Abstract
Pancreatic β cells dedicate much of their protein translation capacity to producing insulin to maintain glucose homeostasis. In response to increased secretory demand, β cells can compensate by increasing insulin production capability even in the face of protracted peripheral insulin resistance. The ability to amplify insulin secretion in response to hyperglycemia is a critical facet of β-cell function, and the exact mechanisms by which this occurs have been studied for decades. To adapt to the constant and fast-changing demands for insulin production, β cells use the unfolded protein response of the endoplasmic reticulum. Failure of these compensatory mechanisms contributes to both type 1 and 2 diabetes. Additionally, studies in which β cells are “rested” by reducing endogenous insulin demand have shown promise as a therapeutic strategy that could be applied more broadly. Here, we review recent findings in β cells pertaining to the metabolic amplifying pathway, the unfolded protein response, and potential advances in therapeutics based on β-cell rest.
Keywords: pancreatic islet beta cell, insulin secretion, unfolded protein response, endoplasmic reticulum stress, beta cell rest
Recent research contributions in the islet biology field, particularly those based on experiments in human pancreatic islets, have dramatically improved our knowledge of the normal physiological mechanisms underlying pancreatic β-cell function, as well as what goes awry in these specialized cells during diabetes pathogenesis (see (1) for an extensive review). In this article, we consider advances in our understanding of the signal transduction mechanisms that fine tune stimulus–secretion coupling for optimal insulin output and glucose homeostasis. We next highlight new findings about the unfolded protein response in β cells and other relevant cell types that may have important implications in pancreatic islets. We expect these lines of research will lead to increased understanding of islet physiology as well as opportunities for therapeutic interventions. Additionally, treatment strategies that involve “resting” β cells have been suggested and pursued in the past and may warrant renewed effort considering the unique secretory demands placed on β cells.
Overview of Metabolic Triggering and Amplifying Pathways of Insulin Secretion
Secreted proteins are simultaneously translated and transported into the endoplasmic reticulum (ER) in the first step of an arduous biochemical journey that enables nascent polypeptides to be folded, posttranslationally modified, proteolytically processed, sorted, and packaged into vesicles that deliver the cargo to the extracellular milieu via fusion with the plasma membrane (2, 3). Dedicated secretory cell types, in particular pancreatic islet β cells, devote much of their protein translation capacity to this process. The coupling of preproinsulin synthesis to nutrient demand (4) and the energy-intensive production of insulin means β cells are potentially under repeated low-level stress every time nutrient levels rise in the circulation. Although β cells are normally adapted to handle this stress over long periods, these adaptive responses may fail or become maladaptive because of chronic stimulation and/or underlying genetic susceptibilities during diabetes pathogenesis. Underscoring the importance of this process, defects in proinsulin folding from mutations in translation factors, as well as within insulin itself, can cause or predispose individuals to different types of diabetes (5, 6).
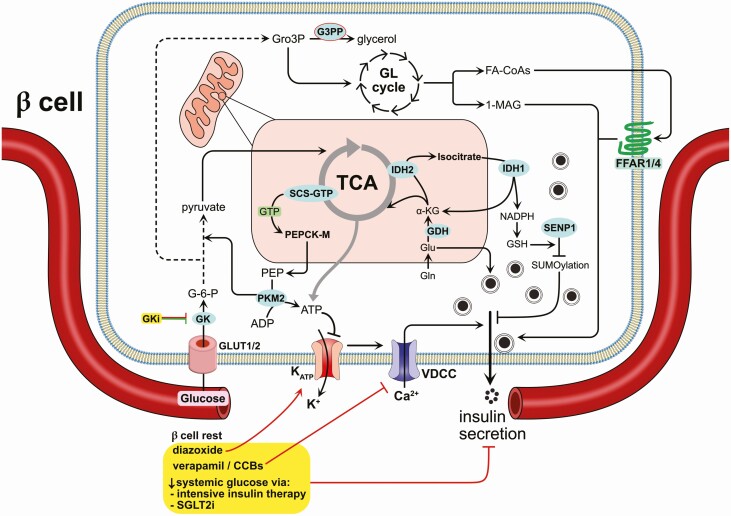
The fundamental purpose of pancreatic islet β cells is to secrete insulin in response to elevated circulating nutrient secretagogues, largely glucose. Secreted insulin then signals to peripheral skeletal muscle and adipose tissue to increase glucose uptake. Circulating glucose enters the β cell through GLUT1/2 transporters and is metabolized, increasing the ATP/ADP ratio and causing the closure of ATP-sensitive potassium (KATP) channels (Fig. 1). Closure of KATP channels depolarizes the plasma membrane, leading to opening of voltage-dependent calcium channels and calcium influx. This calcium influx is the triggering signal for docked and primed insulin secretory vesicles to fuse with the plasma membrane and exocytose their cargo. In addition to the triggering signal, the metabolic amplifying pathway enhances insulin secretion without further increases in calcium influx; our current understanding of this process has been reviewed in detail (7-10). A major result of the metabolic amplifying pathway is an increase in the release competency of insulin granules. This pathway is critical because metabolic amplification is estimated to account for up to 50% of glucose-stimulated insulin secretion (11). Metabolic amplification of insulin secretion is also linked to β-cell maturation because adult β cells gain their functionally mature state during postnatal development (12). During embryonic development, lineage-determining transcription factors (LDTFs) control the formation of distinct endocrine islet cell types, whereas signal-dependent transcription factors (SDTFs) are guided by LDTFs to control actual β-cell function (13). For example, Foxa2 is an LDTF that contributes to β-cell identity and eventual secretory function (14), whereas NFATc1 and NFATc2 are SDTFs activated by calcium influx and promote expression of genes involved in β-cell function, including Gck and Slc2a2 (15, 16). In another example, as β cells mature, synaptotagmin 4 expression is increased because of a loss of repression from the SDTF Myt1; synaptotagmin 4 is an insulin granule membrane protein that controls granule release competency (17). Given the number of metabolic coupling factors and signaling pathways involved, metabolic amplification of insulin secretion occurs as the complex summation of these many contributors (Fig. 1) (10). Even after nearly 30 years since its discovery (18, 19), new mechanisms related to the triggering and metabolic amplifying pathways continue to be discovered (see the following section).
Figure 1.
Intersection of the metabolic amplifying pathway of insulin secretion and β cell rest. Metabolism of nutrients through glycolysis and the tricarboxylic acid (TCA) cycle feed pathways generating metabolites including phosphoenolpyruvate (PEP), ATP, isocitrate, as well as intermediates of the pentose phosphate pathway and nucleotide biosynthesis pathways. The enzyme SCS-GTP generates succinate and GTP; this GTP, combined with oxaloacetate from the TCA cycle, is converted to PEP by PEP carboxykinase (PEPCK-M or Pck2). Recent work showed that membrane-localized pyruvate kinase (PKM2) converts this PEP to ATP, depleting local ADP concentrations, and promoting the closure of KATP channels and subsequent membrane depolarization. Another highlighted pathway is that of glutamate dehydrogenase (GDH), which converts glutamine to α-ketoglutarate (α-KG) in the mitochondria. There, α-KG may fuel the generation of other TCA cycle intermediates or be converted into isocitrate to replenish the downstream reducing equivalent glutathione (GSH), which is required for deSUMOylation of exocytotic proteins. The glycerolipid (GL) cycle also feeds into metabolic amplification via supplying 1-monoacylglycerol, which acts directly on exocytotic machinery to promote secretion. Fatty-acyl-CoAs (FA-CoAs) are also released and/or reach the β cell from the circulation to activate the free fatty acid receptors 1/4 (FFAR1/4), GPCRs that promote ER calcium release. Additionally, glycerol-3-phosphate (Gro3P) derived from glycolysis can be degraded by glycerol-3-phosphate phosphatase (G3PP) to regulate the impact of Gro3P on insulin secretion. There are direct and indirect therapeutic strategies that aim to reduce the insulin secretory demand on β cells (ie, β-cell rest, highlighted in yellow). These include, but are not necessarily limited to: (1) the use of diazoxide to open KATP channels and prevent membrane depolarization even in the face of increased glucose concentrations; (2) targeting of β-cell calcium channels to directly prevent calcium influx-mediated exocytosis; (3) glucokinase inhibitors (GKis) that could reduce metabolic flux of glucose into glycolysis and the TCA cycle, but also lead to improved secretory regulation; and (4) intensive insulin treatments or sodium-glucose transporter 2 inhibitors (SGLT2i), each of which results in a decrease in circulating glucose and therefore indirectly alleviate the demand on β cells. Supporting references are provided in the text. The arrow from GKi to GK is red and green, indicating it suppresses β-cell insulin secretory activity but also enhances β-cell glucose dose-response.
Several new insights into the mechanisms governing metabolic triggering and amplification are derived from studies of β-cell energetic pathways and their linkage to insulin secretion (Fig. 1). Pyruvate kinase was recently demonstrated to control KATP channel closure and insulin secretion via plasma membrane-localized production of ATP from phosphoenolpyruvate (20). However, a small molecule activator of pyruvate kinase also increased glucose-induced metabolic amplification under conditions in which the KATP channel was pharmacologically held in an open state with diazoxide. This finding indicates that pyruvate kinase has a dual role in the metabolic control of both the triggering and amplifying pathways.
Phosphoenolpyruvate may be provided to pyruvate kinase via upstream succinyl-CoA synthetase. The β cell expresses 2 isoforms of succinyl-CoA synthetase, 1 that produces ATP (SCS-ATP) and another that produces GTP (SCS-GTP). In rat islets (and INS-1 832/13 cells), knockdown of SCS-ATP increased glucose-stimulated insulin secretion, whereas suppression of SCS-GTP reduced insulin secretion (21). When SCS-ATP is absent, SCS-GTP generates increased amounts of mitochondrial GTP and enhances downstream Ca2+ influx. GTP generation within the mitochondrial matrix is not a major contributor to the cytosolic pool, so it is unlikely that this source of GTP influences insulin secretion via cytosolic- or plasma membrane-localized small GTPase activity. Accordingly, mitochondrial GTP generated by SCS-GTP drives metabolic amplification of insulin secretion through mitochondrial phosphoenolpyruvate carboxykinase via the phosphoenolpyruvate cycle (22). Furthermore, a reductive tricarboxylic acid (TCA) cycle flux, operating in a “counterclockwise” manner, can influence the amplifying pathway stimulated by glucose and glutamine (23). This process occurs through mitochondrial isocitrate dehydrogenase 2 converting α-ketoglutarate to isocitrate, which is then transported to the cytosol and converted back to α-ketoglutarate by cytosolic isocitrate dehydrogenase 1, generating NADPH, a key factor for metabolic amplification of insulin secretion (9). Interestingly, although the citrate-isocitrate carrier is not required for glucose-stimulated insulin secretion in mouse β cells, cytosolic isocitrate dehydrogenase 1 is indeed required (24).
The mitochondrial enzyme glutamate dehydrogenase (GDH; GLUD1) is also a major contributor to insulin secretion because mutations in this enzyme or in its regulators cause defects in insulin secretion (25-30). GDH converts glutamine-derived glutamate into α-ketoglutarate, which can feed the reductive TCA cycle, increasing downstream generation of cytosolic NADPH, as well as fuel succinyl coenzyme A (CoA) synthetase for mitochondrial GTP production. This key role of GDH has been confirmed using clonal knockout β cells (31) and in β-cell-specific GDH knockout mice (27, 28). Downstream, NADPH is critical for regenerating reduced glutathione and promoting insulin secretion through the removal of inhibitory SUMO modifications on exocytotic proteins (32). Another metabolite, adenylosuccinate within the de novo purine synthesis pathway, has also been linked to the deSUMOylating enzyme SENP1 and promoting metabolic amplification of insulin secretion through a yet-unidentified mechanism (33).
Lipids also influence the metabolic amplifying pathway both intracellularly and extracellularly by serving as a source of metabolic coupling factors and signaling via certain G protein-coupled receptors (GPCRs), respectively (34). Free fatty acids (FFAs), or nonesterified fatty acids, including short-, medium-, and long-chain forms, can signal through the lipid GPCRs GPR40 and GPR120, activating either Gi/o or Gq to modulate insulin secretion (35). Of note, recent work showed that deletion of both GPR40 and GPR120 (FFAR1 and FFAR4, respectively) had a minimal effect on β-cell function and glucose homeostasis (36). Nevertheless, FFAs support and are required for normal nutrient-induced insulin secretion (37-39). The source of lipids that have functional impact on β-cell function may be paracrine/autocrine (40). In response to glucose stimulation, mouse and human islets generate a variety of lipid molecules, including fatty acyl-CoAs, glycerolipids like 1-monoacylglycerol (41), and eicosanoids like 20-HETE (40), each of which contribute to regulated insulin exocytosis (42, 43).
The many paths leading to amplified secretion allow β cells to closely balance insulin production and glucose homeostasis. However, this robust secretory activity could have deleterious effects on long-term β-cell function, particularly in face of prolonged insulin resistance. For this reason, direct or indirect reduction of β-cell insulin secretory activity could be exploited for therapeutic benefit. The continued stress of insulin secretion can activate the unfolded protein response of the ER (UPRER), which is a regulated process necessary for β-cell function and survival. The UPRER and metabolic amplification also have overlapping regulatory pathways, such as the glutathione antioxidant system, which is important for SUMOylation and deSUMOylation in both the UPRER (44, 45) and in the amplifying pathway (32, 46-49). SUMOylation can also contribute to RNA processing and senescence in the β cell (44).
Advances in the Understanding of ER Stress and the UPRER in the β Cell
To maintain sufficient stores of insulin, transcription of preproinsulin mRNA is coupled to nutrient signals and newly synthesized polypeptide enters the ER lumen of the β-cell through cotranslational and posttranslational mechanisms (50). The activities of ER chaperones and catalysts enable proinsulin molecules to attain a mature tertiary conformation that is permitted to enter anterograde transport arriving at the trans-Golgi network for sorting, copackaging into immature secretory granules, and final proteolytic processing (1, 5, 50-52). The ER chaperone binding immunoglobulin (BiP; Hspa5) associates with preproinsulin during the early cotranslational insertion of the nascent polypeptide into the ER (53). Subsequently, protein folding is catalyzed by spontaneous formation of correct intramolecular disulfide bond pairings through spontaneous reactions of proximal cysteine residues (interchain cysteines A20 to B19 and A7 to B7; intrachain cysteines A6 to A11) and rearrangements of incorrect disulfide pairings via the actions of protein disulfide isomerases and oxidoreductases (50). The order of bond formation during folding, identity of aberrant bond pairs, and nature of misfolded aggregated proinsulin are critical parameters governing normal β-cell function and the dysfunction that occurs in diabetes (54). The importance of correct folding is underscored by the identification of numerous proinsulin mutations within or near critical cysteine residues of the protein that cause neonatal diabetes because of ER retention of proinsulin, induction of ER stress, and β-cell death (55-59). Additionally, mutations in translation factors, such as the signal sequence receptor Ssr1 (60) and the tRNA methylthiotransferase Cdkal1 (61), can lead to defective insulin processing and folding and contribute to diabetes development (5, 6).
Increased influx of nascent polypeptides into the ER lumen can exceed the ER capacity and will activate an adaptive stress response through at least 3 well-known signal transducers: ATF6, IRE1α, and PERK. These signaling pathways are part of the UPRER and control a very broad program of translational and transcriptional control that includes chaperone upregulation, misfolded protein degradation, and both survival and death (Fig. 2A). The 3 main UPRER pathways (ATF6, IRE1α, and PERK) are all regulated through direct interactions with a chaperone in the ER lumen, BiP. Upon increased protein flux through the ER-Golgi, BiP is titrated away from ATF6, IRE1α, and PERK to aid in folding and prevent aggregation of secreted proteins (62, 63). This BiP dissociation allows for activation of each of these pathways. Calcium concentrations in the cytosol and ER are tightly regulated (64), with the ER [Ca2+] at ~200 to 500 µM and cytosolic [Ca2+] at ~100 nM; Ca2+ is a key cofactor for many ER chaperones like BiP and protein disulfide isomerases (65). Accordingly, dysregulation of ER Ca2+ either by nutrient overload, cytokines, or pharmacological manipulation also causes activation of these UPRER pathways (64, 66, 67). SERCA2 is an ER membrane-localized ATPase that pumps Ca2+ from the cytosol into the ER. Defects in SERCA2 lead to β-cell failure and diabetes (68). Other non-ER pathways can also activate the UPR and may act in β-cell stress adaptation, as discussed next.
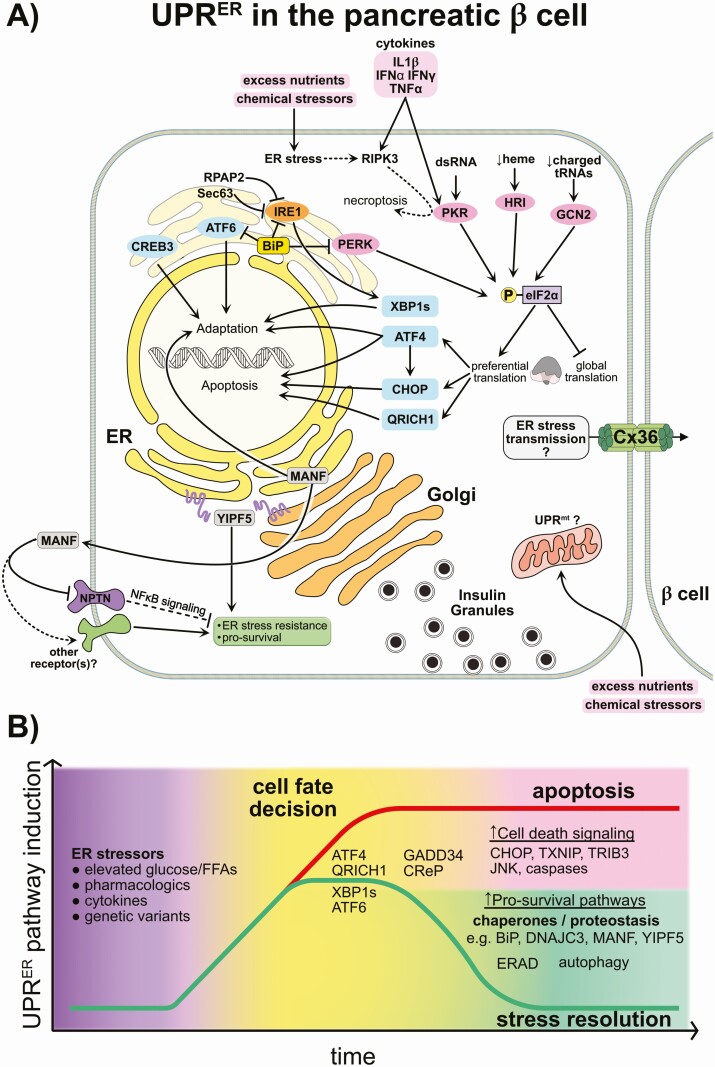
Figure 2.
Overview of endoplasmic reticulum (ER) stress and non-ER stress UPR pathways in β cells and the fate decision of adaptive vs terminal ER stress-induced unfolded protein response (UPR ER ). (A) The β cell repeatedly responds to nutrient fluxes over its lifetime and uses the UPRER as a mechanism to handle this constant demand for insulin production. Increased secretory protein production causes the titration of the ER chaperone binding immunoglobulin (BiP) away from ER membrane resident stress sensors, leading to their activation. These include PERK, IRE1α, and the ATF6/CREB3 family. Each of these sensors activates downstream transcription factors that enable cells to tolerate or mitigate the stress. ATF6/CREB3 are cleaved and release the respective active transcription factors; IRE1α and cofactors catalyze the cleavage and splicing of XBP1 mRNA, causing expression of the transcription factor XBP1s; and the eIF2α kinase PERK phosphorylates eIF2α, inhibiting global translation and causing the preferential translation of additional transcription factors (ATF4, CHOP, QRICH1). These transcription factors induce expression of pro-survival as well as pro-apoptotic genes, depending on the factor and also on the duration/intensity of the stress. Distinct non-ER-related stresses can intersect with UPR signaling through the remaining eIF2α kinases HRI, GCN2, and PKR (in pink), which each similarly inhibit global translation. ER and Golgi localized factors including YIPF5 and MANF promote ER stress resistance, although the mechanisms are under investigation. Finally, other mechanisms may be at play during the β-cell response to stress. For example, it is unknown what the mitochondrial UPR (UPRmt) does, or whether the highly interconnected islet β cells may transmit stress signals via gap junctions, as has been shown in other cell types. Supporting references provided in the text. (B) In response to physiologic, pathophysiologic, or pharmacologic stressors, β cells activate the UPRER (as described in Fig. 2). Genetic variation or predisposal also contributes to baseline stress and the ability of the β cell to respond appropriately. The duration and intensity of the stress response may dictate a cell fate decision wherein β cells progress either toward stress resolution or programmed cell death. Key factors in stress resolution include the early induction of ER chaperones (eg, DNAJC3/p58IPK, GRP78/BiP), protein disulfide isomerases (PDIs), and ER membrane/luminal pro-survival factors (eg, MANF, YIPF5), induction of ER-associated degradation (ERAD) and autophagy, culminating in the clearance of misfolded proteins and restoration of homeostasis. When the stress persists unmitigated, continuous ATF4 expression leads to C/EBP homologous protein (CHOP) production and downstream induction of pro-apoptotic factors (eg, TXNIP, TRIB3). Novel factors like glutamine rich 1 (QRICH1) may play a role in this cell fate decision and progression toward apoptosis. Intense UPRER can induce negative feedback of eIF2α phosphorylation via the expression of eIF2α phosphatases GADD34 and CReP. Attempting to restart translation before sufficient stress mitigation may then antagonize recovery and promote cell death.
Activation of the UPRER is necessary and β cells are normally equipped to handle this repeated rise and fall in secretory demand (69). In this regard, the UPRER helps β cells execute the complicated process of nutrient- and hormone-regulated insulin expression, translation, processing, trafficking, and exocytosis, even under dietary stress (70). However, in disease, β cells are taxed by the combination of multiple factors, among them genetic mutations or polymorphisms (71-73), peripheral insulin resistance (74), nutrient-induced metabolic stress (34, 75), and inflammatory mediators (76, 77). These events can lead to a maladaptive or terminal UPRER, which eventually results in β-cell dysfunction and apoptosis (78, 79). The need for further evidence of β-cell UPRER in diabetes has been noted (80). Such evidence is accumulating through single-cell transcriptomic studies in rodent, primate, and human islets as well as in rodent models of type 2 diabetes progression (81, 82) and histological studies of islets from patients affected by type 2 (83), and type 1 diabetes (84). A recent preprint highlighted the limits of the adaptability of β cells to repeated ER stress, showing that although β cells may survive this process, they eventually lose their plasticity, are less able to mount a full ER stress response, and have reduced insulin gene expression (85). In a simplified view of this process, Fig. 2B illustrates how β cells exposed to ER stressors induce the UPRER in response and, depending on the intensity and duration of the insult, undergo a cell fate decision toward either apoptosis or survival. Other contributors to resolution of ER stress include ER-associated degradation (ERAD) (86) and autophagy (87).
PERK, ATF4, and CHOP
PERK is activated on dissociation of BiP from its ER-luminal domain. This activation allows the oligomerization and transautophosphorylation of PERK, which promotes its kinase activity and consequent phosphorylation of eIF2α (88, 89). Phosphorylation of eIF2α causes a global suppression of protein translation and preferential production of ER stress response genes including the basic leucine zipper (bZIP) transcription factor ATF4. This process occurs because of a decoy upstream open reading frame in the ATF4 mRNA that under normal conditions leads to a nonfunctional product. When translation is suppressed because of eIF2α phosphorylation, the ribosome skips this upstream open reading frame and translates a downstream open reading frame that encodes functional ATF4 (90, 91). During adaptation to ER stress, ATF4 regulates the transcription of genes involved in amino acid metabolism and antioxidant response (92). The induction of these genes by ATF4 contributes to the increase in the folding capacity of the ER, adapting it to the ongoing stress or functional demand. Other functions of this pathway are to enhance the metabolism of the increased amounts of amino acids resulting from the increased protein degradation from ERAD and to adapt the ER to oxidative stress caused by misfolded and unfolded proteins (93, 94). A key gene induced by ATF4 is Ddit3, which encodes the pro-apoptotic transcription factor C/EBP homologous protein (CHOP). Early in the UPRER, CHOP induction may serve as a negative feedback signal to attenuate eIF2α phosphorylation via the induction of the PPP1R15 members GADD34 and CrEP (95). During prolonged or excessive UPRER, CHOP coordinates with ATF4 to induce key elements of β-cell demise (96, 97). Recent findings about CHOP regulation in the β cell have implications for β-cell calcium handing, diabetes, and what is likely insulin-driven liver storage of nutrients leading to steatosis (98).
Deletion of PERK causes diabetes because of β-cell dysfunction in mouse models, and inactivating mutations in PERK cause a genetic form of human diabetes called Wolcott-Rallison syndrome, indicating the essential nature of the UPRER in β cells (99). In these diseases, mutations in PERK abolish its kinase activity, which prevents a physiological response to increased flux of protein production through the ER. On the other hand, partial pharmacological inhibition of PERK has beneficial effects in a mouse model of diabetes (100) and mice missing 1 copy of the Eif2ak2 gene encoding PERK have improved β-cell function, suggesting there is a “goldilocks” zone for PERK in particular and ER stress sensors in general (101). Moreover, cross-talk between PERK and IRE1 influences whether or not cells adapt to UPR in ovarian and colorectal cancer cell lines (102), although whether this particular mechanism also occurs in the β cell is unknown.
IRE1α and XBP1s
IRE1 and its relationship to metabolic regulation has been reviewed in detail (103). IRE1α is an ER membrane-spanning protein with kinase and nuclease domains facing the cytosol. Like PERK and ATF6, the ER luminal N-terminus of IRE1α is bound by BiP under normal conditions. When BiP is titrated away during ER stress, IRE1α oligomerizes and is transautophosphorylated, which activates its nuclease activity. IRE1α nuclease activity combines with associated ligation machinery to act on its most well-known target, Xbp1 mRNA. In this process, unconventional splicing of Xbp1 mRNA removes a short sequence, resulting in translation of the functional bZIP transcription factor XBP1s (104-106). XBP1s translocates to the nucleus and binds to ER stress response elements (ERSEs) and UPR elements to induce transcription of genes encoding ER chaperones and enzymes involved in protein folding (eg, Hspa5), maturation, and degradation, as well as transcription factors such as Ddit3, Atf4, and Xbp1 (107-109). XBP1s also regulates genes involved in ER expansion/biogenesis and the transport and trafficking of vesicles (109-111). IRE1α and XBP1s are required to maintain β-cell function and prevent diabetes because IRE1α deletion in β cells leads to defects in glucose-stimulated induction of genes required for proinsulin processing (112). IRE1α also cleaves other mRNAs in a process termed “regulated IRE1-dependent decay” (RIDD), which decreases the load of mRNAs marked for processing in the ER, including insulin mRNA (113-115). When excessive, RIDD may be a contributing factor to β-cell death (116). IRE1α may also contribute to the regulation of insulin synthesis in response to exposure to high glucose, independent from its XBP1 splicing activity (117).
IRE1α likely integrates many different signaling pathways through participation in a multiprotein regulatory complex called the UPRosome (103, 118), and IRE1α deletion in the β cells protects against type 1 diabetes in a mouse model (119). This effect is proposed to be due to β-cell dedifferentiation and reduced expression of autoantigens, which is in line with findings suggesting that cytokine-induced ER stress signals mostly via the IRE1α-JNK pathway (76). This suggests that diminishing IRE1α activity before the onset of type 1 diabetes could be a strategy to prevent or delay disease.
Relatively less is known about the processes by which adaptive IRE1α signaling is turned off during sustained ER stress. As referenced previously, sustained PERK activation attenuates IRE1α activity via the phosphatase RNA polymerase II-associated protein 2 (RPAP2) (102). RPAP2 dephosphorylates IRE1α leading to reduced RIDD, terminating IREα’s stress-mitigating activity. IRE1α is also shut off by the translocon-associated protein Sec63, which facilitates BiP binding to IRE1α in HEK293 cells (120). RPAP2 and Sec63 appear to be expressed in human pancreatic islets (TIGER Data Portal; http://tiger.bsc.es). Additionally, 2 cytokine-induced proteins, namely N-MYC interactor (121) and ubiquitin D (122), prevent IRE1α-induced JNK activation in β cells. Whether these mechanisms are exploitable therapeutically in β cells is an open question. A recent high-throughput screening campaign successfully identified activators of the IRE1α-XBP1 pathway using a reporter of Xbp1 mRNA splicing (123). The relative specificity of the primary hit IXA4 was confirmed through incorporation of a counter-screen against activators of ATF6 and use of IRE1α inhibitor 4µ8c.
Recent findings from a preprint suggest that in vivo treatment of diet-induced obese mice with IXA4 had beneficial effects in both the liver and pancreatic β cells (124). The potential for targeting IRE1α with such a compound to treat diabetes and/or obesity appears promising. However, care must be taken as the β cell-specific loss of IRE1α (112) or Xbp1 (125), as well as sustained expression of XBP1s (126) leads to β-cell dysfunction.
ATF6α and CREB3
ATF6α is an ER resident protein that transits forward to the Golgi upon ER stress where it is proteolytically cleaved by the same proteases that process SREBPs (127, 128). Cleaved ATF6 is a bZIP transcription factor enters the nucleus and controls a subset of UPR transcription (129, 130).
ATF6α is acutely required for UPRER gene induction in response to thapsigargin or tunicamycin in mouse islets (131). XBP1s-targeted gene expression requires ATF6α, but ATF6α-regulated gene expression seems to be independent of XBP1s. ATF6 and XBP1s also contribute to β-cell proliferation in rodents (132). A recent study discovered a small molecule inhibitor of the SIK kinases that induced a transient UPRER in β cells involving induction of ATF6 and XBP1s and leading to β-cell proliferation in vivo (133). Besides ATF6, the CREB3 family of ER-associated proteins are processed to bZIP transcription factors and regulate expression of secretory pathway genes (134). CREB3 family members aid in ER stress sensing and are expressed in pancreatic β cells; they include CREB3 (aka Luman or LZIP), CREB3L1 (aka OASIS), CREB3L2, CREB3L3, and CREB3L4 (135). During UPRER, these ER transmembrane proteins are processed in a manner similar to ATF6. Expression of CREB3 was enriched in human β cells with elevated levels of UPR (136) and the metabolic stressor palmitate induced expression of CREB3 and CREB3L3 in human islets (137). CREB3 was also recently implicated as part of a Golgi stress signature identified in human islets treated with brefeldin A (138). A protective action of CREB3 was identified because inhibition of CREB3 by a specific siRNA potentiated palmitate-induced apoptosis in rodent and human β cells (137). In β cells, CREB3L1 could be activated by ER stressors, and induced extracellular matrix genes and genes involved in pancreas development, but did not induce typical ER stress response genes in β cells. The other CREB3 family members are also of uncertain value to β-cell UPRER (139).
Unfolded Protein Response-related Pathways in β Cells
eIF2α Kinases
Phosphorylation of eIF2α at Ser51 is a major determinant of β-cell survival (53, 140, 141) and is linked to the ER stress kinase PERK (142, 143). Three additional eIF2α kinases—PKR, GCN2, and heme-regulated inhibitor (HRI)—may also influence β cells under specialized conditions (144). Although the mouse knockout of PERK causes overt diabetes because of β-cell dysfunction and degeneration (142, 143), this does not occur with deletion of the other eIF2α kinases, suggesting they may not be required for glucose homeostasis in the absence of additional genetic or environmental perturbations.
PKR is classically known to be activated in response to double-stranded RNA, such as occurs during certain virus infections (145). PKR can be activated by other stressors as well. Studies of PKR knockout mice did not report metabolic defects (146, 147), but β cells were not put under stress in these models, which may have failed to disclose relevant phenotypes. PKR inhibition with small molecules suppresses ER stress in β cells (148) and has antidiabetic properties in obese mouse models (149). Interestingly, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was recently demonstrated to activate PKR (150). Although controversy exists regarding the potential for SARS-CoV-2 to infect pancreatic islet β cells (151), there have been several reports of infection and subsequent dysfunction/killing of β cells (152-154). In one of these studies, PKR was shown to be activated by SARS-CoV-2 in β cells causing β-to-α cell transdifferentiation (153). This process was blocked by trans-ISRIB, a drug that prevents phospho-eIF2α from binding to eIF2B, thereby alleviating suppression of global translation (155).
GCN2 is an amino acid sensor activated by decreased charged tRNA levels. GCN2 knockout mice maintained on a standard diet did not exhibit any obvious glucose homeostasis phenotypes (156). However, a recent study of GCN2-deficient mice showed that when stressed with a high-fat diet, these mice lose pancreatic β-cell mass (157). Furthermore, humans carrying a type 2 diabetes risk allele of GCN2 have impaired insulin secretion (157). These findings draw renewed attention to GCN2 and its previously recognized intersection with PERK in β-cell function and ER stress. For example, amino acid starvation still causes eIF2α phosphorylation in Gcn2 null mouse embryonic stem cells, although delayed, and conversely phosphorylation of eIF2α can also occur in response to ER stress in Perk null cells (156). Therefore, GCN2 may work in β cells either as an amino acid sensor or as an auxiliary kinase recruited under specific metabolic conditions where PERK is the primary signal transducer.
HRI, as its name suggests, senses heme levels in erythroid cells, but has both heme-dependent and heme-independent actions across multiple tissues (158). Even though HRI is apparently well-expressed in human islets, how it aids the β cell or UPRER is not known. However, a small molecule HRI activator, EPB-53, improved metabolic health in high-fat diet fed mice (159). This effect required FGF21 as no effects of EPB-53 were seen in FGF21 null mice.
Mitochondrial UPR
Stresses that cause increased amounts of unfolded proteins within the mitochondrial matrix or disrupt oxidative phosphorylation can lead to the mitochondrial UPR (UPRmt) (160). UPRmt has been observed in the hypothalamus and adipocytes (161-164). Little is known about the UPRmt in normo- and pathophysiology in the islet. However, transcriptomic data indicate that palmitate induces expression of LonP1, a gene involved in the UPRmt, in human β cells (137). As a result of the UPRmt, specific mitochondrial chaperones and proteases are expressed, distinct from the factors involved in the UPRER, which are presumed to aid in alleviating mitochondrial stress. Phosphorylation of eIF2α is required for the induction of CHOP, ATF4, and ATF5 during UPRmt (165), although it is not clear which eIF2α kinase(s) is/are responsible under different conditions leading to mitochondrial stress and the UPRmt (160). Preliminary findings suggest a role for the UPRmt-related protein ubiquitin-like protein 5 in that β cell-specific ubiquitin-like protein 5 null mice exhibit a diabetic phenotype with β-cell impairment and altered islet UPRmt gene expression (166).
Contributions of Lipids to β-cell UPRER
The UPRER can both influence and be influenced by lipid metabolism and signaling. Treatment of β cells with the saturated fatty acid palmitate induces the UPRER including expression of BiP, ATF6, and XBP1s and causes apoptosis likely through CHOP, JNK, and other signals (167, 168). Treatment with the unsaturated fatty acid oleate had opposing effects and, when combined with palmitate, leads to partial protection against ER stress (167). Multiple mechanisms downstream of palmitate lead to β-cell dysfunction and UPRER, including depletion of β-cell ER calcium stores (169-171), disrupted ER membrane lipid composition (172), altered protein palmitoylation (173), degradation of proinsulin processing enzymes (174), and accumulation of islet amyloid polypeptide (137, 175). In β cells, palmitate can also act through mTORC1 to increase protein translation and ER load, leading to UPRER (176). Subsequent activation of the XBP1s arm of the UPRER can also regulate gluconeogenic and lipogenic genes either directly or in conjunction with FoxO1 or SREBP1c in hepatocytes (177); some of these signaling pathways are likely also functioning in β cells. Lipids also accumulate in droplets inside cells and adult human and mouse islet β cells express the lipid droplet-associated perilipins PLIN2 and PLIN3 (178). The expression of PLIN2/3 changes little with age, but lipid droplets accumulate in human islet β cells as individuals age and are increased further in human type 2 diabetes (178). PLIN2 is upregulated in response to fatty acid or pharmacological UPRER stressors, and knocking out Plin2 partially rescued β-cell failure in a mouse model of ER stress-induced diabetes, potentially via increased autophagic flux (179). Together, these studies highlight the importance of lipids and lipid storage in β-cell homeostasis and the UPRER.
Emerging ER Homeostasis Contributors
Discovery of genes potentially implicated in ER stress in different tissues have emerged in recent years. Here, we highlight a few of these genes with exciting potential; several of these mechanisms are incorporated into an integrated depiction of ER stress signaling (Fig. 2).
QRICH1.
The transcription factor glutamine rich 1 (QRICH1) controls the apoptotic fate decision during a prolonged unfolded protein response in intestinal cells (180). This discovery was made applying single-cell RNA sequencing and a whole-genome CRISPR knockout screen for ER stress regulators in primary mouse intestinal organoids. QRICH1 has an upstream inhibitory short open reading frame, similar to ATF4, which allows preferential translation during the UPR. QRICH1 is a member of the caspase activation and recruitment domain family of proteins, implying a role in cell survival. QRICH1 deletion is protective under pharmacological ER stress conditions, placing it as a negative regulator of cell survival. Additionally, QRICH1 can activate its own transcription and bind some of the same gene promoters as ATF4 (180). This activity up-regulates a program of increased translation and protein secretion. QRICH1 is widely expressed and likely has a conserved function across different cell types. Interestingly, QRICH1 was also a hit in a genome-wide CRISPR knockout screen in β cells (181), where its deletion led to a decrease in insulin content. These results suggest QRICH1 may regulate β-cell insulin production and ER stress. Interestingly, human loss-of-function mutations in QRICH1 have been identified, leading to Ververi-Brady syndrome (182-184), characterized primarily by developmental delay. It is presently unclear whether there is any diabetic phenotype in these individuals.
MANF.
Mesencephalic astrocyte-derived neurotrophic factor (MANF) is an ER protein that can act intracellularly or be secreted. Truncating mutations in MANF have been linked to rare nonautoimmune diabetes in humans (72). β-cell dysfunction in these individuals is presumably due to the loss of the protective effect of MANF in context of the UPRER (185, 186). Recombinant MANF treatment protected human and mouse β cells against cytokine or thapsigargin-induced stress (185), implying an extracellular activity. In β cells, MANF expression is induced in response to ER stress. This is dependent on thrombospondin-1, an adhesive glycoprotein that protects β cells from lipotoxic and ER stressors. MANF binds to BiP in the ER and is released to be secreted during ER stress (187, 188). MANF binding to BiP stabilized BiP–client substrate complexes and inhibited BiP nucleotide exchange (189). Recently, neuroplastin was identified as a receptor for secreted MANF in rat INS-1 β cells, (190). In that study, MANF was found to bind neuroplastin and suppress downstream nuclear factor-κB signaling to partially protect against pharmacological ER stressors. Given its properties, MANF and its receptor(s) represent an exciting frontier for potential disease therapies (191).
YIPF5.
Yip1 domain family member 5 (YIPF5) is an ER transmembrane protein involved in ER-Golgi trafficking (192) as well as ER-Golgi structural maintenance (193). Loss-of-function mutations in YIPF5 in humans result in neonatal diabetes, microcephaly, and epilepsy (71). Diabetes occurs in these individuals because of loss of β cells in response to increased ER stress. YIPF5 is not required for insulin secretion, but it is necessary for resilience against ER stress. ER stressors can induce transcription of YIPF5, suggesting a role in stress adaptation. The related gene YIPF3 was also implicated in β cells because YIPF3 deletion in a genome-wide CRISPR screen resulted in increased insulin content (181). YIPF3 has limited sequence identity with YIPF5 so it is unclear if they share similar tasks in β cells.
RIPK3.
Receptor interacting serine/threonine kinase 3 (RIPK3) is involved in inflammation and regulated necrosis signaling (194). RIPK3 is activated in response to β-cell ER stress induced in zebrafish with muscle insulin resistance (195). In that zebrafish model, RIPK3 activation led to IL-1β induction and β-cell loss. In further studies, human islets were transplanted into transgenic mice, which permit pharmacological β-cell destruction. RIPK3 was activated specifically in human islet β cells when endogenous β cells were ablated, suggesting a conserved role for RIPK3 across multiple model systems and humans. Interferon-induced RIPK-mediated necrosis also requires PKR (196), tying the β-cell UPR to cytokine signaling pathways, an idea that is supported in other cell types. In fibroblasts, ER stress induces necroptosis dependent on RIPK1 and RIPK3 (197), and RIPK3 null mice were protected from ER stress induced by cardiac ischemia-reperfusion (198). Of note, preexposure of β cells to a mild ER stress, particularly caused by depletion of ER Ca2+, potentiates IL-1β-induced nuclear factor-κB activation and the downstream expression of chemokines and other pro-inflammatory genes (199). During necrosis in NIH 3T3 cells, RIPK3 interacts with glutamate dehydrogenase and isocitrate dehydrogenase 1, key players in the metabolic amplification of insulin secretion (194). The contribution of β cell RIPK3 in these responses is yet to be explored.
Technical Advances in β-cell UPRER Understanding
Our knowledge of the UPR in normal biology and disease states continues to evolve as technical advances drive the discovery and understanding of stimuli, new disease implications emerge, and the UPR is described with higher resolution and further assignment of functions and regulators. Cell lines that represent secretory cell types and disease states, advanced gene expression sequencing, pharmacological tools, UPR reporter assays, genetic manipulation of cells by CRISPR, and production of UPR sensor-null mice are some of the experimental approaches that have defined UPR sensors in normal biology and disease. Next, we highlight some of these recent technical advances.
Temporal Analyses to Better Understand UPRER Regulation
The dynamics of transcription, splicing, translation, and posttranslational events during the initiation and progression of β-cell UPRER is not completely understood. Omics experiments measuring outputs in cell types other than β cells could provide some insights. For example, 1 study in HeLa cells used transcriptome, translatome, and proteome readouts to compare tunicamycin and H2O2-induced stress over 0 to 8 hours (200). Many up- and down-regulated pathways were shared between the ER stress and oxidative stress responses, although the data have yet to be compared with dedicated secretory cell types. The extent to which UPRER and alternative splicing transcriptional responses to diverse stresses are present and specific to β cells is an area under active investigation (201). Another example is the recent study identifying the action of QRICH1 in ER stress (180). The authors succeeded in identifying this factor by performing a time-course tunicamycin treatment in primary mouse intestinal organoids followed by single-cell RNA sequencing. Given the secretory parallels in enteroendocrine cells, the alterations observed in the QRICH1 study may also be relevant to β cells.
ATF6α and XBP1 were investigated using time-course pharmacological ER stress experiments in isolated mouse islets (131). The study demonstrated the differing kinetics of UPR gene induction depending on the stressor: thapsigargin (which depletes ER Ca2+) was quicker than tunicamycin (which prevents protein glycosylation in the ER) to induce splicing of XBP1 and induction of Hspa5 (BiP) expression. A time-course analysis of cyclopiazonic acid (a drug that depletes ER Ca2+ in a reversible way) in rat β cells identified an early degradation of insulin mRNA expression, which was paralleled by induction of the UPR proteins CHOP and BiP. Although the pro-apoptotic protein CHOP returned to a basal level 3 hours after the removal of cyclopiazonic acid, the chaperone BiP remained up-regulated, suggesting a long-term β-cell adaptive response (115) that may protect these cells against subsequent stresses (202). Future use of unbiased RNA sequencing analyses will undoubtedly provide additional insights into facets of UPRER regulation specific to the β cell and hopefully provide a path forward that dissociates the deleterious from the protective effects of the UPR in these cells.
Longer time courses associated with RNA sequencing have been performed. For example, an experimental paradigm was applied in which human islets were treated over the duration of days with palmitate, glucose, or both to model nutrient-induced metabolic stress and poststress recovery (203). Although human β cells fully recovered when followed for 4 days after a 48-hour exposure to high glucose or palmitate alone, they remained dysfunctional when exposed to both high glucose and palmitate, which was associated with a severe ER stress-induced mRNA signature (203). Another recent study evaluated the transcriptome during β-cell development and maturation in mice after birth and up to 60 days of age (204). A unique aspect of this study was the application of an algorithm that clusters gene expression changes based upon their respective temporal patterns (205). Applying this approach to temporal UPRER datasets could provide new insights into the UPR in general as well as potentially β cell-specific mechanisms.
Finally, an exciting use of these advanced computational approaches is to compare such changes between datasets from different researchers (206, 207) or target tissues from different diseases with common pathogenic pathways, including ER stress (208). These analyses open promising research areas that will advance our understanding of diabetes susceptibility, UPRER biology, and development of novel therapeutic strategies.
UPR Biosensors
The complexity of the UPRER signal has demanded development of a multitude of reporter assays for use both in vitro and in vivo. Early in the characterization of the UPR, cell-based assays relied upon the coupling of ERSE and UPR element transcriptional control elements of the UPR with expression of colorimetric or fluorescent reporters that were induced in response to pharmacological modulators of the UPR (209). Also, the upstream open reading frames that govern ATF4 translation upon eIF2α phosphorylation enable repression of translation and stress-induced induction of reporter proteins (210, 211). The inhibition of mRNA translation from unspliced XBP1 sequences and the production of a functional mRNA on stress has been incorporated into the design of numerous in vitro and in vivo reporter assays that drive the production of a fluorescent product on IRE1α activation (212, 213). Secreted alkaline phosphatase and fluorescent proteins also report upon the integrity of the ER and anterograde transport under conditions of stress (214, 215). The innovative impact of reporters of ER function is highlighted by the identification of a novel mechanism of ER reflux of material to the cytosol using ER-targeted superfolder GFP and ER-targeted mCherry as biosensor molecules (216). ERSE- and UPRE-driven mCherry has also been used a fluorescent imaging-based readout of UPRER induction, for example, to demonstrate the cell–cell transmissibility of the UPRER in hepatocytes (215).
A powerful approach toward increasing understanding of β-cell UPR is the combination of different reporter assay readouts for ER homeostasis and secretory capability. A dual-luciferase reporter assay was developed for simultaneous readings of both ER calcium and UPR status (217). The assay combines a Gaussia luciferase-based ER calcium-monitoring protein (218) and a new UPRE-driven Nano luciferase. This tool could be used for high-throughput screening in β cells or other islet cell types alone or perhaps in combination with additional reporter assays. For example, a spliced XBP1-luciferase fusion protein was developed (219). Swapping one of these luciferases for a distinct substrate-specific luciferase or an alternate readout could allow assay multiplexing and greater extraction of information from high-throughput experimentation. Advances in the monitoring of preproinsulin processing in β cells using a fusion of insulin and superfolder GFP and mCherry (220) could be combined with measures of ER sensory pathways in experimental designs (especially luciferase-based) and in screening for therapeutic agents for treatment of diabetes. Incorporation of specific biosensors downstream of other stress signaling pathways could also provide valuable insights.
Single-cell Variation and β-cell Heterogeneity Contributions to ER Stress Responses
There may be distinct subtypes of β cells (221-223), but in certain cases these apparent subpopulations may actually be β cells transitioning between different states. Insulin expression and activity also vary between subpopulations of β cells (224), which may involve bursting of insulin gene transcriptional activity (225). β-cell heterogeneity and state transitions have been extensively reviewed (226-230), yet we would like to draw attention to a few key studies that are starting to unveil aspects of ER stress related to β cells transitioning through different states of relatively high and low insulin production and high and low UPRER (136). To handle stress, β cells are able to reduce insulin expression and increase UPR and antioxidant gene expression. Reduced insulin expression in that state was correlated with increased markers of β-cell proliferation and the cells also had increased metabolic gene expression.
In another approach, clusters containing β-like cells were derived from induced pluripotent stem cells (iPSCs) (231) obtained from individuals with neonatal diabetes resulting from mutations in the insulin gene at cysteine codons, which causes misfolding of proinsulin. The authors corrected 1 of these mutations in iPSCs using CRISPR/Cas9 and studied the β-like cells derived from mutant and corrected iPSCs. Applying single-cell RNA sequencing to these iPSC-derived β-like cells indicated subtle gene expression differences among the multiple cell types contained in the clusters after differentiation. β-like cells with mutated insulin had up-regulation of many genes involved in ER stress and ER-associated degradation, and down-regulation of genes involved in mitochondrial respiration, β-cell proliferation, and function. When these clusters were transplanted into mice, the mutant cells exhibited elevated expression of ER stress markers (eg, BiP, MANF) and reduced mTOR signaling, but there was no increase in cell death (231), possibly because the physiology of the normal recipient mice did not challenge the transplant. These findings suggest that early UPRER in differentiating β cells, in this case β cells with mutant insulin, causes dysfunction immediately and results in lower β-cell proliferation.
Single-cell RNA sequencing was applied to pancreatic islets from cynomolgus monkeys to investigate changes that occur with aging (232). β-cell function is known to decline with age (233-235), but because of the heterogeneous nature of islet endocrine cells, it is difficult to determine the major contributing factors. Single-cell transcriptomic analysis of young and aged monkey islets revealed hundreds of up- and down-regulated genes associated with aging in both β and α cells. In particular, there was significant enrichment of UPRER gene expression in aged islet β cells. For example, increased expression of the ER chaperone HSP90B1 with age contributes to inhibition of the β cell’s secretory response to glucose. Aged β cells exhibit down-regulation of genes involved in regulated exocytosis, protein trafficking, translation, and GPCR signaling pathways. Finally, islet amyloid polypeptide expression and amyloid formation is increased in aged islets, concomitant with a switch from adaptive to pro-apoptotic ER stress (232). These alterations combined with the enhanced UPR likely contribute to impaired β-cell function in aging.
Cell–cell Connections and the Spreading of the UPR
Islet β cells are highly interconnected through gap junction proteins, the connexins. In the case of β cells, Cx36 is a major player (236, 237). These junctions are dysfunctional in diabetic mice (238). ER stress can spread from cell to cell, a phenomenon previously observed in cancer (239, 240). Recently, work in hepatocytes established that ER stress can indeed be transmitted intercellularly through gap junctions (215), although via a distinct connexin, namely Cx46. The identity of the small molecule messenger(s) that propagates this stress signal is unknown. Given the interconnectedness of islet β cells, the same property that permits synchronous responses to nutrients and hormones may also predispose β cells to the propagation of stress signals. Whether stressed β cells can induce ER stress in adjacent β cells is an open question.
Potential for β-cell Rest Therapies
Often β cell-targeted therapies in type 2 diabetes aim to enhance the amount of insulin that is produced and secreted in response to hyperglycemia. It has been proposed that such an approach eventually leads to so-called “β-cell burnout” and contributes to progressive β-cell failure in type 2 diabetes (241-243). This scenario does not always occur and is likely influenced by genetic and environmental factors, as suggested by the existence of individuals in whom β-cell function is preserved despite decades of insulin hypersecretion to compensate for obesity-induced insulin resistance (244-248). The chronic use of sulfonylureas, which directly cause closure of the KATP channel to elicit insulin secretion, leads to β-cell dysfunction (242, 249), and β-cell function declines in type 2 diabetes across multiple distinct treatment strategies (250, 251). In contrast, glucagon-like peptide 1 analogs both increase insulin release and protect human β cells against ER stress, by favoring BiP up-regulation (252, 253).
“β-cell rest” involves suppressing endogenous β-cell activity pharmacologically (254), either indirectly by treating with exogenous insulin or by combination therapies that lower blood glucose while increasing insulin sensitivity (Fig. 1). The strategy based on intensive insulin therapy was first proposed and tested in 1940 in patients with type 1 diabetes (255). This intensive insulin treatment approach was later applied to individuals with type 2 diabetes by the 1970s, with promising results (256). Although subsequent trials have been limited in size, a review of the results suggests positive outcomes (250). More recently, the combination therapy approach was used to rest β cells and restore function in a strain of db/db mice with impaired β-cell compensation (257). These mice develop obesity and severe diabetes, but a combination of thiazolidinedione and SGLT2 inhibitor therapies allowed β-cell recovery. Work in db/db mice also demonstrated the benefits of β-cell rest via sequentially inhibiting GIPR signaling during the light cycle and activating GLP-1 receptor signaling during the dark cycle over a 4-week course of treatments (258).
Additionally, metabolically active cells have adaptive mechanisms to maintain the optimal amount of reactive oxygen species generation downstream of glucose metabolism, and this points to an avenue that could lead to therapeutic benefits in β cells (259). One such example is to suppress β-cell function as a therapeutic strategy by reducing the activity of glucokinase (260), the enzyme that controls glucose entry into the glycolytic pathway (261). For example, chronically treating islets from diabetic (db/db) mice with the glucokinase inhibitor mannoheptulose reversed the left-shifted glucose dose-response (262). Although, β cell-specific targeting strategies should be considered given that glucokinase in the α cell may have an opposite action (263). This is in line with previous suggestions that prolonged glucose-stimulated insulin secretion itself is a causative factor in β-cell stress through increased generation of reactive oxygen species (264). In agreement with this hypothesis, resting β-cell lines and rodent islets under lipotoxic stress using diazoxide protects against ER stress and secretory dysfunction (265). Recent evidence supports the idea that even short-term hyperglycemic stress can impair β cells (266). Conversely, a recent study of a subset of Diabetes Remission Clinical Trial participants demonstrated that dietary weight loss provides sustained improvements in β-cell function (267).
Similar to intensive insulin/diazoxide therapies, suppression of β-cell function by other means has therapeutic benefits not only in type 2 diabetes, but also in type 1 diabetes, given the results of studies using the calcium channel blocker verapamil (268, 269). It is possible that preventing β cells from hypersecreting insulin may lower the exposure of potential autoantigens, allowing β cells to be less visible to the immune system and thus delaying disease (270).
Conclusions
The findings reviewed here suggest that further studies are warranted regarding both the mechanisms of the UPR in β cells and mechanisms/strategies to alleviate this stress—potentially through β-cell rest. Targeting these pathways in a β cell-specific manner could be a highly effective approach to improve β-cell health in disease. Toward this end, there are some key questions and topics, among many, that remain to be addressed:
Do stressed β cells transmit UPR stress signals to adjacent β cells within the islet? If so, can this process be modulated pharmacologically for therapy? Given β cells’ interconnection and their juxtaposition to γ and δ cells, whether such signals can propagate between these endocrine cells to affect global islet function is an open question.
Can our current understanding of stimulus-secretion coupling and UPR biology be used in conjunction with β-cell targeting methods to therapeutically and specifically restore β-cell function without affecting other tissues?
IRE1α can be inhibited by Sec63 (120) and RPAP2 (102). However, whether these mechanisms are conserved or potentially exploitable in β cells is unknown. Can IRE1α activity be specifically modulated in β cells through these regulators or through other means? One approach could be to link an IRE1 inhibitor (eg, IXA4) to GLP1 to target β cells, similar to GLP1-linked antisense oligonucleotides (98) and GLP1-linked estrogen (271). For an extensive discussion of pharmacological targeting of the UPRER as a therapeutic strategy in disease, please see (272) and see (273) for a β cell-focused review.
Are there additional MANF receptors in addition to neuroplastin and is their expression altered and/or activity disrupted in diabetes? Can MANF or its receptor(s) be targeted therapeutically in β cells in vivo or ex vivo?
Advanced analysis of dynamic transcriptomic data at both the bulk and single-cell level should continue to be actively pursued to gain new understanding of how β cells handle physiological fluctuations in insulin demand and which aspects go awry in disease. Factors that influence the cell fate decision between survival and apoptosis in the β-cell UPRER (Fig. 2B) are of particular interest.
A better understanding of the potential cross-talk between UPR pathways in the β cell may lead to therapeutically exploitable options. Can “protective pathways” (eg, BiP) be stimulated while inhibiting the pro-apoptotic ones (eg, CHOP, JNK)? PERK-IRE1α cross-talk has been established in non-β cells in the context of UPR adaptation (102), although whether this mechanism also occurs in the β cell is unknown.
Pursuing lines of research that bring us closer to addressing any of these points will undoubtedly result in many new questions. Such pursuits represent critical opportunities to discover therapeutic in-roads for diabetes and other diseases of secretory biology.
Acknowledgments
Thank you to members of the Kalwat laboratory, Fiona Armoo and Gitanjali Roy, for helpful discussions and comments. The authors also thank Andrew Templin (Indiana Biosciences Research Institute) and Scott Soleimanpour (University of Michigan) for critiques and suggestions during the preparation of the manuscript. Human pancreatic islet expression data were accessed via the TIGER Data Portal (http://tiger.bsc.es) and the T2DSystems Consortium, and via the Huising laboratory (http://huisinglab.com/diabetes_2019/index.html) (274).
Financial Support : M.A.K. is supported as a Lilly Scholar in the Lilly Diabetes Center of Excellence at Indiana Biosciences Research Institute (IBRI) and by an award from the Diabetes Research Connection. D.L.S. is supported by the IBRI. M.H.C. is supported by National Institutes of Health R37 DK34128 and Welch I1243. D.L.E. acknowledges the support of grants from the Welbio-FNRS (Fonds National de la Recherche Scientifique; WELBIO-CR-2019C-04), Belgium; the Innovate2CureType1—Dutch Diabetes Research Foundation (DDRF), Holland; the Juvenile Diabetes Research Foundation (JDRF; 2-SRA-2019-834-S-B); the National Institutes of Health (HIRN-CBDS) grant U01 DK127786, USA; startup funds from the IBRI, Indianapolis, IN, USA; and the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement numbers 115797 (INNODIA) and 945268 (INNODIA HARVEST), supported by the European Union’s Horizon 2020 research and innovation program. These Joint Undertakings receive support from the Union’s Horizon 2020 research and innovation program and European Federation of Pharmaceutical Industries and Associations, JDRF, and The Leona M. and Harry B. Helmsley Charitable Trust.
Author Contributions : M.A.K. conceived, researched, and wrote the manuscript. D.S., K.R.-S., and D.L.E. wrote, revised, and edited the manuscript. M.H.C. revised and edited the manuscript.
Conflict of Interest : D.L.E. receives grant support from Eli Lilly and Company, Indianapolis, for research on new approaches to protect pancreatic β cells in type 1 diabetes. The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- BiP
binding immunoglobulin
- bZIP
basic leucine zipper
- CHOP
C/EBP homologous protein
- CoA
coenzyme A
- ER
endoplasmic reticulum
- ERAD
endoplasmic reticulum-associated degradation
- ERSE
endoplasmic reticulum stress response element
- FFA
free fatty acid
- GDH
glutamate dehydrogenase
- GPCR
G protein-coupled receptor
- HRI
heme-regulated inhibitor
- iPSC
induced pluripotent stem cell
- KATP
ATP-sensitive potassium
- LDTF
lineage-determining transcription factor
- MANF
mesencephalic astrocyte-derived neurotrophic factor
- QRICH1
glutamine rich 1
- RIDD
regulated IRE1-dependent decay
- RIPK3
receptor interacting serine/threonine kinase 3
- RPAP2
RNA polymerase II-associated protein 2
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SCS-ATP
succinyl-CoA synthetase ATP
- SCS-GTP
succinyl-CoA synthetase ATP
- SDTF
signal-dependent transcription factor
- TCA
tricarboxylic acid
- UPRER
endoplasmic reticulum stress-induced unfolded protein response
- UPRmt
mitochondrial unfolded protein response
- YIPF5
Yip1 domain family member 5
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Walker JT, Saunders DC, Brissova M, Powers AC. The human islet: mini-organ with mega-impact. Endocr Rev. 2021. doi: 10.1210/endrev/bnab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guerriero CJ, Brodsky JL. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol Rev. 2012;92(2): 537-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):169-181. [DOI] [PubMed] [Google Scholar]
- 4. Joslin EP, Kahn CR.. Joslin’s Diabetes Mellitus. Lippincott Williams & Wilkins;2005. [Google Scholar]
- 5. Liu M, Huang Y, Xu X, et al. Normal and defective pathways in biogenesis and maintenance of the insulin storage pool. J Clin Invest. 2021;131(2). doi: 10.1172/JCI142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez-Calvo T, Chen YC, Verchere CB, et al. Altered β-cell prohormone processing and secretion in type 1 diabetes. Diabetes. 2021;70(5):1038-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalwat MA, Cobb MH. Mechanisms of the amplifying pathway of insulin secretion in the β cell. Pharmacol Ther. 2017;179:17-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49(11):1751-1760. [DOI] [PubMed] [Google Scholar]
- 9. Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013; 18(2):162-185. [DOI] [PubMed] [Google Scholar]
- 10. Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22(2):142-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Henquin JC. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52(5):739-751. [DOI] [PubMed] [Google Scholar]
- 12. Blum B, Hrvatin S, Schuetz C, Bonal C, Rezania A, Melton DA. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol. 2012;30(3):261-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wortham M, Sander M. Transcriptional mechanisms of pancreatic β-cell maturation and functional adaptation. Trends Endocrinol Metab. 2021;32(7):474-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lantz KA, Vatamaniuk MZ, Brestelli JE, Friedman JR, Matschinsky FM, Kaestner KH. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest. 2004;114(4):512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heit JJ, Apelqvist AA, Gu X, et al. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443(7109):345-349. [DOI] [PubMed] [Google Scholar]
- 16. Keller MP, Paul PK, Rabaglia ME, et al. The transcription factor Nfatc2 regulates β-cell proliferation and genes associated with type 2 diabetes in mouse and human islets. Plos Genet. 2016;12(12):e1006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Walker EM, Dadi PK, et al. Synaptotagmin 4 regulates pancreatic beta cell maturation by modulating the Ca(2+) sensitivity of insulin secretion vesicles. Dev Cell. 2021. doi: 10.1016/j.devcel.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gembal M, Gilon P, Henquin JC. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J Clin Invest. 1992;89(4):1288-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato Y, Aizawa T, Komatsu M, Okada N, Yamada T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes. 1992;41(4):438-443. [DOI] [PubMed] [Google Scholar]
- 20. Lewandowski SL, Cardone RL, Foster HR, et al. Pyruvate kinase controls signal strength in the insulin secretory pathway. Cell Metab 2020;32(5):736-750 e735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jesinkey SR, Madiraju AK, Alves TC, et al. Mitochondrial GTP links nutrient sensing to beta cell health, mitochondrial morphology, and insulin secretion independent of OxPhos. Cell Rep. 2019;28(3):759-772 e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang GF, Jensen MV, Gray SM, et al. Reductive TCA cycle metabolism fuels glutamine- and glucose-stimulated insulin secretion. Cell Metab. 2021;33(4):804-817 e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauchle CJ, Rohli KE, Boyer CK, et al. Mitochondrial efflux of citrate and isocitrate is fully dispensable for glucose-stimulated insulin secretion and pancreatic islet beta-cell function. Diabetes. 2021. doi: 10.2337/db21-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luczkowska K, Stekelenburg C, Sloan-Béna F, et al. Hyperinsulinism associated with GLUD1 mutation: allosteric regulation and functional characterization of p.G446V glutamate dehydrogenase. Hum Genomics. 2020;14(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimaldi M, Karaca M, Latini L, Brioudes E, Schalch T, Maechler P. Identification of the molecular dysfunction caused by glutamate dehydrogenase S445L mutation responsible for hyperinsulinism/hyperammonemia. Hum Mol Genet. 2017;26(18):3453-3465. [DOI] [PubMed] [Google Scholar]
- 27. Vetterli L, Carobbio S, Frigerio F, Karaca M, Maechler P. The amplifying pathway of the β-cell contributes to diet-induced obesity. J Biol Chem. 2016;291(25):13063-13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carobbio S, Frigerio F, Rubi B, et al. Deletion of glutamate dehydrogenase in beta-cells abolishes part of the insulin secretory response not required for glucose homeostasis. J Biol Chem. 2009;284(2):921-929. [DOI] [PubMed] [Google Scholar]
- 29. Stanley CA. Two genetic forms of hyperinsulinemic hypoglycemia caused by dysregulation of glutamate dehydrogenase. Neurochem Int. 2011;59(4):465-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Snider KE, Becker S, Boyajian L, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98(2):E355-E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han G, et al. Glutamate is an essential mediator in glutamine-amplified insulin secretion. J Diabetes Investig. 2021. doi: 10.1111/jdi.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferdaoussi M, Dai X, Jensen MV, et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J Clin Invest. 2015;125(10):3847-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gooding JR, Jensen MV, Dai X, et al. Adenylosuccinate is an insulin secretagogue derived from glucose-induced purine metabolism. Cell Rep. 2015;13(1):157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prentki M, Peyot ML, Masiello P, Madiraju SRM. Nutrient-induced metabolic stress, adaptation, detoxification, and toxicity in the pancreatic β-cell. Diabetes. 2020;69(3):279-290. [DOI] [PubMed] [Google Scholar]
- 35. Ghislain J, Poitout V. Targeting lipid GPCRs to treat type 2 diabetes mellitus - progress and challenges. Nat Rev Endocrinol. 2021;17(3):162-175. [DOI] [PubMed] [Google Scholar]
- 36. Croze ML, Guillaume A, Ethier M, et al. Combined deletion of free fatty-acid receptors 1 and 4 minimally impacts glucose homeostasis in mice. Endocrinology 2021;162(3). doi: 10.1210/endocr/bqab002. [DOI] [PubMed] [Google Scholar]
- 37. Hauke S, Keutler K, Phapale P, Yushchenko DA, Schultz C. Endogenous fatty acids are essential signaling factors of pancreatic β-cells and insulin secretion. Diabetes. 2018;67(10): 1986-1998. [DOI] [PubMed] [Google Scholar]
- 38. Dobbins RL, Chester MW, Stevenson BE, Daniels MB, Stein DT, McGarry JD. A fatty acid-dependent step is critically important for both glucose- and non-glucose-stimulated insulin secretion. J Clin Invest. 1998;101(11):2370-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roduit R, Nolan C, Alarcon C, et al. A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes. 2004;53(4):1007-1019. [DOI] [PubMed] [Google Scholar]
- 40. Tunaru S, Bonnavion R, Brandenburger I, et al. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun. 2018;9(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao S, Mugabo Y, Iglesias J, et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 2014;19(6):993-1007. [DOI] [PubMed] [Google Scholar]
- 42. Prentki M, Corkey BE, Madiraju SRM. Lipid-associated metabolic signalling networks in pancreatic beta cell function. Diabetologia. 2020;63(1):10-20. [DOI] [PubMed] [Google Scholar]
- 43. Possik E, Madiraju SRM, Prentki M. Glycerol-3-phosphate phosphatase/PGP: role in intermediary metabolism and target for cardiometabolic diseases. Biochimie. 2017;143:18-28. [DOI] [PubMed] [Google Scholar]
- 44. Li N, Zhang S, Xiong F, Eizirik DL, Wang CY. SUMOylation, a multifaceted regulatory mechanism in the pancreatic beta cells. Semin Cell Dev Biol. 2020. doi: 10.1016/j.semcdb.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 45. Li N, Luo X, Yu Q, et al. SUMOylation of Pdia3 exacerbates proinsulin misfolding and ER stress in pancreatic beta cells. J Mol Med (Berl). 2020;98(12):1795-1807. [DOI] [PubMed] [Google Scholar]
- 46. Davey JS, Carmichael RE, Craig TJ. Protein SUMOylation regulates insulin secretion at multiple stages. Sci Rep. 2019;9(1):2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacDonald PE. A post-translational balancing act: the good and the bad of SUMOylation in pancreatic islets. Diabetologia. 2018;61(4):775-779. [DOI] [PubMed] [Google Scholar]
- 48. Ferdaoussi M, Fu J, Dai X, et al. SUMOylation and calcium control syntaxin-1A and secretagogin sequestration by tomosyn to regulate insulin exocytosis in human ß cells. Sci Rep. 2017;7(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dai XQ, Plummer G, Casimir M, et al. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes. 2011;60(3):838-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu M, Weiss MA, Arunagiri A, et al. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018;20 Suppl 2:28-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2012;9(1):25-53. [PMC free article] [PubMed] [Google Scholar]
- 52. Sims EK, Carr ALJ, Oram RA, DiMeglio LA, Evans-Molina C. 100 years of insulin: celebrating the past, present and future of diabetes therapy. Nat Med. 2021;27(7):1154-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757-764. [DOI] [PubMed] [Google Scholar]
- 54. Haataja L, Arunagiri A, Hassan A, et al. Distinct states of proinsulin misfolding in MIDY. Cell Mol Life Sci. 2021;78(16):6017-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hodish I, Liu M, Rajpal G, et al. Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem. 2010;285(1):685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Edghill EL, Flanagan SE, Patch AM, et al. ; Neonatal Diabetes International Collaborative Group . Insulin mutation screening in 1044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes. 2008;57(4):1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Støy J, Edghill EL, Flanagan SE, et al. ; Neonatal Diabetes International Collaborative Group . Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104(38):15040-15044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park SY, Ye H, Steiner DF, Bell GI. Mutant proinsulin proteins associated with neonatal diabetes are retained in the endoplasmic reticulum and not efficiently secreted. Biochem Biophys Res Commun. 2010;391(3):1449-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dhayalan B, Chatterjee D, Chen YS, Weiss MA. Diabetes mellitus due to toxic misfolding of proinsulin variants. Mol Metab. 2021;101229. doi: 10.1016/j.molmet.2021.101229. [DOI] [PubMed] [Google Scholar]
- 60.Li X, et al. Requirement for translocon-associated protein (TRAP) alpha in insulin biogenesis. Sci Adv. 2019;5(12):eaax0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wei FY, Suzuki T, Watanabe S, et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J Clin Invest. 2011;121(9):3598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326-332. [DOI] [PubMed] [Google Scholar]
- 63. Amin-Wetzel N, Saunders RA, Kamphuis MJ, et al. A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 2017;171(7):1625-1637 e1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Idevall-Hagren O, Tengholm A. Metabolic regulation of calcium signaling in beta cells. Semin Cell Dev Biol. 2020;103:20-30. [DOI] [PubMed] [Google Scholar]
- 65. Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3(6). doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang IX, Raghavan M, Satin LS. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology 2020;161(2). doi: 10.1210/endocr/bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Postić S, Sarikas S, Pfabe J, et al. Intracellular Ca2+ channels initiate physiological glucose signaling in beta cells examined in situ. bioRxiv. 2021. doi: 10.1101/2021.04.14.439796. [DOI] [Google Scholar]
- 68. Tong X, Kono T, Anderson-Baucum EK, et al. SERCA2 deficiency impairs pancreatic β-cell function in response to diet-induced obesity. Diabetes. 2016;65(10):3039-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaufman RJ, Back SH, Song B, Han J, Hassler J. The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in β-cells. Diabetes Obes Metab. 2010;12 Suppl 2:99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Omikorede O, Qi C, Gorman T, et al. ER stress in rodent islets of Langerhans is concomitant with obesity and β-cell compensation but not with β-cell dysfunction and diabetes. Nutr Diabetes. 2013;3:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Franco E, Lytrivi M, Ibrahim H, et al. YIPF5 mutations cause neonatal diabetes and microcephaly through endoplasmic reticulum stress. J Clin Invest. 2020;130(12):6338-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Montaser H, Patel KA, Balboa D, et al. Loss of MANF causes childhood-onset syndromic diabetes due to increased endoplasmic reticulum stress. Diabetes. 2021;70(4):1006-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Locke JM, Hysenaj G, Wood AR, Weedon MN, Harries LW. Targeted allelic expression profiling in human islets identifies cis-regulatory effects for multiple variants identified by type 2 diabetes genome-wide association studies. Diabetes. 2015;64(4):1484-1491. [DOI] [PubMed] [Google Scholar]
- 74. Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98(4):2133-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P. Endoplasmic reticulum stress and eIF2α phosphorylation: the Achilles heel of pancreatic β cells. Mol Metab. 2017;6(9):1024-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349-362. [DOI] [PubMed] [Google Scholar]
- 77. Cosentino C, Regazzi R. Crosstalk between macrophages and pancreatic beta-cells in islet development, homeostasis and disease. Int J Mol Sci. 2021;22(4). doi: 10.3390/ijms22041765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chan JY, Luzuriaga J, Bensellam M, Biden TJ, Laybutt DR. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes. 2013;62(5):1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29(3):317-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Herbert TP, Laybutt DR. A reevaluation of the role of the unfolded protein response in islet dysfunction: maladaptation or a failure to adapt? Diabetes. 2016;65(6):1472-1480. [DOI] [PubMed] [Google Scholar]
- 81. Huang H, Yang K, Wang R, et al. β-Cell compensation concomitant with adaptive endoplasmic reticulum stress and β-cell neogenesis in a diet-induced type 2 diabetes model. Appl Physiol Nutr Metab. 2019;44(12):1355-1366. [DOI] [PubMed] [Google Scholar]
- 82. Yang K, Gotzmann J, Kuny S, Huang H, Sauvé Y, Chan CB. Five stages of progressive β-cell dysfunction in the laboratory Nile rat model of type 2 diabetes. J Endocrinol. 2016;229(3):343-356. [DOI] [PubMed] [Google Scholar]
- 83. Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50(12):2486-2494. [DOI] [PubMed] [Google Scholar]
- 84. Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55(9):2417-2420. [DOI] [PubMed] [Google Scholar]
- 85. Chen C-W, Guan B-J, Alzahrani MR, et al. Adaptation to chronic ER stress enforces pancreatic β-cell plasticity. bioRxiv. 2021. doi: 10.1101/2021.05.24.445193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pearson GL, Gingerich MA, Walker EM, Biden TJ, Soleimanpour SA. A selective look at autophagy in pancreatic β-cells. Diabetes. 2021;70(6):1229-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Marciniak SJ, Garcia-Bonilla L, Hu J, Harding HP, Ron D. Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J Cell Biol. 2006;172(2):201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem. 2002;277(21):18728-18735. [DOI] [PubMed] [Google Scholar]
- 90. Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167(1):27-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099-1108. [DOI] [PubMed] [Google Scholar]
- 92. Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11(3):619-633. [DOI] [PubMed] [Google Scholar]
- 93. Lilley BN, Ploegh HL. Multiprotein complexes that link dislocation, ubiquitination, and extraction of misfolded proteins from the endoplasmic reticulum membrane. Proc Natl Acad Sci U S A. 2005;102(40):14296-14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21(5):500-511. [DOI] [PubMed] [Google Scholar]
- 95. Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A. 2009;106(6):1832-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Han J, Kaufman RJ. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev. 2017;31(14):1417-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yong J, Parekh VS, Nayak J, et al. Chop/Ddit3 depletion in beta cells alleviates ER stress and corrects hepatic steatosis in mice. Sci Transl Med. 2021;13(604). doi: 10.1126/scitranslmed.aba9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Delépine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25(4):406-409. [DOI] [PubMed] [Google Scholar]
- 100. Kim MJ, Kim MN, Min SH, et al. Specific PERK inhibitors enhanced glucose-stimulated insulin secretion in a mouse model of type 2 diabetes. Metabolism. 2019;97:87-91. [DOI] [PubMed] [Google Scholar]
- 101. Wang R, Munoz EE, Zhu S, McGrath BC, Cavener DR. Perk gene dosage regulates glucose homeostasis by modulating pancreatic β-cell functions. Plos One. 2014;9(6):e99684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chang TK, Lawrence DA, Lu M, et al. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol Cell 2021;71(4):629-636 e625. [DOI] [PubMed] [Google Scholar]
- 103. Huang S, Xing Y, Liu Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J Biol Chem. 2019;294(49):18726-18741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jurkin J, Henkel T, Nielsen AF, et al. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. Embo J. 2014;33(24):2922-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kosmaczewski SG, Edwards TJ, Han SM, et al. The RtcB RNA ligase is an essential component of the metazoan unfolded protein response. EMBO Rep. 2014;15(12):1278-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lu Y, Liang FX, Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase RtcB. Mol Cell. 2014;55(5):758-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881-891. [DOI] [PubMed] [Google Scholar]
- 108. Chen CY, Malchus NS, Hehn B, et al. Signal peptide peptidase functions in ERAD to cleave the unfolded protein response regulator XBP1u. Embo J. 2014;33(21):2492-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Acosta-Alvear D, Zhou Y, Blais A, et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell. 2007;27(1):53-66. [DOI] [PubMed] [Google Scholar]
- 110. Sriburi R, Bommiasamy H, Buldak GL, et al. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282(10):7024-7034. [DOI] [PubMed] [Google Scholar]
- 111. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hassler JR, Scheuner DL, Wang S, et al. The IRE1α/XBP1s pathway is essential for the glucose response and protection of β cells. Plos Biol. 2015;13(10):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104-107. [DOI] [PubMed] [Google Scholar]
- 114. Han D, Lerner AG, Vande Walle L, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pirot P, Naamane N, Libert F, et al. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia. 2007;50(5):1006-1014. [DOI] [PubMed] [Google Scholar]
- 116. Ghosh R, Colon-Negron K, Papa FR. Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes. Mol Metab. 2019;27S:S60-S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lipson KL, Fonseca SG, Ishigaki S, et al. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4(3):245-254. [DOI] [PubMed] [Google Scholar]
- 118. Urra H, Pihan P, Hetz C. The UPRosome—decoding novel biological outputs of IRE1alpha function. J Cell Sci. 2020;133(15). doi: 10.1242/jcs.218107. [DOI] [PubMed] [Google Scholar]
- 119.Lee H, et al. Beta cell dedifferentiation induced by IRE1alpha deletion prevents type 1 diabetes. Cell Metab. 2020;31(4):822-836 e825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Li X, Sun S, Appathurai S, Sundaram A, Plumb R, Mariappan M. A molecular mechanism for turning Off IRE1α signaling during endoplasmic reticulum stress. Cell Rep. 2020;33(13):108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Brozzi F, Gerlo S, Grieco FA, et al. A combined “omics” approach identifies N-Myc interactor as a novel cytokine-induced regulator of IRE1 protein and c-Jun N-terminal kinase in pancreatic beta cells. J Biol Chem. 2014;289(30):20677-20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Brozzi F, Gerlo S, Grieco FA, et al. Ubiquitin D regulates IRE1α/c-Jun N-terminal kinase (JNK) protein-dependent apoptosis in pancreatic beta cells. J Biol Chem. 2016;291(23): 12040-12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Grandjean JMD, Madhavan A, Cech L, et al. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat Chem Biol. 2020;16(10):1052-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Madhavan A, Kok BP, Grandjean JMD, et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. bioRxiv. 2020. doi: 10.1101/2020.12.03.408716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108(21):8885-8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Allagnat F, Christulia F, Ortis F, et al. Sustained production of spliced X-box binding protein 1 (XBP1) induces pancreatic beta cell dysfunction and apoptosis. Diabetologia. 2010;53(6):1120-1130. [DOI] [PubMed] [Google Scholar]
- 127. Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ye J, Rawson RB, Komuro R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355-1364. [DOI] [PubMed] [Google Scholar]
- 129. Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136(3):343-350. [DOI] [PubMed] [Google Scholar]
- 130. Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13(3):351-364. [DOI] [PubMed] [Google Scholar]
- 131. Sharma RB, Darko C, Alonso LC. Intersection of the ATF6 and XBP1 ER stress pathways in mouse islet cells. J Biol Chem. 2020;295(41):14164-14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sharma RB, O’Donnell AC, Stamateris RE, et al. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest. 2015;125(10):3831-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Charbord J, Ren L, Sharma RB, et al. In vivo screen identifies a SIK inhibitor that induces β cell proliferation through a transient UPR. Nat Metab. 2021;3(5):682-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fox RM, Hanlon CD, Andrew DJ. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J Cell Biol. 2010;191(3):479-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sampieri L, Di Giusto P, Alvarez C. CREB3 transcription factors: ER-Golgi stress transducers as hubs for cellular homeostasis. Front Cell Dev Biol. 2019;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xin Y, Dominguez Gutierrez G, Okamoto H, et al. Pseudotime ordering of single human β-cells reveals states of insulin production and unfolded protein response. Diabetes. 2018;67(9):1783-1794. [DOI] [PubMed] [Google Scholar]
- 137. Cnop M, Abdulkarim B, Bottu G, et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 2014;63(6):1978-1993. [DOI] [PubMed] [Google Scholar]
- 138. Bone RN, Oyebamiji O, Talware S, et al. A computational approach for defining a signature of β-cell Golgi stress in diabetes. Diabetes. 2020;69(11):2364-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vellanki RN, Zhang L, Guney MA, Rocheleau JV, Gannon M, Volchuk A. OASIS/CREB3L1 induces expression of genes involved in extracellular matrix production but not classical endoplasmic reticulum stress response genes in pancreatic beta-cells. Endocrinology. 2010;151(9):4146-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Scheuner D, Song B, McEwen E, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165-1176. [DOI] [PubMed] [Google Scholar]
- 141. Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153-1163. [DOI] [PubMed] [Google Scholar]
- 143. Zhang P, McGrath B, Li S, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22(11): 3864-3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci. 2013;70(19):3493-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gal-Ben-Ari S, Barrera I, Ehrlich M, Rosenblum K. PKR: a kinase to remember. Front Mol Neurosci. 2018;11:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Abraham N, Stojdl DF, Duncan PI, et al. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274(9):5953-5962. [DOI] [PubMed] [Google Scholar]
- 147. Yang YL, Reis LF, Pavlovic J, et al. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. Embo J. 1995;14(24):6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yalçin A, Şarkici G, Kolaç UK. PKR inhibitors suppress endoplasmic reticulum stress and subdue glucolipotoxicity-mediated impairment of insulin secretion in pancreatic beta cells. Turk J Biol. 2020;44(2):93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nakamura T, Arduini A, Baccaro B, Furuhashi M, Hotamisligil GS. Small-molecule inhibitors of PKR improve glucose homeostasis in obese diabetic mice. Diabetes. 2014;63(2):526-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Y, et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc Natl Acad Sci USA 2021;118(16). doi: 10.1073/pnas.2022643118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kusmartseva I, Wu W, Syed F, et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32(6):1041-1051 e1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Wu CT, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565-1576.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Tang X, Uhl S, Zhang T, et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2020. doi: 10.1016/j.cmet.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Steenblock C, Richter S, Berger I, et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat Commun. 2021;12(1):3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Zyryanova AF, Weis F, Faille A, et al. Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science. 2018;359(6383):1533-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhang P, McGrath BC, Reinert J, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22(19):6681-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kanno A, Asahara S-I, Furubayashi A, et al. GCN2 regulates pancreatic beta cell mass by sensing intracellular amino acid levels. JCI Insight 2020;5(9). doi: 10.1172/jci.insight.128820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Burwick N, Aktas BH. The eIF2-alpha kinase HRI: a potential target beyond the red blood cell. Expert Opin Ther Targets. 2017;21(12):1171-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zarei M, Pujol E, Quesada-López T, et al. Oral administration of a new HRI activator as a new strategy to improve high-fat-diet-induced glucose intolerance, hepatic steatosis, and hypertriglyceridaemia through FGF21. Br J Pharmacol. 2019;176(13):2292-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19(2):109-120. [DOI] [PubMed] [Google Scholar]
- 161. Braga RR, Crisol BM, Brícola RS, et al. Exercise alters the mitochondrial proteostasis and induces the mitonuclear imbalance and UPRmt in the hypothalamus of mice. Sci Rep. 2021;11(1):3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kobayashi M, Nezu Y, Tagawa R, Higami Y. Mitochondrial unfolded protein responses in white adipose tissue: lipoatrophy, whole-body metabolism and lifespan. Int J Mol Sci. 2021;22(6). doi: 10.3390/ijms22062854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Martinus RD, Garth GP, Webster TL, et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem. 1996;240(1):98-103. [DOI] [PubMed] [Google Scholar]
- 164. Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. Embo J. 2002;21(17):4411-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Anderson NS, Haynes CM. Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol. 2020;30(6):428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Haralambous C, Ntouma V. Islet beta-cell specific deletion of UBL5 gene associated with mitochondrial stress leads to diabetes in mice and beta-cell impairment/death. Diabetes 2018;67(Supplement 1). doi: 10.2337/db18-311-LB. [DOI] [Google Scholar]
- 167. Nemecz M, Constantin A, Dumitrescu M, et al. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: the elucidation of associated mechanisms and effector molecules. Front Pharmacol. 2018;9:1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lytrivi M, Castell AL, Poitout V, Cnop M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J Mol Biol. 2020;432(5):1514-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology. 2014;155(3):758-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296(4):E690-E701. [DOI] [PubMed] [Google Scholar]
- 171. Cunha DA, Hekerman P, Ladrière L, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121(Pt 14):2308-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Biden TJ, Boslem E, Chu KY, Sue N. Lipotoxic endoplasmic reticulum stress, β cell failure, and type 2 diabetes mellitus. Trends Endocrinol Metab. 2014;25(8):389-398. [DOI] [PubMed] [Google Scholar]
- 173. Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. A role for aberrant protein palmitoylation in FFA-induced ER stress and β-cell death. Am J Physiol Endocrinol Metab. 2012;302(11):E1390-E1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jeffrey KD, Alejandro EU, Luciani DS, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A. 2008;105(24):8452-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Krizhanovskii C, Fred RG, Oskarsson ME, Westermark GT, Welsh N. Addition of exogenous sodium palmitate increases the IAPP/insulin mRNA ratio via GPR40 in human EndoC-βH1 cells. Ups J Med Sci. 2017;122(3):149-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Hatanaka M, Maier B, Sims EK, et al. Palmitate induces mRNA translation and increases ER protein load in islet β-cells via activation of the mammalian target of rapamycin pathway. Diabetes. 2014;63(10):3404-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Piperi C, Adamopoulos C, Papavassiliou AG. XBP1: a pivotal transcriptional regulator of glucose and lipid metabolism. Trends Endocrinol Metab. 2016;27(3):119-122. [DOI] [PubMed] [Google Scholar]
- 178. Tong X, Dai C, Walker JT, et al. Lipid droplet accumulation in human pancreatic islets is dependent upon both donor age and health. Diabetes. 2019. doi: 10.2337/db19-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chen E, Tsai TH, Li L, Saha P, Chan L, Chang BH. PLIN2 is a key regulator of the unfolded protein response and endoplasmic reticulum stress resolution in pancreatic β cells. Sci Rep. 2017;7:40855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.You K, et al. QRICH1 dictates the outcome of ER stress through transcriptional control of proteostasis. Science 2021;371(6524). doi: 10.1126/science.abb6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Fang Z, Weng C, Li H, et al. Single-cell heterogeneity analysis and CRISPR screen identify key beta-cell-specific disease genes. Cell Rep. 2019;26(11):3132-3144 e3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Baruch Y, Horn-Saban S, Plotsky Y, Bercovich D, Gershoni-Baruch R. A case of Ververi-Brady syndrome due to QRICH1 loss of function and the literature review. Am J Med Genet A. 2021;185(6):1913-1917. [DOI] [PubMed] [Google Scholar]
- 183. Föhrenbach M, Jamra RA, Borkhardt A, et al. QRICH1 variants in Ververi-Brady syndrome-delineation of the genotypic and phenotypic spectrum. Clin Genet. 2021;99(1):199-207. [DOI] [PubMed] [Google Scholar]
- 184. Ververi A, Splitt M, Dean JCS, Brady AF; DDD Study . Phenotypic spectrum associated with de novo mutations in QRICH1 gene. Clin Genet. 2018;93(2):286-292. [DOI] [PubMed] [Google Scholar]
- 185. Cunha DA, Cito M, Grieco FA, et al. Pancreatic β-cell protection from inflammatory stress by the endoplasmic reticulum proteins thrombospondin 1 and mesencephalic astrocyte-derived neutrotrophic factor (MANF). J Biol Chem. 2017;292(36): 14977-14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lindahl M, Danilova T, Palm E, et al. MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep. 2014;7(2):366-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Oh-Hashi K, Tanaka K, Koga H, Hirata Y, Kiuchi K. Intracellular trafficking and secretion of mouse mesencephalic astrocyte-derived neurotrophic factor. Mol Cell Biochem. 2012;363(1-2):35-41. [DOI] [PubMed] [Google Scholar]
- 188. Glembotski CC, Thuerauf DJ, Huang C, Vekich JA, Gottlieb RA, Doroudgar S. Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion. J Biol Chem. 2012;287(31): 25893-25904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yan Y, Rato C, Rohland L, Preissler S, Ron D. MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat Commun. 2019;10(1):541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Yagi T, Asada R, Kanekura K, et al. Neuroplastin modulates anti-inflammatory effects of MANF. Iscience. 2020;23(12):101810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yang S, Li S, Li XJ. MANF: a new player in the control of energy homeostasis, and beyond. Front Physiol. 2018;9:1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kano F, Yamauchi S, Yoshida Y, et al. Yip1A regulates the COPI-independent retrograde transport from the Golgi complex to the ER. J Cell Sci. 2009;122(Pt 13):2218-2227. [DOI] [PubMed] [Google Scholar]
- 193. Yoshida Y, Suzuki K, Yamamoto A, et al. YIPF5 and YIF1A recycle between the ER and the Golgi apparatus and are involved in the maintenance of the Golgi structure. Exp Cell Res. 2008;314(19):3427-3443. [DOI] [PubMed] [Google Scholar]
- 194. Zhang DW, Shao J, Lin J, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332-336. [DOI] [PubMed] [Google Scholar]
- 195. Yang B, Maddison LA, Zaborska KE, et al. RIPK3-mediated inflammation is a conserved beta cell response to ER stress. Sci Adv. 2020;6(51). doi: 10.1126/sciadv.abd7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Thapa RJ, Nogusa S, Chen P, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110(33): E3109-E3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Saveljeva S, Mc Laughlin SL, Vandenabeele P, Samali A, Bertrand MJ. Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis. 2015;6:e1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhu P, Hu S, Jin Q, et al. Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol. 2018;16:157-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56(2):234-241. [DOI] [PubMed] [Google Scholar]
- 200. Rendleman J, Cheng Z, Maity S, et al. New insights into the cellular temporal response to proteostatic stress. Elife 2018;7. doi: 10.7554/eLife.39054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Colli ML, Ramos-Rodríguez M, Nakayasu ES, et al. An integrated multi-omics approach identifies the landscape of interferon-α-mediated responses of human pancreatic beta cells. Nat Commun. 2020;11(1):2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kolb H, Eizirik DL. Resistance to type 2 diabetes mellitus: a matter of hormesis? Nat Rev Endocrinol. 2011;8(3): 183-192. [DOI] [PubMed] [Google Scholar]
- 203. Marselli L, Piron A, Suleiman M, et al. Persistent or transient human β cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with type 2 diabetes. Cell Rep. 2020;33(9):108466. [DOI] [PubMed] [Google Scholar]
- 204. Sanavia T, Huang C, Manduchi E, et al. Temporal transcriptome analysis reveals dynamic gene expression patterns driving β-cell maturation. Frontiers Cell Dev Biol. 2021;9. doi: 10.3389/fcell.2021.648791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Sanavia T, Finotello F, Di Camillo B. FunPat: function-based pattern analysis on RNA-seq time series data. BMC Genomics. 2015;16:S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Cahill KM, Huo Z, Tseng GC, Logan RW, Seney ML. Improved identification of concordant and discordant gene expression signatures using an updated rank-rank hypergeometric overlap approach. Sci Rep. 2018;8(1):9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Plaisier SB, Taschereau R, Wong JA, Graeber TG. Rank-rank hypergeometric overlap: identification of statistically significant overlap between gene-expression signatures. Nucleic Acids Res. 2010;38(17):e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Szymczak F, Colli ML, Mamula MJ, Evans-Molina C, Eizirik DL. Gene expression signatures of target tissues in type 1 diabetes, lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. Sci Adv. 2021;7(2). doi: 10.1126/sciadv.abd7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Iwawaki T, Akai R, Toyoshima T, Takeda N, Ishikawa TO, Yamamura KI. Transgenic mouse model for imaging of ATF4 translational activation-related cellular stress responses in vivo. Sci Rep. 2017;7:46230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(31):11269-11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Jin B, Ishikawa T, Taniguchi M, et al. Development of a rapid in vivo assay to evaluate the efficacy of IRE1-specific inhibitors of the unfolded protein response using medaka fish. Cell Struct Funct. 2020;45(1):23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Alavi MV, Chiang WC, Kroeger H, et al. In vivo visualization of endoplasmic reticulum stress in the retina using the ERAI reporter mouse. Invest Ophthalmol Vis Sci. 2015;56(11):6961-6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kitamura M, Hiramatsu N. Real-time monitoring of ER stress in living cells and animals using ESTRAP assay. Methods Enzymol. 2011;490:93-106. [DOI] [PubMed] [Google Scholar]
- 215. Tirosh A, Tuncman G, Calay ES, et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. 2021;33(2):319-333 e316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Lajoie P, Snapp EL. Size-dependent secretory protein reflux into the cytosol in association with acute endoplasmic reticulum stress. Traffic. 2020;21(6):419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wires ES, Henderson MJ, Yan X, et al. Longitudinal monitoring of Gaussia and Nano luciferase activities to concurrently assess ER calcium homeostasis and ER stress in vivo. Plos One. 2017;12(4):e0175481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Henderson MJ, Wires ES, Trychta KA, Richie CT, Harvey BK. SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Mol Biol Cell. 2014;25(18):2828-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kracht MJL, de Koning EJP, Hoeben RC, Roep BO, Zaldumbide A. Bioluminescent reporter assay for monitoring ER stress in human beta cells. Sci Rep. 2018;8(1):17738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Schifferer M, Yushchenko DA, Stein F, Bolbat A, Schultz C. A ratiometric sensor for imaging insulin secretion in single beta cells. Cell Chem Biol. 2017;24(4):525-531 e524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Bader E, Migliorini A, Gegg M, et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature. 2016;535(7612):430-434. [DOI] [PubMed] [Google Scholar]
- 222. Dorrell C, Schug J, Canaday PS, et al. Human islets contain four distinct subtypes of β cells. Nat Commun. 2016; 7:11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Karaca M, Castel J, Tourrel-Cuzin C, et al. Exploring functional beta-cell heterogeneity in vivo using PSA-NCAM as a specific marker. Plos One. 2009;4(5):e5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Katsuta H, Aguayo-Mazzucato C, Katsuta R, et al. Subpopulations of GFP-marked mouse pancreatic β-cells differ in size, granularity, and insulin secretion. Endocrinology. 2012;153(11):5180-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Modi H, Skovsø S, Ellis C, et al. Ins2 gene bursting activity defines a mature β-cell state. bioRxiv. 2019. doi: 10.1101/702589. [DOI] [Google Scholar]
- 226. Gutierrez GD, Gromada J, Sussel L. Heterogeneity of the pancreatic beta cell. Front Genet. 2017;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Da Silva Xavier G, Rutter GA. Metabolic and functional heterogeneity in pancreatic β cells. J Mol Biol. 2020;432(5):1395-1406. [DOI] [PubMed] [Google Scholar]
- 228. Avrahami D, Wang YJ, Klochendler A, Dor Y, Glaser B, Kaestner KH. β-Cells are not uniform after all-novel insights into molecular heterogeneity of insulin-secreting cells. Diabetes Obes Metab. 2017;19 Suppl 1:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Roscioni SS, Migliorini A, Gegg M, Lickert H. Impact of islet architecture on β-cell heterogeneity, plasticity and function. Nat Rev Endocrinol. 2016;12(12):695-709. [DOI] [PubMed] [Google Scholar]
- 230. Dominguez-Gutierrez G, Xin Y, Gromada J. Heterogeneity of human pancreatic β-cells. Mol Metab. 2019;27S:S7-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Balboa D, Saarimäki-Vire J, Borshagovski D, et al. Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes. Elife 2018;7. doi: 10.7554/eLife.38519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li J, et al. A single-cell transcriptomic atlas of primate pancreatic islet aging. Nat. Sci. Rev. 2021;8(2). doi: 10.1093/nsr/nwaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Kushner JA. The role of aging upon β cell turnover. J Clin Invest. 2013;123(3):990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58(6):1312-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284(1):E7-12. [DOI] [PubMed] [Google Scholar]
- 236. Serre-Beinier V, Le Gurun S, Belluardo N, et al. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49(5):727-734. [DOI] [PubMed] [Google Scholar]
- 237. Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012;61(7):1700-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Carvalho CP, Oliveira RB, Britan A, et al. Impaired β-cell-β-cell coupling mediated by Cx36 gap junctions in prediabetic mice. Am J Physiol Endocrinol Metab. 2012;303(1):E144-E151. [DOI] [PubMed] [Google Scholar]
- 239. Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108(16):6561-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Rodvold JJ, Chiu KT, Hiramatsu N, et al. Intercellular transmission of the unfolded protein response promotes survival and drug resistance in cancer cells. Sci Signal 2017;10(482). doi: 10.1126/scisignal.aah7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Abdulreda MH, Rodriguez-Diaz R, Caicedo A, Berggren PO. Liraglutide compromises pancreatic β cell function in a humanized mouse model. Cell Metab. 2016;23(3):541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. Plos Med. 2008;5(10):e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Donath MY, Ehses JA, Maedler K, et al. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005;54 Suppl 2:S108-S113. [DOI] [PubMed] [Google Scholar]
- 244. Bluher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3). doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Buscemi S, Chiarello P, Buscemi C, et al. Characterization of metabolically healthy obese people and metabolically unhealthy normal-weight people in a general population cohort of the ABCD Study. J Diabetes Res. 2017;2017:9294038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Eckel N, Mühlenbruch K, Meidtner K, Boeing H, Stefan N, Schulze MB. Characterization of metabolically unhealthy normal-weight individuals: risk factors and their associations with type 2 diabetes. Metabolism. 2015;64(8):862-871. [DOI] [PubMed] [Google Scholar]
- 247. Lee SH, Han K, Yang HK, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes. 2015;5:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lee S-H, et al. Prevalence and characteristics of metabolically obese but normal weight and metabolically healthy but obese in middle-aged Koreans: the chungju metabolic disease cohort (CMC) study. Endocrinol Metab. 2011;26(2). doi: 10.3803/EnM.2011.26.2.133. [DOI] [PubMed] [Google Scholar]
- 249. Shin MS, Yu JH, Jung CH, et al. The duration of sulfonylurea treatment is associated with β-cell dysfunction in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14(11):1033-1042. [DOI] [PubMed] [Google Scholar]
- 250. Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9(3 Pt 2):14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Esser N, Utzschneider KM, Kahn SE. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia. 2020;63(10): 2007-2021. [DOI] [PubMed] [Google Scholar]
- 252. Cunha DA, Ladrière L, Ortis F, et al. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58(12):2851-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4(5):391-406. [DOI] [PubMed] [Google Scholar]
- 254. Gier B, Krippeit-Drews P, Sheiko T, et al. Suppression of KATP channel activity protects murine pancreatic beta cells against oxidative stress. J Clin Invest. 2009;119(11):3246-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Jackson RL. Stabilization of the diabetic child. Arch Pediatr Adolesc Med. 1940;59(2):332-341. [Google Scholar]
- 256. Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1(7957):444-447. [DOI] [PubMed] [Google Scholar]
- 257. Boland BB, Brown C Jr, Boland ML, et al. Pancreatic β-cell rest replenishes insulin secretory capacity and attenuates diabetes in an extreme model of obese type 2 diabetes. Diabetes. 2019;68(1):131-140. [DOI] [PubMed] [Google Scholar]
- 258. Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N. Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia. 2015;58(9): 2144-2153. [DOI] [PubMed] [Google Scholar]
- 259. Panieri E, Santoro MM. ROS homeostasis and metabolism: a dangerous liaison in cancer cells. Cell Death Dis. 2016;7(6):e2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Whitticar NB, Nunemaker CS. Reducing glucokinase activity to enhance insulin secretion: a counterintuitive theory to preserve cellular function and glucose homeostasis. Front Endocrinol (Lausanne). 2020;11:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Sweet IR, Li G, Najafi H, Berner D, Matschinsky FM. Effect of a glucokinase inhibitor on energy production and insulin release in pancreatic islets. Am J Physiol. 1996;271(3 Pt 1):E606-E625. [DOI] [PubMed] [Google Scholar]
- 262. Jahan I, Corbin KL, Bogart AM, et al. Reducing glucokinase activity restores endogenous pulsatility and enhances insulin secretion in islets from db/db mice. Endocrinology. 2018;159(11):3747-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Bahl V, Lee May C, Perez A, Glaser B, Kaestner KH. Genetic activation of α-cell glucokinase in mice causes enhanced glucose-suppression of glucagon secretion during normal and diabetic states. Mol Metab. 2021;49:101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Fridlyand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes. 2004;53(8):1942-1948. [DOI] [PubMed] [Google Scholar]
- 265. Sargsyan E, Ortsäter H, Thorn K, Bergsten P. Diazoxide-induced beta-cell rest reduces endoplasmic reticulum stress in lipotoxic beta-cells. J Endocrinol. 2008;199(1):41-50. [DOI] [PubMed] [Google Scholar]
- 266. Merovci A, Tripathy D, Chen X, et al. Effect of mild physiologic hyperglycemia on insulin secretion, insulin clearance, and insulin sensitivity in healthy glucose-tolerant subjects. Diabetes. 2021;70(1):204-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Zhyzhneuskaya SV, Al-Mrabeh A, Peters C, et al. Time course of normalization of functional β-cell capacity in the diabetes remission clinical trial after weight loss in type 2 diabetes. Diabetes Care. 2020;43(4):813-820. [DOI] [PubMed] [Google Scholar]
- 268. Ovalle F, Grimes T, Xu G, et al. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med. 2018;24(8):1108-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Xu G, Chen J, Jing G, Shalev A. Preventing β-cell loss and diabetes with calcium channel blockers. Diabetes. 2012;61(4):848-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Carré A, Mallone R. Making insulin and staying out of autoimmune trouble: the beta-cell conundrum. Front Immunol. 2021;12:639682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Finan B, Yang B, Ottaway N, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18(12): 1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Hetz C, Axten JM, Patterson JB. Pharmacological targeting of the unfolded protein response for disease intervention. Nat Chem Biol. 2019;15(8):764-775. [DOI] [PubMed] [Google Scholar]
- 273. Bilekova S, Sachs S, Lickert H. Pharmacological targeting of endoplasmic reticulum stress in pancreatic beta cells. Trends Pharmacol Sci. 2021;42(2):85-95. [DOI] [PubMed] [Google Scholar]
- 274. Mawla AM, Huising MO. Navigating the depths and avoiding the shallows of pancreatic islet cell transcriptomes. Diabetes. 2019;68(7):1380-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.