Abstract
Cell therapy involves transplantation of human cells to promote repair of diseased or injured tissues and/or cells. Only a limited number of mostly small‐scale trials have studied cell therapy in nonischemic cardiomyopathy (NICM). We performed a meta‐analysis of randomized clinical trials (RCTs) to assess the safety and efficacy of cell therapy in NICM. Electronic databases were searched for relevant RCTs from inception until August 2020. Outcomes assessed were left ventricular ejection fraction (LVEF), left ventricular end‐diastolic diameter or volume (LVEDD), quality of life (QoL) indices, and major adverse cardiac events (MACEs). Weighted mean differences (MDs) and standardized mean differences (SMDs) were calculated using random‐effects methods. Eleven RCTs with 574 participants were included in the analysis. There was a significant increase in mean LVEF (MD, 4.17%; 95% confidence interval [CI] = 1.66‐6.69) and modest decrease in LVEDD (SMD, −0.50; 95% CI = −0.95 to −0.06) in patients treated with cell therapy compared with controls. Cell therapy was also associated with improvement in functional capacity, as assessed by the 6‐minute walking distance (MD, 72.49 m; 95% CI = 3.44‐141.53). No significant differences were seen in MACEs and QoL indices between treated and control groups. This meta‐analysis suggests that cell therapy may improve LV systolic function and may be associated with improvement in LVEDD and functional capacity compared with maximal medical therapy. Cell therapy was safe, with no significant difference in MACEs between treatment and control groups. However, given the limitations of current studies, larger well‐designed RCTs are needed to evaluate the efficacy of cell therapy in patients with NICM.
Keywords: cell therapy, heart failure with reduced ejection fraction, meta‐analysis, nonischemic cardiomyopathy, systematic review
Among 11 studies included in our systematic review, meta‐analysis of nine studies that reported mean change in left ventricular systolic function from baseline to the end of follow‐up after cell therapy in comparison to control group showed significant increase in left ventricular systolic function over time.

Significance statement.
This study provides comprehensive evaluation of efficacy and safety of cell therapy for nonischemic dilated cardiomyopathy (NICM). The results of this meta‐analysis suggest that cell therapy is safe with no increased risk for major adverse cardiac events and may improve left ventricular systolic function, left ventricular diastolic dimensions, and functional capacity in patients with NICM. This supports the concept that cell therapy remains a promising therapeutic option for NICM. Well‐designed, adequately powered randomized trials are needed to conclusively determine the benefits of cell therapy in NICM.
1. INTRODUCTION
In the United States, more than 6 million people are affected by heart failure (HF) and more than a half‐million new cases are reported every year.1 Nonischemic dilated cardiomyopathy (NICM) accounts for approximately one‐third of HF cases.2, 3, 4 The underlying etiology of NICM is heterogeneous, including infections, toxins such as alcohol and anthracyclines, metabolic or endocrine disturbances, and genetic mutations such as those causing idiopathic and familial dilated cardiomyopathy.5 Although progress has been made in the treatment of HF, resulting in prolonged survival and alleviation of symptoms,6, 7 the morbidity and mortality associated with HF continue to be a significant health care problem. Therefore, there remains a need to find new treatments that may improve the prognosis of this syndrome.
A large body of research, both in animal models and human subjects, supports the potential of cell therapy as a promising approach to HF.8, 9, 10, 11 A variety of cell types, including bone marrow (BM)‐derived mesenchymal stromal cells (MSCs), CD34+ cells, adipose‐derived regenerative cells, and umbilical cord‐derived MSCs, have yielded promising results after either intracoronary or intramyocardial delivery.8, 9, 10, 11 Although the majority of cell therapy research has focused on ischemic cardiomyopathy, there is now a growing interest in exploring the use of cell therapy for NICM.8, 11
More than 30 randomized controlled trials (RCTs) have examined the effect of various cell types in ischemic cardiomyopathy as compared with <15 trials for NICM.12 TOPCARE‐DCM was the first study in NICM; this trial showed that left ventricular (LV) ejection fraction (EF) improved after intracoronary delivery of BM‐derived mononuclear cells (BM‐MNCs).13 Most subsequent trials were small, had substantial differences in design, and have yielded mixed results.14, 15, 16, 17, 18, 19, 20 Previous meta‐analyses of clinical trials of cell therapy in NICM suggested that this treatment might improve LVEF but not LV end‐diastolic dimensions (LVEDD).21, 22 Due to lack of analyzable data, these meta‐analyses did not include other important outcomes such as quality of life (QoL) and major adverse cardiac events (MACEs).21, 22, 23, 24 In recent years additional trials have been reported, for a total of 11 RCTs.15, 16, 25, 26 However, the results of published trials remain discordant and, therefore, the therapeutic potential of cell therapy as an intervention for NICM remains unclear. The objective of this study was to perform a comprehensive meta‐analysis of pooled data from all 11 RCTs published to date to better understand the safety and therapeutic efficacy of cell therapy as a treatment for NICM.
2. METHODS
2.1. Eligibility
We included trials that met all of the following inclusion criteria: the study design was a RCT; a clearly stated strategy to include subjects with NICM; autologous or allogeneic cell therapy as the experimental group; either no intervention or placebo for the control group; inclusion of participants with HF symptoms ≥ class II New York Heart Association (NYHA); and either echocardiographic or magnetic resonance imaging (MRI) evidence of LVEF <40%. Only trials published in English were included. We did not include any trials presented only as conference proceedings or abstracts.
2.2. Search strategy and study selection
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta‐analyses (PRISMA) guidelines.27 The search strategy, including initial subject headings and keywords, was developed by two authors (A.T. and M.S.K.). The search was subsequently performed and revised as needed in collaboration with an experienced medical reference librarian (V.M.V.). The following databases were used: Medline/Ovid, Embase, and the Cochrane Central Register of Controlled Trials from inception through August 2020. Results were limited to humans. The full literature search strategy is shown in Supplementary Appendix 1. The search was completed by using references manually extracted from article reference lists. We also reviewed the reference list of previously published systematic reviews in search of potential studies. A.T. and M.S.K. independently reviewed the titles and abstracts of all the articles found initially in the eligibility criteria‐based search; then, they jointly reviewed and cross‐checked notes on their independent selection of the studies. Any discordance and/or disagreements were solved through discussion and review of the full‐text articles to ensure that the eligibility criteria were met.
2.3. Data extraction and quality assessment
Data from selected studies were extracted independently by A.T. and M.S.K. Data included (a) study characteristics (objectives, methods of randomization, and blinding); (b) characteristics of participants, such as demographic data and sample size; and (c) characteristics of the intervention, such as type of intervention in the experimental and control group, the timing of intervention and subsequently timing of outcome evaluation, type and dose of cells, and method of cell delivery. Primary outcomes for this analysis included the change in LVEF and LV end‐diastolic dimension (LVEDD). Secondary outcomes included NYHA classification, quality‐of‐life (QoL) measures, such as the Kansas City Cardiomyopathy Questionnaire (KCCQ), 6‐minute walk distance, and MACEs, which were defined as all‐cause death, myocardial infarction, stroke, life‐threatening arrhythmias, hospitalization for HF, or heart transplant. In studies with multiple follow‐up times, the outcomes at the longest follow‐up were used. In studies where results were presented graphically, and actual mean differences and SDs were not available, values were estimated from the graphs independently by two authors (A.T. and M.S.K.) and average values were used. Two authors (A.T. and M.S.K.) reviewed each selected trial for quality assessment including the risk of bias using the Cochrane criteria for the systematic review of interventions. This methodology explores the adequacy of sequestration, allocation sequence concealment, blinding of participants and study personnel, blinding for outcome assessment, incomplete outcome or selective outcome reporting, and other potential biases. Any disagreement between the authors was resolved with the mutual agreement after discussion.
2.4. Statistical analysis
Outcomes were used in this meta‐analysis only if at least four of the 11 included trials reported usable data (ie, data were presented either as numbers or graphically which allowed for the calculation of mean differences and their associated SDs). Random effect models were used throughout because of likely heterogeneity in studies that included different patient populations and different types of cells in different doses. In studies where measures of variation such as SD were not reported, these were calculated from P values and confidence intervals (CIs). For continuous outcome variables, mean differences between the experimental and control groups from baseline to the longest reported follow‐up period were calculated. For categorical outcome variables such as MACE, odds ratios were calculated. In one trial, the experimental group was divided into two arms, one receiving BM‐MNCs and the other BM‐MSCs.26 We statistically combined the outcomes of these two experimental groups for the analysis. In another study, MRI was used in short‐term follow‐up evaluation of LV function and dimension, and echocardiography was used in long‐term follow‐up.17 We used the outcome at the longest follow‐up irrespective of the imaging modality. For LVEDD, some studies reported chamber diameter while others reported volume. Therefore, the standardized mean difference (SMD) was used to allow the analysis of these measurements on different scales. Similarly, the SMD was used for QoL measurements because some studies used the KCCQ whereas others used the Minnesota Living with Heart Failure Questionnaire (MLWHFQ). I 2 statistics was used to assess heterogeneity among studies. Sensitivity analysis was done to investigate the associated heterogeneity and the effect of individual studies on it. Statistical analysis was performed using RevMan v5.3.5.28
3. RESULTS
3.1. Search results and characteristics of included trials
The literature search yielded 1060 potentially relevant studies, of which 11 were eligible for inclusion in this study after a full read. The PRISMA flow diagram is presented in Figure 1.
FIGURE 1.
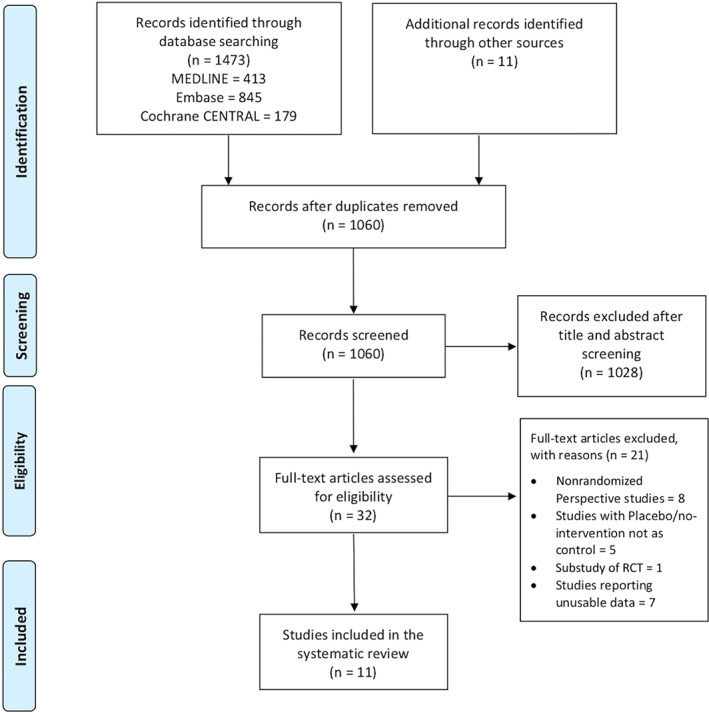
PRISMA flowchart outlining the literature search
The total number of randomized participants was 574 (312 received cells and 262 were in the control group). Out of the 11 trials, one was conducted in India,18 one in China,26 one in United Kingdom,15 two in Slovenia,19, 20 two in the United States,25, 29 three in Brazil,14, 16, 17 and one in three countries (Germany, India, and Peru).30 The trials were conducted from 2006 through 2017. Eight trials recruited participants from a single medical center, while three were multicenter studies.16, 25, 30 One trial had a crossover randomized design, whereby the experimental and placebo groups received alternative treatment in the crossover phase25; for this trial, only the assessments of the pre‐crossover phase period were included in the analysis. The included patients were predominantly male with an average age > 55 years in almost all the studies. Table 1 summarizes the characteristics of the included studies.
TABLE 1.
Characteristics of the included studies
| Study and year | Design | Cell type | Number of patients | LVEF | NYHA | Number of cells delivered | Delivery | Final assessment time | Final assessment modality | Outcomea |
|---|---|---|---|---|---|---|---|---|---|---|
|
Seth et al 2010, India ABCD trial |
RCT | BM‐MNCs | 45 treated; 40 control | ≤35% | ≥II | 1.68 ± 0.96 × 108 | Intracoronary with balloon inflated in the coronary sinus | 28 ± 9 mo | Echo |
↑LVEF ↔LVEDD ↔NYHA ↑QoL ↔MACE |
| Bocchi et al 2010, Brazil | RCT | G‐CSF stimulated, autologous CD34+ PBSCs | 8 treated; 15 control | ≤35% | ≥II | 96 × 106 | Intracoronary without balloon inflation | 468 ± 374 d | Echo |
↑LVEF ↔LVEDD ↔MACE |
| Vrtovec et al 2011, Slovenia | RCT | G‐CSF stimulated, autologous CD34+ PBSCs | 28 treated; 27 control | ≤30% | ≥III | 1.23 ± 0.23 × 108 | Intracoronary without balloon inflation | 1 y | Echo |
↑LVEF ↓LVEDD ↔MACE ↑6MWD ↓Pro‐BNP |
| Vrtovec et al 2013, Slovenia | RCT | G‐CSF stimulated, autologous CD34+ PBSCs | 55 treated; 55 control | ≤30% | III | 1.13 ± 0.26 × 108 | Intracoronary without balloon inflation | 5 y | Echo |
↑LVEF ↔LVEDD ↔MACE ↑6MWD ↓Pro‐BNP |
|
Henry et al 2014, United States IMPACT‐ DCM |
RCT | Expanded cell population enriched in MSCs (CD90+) and activated CD45+ CD14+ macrophages (Ixmyelocel‐T) | 39 (18 NICM) treated; 20 (11 NICM) control | ≤30% | ≥III | 0.35 × 108 to 2.95 × 108 | Intramyocardial either through mini‐thoracotomy or NOGA | 1 y | Echo and SPECT |
↔LVEF ↔LVEDD ↔MACE ↔NYHA ↔QoL ↔6MWD |
| Sant'Anna et al 2014, Brazil, INTRACELL trial | RCT | BM‐MNCs | 19 treated;10 control | <40% | ≥III | 1.06 ± 0.43 × 108 | Intramyocardial through mini thoracotomy | 9–12 mo | Echo and CMRI |
↔LVEF ↔LVEDD ↔MACE ↔NYHA ↔QoL ↔6MWD |
|
Martino et al 2015, Brazil MiHeart Study |
RCT | BM‐MNCs | 82 treated; 78 control | <35% | ≥III | 2.36 × 108 | Intracoronary without balloon inflation | 1 y | Echo |
↔LVEF ↑LVEDV ↔LVSV ↔MACE ↔6MWD ↔QoL |
| Hamshere et al 2015, United Kingdom, REGENERATE‐DCM | RCT | BM‐MNCs | 15 treated; 14 controls (also 14 each in peripheral G‐CSF and IC serum groups) | <45% | ≥II | 2.16 × 108 ± 221.8 | Intracoronary with balloon inflation | 1 y | CMRI |
↑LVEF ↔LVEDD ↔MACE ↓NYHA ↓Pro‐BNP ↑QoL |
| Patel et al 2015, Germany, India, and Peru | RCT | BM‐MNCs | 24 treated; 6 control | <40% | ≥III | 3.7 ± 0.9 × 109 | Retrograde venous delivery via coronary sinus | 1 y | Echo and SPECT |
↔LVEF ↔LVEDD ↔NYHA ↔QoL ↔MACE ↔BNP |
| Butler et al 2017, United States, aMBMC Study | RCT, Crossover design | Allogenic itBM‐MSCs | 10 treated; 12 control | ≤40% | II/III | 1.5 million cells/kg | Intravenous | 180 d | CMRI |
↔LVEF ↔LVEDD ↔MACE ↔6MWD ↔NYHA ↔Pro‐BNP ↔QoL |
| Xiao et al 2017, China | RCT | BM‐MNCs and BM‐MSCs | 33 treated (16 BM‐MNCs, 17 BM‐MSCs); 20 control | <40% | ≥II |
BM‐MNCs: 5.1 ± 2.0 × 108 BM‐MSCs: 4.9 ± 1.7 × 108 |
Intracoronary with balloon inflation | 1 y | Echo and SPECT |
↑LVEF ↔LVEDD ↔MACE ↓NYHA |
The outcome column represents comparison between treatment arm and control arm; Hamshere et al 2015 study had four participant arms, peripheral G‐CSF with intracoronary cells was taken as treatment arm and peripheral placebo (normal saline) was taken as control arm; Henry et al 2014 study had the treatment group divided into two arms, one received stem cells intramyocardially and other received percutaneously, combined outcomes of these two arms were considered as treatment group; Xiao et al 2017 study had the treatment group divided into two arms, one receiving BM‐MNCs and the other BM‐MSCs, combined outcomes of these two arms were considered as treatment group.
Abbreviations: 6MWD 6‐minute walk distance; BM‐MNCs, bone marrow mononuclear cells; BM‐MSCs, bone marrow mesenchymal stem cells; CMRI, contrast magnetic resonance imaging; G‐CSF, granulocyte‐colony‐stimulating factor; IC, intracoronary; itBM‐MSCs, ischemia tolerant bone marrow mesenchymal stem cells; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end diastolic volume; LVEF, left ventricle ejection fraction; LVSV, left ventricular stroke volume; MACEs, major adverse cardiac events; MSCs, mesenchymal stem cells; NICM, nonischemic cardiomyopathy; NYHA, New York Heart Association classification; PBSC, peripheral blood stem cells; QoL, quality of life; RCT, randomized controlled trial; SPECT, single photon emission computed tomography.
In two trials, participants with both ischemic and nonischemic cardiomyopathy were included; however, a separate analysis was conducted for the two groups in these studies.29, 30 Only the analysis and outcomes of participants with nonischemic cardiomyopathy were included in our study. All trials mentioned administration of maximum tolerated medical therapy for HF, including diuretics, beta‐blockers, angiotensin‐converting enzyme inhibitors, or angiotensin receptor blockers, and nitrates for at least 3 months. In one study by Henry et al, the experimental group was divided into two arms: one received stem cells intramyocardially via a mini‐thoracotomy (IMPACT‐DCM trial) while the other arm received them percutaneously using the Noga XP Cardiac Navigation System (Catheter‐DCM trial) (BDS, a Johnson & Johnson company, Irwindale, California).29
Allogeneic cells were used in only one trial25; rest of the studies utilized autologous cells. The trial by Butler et al utilized ischemia‐tolerant allogeneic BM‐MSCs, which were extracted from the BM of young healthy volunteers and grown under hypoxic conditions from the moment of extraction.25 Of the trials with autologous cells, six used BM‐MNCs15, 16, 17, 18, 29, 30; one trial used either BM‐MNCs or BM‐MSCs,26 and three harvested mononuclear cells from the peripheral circulation by leukopheresis after mobilization of BM cells using granulocyte‐colony‐stimulating factors (G‐CSFs).14, 19, 20 In one of the trials that used BM‐MNCs, the BM aspirate was cultured and later harvested to include an expanded cell population enriched in MSCs (CD90+ cells), and alternatively activated CD45+ and CD14+ cells.29 Three trials used G‐CSF in the experimental group,14, 19, 20 while one used G‐CSF for both experimental and control groups.15 All trials tested the viability of cells before delivery and reported it to be greater than 90%. None of the trials used cultured c‐kit positive cells.
In one trial the cells were delivered intravenously25 and in two trials intramyocardially17, 29; in one study retrograde venous delivery of cells via the coronary sinus was performed,30 while in the others cells were delivered intracoronary. Among the trials with intracoronary delivery, an equal volume of cells was delivered into both left and right coronary arteries in three trials15, 16, 18; selective delivery into the right or left system based on target area selection using myocardial perfusion scintigraphy before transplantation was performed in two trials19, 20; and selective delivery into the left main coronary artery was done in two trials.14, 26 Also, among trials with intracoronary delivery, the flow‐stop technique with an intracoronary balloon was used in three studies.15, 18, 26
Three trials used MRI to assess LVEF.15, 17, 25 One study used both MRI and two‐dimensonal transthoracic echocardiography (TTE); however, TTE was used for long‐term assessment at follow‐up.17 In the remaining trials, TTE was used for assessment of cardiac structure and function.
In six trials, cell therapy was compared with optimal medical treatment.17, 18, 19, 20, 29, 30 In one study, the control group was given peripheral injections of G‐CSFs.14 In one trial, participants were divided into four groups: peripheral placebo (normal saline), peripheral G‐CSF, peripheral G‐CSF and intracoronary serum, and peripheral G‐CSF and intracoronary cells.15 Since the majority of included trials comprised control groups that did not receive either placebo or peripheral G‐CSF, we used the peripheral placebo (normal saline) group as the primary control group and compared it with the intracoronary cells group.15
3.2. Evaluation of bias
The risk of bias for the included trials is presented in Figure 2. All trials reported using random sequence generation. Concealment of allocation to the intervention group was reported in only three trials15, 17, 25; therefore, allocation concealment remains unclear in the other trials. Two trials reported a double‐blind study design and therefore had a low risk of performance bias.15, 16 One trial had a single‐blind study design25 and five trials had an open‐label study design17, 19, 20, 29, 30; these studies had a high risk of performance bias. Three trials did not mention any strategy regarding blinding study participants from medical personnel; therefore, the risk of performance bias is not clear.14, 18, 26 Seven trials clearly stated blinding of outcomes assessment and therefore had a lower risk of detection bias,15, 19, 20, 25, 26, 29, 30 whereas the risk of detection bias is unclear in three studies.14, 16, 18 In one trial, the risk of detection bias was deemed high because of the presence of a mini‐thoracotomy scar at the time of the echocardiogram after cell therapy.17
FIGURE 2.
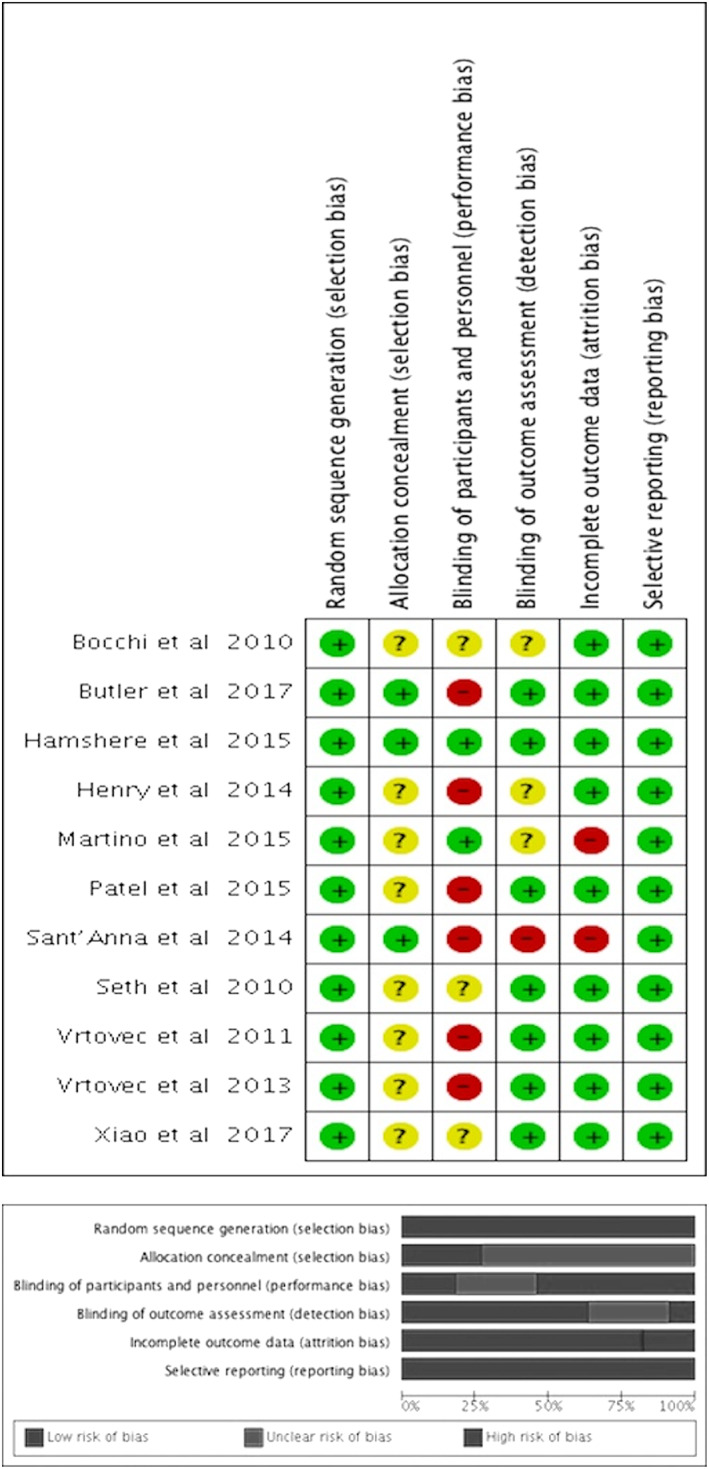
A, Risk of bias summary of included randomized trials. Symbols: (+) low risk of bias; (?) unclear risk of bias; (−) high risk of bias. B, Risk of bias graph of included randomized trials
The risk of attrition bias was deemed high in two trials because of the high attrition rate and failure to use intention‐to‐treat analysis.16, 17 In the study by Martino et al, 21/82 and 24/78 participants were lost to follow‐up in the cell therapy and control/placebo groups, respectively.16 In the study by Sant'Anna et al, ~25% of the participants in the treatment group were lost to follow‐up.17 In the rest of the trials, the risk of attrition bias was low.14, 15, 17, 18, 19, 20, 25, 26, 29, 30 Bias due to selective reporting was deemed high in two studies because data were not presented for some of the secondary outcomes,17, 26 whereas it was deemed low for the remaining trials.14, 15, 16, 18, 19, 20, 25, 29, 30
3.3. Assessment of outcomes
3.3.1. Left ventricular ejection fraction
Changes in LVEF from baseline to end of follow‐up after cell therapy were reported in all trials. Two trials had a long‐term follow‐up of up to 3 and 5 years.18, 19 One study reported a mean follow‐up of 468 ± 374 days,14 one reported results at 180 days post‐stem cell transfusion,25 and the others reported final endpoint assessments at 12 months15, 16, 17, 20, 26, 29, 30 (Table 1).
Six trials showed a significant increase in LVEF in the treatment group as compared to the control group,14, 15, 18, 19, 20, 26 whereas five trials did not.16, 17, 25, 29, 30 In two studies, the mean change in LVEF was reported graphically with no clear details of measure of variation; therefore, these data could not be included in the analysis.29, 30 Pooled data from nine trials14, 15, 16, 17, 18, 19, 20, 25, 26 showed that the weighted mean LVEF was 4.17% greater in the treatment group as compared with the control group (95% CI = 1.66‐6.69; P = .001; I 2 = 48%). A subgroup analysis performed by stratifying trials based on duration of follow‐up (≤1 year vs ≥1 year) suggested that the mean LVEF improvement favored treatment both for follow‐up ≤1 year (MD = 3.52%; 95% CI = 0.57‐6.47; P = .02; I 2 = 51%) and ≥1 year (MD = 6.44%; 95% CI = 2.34‐10.53; P = .002; I 2 = 0%) (Figure 3A). Sensitivity analysis after excluding four trials14, 16, 17, 29 at high risk for detection and/or attrition bias showed increased mean difference in LVEF favoring cell therapy (MD = 5.02%; 95% CI = 2.04‐8.00; P = .0009; I 2 = 63%) (Figure S1A).
FIGURE 3.
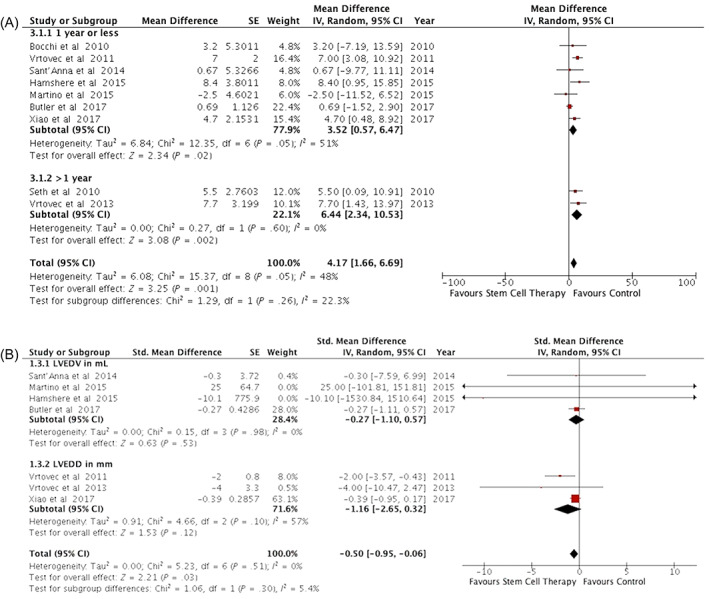
Effect of cell therapy on LV structure and function. A, Forest plot of the effect of cell therapy on LVEF. B, Forest plots of the effect of cell therapy on LV end‐diastolic volume or diameter. LVEF, left ventricular ejection fraction
3.3.2. Left ventricular end‐diastolic dimensions (volume or diameter)
Change in LVEDD (LV end‐diastolic volume and/or diameter) was evaluated in all included trials. Six trials presented LVEDD in milliliters,15, 16, 17, 18, 25, 29 two in centimeters,19, 20 and three in millimeters.14, 26, 30 One study20 suggested a significant decrease in LVEDD, and another16 showed increased LVEDD in the treated group as compared to the control group. Nine trials suggested no significant association of cell therapy with LVEDD changes. Pooled data analysis could be used only for seven trials (with 458 participants) due to lack of information on variance in three trials.15, 16, 17, 19, 20, 25, 26 We used inverse variance methodology with SMD across two subgroups by the measure of LVEDD, that is, volume or diameter. The cumulative weighted SMD was 0.5 points lower in favor of cell therapy as compared to the control group (SMD = −0.50; 95% CI = −0.95 to −0.06; P = .03; I 2 = 0%) (Figure 3B). In this analysis, one trial26 contributed 63% of the weight; removal of this trial rendered the association between cell therapy and LVEDD changes nonsignificant (Figure S2).
3.3.3. Major adverse cardiac events
Safety outcomes were reported in all trials; they varied among trials, but periprocedural life‐threatening adverse events and mortality were reported in all 11 studies. Two trials showed decreased MACE in the treatment group as compared to control,19, 20 whereas the other nine trials did not show any significant difference between the two groups. Meta‐analysis of pooled data showed that the MACE event rate was 69/310 vs 69/259 in the treatment and control groups, respectively (odds ratio = 0.77; 95% CI = 0.48‐1.24; P = .28; I 2 = 17%) (Figure 4). Sensitivity analysis after exclusion of trials at high risk of detection and/or attrition bias resulted in significantly decreased risk of MACE in favor of cell therapy (odds ratio = 0.55; 95% CI = 0.33‐0.90; P = .02; I 2 = 0%) (Figure S1B).
FIGURE 4.
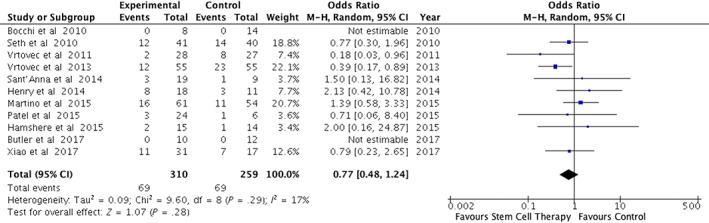
Forest plot of the effect of cell therapy on major adverse cardiac events (MACEs)
3.3.4. Secondary outcomes
Changes in NYHA classification were reported in seven trials.15, 17, 18, 25, 26, 29, 30 Two trials showed significant improvement in NYHA classification in the treatment group,15, 26 while five trials showed nonsignificant association.17, 18, 25, 29, 30 Meta‐analysis for NYHA classification was not feasible because some of the studies reported changes in NYHA classification as mean differences,17, 18, 26, 29 while other trials reported them as categorical variable.15, 25, 30
Changes in QoL measures were evaluated by seven trials using different measures, that is, the MLHFQ,16, 17, 29, 30 the KCCQ,15, 18, 25 and European quality of life‐5 Dimensions.15 Two trials reported an overall improvement in QoL measures in the cell treatment group.15, 18 Pooled analysis was done using the SMD in MLHFQ and KCCQ scores from four distinct trials. The mean difference was not significantly different between treatment and control groups (SMD = 0.13; 95% CI = −0.12 to 0.39; P = .30; I 2 = 0%) (Figure 5A).
FIGURE 5.
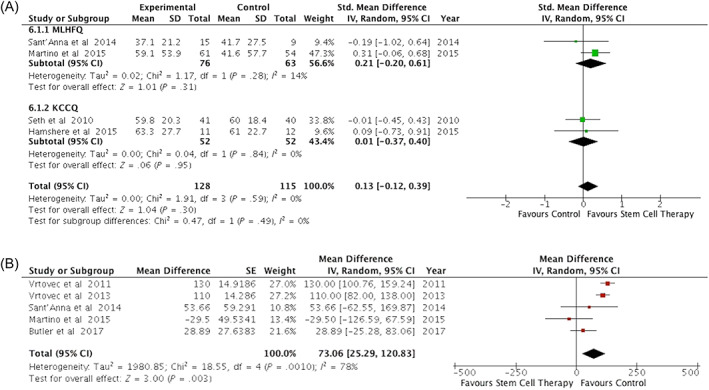
Effect of cell therapy on quality of life measures and functional capacity. A, Forest plot of the effect of cell therapy on changes in quality of life measures assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) and Kansas City Cardiomyopathy Questionnaire (KCCQ). B, Forest plots of the effect of cell therapy on functional capacity assessed by the 6‐minute walk distance
Changes in 6‐minute walk distance were evaluated in six trials.16, 17, 19, 20, 25, 29 Two trials reported a significant improvement in 6‐minute walk distance in the treated group compared with the control group19, 20 while four trials reported no significant change.16, 17, 25, 29 Pooled data analysis was performed in five trials16, 17, 19, 20, 25 because in one trial, the mean change in 6‐minute walk distance was reported graphically with no clear details of measure of variation29 and therefore could not be used in the analysis. There was significant improvement in 6‐minute walk distance with cell treatment (MD = 72.49 m; 95% CI = 3.44‐141.53; P = .04; I 2 = 72%) (Figure 5B).
Comparisons of changes in brain natriuretic protein (BNP) or N‐Terminal pro‐BNP were performed in six trials.14, 15, 19, 20, 25, 30 In one trial, incomplete data are provided14; three trials showed a significant decrease in NT pro‐BNP levels in the cell therapy group,15, 19, 20 while in two trials there were no significant differences in BNP or NT pro‐BNP.25, 30
4. DISCUSSION
The utility of cell therapy for HF due to NICM is unclear because of the paucity of trials reported, their small size, and their inconsistent design. We conducted the most comprehensive review and meta‐analysis of RCTs in this patient population to date, including endpoints (QoL and MACEs) and trials that were not available in previous meta‐analyses.21, 22, 23 The salient findings of this study can be summarized as follows: (a) in patients with NICM who are already on maximal tolerated medical therapy, cell therapy improves LV systolic function and may decrease LV diastolic dimensions; (b) cell therapy is also associated with improvement in functional capacity, as assessed by the 6‐minute walking distance; and (c) importantly, cell therapy appears to be safe, with no increase in MACE compared with the control group. These results not only support the safety and efficacy of cell therapy in patients with NICM, but also strengthen the concept that this approach is a promising avenue of therapeutic intervention to improve morbidity and mortality in this population. The present study provides a strong rationale for the continued investigation of cell therapy in NICM.
Prior meta‐analyses in patients with NICM have suggested the safety and efficacy of cell therapy.12, 21 These studies, however, were limited by several biases that were inherent to the included trials, namely, concealment bias, selection bias, detection bias, and attrition bias, among others. The impact of blinding and concealment bias on our results is not clear because sensitivity analysis was not feasible. However, we did perform sensitivity analyses to assess the risk that detection bias and attrition bias may have affected significant endpoints, that is, changes in LVEF and MACE. The results suggest that after removal of lower quality trials, that is, those with high risk of detection and/or attrition bias, cell therapy was associated with a lower risk of MACE and greater improvement in LVEF. These results suggest that inherent biases in the included trials may obfuscate the true potential of cell therapy in the treatment of HF.
In our study, cell therapy produced a mean increase in LVEF of 4.17%, consistent with previous systematic reviews and meta‐analyses, which have suggested an improvement in LVEF ranging from 3% to 5%.21, 22, 24 Our study also demonstrated an improvement in LVEDD in patients who receive cell therapy, although this association was limited by differences in imaging methodology and units of measurement used in individual studies. Future studies should be designed with standardized quantitative assessment of LV volumes with MRI, which provides the advantage of better spatial and contrast resolution, operator independence, and reproducibility.31
Although our study supports the utility of cell therapy in patients with NICM, the evidence available at this time is insufficient to determine which specific cell type offers the greatest promise. The trials included in our analysis used different types of cells: autologous or allogenic BM‐derived cells and autologous peripheral blood‐derived CD34+ cells obtained after G‐CSF stimulation. Early studies in NICM used BM‐MNCs and yielded varied results.17, 18, 29 Later trials utilizing selected populations of CD 34+ cells showed more consistent improvement in LVEF.19, 20 Recently, MSCs, which are potent modulators of the immune system and exert anti‐inflammatory effects,32 have emerged as a promising cell type for the treatment of cardiomyopathy.8, 10, 11 Allogeneic MSCs show great promise as an off‐the‐shelf therapeutic agent, as they may be able to escape immune recognition and allosensitization.33, 34 They also have the potential to be safely delivered intravenously.10, 25 Various strategies have been proposed to enhance the survival and function of donor cells after transplantation. These include using combinatorial cell therapy, in vitro preconditioning by treatment with growth factors or small molecules or by physical stimulation with hypoxia or heat shock, and genetic modification through overexpression of pro‐survival molecules or knockdown of proapoptotic factors resulting in greater cell survival and paracrine factor secretion.35, 36
As discussed above, many distinct etiologies may account for nonischemic cardiomyopathy, leading to differences in natural history, prognosis, and response to treatment.37 It is conceivable that identification of the underlying pathophysiologic and structural substrate may help select the most appropriate cell therapy modality.38 As an example, anthracycline‐induced cardiomyopathy is strongly linked to an increase in cardiac oxidative stress and accumulation of reactive oxygen species leading to endothelial dysfunction and cardiotoxicity.39 Administration of MSCs can alleviate anthracycline induced oxidative stress and improve endothelial dysfunction.40, 41 Furthermore, a recent study evaluated the role of genetic influences in determining responsiveness to cell therapy in NICM and reported that individuals with certain genetic variants can have greater clinical benefit compared to others.42 Taken together, these factors can help individualize the approach to cell therapy in NICM populations to improve responsiveness.
Several limitations of our study need to be acknowledged. First, significant heterogeneity was observed in the included studies in terms of baseline characteristics of patients, associated comorbidities, etiology of cardiomyopathy, type and number of cells delivered, route of delivery, imaging modality for assessing LV dimensions, QoL measurement indices, and definition of MACEs. Second, our results are limited by the small number of trials included and by the fact that the majority of these trials had a small sample size and short follow‐up. Third, many of the included trials have unclear risk of bias, which limits the ability to interpret their results. A strength of this review is that it provides the most updated analysis of the safety and efficacy of cell therapy in NICM, with the inclusion of recently published RCTs.25, 26 Because of the larger number of trials, it has greater statistical power than previous systematic reviews and meta‐analyses, improving the robustness of the evidence.
5. CONCLUSION
In conclusion, this meta‐analysis suggests that in patients with NICM, cell therapy is not only safe but also associated with improvement in LVEF and, possibly, in LVEDD, functional capacity, and risk of MACE. These results support the concept that cell therapy remains a promising strategy for mitigating the morbidity associated with HF due to NICM, and are in agreement with a multitude of trials and meta‐analyses in patients with ischemic cardiomyopathy43 and refractory angina44 who received cell therapy.11 The main limitation in NICM is the lack of pivotal phase III trials. Our findings provide a rationale for conducting larger, rigorous studies aimed at conclusively determining the efficacy of cell therapy in patients with NICM and identifying the appropriate cell type, dose, and delivery route.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
A.T., M.S.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.R.K.: provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript; V.M.V.: provision of study material or patients, data analysis and interpretation, final approval of manuscript; R.B.: financial support, administrative support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Supporting information
Supplementary Figure 1 Sensitivity analysis Forest plot after excluding four trials with high or unknown risk of detection and attrition bias (A) effect of stem cell therapy on left ventricular ejection fraction (B) effect of stem cell therapy on major adverse cardiac events (MACE).
Supplementary Figure 2: Forest plots of the effect of stem cell therapy on left ventricular end diastolic volume or diameter after excluding the trial with maximum weightage.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health Grants P01 HL078825 and UM1 HL113530.
Tripathi A, Khan MS, Khan AR, Vaughn VM, Bolli R. Cell therapy for nonischemic dilated cardiomyopathy: A systematic review and meta‐analysis of randomized controlled trials. STEM CELLS Transl Med. 2021;10(10):1394‐1405. 10.1002/sctm.21-0094
Avnish Tripathi and Mohammad Saud Khan contributed equally to this study.
Funding information National Institutes of Health, Grant/Award Numbers: UM1 HL113530, P01 HL078825
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113(6):646‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shore S, Grau‐Sepulveda MV, Bhatt DL, et al. Characteristics, treatments, and outcomes of hospitalized heart failure patients stratified by etiologies of cardiomyopathy. JACC Heart Fail. 2015;3(11):906‐916. [DOI] [PubMed] [Google Scholar]
- 4.Seferovic PM, Polovina M, Bauersachs J, et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(5):553‐576. [DOI] [PubMed] [Google Scholar]
- 5.Schultheiss HP, Fairweather D, Caforio ALP, et al. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569‐582. [DOI] [PubMed] [Google Scholar]
- 7.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. N Engl J Med. 1994;331(23):1564‐1575. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee MN, Bolli R, Hare JM. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res. 2018;123(2):266‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113(6):810‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wysoczynski M, Khan A, Bolli R. New paradigms in cell therapy: repeated dosing, intravenous delivery, immunomodulatory actions, and new cell types. Circ Res. 2018;123(2):138‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli R, Solankhi M, Tang XL, Kahlon A. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res. 2021. 10.1093/cvr/cvab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher SA, Doree C, Mathur A, Martin‐Rendon E. Meta‐analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116(8):1361‐1377. [DOI] [PubMed] [Google Scholar]
- 13.Fischer‐Rasokat U, Assmus B, Seeger FH, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow‐derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1‐year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circ Heart Fail. 2009;2(5):417‐423. [DOI] [PubMed] [Google Scholar]
- 14.Bocchi EA, Bacal F, Guimaraes G, et al. Granulocyte‐colony stimulating factor or granulocyte‐colony stimulating factor associated to stem cell intracoronary infusion effects in non ischemic refractory heart failure. Int J Cardiol. 2010;138(1):94‐97. [DOI] [PubMed] [Google Scholar]
- 15.Hamshere S, Arnous S, Choudhury T, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non‐ischaemic dilated cardiomyopathy: the REGENERATE‐DCM clinical trial. Eur Heart J. 2015;36(44):3061‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino H, Brofman P, Greco O, et al. Multicentre, randomized, double‐blind trial of intracoronary autologous mononuclear bone marrow cell injection in non‐ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). Eur Heart J. 2015;36(42):2898‐2904. [DOI] [PubMed] [Google Scholar]
- 17.Sant'Anna RT, Fracasso J, Valle FH, et al. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non‐ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Rev Bras Cir Cardiovasc. 2014;29(3):437‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seth S, Bhargava B, Narang R, et al. The ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial a long‐term follow‐up study. J Am Coll Cardiol. 2010;55(15):1643‐1644. [DOI] [PubMed] [Google Scholar]
- 19.Vrtovec B, Poglajen G, Lezaic L, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5‐year follow‐up. Circ Res. 2013;112(1):165‐173. [DOI] [PubMed] [Google Scholar]
- 20.Vrtovec B, Poglajen G, Sever M, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. J Card Fail. 2011;17(4):272‐281. [DOI] [PubMed] [Google Scholar]
- 21.Marquis‐Gravel G, Stevens LM, Mansour S, Avram R, Noiseux N. Stem cell therapy for the treatment of nonischemic cardiomyopathy: a systematic review of the literature and meta‐analysis of randomized controlled trials. Can J Cardiol. 2014;30(11):1378‐1384. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Wang Y, Lin M, et al. A systematic review of randomised controlled trials examining the therapeutic effects of adult bone marrow‐derived stem cells for non‐ischaemic dilated cardiomyopathy. Stem Cell Res Ther. 2016;7(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rong SL, Wang ZK, Zhou XD, Wang XL, Yang ZM, Li B. Efficacy and safety of stem cell therapy in patients with dilated cardiomyopathy: a systematic appraisal and meta‐analysis. J Transl Med. 2019;17(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia L, Zeng L, Pan J, Ding YM. Effects of stem cells on non‐ischemic cardiomyopathy: a systematic review and meta‐analysis of randomized controlled trials. Cytotherapy. 2020;22(12):699‐711. [DOI] [PubMed] [Google Scholar]
- 25.Butler J, Epstein SE, Greene SJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II‐A randomized trial. Circ Res. 2017;120(2):332‐340. [DOI] [PubMed] [Google Scholar]
- 26.Xiao W, Guo S, Gao C, et al. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. Int Heart J. 2017;58(2):238‐244. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre TCC; 2014. revman.cochrane.org (November 3, 2020, date last accessed).
- 29.Henry TD, Traverse JH, Hammon BL, et al. Safety and efficacy of ixmyelocel‐T: an expanded, autologous multi‐cellular therapy, in dilated cardiomyopathy. Circ Res. 2014;115(8):730‐737. [DOI] [PubMed] [Google Scholar]
- 30.Patel AN, Mittal S, Turan G, et al. REVIVE trial: retrograde delivery of autologous bone marrow in patients with heart failure. Stem Cells Translational Medicine. 2015;4(9):1021‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewey M, Muller M, Eddicks S, et al. Evaluation of global and regional left ventricular function with 16‐slice computed tomography, biplane cineventriculography, and two‐dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol. 2006;48(10):2034‐2044. [DOI] [PubMed] [Google Scholar]
- 32.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116(8):1413‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broughton KM, Sussman MA. Enhancement strategies for cardiac regenerative cell therapy: focus on adult stem cells. Circ Res. 2018;123(2):177‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu R, Hu X, Wang J. Concise review: optimized strategies for stem cell‐based therapy in myocardial repair: clinical translatability and potential limitation. Stem Cells. 2018;36(4):482‐500. [DOI] [PubMed] [Google Scholar]
- 37.Psaltis PJ, Schwarz N, Toledo‐Flores D, et al. Cellular therapy for heart failure. Curr Cardiol Rev. 2016;12(3):195‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez‐Aviles F, Sanz‐Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017;38(33):2532‐2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin‐induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52(6):1213‐1225. [DOI] [PubMed] [Google Scholar]
- 40.Hoeeg C, Frljak S, Qayyum AA, et al. Efficacy and mode of action of mesenchymal stem cells in non‐ischemic dilated cardiomyopathy: a systematic review. Biomedicine. 2020;8(12):1‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Premer C, Wanschel A, Porras V, et al. Mesenchymal stem cell secretion of SDF‐1alpha modulates endothelial function in dilated cardiomyopathy. Front Physiol. 2019;10:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieger AC, Myerburg RJ, Florea V, et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non‐ischemic dilated cardiomyopathy. EBioMedicine. 2019;48:377‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher SA, Doree C, Mathur A, et al. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2016;12:CD007888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan AR, Farid TA, Pathan A, et al. Impact of cell therapy on myocardial perfusion and cardiovascular outcomes in patients with angina refractory to medical therapy: a systematic review and meta‐analysis. Circ Res. 2016;118(6):984‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Sensitivity analysis Forest plot after excluding four trials with high or unknown risk of detection and attrition bias (A) effect of stem cell therapy on left ventricular ejection fraction (B) effect of stem cell therapy on major adverse cardiac events (MACE).
Supplementary Figure 2: Forest plots of the effect of stem cell therapy on left ventricular end diastolic volume or diameter after excluding the trial with maximum weightage.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


