Abstract
Objective:
Apolipoprotein M (ApoM) enriches sphingosine-1-phosphate (S1P) within high density lipoproteins (HDL) and facilitates the activation of the S1P1 receptor by S1P, thereby preserving endothelial barrier function. Many protective functions exerted by HDL in extravascular tissues raise the question how S1P regulates transendothelial HDL transport.
Approach and Results:
HDL were isolated from plasma of wild type mice, Apom knock-out mice, human apoM transgenic mice or humans and radioiodinated to trace its binding, association, and transport by bovine or human aortic endothelial cells (BAECs and HAECs, respectively). We also compared the transport of fluorescently-labeled HDL or Evan’s Blue, which labels albumin, from the tail vein into the peritoneal cavity of apoE-haploinsufficient mice with (S1P1-iECKI) or without (CTRL) endothelium specific knock-in of S1P1. The binding, association, and transport of HDL from Apom knock-out mice and human apoM-depleted HDL by BAECs was significantly lower than that of HDL from wild type mice and human apoM containing HDL, respectively. The binding, uptake, and transport of 125I-HDL by HAECs was increased by an S1P1 agonist but decreased by an S1P1 inhibitor. Silencing of scavenger receptor BI (SR-BI) abrogated the stimulation of 125I-HDL transport by the S1P1 agonist. Compared to CTRL, S1P1-iECKI showed decreased transport of Evan’s Blue but increased transport of HDL from blood into the peritoneal cavity and SR-BI expression in the aortal endothelium.
Conclusions:
ApoM and S1P1 promote transendothelial HDL transport. Their opposite effect on transendothelial transport of albumin and HDL indicates that HDL passes endothelial barriers by specific mechanisms rather than passive filtration.
Keywords: HDL, sphingosine-1-phosphate, apolipoprotein M, endothelium, transcytosis
Introduction
Low plasma levels of high density lipoprotein (HDL) cholesterol are associated with increased risk of coronary heart disease (CHD)1. HDL particles exert many effects, which may protect the organism from chemical or biological harm and thereby explain these inverse associations2. Nevertheless, HDL has not been as yet exploited for prevention or treatment of CHD1. An important reason for this shortfall is the structural and functional complexity of HDL particles, which are heterogeneous and complex macromolecules carrying hundreds of lipid species and proteins2, 3. Among them sphingosine-1-phosphate (S1P) has attracted considerable interest. S1P is the agonist of five G-protein coupled receptors termed S1P1, S1P2, S1P3, S1P4, and S1P5 4. S1P is enriched in HDL due to the presence of apoM5. By providing a binding pocket 5, this lipocalin promotes efflux of S1P from erythrocytes 6, 7 and acts as a chaperone facilitating the interaction of S1P with its cognate receptors, notably S1P1 8–10. The interaction of apoM was shown to be mandatory for the activation of S1P1 by S1P in endothelial cells and the subsequent inhibition of adhesion molecule expression 6, 7. In addition, the apoM/S1P complex stabilizes the endothelial barrier as indicated by the exudation of albumin into the extravascular space and the formation of lung edema in Apom knock-out mice 5, 10. The endothelial barrier was restored by treatment with an S1P1 agonist 10. The endothelium-specific knock-out of the S1p1 receptor in mice also led to increased endothelial permeability upon stimulation with immune complexes 11. Moreover, Apom knockout mice showed a weakened blood brain barrier with increased paracellular permeability in the capillaries and enhanced vesicular transport through the endothelium of arterioles and this phenotype was reversed by S1P1-agonist treatment 12.
The classical anti-atherogenic function of HDL, namely the removal of cholesterol from macrophages for reverse transport of cholesterol to the liver, requires both entry into and exit from the arterial wall, and hence two passages through endothelial cells13. By recording the binding, uptake, degradation, and resecretion of radioiodinated HDL as well as by fluorescence microscopy and electron microscopy of labeled HDL, our laboratory showed that human and bovine aortic endothelial cells (HAECs and BAECs, respectively) internalize HDL without degrading it 14, 15. By using a transwell cell culture system and applying siRNAs or pharmacological inhibitors against candidate genes and proteins, respectively, we found scavenger receptor BI (SR-BI), ATP binding cassette transporters (ABC) A1 and G1, endothelial lipase (LIPG), and the ecto-ATPase/purinergic P2Y-receptor axis to be rate limiting for the transendothelial transport of apoA-I or HDL 16–19. Moreover, SR-BI facilitates the transport of HDL from extravascular tissues into lymphatic vessels as well as for reverse cholesterol transport 20. However, how HDL passes the elastin barrier in the arterial wall is not known.
Any general stabilization of intercellular junctions and general inhibition of transendothelial vesicular transport by HDL-bound apoM and S1P would interfere with the exit of HDL from the blood stream into extravascular compartments and thereby counteract the protective functions exerted by HDL therein 2, 21. We hypothesized that S1P does not interfere with transendothelial transport of HDL and therefore investigated the effects of apoM and the S1P1 receptor on the binding, uptake, and re-secretion of HDL by bovine or human aortic endothelial cells as well as transendothelial transport of HDL in vivo.
Materials and Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Details on animals, cells, antibodies, primers, and small interfering RNA’s are provided in the Major Resource Table.
Mouse models
ApoM-free/S1P-poor and apoM/S1P-enriched HDL were isolated from pooled plasmas of male and female Apom knock-out mice and male and female human APOM overexpressing mice, respectively, which were described previously 20, 22, 23.
Experiments on the effects of the S1P1 receptor agonist SEW 2871 on the transport of HDL and Evan’ Blue were performed in 10 – 12 weeks old female C57Bl/6J mice (Charles River Laboratories, Sulzfeld, Germany)) fed with a regular laboratory diet.
Triple transgenic mice overexpressing murine S1P1 exclusively in endothelial cells were developed by crossing two lines. The C57Bl/6J-Gt(ROSA)26Sortm1(S1pr1)Geno (referred to as S1pr1 LSL line), generated by genOway (Lyon, France) using their patented Rosa26 locus Quick knock-in™ technology, carries a transgenic cassette in the Rosa26 locus, which harbors S1P1 cDNA. It is separated from the synthetic cytomegalovirus early enhancer/chicken β-actin (CAG) strong promoter by a LoxP-STOP-LoxP (LSL) insert (Supplemental Figure Ia). S1pr1LSL mice were crossed to Apoe−/−Cdh5-CreERT2 mice, provided by Dr. Christian Weber (Ludwig Maximillian University, München, Germany). They express Cre recombinase under control of the VE-cadherin promoter, which is in turn induced by tamoxifen treatment 24. In triple transgenic mice, the LSL insert is hence excised only in Cre-expressing cells, which induces cDNA expression driven by the CAG promoter only in these lineages. Transcription thus occurs exclusively in the endothelial cell lineage. These mice are henceforth referred to as S1P1-inducible endothelial cell knock-in S1P1-iECKI mice. Double heterozygous Apoe+/−Cdh5-CreERT2 +/− mice were used as controls (CTRL). Genotyping was performed by classical PCR (Supplemental Figure Ib). The experiments on transport of lipoproteins and Evan’s Blue as well as en face immunostaining of S1P1 and SR-BI were performed on 10 – 12 weeks old female mice fed a regular laboratory diet. For atherosclerosis studies, following induction of S1P1 overexpression, 6-week-old female mice received high-fat atherogenic diet (w/w: 1.25% cholesterol; 16% fat; 0,5% sodium cholate; Altromin, Lage, Germany; corresponding to Research Diets D12109) for 30 weeks 25, 26.We used female mice because of substantial scientific evidence that atherosclerosis development is more prominent in female mice than in male mice including Apoe knock-out mice 27–29 (3 refs). This is even more relevant in Apoe haploinsufficient mice that inherently less susceptible to atherosclerosis than complete Apoe knock-out mice.
Animal experiments were carried out in compliance with the Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes and was approved by authorities in charge of animal protection (LANUV, permission 84–02.04.2011.A351, 84–02.04.2015.A505 and 84–02.04.2016.A545).
Assessment of vascular permeability for lipoproteins and Evan’s Blue
Female S1P1-iESKI or CTRL mice were intravenously administered with Evans blue (600μg/animal), or DyLight™-HDL or DyLight™-LDL (DyL-HDL and DyL-LDL, respectively; 350 μg/animal, prepared as described below) 15 minutes before LPS (25 μg/animal) was injected intraperitoneally. SEW2871 was diluted in PBS (to 0.1mg/ml in 1% DMSO) and administered as a single i.p. bolus injection (15μg/g body weight) 150 minutes prior to albumin or lipoprotein injection12 Mice were sacrificed after 3h and their peritoneal cavities were washed with 10mL of ice-cold heparinized PBS. After spinning down cells, the supernatants were analyzed for Evans Blue with photometry (620nm, FluoStar Optima, BMG LabTech, Ortenberg, Germany) and for DyL-HDL or DyL-LDL with fluorescence spectrometry (560nm/590nm, FluoStar Optima).
En face immunostaining and atherosclerotic lesion analysis
At sacrifice, inferior vena cava was opened, and mice were perfused by intracardiac injection of ice-cold sterile saline followed by neutral buffered formalin (NBF, HT501128, Sigma Aldrich). Hearts and aortae were dissected and processed as follows: Hearts were further fixed in NBF for 30 minutes, incubated overnight at +4°C and successively embedded into Tissue-Tek O.C.T. compound. Arteries were incubated for 18h with primary antibodies diluted in blocking solution at 4°C under constant agitation (After three washes (10 minutes each) in washing solution (PBS, 0.1% Triton X-100), tissues were incubated with fluorescent secondary antibodies (Alexa Fluor dyes, Molecular Probes) for 1.5h, washed, counterstained and mounted with Fluoroshield DAPI mounting medium (Sigma-Aldrich), and then imaged using a Leica TCS SP2 confocal microscope. Atherosclerosis was evaluated in the aortic roots as described30, 31. Aortas were quickly cleaned of adventitial tissue, opened longitudinally and incubated for 1.5h at room temperature in the blocking solution (PBS, 2% BSA, 0.1% Triton X-100).
Cell culture
Primary Bovine aortic endothelial cells (BAECs) were cultured in Dulbecco’s modified eagle’s medium (DMEM) with 5% fetal bovine serum at 37°C in 5% CO2, 95% air incubator. Human aortic endothelial cells (HAECs) were cultured in endothelial cell basal medium (LONZA Clonetics CC-3156 or ATCC PCS-100–030) with 5% fetal bovine serum (Sigma-Aldrich), 100U/mL of penicillin and 100μg/mL streptomycin (Sigma-Aldrich), supplemented with singleQuots (LONZA Clonetics CC-4176 or ATCC PCS-100–041, containing human Fibroblast Growth Factor, hVEGF, human insulin like growth factor 1, human epithelial growth factiorF, hydrocortisone, ascorbic acid, heparin) at 37°C in a humidified 5% CO2, 95% air incubator as described previously 17, 32.
Lipoprotein Isolation and labeling
HDL (1.063<d<1.21kg/L) was isolated from fresh human normolipidemic plasma of blood donors or mouse plasma by sequential ultracentrifugation as described previously 18, 33. ApoM-containing and apoM-free HDL of human plasma were separated by immunoaffinity chromatography as described previously 7. HDL were radioiodinated with Na125I by the McFarlane monochloride procedure modified for lipoproteins 18. Specific activities ranged between 300 and 900cpm/ng of protein. For assessment of vascular permeability of lipoproteins in mice, LDL and HDL were labeled with DyLight™ 550 fluorescent dye (DyL, Thermo Fischer)) according to the manufacturer’s instruction. Briefly, HDL or LDL (2.0mg/mL, pH adjusted to 8.0 with 50mmol/L sodium borate) were mixed with DyLight™ 550 reagent (1:1, v/v), incubated for 60 minutes, and purified using spin columns provided by the manufacturer.
Small Interfering RNA Transfection
Endothelial cells were reverse transfected with small interfering RNA (Silencer Select, Thermo Fisher Scientific) targeted to S1P1 (SR-BI) and non-silencing control (Main Resource Table) at a final concentration of 5nmol/L using Lipofectamine RNAiMAX transfection reagent (Invitrogen,) in an antibiotic-free medium. All experiments were performed 72h post-transfection and efficiency of transfection was confirmed with at least two siRNAs against each gene using quantitative RT-PCR and Western blotting.
Quantitative real time PCR
Total RNA was isolated using TRI reagent (Sigma T9424) according to the manufacturer’s instruction. Genomic DNA was removed by digestion using recombinant DNase I (Roche) and RNase inhibitor (Ribolock, Thermo Scientific). Reverse transcription was performed using M-MLVRT (Invitrogen, 200U/μL) according to the standard protocol provided by the manufacturer. Quantitative PCR was done with Lightcycler FastStart DNA Master SYBR Green I (Roche) using gene specific primers (for sequences, see Major Resource Table).
Lipoprotein Binding, Cell association and Transport
The methods for the quantification of binding, association, and transport of radiolabeled HDL by endothelial cells have been previously described 13,15–18,21,22. All assays were performed in DMEM (Sigma) containing 25mmol/L HEPES and 0.2% BSA instead of serum. Cells were pretreated for 30 minutes at 37 °C with either S1P1 agonist SEW2871 (Cat No:2284, Tocris, 20nM) or S1P1 inhibitor W146 (Cat No:3602, Tocris, 20nM). The drugs were prepared as described by the vendor. After 30 minutes of treatment with either S1P1 agonist SEW2871 or S1P1 inhibitor W146, the cells were incubated with 10μg/mL of 125I-HDL without (total) or with 40 times excess of non-labeled HDL (unspecific) for 1hour at 4°C for cellular binding and at 37°C for association and transport experiments. Specific cellular binding/ association/ transport was calculated by subtracting the values obtained in the presence of excess unlabeled HDL (unspecific) from those obtained in the absence of unlabeled HDL (total).
Inulin permeability
After culturing for 72h in transwells, HAECs were treated with SEW2871 or W146 for 30 minutes before 2 mCi/mL of 3H-inulin were added into the apical compartment. After 1h of incubation, the filtrated radioactivity was harvested from the basolateral compartment 18.
Western Blotting
Endothelial cells were lysed in RIPA buffer (10mmol/L Tris pH 7.4, 150mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, with protease and phosphatase inhibitors (complete EDTA (Roche)). Amount of protein in the lysed samples is quantified using Pierce BCA protein assay kit (Thermo Fisher Scientific) 30μg of protein were separated on SDS-PAGE and trans-blotted onto PVDF membrane (GE Healthcare). Membranes were blocked in appropriate blocking buffer recommended for the antibody (PBS-T supplemented with 5% milk or BSA) and incubated either for 1hour or overnight on shaker at 4 °C with primary antibodies at a dilution of 1:1000 in the same blocking buffer. Membranes were incubated for 1h with HRP-conjugated secondary antibody (Dako) in blocking buffer (antibody concentrations are provided in the Major Resource Table). Membranes were further incubated with chemiluminescence substrate for 1 minute (Pierce ECL plus, Thermo Fisher Scientific) and imaged using Fusion Fx (Vilber). As indicated, TATA binding protein was used as loading control with primary antibody at 1:5000 and secondary antibody at 1:10000 dilutions. The silencing efficiencies of S1P1 and SR-BI were evaluated and compared to TATA binding protein (TBP) (for sources and concentrations of antibodies, see Major Resource Table).
Cell surface expression analysis
Biotinylation of intact cells was performed using 20mg/mL EZ-Link sulfo-NHS-S-S-Biotin (Thermo Fisher Scientific) in the cold for 1 hour with mild shaking and quenched with ice-cold 50mM Tris pH 7.4. Cells were lysed in RIPA buffer (total cell lysate) and 200–500μg of lysates were incubated with 20μL of BSA-blocked streptavidin beads suspension (GE Healthcare) for 16 hours at 4°C and pelleted by centrifugation; the pellet represents surface proteins. Proteins were dissociated from the pellet by boiling with SDS loading buffer and analyzed by SDS-PAGE and immunoblotted with SR-BI antibody, LDL-receptor and TATA binding proteinor Na+/K+- ATPase which were used as loading control (for sources and concentrations of antibodies, see Major Resource Table).
Statistical Analysis
The data sets were analyzed using the GraphPad Prism 5 software. Comparison between two groups was performed using unpaired Mann Whitney U-tests. The data was obtained from at least three independent experiments, performed in triplicates or quadruplets. Values are expressed as mean ± SEM. P<0.05 was regarded as significant.
Results
The content in apoM modulates the uptake and transendothelial transport of HDL by bovine aortic endothelial cells (BAECs) –
HDL isolated from plasma of Apom knock-out mice showed 21% reduced binding, 30% reduced association, and 48% reduced apical-to-basolateral transport by bovine aortic endothelial cells (BAECs) as compared to HDL of wild type mice (Figures 1A, 1B, and 1C). Likewise, human apoM-depleted HDL, which lacks S1P 5,7, showed reduced association with BAECs as compared to total HDL (Figures 1D, 1E, and 1F). Conversely, HDL isolated from mice overexpressing human apoM were more effectively associating with BAECs (Figure 2; P = 0.0052).
Figure 1: Presence of ApoM enhances binding binding, association and transendothelial transport of HDL in bovine aortic endothelial cells (BAECs).
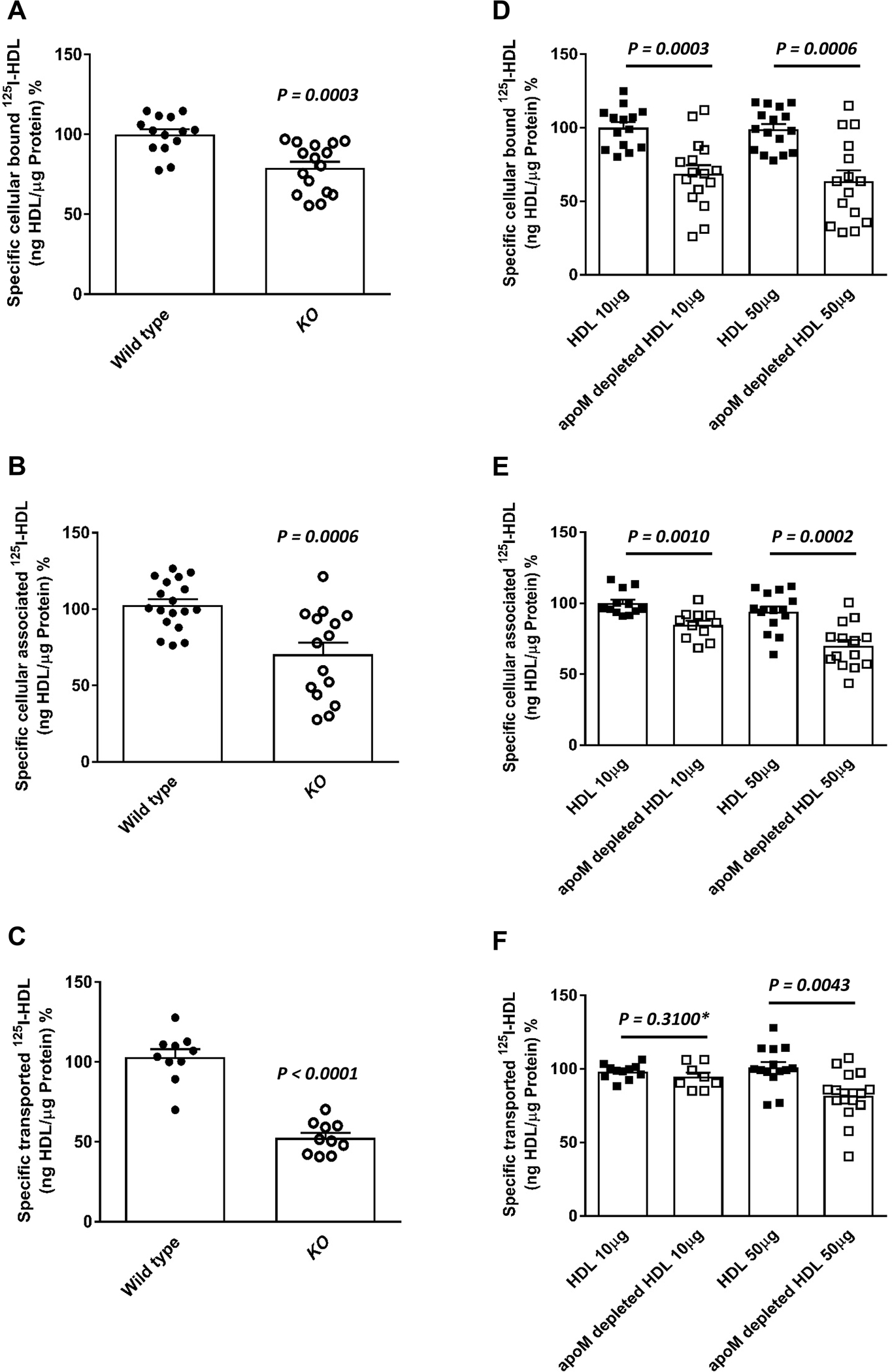
BAECs were cultured for 72 hours before they were incubated for 1 hour with 10μg/mL of 125I-HDL from wild type mice or apom knock-out mice (A- C) or radioioidinated total HDL or radioioidinated apoM-depleted HDL from humans at indicated amounts (D-F) in the absence (total) or in the presence of 40-fold excess of unlabeled HDL, to record nonspecific interactions. Specific binding, association and transport were calculated by subtracting nonspecific values from total values. Specific binding was measured by incubating cells with 125I-HDL (A, D) at 4 °C. To measure specific cell association, cells were incubated with 125I-HDL (B, E) at 37 °C. For the measurement of transport, BAECs were cultured on inserts. The transport of 125I-HDL (C, F) from the apical to basolateral compartment was measured at 37 °C. The results are presented as means ± SEM of three to six independent triplicate experiments (n=3). P was calculated by unpaired Mann Whitney U-test
Figure 2: Enhanced uptake of HDL from human APOM transgenic mice by bovine aortic endothelial cells (BAECs).
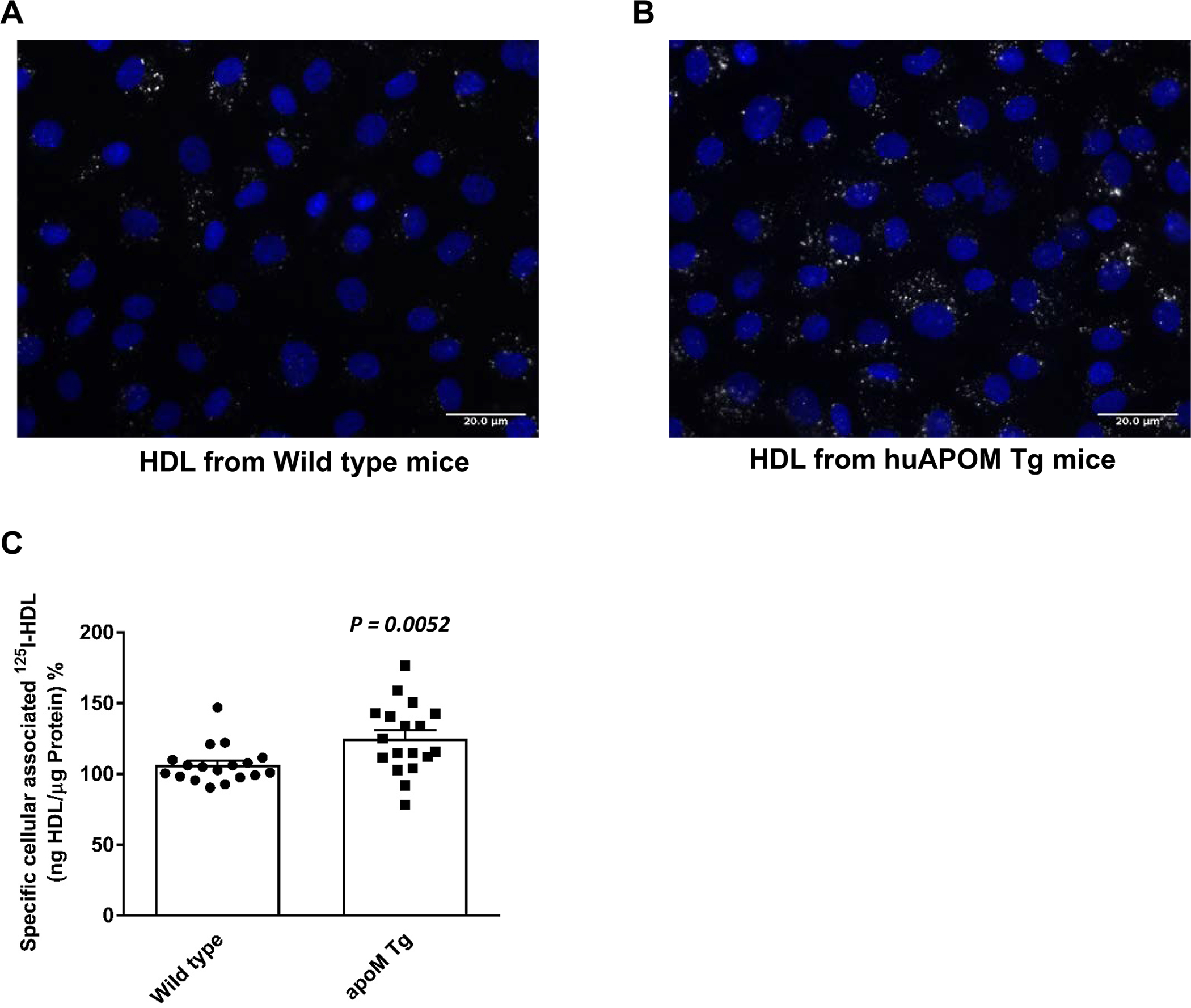
For fluorescence microscopy (A), BAECs were cultured until confluence on cover slips and incubated for 60min at 37° with 50 μg/ml 594Atto-HDL, fixed with 3.75% formaldehyde and counter-stained with DAPI for nuclear staining. (B) BAECs were incubated with 125I-HDL of APOM transgenic mice at 37 °C for 1 hour in the absence (total) or in the presence of 40-fold excess of unlabeled HDL. Specific association was calculated by subtracting unspecific values from total values. The results are presented as means ± SEM of six independent triplicate experiments (n=3). P was calculated by unpaired Mann Whitney U-test.
In human aortic endothelial cells (HAECs), S1P1 regulates cellular binding, association and transendothelial transport of HDL –
We next investigated how the cognate G protein coupled S1P receptor S1P1 regulates the binding, uptake, and transport of HDL by cultivated HAECs. They express S1P1 as analyzed at the protein level (Supplemental Figure IIa). Treatment of HAECs for 30 minutes prior to the assays with 20nM of the S1P1 agonist SEW2871 increased the specific cellular binding of 125I-HDL at 4°C by 95% (Figure 3A), the cellular association of 125I-HDL at 37°C by 39% (Figure 3B), and the transendothelial transport of 125I-HDL from apical to basolateral compartments by 37% (Figure 3C). Conversely, treatment with 20nM of the S1P1 inhibitor W146 (20nM) decreased the specific binding, association, and transport of 125I-HDL by 61% (Figure 3D), 46% (Figure 3E), and 59% (Figure 3F), respectively. Inhibition of S1P1 interfered neither with the permeability of 3H-inulin (Supplemental Figure IIIa) nor the confluence of HAECs as assessed by immunostaining of tight-junction protein 1 (Supplemental Figure IIIb). Like the pharmacological inhibition, silencing of S1P1, decreased the cellular binding, association, and transport of 125I-HDL by 49%, 53% and 42%, respectively (Supplemental Figures IIb to IId).
Figure 3: Agonists (a-c) and inhibitors (d-f) of S1P1 regulate binding, association and transendothelial transport of HDL in human aortic endothelial cells (HAECs).
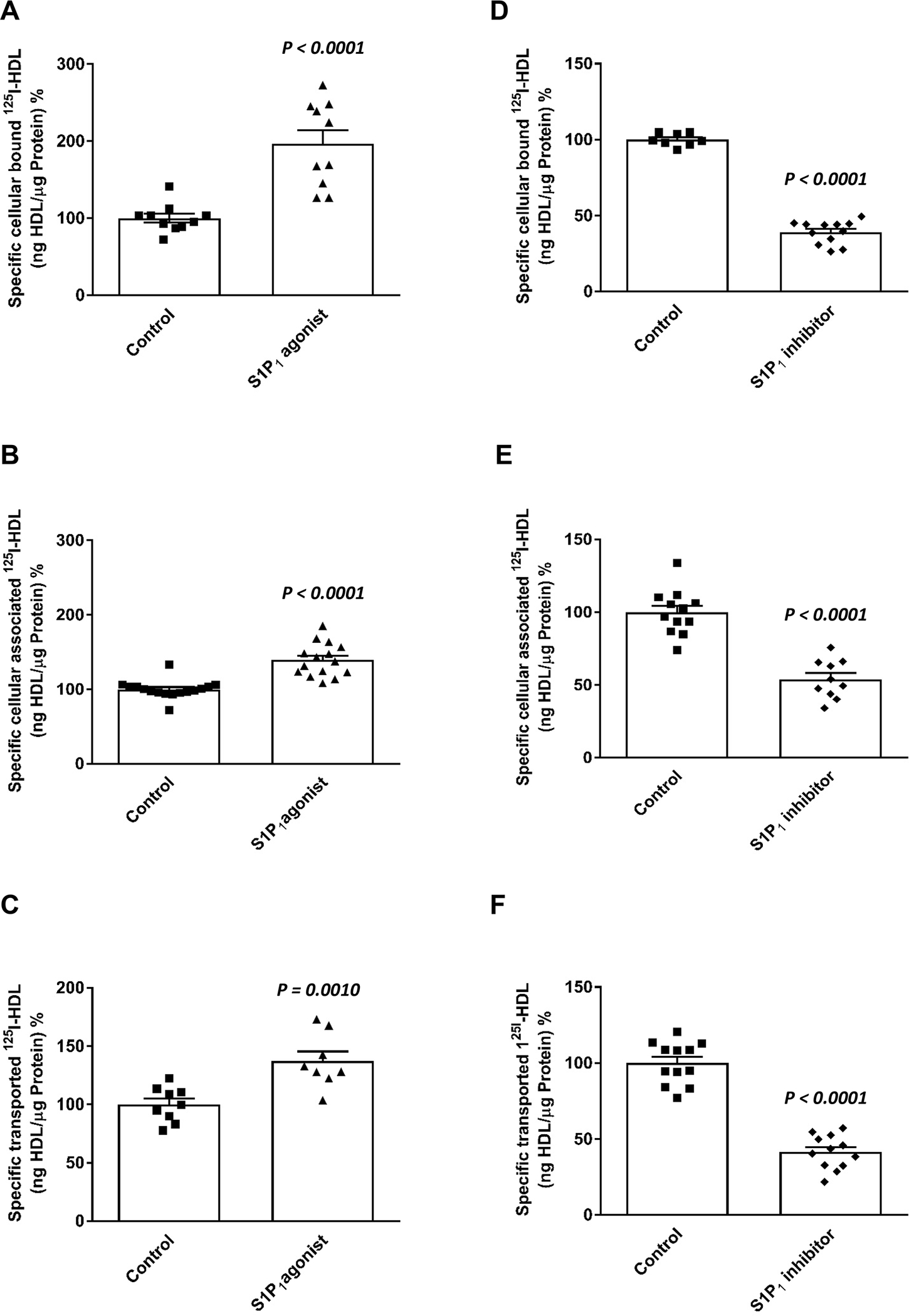
HAECs were cultured for 72h. Cells were then treated with S1P1 agonist (SEW2871, 20nM; A-C) or S1P1 inhibitor (W146, 20nM, D-F) for 30 minutes, at 37 °C as indicated. To study cellular binding, association and transport, HAECs were incubated with 10μg/mL of 125I-HDL for 1 hour in the absence (total) or in the presence of 40-fold excess of unlabeled HDL, to record nonspecific interactions. Specific binding, association and transport were calculated by subtracting nonspecific values from total values. Specific binding was measured by incubating cells with 125I-HDL (A, D) at 4°C. To measure specific cell association, cells were incubated with 125I-HDL (B, E) at 37°C. For the measurement of transport, HAECs were cultured on inserts. The transport of 125I-HDL (C, F) from the apical to basolateral compartment was measured at 37°C. The results are presented as means ± SEM of three independent triplicate experiments (n=3). P was calculated by unpaired Mann Whitney U-test.
S1P1 regulate cellular binding, association and transendothelial transport of HDL via SR-BI
Because SR-BI mediates the binding, uptake and transport of HDL by both BAECs and HAECs 17,22, we next investigated by RNA interference whether it is involved in the S1P1-stimulated trans-endothelial transport of HDL. The knockdown of SR-BI was efficient at protein level (Figure 4A). Specific binding, association, and transport of HDL by HAECs was significantly decreased by 40%, 36%, and 43% after silencing of SR-BI and remained unrestored in the presence of the S1P1 agonist (Figures 4B, 4C, and 4D, respectively). Cell surface biotinylation experiments showed that activation of S1P1 receptor increases the cell surface abundance of SR-BI but not of Na/K ATPase which was included as the loading control (Figure 5
Figure 4: SR-BI is involved in the S1P1 regulated binding, association and transport of HDL by human aortic endothelial cells (HAECs).
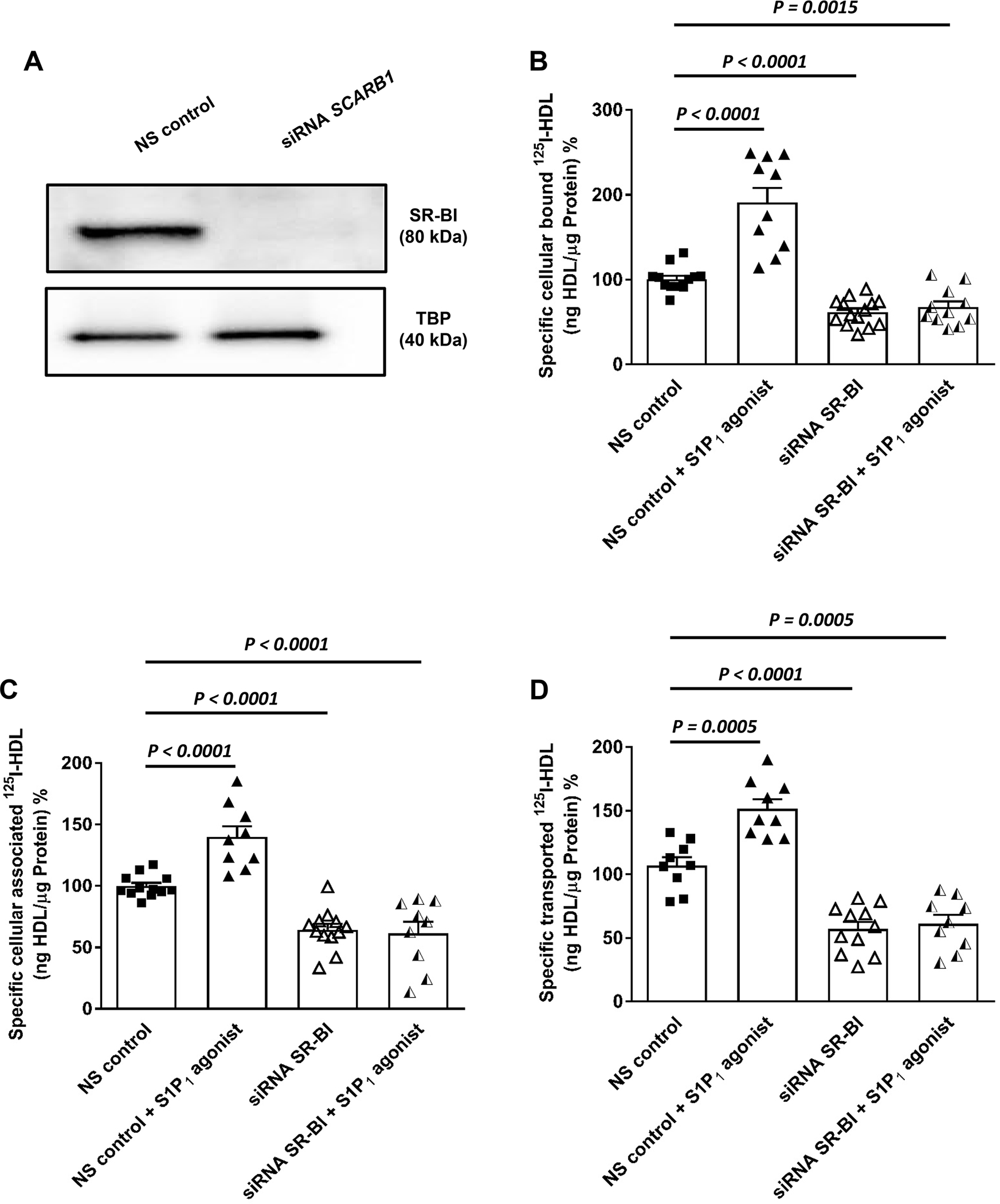
HAECs were transfected with a specific siRNA against SCARB1 or with non-silencing control siRNA (NS control). Assays were performed 72 hours post-transfection. (A) representative Western blot showing the efficacy of the silencing relative to the non-silencing siRNA (NS control) and TATA-binding protein (TBP) used as the loading control. Cells were then treated with S1P1 agonist (SEW2871, 20nM) for 30 minutes at 37 °C. (B) cellular binding of 125I-HDL was measured at 4 °C after pre-treating cells with the S1P1 agonist. (C) cellular association of 125I-HDL was measured at 37 °C. (D) for the measurement of transport of 125I-HDL, HAECs were cultured on inserts. The transport of 125I-HDL was measured after pre-treatment with the S1P1 agonist from the apical to basolateral compartment was measured at 37 °C. The results are presented as means ± SEM of three independent triplicate experiments (n=3). P was calculated by unpaired Mann Whitney U-test).
Figure 5: Effects of S1P1 activation on the cell surface abundance of SR-BI.
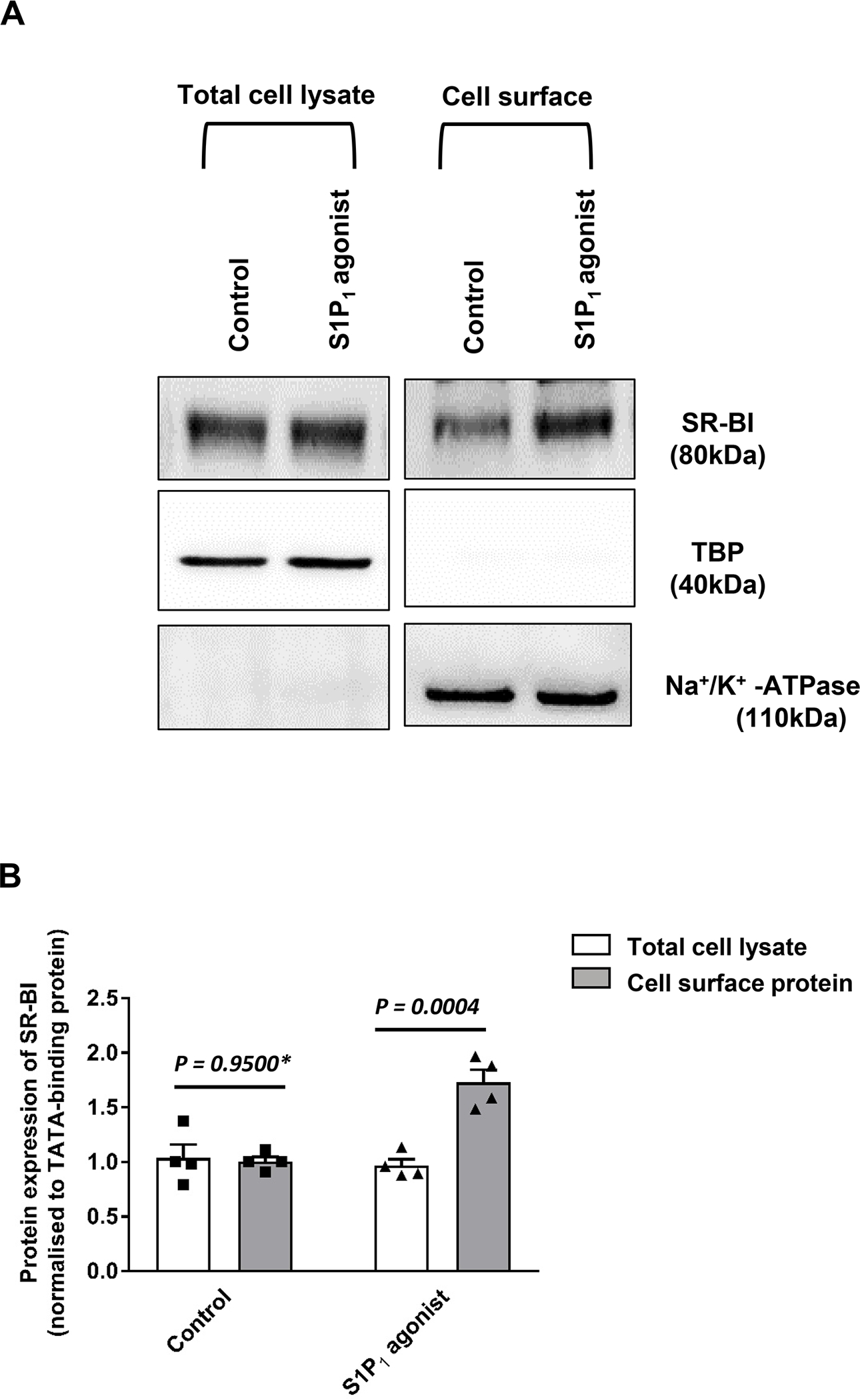
HAECs were cultured for 72 hours. Cells were then treated with 20nM SEW2871 for 30 minutes at 37 °C. Cell surface expression of SR-BI in HAECs was measured using Western blot analysis in total cell lysates (left) and on the cell surface (right). The western blots were probed with anti-SR-BI (82kDa), anti-TBP (40kDa, used as a control for intracellular protein expression) and Na+/K+-ATPase (110 kDa, used as a loading control for cell surface protein expression). (A) shows a representative western blot. (B) shows the summary of four independent experiments quantified by densitometry. P was calculated by unpaired Mann Whitney U-test.
Endothelial overexpression of S1P1 in mice increases SR-BI in the aortic endothelium and endothelial permeability for HDL but decreases endothelial permeability for LDL and Evan’s Blue
Treatment of wild type mice with the S1P1 agonist SEW2871versus saline significantly decreased the occurrence of Evan’s Blue which binds to albumin by 19% but increased the occurrence of DyL-HDL by 37% in the peritoneal lavage (Supplemental Table I). To further investigate the impact of S1P1 on transendothelial lipoprotein transport in vivo, we generated Apoe haploinsufficient mice which overexpress human S1P1 (S1P1-iECKI) receptors under the control of a tamoxifen-inducible VE-cadherin promotor in endothelial cells only. The successful knock-in was demonstrated by genotyping (Supplemental Figure Ib) as well as the indirect immunofluorescence microscopy of aortas (Figure 6 and Supplemental Figure IV). The anti-S1P1 immunoreactivity of the endothelium was rather weak in aortas of CTRL mice (Figure 6A) but much enhanced in aortas of S1P1-iECKI (Figure 6B). Likewise, the anti-SR-BI immunoreactivity was strongly enhanced in the aortic endothelium of S1P1-iECKI mice compared to CTRL mice (Figures 6c and 6d and Supplemental Figure V). While consuming a regular laboratory diet, CTRL and S1P1-iECKI mice had similar plasma levels of cholesterol and triglycerides (Table 1). Upon gel filtration of plasma, no major difference in the distribution of cholesterol and triglycerides among lipoproteins was seen (not shown). Compared to CTRL mice, S1P1 -iECKI mice showed 46% and 40% decreases of Evan’s Blue, and DyL-LDL but a 77% increase of DyL-HDL in the peritoneal lavage (Table 1). After 30 weeks feeding of a 1.25% cholesterol containing Western diet, the S1P1-iECKI mice had developed less pronounced hypercholesterolema as well as 30% less fatty lesions in their sinus aortae than Apoe haploinsufficient CTRL mice (Supplemental Table II).
Figure 6: Demonstration of S1P1 (A, B) or SR-BI (C, D) in the endothelium of aortas from Apoe haploinsufficient mice without (CTRL: A, C) or with overexpression of S1P1 (S1P1-iECKI; B, D).
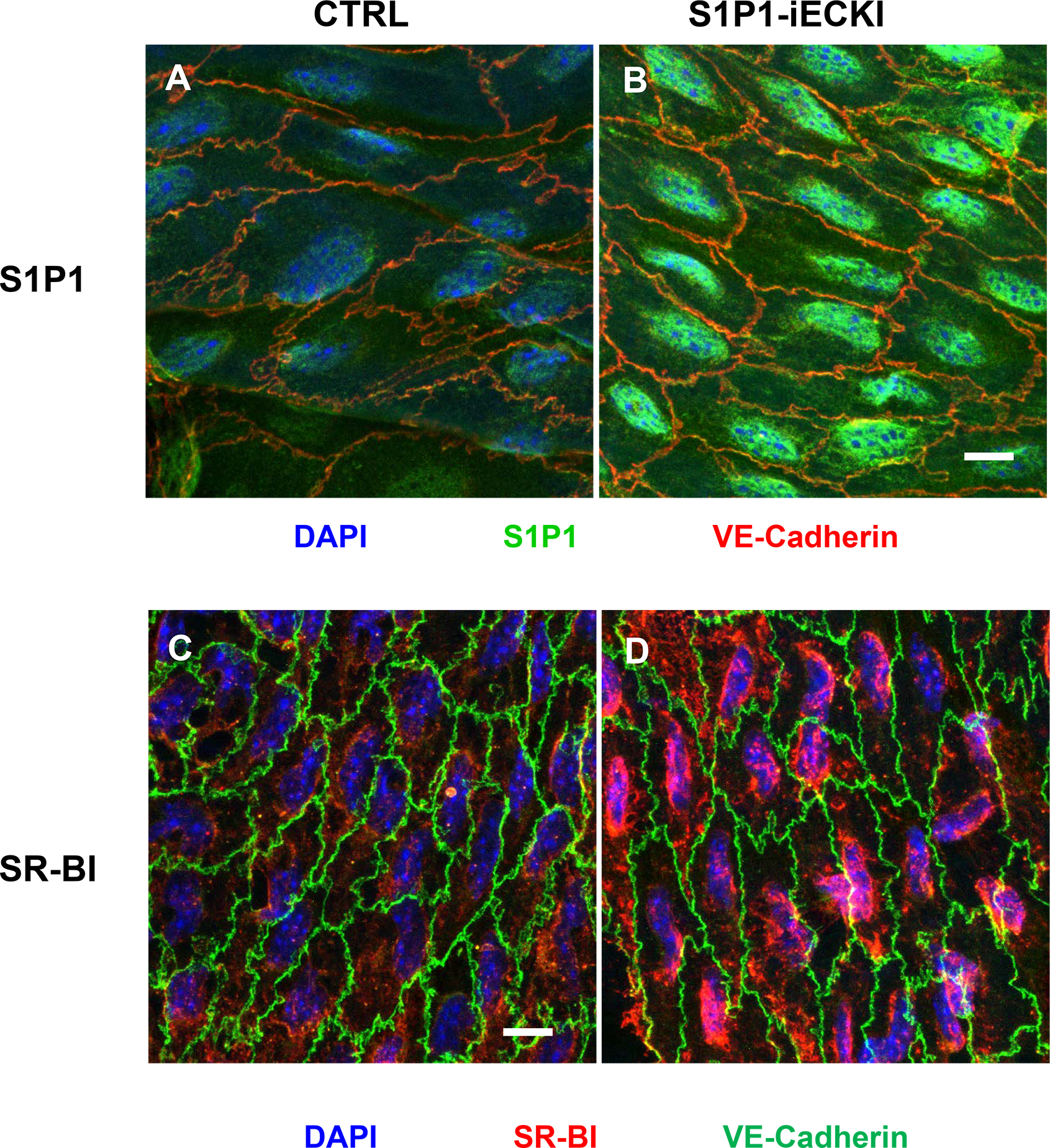
Figure shows en-face prepared aortic immunostainings. Aortas were quickly cleaned of adventitial tissue, opened longitudinally and incubated with primary and secondary antibodies conjugated with green or red fluorescent dyes, as indicated. Nuclei were counterstained with DAPI. Images were captured by confocal microscope and z-axis projections of 14 scanned planes are shown. Scale bar = 10μm. Original micrographs are shown as supplemental figures IV and V.
Table 1: Endothelium-specific overexpression of S1P1 does not alter lipids but differentially regulates endothelial permeability for HDL and Evan’s Blue in Apoe haploinsufficient mice.
i.v. injection of Evan’s Blue or DyLight-HDL and i. p. stimulation with LPS. Collection of peritoneal fluid after 3 hours. (N = 4 per group; results are presented as means ± SEM
| CTRL | S1P1 -iECKI | P* | |
|---|---|---|---|
| Cholesterol (mmol/L) | 1.15 ± 0.16 | 1.05 ± 0.10 | 0.317 |
| Triglycerides (mmol/L) | 0.78 ± 0.11 | 0.65 ± 0.05 | 0.258 |
| Evans’ Blue in the peritoneal fluid (arbU) | 0.13 ± 0.01 | 0.07 ± 0.02 | 0.029 |
| DyL-LDL in the peritoneal fluid (arbU) | 89.0 ± 1.6 | 53.0 ± 6.1 | 0.028 |
| DyL-HDL in the peritoneal fluid (arbU) | 18.5 ± 2.6 | 32.7 ± 2.8 | 0.019 |
:statistical significance calculated by Mann-Whitney U-Test)
Discussion
In mice, the knock-out of the S1P-binding protein apoM caused a strong decrease of S1P levels in plasma and HDL as well as increases in the permeability of lung capillaries and the blood brain barrier for albumin 5, 10, 12. Likewise, the endothelium-specific knock-out of the S1p1 receptor increased the endothelial permeability of albumin 11. Contradicting a general inhibitory effect of S1P on transendothelial macromolecule transport, we here demonstrate that the apoM/S1P/S1P1 interaction promotes rather than inhibits the transendothelial transport of HDL, which may be important in facilitating specific HDL entry into extravascular tissues where the particles exert most of its protective functions 2.
For our in vitro experiments, we cultivated BAECs or HAECs either in monolayers for binding and association experiments or in transwells for the transport experiments. We have used these models previously for the identification and validation of several proteins as facilitating factors of the transendothelial transport of apoA-I or HDL, namely ABCA1 and ABCG1, SR-BI, LIPG and the ecto-ATPase/P2Y receptor axis 16–19. Among them, SR-BI was also identified as a limiting factor of LDL transport by independent laboratories and methods 24, 34, 35.
To study the role of apoM and S1P in transendothelial HDL transport, we compared HDL of wild type mice and Apom knock-out mice for binding, uptake, and transport by BAECs. Christoffersen et al. have shown that the lack of apoM leads to the near disappearance of S1P from HDL5–7. The absence of apoM and S1P caused 25% to 50% decrease in the binding, association, and transport of radioiodinated HDL by BAECs (Figures 1A, 1B, and 1C). Similar significant decreases were seen for apoM-depleted HDL (Figures 1D, E and 1F), which also is completely devoid of S1P 5. Since apoM predominates in smaller HDL3 particles 36, one may wonder whether different particle size rather than difference in apoM and S1P content caused the differences in uptake and transport. However HDL of mice transgenic for human apoM, which have 150% to 200% higher levels of S1P 33, 22, showed a significantly higher endothelial cell association than HDL of wild type control mice (figure 2) despite their increased particle size22, 37. Moreover, we showed previously that HDL3 and HDL2 are equally bound, internalized and transported through aortic endothelial cells and that HDL size rather decreases during the transport 17, 18. Therefore, and because of the presence of apoM in only 5% of HDL particles36, it is very unlikely that the increased transendothelial transport of apoM containing HDL compared to apoM deficient HDL is simply caused by smaller particle size.
Binding, uptake, and transport of HDL by HAECs were promoted by the S1P1 agonist SEW2871 and impaired by the S1P1 inhibitor W146. These drugs were previously used to show the involvement of S1P1 in mediating inhibitory HDL effects on adhesion molecule expression 9. The present study validates the specificity of the drugs as RNA interference mimicked the effects of W146 and abrogated the effects of SEW2871. In addition, interference with SR-BI abrogated the enhanced binding, uptake, and transport of HDL otherwise apparent in the presence of the S1P1-agonist. Likewise, increased cell surface abundance of SR-BI underlies the enhanced transendothelial transport upon stimulation with VEGF-A 32. Activation of either S1P1 and VEGFR-2 induces Akt phosphorylation 38, which promotes SR-BI translocation to the plasma membrane and binding, uptake and transport of HDL by HAECs 32. A recent untargeted proteomics approach found that the expression of S1p1 in murine pulmonary lung endothelial cells alters the apical plasma membrane abundance of several proteins 39. One of them was CD36, which is very homologous to SR-BI. The mechanism by which S1P1 or VEGFR-2 regulate the cell surface abundance of SR-BI is not known. One candidate is the regulation of the retromer that mediates the recycling of endocytosed lipoprotein receptors including SR-BI to the cell surface40. Moreover, it is important to consider that S1P1 might regulate SR-BI by different mechanisms, depending on the time kinetics: Translocation of preformed SR-BI may explain the quick effects of apoM/S1P as well as the pharmacological interventions with S1P1 on HDL uptake and transport. Both the knock-down of S1P1 by RNA interference in vitro as well as the knock-in of S1P1 in vivo, however, may also regulate the production of SR-BI. Indeed, the aortic endothelium of S1P1-iECKI mice presented with increased anti-SR-BI immunoreactivity. Moreover, we previously reported decreased SR-BI mRNA and protein expression of SR-BI in HAECs after knock-down of S1P141.
The in vitro findings regarding S1P1 activation and its role in the transendothelial transport were principally recapitulated under in vivo conditions. In mice, both treatment with the S1P1 agonist and the endothelium-specific overexpression of S1P1 led to increased transport of fluorescent HDL from the blood stream into the peritoneal cavity. Interestingly the transport of Evan’s Blue, which is bound by albumin and hence reflects albumin transport is decreased by S1P1 overexpression. We also found transport of fluorescent LDL into the peritoneal cavity decreased in our model. Likewise, mice with a knock-out of sphingosine kinase 2, resulting in increased S1P levels in plasma, show decreased transport of both dextran beads and LDL into the peritoneal cavity 42. Conversely, endothelium specific knock-out of endothelial S1P1 showed enhanced exudation of Evan’s Blue into the lung 11.
Taken together, the in vivo and in vitro data indicate that transendothelial HDL transport is promoted rather than inhibited by the S1P/S1P1 interaction. Of note, we made these complementary findings in different endothelial cell systems. Our in vivo model like the ones used by others previously on apoM and S1P1 reflects macromolecule transport through capillaries, which is happening either paracellularly or transcellularly through pores 43. The in vitro experiments were done in confluent macrovascular endothelial cells from two different species, which is supposed to involve vesicular transport at least in addition to those described before. Of note, however, apoM/S1P was previously shown to limit the transendothelial transport of albumin through the blood brain barrier through either trafficking route 12. Thus, apoM/S1P/S1P1 interactions facilitate rather than restrict HDL transport from the blood stream into extravascular tissues. At first sight, the effects are even unexpectedly strong in view of the rather low concentration of apoM and S1P in HDL as the consequence of which only five percent of HDL particles are supposed to contain one molecule of apoM and S1P30,33. However, HDL promotes efflux of S1P in both apoM dependent and independent manner 6,7, so that the ex vivo measured S1P may underestimate the local concentration of S1P and thereby also the potential effect on endothelial transport. In addition, the promotion of SR-BI expression and cell surface abundance upon apoM/S1P/S1P receptor interaction will facilitate the binding, uptake, and transport of HDL particles in general, independently of their load of S1P and apoM.
By promoting the transendothelial transport of potentially anti-atherogenic HDL, apoM/S1P and S1P1 may play an important role in the pathogenesis of atherosclerosis. At first sight in agreement, we found atherosclerosis reduced in S1P1-iECKI mice. However, this difference may simply reflect less pronounced dyslipidemia. Moreover, it is important to keep in mind that S1P and S1P1 exert many vasoprotective effects on the endothelium beyond regulating transendothelial transport, for example on the transmigration of leukocytes and nitric oxide production 44. Therefore, further studies are needed to show the pathogenic relevance for the regulation of transendothelial HDL transport through apoM/S1P and its cognate receptor S1P1.
Supplementary Material
Highlights.
The presence of apolipoprotein M increases binding, association, and transport of HDL by bovine aortic endothelial cells
Binding, association, and transport of HDL by human aortic endothelial cells is promoted by agonists of the sphingosine-1-phosphate receptor type 1 (S1P1) but decreased by inhibitors or knock-down of S1P1
The stimulatory effect of S1P1 activation on transendothelial HDL transport depends on the expression of SR-BI
Endothelium-specific overexpression of S1p1 in Apoe haploinsufficient mice increases SR-BI expression in the aortal endothelium
Endothelium-specific overexpression of S1p1 in Apoe haploinsufficient mice decreases transport of albumin but increases transport of HDL from blood into the peritoneal cavity
Acknowledgements
The authors thank Silvija Radosavljevic (Zurich) and Charlotte Wandel (Copenhagen) for lipoprotein isolation and excellent technical assistance.
Sources of Funding
This work was supported by grants from the Zurich Integrative Human Physiology, the Swiss National Science Foundation (31003A-160216 and 310030_166391/1), the 7th Framework Program of the European Commission (Project Transcard 603091), and a Systems X Program Grant MRD 2014/267 to A.v.E. as well as a grant from Deutsche Forschungsgemeinschaft (NO406/3-1) and intramural resources of the Center for Laboratory Medicine University Hospital Münster to J.-R.N., a FIRB-IDEAS grant RBID08777T from the Italian Ministry of Education, University and Research to J.-R.N. and M.S, a fellowship from Peter and Traudl Engelhorn Foundation for the Promotion of Life Sciences to D.W, a National Institutes of Health Grant R01 HL119962 to J.S.P., a Young Investigators Grant GR-2011-02346974 from the Italian Ministry of Health to F.P, and an Excellent grant from Novo Nordisk Foundation (NNF13OC0003898) to CC
Abbreviations:
- ABC
ATP binding cassette transporter
- Apo
apolipoprotein
- BAECs
bovine aortic endothelial cells
- CHD
coronary heart disease
- CTRL
control mice, i.e apoE-haploinsufficient mice without endothelium specific knock-in of S1P1
- HAECs
human aortic endothethelial cells
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LIPG
gene encoding endothelial lipase
- S1P
sphingosine-1-phosphate
- S1P1
sphingosine-1-phosphate receptor 1
- S1P1-iECKI
apoE-haploinsufficient mice with endothelium specific knock-in of S1P1
- SR-BI
scavenger receptor BI
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Disclosure
MS is a scientific advisory board member of Alnylam Pharmaceuticals. The other authors have no conflict of interest to disclose with respect to the work described except the funding mentioned above
References
- 1.Marz W, Kleber ME, Scharnagl H, Speer T, Zewinger S, Ritsch A, Parhofer KG, von Eckardstein A, Landmesser U, Laufs U. Hdl cholesterol: Reappraisal of its clinical relevance. Clin Res Cardiol. 2017;106:663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annema W, von Eckardstein A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J. 2013;77:2432–2448 [DOI] [PubMed] [Google Scholar]
- 3.Cardner M, Yalcinkaya M, Goetze S, Luca E, Balaz M, Hunjadi M, Hartung J, Shemet A, Krankel N, Radosavljevic S, Keel M, Othman A, Karsai G, Hornemann T, Claassen M, Liebisch G, Carreira E, Ritsch A, Landmesser U, Krutzfeldt J, Wolfrum C, Wollscheid B, Beerenwinkel N, Rohrer L, von Eckardstein A. Structure-function relationships of hdl in diabetes and coronary heart disease. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartier A, Hla T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science. 2019;366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by hdl-associated apolipoprotein m. Proc Natl Acad Sci U S A. 2011;108:9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutter I, Park R, Othman A, Rohrer L, Hornemann T, Stoffel M, Devuyst O, von Eckardstein A. Apolipoprotein m modulates erythrocyte efflux and tubular reabsorption of sphingosine-1-phosphate. J Lipid Res. 2014;55:1730–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen PM, Bosteen MH, Hajny S, Nielsen LB, Christoffersen C. Apolipoprotein m mediates sphingosine-1-phosphate efflux from erythrocytes. Sci Rep. 2017;7:14983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, Dahlback B, Kono M, Proia RL, Smith JD, Hla T. Hdl-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor s1p1 to limit vascular inflammation. Sci Signal. 2015;8:ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz M, Frej C, Holmer A, Guo LJ, Tran S, Dahlback B. High-density lipoprotein-associated apolipoprotein m limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler Thromb Vasc Biol. 2017;37:118–129 [DOI] [PubMed] [Google Scholar]
- 10.Christensen PM, Liu CH, Swendeman SL, Obinata H, Qvortrup K, Nielsen LB, Hla T, Di Lorenzo A, Christoffersen C. Impaired endothelial barrier function in apolipoprotein m-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016;30:2351–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 2018;70:1879–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathiesen Janiurek M, Soylu-Kucharz R, Christoffersen C, Kucharz K, Lauritzen M. Apolipoprotein m-bound sphingosine-1-phosphate regulates blood-brain barrier paracellular permeability and transcytosis. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang E, Robert J, Rohrer L, von Eckardstein A, Lee WL. Transendothelial transport of lipoproteins. Atherosclerosis. 2020;315:111–125 [DOI] [PubMed] [Google Scholar]
- 14.Rohrer L, Cavelier C, Fuchs S, Schluter MA, Volker W, von Eckardstein A. Binding, internalization and transport of apolipoprotein a-i by vascular endothelial cells. Biochim Biophys Acta. 2006;1761:186–194 [DOI] [PubMed] [Google Scholar]
- 15.Perisa D, Rohrer L, Kaech A, von Eckardstein A. Itinerary of high density lipoproteins in endothelial cells. Biochim Biophys Acta. 2016;1861:98–107 [DOI] [PubMed] [Google Scholar]
- 16.Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A. The beta-chain of cell surface f(0)f(1) atpase modulates apoa-i and hdl transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:131–139 [DOI] [PubMed] [Google Scholar]
- 17.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor bi and atp-binding cassette transporter g1. Circ Res. 2009;104:1142–1150 [DOI] [PubMed] [Google Scholar]
- 18.Cavelier C, Rohrer L, von Eckardstein A. Atp-binding cassette transporter a1 modulates apolipoprotein a-i transcytosis through aortic endothelial cells. Circ Res. 2006;99:1060–1066 [DOI] [PubMed] [Google Scholar]
- 19.Robert J, Lehner M, Frank S, Perisa D, von Eckardstein A, Rohrer L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arterioscler Thromb Vasc Biol. 2013;33:2699–2706 [DOI] [PubMed] [Google Scholar]
- 20.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by sr-bi-mediated transport of hdl. Cell Metab. 2013;17:671–684 [DOI] [PubMed] [Google Scholar]
- 21.Wolfrum C, Poy MN, Stoffel M. Apolipoprotein m is required for prebeta-hdl formation and cholesterol efflux to hdl and protects against atherosclerosis. Nat Med. 2005;11:418–422 [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Seo J, Allegood J, Bi X, Zhu X, Boudyguina E, Gebre AK, Avni D, Shah D, Sorci-Thomas MG, Thomas MJ, Shelness GS, Spiegel S, Parks JS. Hepatic apolipoprotein m (apom) overexpression stimulates formation of larger apom/sphingosine 1-phosphate-enriched plasma high density lipoprotein. J Biol Chem. 2014;289:2801–2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulya A, Seo J, Brown AL, Gebre AK, Boudyguina E, Shelness GS, Parks JS. Apolipoprotein m expression increases the size of nascent pre beta hdl formed by atp binding cassette transporter a1. J Lipid Res. 2010;51:514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. Ve-cadherin-creert2 transgenic mouse: A model for inducible recombination in the endothelium. Dev Dyn. 2006;235:3413–3422 [DOI] [PubMed] [Google Scholar]
- 25.van Ree JH, van den Broek WJ, Dahlmans VE, Groot PH, Vidgeon-Hart M, Frants RR, Wieringa B, Havekes LM, Hofker MH. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein e-deficient mice. Atherosclerosis. 1994;111:25–37 [DOI] [PubMed] [Google Scholar]
- 26.Zhang SH, Reddick RL, Burkey B, Maeda N. Diet-induced atherosclerosis in mice heterozygous and homozygous for apolipoprotein e gene disruption. J Clin Invest. 1994;94:937–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Scheidt M, Zhao Y, Kurt Z, Pan C, Zeng L, Yang X, Schunkert H, Lusis AJ. Applications and limitations of mouse models for understanding human atherosclerosis. Cell Metab. 2017;25:248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. 2020;126:1297–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppi S, Luscher TF, Stein S. Mouse models for atherosclerosis research-which is my line? Front Cardiovasc Med. 2019;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer JR. Krp-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in ldl-r−/− mice. Arterioscler Thromb Vasc Biol. 2013;33:1505–1512 [DOI] [PubMed] [Google Scholar]
- 31.Poti F, Costa S, Bergonzini V, Galletti M, Pignatti E, Weber C, Simoni M, Nofer JR. Effect of sphingosine 1-phosphate (s1p) receptor agonists fty720 and cym5442 on atherosclerosis development in ldl receptor deficient (ldl-r(−)/(−)) mice. Vascul Pharmacol. 2012;57:56–64 [DOI] [PubMed] [Google Scholar]
- 32.Velagapudi S, Yalcinkaya M, Piemontese A, Meier R, Norrelykke SF, Perisa D, Rzepiela A, Stebler M, Stoma S, Zanoni P, Rohrer L, von Eckardstein A. Vegf-a regulates cellular localization of sr-bi as well as transendothelial transport of hdl but not ldl. Arterioscler Thromb Vasc Biol. 2017;37:794–803 [DOI] [PubMed] [Google Scholar]
- 33.Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, Kuivenhoven JA, Rohrer L, Matile H, Hornemann T, Stoffel M, Rentsch KM, von Eckardstein A. Plasma levels of sphingosine-1-phosphate and apolipoprotein m in patients with monogenic disorders of hdl metabolism. Atherosclerosis. 2011;219:855–863 [DOI] [PubMed] [Google Scholar]
- 34.Armstrong SM, Sugiyama MG, Fung KY, Gao Y, Wang C, Levy AS, Azizi P, Roufaiel M, Zhu SN, Neculai D, Yin C, Bolz SS, Seidah NG, Cybulsky MI, Heit B, Lee WL. A novel assay uncovers an unexpected role for sr-bi in ldl transcytosis. Cardiovasc Res. 2015;108:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang L, Chambliss KL, Gao X, Yuhanna IS, Behling-Kelly E, Bergaya S, Ahmed M, Michaely P, Luby-Phelps K, Darehshouri A, Xu L, Fisher EA, Ge WP, Mineo C, Shaul PW. Sr-b1 drives endothelial cell ldl transcytosis via dock4 to promote atherosclerosis. Nature. 2019;569:565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christoffersen C, Nielsen LB, Axler O, Andersson A, Johnsen AH, Dahlback B. Isolation and characterization of human apolipoprotein m-containing lipoproteins. J Lipid Res. 2006;47:1833–1843 [DOI] [PubMed] [Google Scholar]
- 37.Liu M, Allegood J, Zhu X, Seo J, Gebre AK, Boudyguina E, Cheng D, Chuang CC, Shelness GS, Spiegel S, Parks JS. Uncleaved apom signal peptide is required for formation of large apom/sphingosine 1-phosphate (s1p)-enriched hdl particles. J Biol Chem. 2015;290:7861–7870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. Vegf induces s1p1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A. 2003;100:10664–10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi X, Wang W, Li J, Wang T, Lin Y, Huang S, Kuver A, Chen C, Hla T, Li X, Dai K. Sphingosine 1-phosphate receptor 1 regulates cell-surface localization of membrane proteins in endothelial cells. Biochim Biophys Acta Gen Subj. 2019;1863:1079–1087 [DOI] [PubMed] [Google Scholar]
- 40.Wijers M, Zanoni P, Liv N, Vos DY, Jackstein MY, Smit M, Wilbrink S, Wolters JC, van der Veen YT, Huijkman N, Dekker D, Kloosterhuis N, van Dijk TH, Billadeau DD, Kuipers F, Klumperman J, von Eckardstein A, Kuivenhoven JA, van de Sluis B. The hepatic wash complex is required for efficient plasma ldl and hdl cholesterol clearance. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Rohrer L, von Eckardstein A. Sphingosine-1-phosphate receptors 1 and 3 regulate the expression of scavenger receptor b1 in human aortic endothelial cells. bioRxiv. 2020:2020.2004.2023.058263 [Google Scholar]
- 42.Feuerborn R, Besser M, Poti F, Burkhardt R, Weissen-Plenz G, Ceglarek U, Simoni M, Proia RL, Freise H, Nofer JR. Elevating endogenous sphingosine-1-phosphate (s1p) levels improves endothelial function and ameliorates atherosclerosis in low density lipoprotein receptor-deficient (ldl-r−/−) mice. Thromb Haemost. 2018;118:1470–1480 [DOI] [PubMed] [Google Scholar]
- 43.Waniewski J, Poleszczuk J, Antosiewicz S, Baczynnski D, Galach M, Pietribiasi M, Wannkowicz Z. Can the three pore model correctly describe peritoneal transport of protein? ASAIO J. 2014;60:576–581 [DOI] [PubMed] [Google Scholar]
- 44.Robert J, Osto E, von Eckardstein A. The endothelium is both a target and a barrier of hdl’s protective functions. Cells. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


