Abstract
Background
The COVID-19 pandemic threatens the impact of cervical cancer screening and global cervical cancer elimination goals. As cervical cancer screening programmes were adjusting to the new situation, we evaluated the intensity, quality, and outcomes of cervical cancer screening in Slovenia in the first seven months of the pandemic.
Methods
Historical observational study on data from a population-based cervical cancer screening registry. Number of cervical cytopathology (screening and follow-up), histopathology (diagnostic procedures, invasive procedures and number of newly diagnosed CIN2+ cases) and HPV test results from the entire Slovenian women population between January 1st and September 30th 2020 were compared to a three-year average of the years 2017–19.
Findings
A two-month screening lock-down between March 12th and May 8th 2020 resulted in an epidemic deficit of screening (-92%), follow-up (-70%), and HPV triage tests (-68%), as well as invasive diagnostic (-47%) and treatment (-15%) of cervical lesions. Time to diagnosis and treatment did not increase; times to laboratory results fluctuated but stayed within standards. Slovenia has entered the second epidemic intending to add as little as possible to the pandemic deficit of screening smears (-23%) and yearly CIN2+ cases (-10%). Women aged 30–39 were most affected, with the highest pandemic deficit of screening smears (-26%) and yearly CIN2+ cases (-19%).
Interpretation
The pandemic has deeply affected all levels of our lives. New vulnerable groups and inequalities have emerged that require recognition and action. To prevent long-term increases in the cervical cancer burden due to the COVID-19 pandemic, it is crucial that organised screening is maintained and monitored in settings where it can be safely and comprehensively provided.
Funding
None.
Research in context.
Evidence before this study
On November 17th 2020, we were facing a historic moment of WHO launching the global strategy to accelerate the elimination of cervical cancer as a public health problem in the midst of the COVID-19 pandemic. With the aim to identify data available on the disruption of cervical screening due to the pandemic, we searched PubMed for peer-reviewed articles published until December 21st 2020, using search string ‘(COVID-19) AND (cervical cancer) AND (screening)’. We inspected all 24 results for data on screening intensity, quality and outcomes, as well as heath inequalities and vulnerable subgroups of women. We also searched Google Scholar with the same keywords and scanned the 3.360 results. We found four articles reporting data on screening decline during the COVID-19 pandemic and one reporting intensity of scaling-up after the first COVID-19 wave. We found no studies or institutional webpages reporting the change in cervical precancer detection during the pandemic. We found two studies discussing cervical cancer screening service uptake disparities during the COVID-19 pandemic; both stressed the importance to identify and act upon new pandemic disparities; however, they did not report on new disparities. More research and data from observational studies is needed to understand the dynamics of screening attendance and compliance determinants during the crises, such as the COVID-19 pandemic, and its impact on future cervical cancer burden.
Added value of this study
To the best of our knowledge, this study is the first to analyse the impact of the COVID-19 pandemic on the detection of cervical precancer; and also the first to identify a new vulnerable group of women at higher risk for cervical cancer due to the clinically relevant decrease in screening intensity and CIN2+ detection during the COVID-19 pandemic.
Implications of all the available evidence
Our findings suggest that even relatively short-term cervical cancer screening disruptions can lead to clinically significant decrease in cervical precancer detection and that the deficit can accumulate in specific subgroups of women. In Slovenian example, women aged 30–39 were most affected. The present study's results can be used to advocate for the continuation and/or scaling-up of cervical cancer screening and its monitoring during the pandemic, in local settings where and when it can be safely provided. The study also revealed the need to scale-up the research on how the pandemic interferes with determinants of screening on general and in the specific subgroups of women, with the aim to develop and implement tailored strategies to overcome the new pandemic-related barriers to screening.
Alt-text: Unlabelled box
1. Introduction
In many countries, access to preventive programmes is not a priority during the COVID-19 pandemic. Many organised programmes stopped systematically or were disrupted. The pandemic might also challenge women's decisions and ability to participate in screening, as well as disrupt the quality of screening, diagnostics, treatment, and follow-up. The cervical cancer burden might also increase in countries with long histories of comprehensive cervical cancer control programmes. New vulnerable groups and inequalities may emerge that require recognition and action.
Despite Slovenia was successfully controlling the historically high cervical cancer burden with organised population-based screening, the government halted cervical cancer screening and management of low-grade (LG) lesions, together with other preventive and elective services, during the first epidemic with the regulatory act between March 12th and May 8th 2020. The main reasons for the lockdown of preventive and elective services were lack of personal protective equipment and the relocation of the critical infrastructure and personnel. However, gynaecologists were not relocated to COVID facilities and were available on all healthcare levels, since further diagnostics and treatment of high-grade (HG) changes and symptomatic women were endorsed throughout the pandemic. The public was acquainted via media that their preventive and elective appointments were cancelled and will be rescheduled by the health providers. After the end of the epidemic all screening services were resumed and scaling-up of screening and management of women with LG lesions was endorsed by the central ZORA coordination office and other key stakeholders on the national level. Evidence-based governance of ZORA programme management during different pandemic phases was possible due to monitoring and evaluation of routinely available data in national cervical cancer screening ZORA registry and good collaboration of the key stakeholders.
We are presenting the evaluation of the population impact of COVID-19 pandemic on organised cervical cancer screening in the first seven months of COVID-19 pandemic. We aimed to assess the (i) intensity and quality of cervical cancer screening during the first lockdown and scaling-up afterwards and (ii) the deficit of cervical precancer detection. The additional aim was to identify if new vulnerable groups had emerged during the pandemic.
2. Methods
2.1. Study design, population, and data source
We performed a historical observational routine-data-based study from data derived from a population-based routine data-collection system that was carried out at an individual level information. All cytological, histological, and HPV results with sampling date between January 1st 2017 and September 30th 2020 from the program's target age group 20–64 were obtained from the National Cervical Cancer Screening Registry ZORA (ZORA registry) and managed according to the Healthcare Databases Act, Personal Data Protection Act and WMA Declaration of Helsinki. The ZORA registry is a central national database for all cervical cytology, histology, and HPV test results [1]. It is located and managed by the central coordination office (ZORA office) at the Institute of Oncology Ljubljana. Results are reported by all Slovenian laboratories in standardised forms, either by nightly synchronisation (three cytology laboratories) or in weekly or monthly batches, electronically (two HPV and six cytology laboratories) or in paper copies (11 pathology laboratories). Quality monitoring is performed regularly, and missing or illogical data is subjected to verification claims. Demographic data are obtained by nightly synchronisation with the Central Population Register. The ZORA registry also links data using a unique personal identification number with other national databases, including the Slovenian Cancer Registry. This manuscript follows the STROBE statement for reporting of observational studies.
2.2. Setting
Population-based organised cervical cancer screening in Slovenia started in 2003 for women aged 20 to 64 years in a three-year interval with conventional cytology [1]. The screening policy is currently under revision regarding the screening test and triage, screening interval, as well as women's age and HPV vaccination status [2]. The programme is regulated by the Rules on the Implementation of National Cancer Screening Programmes. Programme services are provided by around 350 gynaecologist teams on the primary healthcare level (invitation, screening, follow-up of women with LG changes and after treatment) and secondary and tertiary level (colposcopy, invasive diagnostic and treatment, multidisciplinary assessment and management of difficult cases), as well as 9 cytology, 2 HPV and 11 histopathology laboratories [1]. All Slovenian personal gynaecologists at the primary healthcare level are responsible for inviting and screening their registered women. The ZORA office monitors the attendance of women within the ZORA registry and sends reminders, it is also responsible for monitoring, evaluation and quality assurance of the programme. An HPV test (Hybrid Capture 2 High-Risk HPV DNA assay, Qiagen, Hilden, Germany) has been used as a triage test for LG changes and as a test of cure since 2011, according to national guidelines it is performed six months after the initial diagnosis, together with FU cervical smear.
2.3. Variables
We performed an extensive analysis of the pandemic's impact on the intensity, quality, and outcomes of cervical cancer screening in Slovenia. The impact on intensity was measured as absolute and relative (%) deficit/excess in the number of screening and follow-up (FU) cytology smears, triage HPV tests, and invasive diagnostics and therapeutic procedures during the lockdown of screening during the first epidemic and the following five months of a relatively good epidemiologic situation during the post first COVID-19 epidemic. The impact on quality was measured by time to diagnosis and time to treatment in women with higher risk, and samples turnaround times. The impact on outcomes was measured as the absolute and relative (%) cumulative deficit of newly detected cervical CIN2+ cases from January 1st to September 30th 2020 compared to the average in 2017–19. We are also presenting age-specific outcomes (Table 1, Figs. 3 and S2) and deficit of treatment according to treatment modality (Table 1).
Table 1.
Screening programme intensity measured as number, relative deficit, and excess (%) of screening and FU smears, HPV triage tests, and invasive diagnostics in two pandemic periods. Observed periods: the first COVID-19 epidemic with screening lockdown (March 12th to May 6th), the five-month period of post first COVID-19 epidemic (May 7th to September 30th) and a cumulative pandemic (March 12th to September 30th). The 2020 data was compared to the average (arithmetic mean) of a three-year period (2017–2019). Data source: National Cervical Cancer Screening Registry ZORA.
| First COVID-19 epidemic (March 12th–May 6th) |
Post first COVID-19 epidemic (May 7th–September 30th) |
Cumulative COVID-19 pandemic (March 12th–September 30th) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Year | Deficit/Excess | Average | Year | Deficit/Excess | Average | Year | Deficit/Excess | |
| 2017–19 | 2020 | in year 2020 | 2017–19 | 2020 | in year 2020 | 2017–19 | 2020 | in year 2020 | |
| n | n | % (95% CI) | n | n | % (95% CI) | n | n | % (95% CI) | |
| Smears (cytology – screening and FU 20–64) | 29.441 | 3.131 | −89·4 (−89·7, −89·0) | 64.049 | 71.312 | +11·3 (10·4, 12·3) | 93.491 | 74.443 | −20·4 (−21·0, −19·7) |
| Screening smears (20–64) | 26.540 | 2.266 | −91·5 (−91·8, −91·1) | 56.793 | 61.608 | +8·5 (7·5, 9·5) | 83.334 | 63.874 | −23·4 (−24·0, −22·7) |
| 20–29 | 5.139 | 595 | −88·4 (−89·4, −87·5) | 11.943 | 12.580 | +5·3 (3·2, 7·6) | 17.082 | 13.175 | −22·9 (−24·3, −21·4) |
| 30–39 | 6.426 | 682 | −89·4 (−90·2, −88·6) | 14.023 | 14.561 | +3·8 (1·9, 5·8) | 20.449 | 15.243 | −25·5 (−26·8, −24·1) |
| 40–49 | 6.530 | 482 | −92·6 (−93·3, −92·0) | 13.731 | 15.265 | +11·2 (9·1, 13·3) | 20.262 | 15.747 | −22·3 (−23·7, −20·9) |
| 50–64 | 8.445 | 507 | −94·0 (−94·5, −93·5) | 17.096 | 19.202 | +12·3 (10·4, 14·2) | 25.541 | 19.709 | −22·8 (−24·0, −21·6) |
| FU smears (20–64) | 2.901 | 865 | −70·2 (−72·2, −68·2) | 7.256 | 9.704 | +33·7 (30·6, 37·0) | 10.157 | 10.569 | +4·1 (1·7, 6·3) |
| 20–29 | 597 | 192 | −67·8 (−72·3, −63·0) | 1.527 | 1.932 | +26·5 (19·8, 33·5) | 2.124 | 2.124 | 0·0 (−4·9, 5·1) |
| 30–39 | 740 | 243 | −67·2 (−71·6, −62·6) | 1.852 | 2.429 | +31·1 (25·2, 37·4) | 2.593 | 2.672 | +3·1 (−1·9, 8·1) |
| 40–49 | 806 | 240 | −70·2 (−74·0, −66·2) | 1.987 | 2.759 | +38·9 (32·8, 45·5) | 2.793 | 2.999 | +7·4 (2·9, 11·7) |
| 50–64 | 758 | 190 | −74·9 (−78·6, −71·2) | 1.890 | 2.584 | +36·7 (30·5, 43·3) | 2.648 | 2.774 | +4·8 (0·3, 9·4) |
| HPV triage (20–64) | 1.859 | 590 | −68·3 (−70·9, −65·6) | 4.908 | 6.700 | +36·5 (32·6, 40·5) | 6.767 | 7.290 | +7·7 (4·8, 10·6) |
| 20–29 | 426 | 131 | −69·2 (−74·6, −63·5) | 1.113 | 1.472 | +32·3 (24·4, 40·6) | 1.539 | 1.603 | +4·2 (−1·7, 10·0) |
| 30–39 | 556 | 166 | −70·1 (−74·8, −65·2) | 1.441 | 1.892 | +31·3 (24·3, 38·7) | 1.997 | 2.058 | +3·1 (−2·0, 8·3) |
| 40–49 | 506 | 164 | −67·6 (−72·6, −62·2) | 1.346 | 1.889 | +40·4 (33·0, 48·1) | 1.852 | 2.053 | +10·9 (5·4, 16·5) |
| 50–64 | 371 | 129 | −65·3 (−71·4, −58·7) | 1.008 | 1.447 | +43·6 (34·6, 52·7) | 1.379 | 1.576 | +14·3 (7·6, 21·0) |
| Invasive procedures (20–64) | 1.126 | 685 | −39·2 (−44·0, −34·2) | 2.808 | 2.775 | −1·2 (−5·3, 3·1) | 3.934 | 3.460 | −12·1 (−15·3, −8·7) |
| Diagnostics (20–64) | 840 | 443 | −47·3 (−52·5, −41·7) | 2.110 | 2.120 | +0·5 (−4·3, 5·5) | 2.950 | 2.563 | −13·1 (−16·9, −9·2) |
| 20–29 | 149 | 93 | −37·6 (−50·8, −22·7) | 398 | 402 | +1·1 (−9·8, 13·1) | 547 | 495 | −9·5 (−18·4, −0·1) |
| 30–39 | 190 | 120 | −37·0 (−48·9, −23·9) | 492 | 487 | −1·1 (−11·0, 9·4) | 683 | 607 | −11·1 (−19·0, −3·0) |
| 40–49 | 263 | 111 | −57·7 (−66·0, −48·9) | 610 | 638 | +4·6 (−4·6, 14·4) | 873 | 749 | −14·2 (−20·9, −7·0) |
| 50–64 | 238 | 119 | −50·1 (−59·2, −39·9) | 610 | 593 | −2·7 (−11·4, 6·3) | 848 | 712 | −16·0 (−22·8, −8·6) |
| Treatment (20–64) | 286 | 242 | −15·4 (−26·7, −2·4) | 698 | 655 | −6·2 (−14·3, 2·2) | 984 | 897 | −8·9 (−15·4, −1·7) |
| 20–29 | 58 | 54 | −6·9 (−33·0, 24·4) | 142 | 128 | −9·9 (−27·0, 9·7) | 200 | 182 | −9·0 (−23·0, 7·5) |
| 30–39 | 101 | 76 | −24·5 (−41·9, −3·8) | 249 | 212 | −15·0 (−27·2, −1·6) | 350 | 288 | −17·7 (−28·0, −6·4) |
| 40–49 | 74 | 71 | −4·1 (−27·6, 23·4) | 176 | 180 | +2·3 (−14·7, 20·7) | 250 | 251 | +0·4 (−13·3, 15·3) |
| 50–64 | 53 | 41 | −23·1 (−47·3, 6·4) | 131 | 135 | +3·1 (−15·9, 24·1) | 184 | 176 | −4·5 (−19·8, 12·6) |
| LLETZ (20–64) | 197 | 168 | −14·9 (−28·7, 0·7) | 473 | 498 | +5·3 (−5·3, 16·4) | 670 | 666 | −0·6 (−9·2, 8·3) |
| Cold knife conisation (20–64) | 61 | 47 | −23·4 (−44·6, 2·9) | 172 | 95 | −44·8 (−56·7, −31·9) | 233 | 142 | −39·1 (−49·7, −28·0) |
| Re–LLETZ/conisation (20–64) | 27 | 27 | −1·2 (−40·7, 47·9) | 53 | 62 | +16·3 (−15·0, 53·1) | 81 | 89 | +10·3 (−15·1, 39·4) |
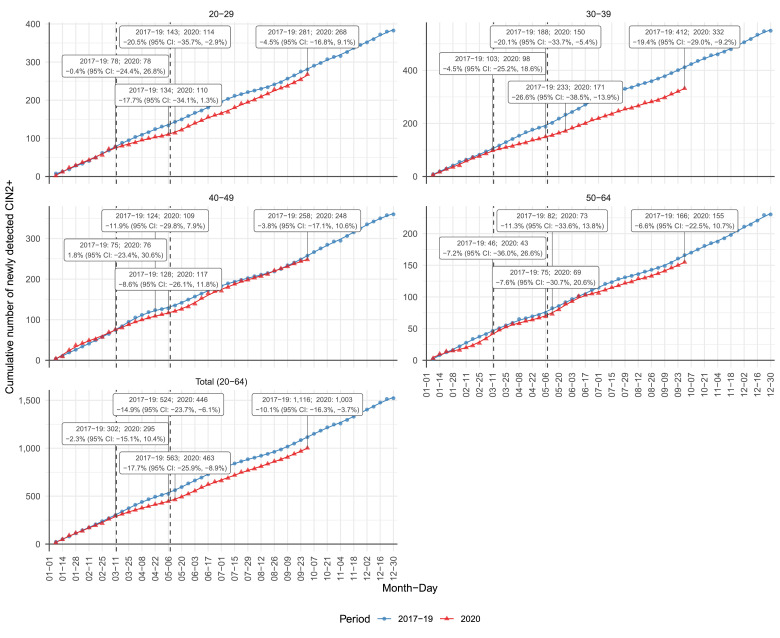
Fig. 3.
Age-specific cumulative number of newly detected CIN2+ in Slovenia by weeks for the period from January 1st to September 30th 2020 (red) in comparison to three-year average during 2017–2019 (blue). Data at the beginning and the end of the first wave and the data at the beginning of the second wave of COVID-19 epidemics are shown; additionally, together with the largest deficit compared to the previous three-year average. Age groups: 20–29, 30–39, 40–49, 50–64. 65+, total 20–64). Data source: National Cervical Cancer Screening Registry ZORA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The category screening smears includes all screening smears, while the category FU smears includes smears after pathological cytology results. Time to test result includes gynaecologist turnaround time (from smear taking to laboratory reception), laboratory turnaround time (from smear reception to releasing a report), and report turnaround time (from smear taking to releasing a report by a laboratory). We also calculated the percentage of women with an invasive procedure within 120 days after the HG change detection, as recommended by the European Federation for Colposcopy [3]. Time to diagnosis was defined as the time between taking the HG screening smear and the first invasive procedure for histopathological verification of lesion afterwards, and time to treatment as the time between confirmed CIN2+ diagnosis and the first invasive procedure. A newly detected CIN2+ case was defined as histologically confirmed CIN2+ case in a woman without such diagnosis in at least three years. Invasive procedures include diagnostics procedures (biopsies, excisions and endocervical curettage) and treatment (LLETZ, cold knife conisation and tracheletomy). Tracheletomies were merged with cold knife conisations in analysis. Hysterectomies and treatments of unknown type (less than 0.3% of registry data) were excluded from the analysis. The Bethesda classification has been used to report cervical cytology since 2011 and the WHO classification for histology since 2015, with specifying CIN2 and CIN3 in HG squamous intraepithelial lesions (HSIL). HG change refers to HG cytology (for which colposcopy is recommended: ASC-H, HSIL, squamous cell carcinoma, all glandular pathology) or HG lesion. HG lesion refers to CIN2+ (CIN2, CIN3, HSIL-CIN cannot be defined, adenocarcinoma in situ; cervical cancer). In the manuscript we refer to HG lesions as precancer as risk for progression of HG lesion to cervical cancer is high enough that treatment is indicated in order to prevent cancer. However, it should be noted that not all HG lesions would progress to cancer if left untreated; the risk for progression is associated with type of HG lesions and age of the women. LG changes include diagnoses in which immediate colposcopy is not recommended (ASC-US and LSIL).
2.4. Methods and statistics
We compared the number of cervical cytopathology, histopathology and HPV tests, as well as number of newly diagnosed CIN2+ from the entire Slovenian population in year 2020 to a three-year average of the years 2017–19. Each result was computed in weekly intervals (week one being the first seven days of each year) as a total number of results and arithmetic mean was calculated for the 2017–19 average. Average weekly turnaround times were calculated as arithmetic mean for each week of each year range. Results are graphically presented, either as a cumulative sum, absolute and relative difference of cumulative sums or mean value in days for each week. The relative difference in cumulative sums was calculated as the absolute cumulative difference between 2020 and 2017–19 average over 2017–19 average. Confidence intervals were derived with the bootstrap method with 10,000 replications. Observed values are marked as points on graphs, and smoothed lines are added for better trend representation. We also present data for different periods regarding the screening lockdown; epidemic refers to the period of first epidemic during which the screening was halted (12th March to 6th May), post first COVID-19 epidemic refers to the period since screening was restarted to the end of our observation (7th May to 30th September 2020) and pandemic refers to the period from the start of the first epidemic to the end of our observation (12th March to 30th September 2020). Since data were computed in weekly intervals, we approximated the period of the screening lockdown. While the start of the screening lockdown was effectively March 12th, which is aligned with our weekly calculations, the end of lockdown was approximated to May 6th; in fact, the last day of lockdown was May 8th. Specific date range from 2020 was always compared to the corresponding date range from the 2017–19. Data analyses and visualisations were done in R (version 4.0.3).
2.5. Role of the funding source
This research received no specific funding.
3. Results
3.1. Intensity of screening programme
The number of all tests and invasive procedures began to decrease at the start of the COVID-19 epidemic (Fig. 1, Supplementary Fig. S1). Treatment stabilised around 10% below the reference value in the 4th week into the epidemic and remained stable during the summer, with a slight increase toward the end of the summer (Fig. 1). The decline in the number of invasive diagnostics was larger than the decline in treatment at the end of the epidemic; the restoration of invasive diagnostics after the epidemic closed this gap at the end of the summer. FU cytology and HPV triage tests had similar dynamic: the number of tests started to increase toward the end of the epidemic and stabilised around 10% above the reference value during the summer. Scaling-up resulted in the excess of screening smears (+8.5%), follow up smears (+33.7%) and HPV triage (+36.5%) in the post first COVID-pandemic period (Table 1). The FU smears and HPV triage tests started to increase and peaked above the past year's average a few weeks before the increase of screening smears (Supplementary Fig. S1).
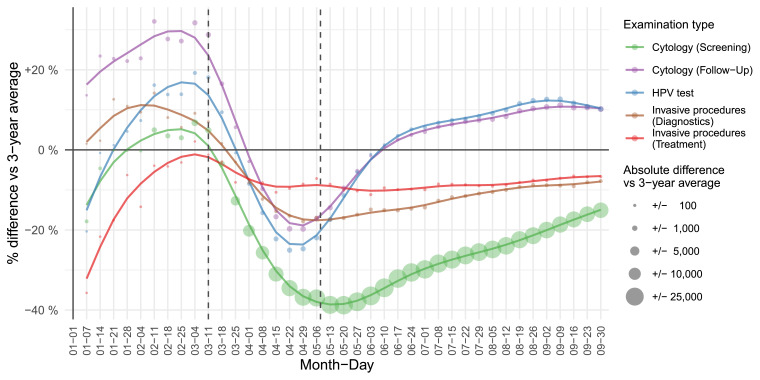
Fig. 1.
Cumulative deficit and excess (%) of cervical tests and invasive procedures for women aged 20–64 from January 1st to September 30th 2020 by weeks compared to 3-year average in 2017–2019 (reference value 0). Screening (green) and follow-up (purple) cervical smears (cervical cytology), HPV triage tests (blue) and invasive diagnostic (brown) and treatment (red) procedures are shown. Vertical deviation from 0% represents cumulative relative (%) difference and point size represents absolute cumulative difference from January 1st 2020 compared to 3-year average. Data source: National Cervical Cancer Screening Registry ZORA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The most profound epidemic decline was observed in screening smears, for which a delayed and less intensive increase was observed in comparison to FU and HPV triage tests, leading to the largest gap in comparison to the past-years average at the end of the summer, just before the second epidemic hit. We entered the second wave of epidemics with a pandemic deficit of −19,460 (−23%) programme screening smears, however with excess in FU smears (412, 4%) and HPV triage tests (523, 8%) (Table 1). The pandemic deficit was smaller, however significant in invasive diagnostics (−387, −13%) and in treatment (−87, −9%). Among treatment, we observed significantly higher deficit in cold-knife conisations (−91, −39%) compared to LLETZ (−4, −1%).
Older women (aged 40 to 64) had significantly larger deficit of screening smears during the epidemic than younger (aged 20 to 39) women did (Table 1, Supplementary Fig. S2). However, due to different intensities of scaling-up during the summer, the pandemic deficit of screening smears was significantly larger in age group 30 to 39 than in older groups and age group 20 to 29 was more similar to older groups than 30 to 39. Also, younger women had a smaller pandemic excess in FU smears and HPV triage tests. The opposite was observed for invasive diagnostics, for which the pandemic deficit was still larger in older women. The most affected were women in the 30–39 age group, who had the largest pandemic deficit of screening smears (−26%), the second-lowest scaling-up of FU tests (+31%), the lowest scaling-up of HPV tests during summer (+31%), and the highest epidemic (−25%) and pandemic (−18%) deficit in treatment.
3.2. Time to invasive procedures and turnaround times
We did not observe disruptions in invasive diagnostics and treatment in women with already diagnosed HG changes during the epidemic. The proportion of women who underwent invasive procedures 120 days after HG screening diagnosis in 2020 was similar than in the past (77% vs 70%); the same was observed for women with CIN2+ (73% vs 76%). In 2017–19, on average 90% of women with CIN2+ were treated within a one-year period. There was also no delay in time to invasive diagnostics and time to treatment; the time to invasive diagnostics decreased from 60 (SD = 26) to 41 days (SD = 15), and the time to treatment decreased from 58 (SD = 25) to 52 (SD = 25) days.
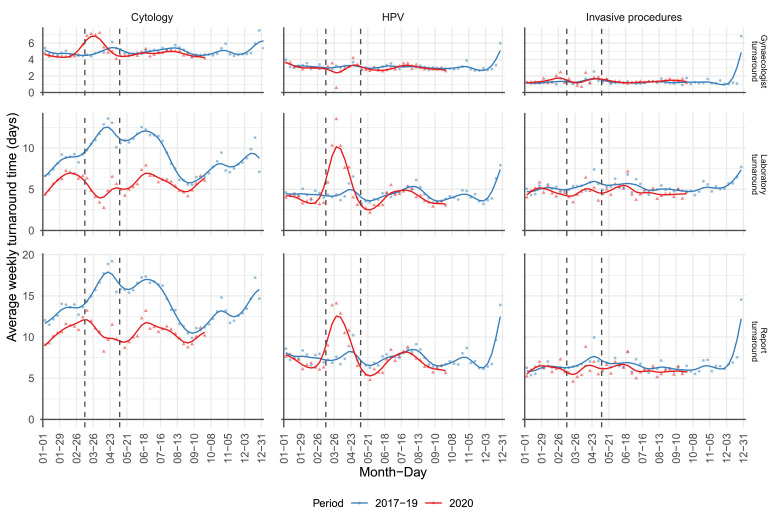
During the screening lockdown, the turnaround times were shortened in cytology laboratories and prolonged in HPV laboratories; however, they remained within the national standards (Fig. 2). In cytology laboratories, the turnaround time remained shortened even after the number of cervical smears significantly increased during the summer.
Fig. 2.
Turnaround time to the results of laboratory investigation. Upper graph: gynaecologist turnaround time, middle graph: laboratory turnaround time, bottom graph: report turnaround time of cytology (left), HPV triage tests (middle) and invasive procedures (right) through weeks for the period from January 1st 2020 to September 30th 2020 (red) in comparison to a three-year average for 2017–2019 (blue). Data source: National Cervical Cancer Screening Registry ZORA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. CIN2+ detection
Fig. 3 shows the cumulative sum of newly detected CIN2+ in Slovenia by weeks in 2020 compared to an average in 2017–2019. In the last three years, on average, 1522 new cases of CIN2+ per year have been detected in the 20–64 age group. After the two-month screening lockdown, 71 (32%) CIN2+ fewer cases were detected than expected. The relative deficit of newly detected CIN2+ cases was the largest one week after the lockdown ended (100 cases, −18%); later, the deficit gradually decreased, and by the end of September 2020, there were 113 missing cases of CIN2+ (−10%), which is a significant difference. The only age group with a significant CIN2+ deficit at the end of September (−19%) was 30–39 years, in which almost one third of all new CIN2+ cases were detected in the previous years. In other age groups, CIN2+ deficit ranged from −4% to −8%.
4. Discussion
Slovenia entered the COVID-19 pandemic as a country with a low cervical cancer burden, which was controlled with the organised population-based cervical cancer screening programme [4]. During the first COVID-19 epidemic the government systematically halted the screening and management of women with LG changes for two months. This lockdown led to a rapid decline in screening and follow-up tests, which resulted in a relatively rapid and profound decline in CIN2+ detection. We did not however observe disruptions in diagnostics and treatment of women at higher risk at colposcopy clinics, nor disruptions in turnaround times in laboratories. Decline in CIN2+ detection was relatively difficult to compensate for, despite the rapid scaling-up of screening and follow-up testing after a programme services were restored. During the five months of scaling-up phase, a new vulnerable group emerged and was most likely related to decreased participation in cervical cancer screening programme ZORA during the pandemic. These were women aged 30–39 who entered the second epidemic wave with a −26% deficit in screening smears and a −19% deficit in cumulative CIN2+. If we fail to detect and treat precancerous cervical lesions in settings in which cervical cancer was successfully controlled by screening, the cervical cancer burden will increase again.
The present study's main strength is that it was performed on the population data from the high-quality registry, which enabled in-depth analysis of screening intensity, quality, and outcomes in different periods of the pandemic year 2020 in comparison to previous years. To the best of our knowledge, this study is the first to analyse the impact of the COVID-19 pandemic on the detection of cervical precancer and the first to identify a new vulnerable group of women at higher risk for cervical cancer due to the pandemic. The main limitation is that its findings cannot directly answer the question of how the pandemic deficit in CIN2+ will be reflected in the future cervical cancer burden due to a complexity in the natural history of cervical cancer, but age-specific results could serve as inputs for further projections [5], [6], [7]. We cannot fully exclude that other factors than COVID-19 pandemic contributed to the observed differences of study outcomes in year 2020 compared to years 2017–19, however we believe this possible bias is not substantial, since there were no other changes in screening programme policy and its implementation within the healthcare system besides those related to COVID-19 pandemic between 2017 and 2020 and the trends of the observed outcomes were stable in the past years. Also, the study does not provide insight into what the reasons were for a new vulnerable group. More research is needed to understand the dynamic of screening determinants during crises like the COVID-19 pandemic.
4.1. Screening reduction in the first epidemic wave
The profound deficit of screening tests at the end of the first epidemic in Slovenia (−92%) is comparable to a few published results from the USA and Europe [8], [9], [10], [11]. The largest reduction was related to services provided within the primary healthcare level. The reduction in invasive procedures, performed mostly at clinics and university medical centres, was smaller and less dynamic (Fig. 1). We did not observe any disruptions in time to diagnosis and treatment in women at higher risk. During the screening lockdown, the turnaround times in HPV laboratories were prolonged due to relocation of personnel to COVID testing; however, they remained within the national standards. One can speculate that systematic reduction of workload at the primary health care level was reflected in the reduction of invasive diagnostics, which again was reflected in the reduction of treatment. The Slovenian experience indicates that good access to primary health care services is critical for population health, including during the pandemic crisis, as emphasised in a recent WHO publication [12]. During the second epidemic in autumn, primary health care was restructured, and good access endorsed. Cervical cancer screening (as well as breast and colorectal) was not stopped systematically, local adjustments were advised. Regional coordinators of gynaecology activities were appointed by the Expert Committee for Gynaecology and Obstetrics at the Ministry of Health. One of the advantages in cervical screening during the pandemic is that the treatment of HG lesions is mostly performed under local anaesthesia and is therefore not compromised by the relocation of anaesthetists and patients beds to COVID wards.
4.2. Scaling-up
Intensive scaling-up of screening as well as FU smears and HPV triage tests, was observed toward the middle of the first epidemic and lasted throughout the next five-months (Supplementary Fig. S1). Scaling-up was also reported in the study of Mast and Munoz del Rio, in which the mid-June volume of cervical cancer screening smears remained 35% lower compared to previous years in the study that assess the data from 60 healthcare organisations in 28 American states [13]. Scaling-up in our study was more intense; FU smears and HPV triage tests had similar dynamics and peaked three weeks after the end of the pandemic and then remained above the past average until the beginning of September. These results indicate that screening providers on the primary healthcare level showed high motivation for scaling-up, that they prioritised management of women at higher risk due to delay in FU smear or HPV triage, and that women responded to their invitations well.
The risk-based scaling-up was endorsed by the central ZORA office, who sent rapid feedback regarding the epidemic screening and FU deficit to all Slovenian gynaecologists, with the recommendation to prioritise services to women at higher risk due to the LG cervical pathology, whose appointments were rescheduled during the first epidemic due to the regulation on national level. Gynaecologists also received two tools; the list of their registered women with pathological cervical changes since June 1st 2019 and a decision aid tool with information about CIN2+ risk based on women's previous tests and procedures results. This information was mailed on May 11th, the first working day after the end of the screening lockdown. Use of a risk stratification approach and/or use of the decision aids was recommended also by other scientific consortiums [9,11,[14], [15], [16], [17], [18]].
The screening deficit during the first epidemic was relatively large, since the epidemic hit in March and April, which are the months of high screening intensity. However, the usual summer reserves due to the summer vacation of women and screening providers gave some space for the intensive scaling-up in summer months. To encourage women to respond to invitations to screening, a joint press release of all three organised screening programmes was sent to the media at the end of May during the European week against cancer, with the main message that screening can save lives and that it can also be provided safely during the pandemic. A new webpage was launched by the Association of Slovenian Cancer Societies, in collaboration with Institute of Oncology, National Institute of Public Health, Ministry of Health and National Cancer Control Plan, providing the information about cancer control in Slovenia during the COVID-19 pandemic, emphasising the importance of 12 recommendations against cancer, including screening attendance, also during the pandemic [19,20]. One can speculate that having an organised screening programme with a central screening database and a good communication with the providers and media support contributed significantly to a relatively successful, risk-based scaling-up in Slovenia.
4.3. A new vulnerable group in cervical cancer screening during the COVID-19 pandemic
We did not expect to observe a much higher CIN2+ deficit in women aged 30–39 years (−19%) compared to women in other age groups (−4% to −9%) (Fig. 3). We were not able to foresee this deficit from the previous decade of routine monitoring of screening intensity in Slovenia, nor CIN3 registration at Slovenian Cancer Registry. Routine monitoring of screening intensity in the previous ten years showed stable three-year coverage by a screening test in the 30–39 years age group (78–79%), that was somewhat lower than in women aged 20–29 years (decreasing trend from 86% to 81%), and higher than in women aged 40–49 years (stable 74–75%) and 50–64 years (increasing trend from 57–63%) [21]. Slovenian Cancer Registry data for the previous ten years showed even slightly increasing trend in the crude CIN3 incidence rate per 100,000 women in the age groups 30–34 (from 256 to 357) and 35–39 (from 224 to 275) [22]. However, our analysis of the pandemic deficit showed that scaling-up of screening was the least successful in the age group of 30–39 years (Table 1). Furthermore, the gap with the other age-groups became more profound in September, the beginning of the school year (Supplementary Fig. S2). These observations indicate that the pandemic deficit in CIN2+ detection in women aged 30–39 years most likely reflects new, pandemic-related barriers to screening participation specific for this age group of women.
Cervical cancer screening participation has many determinants; the most generalisable are women's age, socio-economic status, psychosocial issues, and marital status [23,24]. One could speculate that new barriers to participation, specific to the age group of 30–39 years, observed in our study might be related to social and psychological factors, such us new pandemic challenges in balancing the life course events with career trajectory in combination with pandemic fatigue. The average age of a mother at the birth of her first child in Slovenia is increasing and was 30 years in 2019 [25]. During the pandemic, much has changed for young families. New demands include combining work from home and the home-schooling of children. Intergenerational relationships restructured due to the higher vulnerability of older people to COVID-19, which could lead to lower intergenerational support, especially to young families. Structural barriers, including lack of time due to work and family obligations, have been reported to be associated with non-participation prior to the COVID pandemic [26], [27], [28]. One of the identified barriers in the UK, which could become more prevalent in the COVID pandemic, is the gap between the intent (‘I want to go’) and actual participation (‘not getting around to it’) [26]. Additionally, women who reported a greater number of major life events, who had a higher life stressor score and who reported greater feelings of discrimination were less compliant to screening guidelines in the study of Paskett et al. [29]. However, currently, there is a lack of real-time demographic and socio-economic data for the early identification of screening service uptake disparities during the COVID-19 pandemic [11,30]. More research is needed to understand the dynamics of screening attendance and compliance determinants during the crises, such as the COVID-19 pandemic.
5. Conclusions
In the historical observational study on population-based screening registry data, we found a clinically and statistically significant decrease in the detection of cervical precancer during the first seven months of COVID-19 pandemics in women aged 30–39 years. This is worrisome since one third of all cervical precancers were detected and treated in this age group prior the pandemic, due to the natural history of HPV infection and development of cervical cancer, which peaks in age group 40–49 in the unscreened population. The deficit in the detection and treatment of cervical precancer might led to increased cervical cancer burden in countries, which managed to control it in the past with the screening programmes, if women will not be reinvited and screened timely or will skip screening rounds [7]. Further research is needed to understand the dynamic of screening attendance determinants in such crises as pandemics and to identify feasible solutions to overcome the new barriers. HPV-self sampling is one of the possible approaches[14,31], however it is important that it is implemented in an organised and controlled environment.
Evidence-based steering of the screening programme based on continuous monitoring of screening programme during the COVID-19 pandemic led to informed decision on the national level, that despite much higher burden of confirmed COVID cases, cancer screening was not halted by the government during the second epidemic, which was declared on October 19th 2020. Local adjustments were advised, and screening endorsed. The continuation of screening during the second epidemic was possible also due to better preparedness of healthcare system to the pandemic and good availability of personal protective equipment.
Author Contributions
UI, TJ, UGO and LM: Conceptualisation. UI, MPŽ and TJ: Methodology. TJ and MF: Data curation. TJ and UI: Formal analyses, Data validation. TJ and IJ: Software. TJ: Visualisation. UI: Writing – original draft, Supervision. All authors: Investigation and interpretation, Writing – review and editing.
Data sharing statement
Data on the examination coverage and other data from monitoring and evaluation of cervical cancer screening programme in Slovenia are freely available from ZORA programme website (https://zora.onko-i.si/en/monitoring-and-evaluation). Data on the burden of cervical cancer in Slovenia is freely available from the Slovenian Cancer Registry SLORA website (http://www.slora.si/en/). Anonymised individual data of this observational, population-based study will be available following the publication for at least 5 years together with investigators support, for investigators who will provide a methodological sound proposal, which was approved by the independent review committee, to achieve aims in the approved proposal. Proposals should be directed to the corresponding author.
Declaration of Interests
We declare no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100101.
Appendix. Supplementary materials
References
- 1.Ivanuš U. ZORA programme organisation. ZORA: Slovenian national cervical cancer screening programme and registry. 2020. https://zora.onko-i.si/en/program/organisation (Accessed Dec 20, 2020).
- 2.Jansen E.E.L., Ivanuš U., Jerman T., de Koning H., de Kok I.M.C.M. The optimal HPV-screening protocol in Eastern-Europe: the example of Slovenia. Gynecol Oncol. 2020 doi: 10.1016/j.ygyno.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Ciavattini A., Delli Carpini G., Giannella L. European Federation for Colposcopy (EFC) and European Society of Gynaecological Oncology (ESGO) joint considerations about human papillomavirus (HPV) vaccination, screening programs, colposcopy, and surgery during and after the COVID-19 pandemic. Int J Gynecol Cancer. 2020;30:1097–1100. doi: 10.1136/ijgc-2020-001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Regional Office for Europe. Turning the tide: Slovenia's success story of fighting cervical cancer. 2020. https://www.euro.who.int/en/health-topics/noncommunicable-diseases/cancer/news/news/2020/12/turning-the-tide-slovenias-success-story-of-fighting-cervical-cancer (Accessed Dec 20, 2020).
- 5.McCredie M.R., Sharples K.J., Paul C., Baranyai J., Medley G., Jones R.W., Skegg D.C. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9:425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki A.B., Schiffman M., Burchell A. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30:F24–F33. doi: 10.1016/j.vaccine.2012.05.089. Suppl 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castanon A., Rebolj M., Pesola F., Sasieni P. The impact of COVID-19 disruption to cervical cancer screening in England on excess diagnoses. 2020. 10.1101/2020.11.30.20240754 (Accessed Dec 20, 2020)
- 8.Gorin S.N.S., Jimbo M., Heizelman R., Harmes K.M., Harper D.M. The future of cancer screening after COVID-19 may be at home. Cancer. 2020 doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- 9.Epic Health Research Network. Delayed cancer screenings. 2020. https://ehrn.org/articles/delays-in-preventive-cancer-screenings-during-covid-19-pandemic/ (Accessed Dec 20, 2020).
- 10.de Pelsemaeker M.C., Guiot Y., Vanderveken J., Galant C., Van Bockstal M.R. The impact of the COVID-19 pandemic and the associated Belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology. 2020 doi: 10.1159/000509546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corley D.A., Sedki M., Ritzwoller D.P. Cancer Screening during COVID-19: a perspective from NCI's PROSPR consortium. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Maintaining essential health services: operational guidance for the COVID-19 context interim guidance. 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-essential-health-services-2020.1 (Accessed Dec 20, 2020).
- 13.Epic Health Research Network. Delayed cancer screenings—a second look. 2020. https://ehrn.org/articles/delayed-cancer-screenings-a-second-look/ (Accessed Dec 20, 2020).
- 14.Arbyn M., Gultekin M., Morice P. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int J Cancer. 2021;148:277–284. doi: 10.1002/ijc.33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen M.A., Powell A.M., Coleman J.S., Keller J.M., Livingston A., Anderson J.R. Special ambulatory gynecologic considerations in the era of coronavirus disease 2019 (COVID-19) and implications for future practice. Am J Obstet Gynecol. 2020;223:372–378. doi: 10.1016/j.ajog.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciavattini A., Delli Carpini G., Giannella L. Expert consensus from the Italian Society for Colposcopy and Cervico-Vaginal Pathology (SICPCV) for colposcopy and outpatient surgery of the lower genital tract during the COVID-19 pandemic. Int J Gynecol Obstet. 2020;149:269–272. doi: 10.1002/ijgo.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo I., Zaccarelli E., Del Grande M. ESMO management and treatment adapted recommendations in the COVID-19 era: gynaecological malignancies. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ajenifuja K.O., Belinson J., Goldstein A., Desai K.T., de Sanjose S., Schiffman M. Designing low-cost, accurate cervical screening strategies that take into account COVID-19: a role for self-sampled HPV typing. Infect Agent Cancer. 2020;15:61. doi: 10.1186/s13027-020-00325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Association of Slovenian Cancer Societies. Obvladovanje raka v času epidemije COVID-19 in po njej. 2020. https://priporocila.si/covid-19/ (Accessed Dec 20, 2020).
- 20.World Health Organization. European code against cancer. 2016. https://cancer-code-europe.iarc.fr/index.php/en/ (Accessed Dec 20, 2020).
- 21.Jerman T., Ivanuš U., Florjančič M. ZORA programme monitoring and evaluation. ZORA: Slovenian national cervical cancer screening programme and registry. 2020. https://zora.onko-i.si/en/monitoring-and-evaluation (Accessed Dec 20, 2020).
- 22.Zadnik V., Primic Žakelj M. Institute of Oncology Ljubljana; 2020. SLORA: Slovenia and cancer. epidemiology and cancer registry.http://www.slora.si/en/ (Accessed Dec 20, 2020) [Google Scholar]
- 23.Arbyn M., Anttila A., Jordan J. 2nd edition. Office for Official Publications of the European Communities; Luxembourg: 2008. European guidelines for quality assurance in cervical cancer screening; p. 34. [Google Scholar]
- 24.Limmer K., LoBiondo-Wood G., Dains J. Predictors of cervical cancer screening adherence in the United States: a systematic review. J Adv Pract Oncol. 2014;5:31–41. [PMC free article] [PubMed] [Google Scholar]
- 25.Statistical Office of the Republic of Slovenia. Basic data on live births, Slovenia, annually. 2020. https://pxweb.stat.si/SiStatData/pxweb/en/Data/-/05J1002S.px/ (Accessed Dec 20, 2020).
- 26.Waller J., Bartoszek M., Marlow L., Wardle J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16:199–204. doi: 10.1258/jms.2009.009073. [DOI] [PubMed] [Google Scholar]
- 27.Coronado G.D., Thompson B., Koepsell T.D., Schwartz S.M., McLerran D. Use of Pap test among Hispanics and non-Hispanic whites in a rural setting. Prev Med. 2004;38:713–722. doi: 10.1016/j.ypmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Bennett K.F., Waller J., Chorley A.J., Ferrer R.A., Haddrell J.B., Marlow L.A. Barriers to cervical screening and interest in self-sampling among women who actively decline screening. J Med Screen. 2018;25:211–217. doi: 10.1177/0969141318767471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paskett E.D., McLaughlin J.M., Reiter P.L. Psychosocial predictors of adherence to risk-appropriate cervical cancer screening guidelines: a cross sectional study of women in Ohio Appalachia participating in the community awareness resources and education (CARE) project. Prev Med. 2010;50:74–80. doi: 10.1016/j.ypmed.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancino R.S., Su Z., Mesa R., Tomlinson G.E., Wang J. The impact of COVID-19 on cancer screening: challenges and opportunities. JMIR Cancer. 2020;6:e21697. doi: 10.2196/21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbyn M., Bruni L., Kelly D. Tackling cervical cancer in Europe amidst the COVID-19 pandemic. Lancet Public Health. 2020;5:E425. doi: 10.1016/S2468-2667(20)30122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.