Abstract
Cell physiology relies on metalloenzymes and can be easily disrupted by imbalances in metal ion pools. Bacillus subtilis requires manganese for growth and has highly regulated mechanisms for import and efflux that help maintain homeostasis. Cells defective for manganese (Mn) efflux are highly sensitive to intoxication, but the processes impaired by Mn excess are often unknown. Here, we employed a forward genetics approach to identify pathways affected by manganese intoxication. Our results highlight a central role for the membrane-localized electron transport chain (ETC) in metal intoxication during aerobic growth. In the presence of elevated manganese there is an increased generation of reactive radical species associated with dysfunction of the major terminal oxidase, the cytochrome aa3 heme-copper menaquinol oxidase (QoxABCD). Intoxication is suppressed by diversion of menaquinol to alternative oxidases or by a mutation affecting heme A synthesis that is known to convert QoxABCD from an aa3 to a bo3-type oxidase. Manganese sensitivity is also reduced by derepression of the MhqR regulon, which protects cells against reactive quinones. These results suggest that dysfunction of the cytochrome aa3-type quinol oxidase contributes to metal-induced intoxication.
Keywords: Cytochrome aa3, quinol oxidase, mhqR, respiration, electron transport chain
Introduction
Life relies on metal ions as catalytic cofactors for fundamental processes including respiration, photosynthesis, nitrogen fixation, and most major metabolic pathways (Silva & Williams, 2001). Many enzymes require specific metal ions for function, and these are often accessed from a buffered cytoplasmic pool in the absence of specific chaperones (Foster et al., 2014). Although the levels of accessible metal ions vary widely, cells strive to maintain optimal intracellular metal pools by the deployment of specific import systems, often acting with extracellular metallophores and metal-binding proteins (Chandrangsu et al., 2017). High affinity metal import systems are counterbalanced by efflux systems and metal storage and sequestration mechanisms. These systems are necessary to help avoid mismetallation that can lead to enzyme inhibition or aberrant activity (Barwinska-Sendra & Waldron, 2017, Imlay, 2014, Chandrangsu & Helmann, 2016, Osman et al., 2019).
Metal-sensing transcription factors known as metalloregulatory proteins are the key regulators of both metal import and efflux (Chandrangsu et al., 2017, Osman et al., 2019, Baksh & Zamble, 2020). Metalloregulation is critical during bacterial pathogenesis. Metal sequestration by the innate immune system (nutritional immunity) limits bacterial growth in many host niches (Palmer & Skaar, 2016, Antelo et al., 2020), and in response bacteria deploy high affinity metal import systems. However, these same systems can lead to metal intoxication when metals are reintroduced. In addition, mammalian phagocytes actively employ metal intoxication, most notably of Zn and Cu, as an additional mechanism to impede bacterial growth. As a result, Zn and Cu efflux systems are in some cases critical for virulence (Sheldon & Skaar, 2019, von Pein et al., 2020).
Mn efflux has also been identified as a virulence determinant for several pathogens (Rosch et al., 2009, Grunenwald et al., 2019, Xu et al., 2017, Turner et al., 2015, Johnsrude et al., 2019), but the effects of Mn intoxication on bacterial physiology are not well understood (Juttukonda & Skaar, 2015). Intoxication by elevated intracellular Mn may result from mismetallation of metal-sensing transcription factors and metalloenzymes. In many Gram-negative bacteria, Mn mismetallates the ferric uptake regulator (Fur), which monitors intracellular Fe concentrations (Martin et al., 2015, Hantke, 1987). As a result, Escherichia coli intoxicated by Mn inappropriately represses Fe import, which leads to a cascade of effects including inhibition of ferrochelatase, the Fe insertase that functions in heme synthesis (Martin et al., 2015), inhibition of iron-sulfur cluster biogenesis, and compromised function of the ETC (Kaur et al., 2017).
Bacillus subtilis Mn homeostasis is regulated by MntR, a Mn(II)-activated transcription factor (Que & Helmann, 2000, Helmann, 2014). When Mn is sufficient, MntR represses the expression of the proton-coupled Mn importer MntH and the ATP-binding cassette family uptake system MntABCD. As Mn levels rise further, MntR binds to additional sites to activate transcription of two cation-diffusion facilitator (CDF) efflux pumps, MneP and MneS (Huang et al., 2017). Transient Mn overload upon upshift from limiting (50 nM) to sufficient (2.5 μM) Mn leads to repression of MntR and Fur-regulated genes, and induction of the sigma B (σB) regulon (Guedon et al., 2003). Increased σB activity is dependent on the RsbU Mn-dependent PP2C protein serine phosphatase (Guedon et al., 2003). Hyperactivation of Mn-dependent protein phosphatases is thought to result from an increase in protein metalation, as shown in efflux deficient mutants of Streptococcus pneumoniae where Mn-dependent activation of PhpP leads to the aberrant dephosphorylation of cell division proteins (Martin et al., 2017). Although activation of B. subtilis Fur is specific for Fe under most growth conditions, mismetallation with Mn is observed in cells with slightly elevated levels of Fur (Ma et al., 2012, Faulkner et al., 2012). Thus, proper metal selectivity requires that the levels of the Fur metalloregulator be finely tuned.
Mutants lacking either mntR (Que & Helmann, 2000, Helmann, 2014) or both mneP mneS (designated PS; Huang et al., 2017) are highly sensitive to elevated Mn, but the basis of intoxication is not well understood. In previous genetic studies we identified suppressors of Mn sensitivity that led to decreased Mn import, increased efflux, or compensatory changes in Mg homeostasis. For example, the Mn sensitivity of an mntR mutant is partially suppressed by loss of mntH, which encodes a major Mn importer (Que & Helmann, 2000). The residual Mn sensitivity of an mntR mntH mutant is suppressed by inactivation of mpfA, which encodes a magnesium (Mg) efflux pump (Pi et al., 2020). Increased intracellular Mg is postulated to protect one or more targets from Mn mismetallation, as proposed also for Bradyrhizobium japonicum (Hohle & O’Brian, 2014). Selection for Mn resistant suppressors of the efflux deficient PS strain led to the identification of strains with upregulated expression of YceF, a the TerC family membrane protein implicated in Mn efflux (Paruthiyil et al., 2020). While these results highlight the diverse ways in which cells can balance metal ion levels, and suggest an interplay between Mg and Mn homeostasis, they do not identify the causes of Mn intoxication.
Here, we identified mutations that suppress Mn intoxication in the PS efflux-deficient mutant during growth in minimal medium with different carbon sources. Strong suppression was observed for a qoxA mutation that inactivated the major aerobic respiratory quinol oxidase (QoxABCD). Moreover, elevated expression of other terminal oxidases also suppressed Mn intoxication. Protection is also conferred by expression of MhqR-regulated proteins that reduce or degrade quinones and other reactive electrophiles. Our results support a model in which elevated intracellular Mn leads to a toxic dysfunction of the ETC. These findings provide further insights into how dysregulation of metal homeostasis can perturb cell physiology.
Results
Identification of mutations that suppress intoxication in cells lacking Mn efflux
B. subtilis PS mutants lack the two CDF proteins that export Mn(II) and are unable to grow in the presence of high Mn (Huang et al., 2017). We used mariner transposon (mTn) mutagenesis to select Mn resistant suppressors of a PS strain in minimal medium (MM) with either a glycolytic (MM-glucose) or a gluconeogenic (MM-malate) carbon source (Table S1). Mn sensitivity of the PS mutant was higher on MM-malate (Fig 1C) than on MM-glucose (Fig 1B). On MM-glucose, we recovered Mn resistant suppressors with mTn insertions in mpfA, encoding the MpfA Mg efflux pump (Fig 1B), as also isolated in prior selections done on rich medium (Pi et al., 2020). We also recovered mTn insertions upstream of opuD (glycine betaine importer) and interrupting pyk (pyruvate kinase) (Fig 1B). On MM-malate, we recovered mTn insertions in yqiK (glycerophosphodiester phosphodiesterase), in the intergenic region upstream of sodA (Mn-dependent superoxide dismutase), and in qoxA (a subunit of the QoxABCD menaquinol oxidase) (Fig 1C).
Fig 1. Suppression of Mn toxicity by inactivation of qoxA.

A) The B. subtilis ETC reduces O2 (complex I-IV) to generate a proton gradient for ATP synthesis (complex V). During growth on glucose or malate, quinone reductases (e.g. NADH dehydrogenase, succinate dehydrogenase) reduce menaquinone (MK) to generate menaquinol (MKH2). Cells require either the QoxABCD or CydABCD menaquinol oxidases for aerobic growth. The bar graphs represent the Mn sensitivities of strains containing mariner transposon (mTn) insertions (Table S1) compared with WT and the Mn-sensitive mneP mneS (PS) parent strain by disk diffusion assay on minimal medium (MM) agar with (B) glucose, (C) malate, and (D) glucose plus malate. Checkered patterns in panels (B) and (C) represent mTn insertions originally selected with malate and glucose, respectively. The 6 mm filter disk was saturated with 10 μL of 100 mM MnCl2 and the clear zone of inhibition (ZOI) was measured in millimeter (mm) after incubation. Data represent the means ± the SEM for three biological replicates. **** in panel (B-D) represent P<0.0001 and ns represents no statistical significance, as determined using a one-way ANOVA test with Tukey’s post-hoc analysis. All comparisons are shown with respect to PS.
To determine if genetic suppression was specific to the carbon source, we measured Mn sensitivity for each suppressor strain isolated on MM-glucose when grown on MM-malate, and for those selected on MM-malate when grown on MM-glucose (Fig 1B, 1C; light bars with checkered fill). Inactivation of pyk suppressed Mn sensitivity on MM-glucose (Fig 1B), but not on MM-malate (Fig 1C). This is consistent with the role of pyruvate kinase at the junction between glycolysis and the TCA cycle (Fig 1A, Fig S1 and supplemental discussion). On glucose medium, pyk mutants grow primarily through substrate-level phosphorylation and have restricted ETC activity (Zamboni et al., 2004). The effect of the qoxA::mTn insertion was most dramatic on MM-malate (Fig 1C), where an active ETC is obligatory for growth. This effect is not specific to qoxA, since single and multiple mutations in any of the genes of the qoxABCD operon rescue growth on MM-malate plates amended with 200 μM Mn (Fig S2). We also tested sensitivity on medium containing both glucose and malate, which are co-metabolized in B. subtilis (Kleijn et al., 2010). In this condition, the strongest suppression was noted for the qoxA mutation (Fig 1D). Here, we have focused on the role of the QoxABCD menaquinol oxidase (herein referred to as the Qox complex) during Mn intoxication.
Suppression of Mn intoxication by qoxA does not result from reduced MntH activity
One possibility for the suppression of Mn sensitivity in the PS qoxA strain is that a decrease in proton motive force (PMF) impairs Mn uptake through MntH, a major PMF-dependent Mn importer (Makui et al., 2000, Bozzi et al., 2019). Null mutants lacking Qox have been shown to have an ~30% reduction in O2 consumption and a 50% decrease in vectorial proton pumping, consistent with a modest reduction in the overall rate of respiration (Villani et al., 1995). However, loss of mntH does not confer a high level of Mn resistance in zone-of-inhibition assays, even in an mntR background where it is constitutively expressed (Huang et al., 2017). Consistently, on MM-malate plates the PS mntH strain showed only a slightly reduced zone-of-inhibition with Mn (17 ± 2.5 mm) when compared to the PS strain (21 ± 1.5 mm). Thus, even if a qoxA mutation reduced MntH function, this could not account for the high level of resistance seen here (Fig 1C, 1D).
We have further investigated the effects of the qoxA and mntH mutations on Mn accumulation using ICP-MS (Table 1). For these studies we used cells grown in liquid MM-malate medium with levels of Mn below the 15 μM MIC of the PS parent strain (Table S2). Under these conditions we did not observe a reduction in Mn levels in the PS qoxA mutant (Table 1). We did confirm that PS strains accumulate high levels of Mn when challenged with excess Mn in LB medium, and this accumulation was somewhat reduced in the PS qoxA mutant (Table S3). During these studies, we observed an unexpected increase in Mn levels in the PS mntH strain (Table 1), but not in other metal ions (see Table S4 and associated supplemental discussion). We conclude that a loss of Mn import through MntH is insufficient to account for the suppression of Mn sensitivity in the PS qoxA strain.
Table 1:
Determination of intracellular Mn content (μg g−1 of protein) by ICP-MS.
| Strains | MMM1+ 100 nM Mn | MMM+ 1 μM Mn | MMM+ 10 μM Mn |
|---|---|---|---|
| WT | 17 ± 2.8 | 84 ± 0.5 | 159 ± 22 |
| PS | 17 ± 5.6 | 97.5 ± 4.5 | 133 ± 0.3 |
| PS qoxA | 19 ± 5.1 | 84.2 ± 0.4 | 153 ± 23 |
| PS mhqR | 19 ± 7 | 60.4 ± 4.6 | 98 ± 4.6 |
| PS mntH | 21.5 ± 3.5 | 107.8 ± 0.2 | 542 ± 80 |
| PS mpfA | 17.4 ± 3.5 | 125.3 ± 25 | 160 ± 55 |
MMM = minimal medium with malate.
Suppression of Mn intoxication by qoxA is strongest during growth on plates
In light of the striking effect of the qoxA::mTn insertion mutation on Mn sensitivity, we focused our attention on the Qox complex, an aa3-type heme-copper menaquinol oxidase. B. subtilis contains a branched respiratory chain (Fig 1A) and requires one of two terminal oxidases for aerobic growth (Winstedt & von Wachenfeldt, 2000). The Qox complex is the major oxidase in aerated cultures, whereas the cytochrome bd oxidase (CydABCD) is most important at low oxygen tensions that lead to its transcriptional induction. During growth on plates both terminal oxidases are functional (Winstedt & von Wachenfeldt, 2000).
While qoxA was the strongest suppressor on solid medium, different results were noted when the MIC for Mn was monitored in shaking, aerobic cultures using a plate reader (Table S2). Under these highly aerated conditions, the qoxA and qoxC mutants grew poorly, consistent with the dominant role for the Qox complex in respiration. In liquid cultures, the PS qoxC mutant was even more sensitive to Mn than PS alone, likely due to the previously noted assembly of a dysfunctional Qox complex with increased O2 consumption (Villani et al., 1995). This contrasts with the results seen on plates (Fig S2), where there is sufficient expression of CydABCD to support robust growth. In contrast, a pyk mutation reduced Mn toxicity in liquid culture in MM-glucose and, to a lesser extent, MM-malate. Thus, Mn toxicity is suppressed in strains with decreased flux through the ETC. During growth on MM-glucose plates, this results from a pyk mutation, and on MM-malate from loss of the major quinol oxidase (Fig 1). We therefore hypothesized that elevated Mn triggers a toxic dysfunction of the ETC under conditions with high flux through the Qox complex.
Diversion of menaquinol from the Qox complex suppresses metal intoxication
We next sought to test whether derepression of the alternative cytochrome bd oxidase CydABCD might suppress metal intoxication by diversion of menaquinol from the Qox complex. Rex is an NADH/NAD sensing repressor that controls expression of the cydABCD operon (Wang et al., 2008, Gyan et al., 2006). Indeed, a PS rex triple mutant was significantly more Mn resistant than the PS parent strain, although not as resistant as a PS qoxA mutant (Fig 2A). Consistently, ectopic expression of cydABCD from a xylose-inducible promoter also suppressed Mn sensitivity similarly to a rex mutant (Fig 2A), as did expression of qcrABCD, encoding a cytochrome c dependent bc-type terminal oxidase (Garcia Montes de Oca et al., 2012, Yu et al., 1995) (Fig 2C). Further, simply amending the medium with nitrate, the substrate for the respiratory nitrate reductase (Fig 1A), reduced the Mn sensitivity of the PS mutant (Fig 2D). These results suggest that diversion of menaquinol from the dominant cytochrome aa3 (Qox complex) to any of several alternative electron transfer pathways can suppress Mn toxicity. We therefore hypothesize that Mn intoxication is a specific consequence of flux through the Qox complex, rather than the upstream components of the ETC. A similar dysfunction may contribute to Zn intoxication since inactivation of rex also increases Zn resistance in wild-type cells (Chandrangsu & Helmann, 2016). Here, we extend these findings to show that Zn intoxication is also suppressed by inactivation of qoxA or by ectopic induction of the cydABCD operon (Fig. 2B). Induction of sodA also suppressed Zn intoxication in wild-type cells (Fig. 2B), but failed to suppress Mn intoxication in the PS parent strain.
Fig 2. Diversion of menaquinol (MKH2) from the aa3 quinol oxidase can counteract Mn sensitivity.
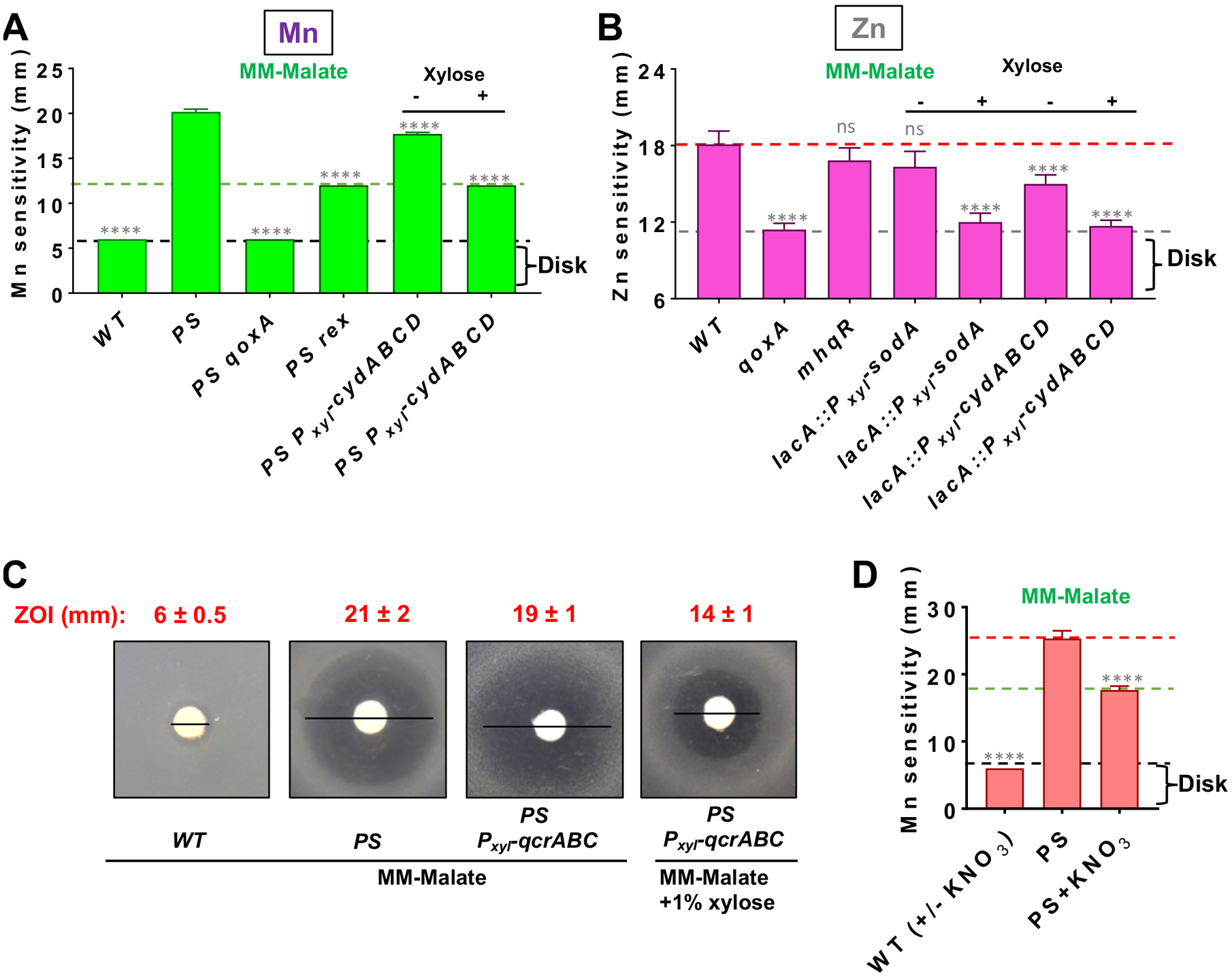
A) Mn sensitivity measured by disk diffusion assay on MM-malate with and without 1% xylose. All strains contain gene replacements with erm or kan as obtained from the BKE/BKK collection, except for mneP and mneS which are unmarked, clean deletions (Table S5). Dotted lines highlight strains with similar levels of Mn sensitivity. All xylose-inducible constructs (including cydABCD) were integrated at lacA. All measurements are mean±SEM (n=3). **** represents P<0.0001 and ns, no statistical significance when compared to the PS parent strain. B) Zn sensitivity monitored on MM-malate (with or without 1% xylose) measured by disk diffusion assay with 10 μl of 100 mM ZnCl2. All measurements are mean±SEM (n=3). **** represent P<0.0001 and ns, no statistical significance relative to the wild-type parent strain. C) Representative images illustrate suppression of Mn sensitivity in PS by induction of qcrABC (cytochrome bc menaquinol-cytochrome c oxidoreductase) (numbers in inset are ZOI diameter; measurements are mean±SEM, n=3). D) Mn sensitivity of PS on MM-malate is suppressed by amendment with 10 mM potassium nitrate (KNO3). All measurements are mean±SEM (n=3). **** represents P<0.0001 and ns, no statistical significance. For all panels, significance was determined using a one-way ANOVA test with Tukey’s post-hoc analysis and all comparisons are shown with respect to PS.
Conversion of Qox into a bo3 heme-copper oxidase suppresses Mn toxicity
Next, we explored the effect of different structural types of heme on Mn sensitivity. The Qox complex provides B. subtilis cells with their characteristic pink color, as monitored in cell culture pellets (Fig 3A). Qox is assembled with two heme A molecules (van der Oos et al., 1991, Contreras-Zentella et al., 2003, Hammer et al., 2016). The initial product of heme biosynthesis, resulting from Fe insertion into protoporphyrin IX by ferrochelatase, is heme B (Fig 3B) (Hederstedt, 2012). Heme B is converted to heme O by CtaB (heme O synthase), and then to heme A by CtaA (heme A synthase) (Fig 3B) (Svensson et al., 1993). Heme B is an essential cofactor for catalase, and, together with heme D, is required for the cytochrome bd quinol oxidase.
Fig 3. Conversion of QoxABCD to a bo3 quinol oxidase suppresses Mn sensitivity.
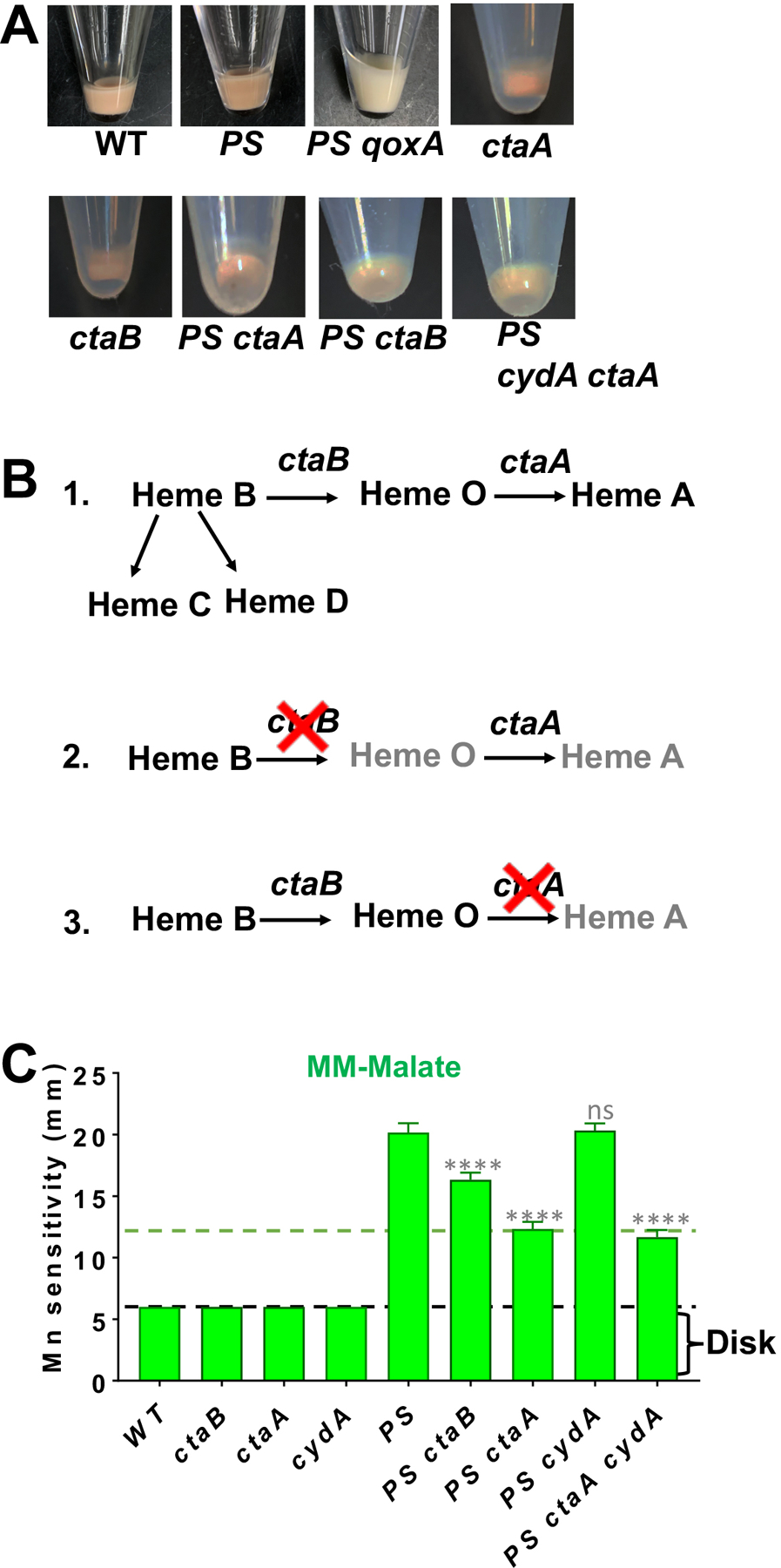
A) QoxABCD is the major cytochrome in WT and PS cells, and mutation of qoxA leads to a loss of characteristic reddish-brown color as observed in cell pellets. B) Pathway of heme B conversion to heme A by the sequential action of CtaB and CtaA. Heme C is generated as thioester linkage of heme B in polypeptides. Additionally, heme D is also produced from heme B. C) Mn sensitivity enumerated on MM-malate plates. Dotted lines highlight strains with similar levels of Mn sensitivity. All measurements are mean±SEM (n=3). **** is P<0.0001 and ns, no statistical significance as determined using one-way ANOVA test with Tukey’s post-hoc analysis. All comparisons are shown with respect to PS.
Introduction of a ctaA mutation into the PS strain led to a significant increase in Mn resistance (Fig 3C), although not quite to the level seen in a PS qoxA strain (Fig 1C). This was surprising since ctaA mutants can still form a functional Qox complex (a cytochrome bo3 quinol oxidase) by incorporation of heme B and O into the QoxB subunit I, as shown previously in both S. aureus and B. cereus (Contreras-Zentella et al., 2003, Hammer et al., 2016). Further, this cytochrome bo3 quinol oxidase has been reported to be as active as the aa3 oxidase (Hammer et al., 2016). The reduced suppression noted for a ctaA mutation relative to a qoxA null mutation is consistent with the idea that this strain retains a functional Qox complex. Consistently, we were able to construct ctaA cydA mutants (lacking heme A and the cytochrome bd oxidase) in both WT and PS strains, and these strains retain the pink coloration associated with the abundant Qox complex in the membrane (Fig 3A). As expected, we were unable to generate a qoxA cydA double mutant, consistent with their reported synthetic lethality (Winstedt & von Wachenfeldt, 2000). The PS ctaA cydA mutant was as resistant to Mn as the PS ctaA mutant (Fig 3C), which demonstrates that cytochrome bd oxidase (CydABCD) is not required for growth or Mn resistance under these conditions. These data suggest that a functional bo3-type heme copper oxidase assembles in the ctaA mutant, and this version of the Qox oxidase is sufficient to support aerobic growth but contributes less to Mn intoxication than the canonical Qox complex assembled as an aa3-type oxidase.
A PS ctaB strain retained a high level of Mn sensitivity (Fig 3C), in contrast to a PS qoxA strain (Fig 1B). Further, this strain (unlike the PS qoxA strain) retains the pink color indicative of cytochromes in the membrane. Thus, a ctaB mutation does not phenocopy a qoxA null mutation. This suggests that a Qox complex assembles in the ctaB strain despite the loss of both hemes O and A, and that this aberrant complex still contributes to Mn toxicity. This strain may accumulate heme B, which has been proposed to assemble in the CydABCD oxidase as a bb’-type cytochrome (Azarkina et al., 1999). Whether the Qox complex can also assemble as bb’-type cytochrome, and whether this complex contributes to Mn intoxication, requires further study. This putative Qox complex is likely not very functional in respiration, since we were unable to generate a PS ctaB cydA mutant, which implies that these cells rely on the cytochrome bd oxidase (CydABCD) for viability.
Derepression of the MhqR electrophile stress response suppresses Mn toxicity
Oxidative stress arising from the ETC has also been linked to toxicity in cells genetically suppressed for peptidoglycan synthesis, and can be a barrier that precludes emergence of cells lacking a cell wall (L-forms) (Kawai et al., 2015, Luo & Helmann, 2012). Mutations that facilitate the transition from vegetative growth to L-forms include those that inactivate the Qox complex and null mutations in mhqR (methylhydroquinone resistance) (Kawai et al., 2019), which encodes one of a family of repressors that coordinate electrophile stress responses (Fig 4A) (Towe et al., 2007, Leelakriangsak et al., 2008). Motivated by these results, we decided to test whether introduction of a mhqR deletion mutation into the PS strain would restore Mn resistance. Indeed, a PS mhqR triple mutant was as Mn resistant as WT as monitored by both a disk diffusion assay (Fig 4B), and in aerated liquid cultures in MM (Fig 4C, 4D, Table S2). This is consistent with the hypothesis that the MhqR regulon protects against intoxication due to dysfunction of the ETC. In contrast, inactivation of mhqR did not suppress Zn toxicity (Fig 2B). In addition to MhqR, the CatR and YodB repressors also control genes important for resistance to reactive electrophiles (Chi et al., 2010). However, deletion of either catR or yodB led to only a modest increase in Mn resistance in PS background (Fig 2B).
Fig 4. Mn toxicity is suppressed by induction of the MhqR regulon.
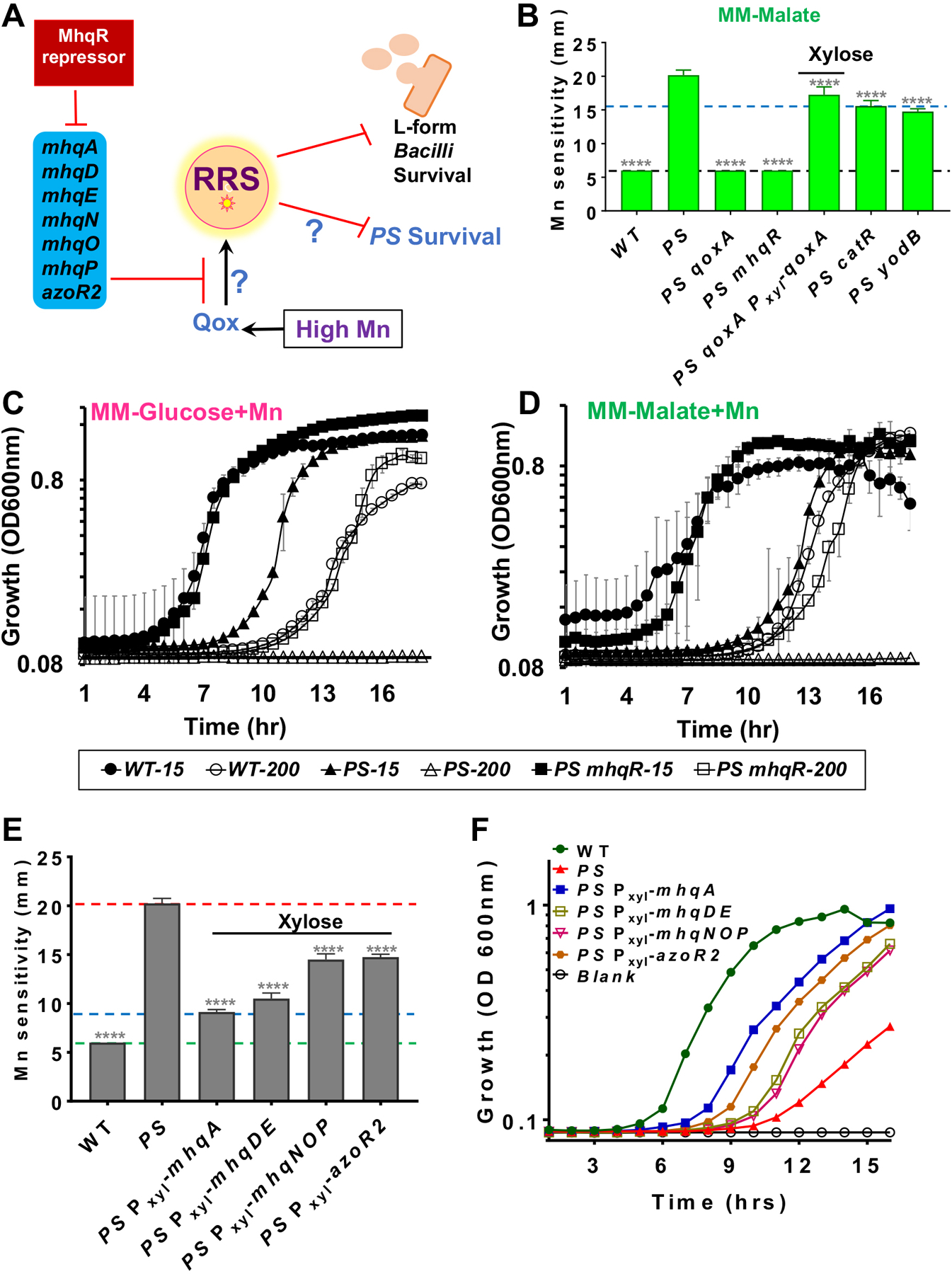
A) MhqR (methyl hydroquinone resistance regulator) represses genes for resistance to toxic quinones, which can function as electrophiles and also generate reactive radical species (RRS). B) Deletion of mhqR restores Mn resistance to WT levels on MM-plates as monitored by a disk diffusion assay. Deletion of catR or yodB had a modest, but still significant, effect (**** = P<0.0001). Representative aerobic growth in MM-glucose (C) or MM-malate (D) with either 15 μM (sufficient) or 200 μM (excess) Mn (n=3) as monitored in a plate reader (BioTek). Sensitivity of PS to Mn can be fully alleviated by derepression of the MhqR-regulated electrophile stress response. E) Mn resistance relative to the PS parent strain was monitored using disk diffusion assays (disk saturated with 10 μl of 100 mM Mn) for strains engineered to have xylose-based (final 1% concentration) expression of MhqR-regulated operons (mhqA, mhqDE, mhqNOP, and azoR2). The green line shows disk diameter, red line shows hyper-sensitivity of PS strain, and blue line shows rescue of Mn resistance upon mhqA expression (**** = P<0.0001 using a one-way ANOVA test with Tukey’s post-hoc analysis). F) Representative growth curves (n=3) of strains monitored in aerobic, shaking cultures in MM-malate with 200 μM Mn. All xylose-based overexpression constructs were induced with 1% xylose in final concentration.
The MhqR regulon includes seven genes organized in four operons: azoR2, mhqA, mhqDE, and mhqNOP (Towe et al., 2007). When these operons are individually induced in the PS strain, each one can confer Mn resistance both on plates (Fig 4E) and in aerobic, shaking cultures (Fig 4F). The strongest effect was noted for induction of mhqA, encoding a dioxygenase that confers resistance to 2-methylhydroquinone (Nguyen et al., 2007). We speculate that MhqA may degrade excess menaquinol that accumulates in cells with an impaired respiratory chain. The other MhqR-regulated operons encode two MhqA paralogs (MhqE and MhqO), that may work similarly, and AzoR2 (an azoreductase similar to quinone NAD(P)H dehydrogenase). These results suggest that the MhqR regulon alleviates stresses imposed by either exogenous electrophiles (e.g. methylhydroquinones) or stress arising from dysfunction of the ETC.
Mn poisoning of cytochrome aa3 oxidase generates reactive radicals
We next investigated the production of reactive radical species (RRS) in the PS strain and its derivatives using the fluorogenic probe 2’,7’-dichlorofluorescein diacetate (DCF) (Winterbourn, 2014). The DCF assay reveals a low level of endogenous radical production in MM-glucose with 15 μM Mn, and this was not further increased in the PS mutant (Fig 5A). However, in cells subjected to 200 μM Mn the level of RRS was elevated ~3-fold in WT, and ~5-fold in the PS mutant. This Mn-dependent increase in the production of RRS was not observed in the PS mutant strain additionally containing mutations in mhqR, rex, pyk, or qoxA (Fig 5A), consistent with the ability of these mutations to suppress Mn sensitivity.
Fig 5. Suppressor mutations that reduce Mn intoxication reduce RRS.
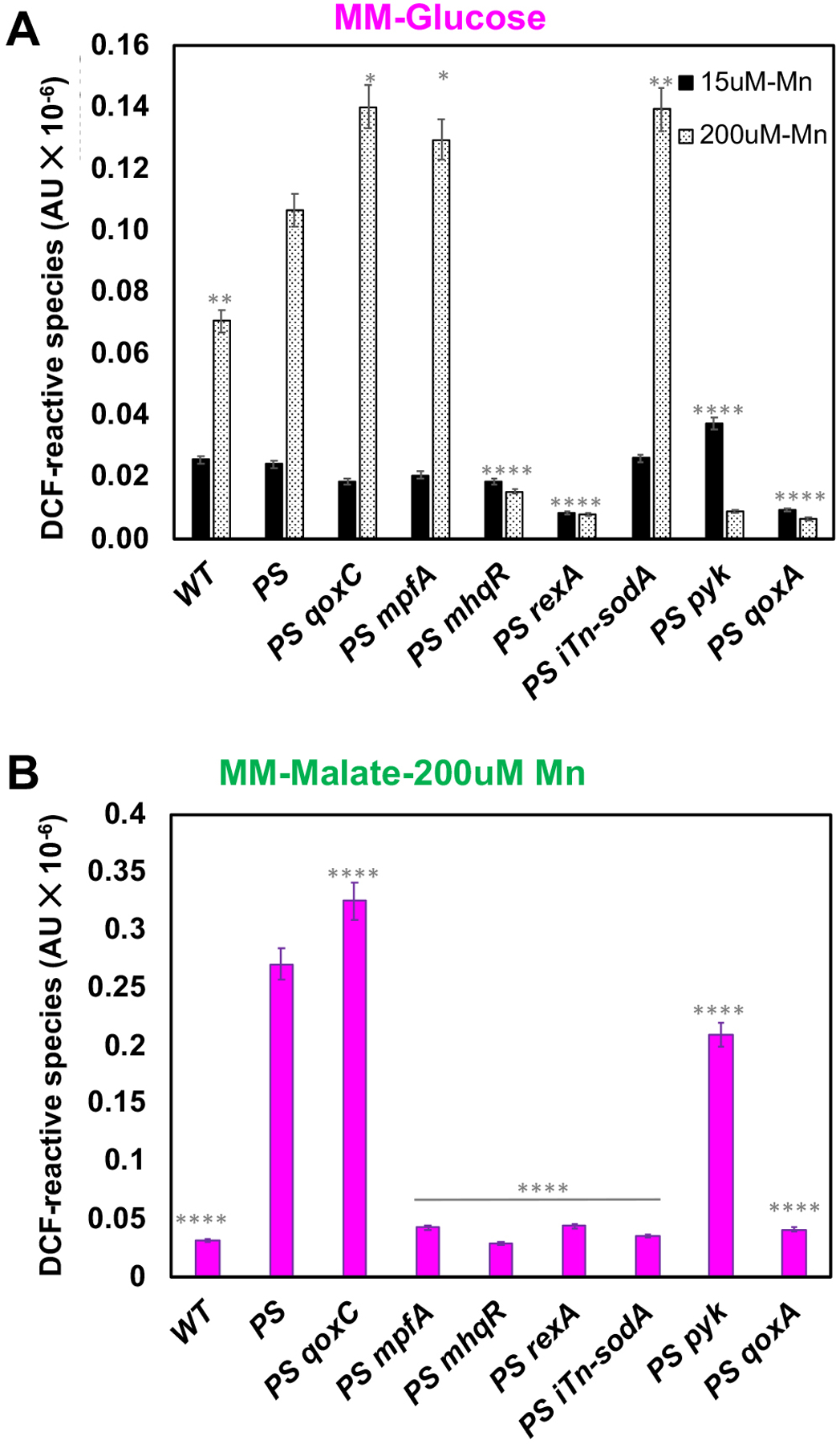
(A) Levels of endogenous RRS were measured using DCFDA during aerobic growth in MM-glucose cultures in a plate reader with 5 or 200 μM Mn supplementation. (B) Levels of endogenous RRS were measured during growth in MM-malate with 200 μM Mn. RRS production in the presence of 200 μM Mn was compared to the PS strain with 200 μM Mn using a one-way ANOVA test with Tukey’s post-hoc analysis (n=3: *P<0.5; **P<0.01; ****P<0.0001).
When cells were subjected to 200 μM Mn in MM-malate, the overall level of RRS in the PS mutant was further increased, and in this condition the pyk mutation was a poor suppressor of radical production (Fig 5B), consistent with the lack of suppression of Mn sensitivity (Fig 1C). The level of RRS was greatly reduced in the iTn-sodA strain (Fig 5B), although, for reasons unclear, this effect was not observed in cells growing on glucose (Fig 5A). This suggests that there may be multiple types of RRS detected by the DCF assay. The mpfA mutation suppresses Mn intoxication in the PS strain by elevation of Mg levels (Pi et al., 2020), and it also retained a high level of RRS (Fig 5A). These results support the notion that a Mn-dependent dysfunction of the Qox complex is correlated with production of RRS, and that many of the isolated suppressors reduce production of RRS. Interestingly, the PS qoxC mutant had an aggravated production of RRS, which contrasts with the effects noted in the PS qoxA strain (Fig 5A, 5B). Previously, it was reported that qoxC null mutants have a ~3-fold reduction in cytochrome aa3 oxidase in the membrane, but have an elevated O2 consumption rate, suggesting that the Qox complex may be inappropriately assembled and have aberrant activity in this strain (Villani et al., 1995). In contrast, qoxA is largely lacking membrane-localized cytochromes (Fig 3A), and has reduced O2 consumption.
One RRS produced by the ETC is superoxide (Wong et al., 2017), which then leads to the production of hydrogen peroxide (H2O2) and hydroxyl radical. Rates of H2O2 production can be monitored in cells engineered to lack peroxidases using the Amplex Red fluorescence assay (Seaver & Imlay, 2004, Li & Imlay, 2018). We monitored H2O2 production from hpx strains lacking catalase (katA::tet) and alkylhydroperoxide reductase (ahpCF::kan) as a function of time in MM-malate medium. In this hpx background, we observed a linear increase in H2O2 over time, which was reduced >23-fold in an hpx qoxA strain (Fig S3). This suggests that even in the absence of Mn stress the vast majority of endogenous H2O2 emanates from the Qox complex, presumably by initial formation of superoxide which then dismutates to generate the measured H2O2. However, we were unable to detect an increase in H2O2 by this assay after challenge of cells with elevated Mn, suggesting that RRS other than superoxide may account for intoxication in the presence of excess Mn. This is consistent with the observation that ectopic induction of SodA reduced Zn intoxication, but not Mn intoxication, as noted above. Conversely, derepression of the MhqR regulon strongly repressed Mn intoxication, but was unable to rescue Zn intoxication. Although the RRS produced under conditions of Mn intoxication are currently unknown, the striking ability of enzymes in the MhqR regulon to suppress toxicity suggests that the RRS may include semiquinones.
Discussion
We here used a genetic suppression approach to investigate the physiological consequences of Mn intoxication in B. subtilis, a model system for understanding metal homeostasis (Chandrangsu et al., 2017, Helmann, 2014). By selection for Mn resistance in a PS efflux deficient strain, we identified the cytochrome aa3 menaquinol oxidase (Qox complex) as an important contributor to Mn intoxication (Fig 6). A PS qoxA triple mutant has a greatly increased tolerance to elevated Mn (Fig 1), and this tolerance is correlated with a reduced production of RRS (Fig 5). Intoxication by Mn was also suppressed by derepression of the MhqR-regulated electrophile stress response, or by diversion of menaquinol to alternative terminal oxidases.
Fig 6. High intracellular Mn triggers a toxic dysfunction of QoxABCD.
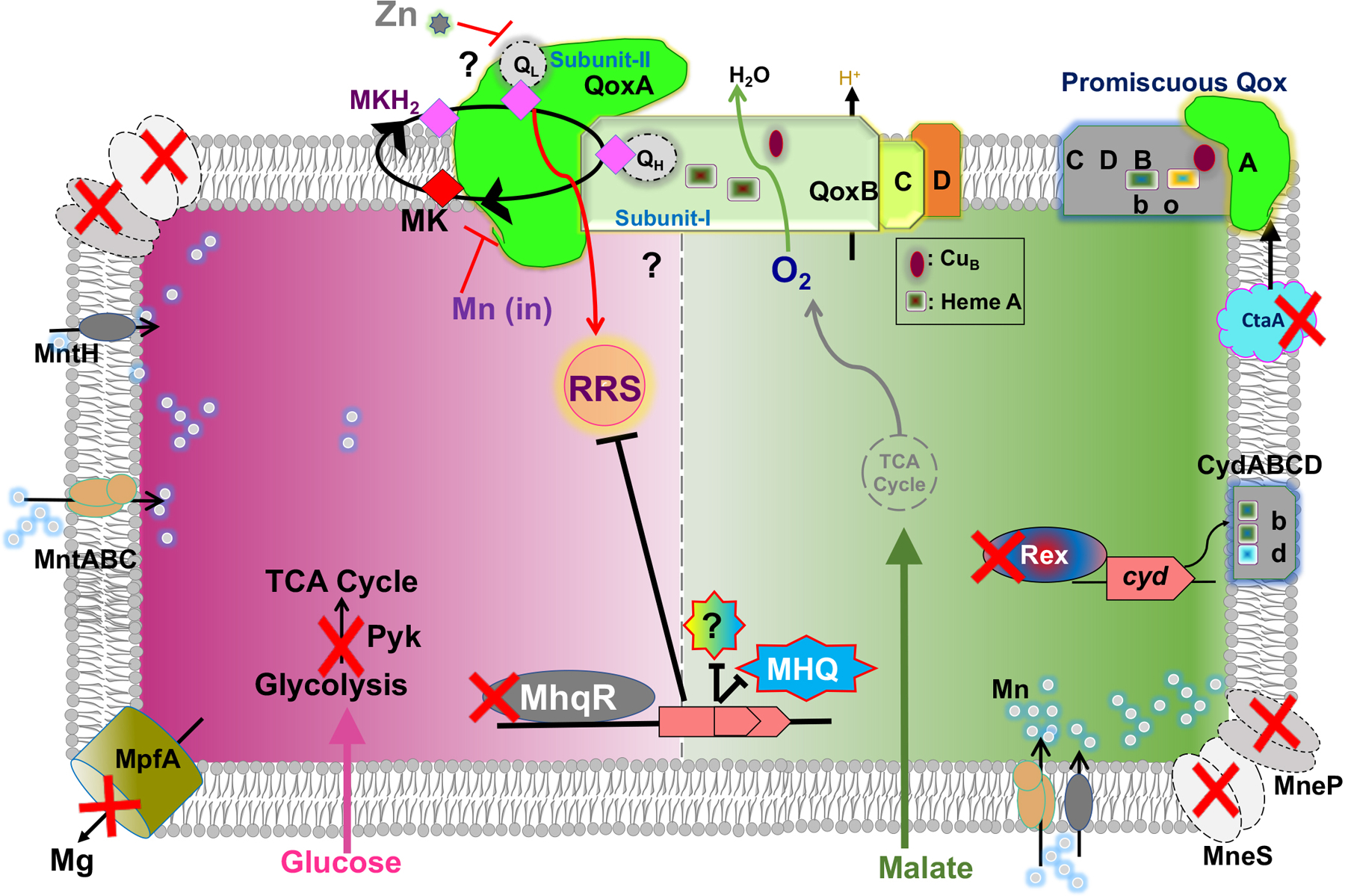
A schematic depiction of cellular responses to high Mn for cells growing in MM-malate (green arrow and shading) or MM-glucose (pink arrow and shading). Intracellular Mn (silver circles) is regulated by both import (MntH and MntABC, left) and efflux (MneP and MneS, upper left and lower right). In the absence of efflux (PS mutant), RRS accumulate, as detected by reaction with DCF. On MM-glucose, loss of pyruvate kinase (Pyk) or a Mg efflux pump (MpfA) is beneficial. On MM-malate, Mn toxicity is suppressed by loss of QoxA (qoxA::mTn), derepression of the MhqR regulon, a mutation of ctaA that converts QoxABCD into a bo3-type oxidase, or expression of CydABCD bd-type oxidases (rex mutation or ectopic induction).
Collectively, our results suggest that Mn intoxication results from a dysfunction of the Qox complex, although the molecular mechanism is not yet known. The Qox complex is a member of a widely conserved family of heme-copper oxidases that includes the E. coli ubiquinone-dependent bo3 oxidase (Xu et al., 2020). B. subtilis QoxB (subunit I) contains two heme A moieties, with the heme a3-CuB center as the site of O2 reduction, and a high affinity menaquinol-binding site (QH) (Xu et al., 2020, Bossis et al., 2014). The QH-bound menaquinol undergoes two sequential one-electron oxidations, with an intermediate menasemiquinone radical (Yi et al., 2010). We do not yet know whether Mn interacts with the Qox complex to reduce the stability of the reactive menasemiquinone intermediate, or instead affects O2 reduction at the heme-Cu center. This dysfunction is sensitive to the nature of the associated heme cofactors. In the ctaA mutant, where Qox assembles as a bo3-type quinol oxidase (Contreras-Zentella et al., 2003), Mn intoxication is intermediate between that of WT cells and those lacking Qox completely.
The ETC is widely appreciated as a potential source of ROS, including superoxide and derivative reactive radicals (e.g. hydroxyl radical). In mitochondria, ROS arises primarily from complexes I and III, with production from complex III (coenzyme Q:cytochrome c reductase) accentuated when downstream electron transfer events are inhibited (Nolfi-Donegan et al., 2020, Wong et al., 2017). Like the Qox complex, mitochondrial complex III is the site of quinol oxidation and proceeds through a reactive semiquinone intermediate (Turrens et al., 1985). Previous work in B. subtilis has implicated the abundant Qox complex in ROS generation during the transition of growing cells to L-forms lacking a cell wall, a condition that leads to a increase in flux through the ETC (Kawai et al., 2019). Other mutations that support L-form emergence included ispA (which decreases menaquinone synthesis), ndh (NADH dehydrogenase), ctaB (heme synthesis), and mhqR (methyhydroquinone resistance). In general, there was good congruence between mutations that allow the establishment of L-forms and those that suppress Mn intoxication.
Although originally defined by its role in regulating resistance to electrophilic compounds such as 2-methylhydroquinone and catechol (Towe et al., 2007, Fritsch et al., 2019), our results suggest that the MhqR stress response may also ameliorate RRS associated with a menaquinol-dependent ETC (Fig 6). MhqR-regulated enzymes are predicted to mediate NADH‐ and flavin-dependent reduction of quinones, and include thiol-dependent dioxygenases thought to catalyze ring cleavage of quinone-S-adducts (Antelmann et al., 2008, Towe et al., 2007). MhqR-regulated enzymes may help maintain the menaquinol pool in a reduced state (quinone reductase) or degrade and remove excess quinones (and their adducts).
In summary, our findings indicate that intoxication by Mn triggers dysfunction of the ETC, and specifically the Qox complex. Inactivation of Qox reduced intoxication, and derepression of the MhqR regulon rescued growth. These studies support a role of ETC dysfunction in RRS generation and lay the groundwork for detailed studies to define sites of Mn mismetalation in the Qox complex and other targets. The Qox complex is also implicated in Zn intoxication (Fig. 2B), however there are notable differences relative to Mn intoxication. Mn intoxication in the PS strain is suppressed by mhqR, but not by induction of SodA, whereas the converse is true for Zn intoxication of wild-type cells. Mn intoxication is correlated with the production of DFC-reactive radicals, but the nature of the reactive compounds is not yet known. The ability of metal intoxication to dysregulate the ETC has been implicated in a variety of human disease states (Harischandra et al., 2019), and high Mn can lead to ROS production by oxidation of quinones bound to complex II (Bonke et al., 2015). Thus, the sites and importance of RRS from the ETC varies dramatically between species and depends on growth conditions and nutrient status.
Experimental Procedures
Bacterial strains, growth conditions, and sequencing.
Mutant strains were obtained from the Bacillus Genetic Stock Center (BGSC) as erythromycin/kanamycin resistance gene disruptants from the BKE/BKK collection (Koo et al., 2017). Mutations were transformed into the desired strain. For the PS mutant, in-frame, markerless deletions (designated with Δ) were generated using plasmid pDR244 as described (Koo et al., 2017). Strains are listed in Table S5, and oligos used in this study are listed in Table S6.
Operon gene deletions for qoxAB, qoxABC, qoxABCD were made by joining PCR and introduced using transformation. Briefly, for joining PCR upstream and downstream fragments were generated and contained homology for integration and replacement of 2 or more genes with erythromycin cassette of BKE collection. For example, for qoxAB::erm we used qoxA-up-F and erm-middle-R primers to generate ~1.2 kb upstream homology PCR from qoxA::erm gDNA as a template and downstream homology fragment involved erm-middle-F and qoxB-down-R primers for amplification from qoxB::erm gDNA as a template. These upstream and downstream fragments were combined by joining PCR using qoxA-up-F and qoxB-down-R primers. The qoxABC::erm and qoxABCD::erm deletion strains were constructed with the same strategy. PCR products were transformed into wild-type (CU1065) cells, were moved into the PS background, and the PS deletions verified using analytical PCR.
Xylose-based overexpression constructs were generated by PCR amplification of open reading frames with high-fidelity Phusion polymerase with primers engineered to have a ribosome-binding site in the 5’-UTR region. The PCR products were restriction digested and ligated with pre-digested pAX01 plasmid (or pPL82) using T4 DNA ligase (NEB). The resultant constructs were maintained in Escherichia coli DH5α strains using ampicillin (100 μg ml−1). Plasmids were transformed into B. subtilis and selected with an appropriate antibiotic on rich medium. The rich medium for inoculum growth and revival of glycerol stocks was lysogeny broth (LB) (Affymetrix) with erythromycin (1 μg ml−1), lincomycin (25 μg ml−1), chloramphenicol (10 μg ml−1), or kanamycin (15 μg ml−1) as needed.
Chemically defined minimal medium (MM) contains: ammonium sulfate (NH4)2SO4 10 g l−1, trisodium citrate Na3C6H5O7·2H2O 5 g l−1, L-glutamic acid potassium salt monohydrate 5 g l−1, 40 mM MOPS buffer (pH 7.4 using KOH), 2 mM KPO4 (pH 7.0), 10 mg l−1 tryptophan, 0.8 mM MgSO4, 4.4 mg l−1 ferric ammonium citrate (15 μM) and 1% malate (pH 7.4) (MM-malate) or 1% glucose (MM-glucose) as carbon source. The medium was amended with MnCl2 to the final concentration indicated for each experiment. For nitrate supplementation experiments the medium was bubbled with argon for 10 min. and potassium nitrate was added to 10 mM. This medium was immediately moved into an anaerobic chamber where gas composition was kept at 95% N2 and 5% H2 and plates were poured. The top agar layer was similarly treated, mixed with bacterial culture, and poured on plates prior to incubation at 37 °C in a sealed chamber to limit oxygen availability.
Transposon library preparation.
Cells containing pMarA (Le Breton et al., 2006, Dempwolff et al., 2020) were grown at 30 °C in LB broth and were plated onto MM agar containing the indicated carbon source (malate or glucose) and incubated at 45 °C on Mn gradient plates containing filter disks saturated with 10 μl of 100 mM Mn solution. Candidate suppressor clones were picked from the zone of clearance and transposon-related antibiotic resistance was confirmed by growth in the presence of kanamycin (15 μg ml−1) and tetracycline (12.5 μg ml−1). Genomic DNA was extracted and purified followed by Taqα1 (NEB) enzyme digestion and libraries were constructed by ligation with T4 DNA ligase enzyme (NEB) to generate circular DNA. Inverse PCR and Sanger-sequencing (Cornell University Biotechnology Resources Center) was used to locate the site of mTn insertions. The extracted genomic DNA from suppressor clones was backcrossed into the parental PS strain to perform linkage experiments to verify that the observed phenotype was due to the mTn insertion. We further confirmed all mTn loss-of-function insertion mutations by introduction of deletion mutations from the BKK/BKE collection (Koo et al., 2017) into the PS mutant.
Defined media preparation and disk diffusion assay.
Mn and Zn sensitivities were monitored either by disk diffusion (zone-of-inhibition) assays or in liquid broth cultures with MM-malate or MM-glucose. For plates, the bottom hard agar was prepared by mixing an equal volume of 3% agar with 2X strength liquid MM plus carbon source, then 15 ml was poured in a petri dish. Similarly, top agar was prepared by mixing an equal volume of 1.5% agar solution with 2X liquid MM containing the desired carbon source. This medium was cooled to 50 °C and was divided into 5 ml tubes (4 ml per tube). To each tube, 100 μL of freshly grown cell-culture was added and further mixed by inversion and was carefully layered onto the bottom agar. Plates were allowed to solidify for 20 min and then a Whatman filter number 4 disk (6 mm in diameter) was overlaid. These disks were saturated with 10 μL of 100 mM MnCl2 solution (or 10 μL of 100 mM ZnCl2). The plates were incubated at 37 °C for 18 h and the zone of clearance was measured.
Spot-dilution method.
Cultures were grown on LB agar with appropriate antibiotics and were used to inoculate 5 ml LB broth grown aerobically at 37 °C to an OD 600nm of 0.4. These cultures were serially diluted in LB broth in a microtiter plate and 5 μl of diluted culture was spotted onto LB and MM-malate plus 15 or 200 μM Mn. These spots were allowed to air dry for 20 min in a laminar hood. Plates were then incubated at 37 °C for 18 h.
Reactive radical species (RRS) measurement.
For inoculum preparation, cultures were streaked and grown on LB agar medium with appropriate antibiotics. Individual colonies were picked and inoculated into 5 ml LB broth (no antibiotics) and grown to an OD 600 nm of 0.4. From these cultures, 200 μL was withdrawn and pelleted at 7000 rpm for 2 min. at room temperature, the pellet was re-suspended and washed with 400 μL 1X liquid MM-malate or MM-glucose (without MnCl2). This solution was further centrifuged at 7000 rpm for 2 min, and the resultant pellet was resuspended in 200 μL 1X liquid MM-malate or MM-glucose (no added MnCl2). In 96 well microtiter well plate 200 μL 1X liquid MM-malate or MM-glucose containing either 5 μM or 200 μM MnCl2 was dispensed. 2 μL of inoculum was added and cells were allowed to grow under shaking condition for 21 hours at 37 °C in a Synergy H1 plate reader (BioTek Instruments Inc, VT) and OD 600 nm was monitored. DCFDA (Sigma) was added to a final concentration of 1.25 mg l−1 (5 μL of 2 mg ml−1 DCFDA into 200 μL), growth was resumed with continuous shaking for another 6–8 hours at 37 °C, with DCF fluorescence measured using excitation and emission wavelengths of 498 and 522 nm, respectively. DCF-reactive RRS levels were calculated by dividing fluorescence intensity (6 hours post DCFDA addition) by the OD600 at the time of DCFDA addition.
Hydrogen peroxide measurement.
Measurement of H2O2 from live cells was performed using Amplex™ Red as described previously (Li & Imlay, 2018). Briefly, cells were collected from LB plate and re-suspended in MM-malate (15 μM Mn) at 37 °C followed by cells were grown to ~0.02 OD600. Then, 250 μL of sample was collected for 60 min at every 2 min interval under aerobic growth conditions. These cells were kept on ice for further processing. In a dark room, a 96 well plate was set consisting of 50 μl of 50 mM K2HPO4 adjusted to pH 7.8, 50 μl of collected samples (in triplicate), 50 μl of Amplex™ Red dye (Life Technologies, 1 mg per 20 ml), 50 μl of horseradish peroxidase enzyme (Sigma, 1 mg per 20 mL) was added and fluorescence was read in a plate reader (BioTek Instruments Inc, VT) using excitation wavelength of 520 nm and emission wavelength of 620 nm. Fluorescence values were converted to H2O2 concentrations by comparing with a peroxide standard curve.
Determination of intracellular metal content.
LB samples:
Bacterial strains were grown in LB to an OD 600 nm of 0.4 at 37 °C under aerobic growth conditions. Cultures were exposed to 150 μM Mn and further incubated for 30 min. and centrifuged to pellet the cells.
Minimal media samples:
strains were grown aerobically in MM-malate with sub-MIC concentrations (100 nM, 1 μM, and 10 μM) of Mn to an OD 600 nm of 0.7–0.8 at 37 °C. Following growth cells were centrifuged and the pellet was collected.
Sample processing:
Cell pellets were washed several times with Chelex-treated PBS. Cells were further mixed with Chelex-treated PBS containing 75 mM NaN3 and 1% Triton X-100 and were incubated at 37 °C for 90 min. Following this, cells were sonicated in a sonicator bath at max power for 5 min at room temperature. Samples were centrifuged and the protein concentration of the supernatant lysate was determined using the Bradford reagent. Supernatant fractions were boiled for 30 min in 5 % nitric and 0.1 % triton X-100 acidified solution. Samples were then centrifuged at 12,000 rpm for 10 min. and supernatant was carefully collected, mixed with 1% nitric acid for a desired dilution.
ICP-MS operation:
samples were injected in ICP-MS analyzer (Perkin-Elmer SCIEX ELAN DRC II ICP-MS instrument with reaction gas as ammonia and collision gas of argon). Specific metal standards and internal Gallium standards were used during the instrument operation, and metal levels were calculated as μg per g−1 (ppm) relative to total protein (mean ± standard deviation [SD]; n=3), except for potassium which is reported as μg per mg−1 (ppt).
Data Availability:
The data that supports the findings of this study are available in the figures and supplementary material of this article, and/or are available from the authors upon request.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health under award number R35GM122461 to JDH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Brian Wendel for ICP-MS instrument operation. We thank Jim Imlay, Lars Hederstedt, and Jim Shapleigh for helpful comments.
Footnotes
Conflict of Interest: The authors declare that they have no conflicts of interest.
REFERENCES
- Antelmann H, Hecker M & Zuber P, (2008) Proteomic signatures uncover thiol-specific electrophile resistance mechanisms in Bacillus subtilis. Expert Rev Proteomics 5: 77–90. [DOI] [PubMed] [Google Scholar]
- Antelo GT, Vila AJ, Giedroc DP & Capdevila DA, (2020) Molecular Evolution of Transition Metal Bioavailability at the Host-Pathogen Interface. Trends Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarkina N, Siletsky S, Borisov V, von Wachenfeldt C, Hederstedt L & Konstantinov AA, (1999) A cytochrome bb’-type quinol oxidase in Bacillus subtilis strain 168. J Biol Chem 274: 32810–32817. [DOI] [PubMed] [Google Scholar]
- Baksh KA & Zamble DB, (2020) Allosteric control of metal-responsive transcriptional regulators in bacteria. J Biol Chem 295: 1673–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barwinska-Sendra A & Waldron KJ, (2017) The Role of Intermetal Competition and Mis-Metalation in Metal Toxicity. Adv Microb Physiol 70: 315–379. [DOI] [PubMed] [Google Scholar]
- Bonke E, Zwicker K & Drose S, (2015) Manganese ions induce H2O2 generation at the ubiquinone binding site of mitochondrial complex II. Arch Biochem Biophys 580: 75–83. [DOI] [PubMed] [Google Scholar]
- Bossis F, De Grassi A, Palese LL & Pierri CL, (2014) Prediction of high- and low-affinity quinol-analogue-binding sites in the aa3 and bo3 terminal oxidases from Bacillus subtilis and Escherichia coli. Biochem J 461: 305–314. [DOI] [PubMed] [Google Scholar]
- Bozzi AT, Zimanyi CM, Nicoludis JM, Lee BK, Zhang CH & Gaudet R, (2019) Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter. Elife 8: e41124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P & Helmann JD, (2016) Intracellular Zn(II) Intoxication Leads to Dysregulation of the PerR Regulon Resulting in Heme Toxicity in Bacillus subtilis. PLoS Genet 12: e1006515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P, Rensing C & Helmann JD, (2017) Metal homeostasis and resistance in bacteria. Nature Reviews Microbiology 15: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi BK, Kobayashi K, Albrecht D, Hecker M & Antelmann H, (2010) The paralogous MarR/DUF24-family repressors YodB and CatR control expression of the catechol dioxygenase CatE in Bacillus subtilis. J Bacteriol 192: 4571–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Zentella M, Mendoza G, Membrillo-Hernandez J & Escamilla JE, (2003) A novel double heme substitution produces a functional bo3 variant of the quinol oxidase aa3 of Bacillus cereus. Purification and paratial characterization. J Biol Chem 278: 31473–31478. [DOI] [PubMed] [Google Scholar]
- Dempwolff F, Sanchez S & Kearns DB, (2020) TnFLX: a third-generation mariner-based transposon system for Bacillus subtilis. Appl Environ Microbiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner MJ, Ma Z, Fuangthong M & Helmann JD, (2012) Derepression of the Bacillus subtilis PerR peroxide stress response leads to iron deficiency. J Bacteriol 194: 1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AW, Osman D & Robinson NJ, (2014) Metal preferences and metallation. J Biol Chem 289: 28095–28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch VN, Loi VV, Busche T, Sommer A, Tedin K, Nurnberg DJ, Kalinowski J, Bernhardt J, Fulde M & Antelmann H, (2019) The MarR-Type Repressor MhqR Confers Quinone and Antimicrobial Resistance in Staphylococcus aureus. Antioxid Redox Signal 31: 1235–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Montes de Oca LY, Chagolla-Lopez A, Gonzalez de la Vara L, Cabellos-Avelar T, Gomez-Lojero C & Gutierrez Cirlos EB, (2012) The composition of the Bacillus subtilis aerobic respiratory chain supercomplexes. J Bioenerg Biomembr 44: 473–486. [DOI] [PubMed] [Google Scholar]
- Grunenwald CM, Choby JE, Juttukonda LJ, Beavers WN, Weiss A, Torres VJ & Skaar EP, (2019) Manganese Detoxification by MntE Is Critical for Resistance to Oxidative Stress and Virulence of Staphylococcus aureus. MBio 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedon E, Moore CM, Que Q, Wang T, Ye RW & Helmann JD, (2003) The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and sigmaB regulons. Mol Microbiol 49: 1477–1491. [DOI] [PubMed] [Google Scholar]
- Gyan S, Shiohira Y, Sato I, Takeuchi M & Sato T, (2006) Regulatory loop between redox sensing of the NADH/NAD(+) ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J Bacteriol 188: 7062–7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer ND, Schurig-Briccio LA, Gerdes SY, Gennis RB & Skaar EP, (2016) CtaM Is Required for Menaquinol Oxidase aa3 Function in Staphylococcus aureus. mBio 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K, (1987) Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12: fur not only affects iron metabolism. Mol Gen Genet 210: 135–139. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V & Kanthasamy AG, (2019) Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci 13: 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L, (2012) Heme A biosynthesis. Biochim Biophys Acta 1817: 920–927. [DOI] [PubMed] [Google Scholar]
- Helmann JD, (2014) Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem 289: 28112–28120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohle TH & O’Brian MR, (2014) Magnesium-dependent processes are targets of bacterial manganese toxicity. Mol Microbiol 93: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Shin JH, Pinochet-Barros A, Su TT & Helmann JD, (2017) Bacillus subtilis MntR coordinates the transcriptional regulation of manganese uptake and efflux systems. Mol Microbiol 103: 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA, (2014) The mismetallation of enzymes during oxidative stress. J Biol Chem 289: 28121–28128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsrude MJ, Pitzer JE, Martin DW & Roop RM 2nd, (2019) The cation diffusion facilitator family protein EmfA confers resistance to manganese toxicity in Brucella abortus 2308 and is an essential virulence determinant in mice. J Bacteriol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juttukonda LJ & Skaar EP, (2015) Manganese homeostasis and utilization in pathogenic bacteria. Mol Microbiol 97: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Kumar V, Arora A, Tomar A, Ashish, Sur R & Dutta D, (2017) Affected energy metabolism under manganese stress governs cellular toxicity. Sci Rep 7: 11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Mercier R, Mickiewicz K, Serafini A, Sorio de Carvalho LP & Errington J, (2019) Crucial role for central carbon metabolism in the bacterial L-form switch and killing by beta-lactam antibiotics. Nat Microbiol 4: 1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai Y, Mercier R, Wu LJ, Dominguez-Cuevas P, Oshima T & Errington J, (2015) Cell growth of wall-free L-form bacteria is limited by oxidative damage. Curr Biol 25: 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn RJ, Buescher JM, Le Chat L, Jules M, Aymerich S & Sauer U, (2010) Metabolic fluxes during strong carbon catabolite repression by malate in Bacillus subtilis. J Biol Chem 285: 1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BM, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, Wapinski I, Galardini M, Cabal A, Peters JM, Hachmann AB, Rudner DZ, Allen KN, Typas A & Gross CA, (2017) Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Syst 4: 291–305 e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Breton Y, Mohapatra NP & Haldenwang WG, (2006) In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leelakriangsak M, Huyen NT, Towe S, van Duy N, Becher D, Hecker M, Antelmann H & Zuber P, (2008) Regulation of quinone detoxification by the thiol stress sensing DUF24/MarR-like repressor, YodB in Bacillus subtilis. Mol Microbiol 67: 1108–1124. [DOI] [PubMed] [Google Scholar]
- Li X & Imlay JA, (2018) Improved measurements of scant hydrogen peroxide enable experiments that define its threshold of toxicity for Escherichia coli. Free Radic Biol Med 120: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y & Helmann JD, (2012) Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol Microbiol 83: 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Faulkner MJ & Helmann JD, (2012) Origins of specificity and cross-talk in metal ion sensing by Bacillus subtilis Fur. Mol Microbiol 86: 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makui H, Roig E, Cole ST, Helmann JD, Gros P & Cellier MF, (2000) Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol Microbiol 35: 1065–1078. [DOI] [PubMed] [Google Scholar]
- Martin JE, Lisher JP, Winkler ME & Giedroc DP, (2017) Perturbation of manganese metabolism disrupts cell division in Streptococcus pneumoniae. Mol Microbiol 104: 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JE, Waters LS, Storz G & Imlay JA, (2015) The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11: e1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VD, Wolf C, Mader U, Lalk M, Langer P, Lindequist U, Hecker M & Antelmann H, (2007) Transcriptome and proteome analyses in response to 2-methylhydroquinone and 6-brom-2-vinyl-chroman-4-on reveal different degradation systems involved in the catabolism of aromatic compounds in Bacillus subtilis. Proteomics 7: 1391–1408. [DOI] [PubMed] [Google Scholar]
- Nolfi-Donegan D, Braganza A & Shiva S, (2020) Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol: 101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman D, Martini MA, Foster AW, Chen J, Scott AJP, Morton RJ, Steed JW, Lurie-Luke E, Huggins TG, Lawrence AD, Deery E, Warren MJ, Chivers PT & Robinson NJ, (2019) Bacterial sensors define intracellular free energies for correct enzyme metalation. Nat Chem Biol 15: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LD & Skaar EP, (2016) Transition Metals and Virulence in Bacteria. Annual Review of Genetics 50: annurev-genet-120215–035146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruthiyil S, Pinochet-Barros A, Huang X & Helmann JD, (2020) Bacillus subtilis TerC Family Proteins Help Prevent Manganese Intoxication. J Bacteriol 202. e00624–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi H, Wendel BM & Helmann JD, (2020) Dysregulation of Magnesium Transport Protects Bacillus subtilis against Manganese and Cobalt Intoxication. J Bacteriol 202. e00711–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que Q & Helmann JD, (2000) Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol Microbiol 35: 1454–1468. [DOI] [PubMed] [Google Scholar]
- Rosch JW, Gao G, Ridout G, Wang Y-D & Tuomanen EI, (2009) Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol 72: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC & Imlay JA, (2004) Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem 279: 48742–48750. [DOI] [PubMed] [Google Scholar]
- Sheldon JR & Skaar EP, (2019) Metals as phagocyte antimicrobial effectors. Curr Opin Immunol 60: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J.J.R.F.d. & Williams RJP, (2001) The Biological Chemistry of the Elements: The Inorganic Chemistry of Life Oxford University Press. [Google Scholar]
- Svensson B, Lubben M & Hederstedt L, (1993) Bacillus subtilis CtaA and CtaB function in haem A biosynthesis. Mol Microbiol 10: 193–201. [DOI] [PubMed] [Google Scholar]
- Towe S, Leelakriangsak M, Kobayashi K, Van Duy N, Hecker M, Zuber P & Antelmann H, (2007) The MarR-type repressor MhqR (YkvE) regulates multiple dioxygenases/glyoxalases and an azoreductase which confer resistance to 2-methylhydroquinone and catechol in Bacillus subtilis. Mol Microbiol 66: 40–54. [DOI] [PubMed] [Google Scholar]
- Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG & Walker MJ, (2015) Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. MBio 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF, Alexandre A & Lehninger AL, (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237: 408–414. [DOI] [PubMed] [Google Scholar]
- van der Oost J, von Wachenfeld C, Hederstedt L, Saraste M. Bacillus subtilis cytochrome oxidase mutants: biochemical analysis and genetic evidence for two aa3-type oxidases. Mol Microbiol 1991; 5(8): 2063–72. [DOI] [PubMed] [Google Scholar]
- Villani G, Tattoli M, Capitanio N, Glaser P, Papa S & Danchin A, (1995) Functional analysis of subunits III and IV of Bacillus subtilis aa3-600 quinol oxidase by in vitro mutagenesis and gene replacement. Biochim Biophys Acta 1232: 67–74. [DOI] [PubMed] [Google Scholar]
- von Pein JB, Stocks CJ, Schembri MA, Kapetanovic R & Sweet MJ, (2020) An alloy of zinc and innate immunity: Galvanising host defence against infection. Cell Microbiol: e13268. [DOI] [PubMed] [Google Scholar]
- Wang E, Bauer MC, Rogstam A, Linse S, Logan DT & von Wachenfeldt C, (2008) Structure and functional properties of the Bacillus subtilis transcriptional repressor Rex. Mol Microbiol 69: 466–478. [DOI] [PubMed] [Google Scholar]
- Winstedt L & von Wachenfeldt C, (2000) Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa(3) or cytochrome bd, is required for aerobic growth. J Bacteriol 182: 6557–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC, (2014) The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys Acta 1840: 730–738. [DOI] [PubMed] [Google Scholar]
- Wong HS, Dighe PA, Mezera V, Monternier PA & Brand MD, (2017) Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 292: 16804–16809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ding Z, Liu B, Yi SM, Li J, Zhang Z, Liu Y, Li J, Liu L, Zhou A, Gennis RB & Zhu J, (2020) Structure of the cytochrome aa3 −600 heme-copper menaquinol oxidase bound to inhibitor HQNO shows TM0 is part of the quinol binding site. Proc Natl Acad Sci U S A 117: 872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zheng C, Cao M, Zeng T, Zhao X, Shi G, Chen H & Bei W, (2017) The manganese efflux system MntE contributes to the virulence of Streptococcus suis serotype 2. Microb Pathog 110: 23–30. [DOI] [PubMed] [Google Scholar]
- Yi SM, Narasimhulu KV, Samoilova RI, Gennis RB & Dikanov SA, (2010) Characterization of the semiquinone radical stabilized by the cytochrome aa3-600 menaquinol oxidase of Bacillus subtilis. J Biol Chem 285: 18241–18251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Hederstedt L & Piggot PJ, (1995) The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J Bacteriol 177: 6751–6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni N, Maaheimo H, Szyperski T, Hohmann HP & Sauer U, (2004) The phosphoenolpyruvate carboxykinase also catalyzes C3 carboxylation at the interface of glycolysis and the TCA cycle of Bacillus subtilis. Metab Eng 6: 277–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that supports the findings of this study are available in the figures and supplementary material of this article, and/or are available from the authors upon request.


