Abstract
Determining the cost-effectiveness of technological interventions is a crucial aspect in assuring these interventions can be adopted. The FamTechCare intervention is an innovative telehealth support that links family caregivers of persons living with dementia to tailored feedback from dementia care experts based on caregiver-initiated videorecordings of challenging care situations. The FamTechCare intervention has demonstrated significant reductions in caregiver depression and increases in caregiver competence when compared to standard telephone support. The purpose of this article is to report on the cost-effectiveness of the FamTechCare telehealth intervention. Process-based costing and a cost-effectiveness analysis using the incremental cost-effectiveness ratio (ICER) was completed with 68 caregiver and person living dementia with dyads. The cost of the 12-week FamTechCare telehealth intervention was found to be greater ($48.43 per dyad per week) due to the telehealth equipment, recording application, and expert panel time compared to the telephone support intervention ($6.96 per dyad per week). The ICER was $18.51 for caregiver depression and $36.31 for caregiver competence indicating that it cost no more than $36.38 per dyad per week over 12 weeks to achieve significant improvement in depression and competence in the FamTechCare caregivers compared to the telephone support caregivers. The FamTechCare intervention appears to be cost-effective when compared to the telephone support intervention and remains near the willingness-to-pay threshold for caregivers providing in-home dementia care support.
Keywords: Behavioral Symptoms, Caregivers, Cost Analysis, Dementia, Telemedicine
Introduction and Background
Cost-effective dementia care that supports quality of life is a worldwide public health priority (World Health Organization, 2012). It is estimated that informal caregivers in the United States provide 18.5 billion hours of unpaid care annually, which contributes to $11.8 billion in caregiver healthcare costs associated with the physical and emotional impact of caregiving (Alzheimer’s Association, 2019). Without cost-effective dementia care that supports caregivers, this number will continue rising as the population of persons living with dementia expands from 47 million in 2015 to an estimated 132 million by 2050 (Prince, Comas-Herrera, Knapp, Guerchet, & Karagiannidou, 2016). Despite the necessity to support dementia caregivers to meet this growing public health need (World Health Organization, 2017), the costs of many dementia care interventions tested through robust research designs are unknown (Jones, Edwards, & Hounsome, 2012; Meiland et al., 2017).
The Kales, Gitlin, Lyketosos (2015) conceptual model for managing behavioral and psychological symptoms of dementia (BPSD) posits that the neurodegeneration associated with dementia combined with factors of the person living with dementia, caregiver, and environment lead to the BPSD (Kales, Gitlin, & Lyketsos, 2015). In order to manage behavioral symptoms, caregivers must be able to identify the underlying factors of the person living with dementia related to unmet needs as posited by the Need-Driven Dementia Compromised Behavior Model (Kovach, Noonan, Schlidt, & Wells, 2005) and environmental stressors as posited by the Progressively Lowered Stress Threshold Model (Smith, Gerdner, Hall, & Buckwalter, 2004). When needs are not met, persons living with dementia may exhibit more challenging dementia behaviors (Kovach et al., 2005). Further, the stress threshold of a person living with dementia is reduced, and this can lead to the production of challenging behaviors due to environmental stressors (Smith et al., 2004).
Addressing caregiver burden through the management of behavioral symptoms in persons living with dementia is an essential component to intervention design because caregiver burden and well-being worsen with the severity of neuropsychiatric symptoms in persons living with dementia (Feast, Moniz-Cook, Stoner, Charlesworth, & Orrell, 2016; Isik, Soysal, Solmi, & Veronese, 2018). Health care and social costs also increase as behavioral symptoms such as agitation increase (Livingston et al., 2014). Creating cost-effective and theoretically-driven interventions that support family caregivers of persons living with dementia is essential because caregiver well-being and satisfaction are directly associated with caregiving competence and stress (Quinn et al., 2019). For dementia care interventions to be effective they need to target the dyadic nature of caregiving rather than focusing only on the individual caregiver or person living with dementia (Gilhooly et al., 2016). Further, interventions cannot simply focus on providing caregivers with general coping, education, and care strategies, but rather need to be address specific caregiver stressors (Caspar, Davis, Douziech, & Scott, 2018; Gilhooly et al., 2016). Not only do few of these targeted dyadic interventions exist (Caspar et al., 2018), but little is known about the cost-effectiveness of interventions that have been tested (Jones et al., 2012; Meiland et al., 2017).
Providing caregivers with dementia care expertise should be an essential aspect of interventions because of the complexity of the theories of dementia behavior and the complexity of recommendations for nonpharmacologic approaches that involve first describing the behavior, identifying the underlying cause, devising a treatment plan, and evaluating the result of the intervention (Gitlin, Kales, & Lyketsos, 2012; Kales et al., 2015). Traditionally, experts are only able to provide care recommendations to caregivers based on retrospective recall rather than direct observation. Yet, historical recall by the caregiver may be limited by the caregiver’s ability to effectively identify and remember the precipitating needs of the person living with dementia and the surrounding environmental factors. Telehealth technology provides a means to not only address these limitations of caregiver retrospective recall but also to reach caregivers through direct observation that can lead to individualized support.
Telehealth technology offers a platform to assist family caregivers who report a desire to use technology specifically for personalized professional consultation and guidance in providing care (American Association of Retired Persons, 2016). Current use of technology to support family caregivers ranges from the simple provision of information, to support programs with peers and/or professionals, to actual training, as well as psychotherapy for caregivers (Bossen, Kim, Williams, Steinhoff, & Strieker, 2015; Egan et al., 2018; Hopwood et al., 2018; Scott et al., 2016; Topo, 2008; Waller, Dilworth, Mansfield, & Sanson-Fisher, 2017). However, many of these technological interventions may not meet the robust recommendations for effective nonpharmacological caregiver support, mainly the need for the intervention be tailored to each individual caregiver-person living with dementia dyad. The Supporting Family Caregivers with Technology clinical trial tested the theory-driven FamTechCare telehealth intervention that connected in-home dementia caregivers to care experts through videorecording of challenging dyadic care interactions (Williams et al., 2018; Williams et al., 2019).
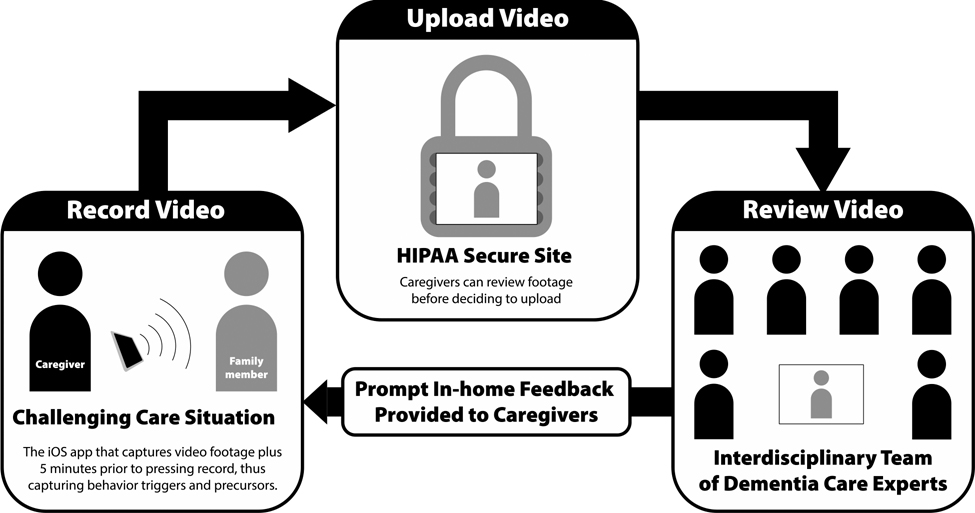
The FamTechCare telehealth intervention used caregiver-initiated videorecordings of challenging care interactions to connect caregivers with dementia care experts (Figure 1). When compared to traditional retrospective recall via a telephone call with a dementia care expert, the FamTechCare telehealth intervention significantly reduced caregiver depression and improved caregiver competence (Williams et al., 2019). Although the FamTechCare intervention was successful in improving caregiver focused outcomes, the cost-effectiveness is an essential feature in understanding the pragmatic nature of the intervention and must be determined.
Figure 1.
FamTechCare Study Procedure
Cost-effectiveness analysis is an important analytic tool used to compare costs of interventions based on patient outcomes (Sanders, Maciejewski, & Basu, 2019). Understanding the costs and efficiency of interventions is critical to transferring interventions from the research setting into the community. Cost-effectiveness analyses have been completed on non-telehealth dementia care interventions but have found inconsistent results related to cost-effectiveness. For example, dementia care mapping has demonstrated both cost-effectiveness (Michalowsky et al., 2019) and ineffectiveness (Meads et al., 2019); and in-home respite was found cost-effective (Vandepitte, Putman, Van Den Noortgate, Verhaeghe, & Annemans, 2020) whereas family meetings were not (Joling et al., 2013). However, cost-effectiveness analyses on assistive and healthcare technologies for community-dwelling persons living with dementia have yet to be completed (Meiland et al., 2017). The purpose of this paper is to determine the cost-effectiveness of the FamTechCare intervention compared to standard telephone-based support using caregiver retrospective recall.
Methods
Design
The effects of FamTechCare intervention on caregiver psychosocial outcomes were evaluated in a multi-site randomized controlled trial. The caregivers receiving the 3-month FamTechCare intervention were compared to caregivers receiving telephone support at two research sites in the Midwest. All study procedures were IRB-approved by both sites. The trial was registered with ClinicalTrials.gov (NCT02483520). The current paper is a report on the cost-effectiveness of the 3-month FamTechCare intervention versus telephone support.
Sample
A convenience sample of caregiver and person living with dementia dyads were recruited between October 2014 and June 2018. Persons living with dementia were included if they were living at home and had a diagnosis of dementia rated as mild or more severe on the Functional Assessment Staging (FAST) scale (Reisberg, 2007; Sclan & Reisberg, 1992). Persons living with dementia were excluded if they had a diagnosis of Huntington’s disease, schizophrenia, manic-depressive disorder, deafness, and/or intellectual disability. There was no exclusion based on the type of dementia. Caregivers were included if they were an adult in-home family caregiver of the person living with dementia. Dyads were recruited in Iowa and Kansas using a variety of strategies including advertisements in local magazines and newspapers, presentations at local caregiving meetings, mass email notifications through the university community, and electronic medical record review using a partial HIPPA waiver. Informed consent was obtained from all participants or their surrogate decision makers, and assent was obtained from persons living with dementia who were unable to consent independently.
Blinded randomization using a quarter-based blocking strategy with 1:1 allocation was used to assign dyads to the experimental group or the attention control group. The dyads and the study personnel were unblinded to group allocation following consent. Multiple caregivers could enroll in the study if the person living with dementia had more than one primary family caregiver. These families were cluster-randomized to the same group. The published FamTechCare protocol (Williams et al., 2018) and main outcome analysis (Williams et al., 2019) provide further detail on intervention development, participant recruitment and eligibility, the protection of human subjects, study procedures, and intervention fidelity.
Supporting Family Caregivers with Technology Clinical Trial
The Supporting Family Caregiver with Technology clinical trial compared the FamTechCare telehealth intervention to traditional telephone support. The innovative FamTechCare intervention provided family caregivers with individualized care recommendations from an interdisciplinary expert team based on caregiver initiated videorecordings of challenging care episodes. To videorecord, caregivers were provided with a video-monitoring unit (VMU) at no cost. The VMU included an iPad Mini with the Behavior Capture videorecording application (https://behaviorimaging.com, Boise, ID), a Bluetooth remote, and an iPad stand. Unlike traditional videorecording, the Behavior Capture application has a buffering capability which allows for video to captured and saved before the recording is initiated by the caregiver. Thus, the application can capture the time leading up to a challenging care situation before the caregiver actually triggers the recording. This feature allows the expert team to identify the antecedents that led to a challenging care encounter. The buffering technology is utilized as long as the application is open and running. An iPad stand was provided to facilitate the buffering feature allowing the iPad to be connected to a power source, upright, and constantly running. The caregiver then reviewed the video on the application and decided to delete it or upload it to the HIPAA-secure Behavior Connect website for review by the expert team. All videorecordings were screened for immediate safety concerns within 24 hours of submission. All VMU materials were returned following the completion of the study.
The uploaded videorecordings were reviewed weekly by a team of interdisciplinary dementia care experts who were from the fields of nursing, geriatric psychiatry, social work, and psychology. Tailored interventions were developed from both evidence-based dementia care protocols and clinical expertise. These recommendations focused on a variety of caregiver needs including managing BPSD, performing ADL care, providing education about dementia, increasing knowledge related to medication administration, improving medical care utilization, increasing home and personal safety, providing caregiver social support, enhancing caregiver self-care, acquiring and utilizing respite services, as well as providing positive reinforcement to the caregiver (Kim, Shaw, Williams, & Hein, 2019). Each study site held separate review meetings and following the expert review, the nurse or social worker interventionist relayed the tailored care interventions to the caregiver during a scheduled weekly phone call.
In the clinical trial, the FamTechCare intervention was compared to traditional telephone support in which caregivers received a weekly phone call from a nurse or social worker interventionist who provided tailored interventions using the same guidelines used for the FamTechCare group. However, the interventions were based solely on caregiver self-report using retrospective recall. The main outcome analysis in the clinical trial demonstrated that the FamTechCare caregivers had reductions in caregiver depression and gains in caregiver competence when compared to the attention control group. The full outcome methodology and results are reported elsewhere (Williams et al., 2019).
Data Collection
Caregiver data for both the FamTechCare and attention control groups were collected at baseline and 3 months. The cost data was collected on both fixed (e.g., equipment) costs and time (e.g., personnel) costs. Fixed costs for the FamTechCare group included the costs of the VMU equipment and the cost to receive and return the VMU equipment via mail. There were no fixed costs associated with the attention control group. Time was recorded for both the FamTechCare and attention control groups. Weekly time records for the FamTechCare group included the video screening time, expert review time, interventionist feedback time, and technology support time. Weekly time records for the attention control group included the interventionist feedback time. Time records for both groups also included the introductory procedure/socialization time along with the initial technology training time for the FamTechCare group.
Analysis
Process-based costing was used to calculate the pragmatic cost of the intervention using the fixed and time cost records. Pragmatic costing generates a realistic cost for the intervention to be applied in the community setting compared to the budgetary costs used to conduct the research study itself. All cost decisions were determined by the study PI and a member of the research team at each site. Table 1 summarizes the pragmatic cost decisions.
Table 1.
Process-based Costing Pragmatic Decisions
| Decision | Reasoning |
|---|---|
| Expert team and interventionist salaries will be based on national salary averages. | National averages of occupational salaries represent the national costs rather than institutional salaries at each site. National averages were determined from the 2017 U.S. Bureau of Labor Statistics National Occupational Employment and Wage Estimates (U.S. Bureau of Labor Statistics, 2018). The hourly salaries were abstracted for the interventionist and expert team. |
| Consistent occupations will be used for the interventionist and expert team costs. | Suggestions for the interventionist and expert team were based on qualitative exit interviews conducted with the expert panel (Williams, Shaw, Perkhounkova, Hein, & Coleman, Under Review). The interventionist will be a registered nurse. The expert team will include the nurse interventionist, a social worker, an occupational therapist, and a nurse practitioner. |
| Initial enrollment time will not include research related procedures. | The time used to complete the study consent and study surveys will be excluded from the time records for both the FamTechCare and attention control groups. The FamTechCare initial enrollment time will include VMU training and support description/socialization. The attention control enrollment time will include support description/socialization. |
| VMU will be mailed to and from each dyad. | To increase access to the intervention, the VMU will be delivered to and returned from the dyad via mail. Mail costs from dyads who used this procedure are averaged and imputed to all dyads. |
| VMU training will occur via the telephone. | To increase access to the intervention, telephone training reduces the need for the interventionist to be near the dyad (i.e., reduces mileage costs and interventionist travel time). The dyad training time will not be imputed for dyads who received in-person training compared to phone training. The time for phone training did not differ significantly from the time for in-person training (p = .353). |
| Each VMU will be used by 20 dyads. | Each set of VMU equipment will be estimated to last 5 years between a total of 20 dyads. In the current trial, one set of VMU equipment was used by four dyads per year. Additionally, all equipment was returned in working order and lasted the entire 4-year trial. No iPads needed replaced throughout the trial and would not have needed replacing at the end of 4-years. |
The cost-effectiveness analysis was completed by calculating the incremental cost-effectiveness ratio (ICER). The ICER is the ratio of the difference in costs between the FamTechCare and attention control groups to the difference in intervention effectiveness (Box 1) (Sanders et al., 2016). The difference in costs between the FamTechCare and attention control groups was calculated using the results from the process-based costing. Effectiveness was determined by calculating the difference of change in the outcomes of caregiver depression and caregiver competence between groups. Caregiver depression and caregiver competence were the selected outcomes for the cost-effectiveness analysis because they were found to have significant changes for the FamTechCare group compared to the attention control group in the main outcome analysis using linear mixed modeling of the Supporting Family Caregivers with Technology clinical trial (Williams et al., 2019).
Box 1. Incremental Cost-effectiveness Ratio (ICER).
Note: FTC = FamTechCare.
The outcome change scores were calculated by averaging the caregiver change scores from baseline to 3 months for each group. Caregiver depression was measured using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The CES-D contains 20 items on a 4-point Likert scale ranging from “rarely or none of the time” to “most or all of the time.” Greater depression is represented by a higher score (range=0 to 60). The CES-D has adequate internal consistency (α=.84-.90), moderate convergent validity with other depression scales (r=.44-.75) (Radloff, 1977), and has effectively measured change in dementia caregivers (Pinquart & Sorensen, 2006). Caregiver competence was measured using the Short Sense of Competence Questionnaire (SSCQ) (Vernooij-Dassen et al., 1999). The SSCQ contains seven negatively worded items (e.g., “I wish I had a better relationship with…”). Each item is rated with a 5-point Likert scale and dichotomized to agree or disagree and the items to which the caregiver disagreed are summed for the total score. A higher sense of competence is represented with a higher score (range=0 to 7). The SSCQ shows adequate internal consistency (α=.76) and concurrent validity with the original Sense of Competence Questionnaire (r=.88) (Vernooij-Dassen et al., 1999).
Results
Sample
This cost analysis was made up of N = 68 dyads from the N = 84 dyads in the Supporting Family Caregivers with Technology clinical trial. Sixteen dyads were not included in this analysis because of incomplete time records that were determined to be missing at random. The 68 dyads included for this analysis were made up of 68 caregivers and 56 persons living with dementia. The FamTechCare group included 31 dyads and the attention control group included 37 dyads. The majority of caregivers were female (72.1%), had a mean age of 65.0±13.2 years (range = 32.0 to 90.0), and cared for their spouse (64.7%). Over half of the persons living with dementia had moderately severe dementia (58.9%). Table 2 reports the demographic characteristics for the caregivers and persons living with dementia.
Table 2.
Demographic Characteristics of Caregivers and Persons Living with Dementia
| FamTechCare | Attention Control | |||
|---|---|---|---|---|
|
| ||||
| Caregiver | ||||
|
| ||||
| n | Mean (SD) | n | Mean (SD) | |
|
| ||||
| Age | 31 | 66.5 (11.7) | 37 | 63.8 (14.4) |
|
| ||||
| n | % 1 | n | % 1 | |
|
| ||||
| Gender | ||||
| Female | 21 | 67.6 | 28 | 75.7 |
| Male | 10 | 32.3 | 9 | 24.3 |
| Race | ||||
| White | 28 | 90.3 | 37 | 100.0 |
| African American | 3 | 9.7 | 0 | 0.0 |
| Ethnicity | ||||
| Not Hispanic/Latino | 30 | 96.8 | 33 | 89.2 |
| Unknown/Not reported | 1 | 3.2 | 4 | 10.8 |
| Relationship to PLWD2 | ||||
| Spouse | 22 | 71.0 | 22 | 59.5 |
| Child/Spouse of child | 8 | 25.8 | 15 | 40.5 |
| Other3 | 1 | 3.2 | 0 | 0.0 |
| Education level | ||||
| Less than Bachelor’s degree | 12 | 38.7 | 14 | 37.8 |
| Bachelor’s degree | 11 | 35.5 | 17 | 45.9 |
| Master’s degree or higher | 8 | 25.8 | 6 | 16.2 |
|
| ||||
| Persons Living with Dementia | ||||
|
| ||||
| n | Mean (SD) | n | Mean (SD) | |
|
| ||||
| Age | 28 | 75.1 (9.0) | 28 | 77.1 (8.3) |
|
| ||||
| n | % 1 | n | % 1 | |
|
| ||||
| Gender | ||||
| Male | 17 | 60.7 | 17 | 60.7 |
| Female | 11 | 39.3 | 11 | 39.3 |
| Race | ||||
| White | 26 | 92.9 | 28 | 100.0 |
| African American | 2 | 7.1 | 0 | 0.0 |
| Ethnicity | ||||
| Not Hispanic/Latino | 27 | 96.4 | 25 | 89.3 |
| Unknown/Not reported | 1 | 3.6 | 3 | 10.7 |
| Education level | ||||
| Less than Bachelor’s degree | 15 | 53.6 | 13 | 46.4 |
| Bachelor’s degree | 4 | 14.3 | 9 | 32.1 |
| Master’s degree or higher | 9 | 32.1 | 6 | 21.4 |
| Type of dementia | ||||
| Alzheimer’s disease | 15 | 53.6 | 14 | 50.0 |
| Other diagnosed dementia | 11 | 39.3 | 8 | 28.6 |
| Unknown | 2 | 7.1 | 6 | 21.4 |
| Dementia Stage (FAST) | ||||
| Mild | 5 | 17.9 | 7 | 25.0 |
| Moderate | 4 | 14.3 | 6 | 21.4 |
| Moderately severe | 18 | 64.3 | 15 | 53.6 |
| Severe | 1 | 3.6 | 0 | 0.0 |
Note.
Percentages may total more than 100% due to rounding
PLWD = Person living with dementia
Other relationship with the person with dementia was girlfriend.
An average of 15.4±12.9 videos (range = 1 to 44) were uploaded for video review by the 31 dyads in the FamTechCare group over the 12-week study. The number of videos declined slightly over time with an average of 2.2±2.1 videos submitted during week 1 and 1.3±3.3 submitted during week 12 (Table 3). Additional details on the utilization of the video intervention by the FamTechCare group is reported elsewhere (Williams et al., Under Review).
Table 3.
Video Utilization by FamTechCare group (N = 31 dyads)
| Week | Total Number of Videos | Average Number of Videos per Dyad (SD) | Number of Dyads Sending At Least 1 Video |
|---|---|---|---|
|
| |||
| Week 1 | 61 | 2.2 (2.1) | 22 |
| Week 2 | 41 | 1.5 (1.7) | 21 |
| Week 3 | 47 | 1.7 (2.1) | 16 |
| Week 4 | 39 | 1.4 (2.2) | 14 |
| Week 5 | 30 | 1.1 (1.4) | 14 |
| Week 6 | 31 | 1.1 (1.3) | 15 |
| Week 7 | 45 | 1.6 (3.2) | 14 |
| Week 8 | 29 | 1.0 (1.5) | 12 |
| Week 9 | 24 | 0.9 (1.4) | 12 |
| Week 10 | 27 | 1.0 (1.5) | 13 |
| Week 11 | 21 | 0.8 (1.3) | 9 |
| Week 12 | 35 | 1.3 (3.3) | 10 |
Process-Based Costing
Based on the process-based costing, the 3-month FamTechCare intervention cost a total of $581.21 per dyad, which equates to $48.43 per dyad per week. The attention control cost a total of $83.57 per dyad for the 3-month trial, which equates to $6.96 per week (Table 4). Nearly one-third of the costs in the FamTechCare group came from the VMU that included the iPad Mini, iPad stand, extension cord, iPad case, Bluetooth remote, screen protector, instructional binder, and Behavior Capture application. The Behavior Capture application and associated server storage on the Behavior Connect web platform made up 85.6% of the VMU costs. The costs of the equipment alone was $25.14 per dyad for the 3-month period. The other primary cost of the FamTechCare group was the expert team salaries, which made up nearly one-third of the total costs at $14.51 per dyad per week. The interventionist feedback call, video screening, and enrollment costs (i.e., training) were all based on the nurse interventionist’s salary and made up the majority of the remaining FamTechCare costs at $182.69 total per dyad or $15.22 per dyad per week. All attention control costs were based on the nurse interventionist’s salary, primarily when completing the interventionist feedback calls.
Table 4.
Cost Breakdown by Group
| Category | FamTechCare | Attention Control | ||
|---|---|---|---|---|
| 3-Month Cost | Percent of Total | 3-Month Cost | Percent of Total | |
|
| ||||
| Video-monitoring unit (VMU) | $175.14 | 30.1% | - | - |
| Expert team | $174.16 | 30.0% | - | - |
| Interventionist feedback call | $86.63 | 14.9% | $76.02 | 91.0% |
| Video screening | $65.69 | 11.3% | $7.54 | 9.0% |
| $49.22 | 8.5% | - | - | |
| Enrollment* | $30.37 | 5.2% | - | - |
Note.
Enrollment for FamTechCare group included technology training and support description/socialization, while enrollment for the attention control group only included support description/socialization.
Cost-Effectiveness Analysis
Based on the linear mixed-modeling primary outcome analysis that is reported elsewhere (Williams et al., 2019), the caregivers in the FamTechCare group demonstrated improvements in levels of depression and competence compared to caregivers in the attention control group who reported greater depression and lower competence at the end of the 3-month trial (Table 5). Further associations between these outcomes at baseline and the change at 3-months and the number of video uploads were not significant at the p<.05 level. However, although all these correlations were nonsignificant, they were all in the hypothesized direction in which a greater number of videos submitted equated to either a worse depression or competence score and a greater improvement in depression or competence scores at 3-months equated to more videos submitted.
Table 5.
Change in Caregiver Depression and Competence by Group
| Depression (CES-D)1 | Competence (SSCQ)2 | |||
|---|---|---|---|---|
| Change Score | SD | Change Score | SD | |
|
| ||||
| FamTechCare | −1.81 | 9.31 | 0.71 | 1.70 |
| Control | 0.43 | 7.47 | −0.43 | 2.44 |
Note.
A decrease in CES-D (0–60) scores indicates improvement in caregiver depression.
An increase in SSCQ (0–7) scores indicates improvement in caregiver competence.
The difference in costs between the FamTechCare and attention control groups was $497.64 per dyad for the 3-month trial or $41.47 per dyad per week, with the FamTechCare intervention being more expensive. The ICER based on depression level was $222.17 per dyad for the 3-month trial or $18.51 per dyad per week (Table 6). Thus, it cost $18.51 per dyad per week over 12 weeks to achieve the significant improvement in depression in the FamTechCare caregivers compared to the attention control caregivers. The ICER based on competence is $436.53 per dyad for the 3-month trial or $36.38 per dyad per week. Thus, it cost $36.38 per dyad per week over 12 weeks to achieve the significant improvement in competence in the FamTechCare caregivers compared to the attention control caregivers.
Table 6.
Incremental Cost-effectiveness Ratio (ICER) for Depression and Competence
| ICER Total | ICER by Week | |
|---|---|---|
|
| ||
| Depression | $222.17 | $18.51 |
| Competence | $436.53 | $36.38 |
Discussion
A cost-effectiveness analysis was used to compare a telehealth intervention to support in-home family caregivers of persons living with dementia to traditional telephone-based support based on caregiver retrospective recall. The FamTechCare telehealth intervention was found to be of greater expense due to the telehealth equipment, innovative recording application, and expert panel time. However, the FamTechCare intervention also produced greater improvements in outcomes for the family caregivers (Williams et al., 2019). In healthcare settings, cost savings do not automatically equate to cost-effectiveness because more cost can provide better health. Thus, an ICER was used to determine the ratio of difference in costs between the FamTechCare and attention control groups. It was demonstrated that it would cost no more than $36.38 per dyad per week to achieve the significant impact on outcomes in the FamTechCare group compared to the attention control group. This equates to the true cost of $48.43 per dyad per week for the FamTechCare intervention.
The caregiver’s willingness-to-pay (WTP) amount can be used as a threshold to determine if interventions are cost-effective based on the ICER (Sanders et al., 2019). Although, the WTP amount is unknown for this specific intervention, a different trial demonstrated that in-home family caregivers of persons living with dementia were willing to pay $36 per session for an intervention focused on training caregivers to provide tailored activities for a person living with dementia (Jutkowitz et al., 2019). The goals of the activity trial and our trial are similar in that both aim to reduce behavioral symptoms of persons living with dementia and improve caregiver outcomes through tailored interventions. However, the $36 based on the other trial was for 8 sessions over a 3-month period, while the FamTechCare trial provided 12 sessions over a 3-month period. The WTP amount in the activity trial was also determined prior to intervention delivery with the authors concluding that WTP may have increased based on satisfaction with the intervention. Thus, considering the WTP threshold, the FamTechCare intervention does appear to be cost-effective based on the ICER. When considering the true cost, the FamTechCare intervention should also be thought cost-effective because the intervention provides 4 additional weeks of expert feedback compared to the 8-session intervention used in the WTP threshold.
Process-based costing was used to determine the pragmatic costs of the FamTechCare intervention. However, further adaptations can be made to continue to reduce these costs. First, the technological costs should continue to be reduced as technology advances. The majority of the technological costs were associated with the application itself and associated server storage. Additional, reductions could be demonstrated if the intervention were also adapted to a mobile health platform in which caregivers used their own mobile device or tablet for video recording. Second, the expert team costs could be reimbursed or covered by a payer (e.g., Medicare), which is consistent with many caregivers’ views that the government should pay over half the costs for technological interventions that support caregiving (Schulz et al., 2016). Third, costs can be based on caregiver location and available equipment. Shipping of equipment alone cost nearly $50 per dyad. For rural dyads, mail may be the most cost-effective option to receive the supplies and training. However, for more urban dyads it may be more cost-effective for the interventionist to deliver of the equipment to and provide the technological training in the caregivers home.
Future research on the FamTechCare intervention should focus on addressing these pragmatic changes. The greatest change that would have the biggest impact on cost would be integrating the intervention into a mature healthcare service. Exit interviews with the expert team addressed ways to adapt the FamTechCare intervention for testing within the healthcare system (Williams et al., Under Review). The experts identified two methods for FamTechCare integration. The first would be implementing FamTechCare directly into the primary care environment. Although experts felt that caregivers may benefit most from this option, they also felt it also posed the greatest challenges with implementation, management, and reimbursement. The other option suggested by experts would be to implement the intervention into existing community-based resources such as the Program of All Inclusive Care for the Elderly (PACE) or through local chapters of groups such as the Alzheimer’s Association.
Despite the ICER for the intervention falling within the WTP threshold, the out-of-pocket cost could be a restrictive for dyads. Having the expert team reimbursed or covered by a payer would be essential to ensuring equal access to the intervention. Prior to the COVID-19 pandemic few telemedicine services were covered by payers in the United States. However, the pandemic has led to the expansion and payment of telehealth services within the United States healthcare system (Bashshur et al., 2020; Weigel et al., 2020). Although the current intervention does not fall within the covered services, the expansion of telehealth is moving within the correct direction to provide this type of support to family caregivers of persons living with dementia.
This cost-effectiveness analysis was based on a pragmatic costing of the intervention and trial-based outcomes. The analysis is limited in its cost generalizability as it does not include extrapolations of costs related to societal costs including informal caregiving hours or additional healthcare outcomes such as emergency room visits (Sanders et al., 2016). This analysis used trial-based outcomes and did not calculate the quality-adjusted life years (QALY) for comparison with other interventions. However, the outcomes used are caregiver-driven whereas the standard QALY does not consistently capture quality of life for a caregiver and person living with dementia (Sopina et al., 2019). This limits the comparison of our interventions to others that use the QALY.
The process-based costing did not include the cost of internet required for video transmission. With few exceptions, dyads had their own internet and the iPad was connected to their personal highspeed internet. However, we provided a hotspot for dyads without internet or without a reliable wireless connection. The hotspot would accrue additional costs estimated at an additional $40/month when required, thus making the intervention significantly less cost-effective. Other limitations include the limited diversity in the sample as it is mostly older non-Hispanic white participants. None of the VMU equipment needed replacing during the 4-year trial and equipment replacement would have also accrued and additional cost. Additionally, the outcomes selected are on different scales, which directly impacts the amount calculated with the ICER.
Determining the cost-effectiveness of interventions is a crucial aspect in assuring interventions can be translated from research to practice. The FamTechCare intervention is an innovative telehealth support that reduces caregiver depression and increases caregiver competence (Williams et al., 2019). In the FamTechCare intervention, in-home family dementia caregivers videorecord challenging care situations and receive feedback from an interdisciplinary expert team. The FamTechCare intervention is cost-effective when compared to a traditional non-telehealth telephone-support intervention based on caregivers WTP.
Financial and Other Support.
This work was supported by the National Institute of Nursing Research of the National Institutes of Health under grant number R01NR014737. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Clinical Trials registration NCT02483520. The University of Kansas Alzheimer’s Disease Center (P30AG035982) provided essential infrastructure and recruitment support. The authors thank other research team members who were involved in the conduct of the FamTechCare study including Yelena Perkhounkova, Maria Hein, Eric Vidoni, Diane Blyler, Denise Seabold, Michelle Cochran, JoEllen Wurth, Ann Arthur, Michelle Niedens, Phyllis Switzer, and Ann Bossen.
Footnotes
Conflict of Interest Statement
The Authors declare no conflict of interests.
References
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 15(3), 321–387. doi: 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- American Association of Retired Persons. (2016). Caregivers & Technology: What they want and need. Retrieved January 25, 2019 from http://www.aarp.org/content/dam/aarp/home-and-family/personal-technology/2016/04/Caregivers-and-Technology-AARP.pdf [Google Scholar]
- Bashshur R, Doarn CR, Frenk JM, Kvedar JC, & Woolliscroft JO (2020). Telemedicine and the COVID-19 pandemic, lessons for the future. Telemedicine and e-Health, 26(5), 571–573. doi: 10.1089/tmj.2020.29040.rb [DOI] [PubMed] [Google Scholar]
- Bossen AL, Kim H, Williams KN, Steinhoff AE, & Strieker M (2015). Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technology and Telehealth, 3, 49–57. doi: 10.2147/shtt.S59500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar S, Davis ED, Douziech A, & Scott DR (2018). Nonpharmacological management of behavioral and psychological symptoms of dementia: What works, in what circumstances, and why? Innovations in Aging, 2(1). doi: 10.1093/geroni/igy001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KJ, Pinto-Bruno AC, Bighelli I, Berg-Weger M, van Straten A, Albanese E, & Pot AM (2018). Online training and support programs designed to improve mental health and reduce burden among caregivers of people with dementia: A systematic review. Journal of the American Medical Directors Association, 19(3), 200–206.e201. doi: 10.1016/j.jamda.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Feast A, Moniz-Cook E, Stoner C, Charlesworth G, & Orrell M (2016). A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. International Psychogeriatrics, 28(11), 1761–1774. doi: 10.1017/s1041610216000922 [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Gilhooly ML, Sullivan MP, McIntyre A, Wilson L, Harding E, . . . Crutch S (2016). A meta-review of stress, coping and interventions in dementia and dementia caregiving. BMC Geriatrics, 16, 106. doi: 10.1186/s12877-016-0280-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Kales HC, & Lyketsos CG (2012). Nonpharmacologic management of behavioral symptoms in dementia. JAMA, 308(19), 2020–2029. doi: 10.1001/jama.2012.36918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J, Walker N, McDonagh L, Rait G, Walters K, Iliffe S, . . . Davies N (2018). Internet-based interventions aimed at supporting family caregivers of people with dementia: Systematic review. Journal of Medical Internet Research, 20(6), e216. doi: 10.2196/jmir.9548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik AT, Soysal P, Solmi M, & Veronese N (2018). Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer’s disease: A narrative review. International Journal of Geriatric Psychiatry. doi: 10.1002/gps.4965 [DOI] [PubMed] [Google Scholar]
- Joling KJ, Bosmans JE, van Marwijk HW, van der Horst HE, Scheltens P, MacNeil Vroomen JL, & van Hout HP (2013). The cost-effectiveness of a family meetings intervention to prevent depression and anxiety in family caregivers of patients with dementia: A randomized trial. Trials, 14, 305. doi: 10.1186/1745-6215-14-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Edwards RT, & Hounsome B (2012). A systematic review of the cost-effectiveness of interventions for supporting informal caregivers of people with dementia residing in the community. International Psychogeriatrics, 24(1), 6–18. doi: 10.1017/s1041610211001207 [DOI] [PubMed] [Google Scholar]
- Jutkowitz E, Scerpella D, Pizzi LT, Marx K, Samus Q, Piersol CV, & Gitlin LN (2019). Dementia family caregivers’ willingness to pay for an in-home program to reduce behavioral symptoms and caregiver stress. Pharmacoeconomics, 37(4), 563–572. doi: 10.1007/s40273-019-00785-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, & Lyketsos CG (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ, 350. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Shaw C, Williams K, & Hein M (2019). Typology of technology-supported dementia care guidance from an in-home telehealth trial. Western Journal of Nursing Research, 41(12), 1724–1746. doi: 10.1177/0193945919825861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CR, Noonan PE, Schlidt AM, & Wells T (2005). A model of consequences of Need-Driven, Dementia-Compromised Behavior. Journal of Nursing Scholarship, 37(2), 134–140. doi: 10.1111/j.1547-5069.2005.00025_1.x [DOI] [PubMed] [Google Scholar]
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, . . . Cooper C (2014). A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technology Assessment, 18(39), 1–226, v-vi. doi: 10.3310/hta18390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads DM, Martin A, Griffiths A, Kelley R, Creese B, Robinson L, . . . Surr CA (2019). Cost-Effectiveness of dementia care mapping in care-home settings: Evaluation of a randomised controlled trial. Applied Health Economics and Health Policy. doi: 10.1007/s40258-019-00531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiland F, Innes A, Mountain G, Robinson L, van der Roest H, Garcia-Casal JA, . . . Franco-Martin M (2017). Technologies to support community-dwelling persons with dementia: A position paper on issues regarding development, usability, effectiveness and cost-effectiveness, deployment, and ethics. JMIR Rehabilitation and Assistive Technologies, 4(1), e1. doi: 10.2196/rehab.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowsky B, Xie F, Eichler T, Hertel J, Kaczynski A, Kilimann I, . . . Hoffmann W (2019). Cost-effectiveness of a collaborative dementia care management-Results of a cluster-randomized controlled trial. Alzheimer’s & Dementia, 15(10), 1296–1308. doi: 10.1016/j.jalz.2019.05.008 [DOI] [PubMed] [Google Scholar]
- Pinquart M, & Sorensen S (2006). Helping caregivers of persons with dementia: Which interventions work and how large are their effects? International Psychogeriatrics, 18(4), 577–595. doi: 10.1017/s1041610206003462 [DOI] [PubMed] [Google Scholar]
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, & Karagiannidou M (2016). World Alzheimer Report 2016: Improving Healthcare for People Living with Dementia. Retrieved January 25, 2019 from https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf
- Quinn C, Nelis SM, Martyr A, Victor C, Morris RG, & Clare L (2019). Influence of positive and negative dimensions of dementia caregiving on caregiver well-being and satisfaction with life: Findings from the IDEAL study. The American Journal of Geriatric Psychiatry. doi: 10.1016/j.jagp.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Reisberg B (2007). Global measures: Utility in defining and measuring treatment response in dementia. International Psychogeriatrics, 19(3), 421–456. doi: 10.1017/s1041610207005261 [DOI] [PubMed] [Google Scholar]
- Sanders GD, Maciejewski ML, & Basu A (2019). Overview of cost-effectiveness analysis. JAMA, 321(14), 1400–1401. doi: 10.1001/jama.2019.1265 [DOI] [PubMed] [Google Scholar]
- Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, . . . Ganiats TG (2016). Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA, 316(10), 1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Matthews JT, Courtney K, De Vito Dabbs A, & Mecca LP (2016). Caregivers’ willingness to pay for technologies to support caregiving. Gerontologist, 56(5), 817–829. doi: 10.1093/geront/gnv033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclan SG, & Reisberg B (1992). Functional assessment staging (FAST) in Alzheimer’s disease: Reliability, validity, and ordinality. International Psychogeriatrics, 4(1), 55–69. doi: 10.1017/S1041610292001157 [DOI] [PubMed] [Google Scholar]
- Scott JL, Dawkins S, Quinn MG, Sanderson K, Elliott KE, Stirling C, . . . Robinson A (2016). Caring for the carer: A systematic review of pure technology-based cognitive behavioral therapy (TB-CBT) interventions for dementia carers. Aging and Mental Health, 20(8), 793–803. doi: 10.1080/13607863.2015.1040724 [DOI] [PubMed] [Google Scholar]
- Smith M, Gerdner LA, Hall GR, & Buckwalter KC (2004). History, development, and future of the Progressively Lowered Stress Threshold: A conceptual model for dementia care. Journal of the American Geriatrics Society, 52(10), 1755–1760. doi: 10.1111/j.1532-5415.2004.52473.x [DOI] [PubMed] [Google Scholar]
- Sopina E, Chenoweth L, Luckett T, Agar M, Luscombe GM, Davidson PM, . . . Goodall S (2019). Health-related quality of life in people with advanced dementia: a comparison of EQ-5D-5L and QUALID instruments. Quality of Life Research, 28(1), 121–129. doi: 10.1007/s11136-018-1987-0 [DOI] [PubMed] [Google Scholar]
- Topo P (2008). Technology studies to meet the needs of people with dementia and their caregivers: A literature review. Journal of Applied Gerontology, 28(1), 5–37. doi: 10.1177/0733464808324019 [DOI] [Google Scholar]
- U.S. Bureau of Labor Statistics. (2018). May 2017 National Occupational Employment and Wage Estimates United States. Retrieved Feburary 12, 2019 from https://www.bls.gov/oes/2017/may/oes_nat.htm.
- Vandepitte S, Putman K, Van Den Noortgate N, Verhaeghe N, & Annemans L (2020). Cost-effectiveness of an in-home respite care program to support informal caregivers of persons with dementia: A model-based analysis. International Journal of Geriatric Psychiatry. doi: 10.1002/gps.5276 [DOI] [PubMed] [Google Scholar]
- Vernooij-Dassen MJ, Felling AJ, Brummelkamp E, Dauzenberg MG, van den Bos GA, & Grol R (1999). Assessment of caregiver’s competence in dealing with the burden of caregiving for a dementia patient: A Short Sense of Competence Questionnaire (SSCQ) suitable for clinical practice. Journal of the American Geriatrics Society, 47(2), 256–257. [DOI] [PubMed] [Google Scholar]
- Waller A, Dilworth S, Mansfield E, & Sanson-Fisher R (2017). Computer and telephone delivered interventions to support caregivers of people with dementia: A systematic review of research output and quality. BMC Geriatrics, 17(1), 265. doi: 10.1186/s12877-017-0654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel G, Ramaswamy A, Sobel L, Salganicoff A, Cubanski J, & Freed M (2020, May 11). Opprotunities and barriers for telemedicine in the U.S. during the COVID-19 emergency and beyond. Retrieved June 14, 2020 from https://www.kff.org/womens-health-policy/issue-brief/opportunities-and-barriers-for-telemedicine-in-the-u-s-during-the-covid-19-emergency-and-beyond/ [Google Scholar]
- Williams K, Blyler D, Vidoni Eric D, Shaw C, Wurth J, Seabold D, . . . Van Sciver A (2018). A randomized trial using telehealth technology to link caregivers with dementia care experts for in-home caregiving support: FamTechCare protocol. Research in Nursing and Health, 41(3), 219–227. doi: 10.1002/nur.21869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KN, Perkhounkova Y, Shaw CA, Hein M, Vidoni ED, & Coleman CK (2019). Supporting family caregivers with technology for dementia home care: A randomized controlled trial. Innovations in Aging, 3(3), 1–19. doi: 10.1093/geroni/igz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KN, Shaw CA, Perkhounkova Y, Hein M, & Coleman CK (In Press). Satisfaction, utilization, and feasibility of a telehealth intervention for in-home dementia care support: A mixed methods study. Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2012). Dementia: A Public Health Priority. Retrieved January 25, 2019 from https://www.who.int/mental_health/publications/dementia_report_2012/en/
- World Health Organization. (2017). Global Action Plan on the Public Health Response to Dementia 2017–2025. Retrieved July 17, 2019 from https://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/