Abstract
The constitutively active receptor (CAR) transactivates a distal enhancer called the phenobarbital (PB)-responsive enhancer module (PBREM) found in PB-inducible CYP2B genes. CAR dramatically increases its binding to PBREM in livers of PB-treated mice. We have investigated the cellular mechanism of PB-induced increase of CAR binding. Western blot analyses of mouse livers revealed an extensive nuclear accumulation of CAR following PB treatment. Nuclear contents of CAR perfectly correlate with an increase of CAR binding to PBREM. PB-elicited nuclear accumulation of CAR appears to be a general step regulating the induction of CYP2B genes, since treatments with other PB-type inducers result in the same nuclear accumulation of CAR. Both immunoprecipitation and immunohistochemistry studies show cytoplasmic localization of CAR in the livers of nontreated mice, indicating that CAR translocates into nuclei following PB treatment. Nuclear translocation of CAR also occurs in mouse primary hepatocytes but not in hepatocytes treated with the protein phosphatase inhibitor okadaic acid. Thus, the CAR-mediated transactivation of PBREM in vivo becomes PB responsive through an okadaic acid-sensitive nuclear translocation process.
Hepatic microsomal cytochromes P450 (CYPs) display diverse functions in the metabolism of biological signaling molecules such as steroid hormones and xenochemicals, including pharmaceutical drugs and environmental contaminants. Inducible gene transcription by exposure to xenochemicals is characteristic for CYPs and increases the organism’s metabolic capabilities against chemical toxicity and carcinogenicity (3). Phenobarbital (PB) is the prototype for a large number of structurally diverse xenochemicals that induce CYPs and other xenochemical-metabolizing enzymes (3, 10, 20). The PB-responsive enhancer module (PBREM), a versatile enhancer capable of responding to numerous PB-type inducers, regulates PB induction of the CYP2B genes in mouse, rat, and human cells (8, 11, 13, 18, 19). PBREM contains two DR-4 nuclear receptor-binding motifs, NR1 and NR2. Acting as a retinoid X receptor (RXR) heterodimer, the liver-enriched constitutively active receptor (CAR) increases its binding to NR1 in PB-treated mice. Moreover, CAR can stimulate transcriptional activity from a cis-linked PBREM-containing reporter plasmid transfected into HepG2 cells (11). CAR-mediated transactivation, however, is constitutive in HepG2 cells, and the remaining key question is how CAR responds to PB in inducing the transcription of the CYP2B gene in the liver.
CAR was originally characterized as a constitutive activator of an empirical set of retinoic acid response elements (1). Forman et al. have recently shown that this activity can be repressed by 3α-androstenol in transfected CV-1 and HepG2 cells (7). These results suggest the presence of ligands which may act positively to confer a regulatory capability to CAR (7, 15). We have demonstrated that 3α-androstenol represses expression of the endogenous CYP2B6 gene in stable HepG2 cells transfected with a CAR-expressing plasmid. Moreover, PB activates (i.e., induces) the repressed CYP gene (19). Similarly, 3α-androstenol-repressed PBREM can be reactivated by PB in HepG2 cells. An activation by PB of repressed CAR may be a mechanism regulating the induction of CYP2B genes. Despite the fact that binding of CAR to PBREM depends on PB treatment in vivo, an in vitro-translated CAR-RXR heterodimer binds to PBREM without the presence of PB (11). Moreover, 3α-androstenol does not directly interfere with the ability of CAR to form a dimer with RXR or to bind to the elements (7). Thus, an additional or alternative mechanism that confers PB responsiveness to CAR may remain undetected.
Binding of CAR to NR1 occurs only after PB induction in liver in vivo (11), indicating that the function of CAR in the liver may differ from that in HepG2 cells. To explore this possibility, we examined the intracellular localization of CAR in mouse liver and primary hepatocytes. Nuclear receptors generally reside in nuclei and can be activated upon ligand binding. Constitutively active CAR may be excluded from nuclei to suppress unwanted gene activation in nontreated mice. If, in fact, CAR localizes to liver nuclei following PB treatment, the induction of CYP2B genes may be regulated through a nuclear translocation process. Western blot and immunohistochemistry studies have been applied to demonstrate the cytoplasmic localization of CAR in nontreated mice. CAR undergoes nuclear translocation in livers of PB-treated mice. PB-induced nuclear translocation appears to be regulated through a phosphorylation-dephosphorylation pathway. Moreover, various PB-type inducers have been tested to see whether nuclear translocation of CAR is a general mechanism involved in CYP2B induction.
MATERIALS AND METHODS
Plasmids.
To construct the green fluorescent protein (GFP)-CAR expression plasmid, CAR cDNA was cloned into the pEGFP-C1 vector (Clontech). (NR1)5-tk (thymidine kinase)-luciferase plasmid was constructed by cloning quintuple NR1 sequences in front of the tk-luciferase promoter (at the BglII site) as described previously (19).
Transfection assays.
HepG2 cells were cultured in minimal essential medium supplemented with 10% fetal bovine serum. (NR1)5-tk-luciferase plasmids (0.1 μg) were cotransfected with GFP-CAR expression plasmids (0.2 μg) and pRL-SV40 (0.1 μg) into HepG2 cells by calcium phosphate coprecipitation. Mouse primary hepatocytes were prepared from 2-month-old Cr1:CD-1(ICR)BR males by a two-step collagenase perfusion and were cultured as previously described (8). (NR1)5-tk-luciferase plasmids (10 μg) were cotransfected with pRL-SV40 (3 μg) into primary hepatocytes by electroporation. Luciferase activity was measured by using the Dual-Luciferase reporter assay system (Promega). To visualize the GFP-CAR fusion protein, HepG2 cells transfected with GFP-CAR expression plasmids were fixed with 4% paraformaldehyde and nuclei were stained with Hoechst S33258. Intracellular localization of GFP-CAR was determined by classifying GFP fluorescence-positive cells into three different categories under a fluorescence microscope: N > C (nucleus-dominant fluorescence) N = C (equal distribution of fluorescence in cytoplasm and nucleus), and N < C (cytoplasm-dominant fluorescence).
Liver nuclear extracts and DNA affinity chromatography.
Forty Cr1:CD-1(ICR)BR males were treated by intraperitoneal injection with PB (100 mg/kg) or various PB-type inducers: 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP; 0.3 mg/kg), chlorpromazine (CPZ; 50 mg/kg), 1,1,1-trichloro-1,2-bis(o,p′-chlorophenyl)ethane (o,p′-DDT; 1 mg/kg), and 2,3,3′,4′,5′,6-hexachlorobiphenyl (PCB; 3 mg/kg). Subsequently, liver nuclear extracts were prepared from 10 mice at each time point after induction as described previously (11). For affinity purification of CAR, 1 mg of the nuclear extracts was incubated with NR1-conjugated Dynabeads as previously described (11). The bound proteins were eluted with 100 μl of 0.5 M NaCl from the beads, and 30-μl aliquots of the elutes were subjected to Western blot analysis.
Whole-liver extracts and immunoprecipitation.
A mouse liver was homogenized with 5 ml of 10 mM Tris-HCl buffer (pH 7.5) containing 0.2 mM sodium orthovanadate, 1% Triton X-100, 0.5% Nonidet P-40, and 0.2 mM phenylmethylsulfonyl fluoride. After incubation for 30 min at 4°C, the homogenate was centrifuged at 50,000 × g for 30 min at 4°C to obtain whole-cell extracts. Anti-CAR antibody was enriched from rabbit antiserum raised against recombinant human CAR by chromatography using recombinant mouse CAR as the affinity ligand. For immunoprecipitation, 1 ml of whole-cell extracts or 50 μg of nuclear extracts was incubated with anti-CAR antibody for 1 h at 4°C and with protein A-Sepharose (Pharmacia) for 1 h at 4°C. Then, the Sepharose was recovered by centrifugation at 1,000 × g for 1 min, washed with homogenizing buffer three times, and subjected to Western blot analysis.
Primary hepatocytes.
Mouse primary hepatocytes were pretreated with okadaic acid (OA; 10 nM) for 30 min and then induced by PB (1 mM), TCPOBOP (50 nM), or the solvent (dimethyl sulfoxide) for 90 min. The nuclear extracts were prepared from these hepatocytes by the method of Dignam et al. (6). Total RNAs were extracted from the hepatocytes for Northern blot analysis at 9 h after PB induction, using TRIZOL reagent (Life Technologies).
Western blot, Northern blot, and gel shift assays.
Nuclear extracts were resolved on a sodium dodecyl sulfate–10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, and incubated with anti-CAR or anti-RXRα antibody (Santa Cruz Biotechnology). After incubation with the secondary anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase conjugate, the immunoreactive bands were visualized with an enhanced chemiluminescence system (Amersham). For Northern blot analysis, 20-μg aliquots of RNAs were separated on a 1% agarose gel containing 2.2 M formaldehyde, transferred to a nylon membrane, and hybridized separately with either a 360-bp Cyp2b10 or 180-bp albumin cDNA probe as described previously (8). Gel shift assays were performed as described previously (11). Reverse transcription-PCR was performed to measure CAR mRNA, using AmpliTaq Gold DNA polymerase (Perkin-Elmer Cetus) and the specific primers 5′-TCTCACTCAACACTACGGTTC-3′ and 5′-TCAACTGCAAATCTCCCCGA-3′.
Immunohistochemistry.
The paraffin-embedded liver from nontreated and PB- or TCPOBOP-treated (for 3 h) mice was fixed with 4% paraformaldehyde for 6 h. Sections (5 to 7 μm thick) were deparaffinized and blocked with goat serum. Colorimetric detection was performed by the Vectastain protocol (Elite-ABC kit; Vector Laboratories), using anti-CAR antibody.
RESULTS AND DISCUSSION
Different regulation of CAR in HepG2 and primary hepatocytes.
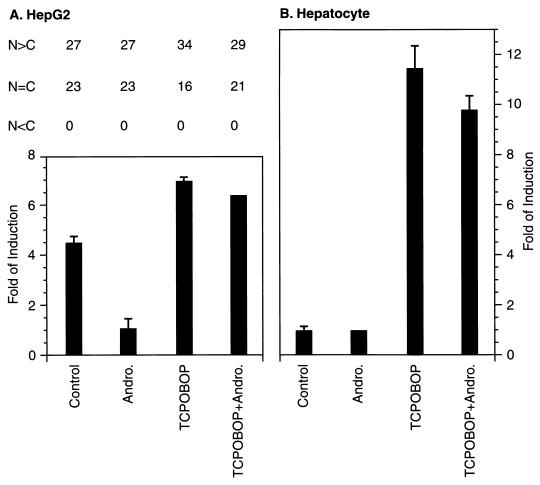
As expected from our previous findings (19), expression of the transfected (NR1)5-tk-luciferase was activated by cotransfection of HepG2 cells with the CAR expression vector (Fig. 1A). 3α-Androstenol repressed this CAR-mediated activation, whereas the potent PB-type inducer TCPOBOP derepressed (NR1)5-tk-luciferase activity. In sharp contrast, the transfected (NR1)5-tk-luciferase gene was not activated in control murine hepatocytes unless the hepatocytes were treated with TCPOBOP (Fig. 1B). Noticeably, 3α-androstenol treatment did not affect (NR1)5-tk-luciferase activity in hepatocytes. To determine how CAR was regulated in HepG2 cells, we examined the intracellular localization of GFP-CAR in HepG2 cells (Fig. 1A). The expressed GFP-CAR was always localized in the nuclei of HepG2 cells, suggesting that CAR spontaneously translocated to nucleus. Moreover, the treatment with either 3α-androstenol or TCPOBOP did not alter the nuclear localization of GFP-CAR in HepG2 cells. Because NR1 was inactive in the absence of TCPOBOP and did not respond to 3α-androstenol in hepatocytes, the regulation of CAR may be different from that in HepG2 cells.
FIG. 1.
Transactivation of NR1 by CAR and intracellular localization of GFP-CAR. (A) (NR1)5-tk-luciferase reporter plasmids were cotransfected with GFP-CAR expression plasmids and pRL-SV40 into HepG2 cells. Sixteen hours later, the transfected cells were treated for another 24 h with 3α-androstenol (Andro.; 4 μM), TCPOBOP (250 nM), or both, and luciferase activity was assayed (bottom). To determine intracellular localization, transfected HepG2 cells were induced for 2 h, and the fluorescence intensity of GFP-CAR was determined by counting 50 HepG2 cells under a microscope and scored as described in the text (top). (B) (NR1)5-tk-luciferase reporter plasmids were cotransfected with pRL-SV40 into primary hepatocytes. The cells were then treated with 3α-androstenol (4 μM), TCPOBOP (50 nM), or both for 24 h, and luciferase activity was assayed.
Nuclear accumulation of CAR in liver following PB treatment.
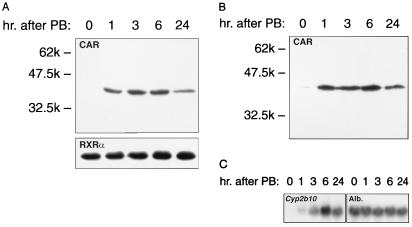
Binding activity of NR1 to CAR is increased extensively by PB treatment but is very low in liver nuclear extracts from nontreated mice (11). We examined whether this increase of binding in response to PB resulted from the nuclear accumulation of CAR or a functional activation of preexisting CAR. For this, total liver nuclear extracts were subjected to Western blot analysis using an affinity-purified anti-CAR antibody. As shown in Fig. 2A, CAR was barely detectable in liver nuclear extracts from nontreated mice, but the nuclear content of CAR was dramatically increased within 1 h after PB treatment. In sharp contrast, RXRα remained at similar levels before and after PB treatment (Fig. 2A). Thus, PB treatment resulted in a specific accumulation of CAR in liver nuclei. An increase of CAR binding activity to NR1 correlated with that of its nuclear accumulation, as indicated by Western blot analysis of the NR1 affinity fractions (Fig. 2B). Following the nuclear accumulation of CAR and its binding to NR1, the Cyp2b10 mRNA began to increase at 1 h and reached its maximum level 6 h after PB treatment (Fig. 2C). These induction kinetics were consistent with our previous findings (11).
FIG. 2.
Nuclear accumulation of CAR after PB treatment. Forty-microgram aliquots of total liver nuclear extracts (A) or 30-μl aliquots of the affinity-purified fractions (B) were used for Western blot analyses. The images were prepared from 3-min (CAR in panel A), 30-s (RXRα in panel A), and 1-min (CAR in panel B) exposures. Prestained Protein Marker Broad Range (New England Biolabs) was used as the molecular mass marker. For the Northern blot (C), 20-μg aliquots of total liver RNAs were electrophoresed, transferred, and hybridized as described in Materials and Methods.
Nuclear accumulation in response to various PB-type inducers.
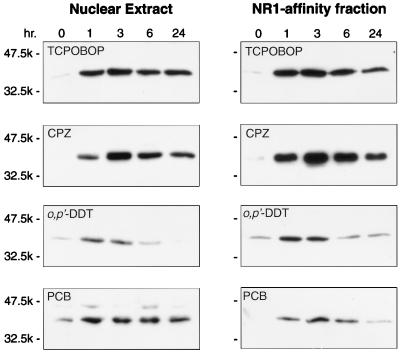
It is known that CAR transactivates PBREM in response to various PB-type inducers (19). Using liver nuclear extracts, we performed Western blotting to see whether these inducers also trigger nuclear accumulation of CAR (Fig. 3). TCPOBOP and CPZ were as effective as PB in producing accumulation of CAR in liver nuclei. Also, o,p′-DDT and PCB induced accumulation of nuclear CAR, although less effectively than PB. Increased levels of nuclear CAR accumulation in livers generally correlated with that of PBREM transactivation in HepG2 cells. This correlation, however, was not perfect; for example, CPZ transactivated PBREM approximately four times more effectively than PB in HepG2 cells (19), whereas the two inducers produced similar levels of nuclear CAR in livers. Pharmacokinetic differences in absorption or metabolism may have contributed to some of these poor correlations. Nevertheless, many, if not all, PB-type inducers elicited nuclear accumulation of CAR in mouse livers.
FIG. 3.
Nuclear accumulation elicited by various PB-type inducers. Liver nuclear extracts were prepared from mice treated with various xenochemicals. Total nuclear extracts or NR1 affinity-purified fractions were subjected to Western blot analysis using anti-CAR antibody. Due to different exposure times, band intensities cannot be accurately compared. In general, exposure times were 3 to 5 min for Western blots of the TCPOBOP and CPZ samples and around 20 min for Western blots of the o,p′-DDT and PCB samples.
Immunochemical evidence for nuclear translocation induced by PB.
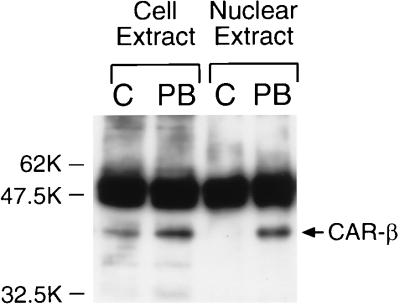
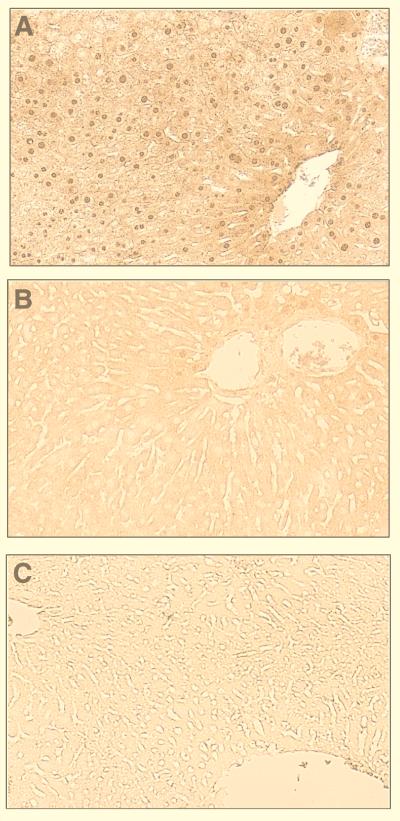
To confirm the presence of CAR in nontreated mouse livers, we performed immunoprecipitation assays of CAR from whole-liver-cell extracts. Immunoprecipitates were subjected to Western blot analysis using an anti-CAR antibody (Fig. 4). An immunostained band that corresponded to CAR was easily detected in whole-cell extracts of both nontreated and PB-treated mouse livers. A slight increase of CAR in whole-cell extracts from PB-treated mice may be within the range of experimental variation. Thus, the extraction efficiency of CAR from whole liver cells may have contributed to this variation. In contrast, but consistent with findings shown in Fig. 2, CAR was immunoprecipitated only from the liver nuclear extracts of PB-treated mice (Fig. 4). Thus, CAR appeared to be present as a cytoplasmic receptor in the livers of nontreated mice and was localized to nuclei following PB treatment. Immunohistochemistry was also used to visualize the intracellular localization of CAR on paraffin-embedded liver sections prepared from nontreated and PB-treated mice. In PB-treated mouse livers, CAR was clearly localized in the nuclei (Fig. 5A). Only a few positively stained nuclei were observed around vessels in the nontreated liver sections; the majority of nuclei were devoid of staining compared with the cytoplasmic regions (Fig. 5B). Taken together, these results lead us to conclude that PB elicits the nuclear translocation of CAR in liver.
FIG. 4.
Cytoplasmic localization of CAR in livers of nontreated mice. Whole-cell and nuclear extracts were prepared from nontreated (control [C]) and PB-treated (for 3 h) mice as described in Materials and Methods. The immunoprecipitate was electrophoresed on a sodium dodecyl sulfate–10% polyacrylamide gel, transferred, and immunostained by anti-CAR antibody. The band corresponding to CAR is indicated by an arrow. Intense bands just above CAR represent heavy-chain IgG. Prestained Protein Marker Broad Range (New England Biolabs) was used as the molecular mass marker.
FIG. 5.
Nuclear localization of CAR after PB treatment. Sections of paraffin-embedded liver tissue from a PB-treated mouse were incubated with either anti-CAR antibody (A) or control IgG (C); that from a nontreated mouse was incubated with anti-CAR antibody (B). Specific immunoreaction of the sections was visualized colorimetrically by diaminobenzidine.
OA inhibition of nuclear translocation in hepatocytes.
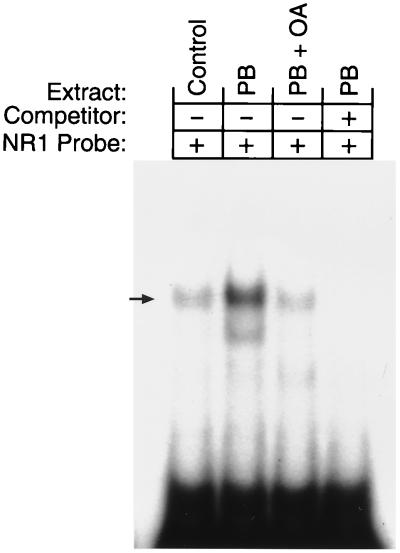
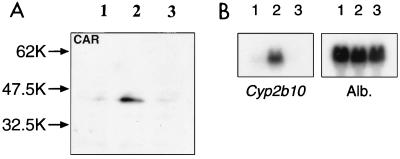
The protein phosphatase inhibitor OA is known to inhibit the PB induction of CYP2B gene expression in rodent primary hepatocytes (9, 17). Therefore, we examined whether OA repressed the PB-elicited nuclear localization of CAR. Nuclear extracts prepared from mouse primary hepatocytes treated with PB for 90 min or pretreated by OA prior to PB induction were subjected to gel shift assays and Western blot analyses. NR1 complex formation in hepatocyte nuclear extracts was markedly increased by PB induction. Conversely, OA pretreatment prevented the increase in the level of the NR1 complex (Fig. 6). Western blot analysis unequivocally showed that the nuclear content of CAR dramatically increased in PB-treated hepatocytes, while it was barely detected in nuclear extracts from OA- plus-PB-treated or nontreated hepatocytes (Fig. 7A). Again, the nuclear level of CAR in OA-pretreated hepatocytes was as low as that in nuclear extracts of nontreated hepatocytes. OA inhibited the PB-induced increase of Cyp2b10 mRNA (Fig. 7B). Thus, OA inhibits the nuclear translocation of CAR in primary hepatocytes, thus suppressing PB induction of the CYP2B gene.
FIG. 6.
OA inhibition of PB-dependent increase in the CAR-NR1 complex. Gel shift assays were performed with the hepatocyte nuclear extracts and 32P-labeled NR1 oligonucleotides. Unlabeled NR1 oligonucleotides (200-fold excess) were included as the competitor.
FIG. 7.
OA inhibition of PB-dependent nuclear localization of CAR. (A) Western blot analysis was performed with anti-CAR antibody and the nuclear extracts prepared from nontreated (lane 1), PB-treated (lane 2), and PB-plus-OA-treated (lane 3) hepatocytes. (B) Total RNAs were subjected to Northern blot analysis for Cyp2b10 and albumin (Alb.) mRNAs as described in Materials and Methods. Lanes 1, 2, and 3, RNA samples from control, PB-treated, and PB-plus-OA-treated hepatocytes, respectively.
General discussion.
CAR is a cytoplasmic receptor in liver and primary hepatocytes which translocates into the nucleus only after treatment with inducers such as PB and TCPOBOP. This PB-elicited nuclear translocation is in sharp contrast to an apparently spontaneous nuclear localization of CAR in transfected HepG2 cells. Recombinant CAR fused with GFP localizes to nuclei in transfected HepG2 cells in the absence of the inducer. 3α-Androstenol does not inhibit the nuclear localization of CAR, even at its extremely high concentrations (4 μM) compared with known plasma concentrations. Seemingly, HepG2 cells cannot retain CAR in the cytoplasm, resulting in constitutive activation of PBREM and expression of the CYP2B6 gene (19). Since CAR does not require ligand to transactivate the cis-acting response elements, it is not surprising to find that CAR is excluded from liver nuclei of nontreated mice. The nuclear translocation of CAR is tightly regulated in liver and primary hepatocytes. Consequently, the constitutively activated CAR can become PB responsive through a tightly regulated nuclear translocation process. Nuclear translocation of CAR is spontaneous in HepG2 cells and does not require binding by inducers, implying that the CAR translocation may be ligand independent. This hypothesis of ligand-independent nuclear localization seems to be supported by the fact that 3α-androstenol does not affect the nuclear translocation of CAR in either HepG2 cells or primary hepatocytes. However, it remains to be proven that negative ligands such as 3α-androstenol and their displacement by PB do not, in fact, regulate CAR at the step of the nuclear translocation.
The PB-induced CAR nuclear translocation appears to be regulated by a dephosphorylation-sensitive signaling cascade. OA treatment inhibits nuclear translocation of CAR as well as induction of Cyp2b10 mRNA. Glucocorticoid and vitamin D3 receptors are known to undergo ligand-dependent nuclear translocation in transfected cells (2, 12, 16, 21). These receptors are phosphoproteins (4), and agonist binding is the key step which initiates a change in the phosphorylation state of the glucocorticoid receptor (14). OA treatment hyperphosphorylates this receptor and decreases its nuclear translocation, although the phosphorylated receptor remains active in enhancing the glucocorticoid enhancer elements. OA also hyperphosphorylates the vitamin D3 receptor and inhibits its ligand-dependent nuclear translocation (5). However, the OA concentration affecting glucocorticoid and vitamin D3 receptors was 100 nM, 10- to 50-fold higher than the concentrations inhibiting the nuclear translocation of CAR. Nuclear translocation of CAR appears to be spontaneous in HepG2 cells and does not require binding of inducer, meaning that the CAR translocation may be ligand independent. Thus, a cellular mechanism of the PB-inducible nuclear translocation of CAR may differ from that regulating the ligand-dependent translocation of glucocorticoid and vitamin D3 receptors. Since the OA-dependent dephosphorylation is essential for the nuclear translocation of CAR and the induction of CYP2B genes, the exact mechanism of this signal transduction pathway remains a question of major interest.
PB displays pleiotropic effects on liver metabolism and physiology, from glucose metabolism to growth regulation and tumor promotion (3, 10, 20). A large number of hepatic genes are up-regulated by PB, including those enzymes involved in xenochemical metabolism. Does CAR play a central role in pleiotropic activation of various genes? The answer to this question may be yes, although only a few genes, including CYP2B, CYP3A (19), and the UDPG glucuronosyl transferase gene UGT1A (13a), are known to be regulated by CAR. CAR localizes to liver nuclei in response to various PB-type inducers that transactivate PBREM to induce CYP2B genes, indicating that nuclear translocation of CAR is a common step in the regulation of these genes by many, if not all, PB-type inducers. CAR is now emerging as the major factor mediating a large number of PB-type inducers and pleiotropically activating numerous genes. Given the fact that CAR is a constitutively activated receptor, the regulatory mechanism such as an OA-sensitive nuclear translocation may help explain how CAR plays the central role in the induction of various genes in respond to numerous xenochemicals.
REFERENCES
- 1.Baes M, Gulick T, Choi H S, Martinoli M G, Simha D, Moore D D. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol. 1994;14:1544–1551. doi: 10.1128/mcb.14.3.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barsony J, Renyi I, McKoy W. Subcellular distribution of normal and mutant vitamin D receptors in living cells. Studies with a novel fluorescent ligand. J Biol Chem. 1997;272:5774–5782. doi: 10.1074/jbc.272.9.5774. [DOI] [PubMed] [Google Scholar]
- 3.Conney A H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 4.DeFranco D B, Qi M, Borror K C, Garabedian M J, Brautigan D L. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol. 1991;5:1215–1228. doi: 10.1210/mend-5-9-1215. [DOI] [PubMed] [Google Scholar]
- 5.Desai R K, van Wijnen A J, Stein J L, Stein G S, Lian J B. Control of 1,25-dihydroxyvitamin D3 receptor-mediated enhancement of osteocalcin gene transcription: effects of perturbing phosphorylation pathways by okadaic acid and staurosporine. Endocrinology. 1995;136:5685–5693. doi: 10.1210/endo.136.12.7588324. [DOI] [PubMed] [Google Scholar]
- 6.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman B M, Tzameli I, Choi H S, Chen J, Simha D, Seol W, Evans R M, Moore D D. Androstane metabolites bind to and deactivate the nuclear receptor CAR-β. Nature. 1998;395:612–615. doi: 10.1038/26996. [DOI] [PubMed] [Google Scholar]
- 8.Honkakoski P, Moore R, Washburn K A, Negishi M. Activation by diverse xenochemicals of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol Pharmacol. 1998;53:597–601. doi: 10.1124/mol.53.4.597. [DOI] [PubMed] [Google Scholar]
- 9.Honkakoski P, Negishi M. Protein serine/threonine phosphatase inhibitors suppress phenobarbital-induced Cyp2b10 gene transcription in mouse primary hepatocytes. Biochem J. 1998;330:889–895. doi: 10.1042/bj3300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honkakoski P, Negishi M. Regulatory DNA elements of phenobarbital-responsive cytochrome P450 CYP2B genes. J Biochem Mol Toxicol. 1998;12:3–9. doi: 10.1002/(sici)1099-0461(1998)12:1<3::aid-jbt2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18:5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Htun H, Barsony J, Renyi I, Gould D L, Hager G L. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kemper B. Phenobarbital alters protein binding to the CYP2B1/2 phenobarbital-responsive unit in native chromatin. J Biol Chem. 1997;272:29423–29425. doi: 10.1074/jbc.272.47.29423. [DOI] [PubMed] [Google Scholar]
- 13a.Kojima, H., T. Sueyoshi, K. Yoshinari, Q.-H. Gong, I. Owens, and M. Negishi. Unpublished data. [DOI] [PubMed]
- 14.Lim-Tio S S, Fuller P J. Intracellular signaling pathways confer specificity of transactivation by mineralocorticoid and glucocorticoid receptors. Endocrinology. 1998;139:1653–1661. doi: 10.1210/endo.139.4.5928. [DOI] [PubMed] [Google Scholar]
- 15.Picard D. Two orphans find a home. Nature. 1998;395:543–544. doi: 10.1038/26850. [DOI] [PubMed] [Google Scholar]
- 16.Picard D, Yamamoto K R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidhu J S, Omiecinski C J. An okadaic acid-sensitive pathway involved in the phenobarbital-mediated induction of CYP2B gene expression in primary rat hepatocyte cultures. J Pharmacol Exp Ther. 1997;282:1122–1129. [PubMed] [Google Scholar]
- 18.Stoltz C, Vachon M H, Trottier E, Dubois S, Paquet Y, Anderson A. The CYP2B2 phenobarbital response unit contains an accessory factor element and a putative glucocorticoid response element essential for conferring maximal phenobarbital responsiveness. J Biol Chem. 1998;273:8528–8536. doi: 10.1074/jbc.273.14.8528. [DOI] [PubMed] [Google Scholar]
- 19.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 20.Waxman D J, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weigel N L. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]