Abstract
Lactate is the main product generated at the end of anaerobic glycolysis or during the Warburg effect and its role as an active signalling molecule is increasingly recognised. Lactate can be released and used by host cells, by pathogens and commensal organisms, thus being essential for the homeostasis of host–microbe interactions. Infection can alter this intricate balance, and the presence of lactate transporters in most human cells including immune cells, as well as in a variety of pathogens (including bacteria, fungi and complex parasites) demonstrates the importance of this metabolite in regulating host–pathogen interactions. This review will cover lactate secretion and sensing in humans and microbes, and will discuss the existing evidence supporting a role for lactate in pathogen growth and persistence, together with lactate's ability to impact the orchestration of effective immune responses. The ubiquitous presence of lactate in the context of infection and the ability of both host cells and pathogens to sense and respond to it, makes manipulation of lactate a potential novel therapeutic strategy. Here, we will discuss the preliminary research that has been carried out in the context of cancer, autoimmunity and inflammation.
Keywords: host–pathogen interactions, immunometabolism, infection, lactate
Introduction
Lactate and its production within the human body
Lactic acid (C3H6O3) was first reported in sour milk in 1780 [1]. Lactate is the anion form of lactic acid and the main product generated at the end of anaerobic glycolysis [2,3] as well as of aerobic glycolysis in highly proliferative cells, also known as the Warburg effect [4]. In this cytosolic central metabolic pathway, one glucose molecule is broken down into two pyruvate molecules, which are converted into lactate by lactate dehydrogenase (LDH). In this process, ATP and NADH molecules are generated, creating usable energy for the cell. Lactate comprises two stereoisomers, d(−) lactate and l(+) lactate, which are metabolised by d-LDH and l-LDH, respectively [5]. Microbial species contain both LDH forms and can, therefore, produce both lactate isoforms, whereas in vertebrates l-LDH and consequently l-lactate prevail [6,7]. Lactate was originally considered a by-product of cell metabolism, however, there is increasing evidence that it can act as a signalling molecule impacting cell behaviour and function [8,9].
Within the human body, lactate is predominantly produced by muscle cells, erythrocytes and the brain, although most tissues are capable of lactate production under anaerobic conditions.
Lactate is present in the blood and tissues in healthy conditions at low concentrations (typically <2 mM) [10]. Pathological levels of lactate were first demonstrated in 1843 in the context of sepsis and haemorrhagic shock [11]; however, the association between hyperlactatemia (>4 mM) and poor outcomes in human disease was not described until 1964 [12].
Today, lactate is routinely measured and used as a biomarker for disease severity in critically ill patients [13]. However, clinical trials targeting clearance of lactate as a marker of resuscitation in sepsis have failed to demonstrate any benefit [14]. The aetiology of hyperlactataemia is mixed and dependent on individual circumstances in body compartments. Hypoxia and hypoperfusion (decreased blood flow) are the main causes of increased lactate, other contributing factors being hypermetabolism, and mitochondrial or liver dysfunction which impact clearance of lactate [15]. Tissue lactate concentrations are often many folds higher than blood lactate, a scenario seen in cancer and inflammatory settings where blood lactate is frequently normal, but tissue lactate may reach 15–40 mM [16–19] (Table 1). These tissue lactate concentrations in solid tumours were measured using mass spectrometry [16] and imaging bioluminescence (measurements in µmol/g) [19], followed by conversion to mM considering the tumour water content [18].
Table 1. The estimated range of lactate levels in healthy and pathological human tissues.
| Disease state | Lactate level (mM) | Reference |
|---|---|---|
| Healthy tissue/blood | <2 | [10] |
| Empyema (human) | 13.8 | [20] |
| Inflammatory bowel disease (quiescent) (human) | 4 | [21] |
| Inflammatory bowel disease (active) | >10 | [21] |
| Bacterial meningitis (human) | 13.6 | [22] |
| Murine S. typhimurium infected gut lumen | 11.7 | [23] |
| Female genital tract in health | 6 | [24] |
| Burn wound exudate | 3.19 | [25] |
| Cystic fibrosis sputum (colonised but not exacerbating) | 9 | [26] |
| Blood derived macrophages infected with M.tb | 0.56–6.7 | [27] |
| Saliva | 0.3–1.3 | [28] |
| Tumour tissue | 30–40 | [16,17] |
The human body is a complex ecosystem, comprised of both human cells and microbial organisms, such as bacteria, viruses and fungi. Many of these commensals produce lactate as part of their life cycle. Important species include lactobacilli, bifidobacteria and enterococci [29,30]. Hence, we harbour lactate-producing bacteria, but also lactate-utilising bacteria [29,31]. Therefore, lactate can be produced and used by both human and commensal cells, creating a complex but necessary network for the homeostasis of host–microbe interactions. This delicate balance can be perturbed by infections, with invading pathogens also having the capacity to secrete, sense and use lactate [32].
Lactate transporters and receptors
Lactate stereoselective transport across plasma membranes is mediated by six described solute carrier transporters that perform proton-lactate symport (monocarboxylate transporters: SLC16A1, SLC16A7, SLC16A8 and SLC16A3, also known as MCT1–4) and sodium-dependent transporters (SLC5A8 and SLC5A12, also known as SMCT1–2) [33,34]. Structurally, these transporters contain two six-helix bundles, which constitute their transmembrane domain [35]. Each transporter prefers import or export of lactate; however, the transport direction of both systems depends on the lactate gradient, favouring lactate import when extracellular lactate is high, such as in inflamed tissues. These transporters display extensive substrate specificity and also transport alternative monocarboxylic metabolites such as pyruvate or ketone bodies [34]. They differ in their expression pattern and lactate affinity, SLC16A7 (MCT2) has the highest affinity for lactate (Km ∼ 0.7 mM) [35] and is predominantly found in liver, kidney, testis and brain [34]. SLC16A8 (MCT3) is restricted to retinal pigment and choroid plexus epithelia, whereas high expression of SLC16A3 (MCT4) is observed in anaerobic and highly glycolytic tissues. SLC5A8 (SMCT1; high affinity) and SLC5A12 (SMCT2; low affinity) expression is enriched in the kidney, and present to a lesser extent in other body tissues such as the intestine, salivary and thyroid glands, brain and retina, and in small intestine and skeletal muscle, respectively [33,36–38]. Inhibitors targeting MCT1-4 transporters currently exist [39,40] and offer great therapeutic potential, as it will be discussed in the Therapeutics section.
Importantly, G-protein-coupled receptors (GPR) have been described for lactate. These include GPR81 (HCAR1), which plays an active role in adipose tissue, muscle and brain [41–43], as well as GPR132, which is highly expressed in blood and immune cells, particularly macrophages, and promotes the M2-like phenotype [44–49].
Multiple lactate transporters are expressed in T cells and macrophages [50]. SCL16A1 and SLC16A3 (MCT1 and MCT4) have been identified in macrophages; SLC16A1 (MCT1) has also been described in the CD8+ T cell compartment, whereas CD4+ T cells mainly express SLC5A12 upon activation [8]. Activation of peripheral blood monocyte cells (PBMCs) via stimulation of CD3 causes indirect up-regulation of SLC5A12 in CD14+ monocytes, and in CD19+ B cells at a lower level [51]. Furthermore, CD20+ B cells and CD68+ macrophages were confirmed to express SLC5A12 in inflamed tonsils [51].
Bone marrow-derived neutrophils predominantly express SLC16A3 (MCT4) and have low levels of SLC16A1 (MCT1) and GPR81 [52], with lipopolysaccharide (LPS) activation or bacterial infection increasing expression of SLC16A3 [52]. Peripheral blood neutrophils also express SLC16A1 and SLC16A3 (MCT1 and 4) [53,54]. Antigen-presenting cells such as dendritic cells (DCs) and macrophages have been shown to express GPR81 [55–57] (Table 2).
Table 2. Expression of lactate transporters in human immune cells and pathogens.
| Cell type | Lactate transporter | Reference |
|---|---|---|
| Macrophages | GPR132 GPR81 MCT1, MCT4 SLC5A12 (activated) |
[44] [56] [51] |
| CD8+ T cells | MCT1 | [8] |
| CD4+ T Cells | SLC5A12 (activated) | [8] |
| B cells | SLC5A12 (activated) | [51] |
| Dendritic cells | GPR81 | [55] [57] |
| Neutrophils | MCT1 MCT4 GPR81 |
[52,53,58] |
| Bacteria | Lactate permeases (lctP) (Gonococcus, Haemophillus and Lactic acid bacteria as examples) Aquaglyceroporins (Lactobacillales) |
[28,59,60] [61] |
| Fungi | Jen transporters Gpr1 |
[62–65] |
| Parasites | FNT Aquaglyceroporins TbPT0 |
[66] (P. falciparum) [67] (T. gondii) [68] (T. brucei), [69] (T. brucei) |
The role of lactate in the context of immunity
The combination of these lactate transporters and receptors in immune cells ensures a sensing capacity to a changing environment, such as in response to infection and inflammation. This enables the adaptation of immune cells to fluctuating environments, and ultimately permits the orchestration of an appropriate and effective immune response.
In bacteria, lactic acid and sodium lactate diffusion can be facilitated by different transporters (Table 2), including aquaglyceroporins, such as GlpF1 and GlpF4 [61], and lactate permeases, such as LctP [59]. Therefore, bacteria have evolved their own means for the import/export of lactic acid and/or sodium lactate, through a different mechanism to eukaryotes and often regulated by operons. The lactate–proton symporter Jen1 was first identified in Saccharomyces cerevisiae [62], and its ortholog has been reported in Candida species [63] (Table 2). They present a more diverse repertoire of lactate transporters, including the evolutionarily conserved receptor Gpr1 [64], which is essential for pathogen survival in certain host niches [65].
There has been relatively little investigation of how lactate impacts immune cell function in infection; however; there is a significant body of evidence of the diverse roles lactate plays in both cancer and inflammatory conditions [50]. All three conditions — infection, cancer and inflammation — are characterised by high lactate levels, and they frequently result in hypoxic and acidic environments [8,16,21]. The diverse roles of lactate in cancer and inflammatory settings have been well reviewed [50]. In brief, the impact of lactate varies according to cell type and disease state. Particularly, effector functions and cytotoxicity are altered by lactate in T cells, macrophages, mast and epithelial cells [9]. In addition to cancer cells [4], stromal and immune cells also contribute to the establishment of a lactate-rich environment within tumours [70]. These conditions suppress immune responses and hence promote tumour growth [9].
The impact of lactate in the tumour microenvironment is outside the scope of this review and has been recently covered [9]. However, valuable lessons can be learned from the cancer field in terms of lactate's immunosuppressive function, which could be translated to hyperlactatemia in the context of infection and inflammation.
Both anti- and proinflammatory responses have been described in response to lactate [9]. Lactate can polarise immune cells to both tolerance states to aid immune evasion, and inflammatory phenotypes, which lead to persistence of inflammation. In cancer cells, an association between GPR81 signalling and up-regulation of PD-L1 was shown, resulting in reduced T cell effector function and proliferation [71]. Although most cancer literature points towards a suppression of immune cell function caused by lactate, evidence also exists for lactate enhancing CD8+ T cell cytotoxic capacity and delaying tumour growth [72]. In the myeloid compartment, high lactate concentrations reduce TNF secretion by human monocytes [73], while promoting M2 polarisation in tumour-associated macrophages [16]. Neutrophils are the first responders to inflammation, they are highly glycolytic and contribute to the accumulation of lactate during infection [52]. Murine studies of bone marrow-derived neutrophils have demonstrated that both LPS and Salmonella. typhimurium infection induce glycolysis in neutrophils which then rapidly accumulate lactate. Mobilisation of neutrophils from the bone marrow is dependent on GPR81 signalling, and administration of sodium lactate results in recruitment of bone marrow neutrophils to peripheral blood and peritoneum suggesting potential for a positive feedback loop for lactate mediated neutrophil recruitment [52].
This review focuses on the role of lactate in host–pathogen interactions. We will discuss lactate release from human and pathogenic sources. We will examine how lactate impacts current anti-microbial treatments, and how targeting lactate sensing could be used therapeutically to influence infection outcomes.
Lactate secretion
In health and disease
In healthy conditions, lactate levels are usually <2 mM in blood and other bodily fluids [10]. Hyperlactataemia during infection results as a complex interplay of tissue hypoxia, enhanced lactate production by activated immune cells, and generation of lactate by both pathogenic and commensal bacteria, combined with reduced clearance of lactate due to hypoperfusion and impaired lactate shuttles [74]. Production of lactate by the human host varies significantly by cell type but is also heavily influenced by environmental factors. In the context of host–pathogen interactions, lactate secretion by immune cells varies hugely. Neutrophils are the most abundant leukocyte and are the first responders to infection. They heavily rely on glycolysis [75,76], while other immune cells have a more flexible metabolism depending on their environment and activation state [77]. Untangling whether lactate is host or pathogen derived is challenging. Many bacteria can produce and utilise both d- and l-lactate, whereas humans generally only produce and utilise l-lactate. In humans, only l-lactate is routinely measured in clinical practice, as d-lactate rarely reaches sufficient levels to cause disease [6].
Lactic acid producing and utilising bacteria
Lactic acid-producing bacteria (LAB) are the archetypal lactate producers and are the predominant contributors to gut lumen lactate levels [55,78]. LAB have long been recognised to promote host immunity [79] both in the gut mucosa and systemically; however, the mechanisms of this relationship remain to be elucidated. Whilst LAB are not frequently pathogenic in humans, important human pathogens such as Streptococcus and Enterobacteriaceae are included in this group [80].
In healthy individuals, lactate levels in the faeces are low, despite an abundance of LAB. The major lactate utilisers in the gut are beneficial Clostridia species [29]. Clostridia produce butyrate, the major fuel for colonocytes and in the absence of butyrate, colonocytes switch from fatty acid metabolism to glucose metabolism, increasing lactate in the gut lumen [32]. Loss of Clostridia species is associated with the use of antibiotics as well as infection with enteric pathogens such as Salmonella enterica typhimurium (S. typhimurium). Pathogens can take advantage of this dysbiosis and use the increased lactate to support their expansion [32], further driving inflammation and lactate generation by host and pathogen. This is also seen in the female genital tract, which is also colonised by LAB [81]; dysbiosis of lactate utilising and producing strains in this body niche enables expansion of pathogens such as Neisseria gonorrhoea [82].
Pathogens
Non-LAB bacteria are also capable of generating lactate as a product of their metabolism by fermentation of glucose or anaerobic respiration. Particularly notable bacteria capable of lactate production are Staphylococcus aureus, Enterobacteria and the LAB discussed above, although many more human pathogens are capable of generating lactate [83], as it will be detailed in the section Lactate in non-bacterial pathogens. In bacteria, lactate transport is controlled by lactate permease (LctP), a symporter that forms an operon with the LDH genes and is highly conserved across lactate utilising species [84].
The source of lactate at tissue sites is likely to vary depending on the degree of bacterial load and infiltration of immune cells, in addition to oxygen tension and availability of nutrients. For example, in an empyema, lactate levels are typically 13 mM [20] and similar levels are also seen in cerebrospinal fluid (CSF) from patients with bacterial meningitis [22]. In these infections pleural fluid and CSF have significant neutrophilic infiltration and high bacterial burden, contributing to lactate production. These conditions — elevated neutrophil count and bacterial numbers — are also seen in sputum samples from patients with cystic fibrosis and respiratory infections where lactate levels correlate with neutrophil burden [26,85].
Lactate in bacterial pathogens
Mechanisms of lactate influence on microbial pathogenicity
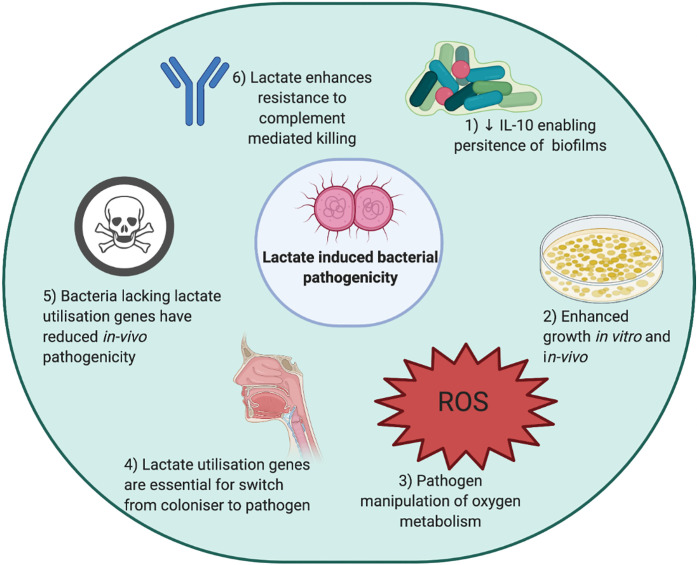
The mechanisms by which lactate can be used to enhance microbial pathogenicity, including its impact on immune surveillance and pathogen growth/survival, the manipulation of oxygen metabolism, the switch from coloniser to invader pathogen and the resistance to complement-mediated killing are outlined in Figure 1.
Figure 1. Mechanisms of lactate-driven bacterial pathogenesis.
Bacterial pathogens have been demonstrated to use lactate in multiple ways to enhance their pathogenicity. (1) S. aureus generated lactate polarises innate immune cells to an immunotolerant state allowing persistence of biofilms. (2) Lactate can be used as a sole carbon source by a variety of bacteria, or bacteria can use lactate as fuel to enhance growth [32,86]. Enhanced growth is seen in Pseudomonas and other bacteria. (3) Reactive oxygen species are a key bactericidal mechanism used by innate immune cells. Lactate enables bacteria to manipulate oxygen metabolism and thus evade killing, a key mechanism for the persistence of S. aureus [87]. (4) Many bacteria including N. meningitidis and S. aureus colonise the nasopharynx, and the ability to invade is crucial in the move to becoming pathogenic. Lactate utilisation genes have been demonstrated to be required for this step [88]. (5) Bacteria lacking lactate utilisation genes have reduced in vivo pathogenicity in a variety of animal models of infection, this has been shown for Neisseria species and H. influenzae [89]. (6) Complement-mediated killing is reduced in the presence of lactate, mediated by lactate permease [90]. Created using Biorender.com.
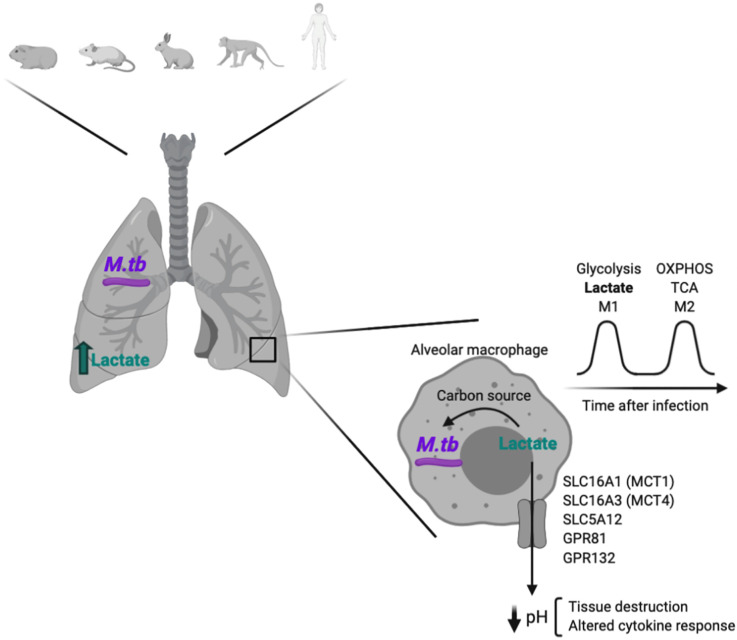
Mycobacterium tuberculosis (M.tb) infection will be used as a specific example to illustrate how infection-induced host-derived lactate can change the extracellular milieu, impacting the pathogen's ability to survive and immune function (Figure 2).
Figure 2. Glycolysis is increased during Mycobacterium tuberculosis infection.
Glycolysis is induced in the lungs of Mycobacterium tuberculosis (M.tb) infected hosts, which results in lactate secretion. This increased in glycolysis upon M.tb infection has been shown in the lungs of guinea pigs, mice, rabbits, non-human primates and humans. Alveolar macrophages are the first M.tb cellular target and, inside them, lactate can be used by M.tb as a carbon source, enhancing pathogen survival and cell growth. Lactate can also be exported through different specific transporters, which results in the acidification of the extracellular milieu. This can promote an altered cytokine response as well as tissue destruction. Created using Biorender.com.
Mycobacterium tuberculosis infection
Lactate's role in manipulating both host and pathogen is exemplified by M.tb infection. The first target of M.tb is the alveolar macrophage (AM) [91]. Upon infection, macrophages switch their metabolism to glycolysis and pyruvate is reduced to lactate [92,93]. Lactate release from macrophages, stimulated with live H37Rv at 10 : 1 multiplicity of infection (MOI) for 24 h, was measured using a fluorescent coupled enzymatic assay [92]. Lactate secretion was also shown to be increased when human monocyte-derived macrophages (hMDMs) and AMs were infected with the live strains of M.tb H37Ra and H37Rv for 24 h [93]. In this case, macrophages were infected at MOI 1–5 bacilli/cell for 3 h and then extracellular bacteria were removed. This metabolic shift was shown to be required for host control of H37Ra M.tb replication, with CFU/ml measured 72 h post-infection.
Using galactose instead of glucose reduced the ability of the macrophage to control infection in hMDMs and hAM and 2-deoxyglucose had the same effect on mBMDM [93].
The importance of glycolysis in infection resolution has also been shown in an elegant comparison between AMs (which mainly rely on fatty acid oxidation) and interstitial macrophages (predominantly glycolytic), and their respective capacities to control bacterial burden [94]. C57BL/6 mice were intranasally inoculated with the Erdman M.tb strain at high CFUs (∼1000).
Interestingly, extracellular flux analysis after 24 h infection of hMDM with H37Rv M.tb using MOI 5 : 1 promoted a general metabolic quiescent state, including decreased glycolysis [95]. This was further demonstrated using radiolabelled 13C-glucose and showing diminished 13C incorporation into several glycolytic intermediates [95]. Dead M.tb or Bacillus Calmette-Guerin (BCG) infections induced a glycolytic phenotype, suggesting that M.tb has evolved strategies to counteract this metabolic shift. Hackett et al. also showed that expression of glycolytic genes was increased when mBMDM were stimulated with heat-killed M.tb compared with live M.tb H37Ra, and that lactate secretion was also higher on mBMDMs and hMDMs treated with heat-killed M.tb compared with live M.tb (H37Ra/H37Rv and H37Ra, respectively). They also demonstrated that upon M.tb infection, microRNA-21 targets PFK-M, restricting host glycolysis [96].
The current literature assessing the metabolic impact of M.tb infection in macrophages uses a variety of models and therefore drawing conclusive answers is challenging. Some of the reported differences are due to the use of murine versus hMDMs following different differentiation protocols, to different strains of live M.tb, dead M.tb or M.tb lysates, different MOIs and/or route and duration of infection.
A biphasic metabolic response for the infected macrophage has been proposed [97], based on transcriptomic analysis of published datasets, including C57BL/6 BMDMs infected with M.tb H37Rv [98], B6D2F1 BMDMs infected with M.tb CDC1551 or HN878 [99] and BALB/c BMDMs infected with M.tb HN878 [100]. This model proposes a first phase (4–8 h post-infection) characterised by a switch to glycolysis, which is accompanied by HIF-1a expression, enabling M1 polarisation and the orchestration of an effective antimicrobial programme, including the production of pro-inflammatory effector molecules. In the second phase (24–48 h post-infection), macrophages transition towards mitochondrial oxidative metabolism with high TCA activity. The dampening of M1 polarisation allows increased M.tb survival and growth [97,101]. According to this biphasic model, important metabolic changes might be happening by ∼24 h post-infection. It is, therefore, possible that measurements of lactate at this time point reflect previous macrophage engagement in glycolysis [92,93], while the strategies of M.tb to supress glycolysis are already in place 24 h post-infection [95]. It is also possible that glycolytic genes are up-regulated at the transcript level, but M.tb has strategies in place to supress glycolysis further down the line (i.e. at the protein level as shown by Hackett et al. [96]. In that case, transcriptional up-regulation of glycolytic genes and decreased glycolysis measured through extracellular flux analyses would not be incompatible. Also, extracellular flux studies [95] oppose the increased TCA activity 24 h post-infection showed using transcriptomic analysis [97].
RNA-sequencing analysis of M.tb H37Rv-infected mice demonstrated up-regulation of glucose transporters and glycolytic enzymes, including Gapdh, Hk3 and Ldha in lung tissue [102]. They also showed increased levels of HK3 and LDHA protein through immunohistochemical staining of M.tb infected mouse lungs. These two glycolytic enzymes partially co-localised with macrophages (IBA-1+) and T cells (CD3+). Using a rabbit aerosol infection model with M.tb HN878, Subbian and colleagues [103] showed up-regulation of gene transcripts related to the Warburg effect between 4 and 12 weeks post-infection, including some glucose transporters, glycolytic enzymes and monocarboxylate transporters [104]. Transcriptomics of lung granulomas from patients with active TB expressed increased Warburg-effect related genes in comparison with non-granulomatous portions of the same lung [104,105]. Furthermore, a recent study using a dual RNA-seq approach to investigate gene expression of both M.tb and the human host described a shift towards host glycolysis in TB sputa, in comparison with non-TB controls [106]. Thus, both at the transcript and protein levels and using different in vivo infection models and human data, the evidence points towards an increase in glycolysis in the lungs of M.tb infected hosts.
Further evidence of this metabolic shift towards glycolysis comes from lactate measurements. Mice infected with M.tb H37Rv via aerosols showed increased lactate in their lungs 4 weeks post-infection, measured by nuclear magnetic resonance (NMR) [107]. Similar findings were reported in a guinea pig model of infection also infected with M.tb H37Rv and using NMR [108]. Here, up-regulation of lactate was described as infection progressed (from day 15 onwards) in granulomatous tissues. High-resolution mass spectrometry has been used to measure the mouse lung metabolome infected with M.tb H37Rv intratracheally. In this case, only marginal and moderate lactate increase was detected at 4- and 9-weeks post-infection, respectively [109]. The different infection route, as well as the dose of M.tb given (10-fold higher in the latter), may account for some of the observed differences. Interestingly, enhanced LDH levels were detected in serum and bronchoalveolar lavage (BAL) from TB patients [110,111].
The impact of lactate sensing on immune cell function in the context of M.tb infection has been limitedly studied. Preliminary studies suggest the enhanced killing of M.tb by human macrophages incubated with sodium l-lactate [112]. Enhanced lactate secretion also lowers tissue pH, and extracellular acidosis has been shown to promote tissue destruction and altered cytokine secretion, impacting the control of infection and patient survival in tuberculosis [113,114].
M.tb is also able to use lactate as a sole carbon source [115], and when actively replicating it is able to use lactate or pyruvate more efficiently than glucose [27]. M.tb has a preference for pyruvate as carbon source, up-regulation of LDHA in infected macrophages being a mechanism to prevent pyruvate access to the pathogen (and consequent growth) by converting it to lactate [116]. It has been shown that M.tb is able to grow in lactate concentrations of up to 44 mM in in vitro cultures [27], however, it has also been described that greater than 20 mM lactate inhibits M.tb growth [117]. Further confirming this, deletion of ldh (lldD2, Rv1872c) in M.tb is detrimental to growth in lactate containing media, suggesting potential lactate toxicity when M.tb cannot metabolise it. Inside the infected macrophage, lactate concentrations have been estimated to be between 0.56 and 6.7 mM [27]. The observed discrepancies may be explained by strain and/or culture conditions differences [27]. The TB granuloma and the tumour microenvironment share relevant similarities such as nutrients and oxygen gradients potentially leading to hypoxia (a driver of anaerobic glycolysis and lactate release) and nutrient scarcity in certain regions [118–120]. As discussed previously, lung tissue from M.tb infected hosts present evidence of high glycolytic rates and lactate production. These factors impact pathogen survival [118,121] as well as immune cell function and infection resolution [112,122]. Furthermore, M.tb as an intra-cellular pathogen is commonly exposed to oxidative stress, and it is thought that lactate oxidation also plays a role in protecting M.tb from NO· [117] by conversion to pyruvate and consequent NAD+ generation, which helps with redox control.
Genetic studies on M.tb strains point towards the presence of lactate in M.tb niches.
Both promoter and non-synonymous gene mutations have been identified in genomic analyses of M.tb lineage 4 genomes within the lldD2 gene, encoding for l-lactate dehydrogenase. These mutations evolved independently more than 100 times and emerged in all continents. Furthermore, some of them promoted increased transmissibility [123]. Another study that analysed publicly available genomes from different lineages found evidence of positive selection in the lldD2 gene [124], suggesting metabolic adaptations to lactate-rich environments. Supporting this same notion, the Colombian clinical isolate UT127 was shown to cope with scarce lipid availability by up-regulating lldD2 (Rv1872c), suggesting the potential use of lactate as an alternative carbon source [125].
In summary, the evidence converges towards increased glycolysis and lactate release in the context of TB disease, with M.tb having developed efficient strategies to dampen this metabolic shift [95,96]. Due to the wide range of models and techniques used, it is still hard to define the precise kinetics of such metabolic rearrangements. Further investigation is also needed to specifically define when and where within the lung lesions glycolysis occurs, whether it happens in aerobic or anaerobic conditions [119], and its consequences in terms of lactate production, immune function and infection resolution. It is also possible that glycolysis and lactate production happen in immune cells other than macrophages during M.tb infection. For instance, neutrophilic infiltration is a well-described phenomenon in TB lung lesions [126,127], and neutrophils highly rely on glycolysis [52,75]. Also, switches towards glycolysis have been described in other lung cells including fibroblasts [128] and lung epithelial cells [129].
Lactate, therefore, acts at multiple levels of the host–pathogen interface and further investigation is required to fully elucidate its detrimental or beneficial effects in the ability of the macrophage to clear M.tb infection, and potentially identify new host-therapeutic targets [130–132]. There are limited studies exploring the role of lactate in other infection settings and disease states. Next, we discuss the evidence that exists for other organisms and the mechanisms employed.
The impact of lactate on bacterial persistence in biofilms
In the field of cancer immunometabolism, lactate has been demonstrated to induce immunotolerance and enable cancer cells to evade detection. Similar mechanisms may support the development of chronic infection.
Staphylococcus aureus (S. aureus) is an important pathogen in human disease and can form biofilms, which are challenging to treat and require prolonged courses of antibiotics, particularly in infections of prosthetic material. Host-derived IL-10 is crucial for the maintenance of the biofilm with anti-inflammatory myeloid-derived suppressor cells and anti-inflammatory macrophages enabling biofilm persistence [133]. Lactate derived from S. aureus has been shown to regulate the inflammatory properties of the innate immune response and drive biofilm persistence. In a prosthetic joint infection model, S. aureus lacking ldh caused less bacterial burden and decreased levels of IL-10 [134]. IL-10 is also a potent suppressor of T cells [135]. The proposed mechanism is that lactate can inhibit histone deacetylase and therefore regulate gene expression in innate immune cells leading to the anti-inflammatory phenotype, high production of IL-10, thereby promoting the persistence of the biofilm. These findings could aid a better understanding of other biofilm infections that cause medical complications, such as Mycobacterium abcessus [136,137].
Lactate promotes enhanced bacterial growth
As seen in M.tb lactate can be used as a sole carbon source, or as a combined fuel by many other microorganisms [86,138]. This flexibility can enhance bacterial growth compared with use of glucose alone. d and l-lactate are likely to be present during infection, and Escherichia coli (E. coli) and Pseudomonas aeruginosa can utilise them both [138,139]. Pseudomonas species in the context of biofilms have been demonstrated to cross-feed d-lactate produced by its own fermentation [139].
Within inflammatory compartments caused by infection, lactate is abundant. Several organisms have adapted to utilise lactate, and further drive inflammation to increase local lactate levels to support their expansion. In a murine model of S. typhimurium infection lactate reached 11.7 mM in the gut lumen [32]. This is thought to be driven by S. typhimurium-induced dysbiosis causing a switch in intestinal epithelial cell metabolism from fatty acid to glucose metabolism. The ability to oxidise lactate by S. typhimurium confers a fitness advantage in the gut, as lactate utilisation genes (lldP, lldR and lldD) are rapidly induced upon exposure to l-lactate [23]. The inflammation induced by expansion of S. typhimurium drives further dysbiosis and correlates with increased availability of lactate [32].
The female genital tract also has high levels of lactate (∼6 mM) [24]. Neisseria gonorrhoea (N. gonorrhea) [140] is a common pathogen in the genital tract, that is able to utilise lactate as a sole fuel and can result in significant morbidity [140,141].
The flexibility to use varied carbon sources enables growth and success in different niches, as exemplified by Neisseria meningitidis (N. meningitidis). N. meningitidis can use lactate as a fuel when glucose is unavailable [24,82]. In bacterial meningitis, CSF glucose levels are initially high but fall rapidly, whereas lactate (13 mM) on average) is replenished, presumably from glycolysis of activated immune cells and bacteria [22]. N. meningitidis growth is enhanced when lactate is added to glucose-containing media [142].
The ability to breakdown pyruvate to lactate and NADH has been shown to be beneficial for the growth of Streptococcus pneumoniae (S. pnuemoniae). S. pnuemoniae possesses a single copy of ldh [143]. Deletion of ldh abolishes lactate production entirely, and ldh mutants demonstrated 2.5-fold lower growth when glucose was the sole carbon source [144] compared with media containing glucose and galactose. The ldh mutant showed a shift to mixed acid fermentation rather than homolactic fermentation with accumulation of pyruvate [144].
Pseudomonas aeruginosa cultured in burn wound exudate rather than LB media demonstrated preferential use of lactate as a carbon source as compared with glucose. Lactate levels in the burn wound exudate were typically 3.19 mM [25] and declined over the 24 h culture period. Pseudomonas’ preference for lactate as a carbon source was confirmed in experiments where a cystic fibrosis like sputum media containing 9 mM lactate demonstrated superior growth [26] compared with conventional media.
Manipulation of oxygen metabolism
Inflammatory nitric oxide (NO·) is a crucial bactericidal mechanism for leukocytes. NO· has a range of cytotoxic effects, however, certain bacterial species, such as S. aureus display resistance to NO·. This resistance may play a role in the ability of S. aureus to persist and colonise the nasopharynx. Host-derived NO· prevents aerobic respiration by S. aureus and induces fermentation of glucose, thereby generating significant levels of l-lactate. l-lactate is then converted to pyruvate generating NAD+, which is used to maintain redox balance in the face of NO· [87]. Additionally, S. aureus possesses NAD independent LDH (iLDH) which oxidises lactate to pyruvate with simultaneous reduction of the respiratory quinone pool [145]. Inactivation of ldh1 impairs S. aureus growth in the presence of NO· and, similarly, the ldh1 mutant S. aureus had significantly reduced virulence in a murine model of sepsis. However, in mice lacking the ability to generate NO· the virulence of the ldh1 mutant was partially restored [87]. This was also replicated in a S. aureus mutant lacking iLDH or Lqo [145]. Suggesting that iLDH and LDH in S. aureus enables the pathogen to use host or pathogen-derived lactate to protect from exogenous NO·. The bactericidal effect of NO· are clearly abrogated in S. aureus, however, it is important to note NO· is also able to influence bacteria by other cytotoxic mechanisms [146] which are not fully explained by this observation.
Competition between species and manipulation of oxygen metabolism has also been shown to enhance the survival of other bacteria. For example, N. meningitidis is able to utilise lactate derived from neutrophil glycolysis to enhance oxygen consumption and therefore reduce oxygen availability for bactericidal mechanisms by neutrophils [141].
Ability to move from coloniser to invasive pathogen
N. meningitidis colonises the nasopharynx, and a crucial step in dissemination and its ability to cause invasive disease is the dispersal of micro-colonies, a process during which bacteria detach and access new sites. This has been shown in a nasal mucosa explant cell culture model to be dependent on both d- and l-lactate metabolism [88], as well as in isolated epithelial cells. It is important to consider that in single pathogen in vitro or ex vivo models there is invariably simplification of the complex interplay taking place in vivo with competition between pathogens and a rapidly changing immune environment, none the less it is increasingly clear that lactate plays an important role in pathogen virulence. A similar effect was seen in a different strain of N. meningitidis and N. gonorrhea [147]. It was confirmed that expression of lactate utilisation genes (lctP) is required for nasopharyngeal colonisation by N. meningitidis [88], despite the mutant having normal adhesion.
Resistance to serum mediated killing
Complement-mediated killing is a crucial host bactericidal mechanism. Lactate is a key virulence factor in protection from complement-mediated killing in N. meningitidis as the presence of lctP is required for avoidance of serum-based killing [142]. The reduced virulence of the lctP mutant strain was restored to normal levels in the absence of serum complement [142]. N. gonorrhea has enhanced resistance to serum-based killing mediated by lactate metabolism, leading to enhanced LPS sialylation, independent of sialyltransferase, which prevents the bactericidal action of antibodies and complement [82].
Haemophilus influenzae strain b (Hib) demonstrates increased resistance to serum mediated killing when grown in either nasopharyngeal aspirate or human serum which contains lactate compared with lactate free culture media [90]. This phenotypic change was inducible by as little as 30 min incubation in human serum. The mechanism underlying this observation is unclear as it was shown that deletion of lctP did not affect survival in human serum [28]. However, these effects may be strain dependent as a strain of lctP mutated non-typeable H. influenzae (NTHi) had impaired growth compared with a wild type strain, but again this was not replicated in a different NTHi strain [28]. Resistance to serum mediated killing could simply relate to a carbon source preference for lactate in Hib increasing growth velocity. Again it is important to acknowledge that these are in vitro findings when bacteria are cultured at densities exceeding those seen in human and animal infections, with tightly regulated nutrient availability not reflecting true infection states.
In vivo survival
In vivo survival is much more complex than the in vitro culture described above, and the central role of lactate utilisation has been demonstrated in animal models of infection. Loss of lactate utilisation genes reduces virulence, attenuates bacteria's ability to colonise and results in less severe infection. N. gonorrhea can utilise both d and l-lactate, and lactate controls key virulence factors as loss of ldh and lctP results in decreased survival in cervical epithelial cells under microaerobic conditions [140].
Ldh mutant pneumococcus was unable to cause bacteriaemia in a mouse model of sepsis and mice had significantly longer survival compared with infection with wild type strains [144]. lctP mutant N. meningitidis has an attenuated ability to cause bacteriaemia compared with wild type strains in a rat model [142]. Signature tagged mutagenesis of H. influenzae identified l-lactate permease as crucial for in vivo survival [89]. lctP mutant N. gonorrhea has reduced ability to colonise the murine vagina compared with WT [82,148].
Lactate in non-bacterial pathogens
Viruses
Viruses are obligate parasites and there is no evidence of direct lactate sensing capacity. However, they often rely on endocytic pathways for cell entry in which a decrease in pH acts as a signal for host penetration [149]. In particular, the requirement for a drop in pH for viral fusion and entry has been described in multiple viruses such as the Semliki forest virus [150] and influenza [151,152]. In Epstein–Barr virus (EBV)-derived lymphomas [153], EBV increases LDH-A expression and, consequently, lactate secretion by B lymphoma cells, resulting in down-regulation of viral microRNA promoting cancer growth. Viruses can also influence cellular processes such as metabolism to impose an environment that favours their replication [154,155]. Adenovirus infection of human cell lines HEK293 and 1G3 resulted in 2-fold and 4-fold increases of glucose consumption and lactate release, respectively [156,157]. Similar trends have been observed with Herpes simplex virus 1 and 2 [158,159], human CMV [158,160,161] and influenza [162–164]. Also, lactate limits retinoic-acid-inducible gene I (RIG-I)-like receptor (RLR) signalling, diminishing type I interferon production and preventing viral clearance [165]. Lactate is directly sensed by the mitochondrial antiviral-signalling (MAVS) protein, blocking MAVS aggregation and downstream RLR signalling. Inactivating LDH-A, therefore, results in increased type I IFN secretion and protection of mice from viral infection. In summary, lactate is closely linked to viral infections, from viruses increasing LDH expression and lactate production, to lactate specifically targeting key proteins in antiviral signalling.
Fungi
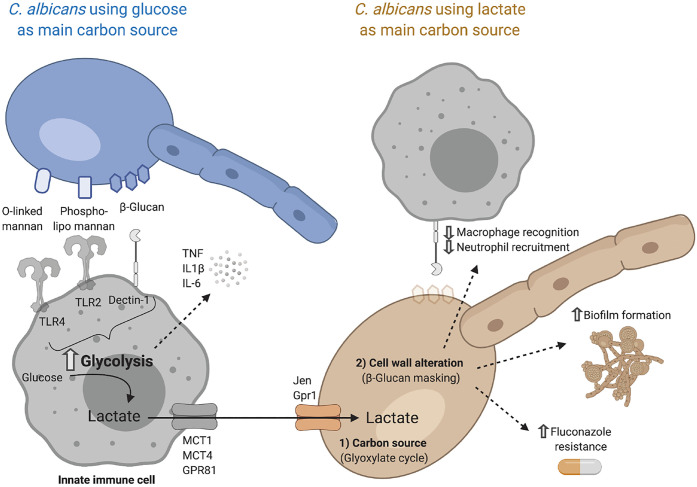
Recognition of fungal cell wall components triggers glycolysis in innate immune cells [166–169]. This metabolic adaptation has been described in response to Candida albicans [166–168], Aspergillus fumigatus [170] and Cryptococcus gatti [171] infection. The shift to glycolysis enables optimal immune responses through the release of proinflammatory cytokines [166,170]. The Jen transporters, which perform proton-lactate symport, are expressed in fungal species [62,63]. Pathogens such as Candida can use lactate as a carbon source to thrive in nutrient-restricted body niches such as the gut or the vagina [172]. The role of lactate in C. albicans infection goes beyond nutrition, as lactate is directly involved in modulating pathogen–phagocyte interactions (Figure 3). Lactate increases biofilm formation, enhances resistance to different host-stressors and diminishes macrophage recognition [64,172,173]. β-glucan, a major cell wall component, can be masked after triggering by lactate which has been described as one of the key immune evasion strategies employed by C. albicans [64,174]. C. albicans grown with lactate as its main carbon source also impacted immune cell function increasing secretion of the immunosuppressive cytokine IL-10, while decreasing IL-17, which plays a key role in protection against invasive candidiasis [175,176].
Figure 3. Lactate cross-talk between Candida albicans and phagocytic immune cells.
The main pattern recognition receptors (PRRs) involved in the recognition of C. albicans are C-type lectin receptors (CLRs) and Toll-like receptors (TLRs) expressed on the surface of innate immune cells with high phagocytic capacity (neutrophils, macrophages and dendritic cells). Sensing of C. albicans triggers a metabolic shift towards glycolysis, which is essential for the effective production and release of proinflammatory cytokines such as TNF, IL1β and IL-6. Active glycolysis results in the production of lactate, which can be exported through different lactate transporters including MCT1, MCT4 and GPR81, depending on cell type (see Table 2). Once this lactate is in the extracellular milieu C. albicans can take it up through the Jen and Gpr1 transporters and use it to thrive in nutrient-restricted body niches. Lactate can not only be used as a carbon source, fuelling metabolic pathways such as the glyoxylate shunt, but it can also alter cell wall composition. These changes largely impact the ability of the host to mount an effective immune response. One of the best-described strategies is the masking of β-glucan, which results in decreased macrophage recognition and neutrophil recruitment. Furthermore, when C. albicans uses lactate as the main carbon source, biofilm formation, as well as resistance to antifungal drugs such as fluconazole, are increased. Created using Biorender.com.
Parasites
Glycolysis is the preferred metabolic pathway for fulfilling the energy requirements of Plasmodium falciparum, the intra-erythrocytic parasite that causes malaria [177]. This process generates large quantities of lactate that is primarily expelled through pfFNT, a surface transporter of the formate-nitrite family [66]. Erythrocytes will in turn release lactate to the bloodstream through SLC16A1 (MCT1) [178]. Toxoplasma gondii is a major zoonotic pathogen that also relies on glycolysis for its growth, despite being metabolically versatile [179,180]. Several studies have shown how Toxoplasma LDH determines bradyzoite (slowly dividing phase) differentiation, virulence and chronic infection [179,181,182]. Whether lactate is directly implicated or whether these findings reflect the insufficiency of oxidative phosphorylation to meet the parasite's energetic requirements is still unknown. In general, the use of and response to lactate in parasites has not been well characterised in detail. However, there is abundant evidence of the evolutionary pressure for all these parasites to have effective lactate transport systems in place.
Therapeutics
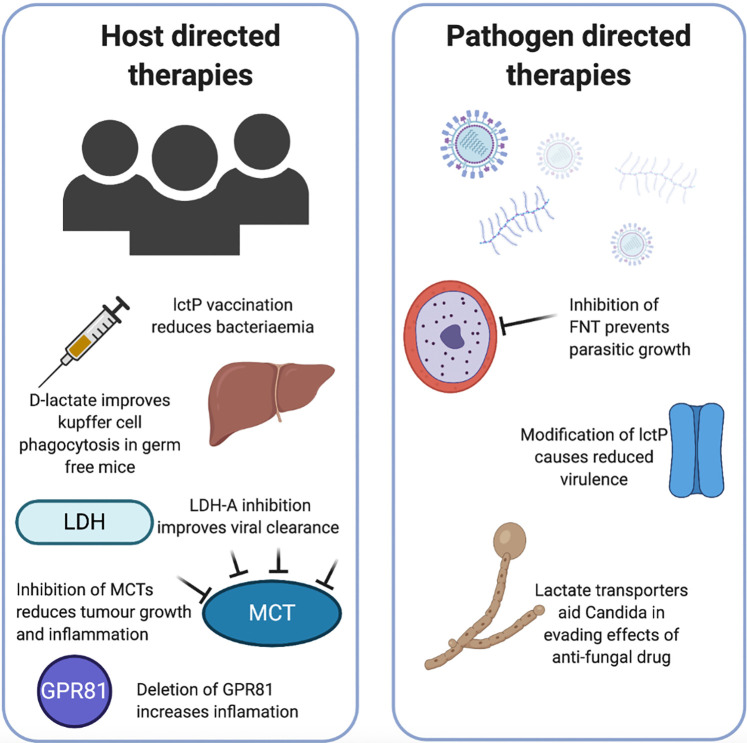
Human studies specifically modulating lactate in infection are scarce, however, research has been done regarding the therapeutic potential of targeting lactate in the context of cancer, autoimmunity and inflammation. The most common approaches include blocking specific lactate transporters, targeting LDH or adding lactate (Figure 4).
Figure 4. Lactate directed host and pathogen therapeutic avenues.
Lactate directed therapeutics can be considered to target host or pathogen utilisation of lactate. In the host, vaccination of mice with recombinant lctP was partially protective against N. meningitidis bacteraemia. Supplementing germ-free mice with either d-lactate or LAB improved the phagocytic capacity of Kupffer cells in the liver preventing bacteriaemia. Inhibition of lactate utilisation tools such as MCT, LDH or the lactate receptor GPR81 has been demonstrated to improve viral clearance, reduce tumour burden while deletion of GPR81 increases susceptibility to inflammation. For pathogen directed therapies inhibition of lactate transporters prevents growth of plasmodium falciparum, and in other bacteria deletion of lctP causes reduced virulence in vivo. Lactate transporters are crucial for Candida to avoid antifungal drugs. LctP, lactate permease; LAB, lactic acid-producing bacteria; MCT, monocarboxylate transporters (lactate transporters in vertebrates) and LDH, lactate dehydrogenase. Created using Biorender.com.
Cancer therapeutics
The potential of MCTs and LDH inhibitors for cancer treatment has been reviewed elsewhere [9]. Both small compounds and RNA silencing strategies have been employed with promising results. For instance, the orally dosed MCT inhibitor AZD3695 is undergoing phase I/II trials in lymphoma (NCT017915950). It is promising that modulators of lactate transport are in use in human studies, suggesting that safety and tolerability will soon be established to enable use in other human diseases. Furthermore, the use of shRNAs to knock down LDH-A resulted in decreased proliferation of tumour cells [183].
The use of oral MCT inhibitors in human studies of lymphoma are promising, but care needs to be taken in translating tolerability and safety to infection. Some infections are chronic and associated with immunosuppression, but initial stages of acute infection including sepsis are associated with overwhelming immune activation.
To begin to therapeutically target lactate we need to first understand the role lactate plays in the diverse infection settings in terms of host response, across organ systems and disease states to prevent inadvertent harm. Developing a clearer understanding of pathogens lactate utilisation but also symbionts may allow directed targeting of their specific transporters avoiding harm to the host.
Autoimmunity and inflammation
The role of lactate as an immune modulator in inflammatory conditions is now well accepted. Studies in a collagen-induced arthritis mouse model showed that silencing of SLC16A3 (MCT4) reduced the severity of arthritis [184]. Also, SLC16A3 (MCT4) transcripts were up-regulated in synovial fibroblasts from rheumatoid arthritis patients compared with osteoarthritis patients, and knockdown of SLC16A3 (MCT4) prevented their proliferation [184]. Furthermore, in other mouse models of arthritis and peritonitis, antibody, or shRNA blockade of SLC5A12 also reduced disease severity, which was accompanied by restored T cell function [8,51].
In sterile models of chemically induced hepatitis and pancreatitis, pre-treatment with lactate reduced inflammation by attenuating TLR induced inflammatory cytokines through GPR81 signalling [185]. The crucial role of lactate sensing by GPR81 was confirmed as deletion of GPR81 increased susceptibility of mice to chemically induced colitis, where GPR81 deficient mice had elevated inflammatory cytokines and up-regulation of inflammatory Th1/Th17 cells [78].
Targeting lactate in the context of infection
Although the therapeutic potential of targeting lactate has not yet been explored in human clinical trials, evidence is accumulating and promising avenues are being investigated.
Viral infection
The in vivo immunosuppressive action of lactate has been demonstrated: lactate is sensed by mitochondrial antiviral signalling proteins reducing RIG-I-like receptor activation to down-regulate type I interferon production providing an immunotolerant state in which the virus was able to evade immune surveillance [165]. Inhibition of LDHA reduced lactate and promoted viral clearance through up-regulation of type I interferon and other inflammatory cytokines [165]. Thus, targeting lactate could directly aid the orchestration of effective antiviral immune responses.
Bacterial infection
As discussed in previous sections, some bacteria can use lactate as a carbon source. Therefore, targeting bacterial utilisation of lactate could potentially prevent the expansion of bacterial communities. Lactate utilisation genes are crucial for the virulence of many organisms, and targeting them with vaccination in vivo, or by in vitro inhibition seems to have some promise [186]. For instance, the expression of lactate permease (lctP) by N. meningitidis is crucial for its pathogenicity, and lctP has been explored for its potential as a vaccine target. Treatment of mice with recombinant lctP partially protected them from N. meningitidis bacteriaemia [186].
Parasitic infection
Targeting lactate transport in parasites has also been trialled in vitro, where inhibition of formate–nitrate transporters impairs growth and leads to parasitic death in both P. falciparum and Toxoplasmosis [67,187]. Targeting lactate transporters in candida species could also offer therapeutic potential since they have been shown to aid C. albicans and C. glabrata in evading the effects of antifungal drugs [65,172].
Based on the reviewed evidence, a degree of immunosuppression induced by lactate in acute infection may be protective in preventing overwhelming inflammation-causing myocardial and associated organ damage. Alternatively, in chronic infections, the immunosuppressive effect of lactate may be more deleterious by allowing the virus to remain undetected and actively replicate. Further investigations are warranted to fully understand the complex interplay between the pathogen and the host in terms of responses to lactate. Only then we will be able to target lactate and improve infection outcome.
Beneficial role of lactate in host–pathogen relations
Evidence also exists for the beneficial roles of lactate. Specifically, in the context of bacterial derived lactate, there is a further challenge to be considered. LAB are the main producers of d-lactate in the gut. Dysbiosis occurs commonly in disease states and is associated with increased risk of sepsis and disseminated infection [188]. Giving critically ill patients probiotics containing LAB protects them from nosocomial acquired infection [189]. This link between loss of intestinal LAB and health is clear, but the mechanism that is able to impact distant immune cells remained unclear until recently [190]. The role of intestinal microbiota-derived d-lactate in maintaining crucial immune surveillance distant to the gut was demonstrated. In germ-free mice, the phagocytic capacity of Kupffer cells was significantly reduced, leading to the persistence of S. aureus bacteriaemia. Supplementing their diet with d-lactate partially restored the ability to clear bacteriaemia, and the same effect was demonstrated when gnotobiotic mice were supplemented with specific LAB which produced high levels of d-lactate. d-lactate levels were shown to be elevated in the gut, and portal vein, but not systemically. It is also important to note that although d-lactate played a crucial role, the phagocytic capacity was not fully restored, likely reflecting the contribution of other metabolites [190]. This study opens new avenues for using lactate itself as a therapeutic molecule.
Further characterisation of lactate utilisation in both symbiotic and pathogenic organisms, and the role of lactate on influencing host immunity during acute and chronic infection, will allow us to understand this complex interplay. Thus, it seems likely that manipulation of lactate in infection, whether host or pathogen derived, will require a balance between influencing pathogenic organisms, while enhancing the beneficial relationship with symbionts.
Acknowledgements
We thank Dr. Patricia Glynn and Dr. Martin Dedicoat for their critical reading of this manuscript.
Abbreviations
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- CSF
cerebrospinal fluid
- DCs
dendritic cells
- EBV
Epstein–Barr virus
- GPR
G-protein-coupled receptors
- Hib
Haemophilus influenzae strain b
- hMDM
human monocyte-derived macrophage
- iLDH
independent LDH
- LAB
lactic acid-producing bacteria
- LctP
lactate permease
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- MAVS
mitochondrial antiviral-signalling
- MOI
multiplicity of infection
- NMR
nuclear magnetic resonance
- NTHi
non-typeable H. influenzae
- RLR
retinoic-acid-inducible gene I (RIG-I)-like receptor
Conclusion
In recent years, we have seen our understanding of lactate evolve from a mere by-product of cellular metabolism to an active signalling molecule playing an active role in cancer, inflammation and host–pathogen interactions. Curating a comprehensive view of the consequences of increased lactate concentrations in the context of infection and how this affects both pathogen and immune function is challenging. Lactate can be released by host cells, as well as pathogenic and commensal microbes. The precise impact of lactate on immune cell function seems to be context dependent, as it is mainly immune-suppressive in the tumour microenvironment, while it can favour chronic inflammation in other settings.
Several pathogenic microbial species have evolved mechanisms to use lactate that enhances their pathogenesis. The presence of lactate transporters in most human cells including immune cells, as well as in a wide range of pathogens including bacteria, fungi and complex parasites, is proof of the importance of this metabolite in regulating host and pathogen function in the context of infection. This makes lactate an attractive therapeutic target, and proof of concept studies have already been developed. We still require a deeper understanding of the role lactate plays in the complex interplay between commensals, pathogens, and the host before we can benefit from these potential novel therapeutic approaches.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
A.L. is supported by the European Commission (H2020-MSCA-IF-2018, 841729). F.S.G. is supported by The Dunhill Medical Trust (RTF1906\86). D.R.T. and A.S. report funding from the British Lung Foundation (PPRG16-12), the Medical Research Council (MR/S002782/1) and the Health Technology Assessment (NIHR129593). C.M. is supported by the Medical Research Council (MR/T016736/1) and by a Professorial Fellowship and Translational Funds from the University of Birmingham.
Open Access Statement
Open access for this article was enabled by the participation of University of Birmingham in an all-inclusive Read & Publish pilot with Portland Press and the Biochemical Society under a transformative agreement with JISC.
References
- 1.Scheele (1931) The collected papers of carl Wilhelm Scheele. Nature 128, 1023–1024 10.1038/1281023a0 [DOI] [Google Scholar]
- 2.Hume, D.A., Radik, J.L., Ferber, E. and Weidemann, M.J. (1978) Aerobic glycolysis and lymphocyte transformation. Biochem. J. 174, 703–709 10.1042/bj1740703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levene, P.A., M.G. and Meyer, (1912) On the action of leucocytes on glucose. J. Biol. Chem. 11, 361––3370. 10.1016/S0021-9258(18)88742-7 [DOI] [Google Scholar]
- 4.Warburg, O., Wind, F. and Negelein, E. (1927) The metabolism of tumors in the body. J. Gen. Physiol. 8, 519–530 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright, M.R. and Jamali, F. (1993) Methods for the analysis of enantiomers of racemic drugs application to pharmacological and pharmacokinetic studies. J. Pharmacol. Toxicol. Methods 29, 1–9 10.1016/1056-8719(93)90044-F [DOI] [PubMed] [Google Scholar]
- 6.Kowlgi, N.G. and Chhabra, L. (2015) D-lactic acidosis: an underrecognized complication of short bowel syndrome. Gastroenterol. Res. Pract. 2015, 476215 10.1155/2015/476215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen, C. (2005) D-lactic acidosis. Nutr. Clin. Pract. 20, 634–645 10.1177/0115426505020006634 [DOI] [PubMed] [Google Scholar]
- 8.Haas, R., Smith, J., Rocher-Ros, V., Nadkarni, S., Montero-Melendez, T., D'Acquisto, F.et al. (2015) Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 10.1371/journal.pbio.1002202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Certo, M., Tsai, C.H., Pucino, V., Ho, P.C. and Mauro, C. (2020) Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 10.1038/s41577-020-0406-2 [DOI] [PubMed] [Google Scholar]
- 10.Kraut, J.A. and Madias, N.E. (2016) Lactic acidosis: current treatments and future directions. Am. J. Kidney Dis.: Off. J. Natl. Kidney Found. 68, 473–482 10.1053/j.ajkd.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 11.Kompanje, E.J.O., Jansen, T.C., van der Hoven, B. and Bakker, J. (1843) The first demonstration of lactic acid in human blood in shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med. 33, 1967–1971 10.1007/s00134-007-0788-7 [DOI]
- 12.Broder, G. and Weil, M.H. (1964) Excess lactate: an index of reversibility of shock in human patients. Science (New York, NY) 143, 1457–1459 10.1126/science.143.3613.1457 [DOI] [PubMed] [Google Scholar]
- 13.Okorie, O.N. and Dellinger, P. (2011) Lactate: biomarker and potential therapeutic target. Crit. Care Clin. 27, 299–326 10.1016/j.ccc.2010.12.013 [DOI] [PubMed] [Google Scholar]
- 14.Investigators, P., Rowan, K.M., Angus, D.C., Bailey, M., Barnato, A.E., Bellomo, R.et al. (2017) Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N. Engl. J. Med. 376, 2223–2234 10.1056/NEJMoa1701380 [DOI] [PubMed] [Google Scholar]
- 15.Andersen, L.W., Mackenhauer, J., Roberts, J.C., Berg, K.M., Cocchi, M.N. and Donnino, M.W. (2013) Etiology and therapeutic approach to elevated lactate levels. Mayo Clin. Proc. 88, 1127–1140 10.1016/j.mayocp.2013.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colegio, O.R., Chu, N.Q., Szabo, A.L., Chu, T., Rhebergen, A.M., Jairam, V.et al. (2014) Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschhaeuser, F., Sattler, U.G.A. and Mueller-Klieser, W. (2011) Lactate: a metabolic key player in cancer. Cancer Res. 71, 6921–6925 10.1158/0008-5472.CAN-11-1457 [DOI] [PubMed] [Google Scholar]
- 18.Goetze, K., Walenta, S., Ksiazkiewicz, M., Kunz-Schughart, L.A. and Mueller-Klieser, W. (2011) Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol. 39, 453–463 10.3892/ijo.2011.1055 [DOI] [PubMed] [Google Scholar]
- 19.Walenta, S., Wetterling, M., Lehrke, M., Schwickert, G., Sundfor, K., Rofstad, E.K.et al. (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 60, 916–921 PMID: [PubMed] [Google Scholar]
- 20.Chavalittamrong, B., Angsusingha, K., Tuchinda, M., Habanananda, S., Pidatcha, P. and Tuchinda, C. (1979) Diagnostic significance of pH, lactic acid dehydrogenase, lactate and glucose in pleural fluid. Respir. Int. Rev. Thorac. Dis. 38, 112–120 10.1159/000194067 [DOI] [PubMed] [Google Scholar]
- 21.Hove, H. and Mortensen, P.B. (1995) Influence of intestinal inflammation (IBD) and small and large bowel length on fecal short-chain fatty acids and lactate. Dig. Dis. Sci. 40, 1372–1380 10.1007/BF02065554 [DOI] [PubMed] [Google Scholar]
- 22.Genton, B. and Berger, J.P. (1990) Cerebrospinal fluid lactate in 78 cases of adult meningitis. Intensive Care Med. 16, 196–200 10.1007/BF01724802 [DOI] [PubMed] [Google Scholar]
- 23.Gillis, C.C., Winter, M.G., Chanin, R.B., Zhu, W., Spiga, L. and Winter, S.E. (2019) Host-derived metabolites modulate transcription of salmonella genes involved in l-lactate utilization during gut colonization. Infect. Immun. 87, e00773-18 10.1128/IAI.00773-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, H., Yates, E.A., Cole, J.A. and Parsons, N.J. (2001) Lactate stimulation of gonococcal metabolism in media containing glucose: mechanism, impact on pathogenicity, and wider implications for other pathogens. Infect. Immun. 69, 6565–6572 10.1128/IAI.69.11.6565-6572.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez, M.R., Ducret, V., Leoni, S., Fleuchot, B., Jafari, P., Raffoul, W.et al. (2018) Transcriptome analysis of pseudomonas aeruginosa cultured in human burn wound exudates. Front. Cell. Infect. Microbiol. 8, 39 10.3389/fcimb.2018.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer, K.L., Aye, L.M. and Whiteley, M. (2007) Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087 10.1128/JB.01138-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serafini, A., Tan, L., Horswell, S., Howell, S., Greenwood, D.J., Hunt, D.M.et al. (2019) Mycobacterium tuberculosis requires glyoxylate shunt and reverse methylcitrate cycle for lactate and pyruvate metabolism. Mol. Microbiol. 112, 1284–1307 10.1111/mmi.14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenegger, S., Bina, I., Roier, S., Bauernfeind, S., Keidel, K., Schild, S.et al. (2014) Characterization of lactate utilization and its implication on the physiology of Haemophilus influenzae. Int. J. Med. Microbiol. 304, 490–498 10.1016/j.ijmm.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan, S.H., Louis, P. and Flint, H.J. (2004) Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 70, 5810–5817 10.1128/AEM.70.10.5810-5817.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessione, E. (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front. Cell. Infect. Microbiol. 2, 86 10.3389/fcimb.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belenguer, A., Duncan, S.H., Holtrop, G., Anderson, S.E., Lobley, G.E. and Flint, H.J. (2007) Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 73, 6526–6533 10.1128/AEM.00508-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillis, C.C., Hughes, E.R., Spiga, L., Winter, M.G., Zhu, W., de Carvalho T, F.et al. (2018) Dysbiosis-associated change in host metabolism generates lactate to support Salmonella growth. Cell Host Microbe 23, 54–64.e6 10.1016/j.chom.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srinivas, S.R., Gopal, E., Zhuang, L., Itagaki, S., Martin, P.M., Fei, Y.J.et al. (2005) Cloning and functional identification of slc5a12 as a sodium-coupled low-affinity transporter for monocarboxylates (SMCT2). Biochem. J. 392(Pt 3), 655–664 10.1042/BJ20050927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halestrap, A.P. (2012) The monocarboxylate transporter family–structure and functional characterization. IUBMB Life 64, 1–9 10.1002/iub.573 [DOI] [PubMed] [Google Scholar]
- 35.Bosshart, P.D., Kalbermatter, D., Bonetti, S. and Fotiadis, D. (2019) Mechanistic basis of L-lactate transport in the SLC16 solute carrier family. Nat. Commun. 10, 2649 10.1038/s41467-019-10566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopal, E., Umapathy, N.S., Martin, P.M., Ananth, S., Gnana-Prakasam, J.P., Becker, H.et al. (2007) Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim. Biophys. Acta 1768, 2690–2697 10.1016/j.bbamem.2007.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez, A.M., Perron, B., Lacroix, L., Caillou, B., Leblanc, G., Schlumberger, M.et al. (2002) Identification and characterization of a putative human iodide transporter located at the apical membrane of thyrocytes. J. Clin. Endocrinol. Metab. 87, 3500–3503 10.1210/jcem.87.7.8797 [DOI] [PubMed] [Google Scholar]
- 38.Ganapathy, V., Thangaraju, M., Gopal, E., Martin, P.M., Itagaki, S., Miyauchi, S.et al. (2008) Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 10, 193–199 10.1208/s12248-008-9022-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puri, S. and Juvale, K. (2020) Monocarboxylate transporter 1 and 4 inhibitors as potential therapeutics for treating solid tumours: a review with structure-activity relationship insights. Eur. J. Med. Chem. 199, 112393 10.1016/j.ejmech.2020.112393 [DOI] [PubMed] [Google Scholar]
- 40.Payen, V.L., Mina, E., Van Hee, V.F., Porporato, P.E. and Sonveaux, P. (2020) Monocarboxylate transporters in cancer. Mol. Metab. 33, 48–66 10.1016/j.molmet.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rooney, K. and Trayhurn, P. (2011) Lactate and the GPR81 receptor in metabolic regulation: implications for adipose tissue function and fatty acid utilisation by muscle during exercise. Br. J. Nutr. 106, 1310–1316 10.1017/S0007114511004673 [DOI] [PubMed] [Google Scholar]
- 42.Abrantes, H.d.C., Briquet, M., Schmuziger, C., Restivo, L., Puyal, J., Rosenberg, N.et al. (2019) The lactate receptor HCAR1 modulates neuronal network activity through the activation of galpha and gbetagamma subunits. J. Neurosci. 39, 4422–4433 10.1523/JNEUROSCI.2092-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morland, C., Lauritzen, K.H., Puchades, M., Holm-Hansen, S., Andersson, K., Gjedde, A.et al. (2015) The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J. Neurosci. Res. 93, 1045–1055 10.1002/jnr.23593 [DOI] [PubMed] [Google Scholar]
- 44.Chen, Y.J., Mahieu, N.G., Huang, X., Singh, M., Crawford, P.A., Johnson, S.L.et al. (2016) Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 10.1038/nchembio.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami, N., Hashidate, T., Harayama, T., Yokomizo, T., Shimizu, T. and Nakamura, M. (2009) Transcriptional regulation of human G2A in monocytes/ macrophages: involvement of c/EBPs, Runx and Pu.1. Genes Cells 14, 1441–1455 10.1111/j.1365-2443.2009.01360.x [DOI] [PubMed] [Google Scholar]
- 46.Chen, P., Zuo, H., Xiong, H., Kolar, M.J., Chu, Q., Saghatelian, A.et al. (2017) Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl Acad. Sci. U.S.A. 114, 580–585 10.1073/pnas.1614035114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kern, K., Schafer, S.M.G., Cohnen, J., Pierre, S., Osthues, T., Tarighi, N.et al. (2018) The G2A receptor controls polarization of macrophage by determining their localization within the inflamed tissue. Front. Immunol. 9, 2261 10.3389/fimmu.2018.02261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parks, B.W., Gambill, G.P., Lusis, A.J. and Kabarowski, J.H. (2005) Loss of G2A promotes macrophage accumulation in atherosclerotic lesions of low density lipoprotein receptor-deficient mice. J. Lipid Res. 46, 1405–1415 10.1194/jlr.M500085-JLR200 [DOI] [PubMed] [Google Scholar]
- 49.Bolick, D.T., Skaflen, M.D., Johnson, L.E., Kwon, S.C., Howatt, D., Daugherty, A.et al. (2009) G2a deficiency in mice promotes macrophage activation and atherosclerosis. Circ. Res. 104, 318–327 10.1161/CIRCRESAHA.108.181131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Certo, M., Marone, G., de Paulis, A., Mauro, C. and Pucino, V. (2020) Lactate: fueling the fire starter. Wiley Interdiscip. Rev. Syst. Biol. Med. 12, e1474 10.1002/wsbm.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pucino, V., Certo, M., Bulusu, V., Cucchi, D., Goldmann, K., Pontarini, E.et al. (2019) Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring. Cell Metab. 30, 1055–1074.e8 10.1016/j.cmet.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khatib-Massalha, E., Bhattacharya, S., Massalha, H., Biram, A., Golan, K., Kollet, O.et al. (2020) Lactate released by inflammatory bone marrow neutrophils induces their mobilization via endothelial GPR81 signaling. Nat. Commun. 11, 3547 10.1038/s41467-020-17402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merezhinskaya, N., Ogunwuyi, S.A., Mullick, F.G. and Fishbein, W.N. (2004) Presence and localization of three lactic acid transporters (MCT1, -2, and -4) in separated human granulocytes, lymphocytes, and monocytes. J. Histochem. Cytochem. 52, 1483–1493 10.1369/jhc.4A6306.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price, N.T., Jackson, V.N. and Halestrap, A.P. (1998) Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem. J. 329(Pt 2), 321–328 10.1042/bj3290321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ranganathan, P., Shanmugam, A., Swafford, D., Suryawanshi, A., Bhattacharjee, P., Hussein, M.S.et al. (2018) GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol. 200, 1781–1789 10.4049/jimmunol.1700604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, L., He, H.W., Xing, Z.Q., Tang, B. and Zhou, X. (2020) Lactate induces alternative polarization (M2) of macrophages under lipopolysaccharide stimulation in vitro through G-protein coupled receptor 81. Chin. Med. J. (Engl.) 133, 1761–1763 10.1097/CM9.0000000000000955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown, T.P., Bhattacharjee, P., Ramachandran, S., Sivaprakasam, S., Ristic, B., Sikder, M.O.F.et al. (2020) The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 39, 3292–3304 10.1038/s41388-020-1216-5 [DOI] [PubMed] [Google Scholar]
- 58.Khatib-Massalha, E., Kumari, A., Golan, K., Massalha, H., Avemaria, F., Gur-Cohen, S.et al. (2017) Lactate release by bone marrow neutrophils promotes their inflammatory mobilization via endothelial GPR81 signaling. Blood 130, 446 10.1038/s41467-020-17402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, Y., Zhang, C., Liu, G., Ju, J., Yu, B. and Wang, L. (2019) Elucidating the role and regulation of a lactate permease as lactate transporter in Bacillus coagulans DSM1. Appl. Environ. Microbiol. 85, e00672-19. 10.1128/AEM.00672-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ayala, J.C. and Shafer, W.M. (2019) Transcriptional regulation of a gonococcal gene encoding a virulence factor (L-lactate permease). PLoS Pathog. 15, e1008233 10.1371/journal.ppat.1008233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bienert, G.P., Desguin, B., Chaumont, F. and Hols, P. (2013) Channel-mediated lactic acid transport: a novel function for aquaglyceroporins in bacteria. Biochem. J. 454, 559–570 10.1042/BJ20130388 [DOI] [PubMed] [Google Scholar]
- 62. Casal, M., Paiva, S., Andrade, R.P and Gancedo, C. doi: 10.1128/jb.181.8.2620-2623.1999. The Lactate–Proton Symport of Saccharomyces cerevisiae is Encoded by JEN1 Downloaded from. 1999. Report No.: 00219193/99. [DOI] [PMC free article] [PubMed]
- 63.Soares-Silva, I., Paiva, S., Kotter, P., Entian, K.D. and Casal, M. (2004) The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol. Membr. Biol. 21, 403–411 10.1080/09687860400011373 [DOI] [PubMed] [Google Scholar]
- 64.Ballou, E.R., Avelar, G.M., Childers, D.S., Mackie, J., Bain, J.M., Wagener, J.et al. (2016) Lactate signalling regulates fungal beta-glucan masking and immune evasion. Nat. Microbiol. 2, 16238 10.1038/nmicrobiol.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alves, R., Sousa-Silva, M., Vieira, D., Soares, P., Chebaro, Y., Lorenz, M.C.et al. (2020) Carboxylic acid transporters in candida pathogenesis. mBio 11, e00156-20 10.1128/mBio.00156-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchetti, R.V., Lehane, A.M., Shafik, S.H., Winterberg, M., Martin, R.E. and Kirk, K. (2015) A lactate and formate transporter in the intraerythrocytic malaria parasite, Plasmodium falciparum. Nat. Commun. 6, 6721 10.1038/ncomms7721 [DOI] [PubMed] [Google Scholar]
- 67.Erler, H., Ren, B., Gupta, N. and Beitz, E. (2018) The intracellular parasite Toxoplasma gondii harbors three druggable FNT-type formate and l-lactate transporters in the plasma membrane. J. Biol. Chem. 293, 17622–17630 10.1074/jbc.RA118.003801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uzcategui, N.L., Figarella, K., Segnini, A., Marsiccobetre, S., Lang, F., Beitz, E.et al. (2018) Trypanosoma brucei aquaglyceroporins mediate the transport of metabolic end-products: methylglyoxal, D-lactate, L-lactate and acetate. Biochim. Biophys. Acta Biomembr. 1860, 2252–2261 10.1016/j.bbamem.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 69.Sanchez, M.A. (2013) Molecular identification and characterization of an essential pyruvate transporter from Trypanosoma brucei. J. Biol. Chem. 288, 14428–14437 10.1074/jbc.M113.473157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Romero-Garcia, S., Moreno-Altamirano, M.M., Prado-Garcia, H. and Sanchez-Garcia, F.J. (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 7, 52 10.3389/fimmu.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng, J., Yang, H., Zhang, Y., Wei, H., Zhu, Z., Zhu, B.et al. (2017) Tumor cell-derived lactate induces TAZ-dependent up-regulation of PD-L1 through GPR81 in human lung cancer cells. Oncogene 36, 5829–5839 10.1038/onc.2017.188 [DOI] [PubMed] [Google Scholar]
- 72.Rundqvist, H., Velica, P., Barbieri, L., Gameiro, P.A., Bargiela, D., Gojkovic, M.et al. (2020) Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. eLife 9, e59996 10.7554/eLife.59996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mendler, A.N., Hu, B., Prinz, P.U., Kreutz, M., Gottfried, E. and Noessner, E. (2012) Tumor lactic acidosis suppresses CTL function by inhibition of p38 and JNK/c-Jun activation. Int. J. Cancer 131, 633–640 10.1002/ijc.26410 [DOI] [PubMed] [Google Scholar]
- 74.Nolt, B., Tu, F., Wang, X., Ha, T., Winter, R., Williams, D.L.et al. (2018) Lactate and immunosuppression in sepsis. Shock 49, 120–125 10.1097/SHK.0000000000000958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borregaard, N. and Herlin, T. (1982) Energy metabolism of human neutrophils during phagocytosis. J. Clin. Investig. 70, 550–557 10.1172/JCI110647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadiku, P., Willson, J.A., Ryan, E.M., Sammut, D., Coelho, P., Watts, E.R.et al. (2021) Neutrophils fuel effective immune responses through gluconeogenesis and glycogenesis. Cell Metab. 33, 411–423.e4 10.1016/j.cmet.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makowski, L., Chaib, M. and Rathmell, J.C. (2020) Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 295, 5–14 10.1111/imr.12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee, Y.S., Kim, T.Y., Kim, Y., Lee, S.H., Kim, S., Kang, S.W.et al. (2018) Microbiota-derived lactate accelerates intestinal stem-cell-mediated epithelial development. Cell Host Microbe 24, 833–846.e6 10.1016/j.chom.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 79.Wells, J.M. and Mercenier, A. (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6, 349–362 10.1038/nrmicro1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Md, T., Huch, M., Cho, G.-S. and Franz, C.M.A.P. (2014) The genus Streptococcus. Lactic Acid Bacteria, Wilhelm H. Holzapfel and Brian J.B. Wood (eds), Ch. 28, 457–505 10.1002/9781118655252.ch28 [DOI] [Google Scholar]
- 81.Amabebe, E. and Anumba, D.O.C. (2020) Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front. Immunol. 11, 2184 10.3389/fimmu.2020.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Exley, R.M., Wu, H., Shaw, J., Schneider, M.C., Smith, H., Jerse, A.E.et al. (2007) Lactate acquisition promotes successful colonization of the murine genital tract by Neisseria gonorrhoeae. Infect. Immun. 75, 1318–1324 10.1128/IAI.01530-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jurtshuk, P.1996. Bacterial metabolism. In Medical microbiology, 4th edn (Baron, S., ed.), University of Texas Medical Branch at Galveston, Galveston, TX: [PubMed] [Google Scholar]
- 84.Jiang, T., Gao, C., Ma, C. and Xu, P. (2014) Microbial lactate utilization: enzymes, pathogenesis, and regulation. Trends Microbiol. 22, 589–599 10.1016/j.tim.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 85.Fredman, G., Kolpen, M., Hertz, F.B., Petersen, P.T., Jensen, A.V., Baunbaek-Egelund, G.et al. (2019) The inflamed sputum in lower respiratory tract infection: l-lactate levels are correlated to neutrophil accumulation. APMIS 127, 72–79 10.1111/apm.12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gao, C., Hu, C., Zheng, Z., Ma, C., Jiang, T., Dou, P.et al. (2012) Lactate utilization is regulated by the FadR-type regulator lldR in Pseudomonas aeruginosa. J. Bacteriol. 194, 2687–2692 10.1128/JB.06579-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson, A.R., Libby, S.J. and Fang, F.C. (2008) A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science (New York, NY) 319, 1672–1676 10.1126/science.1155207 [DOI] [PubMed] [Google Scholar]
- 88.Exley, R.M., Goodwin, L., Mowe, E., Shaw, J., Smith, H., Read, R.C.et al. (2005) Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infect. Immun. 73, 5762–5766 10.1128/IAI.73.9.5762-5766.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Herbert, M.A., Hayes, S., Deadman, M.E., Tang, C.M., Hood, D.W. and Moxon, E.R. (2002) Signature tagged mutagenesis of Haemophilus influenzae identifies genes required for in vivo survival. Microb. Pathog. 33, 211–223 10.1006/mpat.2002.0530 [DOI] [PubMed] [Google Scholar]
- 90.Kuratana, M. and Anderson, P. (1991) Host metabolites that phenotypically increase the resistance of Haemophilus influenzae type b to clearance mechanisms. J. Infect. Dis. 163, 1073–1079 10.1093/infdis/163.5.1073 [DOI] [PubMed] [Google Scholar]
- 91.Cohen, S.B., Gern, B.H., Delahaye, J.L., Adams, K.N., Plumlee, C.R., Winkler, J.K.et al. (2018) Alveolar macrophages provide an early Mycobacterium tuberculosis niche and initiate dissemination. Cell Host Microbe 24, 439–446.e4 10.1016/j.chom.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lachmandas, E., Beigier-Bompadre, M., Cheng, S.C., Kumar, V., van Laarhoven, A., Wang, X.et al. (2016) Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 46, 2574–2586 10.1002/eji.201546259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gleeson, L.E., Sheedy, F.J., Palsson-McDermott, E.M., Triglia, D., O'Leary, S.M., O'Sullivan, M.P.et al. (2016) Cutting edge: Mycobacterium tuberculosis induces aerobic glycolysis in human alveolar macrophages that is required for control of intracellular bacillary replication. J. Immunol. 196, 2444–2449 10.4049/jimmunol.1501612 [DOI] [PubMed] [Google Scholar]
- 94.Huang, L., Nazarova, E.V., Tan, S., Liu, Y. and Russell, D.G. (2018) Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 215, 1135–1152 10.1084/jem.20172020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cumming, B.M., Addicott, K.W., Adamson, J.H. and Steyn, A.J. (2018) Mycobacterium tuberculosis induces decelerated bioenergetic metabolism in human macrophages. eLife 7, e39169 10.7554/eLife.39169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hackett, E.E., Charles-Messance, H., O'Leary, S.M., Gleeson, L.E., Munoz-Wolf, N., Case, S.et al. (2020) Mycobacterium tuberculosis limits host glycolysis and IL-1beta by restriction of PFK-M via MicroRNA-21. Cell Rep. 30, 124–136.e4 10.1016/j.celrep.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi, L., Jiang, Q., Bushkin, Y., Subbian, S. and Tyagi, S. (2019) Biphasic dynamics of macrophage immunometabolism during Mycobacterium tuberculosis infection. mBio 10, e02550-18. 10.1128/mBio.02550-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rothchild, A.C., Sissons, J.R., Shafiani, S., Plaisier, C., Min, D., Mai, D.et al. (2016) MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc. Natl Acad. Sci. U.S.A. 113, E6172–E6E81 10.1073/pnas.1608255113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koo, M.S., Subbian, S. and Kaplan, G. (2012) Strain specific transcriptional response in Mycobacterium tuberculosis infected macrophages. Cell Commun. Signal. 10, 2 10.1186/1478-811X-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roy, S., Schmeier, S., Kaczkowski, B., Arner, E., Alam, T., Ozturk, M.et al. (2018) Transcriptional landscape of Mycobacterium tuberculosis infection in macrophages. Sci. Rep. 8, 6758 10.1038/s41598-018-24509-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rohde, K.H., Veiga, D.F., Caldwell, S., Balazsi, G. and Russell, D.G. (2012) Linking the transcriptional profiles and the physiological states of Mycobacterium tuberculosis during an extended intracellular infection. PLoS Pathog. 8, e1002769 10.1371/journal.ppat.1002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shi, L., Salamon, H., Eugenin, E.A., Pine, R., Cooper, A. and Gennaro, M.L. (2015) Infection with Mycobacterium tuberculosis induces the Warburg effect in mouse lungs. Sci. Rep. 5, 18176 10.1038/srep18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Subbian, S., Tsenova, L., Yang, G., O'Brien, P., Parsons, S., Peixoto, B.et al. (2011) Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 1, 110016 10.1098/rsob.110016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi, L., Eugenin, E.A. and Subbian, S. (2016) Immunometabolism in tuberculosis. Front. Immunol. 7, 150 10.3389/fimmu.2016.00150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Subbian, S., Tsenova, L., Kim, M.J., Wainwright, H.C., Visser, A., Bandyopadhyay, N.et al. (2015) Lesion-specific immune response in granulomas of patients with pulmonary tuberculosis: a pilot study. PLoS One 10, e0132249 10.1371/journal.pone.0132249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lai, R.P.J., Cortes, T., Marais, S., Rockwood, N., Burke, M., Garza-Garcia, A.et al. (2020) Transcriptomic characterization of tuberculous sputum reveals a host Warburg effect and microbial cholesterol catabolism. bioRxiv [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin, J.H., Yang, J.Y., Jeon, B.Y., Yoon, Y.J., Cho, S.N., Kang, Y.H.et al. (2011) (1)H NMR-based metabolomic profiling in mice infected with Mycobacterium tuberculosis. J. Proteome Res. 10, 2238–2247 10.1021/pr101054m [DOI] [PubMed] [Google Scholar]
- 108.Somashekar, B.S., Amin, A.G., Rithner, C.D., Troudt, J., Basaraba, R., Izzo, A.et al. (2011) Metabolic profiling of lung granuloma in Mycobacterium tuberculosis infected Guinea pigs: ex vivo 1H magic angle spinning NMR studies. J. Proteome Res. 10, 4186–4195 10.1021/pr2003352 [DOI] [PubMed] [Google Scholar]
- 109.Fernandez-Garcia, M., Rey-Stolle, F., Boccard, J., Reddy, V.P., Garcia, A., Cumming, B.M.et al. (2020) Comprehensive examination of the mouse lung metabolome following Mycobacterium tuberculosis infection using a multiplatform mass spectrometry approach. J. Proteome Res. 19, 2053–2070 10.1021/acs.jproteome.9b00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Emad, A. and Rezaian, G.R. (1999) Lactate dehydrogenase in bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis. Respiration 66, 41–45 10.1159/000029335 [DOI] [PubMed] [Google Scholar]
- 111.Sharma, P.R., Jain, S., Bamezai, R.N. and Tiwari, P.K. (2010) Utility of serum LDH isoforms in the assessment of mycobacterium tuberculosis induced pathology in TB patients of Sahariya tribe. Indian J. Clin. Biochem. 25, 57–63 10.1007/s12291-010-0012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maoldomhnaigh, C.Ó, Cox, D., McQuaid, K., Gogan, K., Basdeo, S. and Keane, J. (2020) Lactate improves killing of Mycobacterium tuberculosis in human macrophages. Access Microbiol. 2, 1 10.1099/acmi.mim2019.po0025 [DOI] [Google Scholar]
- 113.Whittington, A.M. (2017) The Effect of Extracellular Acidosis on Tissue Destruction in Tuberculosis, Imperial College London, London, UK 10.25560/76364 [DOI] [Google Scholar]
- 114.Malangu, N. and Mogashoa, M. (2012) Tuberculosis and lactic acidosis as causes of death in adult patients from a regional hospital in Johannesburg. Afr. J. Prim. Health Care Fam. Med. 4, 266 10.4102/phcfm.v4i1.266 [DOI] [Google Scholar]
- 115.Youmans, A.S. and Youmans, G.P. (1953) Studies on the metabolism of Mycobacterium tuberculosis. III. The growth of Mycobacterium tuberculosis var. hominis in the presence of various intermediates of the dissimilation of glucose to pyruvic acid. J. Bacteriol. 65, 100–102 10.1128/jb.65.1.100-102.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Osada-Oka, M., Goda, N., Saiga, H., Yamamoto, M., Takeda, K., Ozeki, Y.et al. (2019) Metabolic adaptation to glycolysis is a basic defense mechanism of macrophages for Mycobacterium tuberculosis infection. Int. Immunol. 31, 781–793 10.1093/intimm/dxz048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Billig, S., Schneefeld, M., Huber, C., Grassl, G.A., Eisenreich, W. and Bange, F.C. (2017) Lactate oxidation facilitates growth of Mycobacterium tuberculosis in human macrophages. Sci. Rep. 7, 6484 10.1038/s41598-017-05916-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qualls, J.E. and Murray, P.J. (2016) Immunometabolism within the tuberculosis granuloma: amino acids, hypoxia, and cellular respiration. Semin. Immunopathol. 38, 139–152 10.1007/s00281-015-0534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cumming, B.M., Pacl, H.T. and Steyn, A.J.C. (2020) Relevance of the warburg effect in tuberculosis for host-directed therapy. Front. Cell. Infect. Microbiol. 10, 576596 10.3389/fcimb.2020.576596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kiran, D. and Basaraba, R.J. (2021) Lactate metabolism and signaling in tuberculosis and cancer: a comparative review. Front. Cell. Infect. Microbiol. 11, 624607 10.3389/fcimb.2021.624607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matta, S.K. and Kumar, D. (2016) Hypoxia and classical activation limits Mycobacterium tuberculosis survival by Akt-dependent glycolytic shift in macrophages. Cell Death Discov. 2, 16022 10.1038/cddiscovery.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Prosser, G., Brandenburg, J., Reiling, N., Barry, III, C.E., Wilkinson, R.J. and Wilkinson, K.A. (2017) The bacillary and macrophage response to hypoxia in tuberculosis and the consequences for T cell antigen recognition. Microbes Infect. 19, 177–192 10.1016/j.micinf.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brynildsrud, O.B., Pepperell, C.S., Suffys, P., Grandjean, L., Monteserin, J., Debech, N.et al. (2018) Global expansion of Mycobacterium tuberculosis lineage 4 shaped by colonial migration and local adaptation. Sci. Adv. 4, eaat5869 10.1126/sciadv.aat5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osorio, N.S., Rodrigues, F., Gagneux, S., Pedrosa, J., Pinto-Carbo, M., Castro, A.G.et al. (2013) Evidence for diversifying selection in a set of Mycobacterium tuberculosis genes in response to antibiotic- and nonantibiotic-related pressure. Mol. Biol. Evol. 30, 1326–1336 10.1093/molbev/mst038 [DOI] [PubMed] [Google Scholar]
- 125.Baena, A., Cabarcas, F., Alvarez-Eraso, K.L.F., Isaza, J.P., Alzate, J.F. and Barrera, L.F. (2019) Differential determinants of virulence in two Mycobacterium tuberculosis Colombian clinical isolates of the LAM09 family. Virulence 10, 695–710 10.1080/21505594.2019.1642045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lyadova, I.V. (2017) Neutrophils in tuberculosis: heterogeneity shapes the way? Mediators Inflamm. 2017, 8619307 10.1155/2017/8619307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Muefong, C.N. and Sutherland, J.S. (2020) Neutrophils in tuberculosis-associated inflammation and lung pathology. Front. Immun. 11, 962 10.3389/fimmu.2020.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bueno, M., Calyeca, J., Rojas, M. and Mora, A.L. (2020) Mitochondria dysfunction and metabolic reprogramming as drivers of idiopathic pulmonary fibrosis. Redox Biol. 33, 101509 10.1016/j.redox.2020.101509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rahman, S.M.J., Ji, X., Zimmerman, L.J., Li, M., Harris, B.K., Hoeksema, M.D.et al. (2016) The airway epithelium undergoes metabolic reprogramming in individuals at high risk for lung cancer. JCI Insight 1, e88814 10.1172/jci.insight.88814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hawn, T.R., Matheson, A.I., Maley, S.N. and Vandal, O. (2013) Host-directed therapeutics for tuberculosis: can we harness the host? Microbiol. Mol. Biol. Rev. 77, 608–627 10.1128/MMBR.00032-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hackett, E.E. and Sheedy, F.J. (2020) An army marches on its stomach: metabolic intermediates as antimicrobial mediators in Mycobacterium tuberculosis infection. Front. Cell. Infect. Microbiol. 10, 446 10.3389/fcimb.2020.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sheedy, F.J. and Divangahi, M. (2021) Targeting immunometabolism in host defence against Mycobacterium tuberculosis. Immunology 162, 145–159 10.1111/imm.13276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heim, C.E., Vidlak, D. and Kielian, T. (2015) Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J. leukoc. Biol. 98, 1003–1013 10.1189/jlb.4VMA0315-125RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Heim, C.E., Bosch, M.E., Yamada, K.J., Aldrich, A.L., Chaudhari, S.S., Klinkebiel, D.et al. (2020) Lactate production by Staphylococcus aureus biofilm inhibits HDAC11 to reprogramme the host immune response during persistent infection. Nat. Microbiol. 5, 1271–1284 10.1038/s41564-020-0756-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akdis, C.A. and Blaser, K. (2001) Mechanisms of interleukin-10-mediated immune suppression. Immunology 103, 131–136 10.1046/j.1365-2567.2001.01235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qvist, T., Eickhardt, S., Kragh, K.N., Andersen, C.B., Iversen, M., Hoiby, N.et al. (2015) Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur. Respir. J. 46, 1823–1826 10.1183/13993003.01102-2015 [DOI] [PubMed] [Google Scholar]
- 137.Fennelly, K.P., Ojano-Dirain, C., Yang, Q., Liu, L., Lu, L., Progulske-Fox, A.et al. (2016) Biofilm formation by Mycobacterium abscessus in a lung cavity. Am. J. Respir. Crit. Care Med. 193, 692–693 10.1164/rccm.201508-1586IM [DOI] [PubMed] [Google Scholar]
- 138.Nunez, M.F., Kwon, O., Wilson, T.H., Aguilar, J., Baldoma, L. and Lin, E.C. (2002) Transport of L-Lactate, D-Lactate, and glycolate by the LldP and GlcA membrane carriers of Escherichia coli. Biochem. Biophys. Res. Commun. 290, 824–829 10.1006/bbrc.2001.6255 [DOI] [PubMed] [Google Scholar]
- 139.Lin, Y.C., Cornell, W.C., Jo, J., Price-Whelan, A. and Dietrich, L.E.P. (2018) The Pseudomonas aeruginosa complement of lactate dehydrogenases enables use of d- and l-lactate and metabolic cross-feeding. mBio 9, e00961-18 10.1128/mBio.00961-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen, N.H., Ong, C.Y., O'Sullivan, J., Ibranovic, I., Davey, K., Edwards, J.L.et al. (2020) Two distinct L-lactate dehydrogenases play a role in the survival of Neisseria gonorrhoeae in cervical epithelial cells. J. Infect. Dis. 221, 449–453 10.1093/infdis/jiz468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Britigan, B.E., Klapper, D., Svendsen, T. and Cohen, M.S. (1988) Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. An unrecognized aspect of the oxygen metabolism of phagocytosis. J. Clin. Investig. 81, 318–324 10.1172/JCI113323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Exley, R.M., Shaw, J., Mowe, E., Sun, Y.H., West, N.P., Williamson, M.et al. (2005) Available carbon source influences the resistance of Neisseria meningitidis against complement. J. Exp Med. 201, 1637–1645 10.1084/jem.20041548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tettelin, H., Nelson, K.E., Paulsen, I.T., Eisen, J.A., Read, T.D., Peterson, S.et al. (2001) Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science (New York, NY) 293, 498–506 10.1126/science.1061217 [DOI] [PubMed] [Google Scholar]
- 144.Gaspar, P., Al-Bayati, F.A., Andrew, P.W., Neves, A.R. and Yesilkaya, H. (2014) Lactate dehydrogenase is the key enzyme for pneumococcal pyruvate metabolism and pneumococcal survival in blood. Infect. Immun. 82, 5099–5109 10.1128/IAI.02005-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fuller, J.R., Vitko, N.P., Perkowski, E.F., Scott, E., Khatri, D., Spontak, J.S.et al. (2011) Identification of a lactate-quinone oxidoreductase in Staphylococcus aureus that is essential for virulence. Front. Cell. Infect. Microbiol. 1, 19 10.3389/fcimb.2011.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schairer, D.O., Chouake, J.S., Nosanchuk, J.D. and Friedman, A.J. (2012) The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 3, 271–279 10.4161/viru.20328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sigurlasdottir, S., Engman, J., Eriksson, O.S., Saroj, S.D., Zguna, N., Lloris-Garcera, P.et al. (2017) Host cell-derived lactate functions as an effector molecule in Neisseria meningitidis microcolony dispersal. PLoS Pathog. 13, e1006251 10.1371/journal.ppat.1006251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jerse, A.E., Crow, E.T., Bordner, A.N., Rahman, I., Cornelissen, C.N., Moench, T.R.et al. (2002) Growth of Neisseria gonorrhoeae in the female mouse genital tract does not require the gonococcal transferrin or hemoglobin receptors and may be enhanced by commensal lactobacilli. Infect. Immun. 70, 2549–2558 10.1128/IAI.70.5.2549-2558.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Helenius, A. (2013) Virus entry: what has pH got to do with it? Nat. Cell Biol. 15, 125 10.1038/ncb2678 [DOI] [PubMed] [Google Scholar]
- 150.Helenius, A., Kartenbeck, J., Simons, K. and Fries, E. (1980) On the entry of Semliki forest virus into BHK-21 cells. Report No.: 00219525/80/02/0 [DOI] [PMC free article] [PubMed]
- 151.Stegmannss, T., Booyt, F.P. and Wilschutsii, J. (1987) Effects of low pH on influenza virus activation and inactivation of the membrane fusion capacity of hemagglutin. J. Biol. Chem. 262, 17744–17749 10.1016/S0021-9258(18)45442-7 [DOI] [PubMed] [Google Scholar]
- 152.Costello, D.A., Whittaker, G.R. and Daniel, S. (2015) Variations in pH sensitivity, acid stability, and fusogenicity of three influenza virus H3 subtypes. J. Virol. 89, 350–360 10.1128/JVI.01927-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mo, X., Wei, F., Tong, Y., Ding, L., Zhu, Q., Du, S.et al. (2018) Lactic acid downregulates viral MicroRNA To promote Epstein–Barr virus-immortalized B lymphoblastic cell adhesion and growth. J. Virol. 92, e00033-18. 10.1128/JVI.00033-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Plaza, J.J.G., Hulak, N., Kausova, G., Zhumadilov, Z. and Akilzhanova, A. (2016) Role of metabolism during viral infections, and crosstalk with the innate immune system. Intractable Rare Dis. Res. 5, 90–96 10.5582/irdr.2016.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Thaker, S.K., Ch'ng, J. and Christofk, H.R. (2019) Viral hijacking of cellular metabolism. BMC Biol. 17, 59 10.1186/s12915-019-0678-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Silva AC, A.P.T. and MA, P. (2016) Impact of Adenovirus infection in host cell metabolism evaluated by (1)H-NMR spectroscopy. J. Biotechnol. 231, 16–23 10.1016/j.jbiotec.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 157.Prusinkiewicz, M.A. and Mymryk, J.S. (2019) Metabolic reprogramming of the host cell by human adenovirus infection. Viruses 11, 141 10.3390/v11020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vastag, L., Koyuncu, E., Grady, S.L., Shenk, T.E. and Rabinowitz, J.D. (2011) Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 7, e1002124 10.1371/journal.ppat.1002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Abrantes, J.L., Alves, C.M., Costa, J., Almeida, F.C., Sola-Penna, M., Fontes, C.F.et al. (2012) Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1). Biochim. Biophys. Acta 1822, 1198–1206 10.1016/j.bbadis.2012.04.011 [DOI] [PubMed] [Google Scholar]
- 160.Landini, M.P. (1984) Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 65(Pt 7), 1229–1232 10.1099/0022-1317-65-7-1229 [DOI] [PubMed] [Google Scholar]
- 161.Munger, J., Bennett, B.D., Parikh, A., Feng, X.J., McArdle, J., Rabitz, H.A.et al. (2008) Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 26, 1179–1186 10.1038/nbt.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ritter, J.B., Wahl, A.S., Freund, S., Genzel, Y. and Reichl, U. (2010) Metabolic effects of influenza virus infection in cultured animal cells: intra-and extracellular metabolite profiling. BMC Syst. Biol. 4, 61 10.1186/1752-0509-4-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kohio, H.P. and Adamson, A.L. (2013) Glycolytic control of vacuolar-type ATPase activity: a mechanism to regulate influenza viral infection. Virology 444, 301–309 10.1016/j.virol.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 164.Smallwood, H.S., Duan, S., Morfouace, M., Rezinciuc, S., Shulkin, B.L., Shelat, A.et al. (2017) Targeting metabolic reprogramming by influenza infection for therapeutic intervention. Cell Rep. 19, 1640–1653 10.1016/j.celrep.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang, W., Wang, G., Xu, Z.G., Tu, H., Hu, F., Dai, J.et al. (2019) Lactate is a natural suppressor of RLR signaling by targeting MAVS. Cell 178, 176–189.e15 10.1016/j.cell.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tucey, T.M., Verma, J., Harrison, P.F., Snelgrove, S.L., Lo, T.L., Scherer, A.K.et al. (2018) Glucose homeostasis is important for immune cell viability during candida challenge and host survival of systemic fungal infection. Cell Metabo. 27, 988–1006.e7 10.1016/j.cmet.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Quintin, J., Saeed, S., Martens, J.H.A., Giamarellos-Bourboulis, E.J., Ifrim, D.C., Logie, C.et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 10.1016/j.chom.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cheng, S.-C., Quintin, J., Cramer, R.A., Shepardson, K.M., Saeed, S., Kumar, V.et al. (2014) mTOR- and HIF-1-mediated aerobic glycolysis as metabolic basis for trained immunity. Sci. Adv. 345, 1250684. 10.1126/science.1250684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weerasinghe, H. and Traven, A.) Immunometabolism in fungal infections: the need to eat to compete. Curr. Opin. Microbiol. 58, 32–40 10.1016/j.mib.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 170.Goncalves, S.M., Duarte-Oliveira, C., Campos, C.F., Aimanianda, V., Ter Horst, R., Leite, L.et al. (2020) Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 11, 2282 10.1038/s41467-020-16120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rosa, R.L., Berger, M., Santi, L., Driemeier, D., Barros Terraciano, P., Campos, A.R.et al. (2019) Proteomics of Rat lungs infected by Cryptococcus gattii reveals a potential Warburg-like effect. J. Proteome Res. 18, 3885–3895 10.1021/acs.jproteome.9b00326 [DOI] [PubMed] [Google Scholar]
- 172.Ene, I.V., Cheng, S.C., Netea, M.G. and Brown, A.J. (2013) Growth of Candida albicans cells on the physiologically relevant carbon source lactate affects their recognition and phagocytosis by immune cells. Infect. Immun. 81, 238–248 10.1128/IAI.01092-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Williams, R.B. and Lorenz, M.C. (2020) Multiple alternative carbon pathways combine to promote Candida albicans stress resistance, immune interactions, and virulence. mBio 11, 1–18 10.1128/mBio.03070-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lopes, J.P., Stylianou, M., Backman, E., Holmberg, S., Jass, J., Claesson, R.et al. (2018) Evasion of immune surveillance in low oxygen environments enhances Candida albicans virulence. mBio 9, e02120-18 10.1128/mBio.02120-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gaffen, S.L., Hernandez-Santos, N. and Peterson, A.C. (2011) IL-17 signaling in host defense against Candida albicans. Immunol. Res. 50, 181–187 10.1007/s12026-011-8226-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bar, E., Whitney, P.G., Moor, K., Reis e Sousa, C. and LeibundGut-Landmann, S. (2014) IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity 40, 117–127 10.1016/j.immuni.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 177.MacRae, J.I., Dixon, M.W., Dearnley, M.K., Chua, H.H., Chambers, J.M., Kenny, S.et al. (2013) Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 11, 67 10.1186/1741-7007-11-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Poole, R.C and Halestrap, A.P. N-Terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem. J. 1994. 303:755–759. 10.1042/bj3030755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xia, N., Zhou, T., Liang, X., Ye, S., Zhao, P., Yang, J.et al. (2018) A lactate fermentation mutant of toxoplasma stimulates protective immunity against acute and chronic toxoplasmosis. Front. Immunol. 9, 1814 10.3389/fimmu.2018.01814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shukla, A., Olszewski, K.L., Llinas, M., Rommereim, L.M., Fox, B.A., Bzik, D.J.et al. (2018) Glycolysis is important for optimal asexual growth and formation of mature tissue cysts by Toxoplasma gondii. Int. J. Parasitol. 48, 955–968 10.1016/j.ijpara.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 181.Abdelbaset, A.E., Fox, B.A., Karram, M.H., Abd Ellah, M.R., Bzik, D.J. and Igarashi, M. (2017) Lactate dehydrogenase in Toxoplasma gondii controls virulence, bradyzoite differentiation, and chronic infection. PLoS One 12, e0173745 10.1371/journal.pone.0173745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xia, N., Yang, J., Ye, S., Zhang, L., Zhou, Y., Zhao, J.et al. (2018) Functional analysis of toxoplasma lactate dehydrogenases suggests critical roles of lactate fermentation for parasite growth in vivo. Cell Microbiol. 20, 1–15 10.1111/cmi.12794 [DOI] [PubMed] [Google Scholar]
- 183.Fantin, V.R., St-Pierre, J. and Leder, P. (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434 10.1016/j.ccr.2006.04.023 [DOI] [PubMed] [Google Scholar]
- 184.Fujii, W., Kawahito, Y., Nagahara, H., Kukida, Y., Seno, T., Yamamoto, A.et al. (2015) Monocarboxylate transporter 4, associated with the acidification of synovial fluid, is a novel therapeutic target for inflammatory arthritis. Arthritis Rheumatol. 67, 2888–2896 10.1002/art.39270 [DOI] [PubMed] [Google Scholar]
- 185.Hoque, R., Farooq, A., Ghani, A., Gorelick, F. and Mehal, W.Z. (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774 10.1053/j.gastro.2014.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun, Y., Li, Y., Exley, R.M., Winterbotham, M., Ison, C., Smith, H.et al. (2005) Identification of novel antigens that protect against systemic meningococcal infection. Vaccine 23, 4136–4141 10.1016/j.vaccine.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 187.Hapuarachchi, S.V., Cobbold, S.A., Shafik, S.H., Dennis, A.S., McConville, M.J., Martin, R.E.et al. (2017) The malaria parasite's lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog. 13, e1006180 10.1371/journal.ppat.1006180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Freedberg, D.E., Zhou, M.J., Cohen, M.E., Annavajhala, M.K., Khan, S., Moscoso, D.I.et al. (2018) Pathogen colonization of the gastrointestinal microbiome at intensive care unit admission and risk for subsequent death or infection. Intensive Care Med. 44, 1203–1211 10.1007/s00134-018-5268-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Manzanares, W., Lemieux, M., Langlois, P.L. and Wischmeyer, P.E. (2016) Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Critical care (London, England) 19, 262 10.1186/s13054-016-1434-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McDonald, B., Zucoloto, A.Z., Yu, I.L., Burkhard, R., Brown, K., Geuking, M.B.et al. (2020) Programing of an intravascular immune firewall by the Gut microbiota protects against pathogen dissemination during infection. Cell Host Microbe 28, 660–668.e4 10.1016/j.chom.2020.07.014 [DOI] [PubMed] [Google Scholar]