Abstract
Gastrointestinal (GI) cancers represent a major public health problem worldwide. Due to the late detection and high heterogeneity of GI cancers, traditional treatments, including surgery, radiotherapy, chemotherapy, and targeted therapy, have shown limited effects, and the overall prognosis of these patients remains poor. Recently, immunotherapy, involving programmed cell death-1 (PD-1) and its ligand (PD-L1), has shown promising efficacy in several solid cancers and seems to have become a potential treatment option for GI cancers This review focuses on data on the development of immunotherapy-based clinical trials in esophageal cancer, gastric cancer, and colorectal cancer. The predictive biomarkers and combination strategies in clinical trials and translational medicine are also discussed. Finally, prospects for immunotherapy in the treatment of GI cancers are described. Although only a small proportion of patients with GI cancers respond to PD-1/PD-L1 blockade, we strongly believe that precision immunotherapy might improve the overall survival of many more GI cancer patients in the future.
Keywords: gastrointestinal cancer, immune checkpoint blockade, biomarkers, precision immunotherapy
Graphical Abstract
Public Summary
-
•
Immunotherapy has revolutionized the therapeutic landscape of gastrointestinal cancer, based on a series of clinical trials conducted in recent years.
-
•
The next level of predictive biomarker of the response to immune checkpoint inhibitors (ICIs) should address the integration of current multi-omics biomarkers with an interdisciplinary approach.
-
•
The emerging combination strategies based on ICIs should be more defined in the specific population.
-
•
It is imperative to understand mechanism of acquired resistance to ICIs and explore therapeutic strategies to reverse it.
Main Text
Background
According to the GLOBOCAN database, there were 18.1 million newly diagnosed cases and 9.6 million deaths from cancer worldwide in 2018.1 Notably, more than 15% of cancer incidence and 17% of cancer-caused deaths were attributed to gastrointestinal (GI) tract cancers.2 Moreover, GI malignancies are the most common cancers and have a huge impact on cancer-associated mortality in China.3,4 The mainstreams of therapeutic approaches for such patients are still surgery, radiation therapy, chemotherapy, and targeted therapy. However, the effectiveness of these treatments is not satisfactory due to the late diagnosis.
The emergence of cancer immunotherapy has revolutionized the landscape of cancer treatment, currently driven by the application of monoclonal antibodies targeting immune checkpoint such as programmed cell death-1 (PD-1) and cytotoxic T lymphocyte-associated protein-4 (CTLA-4) that release cellular brakes on T cells. Since the first approval of immune checkpoint inhibitors (ICIs) in melanoma in 2011, and afterward in more than 20 different indications, ICIs have become a promising treatment option for patients with cancer. In the field of GI cancers, despite the response rate of ICIs not being as high as for some immunogenic tumors, immunotherapy was still considered as the potentially curative therapeutic approach evidenced by a series of clinical trials. Here, we discuss the current status and prospects for immunotherapy in the treatment of GI cancers.
Current Status of Immune Checkpoint Blockade in GI Cancers
Esophageal Cancer
Squamous cell carcinoma and adenocarcinoma are the two main histological types of esophageal cancer. Patients with esophageal squamous cell carcinoma (ESCC) seemed to benefit more from ICIs than those with adenocarcinoma.
Regarding esophageal adenocarcinoma (EAC), data on ICI efficacy are limited. In the esophageal cancer cohort of the KEYNOTE-028 trial, the overall response rate (ORR) was 40% in EAC patients (n = 5) with a positive PD-L1 expression.5 However, among patients who progressed after 2 or more lines of systemic therapy, only 5.2% of EAC patients (n = 58) achieved partial response (PR), and no patient achieved complete response (CR) in the phase 2 KEYNOTE-180 trial.6 In the phase 3 KEYNOTE-181 trial, subgroup analysis demonstrated that pembrolizumab had no overall survival (OS) advantage over chemotherapy for EAC patients, even those with a PD-L1 combined positive score (CPS) ≥10 (n = 55).7 Therefore, it is currently unclear whether patients with EAC might benefit from ICIs and whether determination of the PD-L1 status could identify potential candidates for ICI therapy.
Concerning ESCC, three phase 3 trials led to landmark changes in second-line treatment. In the KEYNOTE-181 trial, pembrolizumab did not show a survival benefit in the whole population (n = 628); however, pembrolizumab was superior to chemotherapy in terms of OS (8.2 versus 7.1 months, p = 0.0095) and ORR (16.7% versus 7.4%, p = 0.0022) for patients with ESCC (n = 401).7 Similarly, nivolumab showed a statistically significant extension in OS for PD-L1 unselected advanced ESCC patients in the ATTRACTION-3 trial,8 as did camrelizumab in the ESCORT study9 (Table 1). According to these three trials, it seems that the relationship between PD-L1 status and the response to immunotherapy is still controversial.7, 8, 9
Table 1.
Efficacy of Immune Checkpoint Inhibitors in Esophageal Squamous Cell Carcinoma
| KEYNOTE-1817 | ATTRACTION-38 | ESCORT9 | ||||
|---|---|---|---|---|---|---|
| Regimen | Pembrolizumab | chemotherapy | nivolumab | chemotherapy | camrelizumab | chemotherapy |
| Sample size | 198 | 203 | 210 | 209 | 228 | 220 |
| Prior treatment lines | ≥1 | ≥1 | ≥1 | ≥1 | ≥1 | ≥1 |
| ORR, n (%) | 33 (16.7) | 15 (7.4) | 33/171a (19) | 34/158a (22) | 46 (20.2) | 14 (6.4) |
| PFS, months | 2.2 | 3.1 | 1.7 | 3.4 | 1.9 | 1.9 |
| OS, months | 8.2 | 7.1 | 10.9 | 8.4 | 8.3 | 6.2 |
Randomly assigned patients who had target lesion measurements at baseline.
Gastric Cancer
A randomized, double-blind, placebo-controlled, phase 3 trial, ATTRACTION-2, first showed the efficacy of nivolumab in patients with advanced gastric cancer (AGC) (including gastroesophageal junction cancer) for whom no current standard-of-care therapy was available. The median OS (5.26 versus 4.14 months, p < 0.0001) and ORR (11.2% versus 0%) of nivolumab were both significantly better than those of placebo. The survival benefit from nivolumab was independent of PD-L1 positivity.10
In a later-line setting, 259 patients who failed to respond to at least two lines of systemic therapies were enrolled in the KEYNOTE-059 trial. The ORR was 11.6%, with 2.3% achieving CR. Among patients with a PD-L1 CPS ≥1, the ORR was 15.5%, while the ORR of patients with PD-L1 negative expression was only 6.4%.11 The phase 3 KEYNOTE-061 trial included patients with one prior line of treatment and compared pembrolizumab with paclitaxel. Updated results presented at the ASCO 2020 meeting indicated that pembrolizumab prolongs OS relative to paclitaxel in PD-L1 positive patients. The ORR was higher for pembrolizumab in the CPS ≥10 group, and the response duration was longer in the pembrolizumab group using all PD-L1 CPS cutoff values (CPS ≥1, CPS ≥5, and CPS ≥10).12 Meanwhile, in the JAVELIN Gastric 300 study, avelumab failed to demonstrate its priority as top physicians' choice of chemotherapy as a third-line treatment for unresectable, recurrent, or metastatic gastric or gastroesophageal junction adenocarcinoma patients.13
In the first-line setting, the phase 3 KEYNOTE-062 study demonstrated that pembrolizumab showed clinically meaningful efficacy in patients with a PD-L1 CPS ≥10 as well as more durable responses than chemotherapy.14 Globally, 256 patients received pembrolizumab monotherapy and 250 patients received chemotherapy. Pembrolizumab was non-inferior to chemotherapy for OS in tumors with a PD-L1 CPS ≥1 per prespecified margins (median OS, 10.6 versus 11.1 months; hazard ratio [HR], 0.91; 99.2% confidence interval [CI], 0.69–1.18). The Asian subpopulation analysis demonstrated that OS was longer with pembrolizumab than with chemotherapy regardless of the cutoff value used (CPS ≥1, 22.7 versus 13.8 months; CPS ≥10, 28.5 versus 14.8 months).15
These data indicate that ICIs might be a new option for patients with AGC regardless of treatment lines. Moreover, PD-L1 positivity was associated with a pronounced survival benefit of patients who underwent ICI treatment, especially those with a PD-L1 CPS ≥10.16 However, given the modest additional benefit of pembrolizumab plus chemotherapy versus chemotherapy alone,14 the combinational regimen of an anti-PD-1 antibody plus chemotherapy still needs to be explored.17
Colorectal Cancer
Deficient Mismatch Repair and Microsatellite Instability-High
Microsatellite instability-high (MSI-H)/deficient mismatch repair (dMMR) was first identified as an excellent predictive biomarker of PD-1 blockade in a phase 2 trial with a small sample size.18 Since then, an increasing number of studies have demonstrated the activity of monotherapy anti-PD-1 antibodies (nivolumab and pembrolizumab) in patients with MSI-H/dMMR. In the second- or later-line setting (Table 2), the ORR and disease control rate (DCR) were 32%–52% and 57%–82%, respectively. The survival data showed that the 1-year progression-free survival (PFS) rate ranged from 41% to 44% and the 1-year OS rate ranged from 72% to 76%.19, 20, 21, 22 More recently, durvalumab, an anti-PD-L1 antibody, also showed similar toxicity and comparable efficacy with pembrolizumab or nivolumab as a later-line therapy at a dose of 10 mg/kg repeated every 2 weeks, with ORR from 22% to 27% and the 1-year PFS rate ranging from 36% to 38%.23
Table 2.
Regimens and Efficacy of Immune Checkpoint Inhibitors in Patients with dMMR or MSI-H Tumors
| Regimen | Nivo 3 mg/kg q2w Ipi 1 mg/kg q6w24 |
Pembro 200 mg q3w25 | Nivo 3 mg/kg q2w Ipi 1 mg/kg q3w26,27 |
Nivo 3 mg/kg q2w19,20 | Pembro 200 mg q3w21 | Pembro 10 mg/kg q2w22 | Durva 10 mg/kg q2w23 | Durva 10 mg/kg q2w23 | |
|---|---|---|---|---|---|---|---|---|---|
| (NCT02227667) | (NCT01693562) | ||||||||
| Sample size | 45 | 153 | 119 | 74 | 63 | 40 | 11 | 36 | |
| Prior treatment lines | 0 | 0 | ≥1 | ≥1 | ≥1 | ≥2 | ≥2 | NA | |
| Best overall response, n (%) | |||||||||
| CR | 3 (7) | 17 (11) | 7 (6) | 7 (9) | 2 (3) | 5 (12) | NA | NA | |
| PR | 24 (53) | 50 (33) | 62 (52) | 18 (24) | 18 (29) | 16 (40) | NA | NA | |
| SD | 11 (24) | 32 (21) | 33 (28) | 23 (31) | 16 (25) | 12 (30) | NA | NA | |
| PD | 6 (13) | 45 (30) | 14 (12) | 22 (30) | 25 (40) | 4 (10) | NA | NA | |
| Not evaluable | 1 (2) | 9 (6) | 3 (3) | 4 (5) | 2 (3) | 3 (8) | NA | NA | |
| ORR | 27 (60) | 67 (44) | 69 (58) | 25 (33) | 20 (32) | 21 (52) | 3 (27) | 8 (22) | |
| CR + PR + SD ≥12 weeks | 38 (84) | 99 (65) | 96 (81) | 46 (62) | 36 (57) | 33 (82) | NA | NA | |
| PFS | NR | 16.5 months | NR | 6.6 months | 4.1 months | NR | 6 months | 6 months | |
| 1-year PFS, % | 77 | 55 | 71 | 44 | 41 | NA | 36 | 38 | |
| 2-year PFS, % | NA | 48 | 60 | NA | NA | 59 | NA | NA | |
| OS | NR | NA | NR | NR | NR | NR | NR | NR | |
| 1-year OS, % | 83 | NA | 85 | 72 | 76 | NA | NA | NA | |
| 2-year OS, % | NA | NA | 74 | NA | NA | 72 | NA | 54 | |
| TRAEs, n (%) | |||||||||
| Any | 35 (78) | 122 (80) | 87 (73) | 54 (73) | 40 (64) | NA | NA | NA | |
| Grades 3–5 | 7 (16) | 34 (22) | 37 (31) | 15 (20) | 7 (11) | NA | NA | NA | |
q(n)w, every (n) weeks. Anti-PD-1 antibody: Nivo (nivolumab), Pembro (pembrolizumab). Anti-PD-L1 antibody: Durva (durvalumab). Anti-CTLA-4 antibody: Ipi (ipilimumab). NR, not reached; NA, not available; TRAEs, treatment-related adverse events.
The combination of anti-PD-1 (nivolumab) and anti-CTLA-4 (ipilimumab) blockade seems to be more effective than monotherapy as a second- or later-line treatment. The ORR, DCR, 1-year PFS, and 1-year OS rates were 58%, 81%, 71%, and 85%, respectively.26,27 However, the combination therapy was associated with more grade 3–5 treatment-related adverse events (TRAEs),26,27 which might lead to decreased quality of life and discontinuation of treatment. Lowering the frequency of ipilimumab (1 mg/kg) from repeating every 3–6 weeks seemed to decrease the rate of grade 3–5 TRAEs.20,24,27 However, it is unclear whether the antitumor activity of combination immunotherapy in subsequent lines is influenced by dose modification, considering that 1 mg/kg nivolumab plus 3 mg/kg ipilimumab performed better than 3 mg/kg nivolumab plus 1 mg/kg ipilimumab in terms of the ORR and survival in patients with metastatic esophagogastric cancer.28
In the first-line setting, 45 patients received a doublet of nivolumab (3 mg/kg every 3 weeks [q3w]) plus ipilimumab (1 mg/kg q6w). The ORR for these patients was 64%, with 80% of patients having disease control for at least 12 weeks. The 1-year PFS and 1-year OS rates were 77% and 83%, respectively.24 The survival durations were longer than the pooled PFS (6.2 months) and OS (13.6 months) of patients with dMMR or MSI-H treated with first-line non-immunotherapy in a pooled analysis of four phase 3 studies (CAIRO, CAIRO2, COIN, and FOCUS).29 More recently, the KEYNOTE-177 trial showed that pembrolizumab provided a statistically significant improvement in PFS versus chemotherapy in patients with MSI-H metastatic colorectal cancer (mCRC) (16.5 versus 8.2 months).25 The ORR of pembrolizumab was also significantly higher than that of chemotherapy (43.8% versus 33.1%, p = 0.0275). All the aforementioned regimens were associated with favorable and manageable safety profiles. Therefore, immunotherapy should be a new standard for MSI-H mCRC in the first-line setting. However, it is unknown whether the combination of anti-PD-1 and anti-CTLA-4 therapy is superior to an anti-PD-1 antibody alone.
Proficient MMR and Microsatellite Stable
The proportion of patients with microsatellite stable (MSS) and proficient MMR (pMMR) mCRC accounts for more than 95% of mCRC patients.30 Monotherapy anti-PD-1 antibody was ineffective in this population.18 Anti-VEGF (vascular endothelial growth factor) treatment may enhance the efficacy of immunotherapy by reversing VEGF-mediated immunosuppression to promote T cell infiltration into tumors; however, in the phase 3 MODUL trial in which approximately 2% of tumors were MSI-H, the addition of atezolizumab (an anti-PD-L1 antibody) to fluoropyrimidine and bevacizumab did not improve PFS or OS as a first-line maintenance therapy.31 Similarly, despite the encouraging results from a single-arm phase 2 trial of atezolizumab plus cobimetinib, the phase 3 IMblaze370 trial, with 2%–3% of patients included being MSI-H, did not meet its primary endpoint of improved OS with atezolizumab plus cobimetinib or atezolizumab versus regorafenib.32
The combination of anti-PD-1/PD-L1 and anti-CTLA4 blockade was also investigated in the Checkmate-142 study and the CCTGCO.26 trial. Responses were observed, and median PFS was 1.4 months in MSS patients receiving nivolumab plus ipilimumab.33 Recently, the phase 2 randomized CCTGCO.26 trial, in which only 1%–2% of tumors were MSI-H, showed that durvalumab plus tremelimumab significantly prolonged OS (6.6 months) compared with best supportive care (4.1 months) in mCRC refractory to all available standard treatments. However, there was no difference in PFS between the two arms (1.8 versus 1.9 months), and no details on treatment after disease progression were reported.34 This combination regimen was proved to bring the greatest OS benefit compared with placebo in MSS patients with a tumor mutational burden (TMB) of >28 single nucleotide variations per Mb (21% of MSS patients).35 However, these findings need confirmation in phase 3 trials.
Increasing effort had have been made to identify effective combination immunotherapy regimens for the treatment of pMMR/MSS mCRC (see details in Lee et al.36). Although these preliminary results were promising in early-phase trials, they should be examined in well-designed randomized trials.
Immunotherapy beyond PD-1/PD-L1 Blockade in GI Cancer
Beyond immune checkpoint inhibitors, other immunotherapies, including adoptive T cell therapy and cancer vaccine, are also under active investigation.
Cellular Immunotherapy
Adoptive T cell therapies have produced remarkable responses, especially chimeric antigen receptor (CAR)-T therapy in acute lymphocytic leukemia and tumor-infiltrating lymphocyte (TIL) therapy in melanoma.37,38 These breakthroughs provided new insights into immunotherapy for GI cancer.
In the past decade, research has examined the efficacy of CAR-T cell therapy for use in GI tumors with antigens including EGFR, HER2, CEA, MUC1, and EpCAM.39,40 However, their application has met great challenges. This is thought to be due to limited cancer-specific targets, difficulties in CAR-T cells zoning to tumor sites, and non-persistence of adoptively transferred CAR-T cells.41 For instance, a CAR-T therapy targeting CEA caused severe colitis in a high percentage of patients, as this antigen is also expressed in normal intestinal tissue.42 In another study, fatal pulmonary toxicity was noted in a colon cancer patient received CAR-T cell infusion targeting HER2, which was likely due to on‑target, off-tumor effect resulting from low level of this antigen on pulmonary epithelium .43
However, there are numerous studies under investigation to identify a consistent antigen to serve as a target for CAR-T cell therapy used in solid tumors.44,45 Claudin 18.2 (CLDN18.2) is a tight-junction protein that is overexpressed in approximately 50% of gastric cancers (GCs).46 Recently, a single-arm, open-label, first-in-human phase 1 study (NCT03159819) enrolled 7 AGC and 5 pancreatic adenocarcinoma patients with positive CLDN 18.2 expression, to evaluate the safety and clinical efficacy of CAR-CLDN18.2 T cell treatment. No severe events were observed, and all cytokine release syndromes were noted grade 1 or 2. Among the 11 evaluable patients, The total ORR was 33.3% (1 CR and 2 PR, 5 stable disease [SD], and 2 progressive disease [PD]), with median PFS of 130 days (95% CI [38, 230]).The preliminary results indicated that CAR-CLDN18.2 T cell therapy is a promising therapeutic strategy in CLDN18.2 positive AGC and pancreatic adenocarcinoma patients.47 In the future, an increased number of patients and long-term follow-up are needed to further evaluate both safety and efficacy endpoints.
Therapeutic Vaccines
Therapeutic cancer vaccines is another way to stimulate an anticancer immune response to promote the recognition and eradication of malignant cells.48 Targets for therapeutic cancer vaccines mainly fall into two categories: tumor-associated antigens and overexpressed neoantigens.49 Different from tumor-associated antigens—normal host proteins that are abnormally expressed in cancer cells—neoantigens were resulted from aberrant proteins of cancer cells, harbored with high immunogenicity. However, like mutations, the majority of neoantigens are unique to each patient, and their numbers vary according to the tumor subtypes, and thus a personalized management is required.50
One clinical trial of a personalized neoantigen/cancer testis antigen nanovaccine (PVAC) introduced an amphiphilic nanovaccine loaded with a personalized vaccine encoding multiple neoantigens designed to induce neoantigen-specific T cell responses. Patient-specific mutation-containing neoantigens were selected on the basis of whole-exome sequencing and RNA-sequencing analyses of tumor-specific mutations. Cancer testis antigens were obtained according to immunohistochemical staining and HLA-binding affinity prediction. Thirteen patients with MSS solid tumors were enrolled. Five patients (1 with GC, 1 with liver cancer, 1 with cervical carcinoma, 1 with soft tissue sarcoma, 1 with renal carcinoma) received the PVAC in combination with a PD-1 antibody, and another eight patients (3 with GC, 2 with CRC, 1 with non-small-cell lung carcinoma, and 1 with renal carcinoma) received the PVAC in combination with the anti-PD-1 antibody and anti-angiogenic therapy. No dose-limiting toxicities were reported. The ORR was 53.8% (1 had CR, 6 had PR, 4 had SD, and 2 had PD). Moreover, neoantigen-specific T cell responses were detected.51
The PVAC was also administered to stage IIIb/IIIc GC patients after six cycles of adjuvant chemotherapy in another clinical trial. In clinical practice, 35% of stage III GC patients will experience recurrence after D2 gastrectomy within 1 year. In this study, at the median follow-up time of 12.6 months (8.5–25.0 months), only two patients developed local recurrence at 24.0 months and 10.5 months after surgery. The remaining 23 patients remained disease free. The preliminary results demonstrated that the addition of PVAC may prolong PFS after standard adjuvant chemotherapy.52 A longer follow-up and a larger sample size are needed to confirm the efficacy of the PVAC.
Implications for the Future
As described above, in the GI cancer field, we have not had as much success on cellular immunotherapy as in hematologic malignancies. On one hand, it is clear that choosing the appropriate antigen and designing a CAR-T cell unique to the cancer type is important. On the other hand, further optimization of CAR-T in solid tumors should focus on changing the immunosuppressive environment. Therefore, it is important to further decipher the tumor microenvironment (TME) so that next-generation CAR T cells can be developed.53 Optimization of the CAR design such as the CARs co-express cytokine genes such as interleukin-12 (IL-12), IL-15, and IL-17 has been reported to modulate the immunosuppressive TME.54, 55, 56 In addition, the combination of CAR-T and ICIs may represent an another approach to enhance the immune response, which are being investigated in solid tumors.57 Notably, the toxicity and immunogenicity of CARs need to be addressed in future clinical trials.
To obtain more efficacy in therapeutic vaccine, revolutions in technologies are also needed. Several investigations are ongoing, including better co-stimulatory components, multi-antigen vaccines, and viral vector prime-boost approaches.48 Furthermore, whether combining the vaccine with immune modulators or conventional chemotherapy/radiation could further enhance clinical efficacy will require considerable effort to validate.58,59
Collectively, in GI cancer very little progress has been made on cellular immunotherapy and vaccine development. Solid tumors present some challenges that hematologic malignancies do not have. The highly heterogeneity in GI cancer adds more difficulty in this field. Investigators are currently focusing on several approaches, including developments in the CAR-T cell engineering itself, to overcome the immunosuppression in solid tumors, and its combination with ICIs. These cellular immunotherapy based strategies will likely play critical roles in improving cancer treatment outcomes.
Biomarkers for ICIs in GI Cancer
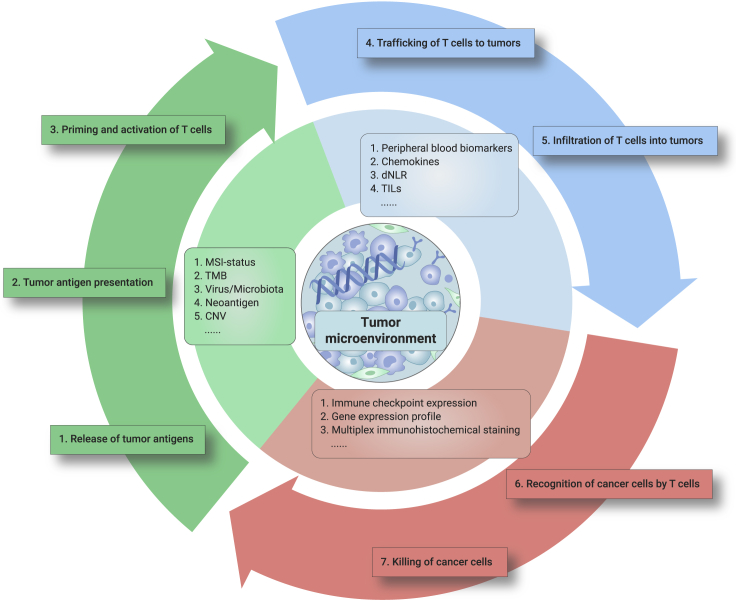
Despite the success of immunotherapy in GI cancers, only approximately 10%–30% of patients benefit from ICIs,60 which highlights the urgent need to identify biomarkers for patient selection (Figure 1).
Figure 1.
Predictive Biomarkers for Immune Checkpoint Inhibitors in GI Cancer.
The current immunotherapeutic biomarkers in GI cancer were developed on the basis of the mechanism of antitumor immunity. Consideration of each step of the cancer immune cycle must be incorporated in the ongoing efforts of biomarker optimization in GI cancer.
Microsatellite Instability
MSI-H/dMMR was first identified as the landmark predictive biomarker of PD-1 blockade in mCRC in a phase 2 trial with a small sample size.18 Since then, an increasing number of studies have demonstrated the activity of monotherapy PD-1 blockade in CRC patients with MSI-H/dMMR. In the second- or later-line setting (Table 2), the ORR and DCR were 32%–52% and 57%–82%, respectively. The survival data showed that 1-year PFS rate ranged from 41% to 44% and the 1-year OS rate ranged from 72% to 76%.19, 20, 21, 22 Moreover, pembrolizumab showed encouraging improved PFS in treatment-naive mCRC patients with MSI-H in the KEYNOTE-177 study (16.5 versus 8.2 months).
Similarly, MSI-H showed its predictive value in GC. A previous study reported an outstanding response to pembrolizumab in patients with MSI-H metastatic GC (85.7%).61 Additionally, the retrospective post hoc analysis of the KEYNOTE-059, -061, and -062 trials demonstrated that the MSI-H status is a predictive biomarker for pembrolizumab monotherapy in AGC patients irrespective of line of therapy.62
PD-L1
As reported at the 2019 ASCO-GI meeting, the KEYNOTE-181 study, which included 401 patients with ESCC and 227 patients with EAC, showed that although pembrolizumab did not benefit survival in the whole population, it exhibited superior OS benefits to chemotherapy as a second-line therapy in patients with a PD-L1 CPS ≥10 (n = 222/628).7 This difference was mainly due to ESCC. At the same time, nivolumab demonstrated a statistically significant extension in OS for PD-L1 unselected advanced esophageal cancer patients in the ATTRACTION-03 trial, as did camrelizumab in the ESCORT study.8,9 Therefore, PD-L1, especially the PD-L1 CPS, may be the first potential biomarker for esophageal cancer.
The PD-L1 CPS also demonstrated its importance in GC. The phase 3 KEYNOTE-061 trial included patients with one prior line of treatment and compared pembrolizumab with paclitaxel. Based on the primary analysis, pembrolizumab did not significantly prolong OS compared with paclitaxel (9.1 versus 8.3 months) in patients with a PD-L1-positive status (CPS ≥1).63 The updated results presented at the ASCO 2020 meeting indicated that the ORR was higher for pembrolizumab in the CPS ≥10 group (24.5% versus 9.1%), and the duration of response was longer with pembrolizumab using all CPS cutoff values (CPS ≥1, CPS ≥5, and CPS ≥10).12 Additionally, in the phase 3 KEYNOTE-062 study, pembrolizumab induced clinically meaningful efficacy in patients with a CPS ≥10, as well as more durable responses than chemotherapy.14 Notably, the Asian subpopulation preferred pembrolizumab to chemotherapy using both CPS cutoff values (median OS, HR [95% CI]: CPS ≥1, 22.7 versus 13.8 months, 0.54 [0.35–0.82]; CPS ≥10, 28.5 versus 14.8 months, 0.43 [0.21–0.89]).15 Collectively, these data indicate that a CPS ≥10 is a meaningful biomarker for first-line, second-line, and third-line and beyond pembrolizumab monotherapy in GC.16 This efficacy may be amplified, particularly in Asian patients.
However, PD-L1 did not demonstrate its predictive value in CRC. According to the analyses of several studies, PD-L1expression was not associated with clinical outcomes in the KEYNOTE-028, KEYNOTE-016, or CheckMate-142 trials.26,64,65 Therefore, based on the results so far, PD-L1 CPS is a promising indicator for immunotherapy in esophagogastric cancer, which may facilitate the selection of patients to some extent.
TMB
TMB is defined as the total number of somatic missense mutations.66 Recently, based on the results of the KEYNOTE-158 trial which reported in ESMO 2019,67 the Food and Drug Administration granted accelerated approval to pembrolizumab for the treatment of adult and pediatric patients with unresectable or metastatic TMB-H (TMB ≥10 mutations/Mb) solid tumors that have progressed following prior treatment and which have no satisfactory alternative treatment options. However, the KEYNOTE-158 trial did not include esophageal cancer and CRC patients, and the cutoff values defined as TMB ≥10 mutations/Mb may not be applicable for GI cancer. According to the broad analysis of the association between TMB and survival outcomes of ICIs, which included 110 CRC patients and 126 esophagogastric cancer patients, a higher TMB was correlated with improved survival.68 The TMB cutoff point for the top 20% of CRC patients was high (52.2 mutations/Mb), while the TMB cutoff point for esophagogastric cancer patients was low (8.8 mutations/Mb), indicating that one universal definition of TMB-H may not fit all cancer types. Moreover, TMB-high (TMB-H) may not be a favorable biomarker for ESCC because of its scarcity.69 In the KEYNOTE-061 trial, pembrolizumab demonstrated an OS benefit compared with paclitaxel in the subgroup with a tissue TMB ≥10 mutations/Mb (accounting for 17% of patients), which also remained when patients with MSI-H disease were excluded.70 Thus, TMB-H is a challenging predictive biomarker for GI cancer receiving ICIs, so further optimization and standardization are warranted.
Epstein-Barr Virus-Positive GC
Epstein-Barr virus (EBV)-positive GC constitutes a unique subgroup of GC in The Cancer Genome Atlas with several distinct clinicopathologic characteristics, including abundant TILs and a high PD-L1/PD-L2 expression.71 Previous studies have found that less than 10% of GC patients were EBV positive,71,72 and one has shown full response (100%) to ICIs in six cases of metastatic GC, with a median response duration of 8.5 months.61 In addition, EBV positivity is mutually exclusive with MSI-H, and the ORR was significantly higher in patients with PD-L1 ≥1% than in patients with PD-L1 negativity (50.0% versus 0.0%, p < 0.001).61 In contrast, Wang et al. noted an ORR of 25% in four EBV-positive GC patients.73 In another study, only 33.3% (3/9) of patients achieved PR after immunotherapy. It is also interesting to note that the patients who achieved PR had positive PD-L1 expression.74 Other clinical trials are continually testing the efficacy of a PD-1 antibody, or with a CTLA-4 antibody, in EBV-positive GC (NCT03755440 and NCT04202601).
Gut Microbiota
Accumulating evidence has suggested that gut microbiota may play an important role in host immune function and may assist the antitumor activities of immunotherapy.64, 65, 66 Experiments in mice have supported that tumor-bearing mice treated with broad-spectrum antibiotics failed to respond to ICI,75 and the presence of particular bacteria within intestinal microbiota could have favorable therapeutic outcomes from immune-based treatment.76,77 Peng et al. performed the first and largest cohort on the gut microbiome of 126 GI cancer patients in China receiving anti-PD-1 treatment, demonstrating that higher abundance of Prevotellaceae and lower presence of Holdemania and Lachnospiraceae correlate with favored clinical outcomes in these patients.78 Further investigations are needed for validation.
TILs
The prognostic value of TILs has been investigated in esophageal cancer, GC, and CRC, reporting that more generalized TILs were associated with improved survival.79, 80, 81 Although ICIs showed outstanding antitumor activity in patients with MSI-H/dMMR, 20%–30% of patients may not benefit from immunotherapy. In addition to the misdiagnosis and heterogeneity of MSI/dMMR, some basic studies have also provided insights into the mechanisms of primary resistance. As reported, 26%–35% of dMMR tumors exhibit CD3+ and CD8+ T cell densities as low as those in the bottom half of pMMR tumors.82 Similarly, Loupakis et al. found that an increasing number of TILs correlated with a higher TMB, and survival outcomes also differed significantly in favor of patients with TIL-high/MSI-H tumors (PFS: HR = 0.42, p = 0.0278; OS: HR = 0.41, p = 0.0463).83 The TIL subtypes that can be used to predict the clinical outcomes of immunotherapy for GI cancer should be determined in the future.
Peripheral Blood Biomarkers
Considering the invasive procedures to obtain tumor tissue, peripheral blood samples are less invasive and easier to obtain. Based on previous studies on melanoma and NSCLC, peripheral inflammation indicators would be helpful to identify biomarkers predicting the outcomes of ICI therapy.84, 85, 86, 87, 88, 89, 90 Recently, Li et al. validated the prognostic value of derived neutrophil-to-lymphocyte ratio (dNLR) in non-colorectal GI cancer patients treated with ICIs and reported that a higher level of the dNLR (cutoff ≥3) was correlated with worse outcomes.91 Lu et al. found that the serum protein level could not only predict the response to ICI in metastatic GI cancer patients but also identify patients who may develop hyperprogressive disease upon receiving ICI.92 Notably, these studies were retrospective, and further prospective studies are needed to validate their findings.
Gene Expression Profile
Bioinformatics analysis of RNA sequencing and gene expression could reflect antitumor immunity and provide insights into the molecular features associated with immunotherapy treatment outcomes. For example, the interferon-γ (IFN-γ) signature or T cell inflamed gene expression profile has been validated in multiple clinical trials.93,94 The KEYNOTE-012 trial showed a non-significant trend toward prolonged PFS and a high ORR with pembrolizumab for the treatment of esophageal cancer and GC characterized by the IFN-γ gene signature.5,95 However, the IFN-γ signature failed to predict the survival of GC patients in a prospective study.96 One important reason was the use of archival tissue rather than fresh tissue, suggesting that timing of tissue acquisition and mRNA quality are the greatest challenges of transcriptomic biomarker utility.
Biomarker Optimization
As mentioned above, extensive efforts have been made to identify potential predictors of the therapeutic response to ICIs. However, each of these biomarkers is still suboptimal, mainly because of the complexity and heterogeneity of the tumor microenvironment (TME) and a lack of standardization among molecular detection assays. With the current understanding of cancer immunology, a single parameter may not be sufficient to capture the TME characteristics, and thus may not precisely identify patients who will likely benefit from immunotherapy. Therefore, the investigations at the proteomic, metabolomic, epigenetic, and even single-cell level would unravel novel aspects of response not yet observed previously. Therefore, the next level of biomarker research should address the integration of multi-omics biomarkers with an interdisciplinary approach. In recent years, machine-learning methodologies have offered a novel approach for selecting tumor-intrinsic and TME features, optimizing the integration process and prediction model building for immunotherapy.97, 98, 99 The implementation of these novel strategies may help develop potential prediction tools that can overcome the challenges of existing biomarkers. Although an improved notion of immune contexture can be harnessed to guide precision immuno-oncology, how certain treatment strategies affect the TME of GI cancer remains poorly understood and may partially explain the failure of the KEYNOTE-062 study. Importantly, it was revealed at the ESMO 2019 meeting that TMB may not be a suitable predictive biomarker for chemotherapy plus ICIs,100,101 implying that the immune context changes according to combinational strategies. These results address the necessity of taking combinational strategies into account when characterizing TME and exploring optimal biomarkers for such combinations. Since a range of trials testing novel ICI-based combinations are ongoing, the corresponding biomarkers should also be adjusted to suit different indications.
Future Perspective of Immunotherapy
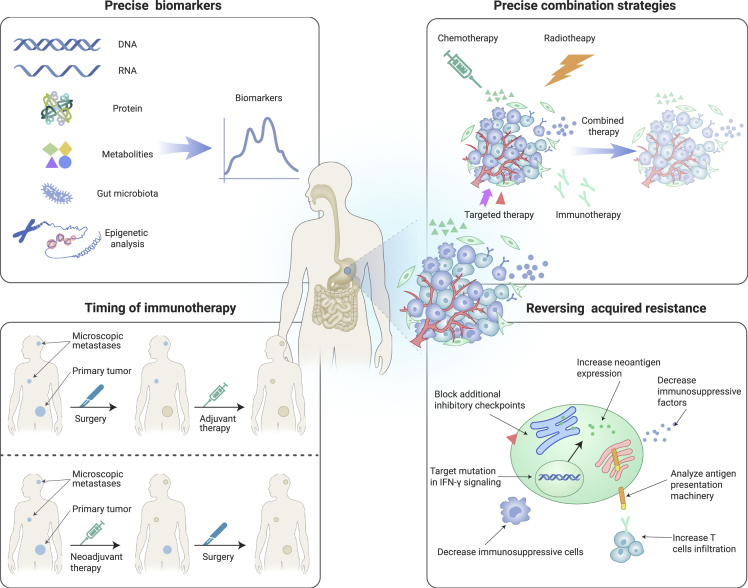
It is evident that precision immunotherapy has been taken the lead to identify the appropriate patients for immunotherapy or combination immunotherapy and can accurately predict the best response to immunotherapy guided by biomarkers. In the future, we should focus on the following (Figure 2).
Figure 2.
Future Perspective of Immunotherapy.
For precision immunotherapy, shown above, the following aspects could be focused upon, based on the tumor environment. (1) Identification of potential precise biomarkers. With the current understanding of cancer immunology, biomarkers could be identified in multi-omics, and combinational strategies taken into account to guide precision immuno-oncology. (2) Exploration of the precise combination strategies. Based on the complexity of TME, the different combination strategies of chemotherapy, radiotherapy, targeted therapy, immunotherapy, and so forth that co-target the function of tumor-specific factors could be considered. (3) Identification of the timing of immunotherapy. Neoadjuvant therapy showed promising prevention of tumor relapse, even better than adjuvant therapy in some preclinical mouse models and clinical trials, providing a new option for patients. Therefore, it is necessary to confirm the appropriate timing of immunotherapy to obtain better efficacy. (4) Identification of the strategy to reverse acquired resistance. Acquired resistance to ICIs always discounts the efficiency of immunotherapy and remains a big challenge, so it is imperative to understand the mechanism of acquired resistance. The strategies that analyze the antigen presentation machinery, target mutation in IFN-γ signaling, block additional inhibitory checkpoints, increase neoantigen expression, decrease immunosuppressive cells or factors, increase T cell infiltration, and so forth could be explored to reverse acquired resistance.
Combination Strategies
To date, the clinical success of immunotherapy in GI cancer has focused primarily on a single agent that targets PD-1/PD-L1. However, there are still many patients who do not respond to ICI monotherapy, and alternative strategies are required for optimal therapeutic benefit. To our best knowledge, multiple immunosuppressive pathways have shown their effects in modulating the TME. Therefore, these pathways in the TME could be co-targeted to restore the effector function of tumor-specific immune cells.102
As novel drugs are continuously being developed, the combination of ICIs and targeted therapy has yielded promising prospects. Previous studies have reported that the administration of trastuzumab plus pembrolizumab can improve HER2-specific T cell responses, enhance dendritic cell and T cell trafficking, and induce the expansion of peripheral memory T cells.103, 104, 105 In a phase 2 trial of triple combination regimen (pembrolizumab, trastuzumab, and chemotherapy) as first-line therapy for HER2-positive AGC, 37 patients were included and 26 (70%; 95% CI 54–83) of 37 treated patients were alive and the PFS was 6 months.106 The ongoing phase III KEYNOTE-811 study (NCT03615326) was based on the protocol of this study. Similarly, there is much interest in the use of other anti-HER2 drugs in combination with checkpoint inhibitors, such as margetuximab in combination with the anti-PD-1 antibody,107 DS-8201 in combination with the anti-PD-1 antibody, and ZW25 in combination with PD-L1 antibody (NCT04082364, NCT04379596, and NCT04276493).
Inhibitors of angiogenic or oncogenic kinases can also be combined with immunotherapy. Regorafenib, a potent inhibitor of VEGF receptor 2, decreases regulatory T cells (Tregs) infilteration in the local tumors of patients with GC.108 Surprisingly, the combination of regorafenib plus nivolumab achieved an ORR of 44% in GC patients and 36% in MSS CRC patients. The REGONIVO study highlighted that targeted angiogenesis combined with anti-PD-1 is promising, and investigations in larger cohorts is needed.108
With the exception of PD-1/PD-L1 and CTLA-4, other immune checkpoint receptors, including lymphocyte gene 3 (LAG 3) and T cell immunoglobulin and mucin protein 3 (TIM 3), are co-expressed on exhausted PD-1+ T cells in the TME, promoting antitumor immune evasion.109 LAG 3 and TIM 3 may correlate with the prognosis of esophagogastric cancer and CRC.110, 111, 112, 113, 114, 115 The clinical response of the combination of anti-LAG 3 or anti-TIM 3 with ICI still needs further investigation (NCT04140500, NCT01968109, NCT03662659, NCT03708328, NCT03652077, and NCT02817633).
The combination of immunotherapy and radiotherapy is another popular aspect in GI cancer, especially for patients with locally advanced disease receiving neoadjuvant treatment. The potential synergistic effects of this combination on local and distant tumor control have been fully discussed in preclinical studies, including activating dendritic cells, enhancing cross-presentation of tumor antigens, increasing the density of TILs, and regulating the expression of immune checkpoint molecules.116 Among clinical studies, Hong et al. reported a pathologic complete response (pCR) rate of 46.1% (12/26) in locally advanced ESCC patients who were administrated with preoperative chemoradiotherapy (CRT) and pembrolizumab, along with acceptable toxicity.117 Meanwhile, neoadjuvant nivolumab combined with concurrent CRT achieved a pCR rate of 40% in patients with stage II/III esophageal/gastroesophageal junction cancer.118 The results were similar in populations who received PD-L1 blockade.119,120 As expected in the Voltage study, both patients with locally advanced MSI-H rectal cancer showed pCR following CRT and nivolumab with subsequent radical surgery, while 30% (11/37) of MSS patients also achieved pCR.121 Of note, translational research found that a higher CD8+ T cell/effector Treg ratio, PD-L1 positivity, Ki67 expression by CD8+ T cells in TILs, consensus molecular subtype 1 or 3, and higher TMB were good predictors of the efficacy of the sequential combination of CRT and nivolumab in MSS patients.122 Along with the mainstream attention, several clinical factors should be taken into consideration. The optimal radiation dose and fractionation for a desired induced immune modulation or synergy with immunotherapy is still under investigation. Also, it is still unclear which therapeutic strategies, concurrent treatment or sequential treatment, could bring better efficacy in GI cancer. Furthermore, the potential higher risk of toxicities is a main concern in many studies.
Importantly, based on the complexity of the TME, a variety of combinations can be considered. However, alternative strategies without patient selection are unwise, and novel techniques to distinguish different TMEs are necessary.
Timing of Immunotherapy
ICI as a neoadjuvant therapy could result in a more effective prevention of tumor relapse than adjuvant therapy, and was recently explored in preclinical mouse models.123 The advantages of neoadjuvant immunotherapy can be attributed to several aspects. First, neoadjuvant immunotherapy induces broad immune activation, which is manifested by different activated T cell clones and dependent on exposure to broad classes of antigens.124,125 Thus, checkpoint inhibition may be more effective when a tumor is present than after complete resection. Second, the abundance of tumor samples collected after neoadjuvant treatment allows an unparalleled opportunity to explore the genetic signatures and molecular mechanisms correlated with therapeutic effects or drug resistance, and options for systemic adjuvant therapies can be reconsidered accordingly.126 In addition, tumor burden reduction before surgery could convert the unresectable disease to resectable disease, improve surgical resectability, and thus lower morbidity.127
With the widespread use of ICIs, more recent trials have focused on the value of these agents in the neoadjuvant setting. The NICHE study demonstrated that short preoperative treatment with nivolumab plus ipilimumab leads to 100% major pathological responses in 20 dMMR colon tumors, with 19 major pathological responses (≤10% residual viable tumor) and 12 pCR.128 Meanwhile, Zhang et al. also observed 100% pathological responses and 83% pCR in four GC and two CRC patients with MSI-H who received neoadjuvant ICIs.129 These excellent data indicate that neoadjuvant immunotherapy is a promising option for GI cancer patients.
In addition to colon cancer, clinical trials in other GI cancers have also been launched to evaluate the potential effect of neoadjuvant immunotherapy in GC (NCT03878472) and esophageal cancer (NCT04225364). A preliminary assessment of efficacy and response in these studies will provide significant insight into clinical outcomes as well as the impact of therapy on the systemic immune response.
Acquired Resistance to ICIs
Among patients who initially respond to ICIs, disease progression ultimately develops in 42%–71% of GI cancer patients.130 Only a small proportion of patients could achieve the long-term and durable clinical benefit from ICIs, while most patients would inevitably develop resistance to the therapy. Over time, patients who develop acquired resistance to ICIs account for a large proportion of the population and represent one of the most challenging problems that oncologists may face. Therefore, it is imperative that we better understand the mechanism of resistance and manage acquired resistance to circumvent or reverse it. Despite the fact that many uncertain mechanisms are still under investigation, those with hypothesized mechanisms of resistance are divided into the following categories: (1) defects in antigen presentation machinery; (2) defects in IFN-γ signaling; (3) neoantigen depletion; (4) tumor-mediated immunosuppression/exclusion; (5) increased immunosuppressive cells or factors; and (6) additional inhibitory checkpoints.130
On the basis of the insights gained,131, 132, 133 several approaches have been proposed and are currently under evaluation in an attempt to overcome the mechanisms of resistance to ICIs. Combination strategies using multiple treatment modalities are emerging to surmount the pre-existing insufficient immune response, ultimately turning “cold” tumors into “hot” tumors. Numerous strategies combining immune modulation of the TME with ICI therapy are currently being tested in clinical trials134 (NCT02263508, NCT02626000, NCT02565992, NCT02043665, and NCT02501473). In addition, a strong clinical and preclinical rationale supports the use of molecular targeted therapy (such as a BRAF inhibitor and anti-VEGF) in combination with immunotherapy, including enhanced antigen presentation,135,136 reduced immunosuppressive cytokines,137,138 and improved T cell infiltration and function.139 In addition, demethylating agents may enable the re-expression of immune-related genes and potentially improve the therapeutic effect, especially combined with immunotherapy.140,141 Apart from combination strategies, promoting the generation or presentation of proper neoantigens in TME is also being exploited through the administration of chemotherapy and radiotherapy (by inducing immunogenetic death),142 with some questions to be solved (e.g., sequencing, timing, and radiation fractionation). Cancer vaccines which perform against the identified neoantigens are also being combined with immunotherapeutic agents, although mature data of the clinical benefit are not available.130
Conclusion
In conclusion, the treatment of advanced GI cancer remains an area of great unmet medical need, and immunotherapy has presented promising effects across all lines of therapy. The ultimate goal of precision immunotherapy for cancer is to select a subset of patients with a common biological basis of cancer who are most likely to benefit from a particular immunotherapy. Hopefully, with the development of precise biomarkers, new immunotherapeutic strategies, and feasible combinations, precision cancer immunology will be applied more widely to treat GI cancer.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2017YFC1308900) and the National Key Research and Development Program of China (2018YFC1313303).
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jian Li, Email: oncogene@163.com.
Lin Shen, Email: shenlin@bjmu.edu.cn.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ren C., Xu R.H. The drug treatment research of gastrointestinal cancer in China. Eur. J. Surg. Oncol. 2020 doi: 10.1016/j.ejso.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Wei K.R., Zeng H., Zheng R., Zhang S., An L., Chen R., Wang S., Sun K., Matsuda T., Bray F., He J. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 5.Doi T., Piha-Paul S.A., Jalal S.I., Saraf S., Lunceford J., Koshiji M., Bennouna J. Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J. Clin. Oncol. 2018;36:61–67. doi: 10.1200/JCO.2017.74.9846. [DOI] [PubMed] [Google Scholar]
- 6.Shah M.A., Kojima T., Hochhauser D., Enzinger P., Raimbourg J., Hollebecque A., Lordick F., Kim S.B., Tajika M., Kim H.T., et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5:546–550. doi: 10.1001/jamaoncol.2018.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima T., Muro K., Francois E., Hsu C.-H., Moriwaki T., Kim S.-B., Lee S.-H., Bennouna J., Kato K., Lin S., et al. Pembrolizumab versus chemotherapy as second-line therapy for advanced esophageal cancer: phase III KEYNOTE-181 study. J. Clin. Oncol. 2019;37(4_suppl):2. [Google Scholar]
- 8.Kato K., Cho B.C., Takahashi M., Okada M., Lin C.Y., Chin K., Kadowaki S., Ahn M.J., Hamamoto Y., Doki Y., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 9.Huang J., Xu J., Chen Y., Zhuang W., Zhang Y., Chen Z., Chen J., Zhang H., Niu Z., Fan Q., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 10.Kang Y.-K., Boku N., Satoh T., Ryu M.H., Chao Y., Kato K., Chung H.C., Chen J.S., Muro K., Kang W.K., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs C.S., Doi T., Jang R.W., Muro K., Satoh T., Machado M., Sun W., Jalal S.I., Shah M.A., Metges J.P., et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs C.S., Özgüroğlu M., Bang Y.-J., Di Bartolomeo M., Mandalà M., Ryu M.-h., Fornaro L., Olesinski T., Caglevic C., Chung H.C., et al. Pembrolizumab versus paclitaxel for previously treated patients with PD-L1-positive advanced gastric or gastroesophageal junction cancer (GC): update from the phase III KEYNOTE-061 trial. J. Clin. Oncol. 2020;38(15_suppl):4503. doi: 10.1007/s10120-021-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bang Y.J., Ruiz E.Y., Van Cutsem E., Lee K.W., Wyrwicz L., Schenker M., Alsina M., Ryu M.H., Chung H.C., Evesque L., et al. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018;29:2052–2060. doi: 10.1093/annonc/mdy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabernero J., Van Cutsem E., Bang Y.-J., Fuchs C.S., Wyrwicz L., Lee K.W., Kudaba I., Garrido M., Chung H.C., Castro Salguero H.R., et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J. Clin. Oncol. 2019;37(18_suppl):LBA4007. [Google Scholar]
- 15.Satake H., Lee K.W., Chung H.C., Lee J., Yamaguchi K., Chen J.-S., Yoshikawa T., Amagai K., Yeh K.-H., Goto M., et al. Pembrolizumab (pembro) versus standard of care chemotherapy (chemo) in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Asian subgroup analysis of KEYNOTE-062. J. Clin. Oncol. 2020;38(15_suppl):4523. doi: 10.1093/jjco/hyac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wainberg Z.A., Fuchs C.S., Tabernero J., Shitara K., Muro K., Van Cutsem E., Bang Y.-J., Chung H.C., Yamaguchi K., Varga E., et al. Efficacy of pembrolizumab (pembro) monotherapy versus chemotherapy for PD-L1-positive (CPS ≥10) advanced G/GEJ cancer in the phase II KEYNOTE-059 (cohort 1) and phase III KEYNOTE-061 and KEYNOTE-062 studies. J. Clin. Oncol. 2020;38(4_suppl):427. [Google Scholar]
- 17.Boku N., Ryu M.H., Kato K., Chung H.C., Minashi K., Lee K.W., Cho H., Kang W.K., Komatsu Y., Tsuda M., et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4) Ann. Oncol. 2019;30:250–258. doi: 10.1093/annonc/mdy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabernero J., Yoshino T., Cohn A.L., Obermannova R., Bodoky G., Garcia-Carbonero R., Ciuleanu T.E., Portnoy D.C., Van Cutsem E., Grothey A., et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 19.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.-J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.-J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): long-term survival according to prior line of treatment from CheckMate-142. J. Clin. Oncol. 2018;36(suppl 4S) abstr 554. [Google Scholar]
- 21.Lee D.T., Kavan P., Kim T.W., Burge M.E., Van Cutsem E., Hara H., McKay Boland P., Van Laethem J.-L., Geva R., Taniguchi H., et al. KEYNOTE-164: pembrolizumab for patients with advanced microsatellite instability high (MSI-H) colorectal cancer. J. Clin. Oncol. 2018;36(15_suppl) doi: 10.1200/JCO.2018.36.15_suppl.3514. abstract 3514. [DOI] [Google Scholar]
- 22.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal N.H., Wainberg Z.A., Overman M.J., Ascierto P.A., Arkenau H.-T., Butler M.O., Eder J.P., Keilholz U., Kim D.-W., Cunningham D., et al. Safety and clinical activity of durvalumab monotherapy in patients with microsatellite instability–high (MSI-H) tumors. J. Clin. Oncol. 2019;37(4_suppl) doi: 10.1200/JCO.2019.37.4_suppl.670. abstract 670. [DOI] [Google Scholar]
- 24.Lenz H.-J.J., Van Cutsem E., Limon M.L., Wong K.Y., Hendisz A., Aglietta M., Garcia-Alfonso P., Neyns B., Luppi G., Cardin D., et al. Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) Ann. Oncol. 2018;29(suppl_8):LBA18_PR. [Google Scholar]
- 25.Andre T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C.J.A., Smith D.M., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 Study. J. Clin. Oncol. 2020;38(18_suppl):LBA4. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 27.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.-J., Gelsomino F., Aglietta M., Morse M., Van Cutsem E., McDermott R.S., Hill A.G., et al. Nivolumab (NIVO) + low-dose ipilimumab (IPI) in previously treated patients (pts) with microsatellite instability-high/mismatch repair-deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC): long-term follow-up. J. Clin. Oncol. 2019;37(4_suppl):635. [Google Scholar]
- 28.Janjigian Y.Y., Bendell J., Calvo E., Kim J.W., Ascierto P.A., Sharma P., Ott P.A., Peltola K., Jaeger D., Evans J., et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J. Clin. Oncol. 2018;36:2836–2844. doi: 10.1200/JCO.2017.76.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venderbosch S., Nagtegaal I.D., Maughan T.S., Smith C.G., Cheadle J.P., Fisher D., Kaplan R., Quirke P., Seymour M.T., Richman S.D., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaeger R., Chatila W.K., Lipsyc M.D., Hechtman J.F., Cercek A., Sanchez-Vega F., Jayakumaran G., Middha S., Zehir A., Donoghue M.T.A., et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33:125–136.e3. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grothey A., Tabernero J., Arnold D., De Gramont A., Ducreux M.P., O'Dwyer P.J., Van Cutsem E., Bosanac I., Srock S., Mancao C., et al. Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): findings from Cohort 2 of MODUL—a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann. Oncol. 2018;29(suppl_8):viii714–viii715. [Google Scholar]
- 32.Eng C., Kim T.W., Bendell J., Argilés G., Tebbutt N.C., Di Bartolomeo M., Falcone A., Fakih M., Kozloff M., Segal N.H., et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20:849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 33.Overman M.J., Kopetz S., Lonardi S., McDermott R., Leone F., Leach J., Lenz H.-J., Hendlisz A., Morse M., Alfonso P.G., et al. Nivolumab ± ipilimumab treatment (Tx) efficacy, safety, and biomarkers in patients (Pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): results from the CheckMate-142 study. Ann. Oncol. 2016;27(suppl_6):479P. [Google Scholar]
- 34.Chen E.X., Jonker D.J., Kennecke H.F., Berry S.R., Couture F., Ahmad C.E., Goffin J.R., Kavan P., Harb M., Colwell B., et al. CCTG CO.26 trial: a phase II randomized study of durvalumab (D) plus tremelimumab (T) and best supportive care (BSC) versus BSC alone in patients (pts) with advanced refractory colorectal carcinoma (rCRC) J. Clin. Oncol. 2019;37(4_suppl):481. [Google Scholar]
- 35.Chen E.X., Jonker D.J., Loree J.M., Kennecke H.F., Berry S.R., Couture F., Ahmad C.E., Goffin J.R., Kavan P., Harb M., et al. CCTG CO.26: updated analysis and impact of plasma-detected microsatellite stability (MSS) and tumor mutation burden (TMB) in a phase II trial of durvalumab (D) plus tremelimumab (T) and best supportive care (BSC) versus BSC alone in patients (pts) with refractory metastatic colorectal carcinoma (rmCRC) J. Clin. Oncol. 2019;37(15_suppl):3512. [Google Scholar]
- 36.Lee J.J., Chu E. Recent advances in the clinical development of immune checkpoint blockade therapy for mismatch repair proficient (pMMR)/non-MSI-H metastatic colorectal cancer. Clin. Colorectal Cancer. 2018;17:258–273. doi: 10.1016/j.clcc.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R., et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miliotou A.N., Papadopoulou L.C. CAR T-cell therapy: a new era in cancer immunotherapy. Curr. Pharm. Biotechnol. 2018;19:5–18. doi: 10.2174/1389201019666180418095526. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Wang Y., Wang H. Use of immunotherapy in the treatment of gastric cancer. Oncol. Lett. 2019;18:5681–5690. doi: 10.3892/ol.2019.10935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma S., Li X., Wang X., Cheng L., Li Z., Zhang C., Ye Z., Qian Q. Current progress in CAR-T cell therapy for solid tumors. Int. J. Biol. Sci. 2019;15:2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakarla S., Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20:151–155. doi: 10.1097/PPO.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C., Wang Z., Yang Z., Wang M., Li S., Li Y., Zhang R., Xiong Z., Wei Z., Shen J., et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol. Ther. 2017;25:1248–1258. doi: 10.1016/j.ymthe.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdul-Latif M., Townsend K., Dearman C., Shiu K.K., Khan K. Immunotherapy in gastrointestinal cancer: the current scenario and future perspectives. Cancer Treat. Rev. 2020;88:102030. doi: 10.1016/j.ctrv.2020.102030. [DOI] [PubMed] [Google Scholar]
- 45.Rao D., Parakrama R., Augustine T., Liu Q., Goel S., Maitra R. Immunotherapeutic advances in gastrointestinal malignancies. NPJ Precis. Oncol. 2019;3:4. doi: 10.1038/s41698-018-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuler M.H., Al-Batran S.-E., Zvirbule Z., Manikhas G., Lordick F., Rusyn A.V., Vynnychenko I., Dudov A., Bazin I., Melichar B., et al. Expression of Claudin 18.2 and HER2 in gastric, gastroesophageal junction, and esophageal cancers: results from the FAST study. J. Clin. Oncol. 2017;35(15_suppl):4038. [Google Scholar]
- 47.Zhan X., Wang B., Li Z., Li J., Wang H., Chen L., Jiang H., Wu M., Xiao J., Peng X., et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma. J. Clin. Oncol. 2019;37(15_suppl):2509. [Google Scholar]
- 48.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melief C.J., van Hall T., Arens R., Ossendorp F., van der Burg S.H. Therapeutic cancer vaccines. J. Clin. Invest. 2015;125:3401–3412. doi: 10.1172/JCI80009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efremova M., Finotello F., Rieder D., Trajanoski Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front. Immunol. 2017;8:1679. doi: 10.3389/fimmu.2017.01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J., Liu Q., Sha H., Qian H., Shao J., Zhu L., Wang L., Li R., Liu B. Personalized neoantigen/cancer testis antigen nanovaccine (PVAC) in combination with PD-1 monoclonal antibody and/or antiangiogenic treatment in patients with metastatic solid tumors. J. Clin. Oncol. 2020;38(15_suppl):3134. [Google Scholar]
- 52.Liu Q., Qian H., Shao J., Xu Q., Sha H., Yang Y., Wang W., Yu L., Liu B., Wei J. Personalized neoantigen/cancer testis antigen nanovaccine (PVAC) mobilize specific therapeutic immunity for high-risk resected gastric cancer. J. Clin. Oncol. 2020;38(15_suppl):4535. [Google Scholar]
- 53.Comoli P., Chabannon C., Koehl U., Lanza F., Urbano-Ispizua A., Hudecek M., Ruggeri A., Secondino S., Bonini C., Pedrazzoli P. Development of adaptive immune effector therapies in solid tumors. Ann. Oncol. 2019;30:1740–1750. doi: 10.1093/annonc/mdz285. [DOI] [PubMed] [Google Scholar]
- 54.Chmielewski M., Abken H. TRUCKs: the fourth generation of CARs. Expert Opin. Biol. Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L., Kerkar S.P., Yu Z., Zheng Z., Yang S., Restifo N.P., Rosenberg S.A., Morgan R.A. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol. Ther. 2011;19:751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chmielewski M., Kopecky C., Hombach A.A., Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 2011;71:5697–5706. doi: 10.1158/0008-5472.CAN-11-0103. [DOI] [PubMed] [Google Scholar]
- 57.Heczey A., Louis C.U., Savoldo B., Dakhova O., Durett A., Grilley B., Liu H., Wu M.F., Mei Z., Gee A., et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 2017;25:2214–2224. doi: 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mougel A., Terme M., Tanchot C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front. Immunol. 2019;10:467. doi: 10.3389/fimmu.2019.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hodge J.W., Ardiani A., Farsaci B., Kwilas A.R., Gameiro S.R. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin. Oncol. 2012;39:323–339. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chau I. Clinical development of PD-1/PD-L1 immunotherapy for gastrointestinal cancers: facts and hopes. Clin. Cancer Res. 2017;23:6002–6011. doi: 10.1158/1078-0432.CCR-17-0020. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.T., Cristescu R., Bass A.J., Kim K.M., Odegaard J.I., Kim K., Liu X.Q., Sher X., Jung H., Lee M., et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 62.Chao J., Fuchs C.S., Shitara K. Pembrolizumab (pembro) in microsatellite instability-high (MSI-H) advanced gastric/gastroesophageal junction (G/GEJ) cancer by line of therapy. J. Clin. Oncol. 2020;38(suppl 4):2020. abstr 430. [Google Scholar]
- 63.Shitara K., Özgüroğlu M., Bang Y.J., Di Bartolomeo M., Mandalà M., Ryu M.H., Fornaro L., Olesiński T., Caglevic C., Chung H.C., et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 64.O'Neil B.H., Wallmark J.M., Lorente D., Elez E., Raimbourg J., Gomez-Roca C., Ejadi S., Piha-Paul S.A., Stein M.N., Abdul Razak A.R., et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12:e0189848. doi: 10.1371/journal.pone.0189848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chalmers Z.R., Connelly C.F., Fabrizio D., Gay L., Ali S.M., Ennis R., Schrock A., Campbell B., Shlien A., Chmielecki J., et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marabelle A., Fakih M.G., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W., et al. Association of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Ann. Oncol. 2019;30(suppl_5):v477–v478. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 68.Samstein R.M., Lee C.H., Shoushtari A.N., Hellmann M.D., Shen R., Janjigian Y.Y., Barron D.A., Zehir A., Jordan E.J., Omuro A., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019;51:202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui Y., Chen H., Xi R., Cui H., Zhao Y., Xu E., Yan T., Lu X., Huang F., Kong P., et al. Whole-genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res. 2020 doi: 10.1038/s41422-020-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shitara K., Özgüroğlu M., Bang Y.-J., Di Bartolomeo M., Mandalà M., Ryu M.-h., Vivaldi C., Olesinski T., Chung H.C., Muro K., et al. The association of tissue tumor mutational burden (tTMB) using the Foundation Medicine genomic platform with efficacy of pembrolizumab versus paclitaxel in patients (pts) with gastric cancer (GC) from KEYNOTE-061. J. Clin. Oncol. 2020;38(15_suppl):4537. [Google Scholar]
- 71.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lordick F., Janjigian Y.Y. Clinical impact of tumour biology in the management of gastroesophageal cancer. Nat. Rev. Clin. Oncol. 2016;13:348–360. doi: 10.1038/nrclinonc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang F., Wei X., Wang F., Xu N., Shen L., Dai G., et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019;30:1479–1486. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie T., Liu Y., Zhang Z., Zhang X., Gong J., Qi C., Li J., Shen L., Peng Z. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J. Immunother. 2020;43:139–144. doi: 10.1097/CJI.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 76.Vetizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng Z., Cheng S., Kou Y., Wang Z., Jin R., Hu N., et al. Predictive Biomarkers for Treatment Efficacy 3 (Virtual Poster Session) American Association for Cancer Research; 2020. The gut microbiome is associated with clinical responses to anti-PD-1 immunotherapy in gastrointestinal cancer patients in China; p. 4298. [Google Scholar]
- 79.Lee J.S., Won H.S., Sun S., Hong J.H., Ko Y.H. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11769. doi: 10.1097/MD.0000000000011769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng X., Song X., Shao Y., Xu B., Hu W., Zhou Q., Chen L., Zhang D., Wu C., Jiang J. Prognostic role of tumor-infiltrating lymphocytes in esophagus cancer: a meta-analysis. Cell. Physiol. Biochem. 2018;45:720–732. doi: 10.1159/000487164. [DOI] [PubMed] [Google Scholar]
- 81.Mei Z., Liu Y., Liu C., Cui A., Liang Z., Wang G., Peng H., Cui L., Li C. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br. J. Cancer. 2014;110:1595–1605. doi: 10.1038/bjc.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon H.H., Shi Q., Heying E.N., Muranyi A., Bredno J., Ough F., Djalilvand A., Clements J., Bowermaster R., Liu W.W., et al. Intertumoral heterogeneity of CD3(+) and CD8(+) T-cell densities in the microenvironment of DNA mismatch-repair-deficient colon cancers: implications for prognosis. Clin. Cancer Res. 2019;25:125–133. doi: 10.1158/1078-0432.CCR-18-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loupakis F., Depetris I., Biason P., Intini R., Prete A.A., Leone F., Lombardi P., Filippi R., Spallanzani A., Cascinu S., et al. Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: role of tumor infiltrating lymphocytes. Oncologist. 2020;25:481–487. doi: 10.1634/theoncologist.2019-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrucci P.F., Ascierto P.A., Pigozzo J., Del Vecchio M., Maio M., Antonini Cappellini G.C., Guidoboni M., Queirolo P., Savoia P., Mandalà M., et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann. Oncol. 2016;27:732–738. doi: 10.1093/annonc/mdw016. [DOI] [PubMed] [Google Scholar]
- 85.Weide B., Martens A., Hassel J.C., Berking C., Postow M.A., Bisschop K., Simeone E., Mangana J., Schilling B., Di Giacomo A.M., et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clin. Cancer Res. 2016;22:5487–5496. doi: 10.1158/1078-0432.CCR-16-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mezquita L., Auclin E., Ferrara R., Charrier M., Remon J., Planchard D., Ponce S., Ares L.P., Leroy L., Audigier-Valette C., et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Delyon J., Mateus C., Lefeuvre D., Lanoy E., Zitvogel L., Chaput N., Roy S., Eggermont A.M., Routier E., Robert C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 2013;24:1697–1703. doi: 10.1093/annonc/mdt027. [DOI] [PubMed] [Google Scholar]
- 88.Kelderman S., Heemskerk B., van Tinteren H., van den Brom R.R., Hospers G.A., van den Eertwegh A.J., Kapiteijn E.W., de Groot J.W., Soetekouw P., Jansen R.L., et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol. Immunother. 2014;63:449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilgenhof S., Du Four S., Vandenbroucke F., Everaert H., Salmon I., Liénard D., Marmol V.D., Neyns B. Single-center experience with ipilimumab in an expanded access program for patients with pretreated advanced melanoma. J. Immunother. 2013;36:215–222. doi: 10.1097/CJI.0b013e31828eed39. [DOI] [PubMed] [Google Scholar]
- 90.Simeone E., Gentilcore G., Giannarelli D., Grimaldi A.M., Caracò C., Curvietto M., Esposito A., Paone M., Palla M., Cavalcanti E., et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol. Immunother. 2014;63:675–683. doi: 10.1007/s00262-014-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S., Zou J., Liu C., Jiao X., Gong J., Li J., Wang Z., Lu M., Lu Z., Shen L. Baseline derived neutrophil-to-lymphocyte ratio as a prognostic biomarker for non-colorectal gastrointestinal cancer patients treated with immune checkpoint blockade. Clin. Immunol. 2020;212:108345. doi: 10.1016/j.clim.2020.108345. [DOI] [PubMed] [Google Scholar]
- 92.Lu Z., Zou J., Hu Y., Li S., Zhou T., Gong J., Li J., Zhang X., Zhou J., Lu M., et al. Serological markers associated with response to immune checkpoint blockade in metastatic gastrointestinal tract cancer. JAMA Netw. Open. 2019;2:e197621. doi: 10.1001/jamanetworkopen.2019.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R., Albright A., Cheng J.D., Kang S.P., Shankaran V., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cristescu R., Mogg R., Ayers M., Albright A., Murphy E., Yearley J., Sher X., Liu X.Q., Lu H., Nebozhyn M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362:eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muro K., Chung H.C., Shankaran V., Geva R., Catenacci D., Gupta S., Eder J.P., Golan T., Le D.T., Burtness B., et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 96.Kelly R.J., Lee J., Bang Y.J., Almhanna K., Blum-Murphy M., Catenacci D.V.T., Chung H.C., Wainberg Z.A., Gibson M.K., Lee K.W., et al. Safety and efficacy of durvalumab and tremelimumab alone or in combination in patients with advanced gastric and gastroesophageal junction adenocarcinoma. Clin. Cancer Res. 2020;26:846–854. doi: 10.1158/1078-0432.CCR-19-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peng J., Zou D., Gong W., Kang S., Han L. Deep neural network classification based on somatic mutations potentially predicts clinical benefit of immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology. 2020;9:1734156. doi: 10.1080/2162402X.2020.1734156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richard C., Fumet J.D., Chevrier S., Derangère V., Ledys F., Lagrange A., Favier L., Coudert B., Arnould L., Truntzer C., et al. Exome analysis reveals genomic markers associated with better efficacy of nivolumab in lung cancer patients. Clin. Cancer Res. 2019;25:957–966. doi: 10.1158/1078-0432.CCR-18-1940. [DOI] [PubMed] [Google Scholar]
- 99.Harel M., Ortenberg R., Varanasi S.K., Mangalhara K.C., Mardamshina M., Markovits E., Baruch E.N., Tripple V., Arama-Chayoth M., Greenberg E., et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell. 2019;179:236–250.e18. doi: 10.1016/j.cell.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Langer C., Gadgeel S., Borghaei H., Patnaik A., Powell S., Gentzler R., Yang J.C., Gubens M., Sequist L., Awad M., et al. OA04.05 KEYNOTE-021: TMB and outcomes for carboplatin and pemetrexed with or without pembrolizumab for nonsquamous NSCLC. J. Thorac. Oncol. 2019;14:S216. [Google Scholar]
- 101.Garassino M., Rodriguez-Abreu D., Gadgeel S., Esteban E., Felip E., Speranza G., Reck M., Hui R., Boyer M., Cristescu R., et al. OA04.06 evaluation of TMB in KEYNOTE-189: pembrolizumab plus chemotherapy vs placebo plus chemotherapy for nonsquamous NSCLC. J. Thorac. Oncol. 2019;14:S216–S217. [Google Scholar]
- 102.Smyth M.J., Ngiow S.F., Ribas A., Teng M.W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016;13:143–158. doi: 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 103.Taylor C., Hershman D., Shah N., Suciu-Foca N., Petrylak D.P., Taub R., Vahdat L., Cheng B., Pegram M., Knutson K.L., Clynes R. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin. Cancer Res. 2007;13:5133–5143. doi: 10.1158/1078-0432.CCR-07-0507. [DOI] [PubMed] [Google Scholar]
- 104.Mortenson E.D., Park S., Jiang Z., Wang S., Fu Y.X. Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin. Cancer Res. 2013;19:1476–1486. doi: 10.1158/1078-0432.CCR-12-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park S., Jiang Z., Mortenson E.D., Deng L., Radkevich-Brown O., Yang X., Sattar H., Wang Y., Brown N.K., Greene M., et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–170. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Janjigian Y.Y., Maron S.B., Chatila W.K., Millang B., Chavan S.S., Alterman C., Chou J.F., Segal M.F., Simmons M.Z., Momtaz P., et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–831. doi: 10.1016/S1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Catenacci D.V.T., Kang Y.K., Park H., Uronis H.E., Lee K.W., Ng M.C.H., Enzinger P.C., Park S.H., Gold P.J., Lacy J., et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066–1076. doi: 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 108.Fukuoka S., Hara H., Takahashi N., Kojima T., Kawazoe A., Asayama M., Yoshii T., Kotani D., Tamura H., Mikamoto Y., et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603) J. Clin. Oncol. 2020;38:2053–2061. doi: 10.1200/JCO.19.03296. [DOI] [PubMed] [Google Scholar]
- 109.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kato R., Yamasaki M., Urakawa S., Nishida K., Makino T., Morimoto-Okazawa A., Kawashima A., Iwahori K., Suzuki S., Ueda R., et al. Increased Tim-3(+) T cells in PBMCs during nivolumab therapy correlate with responses and prognosis of advanced esophageal squamous cell carcinoma patients. Cancer Immunol. Immunother. 2018;67:1673–1683. doi: 10.1007/s00262-018-2225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Zhao E., Zhang Z., Zhao G., Cao H. Association between Tim3 and Gal9 expression and gastric cancer prognosis. Oncol. Rep. 2018;40:2115–2126. doi: 10.3892/or.2018.6627. [DOI] [PubMed] [Google Scholar]
- 112.Kuai W., Xu X., Yan J., Zhao W., Li Y., Wang B., Yuan N., Li Z., Jia Y. Prognostic impact of PD-1 and Tim-3 expression in tumor tissue in stage I-III colorectal cancer. Biomed. Res. Int. 2020;2020:5294043. doi: 10.1155/2020/5294043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Saleh R., Taha R.Z., Toor S.M., Sasidharan Nair V., Murshed K., Khawar M., Al-Dhaheri M., Petkar M.A., Abu Nada M., Elkord E. Expression of immune checkpoints and T cell exhaustion markers in early and advanced stages of colorectal cancer. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02593-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohmura H., Yamaguchi K., Hanamura F., Ito M., Makiyama A., Uchino K., Shimokawa H., Tamura S., Esaki T., Mitsugi K., et al. OX40 and LAG3 are associated with better prognosis in advanced gastric cancer patients treated with anti-programmed death-1 antibody. Br. J. Cancer. 2020;122:1507–1517. doi: 10.1038/s41416-020-0810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W., Chen D., Zhao Y., Zhao T., Wen J., Mao Y., Chen C., Sang Y., Zhang Y., Chen Y. Characterization of LAG-3, CTLA-4, and CD8(+) TIL density and their joint influence on the prognosis of patients with esophageal squamous cell carcinoma. Ann. Transl Med. 2019;7:776. doi: 10.21037/atm.2019.11.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharabi A.B., Lim M., DeWeese T.L., Drake C.G. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 117.Hong M.H., Kim H., Park S.Y., Kim D.J., Lee C.G., Cho J., Kim J.H., Kim H.R., Kim Y.-H., Park S.R., Cho B.C. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC) J. Clin. Oncol. 2019;37(15_suppl):4027. [Google Scholar]
- 118.Kelly R.J., Smith K.N., Anagnostou V., Thompson E., Hales R.K., Battafarano R.J.J., Voong K.R., Yang S.C., Feliciano J.L., Shin E.J., et al. Neoadjuvant nivolumab plus concurrent chemoradiation in stage II/III esophageal/gastroesophageal junction cancer. J. Clin. Oncol. 2019;37(4_suppl):142. [Google Scholar]
- 119.Uboha N.V., Maloney J.D., McCarthy D., Deming D.A., LoConte N.K., Matkowskyj K., Eickhoff J.C., Emmerich P., DeCamp M.M., Lubner S.J., et al. Safety of neoadjuvant chemoradiation (CRT) in combination with avelumab (A) in the treatment of resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J. Clin. Oncol. 2019;37(15_suppl):4041. [Google Scholar]
- 120.van den Ende T., de Clercq N.C., van Berge Henegouwen M.I., Gisbertz S.S., Meijer S.L., Schokker S., Bergman J.J.G.H.M., Mohammad N.H., Ruurda J., van Hillegersberg R., et al. A phase II feasibility trial of neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: the PERFECT trial. J. Clin. Oncol. 2019;37(15_suppl):4045. [Google Scholar]
- 121.Yoshino T., Bando H., Tsukada Y., Inamori K., Yuki S., Komatsu Y., Homma S., Uemura M., Kato T., Kotani D., et al. Voltage: investigator-initiated clinical trial of nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable locally advanced rectal cancer. J. Clin. Oncol. 2019;37(15_suppl):3606. [Google Scholar]
- 122.Inamori K., Togashi Y., Bando H., Tsukada Y., Suzuki A., Suzuki Y., Kotani D., Fukuoka S., Kojima M., Fukui M., et al. Translational research of voltage-A1: efficacy predictors of preoperative chemoradiotherapy and subsequent nivolumab monotherapy in patients with microsatellite-stable locally advanced rectal cancer. J. Clin. Oncol. 2020;38(15_suppl):4073. [Google Scholar]
- 123.Liu J., Blake S.J., Yong M.C., Harjunpää H., Ngiow S.F., Takeda K., Young A., O'Donnell J.S., Allen S., Smyth M.J., Teng M.W. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6:1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- 124.Akondy R.S., Johnson P.L., Nakaya H.I., Edupuganti S., Mulligan M.J., Lawson B., Miller J.D., Pulendran B., Antia R., Ahmed R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. U S A. 2015;112:3050–3055. doi: 10.1073/pnas.1500475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Akondy R.S., Monson N.D., Miller J.D., Edupuganti S., Teuwen D., Wu H., Quyyumi F., Garg S., Altman J.D., Del Rio C., et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tetzlaff M.T., Messina J.L., Stein J.E., Xu X., Amaria R.N., Blank C.U., van de Wiel B.A., Ferguson P.M., Rawson R.V., Ross M.I., et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 2018;29:1861–1868. doi: 10.1093/annonc/mdy226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schermers B., Franke V., Rozeman E.A., van de Wiel B.A., Bruining A., Wouters M.W., van Houdt W.J., Ten Haken B., Muller S.H., Bierman C., et al. Surgical removal of the index node marked using magnetic seed localization to assess response to neoadjuvant immunotherapy in patients with stage III melanoma. Br. J. Surg. 2019;106:519–522. doi: 10.1002/bjs.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chalabi M., Fanchi L.F., Dijkstra K.K., Van den Berg J.G., Aalbers A.G., Sikorska K., Lopez-Yurda M., Grootscholten C., Beets G.L., Snaebjornsson P., et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020;26:566–576. doi: 10.1038/s41591-020-0805-8. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Z., Cheng S., Gong J., Lu M., Zhou J., Zhang X., Li J., Shen L., Peng Z. Efficacy and safety of neoadjuvant immunotherapy in patients with microsatellite instability-high gastrointestinal malignancies: a case series. Eur. J. Surg. Oncol. 2020 doi: 10.1016/j.ejso.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 130.Schoenfeld A.J., Hellmann M.D. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37:443–455. doi: 10.1016/j.ccell.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hugo W., Zaretsky J.M., Sun L., Song C., Moreno B.H., Hu-Lieskovan S., Berent-Maoz B., Pang J., Chmielowski B., Cherry G., et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., Walsh L.A., Postow M.A., Wong P., Ho T.S., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Allen E.M., Miao D., Schilling B., Shukla S.A., Blank C., Zimmer L., Sucker A., Hillen U., Foppen M.H.G., Goldinger S.M., et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J., et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boni A., Cogdill A.P., Dang P., Udayakumar D., Njauw C.N., Sloss C.M., Ferrone C.R., Flaherty K.T., Lawrence D.P., Fisher D.E., et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 136.Bradley S.D., Chen Z., Melendez B., Talukder A., Khalili J.S., Rodriguez-Cruz T., Liu S., Whittington M., Deng W., Li F., et al. BRAFV600E Co-opts a conserved MHC class I internalization pathway to diminish antigen presentation and CD8+ T-cell recognition of melanoma. Cancer Immunol. Res. 2015;3:602–609. doi: 10.1158/2326-6066.CIR-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frederick D.T., Piris A., Cogdill A.P., Cooper Z.A., Lezcano C., Ferrone C.R., Mitra D., Boni A., Newton L.P., Liu C., et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin. Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilmott J.S., Long G.V., Howle J.R., Haydu L.E., Sharma R.N., Thompson J.F., Kefford R.F., Hersey P., Scolyer R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 139.Comin-Anduix B., Chodon T., Sazegar H., Matsunaga D., Mock S., Jalil J., Escuin-Ordinas H., Chmielowski B., Koya R.C., Ribas A. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin. Cancer Res. 2010;16:6040–6048. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Loo Yau H., Ettayebi I., De Carvalho D.D. The cancer epigenome: exploiting its vulnerabilities for immunotherapy. Trends Cell Biol. 2019;29:31–43. doi: 10.1016/j.tcb.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 141.Topper M.J., Vaz M., Marrone K.A., Brahmer J.R., Baylin S.B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 2020;17:75–90. doi: 10.1038/s41571-019-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O'Donnell J.S., Long G.V., Scolyer R.A., Teng M.W., Smyth M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]