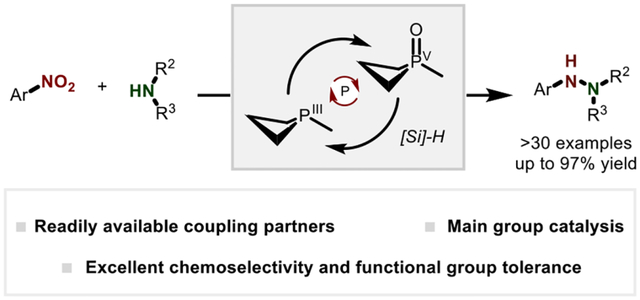
Abstract
An organophosphorus-catalyzed method for the synthesis of unsymmetrical hydrazines by cross-selective intermolecular N–N reductive coupling is reported. This method employs a small ring phosphacycle (phosphetane) catalyst together with hydrosilane as the terminal reductant to drive reductive coupling of nitroarenes and anilines with good chemoselectivity and functional-group tolerance. Mechanistic investigations support an auto-tandem catalytic reaction cascade in which the organophosphorus catalyst drives two sequential and mechanistically-distinct reduction events via PIII/PV=O cycling in order to furnish the target N–N bond.
Graphical Abstract
Hydrazines and related N–N containing derivatives have significant value in organic chemistry,1,2 including as natural products3 and pharmaceuticals (Figure 1A).4,5 Although the NN bond presents a potential strategic site for synthesis—especially within medical chemistry—it is only infrequently targeted for retrosynthetic disconnection. In part, this state of play reflects certain constraints in the methods for N–N bond formation, particularly in an intermolecular sense.6 , 7 Stoichiometric methods employ prefunctionalized N-based reagents whose stability and structural variation are intrinsically bracketed by the high electronegativity of the nitrogen atom.8–14 Complementarily, several notable advances have recently been achieved in catalytic intermolecular N–N bond formation,15 – 18 despite the inherent challenges associated with synthesis of such a weak and nonpolar bond.19 , 20 Transition metal-catalyzed nitrene transfer has successfully been applied to the imination of tertiary amines15–17 and the N–H insertion of acylnitrene equivalents to N-alkylanilines,18 specifically by decomposition of bespoke nitrene donors such as iminoiodinanes and 1,4,2-dioxazol-5-ones (Figure 1B). Among unfunctionalized precursors, simple diarylamine/carbazole substrates have been subject to intermolecular oxidative N–N coupling under aerobic Cu-catalyzed21 – 23, Fe-catalyzed24 or anodic electrochemical conditions,25 although the realization of cross selectivity remains substrate dependent.
Figure 1.
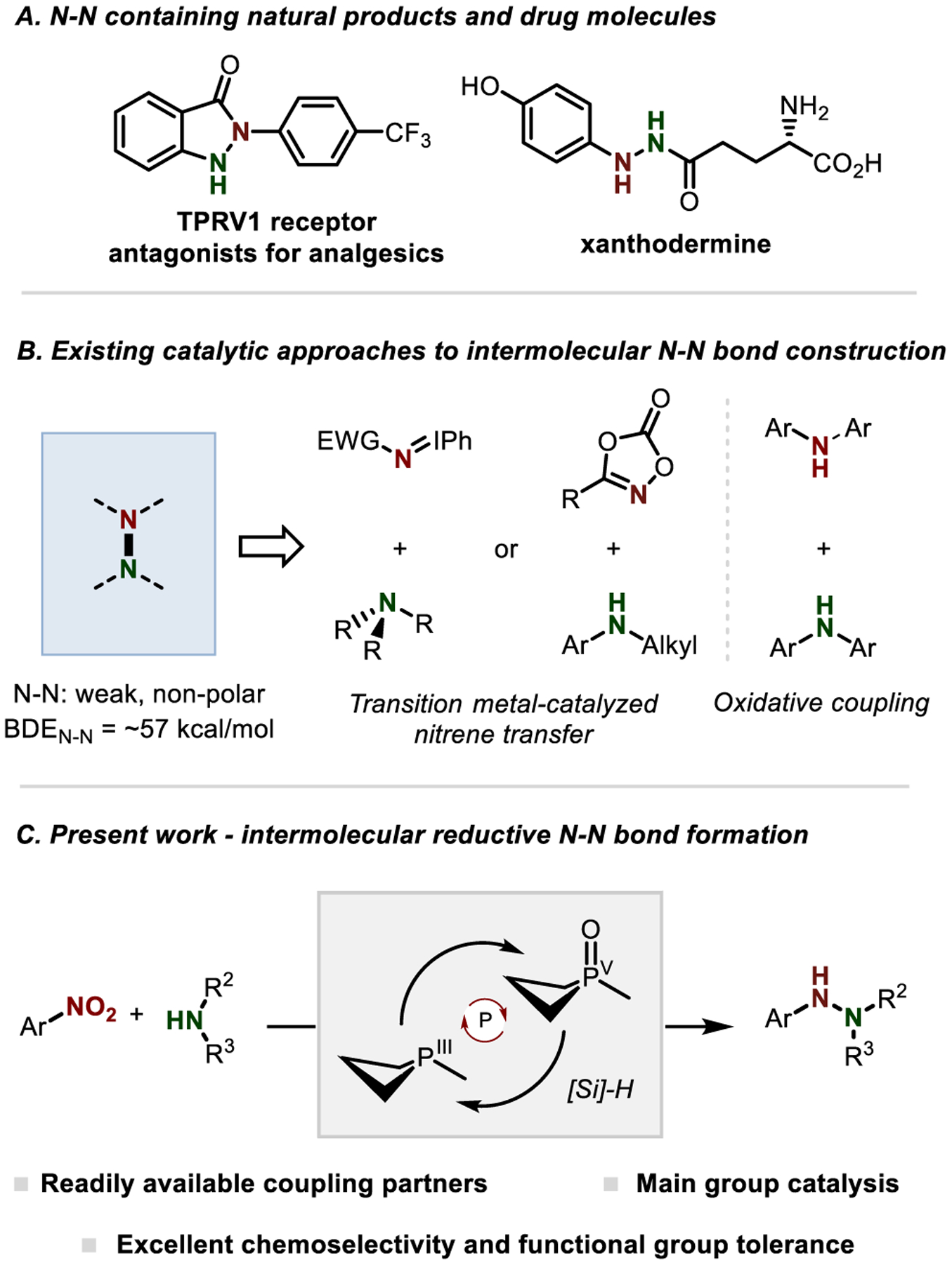
A) N–N containing natural products and drug molecules; B) Current strategies for N–N bond construction; C) Present work: PIII/PV=O-catalyzed reductive N–N coupling of nitroarenes and anilines.
With the goal of advancing the perception of N–N bonds as strategic sites in synthesis, we aimed to develop an approach to N–N bond formation that would leverage simple precursor substrates for precise and selective intermolecular coupling. Nitroarenes are readily-accessed compounds with increasing use as direct amination reagents.26–28 In this vein, prior work has established the viability of organophosphorus-catalyzed reductive N-functionalization of nitroarenes by PIII/PV=O cycling.29 – 36 We considered whether this reactive manifold might enable new cross-selective intermolecular N–N bond forming reactivity by the introduction of an exogenous aniline partner. Herein, we describe the realization of method for the synthesis of unsymmetrical hydrazines by reductive N–N coupling of readily-available nitroarene and aniline substrates (Figure 1C). These results provide a modular and cross-selective N–N coupling strategy enabled by the versatility of the PIII/PV=O redox couple37 – 40 to manage diverse reductive transformations en route to the target N–N bond.
The reaction of 4-nitrobenzonitrile (2) and 4-fluoroaniline (3) to generate hydrazine 4 was selected to evaluate the possibility of reductive intermolecular N–N coupling via PIII/PV=O catalysis. Using 1,2,2,3,4,4-hexamethylphosphetane P-oxide 1·[O]41 as catalyst, diphenylsilane as terminal reductant and 2,4,6-trimethylbenzoic acid as an additive, the desired hydrazine 4 was indeed obtained with complete cross selectivity in 86 % yield after 24 h (Table 1, entry 1). Further experiments show that utilization of phosphetane 1 in the place of phosphetane oxide 1·[O] also smoothly provided hydrazine 4, in line with the notion of PIII/PV=O redox cycling (entry 2). Lower catalyst loading (entry 3) showed similar reaction efficiency, albeit with longer reaction time. No hydrazine is formed in the absence of either phosphetane oxide 1·[O] or hydrosilane (entries 4,5). The carboxylic acid additive proves to be essential to the N–N bond formation process; only trace product is observed when this additive was omitted (entry 6). Despite the reducing conditions, this catalytic reaction could be conducted with similar yield under ambient air (entry 7). Reactions at either higher temperature (entry 8) or higher dilution (entry 9) resulted in erosion of yield. To probe the reaction sensitivity with respect to the interplay of the reaction components and conditions, a five-factor, half-fractional factorial design of experiments (DoE) containing four center point replicates was subsequently performed (see SI). Reaction concentration was identified as a statistically significant factor affecting reaction performance, along with secondary interactions between silane-loading/temperature and silane-loading/2,4,6-trimethylbenzoic acid-loading. The DoE experiments support the prescribed optimal conditions in Table 1 and provide a picture of relative robustness with respect to variables.
Table 1.
Discovery and Optimization of Organophosphorus-Catalyzed N–N cross coupling.a

| ||
|---|---|---|
| Entry | Change from “standard conditions” | Yield (%)a |
| 1 | none | 86 (81) |
| 2 | 1 (15 mol%) | 86 |
| 3 | 1·[O] (5 mol%) | 84b |
| 4 | no catalyst 1·[O] | 0 |
| 5 | no silane | 0 |
| 6 | no 2,4,6-trimethylbenzoic acid | trace |
| 7 | under ambient air | 82 |
| 8 | 100 °C | 79 |
| 9 | THF (0.5 M) | 41 |
Yields were determined through 19F NMR with 4-fluorotoluene as an internal standard.
72 h reaction time. See Supporting Information for full synthetic details.
The optimized N–N reductive coupling protocol provides direct and cross-selective access to hydrazine products from the coupling of widely-available nitroarene starting materials with anilines (Figure 2). A variety of anilines yielded the corresponding hydrazine with high levels of efficiency (4–7). Notably, both electron-poor and electron-rich anilines are transformed smoothly into the desired products with good efficiency (5 and 7, 77% and 90% yields, respectively). Similarly, N-alkylaniline (8), N-alkyl-heterocyclic amines (9, 29) and N-allyl-aniline (10) are all incorporated into corresponding hydrazine products successfully. Moreover, as exemplified by indoline (19, 21), 1,2,3,4-tetrahydroquinoline (20, 31), 2,3,4,5-tetrahydrobenzoazepine (22), 1,4-benzoxazine (23) and 2,3-dihydroquinolin-4-one (24), a variety of arylamines bearing ortho-fused aliphatic rings are all transformed smoothly into the corresponding hydrazine products.
Figure 2.

Synthetic scope and representative examples of hydrazine via PIII/PV=O catalyzed N–N coupling. See SI for full experimental details and conditions.
The organophosphorus-catalyzed reaction exhibits chemoselectivity for the nitro group as a coupling partner, with the preservation of a range of easily-reduced functional groups such as carbonyls (11, 20, 29), esters (12, 31), amides (13, 32), sulfonamides (14) and sulfones (22). Notably, mono-functionalization of 1,4-dinitrobenzene could be achieved with excellent yields, as the second nitro group becomes electronically deactivated after an initial reductive N–N coupling event. Moreover, this method is also applicable to nitroarenes bearing an unsaturated carbon-carbon triple bond (28, 83% yield). Halogen atoms are all maintained (17, 21, 23–27, 51%–90% yields), providing handles for further functionalization by transition-metal catalysis. Other functional groups such as methoxy (23, 26), trifluoromethyl (18–19) and cyano (24) can also be preserved via this approach. Nitropyridines (15, 28) were also amenable to the formation of hydrazine due to the inherently low Lewis acidity of the catalytic components. Compounds with bioactive core structures—as exemplified by flavone (30), novocaine (31), and flutamide (32) derivatives—are similarly applicable.
As a further demonstration of the synthetic utility of this transformation within the context of medicinal chemistry, indazolone 35—an investigational TPRV1 receptor antagonist42—was synthesized in 69% yield in a one pot sequence involving organophosphorus-catalyzed cross-selective N–N coupling of ethyl 2-aminobenzoate (33) and 4-nitrobenzotrifluoride (34), followed by intramolecular cyclization onto the pendent ester group (Figure 3). The modularity of this entry to the indazole core suggests numerous additional opportunities within medicinal chemistry campaigns.
Figure 3.

One pot synthesis of TPRV1 receptor antagonists for analgesics. Reaction conditions: 33 (2.0 equiv), 34 (1.0 equiv), Ph2SiH2 (4.0 equiv), 1·[O] (15 mol %), THF (1 M), 80 °C; then NaHMDS, rt.
Concerning the catalytic mechanism, in situ 19F NMR spectroscopy of the model reaction of 4-cyanonitrobenzene (2) with 4-fluoroaniline (3) revealed that 3 (δ −128.2 ppm) was converted to the desired hydrazine product 4 (δ −125.3 ppm) with the observation of an additional minor species (δ −107.5 ppm), identified as the corresponding azoarene 36 (see SI, Figure S2). Curiously, subjection of independently-prepared azoarene 36 to the nominal catalytic conditions resulted in poor conversion and yield of hydrazine 4 (47% conversion, 23% yield, Figure 4A, eq 1). However, the deliberate addition of H2O as an additive to the initial catalytic conditions significantly improved the conversion from azoarene 36 to the hydrazine 4 (>99% conversion, 96% yield, Figure 4A, eq 2), pointing to an important role for adventitious water in the azoarene reduction.43 A similarly efficient reduction of azoarene 36 is achieved under stoichiometric conditions starting from PIII phosphetane 1, illustrating the importance of the PIII oxidation state for this transformation (Figure 4A, eq 3). Additionally, a crossover experiment in which azoarene 36 is added to an intermolecular reductive coupling of 4-nitrobenzotrifluoride (34) and 4-chloroaniline (37) under catalytic conditions provided 4 (70%) along with the intermolecular N-N coupling product 38 (53%) without scrambling (Figure 4B).
Figure 4.

Mechanistic studies of catalytic reductive N–N coupling. (A) Azoarene reduction investigation (B) Crossover competition.
Taken together with literature precedent, these experimental findings are best accommodated by an auto-tandem catalytic reaction mechanism for the intermolecular N–N reductive coupling involving two sequential and mechanistically-distinct reduction events, as illustrated in Figure 5.44 In a first catalytic stage, the nitroarene substrate is deoxygenated by PIII phosphetane 1, giving nitrosoarene intermediate (Int-2) by a [3+1]/retro-[2+2] sequence via Int-1 as previously described.29,34 Once formed, nitrosoarene Int-2 advances off-cycle to azoarene Int-3 by dehydrative condensation.45 – 47 The equivalent of water thus released proves critical for the second catalyzed reduction event; azoarene Int-3 is reduced by 1 in the presence of carboxylic acid via hydrazinylphosphonium Int-4,48–50 whose hydrolytic decomposition depends on adventitious water for release of the desired hydrazine product and phosphetane oxide 1·[O].51 A common feature uniting both catalytic cycles is the hydrosilane-mediated reductive turnover of the PV=O catalyst 1·[O] to regenerate the reactive PIII state (1), a mechanistic step which is kinetically facilitated by the presence of the small-ring in the organophosphorus catalyst.
Figure 5.

Proposed reaction mechanism for the organophosphorus-catalyzed cross-selective reductive N–N coupling. Methyl groups on catalyst omitted for clarity.
In summary, the results described above constitute a new and robust organophosphorus-catalyzed protocol for cross-selective intermolecular N–N coupling via PIII/PV=O redox cycling that makes use of readily accessible coupling partners in the construction of valuable hydrazine products. Despite the potential lability of the N-N bond under reducing conditions, the high chemoselectivity of the catalytic system reductive allows the synthesis of the target bond with excellent functional group tolerance even among other reductively sensitive functionalities. Critical to the success of this method is the versatility of the PIII/PV=O redox couple to manage diverse reductive transformations, which is manifest in the two-stage, auto-tandem catalytic reaction process. Given the ready-accessibility of nitroarene and aniline partners, we envision that these results might enable a fragment coupling approach to the preparation of highly functionalized hydrazines and related N–N containing derivatives with potential utility in medicinal chemistry and other applications.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by NIH NIGMS (GM114547) and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. G.L. thanks Bristol Myers Squibb for a graduate fellowship. The authors thank Prof. M. Movassaghi (MIT) for initial discussions, Drs. J. M. Lipshultz (MIT), A. M. Hyde (Merck), and B. D. Sherry (Merck) for critical feedback, and Dr. J. Kim (Merck) for assistance with DoE analysis. We are grateful to Drs. S. J. Hwang, G. T. Cleveland, and A. Tanushi (MIT) for assistance with crystallographic data collection and refinement.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
General methods and synthetic procedures (.pdf)
1H, 13C, 19F and 31P NMR spectra (.pdf).
The authors declare no competing financial interests.
REFERENCES
- 1.Jain SR; Sridhara K; Thangamathesvaran PM N–N bonded polymers. Prog. Polym. Sci 1993, 18, 997. [Google Scholar]
- 2.Merino E Synthesis of azobenzenes: the coloured pieces of molecular materials, Chem. Soc. Rev 2011, 40, 3835. [DOI] [PubMed] [Google Scholar]
- 3.Blair LM; Sperry J Natural products containing a nitrogen–nitrogen bond, J. Nat. Prod 2013, 76, 794. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C-H; Wang Y Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem 2012, 19, 239. [DOI] [PubMed] [Google Scholar]
- 5.Küçükgüzel ȘG; Șenkardes S Recent advances in bioactive pyrazoles. Eur. J. Med. Chem 2015, 97, 786. [DOI] [PubMed] [Google Scholar]
- 6.Ragnarsson U Synthetic Methodology for Alkyl Substituted Hydrazines. Chem. Soc. Rev 2001, 30, 205. [Google Scholar]
- 7.Guo Q; Lu Z Recent Advances in Nitrogen-Nitrogen Bond Formation. Synthesis. 2017, 49, 3835. [Google Scholar]
- 8.Cahn JW; Powell RE The Raschig synthesis of hydrazine. J. Am. Chem. Soc 1954, 76, 2565. [Google Scholar]
- 9.Vidal J; Hannachi JC; Hourdin G, Mulatier JC; Collet A N-Boc-3-trichloromethyloxaziridine: a new, powerful reagent for electrophilic amination. Tetrahedron Lett. 1998, 39, 8845. [Google Scholar]
- 10.Armstrong A; Jones LH; Knight JD; Kelsey RD; Ct K Oxaziridine-Mediated Amination of Primary Amines : Scope and Application to a One-Pot Pyrazole Synthesis. Org. Lett, 2005, 7, 713. [DOI] [PubMed] [Google Scholar]
- 11.Nelsen SF; Jalilov AS; Guzei IA O-Capped heteroadamantyl-substituted hydrazines and Their oxidation products. J. Org. Chem 2010, 75, 2445. [DOI] [PubMed] [Google Scholar]
- 12.Polat DE; Brzezinski DD; Beauchemin AM Formation of Complex Hydrazine Derivatives via Aza-Lossen Rearrangement. Org. Lett, 2019, 21, 4849. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y; Duan D; Zhong Y; Guo XA; Guo J; Gou J; Gao Z; Yu B Fe(III)-Catalyzed Aerobic Intramolecular N-N Coupling of Aliphatic Azides with Amines. Org. Lett 2019, 21, 4960. [DOI] [PubMed] [Google Scholar]
- 14.For an example of TiCl3 mediated intramolecular N–N bond formation with nitroalkane, see:; Pangerl M; Hughes CC; Trauner D Total Synthesis of Newbouldine via Reductive N–N Bond Formation. Tetrahedron 2010, 66, 6626. [Google Scholar]
- 15.Trost BM; Boyle O; Torres W; Ameriks MK Development of a flexible strategy towards FR900482 and mitomycins. Chem. Eur. J 2011, 17, 7890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maestre L; Dorel R; Pablo Ó; Escofet I; Sameera WMC; Álvarez E; Maseras F; Díaz-Requejo MM; Echavarren AM; Pérez PJ Functional-Group-Tolerant, Silver-Catalyzed N-N Bond Formation by Nitrene Transfer to Amines. J. Am. Chem. Soc 2017, 139, 2216. [DOI] [PubMed] [Google Scholar]
- 17.Kono M; Harada S; Nemoto T Chemoselective Intramolecular Formal Insertion Reaction of Rh–Nitrenes into an Amide Bond Over C–H Insertion. Chem. Eur. J 2019, 25, 3119. [DOI] [PubMed] [Google Scholar]
- 18.Wang H; Jung H; Song F; Zhu S; Bai Z; Chen D; He G; Chang S; Chen G Nitrene-Mediated Intermolecular N–N Coupling for Efficient Synthesis of Hydrazides. Nat. Chem 2021, 13, 378. [DOI] [PubMed] [Google Scholar]
- 19.For an example of stoichiometric metal-mediated N-N reductive elimination, see:; Diccianni JB; Hu C; Diao T N–N Bond Forming Reductive Elimination via a Mixed-Valent Nickel(II)–Nickel(III) Intermediate. Angew. Chem. Int. Ed 2016, 55, 7534. [DOI] [PubMed] [Google Scholar]
- 20.For an example of intramolecular N-N bond formation in catalysis, see:; Pearce AJ; Harkins RP; Reiner BR; Wotal AC; Dunscomb RJ; Tonks IA Multicomponent Pyrazole Synthesis from Alkynes, Nitriles, and Titanium Imido Complexes via Oxidatively Induced N-N Bond Coupling. J. Am. Chem. Soc 2020, 142, 4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X-M; Chen Z-M; Yang F; Huang Z-Z A dehydrogenative homocoupling reaction for the direct synthesis of hydrazines from N-alkylanilines in air. Synlett 2011, 2011, 569. [Google Scholar]
- 22.Ryan MC; Kim YJ; Gerken JB; Wang F; Aristov MM; Martinelli JR; Stahl SS Mechanistic Insights into Copper-Catalyzed Aerobic Oxidative Coupling of N–N Bonds. Chem. Sci 2020, 11, 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MC; Martinelli JR; Stahl SS Cu-Catalyzed Aerobic Oxidative N–N Coupling of Carbazoles and Diarylamines Including Selective Cross-Coupling. J. Am. Chem. Soc 2018, 140, 9074–9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritsche RF; Theumer G; Kataeva O; Knölker H-J Iron‐Catalyzed Oxidative C–C and N–N Coupling of Diarylamines and Synthesis of Spiroacridines. Angew. Chem., Int. Ed 2017, 56, 549. [DOI] [PubMed] [Google Scholar]
- 25.Rosen BR; Werner EW; O’Brien AG; Baran PS Total Synthesis of Dixiamycin B by Electrochemical Oxidation. J. Am. Chem. Soc 2014, 136, 5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tafesh AM; Weiguny J A Review of the Selective Catalytic Reduction of Aromatic Nitro Compounds into Aromatic Amines, Isocyanates, Carbamates, and Ureas Using CO. Chem. Rev 1996, 96, 2035. [DOI] [PubMed] [Google Scholar]
- 27.Ferretti F; Ramadan DR; Ragaini F Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450. [Google Scholar]
- 28.Gao Y; Yang S; Huo Y; Hu XQ Recent Progress on Reductive Coupling of Nitroarenes by Using Organosilanes as Convenient Reductants. Adv. Synth. Catal 2020, 362, 3971. [Google Scholar]
- 29.Nykaza TV; Harrison TS; Ghosh A; Putnik RA; Radosevich AT A Biphilic Phosphetane Catalyzes N–N Bond-Forming Cadogan Heterocyclization via PIII/PV=O Redox Cycling. J. Am. Chem. Soc 2017, 139, 6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nykaza TV; Ramirez A; Harrison TS; Luzung MR; Radosevich AT Biphilic Organophosphorus-Catalyzed Intramolecular Csp2–H Amination: Evidence for a Nitrenoid in Catalytic Cadogan Cyclizations. J. Am. Chem. Soc 2018, 140, 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nykaza TV; Cooper JC; Li G; Mahieu N; Ramirez A; Luzung MR; Radosevich AT Intermolecular Reductive C–N Cross Coupling of Nitroarenes and Boronic Acids by PIII/PV=O Catalysis. J. Am. Chem. Soc 2018, 140, 15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecomte M; Lipshultz JM; Kim-Lee S-H; Li G; Radosevich AT Driving Recursive Dehydration by PIII/PV Catalysis: Annulation of Amines and Carboxylic Acids by Sequential C–N and C–C Bond Formation. J. Am. Chem. Soc 2019, 141, 12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nykaza TV; Li G; Yang J; Luzung MR; Radosevich AT PIII/PV=O Catalyzed Cascade Synthesis of N-Functionalized Azaheterocycles. Angew. Chem., Int. Ed 2020, 59, 4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G; Nykaza TV; Cooper JC; Ramirez A; Luzung MR; Radosevich AT An Improved PIII/PV=O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- And Product-Determining Steps. J. Am. Chem. Soc 2020, 142, 6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G; te Grotenhuis C; Radosevich AT Reductive Csp2–N Coupling by PIII/PV=O–Catalysis. Trends Chem. 2021, 3, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G; Qin Z; Radosevich AT P(III)/P(V)-Catalyzed Methylamination of Arylboronic Acids and Esters: Reductive C–N Coupling with Nitromethane as a Methylamine Surrogate. J. Am. Chem. Soc 2020, 142, 16205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsden SP Catalytic Variants of Phosphine Oxide-Mediated Organic Transformations in Sustainable Catalysis; Dunn PJ, Hii KK, Krische MJ, Williams MT, Eds.; John Wiley & Sons, Inc.: New York, 2013; pp 339–361. [Google Scholar]
- 38.Guo H; Fan YC; Sun Z; Wu Y; Kwon O Phosphine Organocatalysis. Chem. Rev 2018, 118, 10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipshultz JM; Li G; Radosevich AT Main Group Redox Catalysis of Organopnictogens: Vertical Periodic Trends and Emerging Opportunities in Group 15. J. Am. Chem. Soc 2021, 143, 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie C; Smaligo AJ; Song XR; Kwon O Phosphorus-Based Catalysis. ACS Cent. Sci 2021, 7, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.For a preparation of 1•[O], see:; Nykaza TV; Cooper JC; Radosevich AT Org. Synth 2019, 96, 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fletcher SR; McIver E; Lewis S; Burkamp F; Leech C; Mason G; Boyce S; Morrison D; Richards G; Sutton K; Jones AB The search for novel TRPV1-antagonists: From carboxamides to benzimidazoles and indazolones. Bioorg. Med. Chem. Lett, 2006, 16, 2872. [DOI] [PubMed] [Google Scholar]
- 43. In situ NMR studies with 4-nitrobenzotrifluoride and N-methylaniline were also conducted but with no long-lived intermediate observed (Figures S4–5), presumably due to fast reduction of the corresponding diazenium to hydrazine product.
- 44. The intervention of nitrene or nitrenoid intermediates in this N–N bond construction is not supported by experimental data. Arylnitrenes are known to be trapped with amines to give 2-aminoazepines by ring expansion (Huisgen, R.; Vossius, D.; Appl, M. Die Thermolyse des Phenylazids in primären Aminen; die Konstitution des Dibenzamils. Chem. Ber. 1958, 91, 1–12). No such 2-aminoazepine product was observed under organophosphorus-catalyzed conditions. For comparison, see Ref. 32.
- 45.Ramakumar K; Tunge JA Synthesis of N-Aryl-1-Aminoindoles via Intermolecular Redox Amination. Chem. Commun 2014, 50, 13056. [DOI] [PubMed] [Google Scholar]
- 46.Purkait A; Jana CK N-Aminations of Benzylamines and Alicyclic Amines with Nitrosoarenes to Hydrazones and Hydrazides. Synthesis. 2019, 51, 2687. [Google Scholar]
- 47.Roy SK; Purkait A; Aziz SMT; Jana CK Acid Mediated Coupling of Aliphatic Amines and Nitrosoarenes to Indoles. Chem. Commun 2020, 56, 3167. [DOI] [PubMed] [Google Scholar]
- 48.Hughes DL Progress in the Mitsunobu reaction: A review. Org. Prep. Proced. Int 1996, 28, 127. [Google Scholar]
- 49.But TYS; Toy PH The Mitsunobu Reaction: Origin, Mechanism, Improvements, and Applications. Chem. Asian J 2007, 2, 1340. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher S The Mitsunobu Reaction in the 21st Century. Org. Chem. Front 2015, 2, 739. [Google Scholar]
- 51.Longwitz L; Werner T Reduction of Activated Alkenes by P(III)/P(V) Redox Cycling Catalysis. Angew. Chem., Int. Ed 2020, 59, 2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


