitoring of drinking water quality to inform national efforts to achieve SMDW services by 2030.
WHO guidelines recommend that drinking water should be free from pathogens and elevated levels of harmful substances at all times (WHO 2017). Globally, the highest priority parameters for health are fecal contamination, arsenic, and fluoride. The presence of E. coli is considered a reliable indicator of fecal contamination, but brief events may escape detection even with regular testing, so its absence indicates low risk but does not guarantee safety (Charles et al. 2020). For the purposes of SDG monitoring, “free from contamination” implies that drinking water does not contain E. coli or thermotolerant coliforms (in a sample) or elevated levels of arsenic (), or fluoride ().
Data on drinking water quality primarily come from administrative sources, including regulators that collect information from utilities. Regulatory coverage varies but is generally better for urban than for rural populations and for those who do not use piped water (Kumpel et al. 2016). As a result, data on water quality are often lacking for the majority of the population in many LMICs. To address this data gap, the JMP has supported the piloting and scale-up of water quality testing in nationally representative multitopic household surveys (WHO/UNICEF 2020). This approach enables the collection of data at comparatively low cost that can be linked with information on household characteristics (e.g., health, nutrition, education, wealth, ethnicity). Data from household surveys were critical to MDG WASH monitoring and are expected to continue to be a major data source for SDG monitoring alongside administrative sources and regulators (Bartram et al. 2014; WHO/UNICEF 2017b).
In collaboration with the UNICEF-supported Multiple Indicator Cluster Surveys (MICS), a new water quality module was developed during the fifth round of surveys (MICS5 2013–2017) and offered as a standard module in the sixth round of surveys (MICS6 2017–present) (WHO/UNICEF 2020). To date, 27 countries have published nationally representative data collected through the MICS, providing an unprecedented opportunity to examine risk factors for fecal contamination of drinking water in LMICs. The MICS used a standardized approach to achieve robust random selection of households and collect quantitative information on E. coli levels and samples at both the point of collection (PoC) and point of use (PoU). As such, these data address several key limitations identified by systematic reviews of water quality in LMICs (Bain et al. 2014; Shields et al. 2015; Wright et al. 2004) and enable a multicountry assessment informed by prior research on household- and community-level risk factors for E. coli contamination (Cronin et al. 2017; Harris et al. 2017; Kandel et al. 2017; Kirby et al. 2016; Kumpel et al. 2016; Pickering et al. 2010; Wang et al. 2017b; Wardrop et al. 2018; Yang et al. 2013).
The objectives of this study were to describe the main features of the new water quality module; to explore patterns in water quality by country, type of water source, and wealth; and to compare coverage of the MDG “improved” and SDG “safely managed” indicators for drinking water. Multivariable regression was used to examine household- and community-level risk factors for fecal contamination, including accessibility and availability of drinking water.
Methods
MICS Surveys
The MICS is a survey program supported by UNICEF and is one of the largest sources of data on populations, having covered over 120 countries with over 300 surveys in the past 25 y (Khan and Hancioglu 2019). The surveys are implemented by national governments with technical assistance from UNICEF for all stages of implementation. Surveys are cross sectional and use a multistage stratified sampling approach where enumeration areas (often between 100 and 250 households) in the national census are selected randomly, and then clusters of households (usually 20–25) are randomly selected within these enumeration areas. Each household has a known probability of selection, enabling national estimates to be generated. To ensure an adequate sample size for subnational regions, the sample is usually stratified (e.g., urban/rural and geographic regions). The MICS cover a range of topics, including household assets, water and sanitation, child labor and protection issues, health of children, women and men, and nutrition. Interview teams are composed of female and male interviewers to perform same-sex interviewers, a van driver, a supervisor, and a measurer who is tasked with taking the height and weight of children for the anthropometry module. The MICS is conducted in rounds, and countries that have implemented surveys in each round typically conduct a survey once every 3–5 y.
The water quality module was developed as a collaboration between the JMP and the global MICS program. It was first piloted in Bogra and Sirajgani districts, Bangladesh, in April/May 2012 prior to being adopted in the national MICS Bangladesh survey in 2012–2013. The procedures have evolved since the pilot study and further details are provided in the respective survey final reports (https://mics.unicef.org/surveys).
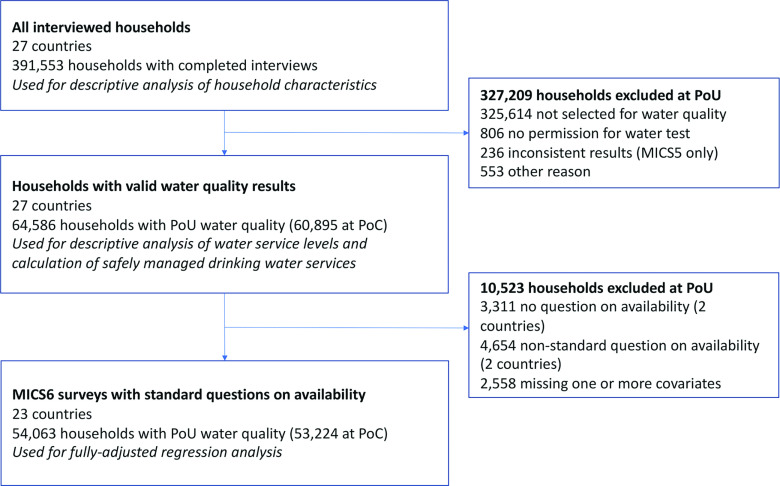
In this study, we used the most recent MICS survey in each country that integrated water quality testing in MICS between 2014 and 2020 and for which data are publicly available (Table 1; Figure 1). In total 27 surveys were included.
Table 1.
Nationally representative Multiple Indicator Cluster Surveys (MICS) that had integrated water testing, 2014–2020.
| Country (MICS round) | Fieldwork | Households interviewed { [response rate (%)]}a | Samples at PoC { [response rate (%)]}b | Samples at PoU { [response rate (%)]}c | Clusters | Teams | Implementing agencies | Technical assistance for water quality testing | Training duration (d) |
|---|---|---|---|---|---|---|---|---|---|
| Algeria (MICS6) | December 2018–April 2019 | 29,919/30,930 (96.7) | 3,137/4,097 (76.6) | 4,076/4,097 (99.5) | 1,253 | 45 | Ministère de la Santé, de la Population et de la Réforme Hospitalière | Laboratory staff from Ministère de la Santé, de la Population et de la Réforme Hospitalière | 5 |
| Bangladesh (MICS6) | January–June 2019 | 61,242/61,602 (99.4) | 6,069/6,150 (98.7) | 6,140/6,150 (99.8) | 3,220 | 34 | Bangladesh Bureau of Statistics | International Center for Diarrhoeal Disease Research, Bangladesh | 5 |
| Central African Republic (MICS6) | December 2018–June 2019 | 8,133/8,302 (98.7) | 1,048/1,228 (85.3) | 1,212/1,228 (98.7) | 451 | 20 | Institut Centrafricain des Statistiques et des Etudes Economiques et Sociales | Direction Générale de l’Hydraulique | 6 |
| Chad (MICS6) | May–December 2019 | 18,967/19,034 (99.6) | 2,166/2,284 (94.8) | 2,274/2,284 (99.6) | 769 | 25 | Institut National de la Statistique, des Etudes Economiques et Démographiques | Ministère des Ressources en Eau | 4 |
| Congo (MICS5) | November 2014–February 2015 | 12,811/12,868 (99.6) | 1,277/1,573 (81.2) | 1,507/1,573 (95.8) | 531 | 19 | Institut National de la Statistique | Société nationale de distribution d’eau | 5 |
| Côte d’Ivoire (MICS5) | April–July 2016 | 11,879/12,303 (96.6) | 1,782/1,926 (92.5) | 1,908/1,926 (99.1) | 511 | 21 | Institut National de la Statistique | Institut National d’Hygiène Publique and Laboratoire National de Santé Publique | 5 |
| Gambia (MICS6) | January–April 2018 | 7,405/7,517 (98.5) | 1,764/1,879 (93.9) | 1,865/1,879 (99.3) | 390 | 8 | Gambia Bureau of Statistics | Ministry of Water Resources | 5 |
| Georgia (MICS6) | September–December 2018 | 12,270/13,030 (94.2) | 2,429/3,059 (79.4) | 2,699/3,059 (88.2) | 706 | 13 | National Statistics Office of Georgia | National Center for Disease Control and Public Health | 3 |
| Ghana (MICS6) | October 2017–January 2018 | 12,886/12,960 (99.4) | 3,161/3,222 (98.1) | 3,219/3,222 (99.9) | 660 | 25 | Ghana Statistical Service | Water Research Institute and Ghana Water Company | 4 |
| Guinea-Bissau (MICS6) | November 2018–March 2019 | 7,379/7,394 (99.8) | 1,784/1,829 (97.5) | 1,828/1,829 (99.9) | 375 | 8 | Instituto Nacional de Estatística | — | 6 |
| Iraq (MICS6) | March–May 2018 | 20,214/20,318 (99.5) | 6,687/6,733 (99.3) | 6,724/6,733 (99.9) | 1,710 | 39 | Central Statistical Organization and Kurdistan Region Statistical Office | Central Public Health Laboratory | 2 |
| Kiribati (MICS6) | November 2018–January 2019 | 3,071/3,113 (98.7) | 589/626 (94.1) | 622/626 (99.4) | 164 | 9 | Kiribati National Statistics Office | Ministry of Health and Ministry of Infrastructure and Sustainable Energy | 4 |
| Lao People’s Democratic Republic (MICS6) | July–November 2017 | 22,287/22,443 (99.3) | 3,292/3,360 (98.0) | 3,346/3,360 (99.6) | 1,165 | 25 | Lao Statistics Bureau | Center for Environmental Health and Water Supply (Nam Saat) | 4 |
| Lesotho (MICS6) | April–September 2018 | 8,847/9,227 (95.9) | 1,339/1,376 (97.3) | 1,373/1,376 (99.8) | 400 | 15 | Bureau of Statistics | Ministry of Health and Ministry of Water Affairs | 3 |
| Madagascar (MICS6) | August–November 2018 | 17,870/18,291 (97.7) | 3,265/3,439 (94.9) | 3,433/3,439 (99.8) | 774 | 30 | L’Institut National de la Statistique de Madagascar | Ministre de Santé and the Ministre de l’Eau | 3 |
| Mongolia (MICS6) | September–December 2018 | 13,798/14,041 (98.3) | 2,598/2,764 (94.0) | 2,736/2,764 (99.0) | 580 | 19 | National Statistical Office | UNICEF Mongolia | 3 |
| Nepal (MICS6) | May–November 2019 | 12,655/12,687 (99.5) | 2,445/2,547 (96.0) | 2,536/2,547 (99.6) | 512 | 16 | Central Bureau of Statistics | Environment and Public Health Organization | 5 |
| Nigeria (MICS5) | September 2016–January 2017 | 33,901/34,289 (98.9) | 2,722/3,058 (89.0) | 3,053/3,058 (99.8) | 2,239 | 78 | National Bureau of Statistics | Federal Ministry of Water Resources | 5 |
| Palestine (MICS6) | December 2019–January 2020 | 9,326/9,751 (95.6) | 1,819/1,909 (95.3) | 1,848/1,909 (96.8) | 420 | 36 | Palestinian Central Bureau of Statistics | Palestinian Water Authority | 5 |
| Paraguay (MICS5) | June–September 2016 | 7,313/7,594 (96.3) | 1,750/1,814 (96.5) | 1,790/1,814 (98.7) | 499 | 15 | Dirección General de Estadística, Encuestas y Censos | Ente Regulador de Servicios Sanitarios | 2 |
| Sao Tome and Principe (MICS6) | August–October 2019 | 3,426/3,469 (98.8) | 342/572 (59.8) | 571/572 (99.8) | 127 | 8 | Instituto Nacional de Estatística | — | 6 |
| Sierra Leone (MICS6) | May–August 2017 | 15,309/15,364 (99.6) | 1,748/1,784 (98.0) | 1,780/1,784 (99.8) | 600 | 24 | Statistics Sierra Leone | Ministry of Water Resources | 5 |
| Suriname (MICS6) | March–August 2018 | 7,915/8,771 (90.2) | 1,619/1,982 (81.7) | 1,701/1,982 (85.8) | 470 | 10 | General Bureau of Statistics | Suriname Water Supply Company and the Bureau of Public Health | 4 |
| Togo (MICS6) | July–October 2017 | 7,916/8,065 (98.2) | 1,088/1,157 (94.0) | 1,153/1,157 (99.7) | 420 | 16 | Institut National de la Statistique et des Etudes Economiques et Démographiques | Institut National d’Hygiène | 5 |
| Tonga (MICS6) | October–December 2019 | 2,498/2,543 (98.2) | 543/628 (86.5) | 613/628 (97.6) | 139 | 8 | Tonga Statistics Department | Ministry of Health | 4 |
| Tunisia (MICS6) | March–May 2018 | 11,225/11,473 (97.8) | 2,664/2,805 (95.0) | 2,769/2,805 (98.7) | 600 | 32 | Institut National de la Statistique | Ministry of Health, Department of Environmental Hygiene and Environmental Protection | 3 |
| Zimbabwe (MICS6) | January–April 2019 | 11,091/11,313 (98) | 2,043/2,138 (95.6) | 2,124/2,138 (99.3) | 462 | 17 | Zimbabwe National Statistics Agency | Ministry of Health and Child Care | 4 |
| Total | — | 391,553/398,692 (98.2) | 61,170/65,939 (92.8) | 64,900/65,939 (98.4) | 20,147 | 615 | — | — | — |
Note: —, not applicable; MICS5, fifth round of Multiple Indicator Cluster Surveys; MICS6, sixth round of Multiple Indicator Cluster Surveys; PoC, point of collection; PoU, point of use; UNICEF, United Nations Children’s Fund.
Household response rate: proportion of completed household interviews of all occupied households.
PoC response rate: Proportion of households with water quality samples at PoC of occupied households selected for water quality testing. Number of samples does not exclude invalid test results. See Figure 1 for the number of samples excluded from analysis.
PoU response rate: proportion of households with water quality samples at PoU of occupied households selected for water quality testing. Number of samples does not exclude invalid test results. See Figure 1 for the number of samples excluded from analysis.
Figure 1.
Study flow diagram. Note: MICS5, fifth round of the Multiple Indicator Cluster Surveys; MICS6, sixth round of the Multiple Indicator Cluster Surveys; PoC, point of collection; PoU, point of use.
Water Quality Module
The procedures for the standardized module are described in the “MICS Manual for Water Quality” and “MICS Water Quality Testing Questionnaire,” both available on the MICS website (https://mics.unicef.org/tools). The standardized module is available in Arabic, English, French, Russian and Spanish.
The module was conducted in a subsample of households (between 3 and 5) within each cluster. Not every household was selected in order to minimize the cost and workload and because it was anticipated that the marginal gains of increasing the sample size would be small owing to intra-cluster correlation (households in the same cluster having similar water quality).
In households selected for water quality testing, the interviewer requested permission to collect samples of drinking water, asking the respondent for a “glass of drinking water” (i.e., the PoU) and to be shown the location of the source of the drinking water (i.e., the PoC) to take a sample from that location. The two samples were analyzed to examine differences in quality between the PoC and the PoU (Shields et al. 2015; Wright et al. 2004). The water quality testing questionnaire included sections for the interviewer to record test results. It also included specific questions on the source of the glass of drinking water and an observation of water storage practices.
The field team member responsible for water quality testing differed between surveys depending on the distribution of other tasks. The measurer who was responsible for anthropometry usually conducted the water quality test. In some countries, either the male interviewer or the supervisor was selected to distribute workload more evenly across the field team.
An international water quality trainer working alongside national experts led the training of the field teams. A standard training program was used and adapted depending on the size of the survey and number of field teams. For example, in Nigeria a centralized training of trainers was followed by zonal trainings by microbiologists selected by the Federal Ministry of Water Resources. A hands-on training course lasting between 2 and 6 d usually included field practice, a strong focus on aseptic procedure, and understanding the different scenarios that testers might face through role-play. National experts from regulatory agencies, research laboratories, or ministries of health provided technical input to the training and oversight during the fieldwork through a small number of field visits to allow for observation of the testing procedure.
Water Quality Assessment
Water samples were analyzed for E. coli using a portable membrane filtration apparatus (Figure S1). E. coli was measured by field teams, who filtered of sample through a filter (Millipore Microfil), which was then placed onto CompactDry EC growth media plates (Nissui) that had been rehydrated with of sample water. In MICS5 surveys, an additional sample was also tested directly on a second media plate. Water samples were either collected directly into the preassembled membrane filtration apparatus or using sterile Whirl-Pak sample collection bags (Enasco). Samples were analyzed on-site within 30 min and incubated overnight (24–48 h) in either an electric incubator (Lynd) or incubation belt/pouch specifically designed to keep samples at close to body temperature. The number of E. coli (blue) and total coliform (red/purple) colonies were enumerated by eye the following day and recorded in the water quality testing questionnaire. Survey teams recorded cases where there were colonies on a given plate and in cases where the plate turned red/purple or blue as “too numerous to count.” For more recent surveys (i.e., MICS6), “E. coli only” CompactDry plates were used, with the advantage that the second media plate was not needed to count up to 100 E. coli. The levels of E. coli were classified according to the number of colony forming units (CFUs) per as follows: , low risk; 1–10, moderate risk; 11–100, high risk; and , very high risk (WHO/UNICEF 2012; WHO 2017).
The use of 100- and samples in MICS5 allowed for inconsistency checks to examine whether the combination of results was improbable and suspected to be the result of errors in the test procedure or data recording. Results were excluded from analysis if the test was E. coli CFUs and test had at least one CFU (Flag I) or the sample count was greater than the sample count, provided both were nonzero and countable (Flag II). The proportion of samples recorded as potentially inconsistent and excluded from analysis was found to range from 1.6% in Côte d’Ivoire to 4.9% in Nigeria (Table S1). Inconsistency checks were not required for MICS6 surveys owing to the use of “E. coli only” CompactDry plates, which meant only a 100-mL sample was assessed.
A key measure of quality control used in water quality analysis is a blank test, a sample used to ensure that the analyst has not inadvertently contaminated the sample and is able to consistently produce the expected negative results. In each survey, blank tests (usually one per cluster) were conducted by the field teams to provide confidence in the results. Water for the blank test was obtained from a reliable brand of mineral water or distilled/deionized water. The proportion of positive blanks was in 24 of 27 countries, indicating high levels of compliance with testing procedures, but there were elevated proportions in Côte d’Ivoire (8.2%), Gambia (6.2%), and Chad (3.6%) (Table S2).
Accessibility and Availability of Drinking Water Services
The WASH module in the MICS started with a question enquiring what type of drinking water source is usually used by household members. Households were then asked where the water source is located (inside dwelling, in yard/plot, elsewhere) and, for water not located on premises, how long it takes to collect drinking water from the main source. Drinking water “accessible on premises” refers to water sources that were located on premises by definition (piped to dwelling, plot, or yard) or reported as being located within the dwelling or in the yard/plot. Households reporting using a source located elsewhere but where members did not collect water themselves were considered to have water accessible on premises.
The MICS6 household questionnaire (https://mics.unicef.org/tools) was adapted to include a new question on the availability of drinking water aligned with the JMP core questions (WHO/UNICEF 2018a): “In the last month, has there been any time when your household did not have sufficient quantities of drinking water?” In MICS5, different availability questions were asked in Nigeria (“Was the water from this source not available for at least one full day?”) and Paraguay (“In the last 15 days has there been any time you have not been able to access water in sufficient quantities?”). Households without sufficient water in the preceding month were then asked to identify the main reason (water not available from source, water too expensive, source not accessible or other reason).
SMDW services were calculated based on the household’s main source of drinking water. Households were asked whether the glass of drinking water provided by the respondent was from their main source (“Is this water from the main source of drinking water used by members of your household?”). In all countries, the main source of drinking water and the source of the water quality sample (where this differed) were recorded. Overall, 93% of samples were from the same category of water source, but concordance was lower for Kiribati (73%) and Chad (79%) (Table S3). Multiple source use has previously been documented (Daly et al. 2021), including in the Pacific (Elliott et al. 2017), and may be the reason for these differences.
Calculation of SMDW Services
SDMW services are defined as improved sources of drinking water, accessible on premises, available when needed, and free from fecal and priority chemical contamination (United Nations 2017). For the countries with information on accessibility, availability, and quality () we estimated the proportion of the national population using improved drinking water sources (MDG indicator) and SMDW services (SDG indicator) taking into consideration all three criteria. For the remaining countries (, Congo and Côte d’Ivoire) all improved water sources meeting the quality and accessibility criteria were assumed to be available when needed.
We calculated estimates for SMDW services based on information from individual households (household-level) and also using the approach taken by the JMP whereby a minimum of the three criteria for whether drinking water services are safely managed is used at the domain-level. For global monitoring the JMP uses the domain-level approach to accommodate estimates drawn from different data sources because relatively few countries have all of the information required to calculate the new SMDW indicator from a single data source (WHO/UNICEF 2018b). The JMP calculates SMDW as the minimum of improved and accessible, improved and available, and improved and free from E. coli contamination. When information on all of the criteria for SMDW are available from a single data source, as is the case in the MICS, they can be combined at the household level, which produces substantially lower estimates.
Data Analysis
Descriptive Analysis
Data were downloaded from the MICS website (https://mics.unicef.org/surveys). Data analysis was conducted using Stata (release 16; StataCorp). For each survey, descriptive statistics and 95% CIs were calculated using appropriate sample weights and taking into account the stratification and clustering using the svy command. Separate sample weights were calculated for the source and household water quality samples because of the different subsamples used and the response rates. Normalized sample weights for each country and indicator were used to calculate pooled mean estimates. Information on drinking water quality was combined with data from the household questionnaire (type of water source, household assets) to examine patterns in water quality for different population subgroups and to calculate the proportion of the population using SMDW services. To examine differences in water quality between the PoC and the PoU, we calculated the difference in E. coli risk level for households with samples at both locations to determine whether the risk level was lower, higher, or the same between the PoC and the PoU. This analysis was repeated for the subset of households with E. coli detected at the PoC and self-reporting appropriate household water treatment practices (i.e., boiling, filtration, adding chlorine, and solar disinfection). As a measure of absolute inequalities, we calculated risk differences (RDs) in E. coli contamination between the richest and poorest quintiles at the PoC and the PoU.
Risk Factors for Contamination
To explore risk factors for fecal contamination of drinking water at the PoC and the PoU, we used bivariate and multivariable modified Poisson regression models, with the results reported as risk ratios (RRs) (Zou 2004). Modified Poisson regression is particularly suited to indicators with a high prevalence and has been used in recent studies of handwashing and the association between water and sanitation and trachoma (Garn et al. 2018; Wolf et al. 2019). The binary dependent variables in these models were the detection of any E. coli contamination () or very high E. coli contamination () at the PoC or the PoU. Pooled estimates were generated using a multilevel model with random intercepts for each country (Gelman and Hill 2007). Random intercepts for clusters were not included in order to improve convergence, and we used equiprobability weights for the regression analysis. Separate country-level models with cluster random intercepts were fit to examine their contributions to the pooled estimates and to calculate unadjusted and adjusted RRs for each country. Covariates were excluded from country-level models where there were households in a response category (e.g., for open defecation in Georgia).
To examine the relationships between the criteria for SMDW services and water quality, we modeled the presence of E. coli (any and very high) in PoC or PoU samples in relation to improved water source (yes/no), drinking water source accessible on premises (yes/no), and available when needed (yes/no; based on the standardized questions in MICS6 surveys). These models were adjusted for household-level covariates: rural or urban residence, wealth index quintiles (poorest 20% through richest 20%, as defined in MICS), improved sanitation (yes/no), shared sanitation (yes/no), education (head of household; none/primary vs. secondary or higher), female head of household (yes/no), livestock ownership (yes/no), and wet season (yes/no). In addition, we adjusted for cluster-level indicators of improved sanitation ( of households vs. ), and open defecation ( household in cluster or none). Models of contamination at the PoU were also adjusted for household-level natural flooring (earth, sand, or dung; yes/no), handwashing (observed facilities with soap and water; yes/no), water storage (storage in covered or uncovered containers vs. direct from source; yes/no), and appropriate water treatment practices (self-reported boiling, filtration, addition of chlorine/bleach, or solar disinfection; yes/no.). Wet seasons were defined based on a simplified version of the accumulation method (Wright et al. 2012). For each country, mean monthly precipitation data 2000–2020 were obtained from the Climatic Research Unit (Harris et al. 2020). We defined the wet season as consecutive months with the highest cumulative precipitation above the yearly average (Table S4). For Congo and Sao Tome and Principe, we identified two distinct wet seasons.
In a second set of models, we modeled fecal contamination in association with specific improved water sources (piped, packaged, boreholes/tube wells, rainwater collection, delivered water, protected wells and springs), using unimproved sources as a common reference group instead of modeling a dichotomous indicator for water from any improved source (yes/no). These models also included indicators for water accessibility (yes/no) and availability (yes/no), as well as the covariates listed above.
Sensitivity Analysis
We conducted sensitivity analyses to assess the robustness of the pooled regression models to alternative specifications. First, we examined the impact of excluding MICS6 surveys with the highest proportion of positive blank tests (Chad, Gambia) and excluding clusters with positive blank test results from the regression models. Second, we constructed custom wealth index quintiles, excluding WASH variables from the principal component analysis to account for potential tautology between the definition of wealth and other predictors of water quality (Martel 2017). The custom wealth quintiles were prepared in SPSS by removing all WASH assets from existing MICS wealth quintile scripts. It was hypothesized that inclusion of WASH variables in the definition of wealth might influence the association between contamination and wealth. Third, we restricted the analysis at the PoU to samples that were contaminated at the PoC in order to assess whether self-reported household water treatment was associated with reduced risk of contamination at the PoU for the subset of households where drinking water was contaminated at the PoC. Last, we reran the pooled regression models with weights based on the inverse of the sample size for each country to assess whether giving equal weight to each country would impact the pooled estimates.
Results
Survey Characteristics
The total number of households interviewed ranged from 2,498 (Tonga) to 61,242 (Bangladesh), with a total sample size of households (Table 1). Most households selected for the water quality module provided a sample of drinking water at the PoU (; range: 86–100%) and at the PoC (; range: 60–100%). The main reasons 5% of households did not show the interviewer their source of drinking water were that the source was too far (37%), not functional (22%), and not accessible (20%) (Table S5). The number of water quality samples collected per country ranged from 571 to 6,724 at the PoU ( in total) and 342 to 6,687 at PoC ( in total) (Table 1).
The proportion of the population using improved drinking water sources (the MDG indicator for drinking water) ranged from 43% in Madagascar to in 10 countries (Excel Table S1). Drinking water was accessible on premises for of the population in 12 countries and for in Paraguay, Suriname, and Tonga. Availability of drinking water when needed ranged from 61% in Central African Republic (CAR) and 68% in Kiribati to in Bangladesh and Lao People’s Democratic Republic (Lao PDR). The main reasons given for water being unavailable in the past month (18%) were that the water was not available from the source (75%) and that the source was not accessible (10%) (Table S6). In 16 countries, of the population self-reported appropriate treatment practices, but self-reported treatment practices were common in Kiribati (88%) and Mongolia (83%) (Excel Table S1). Where respondents were observed filling a glass of drinking water, this was often from a storage container ( in 17 of 24 countries), but in 5 countries (Georgia, Iraq, Palestine, Tunisia, and Suriname) obtained water directly from the source.
Use of improved sanitation facilities ranged from 16% in Chad and 22% in CAR to of the population in Algeria, Palestine, Tonga, and Tunisia (Excel Table S1). The proportion of the population with handwashing facilities with soap and water ranged from 12% in Nigeria to 97% in Iraq and was in 12 countries. The proportion of household heads having a secondary education or higher ranged from 13% in CAR to 90% in Georgia. Livestock ownership ranged from 10% in Algeria to 89% in Kiribati. Natural flooring ranged from in 6 countries to 61% in Bangladesh. Surveys were conducted primarily or exclusively in the dry season (overall, 8.1% of samples were conducted in the wet season).
Water Quality Results at the National Level
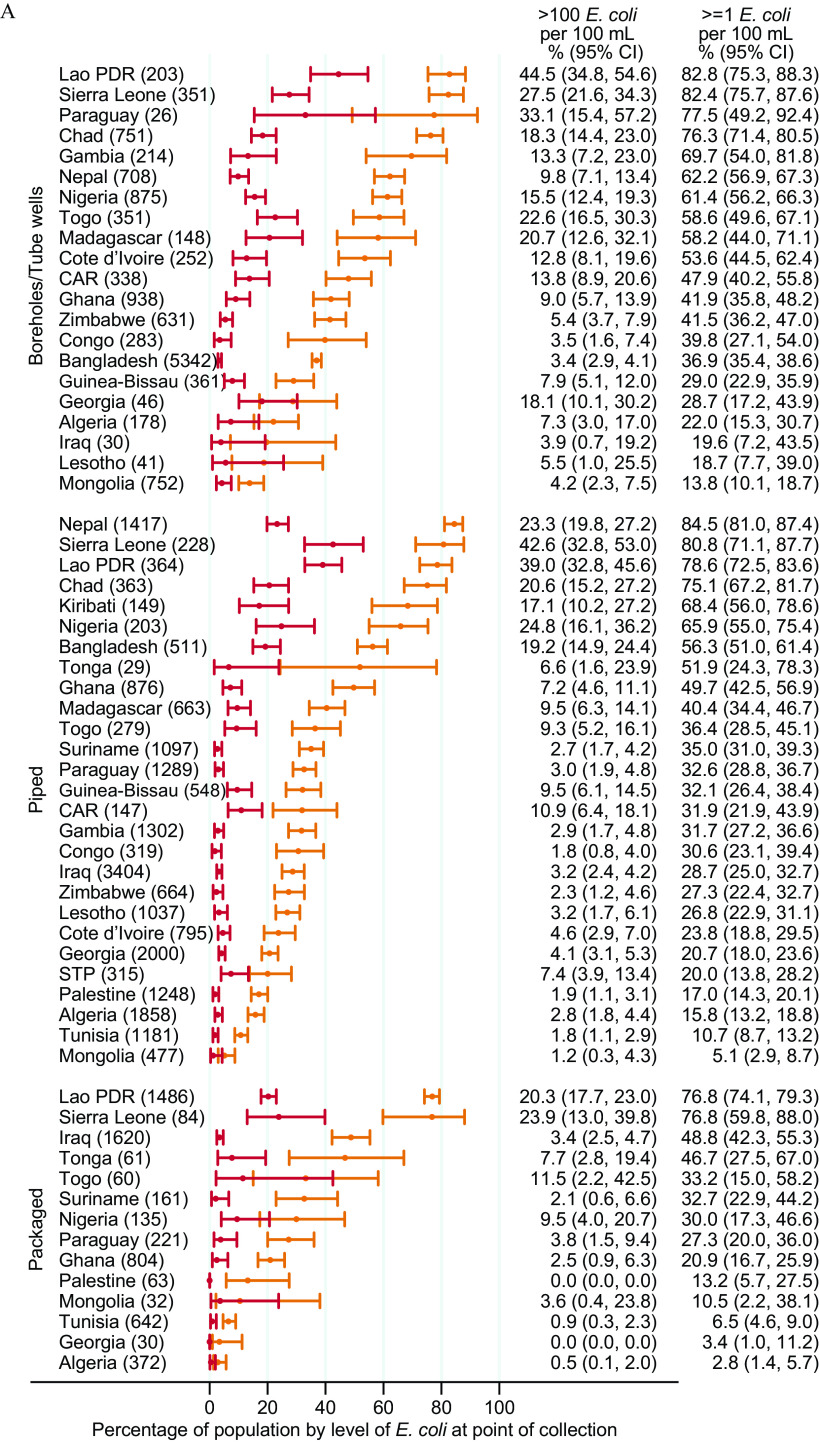
The proportion of the population using a drinking water source (either improved or unimproved) with detectable E. coli ( E. coli CFUs/) ranged from to 16% (95% CI: 13, 18) in Mongolia and 16% (95% CI: 14, 19) in Algeria to 86% (95% CI: 84, 88) in Chad and 90% (95% CI: 87, 92) in Sierra Leone (Figure 2). More than one-third of the population used very high risk ( E. coli CFUs/) drinking water sources ( E. coli CFUs/) in CAR, Chad, Côte d’Ivoire, Kiribati, Lao PDR, Madagascar, Nigeria, Sierra Leone, and Togo.
Figure 2.
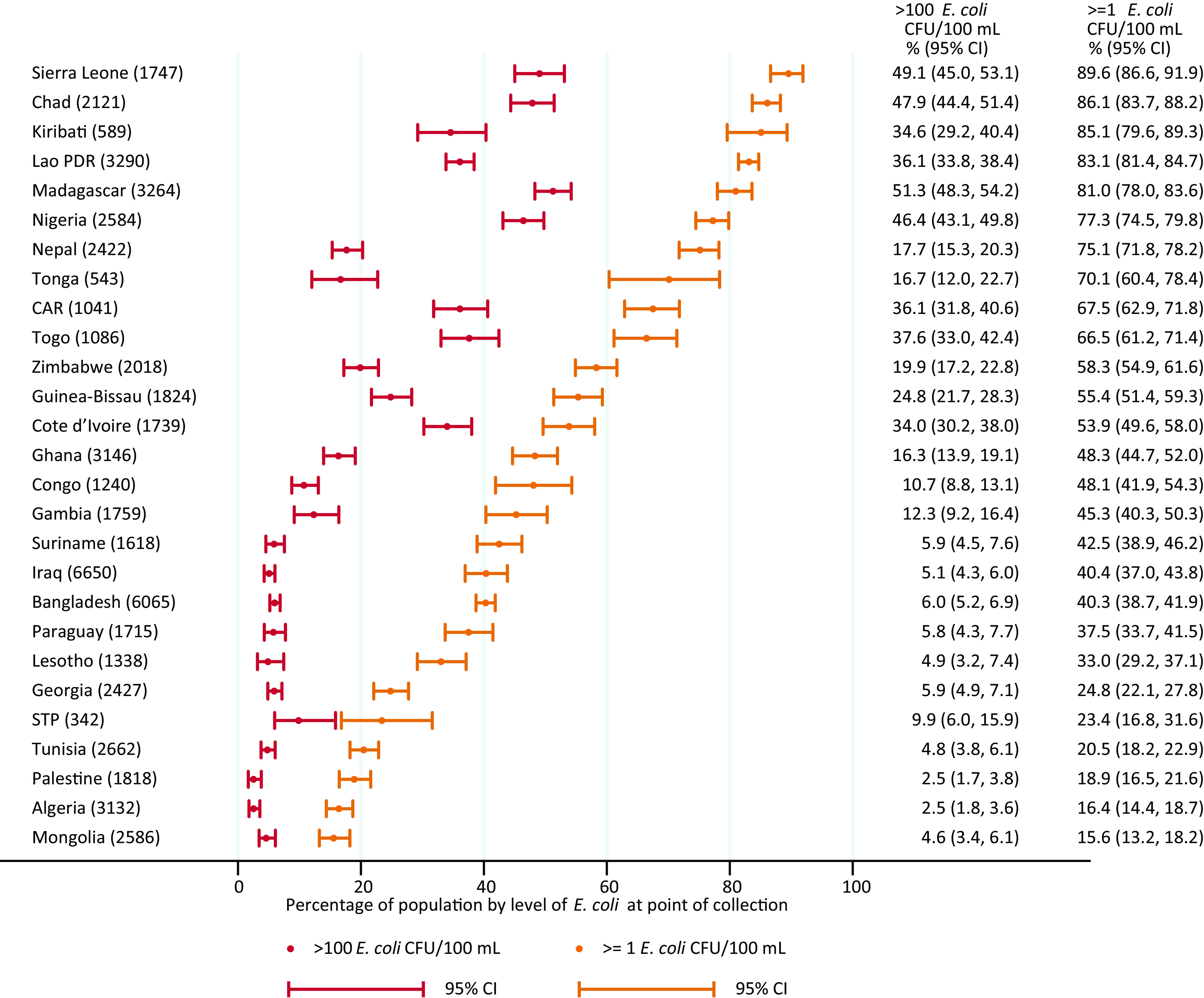
Proportion of population by level of E. coli in drinking water at point of collection in 27 low- and middle-income countries, 2014–2020. Corresponding numeric data are provided in Excel Table S2. Note: CAR, Central African Republic; CFU, colony forming unit; E. coli, Escherichia coli; Lao PDR, Lao People’s Democratic Republic; STP, Sao Tome and Principe.
The proportion of the population consuming drinking water with detectable E. coli at the PoU ranged from 19% (95% CI: 17, 22) in Mongolia to 99% (95% CI: 99, 100) in Chad. Over half the population were exposed to very high risk drinking water at the PoU in Chad, Madagascar, Nigeria, Sierra Leone, and Togo (Figure 3).
Figure 3.
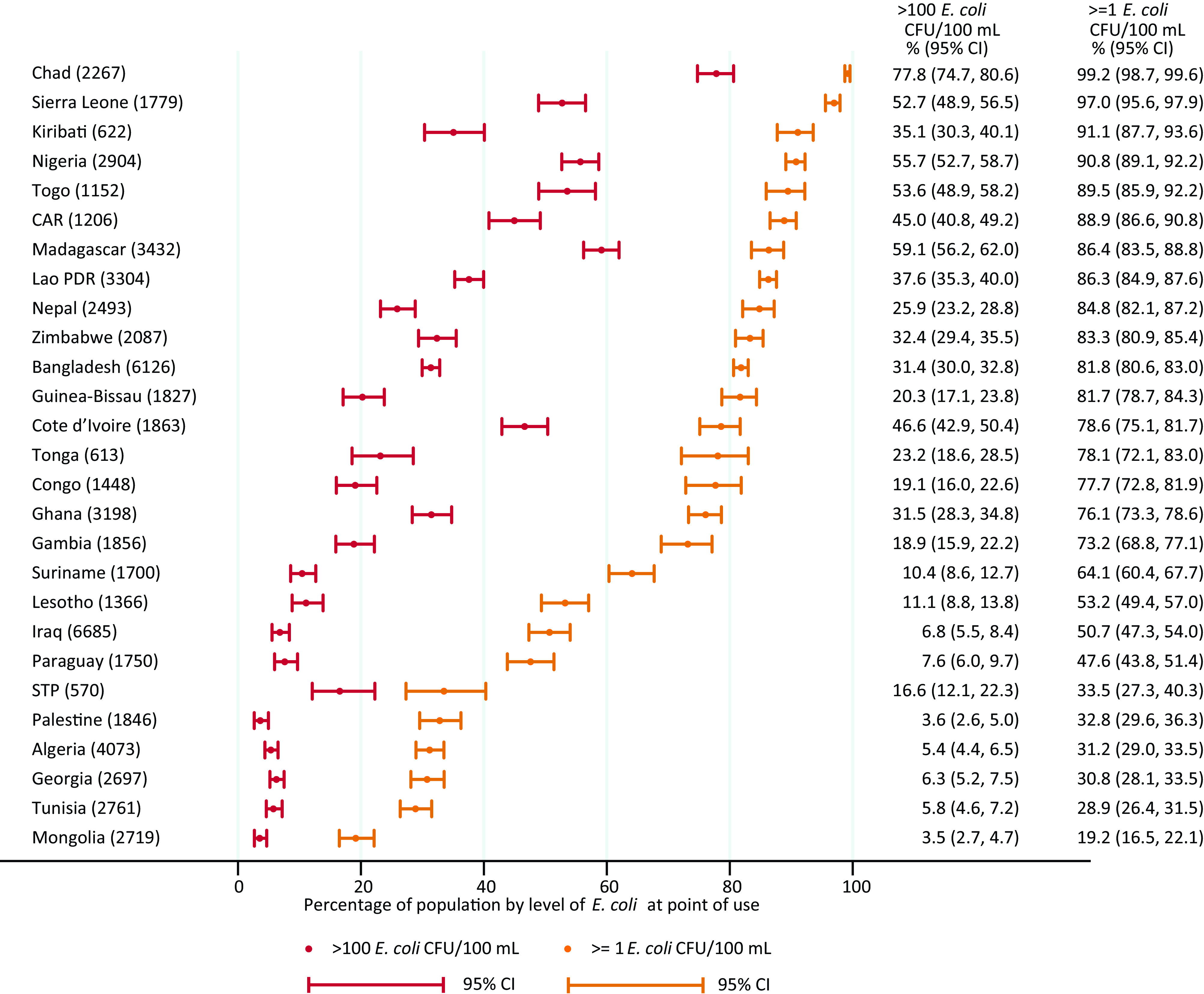
Proportion of population by level of E. coli in drinking water at point of use in 27 low- and middle-income countries, 2014–2020. Corresponding numeric data are provided in Excel Table S2. Note: CAR, Central African Republic; CFU, colony forming unit; E. coli, Escherichia coli; Lao PDR, Lao People’s Democratic Republic; STP, Sao Tome and Principe.
Differences in Water Quality between the PoC and the PoU
For the households with paired samples at the PoC and the PoU, it was possible to assess differences in risk level between the two sampling locations. A similar pattern was seen across the countries: The risk level was more often higher (10–66% of households) than lower (3–20% households) at the PoU; however, for most households, there was no difference in the risk level between the paired PoC and PoU samples (Figure S2).
For the majority of households with paired samples that used a contaminated drinking water source and self-reported appropriate water treatment practices, the risk level was the same or higher at the PoU than the PoC, except in Mongolia, where 72% of samples had a lower risk level at the PoU (Figure S3). For these households, E. coli was often detected at the PoU ( in 14 of 25 countries) and often very high risk ( in 15 of 25 countries) (Figure S4).
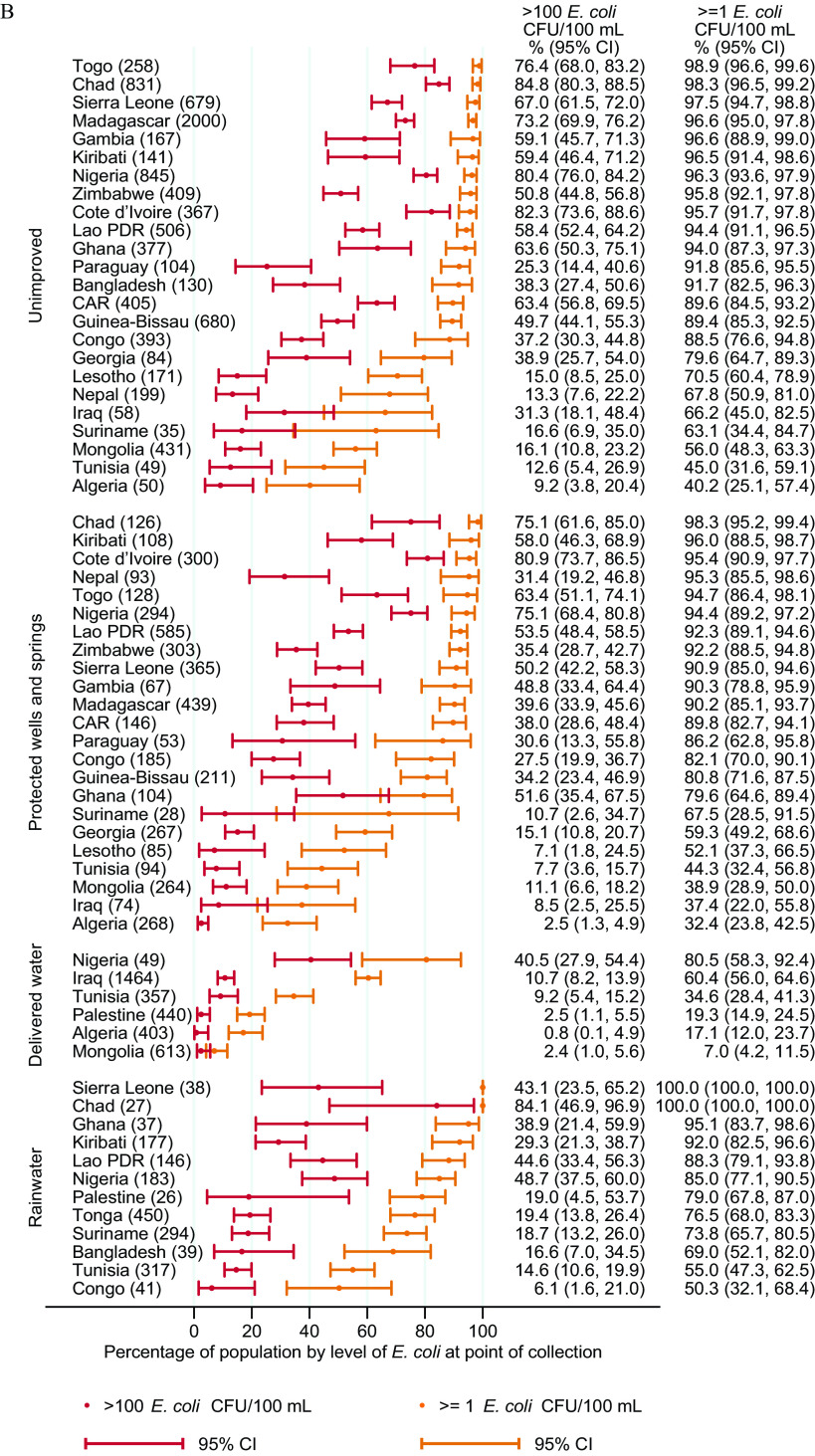
Water Quality by Water Source Type
E. coli contamination of PoC samples varied between water source types and between countries, but contamination in all source types ranged from low ( E. coli ) to very high ( E. coli ) (Figure 4; Excel Table S2). The general pattern suggests that piped water was often the most likely be free from E. coli contamination, followed by water from boreholes/tube wells then by packaged and delivered water. The quality of piped water varied considerably between countries, with of the population using piped water that exceeded 100 E. coli CFUs/ in Chad, Lao PDR, Nepal, Nigeria, and Sierra Leone. In Bangladesh, boreholes provided considerably higher quality water than piped water supplies (63% vs. 44% free from E. coli). Rainwater and protected wells and springs consistently had significant levels of contamination but appeared to be less frequently contaminated than unimproved sources. In countries with samples for delivered water, it was usually free from E. coli in Algeria, Mongolia, Palestine, and Tunisia, but this was not the case in Iraq and Nigeria. Patterns at the PoU were similar, but risk levels were often higher (Excel Table S2).
Figure 4.
E. coli contamination of drinking water at point of use, by type of water source in 27 low- and middle-income countries, 2014–2020 for source types with at least 25 samples: (A) packaged, piped, and boreholes/tube wells; and (B) rainwater, delivered water, protected wells and springs, and unimproved water sources. Numbers in parentheses are the unweighted number of water sources tested for E. coli at point of collection. Corresponding numeric data are provided in Excel Table S2. Note: CAR, Central African Republic; CFU, colony forming unit; E. coli, Escherichia coli; Lao PDR, Lao People’s Democratic Republic; STP, Sao Tome and Principe.
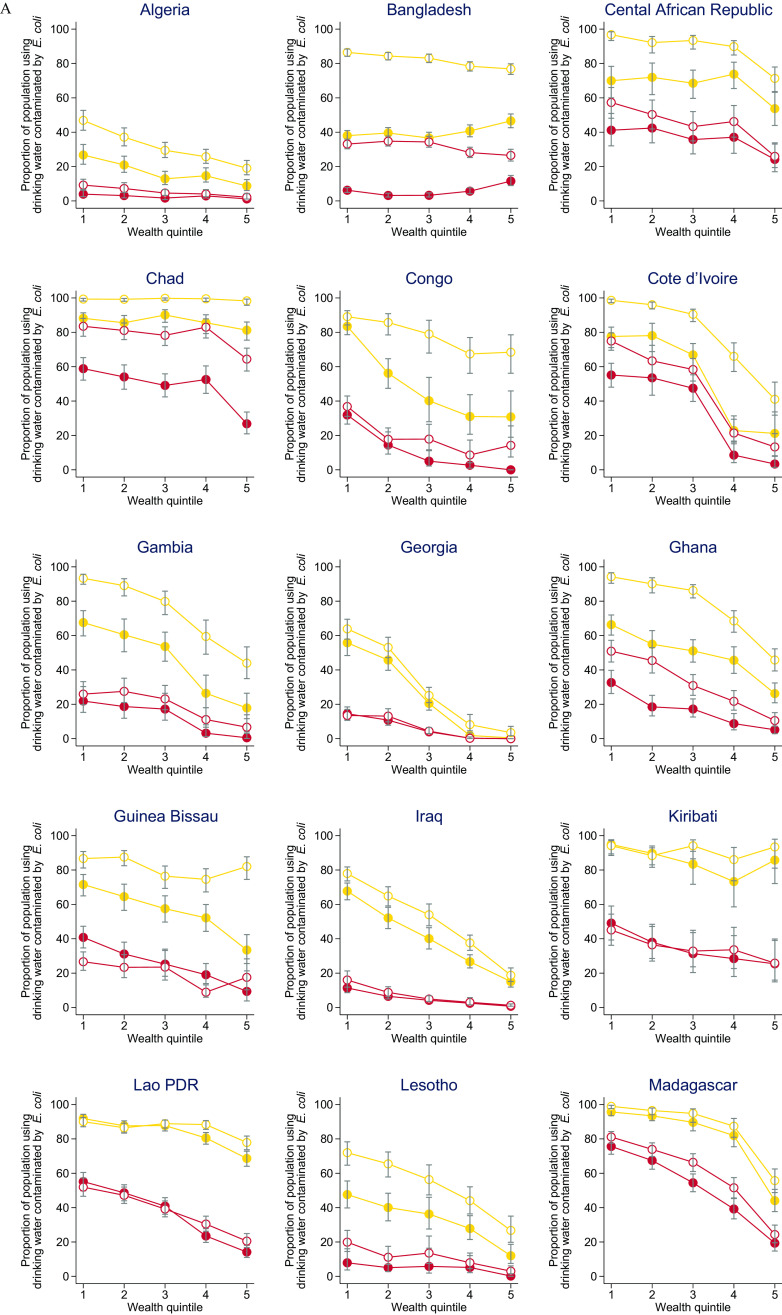
Inequalities in Water Quality by Wealth Quintile
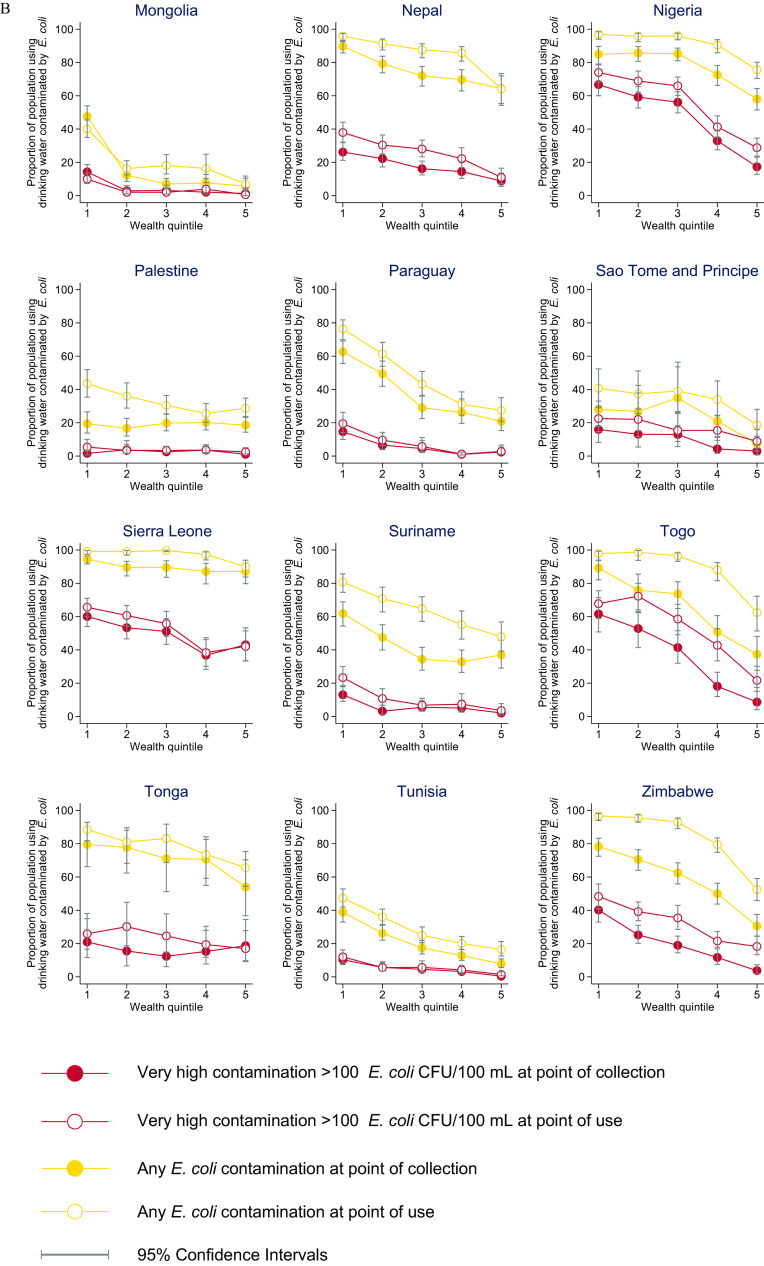
In all countries except Bangladesh, Kiribati, and Palestine, households in the poorest quintile were significantly more likely to use sources with E. coli at the PoC than households in the richest quintile (Figure 5; Excel Table S3). In Bangladesh, households in the poorest quintile were less likely than those in the richest quintile to use a contaminated drinking water source (; 95% CI: , ) but more likely at the PoU (; 95% CI: 5.9, 13.4). Differences in proportion of the population with E. coli at the PoC between the richest and poorest quintiles exceeded 25% points in 17 countries (16 at the PoU) and 50% points in 7 countries (3 at the PoU). For example, in Georgia, 0.5% (95% CI: 1.7, 7.2) of PoC samples were contaminated among households in the richest quintile compared with 55.8% (95% CI: 58.0, 69.5) of samples among those in the poorest quintile.
Figure 5.
E. coli contamination of drinking water at point of collection and point of use by wealth quintile in 27 low- and middle-income countries, 2014–2020: (A) Algeria to Madagascar, and (B) Mongolia to Zimbabwe. Wealth quintiles from 1 (poorest) through 5 (richest). Wealth quintiles reflect a relative measure of inequality within each country based on asset ownership. Corresponding numeric data are provided in Excel Table S3. Note: CFU, colony forming unit; E. coli, Escherichia coli; Lao PDR, Lao People’s Democratic Republic.
Across all wealth quintiles, the proportion of samples with very high E. coli contamination was lower in PoC than PoU samples (Figure 5; Excel Table S3). In countries with lower national contamination levels (e.g., Palestine), absolute inequalities were generally larger for any contamination than for very high risk drinking water. In countries with higher national contamination levels (e.g., Chad), absolute inequalities were generally larger for very high risk drinking water than for any contamination.
Safely Managed Drinking Water Services
In 15 of 27 countries, the quality criterion for SMDW services was the least likely to be met, and in most other countries, accessibility on premises was the limiting factor (Table 2). The adjustment for accessibility, availability, and quality resulted in a substantial reduction in all countries, including countries with a higher coverage of improved drinking water sources.
Table 2.
Proportion of population using improved and safely managed drinking water services in 27 Multiple Indicator Cluster Surveys (MICS) in low- and middle-income countries.
| Country | Improved | Improved and accessible on premises | Improved and available when needed | Improved and free from E. coli contamination | Safely managed calculated at domain levela | Safely managed calculated at household levelb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (95% CI) | Percentage (95% CI) | Percentage (95% CI) | Percentage (95% CI) | Percentage (95% CI) | Percentage (95% CI) | |||||||
| Algeria | 99.1 (98.9, 99.3) | 29,919 | 75.3 (73.3, 77.3) | 29,893 | 72.5 (70.8, 74.1) | 29,882 | 82.7 (80.5, 85) | 3,132 | 72.5 (70.8, 74.1) | 3,132 | 53.8 (50.8, 56.7) | 3,129 |
| Bangladesh | 98.5 (98.2, 98.8) | 61,242 | 82.5 (81.8, 83.1) | 61,241 | 95.6 (95.2, 95.9) | 61,230 | 59.6 (58.0, 61.1) | 6,065 | 59.6 (58.0, 61.1) | 6,065 | 49.1 (47.5, 50.6) | 6,065 |
| Central African Republic | 58.7 (55.3, 62.1) | 8,133 | 6.9 (5.8, 8.0) | 8,132 | 35.7 (33.2, 38.3) | 8,131 | 28.2 (23.9, 32.5) | 1,041 | 6.9 (5.8, 8.0) | 1,041 | 1.8 (1.0, 2.6) | 1,040 |
| Chad | 61.8 (58.7, 64.9) | 18,967 | 9.6 (8.2, 11.0) | 18,949 | 49.2 (46.5, 52.0) | 18,964 | 13.2 (10.9, 15.5) | 2,121 | 9.6 (8.2, 11.0) | 2,121 | 1.6 (0.8, 2.3) | 2,116 |
| Congo | 84.6 (82.3, 86.9) | 12,811 | 55.9 (51.2, 60.5) | 12,804 | NA | 0 | 50.3 (43.7, 56.9) | 1,240 | 50.3 (43.7, 56.9) | 1,240 | 32.4 (26.1, 38.6) | 1,240 |
| Côte d’Ivoire | 80.9 (78.3, 83.6) | 11,879 | 53.1 (49.6, 56.6) | 11,877 | NA | 0 | 45.3 (40.8, 49.9) | 1,739 | 45.3 (40.8, 49.9) | 1,739 | 34.2 (29.7, 38.8) | 1,739 |
| Gambia | 90.4 (88.0, 92.9) | 7,405 | 45.2 (40.8, 49.6) | 7,404 | 78.7 (76.1, 81.3) | 7,402 | 54.1 (48.8, 59.4) | 1,759 | 45.2 (40.8, 49.6) | 1,759 | 27.7 (23.4, 32.0) | 1,759 |
| Georgia | 97.5 (97.0, 98.0) | 12,270 | 91.6 (90.4, 92.8) | 12,265 | 75.6 (73.5, 77.7) | 12,240 | 74.6 (71.5, 77.7) | 2,427 | 74.6 (71.5, 77.7) | 2,427 | 56.0 (52.1, 59.9) | 2,426 |
| Ghana | 86.0 (83.0, 88.9) | 12,886 | 24.5 (22.4, 26.7) | 12,884 | 75.8 (73.0, 78.6) | 12,885 | 50.9 (47.0, 54.7) | 3,146 | 24.5 (22.4, 26.7) | 3,146 | 15.7 (13.4, 18.0) | 3,146 |
| Guinea-Bissau | 66.8 (63.4, 70.2) | 7,379 | 26.8 (22.9, 30.8) | 7,379 | 54.7 (51.7, 57.7) | 7,379 | 41.1 (36.9, 45.3) | 1,824 | 26.8 (22.9, 30.8) | 1,824 | 11.3 (8.3, 14.3) | 1,824 |
| Iraq | 86.4 (83.5, 89.4) | 20,214 | 65.7 (62.5, 68.9) | 20,210 | 65.9 (63.0, 68.7) | 20,190 | 54.7 (51.0, 58.3) | 6,650 | 54.7 (51, 58.3) | 6,650 | 35.5 (31.9, 39.1) | 6,634 |
| Kiribati | 82.1 (78.8, 85.4) | 3,071 | 59.9 (55.8, 64.0) | 3,071 | 51.6 (48.7, 54.6) | 3,070 | 14.3 (9.4, 19.1) | 589 | 14.3 (9.4, 19.1) | 589 | 4.0 (1.9, 6.1) | 589 |
| Lao People’s Democratic Republic | 83.9 (82.1, 85.7) | 22,287 | 77.5 (75.5, 79.4) | 22,287 | 80.9 (79.1, 82.7) | 22,282 | 16.0 (14.3, 17.7) | 3,290 | 16.0 (14.3, 17.7) | 3,290 | 15.1 (13.5, 16.7) | 3,290 |
| Lesotho | 88.9 (86.6, 91.1) | 8,847 | 34.0 (30.5, 37.5) | 8,846 | 74.3 (71.8, 76.8) | 8,844 | 63.6 (59.3, 67.9) | 1,338 | 34.0 (30.5, 37.5) | 1,338 | 24.5 (20.7, 28.2) | 1,338 |
| Madagascar | 43.0 (40.1, 45.9) | 17,870 | 15.4 (13.7, 17.1) | 17,869 | 35.8 (33.3, 38.3) | 17,866 | 17.1 (14.3, 20.0) | 3,264 | 15.4 (13.7, 17.1) | 3,264 | 5.7 (4.4, 7.0) | 3,264 |
| Mongolia | 88.2 (86.0, 90.4) | 13,798 | 33.8 (28.5, 39.1) | 13,787 | 74.5 (71.9, 77.2) | 13,793 | 79.4 (76.0, 82.7) | 2,586 | 33.8 (28.5, 39.1) | 2,586 | 22.7 (18.7, 26.7) | 2,581 |
| Nepal | 90.8 (89.1, 92.6) | 12,655 | 72.9 (70.3, 75.4) | 12,654 | 74.6 (72.1, 77.1) | 12,654 | 21.6 (18.8, 24.3) | 2,422 | 21.6 (18.8, 24.3) | 2,422 | 17.5 (14.9, 20.1) | 2,422 |
| Nigeria | 69.0 (67.0, 71.0) | 33,901 | 23.1 (21.8, 24.4) | 33,886 | 56.8 (54.9, 58.7) | 32,135 | 21.5 (18.8, 24.2) | 2,584 | 21.5 (18.8, 24.2) | 2,584 | 3.8 (2.8, 4.9) | 2,482 |
| Palestine | 99.1 (98.8, 99.3) | 9,326 | 92.5 (91, 93.9) | 9,322 | 88.4 (87.1, 89.6) | 9,319 | 80.1 (77.3, 82.9) | 1,818 | 80.1 (77.3, 82.9) | 1,818 | 66.8 (63.5, 70.2) | 1,813 |
| Paraguay | 95.4 (94.2, 96.5) | 7,313 | 94.1 (92.8, 95.4) | 7,312 | 78.9 (76.7, 81.2) | 7,313 | 62.1 (58.1, 66.2) | 1,715 | 62.1 (58.1, 66.2) | 1,715 | 53.0 (49.1, 57) | 1,715 |
| Sao Tome and Principe | 97.5 (96.4, 98.6) | 3,426 | 57.5 (52.9, 62.0) | 3,426 | 68.3 (64.9, 71.6) | 3,426 | 76.3 (68.2, 84.5) | 342 | 57.5 (52.9, 62.0) | 342 | 37.5 (28.5, 46.5) | 342 |
| Sierra Leone | 67.8 (65.3, 70.2) | 15,309 | 14.5 (13.2, 15.8) | 15,306 | 47.4 (45.2, 49.6) | 15,306 | 9.6 (7.2, 12.0) | 1,747 | 9.6 (7.2, 12.0) | 1,747 | 1.4 (0.7, 2.1) | 1,747 |
| Suriname | 98.2 (97.6, 98.7) | 7,915 | 96.7 (95.8, 97.6) | 7,909 | 82.1 (80.3, 83.9) | 7,904 | 57 (53.3, 60.7) | 1,618 | 57 (53.3, 60.7) | 1,618 | 48.3 (44.6, 52.0) | 1,617 |
| Togo | 74.6 (70.9, 78.3) | 7,916 | 17.3 (15.2, 19.4) | 7,916 | 62.4 (59.1, 65.8) | 7,916 | 33.2 (28.0, 38.5) | 1,086 | 17.3 (15.2, 19.4) | 1,086 | 6.2 (4.0, 8.5) | 1,086 |
| Tonga | 99.2 (98.7, 99.8) | 2,498 | 98.6 (97.9, 99.3) | 2,497 | 90.5 (88.8, 92.2) | 2,498 | 29.8 (20.7, 38.9) | 543 | 29.8 (20.7, 38.9) | 543 | 26.0 (17.6, 34.5) | 543 |
| Tunisia | 98.0 (97.4, 98.6) | 11,225 | 89.8 (88.4, 91.2) | 11,223 | 79.6 (77.8, 81.3) | 11,223 | 78.5 (76.2, 80.9) | 2,662 | 78.5 (76.2, 80.9) | 2,662 | 58.8 (56, 61.6) | 2,662 |
| Zimbabwe | 77.1 (74.3, 79.9) | 11,091 | 29.3 (26.4, 32.2) | 11,091 | 61.5 (58.9, 64.1) | 11,091 | 40.1 (36.5, 43.7) | 2,018 | 29.3 (26.4, 32.2) | 2,018 | 10.1 (7.8, 12.5) | 2,018 |
| Meanc | 84.3 (83.8, 84.8) | 391,553 | 51.1 (50.3, 51.8) | 391,440 | 68.7 (68.2, 69.3) | 364,943 | 46.7 (45.8, 47.7) | 60,766 | 46.7 (45.8, 47.7) | 60,766 | 30.5 (29.3, 31.6) | 60,627 |
Note: CI, confidence interval; E. coli, Escherichia coli; JMP, Joint Monitoring Program; NA: availability questions not included in Congo and Côte d’Ivoire; UNICEF, United Nations Children’s Fund; SMDWs, safely managed drinking water services; WHO, World Health Organization.
Domain-level calculation of SMDWs based on the minimum of the safely managed criteria (improved and accessible, improved and available, and improved and free from E. coli contamination) assessed at the national level. The domain-level assessment is used by the WHO/UNICEF JMP when data must be combined from different sources to estimate SMDWs either at the national level or separately for urban and rural areas.
Household-level calculation of SMDWs is based on the individual household responses. Only households that meet all criteria are considered to use SMDWs. The household-level calculation is possible in MICS because the surveys collect information on the accessibility, availability, and quality of drinking water for the same households.
Mean values based on equality weighting for each country.
On average, 84.3% (95% CI: 83.8, 84.8) of households used an improved drinking water source, and 30.5% (95% CI: 29.3, 31.6) met all of the SMDW criteria (household-level SMDW). SMDW calculated at the domain level was higher than calculated at the household level.
Risk Factors for E. coli Contamination
Regression analyses were restricted to MICS6 surveys with standardized questions on availability of drinking water. Unadjusted (bivariate) regression analyses of E. coli contamination (any or very high, in PoC and PoU samples) showed that the three other SMDW criteria (improved, accessible, and available drinking water) and every covariate included in the fully adjusted multivariable models was a significant predictor of contamination in at least one country (Excel Table S4). In the pooled unadjusted estimates, E. coli at the PoC was strongly associated with improved water (; 95% CI: 0.53, 0.74), and accessibility (; 95% CI: 0.72, 0.90) but not availability (; 95% CI: 0.87, 1.04).
Fully adjusted pooled estimates of associations between the individual SMDW criteria and fecal contamination indicated that the prevalence of any E. coli contamination in PoC samples was significantly lower in association with improved water sources (; 95% CI: 0.64, 0.85), whereas the associations with accessibility (; 95% CI: 0.94, 1.05) and availability (; 95% CI: 0.88, 1.02) were close to the null and not significant (Table 3). Results for any contamination in PoU samples indicated an association with accessibility (; 95% CI: 0.90, 0.99), but associations with improved water (; 95% CI: 0.92, 1.01) and availability (; 95% CI: 0.91, 1.01) were close to null and not significant. Corresponding associations with the prevalence of very high contamination ( E. coli ) in PoC and PoU samples were stronger for improved water sources [ (95% CI: 0.33, 0.56) and (95% CI: 0.66, 0.83) for the PoC and the PoU, respectively], but similar to associations with any contamination for accessibility and availability. All country-specific RRs for any contamination of PoC and PoU samples were for improved water (except for PoC samples in Nepal and PoU samples in Mongolia and Nepal, all of which were nonsignificant) (Excel Tables S5 and S6). Country-specific estimates for accessibility and availability were heterogeneous, ranging from (95% CI: 0.42, 1.01) for Lesotho to (95% CI: 0.82, 2.10) for Tonga and (95% CI: 0.50, 0.74) for Algeria to (95% CI: 0.96, 1.90) for Sao Tome and Principe, at PoC. Country-specific estimates for very high contamination generally had lower RRs for improved water but were similar for accessibility and availability (Excel Tables S7 and S8).
Table 3.
Adjusted RRs for E. coli contamination at the PoC and the PoU in 23 MICS6 in low- and middle-income countries (improved water sources only).
| Variable | PoC E. coli CFUs/ | PoC E. coli CFUs/ | PoU E. coli CFUs/ | PoU E. coli CFUs/ |
|---|---|---|---|---|
| Improved | 0.74 (0.64, 0.85) | 0.43 (0.33, 0.56) | 0.96 (0.92, 1.01) | 0.74 (0.66, 0.83) |
| Accessible | 0.99 (0.94, 1.05) | 1.08 (0.96, 1.21) | 0.94 (0.90, 0.99) | 0.96 (0.88, 1.06) |
| Available | 0.95 (0.88, 1.02) | 0.95 (0.88, 1.02) | 0.96 (0.91, 1.01) | 0.94 (0.89, 0.99) |
| Observations | 53,224 | 53,224 | 54,063 | 54,063 |
Note: Data are shown as exponentiated coefficients from modified Poisson regression with random intercepts for each country and 95% confidence intervals. Regression models were based on MICS6 and are unweighted. Adjusted for PoC and PoU wealth quintile, education of household head, sex of household head, rural residence, improved sanitation, cluster improved sanitation , open defecation in cluster, livestock ownership, and season (PoU only) handwashing, water storage, household water treatment, natural flooring. Covariates and country-specific models are included in Excel Tables S5 and S7. CFU, colony forming unit; E. coli, Escherichia coli; MICS6, sixth round of Multiple Indicator Cluster Surveys; PoC, point of collection; PoU, point of use; RR, risk ratio.
Compared with unimproved water sources, pooled estimates suggest that for PoC samples, piped water (; 95% CI: 0.55, 0.76) and boreholes/tube wells (; 95% CI: 0.48, 0.72) were associated with a significantly lower prevalence of any E. coli contamination (Table 4). There was no clear difference in any contamination at the PoC for rainwater, delivered water, and protected wells and springs compared with unimproved sources (Table 4). In PoU samples, only piped water was associated with a significantly lower prevalence of E. coli contamination compared with unimproved sources (; 95% CI: 0.82, 0.93). Compared with unimproved sources, packaged water, piped water, and boreholes were also associated with a significantly lower prevalence of very high risk drinking water ( E. coli CFUs/mL) for both PoC and PoU samples (Table 4).
Table 4.
Adjusted RRs for E. coli contamination at the PoC and PoU in 23 MICS6 in low- and middle-income countries (full model including water supply types).
| Variable | PoC E. coli CFUs/ | PoC E. coli CFUs/ | PoU E. coli CFUs/ | PoU E. coli CFUs/ |
|---|---|---|---|---|
| Main source of drinking water (Ref: unimproved) | ||||
| Packaged water | 0.74 (0.52, 1.04) | 0.34 (0.20, 0.58) | 0.89 (0.66, 1.21) | 0.55 (0.32, 0.93) |
| Piped water | 0.65 (0.55, 0.76) | 0.34 (0.23, 0.51) | 0.87 (0.82, 0.93) | 0.63 (0.53, 0.75) |
| Boreholes/tube wells | 0.59 (0.48, 0.72) | 0.24 (0.16, 0.34) | 0.95 (0.89, 1.01) | 0.69 (0.61, 0.79) |
| Rainwater | 1.13 (0.90, 1.42) | 0.77 (0.59, 1.01) | 1.14 (0.93, 1.39) | 0.97 (0.77, 1.21) |
| Delivered water | 0.87 (0.66, 1.16) | 0.46 (0.31, 0.69) | 1.16 (0.96, 1.40) | 0.91 (0.70, 1.18) |
| Protected wells and springs | 1.02 (0.95, 1.09) | 0.82 (0.75, 0.91) | 1.05 (1.00, 1.11) | 0.92 (0.84, 1.00) |
| Accessibility and availability | ||||
| Accessible | 0.98 (0.92, 1.04) | 1.02 (0.92, 1.13) | 0.95 (0.91, 1.00) | 0.96 (0.88, 1.04) |
| Available | 0.94 (0.86, 1.02) | 0.96 (0.89, 1.03) | 0.95 (0.89, 1.01) | 0.93 (0.88, 0.98) |
| Wealth quintile (Ref: poorest) | ||||
| Second | 0.94 (0.90, 0.99) | 0.91 (0.88, 0.95) | 0.93 (0.90, 0.97) | 0.91 (0.84, 0.97) |
| Middle | 0.89 (0.81, 0.99) | 0.87 (0.81, 0.95) | 0.89 (0.81, 0.97) | 0.86 (0.77, 0.97) |
| Fourth | 0.84 (0.73, 0.98) | 0.76 (0.68, 0.86) | 0.81 (0.71, 0.94) | 0.77 (0.66, 0.88) |
| Richest | 0.73 (0.56, 0.95) | 0.68 (0.52, 0.89) | 0.70 (0.55, 0.88) | 0.66 (0.53, 0.84) |
| Other household and cluster-level characteristics | ||||
| Education of household head | 0.97 (0.93, 1.01) | 0.94 (0.87, 1.01) | 0.98 (0.96, 1.00) | 0.93 (0.87, 0.99) |
| Sex of household head | 0.98 (0.95, 1.01) | 0.99 (0.93, 1.05) | 1.00 (0.99, 1.02) | 0.97 (0.93, 1.02) |
| Rural | 1.17 (1.09, 1.26) | 1.24 (1.07, 1.42) | 1.10 (1.04, 1.16) | 1.21 (1.10, 1.32) |
| Improved sanitation | 1.03 (0.97, 1.09) | 0.99 (0.93, 1.06) | 1.04 (1.00, 1.07) | 0.97 (0.93, 1.03) |
| Shared sanitation | 1.00 (0.97, 1.03) | 0.96 (0.91, 1.02) | 1.02 (0.99, 1.05) | 1.01 (0.97, 1.05) |
| cluster improved sanitation | 0.91 (0.85, 0.97) | 0.82 (0.73, 0.92) | 0.94 (0.90, 0.97) | 0.90 (0.83, 0.97) |
| Any open defecation in cluster | 0.95 (0.88, 1.03) | 1.04 (0.94, 1.15) | 0.98 (0.95, 1.02) | 1.01 (0.89, 1.14) |
| Livestock ownership | 1.10 (1.03, 1.18) | 1.15 (1.03, 1.28) | 1.08 (1.04, 1.13) | 1.15 (1.07, 1.24) |
| Wet season | 1.05 (0.96, 1.16) | 1.13 (1.01, 1.25) | 1.07 (1.01, 1.15) | 1.24 (1.01, 1.51) |
| Handwashing | — | — | 1.00 (0.97, 1.03) | 1.00 (0.96, 1.04) |
| Water storage | — | — | 0.91 (0.80, 1.04) | 0.89 (0.77, 1.03) |
| Household water treatment | — | — | 0.92 (0.83, 1.02) | 0.91 (0.81, 1.03) |
| Natural floor | — | — | 0.93 (0.87, 0.99) | 0.97 (0.91, 1.05) |
| Observations | 53,224 | 53,224 | 54,063 | 54,063 |
Note: Data are shown as exponentiated coefficients from modified Poisson regression with random intercepts for each country and 95% confidence intervals. Regression models were based on MICS6 and are unweighted. Country-specific models are included in Excel Tables S6 and S8. —, Not applicable; CFU, colony forming unit; E. coli, Escherichia coli; MICS6, sixth round of Multiple Indicator Cluster Surveys; PoC, point of collection; PoU, point of use; Ref, reference; RR, risk ratio.
Several covariates were also associated with the prevalence of E. coli contamination in PoC or PoU samples based on pooled estimates (Table 4). The prevalence of any contamination decreased as the wealth index increased, with (95% CI: 0.55, 0.88) for PoU contamination in the wealthiest quintile. Of the sanitation and hygiene indicators, only the cluster-level indicator for improved sanitation was a significant predictor (PoU ; 95% CI: 0.90, 0.97). Having water storage on-site and self-reported use of appropriate water treatment were also associated with a lower prevalence of contamination, although associations were not significant [PoU (95% CI: 0.80, 1.04) and (95% CI: 0.83, 1.02), respectively]. Rural vs. urban residence and livestock ownership were positively associated with contamination [PoU (95% CI: 1.04, 1.16) and (95% CI: 1.04, 1.13), respectively]. Samples collected in the wet season were also positively associated with contamination at the PoU (; 95% CI: 1.01, 1.15) but this was not significant at the PoC (; 95% CI: 0.96, 1.16). Associations between covariates and any contamination of PoC samples, and very high contamination of PoC and PoU samples, were generally consistent with patterns for any contamination of PoU samples. Country-specific models showed that all selected covariates were significantly associated with contamination at the PoC and the PoU in the fully adjusted models in at least one country (Excel Tables S9–S12). Self-reported household water treatment was significantly associated with a lower prevalence of any E. coli at the PoU in Lao PDR, Mongolia, Nepal, and Suriname, and Zimbabwe (Excel Table S11) and a lower prevalence of very high risk water in Lao PDR, Mongolia, and Sierra Leone (Excel Table S12).
Sensitivity Analysis
Excluding countries with the worst blank test results (Gambia, Chad) or clusters where blank tests were positive had a negligible impact on the RRs (Excel Table S13). Use of a custom wealth index excluding WASH variables marginally decreased the RRs for water supply types [e.g., (95% CI: 0.55, 0.76) to (95% CI: 0.54, 0.75) for piped water] and decreased the strength of the association with wealth [e.g., (95% CI: 0.56, 0.95) to (95% CI: 0.61, 0.96) for the richest quintile] for any E. coli at the PoC (Excel Table S14). Restricting the PoU model to samples contaminated at the PoC narrowed the CIs for the RR for self-reported water treatment, which was significant for this subset of households [from (95% CI: 0.83, 1.02) to (95% CI: 0.87, 0.97)] (Excel Table S15). Equal weighting for countries had a marginal impact on RRs; for example, decreasing the strength of the association with wealth [from (95% CI: 0.56, 0.95) to (95% CI: 0.70, 0.92) for the richest quintile] for any E. coli at the PoC (Excel Table S16).
Discussion
The new SDG indicator for drinking water “safely managed services” reflects consensus on the need to go beyond monitoring the types of water sources used by households and to monitor the level of service received. Integration of water testing in multitopic household surveys has demonstrated the feasibility of this approach for collecting national data on drinking water service levels and the ability to link them with information on the socioeconomic characteristics of the population. Integrating water testing into existing surveys provides a cost-effective approach compared with running a dedicated water quality survey.
Water Quality and Risk Factors for Contamination
The findings in the countries included in the analysis show that large proportions of the population are exposed to fecal contamination through their drinking water. This includes many people who are exposed to very high levels of contamination.
In line with a systematic review of the quality of water from different sources (Bain et al. 2014) and large-scale assessments of regulatory water quality data from sub-Saharan Africa (Kumpel et al. 2016), we find that piped water, packaged water, and boreholes are generally providing higher quality water than unimproved sources and that they offer substantial protection against very high risk drinking water. Rainwater harvesting, delivered water, and protected wells and springs often do not provide drinking water that is less likely to be contaminated than unimproved sources. Piped water was the only source type significantly associated with water free from E. coli contamination at the PoU in the adjusted models as found in a systematic review of PoC to PoU contamination (Shields et al. 2015). Unlike previous research on accessibility (Brown et al. 2013; Swerdlow et al. 1992) and availability (Jeandron et al. 2015; Kumpel and Nelson 2013), we did not find significant associations between E. coli and availability (at the PoC and the PoU) and accessibility (at the PoC) in the pooled fully adjusted models.
By combining data on water quality with information on the socioeconomic status of households, it was possible to explore patterns by wealth quintile. We find pronounced differences between rich and poor in almost all countries, as documented elsewhere (Graham et al. 2018; Yang et al. 2013). Differences were smaller in Bangladesh, where the richest were found to be more likely to use water from a contaminated source. This reflects the high degree of contamination of piped water supplies in Bangladesh disproportionately used by the wealthy, compared with the less contaminated boreholes used by most of the population.
The surveys also find substantial differences in quality between the PoC and the PoU (Shields et al. 2015; Wright et al. 2004). In many countries, households stored water in containers prior to consumption, but self-reported appropriate household water treatment practices were relatively uncommon except in Kiribati and Mongolia. Our analysis suggests that self-reported household water treatment may provide limited protection (Table 4; Excel Table S15), and that many households reporting these practices are still exposed to E. coli contamination at the PoU (Figure S4). These findings may be attributable to a lack of correct and consistent water treatment practices, ineffective treatment technologies, or self-reporting bias (which has been documented in other countries) (Pickering et al. 2010; Rosa et al. 2016; Rosa and Clasen 2017). We find that livestock ownership (Wardrop et al. 2018), rural residence (Kirby et al. 2016), and high community sanitation coverage (Harris et al. 2017) are significantly associated with contamination at the PoC and the PoU in the adjusted regression models.
We attempted to control for seasonality using a simplified definition of wet and dry seasons (Wright et al. 2012). Our finding that the wet season was associated with any E. coli contamination at the PoU in the fully adjusted models supports the findings of a systematic review (Kostyla et al. 2015) and more recent studies examining seasonality (Kumpel et al. 2017; Nguyen et al. 2021) or the impact of rainfall on water quality (Kirby et al. 2016). Integration of water quality testing in large-scale longitudinal surveys may offer insights into the dynamics of water quality at a national scale. The cross-sectional nature of the MICS water quality data, and the predominance of samples from the dry season may understate the importance of some risk factors. Geolocation of household survey clusters would also enable a wider range of environmental risk factors to be considered (Poulin et al. 2020).
The risk factors for contamination vary considerably between countries, and this heterogeneity is reflected in the range of effect sizes in the individual country models. Further work is required to understand the relationship between household- and community-level risk factors and the extent to which these can be mitigated through interventions to improve infrastructure and change behaviors. Future studies could examine changes in risk levels between the PoC and the PoU and examine alternative modeling approaches (Harris et al. 2019). Many potential risk factors for water quality were not considered in the regression analysis either because they are not collected in or could not be combined with MICS (e.g., high-resolution meteorological data, sanitary risk assessments) or because the number of missing values was large (e.g., child feces disposal practices). We chose not to include variables that might plausibly be considered outcomes of poor water quality (e.g., child anthropometry or diarrhea) and did not include country-level covariates (e.g., income classification). Our main regression results were not weighted to reflect population sizes, and the contribution of each survey to the pooled estimates reflects the varying number of samples taken in each country. The sample size, especially in Kiribati, Sao Tome and Principe, and Tonga, limits the precision of estimates for subpopulations and inference from country-specific regression models.
Monitoring SMDW Services
In addition to integrating the water quality module, questions that address the availability and accessibility of drinking water are required to monitor SMDW services. Questions on the location and time to collect drinking water have been included since the second round of MICS in 1999–2003 and form part of the JMP’s updated guidance on monitoring WASH in household surveys (WHO/UNICEF 2018a). New questions to assess the availability of drinking water services were asked in 25 of 27 countries to establish whether the population was lacking sufficient drinking water when needed. In MICS6 surveys with standardized questions on availability, the proportion of the population drinking water that was not accessible on premises or which was contaminated with E. coli was generally much higher than the proportion reporting lacking sufficient water in the past month. A notable exception was Algeria, where availability was the limiting factor for SMDW.
Our study documents substantial differences between the MDG and SDG indicators, underscoring the importance of accounting for accessibility, availability, and quality of drinking water. We also find considerable differences between SMDW calculated at the domain and household levels (Table 2). The domain-level approach is used by the JMP for SDG monitoring at the global level to accommodate information from multiple data sources (e.g., combining household surveys and administrative data). A key advantage of integrating water quality testing and new questions on availability of drinking water in household surveys is that all criteria for SMDW are available from the same data source. The overestimate of SMDW coverage using the domain-level approach will depend on the extent to which individual SMDW criteria are met for the same populations. The multivariable regression analysis suggests that sources meeting the criteria for accessibility and availability are not necessarily more likely to be free from E. coli contamination in LMICs.
We focused on fecal contamination as indicated by E. coli and did not consider contamination from priority chemical contaminants, including arsenic and fluoride, which are widespread in many LMICs (Amini et al. 2008; Podgorski and Berg 2020). In Bangladesh and Nepal, arsenic contamination was also measured in the MICS using field test kits. E. coli remained the limiting factor for SMDW in both countries, with arsenic exceeding the WHO guideline value of at the PoC for 18.6% and 2.8% of the population (vs. 40.3% and 75.3% with E. coli) in Bangladesh and Nepal, respectively.
Water quality testing in household surveys should complement, not compete with, ongoing efforts to strengthen surveillance of water quality by regulatory authorities. A single measurement of water quality, often during a season when weather is favorable for fieldwork, is not a substitute for routine monitoring by the responsible authorities in each country. Data collected through household surveys are more likely to be representative of the full range of water sources used by different population groups, but as a snapshot of quality, they will likely underestimate exposure to fecal indicator bacteria at all times. Furthermore, E. coli as an indicator of fecal contamination has known limitations (Charles et al. 2020; Gleeson and Grey 1996), including greater sensitivity to chlorine compared with pathogens such as Cryptosporidium (WHO 2017). In many LMICs, water quality data from regulatory authorities are limited, especially for rural areas and nonpiped supplies (Kumpel et al. 2016). Nationally representative data from household surveys can provide a cost-effective means of filling these data gaps in the short term and draw attention to inequalities in service levels in the absence of regulation. Furthermore, the responsibility of the service provider usually ends at the household connection or public tap; as a result, regulators rarely collect samples from within the home (Kumpel et al. 2016).
Recommendations for Water Testing in Future Household Surveys
The water quality module has been integrated into an increasing number of MICS and other national and subnational surveys with support from the JMP team, including national household surveys in Ghana, Democratic People’s Republic of Korea, Ethiopia, Ecuador, Lebanon, and the Philippines (WHO/UNICEF 2020). There have also been subnational pilots in the Demographic and Health Survey in Peru (Wang et al. 2017a), the National Socioeconomic Survey in Indonesia (Cronin et al. 2017), and the Afghanistan Living Conditions Survey (Saboor et al. 2021). This illustrates the growing demand for understanding the quality of services and the willingness of national authorities to shine a light on this important but politically sensitive issue.
Several key lessons from these experiences of taking water testing to scale could inform the design and implementation of future surveys. Testing drinking water in a subsample of households for a limited number of priority parameters is critical to the cost effectiveness and practicability of this approach. Further work is required to support the JMP recommendation for selecting 3–5 households per cluster (WHO/UNICEF 2020). Involvement of regulatory authorities is strongly recommended given their mandate for oversight of water service provision. Quality control measures are important during fieldwork and to build confidence in the results, and blank tests are especially valuable to confirm that detection of E. coli is not the result of poor hygiene by field teams. Further work could examine the associations between positive blank tests and risk factors for contamination. Field duplicate tests were conducted in Bangladesh MICS 2012–2013, with the same risk level recorded for 71% of duplicate samples () conducted by the MICS teams and the International Centre for Diarrhoeal Disease Research, Bangladesh laboratory staff and 85% of samples within one risk level (Bangladesh Bureau of Statistics and UNICEF 2017). The interpretation of duplicates is challenging given that some inconsistency is expected purely due to inherent sampling variability (McBride 2005). Duplicates are thus less actionable during fieldwork than blank tests. Close attention is needed for the training which, depending on the number of teams and facilitators, should last 3–5 d and include sufficient practice (preferably at least 15 tests) for each step in the process. In a few countries, leaflets explaining contamination risks and methods for safe water treatment and storage were provided to households selected for the water quality module. Further work is needed to determine how best to share information on water quality with households and their communities in order to inform decisions on data sharing by implementing agencies (Khan et al. 2017).
There are several E. coli tests that could potentially be integrated into household surveys (Bain et al. 2012). In the MICS, an adapted membrane filtration process has been used, yielding reliable quantitative results (Brown et al. 2020). The costs are per test using the new “E. coli only” Compact Dry plates and per team for hardware [mainly the filtration equipment (Figure S1)], but these costs are expected to decrease over time. For example, a low-cost kit () has successfully been piloted in the Afghanistan Living Conditions Survey (Saboor et al. 2021). In the longer term, it is hoped that novel, rapid tests will replace the culture-based approaches that dominate the water quality testing market (Rompré et al. 2002; UNICEF 2019).
Integration of water testing into the MICS has demonstrated that it is feasible to include water testing in nationally representative household surveys and that the data generated can be used to monitor the SDG SMDW services indicator. We find that water quality is often the limiting factor for SMDW services, and there was a substantial difference between the MDG and SDG indicators for all countries in the present study. The large proportions of the population exposed to very high levels of E. coli and the deterioration in quality between the PoC and the PoU within the home suggest that interim targets and approaches are needed to reduce risks and to strengthen water quality surveillance. Countries should be supported to localize and adapt the SDG targets to the national context and to develop plans to progressively improve drinking water quality and to identify and target populations at greatest risk.
Supplementary Material
Acknowledgments
We are grateful to the implementing agencies who integrated water testing in the national household surveys. We thank the entire global Multiple Indicator Cluster Surveys (MICS) team for their excellent collaboration in the development of the new water quality module and the World Health Organization and United Nations Children’s Fund Joint Monitoring Program water quality trainers for providing technical support to countries implementing the water quality module. We thank the following international consultants who supported water quality testing in the MICS: A. Saboor, A. Shantz, C. Dorea, E. Lictevout, L. Osterwalder, L. Hidmi, M. Djerma, and P. van Maanen, as well as Y. Coskun, who provided data processing support on the custom wealth indices; J. Wolf, M. Freeman, A. Mertens, and A. MacDougall, who provided guidance on multilevel regression modeling; J. Wright, who provided guidance on modeling seasonality; and J. de France and A. Reinhold, who kindly reviewed a draft of the study.
Funding for the integration of water testing in household surveys was provided by the Netherlands Directorate-General of International Cooperation, the UK Foreign, Commonwealth and Development, and the U.S. Agency for International Development.
References
- Amini M, Mueller K, Abbaspour KC, Rosenberg T, Afyuni M, Moller KN, et al. . 2008. Statistical modeling of global geogenic fluoride contamination in groundwaters. Environ Sci Technol 42(10):3662–3668, PMID: 18546705, 10.1021/es071958y. [DOI] [PubMed] [Google Scholar]
- Bain R, Bartram J, Elliott M, Matthews R, McMahan L, Tung R, et al. . 2012. A summary catalogue of microbial drinking water tests for low and medium resource settings. Int J Environ Res Public Health 9(5):1609–1625, PMID: 22754460, 10.3390/ijerph9051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain R, Cronk R, Wright J, Yang H, Slaymaker T, Bartram J. 2014. Fecal contamination of drinking-water in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 11(5):e1001644, PMID: 24800926, 10.1371/journal.pmed.1001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics, UNICEF (United Nations Children’s Fund). 2017. Bangladesh MICS 2012–2013 Water Quality Thematic Report. https://washdata.org/report/bangladesh-mics-2012-2013-water-quality-thematic-report-final [accessed 1 June 2021].
- Bartram J, Brocklehurst C, Fisher MB, Luyendijk R, Hossain R, Wardlaw T, et al. . 2014. Global monitoring of water supply and sanitation: history, methods and future challenges. Int J Environ Res Public Health 11(8):8137–8165, PMID: 25116635, 10.3390/ijerph110808137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Bir A, Bain RES. 2020. Novel methods for global water safety monitoring: comparative analysis of low-cost, field-ready E. coli assays. NPJ Clean Water 3(1):9, 10.1038/s41545-020-0056-8. [DOI] [Google Scholar]
- Brown J, Hien VT, McMahan L, Jenkins MW, Thie L, Liang K, et al. . 2013. Relative benefits of on-plot water supply over other ‘improved’ sources in rural Vietnam. Trop Med Int Health 18(1):65–74, PMID: 23107456, 10.1111/tmi.12010. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Nowicki S, Bartram JK. 2020. A framework for monitoring the safety of water services: from measurements to security. NPJ Clean Water 3(1):36, 10.1038/s41545-020-00083-1. [DOI] [Google Scholar]
- Cronin AA, Odagiri M, Arsyad B, Nuryetty MT, Amannullah G, Santoso H, et al. . 2017. Piloting water quality testing coupled with a national socioeconomic survey in Yogyakarta Province, Indonesia, towards tracking of Sustainable Development Goal 6. Int J Hyg Environ Health 220(7):1141–1151, PMID: 28743592, 10.1016/j.ijheh.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Daly SW, Lowe J, Hornsby GM, Harris AR. 2021. Multiple water source use in low- and middle-income countries: a systematic review. J Water Health 19(3):370–392, PMID: 34152293, 10.2166/wh.2021.205. [DOI] [PubMed] [Google Scholar]
- Elliott M, MacDonald MC, Chan T, Kearton A, Shields KF, Bartram JK, et al. . 2017. Multiple household water sources and their use in remote communities with evidence from Pacific Island Countries. Water Resour Res 53(11):9106–9117, 10.1002/2017WR021047. [DOI] [Google Scholar]
- Garn JV, Boisson S, Willis R, Bakhtiari A, Al-Khatib T, Amer K, et al. . 2018. Sanitation and water supply coverage thresholds associated with active trachoma: modeling cross-sectional data from 13 countries. PLoS Negl Trop Dis 12(1):e0006110, PMID: 29357365, 10.1371/journal.pntd.0006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J. 2007. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Gleeson C, Grey N. 1996. The Coliform Index and Waterborne Disease: Problems of Microbial Drinking Water Assessment. London, UK: CRC Press. [Google Scholar]
- Graham JP, Kaur M, Jeuland MA. 2018. Access to environmental health assets across wealth strata: evidence from 41 low- and middle-income countries. PLoS One 13(11):e0207339, PMID: 30444899, 10.1371/journal.pone.0207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AR, Pickering AJ, Boehm AB, Mrisho M, Davis J. 2019. Comparison of analytical techniques to explain variability in stored drinking water quality and microbial hand contamination of female caregivers in Tanzania. Environ Sci Process Impacts 21(5):893–903, PMID: 31017132, 10.1039/c8em00460a. [DOI] [PubMed] [Google Scholar]
- Harris I, Osborn TJ, Jones P, Lister D. 2020. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci Data 7(1):109, PMID: 32246091, 10.1038/s41597-020-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Alzua ML, Osbert N, Pickering A. 2017. Community-level sanitation coverage more strongly associated with child growth and household drinking water quality than access to a private toilet in rural Mali. Environ Sci Technol 51(12):7219–7227, PMID: 28514143, 10.1021/acs.est.7b00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeandron A, Saidi JM, Kapama A, Burhole M, Birembano F, Vandevelde T, et al. . 2015. Water supply interruptions and suspected cholera incidence: a time-series regression in the Democratic Republic of the Congo. PLoS Med 12(10):e1001893, PMID: 26506001, 10.1371/journal.pmed.1001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel P, Kunwar R, Lamichhane P, Karki S. 2017. Extent of fecal contamination of household drinking water in Nepal: further analysis of Nepal Multiple Indicator Cluster Survey 2014. Am J Trop Med Hyg 96(2):446–448, PMID: 27821687, 10.4269/ajtmh.16-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Bain RES, Lunze K, Unalan T, Beshanski-Pedersen B, Slaymaker T, et al. . 2017. Optimizing household survey methods to monitor the Sustainable Development Goals targets 6.1 and 6.2 on drinking water, sanitation and hygiene: a mixed-methods field-test in Belize. PLoS One 12(12):e0189089–18, PMID: 29216244, 10.1371/journal.pone.0189089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Hancioglu A. 2019. Multiple indicator cluster surveys: delivering robust data on children and women across the globe. Stud Fam Plann 50(3):279–286, PMID: 31486080, 10.1111/sifp.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby MA, Nagel CL, Rosa G, Iyakaremye L, Zambrano LD, Clasen TF. 2016. Faecal contamination of household drinking water in Rwanda: a national cross-sectional study. Sci Total Environ 571:426–434, PMID: 27470017, 10.1016/j.scitotenv.2016.06.226. [DOI] [PubMed] [Google Scholar]
- Kostyla C, Bain R, Cronk R, Bartram J. 2015. Seasonal variation of fecal contamination in drinking water sources in developing countries: a systematic review. Sci Total Environ 514:333–343, PMID: 25676921, 10.1016/j.scitotenv.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kumpel E, Cock-Esteb A, Duret M, de Waal D, Khush R. 2017. Seasonal variation in drinking and domestic water sources and quality in Port Harcourt, Nigeria. Am J Trop Med Hyg 96(2):437–445, PMID: 27821689, 10.4269/ajtmh.16-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpel E, Nelson KL. 2013. Comparing microbial water quality in an intermittent and continuous piped water supply. Water Res 47(14):5176–5188, PMID: 23866140, 10.1016/j.watres.2013.05.058. [DOI] [PubMed] [Google Scholar]
- Kumpel E, Peletz R, Bonham M, Khush R. 2016. Assessing drinking water quality and water safety management in sub-Saharan Africa using regulated monitoring data. Environ Sci Technol 50(20):10869–10876, PMID: 27559754, 10.1021/acs.est.6b02707. [DOI] [PubMed] [Google Scholar]
- Martel P. 2017. Review of Options for Reporting Water, Sanitation and Hygiene Coverage by Wealth Quintile. New York, NY: United National Children’s Fund. https://mics.unicef.org/files?job=W1siZiIsIjIwMTcvMDYvMTUvMTYvMzMvMzAvMzE2L01JQ1NfTWV0aG9kb2xvZ2ljYWxfUGFwZXJfNC5wZGYiXV0&sha=adfd855d58aa27ea [accessed 1 June 2021]. [Google Scholar]
- McBride GB. 2005. Using Statistical Methods for Water Quality Management: Issues, Problems and Solutions. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Nguyen KH, Operario DJ, Nyathi ME, Hill CL, Smith JA, Guerrant RL, et al. . 2021. Seasonality of drinking water sources and the impact of drinking water source on enteric infections among children in Limpopo, South Africa. Int J Hyg Environ Health 231:113640, PMID: 33115698, 10.1016/j.ijheh.2020.113640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda K, LoBuglio J, Bartram J. 2012. Global access to safe water: accounting for water quality and the resulting impact on MDG progress. Int J Environ Res Public Health 9(3):880–894, PMID: 22690170, 10.3390/ijerph9030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering AJ, Davis J, Walters SP, Horak HM, Keymer DP, Mushi D, et al. . 2010. Hands, water, and health: fecal contamination in Tanzanian communities with improved, non-networked water supplies. Environ Sci Technol 44(9):3267–3272, PMID: 20222746, 10.1021/es903524m. [DOI] [PubMed] [Google Scholar]
- Podgorski J, Berg M. 2020. Global threat of arsenic in groundwater. Science 368(6493):845–850, PMID: 32439786, 10.1126/science.aba1510. [DOI] [PubMed] [Google Scholar]
- Poulin C, Peletz R, Ercumen A, Pickering AJ, Marshall K, Boehm AB, et al. . 2020. What environmental factors influence the concentration of fecal indicator bacteria in groundwater? Insights from explanatory modeling in Uganda and Bangladesh. Environ Sci Technol 54(21):13566–13578, PMID: 32975935, 10.1021/acs.est.0c02567. [DOI] [PubMed] [Google Scholar]
- Rompré A, Servais P, Baudart J, de-Roubin MR, Laurent P. 2002. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods 49(1):31–54, PMID: 11777581, 10.1016/s0167-7012(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Rosa G, Clasen T. 2017. Consistency of use and effectiveness of household water treatment among Indian households claiming to treat their water. Am J Trop Med Hyg 97(1):259–270, PMID: 28719314, 10.4269/ajtmh.16-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa G, Kelly P, Clasen T. 2016. Consistency of use and effectiveness of household water treatment practices among urban and rural populations claiming to treat their drinking water at home: a case study in Zambia. Am J Trop Med Hyg 94(2):445–455, PMID: 26572868, 10.4269/ajtmh.15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboor A, Amarkhel AK, Hakimi E, Bain R, Luyendijk R. 2021. Inclusion of water quality testing in the Afghanistan living conditions survey and status of bacteriological contamination of drinking water in 10 provinces of Afghanistan. J Water Sanit Hyg Dev 11(4):600–611, 10.2166/washdev.2021.046. [DOI] [Google Scholar]
- Shields KF, Bain RES, Cronk R, Wright JA, Bartram J. 2015. Association of supply type with fecal contamination of source water and household stored drinking water in developing countries: a bivariate meta-analysis. Environ Health Perspect 123(12):1222–1231, PMID: 25956006, 10.1289/ehp.1409002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow DL, Mintz ED, Rodriguez M, Tejada E, Ocampo C, Espejo L, et al. . 1992. Waterborne transmission of epidemic cholera in Trujillo, Peru: lessons for a continent at risk. Lancet 340(8810):28–33, PMID: 1351608, 10.1016/0140-6736(92)92432-f. [DOI] [PubMed] [Google Scholar]
- UNICEF. 2019. Target product profile: rapid water quality detection method or portable kit. https://www.unicef.org/supply/documents/target-product-profile-rapid-e-coli-detection-tests [accessed 1 June 2021].
- United Nations. 2017. Goal 6: ensure availability and sustainable management of water and sanitation for all. Target 6.1: by 2030, achieve universal and equitable access to safe and affordable drinking water for all. Indicator 6.1.1: proportion of population using safely managed drinking water services. https://unstats.un.org/sdgs/metadata/files/Metadata-06-01-01.pdf [accessed 1 June 2021].
- Wang A, McMahan L, Rutstein S, Stauber C, Reyes J, Sobsey MD. 2017a. Household microbial water quality testing in a Peruvian Demographic and Health Survey: evaluation of the compartment bag test for Escherichia coli. Am J Trop Med Hyg 96(4):970–975, PMID: 28500818, 10.4269/ajtmh.15-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Moe CL, Null C, Raj SJ, Baker KK, Robb KA, et al. . 2017b. Multipathway quantitative assessment of exposure to fecal contamination for young children in low-income urban environments in Accra, Ghana: the Sanipath analytical approach. Am J Trop Med Hyg 97(4):1009–1019, PMID: 29031283, 10.4269/ajtmh.16-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardrop NA, Hill AG, Dzodzomenyo M, Aryeetey G, Wright JA. 2018. Livestock ownership and microbial contamination of drinking-water: evidence from nationally representative household surveys in Ghana, Nepal and Bangladesh. Int J Hyg Environ Health 221(1):33–40, PMID: 29031736, 10.1016/j.ijheh.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. Guidelines for Drinking-water Quality, Fourth Edition, Incorporating the First Addendum. Geneva, Switzerland: WHO. https://apps.who.int/iris/rest/bitstreams/1080656/retrieve [accessed 1 June 2021]. [PubMed] [Google Scholar]
- WHO/UNICEF. 2012. Rapid Assessment of Drinking-Water Quality: a Handbook for Implementation. Geneva, Switzerland: WHO. https://apps.who.int/iris/bitstream/handle/10665/331485/9789241504683-eng.pdf?sequence=1&isAllowed=y [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2017a. Progress on Drinking Water, Sanitation and Hygiene—2017 Update and SDG Baselines. Geneva, Switzerland: WHO. https://washdata.org/report/jmp-2017-report-final [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2017b. Safely Managed Drinking Water—Thematic Report on Drinking Water 2017. New York, USA: UNICEF. https://washdata.org/report/jmp-2017-tr-smdw [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2018a. Core Questions on Water, Sanitation and Hygiene for Household Surveys: 2018 Update. Geneva, Switzerland: WHO. https://washdata.org/report/jmp-2018-core-questions-household-surveys [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2018b. JMP Methodology: 2017 Update & SDG Baselines. Geneva, Switzerland: WHO. https://washdata.org/report/jmp-methodology-2017-update [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2019. Progress on Household Drinking Water, Sanitation and Hygiene 2000’2017: Special Focus on Inequalities. New York, NY: UNICEF. https://washdata.org/report/jmp-2019-wash-households [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2020. Integrating Water Quality Testing into Household Surveys. New York, USA: UNICEF. https://washdata.org/report/jmp-2020-water-quality-testing-household-surveys [accessed 1 June 2021]. [Google Scholar]
- WHO/UNICEF. 2021. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2020: Five Years into the SDGs. Geneva, Switzerland: WHO. https://washdata.org/sites/default/files/2021-07/jmp-2021-wash-households.pdf [accessed 1 August 2021]. [Google Scholar]
- Wolf J, Johnston R, Freeman MC, Ram PK, Slaymaker T, Laurenz E, et al. . 2019. Handwashing with soap after potential faecal contact: global, regional and country estimates. Int J Epidemiol 48(4):1204–1218, PMID: 30535198, 10.1093/ije/dyy253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J, Gundry S, Conroy R. 2004. Household drinking water in developing countries: a systematic review of microbiological contamination between source and point-of-use. Trop Med Int Health 9(1):106–117, PMID: 14728614, 10.1046/j.1365-3156.2003.01160.x. [DOI] [PubMed] [Google Scholar]
- Wright JA, Yang H, Walker K. 2012. Do international surveys and censuses exhibit ‘dry season’ bias? Popul Space Place 18(1):116–126, 10.1002/psp.681. [DOI] [Google Scholar]
- Yang H, Bain R, Bartram J, Gundry S, Pedley S, Wright J. 2013. Water safety and inequality in access to drinking-water between rich and poor households. Environ Sci Technol 47(3):1222–1230, PMID: 23276231, 10.1021/es303345p. [DOI] [PubMed] [Google Scholar]
- Zou G. 2004. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706, PMID: 15033648, 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.