Abstract
Postural orthostatic tachycardia syndrome (POTS) is a chronic and often disabling disorder characterized by orthostatic intolerance with excessive heart rate increase without hypotension during upright posture. Patients often experience a constellation of other typical symptoms including fatigue, exercise intolerance and gastrointestinal distress. A typical patient with POTS is a female of child-bearing age, who often first displays symptoms in adolescence. The onset of POTS may be precipitated by immunological stressors such as a viral infection. A variety of pathophysiologies are involved in the abnormal postural tachycardia response; however, the pathophysiology of the syndrome is incompletely understood and undoubtedly multifaceted.
Clinicians and researchers focused on POTS convened at the National Institutes of Health in July 2019 to discuss the current state of understanding of the pathophysiology of POTS and to identify priorities for POTS research. This article, the first of two articles summarizing the information discussed at this meeting, summarizes the current understanding of this disorder and best practices for clinical care.
The evaluation of a patient with suspected POTS should seek to establish the diagnosis, identify co-morbid conditions, and exclude conditions that could cause or mimic the syndrome. Once diagnosed, management typically begins with patient education and non-pharmacologic treatment options. Various medications are often used to address specific symptoms, but there are currently no FDA-approved medications for the treatment of POTS, and evidence for many of the medications used to treat POTS is not robust.
Keywords: Postural orthostatic tachycardia syndrome, Pathophysiology, Treatment, Workshop, Expert consensus
1. Background
Postural orthostatic tachycardia syndrome (POTS) is common but the exact prevalence has not been determined, and the cause of this condition remains unclear. Since POTS predominantly affects adolescent or young adult women who would typically be in the midst of education or early careers, the condition can be severely debilitating and economically devastating (Bagai et al., 2011; Bourne et al., 2021a). Although there is a consensus clinical definition for POTS, misdiagosis is common. The syndrome is heterogeneous in the sense that the spectrum of clinical features varies among patients, multiple etiologies may produce similar clinical phenotype and there is overlap with other clinically-defined syndromes. The clinical evaluation of patients with suspected POTS is not standardized, nor are treatment approaches. There is a lack of familiarity with POTS in the medical community, and the epidemiology of the disorder and natural history are not known (Raj and Robertson, 2018).
The US Congress directed the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Neurological Disorders and Stroke (NINDS) to provide a forum for leading POTS experts to meet and discuss the current state of POTS research and clinical care (U.S. Senate Committee on Appropriations, 2018). A workshop including a broad representation of clinicians and researchers took place on July 29, 2019 at the NIH Intramural campus. To supplement the report prepared by NIH, the workshop participants prepared two independent manuscripts to summarize their findings. This manuscript outlines the current state of the science and clinical care for POTS. This is not meant to be a comprehensive review of the literature but rather an overview from the workshop participants. The accompanying manuscript addresses the current knowledge gaps, clinical needs and research priorities for POTS (Raj et al., 2021). We hope that these manuscripts will raise awareness in the medical community, stimulate research and improve clinical care for POTS.
2. Clinical description
POTS is a complex, multi-system, chronic disorder of the autonomic nervous system characterized by orthostatic intolerance with excessive heart rate (HR) increase and symptoms on standing while blood pressure is maintained. Orthostatic symptoms improve rapidly after return to a supine position. Apart from the abnormal circulatory response to standing, patients with POTS may experience a variety of other persistent symptoms such as low energy, headache, cognitive impairment, muscle fatigue, chest pain, non-specific generalized weakness, along with numerous gastrointestinal symptoms (Benarroch, 2012; Brignole et al., 2018; Fedorowski, 2019; Freeman et al., 2011; Raj, 2013; Sheldon et al., 2015).
POTS has been defined by major international neurologic, autonomic, cardiac and pediatric societies (Freeman et al., 2011; Raj et al., 2020; Sheldon et al., 2015) as requiring:
A sustained HR increment of not less than 30 beats/minute within 10 min of standing or head-up tilt. For individuals who are 12 to 19 years old, the required HR increment is at least 40 beats/minute; and
An absence of orthostatic hypotension (i.e. no sustained systolic blood pressure [BP] drop of 20 mmHg or more); and
Frequent symptoms of orthostatic intolerance during standing, with rapid improvement upon return to a supine position. Symptoms may include lightheadedness, palpitations, tremulousness, generalized weakness, blurred vision, and fatigue; and
Duration of symptoms for at least 3 months; and
Absence of other conditions explaining sinus tachycardia such as anorexia nervosa, primary anxiety disorders, hyperventilation, anemia, fever, pain, infection, dehydration, hyperthyroidism, pheochromocytoma, use of cardioactive drugs (e.g. sympathomimetics, anticholinergics) or severe deconditioning caused by prolonged bed rest.
3. Epidemiology & natural history
The prevalence of POTS has not been properly studied. Estimates, based largely on clinical experience, range from 0.2% and 1% of the US population, which would suggest approximately 1–3 million affected persons (Arnold et al., 2018; Low et al., 2009; Sheldon et al., 2015). A typical patient with POTS is a 12 to 50 years old female (F:M ratio > 4:1) (Arnold et al., 2018; Sheldon et al., 2015). Similarly, our current understanding of the natural history of POTS is derived from incomplete data from clinical experience, small case series or uncontrolled patient- reported data. In a large survey of over 4800 patients with a self- reported POTS diagnosis, the most common age at onset was 14 years (Shaw et al., 2019). The onset of POTS may be precipitated by a typical immunological stressor such as viral syndrome (often upper respiratory or gastrointestinal), physical trauma (such as concussion), menarche, pregnancy, or surgery (Boris and Bernadzikowski, 2018; Low et al., 1995; Mathias et al., 2011; Sandroni et al., 1999; Thieben et al., 2007; Watari et al., 2018). An antecedent history of suspected viral infection is reported in 20–50% of patients (Sandroni et al., 1999; Shaw et al., 2019; Watari et al., 2018). The presentation seems to have two patterns – acute onset after one of the above triggers or with slowly progressive symptoms over a longer period of time (Thieben et al., 2007). Significant symptomatic recovery has been reported by a subset of patients, but a majority report chronic symptoms with recurrent exacerbations. The natural history of POTS over the later decades of life has not been studied.
4. Clinical associations & co-morbidities
A variety of other clinical diagnoses may coexist with POTS, but it is largely unclear whether the presence of one of these other diagnoses defines a unique pathophysiological subset of POTS. Patients with POTS may simultaneously meet the diagnostic criteria for migraine, hypermobile Ehlers-Danlos syndrome (hEDS), mast cell activation syndrome (MCAS) or chronic fatigue syndrome (CFS) (Kavi et al., 2016; McDonald et al., 2014; Okamoto et al., 2012; Shaw et al., 2019). The estimated frequencies of these clinical associations vary, and careful systematic assessments to identify these other disorders have not been done in a large POTS population. Current estimates derived from small samples or uncontrolled survey data suggest that approximately 40% of POTS patients experience migraine, 20–30% meet the diagnostic criteria for hypermobile Ehlers-Danlos syndrome (Miller et al., 2020; Roma et al., 2018), and about 15% carry a diagnosis of a co-morbid autoimmune disease. Some POTS patients endorse symptoms suggestive of abnormal mast cell activation but again accurate data on the frequency of MCAS in POTS is not available (Shaw et al., 2019). This subset of POTS patients commonly report episodes of flushing, urticaria, dyspnea, headache, excessive diuresis, and gastrointestinal symptoms such as diarrhea, nausea, and vomiting, which may be accompanied by elevated urine methylhistamine or 11-ß-Prostaglandin F2 excretion or elevation of other mast cell mediators (Shibao et al., 2005; Weinstock et al., 2020). Conversely, orthostatic intolerance and tachycardia can be found in up to 40% of patients with hypermobility spectrum disorder or hypermobile Ehlers-Danlos Syndrome (Miller et al., 2020; Roma et al., 2018).
A summary of comorbid conditions identified in the large survey of self-identified POTS patients16 is shown in Table 1.
Table 1.
Co-morbidities in patients diagnosed with Postural Orthostatic Tachycardia Syndrome.
| Comorbidity | Number (%) (of 3933 respondents) |
|---|---|
|
| |
| Migraine headaches | 1557 (40%) |
| Irritable bowel syndrome | 1192 (30%) |
| Ehlers-Danlos syndrome | 994 (25%) |
| Chronic Fatigue Syndrome | 809 (21%) |
| Asthma | 798 (20%) |
| Fibromyalgia | 786 (20%) |
| Raynaud’s phenomena | 610 (16%) |
| Iron deficiency anemia | 628 (16%) |
| Gastroparesis | 548 (14%) |
| Vasovagal syncope | 499 (13%) |
| Inappropriate sinus tachycardia | 448 (11%) |
| Mast cell activation disorder | 353 (9%) |
| Autoimmune disease | 616 (16%) |
| Hashimoto’s thyroiditis | 228 (6%) |
| Celiac Disease | 133 (3%) |
| Sjogren’s Syndrome | 112 (3%) |
| Rheumatoid Arthritis | 93 (2%) |
| Lupus | 81 (2%) |
| Other | 160 (4%) |
5. Gastrointestinal dysfunction in POTS
Gastrointestinal functions are intricately controlled and regulated by the autonomic nervous system and its specialized gastrointestinal specific subsystem, the enteric nervous system (ENS) (Furness, 2012).
Gastrointestinal symptoms are commonly reported by individuals with autonomic disorders, including POTS (Sullivan et al., 2005). Clinical diagnostic studies to identify gastrointestinal dysfunction are limited; however, gastrointestinal dysmotility is frequently diagnosed in POTS (DiBaise et al., 2018; Zhang et al., 2019). Patients with mild dysmotility as well as those with complete intestinal failure requiring total parental nutrition or surgery have been observed. The pathophysiology and natural history of gastrointestinal dysfunction in POTS has not been studied. It is intuitive to speculate that dysautonomia may disrupt the intricate neuroimmunophysiology of the gastrointestinal system and gastrointestinal dysfunction may ensue. Further research is needed about how the autonomic, ENS and immune systems interact with each other and with the host microbiota, in order to develop better diagnostic tests and potential new treatments.
6. Potential pathophysiological mechanisms
Clinical observations may show a post-viral onset (Low et al., 1995; Schondorf and Low, 1993) suggesting the possibility of an immunological cause. Positive (though often non-specific) autoantibody tests are frequently seen among POTS patients, and about 20% report a history of an autoimmune disorder such as Hashimoto’s thyroiditis, rheumatoid arthritis or Sjogren syndrome (¨ Blitshteyn, 2015; Shaw et al., 2019). In recent years, a focus of POTS research has been on autoantibodies to cardiovascular G-protein coupled membrane receptors (see below) (Blitshteyn, 2015; Dahan et al., 2016; Fedorowski et al., 2017; Gunning et al., 2019; Li et al., 2014; Ruzieh et al., 2017a; Schofield and Chemali, 2019; Vernino and Stiles, 2018; Wang et al., 2013; Watari et al., 2018; Xichun et al., 2020).
Other proposed pathophysiological mechanisms include abnormally increased sympathetic activity and circulating catecholamine excess (“hyperadrenergic POTS”) (Jacob and Biaggioni, 1999; Schondorf and Low, 1993), peripheral sympathetic noradrenergic denervation leading to venous pooling and relative central hypovolemia (“neuropathic POTS”) (Istvan and Roy, 2004; Jacob et al., 2000; Peltier et al., 2010), and low blood volume (absolute hypovolemia). In the “hyperadrenergic” subtype, patients often complain of tremor, anxiety, migraine and angina-like chest pain (Grubb, 2008). In the “neuropathic” subtype, clinical features of small fiber neuropathy may be present while reduced cardiac preload may lead to a compensatory HR increase during orthostatic challenge. Many POTS patients have a blood volume that is lower than expected for their size and sex, suggesting that hypovolemia may underlie some of the hemodynamic findings of POTS (Fu et al., 2010; Mar and Raj, 2014; Raj et al., 2005).
Cardiovascular deconditioning (objectively demonstrated by low cardiac stroke volume and reduced cardiac mass) may also contribute in a significant way to the pathophysiology of POTS and exacerbate the symptomatology (Fu et al., 2010). Prolonged bed rest from any cause can itself result in cardiovascular deconditioning and orthostatic intolerance, and should be considered before making a diagnosis of POTS.
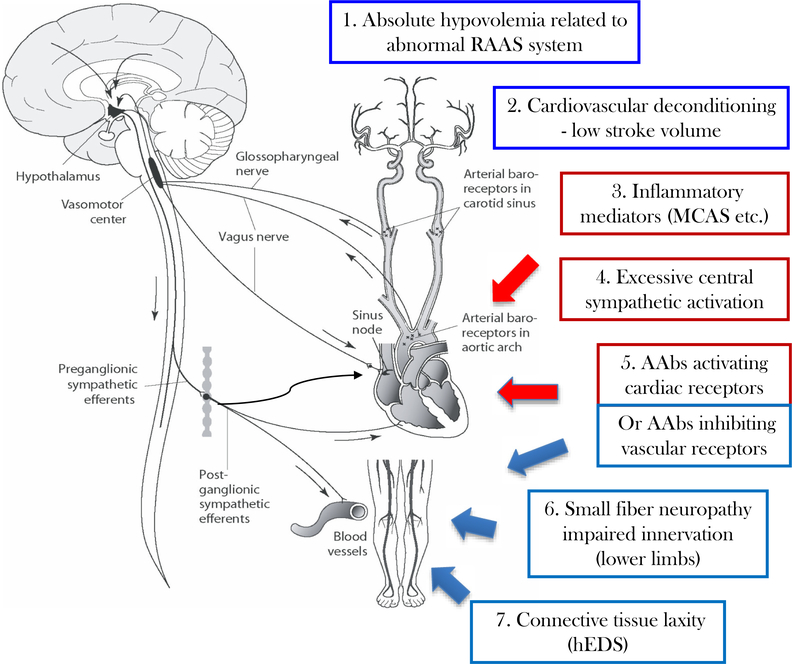
These pathophysiological mechanisms are neither fully inclusive, nor completely exclusive. Fig. 1 summarizes the heterogenous and overlapping potential pathophysiological mechanisms in POTS. Many patients have features indicative of more than one mechanism. At this time, there are no data that long-term prognosis or response to therapies differ based on specific pathophysiological features.
Fig. 1.
Schematic of possible mechanisms leading to orthostatic intolerance and tachycardia in POTS. During upright posture, there is a gravitational shift of plasma volume toward the lower parts of the body which if unopposed would result in reduced cardiac preload and a fall in blood pressure. The autonomic baroreflex serves to prevent orthostatic hypotension and preserve cardiac output through sympathetic activation (peripheral vasoconstriction and increased heart rate). In POTS, excessive orthostatic tachycardia may result from a combination of appropriate autonomic responses to various physiological changes (shown in blue) or an inappropriate exaggeration of the sympathetic response to orthostatic stress (shown in red).
Abnormal cardiovascular physiology could include (1) absolute hypovolemia due to impaired regulation of plasma volume or (2) cardiovascular deconditioning resulting in reduced cardiac mass and low stroke volume. Excessive sympathetic activation may occur in the context of (3) a systemic inflammatory state with increased inflammatory mediators (for example, increased histamine in conditions of mast cell overactivity) or (4) increased sympathetic tone driven by central nervous system (e.g. anxiety or chronic pain). Autoantibodies targeting G-protein coupled autonomic receptors (5) could produce mixed effects by acting as partial agonists that both augment cardiac sympathetic signals and reduce the efficacy of norepinephrine-induced peripheral vasocontriction. Finally, abnormal peripheral vascular function may result from (6) peripheral small fiber neuropathy causing partial denervation in the lower extremities or (7) tissue laxity resulting in increased dependent venous pooling (which might explain an association of POTS with hEDS).
RAAS = renin-angiotensin-aldosterone system; MCAS = mast cell activation syndrome; AAbs = autoantibodies; hEDS = hypermobile form of Ehlers-Danlos syndrome. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
7. Cardiovascular pathophysiology
A wide variety of cardiovascular pathophysiological mechanisms may lead to an abnormal postural tachycardia response. Importantly, these mechanisms may not be mutually exclusive. The hemodynamic properties of POTS identify closely with those seen in moderate hemorrhage in that central hypovolemia, reduced cardiac output, and increased adrenergic-mediated vasoconstriction occur (Fu et al., 2010; Stewart, 2012), while BP is maintained and may even increase (orthostatic hypertension) (Grubb et al., 1997). Reduced systemic venous return and reduced cardiac output contribute to reduced central blood volume when upright,(Barcroft et al., 1944) and absolute hypovolemia can be found (Fouad et al., 1986; Raj and Robertson, 2007; Raj et al., 2005) similar to bed-rested patients or astronauts with gravitational deconditioning (Montgomery et al., 1993). In others, redistributive central hypovolemia when upright occurs due to decreased lower extremity venous tone (Stewart and Montgomery, 2004), or splanchnic pooling (Stewart et al., 2006a). These cases may correlate with “neuropathic POTS”(Stewart et al., 2006b; Tani et al., 2000), in which local sympathetic noradrenergically-mediated vasoconstriction is impaired because of autonomic denervation or a biochemical milieu that locally reduces vasoconstriction, causing baroreflex unloading, decreased baroreflex-cardiovagal function, increased reflexive sympathetic noradrenergic excitation at other sites (Istvan and Roy, 2004), and augmented reflex tachycardia (Stewart and Weldon, 2001).
While a “hyperadrenergic” state with orthostatic hypertension is defined in some cases, there is scant evidence for enhanced sympathetic outflow from the central nervous system as a primary cause. Most investigations show exaggerated skeletal muscle sympathetic nerve activity while upright (Muenter Swift et al., 2005), consistent with reflexive activation. In addition, cardiac parasympathetic deficits (Jacob et al., 2019; Stewart, 2000) may be found in many individuals with POTS leading to relative excess of “sympatho-vagal balance” based on various measures including power spectral analysis of heart rate variability.
8. Pathophysiology of plasma volume and renin-angiotensin system
Absolute hypovolemia is commonly observed in POTS, with up to 70% of patients exhibiting deficits in plasma volume and red blood cell volume (Fu et al., 2010; Raj et al., 2005; Sheldon et al., 2015; Stewart et al., 2006a). This hypovolemia can reduce stroke volume and lead to compensatory tachycardia to maintain cardiac output and BP. The importance of hypovolemia in POTS pathophysiology is illustrated by the finding that some patients have reduced orthostatic tachycardia and improved symptoms after acute plasma volume expansion (e.g. intravenous saline, the vasopressin analog desmopressin, exercise training) (Coffin et al., 2012; Fu et al., 2010; Jacob et al., 1997; Ruzieh et al., 2017b). Ongoing studies are examining the impact of increasing plasma volume with dietary sodium, chronic intravenous saline, or albumin infusions in POTS. While useful as rescue therapy in clinically decompensated patients, long-term intravenous saline infusions are not currently recommended for routine care due to complications associated with chronic venous catheterization (Sheldon et al., 2015).
A normal compensatory response to hypovolemia includes activation of the renin-angiotensin aldosterone system (RAAS) to increase angiotensin II, which stimulates aldosterone release to promote renal sodium and water reabsorption and restore blood volume. While circulating angiotensin II levels are high, POTS patients have impaired ability of the RAAS to expand blood volume (Li et al., 2015a; Mustafa et al., 2011; Raj et al., 2005; Stewart et al., 2006b). Some POTS patients have reduced plasma renin activity, reduced aldosterone and normal BP, suggesting a primary abnormality of the RAAS or of renal sodium and water homeostasis (Garland et al., 2007; Mustafa et al., 2012, 2011; Raj et al., 2005). Initial studies also show reduced levels and actions of angiotensin converting enzyme 2 (ACE2) and angiotensin-(1–7) in POTS, components of the RAAS that oppose deleterious cardiovascular actions of angiotensin II (Raj et al., 2005; Stewart et al., 2009). While these findings suggest RAAS perturbations contribute to underlying POTS pathophysiology, it remains unknown whether targeting this hormone system restores blood volume and improves orthostatic tolerance in patients with hypovolemia in the setting of POTS.
9. Antibodies and immunology of POTS
Associations between immune dysfunction and POTS remains poorly understood. Both pediatric and adult POTS patients may report an infectious prodrome before the emergence of orthostatic symptoms (Boris and Bernadzikowski, 2018; Shaw et al., 2019). A variety of infectious pathogens have been proposed to be linked to POTS, including Epstein Barr virus (Pohlgeers and Stumbo, 2016; Yaxley, 2020), Mycoplasma pneumoniae (Kasmani et al., 2009), and recently SARS-CoV2 (Goldstein, 2020; Kanjwal et al., 2020; Miglis et al., 2020).
POTS patients (Vernino and Stiles, 2018) and their close relatives (Boris et al., 2020; Shaw et al., 2019) have a higher than expected prevalence of autoimmune disorders including celiac disease (Penny et al., 2016), Hashimoto’s thyroiditis (Blitshteyn, 2015), Sjögren’s syndrome (Goodman et al., 2017), and systemic lupus erythematosus (SLE) (Tang et al., 2004). Anecdotal reports and small open-label clinical studies (Weinstock et al., 2018) suggest a beneficial effect of intravenous immunoglobulin (IVIG) therapy in POTS, suggesting that immunomodulatory therapy can play a role in treatment, but there have been no controlled clinical trial data published to date.
Serum immunoglobulins isolated from POTS patients have been reported to act as partial agonists to both α- and β-adrenergic receptors in vitro (Fedorowski et al., 2017; Li et al., 2014), a finding independently confirmed by several other groups (Vernino and Stiles, 2018) and expanded to include a wider range of G-protein coupled receptors (GPCRs) as targets (Kharraziha et al., 2020). These reports have employed transfected cell assays and enzyme-linked immunoassays, as well as functional tests utilizing rat cardiomyocytes and smooth muscle, to detect antibodies that target cellular receptors for norepinephrine (Fedorowski et al., 2017; Gunning et al., 2019; Li et al., 2014; Watari et al., 2018), acetylcholine (Fedorowski et al., 2017; Gunning et al., 2019; Li et al., 2015b; Watari et al., 2018), and angiotensin II (Xichun et al., 2020). In most of these studies, similarly reactive antibodies have been found in healthy control groups, although less frequently or at lower levels than in POTS patients. Preliminary studies using active immunization animal models have supported a potential role of the adrenergic autoantibodies in the pathophysiology of POTS (Li et al., 2019). Despite these observations, a causal role of these autoantibodies in POTS is not established.
10. Genetics
Although there is no evidence for a monogenetic cause of POTS, increased POTS prevalence in family members suggests the presence of genetic predisposition. Case series have identified both familial cases (Shannon et al., 2000) and associated polymorphisms in several candidate genes (Garland et al., 2005; Jacob et al., 2006). As many as 14% of POTS patients have a family member with POTS, 31% have a family member with orthostatic intolerance, 20% have a family member with joint hypermobility, and 45% have a family member with autoimmune disease (Boris et al., 2020). Genome-wide association studies in POTS have not been reported. In one family, a rare mutation of norepinephrine-transporter gene was found, resulting in reduced synaptic norepinephrine reuptake and excessive sympathetic activation (Shannon et al., 2000). In a small study, a single-nucleotide polymorphism of the G-protein β3 subunit C825T was more frequently found in POTS (45.8%) than in controls (20.0%) and was associated with a greater increase in HR on standing.(Nakao et al., 2012) Human- leukocyte antigen variants associated with autoimmune diseases have been overrepresented in POTS (Orban et al., 2020; Shin et al., 2019). Some POTS patients have a single nucleotide polymorphism in the endothelial nitric oxide synthase gene (Garland et al., 2005).
11. Pediatric considerations
Although clinical manifestations of POTS are similar in children compared to adults, there are a few important differences. The sensitivity and specificity of the pediatric heart rate criteria are not established, and POTS remains undefined under age 12 years despite patients under 12 presenting with similar syndromic findings, particularly in the setting of a familial predisposition. Some patients anecdotally report chronic symptoms since infancy or toddlerhood, raising the question of additional genetic factors. A unique factor of all chronic medical illnesses in childhood is parental involvement (Padilla-Walker and Nelson, 2012). Parents of these patients can be strong advocates but may also may have their own medical and/or psychosocial issues that impact the treatment of their child (Boris, 2018).
12. Clinical evaluation of POTS
The evaluation of a patient with suspected POTS should establish the diagnosis, identify co-morbid conditions, and exclude conditions that could cause or mimic the autonomic syndrome. Detailed clinical history must establish the symptoms of orthostatic intolerance, the severity and extent of other autonomic issues, other possible arrhythmia, possible contributory effects of drugs or dietary supplement, and associated comorbid disorders (Goodman, 2018). The physical examination should include postural vital signs in order to confirm exaggerated orthostatic tachycardia (and absence of orthostatic hypotension), careful cardiac examination to exclude gross structural heart disease, neurological examination to identify any features of peripheral neuropathy, and an assessment for clinical features of hypermobile Ehlers-Danlos syndrome. Laboratory studies should, at a minimum, include a complete blood count, electrolytes, thyroid function tests, and an electrocardiogram. Other laboratory tests will often be required, but this should be driven by the patient’s clinical presentation.
While POTS can usually be identified by recording HR and BP while supine and during a 10-minute standing test (with minimal stimulation), more detailed autonomic function testing including tilt table testing can be useful, particularly when other conditions such as neurocardiogenic syncope or peripheral autonomic neuropathy are suspected, or when a patient is unable to perform a 10-minute standing test. Tests that measure the integrity of the cardiovagal and peripheral vasomotor adrenergic responses will be normal in most, but not all, POTS patients (Goodman, 2018). Abnormal sudomotor tests, such as the quantitative sudomotor axon reflex test (QSART) or abnormal intraepidermal nerve fiber density on skin biopsy may be seen in patients with underlying small fiber neuropathy (“neuropathic POTS”). The significance of these findings in terms of prognosis and treatment merits further research.
13. Non-pharmacologic management of POTS
Patient education is a key aspect of the management of POTS. Acute worsening of symptoms may be avoided by appropriate attention to environmental temperature, excessive exercise, or in the post-prandial period after eating. Medications that can worsen sinus tachycardia and orthostatic tachycardia should be withdrawn when possible. These include stimulant medications (Fedorowski et al., 2017), and norepinephrine reuptake inhibitors (Green et al., 2013), among others. Measures to increase effective blood volume and exercise to increase cardiovascular conditioning are very important (Miller and Raj, 2018). In some cases, non-pharmacological interventions (Table 2) are sufficiently effective (Raj et al., 2020).
Table 2.
Treatments for postural tachycardia syndrome.
| Drug | Dosing information | Side effects | Precautions |
|---|---|---|---|
|
| |||
| Non-pharmacological treatments | |||
| Withdraw exacerbating medications | Stop drugs that decrease blood volume or directly increase heart rate | ||
| Increased oral water intake | Target 2–3 L/day | Frequent urination | |
| Increased oral NaCl intake | Target 8–10 g/day | Hypertension, peripheral edema | Buffered NaCl tablets/solutions if cannot be done with diet alone |
| Lower body compression garments | 20–40 mmHg compression; focus on abdomen +/− legs | Can be hot, tight, and itchy | |
| Exercise training | Aerobic: 30+ min 4 days/week with some leg resistance training | Will initially feel poorly/worse for up to 6 weeks | Initial recumbent exercise (rowing, recumbent cycle or swimming) |
| Pharmacological treatments | 0.1 to 0.2 mg daily | Hypokalemia, edema, headache | Electrolytes must be monitored |
| Blood volume expanders | |||
| Fludrocortisone | |||
| Desmopressin (DDAVP) | 0.1 to 0.2 mg as needed | Hyponatremia, edema | Monitor electrolytes |
| Acute IV saline | 2 L Intravenous over 2–3 h | Venous thrombosis, infection | |
| Chronic IV saline | 2 L given intravenously once weekly | Infection/thrombosis risk of central venous catheters | Avoid long-term use; avoid placement of central catheters |
| Erythropoietin | 10,000 IU weekly | Increased risk of cardiovascular death | Monitor Hematocrit |
| Heart rate inhibitors | |||
| Propranolol | 10–20 mg orally up to 4 times daily | Hypotension, bradycardia, bronchospasm | Can worsen asthma or exercise tolerance |
| Ivabradine | 2.5–7.5 mg orally twice daily | headaches, palpitations, hypertension, visual disturbances | |
| Pyridostigmine | 30–60 mg orally up to 3 times daily | Abdominal cramps, diarrhea | Can worsen asthma |
| Vasoconstrictors | |||
| Midodrine | 2.5–15 mg orally 3 times daily | Headache, scalp tingling, hypertension | |
| Octreotide | Long-acting intramuscular injection 10–30 mg | Nausea, stomach cramps, diarrhea | |
| Methylphenidate | 10 mg orally 2–3 times a day. Last dose should be avoided before bed. | Tachycardia, insomnia, nausea, headache, dizziness | |
| Sympatholytic drugs | |||
| Alpha-2 adrenergic agonists, such as clonidine | 0.1–0.2 mg orally 2–3 times daily or long acting patch | Hypotension, fatigue, brain fog | |
| Methyldopa | 125–250 mg orally twice daily | Hypotension, fatigue, brain fog | |
| Other | |||
| Modafinil | 50–200 mg orally 1–2 times daily | Tachycardia | |
Modified with permission from Miller and Raj (2018).
To expand blood volume, patients should have a minimum intake of 2 to 3 L of water per day along with increased sodium intake (Raj et al., 2020; Sheldon et al., 2015). Most clinicians recommend oral sodium intake to avoid potential complications of intravenous access. Sodium intake can be increased to 3 to 10 g daily using ordinary table salt (1 tsp. is approximately 2.3 g sodium), salt tablets or electrolyte solutions (Raj et al., 2020). Further research is needed to establish the true therapeutic benefit of salt loading and the optimal sodium intake. In addition to increased water and sodium intake, measures to reduce venous pooling in the lower limbs or splanchnic circulation can be accomplished using waist-high compression stockings with or without abdominal compression (Bourne et al., 2021b), or with an abdominal binder alone (Smith et al., 2020).
Exercise training should be a component of any POTS treatment plan. Along with increased water and salt intake, a progressive exercise program with recumbent aerobic exercise and leg resistance training transitioning to upright exercise has been shown to improve quality of life and reduce orthostatic HR in many patients (Fu and Levine, 2018, 2015; George et al., 2016; Sheldon et al., 2015; Shibata et al., 2012). Best practices for long-term management of POTS patients after improvement have not been determined.
14. Pharmacologic management of POTS
Pharmacologic therapy (Table 2) has largely been directed toward improving orthostatic tolerance in POTS. Therapies may also be directed at disabling autonomic and non-autonomic symptoms as well as comorbid conditions such as gastrointestinal dysmotility, immune disorders, sleep, pain, cognitive dysfunction (“brain fog”) and headache. Therapies directed at improving orthostatic tolerance can be broadly characterized as targeting blood volume expansion, HR modulation, and BP stabilization. At this point, there are no FDA-approved medications for the treatment of POTS, and more importantly evidence of efficacy for many of the medications used to treat POTS is not robust.
Fludrocortisone is frequently used in the treatment of POTS patients for the purposes of volume expansion, though evidence supporting its use is quite limited (Fortunato et al., 2014). There is no evidence that POTS patients who are hypovolemic respond better to fludorocortisone than do POTS patients who are normovolemic. There is also no evidence that fludrocortisone actually increases blood volume. For fludrocortisone to work, the patient must be on a high sodium diet, with appropriate monitoring of serum potassium. Beta-adrenoreceptor antagonists have often been used to treat POTS, with propranolol (Raj et al., 2009), metoprolol, bisoprolol, and atenolol used most commonly (Abe et al., 2000; Chen et al., 2011; Freitas et al., 2000; Lai et al., 2009; Lin et al., 2015; Zhang et al., 2014; Zhao et al., 2014). A recent clinical trial found propranolol and bisoprolol to have equal efficacy, with no significant benefit of pyridostigmine added to the beta-blocker (Moon et al., 2018). A recent meta-analysis of ivabradine, a “funny channel” blocker that modulates cardiac sinus node activity, suggested benefit in lowering HR and providing symptomatic relief in POTS patients (Gee et al., 2018). Midodrine, an oral alpha-adrenoceptor agonist prodrug, is used to promote venoconstriction and vasoconstriction in POTS patients and has shown benefit in small studies (Lai et al., 2009; Zhang et al., 2012). Additional studies are necessary to provide better evidence of the efficacy of these medications in the treatment of POTS and whether particular clinical phenotypes favor certain therapies. Further research is needed to explore novel pharmacological interventions that have not yet been studied in POTS, and to explore treatments for the non- cardiovascular symptoms seen in POTS.
15. Role of immunotherapy
Several lines of evidence point to an autoimmune mechanism in at least some POTS patients as outlined above. Further, a number of case reports, and retrospective case series have provided anecdotal evidence suggesting efficacy of IVIG in the treatment of refractory POTS and other forms of suspected autoimmune dysautonomia (Goodman, 2014; Schofield and Chemali, 2019; Weinstock et al., 2018). These observations are not sufficient however. Beyond the expected placebo effects, intravenous infusion might provide a temporary increase in plasma volume independent of immunomodulatory effects. A small, randomized, double blind clinical trial is now underway to evaluate this treatment (NCT03919773). At this point, the clinical, laboratory, or autonomic features that should prompt consideration of immunotherapy are unknown.
16. Summary
POTS is a chronic multi-system disorder involving the autonomic nervous system that is characterized by an exaggerated sinus tachycardia, and symptoms upon standing. POTS primarily affects females starting around puberty and through their child-bearing age and is associated with significant functional disability, such as decreased ability to participate in education, limited ability to work and generate an income, and decreased quality of life. The pathophysiology is incompletely understood, which is likely responsible for limited data on effective treatments. Focused research studies on the pathophysiology and treatment of POTS have the potential to improve the lives of patients with POTS.
Funding
This NIH 2019 POTS Conference was supported by the US National Institutes of Health (NIH), Bethesda MD, USA.
Dr. Goldstein’s research is supported by the Division of Intramural Research, NINDS, NIH, Bethesda, MD, USA. The views expressed are the author’s own and do not necessarily reflect those of the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
Declaration of competing interest
SV – Consultant (topics unrelated to POTS) for Sage Therapeutics, Alterity, Catalyst Pharmaceuticals. Research Support from Dysautonomia International, Grifols, Genentech, BioHaven Pharmaceuticals.
KMB – Vanier Scholar, Canadian Institutes of Health Research (CIHR; Ottawa, Canada).
LES – None.
BPGr – None.
AF – Personal fees from Medtronic Inc. and Biotronik SE & Co AG, both unrelated to POTS.
JMS – Research grant from the National, Heart, Lung, and Blood institute (NHLBI) of the National Institutes of Health (NIH; Bethesda, MD, USA); Research grant from Lundbeck NA Ltd.
ACA – Consultant for National Vaccine Injury Compensation Program, U.S. Department of Health and Human Services; Research grant from Dysautonomia International.
LAP – Research grants from the National Institutes of Health (NIH); Research grants from Dysautonomia International.
JA – Founder and financial beneficiary of Ameliekliniken in Stockholm AB, a provider of outpatient healthcare (including to patients with POTS).
JRB – Consultant for National Vaccine Injury Compensation Program, U.S. Department of Health and Human Services.
JPM - None.
BPGo – None.
KRC – Research Grants from Dysautonomia International.
THC – None.
DSG – None.
ADi - None.
MGM – Consultant for Med-IQ CME and Infinite MD, Royalties from Elsevier, Expert Witness in POTS-related cases.
MMC – None.
AJM – None.
RF – None.
IB - Consultant for Theravance Biopharma and Takeda Pharmaceutical. Patent holder, Automated binder for treatment of Orthostatic Hypotension. PCR – None.
RSS - None.
CAS - Consultant for Theravance Biopharma.
DMS – None.
GAC – None.
TAD - Research support from Equillium Inc. HIA - None.
ADa – None.
SRR - Consultant for Lundbeck NA Ltd. and Theravance Biopharma; Chair, Data Safety and Monitoring Board for Arena Pharmaceuticals; Research Grant from Canadian Institutes of Health Research (CIHR; Ottawa, Canada); Research Grants from Dysautonomia International; Cardiac Arrhythmia Network of Canada (CANet; London, Ontario, Canada) Network Investigator.
References
- Abe H, Nagatomo T, Kohshi K, Numata T, Kikuchi K, Sonoda S, Mizuki T, Kuroiwa A, Nakashima Y, 2000. Heart rate and plasma cyclic AMP responses to isoproterenol infusion and effect of beta-adrenergic blockade in patients with postural orthostatic tachycardia syndrome. J. Cardiovasc. Pharmacol. 36 (Suppl. 2), S79–S82. 10.1097/00005344-200000006-00017. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Ng J, Raj SR, 2018. Postural tachycardia syndrome – diagnosis, physiology, and prognosis. Auton. Neurosci. 215, 3–11. 10.1016/j.autneu.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, Robertson D, Raj SR, 2011. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J. Clin. Sleep Med. 7, 204–210. [PMC free article] [PubMed] [Google Scholar]
- Barcroft H, Edholm OG, McMichael J, Sharpey-Shafer EP, 1944. Posthaemorrhagic fainting: study by cardiac output and forearm flow. Lancet 1, 489–491. [Google Scholar]
- Benarroch EE, 2012. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin. Proc. 87, 1214–1225. 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitshteyn S, 2015. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS). Lupus 24, 1364–1369. 10.1177/0961203315587566. [DOI] [PubMed] [Google Scholar]
- Boris JR, 2018. Postural orthostatic tachycardia syndrome in children and adolescents. Auton. Neurosci. 215, 97–101. 10.1016/j.autneu.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Boris JR, Bernadzikowski T, 2018. Demographics of a large paediatric postural orthostatic tachycardia syndrome program. Cardiol. Young 28, 668–674. 10.1017/s1047951117002888. [DOI] [PubMed] [Google Scholar]
- Boris JR, Huang J, Shuey T, Bernadzikowski T, 2020. Family history of associated disorders in patients with postural tachycardia syndrome. Cardiol. Young 30, 388–394. 10.1017/S1047951120000165. [DOI] [PubMed] [Google Scholar]
- Bourne KM, Chew DS, Stiles LE, Shaw BH, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Peltier A, Diedrich A, Biaggioni I, Sheldon RS, Robertson D, Raj SR, 2021a. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. J. Intern. Med. 10.1111/joim.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne KM, Sheldon RS, Hall J, Lloyd M, Kogut K, Sheikh N, Jorge J, Ng J, Exner DV, Tyberg JV, Raj SR, 2021b. Compression garment reduces orthostatic tachycardia and symptoms in patients with postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 77, 285–296. 10.1016/j.jacc.2020.11.040. [DOI] [PubMed] [Google Scholar]
- Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martiın A, Probst V, Reed MJ, Rice CP, Sutton R, Ungar A, van Dijk JG, 2018. 2018 ESC guidelines for the diagnosis and management of syncope. Kardiol. Pol. 76, 1119–1198. 10.5603/KP.2018.0161. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang L, Sun J, Qin J, Tang C, Jin H, Du J, 2011. Midodrine hydrochloride is effective in the treatment of children with postural orthostatic tachycardia syndrome. Circ. J. 75, 927–931. 10.1253/circj.cj-10-0514. [DOI] [PubMed] [Google Scholar]
- Coffin ST, Black BK, Biaggioni I, Paranjape SY, Orozco C, Black PW, Dupont WD, Robertson D, Raj SR, 2012. Desmopressin acutely decreases tachycardia and improves symptoms in the postural tachycardia syndrome. Heart Rhythm. 9, 1484–1490. 10.1016/j.hrthm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan S, Tomljenovic L, Shoenfeld Y, 2016. Postural orthostatic tachycardia syndrome (POTS)–A novel member of the autoimmune family. Lupus. 10.1177/0961203316629558. [DOI] [PubMed] [Google Scholar]
- DiBaise JK, Harris LA, Goodman B, 2018. Postural tachycardia syndrome (POTS) and the GI tract: A primer for the gastroenterologist. Am. J. Gastroenterol. 113, 1458–1467. 10.1038/s41395-018-0215-4. [DOI] [PubMed] [Google Scholar]
- Fedorowski A, 2019. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J. Intern. Med. 285, 352–366. 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- Fedorowski A, Li H, Yu X, Koelsch KA, Harris VM, Liles C, Murphy TA, Quadri SMS, Scofield RH, Sutton R, Melander O, Kem DC, 2017. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 19, 1211–1219. 10.1093/europace/euw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato JE, Wagoner AL, Harbinson RL, D’Agostino RBJ, Shaltout HA, Diz DI, 2014. Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J. Pediatr. Gastroenterol. Nutr. 59, 39–43. 10.1097/MPG.0000000000000305. [DOI] [PubMed] [Google Scholar]
- Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC, 1986. Idiopathic hypovolemia. Ann. Intern. Med. 104, 298–303. 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, Goldstein DS, Hainsworth R, Hilz MJ, Jacob G, Kaufmann H, Jordan J, Lipsitz LA, Levine BD, Low PA, Mathias C, Raj SR, Robertson D, Sandroni P, Schatz IJ, Schondorf R, Stewart JM, van Dijk JG, 2011. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. Basic Clin. 161, 46–48. 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Freitas J, Santos R, Azevedo E, Costa O, Carvalho M, de Freitas AF, 2000. Clinical improvement in patients with orthostatic intolerance after treatment with bisoprolol and fludrocortisone. Clin. Auton. Res. 10, 293–299. 10.1007/BF02281112. [DOI] [PubMed] [Google Scholar]
- Fu Q, Levine BD, 2015. Exercise in the postural orthostatic tachycardia syndrome. Auton. Neurosci. 188, 86–89. 10.1016/j.autneu.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Levine BD, 2018. Exercise and non-pharmacological treatment of POTS. Auton. Neurosci. 215, 20–27. 10.1016/j.autneu.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, Bhella PS, Levine BD, 2010. Cardiac origins of the postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 55, 2858–2868. 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, 2012. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294. 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Garland EM, Winker R, Williams SM, Jiang L, Stanton K, Byrne DW, Biaggioni I, Cascorbi I, Phillips JA 3rd, Harris PA, Rüdiger H, Robertson D, 2005. Endothelial NO synthase polymorphisms and postural tachycardia syndrome. Hypertension 46, 1103–1110. 10.1161/01.HYP.0000185462.08685.da. [DOI] [PubMed] [Google Scholar]
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D, 2007. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 69, 790–798. 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- Gee ME, Watkins AK, Brown JN, Young EJA, 2018. Ivabradine for the treatment of postural orthostatic tachycardia syndrome: A systematic review. Am. J. Cardiovasc. Drugs 12, 12. [DOI] [PubMed] [Google Scholar]
- George SA, Bivens TB, Howden EJ, Saleem Y, Galbreath MM, Hendrickson D, Fu Q, Levine BD, 2016. The international POTS registry: evaluating the efficacy of an exercise training intervention in a community setting. Heart Rhythm. 13, 943–950. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, 2020. The possible association between COVID-19 and postural orthostatic tachycardia syndrome. Heart Rhythm. 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman BP, 2014. Immunoresponsive Postinfectious autonomic neuropathy. Am. J. Ther. 21. [DOI] [PubMed] [Google Scholar]
- Goodman BP, 2018. Evaluation of postural tachycardia syndrome (POTS). Auton. Neurosci. 215, 12–19. 10.1016/j.autneu.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Goodman BP, Crepeau A, Dhawan PS, Khoury JA, Harris LA, 2017. Spectrum of autonomic nervous system impairment in Sjogren syndrome. Neurologist 22, ¨ 127–130. 10.1097/NRL.0000000000000134. [DOI] [PubMed] [Google Scholar]
- Green EA, Raj V, Shibao CA, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR, 2013. Effects of norepinephrine reuptake inhibition on postural tachycardia syndrome. J. Am. Heart Assoc. 2, e000395. 10.1161/JAHA.113.000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BP, 2008. Postural tachycardia syndrome. Circulation 117, 2814–2817. 10.1161/CIRCULATIONAHA.107.761643. [DOI] [PubMed] [Google Scholar]
- Grubb BP, Kosinski DJ, Boehm K, Kip K, 1997. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin. Electrophysiol. 20, 2205–2212. 10.1111/j.1540-8159.1997.tb04238.x. [DOI] [PubMed] [Google Scholar]
- Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP, 2019. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J. Am. Heart Assoc. 8, e013602. 10.1161/JAHA.119.013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan B, Roy F, 2004. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation 110, 3193–3198. 10.1161/01.CIR.0000147280.90339.E9. [DOI] [PubMed] [Google Scholar]
- Jacob G, Biaggioni I, 1999. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 317, 88–101. 10.1016/S0002-9629(15)40482-3. [DOI] [PubMed] [Google Scholar]
- Jacob G, Shannon JR, Black B, Biaggioni I, Mosqueda-Garcia R, Robertson RM, Robertson D, 1997. Effects of volume loading and pressor agents in idiopathic orthostatic tachycardia. Circulation 96, 575–580. 10.1161/01.cir.96.2.575. [DOI] [PubMed] [Google Scholar]
- Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B, Robertson D, 2000. The neuropathic postural tachycardia syndrome. N. Engl. J. Med. 343, 1008–1014. [DOI] [PubMed] [Google Scholar]
- Jacob G, Garland EM, Costa F, Stein CM, Xie H-G, Robertson RM, Biaggioni I, Robertson D, 2006. Beta2-adrenoceptor genotype and function affect hemodynamic profile heterogeneity in postural tachycardia syndrome. Hypertension 47, 421–427. 10.1161/01.HYP.0000205120.46149.34. [DOI] [PubMed] [Google Scholar]
- Jacob G, Diedrich L, Kyoko S, B.R. J, R.S. R, David R, Italo B, André D, 2019. Vagal and sympathetic function in neuropathic postural tachycardia syndrome. Hypertension 73, 1087–1096. 10.1161/HYPERTENSIONAHA.118.11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjwal K, Jamal S, Kichloo A, Grubb BP, 2020. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J. Innov. Card. Rhythm Manag. 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasmani R, Elkambergy H, Okoli K, 2009. Postural orthostatic tachycardia syndrome associated with mycoplasma pneumoniae. Infect. Dis. Clin. Pract. 17. [Google Scholar]
- Kavi L, Nuttall M, Low DA, Opie M, Nicholson LM, Caldow E, N.J, 2016. A profile of patients with postural tachycardia syndrome and their experience of healthcare in the UK. Br. J. Cardiol. 23, 1–6. [Google Scholar]
- Kharraziha I, Axelsson J, Ricci F, Di Martino G, Persson M, Sutton R, Fedorowski A, Hamrefors V, 2020. Serum activity against G protein-coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J. Am. Heart Assoc. 9, e015989. 10.1161/JAHA.120.015989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Fischer PR, Brands CK, Fisher JL, Porter C-BJ, Driscoll SW, Graner KK, 2009. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin. Electrophysiol. 32, 234–238. 10.1111/j.1540-8159.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- Li H, Yu X, Liles C, Khan M, Vanderlinde-Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, Hill MA, Raj SR, Okamoto LE, Cunningham MW, Aston CE, Kem DC, 2014. Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 3, e000755. 10.1161/jaha.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liao Y, Du J, Zhang Q, 2015a. Relationship between 24-hour urinary sodium and renin-angiotensin-aldosterone system in children with postural tachycardia syndrome. Zhonghua Yi Xue Za Zhi 95, 2928–2932. [PubMed] [Google Scholar]
- Li J, Zhang Q, Liao Y, Zhang C, Hao H, Du J, 2015b. The value of acetylcholine receptor antibody in children with postural tachycardia syndrome. Pediatr. Cardiol. 36, 165–170. 10.1007/s00246-014-0981-8. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang G, Zhou L, Nuss Z, Beel M, Hines B, Murphy T, Liles J, Zhang L, Kem DC, Yu X, 2019. Adrenergic autoantibody-induced postural tachycardia syndrome in rabbits. J. Am. Heart Assoc. 8, e013006. 10.1161/jaha.119.013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Han Z, Li H, Chen SY, Li X, Liu P, Wang Y, Tang C, Du J, Jin H, 2015. Plasma C-type natriuretic peptide as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. PLoS One 10, e0121913. 10.1371/journal.pone.0121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA, Rummans TA, 1995. Postural tachycardia syndrome (POTS). Neurology 45, S19–S25. [PubMed] [Google Scholar]
- Low PA, Sandroni P, Joyner M, Shen W-K, 2009. Postural tachycardia syndrome (POTS). J. Cardiovasc. Electrophysiol. 20, 352–358. 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar PL, Raj SR, 2014. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front. Physiol. 5, 220. 10.3389/fphys.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CJ, Low DA, Iodice V, Owens AP, Kirbis M, Grahame R, 2011. Postural tachycardia syndrome–current experience and concepts. Nat. Rev. Neurol. 8, 22–34. 10.1038/nrneurol.2011.187. [DOI] [PubMed] [Google Scholar]
- McDonald C, Koshi S, Busner L, Kavi L, Newton JL, 2014. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open 4, e004127–e004127. 10.1136/bmjopen-2013-004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn D-I, Jaradeh S, 2020. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Raj SR, 2018. Pharmacotherapy for postural tachycardia syndrome. Auton. Neurosci. 215, 28–36. 10.1016/j.autneu.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Stiles LE, Sheehan T, Bascom R, Levy HP, Francomano CA, Arnold AC, 2020. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Auton. Neurosci. 224, 102637. 10.1016/j.autneu.2020.102637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery LD, Parmet AJ, Booher CR, 1993. Body volume changes during simulated microgravity: auditory changes, segmental fluid redistribution, and regional hemodynamics. Ann. Biomed. Eng. 21, 417–433. 10.1007/BF02368634. [DOI] [PubMed] [Google Scholar]
- Moon J, Kim D-Y, Lee W-J, Lee HS, Lim J-A, Kim T-J, Jun J-S, Park B, Byun J-I, Sunwoo J-S, Lee S-T, Jung K-H, Park K-I, Jung K-Y, Kim M, Lee SK, Chu K, 2018. Efficacy of propranolol, bisoprolol, and pyridostigmine for postural tachycardia syndrome: a randomized clinical trial. Neurother. J. Am. Soc. Exp. Neurother. 15, 785–795. 10.1007/s13311-018-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenter Swift N, Charkoudian N, Dotson RM, Suarez GA, Low PA, 2005. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 289, H1226–H1233. 10.1152/ajpheart.01243.2004. [DOI] [PubMed] [Google Scholar]
- Mustafa HI, Garland EM, Biaggioni I, Black BK, Dupont WD, Robertson D, Raj SR, 2011. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 8, 422–428. 10.1016/j.hrthm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa HI, Raj SR, Diedrich A, Black BK, Paranjape SY, Dupont WD, Williams GH, Biaggioni I, Robertson D, 2012. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ. Arrhythm. Electrophysiol. 5, 173–180. 10.1161/CIRCEP.111.965343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao R, Tanaka H, Takitani K, Kajiura M, Okamoto N, Kanbara Y, Tamai H, 2012. GNB3 C825T polymorphism is associated with postural tachycardia syndrome in children. Pediatr. Int. 54, 829–837. 10.1111/j.1442-200X.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- Okamoto LE, Raj SR, Peltier A, Gamboa A, Shibao C, Diedrich A, Black BK, Robertson D, Biaggioni I, 2012. Neurohumoral and haemodynamic profile in postural tachycardia and chronic fatigue syndromes. Clin. Sci. (Lond.) 122, 183–192. 10.1042/CS20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban Z, Miller A, Bourne K, Nitis J, Hu W, Reinsel RA, Stiles LE, Fedorowski A, Axelsson J, 2020. Immunogenetic risk markers in postural orthostatic tachycardia syndrome [abstract]. Clin. Auton. Res 30, 492. [Google Scholar]
- Padilla-Walker LM, Nelson LJ, 2012. Black Hawk down? Establishing helicopter parenting as a distinct construct from other forms of parental control during emerging adulthood. J. Adolesc. 35, 1177–1190. 10.1016/j.adolescence.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Peltier AC, Garland E, Raj SR, Sato K, Black B, Song Y, Wang L, Biaggioni I, Diedrich A, Robertson D, 2010. Distal sudomotor findings in postural tachycardia syndrome. Clin. Auton. Res. 20, 93–99. 10.1007/s10286-009-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny HA, Aziz I, Ferrar M, Atkinson J, Hoggard N, Hadjivassiliou M, West JN, Sanders DS, 2016. Is there a relationship between gluten sensitivity and postural tachycardia syndrome? Eur. J. Gastroenterol. Hepatol. 28, 1383–1387. 10.1097/MEG.0000000000000740. [DOI] [PubMed] [Google Scholar]
- Pohlgeers KM, Stumbo JR, 2016. Syncope in an athlete: a case of infectious mononucleosis-induced postural tachycardia syndrome. Curr. Sports Med. Rep. 15, 41–45. [DOI] [PubMed] [Google Scholar]
- Raj R, Satish, 2013. Postural tachycardia syndrome (POTS). Circulation 127, 2336–2342. 10.1161/CIRCULATIONAHA.112.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj RS, Robertson RD, 2007. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 334, 57–60. 10.1097/MAJ.0b013e318063c6c0. [DOI] [PubMed] [Google Scholar]
- Raj SR, Robertson D, 2018. Moving from the present to the future of postural tachycardia syndrome - what we need. Auton. Neurosci. 215, 126–128. 10.1016/j.autneu.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, Robertson D, 2005. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 111, 1574–1582. 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, Robertson D, 2009. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 120, 725–734. 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau- Jarry N, Sheldon RS, 2020. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can. J. Cardiol. 36, 357–372. 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- Raj SR, Bourne KM, Stiles L, Miglis MG, Boris J, Cortez M, Miller A, Vernino S, 2021. Postural tachycardia syndrome (POTS); advancing POTS care and research from a 2019 national institute of Health Expert Consensus Meeting. Auton. Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roma M, Marden CL, De Wandele I, Francomano CA, Rowe PC, 2018. Postural tachycardia syndrome and other forms of orthostatic intolerance in Ehlers-Danlos syndrome. Auton. Neurosci. 215, 89–96. 10.1016/j.autneu.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Ruzieh M, Batizy L, Dasa O, Oostra C, Grubb B, 2017a. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand. Cardiovasc. J. 51, 243–247. 10.1080/14017431.2017.1355068. [DOI] [PubMed] [Google Scholar]
- Ruzieh M, Baugh A, Dasa O, Parker RL, Perrault JT, Renno A, Karabin BL, Grubb B, 2017b. Effects of intermittent intravenous saline infusions in patients with medication-refractory postural tachycardia syndrome. J. Interv. Card. Electrophysiol. 48, 255–260. [DOI] [PubMed] [Google Scholar]
- Sandroni P, Opfer-Gehrking TL, McPhee BR, Low PA, 1999. Postural tachycardia syndrome: clinical features and follow-up study. Mayo Clin. Proc. 74, 1106–1110. 10.4065/74.11.1106. [DOI] [PubMed] [Google Scholar]
- Schofield JR, Chemali KR, 2019. Intravenous immunoglobulin therapy in refractory autoimmune dysautonomias: a retrospective analysis of 38 patients. Am. J. Ther. 26, 570–582. 10.1097/MJT.0000000000000778. [DOI] [PubMed] [Google Scholar]
- Schondorf R, Low PA, 1993. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 43, 132–137. 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD, Robertson D, 2000. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N. Engl. J. Med. 342, 541–549. 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, Garland EM, Gamboa A, Diedrich A, Raj V, Sheldon RS, Biaggioni I, Robertson D, Raj SR, 2019. The face of postural tachycardia syndrome – insights from a large cross-sectional online community-based survey. J. Intern. Med. 286 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon RS, Grubb BP, Olshansky B, Shen W-K, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK, Kanjwal K, 2015. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 12, e41. 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibao C, Arzubiaga C, Roberts IILJ, Raj S, Black B, Harris P, Biaggioni I, 2005. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 45, 385–390. 10.1161/01.hyp.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD, 2012. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J. Physiol. 590, 3495–3505. 10.1113/jphysiol.2012.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y-W, Moon J, Kim T-J, Kim D-Y, Chang H, Jun J-S, Lee S-T, Jung K-H, Park K-I, Jung K-Y, Kim M, Lee SK, Chu K, 2019. Human leukocyte antigen associations in postural tachycardia syndrome. Ann. Clin. Transl. Neurol. 6, 962–967. 10.1002/acn3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EC, Diedrich A, Raj SR, Gamboa A, Shibao CA, Black BK, Peltier A, Paranjape SY, Biaggioni I, Okamoto LE, 2020. Splanchnic venous compression enhances the effects of ß-blockade in the treatment of postural tachycardia syndrome. J. Am. Heart Assoc. 9 10.1161/JAHA.120.016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, 2000. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr. Res. 48, 218–226. 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- Stewart JM, 2012. Mechanisms of sympathetic regulation in orthostatic intolerance. J. Appl. Physiol. 113, 1659–1668. 10.1152/japplphysiol.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Montgomery LD, 2004. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 287, H1319–H1327. 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Weldon A, 2001. Reflex vascular defects in the orthostatic tachycardia syndrome of adolescents. J. Appl. Physiol. 90, 2025–2032. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Glover JL, Medow MS, 2006a. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin. Sci. (Lond.) 110, 255–263. 10.1042/CS20050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Glover JL, Montgomery LD, 2006b. Persistent splanchnic hyperemia during upright tilt in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 290, H665–H673. 10.1152/ajpheart.00784.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Ocon AJ, Clarke D, Taneja I, Medow MS, 2009. Defects in cutaneous angiotensin-converting enzyme 2 and angiotensin-(1–7) production in postural tachycardia syndrome. Hypertens. (Dallas, Tex. 1979) 53, 767–774. 10.1161/hypertensionaha.108.127357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SD, Hanauer J, Rowe PC, Barron DF, Darbari A, Oliva-Hemker M, 2005. Gastrointestinal symptoms associated with orthostatic intolerance. J. Pediatr. Gastroenterol. Nutr. 40, 425–428. 10.1097/01.mpg.0000157914.40088.31. [DOI] [PubMed] [Google Scholar]
- Tang S, Calkins H, Petri M, 2004. Neurally mediated hypotension in systemic lupus erythematosus patients with fibromyalgia. Rheumatology (Oxford) 43, 609–614. 10.1093/rheumatology/keh132. [DOI] [PubMed] [Google Scholar]
- Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, Low PA, 2000. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS). Auton. Neurosci. 86, 107–113. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK, Low PA, 2007. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin. Proc. 82, 308–313. 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- U.S. Senate Committee on Appropriations, 2018. Departments of labor, health and human services, and education, and related agencies appropriation bill, 2019, (to accompany S. 3158) [WWW Document]. URL. https://www.congress.gov/115/crpt/srpt289/CRPT-115srpt289.pdf. (Accessed 1 August 2021).
- Vernino S, Stiles LE, 2018. Autoimmunity in postural orthostatic tachycardia syndrome: current understanding. Auton. Neurosci. 215, 78–82. 10.1016/j.autneu.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Wang X-L, Ling T-Y, Charlesworth MC, Figueroa JJ, Low P, Shen W-K, Lee HC, 2013. Autoimmunoreactive IgGs against cardiac lipid raft-associated proteins in patients with postural orthostatic tachycardia syndrome. Transl. Res. 162, 34–44. 10.1016/j.trsl.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari M, Nakane S, Mukaino A, Nakajima M, Mori Y, Maeda Y, Masuda T, Takamatsu K, Kouzaki Y, Higuchi O, Matsuo H, Ando Y, 2018. Autoimmune postural orthostatic tachycardia syndrome. Ann. Clin. Transl. Neurol. 5, 486–492. 10.1002/acn3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock LB, Brook JB, Myers TL, Goodman B, 2018. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018. 10.1136/bcr-2017-221405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock LB, Pace LA, Rezaie A, Afrin LB, Molderings GJ, 2020. Mast cell activation syndrome: a primer for the gastroenterologist. Dig. Dis. Sci. 10.1007/s10620-020-06264-9. [DOI] [PubMed] [Google Scholar]
- Xichun Y, Hongliang L, M.T. A, Zachary N, Jonathan L, Campbell L, A.C. E, R.S. R, Artur F, K.D. C, 2020. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J. Am. Heart Assoc. 7, e008351. 10.1161/JAHA.117.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaxley KL, 2020. Infectious mononucleosis complicated by peritonsillar abscess and postural orthostatic tachycardia syndrome: a case report. SAGE Open Med. Case Rep. 10.1177/2050313X20915413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Li X, Ochs T, Chen L, Liao Y, Tang C, Jin H, Du J, 2012. Midregional pro-adrenomedullin as a predictor for therapeutic response to midodrine hydrochloride in children with postural orthostatic tachycardia syndrome. J. Am. Coll. Cardiol. 60, 315–320. 10.1016/j.jacc.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen X, Li J, Du J, 2014. Orthostatic plasma norepinephrine level as a predictor for therapeutic response to metoprolol in children with postural tachycardia syndrome. J. Transl. Med. 12, 249. 10.1186/s12967-014-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LN, Moak JP, Desbiens J, Hanumanthaiah S, Fabian RR, Clarke L, Sahay RD, Darbari A, 2019. Utility of diagnostic studies for upper gastrointestinal symptoms in children with orthostatic intolerance. J. Pediatr. 205, 138–144. 10.1016/j.jpeds.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Zhao J, Du S, Yang J, Lin J, Tang C, Du J, Jin H, 2014. Usefulness of plasma copeptin as a biomarker to predict the therapeutic effectiveness of metoprolol for postural tachycardia syndrome in children. Am. J. Cardiol. 114, 601–605. 10.1016/j.amjcard.2014.05.039. [DOI] [PubMed] [Google Scholar]