This cohort study evaluates the association between low-density lipoprotein cholesterol and development of cardiovascular disease later in life.
Key Points
Question
Is cumulative exposure to low-density lipoprotein cholesterol (LDL-C) during young adulthood and middle age a stronger risk factor of future cardiovascular disease risk than LDL-C level at middle age?
Findings
In this cohort study of 18 288 participants in 4 US cohorts, cumulative LDL-C exposure during young adulthood and middle age was associated with the risk of incident coronary heart disease events, independent of midlife LDL-C level.
Meaning
The findings suggest that maintaining an optimal level of LDL-C throughout young adulthood and middle age can minimize the lifetime risk for atherosclerotic cardiovascular disease; understanding young adult levels of LDL-C may help inform strategies for preventing coronary heart disease.
Abstract
Importance
Low-density lipoprotein cholesterol (LDL-C) is a major risk factor for cardiovascular disease (CVD). Most observational studies on the association between LDL-C and CVD have focused on LDL-C level at a single time point (usually in middle or older age), and few studies have characterized long-term exposures to LDL-C and their role in CVD risk.
Objective
To evaluate the associations of cumulative exposure to LDL-C, time-weighted average (TWA) LDL-C, and the LDL-C slope change during young adulthood and middle age with incident CVD later in life.
Design, Setting, and Participants
This cohort study analyzed pooled data from 4 prospective cohort studies in the US (Atherosclerosis Risk in Communities Study, Coronary Artery Risk Development in Young Adults Study, Framingham Heart Study Offspring Cohort, and Multi-Ethnic Study of Atherosclerosis). Participants were included if they had 2 or more LDL-C measures that were at least 2 years apart between ages 18 and 60 years, with at least 1 of the LDL-C measures occurring during middle age at 40 to 60 years. Data from 1971 to 2017 were collected and analyzed from September 25, 2020, to January 10, 2021.
Exposures
Cumulative exposure to LDL-C, TWA LDL-C, and LDL-C slope from age 18 to 60 years.
Main Outcomes and Measures
Incident coronary heart disease (CHD), ischemic stroke, and heart failure (HF).
Results
A total of 18 288 participants were included in this study. These participants had a mean (SD) age of 56.4 (3.7) years and consisted of 10 309 women (56.4%). During a median follow-up of 16 years, 1165 CHD, 599 ischemic stroke, and 1145 HF events occurred. In multivariable Cox proportional hazards regression models that adjusted for the most recent LDL-C level measured during middle age and for other CVD risk factors, the hazard ratios for CHD were as follows: 1.57 (95% CI, 1.10-2.23; P for trend = .01) for cumulative LDL-C level, 1.69 (95% CI, 1.23-2.31; P for trend <.001) for TWA LDL-C level, and 0.88 (95% CI, 0.69-1.12; P for trend = .28) for LDL-C slope. No association was found between any of the LDL-C variables and ischemic stroke or HF.
Conclusions and Relevance
This cohort study showed that cumulative LDL-C and TWA LDL-C during young adulthood and middle age were associated with the risk of incident CHD, independent of midlife LDL-C level. These findings suggest that past levels of LDL-C may inform strategies for primary prevention of CHD and that maintaining optimal LDL-C levels at an earlier age may reduce the lifetime risk of developing atherosclerotic CVD.
Introduction
Low-density lipoprotein cholesterol (LDL-C) is a major modifiable risk factor for cardiovascular disease (CVD).1 Evidence from mendelian randomization studies and randomized clinical trials suggests that the role that LDL-C plays in CVD risk is associated with both the current level and the cumulative burden of LDL-C exposure over time.2 However, most observational studies on the association between high LDL-C levels and CVD risk have focused on LDL-C levels that were measured at a single time point (usually in middle or older age), and few studies have characterized the long-term exposures to LDL-C and their implications for CVD risk.3,4,5,6 A likely reason for this focus on a single time point is that most cohort studies have a restricted enrollment age range and few studies measure LDL-C levels repeatedly in both early and later life.
High LDL-C level that is measured once in middle age might reflect a rapidly increasing LDL-C (ie, a greater slope of change in LDL-C) level or an LDL-C level that has been persistently high (ie, a greater cumulative exposure to LDL-C). It remains unclear whether LDL-C slope or cumulative LDL-C is a stronger risk factor in future CVD risk. In addition to cumulative LDL-C, time-weighted average (TWA) LDL-C, calculated as the cumulative exposure to LDL-C divided by the duration of exposure, captures the cumulative burden of LDL-C and has been associated with incident CVD risk.4,7 An appealing feature of TWA LDL-C is that it has the same unit as a 1-time LDL-C measure that is familiar to clinicians and patients. However, it is unclear whether cumulative LDL-C and TWA LDL-C share similar or exhibit different associations with incident CVD events.
Using pooled data from 4 prospective cohort studies with repeated LDL-C measurements, we conducted a study to evaluate the associations of cumulative exposure to LDL-C, TWA LDL-C, and the LDL-C slope change during young adulthood and middle age with incident CVD later in life. To this end, we (1) modeled LDL-C trajectories from age 18 years to the end of follow-up for all participants; (2) used those trajectories to estimate the cumulative exposure to LDL-C, the TWA LDL-C, and the LDL-C slope change from age 18 to 60 years; and (3) assessed the independent associations between these LDL-C exposures with subsequent risks of coronary heart disease (CHD), ischemic stroke, and heart failure (HF).
Methods
This cohort study analyzed data from 4 large, community-based prospective cohort studies in the US: (1) Atherosclerosis Risk in Communities Study,8 which involved individuals aged 45 to 64 years who had a baseline visit from 1987 to 1989 and 6 in-person follow-up visits; (2) Coronary Artery Risk Development in Young Adults Study,9 which included individuals aged 18 to 30 years with a baseline visit taking place between 1985 and 1986 followed by 8 in-person visits; (3) Framingham Heart Study Offspring Cohort,10 which focused on the young or adult children (aged 5 to 70 years) of the original Framingham Heart Study cohort with baseline visits starting in 1971 and 8 in-person visits therafter; and (4) Multi-Ethnic Study of Atherosclerosis,11 which included individuals aged 45 to 84 years without CVD and with baseline visits occurring from 2000 to 2002 followed by 5 in-person visits. The design of each study is reported in the eMethods in the Supplement. The protocols for the 4 studies were approved by the institutional review boards at participating institutions, and all participants in these studies provided written informed consent. The institutional review board of Columbia University approved the present study, and data use agreements were obtained from each parent study.
We restricted study inclusion to participants who underwent 2 or more LDL-C measures that were at least 2 years apart between 18 and 60 years of age, with at least 1 of the LDL-C measures occurring during middle age at 40 to 60 years (eFigure 1 in the Supplement). The index visit was defined as the last study visit between 40 and 60 years of age with an observed LDL-C measure (eFigure 2 in the Supplement). Participants who had an existing CVD before the index visit were excluded from the analysis. We also excluded individuals with missing covariate data (eg, body mass index, smoking status, and blood pressure). The final sample for this study comprised 18 288 individuals.
Demographic characteristics, LDL-C levels, and other CVD risk factors were measured in each cohort study with standardized protocols.8,9,10,11 Most CVD risk factors were ascertained at every study visit using similar standardized and validated methods. Data were pooled and harmonized for covariates, including self-reported race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, and use of lipid-lowering and antihypertensive medications. Data from 1971 to 2017 were collected for this analysis.
Cumulative LDL-C, TWA LDL-C, and LDL-C Slope
The primary exposures of interest were cumulative LDL-C, TWA LDL-C, and LDL-C slope during young adulthood and middle age before the index visit. Given that most of the 4 cohort studies had an age range restriction and did not directly measure LDL-C levels during both early and later life (for example, participants in the Atherosclerosis Risk in Communities Study were enrolled after 45 years of age and, therefore, their LDL-C levels before age 45 years were not observed), we used a previously developed multiple imputation method to impute LDL-C values from age 18 years for each participant.7,12 This multiple imputation method is described elsewhere12 as well as in the eMethods and eFigure 2 in the Supplement.
We calculated the cumulative LDL-C exposure as the area under the LDL-C vs age trajectory from age 18 years to the index visit (expressed as mg/dL × years). The TWA LDL-C was calculated as the cumulative LDL-C divided by the total years between age 18 years and the index visit. To estimate the LDL-C slope for each participant, we fitted a linear mixed-effects model of the imputed LDL-C values before the index visit against age, with age modeled as restricted cubic splines with random intercepts and slopes. The final LDL-C slope for each participant was calculated as the population mean slope plus the individual random slope.
Follow-up and CVD Events
All 4 cohort studies prospectively ascertained incident CVD events.8,9,10,11 The primary outcomes of interest for the present analysis were CHD (defined as myocardial infarction or CHD death), ischemic stroke, and HF. Events were ascertained and adjudicated using each cohort’s specific protocol, the details of which are provided in the eMethods and eTable 1 in the Supplement.
Statistical Analysis
Participant characteristics at the index visit were identified for the overall pooled cohort and by individual study. We used Pearson correlation coefficient (Pearson r) to assess the correlation between LDL-C levels at the index visit and cumulative LDL-C level, TWA LDL-C level, and LDL-C slope during young adulthood and middle age before the index visit.
We used Cox proportional hazards regression models to examine the association between LDL-C exposures and incident CVD events. Age was used as the time scale, with left truncation on age at the index visit. We used several approaches to provide detailed dose-response analyses of the association between LDL-C levels and CVD outcomes. First, we categorized each LDL-C variable into quartiles based on their sample distributions. The cutoffs for quartiles were less than 4025 mg/dL × years (to convert millimoles per liter, multiply by 0.0259), 4025 to 4796 mg/dL × years, 4797 to 5603 mg/dL × years, and 5604 mg/dL × years or greater for cumulative LDL-C level; less than 107 mg/dL, 107 to 123 mg/dL, 124 to 141 mg/dL, and 142 mg/dL or greater for TWA LDL-C level; less than 0.4 mg/dL/y, 0.4 to 0.6 mg/dL/y, 0.7 to 0.9 mg/dL/y, and 1.0 mg/dL/y or greater for LDL-C slope; and less than 99 mg/dL, 99 to 120 mg/dL, 121 to 144 mg/dL, and 145 mg/dL or greater for LDL-C levels at the index visit. Second, we used restricted cubic splines to provide a flexible description of the association between each LDL-C variable and CVD outcomes.
To adjust for potential confounders, we used 2 models with progressively greater degrees of adjustment. The first model was adjusted for race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, and use of lipid-lowering and antihypertensive medications at the index visit. The second model was further adjusted for LDL-C levels at the index visit. All models were also stratified by study cohort, allowing the baseline hazard function to vary across different cohorts. The proportional hazards assumption was checked by plotting the log(–log(survival)) vs log(survival time) and by using Schoenfeld residuals.
To account for estimation error in imputed LDL-C values, we used multiple imputation techniques that were based on parametric bootstrap to obtain 30 imputed data sets. Survival analyses of the associations between LDL-C exposures and CVD outcomes were performed on each imputed data set, and summary hazard ratios (HRs) and corresponding 95% CIs were calculated across all 30 imputations using established methods.13
To examine the robustness and consistency of our findings, we performed several sensitivity analyses. These sensitivity analyses included stratifying by sex and self-reported race and ethnicity, excluding individuals who ever used lipid-lowering medications, and leaving out 1 cohort at a time to confirm that the findings were not driven by any single study.
The threshold for statistical significance was a 2-sided P < .05. All analyses were performed with Stata software, version 16 (StataCorp LLC). Data were analyzed from September 25, 2020, to January 10, 2021.
Results
A total of 18 288 participants from the 4 cohort studies are included in this study. These participants had a mean (SD) age of 56.4 (3.7) years at their index visit, 10 309 were women (56.4%), 7979 were men (43.6%), and 12 980 individuals self-identified as non-Hispanic White (71.0%) (Table). Moderate correlations were found between LDL-C levels at the index visit and cumulative LDL-C level, TWA LDL-C level, and LDL-C slope (Pearson r range, 0.6-0.7) (eTable 2 in the Supplement).
Table. Characteristics of Study Participants at the Index Visita.
| Characteristics | No. (%) | ||||
|---|---|---|---|---|---|
| Total | ARIC Study | CARDIA Study | FHS-O | MESA | |
| No. of participants | 18 288 | 8235 | 3892 | 3827 | 2334 |
| Age, mean (SD), y | 56.4 (3.7) | 57.3 (2.5) | 53.4 (4.8) | 56.8 (3.6) | 57.4 (2.3) |
| Race and ethnicity | |||||
| Hispanic | 535 (2.9) | 0 | 0 | 0 | 535 (22.9) |
| non-Hispanic Black | 4465 (24.4) | 1975 (24.0) | 1865 (47.9) | 0 | 625 (26.8) |
| non-Hispanic White | 12 980 (71.0) | 6235 (75.7) | 2027 (52.1) | 3827 (100.0) | 891 (38.2) |
| Otherb | 308 (1.7) | 25 (0.3) | 0 | 0 | 283 (12.1) |
| Sex | |||||
| Female | 10 309 (56.4) | 4800 (58.3) | 2203 (56.6) | 2046 (53.5) | 1260 (54.0) |
| Male | 7979 (43.6) | 3435 (41.7) | 1689 (43.4) | 1781 (46.5) | 1074 (46.0) |
| Smoking status | |||||
| Never | 7497 (41.0) | 3132 (38.0) | 1967 (50.5) | 1283 (33.5) | 1115 (47.8) |
| Former | 7301 (39.9) | 3396 (41.2) | 1280 (32.9) | 1749 (45.7) | 876 (37.5) |
| Current | 3490 (19.1) | 1707 (20.7) | 645 (16.6) | 795 (20.8) | 343 (14.7) |
| BMI, mean | 28.8 (6.1) | 28.5 (5.7) | 30.4 (7.3) | 27.7 (5.2) | 29.1 (6.1) |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic | 122.4 (17.8) | 122.6 (18.1) | 119.7 (15.9) | 127.3 (17.4) | 118.1 (18.3) |
| Diastolic | 73.7 (10.5) | 72.5 (10.2) | 73.6 (11.0) | 77.9 (9.7) | 71.1 (10.2) |
| Cholesterol, mean (SD) | |||||
| HDL, mg/dL | 53.5 (17.5) | 51.3 (17.2) | 59.2 (18.6) | 53.4 (17.2) | 52.2 (15.5) |
| LDL, mean (SD) | |||||
| At index visit, mg/dL | 123.0 (35.6) | 127.1 (35.7) | 111.7 (33.3) | 129.5 (36.1) | 116.4 (32.9) |
| Cumulative, mg/dL × years | 4837 (1181) | 5143 (1111) | 4007 (1032) | 5102 (1191) | 4704 (926 |
| TWA, mg/dL | 125.6 (26.6) | 130.7 (26.2) | 113.0 (24.3) | 131.2 (27.0) | 119.4 (22.2) |
| Slope, mg/dL/y | 0.7 (0.5) | 0.8 (0.4) | 0.5 (0.6) | 0.7 (0.5) | 0.7 (0.4) |
| Diabetes status | 2417 (13.2) | 1223 (14.9) | 517 (13.3) | 298 (7.8) | 379 (16.2) |
| Medication use | |||||
| Lipid-lowering | 2254 (12.3) | 634 (7.7) | 670 (17.2) | 469 (12.3) | 481 (20.6) |
| Antihypertensive | 5440 (29.7) | 2561 (31.1) | 1156 (29.7) | 955 (25.0) | 768 (32.9) |
Abbreviations; ARIC, Atherosclerosis Risk in Communities Study; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CARDIA, Coronary Artery Risk Development in Young Adults study; FHS-O, Framingham Heart Study Offspring Cohort; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MESA, Multi-Ethnic Study of Atherosclerosis; TWA, time-weighted average.
SI unit conversion: To convert mg/dL to millimoles per liter, multiply by 0.0259.
Values are mean (SD) or No. (%) based on observed data at the index visit.
Other includes participants with self-reported race and ethnicity other than Hispanic, non-Hispanic Black, or non-Hispanic White (ie, Asian, American Indian, Pacific Islander).
During a median follow-up of 16 years, there were a total of 1165 incident CHD, 599 ischemic stroke, and 1145 HF events (eTables 3 and 4 in the Supplement). In the multivariable Cox proportional hazards regression model that adjusted for demographic and clinical risk factors, the HRs for CHD that compared the top with bottom quartiles of the LDL-C variables were 1.97 (95% CI, 1.50-2.58; P for trend <.001) for cumulative LDL-C level, 1.95 (95% CI, 1.54-2.46; P for trend <.001) for TWA LDL-C level, 1.26 (95% CI, 1.03-1.55; P for trend = .02) for LDL-C slope, and 1.79 (95% CI, 1.49-2.14; P for trend <.001) for LDL-C levels at the index visit (eFigure 3 in the Supplement). In models that further adjusted for LDL-C levels at the index visit, the HRs for CHD that compared the top with bottom quartiles of the LDL-C variables were 1.57 (95% CI, 1.10-2.23; P for trend = .01) for cumulative LDL-C level, 1.69 (95% CI, 1.23-2.31; P for trend <.001) for TWA LDL-C level, and 0.88 (95% CI, 0.69-1.12; P for trend = .28) for LDL-C slope (Figure 1). No association was observed between any of the LDL-C variables and the risk of ischemic stroke or HF (Figure 2 and Figure 3; eFigures 4 and 5 in the Supplement).
Figure 1. Associations of Cumulative Low-Density Lipoprotein Cholesterol (LDL-C), Time-Weighted Average (TWA) LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Coronary Heart Disease (CHD).
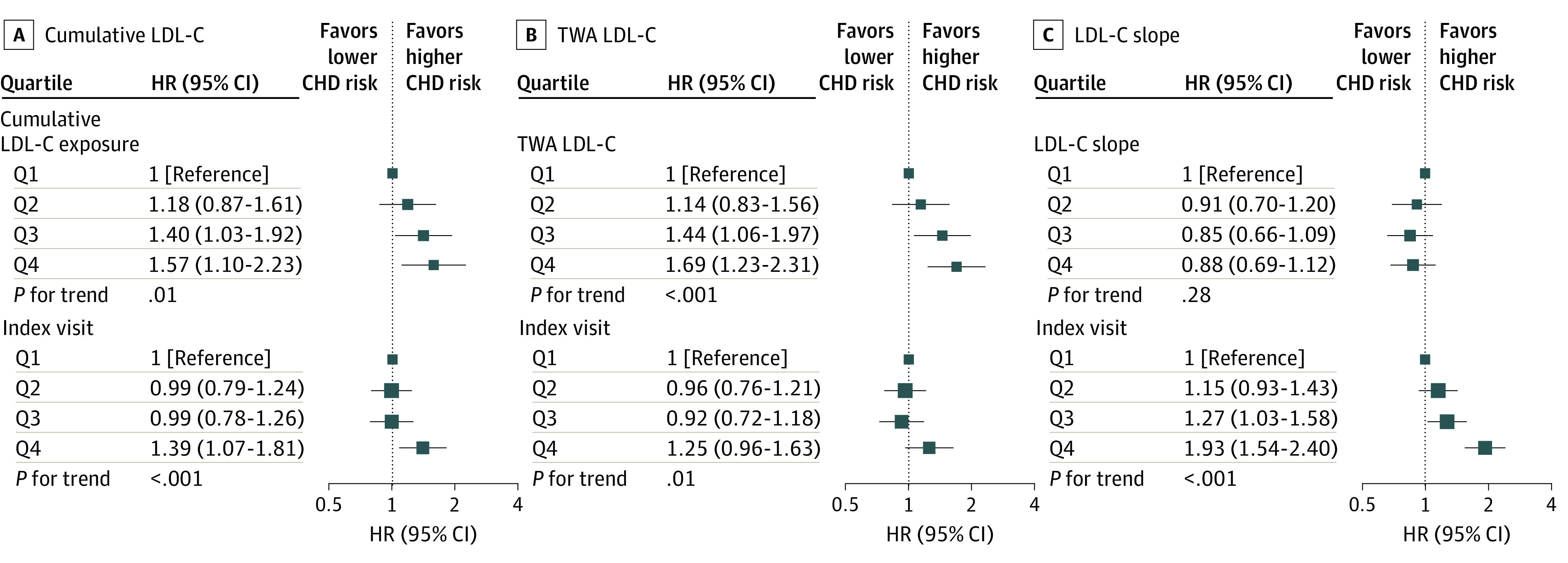
Models were stratified by study cohort and adjusted for race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, use of lipid-lowering and antihypertensive medications, and LDL-C levels at the index visit. HR indicates hazard ratio; Q, quartile.
Figure 2. Associations of Cumulative Low-Density Lipoprotein Cholesterol (LDL-C), Time-Weighted Average (TWA) LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke.
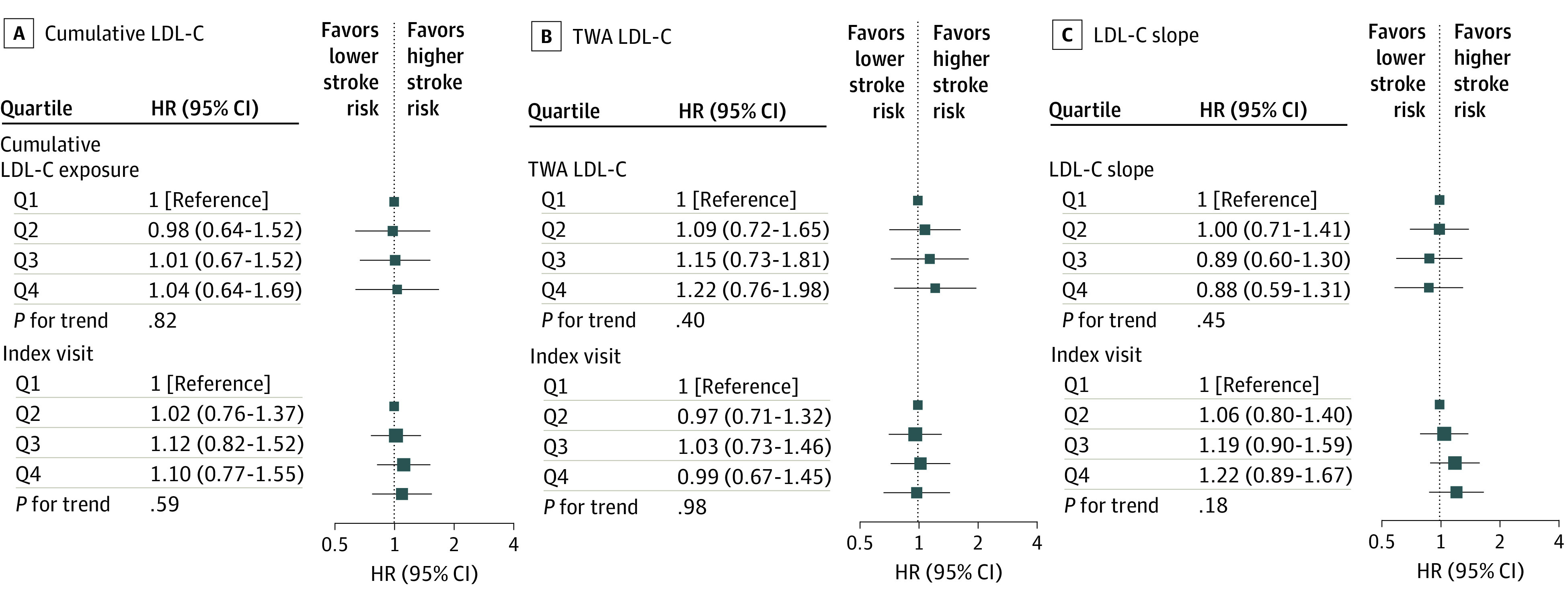
Models were stratified by study cohort and adjusted for race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, use of lipid-lowering and antihypertensive medications, and LDL-C levels at the index visit. HR indicates hazard ratio; Q, quartile.
Figure 3. Associations of Cumulative Low-Density Lipoprotein Cholesterol (LDL-C), Time-Weighted Average (TWA) LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure (HF).
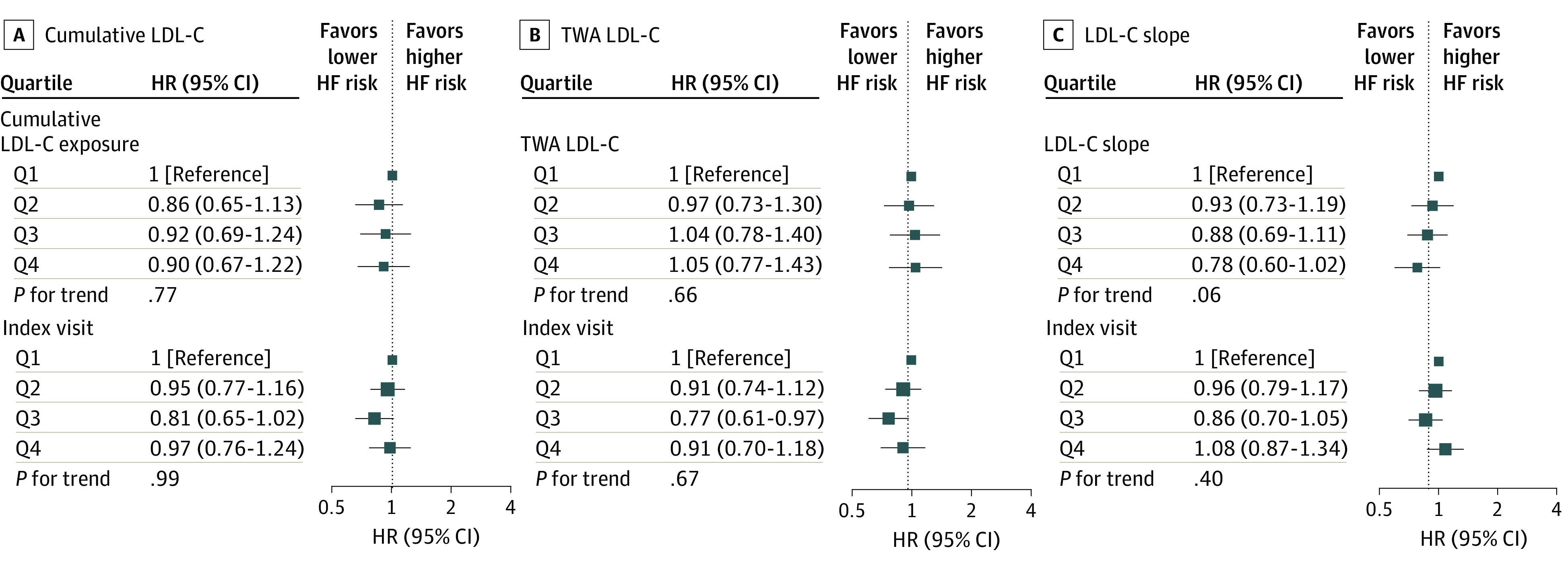
Models were stratified by study cohort and adjusted for race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, use of lipid-lowering and antihypertensive medications, and LDL-C levels at the index visit. HR indicates hazard ratio; Q, quartile.
The associations of cumulative LDL-C and TWA LDL-C levels with incident CHD were also present in spline regression analyses (Figure 4). The patterns of the associations were similar among men and women (eg, the HRs for CHD that compared the top with bottom quartiles of TWA LDL-C were 1.82 [95% CI, 1.07-3.10] for women vs 1.67 [95% CI, 1.06-2.64] for men; all P for interaction by sex >.10) (eFigures 6-8 in the Supplement). The associations of cumulative LDL-C and TWA LDL-C levels with CHD appeared to be stronger in participants who self-identified as non-Hispanic White individuals than in participants who self-identified as non-Hispanic Black individuals (eg, the HRs for CHD that compared the top with bottom quartiles of TWA LDL-C were 1.86 [95% CI, 1.28-2.72] for non-Hispanic White individuals vs 1.58 [95% CI, 0.76-3.27] for non-Hispanic Black individuals; all P for interaction by race and ethnicity >.10). However, the test for interaction did not show evidence of difference by race and ethnicity (eFigures 9-11 in the Supplement).
Figure 4. Adjusted Hazard Ratios (HRs) for Coronary Heart Disease (CHD) by Levels of Cumulative Low-Density Lipoprotein Cholesterol (LDL-C), Time-Weighted Average (TWA) LDL-C, and LDL-C Slope During Young Adulthood and Middle Age.
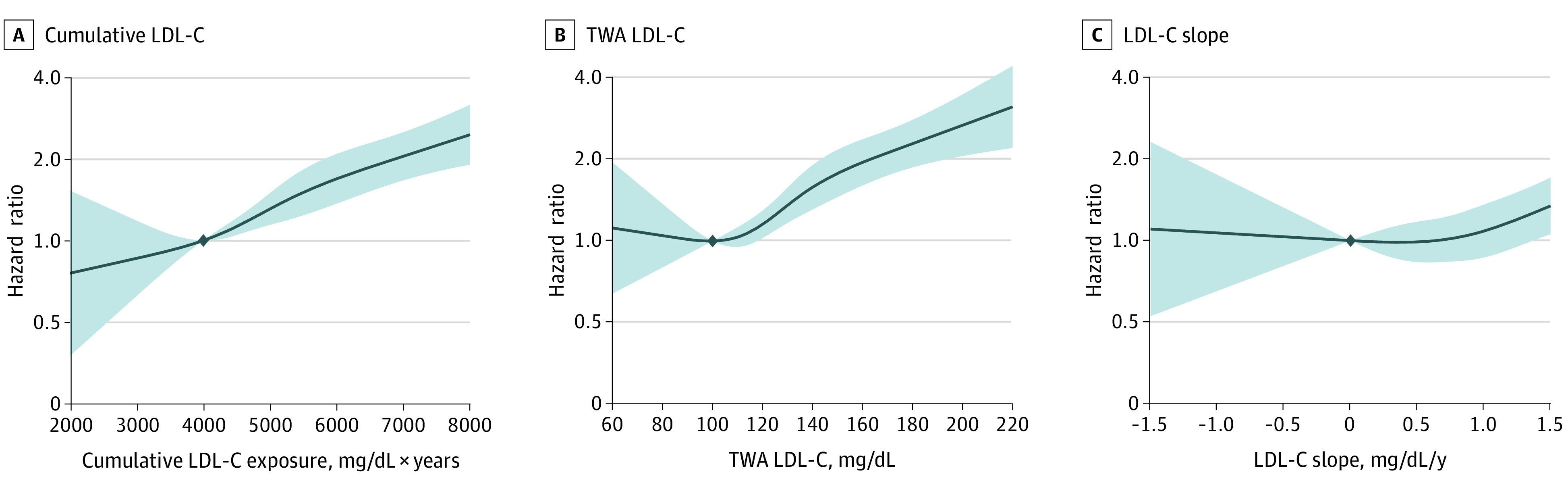
The solid curve represents the adjusted HR for CHD associated with restricted cubic splines for cumulative LDL-C, TWA LDL-C, and LDL-C slope. Shaded areas represent 95% CIs. The reference values (diamond dots) were set at 4000 mg/dL × years for cumulative LDL-C, 100 mg/dL for TWA LDL-C, and 0 mg/dL/y for LDL-C slope. Models were stratified by study cohort and adjusted for race and ethnicity, sex, birth year, body mass index, smoking status, high-density lipoprotein cholesterol level, systolic blood pressure, diastolic blood pressure, diabetes status, and use of lipid-lowering and antihypertensive medications.
Among the 15 626 participants who never used lipid-lowering medication, TWA LDL-C level remained significantly associated with incident CHD (the HR for CHD that compared the top with bottom quartiles of TWA LDL-C was 1.54 [95% CI, 1.08-2.18]) (eFigures 12-14 in the Supplement). The analyses that left out 1 study at a time found results consistent with the main analysis, and there was no indication that the findings of this study were affected by any single cohort (eFigures 15-17 in the Supplement).
Discussion
In this analysis of pooled data from 4 large prospective cohort studies performed in the US, we found that higher levels of exposure to cumulative LDL-C and TWA LDL-C during young adulthood and middle age were associated with an increased risk of incident CHD events, even after adjusting for the most recent LDL-C level during middle age. In contrast, we found no association between LDL-C slope and CHD after adjusting for midlife LDL-C level or measures of cumulative LDL-C level and other cardiovascular events, such as ischemic stroke and HF. These findings suggest that current LDL-C level and cumulative LDL-C burden are independently associated with CHD risk.
Previous observational studies on the association between LDL-C levels and CVD outcomes focused on LDL-C level at a single time point, usually in middle or older age. However, multiple lines of evidence support that a lifelong exposure to elevated LDL-C is associated with atherosclerotic CVD.3,4,5,6,14,15 Mendelian randomization studies have shown that long-term exposure to lower LDL-C beginning early in life because of genetic variation is associated with an up to 3-fold greater reduction in CHD risk compared with shorter-term LDL-C–lowering medication that is started later in life.14 Emerging evidence from longitudinal epidemiological studies also suggest an association between long-term elevated LDL-C level and later-life CVD risk. Duncan et al3 followed 3875 participants in the Framingham Heart Study Offspring Cohort for 35 years and identified 5 distinct patterns of LDL-C trajectories during middle age. Duncan et al3 found that participants with long-term exposure in the highest LDL-C level group had 5 times the risk of CVD and 4 times the risk of total mortality compared with those in the optimal LDL-C level group (80-90 mg/dL).3 A recent study of the Coronary Artery Risk Development in Young Adults Study reported that a greater cumulative exposure to LDL-C during young adulthood was associated with an increased risk of CVD, whereas a more rapidly rising LDL-C slope was inversely associated with CVD.5 Furthermore, it has been suggested that a higher cumulative LDL-C exposure early in adulthood might have a stronger association with CVD risk than cumulative exposures during later adulthood.4,5
The current study extends these previous reports by using different metrics of LDL-C (ie, cumulative LDL-C level, TWA LDL-C level, and LDL-C slope during young adulthood and middle age) with risk of specific subtypes of CVD (ie, CHD, ischemic stroke, and HF). We found that cumulative LDL-C and TWA LDL-C levels were associated with an increased risk of incident CHD events, even after adjusting for the most recent LDL-C level during middle age. However, we did not observe an apparent association between LDL-C slope and incident CHD after adjusting for midlife LDL-C level, likely because at a given LDL-C level during middle age, individuals who reached a steeper slope may have a smaller area under the curve or cumulative LDL-C exposure compared with those with a shallower slope. The findings from this study suggest that the role of LDL-C level in the risk of CHD is associated with both the current level and the long-term cumulative burden of LDL-C exposures.16
The pathophysiological processes of atherosclerosis suggest a mechanistic association between CHD and cumulative LDL-C and TWA LDL-C levels. Low-density lipoprotein cholesterol and other apolipoprotein B–containing lipoproteins that are smaller than 70 nm in diameter freely enter and exit the endothelial barrier, where they can interact with extracellular structures, such as proteoglycans, to become retained in the extracellular matrix.2,17 The LDL-C particles that are retained in the arterial wall are susceptible to various modifications, including oxidation. Oxidized LDL-C elicits an inflammatory response, which results in vascular injury and atheroma formation.18 With continued exposure to LDL-C, additional LDL-C particles accumulate in the arterial wall, leading to the development of atherosclerotic plaque.18 Therefore, an individual’s total atherosclerotic burden is believed to be associated with both the circulating LDL-C levels and the total duration of exposure (ie, the cumulative exposure to LDL-C).2 A total of 5000 mg/dL × years of LDL-C has been estimated to be the minimum threshold of cumulative LDL-C exposure needed to develop a sufficiently large atherosclerotic burden to increase the risk of CHD.2 According to results from the spline regression that we performed, a dose-response association exists between cumulative exposure to LDL-C and CHD risk without evidence for a clear threshold, suggesting that lower cumulative LDL-C throughout young adulthood and middle age is associated with lower lifetime incidence of CHD, at all levels of LDL-C at middle age.
These findings have substantial clinical implications. Clinical decisions are currently guided by contemporary LDL-C values, whereas our findings suggest that incorporating serial LDL-C measures and cumulative LDL-C burden into clinical practice may further refine CVD risk assessment and help inform strategies for primary prevention of CHD. Moreover, given that the risk of developing atherosclerotic plaques and CHD events is associated with the cumulative exposure to LDL-C, it is plausible that achieving optimal lipid levels early in life and maintaining those optimal levels throughout adulthood may prevent incident CHD events better than the current paradigm of deferring lowering LDL-C levels to later in life when atherosclerosis is likely already advanced.2,19 The safest method of maintaining optimal LDL-C levels throughout life is to engage in behaviors that lead to ideal cardiovascular health, particularly adopting a heart-healthy diet, which has been shown to have a relatively large benefit for LDL-C level.20 However, for many people, diet and other lifestyle modifications may not be sufficient to maintain optimal LDL-C levels throughout life.2 For these individuals, pharmacological therapy to lower LDL-C levels earlier in the life course when atherosclerosis is less advanced may be a more successful way to reverse the disease course and prevent future CHD events.19
Because of the lack of direct evidence from clinical trials and observational studies in children and young adults, current guidelines mostly rely on data from cohort studies and clinical trials involving middle-aged and older adults, leading to an emphasis on LDL-C–lowering therapy in these age groups.1 The 2018 American College of Cardiology/American Heart Association cholesterol guideline recommended statin treatment for young adults aged 20 to 39 years with LDL-C less than 190 mg/dL only if they have a family history of premature atherosclerotic CVD, long-standing diabetes, or a concomitant higher-risk condition.1 The ongoing Eliminate Coronary Artery Disease trial may provide much-needed data to answer the question of whether atherosclerotic cardiovascular events can be prevented by early initiation of statin-based LDL-C–lowering medication in healthy young and middle-aged adults who are not yet candidates for guideline-based pharmacological therapy.21
Strengths and Limitations
This study has several strengths. The main strength is the unique study design of pooling data from 4 large prospective cohort studies, allowing us to model long-term LDL-C trajectories and to assess the associations of cumulative LDL-C, TWA LDL-C, and LDL-C slope with future CVD risk while adjusting for LDL-C levels in middle age. Using data from these cohort studies not only permitted high-quality risk factor and outcome assessments but also provided a large sample size and long follow-up duration. These advantages allowed us to more reliably estimate the associations between various metrics of LDL-C exposure and specific subtypes of CVD (including ischemic stroke and HF, which are less frequent than CHD outcomes) after controlling for confounding from a comprehensive set of variables.
This study also has several limitations. We used multiple imputation to estimate long-term LDL-C exposures during young adulthood and middle age, given that the 4 cohort studies had a restricted enrollment age range and did not directly measure LDL-C levels during both young adulthood and middle age. The LDL-C trajectories were subject to imputation error; however, any imputation error was likely nondifferential and biased toward sample means.7 Therefore, our estimates of the association between cumulative LDL-C exposure and CVD outcomes were likely conservative and biased toward the null. We did not find an association between any of the LDL-C variables and ischemic stroke, potentially because of the relatively small number of ischemic stroke events compared with incident CHD events (599 vs 1165). Despite the large sample size, this study may still have been underpowered to detect associations in racial and ethnic minority populations.
Conclusions
This cohort study found that greater exposures to cumulative LDL-C and TWA LDL-C during young adulthood and middle age were associated with an increased risk of CHD, even after adjusting for the most recent LDL-C level during middle age. These findings suggest that previous levels of LDL-C may inform strategies for primary prevention of CHD and that maintaining optimal LDL-C levels throughout young adulthood and middle age may reduce the lifetime risk of developing atherosclerotic CVD.
eMethods
eTable 1. Definitions of Outcomes by Study Cohorts
eTable 2. Correlations Between LDL-C Variables
eTable 3. Study Observation Period and Number of Events
eTable 4. Number of Participants and Events by LDL-C Quartiles
eFigure 1. Study Flow Chart
eFigure 2. Example LDL-C Trajectories During Young Adulthood and Middle Age (From Age 18 Years Up to the Index Visit) for 12 Randomly Selected Participants (3 Participants per Study)
eFigure 3. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident CHD
eFigure 4. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident Ischemic Stroke
eFigure 5. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident Heart Failure
eFigure 6. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Stratified by Sex
eFigure 7. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Stratified by Sex
eFigure 8. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Stratified by Sex
eFigure 9. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Stratified by Race
eFigure 10. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Stratified by Race
eFigure 11. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Stratified by Race
eFigure 12. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Among Participants Who Never Used Lipid Lowering Medications
eFigure 13. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Among Participants Who Never Used Lipid Lowering Medications
eFigure 14. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Among Participants Who Never Used Lipid Lowering Medications
eFigure 15. Associations Between Cumulative LDL-C During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eFigure 16. Associations Between TWA LDL-C During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eFigure 17. Associations Between LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eReferences
References
- 1.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. 2018;72(10):1141-1156. doi: 10.1016/j.jacc.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 3.Duncan MS, Vasan RS, Xanthakis V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: observations from the Framingham Study over 35 years. J Am Heart Assoc. 2019;8(11):e011433. doi: 10.1161/JAHA.118.011433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74(3):330-341. doi: 10.1016/j.jacc.2019.03.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domanski MJ, Tian X, Wu CO, et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76(13):1507-1516. doi: 10.1016/j.jacc.2020.07.059 [DOI] [PubMed] [Google Scholar]
- 6.Navar-Boggan AM, Peterson ED, D’Agostino RB Sr, Neely B, Sniderman AD, Pencina MJ. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation. 2015;131(5):451-458. doi: 10.1161/CIRCULATIONAHA.114.012477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins-Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: the Framingham Offspring Study. PLoS One. 2016;11(5):e0154288. doi: 10.1371/journal.pone.0154288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 10.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring study. Design and preliminary data. Prev Med. 1975;4(4):518-525. doi: 10.1016/0091-7435(75)90037-7 [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 12.Zeki Al Hazzouri A, Vittinghoff E, Zhang Y, et al. Use of a pooled cohort to impute cardiovascular disease risk factors across the adult life course. Int J Epidemiol. 2019;48(3):1004-1013. doi: 10.1093/ije/dyy264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 14.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol. 2012;60(25):2631-2639. doi: 10.1016/j.jacc.2012.09.017 [DOI] [PubMed] [Google Scholar]
- 15.Shapiro MD, Bhatt DL. “Cholesterol-years” for ASCVD risk prediction and treatment. J Am Coll Cardiol. 2020;76(13):1517-1520. doi: 10.1016/j.jacc.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 16.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459-2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832-1844. doi: 10.1161/CIRCULATIONAHA.106.676890 [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161(1):161-172. doi: 10.1016/j.cell.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gidding SS, Allen NB. Cholesterol and atherosclerotic cardiovascular disease: a lifelong problem. J Am Heart Assoc. 2019;8(11):e012924. doi: 10.1161/JAHA.119.012924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckel RH, Jakicic JM, Ard JD, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 21.Domanski MJ, Fuster V, Diaz-Mitoma F, et al. Next steps in primary prevention of coronary heart disease: rationale for and design of the ECAD trial. J Am Coll Cardiol. 2015;66(16):1828-1836. doi: 10.1016/j.jacc.2015.08.857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Definitions of Outcomes by Study Cohorts
eTable 2. Correlations Between LDL-C Variables
eTable 3. Study Observation Period and Number of Events
eTable 4. Number of Participants and Events by LDL-C Quartiles
eFigure 1. Study Flow Chart
eFigure 2. Example LDL-C Trajectories During Young Adulthood and Middle Age (From Age 18 Years Up to the Index Visit) for 12 Randomly Selected Participants (3 Participants per Study)
eFigure 3. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident CHD
eFigure 4. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident Ischemic Stroke
eFigure 5. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age and LDL-C at the Index Visit With Incident Heart Failure
eFigure 6. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Stratified by Sex
eFigure 7. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Stratified by Sex
eFigure 8. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Stratified by Sex
eFigure 9. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Stratified by Race
eFigure 10. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Stratified by Race
eFigure 11. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Stratified by Race
eFigure 12. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Among Participants Who Never Used Lipid Lowering Medications
eFigure 13. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Ischemic Stroke, Among Participants Who Never Used Lipid Lowering Medications
eFigure 14. Associations Between Cumulative LDL-C, TWA LDL-C, and LDL-C Slope During Young Adulthood and Middle Age With Incident Heart Failure, Among Participants Who Never Used Lipid Lowering Medications
eFigure 15. Associations Between Cumulative LDL-C During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eFigure 16. Associations Between TWA LDL-C During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eFigure 17. Associations Between LDL-C Slope During Young Adulthood and Middle Age With Incident CHD, Leaving Out One Study At a Time
eReferences


