Abstract
FoxOs and their post-translational modification by phosphorylation, acetylation, and methylation can affect epigenetic modifications and promote the expression of downstream target genes. Therefore, they ultimately affect cellular and biological functions during aging or occurrence of age-related diseases including cancer, diabetes, and kidney diseases. As known for its key role in aging, FoxOs play various biological roles in the aging process by regulating reactive oxygen species, lipid accumulation, and inflammation. FoxOs regulated by PI3K/Akt pathway modulate the expression of various target genes encoding MnSOD, catalases, PPARγ, and IL-1β during aging, which are associated with age-related diseases. This review highlights the age-dependent differential regulatory mechanism of Akt/FoxOs axis in metabolic and non-metabolic organs. We demonstrated that age-dependent suppression of Akt increases the activity of FoxOs (Akt/FoxOs axis upregulation) in metabolic organs such as liver and muscle. This Akt/FoxOs axis could be modulated and reversed by antiaging paradigm calorie restriction (CR). In contrast, hyperinsulinemia-mediated PI3K/Akt activation inhibited FoxOs activity (Akt/FoxOs axis downregulation) leading to decrease of antioxidant genes expression in non-metabolic organs such as kidneys and lungs during aging. These phenomena are reversed by CR. The results of studies on the process of aging and CR indicate that the Akt/FoxOs axis plays a critical role in regulating metabolic homeostasis, redox stress, and inflammation in various organs during aging process. The benefical actions of CR on the Akt/FoxOs axis in metabolic and non-metabolic organs provide further insights into the molecular mechanisms of organ-differential roles of Akt/FoxOs axis during aging.
Keywords: Aging, Akt/FoxOs axis, metabolic organs, non-metabolic organs, inflammation, CR
Review
1. Introduction
Aging causes detrimental changes at the molecular and cellular level that accumulate over time, and ultimately leads to deterioration of tissues and organs, leading to onset of age-related diseases and increased risk of morbidity and mortality [1]. The inflammatory response is associated with age-related diseases such as atherosclerosis, sarcopenia, Alzheimer’s disease, cancer, kidney diseases and fatty liver diseases. Furthermore, many studies have demonstrated that aging is closely related to the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway [2, 3]. Aging differentially expresses Akt-mediated Forkhead box O (FoxO) levels depending on metabolic- and non-metabolic organs.
The FoxOs protein family consists of FoxO1, FoxO3, FoxO4, and FoxO6, and these are structurally characterized by the presence of a forkhead box DNA binding domain [4]. FoxOs are expressed in ovary, prostate, skeletal muscle, brain, heart, lung, liver, pancreas, spleen, thymus, and testes [5-7]. Key roles of FoxOs transcription factors in induction of downstream target genes involved in regulation of cellular metabolic pathways in the cell cycle, cell death, and oxidative stress response have been reported [8]. Several studies suggest that FoxOs may modulate the aging process for initiation and progression of age-related diseases. However, little information is available on the organ-specific roles of FoxOs and their underlying action mechanisms. Phosphorylation of Akt inactivates FoxOs by inducing their shuttling from the nuclear fraction to the cytoplasm [8-10]. In contrast, suppressed Akt activity leads to elevated nuclear translocation and phosphorylation of FoxOs in aged liver [11]. Increased phosphorylation of FoxOs owing to the activated PI3K/Akt signaling in the aged kidney results in significant changes in insulin levels during the aging process along with other alterations. This observation can be attributed to various functions of FoxOs during aging, which is phosphorylated by Akt activation or inactivation in various aging organs.
Often, the process of aging is associated with many chronic pathological conditions such as vascular diseases, diabetes mellitus, cancer, and metabolic syndrome [12]. The occurrence of diabetes and obesity is associated with insulin resistance [13], which leads to the downregulation of Akt and upregulation of FoxOs, eventually resulting in lipid accumulation in aged liver [11]. FoxOs proteins have various functions in age-associated diseases. For instance, regulation of protein homeostasis during aging progression directly affects the pathogenesis of neuro-degenerative disorders [14-16]. However, protective effects mediated by FoxOs in the process of aging have not been well-documented. It is also unknown whether their signaling pathway and biological effects differ by tissues, disease conditions, or age. FoxOs play a pivotal role in diverse metabolic diseases including obesity, insulin resistance, hyperlipidemia, type 2 diabetes mellitus, and non-alcoholic fatty liver disease (NAFLD) [17]. Most of these conditions are age-related metabolic disorders that are rather associated with dietary factors than aging [18]. The aging process is accompanied by an inflammatory response and metabolic disorders [19]. In addition, insulin resistance is potentially caused by increased secretion of proinflammatory cytokines during aging [20]. Hyperinsulinemia-mediated Akt phosphorylation is increased in aged kidney but decreased in aged liver [21]. Subsequently, FoxOs, a well-known substrate of Akt, becomes suppressed in non-metabolic organs, whereas they are activated in metabolic organ during aging. We summarized the underlying mechanisms responsible for the association of aging with insulin resistance by defining the organ-specific function of the Akt/FoxOs axis in aging.
Calorie restriction (CR) modulates stress responses at cellular as well as physiological levels and extends the lifespan of rodents [22]. Subsequently, many studies conducted in various species have shown that CR modulates the aging progression by regulating the numbers of signaling pathways [23]. Additionally, CR increases genomic stability by reversing DNA methylation changes that occur during aging [24]. The role of FoxOs transcription factors in CR was explored in various organs in the previous reports [25]. The aged mice (24-month-old) demonstrated higher levels of phosphorylated FoxO1 and NF-κB than young mice, and the PI3K/Akt pathway was upregulated during aged kidney. Furthermore, the involvement of FoxO3 in extending the lifespan during CR has been described in a mouse model by Shimokawa et al. [26].
This review highlights the importance of the modifications of FoxOs associated with Akt in various organs such as metabolic organs as well as some in non-metabolic organs. In addition, evidence of the role of the Akt/FoxOs axis as a bridge between various organs is presented, and organ-dependent alterations of Akt/FoxOs axis during the aging progression and CR have also been described.
2. Differential roles of Akt/FoxOs axis in metabolic- and non-metabolic organs
2.1 The function of Akt/FoxOs axis in metabolic organs
FoxO1 is abundantly expressed in metabolic organs such as the liver, white and brown adipose tissues, skeletal muscle, pancreas, and hypothalamus. FoxO1 is a novel regulator of energy metabolism and is highly expressed in the skeletal muscle, which has been identified as a molecular target for insulin signaling modulation [27-29]. FoxO1 promotes glucose production in the liver along with the conversion of carbohydrate oxidation to lipid oxidation in fasting muscle [30]. In the fasting state, hepatic FoxOs [31] are activated owing to the decrease in Akt levels [32]. FoxOs mediates glucose metabolism by converting glucose to acetate for oxidation or to fatty acids [33]. FoxO1 maintains glucose homeostasis by increasing gluconeogenic gene expression in liver, thereby decreasing insulin secretion and insulin sensitivity [34]. Of all the FoxOs family isoforms, FoxO6 is a critical mediator of the production of inflammatory cytokines, such as IL-1β in aged liver [35].
Telomere size and telomerase activity were significantly lower in the FoxO1-KO than those in WT in aged liver [36]. Circulating 17β-estradiol suppressed hepatic glucose production in hepatocytes of mice but failed in Liver-FoxO1-KO mice, suggesting that FoxO1 is required for the inhibition of gluconeogenesis by circulating 17β-estradiols [37]. Additionally, FoxO3-KO mice, although viable, demonstrate infertility due to dysfunctional ovarian follicular development during aging [38] along with dysfunctional muscle regeneration [39]. FoxO3-KO mice downregulate MyoD transcription, a major factor involved in the regulation of myogenesis in myoblasts [39].
The expression of FoxOs inhibits muscle fiber atrophy by inhibiting muscle atrophy F-box (MAFbx)/atrogin-1, muscle RING finger 1 (MuRF1), BNIP3, and cathepsin L mRNA associated with cancer cachexia and sepsis [40]. In addition, the induction of the Mad/Mxd protein facilitates the inhibition of the transcription of Myc target genes, which is required for cell cycle arrest against FoxO3 mediated by the PI3K/Akt signaling pathway [41]. Akt-induced FoxOs phosphorylation leads to nuclear exclusion and prevents atrophy [42]. In addition, Akt/FoxO1, 3/MuRF1 pathway was upregulated in muscle of old mice leading to sarcopenia [43].
FoxO1 suppresses adipogenesis, and FoxO1 haploinsufficiency recovers the numbers and sizes of adipocytes in high-fat diet (HFD)-fed mice [44]. Inhibition of FoxO1 activity ameliorates glucose tolerance and insulin sensitivity and generates energy expenditure under the white adipose tissue of transgenic mice [45]. The inhibition of FoxO1 selectively enhances the expression of PPARγ1 and UCP1 genes that promote oxygen consumption and mitochondrial metabolism in brown adipose tissue. FoxO1 mediates anti-adipogenic actions in response to insulin signaling in the absence of insulin receptor, or insulin receptor substrate, or insulin targeting Akt in cells as well as dysregulated differentiation [46-48]. FoxO1 inhibits apoptosis by cell cycle arrest in the early phase of adipose cell differentiation and terminal differentiation via increased expression of cell cycle inhibitor p21. These observations indicate that FoxO1 demonstrates a dual role in both brown and white adipose tissue.
2.2 The function of Akt/FoxOs axis in non-metabolic organs
Diabetic nephropathy is associated with the inhibition of FoxOs, NADPH oxidases, and antioxidant enzymes, which contribute to its pathology [49, 50]. FoxO1 also suppresses redox stress, inhibits cell death, and regulates TGF-β signaling in keratinocytes. The role of FoxOs in the wound healing process differs significantly in the in vivo diabetic mouse model. FoxO1 exerts beneficial effects in the wound healing process in control mice [51]. FoxO1 modulates wound healing by increasing the expression of TGF-β1 and downstream target genes that are required for keratinocyte migration. In contrast, FoxO1 impairs wound healing in diabetic mice with high levels of oxidative stress. TNFα-driven FoxO1 activity is associated with higher levels of apoptosis and decreased fibroblast proliferation [52]. Moreover, FoxO3 downregulation has also been implicated in the development of hyperplasia in kidney fibroblasts [53]. FoxO1 downregulation increased cellular accumulation of reactive oxygen species (ROS) in response to high levels of glucose in kidney cells [54]. However, increased levels of Beclin-1, Ulk1, Atg4b, Atg9a, and Bnip3 mRNA in the kidney of FoxO3 knockout mice with prolonged occlusion periods have also been demonstrated. The deletion of FoxO3 resulted in a dull autophagy reaction, characterized by lower levels of Atg protein that required for the initiation, nucleation, and elongation of vesicles involved in autophagy [55].
FoxOs are generally activated by relatively small changes in cellular redox levels. These differences are commonly observed in studies using transgenic mice [56]. However, the progression of cellular senescence contributes to aging [57, 58]. As FoxOs are known to be involved in the extension of lifespan, they are expected to reduce senescence. Skurk et al. [59] observed that insulin suppresses cardiac muscle atrophy by Akt-dependent inhibition of FoxO3 in the skeletal muscle. In addition, insulin significantly increases Akt phosphorylation in the lung tissue of lean rats, but not in obese mice, indicating that this tissue does not respond to insulin after 12 weeks of HFD [60]. Additionally, Akt expression and activation in the mouse skin increases with age [61]. Smoking inactivates FoxO3, which accelerates the aging of lung tissue during chronic obstructive pulmonary disease [62]. Cells with depletion of FoxO1 exhibited a change in the usage of metabolic substrates from free fatty acids to glucose, which is associated with decreasing lipid accumulation in the heart. Furthermore, keratinocyte-specific FoxO1 deletion downregulates VEGFA gene expression in mucosal and skin wounds, which leads to decreased proliferation of endothelial cell and angiogenesis, re-epithelialization, and dysfunctional granulation [63]. Also, FoxO6 was decreased in aged skin exhibited an increase in melanogenesis, which promote transcription of antioxidant gene that prevented oxidative stress-induced melanogenesis [64].
As shown in Table 1, the Akt/FoxOs axis was upregulated in metabolically active organs including the muscles, liver, and adipose tissue, which was downregulated in metabolic inactive organs including the lungs, kidney, and skin. Furthermore, our results demonstrated that the Akt/FoxOs axis was upregulated in the liver during aging, while it was downregulated in the kidney and lungs in SD rats in an age-dependent manner (unshown data). We previously reported that Akt-induced FoxO1 phosphorylation was reduced in the livers of aged rats, whereas it was increased in the kidney [65]. Different trends of changes in the Akt/FoxOs axis in metabolic and non-metabolic organs during aging are shown in Table 1 [31, 32, 60-62, 66-73].
Table 1.
Changes in the Akt/FoxOs axis in various organs.
The inhibited Akt activity leads to elevated nuclear activity of FoxOs in metabolic tissue such as the liver and muscle, whereas insulin-mediated Akt activation blunted FoxOs activation in the kidney and lungs during aging. Additionally, reduced growth factor signals activate FoxOs as the Akt-induced FoxOs inhibition axis is disturbed. However, the increase in ROS levels in cells induces the activation of FoxOs via specific mechanisms including the c-Jun N-terminal kinase (JNK) pathway [74] and extends the lifespan via mitohormesis in muscles [75]. Conversely, FoxOs activation modes do not always lead to the activation of the downstream signaling molecules. For example, Evans-Anderson et al. [76] demonstrated that the activation of FoxO4 by ROS and growth factor upregulates the expression of p21 or p27.
FoxO1 inactivation of osteoblasts reduces osteoblast count, bone volume, and the rate of bone formation. In an experimental animal model, the phenotype of osteoblast leading to bone formation in FoxO1-KO mice can be attributed to a suppressed mechanism for antioxidant defense. Elevated ROS activates the p53 signaling pathway leading to cell cycle arrest and limited proliferation of osteoblast cells. N-acetyl cysteine in antioxidative optimal redox levels normalized osteoblast proliferation and bone formation process [77, 78]. The induction of osteoclast formation by parathyroid hormone and IL-1β was followed by an increase in superoxide levels, suggesting that ROS existence is required for osteoclast cell differentiation and resorption of bone in vitro [79]. Both M-CSF and RANKL increase ROS levels and enhance osteoclast formation and activation in osteoclast progenitors [80, 81]. The involvement of redox stress has been indicated in the disease-like bone resorption process associated with estrogen insufficiency [82]. Animal studies with conditional loss or gain of function of FoxOs mutants or mitochondrial catalase in osteoclast cells have demonstrated that FoxOs suppresses the differentiation process in osteoclast cells by stimulating catalase production resulting in the downregulation of H2O2 levels [83]. In addition, PPARα is activated by MHY908-mediated age-related inflammation via modulation of the ROS/Akt/FoxO1 axis in the kidney [84].
In mice and humans, the expression and nuclear localization of FoxO1 and FoxO3 in cartilage decrease at the margins of cartilage exposed to maximum body mass. This may be due to increased secretion of inflammation-inducing cytokines [85]. However, knockdown of FoxO1 and FoxO3 markedly reduced the concentrations of catalase, glutathione peroxidase 1, Sirt1, Beclin-1, and light chain 3 in human articular chondrocytes [86]. The results of this study indicate that aging chondrocyte cells inhibit antioxidants levels and promote susceptibility to cell death associated with ROS [87].
2.3 Changes of phenotypes of Akt/FoxOs axis with respect to organ specificity during aging
FoxO1 plays a role in glucose production by insulin via metabolic pathways. This process primarily takes place in the liver to promote glucose generation from non-carbohydrate substrates such as glycerol, lactate, and amino acids. As a life-sustaining process, glucose production acts as the sole fuel source for the brain, testes, and erythrocytes during a lengthened fasting period or exercise. Gluconeogenesis primarily occurs in the liver, with small amounts in the kidney [8, 88, 89]. Even though the regulatory role of FoxO1 in the gluconeogenesis-associated gene transcription and expression is widely known, its potential regulatory role in hepatic lipid metabolism is not known.
Age has a significant influence on the clinical characteristics of thyroid dysfunction (TD), i.e. hyperthyroidism and hypothyroidism, which cause under-symptoms TD to appear frequently in the elderly [90-93]. As a result, hyperthyroidism or hypothyroidism may be wrongly diagnosed or symptoms may be mistakenly attributed to old age. These results are important for two reasons: i) the prevalence of hyperthyroidism and hypothyroidism increases with age [94, 95], and ii) TD is more likely to generate harmful effects in an aged patient with comorbidities and symptoms including osteoporosis and coronary heart disease [95, 96]. Cardiac aging is characterized by reduced stress tolerance, as the expression of Sur2a, a critical subunit of ATP-sensitive potassium (KATP) channels, decreases with age, resulting in a decrease in the amounts of KATP channels in the sarcolemma between cardiac myocytes [97]. Additionally, cells that express p16Ink4a are key promoters of such characteristics of age-associated cardiac diseases [98].
Chronic kidney disease is associated with energy balance, maximum aerobic exercise, as well as tissue glucose uptake [99, 100]. Overnutrition and obesity induce the expression of proinflammatory molecules, such as IL-1β, TNFα, and IL-12, which are related to diverse metabolic diseases [101]. Recently, our research indicated that the downregulation of the expression of enzymes involved in fatty acid oxidation and anti-inflammatory activity of PPARα results in lipid accumulation and renal fibrosis during aging [102]. Indeed, elevated fatty acid can lead to physiological aging [103].
Recent studies have demonstrated that FoxO4 is elevated in aging cells and sustains cell viability by inhibiting p53-mediated cell death. Suppressed interaction of FoxO4 and p53 transcription factors by the designed peptide FoxO4-DRI not only induced p53-mediated cell apoptosis in senescent cells but also promoted fitness, increased amounts of fur, and kidney function in the chronologically aged mouse (XpdTTD/TTD) model [104]. Skin changes such as epidermal thinning as well as reduced dermal elasticity and subdermal fat increase the occurrence of stress trauma and skin infection [105].
Aging mediates changes in the digestive process, liver, and endocrine systems in different ways. Liver mass decreases approximately 20-40% during aging, with reduced blood flow [106]. Serum albumin may be degraded, but typically does not change over time in liver chemistry [107]. Aged liver exhibits suppressed synthesis of clotting factor synthesized from vitamin K [108]. Metabolism changes affect the longevity of the experimental animal models, and translational targets can be implemented. Aging is characterized by insulin resistance and suppressed levels of circulating insulin-like growth factor [109]. In addition, aging reduces β-cell regeneration in pancreatic islet cells [110]. Metabolomics methods have distinguished potential longevity characteristics, such as the upregulation of circulating citric acid cycle mediators [111]. Several studies have demonstrated triglycerides (TG) as a nutrient of metabolically active organs that regulated immune and inflammatory effects in adipose tissue [112, 113]. However, inflammatory IL-1β activated by inflammasomes [114] induced lipid accumulation via inhibiting PPARα-mediated β-oxidation in the liver [115]. Such insulin resistance conditions stimulate hyperinsulinemia and subsequently activate inflammatory response by inducing Akt signaling pathway in non-metabolic organs such as kidneys during aging (Fig. 1).
Figure 1.
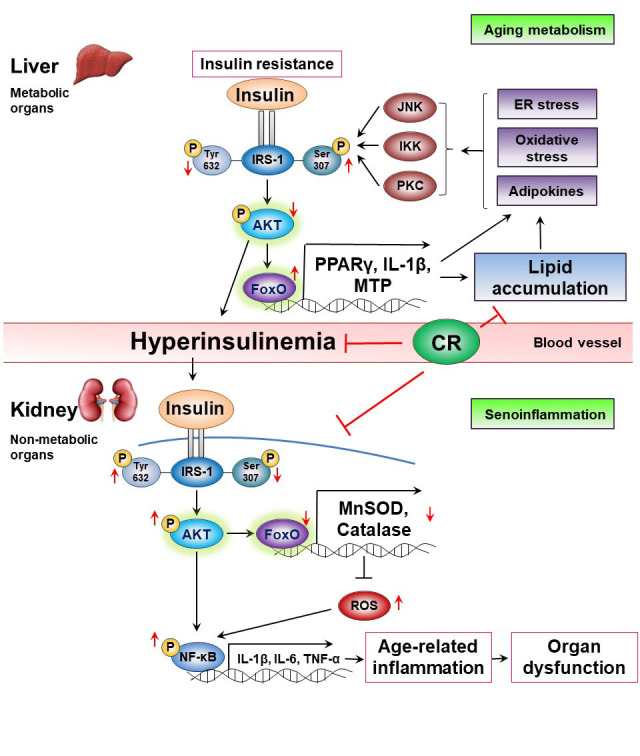
Organ responses based on the Akt/FoxOs axis during aging. Impaired insulin signaling, insulin resistance induces metabolic changes called “aging metabolism” in metabolic organs during aging. The insulin resistance in metabolic organs and tissues such as the liver, muscles, and adipose tissues causes hyperinsulinemia accompanied by Akt inactivation, which increases FoxOs activity (Akt/FoxOs/PPARγ axis upregulation) leading to lipid accumulation. In contrast, the hyperinsulinemia induces Akt activation and inhibits FoxOs activity (Akt/FoxOs/MnSOD axis downregulation) leading to decreases the expression of FoxOs-dependent antioxidant genes such as MnSOD and catalase in non-metabolic organs including the kidneys and the lungs failing to suppress oxidative stress and age-related inflammation. However, CR modulates insulin resistance and hyperinsulinemia, and alleviates age-related inflammation. CR, Calorie restriction; MTP, Microsomal triacylglycerol transfer protein; SOD, Superoxide dismutase; ROS, Reactive oxygen species.
Muscle mass and contractility may be suppressed, and muscle mobility may be limited during aging [116]. Age-associated reduction in muscle mass (sarcopenia) is accompanied by reduced muscle quality, as indicated by the infiltration of fat and connective tissue. Inhibition of MuRF1 and MAFbx function is reduced following the inhibition of muscle loss and subsequent attenuation of the pathology associated with muscular atrophy [117]. MuRF1 and MAFbx are expressed during Akt/FoxOs signaling in aged muscle [43]. The data from the current studies demonstrate aging-induced muscle degradation via elevation of the Akt/FoxOs axis in muscles.
3. Age-related changes and target genes of FoxOs
Drosophila melanogaster and Caenorhabditis elegans model species have been studied extensively in aging research. FoxOs activation mediates the lifespan extension due to reduced insulin/insulin-like growth factor-like signaling in worms, flies, yeast, and mice. This notable evolutionary preservation has also been observed in humans due to the association of specific genetic variations.
Lack of FoxO3 affects lymph proliferation and inflammation in diverse tissues [118] and is also associated with age-associated infertility [45] and decreased number of neural stem cells [119]. Global deletion of FoxO4 exacerbates colitis in response to inflammatory stimuli [120]. The complete deletion of FoxO6 contributes to memory weakening. The correct synaptic number function regulates gene expression and results in sound neural connectivity [121]. FoxOs can accelerate aging via insulin signaling and have been hypothesized to affect longevity by reducing ROS generation and decelerating the extent of redox damage [122]. These findings indicate that FoxOs are involved in the progression of aging and age-associated disorders.
Recent research studies have indicated that FoxOs control a variety of target genes located downstream that are responsible for cell cycle, cell death, and redox stress response [4,8]. One of the major effects of the regulation of FoxOs is phosphorylation by Akt by insulin, growth factors, and its consequential transfer from the nuclear fraction to the cytoplasm [8-10]. FoxO4 appears to suppress cellular oxidative stress levels by directly upregulating gene expression of manganese superoxide dismutase (MnSOD) and catalase in the kidney [123].
Mammalian FoxOs promote hepatic glucose production during the starvation period [9, 124, 125], along with the inhibitory effects of insulin on glucose production in the liver. However, due to the lack of data on FoxOs, little is known about its regulatory role in metabolism or its effect in diabetic conditions. Some studies [126] demonstrated the overexpression of the constitutively active form of FoxO1 in the liver of metabolic organs facilitated the gene expression of lipogenic SREBP-1c and hepatic TG build-up. Furthermore, the regulatory action of FoxO1 was also demonstrated in hepatic lipid metabolism during aging [11]. However, FoxO4 is the critical isoform that acts in FoxOs-mediated transcriptional regulation, which is the ground for skeletal muscle mass reduction in aging diaphragm anabolic syndrome. The Akt activation by the IGF-1 receptor leads to FoxO4 inactivation and the sequential inhibition of the expression of MAFbx/atrogin-1 and MuRF1 genes [127]. Additionally, treatment with β-hydroxy β-methylbutyrate, a metabolite of leucine that modulates muscular atrophy, suppressed dexamethasone-induced muscle wasting by regulating FoxO1 transcription factor and subsequent MuRF1 expression [128].
Available data demonstrate the intricate functions of the FoxOs transcription factors, for instance, under oxidative stress conditions where it not only acts as a positive or negative regulator of cellular function, but also as a cellular modulator of apoptosis, lipogenesis, and inflammation. As summarized in Table 2, accumulated evidence indicates that the FoxOs functions and the expression of their target genes were differential shown according to species and tissues, leading to changes in many physiological phenomena.
Table 2.
Functions of FoxOs and expression of target genes in various organs.
| Organs | Function | Genes |
|---|---|---|
| Pancreatic β-cells | Repression of β-cell proliferation | PDX-1, NGN3, NKX61, CyclinD1 |
| Protection against oxidative stress | MafA, NeuroD | |
| Liver | Increase of gluconeogenesis in mice | G6P, PEPCK, PGC-1a |
| Reduced triglyceride levels in pigs, mice | ApoCIII, MTP | |
| Adipose tissue | Control of differentiation | p21, PPARγ |
| Hypothalamus | Acute orexigenic effect | Agrp, Npy |
| Brain | Protection against neuronal death | Bim, Fas ligand |
| Skeletal muscle | Repression of differentiation | Atrogin-1, MuRF1 |
| Induction of muscle atrophy | MAFbx | |
| Vascular endothelial cells | Regulation of endothelial stability | Ang-2, sprouty2 |
| Smooth muscle cells | Repression of differentiation | Myocardin |
| Kidney | Protection of lipotoxicity and disease | Bcl-2, Bax, MnSOD, Bim |
| Testis | Regulation of apoptosis | HOX genes |
| Heart | Protection of heart against ischemia in mouse | MnSOD, Catalase |
| Inhibition of cardiac mass loss in rat | Autophagy genes | |
| Thymus | Regulation of lymphocyte homeostasis | p27 |
| Control of Treg cell differentiation | FoxP3 | |
| Lung | Regulation of lung tumor in mice | p27 |
| Suppression of lung adenocarcinoma in humans | GADD45 |
4. Modulation of Akt-mediated FoxOs activity by CR for a better understanding of organ based-differential roles of Akt/FoxOs axis
The ROS-mediated regulation of FoxOs may explain its distinct involvement in aging. FoxOs are generally activated by relatively minor alternations in cellular ROS levels and are inactivated with high ROS levels. These changes were clearly observed in transgenic mouse models [56]. Similarly, cellular senescence is a risk factor for aging [57, 58], and activation of FoxOs may extend lifespan through delaying aging process.
Metabolism rates regulate the progression of aging in animals stems from the recognition of the critical link between energy metabolism and homeostasis maintenance. Increased energy expenditure expedites the aging process. It is well established that CR slows down the development of pathologies associated with aging its progression and prolongs lifespan [22, 129-131]. In C. elegans and Drosophila models, CR increases the lifespan independent of FoxOs regulation [132]. Previous studies on CR have demonstrated its efficacy against the aging progression and have identified several major regulatory signaling pathways [23]. Previous epigenetic studies have indicated that chromosomal or gene promoter regions corresponding to DNA methylation and histone modifications exert functional effects on aging [133]. CR epigenetically modulates the aging process [134] by methods such as mediating an increase in genomic stability by reversing age-associated histone acetylation and alterations in DNA methylation [24]. CR upregulates the expression of certain genes that are involved in cell cycle arrest, such as p21, DNA repair (i.e. Gadd45α), apoptosis (i.e. Bim), and the response to redox stress (i.e. MnSOD) in metabolic organs such as the liver. CR promotes FoxO1 binding in the liver in response to glucose-mediated insulin signal activation [135]. However, Edström et al. [136] demonstrated that unlike acute atrophy induced by CR, chronic atrophy caused by diseases, disuse or denervation leads to MAFbx/atrogin-1 and MuRF1 downregulation in the skeletal muscle of 30-month-old rats.
The inhibition of insulin by CR interrupted age-related FoxO6 and FoxO3 reduction by blocking the PI3K/Akt pathway in non-metabolic organs such as the kidney [137, 138]. Subsequently, several studies have supported that CR could slow the aging process in diverse species and regulated several regulatory signaling pathways [23]. Kim et al. [25] reported that FoxO1 was activated and NF-κB was inactivated by CR as observed in aged kidney tissues obtained from ad libitum fed rats and rats subjected to 40% CR.
In a recent study, the activation of Akt under insulin signaling increased, resulting in an increased inactivation of phosphorylated FoxOs, whereas it was increased by CR in metabolic organs. In contrast, the activation of FoxO1 via the inhibition of the PI3K/Akt pathway in non-metabolic organs was suppressed by CR [13, 65, 84, 139]. These results suggest that the Akt/FoxOs axis demonstrates differential actions by organs. This reviews the important findings on the alterations of FoxOs in association with aging and explains its modulation by CR as a potential underlying mechanism affecting the progression of aging.
5. Altered Akt/FoxOs signaling pathway in diabetic conditions and aging tissues
In obese patients, excessive lipids accumulation in tissues other than adipose tissue adds to organ damage via adipogenic toxicity [140]. This toxic process is complex but can be described by the adipose tissue expandability hypothesis [141-143]. When the capacity of adipose tissue storage is exceeded, the lipid flux increases toward the non-adipose tissues, and lipids begin to accumulate in ectopic sites. Ectopic lipid deposits in different cell types, such as myocytes, hepatocytes, and β-cells, initiate adverse physiological effects such as insulin resistance and apoptosis. Recently, studies have demonstrated that the renal deposits and detrimental effects of lipids may lead to kidney pathology [144, 145]. In particular, saturated fatty acids lead to insulin resistance in podocytes that maintain the integrity of the glomerular filtration barrier in the normal kidney in non-metabolic organ [146], and in proximal tubular cells that lead to cellular dysfunction and cell death via apoptosis and necrosis [147].
The association of diabetes with dysfunctional mitochondrial respiratory system in the liver, heart, and kidney of diabetic animals has been demonstrated over 35 years [148-154]. Despite this long history, little is known about mitochondrial dysfunction in diabetics or the mechanisms bridging primary metabolic dysfunction in insulin and blood glucose to metabolic diseases.
Zhang et al. [155] demonstrated genetic and physiological evidence indicating that FoxO1 acts as a crucial transcription factor for the IRE as FoxO1 inactivation reduced the transcription of genes encoding gluconeogenic enzymes (PEPCK and G6pc) and suppressed the blood glucose concentrations in the animal model. In contrast, inactivation of FoxO3 promoted the expression of genes encoding lipogenic enzymes, fatty acid synthase, and hydroxy-3-methylglutaryl-CoA reductase. Simultaneous inactivation of both FoxO1 and FoxO3 synergistically induced the expression of lipogenic enzymes including glucokinase (Gck) and further promoted the serum levels of TG, cholesterol, and lipid secretion and that might result in hepatosteatosis. Recently, Dong’s group highlighted the importance of FoxO6 dysregulation in the dual pathogenesis of fasting hyperglycemia and hyperlipidemia in diabetes [156].
Targeting the hepatic insulin/Akt/FoxO1 signaling pathway could be a strategy in impeding the progression of diabetes mellitus. High glucose-induced cell apoptosis in human kidney 2 (HK-2) cells acts by blocking the ROS-responsive Akt/FoxOs signaling pathway in diabetic nephropathy [157]. Several studies have demonstrated that Akt is associated with the expansion of the glomerular matrix [158], apoptosis in podocytes [159], and metastasis of mature tubular epithelial cells by mediating epithelial-to-mesenchymal transition [160]. The Akt kinase exhibits an anti-apoptotic effect in HK-2 cells via post-translational regulation of different signaling molecules, where FoxO3 acts as a critical downstream transcription factor [161].
Akt-induced phosphorylation of FoxOs family isoforms, namely, FoxO1 and FoxO3, could promote translocation from the nucleus to the cytoplasm, and consequently inhibit the transcription of downstream target genes such as Bim and Fas-Ligand (Fas-L) that are involved in apoptosis [162]. Decreased phosphorylation of Akt and FoxOs family proteins is related to cellular apoptosis in high glucose-treated kidney cells [163, 164]. However, diabetes nephropathy can inhibit the expression of Fas ligand by increasing FoxO3 phosphorylation and transcriptional inactivation via stimulation of the PI3K/Akt pathway [164].
6. Discussion
The potential regulatory roles of FoxOs family isoforms during the aging process and the effects of CR on FoxOs activities provide an interesting insight into the participation of FoxOs in the aging process. Inhibition of FoxO1 affects age-associated insulin resistance and energy metabolism, particularly under normal dietary conditions. However, the pivotal role of FoxOs is observed only under CR conditions.
FoxOs are controlled by a variety of growth factors and paracrine hormones, whose activity is securely regulated by post-translational modifications such as phosphorylation, acetylation, methylation, and ubiquitination, as well as physical interactions with different proteins and transcription factors. Additional studies on the post-translational modifications and protein-protein interactions will help elucidate how the FoxOs family isoforms lead to environmental stimuli that mediate the transcription and expression of specific genes and cellular functions to impede age-associated pathological conditions. This paper reviewed the regulatory roles of FoxOs family members during aging to provide strategic insight into potential intervention strategies for the promotion of health.
Numbers of hypotheses have been suggested for aging in past decades, including the molecular inflammation hypothesis. Such a hypothesis involving molecular insulin resistance and inflammation is suggested based on the activity of key FoxOs and its downstream signaling pathway, which plays an important regulatory role in activating systemic inflammation during the aging process upon the induction of adiposity. Chronic inflammation caused by ectopic fat in obese conditions as well as liver and muscle lipid accumulation further deteriorate the insulin resistance conditions. Chronic inflammation prolongs an insulin resistant state, and the association between chronic inflammation and adiposity likely accelerates the aging process. However, redox stress, ER stress, and age-related metabolites impair insulin signaling via JNK, IKK, and PKC pathways in metabolically active organs, leading to insulin resistance, hyperinsulinemia, and hyperlipidemia through Akt/FoxOs axis upregulation, which we defined as “aging metabolism or senometabolism” meaning metabolic changes in aging process. The insulin resistance initiates the induction of hyperinsulinemia and enhances the expression of proinflammatory genes encoding for factors such as cytokines and chemokines via Akt/FoxOs axis downregulation and NF-κB activation, leading to age-related inflammation (senoinflammation) in non-metabolic organs (Fig. 1). Our previous study confirmed that FoxO6-mediated IL-1β is involved in hepatic inflammation and insulin resistance via TF/PAR2/Akt pathway in aging and diabetic liver [35].
The data presented in this review indicate that insulin resistance, change of Akt/FoxOs axis in metabolic organs such as the liver and muscles during aging leads to aging metabolism such as hyperinsulinemia, hyperlipidemia, and age-related metabolic changes. The hyperinsulinemia induces age-related senoinflammation via insulin-dependent Akt activation leading to organ dysfunction in non-metabolic organs, namely, the kidneys and lungs. Elucidation of the molecular mechanisms in metabolic organs and non-metabolic organs based on the Akt/FoxOs axis and examination of the regulatory role of CR will provide insights for the development of potential anti-aging interventions.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2018R1A2A3075425). This research was also supported by the Basic Science Research Program through the NRF, funded by the Ministry of Education (NRF-2018R1A6A3A11046180). This work was supported by a 2-year Research Grant of Pusan National University. We thank professor Jeong-Hyun Yoon in Pusan National University for the careful review of manuscript.
Footnotes
Conflict of interest
The authors have no conflicts of interests.
References
- [1].Tang P, Low HB, Png CW, Torta F, Kumar JK, Lim HY, et al. (2019). Protective Function of Mitogen-Activated Protein Kinase Phosphatase 5 in Aging- and Diet-Induced Hepatic Steatosis and Steatohepatitis. Hepatol Commun, 3:748-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krasilnikov MA (2000). Phosphatidylinositol-3 kinase dependent pathways: The role in control of cell growth, survival, and malignant transformation. Biochemistry, 65:59-67. [PubMed] [Google Scholar]
- [3].Jeong H, Liu Y, Kim HS (2017). Dried plum and chokeberry ameliorate d-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Exp Gerontol, 95:16-25. [DOI] [PubMed] [Google Scholar]
- [4].van der Heide LP, Hoekman MFM, Smidt MP (2004). The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J, 380:297-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Spurthi KM, Sarikhani M, Mishra S, Desingu PA, Yadav S, Rao S, et al. (2018). Toll-like receptor 2 deficiency hyperactivates the FoxO1 transcription factor and induces aging-associated cardiac dysfunction in mice. J Biol Chem, 293:13073-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N (2002). Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech, 59:331-334. [DOI] [PubMed] [Google Scholar]
- [7].Gao HE, Wu DS, Sun L, Yang LD, Qiao YB, Ma S, et al. (2020). Effects of lifelong exercise on age-related body composition, oxidative stress, inflammatory cytokines, and skeletal muscle proteome in rats. Mech Ageing Dev, 189:111262-111275. [DOI] [PubMed] [Google Scholar]
- [8].Accili D, Arden KC (2004). FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell, 117:421-426. [DOI] [PubMed] [Google Scholar]
- [9].Barthel A, Schmoll D, Unterman TG (2005). FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab, 16:183-189. [DOI] [PubMed] [Google Scholar]
- [10].Biggs WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999). Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA, 96:7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim DH, Ha S, Choi YJ, Dong HH, Yu BP, Chung HY (2019). Altered FoxO1 and PPARγ interaction in age-related ER stress-induced hepatic steatosis. Aging (Albany NY), 11:4125-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lim K, Halim A, Lu TS, Ashworth A, Chong I (2019). Klotho: A Major Shareholder in Vascular Aging Enterprises. Int J Mol Sci, 20:4637-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Park MH, Kim DH, Lee EK, Kim ND, Im DS, Lee J, et al. (2014). Age-related inflammation and insulin resistance: a review of their intricate interdependency. Arch Pharm Res, 37:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A (2006). Opposing activities protect against age-onset proteotoxicity. Science, 313:1604-1610. [DOI] [PubMed] [Google Scholar]
- [15].Chang ZS, Xia JB, Wu HY, Peng WT, Jiang FQ, Li J, et al. (2019). Forkhead box O3 protects the heart against paraquat-induced aging-associated phenotypes by upregulating the expression of antioxidant enzymes. Aging Cell, 18:e12990-e13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morley JF, Brignull HR, Weyers JJ, Morimoto RI (2002). The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA, 99:10417-10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jiramongkol Y, Lam EW (2020). FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev, 39:681-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Galgani JE, Uauy RD, Aguirre CA, Diaz EO (2008). Effect of the dietary fat quality on insulin sensitivity. Br J Nutr, 100:471-479. [DOI] [PubMed] [Google Scholar]
- [19].Feng Z, Cao J, Zhang Q, Lin L (2020). The drug likeness analysis of anti-inflammatory clerodane diterpenoids. Chin Med, 15:126-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fuster JJ, Zuriaga MA, Zorita V, MacLauchlan S, Polackal MN, Viana-Huete V, et al. (2020). TET2-Loss-of-Function-Driven Clonal Hematopoiesis Exacerbates Experimental Insulin Resistance in Aging and Obesity. Cell Rep, 33:108326-108349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Park MH, Kim DH, Kim MJ, Lee EK, An HJ, Jeong JW, et al. (2016). Effects of MHY908, a New Synthetic PPARα/γ Dual Agonist, on Inflammatory Responses and Insulin Resistance in Aged Rats. J Gerontol A Biol Sci Med Sci, 71:300-309. [DOI] [PubMed] [Google Scholar]
- [22].Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang S, et al. (2017). Caloric restriction delays age-related methylation drift. Nat Commun, 8:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fontana L, Partridge L, Longo VD (2010). Extending healthy life span-from yeast to humans. Science, 328:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vaquero A, Reinberg D (2009). Calorie restriction and the exercise of chromatin. Genes Dev, 23:1849-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim DH, Kim JY, Yu BP, Chung HY (2008). The activation of NF-κB through Akt-induced FoxO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology, 9:33-47. [DOI] [PubMed] [Google Scholar]
- [26].Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, et al. (2015). The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell, 14:707-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. (2007). Endocrine regulation of energy metabolism by the skeleton. Cell, 130:456-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu J, Xie X, Yan D, Wang Y, Yuan H, Cai Y, et al. (2020). Up-regulation of FoxO1 contributes to adverse vascular remodelling in type 1 diabetic rats. J Cell Mol Med, 24:13727-13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Russell SJ, Schneider MF (2020). Alternative signaling pathways from IGF1 or insulin to AKT activation and FOXO1 nuclear efflux in adult skeletal muscle fibers. J Biol Chem, 295:15292-15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bastie CC, Nahle Z, McLoughlin T, Esser K, Zhang W, Unterman T, Abumrad NA (2005). FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem, 280:14222-14229. [DOI] [PubMed] [Google Scholar]
- [31].Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, et al. (2012). Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat Med, 18:388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang H, Yan P, Sun T, Mo X, Yin J, Li P, et al. (2018). Procyanidins Extracted from Lotus Seedpod Ameliorate Amyloid-β-Induced Toxicity in Rat Pheochromocytoma Cells. Oxid Med Cell Longev, 2018:4572893-4572906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sato T, Watanabe Y, Nishimura Y, Inoue M, Morita A, Miura S (2019). Acute fructose intake suppresses fasting-induced hepatic gluconeogenesis through the AKT-FoxO1 pathway. Biochem Biophys Rep, 18:100638-100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheng Z (2015). FoxO1: mute for a tuned metabolism? Trends Endocrinol Metab, 26:402-403. [DOI] [PubMed] [Google Scholar]
- [35].Kim DH, Lee B, Lee J, Kim ME, Lee JS, Chung JH, et al. (2019). FoxO6-mediated IL-1β induces hepatic insulin resistance and age-related inflammation via the TF/PAR2 pathway in aging and diabetic mice. Redox Biol, 24:101184-101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Makino N, Oyama J, Maeda T, Koyanagi M, Higuchi Y, Shimokawa I, et al. (2016). FoxO1 signaling plays a pivotal role in the cardiac telomere biology responses to calorie restriction. Mol Cell Biochem, 412:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan H, Yang W, Zhou F, Li X, Pan Q, Shen Z, et al. (2019). Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes, 68:291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hosaka T, Biggs WH III, Tieu D, Boyer AD, Varki NM, Cavenee WK, et al. (2004). Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA, 101:2975-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu P, Geles KG, Paik JH, DePinho RA, Tjian R (2008). Codependent activators direct myoblast-specific MyoD transcription. Dev Cell, 15:534-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Son YH, Jang EJ, Kim YW, Lee JH (2017). Sulforaphane prevents dexamethasone-induced muscle atrophy via regulation of the Akt/Foxo1 axis in C2C12 myotubes. Biomed Pharmacother, 95:1486-1492. [DOI] [PubMed] [Google Scholar]
- [41].Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, et al. (2007). Induction of Mxi1-SR(alpha) by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol, 27:4917-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang J, Fry CME, Walker CL (2019). Carboxyl-terminal modulator protein regulates Akt signaling during skeletal muscle atrophy in vitro and a mouse model of amyotrophic lateral sclerosis. Sci Rep, 9:3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wagatsuma A, Shiozuka M, Takayama Y, Hoshino T, Mabuchi K, Matsuda R (2016). Effects of ageing on expression of the muscle-specific E3 ubiquitin ligases and Akt-dependent regulation of Foxo transcription factors in skeletal muscle. Mol Cell Biochem, 412:59-72. [DOI] [PubMed] [Google Scholar]
- [44].Nakae J, Kitamura T, Kitamura Y, Biggs WHIII, Arden KC, Accili D (2003). The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell, 4:119-129. [DOI] [PubMed] [Google Scholar]
- [45].Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, et al. (2008). Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes, 57:563-576. [DOI] [PubMed] [Google Scholar]
- [46].Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, et al. (2008). Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes, 57:563-576. [DOI] [PubMed] [Google Scholar]
- [47].Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, Kubota N, et al. (2001). Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol, 21:2521-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR (2004). Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol, 24:1918-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].ALTamimi JZ, AlFaris NA, Al-Farga AM, Alshammari GM, BinMowyna MN, Yahya MA (2021). Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p 66 Shc axis and activation of FOXO-3a. J Nutr Biochem, 87:108515. [DOI] [PubMed] [Google Scholar]
- [50].Lu Q, Zhai Y, Cheng Q, Liu Y, Gao X, Zhang T, et al. (2013). The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp Physiol, 98:934-945. [DOI] [PubMed] [Google Scholar]
- [51].Ponugoti B, Xu F, Zhang C, Tian C, Pacios S, Graves DT (2013). FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J Cell Biol, 203:327-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ponugoti B, Dong G, Graves DT (2012). Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res, 2012:939751-939757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bijkerk R, de Bruin RG, van Solingen C, van Gils JM, Duijs JM, van der Veer EP, et al. (2016). Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int, 89:1268-1280. [DOI] [PubMed] [Google Scholar]
- [54].Ji L, Wang Q, Huang F, An T, Guo F, Zhao Y, et al. (2019). FOXO1 Overexpression Attenuates Tubulointerstitial Fibrosis and Apoptosis in Diabetic Kidneys by Ameliorating Oxidative Injury via TXNIP-TRX. Oxid Med Cell Longev, 2019:3286928-3286941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Li L, Zviti R, Ha C, Wang ZV, Hill JA, Lin F (2017). Forkhead box O3 (FoxO3) regulates kidney tubular autophagy following urinary tract obstruction. J Biol Chem, 292:13774-13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yalcin S, Marinkovic D, Mungamuri SK, Zhang X, Tong W, Sellers R, et al. (2010). ROS-mediated amplification of Akt/mTOR signaling pathway leads to myeloproliferative syndrome in Foxo3-/- mice. EMBO J, 29:4118-4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nowaczyk M, Malcher A, Zimna A, Łabędź W, Kubaszewski Ł, Fiedorowicz K, et al. (2020). Transient and Stable Overexpression of Extracellular Superoxide Dismutase Is Positively Associated with the Myogenic Function of Human Skeletal Muscle-Derived Stem/Progenitor Cells. Antioxidants (Basel), 9:817-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhu S, Tian Z, Torigoe D, Zhao J, Xie P, Sugizaki T, et al. (2019). Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS One, 14:e0221366-e0221383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, et al. (2005). The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem, 280:20814-20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].André DM, Calixto MC, Sollon C, Alexandre EC, Tavares EBG, Naime ACA, et al. (2017). High-fat diet-induced obesity impairs insulin signaling in lungs of allergen-challenged mice: Improvement by resveratrol. Sci Rep, 7:17296-17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chen H, Wang X, Han J, Fan Z, Sadia S, Zhang R, et al. (2017). AKT and Its Related Molecular Feature in Aged Mice Skin. PLoS One, 12:e0178969-e0178980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yuan YM, Luo L, Guo Z, Yang M, Lin YF, Luo C (2015). Smoking, aging, and expression of proteins related to the FOXO3 signaling pathway in lung tissues. Genet Mol Res, 14:8547-8554. [DOI] [PubMed] [Google Scholar]
- [63].Jeon HH, Yu Q, Lu Y, Spencer E, Lu C, Milovanova T, et al. (2018). FOXO1 regulates VEGFA expression and promotes angiogenesis in healing wounds. J Pathol, 245:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Moon KM, Lee B, Kim DH, Chung HY (2020). FoxO6 inhibits melanogenesis partly by elevating intracellular antioxidant capacity. Redox Biol, 36:101624-101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Park MH, Kim DH, Kim MJ, Lee EK, An HJ, Jeong JW, et al. (2016). Effects of MHY908, a New Synthetic PPARα/γ Dual Agonist, on Inflammatory Responses and Insulin Resistance in Aged Rats. J Gerontol A Biol Sci Med Sci, 71:300-309. [DOI] [PubMed] [Google Scholar]
- [66].Smith U (2002). Impaired (’diabetic’) insulin signaling and action occur in fat cells long before glucose intolerance--is insulin resistance initiated in the adipose tissue? Int J Obes Relat Metab Disord, 26:897-904. [DOI] [PubMed] [Google Scholar]
- [67].Ejaz A, Mitterberger MC, Lu Z, Mattesich M, Zwierzina ME, Hörl S, et al. (2016). Weight Loss Upregulates the Small GTPase DIRAS3 in Human White Adipose Progenitor Cells, Which Negatively Regulates Adipogenesis and Activates Autophagy via Akt-mTOR Inhibition. EbioMedicine, 6:149-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Calabuig-Navarro V, Yamauchi J, Lee S, Zhang T, Liu YZ, Sadlek K, et al. (2015). Forkhead Box O6 (FoxO6) Depletion Attenuates Hepatic Gluconeogenesis and Protects against Fat-induced Glucose Disorder in Mice. J Biol Chem, 290:15581-15594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Huang JP, Hsu SC, Li DE, Chen KH, Kuo CY, Hung LM (2018). Resveratrol Mitigates High-Fat Diet-Induced Vascular Dysfunction by Activating the Akt/eNOS/NO and Sirt1/ER Pathway. J Cardiovasc Pharmacol, 72:231-241. [DOI] [PubMed] [Google Scholar]
- [70].Li F, Li X, Peng X, Sun L, Jia S, Wang P, et al. (2017). Ginsenoside Rg1 prevents starvation-induced muscle protein degradation via regulation of AKT/mTOR/FoxO signaling in C2C12 myotubes. Exp Ther Med, 14:1241-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sun Y, Dai S, Tao J, Li Y, He Z, Liu Q, et al. (2020). Taurine suppresses ROS-dependent autophagy via activating Akt/mTOR signaling pathway in calcium oxalate crystals-induced renal tubular epithelial cell injury. Aging (Albany NY), 12:17353-17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chen W, Zhang L, Zhou ZQ, Ren YQ, Sun LN, Man YL, et al. (2018). Effects of Long Non-Coding RNA LINC00963 on Renal Interstitial Fibrosis and Oxidative Stress of Rats with Chronic Renal Failure via the Foxo Signaling Pathway. Cell Physiol Biochem, 46:815-828. [DOI] [PubMed] [Google Scholar]
- [73].Tsitsipatis D, Klotz LO, Steinbrenner H (2017). Multifaceted functions of the forkhead box transcription factors FoxO1 and FoxO3 in skin. Biochim Biophys Acta Gen Subj, 1861:1057-1064. [DOI] [PubMed] [Google Scholar]
- [74].Yang Y, Yu S, Liu N, Xu H, Gong Y, Wu Y, et al. (2018). Transcription Factor FOXO3a Is a Negative Regulator of Cytotoxicity of Fusarium mycotoxin in GES-1 Cells. Toxicol Sci, 166:370-381. [DOI] [PubMed] [Google Scholar]
- [75].Owusu-Ansah E, Song W, Perrimon N (2013). Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell, 155:699-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Evans-Anderson HJ, Alfieri CM, Yutzey KE (2008). Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ Res, 102:686-694. [DOI] [PubMed] [Google Scholar]
- [77].Yao H, Yao Z, Zhang S, Zhang W, Zhou W (2018). Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Mol Med Rep, 17:6681-6690. [DOI] [PubMed] [Google Scholar]
- [78].Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. (2010). FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab, 11:147-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990). Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest, 85:632-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhou L, Song H, Zhang Y, Ren Z, Li M, Fu Q (2020). Polyphyllin VII attenuated RANKL-induced osteoclast differentiation via inhibiting of TRAF6/c-Src/PI3K pathway and ROS production. BMC Musculoskelet Disord, 21:112-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].An J, Hao D, Zhang Q, Chen B, Zhang R, Wang Y, et al. (2016). Natural products for treatment of bone erosive diseases: The effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol, 36:118-131. [DOI] [PubMed] [Google Scholar]
- [82].Manolagas SC (2010). From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev, 31:266-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Serra Ucer S, et al. (2014). FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun, 5:3773-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kim YR, Lee EK, Kim DH, Kim KM, Lee B, An HJ, et al. (2017). PPARα activation by MHY908 attenuates age-related renal inflammation through modulation of the ROS/Akt/FoxO1 pathway. Exp Gerontol, 92:87-95. [DOI] [PubMed] [Google Scholar]
- [85].Akasaki Y, Hasegawa A, Saito M, Asahara H, Iwamoto Y, Lotz MK (2014). Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthritis Cartilage, 22:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Akasaki Y, Alvarez-Garcia O, Saito M, Carames B, Iwamoto Y, Lotz MK (2014). FoxO transcription factors support oxidative stress resistance in human chondrocytes. Arthritis Rheumatol, 66:3349-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bolduc JA, Collins JA, Loeser RF (2019). Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med, 132:73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Aljaylani A, Fluitt M, Piselli A, Shepard BD, Tiwari S, Ecelbarger CM (2020). Acid Loading Unmasks Glucose Homeostatic Instability in Proximal-Tubule-Targeted Insulin/Insulin-Like-Growth-Factor-1 Receptor Dual Knockout Mice. Cell Physiol Biochem, 54:682-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang W, Bu SY, Mashek MT, O-Sullivan I, Sibai Z, Khan SA, et al. (2016). Integrated Regulation of Hepatic Lipid and Glucose Metabolism by Adipose Triacylglycerol Lipase and FoxO Proteins. Cell Rep, 15:349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wildisen L, Del Giovane C, Moutzouri E, Beglinger S, Syrogiannouli L, Collet TH, et al. (2020). An individual participant data analysis of prospective cohort studies on the association between subclinical thyroid dysfunction and depressive symptoms. Sci Rep, 10:19111-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Alvarado-Esquivel C, Ramos-Nevarez A, Guido-Arreola CA, Cerrillo-Soto SM, Pérez-Álamos AR, Estrada-Martínez S, et al. (2019). Association between Toxoplasma gondii infection and thyroid dysfunction: a case-control seroprevalence study. BMC Infect Dis, 19:826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goichot B, Caron P, Landron F, Bouée S (2016). Clinical presentation of hyperthyroidism in a large representative sample of outpatients in France: relationships with age, aetiology and hormonal parameters. Clin endocrinol (Oxf), 84:445-451. [DOI] [PubMed] [Google Scholar]
- [93].Laurberg P, Andersen S, Pedersen I (2005). Hypothyroidism in the Elderly: Pathophysiology, Diagnosis and Treatment. Drugs Aging, 22:23-38. [DOI] [PubMed] [Google Scholar]
- [94].Waring AC, Arnold AM, Newman AB, Buzkova P, Hirsch C, Cappola AR (2012). Longitudinal Changes in Thyroid Function in the Oldest Old and Survival: The Cardiovascular Health Study All-Stars Study. J Clin Endocrinol Metab, 97:3944-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Biondi B, Cooper DS (2008). The clinical significance of subclinical thyroid dysfunction. Endocr Rev, 29:76-131. [DOI] [PubMed] [Google Scholar]
- [96].Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA (2001). Prediction of all cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet, 358:861-865. [DOI] [PubMed] [Google Scholar]
- [97].Sudhir R, Sukhodub A, Du Q, Jovanovic S, Jovanovic A (2011). Ageing-induced decline in physical endurance in mice is associated with decrease in cardiac SUR2A and increase in cardiac susceptibility to metabolic stress: therapeutic prospects for up-regulation of SUR2A. Biogerontology, 12:147-155. [DOI] [PubMed] [Google Scholar]
- [98].Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. (2016). Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature, 530:184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cao W, Shi M, Wu L, Yang Z, Yang X, Liu H, et al. (2018). A renal-cerebral-peripheral sympathetic reflex mediates insulin resistance in chronic kidney disease. EBioMedicine, 37:281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Franssen FM, Akkermans MA, Janssen PP, van Hooff JP (2005). Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am J Transplant, 5:1957-1965. [DOI] [PubMed] [Google Scholar]
- [101].Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B (2018). Macrophage polarization and meta-inflammation. Transl Res, 191:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Chung KW, Lee EK, Lee MK, Oh GT, Yu BP, Chung HY (2018). Impairment of PPARα and the Fatty Acid Oxidation Pathway Aggravates Renal Fibrosis during Aging. J Am Soc Nephrol, 29:1223-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL (2011). Aging and regional differences in fat cell progenitors - a mini-review. Gerontology, 57:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Baar MP, Brandt RM, Putavet DA, Klein JD, Derks KW, Bourgeois BR, et al. (2017). Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell, 169:132-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Elewa RM, Abdallah MA, Zouboulis CC (2015). Age-associated skin changes in innate immunity markers reflect a complex interaction between aging mechanisms in the sebaceous gland. J Dermatol, 42:467-476. [DOI] [PubMed] [Google Scholar]
- [106].Ponticelli C, Sala G, Glassock RJ (2015). Drug management in the elderly adult with chronic kidney disease: a review for the primary care physician. Mayo Clin Proc, 90:633-645. [DOI] [PubMed] [Google Scholar]
- [107].Rahmioglu N, Andrew T, Cherkas L, Surdulescu G, Swaminathan R, Spector T, et al. (2009). Epidemiology and genetic epidemiology of the liver function test proteins. PLoS One, 4:e4435-e4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Froom P, Miron E, Barak M (2003). Oral anticoagulants in the elderly. Br J Haematol, 120:526-528. [DOI] [PubMed] [Google Scholar]
- [109].Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, et al. (2014). Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell, 13:769-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sartori DJ, Wilbur CJ, Long SY, Rankin MM, Li C, Bradfield JP, et al. (2014). GATA factors promote ER integrity and β-cell survival and contribute to type 1 diabetes risk. Mol Endocrinol, 28:28-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cheng S, Larson MG, McCabe EL, Murabito JM, Rhee EP, Ho JE, et al. (2015). Distinct metabolomic signatures are associated with longevity in humans. Nat Commun, 6:6791-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Borges MD, Franca EL, Fujimori M, Silva SMC, de Marchi PGF, Deluque AL, et al. (2018). Relationship between Proinflammatory Cytokines/Chemokines and Adipokines in Serum of Young Adults with Obesity. Endocr Metab Immune Disord Drug Targets, 18:260-267. [DOI] [PubMed] [Google Scholar]
- [113].Ertunc ME, Hotamisligil GS (2016). Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. J Lipid Res, 57:2099-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chung KW, Lee EK, Kim DH, An HJ, Kim ND, Im DS, et al. (2015). Age-related sensitivity to endotoxin-induced liver inflammation: Implication of inflammasome/IL-1β for steatohepatitis. Aging Cell, 14:524-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, et al. (2019). Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis, 10:367-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. (2020). Sarcopenia - Molecular mechanisms and open questions. Ageing Res Rev, 65:101200-101216. [DOI] [PubMed] [Google Scholar]
- [117].Delbono O (2011). Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci, 4:248-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lin L, Hron JD, Peng SL (2004). Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity, 21:203-213. [DOI] [PubMed] [Google Scholar]
- [119].Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell, 5:527-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhu M, Goetsch SC, Wang Z, Luo R, Hill JA, Schneider J, et al. (2015). FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circ Res, 117:967-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Salih DA, Rashid AJ, Colas D, de la Torre-Ubieta L, Zhu RP, Morgan AA, et al. (2012). FoxO6 regulates memory consolidation and synaptic function. Genes Dev, 26:2780-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Zhang XS, Wang T, Lin XW, Denlinger DL, Xu WH (2017). Reactive oxygen species extend insect life span using components of the insulin-signaling pathway. Proc Natl Acad Sci USA, 114:E7832-E7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Balaban R, Nemoto S, Finkel T (2005). Mitochondria, oxidants, and aging. Cell, 120:483-495. [DOI] [PubMed] [Google Scholar]
- [124].Langlet F, Haeusler RA, Lindén D, Ericson E, Norris T, Johansson A, et al. (2017). Selective Inhibition of FOXO1 Activator/Repressor Balance Modulates Hepatic Glucose Handling. Cell, 171:824-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, et al. (2011). FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes, 60:2763-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bose SK, Kim H, Meyer K, Wolins N, Davidson NO, Ray R (2014). Forkhead box transcription factor regulation and lipid accumulation by hepatitis C virus. J Virol, 88:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Sanchez AM, Candau RB, Bernardi H (2014). FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci, 71:1657-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Noh KK, Chung KW, Choi YJ, Park MH, Jang EJ, Park CH, et al. (2014). β-Hydroxy β-methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS One, 9:e102947-e102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Martel J, Chang SH, Wu CY, Peng HH, Hwang TL, Ko YF, et al. (2020). Recent advances in the field of caloric restriction mimetics and anti-aging molecules. Ageing Res Rev, 18:101240-101245. [DOI] [PubMed] [Google Scholar]
- [130].Jiang Y, Yan F, Feng Z, Lazarovici P, Zheng W (2019). Signaling Network of Forkhead Family of Transcription Factors (FOXO) in Dietary Restriction. Cells, 9:100-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Erbaba B, Arslan-Ergul A, Adams MM (2020). Effects of caloric restriction on the antagonistic and integrative hallmarks of aging. Ageing Res Rev, 466:101228-101236. [DOI] [PubMed] [Google Scholar]
- [132].Ostojic I, Boll W, Waterson MJ, Chan T, Chandra R, Pletcher SD, et al. (2014). Positive and negative gustatory inputs affect Drosophila lifespan partly in parallel to dFOXO signaling. Proc Natl Acad Sci USA, 111:8143-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mahmood W, Erichsen L, Ott P, Schulz WA, Fischer JC, Arauzo-Bravo MJ, et al. (2020). Aging-associated distinctive DNA methylation changes of LINE-1 retrotransposons in pure cell-free DNA from human blood. Sci Rep, 10:22127-22138. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [134].Evans LW, Stratton MS, Ferguson BS (2020). Dietary natural products as epigenetic modifiers in aging-associated inflammation and disease. Nat Prod Rep, 37:653-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hayashi H, Yamaza H, Komatsu T, Park S, Chiba T, Higami Y, et al. (2008). Calorie restriction minimizes activation of insulin signaling in response to glucose: potential involvement of the growth hormone-insulin-like growth factor 1 axis. Exp Gerontol, 43:827-832. [DOI] [PubMed] [Google Scholar]
- [136].Edstrom E, Altun M, Hagglund M, Ulfhake B (2006). Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J Gerontol A Biol Sci Med Sci, 61:663-674. [DOI] [PubMed] [Google Scholar]
- [137].Kim DH, Park MH, Chung KW, Kim MJ, Jung YR, Bae HR, et al. (2014). The essential role of FoxO6 phosphorylation in aging and calorie restriction. Age (Dordr), 36:9679-9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. (2013). Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science, 339:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Kim DH, Park MH, Lee EK, Choi YJ, Chung KW, Moon KM, et al. (2015). The roles of FoxOs in modulation of aging by calorie restriction. Biogerontology, 16:1-14. [DOI] [PubMed] [Google Scholar]
- [140].Meex RCR, Blaak EE, van Loon LJC (2019). Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev, 20:1205-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Livshits G, Kalinkovich A (2019). Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev, 56:100980-100998. [DOI] [PubMed] [Google Scholar]
- [142].Warfel JD, Bermudez EM, Mendoza TM, Ghosh S, Zhang J, Elks CM, et al. (2016). Mitochondrial fat oxidation is essential for lipid-induced inflammation in skeletal muscle in mice. Sci Rep, 6:37941-37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Raja Gopal Reddy M, Mahesh M, Manne M, Putcha UK, Jeyakumar SM (2019). Vitamin A and its metabolic pathway play a determinant role in high-fructose-induced triglyceride accumulation of the visceral adipose depot of male Wistar rats. Cell Biochem Funct, 37:578-590. [DOI] [PubMed] [Google Scholar]
- [144].Mehmood R, Sheikh N, Khawar MB, Abbasi MH, Tayyeb A, Ashfaq I, et al. (2020). High-Fat Diet Induced Hedgehog Signaling Modifications during Chronic Kidney Damage. Biomed Res Int, 2020:8073926-8073934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Inacio MD, Costa MC, Lima TFO, Figueiredo ID, Motta BP, Spolidorio LC, et al. (2020). Pentoxifylline mitigates renal glycoxidative stress in obese mice by inhibiting AGE/RAGE signaling and increasing glyoxalase levels. Life Sci, 258:118196-118209. [DOI] [PubMed] [Google Scholar]
- [146].Zhou H, Urso CJ, Jadeja V (2020). Saturated Fatty Acids in Obesity-Associated Inflammation. J Inflamm Res, 13:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Cobbs A, Chen X, Zhang Y, George J, Huang MB, Bond V, et al. (2019). Saturated fatty acid stimulates production of extracellular vesicles by renal tubular epithelial cells. Mol Cell Biochem, 458:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Hall JC, Sordahl LA, Stefko PL (1960). The effect of insulin on oxidative phosphorylation in normal and diabetic mitochondria. J Biol Chem, 235:1536-1539. [PubMed] [Google Scholar]
- [149].Mokhtar N, Lavoie JP, Rousseau-Migneron S, Nadeau A (1993). Physical training reverses defect in mitochondrial energy production in heart of chronically diabetic rats. Diabetes, 42:682-687. [DOI] [PubMed] [Google Scholar]
- [150].Tanaka Y, Konno N, Kako KJ (1992). Mitochondrial dysfunction observed in situ in cardiomyocytes of rats in experimental diabetes. Cardiovasc Res, 26:409-414. [DOI] [PubMed] [Google Scholar]
- [151].Brignone JA, de Brignone CMC, de Mignone IR, Ricci CR, Susemihl MC, Rodriguez RR (1991). Improving effects obtained by the ovariectomy or treatment with tamoxifen of female diabetic rats over the function and enzyme activities of liver mitochondria. Horm Metab Res, 23:51-61. [DOI] [PubMed] [Google Scholar]
- [152].Bedetti CD, Montero GA, Stoppani AOM (1987). Effect of diabetes, adrenalectomy, and hypophysectomy on D-3-hydroxybutyrate dehydrogenase activity and substrate oxidation by rat mitochondria. Biochem Int, 14:45-54. [PubMed] [Google Scholar]
- [153].Churchill P, McIntyre JO, Vidal JC, Fleischer S (1983). Basis for decreased D-beta-hydroxybutyrate dehydrogenase activity in liver mitochondria from diabetic rats. Arch Biochem Biophys, 224:659-670. [DOI] [PubMed] [Google Scholar]
- [154].Rinehart RW, Roberson J, Beattie DS (1982). The effect of diabetes on protein synthesis and the respiratory chain of rat skeletal muscle and kidney mitochondria. Arch Biochem Biophys, 213:341-352. [DOI] [PubMed] [Google Scholar]
- [155].Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, et al. (2012). Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology, 153:631-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Lee S, Dong HH (2017). FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol, 233:R67-R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Jiao X, Li Y, Zhang T, Liu M, Chi Y (2016). Role of Sirtuin3 in high glucose-induced apoptosis in renal tubular epithelial cells. Biochem Biophys Res Commun, 480:387-393. [DOI] [PubMed] [Google Scholar]
- [158].Chen Z, Yuan Y, Zou X, Hong M, Zhao M, Zhao Y, et al. (2017). Radix Puerariae and Fructus Crataegi mixture inhibits renal injury in type 2 diabetes via decreasing of AKT/PI3K. BMC Complement Altern Med, 17:454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Takeshita A, Yasuma T, Nishihama K, D’Alessandro-Gabazza CN, Toda M, Totoki T, et al. (2020). Thrombomodulin ameliorates transforming growth factor-β1-mediated chronic kidney disease via the G-protein coupled receptor 15/Akt signal pathway. Kidney Int, 98:1179-1192. [DOI] [PubMed] [Google Scholar]
- [160].Xing L, Liu Q, Fu S, Li S, Yang L, Liu S, et al. (2015). PTEN inhibits high glucose-induced phenotypic transition in podocytes. J Cell Biochem, 116:1776-1784. [DOI] [PubMed] [Google Scholar]
- [161].Wang Z, Li Y, Wang Y, Zhao K, Chi Y, Wang B (2019). Pyrroloquinoline quinine protects HK-2?cells against high glucose-induced oxidative stress and apoptosis through Sirt3 and PI3K/Akt/FoxO3a signaling pathway. Biochem Biophys Res Commun, 508:398-404. [DOI] [PubMed] [Google Scholar]
- [162].Gorgani-Firuzjaee S, Adeli K, Meshkani R (2015). Inhibition of SH2-domain-containing inositol 5-phosphatase (SHIP2) ameliorates palmitate induced-apoptosis through regulating Akt/FOXO1 pathway and ROS production in HepG2 cells. Biochem Biophys Res Commun, 464:441-446. [DOI] [PubMed] [Google Scholar]
- [163].Katsoulieris EN, Drossopoulou GI, Kotsopoulou ES, Vlahakos DV, Lianos EA, Tsilibary EC (2016). High glucose impairs insulin signaling in the glomerulus: an in vitro and ex vivo approach. PLoS One, 11:e0158873-e0158890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Hong YA, Lim JH, Kim MY, Kim Y, Park HS, Kim HW, et al. (2018). Extracellular Superoxide Dismutase Attenuates Renal Oxidative Stress Through the Activation of Adenosine Monophosphate-Activated Protein Kinase in Diabetic Nephropathy. Antioxid Redox Signal, 28:1543-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]


