Abstract
We defined the time course of ionizing radiation-induced senescence in lung compared to bone marrow of p16+/LUC mice in which the senescence-induced biomarker (p16) is linked to a luciferase reporter gene. Periodic in situ imaging revealed increased luciferase activity in the lungs of 20 Gy thoracic irradiated, but not 8 Gy total-body irradiated (TBI) mice beginning at day 75 and increasing to day 170. In serial sections of explanted lungs, senescent cells appeared in the same areas as did fibrosis in the 20 Gy thoracic irradiated, but not the 8 Gy TBI group. Lungs from 8 Gy TBI mice at one year did show increased RNA levels for p16, p21, p19 and TGF-β. Individual senescent cells in 20 Gy irradiated mouse lung included those with epithelial, endothelial, fibroblast and hematopoietic cell biomarkers. Rare senescent cells in the lungs of 8 Gy TBI mice at one year were of endothelial phenotype. Long-term bone marrow cultures (LTBMCs) were established at either day 60 or one year after 8 Gy TBI. In freshly removed marrow at both times after irradiation, there were increased senescent cells. In LTBMCs, there were increased senescent cells in both weekly harvested single cells and in colonies of multilineage hematopoietic progenitor cells producing CFU-GEMM (colony forming unit-granulocyte, erythrocyte, monocyte/macrophage, megakaryocyte) that were formed in secondary cultures when these single cells were plated in semisolid media. LTBMCs from TBI mice produced fewer CFU-GEMM; however, the relative percentage of senescent cell-containing colonies was increased as measured by both p16-luciferase and β-galactosidase. Therefore, 20 Gy thoracic radiation, as well as 8 Gy TBI, induces senescent cells in the lungs. With bone marrow, 8 Gy TBI induced senescence in both hematopoietic cells and in colony-forming progenitors. The p16+/LUC mouse strain provides a valuable system in which to compare the kinetics of radiation-induced senescence between organs in vivo, and to evaluate the potential role of senescent cells in irradiation pulmonary fibrosis.
INTRODUCTION
The role of cellular senescence in carcinogenesis (1-4), inflammation (5-12), organ failure including fibrosis (10-17) and aging (18-21) are subjects of intense interest. Senescent cells reside in an irreversibly non-proliferative state in tissues, which distinguishes them from quiescent cells (3), which retain the ability to resume proliferation when stimulated by cellular or humoral factors (18-19). Senescent cells, which are induced by autophagy (20), ionizing radiation (17) or metabolic deprivation (21-23) do not proliferate (24). Normally, oncogenic induced senescent cells cannot enter the cell cycle; however, some of these cells may escape from senescence and resume cell division and reactivate some transcriptional programs (25-26). The inflammatory cytokines and other gene products released by senescent cells have been termed the senescence-associated secretory phenotype (SASP) (12, 27). Recent evidence indicates that there are differences in the SASP released by senescent cells that arise after proliferation-induced aging, or oncogene induction compared to radiation-induced senescence (3, 28). A widely accepted biomarker of senescent cells is the age-associated beta-galactosidase (β-gal) enzyme, which usually correlates with levels of p16, p21 and telomere shortening (3, 16, 27, 29-34). Biomarkers of senescence such as p16 and p21 (3) are also transiently induced in irradiated cells, which can recover and proliferate independent of the appearance of β-gal (17). The induction by ionizing radiation of senescent cells has been previously associated with lung fibrosis (7-9).
The anatomic proximity of stereotactic radiation-induced senescent cells has been correlated with areas of pulmonary fibrosis (15). One problem with using in situ biomarkers for senescent cells is the inability to correlate their appearance over time with other cell phenotypes and physiologic functions within a particular tissue. Furthermore, the absence of cell surface markers on senescent cells that would facilitate their sorting and removal from irradiated tissues, limits in vitro studies of production and functions of profibrotic factors including those in the SASP (16-17).
In the current studies, we measured the time course of appearance of senescent cells in the lungs of irradiated C57BL/6 background p16+/LUC mice (1) using serial in situ imaging for p16-induced luciferase. We quantitated lung cellular senescence after 20 Gy thoracic irradiation or 8 Gy total-body irradiation (TBI), determined the phenotype of senescent cells in explanted tissue, and compared lung cellular senescence with that detected in bone marrow from TBI mice. We measured the total number and relative percentage of senescent single cells and senescent cell-containing multilineage hematopoietic colonies formed by progenitors in freshly explanted marrow at 60 days or one year after 8 Gy TBI and in weekly cell harvests over 23 weeks in long-term bone marrow cultures (LTBMCs) with bone marrow harvested from nonirradiated mice.
The data showed that the lungs of 20 Gy thoracic irradiated, as well as 8 Gy TBI, p16+/LUC mice displayed senescent cells. In the 20 Gy thoracic irradiated group, there was anatomic correlation of senescence with developing fibrosis over 75–150 days by histopathologic examination of serial sections of explanted lungs. While the bone marrow from 8 Gy TBI mice in LTBMCs produced fewer overall cell numbers, and fewer CFU-GEMM, there was increased production of both senescent cells and senescent cell-containing CFU-GEMM.
MATERIALS AND METHODS
Mice
C57BL/6J, and p16+/LUC mice on the C57BL/6J background were obtained from Dr. Norman Sharpless (1). Mice were housed 4 per cage and fed standard laboratory chow and deionized sterile water according to University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) regulations. We bred mice heterozygous for the p16-Luc transgene to derive homozygous mice expressing two copies of the p16-Luc transgene (p16LUC/LUC), heterozygous mice expressing one copy of the p16 gene and one copy of the p16-Luc transgene (p16+/LUC) or homozygous mice expressing both p16 genes (C57BL/6). Mice were genotyped by extracting DNA from the tail using TRIzol™ (Thermo Scientific™ Inc., Waltham, MA), polymerase chain reaction (PCR) was performed using primers (0.15 mM) specific for the p16-Luc transgene (p16-LUC-F 5′, -CTATGGCGGGCTGTGGAG-3′; p16-LUC-R 5′, -CACGGTAGGCTGCGAAATG-3′) using the following conditions: 95°C for 15 min 363 [94°C for 30 s, 58°C for 30 s, and 72°C for 45 s], 72°C for 2 min. The resulting PCR products are 312 (wild-type) and 543 (p16LUC) base pairs long (1).
Irradiation
Animals received 20 Gy thoracic irradiation using a 6-MV linear accelerator (6) or 8.0 Gy TBI using a cesium137 Model 68 irradiator (JL Shepherd & Associates, San Fernando, CA) at 3 Gy per min, as described elsewhere (17). Radiation dose, dose rate and homogeneity were confirmed, as described elsewhere (6, 35).
Long-Term Bone Marrow Cultures
The methods for establishment of long-term bone marrow cultures have been published elsewhere (36). The femur and tibia bone marrow from TBI, or control nonirradiated mice of each strain was flushed into tissue culture flasks according to methods described elsewhere (36) in media containing 25% horse serum, penicillin, and streptomycin. Cultures were demi-depopulated of hematopoietic non-adherent cells weekly with a replacement of a fresh volume of serum containing Fisher’s culture media. At week 4, 25% of horse serum was substituted with 25% fetal calf serum according to methods described elsewhere (36).
Hematopoietic Colony Forming Assays
Hematopoietic cells that were removed in the non-adherent cells harvested weekly from long-term culture or from single cell populations that were prepared from freshly explanted bone marrow were cultured at several densities ranging from 1 × 104 to 5 × 105 cells per ml in 0.8 methylcellulose containing McCoys 5A culture media and incubated in a high-humidity incubator 7% CO2, 37°C. Colonies containing greater than 50 cells were scored at day 7 and day 14, respectively (36). Bone marrow stromal cells were plated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and colonies of greater than 50 cells were scored at day 7 (36). Single cell cloning of hematopoietic and stromal cells was performed by sorting single cells into a 96-well plate using a flow cytometer and cell depositor according to methods described elsewhere (36). Colonies in semisolid media in 1.0 cm-diameter plates and containing >50 cells per colony did emit enough luciferase-stimulated light for imaging. These plates were imaged for luciferase activity in colonies by adding luciferin to the culture plates at day 7 or day 14. Colonies of >50 cells were counted at each time point.
Immunohistochemistry
C57BL/6 and p16+/LUC mice received 8 Gy TBI (14) or 20 Gy irradiation to the thoracic cavity (6). A subset of mice that received 8 Gy irradiation was sacrificed at day 60 for establishment of LTBMCs, while the remaining mice were held for one year postirradiation. The 20 Gy thoracic irradiated and 8 Gy TBI mice were imaged using the Lumina XR System (PerkinElmer® Inc., Waltham, MA) for areas of senescence by viewing positive areas at serial intervals. Thoracic irradiated mice were sacrificed at day 170 postirradiation, while TBI mice were held for either 60 days or one year at which time they were sacrificed. Explanted tissues were frozen in optimal cutting temperature (OCT), sectioned, with some sections stained with hematoxylin and eosin stained (H&E) or with Masson’s Trichrome for collagen. Immunochemistry was performed using antibodies for β-galactosidase [Rabbit monoclonal (EPR8250); Abcam, Cambridge, UK]; p16 (JC8) anti-mouse-AF488 (Santa Cruz Biotechnology® Inc., Dallas, TX); p16 [Rabbit monoclonal (EPR20418); Abcam], p19ARF Rabbit anti-mouse (Novus Biologicals LLC, Littleton, CO); luciferase [Rabbit monoclonal (EPR17789); Abcam]; CD31(PECAM-1) anti-mouse (BD Pharmingen™, San Diego, CA); CD16/32 anti-mouse (BD Pharmingen); F4/80 rat anti-mouse-RPE (AbD Serotec/Bio-Rad® Laboratories Inc., Hercules, CA); CD326 (EpCAM) anti-mouse-APC-eFlour780 (Invitrogen™, Carlsbad, CA); collagen I Rabbit anti-mouse (Abcam); Elastin Rabbit anti-mouse (Abcam); SMC1 Rabbit anti-mouse (Novus Biologicals). Secondary antibodies included: Donkey anti-rabbit IgG, AF594 (Invitrogen), Chicken anti-mouse IgG, AF647 (Invitrogen), rat IgG2b R-phycoerythrin, R2b04; and goat anti-rabbit antibody (Abcam). Also used was the CellEvent™ Senescence Green Flow Cytometry Assay Kit (Invitrogen).
Identification of the Phenotype of Senescent Cells in the Lungs of 20 Gy Thoracic Irradiated and 8 Gy TBI Irradiated p16+/LUC Mice
Histologic sections from mice at day 170 after 20 Gy irradiation to the thoracic cavity or one year after 8 Gy TBI were prepared for immunostaining according to methods described elsewhere (6). Briefly, histologic sections were prepared for serial immunostaining, then stained for p16, β-gal, and for biomarkers associated with each of several cell phenotypes, including endothelial cells (anti-CD31) (CD31 is normally found on endothelial cells, platelets, macrophages, and Kupffer cells, granulocytes, lymphocytes including T cells, B cells, and NK cells, megakaryocytes and osteoclasts); CD326 (EpCAM) found on epithelial cells; and CD16/32 found on hematopoietic cells. These antibodies target the Fc gamma III receptor and CD32 (Fc gamma II receptors, which include low-affinity receptors for the mouse lgG Fc portion that is expressed by B cells, monocyte/macrophages, NK cells and neutrophils); F4/80 on monocytes; collagen I found on fibroblasts; SMC1 found on smooth muscle cells; and elastin, which is found on stromal cells. For dual imaging, secondary antibodies were labeled with either green fluorescent protein (GFP) or the red fluorochrome phycoerythrin (PE). These antibodies were added according to methods described elsewhere (6). Fusion images were taken according to methods described elsewhere (6).
Lumina XR System Imaging of p16-Luciferase in Lungs In Situ, Scoring of Fibrosis, and Senescence in Explanted Lung and Bone Marrow
To quantitate radiation induction of senescence in lungs, we imaged the organs in situ at serial time points after irradiation. At each imaging time point, mice were injected intravenously with 100 μl of luciferin (1 mg/ml), anesthetized with isoflurane, and imaged using the Lumina XR System. To quantitate marrow senescence, the number of non-adherent single cells or cells within hematopoietic progenitor cell derived multilineage colonies containing colony forming unit-granulocyte/erythroid/macrophage/monocytes (CFU-GEMM) that were derived from the weekly harvests of cells from LTBMCs were histochemically stained for β-gal or for p16-luciferase, and then counted (1, 17). For imaging of CFU-GEMM colonies, 120 μl of luciferin (1 mg/ml) was added to each culture plate at either day 7 or day 14, and 15 min later the cultures were imaged for luciferase-positive colonies using the Lumina XR System.
Detection of Senescent Cells in the Lungs of p16+/LUC Mice at One Year after 8 Gy TBI by RT-PCR
Lung specimens from irradiated and control mice were prepared for real-time polymerase chain reaction (RT-PCR) according to published methods. RNA was extracted from whole lung specimens. Quantitative RT-PCR was performed according to methods described elsewhere (6). qPCR was performed to quantitate RNA expression for each gene by RT-PCR, as described elsewhere (6). Ninety-six-well plates were prepared with 10 uI of TaqMan® Gene Expression Master mix, 5 uI of RNase-free water, 1 uI of the corresponding TaqMan Gene Expression probe, and 4 uI of cDNA (totaling 2 ug cDNA) using the Eppendorf epMotion® 5070 automated pipetting system (Westbury, NY). The cDNA was amplified with 40 cycles of 95°C (denaturation) for 15 s and 60°C (annealing and elongation) for 1 min using the Eppendorf Realplex®2 Mastercycler. Probes for RNA moieties associated with senescence included those for p16, p19 (Arf), p21, as well as fibrosis-associated RNA moieties, TGF-β and IL-6. Triplicate samples from at least three lung specimens at each time point were subjected to RT-PCR and results presented as fold-increase or decrease over baseline levels from unirradiated mice of the same age.
Results of RT-PCR were compared to β-gal staining of sections of the same lung specimens used for RT-PCR to attempt to identify those cells that were expressing senescence markers.
Statistics
Analysis of the lung for correlation of areas of senescence with areas of fibrosis at day 170 after 20 Gy thoracic irradiation was performed by scoring five serial sections from each of the five lung lobes of at least 5 mice per genotype (25 lobes) and at each radiation dose (8 Gy TBI or 20 Gy thoracic irradiation), compared to a similar number from control nonirradiated mice. Serial sections were stained for fibrosis (Masson’s trichrome), β-gal or luciferase.
Analysis of LTBMC data was performed using methods described elsewhere (36). Briefly, we collected data for parameters of hematopoiesis (36) including weekly cobblestone island numbers (as indication of the number of primitive hematopoietic stem cell islands), non-adherent cell numbers, percentage confluence of the adherent cell layer, day 7 colony-forming cells, and day 14 colony-forming cells, all counted each week, and at each weekly non-adherent cell harvest. Data were summarized as mean ± standard deviation (SD), and P values were calculated at each week using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests (37) to compare marrow of nonirradiated with 8 Gy irradiated p16+/LUC and C57BL/6 mice. Comparisons between genotypes was performed for 8 Gy irradiated p16+/LUC and the C57BL/6 control group.
The analysis of production of senescent cells within day 7 and day 14 CFU-GEMM, which were derived from the non-adherent cells that were removed from LTBMCs of 8 Gy TBI p16+/LUC and C57BL/6 mice, was performed as follows. For each of the genotypes (p16+/LUC and C57BL/6), at each day of scoring, colonies were compared with other groups for the percentage of day 7 luciferase-positive colonies calculated as the percentage of total number of day 7 colonies, and this percentage was compared between each 0 Gy and 8 Gy irradiated group. We used the two-sample proportion test (36, 37). The analysis for day 14 colonies was performed in the same manner.
RESULTS
Senescence Precedes Fibrosis in Lungs of 20 Gy Thoracic Irradiated p16+/LUC Mice Over 170 Days Postirradiation
C57BL/6 and p16+/LUC mice received 20 Gy irradiation to the thoracic cavity or 8 Gy TBI. The mice were imaged every two weeks for the percentage of lung showing luciferase activity. The 8 Gy TBI mice showed no detectable luciferase activity over one year after TBI. In contrast, the 20 Gy thoracic irradiated p16+/LUC, but not C57BL/6 mice, showed luciferase positive areas in the lungs beginning at day 75 postirradiation and increasing to day 170 (Fig. 1A and B). These results confirm and extend the findings of a previously published study, which demonstrated that C57BL/6 mice that received 20 Gy irradiation to the pulmonary cavity have increased inflammatory cytokine production and demonstrate migration of bone marrow stromal cell progenitors of fibrosis into the lungs beginning at day 110 postirradiation at the time of first detection of fibrosis (6). These results also confirm prior studies showing that cellular senescence was detected at day 75, significantly before the onset of fibrosis, which has been reported to be detectable at day 110 (6-8). The current results were in contrast to those with nonirradiated p16+/LUC mice in which no evidence of senescence was detectable by in vivo imaging or by histochemical staining for β-gal, and with irradiated C57BL/6 mice that showed no evidence by imaging of luciferin stimulated p16-activated luciferase (Fig. 1C and D).
FIG. 1.
Radiation-induced senescence in areas of developing fibrosis in the lungs of 20 Gy thoracic irradiated p16+/LUC mice over 170 days. C57BL/6J, and p16+/LUC mice received 20 Gy irradiation to the thoracic cavity or 8 Gy TBI, as described in the Materials and Methods. Mice were serially imaged using the IVIS Imaging System for luciferase+ areas in the lungs, at 10 min after injection of luciferin. Serial images demonstrated the appearance of senescent areas throughout the lungs. Panel A: Serial increase in luciferase+ regions in the 20 Gy irradiated lungs of p16+/LUC, but not C57BL/6 mice beginning at day 75. Panel B: Explanted lung and overlying thoracic skin from mice in panel A at day 170. Skin was removed prior to imaging the lungs, skin intact in images on left, skin removed in images on the right, demonstrating senescence-positive areas in both skin and lung. Panel C: IVIS imaging on mice at one year after 8 Gy TBI. Panel D: p16+/LUC and C57BL/6 lung and skin from representative 8 Gy TBI mouse one year postirradiation. (Results are representative of n = 12 mice per group.)
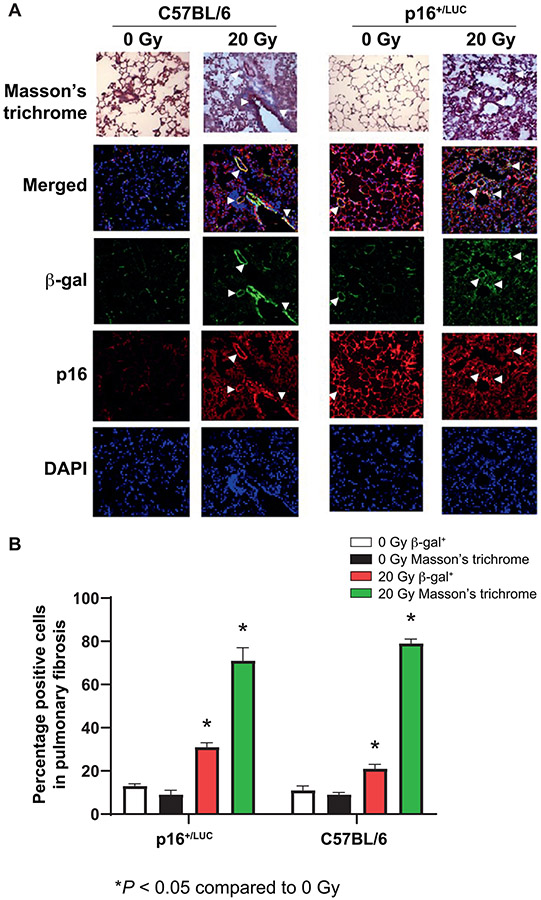
Senescent Cells are Co-Localized with Fibrotic Cells in the Lungs of Mice at Day 170 after 20 Gy Thoracic Irradiation
Fibrotic areas in the lungs showed increased senescent cells at day 170 (Fig. 2A and B). There were also senescent cells in proximity to fibrotic areas in 20 Gy irradiated thoracic skin from the same mice at the same time point. Quantitation of the relative lung volumes and common anatomic areas of senescence and fibrosis is shown in Fig. 2B.
FIG. 2.
Histopathologic demonstration of senescent cells in 20 Gy and 8 Gy TBI lungs and fibrosis in same anatomic areas as senescence in serial sections of 20 Gy thoracic irradiated p16+/LUC mice at 150 days and detection of biomarkers of senescence in lungs of 8 Gy TBI mice at 1 year. Panel A: Serial sections of lung were stained for Masson’s trichrome for collagen, β-gal and p16. Single cells showing senescence marker β-gal, in close proximity to cells showing fibrosis markers of collagen type I at 170 days after 20 Gy thoracic irradiation. Histopathologic display of merged collagen-positive fibrotic cells in proximity to p16+ senescent cells in lungs of p16+/LUC mice. Panel B: Quantitation of areas of fibrosis and senescence by overlay of serial sections of luciferase+ areas of lung with (collagen type I in areas of fibrosis with β-gal). Results are for lungs at day 170 after 20 Gy thoracic irradiation for 5 lobes per mouse, 5 sections per lobe (25 sections per mouse) for 3 mice at each time point (total 75 slides) (500×). Panel C: To identify the phenotype of lung senescent cells, lungs removed from mice at day 150 after 20 Gy thoracic irradiation or at one year after 8 Gy TBI were sectioned and stained for: p16 (senescent cells); CD31 (endothelial cells); CD326 (epithelial cells); CD32 (immune cells); F480 (macrophages); collagen 1 (fibroblasts); and SMC1 (smooth muscle). Green cells were p16+ senescent cells, red cells were positive for one of the cell markers listed above and yellow cells were fusion images that were positive for both senescence and each cell phenotype marker (white arrow). Senescent cells from 20 Gy irradiated lungs were in phenotypes that were positive for CD31, CD326, CD32, F480 and collagen 1. No smooth muscle cells were detectably senescent. Panel D: Expression of p16, p19 (Arf), p21 and senescence-associated secretory phenotype (SASP) (including TGF-β) in lungs of 8 Gy TBI mice at one year by qPCR (n = 3). RNA was extracted from the lungs using TRIzol and amplified by RT-PCR, and assayed as described in Materials and Methods. Panel E: To demonstrate the presence of senescent cells, removed from mice at one year after 8 Gy TBI, C57BL/6 and p16+/LUC mice were sacrificed one year after 8 Gy TBI. The lungs were removed and frozen in OCT, sectioned and stained for CD31+ endothelial cells (red) and p16+ senescent (green) cells. Cells that were CD31+ and p16+ by fusion appear yellow. No senescent cell phenotypes were detected in nonirradiated mice. Rare senescent p16+ CD31+ endothelial cells were detected in the lungs of mice at one year after 8 Gy TBI (400×).
Phenotype of Senescent Cells in the Areas of Fibrosis at Day 170 after 20 Gy Thoracic Irradiation
We next performed immunohistochemical staining of the lungs of 20 Gy thoracic irradiated mice at 170 days, to identify the phenotype of those cells that were p16-positive by immunostaining. Sections of lung were prepared for immunohistochemical staining and assayed for biomarkers of endothelial cell phenotype (CD31), hematopoietic cell phenotype (CD16/CD32) and epithelial cell phenotype (EpCAM). As shown in Fig. 2C, there were endothelial (CD31), epithelial (CD326), hematopoietic (CD32, F4/80) and collagen 1-positive cells, which were p16 positive (Fig. 2C). No p16-positive smooth muscle cells were detected (Fig. 2C). These results establish that the phenotype of p16-positive senescent cells in the areas of fibrosis in the lungs of p16+/LUC mice at 170 days were of multiple lineages, but predominantly of the endothelial cell lineage.
Detection of Senescent Cells in the Lungs of 8 Gy TBI p16+/LUC Mice
In view of the negative results with Lumina XR Imaging of the lungs of 8 Gy TBI mice, tested at one year postirradiation, we next used more sensitive assays to detect senescent cells in these lung samples. We measured levels of senescent cell-associated proteins in these lungs by quantitative RT-PCR. Mouse lung specimens were prepared for RT-PCR as described in the Materials and Methods. Lung specimens were assayed for RNA moieties associated with senescence, including p16, p19 (Arf), p21, and for biomarkers of the senescence-associated secretory phenotype (SASP) proteins, including TGF-β and IL-6. As shown in Fig. 2D increased levels of p16, p21, p19 and TGF-β biomarkers were detected by RT-PCR in lungs of 8 Gy TBI p16+/LUC mice at one year postirradiation (Fig. 2D). Sections of lung from the same time point used for the RT-PCR assay were stained for the detection of p16-positive senescent cells. Rare p16-positive cells were detected, which also displayed the CD31 endothelial cell phenotypic marker (Fig. 2E). These data establish that senescent cells are detected in the lungs of 8 Gy TBI p16+/LUC mice, despite the negative results using the Lumina XR System (IVIS) imaging for luciferase at this time point.
Hematopoiesis in Long-Term Bone Marrow Cultures from the Marrow of 8 Gy TBI p16+/LUC Mice
We explanted bone marrow at day 60 or one year after 8 Gy TBI from C57BL/6 and p16+/LUC mice and examined single cells for the percentage senescence by β-gal and luciferase staining. We also established LTBMCs from the marrow of mice of each genotype. We chose 60 days after 8 Gy TBI for explant of marrow, as this was associated not only with the return of peripheral blood counts to the normal range after this sublethal TBI dose, but also with the return of bone marrow cellularity to that of the preirradiation level (17). In LTBMCs, the weekly and cumulative production of stromal cells and hematopoietic islands on the adherent layer in long-term marrow cultures was similar between the genotypes that were irradiated to 8 Gy (as a group) and the nonirradiated genotypes (as a group) with respect to confluence (Fig. 3A, Supplementary Table S1; https://doi.org/10.1667/RADE-20-00286.1.S1), and number and cumulative production of primitive stem cell-containing cobblestone islands (Fig. 3B and C, Supplementary Table S2) had similar levels. Marrow from 8 Gy TBI mice showed reduced production of hematopoietic cells compared to marrow from nonirradiated mice (Fig. 3D and E, Supplementary Table S3). These parameters were similar between the groups of irradiated compared to control mice. The weekly and cumulative production of total non-adherent cells by long-term marrow cultures (Fig. 3D and E, Supplementary Table S3), as well as the weekly and cumulative production of hematopoietic progenitor cells forming day 7 colonies (Fig. 3F and G, Supplementary Table S4), and the weekly and cumulative production of hematopoietic progenitor cells forming the more primitive day 14 colonies (Fig. 3H and I, Supplementary Table S5) showed a decrease in the 8 Gy irradiated compared to control nonirradiated marrow explanted from all three genotypes. These results confirm a prior published study with C57BL/6 mice showing a decreased cumulative production of hematopoietic cells and hematopoietic progenitor cells from bone marrow explanted at 60 days after 8 Gy TBI (17) and extend the results to now include p16+/LUC mice. Detailed weekly analysis of the parameters of hematopoiesis in p16+/LUC LTBMCs compared to those from control C57BL/6 mouse long-term cultures is shown in Supplementary Tables S1-5. The results establish that marrow from 8 Gy TBI p16+/LUC mice produced fewer hematopoietic cells in vitro in patterns similar to that from C57BL/6 mice.
FIG. 3.


Parameters of continuous hematopoiesis in long-term bone marrow cultures derived from 8 Gy TBI p16+/LUC and C57Bl/6J mice. Eight weeks after TBI, long-term bone marrow cultures were established from femur and tibia bone marrow of four C57BL/6 and four p16+/LUC mice and observed weekly for parameters of hematopoiesis. Panel A: Weekly quantitation of percentage confluence of adherent layer of long-term bone marrow cultures. Panel B: Weekly quantitation of cobblestone islands in the adherent layer of long-term bone marrow cultures representing hematopoietic stem cell containing islands. Panel C: Cumulative production of cobblestone islands over 20 weeks in culture. Panel D: Weekly production of non-adherent cells by long-term bone marrow cultures. Panel E: Cumulative production of non-adherent cells in long-term bone marrow cultures. Panel F: Weekly production of colony-forming hematopoietic progenitor cells forming day 7 CFU-GEMM (50 cell or greater) colonies. Panel G: Cumulative production of day 7 colony-forming hematopoietic progenitors forming day 7 CFU-GEMM. Panel H: Weekly production of colony-forming hematopoietic progenitors capable of forming ≥50 cell colonies scored at day 14 in secondary semisolid medium culture. Panel I: Cumulative production of day 14 colony-forming hematopoietic progenitor cells forming day 14 CFU-GEMM. The results are presented for control nonirradiated marrow cultures, and 8 Gy irradiated mouse long-term marrow cultures established 8 weeks after TBI. Statistical analysis for all data in Fig. 3 is presented in Supplementary Tables S1-9 (https://doi.org/10.1667/RADE-20-00286.1.S1). *Significant difference from control nonirradiated cultures. Supplementary Tables S1-7 provide a detailed analysis, and explanation of the (*) significant difference relative to each figure and the significance.
Increased Relative Numbers of Senescent Cells and Senescent Cells within Colonies Formed by Hematopoietic Progenitors in Freshly Explanted Marrow and Produced by LTBMCs from 8 Gy TBI Mice
LTBMCs have been shown as a reliable model system in which to study the effects of accelerated aging on marrow stem cell populations in vitro (36). We quantitated the production of senescent cells over 20 weeks during hematopoiesis in long-term marrow cultures from 8 Gy TBI p16+/LUC and C57BL/6 mice (Fig. 4, Tables 1-3). We also counted total numbers and percentage of senescent cell containing day 7 and day 14 CFU-GEMM derived from freshly explanted marrow at 60 days after TBI from each mouse strain (Table 4). We counted luciferase-positive colonies by imaging the culture plates (Fig. 5), and also performed β-gal staining of colonies to allow correlation of p16+/LUC with C57BL/6 mice (Table 4).
FIG. 4.

Percentage of luciferase+ CFU-GEMM colonies (containing multilineage cells), formed from the progenitors in weekly harvested non-adherent cells from LTBMCs that were established at day 60 after 8 Gy TBI. Results show the weekly percentage luciferase+ cell-containing colonies formed by hematopoietic progenitor cells harvested from LTBMCs that were derived from 8 Gy TBI p16+/LUC and C57BL/6 mice at day 60 postirradiation. Results are from control nonirradiated mice and irradiated mouse long-term marrow cultures established at day 60 after 8 Gy TBI of p16+/LUC mice compared to control C57BL/6J mice. (Total colonies produced each week are shown in Supplementary Tables S6-7; https://doi.org/10.1667/RADE-20-00286.1.S1.)
TABLE 1.
Production of Senescent Cell-Containing Day 7 CFU-GEMM by Non-adherent Cells Removed from LTBMCs from 8 Gy TBI p16+/LUC and C57BL/6 control mice
| Day of cell plating |
p16+/LUC |
C57BL/6 |
||||
|---|---|---|---|---|---|---|
| Weekly no. of day 7 luciferase+ colonies/ no. of colonies |
P value | Weekly no. of day 7 luciferase+ colonies/ no. of colonies |
P value | |||
| 0 Gy | 8 Gy | 0 Gy | 8 Gy | |||
| 7 | 0/8 (0%) | 3/17 (18%) | 0.2053 | 0/19 (0%) | 0/28 (0%) | 1.0000 |
| 21 | 3/127 (2%) | 1/53 (2%) | 0.8437 | 0/78 (0%) | 0/120 (0%) | 1.0000 |
| 28 | 9/104 (9%) | 1/62 (2%) | 0.0651 | 0/106 (0%) | 0/65 (0%) | 1.0000 |
| 35 | 9/131 (7%) | 14/213 (7%) | 0.9146 | 0/126 (0%) | 0/86 (0%) | 1.0000 |
| 42 | 3/103 (3%) | 13/109 (12%) | 0.0130 | 0/104 (0%) | 0/52 (0%) | 1.0000 |
| 49 | 1/138 (1%) | 4/74 (5%) | 0.0323 | 0/187 (0%) | 0/112 (0%) | 1.0000 |
| 56 | 8/211 (4%) | 8/55 (15%) | 0.0028 | 0/184 (0%) | 0/95 (0%) | 1.0000 |
| 63 | 3/177 (2%) | 7/56 (13%) | 0.0005 | 0/226 (0%) | 0/104 (0%) | 1.0000 |
| 70 | 2/137 (1%) | 0/249 (0%) | 0.0559 | 0/77 (0%) | 0/156 (0%) | 1.0000 |
| 77 | 0/119 (0%) | 0/172 (0%) | 1.0000 | 0/109 (0%) | 0/50 (0%) | 1.0000 |
| 84 | 0/151 (0%) | 0/26 (0%) | 1.0000 | 0/254 (0%) | 0/52 (0%) | 1.0000 |
| 91 | 1/100 (1%) | 0/59 (0%) | 0.4410 | 0/120 (0%) | 0/56 (0%) | 1.0000 |
| 98 | 0/49 (0%) | 0/49 (0%) | 1.0000 | 0/137 (0%) | 0/11 (0%) | 1.0000 |
| 105 | 1/55 (2%) | 0/73 (0%) | 0.2474 | 0/161 (0%) | 0/4 (0%) | 1.0000 |
| 112 | 1/39 (3%) | 4/124 (3%) | 0.8344 | 0/171 (0%) | 0/3 (0%) | 1.0000 |
| 119 | 0/36 (0%) | 5/235 (2%) | 0.3770 | 0/183 (0%) | 0/2 (0%) | 1.0000 |
| 126 | 1/73 (1%) | 1/193 (1%) | 0.4730 | 0/118 (0%) | ||
| 133 | 0/20 (0%) | 0/89 (0%) | 1.0000 | 0/118 (0%) | ||
| 140 | 2/18 (11%) | 1/75 (1%) | 0.0350 | 0/111 (0%) | ||
Notes. Marrow was explanted at day 60 after 8 Gy TBI and LTBMCs established as described in Materials and Methods. Harvested non-adherent cells were assayed for CFU-GEMM scored at day 7 or by IVIS imaging for number and percentage of p16+/LUC colonies. For p16+/LUC colonies, on days 42–63, the irradiated group had a significantly larger percentage of luciferase+ colonies than the nonirradiated mice. For each of the genotypes (p16+/LUC and C57BL/6), at each day, the percentage of day 7 luciferase+ colonies was calculated as the ratio of “total number of day 7 luciferase+ colonies/total number of day 7 colonies”, and compared between 0 Gy and 8 Gy, using the two-sample proportion test. The analysis for day 14 colonies was performed in the same manner as day 7 results. For each of the cell types (p16+/LUC and C57BL/6), the percentage of luciferase+ colonies was compared between 0 Gy and 8 Gy, using the two-sample proportion test.
TABLE 3.
Individual Senescent Cell Containing Colonies (Scored on Day 7 and Day 14 CFU-GEMM) from Freshly Explanted Marrow at 60 Days or One Year after 8 Gy TBI from p16+/LUC compared to C57BL/6 mice
| Time after 8 Gy TBI | ||||
|---|---|---|---|---|
| Control | 60 days | 1 year | ||
| Day 7 CFU-GEMM | ||||
| p16+LUC | LUC+ colonies | 2.3 ± 1.5 | 3.0 ± 1.1 | 2.3 ± 0.9 |
| No. of colonies | 34.0 ± 5.0 | 37.0 ± 0.6 | 45.7 ± 3.5 | |
| C57BL/6 | LUC+ colonies | 0 | 0 | 0 |
| No. of colonies | 60.7 ± 3.7 | 64.0 ± 1.5 | 33.7 ± 0.9 | |
| Day 14 CFU-GEMM | ||||
| p16+LUC | LUC+ colonies | 2.7 ± 0.7 | 2.3 ± 1.5 | 2.7 ± 1.2 |
| No. of colonies | 54.0 ± 4.0 | 34.0 ± 5.0 (P = 0.0363) | 39.3 ± 2.6 (P = 0.0322) | |
| C57BL/6 | LUC+ colonies | 0 | 0 | 0 |
| No. of colonies | 46.7 ± 1.4 | 62.7 ± 2.3 (P = 0.043) | 38.3 ± 2.2 (P = 0.0437) | |
Notes. Significance (P ≤ 0.05) compared to control is indicated in bold face. Cells were plated in CFU-GEMM colony assay as described in the Materials and Methods with four culture plates per data point. (1 × 104 nucleated cell, plates per plate) (n = 4 plate per point). Results are presented as number of colonies ≥50 cells in 104 cell plates. Number of senescent single cells were similar for marrow harvested at 60 days compared to one year after TBI.
TABLE 4.
Individual Beta-gal-positive Senescent Cells and Luciferase Positive Senescent Cell Containing Colonies (Scored on Day 7 and Day 14 CFU-GEMM) from Freshly Removed Bone Marrow Isolated from p16+/LUC and C57BL/6 mice 60 Days after 8 Gy TBI Compared to Nonirradiated Mice
| Mouse strain | Dose (Gy) | Percentage (%) senescent (β-gal) NA single cells |
No. of luciferase+ colonies/total no. of colonies | |
|---|---|---|---|---|
| Day 7 | Day 14 | |||
| p16+Luc | 0 | 7.3 ± 1.1 | 5/53 (9%) | 3/54 (5%) |
| 8 | 43.7 ± 2.8 (P < 0.0001) | 3/37 (8%) | 2/34 (6%) | |
| C57BL/6 | 0 | 5.5 ± 1.5 | 0/64 (0%) | 0/47 (0%) |
| 8 | 39.3 ± 2.1 (P < 0.0001) | 0/35 (0%) | 0/64 (0%) | |
Notes. Significant differences between 0 and 8 Gy are indicated in bold face. Cells were removed from marrow at 60 days or one year after 8 Gy TBI and single cells stained for β-gal, then placed into CFU-GEMM colony assay, as described in Materials and Methods. Results are for percentage positive cells out of 1,000 cells scored from at least 3 mice per group at each time point. P values compared with nonirradiated marrow of each representative genotype. For C57BL/6 mice, the numbers of β-gal-positive/total colonies per 104 cells were, for day 7 colonies for 0 Gy and 8 Gy TBI C57BL/6 mice, 1/54 and 3/35 and for day 14 colonies were 1/47 and 5/64, respectively, based on histochemical staining of removed and stained colonies for β-gal.
FIG. 5.
Luciferase+ senescent cell-containing colonies CFU-GEMM formed from non-adherent hematopoietic cells removed from C57BL/6, and p16+/LUC mouse LTBMCs that have been established from marrow harvested at 60 days after 8 Gy TBI compared to marrow cultures from nonirradiated control mice. Senescent cells containing imaged luciferase p16+ CFU-GEMM were imaged by adding luciferin to the culture plates, as described in the Materials and Methods, and were then scored in separate plates (n = 4) at day 7 (left side) or at day 14 (right side). Non-adherent (NA) cells were harvested weekly from long-term bone marrow cultures that were established from marrow of mice of each genotype at 60 days after 8 Gy TBI, and cells were plated in semisolid media containing hematopoietic growth factors, as described in the Materials and Methods. The culture was scored, as those containing >50 cells/colony at day 7 or at day 14, and then luciferin was added to the culture media. The culture plates were then imaged: Comparison of colonies detected in NA cells harvested at day 7, 14, 56 or 119 from LTBMCs of each genotype mouse strain 8 Gy TBI with day 14 colonies derived from LTBMCs from nonirradiated mice of each genotype. Luciferase+ colonies are blue in color. Quantitative data are shown in Tables 1 and 2.
The results showed that senescent cells and senescent cell-containing colonies were present in low numbers, and at similar frequency in both mouse genotypes (Tables 1-4).
Both mouse strains showed reduced colony numbers after TBI. While there were decreased numbers of hematopoietic colony-forming cells produced by cells from 8 Gy TBI p16+/LUC, as well as C57BL/6 mouse long-term bone marrow cultures (LTBMCs) (Table 1: Day 7 colonies and Table 2: Day 14 colonies), there were increased numbers of β-gal-positive cells (by immunostaining) removed from LTBMCs. We used p16 staining (Fig. 6) and β-gal staining (Fig. 7) to confirm that C57BL/6 mice showed relative numbers of senescent cells comparable to those detected in p16+/LUC mice. The results establish that radiation induction of senescence in p16+/LUC marrow cells was similar to that detected with C57BL/6 mouse marrow.
TABLE 2.
Production of Senescent Cell Containing Day 14 CFU-GEMM by Non-adherent Cells Removed from LTBMCs from 8 Gy TBI p16+/LUC and C57BL/6 Mice
| Day of cell plating |
p16+/LUC |
C57BL/6 |
||||
|---|---|---|---|---|---|---|
| Weekly no. of day 14 luciferase+ colonies/ no. of colonies |
P value | Weekly no. of day 14 luciferase+ colonies/ no. of colonies |
P value | |||
| 0 Gy | 8 Gy | 0 Gy | 8 Gy | |||
| 7 | 0/35(0%) | 0/63 (0%) | 1.0000 | 0/60 (0%) | 0/65 (0%) | 1.0000 |
| 14 | 0/459 (0%) | 33/83 (40%) | <0.0001 | 0/342 (0%) | 0/140 (0%) | 1.0000 |
| 21 | 0/305 (0%) | 0/120 (0%) | 1.0000 | 0/273 (0%) | 0/170 (0%) | 1.0000 |
| 28 | 1/267 (0%) | 14/119 (12%) | <0.0001 | 0/279 (0%) | 0/126 (0%) | 1.0000 |
| 35 | 9/152 (6%) | 1/154 (1%) | 0.0095 | 0/157 (0%) | 0/92 (0%) | 1.0000 |
| 42 | 0/156 (0%) | 0/56 (0%) | 1.0000 | 0/143 (0%) | 0/67 (0%) | 1.0000 |
| 49 | 1/246 (0%) | 13/111 (12%) | <0.0001 | 0/184 (0%) | 0/133 (0%) | 1.0000 |
| 56 | 2/198 (1%) | 6/55 (11%) | 0.0002 | 0/208 (0%) | 0/76 (0%) | 1.0000 |
| 63 | 1/215 (0%) | 1/71 (1%) | 0.4082 | 0/270 (0%) | 0/119 (0%) | 1.0000 |
| 70 | 3/174 (2%) | 0/151 (0%) | 0.1050 | 0/199 (0%) | 0/152 (0%) | 1.0000 |
| 77 | 3/141 (2%) | 3/109 (3%) | 0.7490 | 0/153 (0%) | 0/51 (0%) | 1.0000 |
| 84 | 0/146 (0%) | 3/31 (10%) | 0.0002 | 0/209 (0%) | 0/96 (0%) | 1.0000 |
| 91 | 2/127 (2%) | 0/33 (0%) | 0.4682 | 0/231 (0%) | 0/58 (0%) | 1.0000 |
| 98 | 7/179 (4%) | 5/27 (19%) | 0.0025 | 0/190 (0%) | 0/2 (0%) | 1.0000 |
| 105 | 0/118 (0%) | 3/29 (10%) | 0.0004 | 0/190 (0%) | 0/5 (0%) | 1.0000 |
| 112 | 0/166 (0%) | 2/124 (2%) | 0.1006 | 0/247 (0%) | 0/1 (0%) | 1.0000 |
| 119 | 0/55 (0%) | 19/96 (20%) | 0.0004 | 0/174 (0%) | ||
| 126 | 0/91 (1%) | 0/139 (0%) | 0.2155 | 0/204 (0%) | ||
Notes. Marrow was explanted at day 60 after 8 Gy TBI and results were scored, as described in Table 1, but for day 14. For p16+/LUC colonies scored weekly for cells harvested between day 14 and day 119 of LTBMC, the irradiated group had a significantly larger percentage of luciferase+ cell-containing colonies than the nonirradiated group. For each of the cell genotypes in this assay of colonies scored at day 14 (p16+/LUC and C57BL/6), the percentage of luciferase+ cell-containing colonies was compared between 0 Gy and 8 Gy, using the two-sample proportion test.
FIG. 6.
Morphologic appearance of single senescent cells stained for both β-gal and p16 using non-adherent cells removed at 23 weeks from LTBMCs that had been established from explanted marrow from nonirradiated mice or mice at day 60 after 8 Gy TBI. Results show merging of images, showing that same cells were pictured for β-gal and p16 (500×).
FIG. 7.

Quantitation of the percentage of β-gal positively stained cells in non-adherent single cells that were harvested at 21 weeks from LTBMCs that had been established from explanted marrow from p16+/LUC and C57BL/6 mice at either day 60 after 8 Gy TBI compared to nonirradiated control mice of the same age.
Comparison of Senescent Cells in CFU-GEMM Colonies that were Derived from Freshly Explanted Marrow at One Year after 8 Gy TBI of p16+/LUC Mice
Marrow was harvested from other TBI mice at one year after 8 Gy TBI and assayed for senescent single cells and senescent cell-containing CFU-GEMM in vitro. We compared senescence in freshly removed marrow cells and derived CFU-GEMM colonies from p16+/LUC mice in the marrow explants at one year with marrow harvested at 60 days and marrow from nonirradiated mice. The results showed that similar numbers of senescent cells and cells that were detected in CFU-GEMM that were derived by holding marrow in situ for one year compared to 60 days (Tables 3-4). Using the p16 marker of senescence, or the β-gal biomarker, there was a clear induction by 8 Gy TBI of more senescent cells in bone marrow both in vivo and in explanted irradiated marrow in vitro to LTBMCs (Figs. 6 and 7).
DISCUSSION
Ionizing radiation induces life shortening in experimental animals (38, 39) and irradiated tissues lose physiologic functions, and display molecular biological and biochemical changes that are consistent with accelerated aging (23). The relationship of cellular senescence to aging is a topic of great interest, particularly since many assays for senescence show increases with age (25, 40-56). While p16, p21, p53, and other regulatory proteins have been associated with senescence (33-35, 53), the stable expression of these biomarkers in senescent cells is different from the transient expression of these same proteins after irradiation (6, 14, 35). Irradiated cell lines in culture also can show transient elevation of p16 and p21 (35). Cells that become senescent after exposure to a cytotoxic agent or by multiple cell passage also show stably elevated levels of p16 and p21, as well as β-gal (3, 12, 13, 17). Senescent cells, which accumulate β-gal, increase in frequency during aging and reside in tissues with reduced capacity for self-renewal or differentiation (12, 13). Removing senescent cells by administration of senolytic drugs has been suggested as a method of slowing the aging process (57, 58), as well as decreasing carcinogenesis (59) and tissue damage (13).
Quiescent cells, including hematopoietic stem cells (18, 19), also remain non-dividing within tissues in situ, but retain the capacity to self-renew and/or differentiate at later times (41, 54). The study of quiescent cells in bone marrow has been greatly facilitated by detection of multiple cell surface markers on hematopoietic stem cells, which allow both flow analysis and cell sorting (41, 54). Intravenously injected purified bone marrow hematopoietic stem cells home to the bone marrow and can return to non-proliferative state for the life of the animal (41). In contrast, senescent cells do not express reliable surface biomarkers for labeling and cell sorting. Furthermore, the activator protein AP-1 can reverse oncogene RAS-induced senescence (26). Whether radiation-induced senescent cells can resume cell division, and whether they are associated with late radiation effects including fibrosis and oncogene-induced transformation is unknown (3).
In the current studies, we quantitated the appearance of senescent cells in the lungs and bone marrow of p16+/LUC mice, a strain which demonstrates p16 linked to the luciferase enzyme that converts the target protein luciferin to a fluorescent molecule. Luciferase is not expressed on the cell surface to facilitate fluorescent cell sorting, so luciferase-positive cells must be visualized in situ by immunohistochemical staining, a technique similar to that required for enzymatic staining of β-gal-positive cells. The level of light emitted by luciferase can be visualized in organs by imaging of live mice (1); however, single p16+/LUC cells do not emit enough light to easily allow fluorochrome-based cell sorting, nor visualization of individual cells in vitro. Serial imaging was performed with the same animals at sequential time points after 20 Gy irradiation of the thoracic cavity or 8 Gy TBI without the need for sacrifice of animals, sectioning of tissues and staining for β-gal. At day 170 after 20 Gy to the lungs, there was a correlation of senescence with areas of fibrosis in serial sections of explanted lung and in skin.
We compared the results of generation of luciferase-positive multilineage colonies from p16+/LUC mouse long-term marrow cultures derived from marrow explanted at 60 days after 8 Gy TBI with that from marrow from other mice removed at one year after 8 Gy sublethal TBI. While the lungs of 8 Gy TBI mice showed no evidence of senescence at one year using Lumina XR imaging, the excised lung specimens did demonstrate increased levels of RNA for senescence-associated proteins p16, p19, p21, as well as SASP proteins TGF-β and IL-6. These protein levels were significantly elevated, although, they were not clearly associated with areas of fibrosis by Masson’s trichrome staining. Rare β-gal-positive cells were detected in stained sections of lungs from 8 Gy TBI mice at one year with numbers too small to allow analysis of the phenotype of senescent cells. In contrast, the lungs of 20 Gy thoracic irradiated mice at day 70 showed significant numbers of p16-positive senescent cells in areas of pulmonary fibrosis, and phenotyping of senescent cells by immunohistochemistry showed them to be predominantly of the endothelial lineage; however, senescent cells were also detected in other cell populations including epithelial, hematopoietic, macrophage and fibroblasts. Further studies are needed to determine whether radiation-induced senescent cells include other lineages in the mouse lung.
At day 60 after 8 Gy TBI, peripheral blood counts had returned to normal, and marrow cellularity was restored to the preirradiation level. LTBMCs from mice irradiated at day 60 showed reduced hematopoietic capacity, reduced production of hematopoietic cells, and reduced numbers of multilineage colony-forming progenitor cells released into the non-adherent layer after 8 weeks in culture. However, the conditions for maintenance of hematopoietic stem cells in long-term marrow culture induced senescence in single cells and colony-forming cells compared to leaving marrow in situ for one year after TBI. Senescent cells within in CFU-GEMM colonies of greater than 50 cells from p16+/LUC mice were easily counted by adding luciferin to the culture plates at day 7 or day 14. Previously reported studies have shown that senescence of hematopoietic stem cells is induced by TBI (61, 62) and is expressed long after irradiation (63). These results also confirm and extend those in our previously published study (17). The current studies showed that the cumulative production of luciferase-positive cell-containing colonies was clearly increased in marrow cultures from irradiated p16+/LUC compared to control nonirradiated animals. In other studies, LTBMCs established from homozygous p16LUC/LUC mice produced fewer senescent cells than did those from p16+/LUC mouse marrow (data not shown). The data with p16LUC/LUC mouse marrow may reflect a genotoxic effect of two copies of the manipulated p16 gene or a carcinogenic effect of radiation on this genotype, as reported previously (1).
The marrow mesenchymal stem cell (stromal cell) compartment has been shown to be different from the hematopoietic stem cell compartment with respect to biologic changes and senescence after explant of marrow at one year after TBI. Stromal cells express radiation damage in vitro with respect to decreased hematopoietic cell support and inability to proliferate in vitro. These changes are not reflected in the peripheral blood or marrow cellularity at the time of marrow explant (17).
While LTBMCs from 8 Gy TBI p16+/LUC mice generated overall fewer hematopoietic colony-forming cells in vitro, there were more senescent non-adherent cells and more senescent cell-containing colonies that were detected over the 22 weeks of culture compared to the results with marrow from nonirradiated mice. Since the day 14 colonies are derived from a more primitive hematopoietic progenitor cell (41), the data indicate that the progenitors of both populations sustained radiation damage in vivo leading to senescence that was expressed in vitro.
Since we were unable to serially image single luciferase-positive cells within CFU-GEMM colonies, as they formed in vitro due to the low level of light emitted at the wavelength of luciferase fluorescence, we could not determine when senescent cells first appeared within each individual CFU-GEMM, as it developed over 7 or 14 days in vitro. Whether senescent cells appeared after the first cell division and increased in number continually or all appeared after a later cell division is not known. Scored colonies were uniformly >50 cells and represent those having had a minimum of seven cell divisions. If senescent cells within p16+/LUC mouse marrow-derived colonies were incapable of cell division, then such cells either accumulated in the colony over the 7 to 14 days or simultaneously appeared in colonies during late cell divisions. Whether a subpopulation of cells within a 50-cell colony became senescent and remained intact, while other cells continued to divide, and then later became senescent at several time points over the 7 days or 14 days in secondary colony culture, is also unknown. Previously published studies have demonstrated that 6 Gy and 8 Gy TBI induces senescence in many tissues and organs including the lungs of mice (62–64). Further studies on the kinetics of appearance of senescent cells within organoids in vitro (including LTBMCs), and in different organs in vivo, will be required to more precisely link senescence with fibrosis (64). Interaction between cell phenotypes within organs may require a different model system, such as that found in tdTOMp16+ mice (2).
The current data indicate that p16+/LUC mice are potentially valuable as a model system in which to study the kinetics of radiation induction of senescence in different organs and the effect of senolytic drugs in the same animals over time. Furthermore, the role of senescent cells in the effectiveness of drugs to treat radiation pulmonary fibrosis can now be quantitated.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID grant no. U19A1068021). This project used the Hillman Animal Facility and Tissue and Research Pathology Services, which are supported in part by the NIH/National Cancer Institute (NCI award no. P30CA047904).
Footnotes
Editor’s note. The online version of this article (DOI: https://doi.org/10.1667/RADE-20-00286.1) contains supplementary information that is available to all authorized users.
REFERENCES
- 1.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell 2013; 152:340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sessions GA, Copp ME, Liu J-Y, Sinker MA, D’Costa S, Diekman BO. Controlled induction and targeted elimination of p16(INK4a)-expressing chondrocyte in cartilage explant culture. FASEB J 2019; 33:12364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking transcriptional heterogeneity in senescent cells. Curr Biol 2017; 27:2652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasso D, Garcia MN, Hamidi T, Cano C, Lomberk G, Urrutia R, et al. Genetic inactivation of the pancreatitis-inducible gene Nupr1 impairs PanIN formation by modulating Kras(G12D)-induced senescence. Cell Death Differ 2014; 21:1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr., Hogaboam CM Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol 2019; 60:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalash R, Epperly MW, Goff J, Dixon T, Sprachman MM, Zhang X, et al. Amelioration of radiation-induced pulmonary fibrosis by a water-soluble bifunctional sulfoxide radiation mitigator (MMS350). Radiat Res 2013; 180:474–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citrin DE, Shankavaram U, Horton JA, Shield W III, Zhao S, Asano H, et al. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst 2013; 105:1474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M, et al. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiation Oncol Biol Phys 2017; 99:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Thummuri D, Zheng G, Okunieff P, Citrin DE, Vujaskovic Z, et al. Cellular senescence and radiation-induced pulmonary fibrosis. Transl Res 2019; 209:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyer C, Distler JH. Tyrosine kinase signaling in fibrotic disorders: translation of basic research to human disease. Biochim Biophys Acta 2013; 1832:897–904. [DOI] [PubMed] [Google Scholar]
- 11.Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J 2015; 45:1426–33. [DOI] [PubMed] [Google Scholar]
- 12.Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. Cellular senescence: defining a path forward. Cell 2019; 179:813–25. [DOI] [PubMed] [Google Scholar]
- 13.Chandra A, Lagnado AB, Farr JN, Monroe DG, Park S, Hachfeld C, et al. Targeted reduction of senescent cell burden alleviates focal radiotherapy-related bone loss. J Bone Miner Res 2020; 35:1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalash R, Berhane H, Au J, Rhieu BH, Epperly MW, Goff J, et al. Differences in irradiated lung gene transcription between fibrosis-prone C57BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo 2014; 28:147–72. [PMC free article] [PubMed] [Google Scholar]
- 15.Soysouvanh F, Benadjaoud MA, Santos MD, Mondini M, Lavigne J, Bertho A, et al. Stereotactic lung irradiation in mice promotes long-term senescence and lung injury. Int J Radiation Oncol Biol Phys 2020; 106:1017–27. [DOI] [PubMed] [Google Scholar]
- 16.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 2017; 8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivananthan A, Shields D, Fisher R, Hou W, Zhang X, Franicola D, et al. Continuous 1-year oral administration of the radiation mitigator, MMS350, after total body irradiation restores bone marrow stromal cell proliferative capacity and reduces age-related senescence in Fanconi Anemia (Fanca−/−) mice. Radiat Res 2019; 191:139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimaki K, Li R, Chen H, Croce KD, Zhang HH, Xing J, et al. Graded regulation of cellular quiescence depth between proliferation and senescence by a lysosomal dimmer switch. Proc Natl Acad Sci, USA 2019; 116:22624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang R, Arif T, Kalmykova S, Kasianov A, Lin M, Menon V, et al. Restraining lysosomal activity preserves hematopoietic stem cell quiescence and potency. Cell Stem Cell 2020; 26:359–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slobodnyuk K, Radic N, Ivanova S, Llado A, Trempolec N, Zorzano A, et al. Autophagy-induced senescence is regulated by p38a signaling. Cell Death Dis 2019; 10:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada A, Nangaku M, Jao T-M, Maekawa H, Ishimono Y, Kawakami T, et al. D-serine, a novel uremic toxin, induces senescence in human renal tubular cells via GCN2 activation. Sci Rep 2017; 7:11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielak-Zmijewska A, Wnuk M, Przybylska D, Grabowska W, Lewinska A, Alster O, et al. A comparison of replicative senescence and doxorubicin-induced premature senescence of vascular smooth muscle cells isolated from human aorta. Biogerontology 2014; 15:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosteiro L, Pantoja C, Alcazar N, Marion RM, Chondronasiou D, Rovira M, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 2016; 354:1020–5. [DOI] [PubMed] [Google Scholar]
- 24.An S, Cho S-Y, Kang J, Lee S, Kim H-S, Min D-J, et al. Inhibition of 3-phosphoinositide-dependent protein kinase 1 (PDK1) can revert cellular senescence in human dermal fibroblasts. Proc Natl Acad Sci U S A 2020; 117:31535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson DM, McBryan T, Jeyapalan JC, Sedivy JM, Adams PD. A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age 2014; 36:1049–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Zamudio RI, Roux P-F, de Freitas JANLF, Robinson L, Dore G, Sun B, et al. AP-1 imprints a reversible transcriptional programme of senescent cells. Nat Cell Biol 2020; 22:842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 20l0; 5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel NH, Sohal SS, Manjili MH, Harrell JC, Gewirtz DA. The roles of autophagy and senescence in the tumor cell response to radiation. Radiat Res 2020; 194:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae SY, Park SY, Park JO, Lee KJ, Park G. Gardenia jasminoides extract-capped gold nanoparticles reverse hydrogen peroxide-induced premature senescence. J Photochem Photobiol B 2016; 164:204–11. [DOI] [PubMed] [Google Scholar]
- 30.Scott RW, Arostegui M, Schweitzer R, Rossi FMV, Underhill TM. Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell 2019; 25:797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, LeBoff MS, et al. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell 2008; 7:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamakoshi K, Takahashi A, Hirota F, Nakayama R, Ishimaru N, Kubo Y, et al. Real-time in vivo imaging of p16INK4a reveals cross talk with p53. J Cell Biol 2009; 186:393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J-Y, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, et al. Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A 2019; 116:2603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosteiro L, Pantoja C, de Martino A, Serrano M. Senescence promotes in vivo reprogramming through p16INK4a and IL-6. Aging Cell 2018; 17:e12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinman J, Epperly M, Hou W, Willis J, Wang H, Fisher R, et al. Improved total-body irradiation survival by delivery of two radiation mitigators that target distinct cell death pathways. Radiat Res 2018; 189:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berhane H, Epperly MW, Goff J, Kalash R, Cao S, Franicola D, et al. Radiobiologic differences between bone marrow stromal and hematopoietic progenitor cell lines from Fanconi Anemia (Fancd2−/−) mice. Radiat Res 2014; 181:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc 1955; 50:1096–121. [Google Scholar]
- 38.Epperly MW, Smith T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of total body irradiation induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Radiat Res 2008; 170:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epperly MW, Wang H, Jones J, Dixon T, Montesinos C, Greenberger JS. Antioxidant-chemoprevention diet ameliorates late effects of total body irradiation and supplements radioprotection by MnSOD-plasmid liposome administration. Radiat Res 2011; 175:759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calimport SRG, Bentley BL, Stewart CE, Pawelec G, Scuteri A, Vinciguerra M, et al. To help aging populations, classify organismal senescence. Science 2019; 366:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal M, Ebert BL. Clonal hematopoiesis in human aging and disease. Science 366:4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melzer D, Pilling LC, Ferrucci L. The genetics of human ageing. Nat Commun 2020; 21:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters-Hall JR, Min J, Tedone E, Sho S, Siteni S, Mender I, et al. Proliferation of adult human bronchial epithelial cells without a telomere maintenance mechanism for over 200 population doublings. FASEB J 2020; 34:386–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan X, Jiang B, Wu X, Xu H, Cao S, Bai N, et al. Accumulation of prelamin A induces premature aging through mTOR overactivation. FASEB J 2020; 34:7905–14. [DOI] [PubMed] [Google Scholar]
- 45.Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 2020; 180:968–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavallaie M, Voshtani R, Deng X, Qiao Y, Jiang F, Collman JP, et al. Moderation of mitochondrial respiration mitigates metabolic syndrome of aging. Proc Natl Acad Sci U S A 2020; 117:9840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YY, Um J-H, Yoon J-H, Lee D-Y, Lee YJ, Kim DH, et al. p53 regulates mitochondrial dynamics by inhibiting Drp1 translocation into mitochondria during cellular senescence. FASEB J 2020; 34:2451–64. [DOI] [PubMed] [Google Scholar]
- 48.Desdin-Mico G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabande-Rodriguez E, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020; 368:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer 2020; 20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge Y, Miao Y, Gur-Cohen S, Gomez N, Yang H, Nikolova M, et al. The aging skin microenvironment dictates stem cell behavior. Proc Natl Acad Sci U S A 2020; 117:5339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rymut N, Heinz J, Sadhu S, Hosseini Z, Riley CO, Marinello M, et al. Resolvin D1 promotes efferocytosis in aging by limiting senescent cell induced MerTK cleavage. FASEB J 2020; 34:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 2017; 23:775–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16INK4a-positive senescent cells delays ageing-associated disorders. Nature 2011; 479:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorshkind K, Hofer T, Montecino-Rodriguez E, Pioli PD, Rodewald H-R. Do haematopoietic stem cells age? Nat Rev Immunol 2020; 20:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaum N, Lehallier B, Hahn O, Palovics R, Hosseinzadeh S, Lee SE, et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 2020; 583:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow H-M, Shi M, Cheng A, Gao Y, Chen G, Song X, et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci 2019; 22:1806–19. [DOI] [PubMed] [Google Scholar]
- 57.Amor C, Feucht J, Leibold J, Ho Y-J, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020; 583:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Overholtzer M Senescent cells feed on their neighbors. Nature 2019; 574:635–6. [DOI] [PubMed] [Google Scholar]
- 59.Li F, Huangyang P, Burrows M, Guo K, Riscal R, Godfrey J, Lee KE, et al. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat Cell Biol 2020; 22:728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shao L, Feng W, Li H, Gardner D, Luo Y, Wang Y, et al. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood 2014; 123:3105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang J, Wang Y, Shao L, Laberge R-M, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016; 22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Le ONL, Rodier F, Fontaine F, Coppe J-P, Campisi J, DeGregori J, et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell 2010; 9:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epperly MW, Sikora CA, Defilippi S, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Molecular Cell Biology 2003; 29:213–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.