Abstract
We investigated the hypothesis that acetate ameliorates brain-adipose metabolic dysfunction (BAMED) in high fat diet (HFD)-induced obesity, possibly by modulation of peroxisome proliferator-activated receptor-γ (PPAR-γ). Ten-week-old male Wistar rats were randomly assigned into four groups (n = 6/group): Control, acetate and obese with or without acetate groups received vehicle (distilled water; po), acetate (200 mg/kg, po) and 40% HFD with or without acetate respectively. The treatments lasted for 12 weeks. Obese animals showed increase in body weight, visceral fat mass, insulin and triglyceride-glucose index and a reduction in insulin sensitivity. In addition, obese animals also showed increase in plasma/hypothalamic and adipose pyruvate dehydrogenase kinase-4, lactate-pyruvate ratio, malondialdehyde, γ-glutamyl transferase, and a decrease in glucose-6-phosphate dehydrogenase, glutathione, nitric oxide and PPAR-γ. HFD also elevated plasma/hypothalamic lipid and decreased adipose lipid profile, increased hypothalamic and adipose tumor necrosis factor-α, interleukin-6 and histone deacetylase (HDAC), and elevated plasma/adipose leptin. These alterations were reversed by concomitant administration of acetate. The present results demonstrate that obesity is characterized by BAMED, which is accompanied by altered HDAC/PPAR-γ. The results in addition suggest that acetate, an HDAC inhibitor rescues BAMED with consequent normalization of body weight and visceral fat mass by modulation of PPAR-γ and suppression of oxidative stress.
Subject terms: Physiology, Endocrinology
Introduction
Obesity is a complex multifactorial metabolic disease, affecting more than 30% of global population as overweight or obese, and attributing to 8% of the annual worldwide mortality1. Based on the current prevalence, over 38% of the global population is estimated to be overweight or obese by 20302,3. Obesity was initially evident to be a problem of the developed countries and even as it continues to rise in many of these countries, its prevalence continues to trend upward in developing countries, including among the children and adolescents2,4. It is usually characterized with excessive adiposity and any of the following: insulin resistance, hyperglycemia and overweight1,5. Obesity constitutes a risk factor for a number of chronic diseases including type 2 diabetes mellitus (T2DM), cardiovascular diseases (CVDs), depression, renal disease, liver disease, reproductive dysfunction and cancer, and has adverse effects on overall health as well as global public health5–7. In fact, the economic and psychosocial burden of obesity coupled with its comorbidities is rapidly escalating.
The cause of obesity has been linked to behavioral (including sedentary lifestyle and consumption of high calorie-rich nutrients), environmental and genetic factors8,9. Previous studies reported that genetics influence the body mass index (BMI) which is a commonly used indicator of obesity and accounts for energy requirements, fuel utilization and muscle metabolic characteristics10–12, however, the current obesity epidemic has been shown to be principally driven by behavioral and environmental factors1. Sedentary lifestyle and consumption of high energy diet are the most common contributors to the energy imbalance that causes overweight and excessive fat deposition7,9. Nevertheless, the pathogenic processes underlying obesity are multifactorial and still under elucidation which posit a barrier for designing effective strategies towards prevention and management.
However, a number of previous investigations have documented leptin resistance as a critical factor in the development of obesity13. Physiologically, leptin is secreted by adipocytes in proportion to total fat mass14 and regulates energy balance by reducing energy intake or increasing energy expenditure13. It reduces energy intake by decreasing food or caloric intake through appetite inhibition in the hypothalamus, thereby preventing excess body weight15. It also increases energy expenditure by elevating lipid-glucose utilization, thus limiting excess energy storage or excessive fat deposition in the adipose tissue13,16. The regulatory functions of leptin occur by binding to hypothalamic leptin receptors, which signals to downstream effectors through the Janus kinase/signal transducers and activators of transcription factors (JAK/STAT) signaling pathway13. On the other hand, impaired leptin tolerance elicits metabolic signals that promote energy storage inform of fat deposition and caloric intake, which are crucial features of obesity, thus associating obesity with high leptin concentration15. Earlier studies have demonstrated leptin resistance in experimental rodents fed with high fat diet (HFD)17, suggesting that HFD consumption limits the action of leptin in the regulation of energy balance regardless of its concentration. Similarly, hyperleptinemia could also induce or aggravate leptin insensitivity by upregulating the suppressor of cytokine signalling-3 (SOCS-3) with consequent blockade of signal transduction of leptin17,18.
In addition, the development of obesity which is characterized by leptin resistance has been associated with inflammation both in peripheral tissues including adipose tissue and in hypothalamic areas critical for energy homeostasis19–21. Study by Thaler et al., reported the evidence of hypothalamic inflammatory signaling in rodents within 1 to 3 days of HFD exposure, prior to substantial weight gain22, suggesting the possible involvement of hypothalamic inflammation in the pathogenesis of obesity, unlike inflammation in the peripheral tissues which develops as a consequence of obesity. This study added that both reactive gliosis and markers suggestive of neuron injury were evident in the hypothalamic arcuate nucleus of rodents within the first week of HFD exposure and extends to mediobasal hypothalamus with chronic exposure22. Nevertheless, evidence reveals hypothalamic lesion in obese humans as assessed by magnetic resonance imaging23, suggesting that obesity is critically associated with hypothalamic injury in both humans and experimental rodents. Similarly, more investigations have demonstrated elevated level of circulating pro-inflammatory cytokines, proteases and growth factors derived from adipose tissue of obese rodents, which possibly contributes to an increased hypothalamic inflammatory signaling that mediates leptin resistance and weight gain17,20,21. However, the cellular interactions underlying the brain-adipose inflammatory response in the development of obesity is not clear.
Peroxisome proliferator-activated receptor-γ (PPAR- γ) is among the three isoforms of PPARS, which are nuclear hormone receptors that exert transcriptional control on genes housing PPAR-responsive regulatory elements and heterodimerize with retinoid X receptors24,25. PPARS are activated or repressed in response to regulatory networks of genes controlling various physiological processes that involve inflammation, adipogenesis, lipid-glucose metabolism and insulin sensitivity26,27. Defects in PPARs have been linked to metabolic stress and related syndrome, including the impaired adipose tissue remodeling and function28, which are detrimental during nutrient deprivation when adipose tissue remodeling is required for cellular survival. Earlier studies have demonstrated PPARs as lipid sensors that are capable of improving energy homeostasis when optimally activated29. Nonetheless, considering the therapeutic potential of PPARS activation in metabolic stress and related disorders, it would be of clinical relevance and public health interest to investigate the possible involvement of PPARs, especially PPAR-γ in brain-adipose inflammatory events underlying obesity.
Epigenetic modulation of key metabolic enzymes contributes to the pathogenesis of obesity30,31. Post-translational modification of proteins involving acetylation or deacetylation are governed by histone acetyltransferases (HAT) and histone deacetylases (HDACs) which transfers acetyl group to histone proteins and causes reversal of acetylation respectively. Evidence exists that the PPAR-γ gene is not only genetically regulated, but also regulated by epigenetic modulation, including histone modification and its regulatory enzymes such as HAT and HDACs32,33. Similarly, the functional roles of epigenetic enzymes, including HDACs in the PPAR-γ transcriptional pathway have been suggested34,35. In addition, certain post-translational modifications could also be mediated by pro-inflammatory and pro-oxidant species leading to suppression of gene expression36. Therefore, therapies targeting HDACs might be a potential promoter of PPAR-γ with possible attenuation of visceral adiposity, and in addition be a novel safer and effective intervention for the management of obesity. Acetate is a short chain fatty acid (SCFA) metabolite produced from distal gut microbiota that has been attributed to a wide range of protective health benefits37. Acetate is known to regulate metabolic activities of cells by inhibiting HDAC and/or activating G-protein coupled receptors signaling. It improves intestinal mucosa integrity/immunity, enhance insulin sensitivity and exert anti-inflammatory effects. Earlier studies demonstrated that oral supplementation with acetate exerts recovery from hepatic lipotoxicity38,39, neuroinflammation40, and hypertension41. However, the effects of acetate on disrupted brain-adipose metabolic link and/or brain-adipose inflammatory events underlying obesity was not known. The present study was therefore designed to test the hypothesis that SCFAs, acetate ameliorates defective brain-adipose metabolic network associated with high fat diet (HFD)-induced obesity in male Wistar rats, possibly by modulation of PPAR-γ.
Results
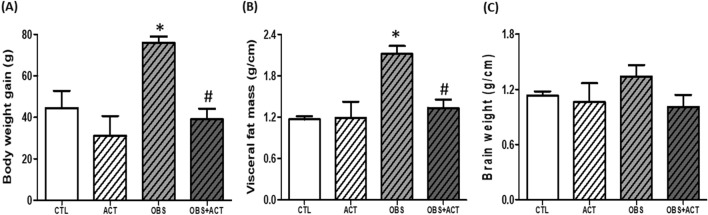
Acetate attenuates excess body weight gain and visceral fat mass in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in body weight and visceral fat mass in obese animals compared with control animals. However, the administration of acetate to obese plus acetate (OBS + ACT) group significantly reduced (p < 0.05) the body weight and visceral fat mass compared to the untreated obese animals and no significant difference when compared to the control group (Fig. 1A,B). Although, the brain weight did not significantly change in all the experimental groups compared to the control group (Fig. 1C).
Figure 1.
Effects of acetate on body weight gain (A), visceral fat mass (B) and brain weight (C) in high fat diet-induced obese Wistar rats. Body weight gain and visceral fat mass but not brain weight increased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control; OBS Obesity, ACT Acetate.
Acetate restores glucose homeostasis in high fat diet-induced obese male rats
Though the fasting blood glucose remained unchanged (p < 0.05) but a significant decrease in glucose tolerance and insulin sensitivity were observed in obese animals compared with control animals and these were significantly increased (p < 0.05) following the administration of acetate to OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group. In addition, there was a significant increase (p < 0.05) in fasting plasma insulin and triglyceride-glucose (TyG) ratio in obese animals compared with control animals and these were normalized following the administration of acetate to OBS + ACT group compared to the untreated obese group (Fig. 2).
Figure 2.
Effects of acetate on fasting blood glucose (A), oral glucose tolerance (B), fasting insulin (C), insulin sensitivity (D) and triglyceride-glucose index (E) in high fat diet-induced obese Wistar rats. The fasting blood glucose did not change, glucose tolerance was impaired, fasting insulin increased, insulin sensitivity decreased and TyG (surrogate marker of insulin resistance) increased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA, except for the oral glucose tolerance, where data were analyzed by repeated measures ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, QUICKI Quantitative check for insulin sensitivity index, TyG triglyceride-glucose index.
Acetate improves glucoregulatory enzymes in high fat diet-induced obese male rats
There was a significant decrease (p < 0.05) in plasma, hypothalamic and adipose glucose 6 phosphate dehydrogenase (G6PD) with corresponding increase in pyruvate dehydrogenase kinase-4 (PDK4) in obese animals compared with control animals. However, administration of acetate significantly increased (p < 0.05) the plasma, hypothalamic and adipose G6PD with corresponding decrease in PDK4 in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 3).
Figure 3.
Effects of acetate on plasma, hypothalamic and adipose Glucose-6-phosphate dehydrogenase (A–C) and Pyruvate dehydrogenase kinase-4 (D–F) in high fat diet-induced obese Wistar rats. Plasma, hypothalamic and adipose G6PD decreased while PDK4 increased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, G6PD Glucose-6-phosphate dehydrogenase, PDK-4 Pyruvate dehydrogenase kinase-4.
Acetate decreases lactate-pyruvate ratio and γ-glutamyl transferase (GGT) activity in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in plasma, hypothalamic and adipose lactate-pyruvate ratio with corresponding increase in GGT activity in obese animals compared with control group. Nevertheless, administration of acetate significantly decreased (p < 0.05) the plasma, hypothalamic and adipose lactate-pyruvate ratio and GGT activity in OBS + ACT group compared to the untreated obese animals and no significant difference when compared to the control group. Nonetheless, hypothalamic lactate-pyruvate ratio was significantly higher OBS + ACT group when compared to the control group (Fig. 4).
Figure 4.
Effects of acetate on plasma, hypothalamic and adipose lactate-pyruvate ratio (A–C) and γ-Glutamyl transferase activity (D–F) in high fat diet-induced obese Wistar rats. Plasma, hypothalamic and adipose lactate-pyruvate ratio and GGT increased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, GGT γ-Glutamyl transferase.
Acetate normalizes lipid profile in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in plasma triglyceride/high-density lipoprotein cholesterol (TG/HDLc) and total cholesterol/HDLc (TC/HDLc) and hypothalamic TG and TC in obese animals compared with control animals. However, administration of acetate attenuated dyslipidemia and hypothalamic lipid accumulation in OBS + ACT group compared to the untreated obese group. Besides, adipose TG and TC decreased significantly (p < 0.05) in obese animals compared with control animals, which were attenuated following the administration of acetate in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 5).
Figure 5.
Effects of acetate on plasma, hypothalamic and adipose lipid profile (A–F) in high fat diet-induced obese Wistar rats. Plasma TG/HDLc and TC/HDLc and hypothalamic TG and TC increased while adipose TG and TC decreased in obese rats, which were reversed following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, TG Triglyceride, TC total cholesterol, HDLc High-density lipoprotein cholesterol.
Acetate attenuates lipid peroxidation and glutathione depletion in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in plasma, hypothalamic and adipose malondialdehyde (MDA) and a decrease in glutathione (GSH) concentration in obese animals compared with control animals. Nevertheless, administration of acetate significantly decreased plasma, hypothalamic and adipose MDA with corresponding increase in GSH concentration in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 6).
Figure 6.
Effects of acetate on plasma, hypothalamic and adipose malondialdehyde (A–C) and glutathione (D–F) in high fat diet-induced obese Wistar rats. Plasma, hypothalamic and adipose MDA increased while glutathione decreased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, MDA Malondialdehyde, GSH Glutathione.
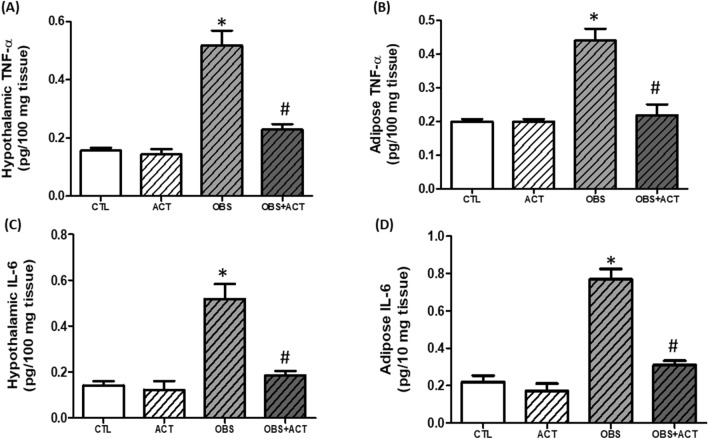
Acetate decreases hypothalamic and adipose inflammatory biomarkers in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in hypothalamic and adipose tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in obese animals compared with control animals and these alterations were reversed following the administration of acetate to OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 7).
Figure 7.
Effects of acetate on hypothalamic and adipose TNF-α (A–C) and IL-6 (D–F) in high fat diet-induced obese Wistar rats. Hypothalamic and adipose TNF-α and IL-6 increased in obese rats, which were attenuated following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, TNF-α Tissue necrosis factor-α, IL-6 Interleukin-6.
Acetate increases circulating, hypothalamic and adipose nitric oxide concentration in high fat diet-induced obese male rats
There was a significant decrease (p < 0.05) in plasma, hypothalamic and adipose nitric oxide concentration in obese animals compared with control animals. However, administration of acetate significantly increased (p < 0.05) the plasma, hypothalamic and adipose nitric oxide (NO) concentration in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 8).
Figure 8.
Effects of acetate on plasma, hypothalamic and adipose nitric oxide concentration (A–C) in high fat diet-induced obese Wistar rats. Plasma, hypothalamic and adipose NO increased in obese rats, which were reduced following the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, NO Nitric oxide.
Acetate decreases circulating and adipose leptin concentration in high fat diet-induced obese male rats
There was a significant increase (p < 0.05) in plasma and adipose leptin concentration in obese animals compared with control animals. However, administration of acetate normalized the circulating and adipose leptin concentration in OBS + ACT group compared to the untreated obese group. Nevertheless, plasma ghrelin concentration was not altered in obese group compared to the control group (Fig. 9).
Figure 9.
Effects of acetate on plasma and adipose leptin (A,B) and plasma ghrelin concentration in high fat diet-induced obese Wistar rats. Plasma and adipose leptin increased while ghrelin concentration remained unchanged and the alteration in leptin concentration was attenuated by the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate.
Acetate decreases hypothalamic and adipose HDAC in high fat diet-induced obese male rats
The hypothalamic and adipose levels of HDAC significantly increased (p < 0.05) in obese animals compared with control animals. Nevertheless, administration of acetate significantly decreased (p < 0.05) the hypothalamic and adipose HDAC levels in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 10).
Figure 10.
Effects of acetate on hypothalamic and adipose histone deacetylase (A,B) in high fat diet- induced obese Wistar rats. Hypothalamic and adipose HDAC, which were restored by the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, HDAC Histone deacetylase.
Acetate increases circulating, hypothalamic and adipose PPAR-γ in high fat diet-induced obese male rats
The plasma, hypothalamic and adipose levels of PPAR-γ significantly increased (p < 0.05) in obese animals compared with control animals. Nonetheless, administration of acetate significantly increased (p < 0.05) the plasma, hypothalamic and adipose levels of PPAR-γ in OBS + ACT group compared to the untreated obese group and no significant difference when compared to the control group (Fig. 11).
Figure 11.
Effects of acetate on plasma, hypothalamic and adipose Peroxisome proliferator-activated receptor-γ (A–C) in high fat diet-induced obese Wistar rats. Plasma, hypothalamic and adipose PPAR-γ decreased in obese rats, which were elevated by the administration of acetate. Data are expressed as mean ± SD. n = 6 and analyzed by one-way ANOVA followed by Bonferroni post hoc test. (*p < 0.05 vs. CTL; #p < 0.05 vs. OBS). CTL Control, OBS Obesity, ACT Acetate, PPAR-γ Peroxisome proliferator-activated receptor-γ.
Discussion
The key finding from the present study is that acetate as HDAC inhibitor attenuates defective hypothalamic-adipose metabolic network in HFD-induced obesity and this beneficial effect of acetate is accompanied by elevated PPAR-γ and suppressed oxidative stress. The results of the study in general showed that HFD induces obesity, which is characterized with increased body weight, visceral fat mass, fasting insulin and triglyceride-glucose index and decreased insulin sensitivity with unaltered blood glucose when compared to the control group. In addition, obese animals also showed increased plasma, hypothalamic and adipose PDK4, lactate-pyruvate ratio, GGT, lipid peroxidation (MDA) and decreased antioxidant capacity (G6PD and GSH), NO and PPAR-γ, elevated plasma and hypothalamic lipid and decreased adipose lipid profile, increased hypothalamic and adipose inflammation (TNF-α, IL-6) and HDAC with corresponding increase in adipose and circulating leptin compared to the control group. However, administration of acetate reversed these alterations with consequent normalization of body weight and visceral fat mass by suppression of oxidative stress and upregulation of PPAR-γ (Fig. 12).
Figure 12.
Schematic diagram showing the possible effects of acetate and the involvement of PPAR-γ in brain-adipose network in the development of obesity. HFD High fat diet, ACT Acetate, PPAR-γ Peroxisome proliferator-activated receptor-γ, HDAC Histone deacetylase.
Obesity has been well characterized by excess body weight and visceral fat mass with or without insulin resistance in both experimental rodents and humans5,7,9. The present results that high fat diet induced obesity with evidence of increased body weight gain and visceral adiposity when compared to the control group show consistency with earlier observations. As extension, the results showed that the present model of obesity is associated with insulin resistance and hyperinsulinemia as revealed with elevated TyG/reduced insulin sensitivity and increased fasting insulin concentration respectively. Although the fasting blood glucose was not affected when compared to control group despite the impaired glucose tolerance observed in obese animals. The findings here suggest that in addition to excess body weight or visceral adiposity, the obese animals are characterized with disrupted glucose homeostasis regardless of the normal blood glucose level that might have resulted from the compensatory hyperinsulinemia. Besides, the findings also imply that the obese animals are pre-diabetic, which is a dysmetabolic state of glucose level that is primarily characterized by impaired glucose tolerance, thus validating earlier observations that obesity constitutes an independent risk factor to T2DM and attendant CVDs5–7. Nevertheless, the brain weight of obese animals was not affected when compared to the control group, although the development of obesity has been associated with changes in organ weight, particularly, the liver, kidney and heart42,43. However, other studies have reported that changes in organ weight is dependent on the etiology of obesity44,45.
Disruption of glucose homeostasis observed in obese animals is a reflection of impaired glucose metabolism46,47 which is corroborated by dysregulated glucoregulatory enzymes, especially G6PD and PDK4, an isoform of PDK. Glucose 6 phosphate dehydrogenase and PDK4 are critical metabolic enzymes that are involved in energy production inform of ATP either through glycolysis or pentose phosphate pathway (PPP)48,49. Glucose 6 phosphate dehydrogenase is the first step and rate-limiting enzyme in the oxidative stage of PPP, which results in production of ribose-5-phosphate49, while PDK, including PDK4 inhibits pyruvate dehydrogenase, an enzyme that regulates the oxidative metabolism of glucose through pyruvate dehydrogenase complex that promotes the conversion of pyruvate to acetyl coA50,51. In the present study, there was a significant decrease in plasma, hypothalamic and adipose G6PD with corresponding increase in PDK4 in obese animals compared to control group. These results therefore indicate that glucose metabolism or utilization is impaired in obese animals, which seems similar to previous studies, including studies from our laboratory that reported elevated PDK in animal models of obesity52,53 and in obese humans54. In addition, impaired glucose metabolism or utilization for ATP production favors conversion of pyruvate into lactate as revealed by elevated plasma, hypothalamic and adipose lactate-pyruvate ratio in obese animals compared with control group. Lactate-pyruvate ratio is a marker of cytosolic redox status55, and its increased level in obese animals possibly triggered hypothalamic and adipose tissue ischemia which moved both tissues toward proinflammatory phenotypes (elevated TNF-α and IL-6) and oxidative stress (increased MDA and decreased GSH) and aggravated by NO depletion. These perhaps led to tissue injuries as biochemically characterized by elevated circulating, hypothalamic and adipose GGT activity in obese animals compared to the control group. Therefore, the present findings suggest that impaired glucose metabolism or utilization at least in part promotes hypothalamic-adipose tissue injuries in obese animals.
Furthermore, the development of insulin resistance in obese animal reduced sensitivity of metabolic tissues including adipose tissue to insulin action as revealed by QUICKI, this possibly increased lipolysis with corresponding increase in circulating lipids such as TG/HDLc and TC/HDLc resulting in excess lipid influx into the non-metabolic tissues, including hypothalamic tissue with correspondent elevated TG and TC compared to the control group. The deposition of excess lipid in the hypothalamus has been earlier reported to reduce insulin sensitivity with corresponding decrease in hypothalamic insulin-mediated glucose uptake56. This possibly caused hypothalamic metabolic stress or lipotoxicity that triggered hypothalamic inflammatory response as revealed by elevated levels of hypothalamic TNF-α and IL-6 in obese animals compared to the control group. The observation here is in consonance with previous studies that reported the evidence of hypothalamic inflammatory signaling in rodents within 1 to 3 days of HFD exposure, prior to substantial weight gain due to energy imbalance22. Besides, hypothalamic metabolic stress could also be responsible for increased lipid peroxidation (MDA) with corresponding decrease in GSH, which possibly triggered hypothalamic oxidative stress with consequent injuries as revealed by elevated GGT activity in obese animals compared to the control group. On the other hand, adipose TG and TC significantly decreased in obese animals compared to control group. This observation is consistent with earlier studies, including a recent study from our laboratory, which reported that insulin-resistant adipose tissue is characterized with increased white visceral adiposity, that remodels and expands causing adipose dysfunction with corresponding decrease in adipose lipids including TG and FFA57,58 due to increased lipolysis. This further aggravates systemic and peripheral insulin resistance that underlie complications associated with obesity5–7. In addition, previous studies reported that HFD provoked insulin resistance-driven adipose remodeling, which often overwhelmed in chronic condition causing numerous macrophages invasion surrounding the dead adipocytes, thus triggering a crosstalk between the lipid, macrophages and toll like receptors located on the macrophages with consequent induction of proinflammatory cytokines59,60 as validated in the present study with elevated levels of circulating and adipose TNF-α and IL-6 in obese animals compared to the control group. The elevated levels of TNF-α and IL-6 in the adipose tissue are due to the enlargement of white adipose tissue (increased adiposity), which characterized obese animals causing the recruitment and activation of immune cell subsets in the white adipose tissue, which systemically induces the low-grade inflammation that in turns aggravate systemic and peripheral insulin resistance, contributing to the complications of obesity as earlier reported25,60. Therefore, the results herein imply that hypothalamic-adipose-lipid dysmetabolism drives hypothalamic/adipose inflammatory response that characterized obesity.
Besides, increase in visceral adipocyte possibly contributed to elevated leptin production in obese animals compared with control group. This observation is in consonance with earlier studies that reported a positive correlation between leptin and adipocytes in obese humans14,61,62. However, hypothalamic inflammatory response reduced the sensitivity of the hypothalamus to leptin (leptin resistance) leading to an increase in the plasma concentration of leptin in obese animals compared to control group. Hence the present results are consistent with earlier investigations by Thaler and Schwartz and Bittencourt et al., who demonstrated hyperleptinemia with consequent energy dysregulation in obese rodents that suffer hypothalamic lesions predominant with inflammation20,21. In addition, the results of the present study did not show significant alteration in the circulating level of ghrelin in obese animals compared to the control group, though earlier studies have shown inconsistent results in the status of ghrelin in obese individuals63–65. Therefore, the findings from the present data suggest that obesity may be independent of ghrelin concentration but is characterized by hyperleptinemia, which is attributable to increased visceral adiposity and leptin resistance invoked by hypothalamic inflammatory mediators.
Notably, there was a significant reduction in the plasma, hypothalamic and adipose concentration of PPARS-γ in obese animals compare to the control group. This reduction in PPAR-γ corresponded with increased visceral adiposity and bodyweight as well as impaired hypothalamic and adipose gluco-lipid metabolism, excessive lipid peroxidation, G6PD/GSH and NO depletion, elevated TNF-α/IL-6 and plasma or adipose leptin concentration that characterized obese animals. Similar to the present observations, deficiency of PPARs has been demonstrated in metabolic stress with consequent impaired adipogenic remodeling28, a pathological process that also occurs during adipose expansion and often results in nitric oxide deficiency and elevated inflammatory mediators as observed in obese animals compared to control group. Besides, earlier studies have demonstrated that administration of PPAR-γ ligands such as thiazolidinediones exert insulin sensitizing and anti-inflammatory effects through action on adipocytes and are thus widely used to treat metabolic-related disorders such as T2DM and atherosclerosis among others25. The present results in addition showed a significant increase in hypothalamic and adipose HDAC in obese animals when compared to the control group, which seems to be in agreement with earlier studies that identified many epigenetic regulators, including HDAC as an interacting partner with PPAR-γ35,66. Similarly, the unliganded PPAR-γ heterodimeric complex is reported to be associated with a multicomponent corepressor complex that contains HDAC activity. After binding to the PPAR-γ ligand, the corepressor complex is dismissed, and the coactivator complex that possesses histone acetylase activity is recruited to the PPAR-γ heterodimer. This leads to chromatin remodeling, which facilitates active transcription67. Therefore, HDAC inhibitor could be a potential agonist of PPAR-γ and a possible suitable therapeutic intervention to obesity.
Intriguingly, administration of acetate, an inhibitor of HDAC significantly increased plasma, hypothalamic and adipose PPAR-γ concentration with corresponding improvement in insulin sensitivity and glucose regulation as well as hypothalamic and adipose lipid metabolism, lipid peroxidation, G6PD/GSH-dependent antioxidant capacity, NO, inflammatory mediators and normalized leptin concentration, visceral adiposity and body weight in OBS + ACT group compared to untreated obese group. A number of studies have documented acetate as HDAC inhibitor39,68,69, and acetate has been earlier shown to improve insulin sensitivity and exert anti-inflammatory effects against neuroinflammation, hypertension and hyperandrogenism among others38,40,41,70. Similarly, the present results are also in line with the observation of Lee et al., who revealed that the inhibition of HDAC by MPT0E014 attenuated cardiomyopathy by upregulation of PPAR-γ71. However, to the best of our knowledge, the results of the present study suggest for the first time that inhibition of HDAC by acetate ameliorates disrupted hypothalamic-adipose metabolic network with consequent normalization of visceral adiposity in HFD-induced obesity by modulation of PPAR-γ. Although the present study is not without a limitation in such that the molecular mechanism underlying the regulatory role of acetate was not fully investigated. Nevertheless, the present data provides justification for future investigation of molecular mechanism and possibly holds public health interest or clinical relevance for prevention and management of obesity and its comorbidities.
In conclusion, the present results demonstrate that obesity is characterized with aberrant brain-adipose metabolic network, which is accompanied by altered HDAC and PPAR-γ. The results in addition suggest that acetate, an HDAC inhibitor rescues defective brain-adipose metabolic network with consequent normalization of body weight/visceral fat mass and metabolic homeostasis by modulation of PPAR-γ and suppression of oxidative stress. Hence, nutritional modifications that incorporate acetate engendered high fiber diet might be beneficial to obese individuals.
Materials and methods
Experimental animals and grouping
The study was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the protocol was approved by the Ethical Review Board of Afe Babalola University, Ado-Ekiti, Nigeria (ABUADERC/2020/06), and every effort was made to minimize both the number of animals used and their suffering. All the experiments were also performed and reported in accordance with the ARRIVE guidelines 2.0. Ten-week-old male Wistar rats weighing 160–180 g were procured from the animal house of the College of Health Sciences, Afe Babalola University, Ado-Ekiti. Rats had unhindered access to standard rat chow and tap water. After two weeks of acclimatization, the animals were randomly assigned into four groups (n = 6 per group) namely: Control (CTL), ACT-treated group, OBS group and OBS + ACT group. Rats were maintained in a colony under standard environmental conditions of temperature (22–26 °C), relative humidity (50–60%), and 12-h dark/light cycle.
Induction of obesity
Obesity was experimentally induced by exposing the animals to 40% high fat diet prepared as described previously72 and administered for 12 weeks.
Treatment
Control group received vehicle (standard rat chow and distilled water; po), ACT-treated group received standard rat chow and sodium acetate (200 mg/kg; po), OBS group receive 40% HFD and distilled water (po0 and OBS + ACT-treated group received 40% high fat diet plus 200 mg/kg of sodium acetate. The treatment lasted for 12 weeks. Initial and final body weights were determined as shown in the supplementary table 1, and weight gain was estimated.
Determination of visceral fat mass
After dissection, visceral fat mass was assessed as previously described58,73 by isolating and weighing the total perirenal, retroperitoneal, and abdominal fat pad and corrected to tibial length to eliminate variability.
Oral glucose tolerance test (OGTT) and insulin resistance (IR)
The OGTT was performed 48 h prior to the sacrifice of the rats. The rats had 12-h overnight fast. Animals were loaded with glucose (2 g/kg; po). Blood was obtained from the tail before glucose load and then sequentially at 30, 60, 90 and 120 min. Glycemic levels were determined with a hand-held glucometer (ONETOUCH-LIFESCAN, Inc., Milpitas). Insulin sensitivity was determined using quantitative insulin sensitivity check index (QUICKI), which is expressed as 1/[log (fasting insulin) + log (fasting glucose)73,74, while IR was determined using its surrogate, triglyceride-glucose index, which is expressed as TyG = Ln (TG (mg/dl) × FPG (mg/dl)/2)51,53,75.
Sample preparation
At the end of the treatment, the rats were anesthetized with sodium pentobarbital (50 mg/kg; iv). Blood was collected by cardiac puncture into heparinized tube and was centrifuged at 704 g for 5 min at room temperature. Plasma was stored frozen until it is required for biochemical assay.
Preparation of brain and adipose tissue homogenates
After weighing the brain and visceral fat mass, 100 mg section of the hypothalamus and visceral fat tissue were carefully removed and homogenized with a glass homogenizer in phosphate buffer solution, centrifuged at 8000 g for 10 min at 4 °C.
Biochemical analysis
Insulin concentration
The plasma level of insulin was determined using Rat ELISA kits with Catalog No. IS130D obtained from Calbiotech Inc. (Cordell Ct., El Cajon, CA 92,020, USA). This method is based on the direct sandwich technique in which two monoclonal antibodies are directed against separate antigenic determinants on the insulin molecule.
Leptin and ghrelin
The plasma and tissue concentration of leptin was determined using Rat ELISA kits with Catalog No. E-EL-R0582 obtained from Elabscience Biotechnology Inc. (Wuhan, Hubei, P.R.C., China). Similarly, plasma concentration of ghrelin was determined using Rat ELISA kits with Catalog No. E-EL-R0842 obtained from Elabscience Biotechnology Inc. (Wuhan, Hubei, P.R.C., China).
Lipid profile and atherogenic indices
Triglycerides, TC and HDLc were estimated in the plasma, adipose and hypothalamus by standardized colorimetric methods using kits with Catalog No. BXC0271, BXC0261 and BXC0422A respectively obtained from Fortress Diagnostics Ltd. (Antrim, UK). Atherogenic indices were estimated as TG/HDLc and TC/HDLc in line with earlier description51,53,76.
Lipid peroxidation and antioxidant capacity
Malondialdehyde is a marker of lipid peroxidation and GSH reflect cellular antioxidant capacity of the cells. Malondialdehyde was determined from the plasma, adipose tissue and hypothalamus by standard non-enzymatic spectrophotometric method using assay kits from Randox Laboratory Ltd. (Co. Antrim, UK). This method involves the reaction of MDA in the sample with thiobarbituric acid (TBA) to generate MDA-TBA adduct, which was quantified spectrophotometrically, whereas GSH was determined using a non-enzymatic spectrophotometric method with assay kits obtained from Oxford Biomedical Research Inc. (Oxford, USA). Glutathione determination by spectrophotometric method was based on the oxidation of GSH in the sample by the sulfhydryl reagent 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) to form the yellow derivative 5′-thio-2-nitrobenzoic acid (TNB), measured at 412 nm.
Glucoregulatory enzymes
Glucose-6-phosphate dehydrogenase activity was determined from the plasma, adipose tissue and hypothalamus using standard spectrophotometric method, whereas PDK4 level was determined from the plasma, visceral fat and hypothalamus using Rat ELISA kits obtained from Calbiotech Inc. (El Cajon, USA).
Nitric oxide concentration
Plasma and tissue NO were assayed spectrophotometrically by measuring the accumulation of its stable degradation products, nitrate and nitrite using kits from Oxford Biomedical Research Inc., (Oxford, UK). This kit employs the NADH-dependent enzyme nitrate reductase for conversion of nitrate to nitrite prior to the quantification of nitrite using Griess reagent—thus providing for accurate determination of total NO production.
Tumor necrosis factor-α and interleukin-6
Plasma and tissue concentration of TNF- α and IL-6 were determined by the quantitative standard sandwich rat ELISA technique using monoclonal antibody specific for these parameters using assay kits with Catalog No. E-EL-R0019 and Catalog No: E-EL-R0015 respectively, obtained from Elabscience Biotechnology Inc. (Wuhan, Hubei, P.R.C., China).
Histone deacetylase
The adipose and hypothalamic tissue levels of HDAC were determined using Rat ELISA kits with Catalog No. E0791Ra, obtained from Bioassay Technology Laboratory (Yangpu Dist. Shanghai 200,090, China), in compliance with the manufacturer’s procedure.
Peroxisome proliferator-activated receptor-γ
Plasma and tissue concentration of PPAR- γ was determined by the quantitative standard sandwich ELISA technique using monoclonal antibody specific for this parameter with rat assay kit (Catalog No. E-EL-R072) obtained from Elabscience Biotechnology Inc. (Wuhan, Hubei, P.R.C., China).
Pyruvate, lactate and γ-glutamyl transferase
Plasma and tissue concentration of pyruvate and lactate were determined by non-enzymatic standard colorimetric method using assay reagent kits obtained from Randox Laboratory Ltd. (Co. Antrim, UK) respectively. Whereas, plasma, adipose and hypothalamic GGT activity was measured by standardized enzymatic colorimetric method using assay kit obtained from Randox Laboratory Ltd. (Co. Antrim, UK).
Data analysis and statistics
The data were confirmed to be normally distributed using Shapiro–Wilk test and. All data were represented as means ± SD. Graphpad prism 9.2.0 was used to perform the statistical group analysis, and the mean values were compared using one-way ANOVA except for the oral glucose tolerance, where data were analyzed by repeated measures ANOVA. Then post hoc analysis was performed by Bonferroni’s test and statistically significant difference was determined at p < 0.05.
Ethical statement
The study was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the protocol was approved by the Ethical Review Board of Afe Babalola University, Ado-Ekiti, Nigeria (ABUADERC/2020/06), and every effort was made to minimize both the number of animals used and their suffering.
Supplementary Information
Acknowledgements
The authors appreciate the technical support of Bridge Biotech, Ilorin, Nigeria.
Author contributions
The study was conceived and designed by K.S.O. The experiment was conducted by K.S.O., M.N.O., C.L.A. and T.B.A. The data were analyzed and interpreted by K.S.O. The manuscript was drafted by K.S.O., C.L.A., M.N.O., T.B.A. and B.Y.A. and all the authors read, revised and approved the final manuscript for submission.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98605-5.
References
- 1.World Health Organization. Overweight and obesity. Geneva: World Health Organization; 2018. Available.from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2018).
- 2.Hruby A, Hu FB. The epidemiology of obesity: A big picture. Pharmacoecon. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health. 2017;14(4):435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacha F, Gidding SS. Cardiac abnormalities in youth with obesity and type 2 diabetes. Curr. Diab. Rep. 2016;16(7):62. doi: 10.1007/s11892-016-0750-6. [DOI] [PubMed] [Google Scholar]
- 5.Ng M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J. Am. Col. Cardiol. 2013;62(15):1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 7.Forouzanfar MH, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. June 3–5, 1997. WHO/NUT/NCD/ 98.1, i-xv, 1–276. 1998. Geneva, Switzerland: World Health Organization. Available at: http://dosei.who.int/uhtbin/cgisirsi/WedNov 2123:21:20MET2001/0/49 (2002). [PubMed]
- 9.Racette SB, Deusinger SS, Deusinger RH. Obesity: Overview of prevalence, etiology, and treatment. Phys. Ther. 2003;83(3):276–288. doi: 10.1093/ptj/83.3.276. [DOI] [PubMed] [Google Scholar]
- 10.Stunkard AJ, Foch TT, Hrubec Z. A twin study of human obesity. JAMA. 1986;256(1):51–54. doi: 10.1001/jama.1986.03380010055024. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian PS, Goldenberg-Cohen N, Shukla S, Cheskin LJ, Miller NR. Plasma ghrelin levels are normal in obese patients with idiopathic intracranial hypertension (pseudotumor cerebri) Am. J. Ophthal. 2004;138(1):109–113. doi: 10.1016/j.ajo.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Sengier A. Multifactorial etiology of obesity: Nutritional and central aspects. Revue Med. de Bruxell. 2005;26(4):S211–S214. [PubMed] [Google Scholar]
- 13.Fam BC, Thorburn AW, et al. Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diab. Obes. Metab. 2007;6:840–852. doi: 10.1111/j.1463-1326.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 14.Frederich RC, et al. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 15.Chrysafi P. Leptin alters energy intake and fat mass but not energy expenditure in lean subjects. Nat. Commun. 2020;11(1):1–5. doi: 10.1038/s41467-020-18885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CZ, et al. Leptin selectively regulates nutrients metabolism in Nile tilapia fed on high carbohydrate or high fat diet. Front. End. 2018;9:574. doi: 10.3389/fendo.2018.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch CE, et al. High-fat diet induces leptin resistance in leptin-deficient mice. J. Neuroendocrinol. 2014;26(2):58–67. doi: 10.1111/jne.12131. [DOI] [PubMed] [Google Scholar]
- 18.El-Haschimi K, Pierroz DD, Hileman SM, Bjørbæk C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Inves. 2000;105(12):1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison CD, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased Nrf2 signaling. J. Neurochem. 2010;14(6):1581–1589. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaler JP, Schwartz MW. Minireview: Inflammation and obesity pathogenesis: The hypothalamus heats up. Endocrinol. 2010;151(9):4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittencourt A, et al. High fat diet-induced obesity causes a reduction in brain tyrosine hydroxylase levels and non-motor features in rats through metabolic dysfunction, neuroinflammation and oxidative stress. Nutr. Neurosci. 2020;78:1–5. doi: 10.1080/1028415X.2020.1831261. [DOI] [PubMed] [Google Scholar]
- 22.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin Inves. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth CL, et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obes. 2015;23(6):1226–1233. doi: 10.1002/oby.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venteclef N, Jakobsson T, Steffensen KR, Treuter E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metab. 2011;22(8):333–343. doi: 10.1016/j.tem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Corrales P, Vidal-Puig A, Medina-Gómez G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int. J. Mol. Sci. 2018;19(7):2124. doi: 10.3390/ijms19072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubrac S, et al. Peroxisome proliferator-activated receptor-α activation inhibits Langerhans cell function. J. Immunol. 2007;178(7):4362–4372. doi: 10.4049/jimmunol.178.7.4362. [DOI] [PubMed] [Google Scholar]
- 27.Vitale SG, et al. Peroxisome proliferator-activated receptor modulation during metabolic diseases and cancers: master and minions. PPAR Res. 2016;651:7313. doi: 10.1155/2016/6517313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tontonoz P, Spiegelman BM. Fat and beyond: The diverse biology of PPARγ. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto Y, et al. Activation of peroxisome proliferator-activated receptor gamma/small heterodimer partner pathway prevents high fat diet-induced obesity and hepatic steatosis in Sprague-Dawley rats fed soybean meal. J. Nutr. Biochem. 2020;75:108250. doi: 10.1016/j.jnutbio.2019.108250. [DOI] [PubMed] [Google Scholar]
- 30.Huang Q, et al. Mechanistic insights into the interaction between transcription factors and epigenetic modifications and the contribution to the development of obesity. Front. Endocrinol. 2018;9:370. doi: 10.3389/fendo.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsen MJ, Guerin M, et al. Epigenetic and transcriptomic characterization of pure adipocyte fractions from obese pigs identifies candidate pathways controlling metabolism. Front. Genet. 2019;10:1268. doi: 10.3389/fgene.2019.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin LC, Hsu SL, Wu CL, Liu WC, Hsueh CM. Peroxisome proliferator-activated receptor γ (PPARγ) plays a critical role in the development of TGFβ resistance of H460 cell. Cell Signal. 2011;23(10):1640–1650. doi: 10.1016/j.cellsig.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Cui TT, Xing TY, Chu YK, Li H, Wang N. Genetic and epigenetic regulation of PPARγ during adipogenesis. Yi Chuan Hereditas. 2017;39(11):1066–1077. doi: 10.16288/j.yczz.17-121. [DOI] [PubMed] [Google Scholar]
- 34.Wu SC, Zhang Y. Minireview: Role of protein methylation and demethylation in nuclear hormone signaling. Mol. Endocrinol. 2009;23(9):1323–1334. doi: 10.1210/me.2009-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugii S, Evans RM. Epigenetic codes of PPARγ in metabolic disease. FEBS Lett. 2011;585(13):2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283(32):21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama M, Kurebayashi R, Kawabe K, Takano K, Nakamura Y. Acetate attenuates lipopolysaccharide-induced nitric oxide production through an antioxidative mechanism in cultured primary rat astrocytes. Neurochem. Res. 2016;41:3138–3146. doi: 10.1007/s11064-016-2038-2. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olaniyi KS, Amusa OA. Sodium acetate-mediated inhibition of histone deacetylase alleviates hepatic lipid dysregulation and its accompanied injury in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2020;128:110226. doi: 10.1016/j.biopha.2020.110226. [DOI] [PubMed] [Google Scholar]
- 40.Soliman ML, Smith MD, Houdek HM, Rosenberger TA. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. J. Neuroinflamm. 2012;9(1):1–4. doi: 10.1186/1742-2094-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marques FZ, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circ. 2017;135(10):964–977. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 42.Waxler SH, Enger M. Organ weights and obesity in mice. J. Nutr. 1954;54(2):209–214. doi: 10.1093/jn/54.2.209. [DOI] [PubMed] [Google Scholar]
- 43.Marshall NB, Andrus SB, Mayer J. Organ weights in three forms of experimental obesity in the mouse. Am. J. Physiol.-Legacy Cont. 1957;189(2):343–346. doi: 10.1152/ajplegacy.1957.189.2.343. [DOI] [PubMed] [Google Scholar]
- 44.Mayer JRG, French CZ, Barknett RJ. Hypothalamic obesity. Am. J. Physiol. 1955;182:2. doi: 10.1152/ajplegacy.1955.182.1.75. [DOI] [PubMed] [Google Scholar]
- 45.Debons AF, et al. Localization of gold in mouse brain in relation to gold thioglucose obesity. Am. J. Physiol.-Legacy Cont. 1962;202(4):743–750. doi: 10.1152/ajplegacy.1962.202.4.743. [DOI] [PubMed] [Google Scholar]
- 46.Sacks DB. A1C versus glucose testing: A comparison. Diab. Care. 2011;34(2):518–523. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colak A, Diniz G. Impaired glucose tolerance, obesity and inflammatory mediators. Glucose Tol. 2012;27:2. [Google Scholar]
- 48.Wood T. Physiological functions of the pentose phosphate pathway. Cell Biochem. Funct. 1986;4(4):241–247. doi: 10.1002/cbf.290040403. [DOI] [PubMed] [Google Scholar]
- 49.Stanton RC. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life. 2012;64(5):362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chambers KT, et al. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J. Biol. Chem. 2011;286(13):11155–11162. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olaniyi KS, Olatunji LA. Oral ethinylestradiol-levonorgestrel attenuates cardiac glycogen and triglyceride accumulation in high fructose female rats by suppressing pyruvate dehydrogenase kinase-4. Naunyn-Schmiedeberg's Arch. Pharmacol. 2019;392(1):89–101. doi: 10.1007/s00210-018-1568-3. [DOI] [PubMed] [Google Scholar]
- 52.Wu CY, et al. A novel inhibitor of pyruvate dehydrogenase kinase stimulates myocardial carbohydrate oxidation in diet-induced obesity. J. Biol. Chem. 2018;293(25):9604–9613. doi: 10.1074/jbc.RA118.002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olaniyi KS, Olatunji LA. Inhibition of pyruvate dehydrogenase kinase-4 by l-glutamine protects pregnant rats against fructose-induced obesity and hepatic lipid accumulation. Biomed. Pharmacother. 2019;10:59–67. doi: 10.1016/j.biopha.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 54.Majer M, Popov KM, Harris RA, Bogardus C, Prochazka M. Insulin downregulates pyruvate dehydrogenase kinase (PDK) mRNA: Potential mechanism contributing to increased lipid oxidation in insulin-resistant subjects. Mol. Genet. Metab. 1998;65(2):181–186. doi: 10.1006/mgme.1998.2748. [DOI] [PubMed] [Google Scholar]
- 55.Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diab. 2013;62(5):1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Posey KA, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol.-Endocrinol. Metab. 2009;296(5):E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Inves. 2019;129(10):4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olaniyi KS, Olatunji LA. L-glutamine ameliorates adipose-hepatic dysmetabolism in OC-treated female rats. J. Endocrinol. 2020;246(1):1–12. doi: 10.1530/JOE-19-0582. [DOI] [PubMed] [Google Scholar]
- 59.Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diab. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 60.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J. Clin. Inves. 2011;121(6):094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Harmelen V, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diab. 1998;47(6):913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- 62.Song SO, et al. Leptin and adiponectin concentrations independently predict future accumulation of visceral fat in nondiabetic Japanese Americans. Obes. 2021;29(1):233–239. doi: 10.1002/oby.23035. [DOI] [PubMed] [Google Scholar]
- 63.Wren AM, et al. Ghrelin causes hyperphagia and obesity in rats. Diab. 2001;50(11):2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 64.Subramanian PS, et al. Plasma ghrelin levels are normal in obese patients with idiopathic intracranial hypertension (pseudotumor cerebri) Am. J. Ophthal. 2004;138(1):109–113. doi: 10.1016/j.ajo.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 65.Zhang CS, et al. The correlation between circulating ghrelin and insulin resistance in obesity: A meta-analysis. Front. Physiol. 2018;9:1308. doi: 10.3389/fphys.2018.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gadde KM, Martin CK, Berthoud HR, Heymsfield SB. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018;71(1):69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14(2):121–141. [PubMed] [Google Scholar]
- 68.Bolduc JF, Hany L, Barat C, Ouellet M, Tremblay MJ. Epigenetic metabolite acetate inhibits class I/II histone deacetylases, promotes histone acetylation, and increases HIV-1 integration in CD4+ T cells. J. Virol. 2017;91(16):e01943–e2016. doi: 10.1128/JVI.01943-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fellows R, et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018;9(1):1–5. doi: 10.1038/s41467-017-02651-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olatunji LA, Areola ED, Usman TO, Badmus OO, Olaniyi KS. Treatment with acetate during late pregnancy protects dams against testosterone-induced renal dysfunction. Heliyon. 2021;7(1):e05920. doi: 10.1016/j.heliyon.2021.e05920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee TI, et al. HDAC inhibition modulates cardiac PPARs and fatty acid metabolism in diabetic cardiomyopathy. PPAR Res. 2016;2:2. doi: 10.1155/2016/5938740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirai T, et al. High dietary fat–induced obesity in Wistar rats and type 2 diabetes in nonobese Goto-Kakizaki rats differentially affect retinol binding protein 4 expression and vitamin A metabolism. Nutr. Res. 2016;36(3):262–270. doi: 10.1016/j.nutres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 73.Schiavone S, et al. Visceral fat dysfunctions in the rat social isolation model of psychosis. Front. Pharmacol. 2017;8:787. doi: 10.3389/fphar.2017.00787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katz A, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 75.Guerrero-Romero F, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 76.Cheung AK, Wu LL, Kablitz C, Leypoldt JK. Atherogenic lipids and lipoproteins in hemodialysis patients. Am. J. Kid. Dis. 1993;22(2):271–276. doi: 10.1016/S0272-6386(12)70318-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.