Summary
Ambient air pollution has been shown to be associated with the pathogenesis of dementia and mild cognitive impairment (MCI). However, few studies have examined these associations in well-characterized populations with low residential mobility, similar living habits, and a standardized assessment of both air pollution exposure and clinical outcome. This study examined the associations of long-term exposure to particulate matter (PM) air pollution with dementia and MCI, using data from the Chinese Veteran Clinical Research Platform. The cognitive function of elderly veterans from 277 communities in 18 Chinese cities was examined. Participants' daily exposures to aerodynamic diameters ≤2.5 μm (PM2.5) and ≤10 μm (PM10) during the 3 years prior to the survey were estimated using a satellite-based prediction. The adjusted odds ratios (ORs) and 95% confidence intervals of MCI associated with each 10 μg/m3 increase in PM2.5 and PM10 were 1.52 (1.39, 1.67) and 1.04 (1.00, 1.08), and those of dementia associated with PM2.5 and PM10 were 1.27 (1.11, 1.46) and 1.13 (1.05, 1.21), respectively. This demonstrates that long-term exposure to PM2.5 and PM10 can increase the prevalence of dementia/MCI among veterans in China. Higher ORs were observed for those with ≤9 years of educational attainment, those who actively attended physical activities, those who never smoked, former drinkers, and those who did not suffer from cerebral infarction. Improvement of ambient air quality, especially decreasing levels of PM2.5, may help to decrease the risk of dementia/MCI. Given the statistically significant association between PM and cognitive impairment demonstrated here, future studies should focus on examining the causal effect of PM pollution on dementia and MCI.
Keywords: particulate matter, dementia, MCI, air pollution
Graphical abstract
Public summary
-
•
Dementia and MCI are emerging as major public health problems, and PM2.5 is hypothesized to be associated with dementia and MCI
-
•
We examined the cognitive function of elderly veterans from 277 communities (a well-characterized population with low residential mobility, similar living habits, standardized assessment of both exposure and outcome), in 18 Chinese cities from December 2009 to December 2011
-
•
Long-term exposure to PM, especially PM2.5 showed associations with dementia and MCI
-
•
The effect was more pronounced for people with no more than nine years of education
-
•
Besides, people with fewer other risk factors (lack of physical activities, smoking, drinking, cerebral infarction) are more susceptible to PM2.5
-
•
Improvement of ambient air quality, especially PM2.5, might be helpful to decrease the risk of dementia and MCI
Introduction
Dementia, a neuropsychiatric disease characterized by cognitive impairment, imposes a heavy burden on patients, their families, and society as a whole.1 Dementia has become a major public health concern in China due to the rapid growth of the elderly population2; it was estimated in 2016 that among 23 million Chinese aged 60 years and over, almost 9.5 million, were suffering from dementia, and this figure was projected to double by 2030.3 Alzheimer disease, a progressive and irreversible neurodegenerative disorder, is the most common subtype of dementia, accounting for 60%–70% of dementia cases.4 The causes of dementia remain unclear, although genetic and environmental factors may play important roles in the etiology. Dementia is currently incurable,5 and therefore mild cognitive impairment (MCI), which is diagnosable prior to more severe forms of cognitive impairment such as dementia, has been proposed as an early intervention target.
Recent research has indicated that ambient air pollution, such as airborne particulate matter (PM) with a diameter of 2.5 μm or less (PM2.5), is potentially associated with the pathogenesis of dementia.6 Some studies have also demonstrated that chronic exposure to PM air pollution can induce inflammation in the brain and accelerate β-amyloid deposition and neurodegeneration, which are associated with brain damage, cognitive decline, and the development of dementia.7, 8, 9 In addition, some recent studies from Western countries (e.g., the United States and Europe) have suggested an association between traffic pollution and decreased cognitive function in adults.10,11
Although a great deal of evidence has suggested that PM is associated with a wide range of negative health outcomes, such as cardiovascular and respiratory diseases,12, 13, 14, 15 limited attention has been paid to neurological and mental effects of PM pollution. In particular, there are few studies investigating the impact of air pollution on dementia and MCI, and the studies that have been done share several important limitations.16, 17, 18 First, participants may have been misclassified with respect to the type or level of PM exposure as a result of living in different areas over time, and high-level exposure to PM2.5 (more than 33.0 mg/m3) was not considered. Second, cognitive conditions were identified with screening tests or ICD-9 (Ninth Revision of International Classification of Diseases) codes, which may lead to outcome misclassification. Third, a number of potentially confounding factors were not accounted for, including physical inactivity, unhealthy diet, social isolation, cognitive inactivity, sleep deprivation, and medical services such as access and quality. Finally, few old-old (70–79 years old) and oldest-old (≥80 years old) participants were included. Therefore, the present study aimed to examine the associations of long-term exposure to PM air pollution with dementia and MCI, using data from the Chinese Veteran Clinical Research (CVCR) platform to address the limitations of prior studies.
Results
Sociodemographic characteristics and prevalence of dementia and MCI
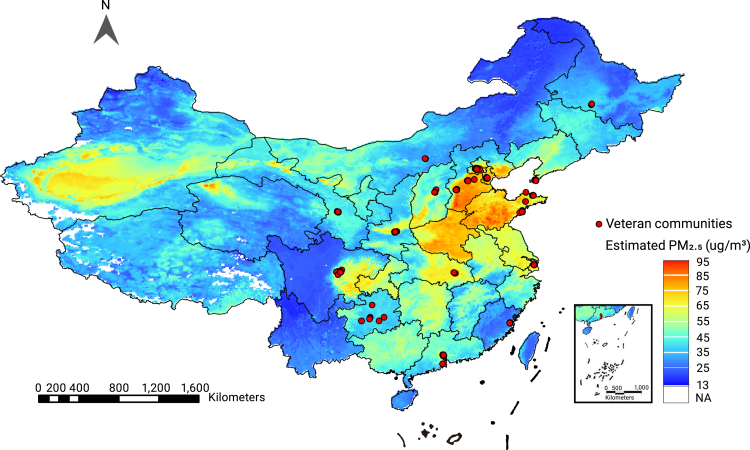
A total of 277 veteran communities (Figure 1), with 9,676 Chinese veterans aged 60 years and older, were recruited via the CVCR platform. Among these veterans, 7,445 had complete information regarding the neuropsychological tests and clinical diagnoses. The sex information data of 84 participants were missing. Data from 7,040 male veterans were included in the analysis after female participant data (n = 321) were excluded (Figure S1). As a result, 921 cases of dementia and 2,180 cases of MCI were identified with prevalence rates of 13.1% and 31.0%, respectively. The prevalence of dementia in our population was comparable with the age-standardized prevalence of dementia (15.2%) (Table S2). The basic sociodemographic characteristics of the veterans are summarized in Table 1.
Figure 1.
Locations of veteran communities included in this study
Table 1.
Basic sociodemographic characteristics of participants and their exposure to PM2.5 and PM10 (μg/m3) during the 3 years prior to the survey
| Factors | N | % | PM2.5 median (Q1, Q3) | PM10 median (Q1, Q3) |
|---|---|---|---|---|
| Age (years) | ||||
| <80 | 2,024 | 28.8 | 54.70 (48.02, 71.27) | 100.16 (84.59, 128.34) |
| ≥80 | 4,946 | 70.3 | 55.23 (48.15, 71.46) | 100.16 (83.66, 128.34) |
| Missing values | 70 | 1.0 | 56.25 (46.29, 73.49) | 100.23 (75.84, 131.79) |
| Years of education | ||||
| ≤9 | 4,564 | 64.8 | 55.32 (48.31, 71.70) | 101.12 (84.21, 128.78) |
| >9 | 2,476 | 35.2 | 54.70 (47.24, 59.88) | 99.95 (83.56, 111.47) |
| Physical activities | ||||
| Yes | 6,000 | 85.2 | 54.89 (48.14, 71.46) | 100.16 (83.76, 128.34) |
| No | 1,009 | 14.3 | 55.30 (48.73, 71.46) | 100.31 (83.66, 128.34) |
| Missing values | 31 | 0.4 | 53.77 (45.07, 55.95) | 91.41 (69.68, 100.98) |
| Social activities | ||||
| Yes | 2,710 | 38.5 | 55.24 (48.23, 71.62) | 99.95 (82.82, 128.78) |
| No | 4,170 | 59.2 | 54.82 (48.03, 71.46) | 100.34 (84.19, 128.34) |
| Missing values | 160 | 2.3 | 55.95 (54.70, 69.88) | 100.98 (99.95, 125.37) |
| Smoking | ||||
| Current | 561 | 8.0 | 54.33 (47.25, 70.89) | 100.31 (84.07, 127.69) |
| Former | 2,328 | 33.1 | 54.89 (48.03, 71.27) | 100.15 (83.66, 127.87) |
| Never | 3,920 | 55.7 | 55.21 (48.15, 71.49) | 100.16 (83.71, 128.34) |
| Missing values | 231 | 3.3 | 56.08 (54.11, 72.30) | 100.98 (94.63, 131.61) |
| Drinking | ||||
| Current | 513 | 7.3 | 55.21 (48.14, 71.27) | 98.82 (82.55, 128.34) |
| Former | 1,115 | 15.8 | 55.24 (48.15, 71.27) | 99.95 (82.67, 127.23) |
| Seldom | 1,689 | 24.0 | 55.21 (48.15, 71.27) | 100.09 (83.69, 128.34) |
| Never | 3,510 | 49.9 | 54.89 (48.03, 71.46) | 100.35 (84.17, 128.34) |
| Missing values | 213 | 3.0 | 55.95 (53.07, 72.53) | 100.98 (95.00, 133.39) |
| Diabetes mellitus | ||||
| Yes | 1,892 | 26.9 | 55.23 (48.31, 71.46) | 100.09 (84.17, 128.34) |
| No | 5,089 | 72.3 | 54.89 (48.03, 71.46) | 100.31 (83.66, 128.34) |
| Missing values | 59 | 0.8 | 55.95 (55.27, 58.72) | 100.98 (100.32, 106.5) |
| Hypertension | ||||
| Yes | 4,713 | 66.9 | 55.21 (48.15, 71.46) | 100.15 (83.66, 128.34) |
| No | 2,282 | 32.4 | 55.21 (48.03, 71.55) | 101.12 (84.19, 129.69) |
| Missing values | 45 | 0.6 | 55.95 (53.77, 59.14) | 100.98 (96.90, 107.10) |
| Hyperlipidemia | ||||
| Yes | 2,631 | 37.4 | 55.21 (48.73, 71.27) | 99.02 (83.56, 127.69) |
| No | 4,311 | 61.2 | 55.21 (48.02, 71.60) | 103.43 (84.21, 128.78) |
| Missing values | 98 | 1.4 | 55.95 (53.21, 60.27) | 100.98 (86.30, 107.70) |
| Cerebral infarction | ||||
| Yes | 1,468 | 21.9 | 54.89 (48.34, 70.90) | 99.96 (83.67, 125.37) |
| No | 5,503 | 78.2 | 55.21 (48.07, 71.60) | 100.31 (83.68, 128.78) |
| Missing values | 69 | 1.0 | 55.95 (55.27, 59.53) | 100.98 (100.32, 106.50) |
| Total | 7,040 | 100 | – | – |
Exposure to PM pollution during the study period
Participants' historical exposure to air pollution is summarized in Table S3. Mean levels of participants' exposures to PM2.5 and PM10 (minimum, maximum) were 56.92 (30.46, 84.23) μg/m3 and 102.71 (53.38, 143.14) μg/m3. The mean ratio of PM2.5/PM10 during the study period was 0.56 (0.40, 0.60).
PM pollution and the prevalence of dementia and MCI
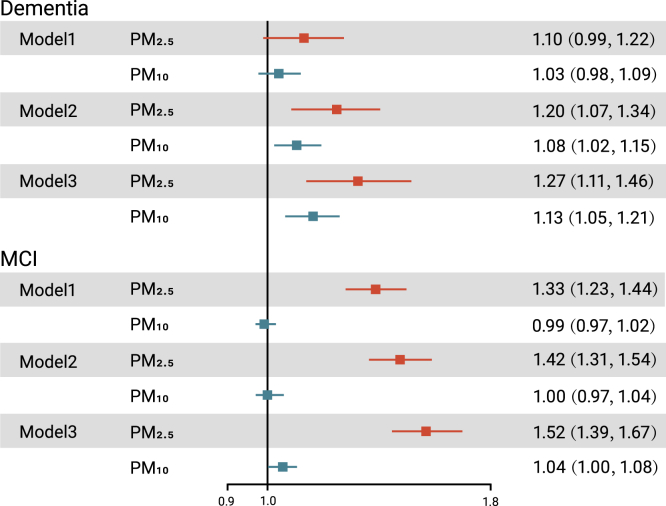
Long-term exposure to PM2.5 was significantly associated with a higher proportion of MCI and dementia in both the crude and adjusted models, while significant associations of PM10 with MCI and dementia were only found in the fully adjusted models (Figure 2). In the crude models, the ORs (and 95% confidence intervals [CIs]) of MCI and dementia associated with each 10-μg/m3 increase in PM2.5 were 1.33 (95% CI: 1.23, 1.44) and 1.10 (95% CI: 0.99, 1.22), respectively. After full adjustment, the ORs (and 95% CIs) of MCI associated with each 10-μg/m3 increase in PM2.5 and PM10 were 1.52 (1.39, 1.67) and 1.04 (1.00, 1.08). Moreover, the ORs (and 95% CIs) of dementia in relation to per-10 μg/m3 increase in PM2.5 and PM10 were 1.27 (1.11, 1.46) and 1.13 (1.05, 1.21), respectively.
Figure 2.
The ORs (and 95% CIs) of MCI and dementia associated with per-10 μg/m3 increase in PM2.5 or PM10
Effect modification by potential covariates
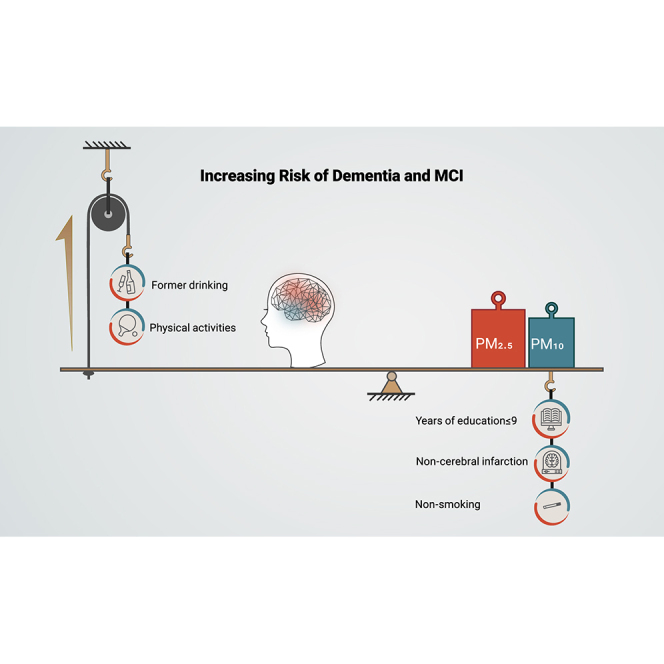
Some basic sociodemographic factors remarkably modified the relationships between PM2.5 exposure and the prevalence of dementia or MCI (Table 2). The association between PM2.5 exposure and MCI was strengthened in participants with ≤9 years of educational attainment (OR = 1.62 [95% CI:1.45,1.81]) compared with those with >9 years (OR = 1.33 [95% CI:1.15,1.54], p = 0.035). The association between PM10 exposure and MCI was strengthened in participants aged ≥80 years (OR = 1.07 [95% CI:1.01,1.12]) compared with those <80 years (OR = 0.97 [95% CI:0.92,1.02], p = 0.008). The association between PM2.5 exposure and dementia was strengthened in non-smokers compared with former smokers. In addition, compared with never drinking, former drinkers showed significantly higher ORs of dementia (Table 2). Suffering from hypertension strengthened the association between PM10 and MCI, while suffering from hyperlipidemia strengthened the association between PM10 and the prevalence of both MCI and dementia (Table 2). The association between PM2.5 exposure and MCI was strengthened in participants without cerebral infarction. The association between PM2.5 exposure and dementia was strengthened in participants who were physically active (Table 2). The ORs of MCI or dementia for participants with social activities were lower than those without social activities, but the difference was not statistically significant. No significant effect modifications were found for a history of diabetes mellitus.
Table 2.
The results of stratified analyses for the modification effects on the association between PM pollution and dementia/MCI
| Factors | PM2.5 (ORs and 95%CIs)a,b |
PM10 (ORs and 95%CIs)a,b |
||
|---|---|---|---|---|
| MCI | Dementia | MCI | Dementia | |
| Age (years) | ||||
| <80 | 1.57 (1.29, 1.90) | 1.02 (0.69, 1.49) | 0.97 (0.92, 1.02)∗∗ | 1.02 (0.84, 1.24) |
| ≥80 | 1.51 (1.36, 1.67) | 1.28 (1.11, 1.47) | 1.07 (1.01, 1.12) | 1.13 (1.05, 1.22) |
| Years of education | ||||
| ≤9 | 1.62 (1.45, 1.81)∗ | 1.34 (1.14, 1.57) | 1.03 (0.98, 1.08) | 1.12 (1.04, 1.22) |
| >9 | 1.33 (1.15, 1.54) | 1.06 (0.83, 1.37) | 1.04 (0.96, 1.12) | 1.12 (0.97, 1.28) |
| Physical activities | ||||
| Yes | 1.54 (1.40, 1.69) | 1.37 (1.17, 1.61)∗∗ | 1.04 (0.99, 1.08) | 1.15 (1.06, 1.25) |
| No | 1.42 (1.08, 1.87) | 0.91 (0.70, 1.18) | 1.04 (0.93, 1.17) | 1.04 (0.90, 1.21) |
| Social activities | ||||
| Yes | 1.40 (1.22, 1.60) | 1.09 (0.85, 1.40) | 1.08 (1.01, 1.16) | 1.13 (0.99, 1.29) |
| No | 1.63 (1.45, 1.84) | 1.35 (1.15, 1.58) | 1.01 (0.97, 1.04) | 1.12 (1.03, 1.22) |
| Smoking | ||||
| Currentc | 1.47 (1.30, 1.66) | 1.07 (0.90, 1.28) | 1.02 (0.97, 1.07) | 1.06 (0.97, 1.16) |
| Formerc | 1.61 (1.39, 1.86) | 1.50 (1.20, 1.87)∗ | 1.08 (1.01, 1.16) | 1.19 (1.07, 1.34) |
| Never | 1.46 (1.07, 1.98) | 1.73 (0.98, 3.04) | 0.96 (0.87, 1.05) | 1.23 (0.93, 1.62) |
| Drinking | ||||
| Currentd | 1.62 (1.19, 2.19) | 1.66 (0.97, 2.86) | 1.09 (0.95, 1.26) | 1.31 (1.00, 1.71) |
| Formerd | 1.50 (1.26, 1.79) | 1.61 (1.18, 2.21)∗ | 1.08 (0.99, 1.18) | 1.53 (1.28, 1.84)∗∗ |
| Seldomd | 1.41 (1.14, 1.74) | 1.19 (0.88, 1.60) | 1.08 (0.97, 1.20) | 1.05 (0.90, 1.24) |
| Never | 1.58 (1.39, 1.80) | 1.10 (0.91, 1.32) | 1.00 (0.97, 1.04) | 1.01 (0.92, 1.10) |
| Diabetes mellitus | ||||
| Yes | 1.58 (1.34, 1.88) | 1.27 (0.99, 1.62) | 1.10 (1.02, 1.20) | 1.07 (0.94, 1.21) |
| No | 1.51 (1.36, 1.68) | 1.26 (1.07, 1.48) | 1.01 (0.97, 1.06) | 1.15 (1.06, 1.25) |
| Hypertension | ||||
| Yes | 1.48 (1.33, 1.65) | 1.26 (1.08, 1.48) | 1.08 (1.02, 1.14)∗ | 1.16 (1.07, 1.26) |
| No | 1.60 (1.35, 1.89) | 1.26 (0.97, 1.63) | 0.99 (0.95, 1.04) | 1.05 (0.92, 1.19) |
| Hyperlipidemia | ||||
| Yes | 1.50 (1.31, 1.71) | 1.42 (1.14, 1.76) | 1.19 (1.11, 1.28)∗∗ | 1.24 (1.10, 1.40)∗ |
| No | 1.53 (1.35, 1.72) | 1.15 (0.97, 1.37) | 0.97 (0.94, 1.00) | 1.05 (0.96, 1.14) |
| Cerebral infarction | ||||
| Yes | 1.16 (1.01, 1.33)∗∗ | 1.11 (0.90, 1.38) | 1.02 (0.96, 1.08) | 1.02 (0.92, 1.14) |
| No | 1.61 (1.46, 1.79) | 1.34 (1.13, 1.58) | 1.06 (1.01, 1.11) | 1.16 (1.07, 1.27) |
∗p < 0.05 in the two-sample test. ∗∗p < 0.01 in the two-sample test. Detailed results are shown in Table S3 in the supplemental information.
Fully adjusted models controlling age, education years, smoking, drinking, family history of dementia, and history of NCDs. In all models, city was modeled as random effect. ORs and 95% CIs were associated with per-10 μg/m3 increase in each pollutant.
The significance of difference in effect estimates between different subgroups was examined using a two-sample test.
The never-smoke group was set as the reference.
The never-drink group was set as the reference.
Discussion
This multicenter cross-sectional study demonstrated that long-term exposure to PM air pollution was significantly associated with dementia and MCI among Chinese veterans. The associations from the fully adjusted model are more reliable since this model controlled for more confounding factors. Higher ORs were observed for those with ≤9 years of educational attainment, those who were physically active, former drinkers, those who had never smoked, and those who had not suffered from cerebral infarction. In addition, long-term exposure to PM2.5 was found to have a greater impact on MCI and dementia than PM10. Overall, our results suggest that exposure to ambient particulate matter, especially to PM2.5, has significant adverse impacts on brain health and cognitive function.
Current experimental evidence and postmortem studies on the harmful effects of PM pollution in relation to dementia pathogenesis and cognitive impairment are insufficient and inconsistent. Some epidemiological studies have demonstrated significant associations of long-term exposure to PM2.5 or PM10 and dementia/MCI in the elderly and middle-aged population,19, 20, 21, 22 with some specifically reporting that PM from wood-burning and traffic-related sources were associated with dementia and MCI.23,24 However, some studies have reported that PM2.5 exposure had no impact on the incidence of cognitive impairment and that long-term exposure to PM10 was not related to reduced cognitive functions among adults.25,26 One explanation for the inconsistent findings is that there are a number of risk factors that lead to dementia/MCI (e.g., PM air pollution, stroke, APOE gene variants) and some of those risk factors might be more or less important depending on the circumstances. In a diverse population, the association between PM and dementia/MCI may not be significant if other risk factors are more important.
Identification of potentially susceptible subgroups is crucial to reducing the adverse impacts of PM air pollution. We examined the effect modifications of several potential factors, most of which were not considered in previous studies.27,28
The stratified analyses indicated that those who received less education, those who were physically active, non-smokers, former drinkers, and those without a history of stroke were more susceptible to the adverse impact of PM air pollution on dementia and MCI. One explanation for the substantial difference in the subgroup analysis is that a heightened baseline risk profile may mask additive effect from pollution,25 and thus PM2.5 showed a stronger effect on dementia/MCI risk when patients had a lower risk of dementia/MCI. For example, it has been found in previous studies that people who exercise properly, have no history of cerebral infarction, do not smoke, and drink a little alcohol have a lower risk of dementia,29 while PM2.5 has a stronger effect in these people. Cognitive reserve is an exception30; we found that people with higher cognitive reserve (associated with higher education) were less likely to be affected by PM2.5 levels. This may be related to the fact that cognitive reserve is a part of cognitive function, and people with higher overall cognitive reserve are less likely to progress to dementia/MCI even though their cognitive function may decline after exposure to PM2.5.
The present study did not observe an effect modification by a history of other non-communicable diseases (NCDs), including cardiovascular disease, transient ischemic attack, depression, metabolic syndrome, chronic obstructive pulmonary disease (COPD), anemia, cataract, glaucoma, and hearing disorders. Some of our results are comparable with those of previous studies. For example, Tzivian et al. found depression has no modification effect on the association between PM2.5 and MCI,21 consistent with our results, but also found that associations between PM2.5 and MCI were stronger in participants with no or moderate alcohol consumption than in those with high alcohol consumption, which is inconsistent with our findings. Other effect modifiers examined in this research have not been discussed in previous studies,21,28,31,32 which limits the ability to make direct comparisons.
Although the biological mechanisms by which PM causes cognitive impairment are far from clear, some plausible mechanisms have been proposed. Several experiments in non-human animals and human postmortem studies have indicated that PM could infiltrate the brain through circulation or translocation via the olfactory nerve.33,34 After penetration to the brain, PM may lead to systemic inflammation, oxidative stress, and a cascade of neuropathological changes. Such changes include the accumulation of amyloid-β42, the presence of hyperphosphorylated tau and neurofibrillary tangles, and neuroinflammation. The neuropathological changes may further lead to neural degeneration and cerebral atrophy, which are consistent with changes in the progression of Alzheimer disease, the most comment type of dementia.5,7,33,35, 36, 37 In addition, existing evidence suggests that PM-related cardiovascular and cerebrovascular disease may accelerate white matter loss, lower the total cerebral brain volume, and lead to more covert brain infarcts in older adults.14,38, 39, 40 All of the above changes associated with PM exposure may contribute to cognitive impairment and the development of dementia or MCI among the elderly.
Some limitations of this study should be noted. The main limitation is that the cross-sectional study design prevented us from examining the causal effect of PM pollution on dementia or MCI. We were also not able to control for some potential confounding factors such as genetic information, coincident gaseous pollutants, and different occupations (work before retirement) due to the unavailability of these data. The spatial resolution of satellite-based exposure estimation could be improved by using new satellite-retrieved aerosol optical depth data.41,42 With an improved spatial resolution of exposure assessment, ORs for PM2.5 and PM10 tend to be higher, due to more remarkable variations of individual-level exposure.43,44 In this study, we used a bilinear interpolation method. The value of predicted PM2.5 is the linear combination of four nearest 10-km grid cells (PM in sites 1–4) weighted by distance from the site to each of the grid cells, that is to say, . Points located in the same grid cell are of varying distance to the four nearest 10-km grid cells, and thus points are typical of different weights. Therefore, points located in the same grid cell have different values of PM2.5. Apart from particle size, the source and chemical composition of PM play important roles in its health effects, but we were unable to consider these issues in this study due to the unavailability of the data. Moreover, this study only included male veterans, which limits the generalizability of the findings to other populations.
Despite the limitations described above, this study has several strengths compared with prior research in this area. First, the participants of this study are from veteran communities, which is a special population group including many old-old and oldest-old participants. Their residential places are stable and their living habits are very similar, which helps to reduce exposure misclassification and reduce unmeasured or residual confounding factors. Second, the clinical diagnoses of dementia or MCI in this study were based on the results of clinical examination and systematic neuropsychological tests. This effectively reduced the misdiagnosis of dementia or MCI compared with other studies in which the diagnosis relied only on the disease code or neuropsychological evaluations.45,46 In addition, the large sample size of this study and the satellite-based estimation of PM in 18 cities ensures representativeness of the data and minimizes exposure misclassification.47 Finally, due to the relatively high mean levels of participants' exposures to PM2.5 and PM10 (56.92 μg/m3 and 102.71μg/m3, respectively), this study provides evidence about the association between PM and dementia/MCI in more seriously polluted regions. Overall, to our knowledge, this is the first epidemiological study examining the association between long-term exposure to PM and MCI/dementia in China, and thus represents an important step in understanding the role of PM in cognitive impairment.
Conclusion
Our study shows a significant association between PM air pollution exposure and development of dementia or MCI. This relationship was modified by the participant's educational level, physical activities, smoking and drinking habits, and prior incidence of cerebral infarction. Improvement of ambient air quality, especially PM2.5, is expected to help decrease the risk of dementia and MCI in the elderly. In addition to reducing the level of pollutants, it is also the future research direction to look for other interventional environmental factors to reduce the influence of PM on cognitive function.
Materials and methods
Study population
This multicenter cross-sectional survey was conducted using the CVCR platform, which was established to investigate the prevalence of NCDs among Chinese veterans from December 2009 to December 2011.48,49 Participants were selected using a multi-stage cluster sampling method. In stage 1, from all areas where there are veteran communities, we chose three municipalities (Beijing, Tianjin, and Shanghai) and six provinces (Liaoning, Hebei, Shandong, Jiangsu, Fujian, and Guangdong) in the developed eastern region, and eight provinces (Heilongjiang, Inner Mongolia, Shanxi, Hubei, Shanxi, Gansu, Sichuan, and Guizhou) in the less developed central and western regions. In stage 2, we selected four first-tier cities (Beijing, Shanghai, Tianjin, and Guangzhou), five second-tier cities (Qingdao, Dalian, Fuzhou, Shijiazhuang, and Yantai), and one third-tier city (Baoding) in the developed eastern region, and eight second-tier cities (Wuhan, Xi'an, Chengdu, Harbin, Lanzhou, Taiyuan, Hohhot, and Guiyang) in the less developed central and western regions. In stage 3, veteran communities located in the selected cities participated in this platform. Finally, we selected all 277 veteran communities from 18 cities, covering 24.36% of all veteran communities in China. The veterans aged ≥60 years (or their legal representatives) in selected communities were investigated in this study. Community workers and veterans' spouses who were not veteran were excluded. The study was approved by the Institutional Review Board of the Chinese People's Liberation Army General Hospital and informed consent was obtained from each participant or their legal representative.
Evaluation of dementia and mild cognitive impairment
All of the medical staff from the participating hospitals in the CVCR platform who have qualified to assess cognitive impairment through training performed a face-to-face, two-stage screening and diagnostic assessment of dementia and MCI with elderly participants in the clinics of veteran communities. The first phase was case screening, where we employed a series of rating scales to evaluate cognitive function, functional dependence, depressive symptoms, and sleep disorders for the eligible participants.48 The scales we used included the Chinese version of the Mini-Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA-P), Activities of Daily Living (ADL), and the Center for Epidemiological Studies Depression Scale (CED-S).50, 51, 52, 53, 54
The second phase was case evaluation. For the veterans whose scores in phase 1 indicated cognitive impairment, we implemented a comprehensive neuropsychological assessment of memory, language, visuospatial perception, calculation, abstract reasoning, and executive function. The assessments included paired-associate word learning of The Clinical Memory Test, episodic memory of modified Wechsler Memory Scale, category verbal fluency, the Clock Drawing Test, the trail-making test A of Halstead-Reitan Neuropsychological Battery for Adults, the symbol-digit modalities test, similarity and calculations of the Wechsler Adult Intelligence Scale, and Aphasia Battery of Chinese (ABC) test.55, 56, 57, 58, 59, 60, 61 The spontaneous speech, auditory comprehension, repetition, and naming subtests from ABC were also used for language evaluations.61 In addition, the Neuropsychiatric Inventory (NPI) and the Global Deterioration Scale (GDS) were used to evaluate the neuropsychiatric symptoms and severity of cognitive impairment.62 All neuropsychological instruments used in this study have been validated in the Mandarin language among the Chinese population.50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 In addition to neuropsychological assessment, cases of dementia or MCI were further confirmed by a series of clinical examinations, including the collection of medical history, physical examinations, neurological assessments, laboratory tests (hepato-renal function, folic acid and vitamin B12 levels, thyroid function test, and syphilis antibody test), and neuroimaging scan (computed tomography [CT] or magnetic resonance imaging [MRI]).
A clinical diagnosis of MCI was made using the core clinical criteria recommended by the National Institute on Aging and the Alzheimer's Association workgroup (NIA-AA): (1) cognitive decline compared with the participant's previous level (obtained from the subject or an informant); (2) impairment in one or more cognitive domains (the cutoff scores of neuropsychological tests were 1.5 SD below norms); (3) preservation of independence in functional abilities (ADL score <26, GDS stage 2–3); and (4) not meeting the criteria for dementia.63 The standard diagnostic criteria of dementia were in accordance with the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV)64: impairment in one or more cognitive domains at a level of 2.5 SD or more below norms, and impairment in daily and social functioning (ADL score ≥26, GDS stage 4–7).
Data of PM air pollution/PM exposure assessment
Daily concentrations of PM2.5 and PM10 across China during the study period were estimated at a resolution of 0.1° (≈10 km) using a machine learning method (random forest model) with ground monitoring data, satellite-retrieved aerosol optical depth, and information on other spatial and temporal predictors (see supplementary material). Satellite-based estimation was conducted as previously described.43,44 We estimated participants' daily exposures to PM2.5 and PM10 according to their addresses (latitude and longitude) during the 3 years prior to the survey. Daily estimations were aggregated into the 3-year averages used in this study (see Figure S3 for additional sub-analyses of timescales for PM exposures). The results of 10-fold cross-validation (CV) showed the CV R2 for estimated annual PM2.5 and PM10 concentrations were 86% and 81%, respectively (Table S1).
Measurement of potential covariates
A range of covariates were considered and adjusted for in our analyses. The sociodemographic characteristics collected included age, gender, and educational attainment. Education was measured in years of schooling, which includes both regular and adult education. In descriptive analyses, this was treated as a categorical variable for each individual: years of education >9, or years of education ≤9. Behavioral factors were participation in regular physical and social activities (yes or no), smoking and alcohol consumption (current, former, or never a smoker/drinker). Current drinking was further divided into current drinking and current heavy drinking (average daily alcohol intake greater than five standard drink units) although there were no heavy drinkers found in this population. Physical activity was defined as doing physical exercise for at least 30 min every day at an intensity greater than or equal to walking. Social activity referred to participating in organized activities involving social contacts. Information about patient history of NCDs was also collected, including cardiovascular disease (angina pectoris, myocardial infarction, hypertension, arrhythmia), stroke (cerebral infarction or hemorrhage), transient ischemic attack, depression, metabolic syndrome including diabetes mellitus and hyperlipidemia, COPD, anemia, cataract, glaucoma, hearing disorders, and dementia. Data on sociodemographic factors, history of NCDs, and family history of neuropsychiatric diseases were collected through medical records in the community clinics or by face-to-face interviews conducted by the medical staff who performed dementia and MCI screening as described above.
Statistical analysis
We used logistic regression models to assess the associations of long-term exposure to PM with dementia and MCI. Due to a limited sample size (n = 321), female cases were excluded. Our initial analyses showed that the associations between PM and dementia/MCI were linear (Figure S2). We therefore applied a linear function for PM in the analyses. Three models, which adjusted for different sets of confounding factors, were performed to estimate the associations of different levels of PM with dementia and MCI. Model 1 (referred to as the crude model) includes only one air pollutant (PM2.5 or PM10). Model 2 (partially adjusted model) controls for age, years of education, smoking, and drinking. Model 3 (fully adjusted model) controls for age, years of education, smoking, drinking, family history of dementia, and history of NCDs other than dementia. In all models, the city was also incorporated as a random-effect term to control for the potential regional difference in the association between PM and dementia/MCI. Additional analyses were performed stratified by age, education, smoking, alcohol consumption, physical and social activity, history of diabetes mellitus, hypertension, hyperlipidemia, and cerebral infarction. An interaction term was added to the regression models to assess the significance of interactions. Participants with any of the above variables missing were excluded from the analysis.
Acknowledgments
This work was supported by Special Research Project on Health Care, Health Sector of the General Logistics Department of Peoples Liberation Army (project numbers: 07BJZ04, 10BJZ19, 11BJZ09, and 12BJZ46), the National Natural Science Foundation of China (project number: 81701067) and the National Science and Technology Support Program (project number: 2013BAI09B14). S.L. was supported by an Early Career Fellowship of the Australian National Health and Medical Research Council (APP1109193). Y.G. was supported by Career Development Fellowships of the Australian National Health and Medical Research Council (APP1163693).
Author contributions
J.T. designed and carried out the study, collected the data, and reviewed and revised the manuscript. N.L. and X.W. conducted the statistical analysis and drafted, reviewed, and revised the manuscript. G.C. conducted satellite-based estimation. L.Y. and Y.Z. critically reviewed and revised the manuscript. S.L. conducted the map drawing work. L.-n.W. and Y.G. conceptualized and designed the study, coordinated and supervised the data collection, and critically reviewed and revised the manuscript.
Declaration of interests
The authors declare no competing interests.
Published Online: July 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xinn.2021.100147.
Contributor Information
Luning Wang, Email: lnw_301@163.com.
Yuming Guo, Email: yuming.guo@monash.edu.
Lead contact website
https://research.monash.edu/en/persons/yuming-guo
Supplemental information
References
- 1.Edelman P., Satcher D. Violence prevention as a public health priority. Health Aff. 1993;12:123–125. doi: 10.1377/hlthaff.12.4.123. [DOI] [PubMed] [Google Scholar]
- 2.Gong P., Liang S., Carlton E.J., et al. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson C. Martin Prince, Adelina Comas-Herrera, Martin Knapp, et al. World Alzheimer Report 2016: Improving healthcare for people living with dementia. Coverage, quality and costs now and in the future. London, England: Alzheimer’s Disease International. 2016 [Google Scholar]
- 4.Porcelli S., Calabro M., Crisafulli C., et al. Alzheimer's disease and neurotransmission gene variants: focus on their effects on psychiatric comorbidities and inflammatory parameters. Neuropsychobiology. 2019;78:79–85. doi: 10.1159/000497164. [DOI] [PubMed] [Google Scholar]
- 5.Moulton P.V., Yang W. Air pollution, oxidative stress, and Alzheimer's disease. J. Environ. Public Health. 2012;2012:472751. doi: 10.1155/2012/472751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heusinkveld H.J., Wahle T., Campbell A., et al. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology. 2016;56:94–106. doi: 10.1016/j.neuro.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Calderon-Garciduenas L., Reed W., Maronpot R.R., et al. Brain inflammation and Alzheimer's-like pathology in individuals exposed to severe air pollution. Toxicol. Pathol. 2004;32:650–658. doi: 10.1080/01926230490520232. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraj R.L., Rodriguez E.A., Wang Y., Block M.L. Outdoor ambient air pollution and neurodegenerative diseases: the neuroinflammation hypothesis. Curr. Environ. Health Rep. 2017;4:166–179. doi: 10.1007/s40572-017-0142-3. [DOI] [PubMed] [Google Scholar]
- 9.Calderón-Garcidueñas L., de la Monte S.M. Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: recipe for Alzheimer's disease development in Mexico city young females. J. Alzheimers Dis. 2017;58:613–630. doi: 10.3233/JAD-161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oudin A., Forsberg B., Adolfsson A.N., et al. Traffic-related air pollution and dementia incidence in northern Sweden: a longitudinal study. Environ. Health Perspect. 2016;124:306–312. doi: 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calderón-Garcidueñas L., Villarreal-Ríos R. Living close to heavy traffic roads, air pollution, and dementia. Lancet. 2017;389:675–677. doi: 10.1016/S0140-6736(16)32596-X. [DOI] [PubMed] [Google Scholar]
- 12.Bourdrel T., Bind M.A., Bejot Y., et al. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017;110:634–642. doi: 10.1016/j.acvd.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crichton S., Barratt B., Spiridou A., et al. Associations between exhaust and non-exhaust particulate matter and stroke incidence by stroke subtype in south London. Sci. Total Environ. 2016;568:278–284. doi: 10.1016/j.scitotenv.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Hoek G., Krishnan R.M., Beelen R., et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. J. Environ. Health Perspect. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Y., Qi J., Ruan Z., et al. Changes in life expectancy of respiratory diseases from attaining daily PM2.5 standard in China: a nationwide observational study. Innovation. 2020;1:100064. doi: 10.1016/j.xinn.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russ T.C., Reis S., van Tongeren M. Air pollution and brain health: defining the research agenda. Curr. Opin. Psychiatry. 2019;32:97–104. doi: 10.1097/YCO.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 17.Paul K.C., Haan M., Mayeda E.R., Ritz B.R. Ambient air pollution, noise, and late-life cognitive decline and dementia risk. Annu. Rev. Public Health. 2019;40:203–220. doi: 10.1146/annurev-publhealth-040218-044058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters R., Ee N., Peters J., et al. Air pollution and dementia: a systematic review. J. Alzheimers Dis. 2019;70:S145–S163. doi: 10.3233/JAD-180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung C.R., Lin Y.T., Hwang B.F. Ozone, particulate matter, and newly diagnosed Alzheimer's disease: a population-based cohort study in Taiwan. J. Alzheimers Dis. 2015;44:573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 20.Ranft U., Schikowski T., Sugiri D., et al. Long-term exposure to traffic-related particulate matter impairs cognitive function in the elderly. J. Environ. Res. 2009;109:1004–1011. doi: 10.1016/j.envres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Tzivian L., Dlugaj M., Winkler A., et al. Long-term air pollution and traffic noise exposures and mild cognitive impairment in older adults: a cross-sectional analysis of the Heinz Nixdorf recall study. Environ. Health Perspect. 2016;124:1361–1368. doi: 10.1289/ehp.1509824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y.C., Lin Y.C., Yu H.L., et al. Association between air pollutants and dementia risk in the elderly. J. Alzheimers Dement. (Amst). 2015;1:220–228. doi: 10.1016/j.dadm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudin A., Segersson D., Adolfsson R., Forsberg B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in northern Sweden. PLoS One. 2018;13:e0198283. doi: 10.1371/journal.pone.0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H., Kwong J.C., Copes R., et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet. 2017;389:718–726. doi: 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- 25.Loop M.S., Kent S.T., Al-Hamdan M.Z., et al. Fine particulate matter and incident cognitive impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. J. PLoS One. 2013;8:e75001. doi: 10.1371/journal.pone.0075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schikowski T., Vossoughi M., Vierkötter A., et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. J. Environ. Res. 2015;142:10–16. doi: 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Ailshire J.A., Clarke P. Fine particulate matter air pollution and cognitive function among U.S. older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2015;70:322–328. doi: 10.1093/geronb/gbu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ailshire J.A., Crimmins E.M. Fine particulate matter air pollution and cognitive function among older US adults. Am. J. Epidemiol. 2014;180:359–366. doi: 10.1093/aje/kwu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgart, M., Snyder, H.M., Carrillo, M.C., et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement.. 11, 718-726. [DOI] [PubMed]
- 30.Amanollahi M., Amanollahi S., Anjomshoa A., Dolatshahi M. Mitigating the negative impacts of aging on cognitive function; modifiable factors associated with increasing cognitive reserve. Eur. J. Neurosci. 2021;53:3109–3124. doi: 10.1111/ejn.15183. [DOI] [PubMed] [Google Scholar]
- 31.Chen R., Wilson K., Chen Y., et al. Association between environmental tobacco smoke exposure and dementia syndromes. Occup. Environ. Med. 2013;70:63–69. doi: 10.1136/oemed-2012-100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orsitto G., Turi V., Venezia A., et al. Relation of secondhand smoking to mild cognitive impairment in older inpatients. ScientificWorldJournal. 2012;2012:726948. doi: 10.1100/2012/726948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Block M.L., Elder A., Auten R.L., et al. The outdoor air pollution and brain health workshop. Neurotoxicology. 2012;33:972–984. doi: 10.1016/j.neuro.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderón-Garcidueñas L., Solt A.C., Henríquez-Roldán C., et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 35.Calderón-Garcidueñas L., Mora-Tiscareño A., Ontiveros E., et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. J. Brain Cogn. 2008;68:117–127. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Block M.L., Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S.H., Knight E.M., Saunders E.L., et al. Rapid doubling of Alzheimer's amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F Res. 2012;1:70. doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cesaroni G., Forastiere F., Stafoggia M., et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Künzli N., Perez L., von Klot S., et al. Investigating air pollution and atherosclerosis in humans: concepts and outlook. Prog. Cardiovasc. Dis. 2011;53:334–343. doi: 10.1016/j.pcad.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Wilker E.H., Preis S.R., Beiser A.S., et al. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46:1161–1166. doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Q., Amini H., Shi L., et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ. Int. 2019;130:104909. doi: 10.1016/j.envint.2019.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei J., Li Z., Cribb M., et al. Improved 1 km resolution PM2.5 estimates across China using enhanced space–time extremely randomized trees. Atmos. Chem. Phys. 2020;20:3273–3289. [Google Scholar]
- 43.Chen G., Li S., Knibbs L.D., et al. A machine learning method to estimate PM2.5 concentrations across China with remote sensing, meteorological and land use information. Sci. Total Environ. 2018;636:52–60. doi: 10.1016/j.scitotenv.2018.04.251. [DOI] [PubMed] [Google Scholar]
- 44.Chen G., Wang Y., Li S., et al. Spatiotemporal patterns of PM10 concentrations over China during 2005–2016: a satellite-based estimation using the random forests approach. Environ. Pollut. 2018;242:605–613. doi: 10.1016/j.envpol.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Power M.C., Adar S.D., Yanosky J.D., Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: a systematic review of epidemiologic research. Neurotoxicology. 2016;56:235–253. doi: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salinas-Rodriguez A., Fernandez-Nino J.A., Manrique-Espinoza B., et al. Exposure to ambient PM2.5 concentrations and cognitive function among older Mexican adults. Environ. Int. 2018;117:1–9. doi: 10.1016/j.envint.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 47.Chen G., Wang A., Li S., et al. Long-term exposure to air pollution and survival after ischemic stroke: the China National Stroke Registry cohort. Stroke. 2019;50:563–570. doi: 10.1161/STROKEAHA.118.023264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan J., Li N., Gao J., et al. Construction of the Chinese Veteran Clinical Research (CVCR) platform for the assessment of non-communicable diseases. Chin Med. J. (Engl). 2014;127:448–456. [PubMed] [Google Scholar]
- 49.Tan J., Li N., Gao J., et al. Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. J. Alzheimers Dis. 2015;43:1403–1412. doi: 10.3233/JAD-141278. [DOI] [PubMed] [Google Scholar]
- 50.Nasreddine Z.S., Phillips N.A., Bedirian V., et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M., Yu E., He Y. Tools for dementia epidemiological investigations and their applications. Shanghai Arch. Psychiatry. 1995;7:1–62. [Chinese] [Google Scholar]
- 52.Zhang Z., Hong X., Li H. The mini-mental state examination in the Chinese residents population aged 55 years and over in the urban and rural areas of Beijing. Chin. J. Neurol. 1999;32:149–153. [Chinese] [Google Scholar]
- 53.Wu W., Zhang M., Yu Q., et al. The applications of the Center for Epidemiological Studies Depression Scale (CES-D) among the elderly population in the communities. J. Shanghai Arch. Psychol. 1989;7:139–142. [Chinese] [Google Scholar]
- 54.Li H., Wang Y., Huang S., et al. Application of Montreal Cognitive Assessment in screening mild cognitive impairment in elderly patients. Zhonghua Shen Jing Yi Xue Za Zhi. 2009;8:376–379. [Chinese] [Google Scholar]
- 55.Gong Y., Jiang D., Deng J. Hunan Med College; 1989. Manual of Modified Wechsler Memory Scale (WMS) [Chinese] [Google Scholar]
- 56.Guo Q.H., Jin L.L., Hong Z. A specific phenomenon of animal fluency test in Chinese elderly. Chin J. Ment. Health. 2007;21:622–625. [Chinese] [Google Scholar]
- 57.Gong Y. The Chinese revision of Halstead-Reitan neuropsychological test battery for adults. Acta Psychol. Sin. 1986;4:433–442. [Chinese] [Google Scholar]
- 58.Gong Y. Hunan Med College; 1982. Manual of Modified Wechsler Adult Intelligence Scale (WAIS-RC) [Chinese] [Google Scholar]
- 59.The Clinical Memory Test (CMT) group The construction of “the clinical memory test”. Acta Psychol. Sin. 1986;1:100–108. [Chinese] [Google Scholar]
- 60.Meng C., Z X., Wang H., et al. The clock drawing test for detecting cognitive impairment. Chin J. Nerv Ment. Dis. 2004;6:452–454. [Chinese] [Google Scholar]
- 61.Gao S.R. Peking University Medical Press; 2006. Aphasia. [Chinese] [Google Scholar]
- 62.Xie H.G., Wang L.N., Yu X., et al. Neuropsychiatric symptoms in dementia and elderly people in the community: results from the Beijing Dementia Cooperative Study. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25:829–832. [Chinese] [PubMed] [Google Scholar]
- 63.Albert M.S., DeKosky S.T., Dickson D., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.American Psychiatric Association . American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.