Abstract
Aging can alter immunity affecting host defense. COVID-19 has the most devastating clinical outcomes in older adults, raising the implication of immune aging in determining its severity and mortality. We investigated biological predictors for clinical outcomes in a dataset of 13,642 ambulatory and hospitalized adult COVID-19 patients, including younger (age < 65, n = 566) and older (age ≥ 65, n = 717) subjects, with in-depth analyses of inflammatory molecules, cytokines and comorbidities. Disease severity and mortality in younger and older adults were associated with discrete immune mechanisms, including predominant T cell activation in younger adults, as measured by increased soluble IL-2 receptor alpha, and increased IL-10 in older adults although both groups also had shared inflammatory processes, including acute phase reactants, contributing to clinical outcomes. These observations suggest that progression to severe disease and death in COVID-19 may proceed by different immunologic mechanisms in younger versus older subjects and introduce the possibility of age-based immune directed therapies.
Keywords: Human, COVID-19, Aging, Immune, Clinical outcome
1. Introduction
Two prominent immune changes that occur with age are expansion of memory T cells with senescent features and persistence of a state of chronic low grade inflammation called inflammaging, which is associated with an increase in circulating levels of innate cytokines [[1], [2], [3]]. Age-related changes in the adaptive immunity, including T cell responses, can contribute to the increased susceptibility of older individuals to infection, while a dysregulation in innate immunity and its inflammatory pathways may promote more severe collateral end-organ damage [[1], [2], [3], [4]]. The possible detrimental impact of immune aging on host defense against microorganism seeks its support from the observation of the increased mortality in older adults with COVID-19.
Studies have shown the association of disease severity and mortality of COVID-19 with patient demographics, clinical characteristics, and various laboratory values and immunological profiles. In fact, advanced age has emerged as the most important clinical predictor for poor outcome and death in COVID-19 [5,6]. Although the exact mechanisms for this observation remains unclear, there is evidence for associations with a heightened inflammatory response leading to multi-organ dysfunction and mortality in patients with COVID-19. There is also evidence that immunosenescence changes contribute to the development and the progression of such age-associated conditions as atherosclerosis, cardiovascular disease, and type 2 DM [4,7], which are all chronic diseases associated with poor clinical outcomes of COVID-19 [6,[8], [9], [10]]. These observations raise the possibility of a role for immune system aging in the development of distinct inflammatory responses in older versus younger subjects that may influence clinical outcome from COVID-19.
Prior studies investigated the relationship of age and comorbidities with inflammatory status and clinical outcomes in patients with COVID-19 [8,9,11], however these studies were generally conducted in a small number of subjects, lacked a direct comparison between younger and older patients, explored only a limited number of comorbidities, and did not include a detailed analysis of inflammatory markers. To better address the influence of age, especially in the context of pre-existing comorbidities and other risk factors, in the development of unique inflammatory responses that may be associated with different clinical outcomes in COVID-19, we investigated clinical and biological predictors for outcomes in a dataset of 13,642 ambulatory and hospitalized adult patients from a single health care center, including younger (age < 65, n = 566) and older (age ≥ 65, n = 717) subjects, by conducting an in-depth analyses of inflammatory molecules, cytokines, and comorbidities collected on the basis of the Elixhauser comorbidity index [12].
2. Materials and methods
2.1. Data collection and ethics
Subjects within the Yale New Haven Health System (YNHHS) who had the positive COVID19 result based on a reverse transcription quantitative polymerase chain reaction assay between March and October 2020 were eligible for inclusion in the study. Medical records were from the EPIC electronic medical record (Epic Systems Corporation Verona, Wisconsin USA), and the study was approved by the Institutional Review Board (IRB) of Yale University. Clinical data were obtained from the Department of Medicine COVID Explorer (DOM-CovX) research database and the YNHHS Joint Data Analytics Team. A subset of admitted patients also had extensive laboratory parameters available; admitted patients had complete blood count with differential completed daily and cytokine levels obtained every other day starting with the day of admission and continuing through discharge or death during their COVID-19 hospitalization. Minimum and maximum laboratory parameter values were based on all of the available results from COVID-19 hospitalization Comorbidities were assessed using coding from the International Classification of Diseases 10 (ICD-10) codes and the Elixhauser comorbidity classification schema [12]. The severity of COVID-19 at admission was graded as severe vs. non-severe disease [13]. Patients with severe COVID-19 had one of the following: (1) tachypnea, respiratory rate ≥ 30/min or (2) resting O2 Sat ≤93% (finger pulse oximetry) at the first presentation to the hospital. A set of cytokines was analyzed as a part of clinical care using a quantitative multiplex bead assay by a CLIA-certified diagnostic lab in Salt Lake City, UT. Lower limit of detection was 5 pg/mL.
2.2. Clustering and principal component analyses
Unbiased hierarchical clustering and principal component analyses were conducted using the publicly available R-based Pheatmap (version 1.0.12), R Stats (version 4.0.2), ggbiplot (version 0.55), factorextra (version 1.0.7), and FactoMineR (version 2.4) packages.
2.3. Statistical analysis
A descriptive analysis for demographic characteristics was performed using the two-sample t-test and chi-square test. Receiver Operating Characteristic (ROC) Curve analysis was performed to determine the discriminability of demographic characteristics on hospitalization and mortality. We selected the demographic characteristics with significant bivariate correlations and developed a multivariable logistic model to examine adjusted effects on hospitalization and mortality. Interaction between significant characteristics were also examined. We estimated odds ratio (OR) to measure the risk of hospitalization and mortality with and without adjusting for covariates. The multivariable logistic regression analysis was used to determine the association of variables with disease outcomes. All statistical tests were performed at 5% significance level. The ANOVA with the Sidak multiple comparison test was used for comparing continuous variables in multiple groups. Data were analyzed with SAS (SAS Institute, Cary, North Carolina), SPSS version 26 (IBM, Armonk, New York) and Prism 8 software (GraphPad Software, Inc., San Diego, California).
3. Results
3.1. The number and types of preexisting comorbidities directly relate to hospitalization and mortality in COVID-19, and are enhanced by age
We first determined whether the interface of age and comorbidities directly related to hospitalization and mortality in a large data set of COVID-19 cohort (n = 13,642). Age and the number of comorbidities were strong discriminable factors for hospitalization and mortality even after controlling other risk factors including body mass index, gender and race (Table 1 , Supplementary Table 1, Supplementary Fig. 1). The greater risk of death was observed in those aged 65 or older (OR = 17.8, 95% CI = [14.5, 21.8]) and those with 7 or more comorbidities (OR = 9.7, 95% CI = [8.1, 11.6]). The patients who were aged 65 or older with 7 or more comorbidities were at a higher risk of death (OR = 20.7, 95% CI = [17.4, 24.6]) compared to those who had only one or none of the conditions. We explored the categories of pre-existing comorbidities that were significantly associated with hospitalization and mortality after controlling for age, gender and the number of comorbidities. Electrolyte disorder, neurologic disease, uncomplicated DM and weight loss were significantly associated with both hospitalization and mortality while complicated DM and renal failure were additionally associated with hospitalization and mortality, respectively (Supplementary Table 2). Overall, our data indicate that age, number and types of preexisting comorbidities are associated with hospitalization and mortality in COVID-19.
Table 1.
Logistic Regression for Hospitalization and Mortality.
| Prediction of Hospitalization |
Prediction of Death |
|||
|---|---|---|---|---|
| Odds Ratio [95% CI] | P-value | Odds Ratio [95% CI] | P-value | |
| Age (per 10 years) | 1.390 [1.349, 1.431] | <0.0001 | 2.133 [1.987, 2.289] | <0.0001 |
| BMI | 0.996 [0.990, 1.002] | 0.2324 | 1.006 [0.993, 1.020] | 0.3445 |
| N. of Chronic Conditions | 1.138 [1.128, 1.148] | <0.0001 | 1.075 [1.426, 2.039] | <0.0001 |
| Gender: (Male vs. Female) | 1.474 [1.345, 1.615] | <0.0001 | 1.705 [1.426, 2.039] | <0.0001 |
| Race | ||||
| Black vs. Non-Hispanic White | 0.701 [0.622, 0.790] | <0.0001 | 1.011 [0.801, 1.275] | 0.1217 |
| Hispanic vs. Non-Hispanic White | 1.107 [0.975, 1.257] | 0.997 [0.739, 1.345] | ||
| Asian vs. Non-Hispanic White | 1.092 [0.790, 1.509] | 2.117 [1.140, 3.932] | ||
| Other vs. Non-Hispanic White | 0.943 [0.734, 1/210] | 0.704 [0.352, 1.409] | ||
| Area Under ROC | 0.763 [0.754, 0.773] | 0.884 [0.873, 0.894] | ||
3.2. Global relationship of inflammatory responses with age, gender, race, comorbidities, clinical course and outcome
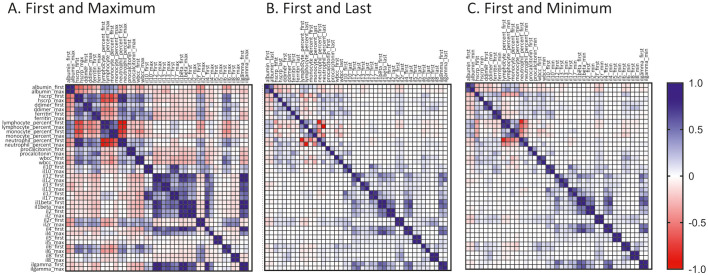
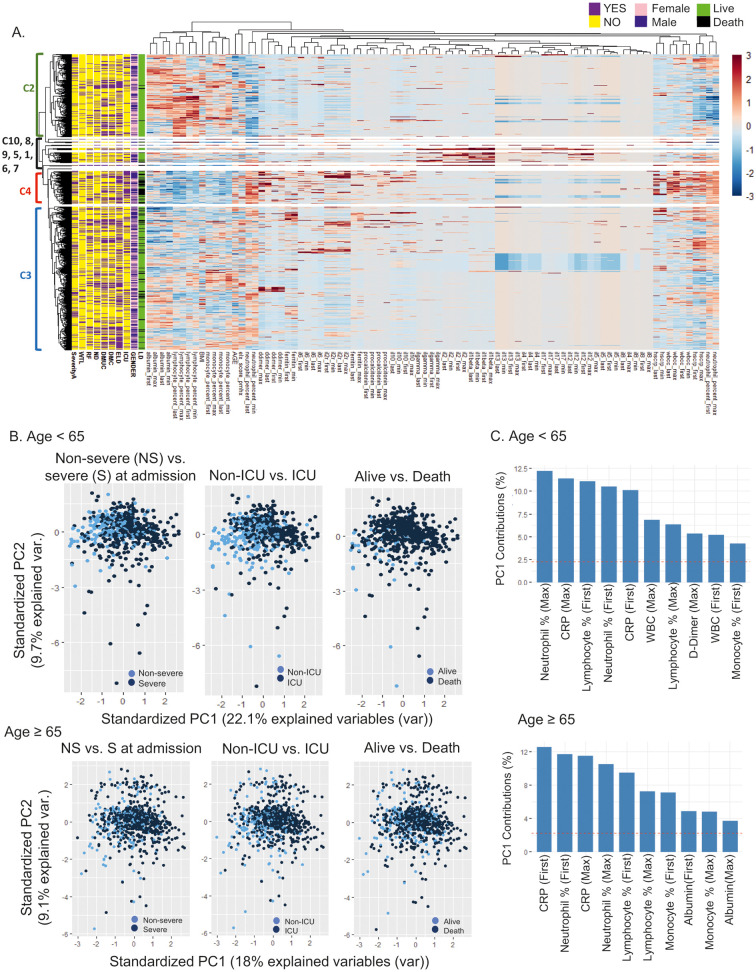
A subset of hospitalized patients (n = 1283) in our cohort had in-depth clinical laboratory testing for inflammation and circulating cytokines at multiple time points during hospitalization. We explored whether age, gender, number, race and types of comorbidities had any relationship with the first, maximum, minimum, and last values of laboratory tests in the context of clinical outcome. The relationship of first and maximum values of individual measurements appeared stronger than those of first and last or minimum values (Fig. 1 ). An unbiased hierarchical clustering analysis showed clustering of different laboratory measurements and individual patients based on the levels of such measurements (Fig. 2A, heatmap). A cluster of deceased patients who presented with severe disease at admission and were admitted to the ICU (Cluster 4, n = 154, mean age ± SD = 69.2 ± 15.9) was associated with low lymphocyte and monocyte percentages but high neutrophil percentages and high total white blood cell (WBC) count. Low circulating levels of albumin and high circulating levels of d-dimer, ferritin, procalcitonin, and CRP also were associated with this cluster. Among measured inflammatory mediators, high levels of IL-6, sIL-2Rα and IL-10 but relatively low levels of IL-4, IL-5 and IL-13 were evident in Cluster 4. Of note, patients in Cluster 2 (n = 387) who often had severe disease at admission and were frequently admitted to the ICU but survived appeared to be relatively young (mean age ± SD = 58.8 ± 16.6) and to have fewer comorbidities, with higher lymphocyte and monocyte percentages, a lower neutrophil percentages, lower levels of total WBC, and lower levels of CRP when compared to Cluster 4. Cluster 3 (n = 673, mean age ± SD = 69.3 ± 15.7) had the largest number of both survived and deceased patients. In this cluster, the levels of inflammatory mediators were mostly between Clusters 2 and 4, suggesting heterogeneity in the expression of these molecules. The rest of the clusters generated by an unbiased hierarchical analysis is a small group of patients (n = 69, mean age ± SD = 68.5 ± 15.5) with high levels of circulating IFN-γ, IL-12, IL-1β, IL-17 and IL-2 who had mixed clinical courses and outcomes. Overall, a large set of patients with different clinical outcomes can be clustered into two groups (e.g., Clusters 2 and 4) based on contrasting patterns of age, number of comorbidities, and altered circulating inflammatory markers although there is also a group of patients (Cluster 3) with heterogeneous levels of these parameters.
Fig. 1.
The relationships of first, maximum, minimum and last values of clinical laboratory tests and circulatory cytokines in a set of 1283 hospitalized adult patients with COVID-19. Pearson correlation coefficients were obtained to assess the relationship of first, maximum, minimum and last values of indicated laboratory tests and circulatory cytokines in 1283 hospitalized adult patients. The correlation coefficients were shown in matrix plots for (A) first and maximum values, (B) first and last values, and (C) first and minimum values.
Fig. 2.
A set of inflammatory tests and circulatory cytokines segregates patients with COVID-19 into subsets with distinct clinical characteristics. (A) Heatmap showing the results of an unbiased hierarchical clustering analysis of inflammatory tests and cytokines in 1283 hospitalized COVID-19 patients with distinct clinical characteristics, including mortality (LD, live vs. death), gender, ICU care (ICU), electrolyte disorders (ELD), diabetes mellitus complicated (DMC), DM uncomplicated (DMUC), neurologic diseases (ND), renal failure (RF), weight loss (WTL) and severity at admission (severityA, severe vs. not severe). Y, yes. N, No. F, female. M, male. L, live. D, dead. C, cluster. The cluster number of 10 was pre-selected. (B) Principal component analyses were done on younger (age < 65, n = 566) and older (age ≥ 65, n = 717) patients with COVID-19, respectively, based on age, BMI, the Elixhauser comorbidity index, clinical laboratory tests and cytokines (first and maximum values). PCA plots showing segregations of survived vs. deceased, non-ICU vs. ICU, and non-severe vs. severe patients in younger and older patients. (C) Top 10 contributing variables to PC 1 in younger and older adults. Red lines indicate the averages of the contributions of the top 10 variables. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Younger and older COVID-19 patients have both shared and distinct inflammatory processes contributing to clinical outcomes
Age is a strong risk factor for COVID-19 hospital admission and mortality [5]. Notably, aging is associated with both immunosuppression and a state of chronic, low-grade inflammation or inflammaging [[1], [2], [3], [4]]. Accordingly, we explored the relationship between individual inflammatory markers and clinical outcomes from COVID-19 in our cohort of 1283 patient cohort of older (age ≥ 65) and younger (age < 65) subjects. We selected age 65 as the cutoff dividing older vs. younger subjects given its frequent use as a demarcator in aging studies [14] and our finding on the relationship of aging with mortality in COVID-19. The numbers of patients who received hydroxychloroquine, glucocorticoids and/or tocilizumab were similar between the younger and older groups (Supplementary Table 3) although remdesivir was more frequently used in younger patients; this may reflect the higher frequency of relative contraindications such as impaired renal function in the older adults. We first performed PCA analysis to determine the relationship of individual laboratory tests and cytokine levels with disease severity at admission, intensive care unit (ICU) care and mortality in younger and older adults. In both age groups, patients who had severe disease at admission, required ICU care and/or died were largely segregated from other patients based on PC1 (Fig. 2B). The top 10 variables contributing to PC1 in both younger and older groups were similar, including CRP levels and neutrophil, lymphocyte, and monocyte percentages, although WBC counts (first and max), d-dimer (max) and albumin (first and max) were distinct in the two groups (Fig. 2C). The variables contributing to PC2 included cytokines; however, PC2 hardly separated patients with different clinical outcomes (Fig. 2B).
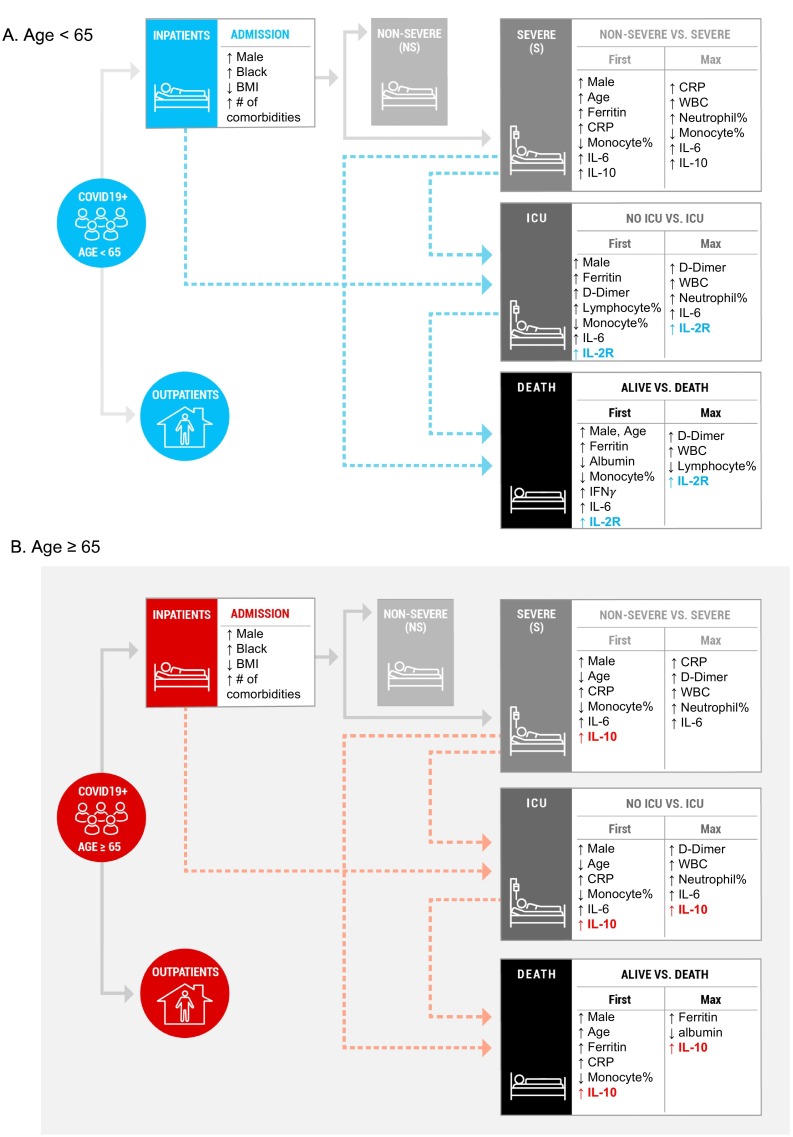
We next investigated the relationship of disease severity at admission, ICU care and mortality with clinical and laboratory parameters in younger and older adults using multivariable logistic regression analysis (Fig. 3 , Supplementary Tables 4–5). Of interest, lower scores of the Elixhauser comorbidity index were associated with severe disease at admission in younger adults but not in older adults; this could be due to different thresholds for younger patients in seeking medical care. In younger and older adults, high levels of first and maximum CRP values were associated with disease severity at admission. However, the relationship of high levels of first CRP values with ICU care and mortality was found in older but not in younger adults.
Fig. 3.
Relationship of disease severity at admission, ICU care and mortality with clinical and laboratory data in younger and older groups. Multivariable logistic regression analysis was done with for five sets of data between non-severe vs. severe at admission, no ICU care vs. ICU care and alive vs. death in (A) younger (age < 65, n = 566) and (B) older (age ≥ 65, n = 717) adults. Five sets included (1) age, gender, number and types of commodities, (2) first and (3) maximum values of WBC, % of neutrophils, monocytes and lymphocytes, albumin, CRP, ferritin, d-dimer, procalcitonin, and (4) first and (5) maximum levels of 12 cytokines (included IL-1β, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, Il-17, IFN-γ) . Only significantly increased or decreased values are shown in the figure. Arrows directing up and down indicate increased and decreased levels, respectively, in patients with severe disease at admission, ICU care, and mortality.
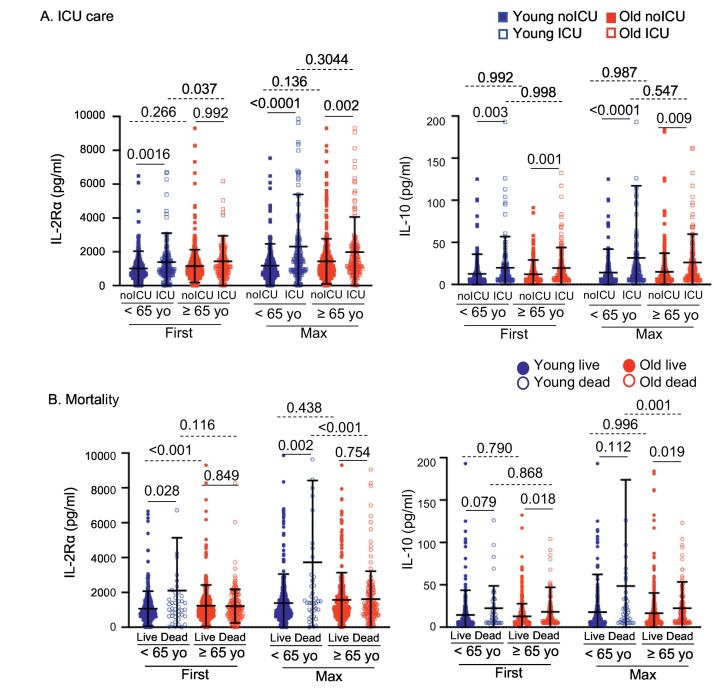
With respect to cytokine analysis, high levels of first IL-10 values in older adults were associated with disease severity at admission, ICU care and mortality, and there was a further association between high levels of maximum IL-10 values and ICU care and mortality. However, in younger adults, high levels of first and maximum IL-10 values were associated only with disease severity at admission but not with other clinical outcomes. In younger but not in older adults, high levels of first and/or maximum sIL-2Rα were associated with ICU care and mortality. Indeed, increased levels of maximum sIL-2Rα in deceased patients were found only in younger patients but not in older patients while increased IL-10 levels in deceased patients were observed only in older patients but not in younger patients (Fig. 4 ). In addition, survived and deceased patients in the older but not the younger group had similar levels of IL-6 (Supplementary Fig. 2). The levels of CRP, d-dimer, ferritin and procalcitonin were higher in younger deceased patients than in older deceased patients while the differences in these molecules between younger and older survived patients were either less or not significant (Supplementary Fig. 2). Overall, these findings suggest that younger and older adults have not only shared but also distinct immunologic responses to SARS-Cov-2 infection that may contribute to disease severity, ICU care and mortality.
Fig. 4.
Circulating levels of soluble IL-2Rα and IL-10 show distinctive associations with ICU care and mortality in younger and older survived and deceased patients with COVID-19. Scatter graphs showing circulating levels of sIL-2Rα and IL-10 in (A) ICU admitted and non-admitted younger and older patients with COVID-19, and (B) survived and deceased younger (age < 65, n = 566) and older (age ≥ 65, n = 717) patients with COVID-19. P-values were obtained by the ANOVA with the Sidak multiple comparison test as a post-hoc analysis.
4. Discussion
The number of individuals over age 65 is increasing rapidly worldwide with a substantial impact on public health, especially with respect in this population to enhanced susceptibility to infectious and inflammatory diseases. Age-associated changes in the immune system contribute to increased risk of infection and subsequent mortality [[1], [2], [3], [4]]. The frequency of naïve T cells decreases with age while the frequency of memory T cells, especially those with the senescent features, increases [2,3]. Notably, a dysregulated inflammatory response or inflammaging also occurs in older subjects [1,4,7]. Although many hospitalized and deceased COVID-19 patients are older adults, the effect of aging on the development of inflammatory responses and its relationship with clinical outcomes in the context of multiple comorbidities in COVID-19 is largely unknown. To better address this point, we investigated clinical and biological predictors for clinical outcomes in a dataset of 13,642 ambulatory and hospitalized adult COVID-19 patients, including younger (age < 65, n = 566) and older (age ≥ 65, n = 717) subjects, with in-depth analyses of circulatory inflammatory molecules, cytokines and comorbidities. The results of our study suggest that progression to severe disease and death in COVID-19 may proceed by different immunologic mechanisms in younger versus older subjects, and introduce the possibility of age-based immune directed therapies.
In COVID-19, advanced age has been shown in multiple studies to be the strongest risk factor for poor clinical outcomes [5,8,9,11]. Several clinical laboratory tests were found to be altered in patients with COVID-19, often correlating with clinical outcomes. These include acute phase reactants such as CRP, ferritin, albumin, and d-dimer as well as WBC count and differentials, including neutrophils, lymphocytes and monocytes [6,[15], [16], [17]]. Circulatory levels of IL-6 and IL-10 are higher in severe than in mild COVID-19, and there is evidence that IL-6 and TNF-α levels are independent predictors of disease severity and death [18,19]. The results of our study support these findings although a combination of such measurements can divide patients into additional groups, even after considering age and comorbidities, as shown in our clustering analysis. The contribution of individual clinical laboratory and cytokine measurements thus appear to be different for predicting clinical outcomes. In both younger and older adults, CRP, neutrophil and lymphocyte percent were tests with the highest level of contribution to the PC1 that separated patients with distinct outcomes based on ICU care and mortality. This relationship suggests the utility of these laboratory measurements for predicting clinical outcomes and potentially in assessing the activation of inflammatory pathways that may influence these outcomes irrespective of patient age. Notably, the first and maximum values of inflammatory laboratory tests and cytokines correlated strongly, suggesting that the overall course and prognosis of COVID-19 may be determined early in the course of the disease, which is of important clinical utility.
Activated T cells upregulate their cell surface expression of the high-affinity IL-2Rα, which is then proteolytically cleaved to release a soluble form (e.g., sIL-2Rα) [20]. Circulating sIL-2Rα can attenuate the activity of the T cell growth factor IL-2 [20]. Increased circulating levels of sIL-2Rα are observed in diseases such as hemophagocytic lymphohistiocytosis and macrophage activation syndrome [20]. Notably, in both hemophagocytic lymphohistiocytosis and macrophage activation syndrome, excessive T cell activation underlies the development of a lethal hyperinflammatory response. Elevated levels of sIL-2Rα have been reported to be associated with disease severity, duration of illness, and prolonged viral shedding in COVID-19 patients, and may be expected in the context of prolonged T cell activation and subsequent apoptosis, leading to lymphopenia and ultimately, the lymphoid atresia reported in terminal disease [[21], [22], [23]]. Notably, we found the relationship of sIL-2Rα with ICU care and mortality in younger but not older adults, suggesting the possible pathologic implication of sustained T cell activation in younger adults.
In our study, high levels of first and maximum IL-10 values in older adults were associated with disease severity at admission (only first values), ICU care and mortality. Our results are in line with a recent meta-analysis showing IL-10 as a covariate predicting disease severity [24]. A study reported an association of circulating IL-10 levels with age in 142 adult patients with COVID-19 after adjusting for comorbidity and disease severity [25]. The exact biological significance of such a relationship in COVID-19 is yet to be elucidated although several possibilities exist. IL-10 is generally considered an anti-inflammatory cytokine with the capacity to limit pro-inflammatory responses and inhibit the activity of T cells, natural killer (NK) cells and macrophages that play an important role in clearing a viral infection [26]. Increased IL-10 levels in COVID-19 could be a counter-regulating phenomenon in response to excessive inflammation, which also in turn may limit the ability of the host to eliminate the virus. This notion can be supported by an increase in the proportion of IL-10-secreting regulatory T cells correlating with disease severity in COVID-19 patients [27]. On the other hand, IL-10 also has been suggested as an immune activating and proinflammatory cytokine in autoimmunity and cancers [[28], [29], [30]]. Of note, pegylated IL-10 infusion enhanced CD8+ T cell activity with elevation of IFN-γ and granzyme B, expansion of peripheral and intratumoral IFN-γ+CD8+ T cells, and proliferation and expansion of peripheral LAG-3+PD-1+ CD8+ T cells in patients with renal cell cancer [29]. In a human endotoxemia study of healthy subjects, recombinant IL-10 was shown to enhance lipopolysaccharide (LPS)-induced IFN-γ release as well as increase the soluble granzymes reflecting enhanced activation of cytotoxic CD8+ T cells and NK cells [31]. A potential pathological role of increased IL-10 levels in COVID-19 has been suggested based on these findings [30]. Aging is accompanied by an expansion of cytotoxic and inflammatory effector memory CD8+ T cells with high levels of granzyme B, perforin and IFN-γ expression which can contribute to aging gene signature in peripheral blood [14,32,33]. Thus, the possible proinflammatory effect of IL-10 on the immune system, especially CD8+ T cells, could be greater in older adults.
A recent meta-analysis identified that IL-6 and IL-10 levels were covariates for predicting disease severity [24]. We also found an association of increased levels of IL-6 with disease severity at admission and ICU care in both younger and older COVID-19 patients. However, IL-6 levels were similar between survived and deceased patients in the older but not younger group, suggesting the reduced ability of this cytokine to discriminate between clinical outcomes in younger versus older subjects. Taken together, younger and older adults with COVID-19 express both shared and distinct features of the immune response that may be associated with poor clinical outcomes, especially ICU care and subsequent mortality.
We recognize limitations of our study. The study subjects are from a single health system, which could confer biases. Our conclusions are limited to laboratory markers that were available for analysis, and additional inflammatory molecules may participate in influencing age-related clinical outcomes in COVID-19. Additional studies are warranted to further these points.
In summary, an analysis of a dataset of 13,642 patients with COVID-19, including 566 younger and 717 older ones with an in-depth assessment of inflammatory molecules, verify the importance of age, comorbidities, and discrete inflammatory markers in predicting hospitalization and subsequent mortality. Our results show that increased levels of circulatory sIL-2Rα and IL-10 which are associated with disease progression to ICU care and mortality in younger and older adults, respectively, may distinguish the immunologic responses of younger versus older patients with COVID-19. These findings raise the consideration of selected immune therapies for COVID-19 in patients at risk for clinical disease progression and mortality based on their age.
Authorship contributions
JJS performed the chart review, designed the study, analyzed and interpreted the results, participated in writing the manuscript; SJ designed the study, analyzed and interpreted and participated in writing the manuscript, SU, JP-U, JK, YA, YK, MS performed the chart review and participated in writing the manuscript, MSS analyzed and interpreted the results and participated in writing the manuscript, GB and RB participated in designing the study and writing the manuscript, IK designed the study, analyzed and interpreted the results, participated in writing the manuscript and supervised the research.
Research funding
This work was supported in part by grants from the National Institutes of Health (1R01AG056728 and R01AG055362 to IK).
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgements
Special thanks go to Soundari Sureshanand and Richard Hintz from the Joint Data Analytics Team at the Yale Center for Clinical Investigation for their proficient and efficient handling of data extraction and reporting.
Data extraction was also supported by the Yale School of Medicine, Department of Internal Medicine, Clinical and Translational Research Accelerator. The content is solely the responsibility of the authors and does not necessarily represent the official views of the members of the Clinical and Translational Research Accelerator.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108857.
Appendix A. Supplementary data
Supplementary material
References
- 1.Shaw A.C., Goldstein D.R., Montgomery R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee N., Shin M.S., Kang I. T-cell biology in aging, with a focus on lung disease. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:254–263. doi: 10.1093/gerona/glr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikolich-Zugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- 4.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018;15:505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Team C.C.-R., Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) —United States, February 12–March 16 MMWR Morb mortal Wkly rep. Volume. 2020;69(2020) doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prattichizzo F., De Nigris V., La Sala L., Procopio A.D., Olivieri F., Ceriello A. “Inflammaging” as a Druggable target: a senescence-associated secretory phenotype-centered view of type 2 diabetes. Oxidative Med. Cell. Longev. 2016;2016:1810327. doi: 10.1155/2016/1810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and Meta-analysis. Intervirology. 2020:1–12. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 2020;382:2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santesmasses D., Castro J.P., Zenin A.A., Shindyapina A.V., Gerashchenko M.V., Zhang B., Kerepesi C., Yim S.H., Fedichev P.O., Gladyshev V.N. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19 doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med. Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. (e1415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park H.J., Shin M.S., Kim M., Bilsborrow J.B., Mohanty S., Montgomery R.R., Shaw A.C., You S., Kang I. Transcriptomic analysis of human IL-7 receptor alpha (low) and (high) effector memory CD8(+) T cells reveals an age-associated signature linked to influenza vaccine response in older adults. Aging Cell. 2019;18(4):1–14. doi: 10.1111/acel.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paranjpe I., Russak A.J., De Freitas J.K., Lala A., Miotto R., Vaid A., Johnson K.W., Danieletto M., Golden E., Meyer D., Singh M., Somani S., Kapoor A., O'Hagan R., Manna S., Nangia U., Jaladanki S.K., O'Reilly P., Huckins L.M., Glowe P., Kia A., Timsina P., Freeman R.M., Levin M.A., Jhang J., Firpo A., Kovatch P., Finkelstein J., Aberg J.A., Bagiella E., Horowitz C.R., Murphy B., Fayad Z.A., Narula J., Nestler E.J., Fuster V., Cordon-Cardo C., Charney D., Reich D.L., Just A., Bottinger E.P., Charney A.W., Glicksberg B.S., Nadkarni G.N., Sinai C.I.C. Mount. Retrospective cohort study of clinical characteristics of 2199 hospitalised patients with COVID-19 in New York City. BMJ Open. 2020;10:e040736. doi: 10.1136/bmjopen-2020-040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., Xiong L., Guo C., Tian J., Luo J., Yao J., Pang R., Shen H., Peng C., Liu T., Zhang Q., Wu J., Xu L., Lu S., Wang B., Weng Z., Han C., Zhu H., Zhou R., Zhou H., Chen X., Ye P., Zhu B., Wang L., Zhou W., He S., He Y., Jie S., Wei P., Zhang J., Lu Y., Wang W., Zhang L., Li L., Zhou F., Wang J., Dittmer U., Lu M., Hu Y., Yang D., Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin M., Park S., Hayden A., Giustini D., Trinkaus M., Pudek M., Mattman A., Schneider M., Chen L.Y.C. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann. Hematol. 2017;96:1241–1251. doi: 10.1007/s00277-017-2993-y. [DOI] [PubMed] [Google Scholar]
- 21.Ma A., Zhang L., Ye X., Chen J., Yu J., Zhuang L., Weng C., Petersen F., Wang Z., Yu X. High levels of circulating IL-8 and soluble IL-2R are associated with prolonged illness in patients with severe COVID-19. Front. Immunol. 2021;12:626235. doi: 10.3389/fimmu.2021.626235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y., Wang X., Li X., Xi D., Mao R., Wu X., Cheng S., Sun X., Yi C., Ling Z., Ma L., Ning Q., Fang Y., Sun B., Wu D. Potential contribution of increased soluble IL-2R to lymphopenia in COVID-19 patients. Cell. Mol. Immunol. 2020;17:878–880. doi: 10.1038/s41423-020-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q., Shi Y., Cai J., Duan Y., Wang R., Zhang H., Ruan Q., Li J., Zhao L., Ping Y., Chen R., Ren L., Fei X., Zhang H., Tang R., Wang X., Luo T., Liu X., Huang X., Liu Z., Ao Q., Ren Y., Xiong J., He Z., Wu H., Fu W., Zhao P., Chen X., Qu G., Wang Y., Wang X., Liu J., Xiang D., Xu S., Zhou X., Li Q., Ma J., Li H., Zhang J., Huang S., Yao X., Zhou Y., Wang C., Zhang D., Wang G., Liu L., Bian X.-W. Pathological changes in the lungs and lymphatic organs of 12 COVID-19 autopsy cases. Natl. Sci. Rev. 2020;7:1868–1878. doi: 10.1093/nsr/nwaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhar S.K., Damodar S., Gujar S., Das M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: results from meta-analysis and regression. Heliyon. 2021;7:e06155. doi: 10.1016/j.heliyon.2021.e06155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luporini R.L., Rodolpho J.M.A., Kubota L.T., Martin A., Cominetti M.R., Anibal F.F., Pott-Junior H. IL-6 and IL-10 are associated with disease severity and higher comorbidity in adults with COVID-19. Cytokine. 2021;143:155507. doi: 10.1016/j.cyto.2021.155507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojas J.M., Avia M., Martin V., Sevilla N. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017;2017:6104054. doi: 10.1155/2017/6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann J., Prezzemolo T., Vanderbeke L., Roca C.P., Gerbaux M., Janssens S., Willemsen M., Burton O., Van Mol P., Van Herck Y., Wauters J., Wauters E., Liston A., Humblet-Baron S. Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin Transl Immunol. 2020;9:e1204. doi: 10.1002/cti2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian G., Li J.L., Wang D.G., Zhou D. Targeting IL-10 in auto-immune diseases. Cell Biochem. Biophys. 2014;70:37–49. doi: 10.1007/s12013-014-9903-x. [DOI] [PubMed] [Google Scholar]
- 29.Naing A., Infante J.R., Papadopoulos K.P., Chan I.H., Shen C., Ratti N.P., Rojo B., Autio K.A., Wong D.J., Patel M.R., Ott P.A., Falchook G.S., Pant S., Hung A., Pekarek K.L., Wu V., Adamow M., McCauley S., Mumm J.B., Wong P., Van Vlasselaer P., Leveque J., Tannir N.M., Oft M. PEGylated IL-10 (Pegilodecakin) induces systemic immune activation, CD8(+) T cell invigoration and polyclonal T cell expansion in Cancer patients. Cancer Cell. 2018;34:775–791. doi: 10.1016/j.ccell.2018.10.007. (e773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L., Zhang H., Dauphars D.J., He Y.W. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42:3–5. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauw F.N., Pajkrt D., Hack C.E., Kurimoto M., van Deventer S.J., van der Poll T. Proinflammatory effects of IL-10 during human endotoxemia. J. Immunol. 2000;165:2783–2789. doi: 10.4049/jimmunol.165.5.2783. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.R., Hong M.S., Dan J.M., Kang I. Altered IL-7R{alpha} expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood. 2006;107:2855–2862. doi: 10.1182/blood-2005-09-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin M.S., Yim K., Moon K., Park H.J., Mohanty S., Kim J.W., Montgomery R.R., Shaw A.C., Krishnaswamy S., Kang I. Dissecting alterations in human CD8+ T cells with aging by high-dimensional single cell mass cytometry. Clin. Immunol. 2019;200:24–30. doi: 10.1016/j.clim.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material