Abstract
The fluid in the 14 distinct segments of the renal tubule undergoes sequential transport processes that gradually convert the glomerular filtrate into the final urine. The solute carrier (SLC) family of proteins is responsible for much of the transport of ions and organic molecules along the renal tubule. In addition, some SLC family proteins mediate housekeeping functions by transporting substrates for metabolism. Here, we have developed a curated list of SLC family proteins. We used the list to produce resource webpages that map these proteins and their transcripts to specific segments along the renal tubule. The data were used to highlight some interesting features of expression along the renal tubule including sex-specific expression in the proximal tubule and the role of accessory proteins (β-subunit proteins) that are thought to be important for polarized targeting in renal tubule epithelia. Also, as an example of application of the data resource, we describe the patterns of acid-base transporter expression along the renal tubule.
Keywords: acid-base, amino acid, proteomics, RNA-Seq, sugar
INTRODUCTION
The kidney is a multifunctional organ, but arguably, its chief function is transport. Classically, the excretion of a substance by the kidney is determined by its rates of glomerular filtration, of its reabsorption along the renal tubule, and of its secretion into the tubule lumen (1). The renal tubule is made up of at least 14 distinct segments, each with distinct transport processes. These transport processes are determined by which transporter genes are expressed. Transporters include ATPases, ion channels, and solute carriers (SLCs). The SLCs comprise a large family of transporters that have been previously catalogued on a publicly accessible web page (http://slc.bioparadigms.org/) (2). With the advent of large-scale genome sequencing projects circa 2000, new methods for transcriptomics and proteomics are being used to map every cell type in the body with regard to gene expression (3). Such mapping has moved forward in the kidney, both at the single-cell level (4–11) and the single-tubule level, the latter using renal tubule microdissection to isolate each of the 14 segments and carrying out RNA sequencing (RNA-Seq) for mRNA abundance profiling (12) and protein mass spectrometry for protein abundance profiling (13). These analyses have been published, although with relatively superficial attention to the distribution of SLC gene products. Here, we integrate data from multiple transcriptomic and proteomic studies of kidney tubules and cells to create maps of the distributions of each SLC along the renal tubule. The information is now provided as publicly accessible, user-friendly resource web pages.
SLC GENE LIST WEBPAGE
The primary goal of this work is to map the distributions of all SLC family proteins along the renal tubule from the S1 proximal tubule to the inner medullary collecting duct (IMCD) (Fig. 1A). As a prerequisite, we created a comprehensive list of SLC genes by collating information from Bioparadigms (https://www.bioparadigms.org/) and the UniProt Knowledge Base (https://www.uniprot.org/uniprot/). This list does not include active transporters (ATPases) or ion channels. To cast this list of SLC genes as a resource, we have established a publicly accessible web page that can be browsed or searched (https://esbl.nhlbi.nih.gov/Databases/SLC-list/). There are 431 SLC family genes in this database. We used this list to extract transcriptomic and proteomic data from genome-wide data sets as described in the following. We use official gene symbols to refer to individual proteins and transcripts as described in the Nomenclature Appendix, but also provide common name abbreviations throughout the text.1
Figure 1.
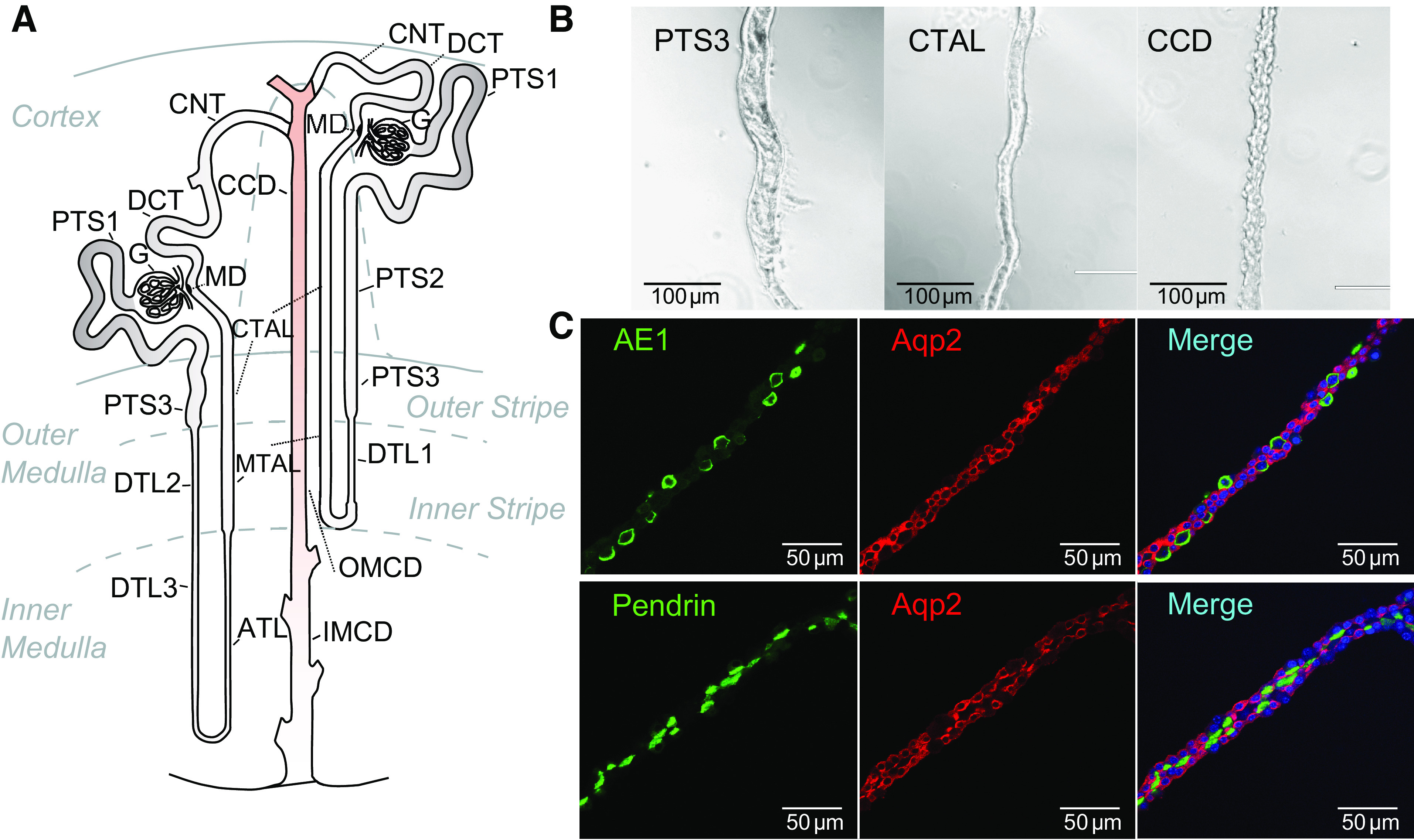
Mouse renal tubule nomenclature. A: diagram of renal tubule labeling all 14 segments (14–16). B: microscopic appearance of microdissected PTS3, CTAL, and CCD segments from mouse kidney. Segments can be readily identified under a zoom stereomicroscope with illumination from below the specimen. C: collecting ducts have a heterogeneous cell population as evidenced by immunofluorescence, including principal cells [expressing AQP2 (red)], type A intercalated cells [expressing AE1 (green, top)], and type B intercalated cells [expressing pendrin (green, bottom)]. Nuclei, labeled with DAPI are blue. ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; IMCD, inner medullary collecting duct; MD, macula densa; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct; PTS1, the initial segment of the proximal tubule; PTS2, proximal straight tubule in cortical medullary rays; PTS3, last segment of the proximal straight tubule in the outer stripe of outer medulla.
MAP OF mRNA LEVELS FOR ALL SOLUTE CARRIER FAMILY TRANSPORTERS ALONG THE RENAL TUBULE
We used a data set from experiments in which RNA-Seq was carried out in 14 different microdissected renal tubule segments of mouse (Fig. 1A) to map mRNA expression across the genome (14). Data for all SLCs were mined from this data set and are provided as a resource at https://esbl.nhlbi.nih.gov/Databases/SLC-kidney/. In all, 380 of the 431 SLC mRNAs were detected above a transcripts-per-million (TPM) level of 1 in at least one segment. Figure 1B shows features of 3 of the 14 microdissected segments, the S3 segment of the proximal tubule (PTS3), the cortical thick ascending limb (CTAL), and the cortical collecting duct (CCD). As shown in Fig. 1C, CCDs contain three distinct cell types and the transcriptomes of this segment as well as connecting tubule (CNT) and outer medullary collecting duct (OMCD) represent the combined transcriptomes of the three cell types. Targeted single-cell RNA-Seq (8) has been applied to resolve separate transcriptomes of the three cell types (https://esbl.nhlbi.nih.gov/Databases/scRNA-Seq/).
MAP OF PROTEIN LEVELS FOR ALL SOLUTE CARRIER FAMILY TRANSPORTERS ALONG THE RENAL TUBULE
We used a data set from experiments in which LC-MS/MS proteomics was carried out in 14 different renal tubule segments to map protein expression across the genome (13). Data for all SLCs were mined from this data set and are provided as a resource at https://esbl.nhlbi.nih.gov/Databases/SLC_Protein/. In all, 175 of the 431 SLC proteins were detected in at least one segment. The lower yield for proteomic identification versus transcriptomic identification is consistent with the lower sensitivity of the proteomic method. The protein levels may be, however, a better indication of gene expression as detected by immunochemical methods. RNA-Seq appears to be exquisitely sensitive, and it is conceivable that detectable transcripts can be present even in cells that do not express the corresponding protein at a functional level, especially if they have short half-lives. Conversely, in almost every case, gene expression detectable at a protein level is also detectable at a transcript level.
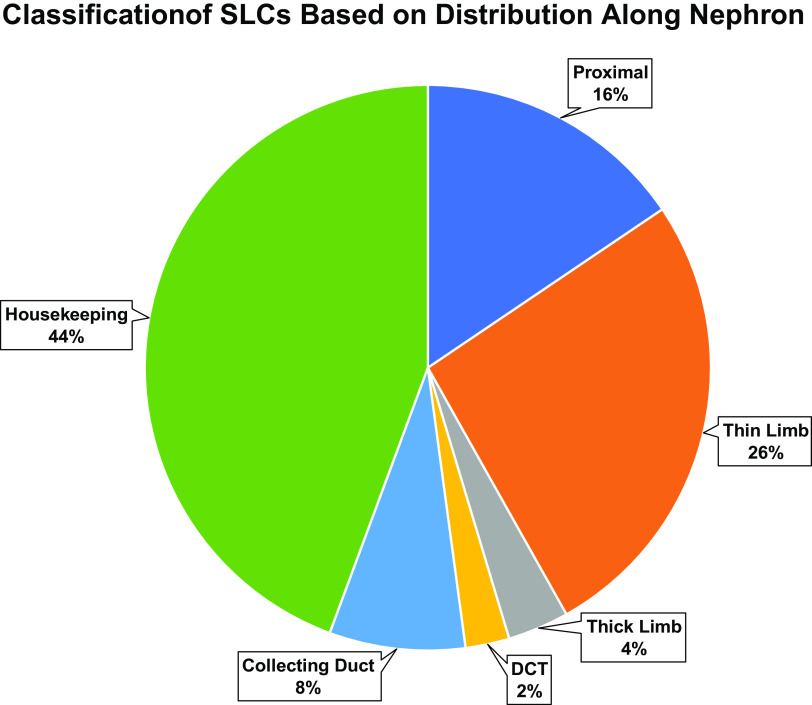
SPECIALIZED VERSUS HOUSEKEEPING TRANSPORTERS
“Housekeeping” proteins are those essential to the generalized function of all cells. We divided the kidney-expressed SLC proteins into housekeeping versus specialized, based on the distribution along the renal tubule. This classification is based on the idea that a housekeeping protein must be expressed in all cells. Of the 380 SLC mRNA species expressed in kidney tubule segments, 164 are expressed in all segments with a TPM of at least 1 using the data of Chen et al. (14). Of the remaining 216 transporters, 62 are selectively expressed in proximal tubule segments, 105 are selectively expressed in thin limb loop of Henle segments, 14 are selectively expressed in thick limb loop of Henle segments, 10 are selectively expressed in the distal convoluted tubule (DCT), and 31 are selectively expressed in collecting duct segments (Fig. 2). Perhaps most surprising about these data is the large number of SLCs expressed selectively in thin limb segments of the loop of Henle. In general, relative to the other segments, we know little about transport in the thin limbs. The most abundant of these are also expressed in the inner medullary collecting ducts, suggesting a role in osmotic regulation in the inner medulla, where solutes are concentrated to levels many fold greater than in circulating blood. An example is Slc6a12 (common term: BGT1; definition: betaine/GABA transporter 1), which transports betaine, one of the main stabilizing organic osmolytes of renal medullary cells (17).
Figure 2.
Distribution of SLC transporters among renal tubule regions. See text for details. ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; IMCD, inner medullary collecting duct; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct; PTS1, the initial segment of the proximal tubule; PTS2, proximal straight tubule in cortical medullary rays; PTS3, last segment of the proximal straight tubule in the outer stripe of outer medulla; SLC, solute carrier.
The macula densa is a specialized patch of the late cortical thick ascending limb of Henle that mediates tubuloglomerular feedback (TGF). Transcriptomic analysis of these cells using single-cell RNA-Seq has revealed their neuroepithelial character (18). Many SLCs are expressed in macula densa cells. Among the most abundant are Slc12a1 (common term: NKCC2; definition: sodium-potassium-chloride cotransporter 2), Slc5a1 (common term: SGLT1; definition: sodium-glucose transporter 1), Slc9a2 (common term: NHE2; definition: sodium-hydrogen exchanger 2), Slc16a7 (common term: MCT2; definition: monocarboxylate transporter 2), Slc29a1 (common term: ENT1; definition: equilibrative nucleoside transporter 1), and Slc3a2 (common term: 4F2HC; definition: 4F2 cell-surface antigen heavy chain).
The separate roles of housekeeping versus segment-selective SLC transporters are illustrated by the distribution of sugar transporters along the renal tubule.
SUGAR TRANSPORTER EXPRESSION ALONG THE RENAL TUBULE
Sugar transporters are important in the kidney for fulfilling two major roles. First, some sugar transporters mediate reabsorption of sugars that pass the glomerular filtration barrier and need to be retrieved to maintain normal homeostasis (19). This retrieval process occurs predominantly in the proximal tubule. Second, other sugar transporters play a housekeeping role in moving sugars across the plasma membrane of nonproximal epithelial cells to provide fuel for energy metabolism. Proximal tubule cells cannot metabolize glucose because of an absence of hexokinases (20) and, therefore, do not require these housekeeping sugar transporters. In addition, modern diets are rich in fructose, which is converted to glucose largely in the proximal tubule via 1-phosphorylation by ketohexokinase (Khk) (21), requiring proximal fructose transporter proteins. Other sugars such as mannose, galactose, and sialic acid are required for protein N-glycosylation in most cell types (22).
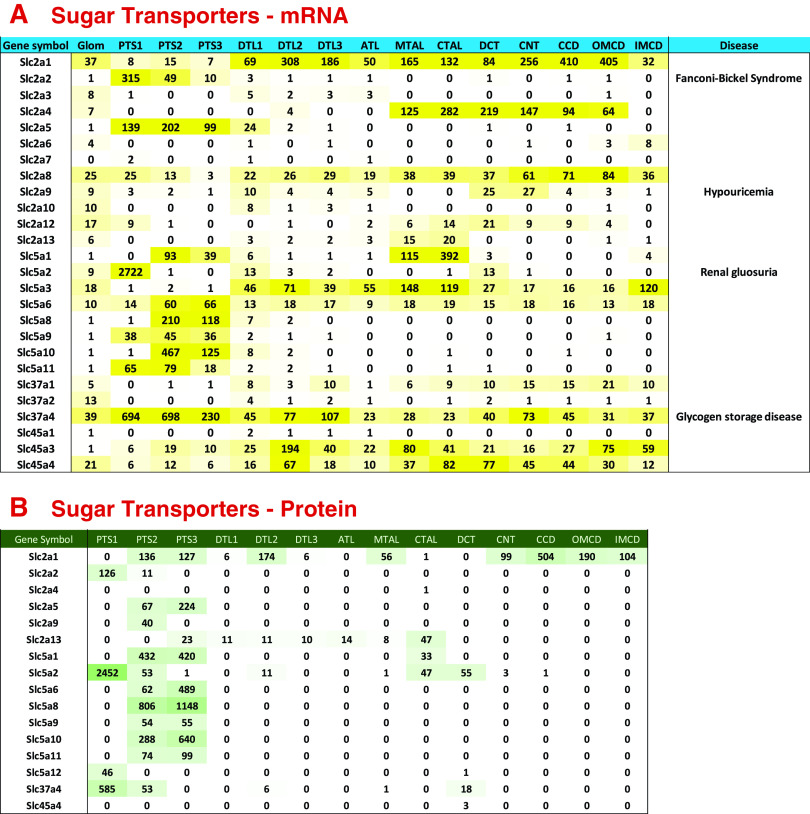
Figure 3A shows the distributions of mRNAs coding for sugar transporting SLC family members, specifically SLC2, SLC5, SLC37, and SLC45 subfamily proteins. Figure 3B shows the distribution of the SLC proteins measured by mass spectrometry. In general, SLC2 family members are ion-independent sugar carriers. Two of them are prominent in glucose metabolism, viz. Slc2a1 (common term: GLUT1; definition: insulin-independent glucose transporter) and Slc2a4 (GLUT4 or the insulin-regulated glucose transporter). The former is expressed throughout the renal tubule, whereas the latter is expressed in the thick ascending limb, distal convoluted tubule, and early parts of the collecting duct, sites of insulin-regulated ion transport (19, 23–27). Slc2a5 (common term: GLUT5; definition: facilitated glucose/fructose transporter) is selective for fructose uptake and is expressed chiefly in proximal tubule segments where fructose is converted to glucose. Another SLC2 transporter is also selectively expressed in the proximal tubule, namely, Slc2a2 (common term: GLUT2; definition: facilitated glucose transporter member 2), which carries multiple monosaccharides across the plasma membrane and in humans is mutated in Fanconi–Bickel syndrome, a glycogen storage disease (Fig. 3A).
Figure 3.
Sugar transporter expression along renal tubule of mouse. A: mRNA levels in TPM (transcripts per million). Genetic disease entities labeled at right. B: protein levels in copies per cell divided by 1,000. See text for data sources. ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; IMCD, inner medullary collecting duct; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct; PTS1, the initial segment of the proximal tubule; PTS2, proximal straight tubule in cortical medullary rays; PTS3, last segment of the proximal straight tubule in the outer stripe of outer medulla.
The SLC5 subfamily mediates sodium-dependent sugar transport. Slc5a2 (common term: SGLT2; definition: sodium-glucose transporter 2) (28) is a sodium-glucose cotransporter and an important drug target for treatment of diabetes mellitus (gliflozins: canagliflozin, dapagliflozin, and empagliflozin). SGLT2 is expressed exclusively in kidney and testis (see https://www.proteinatlas.org/ENSG00000140675-SLC5A2/tissue). Within the kidney, SGLT2 is virtually exclusively expressed in the S1 portion of the proximal tubule, whereas another sodium-coupled glucose transporter SGLT1 (29) is expressed in the late portion of the proximal tubule and even further downstream in the thick ascending limb of Henle (Fig. 3, A and B) and in the macula densa as noted earlier (30). SGLT1 can decrease glucose in the lumen to a lower level than SGLT2 because of a higher sodium:glucose coupling ratio, thus mopping up the last remnants of glucose and preventing glycosuria (31–32). Another proximal specific SLC5 subfamily member is Slc5a9 (common term: SGLT4; definition: sodium-glucose cotransporter 4), which has a broader specificity for monosaccharides (33). Slc5a3, a sodium-inositol transporter (common term: SMIT1; definition: sodium-myo-inositol transporter 1), is expressed at an mRNA level in all segments other than the proximal tubule. Like Slc6a12 (discussed earlier) this transporter plays a key role in protection of renal medullary cells against the effects of high NaCl and urea concentrations (34).
The SLC37 subfamily of SLCs transport glucose 6-phosphate in exchange for inorganic phosphate. Slc37a4 (common term: G6PT; definition: glucose-6-phosphate translocase) is expressed broadly along the renal tubule (Fig. 3, A and B), where it forms a complex with glucose-6-phosphatase (35), and thus is important for dephosphorylation of glucose and release of unphosphorylated glucose from the cells after gluconeogenesis. Inactivating mutations in the SLC37A4 gene in humans cause glycogen storage disease type 1B (36).
The SLC45 proteins are proton-dependent sugar transporters. Slc45a3 (common term: PRST; definition: prostein) and Slc45a4 are broadly expressed along the renal tubule but have not been studied extensively in the kidney. Like Slc6a12 and Slc5a3, Slc45a3 has been proposed to function as an osmolyte transporter in the medullary collecting duct (37).
MALE/FEMALE DIFFERENCES IN SLC FAMILY TRANSPORTERS IN PROXIMAL TUBULE
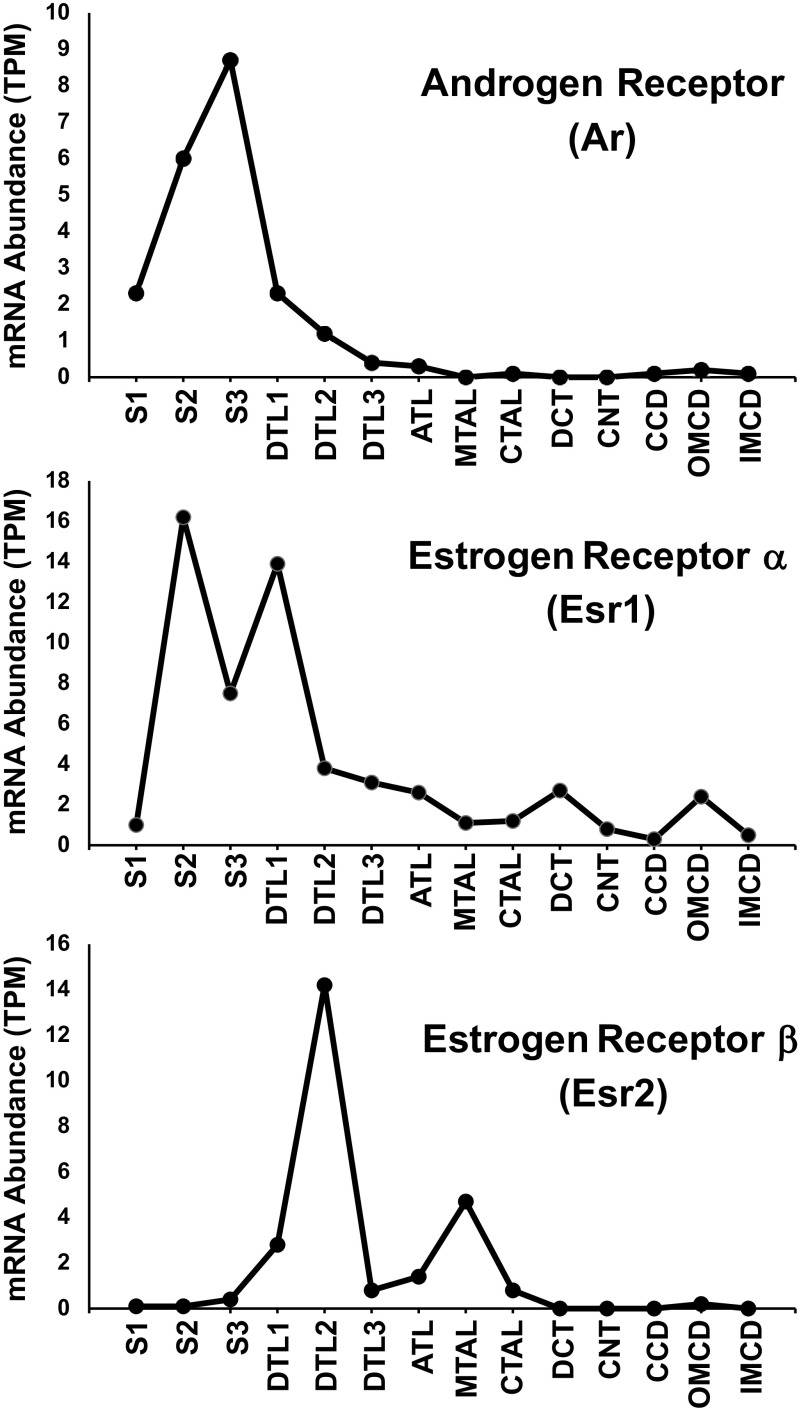
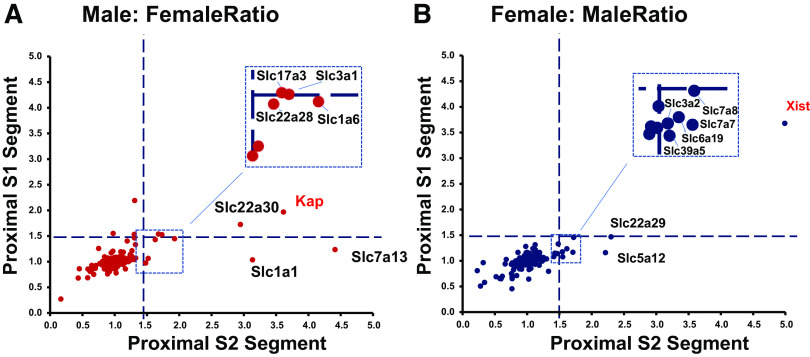
Figure 4 shows the distribution of mRNA for the androgen receptor (Ar) and the two estrogen receptors (Esr1 and Esr2) along the renal tubule as reported in male mice by Chen et al. (14). The peak expression of Ar and Esr1 was seen in the S2 and S3 proximal tubules. In contrast, Esr2 is mainly expressed in loop of Henle segments. Expression of all three receptors in the S1 proximal tubule is very low. Ransick and colleagues (10) have carried out single-cell RNA-Seq studies in mouse kidneys in which they have compared transcriptomes for individual cell types in male versus female mice. They reported that across the transcriptome, the main differences are seen in the S2 segment of the proximal tubule. Similar observations were made by Wu et al. (38) and Huang et al. (39). Figure 5 summarizes expression of SLC proteins in S1 versus S2 proximal tubule cells from male versus female mice as reported by Ransick et al. (10). Figure 5A shows the eight SLCs that were more strongly expressed in males versus females, plus the androgen-specific marker, Kap, included as a positive control. These are predominantly organic ion transporters [Slc1a1 (common term: OATP1; definition: sodium-independent organic anion-transporting polypeptide 1), Slc1a6 (common term: OATP5; definition: sodium-independent organic anion-transporting polypeptide 5), Slc3a1 (common term: OATP3; definition: organic anion transporter polypeptide-related protein 3), Slc22a30 (common term: UST6; definition: organic anion transporter), and Slc22a28 (putative organic ion transporter)]. Figure 5B shows the seven SLCs that were more strongly expressed in females versus males, plus the estrogen-specific marker, Xist, included as a positive control. Four of the seven are amino acid transporters [Slc6a19 (common term: B0AT1; definition: system B0 neutral amino acid transporter), Slc3a2 (common term: 4F2HC; definition: 4F2 cell-surface antigen heavy chain), Slc7a7 (common term: MOP2; definition: monocyte amino acid permease 2), and Slc7a8 (common term: LAT2; definition: L-type amino acid transporter 2)]. The male/female differences described here do not account for the possibility that individual segments could be longer or shorter in the two sexes. Indeed, proximal tubule length and transport surface area appear to be smaller in females, correlating with a lower fractional fluid reabsorption in females (40).
Figure 4.
Distribution of androgen receptor and two estrogen receptors along the renal tubule of male mice. Figure drawn using data from https://esbl.nhlbi.nih.gov/MRECA/Nephron/. TPM, transcripts-per-million.
Figure 5.
Sex-selective expression of SLC transporters in proximal tubule S2 vs. S1 segments. A: male-selective transcripts. B: female-selective transcripts. Figure drawn using data recalculated from Gene Expression Omnibus record GSE129798, originally published by Ransick and colleagues (10).
ACCESSORY SUBUNITS OF SLC FAMILY TRANSPORTERS
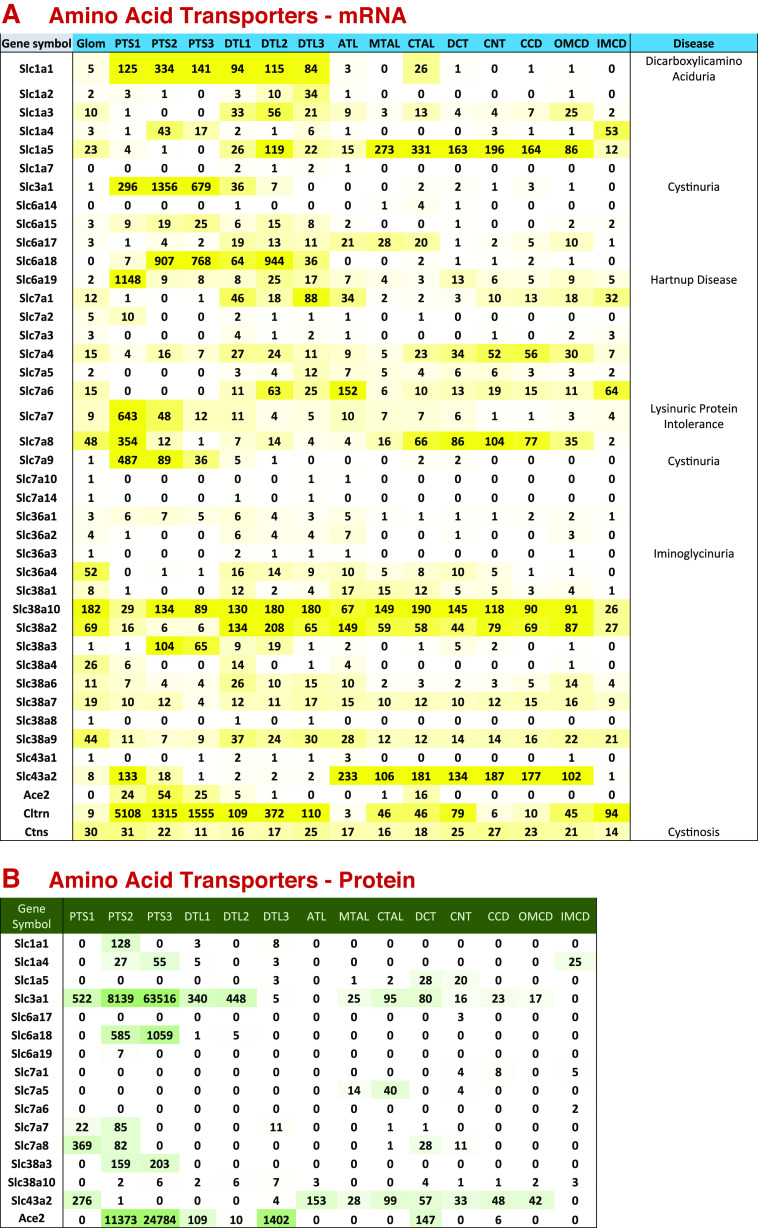
Many SLC family transporters have accessory subunits (“Beta subunits”) that are single-pass integral membrane proteins (41). Table 1 lists some common examples of these accessory proteins that are expressed in kidney epithelia. They are thought to be involved in targeting the transporters to apical or basolateral plasma membranes in polarized cells. For example, collectrin (Cltrn or Tmem27) and a closely related protein, Ace2, function as accessory proteins for several SLC6-family amino acid transporters and are important to their targeting to the apical plasma membrane (42). Figure 6 summarizes the distributions of amino acid transporters along the renal tubule and shows that Ace2 is selectively expressed in proximal tubule segments, whereas collectrin is expressed in all 14 renal tubule segments. Ace2 also plays a role in degrading angiotensin-II and is recognized to be a receptor protein for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus (46–48). Additional transport accessory proteins shown in Table 1 are rBAT, Cd98, embigin, basigin, Slc51b, and barttin. It is likely that other accessory proteins exist in kidney but have not yet been characterized, as this is an understudied area of transporter physiology.
Table 1.
Examples of transporter accessory proteins expressed along the renal tubule
| Accessory Protein | Common Name | Regulated Transporter(s) | Renal Tubule Distribution | Polarity |
|---|---|---|---|---|
| Cltrn | Collectrin | Slc6a18, Slc6a19 | All segments | Apical |
| Ace2 | Angiotensin converting enzyme 2 | Slc6a19, other amino acid transporters | Proximal tubule | Apical |
| Slc3a1 | rBAT | Slc7a9 | Proximal tubule | Apical |
| Slc3a2 | CD98 | Slc7a5, Slc7a6, Slc7a8, Slc7a10, Slc7a11 | All segments | Basolateral |
| Emb | Embigin | Slc16a1, Slc16a7 | All segments beyond proximal tubule | Basolateral |
| Bsg | Basigin | Slc16a1, Slc16a3 and Slc16a8 | All segments | Basolateral |
| Slc51b | Organic solute transporter subunit beta | Slc51a | Proximal tubule | Basolateral |
| Bsnd | Barttin | Clckna, Clcknb | ATL, MTAL, CTAL, DCT, CNT, CCD, OMCD | Basolateral |
ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct.
Figure 6.
Amino acid transporter expression along renal tubule of mouse. A: mRNA levels in TPM (transcripts per million). Genetic disease entities labeled at right. B: protein levels in copies per cell divided by 1,000. Amino acid transporters are important in the kidney for fulfilling two major roles similar to those described earlier for sugar transporters. First, they mediate reabsorption of amino acids that pass the glomerular filtration barrier and must be retrieved to maintain homeostasis. Second, they are required in all cells for uptake of essential amino acids that cannot be synthesized within the cell, namely, valine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, histidine, and lysine. We expect these to be expressed in every tubule segment. A and B show the renal tubular distributions of amino acid transporting SLC subfamily mRNAs and proteins, respectively (subfamilies SLC1, 3, 6, 7, 36, 38, and 43), as well as accessory proteins Ace2, collectrin, and cystinosin. The transporters selectively expressed in proximal tubule are Slc1a1, Slc1a4, Slc3a1, Slc6a15, Slc6a18, Slc6a19, and Slc7a9. These, working in tandem, are responsible for reabsorption of filtered amino acids, each showing different substrate specificities. Slc1a1 mediates reabsorption of acidic amino acids glutamate and aspartate (43). Slc1a4 mediates reabsorption of alanine, serine, threonine, and cysteine (44). Slc3a1 and Slc7a9 function together as a complex that reabsorbs the basic amino acids lysine and arginine, as well as cystine (45) and disruptive mutations in either result in cystinuria (left). Slc6a15, Slc6a18, and Slc6a19 are chiefly involved in reabsorption of neutral amino acids. Note that several of these transporters are associated with genetic aminoacidurias. As documented in the Genotype-Tissue Expression (GTEx) database (https://www.gtexportal.org/home/), Slc3a1, Slc6a18, Slc6a19, and Slc7a9 are highly selectively expressed in kidney and small intestine consistent with their specialized roles in apical amino acid uptake in epithelia. Collectrin (Cltrn or Tmem27) and a closely related protein, Ace2, function as β-subunits of several of these amino acid transporters that are important to their targeting to the apical plasma membrane (42). Ace2 also plays a role in degrading angiotensin-II and is recognized to be a receptor protein for the SARS-CoV-2 virus (46–48). Cystinosin (Ctns) plays an essential role in all cells in removing oxidized cysteine (i.e., cystine) from lysosomes and is responsible for cystinosis when mutated (49). Several amino acid transporters are expressed throughout the renal tubule (A) and are likely to be housekeeping genes necessary for the uptake of essential amino acids. These transporters include Slc38a2, Slc38a7, Slc38a9, and Slc38a10, whose specificities overlap the 9 essential amino acids. Based on GTEx data (GTEx database: https://www.gtexportal.org/home/), these transporters are broadly expressed among tissues and are not specific to the kidney. ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; IMCD, inner medullary collecting duct; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct; PTS1, the initial segment of the proximal tubule; PTS2, proximal straight tubule in cortical medullary rays; PTS3, last segment of the proximal straight tubule in the outer stripe of outer medulla; SARS-CoV-2; severe acute respiratory syndrome coronavirus 2.
ACID-BASE TRANSPORTERS ALONG THE RENAL TUBULE
Of particular interest is the acid-base transport SLC families. The SLC4, SLC9, SLC42, and some members of the SLC26 subfamilies are the main solute carriers involved in renal control of acid-base balance. The distributions of the members of these subfamilies along the renal tubule are shown in Fig. 7.
Figure 7.
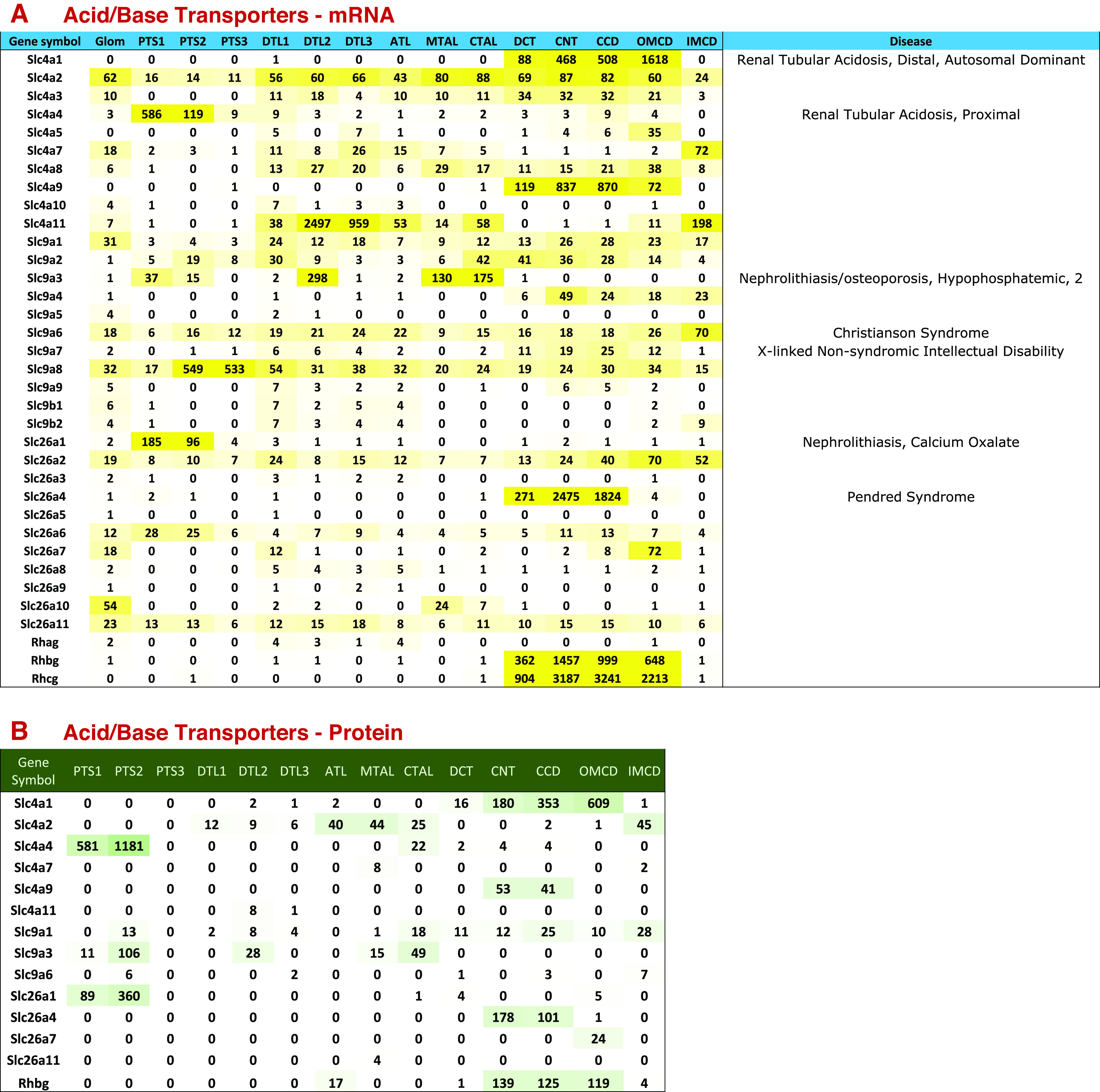
Acid-base transporter expression along renal tubule of mouse. A: mRNA levels in TPM (transcripts per million). Genetic disease entities labeled at right. B: protein levels in copies per cell divided by 1,000. See text for data sources. ATL, thin ascending limb of the loop of Henle; CCD, cortical collecting duct; CNT, connecting tubule; CTAL, cortical thick ascending limb of the loop of Henle; DCT, distal convoluted tubule; DTL1, the short descending limb of the loop of Henle; DTL2, long descending limb of the loop of Henle in the outer medulla; DTL3, long descending limb of the loop of Henle in the inner medulla; IMCD, inner medullary collecting duct; MTAL, medullary thick ascending limb of the loop of Henle; OMCD, outer medullary collecting duct; PTS1, the initial segment of the proximal tubule; PTS2, proximal straight tubule in cortical medullary rays; PTS3, last segment of the proximal straight tubule in the outer stripe of outer medulla.
The SLC4 subfamily consists of bicarbonate transporters, which move bicarbonate either in exchange for chloride ions [Slc4a1 (common term: AE1; anion exchanger 1), Slc4a2 (common term: AE2; anion exchanger 2) and Slc4a3 (common term: AE3; anion exchanger 3)], or as a cotransport process with sodium ions at various stoichiometries [Slc4a4, Slc4a5, and Slc4a6-Slc4a10 (NBC1, NBCe2, NBC3, NDCBE1, AE4, and NCBE, respectively)]. Slc4a11 (common term: BTR1; bicarbonate-transporter-related protein 1), originally thought to be a sodium-borate transporter, functions as an electrogenic H+ or OH− permeation channel, which may or may not be coupled to ammonium or sodium ions (6, 50–53). Among the chloride-bicarbonate exchangers, Slc4a1 (AE1) is the best studied having been identified originally as “Band 3” in erythrocytes (54). In kidney, it is very selectively expressed in α-intercalated cells, where it mediates basolateral base exit as a key element of the luminal acidification mechanism of the renal collecting duct (56). This gene is mutated in autosomal distal renal tubular acidosis (Fig. 7A). In contrast to the very restricted distribution of Slc4a1, Slc4a2 and Slc4a3 are more widely distributed among renal tubule cells indicative of possible housekeeping roles (Fig. 7). Untargeted deletion of Slc4a2 results in a severe phenotype marked by early death of the mice and gastric achlorhydria (56). The distribution of Slc4a2 protein (Fig. 7B) suggests a possible role in the thick ascending limb of Henle’s loop, and hypothetically could be the main base exit pathway associated with luminal acidification in the thick ascending limb (57, 58). This hypothesis could be tested through targeted deletion of Slc4a2 in mice, but we are unaware of such experiments so far. Deletion of Slc4a3 in mice had no effect on acid-base balance (59), and this protein may instead be involved in magnesium handling.
Slc4a4, Slc4a5, and Slc4a7-Slc4a10 are sodium-bicarbonate cotransporters. Slc4a4 is expressed chiefly in proximal tubule segments (Fig. 7), where it is present in the basolateral plasma membrane and appears to be responsible for base exit as part of the luminal acidification mechanism. The gene is mutated in the most common form of proximal renal tubule acidosis (Fig. 7A). Slc4a8 is a sodium-coupled chloride-bicarbonate exchanger whose mRNA is broadly distributed in segments beyond the proximal tubule. This transporter has been implicated in Na-Cl transport in β-intercalated cells, working in parallel with pendrin (Slc26a4) (60). Sodium exit is thought to be mediated by Slc4a9, aka AE4 (61), a sodium-bicarbonate cotransporter (62). Slc4a9 is strongly expressed in both α- and β-intercalated cells (8). Slc4a11 appears to be distributed to medullary segments (both thin limbs of Henle and collecting ducts) matching localizations from immunocytochemistry (63) (Fig. 7). It has been proposed to be involved in countercurrent recycling of ammonium and accumulation of ammonium in the medulla (63).
The SLC9 subfamily consists of sodium-proton exchangers. Slc9a1 (common term: NHE1; definition: sodium/hydrogen exchanger 1), Slc9a6 (common term: NHE6; definition: sodium/hydrogen exchanger 6), and Slc9a8 (common term: NHE8; definition: sodium/hydrogen exchanger 8) are widely distributed along the renal tubule and play roles in pH regulation in different intracellular compartments (Fig. 7A). NHE1 is a basolateral plasma membrane transporter involved in intracellular pH regulation (64). NHE6 is localized to early and recycling endosomes and is important for maintenance of endosomal pH (65). NHE8 is present mainly in the Golgi apparatus and in post-Golgi vesicles (66).
Other SLC9 subfamily members are selectively expressed in certain tubule segments and are important in transepithelial sodium and proton transport, viz. Slc9a3 (common term: NHE3; definition: sodium/hydrogen exchanger 3), Slc9a4 (common term: NHE4; definition: sodium/hydrogen exchanger 6), and Slc9a7 (common term: NHE7; definition: sodium/hydrogen exchanger 7) (Fig. 7A). NHE3 is an apical Na-H exchanger that mediates luminal acidification in the thick ascending limb of Henle (67, 68), the proximal tubule (69), and the descending limb of Henle’s loop in the outer medulla (67). NHE3 is also thought to carry ammonium ions into the lumen in proximal tubule, by substitution of H+ (70). Slc9a4 and Slc9a7 are both selectively expressed in collecting ducts (Fig. 7A). Slc9a4 has been proposed to have a role in cell volume regulation (71). It is expressed in type A intercalated cells and principal cells of the collecting duct, but not type B intercalated cells (8). Slc9a7 has been reported to be localized predominantly to the trans-Golgi network (72).
SLC42 subfamily proteins are thought to function as ammonia or ammonium transporters. Rhag is an erythrocyte-specific protein responsible for ammonia transport in the red blood cell (73) and is expressed at only very low levels in thin limb segments (Fig. 7A). In contrast, both Rhbg and Rhcg are heavily expressed in collecting ducts (Fig. 7, A and B). Single-cell RNA-Seq studies show that both are expressed in DCT cells, principal cells, A type intercalated cells, and B type intercalated cells (8, 14). Immunofluorescence localization showed that Rhcg is apically located in these cell types (74, 75). In contrast, Rhbg was distributed to the basolateral plasma membrane in these cell types (75–77). Ammonia excretion is generally thought to be a result of ammonia production in proximal tubule cells with transport processes that channel the ammonia to the final urine (70). Based on this model, it is unclear what advantage having ammonia transporters in the cortical distal nephron would provide. However, studies by Good and Burg (78) showed that distal tubules also have substantial capacity for ammonia production, and we speculate that Rhcg is needed to deliver the distally produced ammonia to the urine.
Some of the members of the SLC26 family are capable of transporting bicarbonate, viz. Slc26a1 (common term: SAT1; definition: sulfate/anion transporter 1), Slc26a3 (common term: DRA; definition: chloride anion exchanger), Slc26a4 (common term: PDS; definition: pendrin), Slc26a6 (common term: PDS-L1; definition: pendrin-like protein 1), Slc26a7 (common term: SUT2; definition: sulfate anion transporter), and Slc26a9 (anion transporter/exchanger-9) (Fig. 7). Of these, Slc26a4 (pendrin) may be the best studied. It is selectively expressed in β-intercalated cells where it is responsible for bicarbonate secretion (79) and is upregulated in response to alkali in the diet or adrenal steroids (80). In contrast, Slc26a1 is selectively expressed in proximal tubule segments (Fig. 7), where it mediates sulfate reabsorption but can also carry oxalate or bicarbonate (81, 82). Slc26a6 is a chloride-formate exchanger that is an integral element of the Na-Cl reabsorption process in the proximal tubule (83). Slc26a7 has been identified as an endosomal chloride-bicarbonate exchanger in the medullary collecting duct (84).
CONCLUSIONS
This review presents a new database listing all solute carrier proteins in the mouse genome and maps the SLC gene list to transcriptomic and proteomic data for each of 14 renal tubule segments. The gene list database (https://esbl.nhlbi.nih.gov/Databases/SLC-list/) has essentially the same information as on the original SLC tables page at http://slc.bioparadigms.org/, but is set up in a format that allows easy downloading of the information in a single operation. The SLC gene list database was used to generate databases of SLC expression mapped along the renal tubule and are provided as online resources at https://esbl.nhlbi.nih.gov/Databases/SLC-kidney/ and at https://esbl.nhlbi.nih.gov/Databases/SLC_Protein/ that users can interrogate for their own studies. For example, these databases may provide a resource for experimental design and mathematical modeling of transport processes in the kidney. In addition, we have illustrated the use of the databases, choosing three categories of SLC proteins corresponding to sugar transport, amino acid transport, and acid-base transport. An important aspect of the presentation is the tying together of the omics data with functional data derived from classical physiological techniques such as brush border membrane vesicle preparations and from microdissected tubules done chiefly in the 20th century. Several SLC categories were not included but are nonetheless important to the overall physiology of the kidney, for example, the SLC12 subfamily of ion cotransporters, various organic ion transporters, and divalent ion transporters. The online databases give readers opportunities to examine the patterns of expression of these additional subfamilies. Several recent reviews provide comprehensive information about these additional SLC family members (85–88).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.L., V.R., S.J.K., C.-L.C., C.-R.Y., and M.A.K. analyzed data; L.C. and C.-L.C. interpreted results of experiments; S.L., L.C., and M.A.K. prepared figures; S.L., L.C., and M.A.K. drafted manuscript; S.L., L.C., V.R., S.J.K., C.-L.C., C.-R.Y., and M.A.K. edited and revised manuscript; S.L., L.C., V.R., S.J.K., C.-L.C., C.-R.Y., and M.A.K. approved final version of manuscript.
Footnotes
Nomenclature Appendix: nonitalicized gene symbols are used to designate proteins or transcripts. When referring specifically to the gene rather than the gene products, the gene symbols are italicized. Human gene symbols are given in “all caps,” whereas rodent gene symbols are capitalized in the first letter only. Official gene symbols are archived by UniProt (https://www.uniprot.org/). Common name abbreviations are also given for the SLC proteins discussed in the text.
REFERENCES
- 1.Hoenig MP, Zeidel ML. Homeostasis, the milieu intérieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9: 1272–1281, 2014. doi: 10.2215/CJN.08860813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hediger MA, Clémençon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med 34: 95–107, 2013. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, et al. Human cell atlas meeting participants. The human cell atlas. Elife 6: e27041, 2017. doi: 10.7554/eLife.27041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam M, Potter AS, Potter SS. Psychrophilic proteases dramatically reduce single-cell RNA-seq artifacts: a molecular atlas of kidney development. Development 144: 3625–3632, 2017. doi: 10.1242/dev.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young MD, Mitchell TJ, Vieira Braga FA, Tran MGB, Stewart BJ, Ferdinand JR, et al. Single-cell transcriptomes from human kidneys reveal the cellular identity of renal tumors. Science 361: 594–599, 2018. doi: 10.1126/science.aat1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol 30: 23–32, 2019. doi: 10.1681/ASN.2018090912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lake BB, Chen S, Hoshi M, Plongthongkum N, Salamon D, Knoten A, Vijayan A, Venkatesh R, Kim EH, Gao D, Gaut J, Zhang K, Jain S. A single-nucleus RNA-sequencing pipeline to decipher the molecular anatomy and pathophysiology of human kidneys. Nat Commun 10: 2832, 2019. doi: 10.1038/s41467-019-10861-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Lee JW, Chou C-L, Nair AV, Battistone MA, Păunescu TG, Merkulova M, Breton S, Verlander JW, Wall SM, Brown D, Burg MB, Knepper MA. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci USA 114: E9989–E9998, 2017. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karaiskos N, Rahmatollahi M, Boltengagen A, Liu H, Hoehne M, Rinschen M, Schermer B, Benzing T, Rajewsky N, Kocks C, Kann M, Müller RU. A single-cell transcriptome atlas of the mouse glomerulus. J Am Soc Nephrol 29: 2060–2068, 2018. doi: 10.1681/ASN.2018030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ransick A, Lindström NO, Liu J, Zhu Q, Guo JJ, Alvarado GF, Kim AD, Black HG, Kim J, McMahon AP. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 51: 399–413.e7,2019. doi: 10.1016/j.devcel.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, Shrestha R, Qiu C, Kondo A, Huang S, Werth M, Li M, Barasch J, Suszták K. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360: 758–763, 2018. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limbutara K, Chou CL, Knepper MA. Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol 31: 1255–1266, 2020. doi: 10.1681/ASN.2020010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Chou C-L, Knepper MA. A comprehensive map of mRNAs and their isoforms across all 14 renal tubule segments of mouse. J Am Soc Nephrol 32: 897–912, 2021. doi: 10.1681/ASN.2020101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Clark JZ, Nelson JW, Kaissling B, Ellison DH, Knepper MA. Renal-tubule epithelial cell nomenclature for single-cell RNA-sequencing studies. J Am Soc Nephrol 30: 1358–1364, 2019[Erratum inJ Am Soc Nephrol30: 2475, 2019]. doi: 10.1681/ASN.2019040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriz W, Bankir L. A standard nomenclature for structures of the kidney. The Renal Commission of the International Union of Physiological Sciences (IUPS). Kidney Int 33: 1–7, 1988. doi: 10.1038/ki.1988.1. [DOI] [PubMed] [Google Scholar]
- 17.Burg MB, Ferraris JD. Intracellular organic osmolytes: function and regulation. J Biol Chem 283: 7309–7313, 2008. doi: 10.1074/jbc.R700042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Chou CL, Knepper MA. Targeted single-cell RNA-seq identifies minority cell types of kidney distal nephron. J Am Soc Nephrol 32: 886–896, 2021. doi: 10.1681/ASN.2020101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddox DA, Gennari FJ. The early proximal tubule: a high-capacity delivery-responsive reabsorptive site. Am J Physiol Renal Physiol 252: F573–F584, 1987. doi: 10.1152/ajprenal.1987.252.4.F573. [DOI] [PubMed] [Google Scholar]
- 20.Keljo DJ, Kleinzeller A, Murer H, Kinne R. Is hexokinase present in the basal lateral membranes of rat kidney proximal tubular epithelial cells? Biochim Biophys Acta 508: 500–512, 1978. doi: 10.1016/0005-2736(78)90095-0. [DOI] [PubMed] [Google Scholar]
- 21.Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, Markham AF, Hayward BE, Asipu A, Bonthron DT. Ketohexokinase: expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem 57: 763–774, 2009. doi: 10.1369/jhc.2009.953190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem 16: 21–49, 1984. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- 23.Helms MN, Fejes-Toth G, Naray-Fejes-Toth A. Hormone-regulated transepithelial Na+ transport in mammalian CCD cells requires SGK1 expression. Am J Physiol Renal Physiol 284: F480–F487, 2003. doi: 10.1152/ajprenal.00299.2002. [DOI] [PubMed] [Google Scholar]
- 24.Ito O, Kondo Y, Takahashi N, Kudo K, Igarashi Y, Omata K, Imai Y, Abe K. Insulin stimulates NaCl transport in isolated perfused MTAL of Henle’s loop of rabbit kidney. Am J Physiol Renal Physiol 267: F265–F270, 1994. doi: 10.1152/ajprenal.1994.267.2.F265. [DOI] [PubMed] [Google Scholar]
- 25.Mandon B, Siga E, Chabardes D, Firsov D, Roinel N, De Rouffignac C. Insulin stimulates Na+, Cl−, Ca2+, and Mg2+ transports in TAL of mouse nephron: cross-potentiation with AVP. Am J Physiol Renal Physiol 265: F361–F369, 1993. doi: 10.1152/ajprenal.1993.265.3.F361. [DOI] [PubMed] [Google Scholar]
- 26.Pavlov TS, Ilatovskaya DV, Levchenko V, Li L, Ecelbarger CM, Staruschenko A. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J 27: 2723–2732, 2013. doi: 10.1096/fj.12-223792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tallini NY, Stoner LC. Amiloride-sensitive sodium current in everted Ambystoma initial collecting tubule: short-term insulin effects. Am J Physiol Cell Physiol 283: C1171–C1181, 2002.doi: 10.1152/ajpcell.00606.2001. [DOI] [PubMed] [Google Scholar]
- 28.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93: 397–404, 1994. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda TS, Hwang ES, Coady MJ, Hirayama BA, Hediger MA, Wright EM. Characterization of a Na+/glucose cotransporter cloned from rabbit small intestine. J Membr Biol 110: 87–95, 1989. doi: 10.1007/BF01870995. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Wei J, Jiang S, Xu L, Wang L, Cheng F, Buggs J, Koepsell H, Vallon V, Liu R. Macula densa SGLT1-NOS1-tubuloglomerular feedback pathway, a new mechanism for glomerular hyperfiltration during hyperglycemia. J Am Soc Nephrol 30: 578–593, 2019. doi: 10.1681/ASN.2018080844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuhara Y, Turner RJ. The static head method for determining the charge stoichiometry of coupled transport systems. Applications to the sodium-coupled D-glucose transporters of the renal proximal tubule. Biochim Biophys Acta 770: 73–78, 1984. doi: 10.1016/0005-2736(84)90075-0. [DOI] [PubMed] [Google Scholar]
- 32.Turner RJ, Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol Renal Physiol 242: F406–F414, 1982. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- 33.Tazawa S, Yamato T, Fujikura H, Hiratochi M, Itoh F, Tomae M, Takemura Y, Maruyama H, Sugiyama T, Wakamatsu A, Isogai T, Isaji M. SLC5A9/SGLT4, a new Na+-dependent glucose transporter, is an essential transporter for mannose, 1,5-anhydro-D-glucitol, and fructose. Life Sci 76: 1039–1050, 2005[Erratum inLife Sci87: 514, 2010]. doi: 10.1016/j.lfs.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev 87: 1441–1474, 2007. doi: 10.1152/physrev.00056.2006. [DOI] [PubMed] [Google Scholar]
- 35.Chou JY, Mansfield BC. The SLC37 family of sugar-phosphate/phosphate exchangers. Curr Top Membr 73: 357–382, 2014. doi: 10.1016/B978-0-12-800223-0.00010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange AJ, Arion WJ, Beaudet AL. Type Ib glycogen storage disease is caused by a defect in the glucose-6-phosphate translocase of the microsomal glucose-6-phosphatase system. J Biol Chem 255: 8381–8384, 1980. [PubMed] [Google Scholar]
- 37.Vitavska O, Edemir B, Wieczorek H. Putative role of the H+/sucrose symporter SLC45A3 as an osmolyte transporter in the kidney. Pflugers Arch 468: 1353–1362, 2016. doi: 10.1007/s00424-016-1841-6. [DOI] [PubMed] [Google Scholar]
- 38.Wu H, Lai CF, Chang-Panesso M, Humphreys BD. Proximal tubule translational profiling during kidney fibrosis reveals proinflammatory and long noncoding RNA expression patterns with sexual dimorphism. J Am Soc Nephrol 31: 23–38, 2020. doi: 10.1681/ASN.2019040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L, Liao J, He J, Pan S, Zhang H, Yang X, Cheng J, Chen Y, Mo Z. Single-cell profiling reveals sex diversity in human renal proximal tubules. Gene 752: 144790, 2020. doi: 10.1016/j.gene.2020.144790. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, McDonough AA, Layton HE, Layton AT. Functional implications of sexual dimorphism of transporter patterns along the rat proximal tubule: modeling and analysis. Am J Physiol Renal Physiol 315: F692–F700, 2018. doi: 10.1152/ajprenal.00171.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fairweather SJ, Shah N, Brӧer S. Heteromeric solute carriers: function, structure, pathology and pharmacology. Adv Exp Med Biol 21: 13–127, 2021. doi: 10.1007/5584_2020_584. [DOI] [PubMed] [Google Scholar]
- 42.Camargo SM, Singer D, Makrides V, Huggel K, Pos KM, Wagner CA, Kuba K, Danilczyk U, Skovby F, Kleta R, Penninger JM, Verrey F. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 136: 872–882, 2009. doi: 10.1053/j.gastro.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai Y, Stelzner M, Nussberger S, Khawaja S, Hebert SC, Smith CP, Hediger MA. The neuronal and epithelial human high affinity glutamate transporter. Insights into structure and mechanism of transport. J Biol Chem 269: 20599–20606, 1994. doi: 10.1016/S0021-9258(17)32035-5. [DOI] [PubMed] [Google Scholar]
- 44.Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem 268: 15329–15332, 1993. doi: 10.1016/S0021-9258(18)82257-8. [DOI] [PubMed] [Google Scholar]
- 45.Verrey F, Ristic Z, Romeo E, Ramadan T, Makrides V, Dave MH, Wagner CA, Camargo SM. Novel renal amino acid transporters. Annu Rev Physiol 67: 557–572, 2005. doi: 10.1146/annurev.physiol.67.031103.153949. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Lu G, Qiao C, Hu Y, Yuen KY, Wang Q, Zhou H, Yan J, Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 181: 894–904.e9, 2020. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581: 215–220, 2020. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 48.Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature 581: 221–224, 2020. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van’t Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet 18: 319–324, 1998. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 50.Jalimarada SS, Ogando DG, Vithana EN, Bonanno JA. Ion transport function of SLC4A11 in corneal endothelium. Invest Ophthalmol Vis Sci 54: 4330–4340, 2013. doi: 10.1167/iovs.13-11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kao L, Azimov R, Abuladze N, Newman D, Kurtz I. Human SLC4A11-C functions as a DIDS-stimulatable H+(OH−) permeation pathway: partial correction of R109H mutant transport. Am J Physiol Cell Physiol 308: C176–C188, 2015. doi: 10.1152/ajpcell.00271.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Myers EJ, Marshall A, Jennings ML, Parker MD. Mouse Slc4a11 expressed in Xenopus oocytes is an ideally selective H+/OH- conductance pathway that is stimulated by rises in intracellular and extracellular pH. Am J Physiol Cell Physiol 311: C945–C959, 2016. doi: 10.1152/ajpcell.00259.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogando DG, Jalimarada SS, Zhang W, Vithana EN, Bonanno JA. SLC4A11 is an EIPA-sensitive Na+ permeable pHi regulator. Am J Physiol Cell Physiol 305: C716–C727, 2013. doi: 10.1152/ajpcell.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabantchik ZI, Knauf PA, Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of ‘probes’. Biochim Biophys Acta 515: 239–302, 1978. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- 55.Schuster VL, Bonsib SM, Jennings ML. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol Cell Physiol 251: C347–C355, 1986. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- 56.Gawenis LR, Ledoussal C, Judd LM, Prasad V, Alper SL, Stuart-Tilley A, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Mice with a targeted disruption of the AE2 Cl−/HCO−3 exchanger are achlorhydric. J Biol Chem 279: 30531–30539, 2004. doi: 10.1074/jbc.M403779200. [DOI] [PubMed] [Google Scholar]
- 57.Bourgeois S, Massé S, Paillard M, Houillier P. Basolateral membrane Cl−, Na+, and K+-coupled base transport mechanisms in rat MTALH. Am J Physiol Renal Physiol 282: F655–F668, 2002. doi: 10.1152/ajprenal.00220.2000. [DOI] [PubMed] [Google Scholar]
- 58.Quentin F, Eladari D, Frische S, Cambillau M, Nielsen S, Alper SL, Paillard M, Chambrey R. Regulation of the Cl−/HCO−3 exchanger AE2 in rat thick ascending limb of Henle's loop in response to changes in acid-base and sodium balance. J Am Soc Nephrol 15: 2988–2997, 2004. doi: 10.1097/01.ASN.0000146426.93319.16. [DOI] [PubMed] [Google Scholar]
- 59.Kampik NB, Gehring N, Schnitzbauer U, Hennings JC, Hübner CA, Wagner CA. The murine Cl−/HCO−3 exchanger Ae3 (Slc4a3) is not required for acid-base balance but is involved in magnesium handling by the kidney. Cell Physiol Biochem 34: 1566–1577, 2014. doi: 10.1159/000366360. [DOI] [PubMed] [Google Scholar]
- 60.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Hatim H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010[Erratum inJ Clin Invest121: 1668, 2011]. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko SB, Luo X, Hager H, Rojek A, Choi JY, Licht C, Suzuki M, Muallem S, Nielsen S, Ishibashi K. AE4 is a DIDS-sensitive Cl−/HCO−3 exchanger in the basolateral membrane of the renal CCD and the SMG duct. Am J Physiol Cell Physiol 283: C1206–C1218, 2002. doi: 10.1152/ajpcell.00512.2001. [DOI] [PubMed] [Google Scholar]
- 62.Peña-Münzenmayer G, George AT, Shull GE, Melvin JE, Catalán MA. Ae4 (Slc4a9) is an electroneutral monovalent cation-dependent Cl−/HCO−3 exchanger. J Gen Physiol 147: 423–436, 2016. doi: 10.1085/jgp.201611571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gee MT, Kurtz I, Pannabecker TL. Expression of SLC4A11 protein in mouse and rat medulla: a candidate transporter involved in outer medullary ammonia recycling. Physiol Rep 7: e14089, 2019. doi: 10.14814/phy2.14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biemesderfer D, Reilly RF, Exner M, Igarashi P, Aronson PS. Immunocytochemical characterization of Na+-H+ exchanger isoform NHE-1 in rabbit kidney. Am J Physiol Renal Physiol 263: F833–F840, 1992. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 65.Brett CL, Wei Y, Donowitz M, Rao R. Human Na+/H+ exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol 282: C1031–C1041, 2002. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 67.Amemiya M, Loffing J, Lötscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995. doi: 10.1038/ki.1995.404. [DOI] [PubMed] [Google Scholar]
- 68.Good DW. Sodium-dependent bicarbonate absorption by cortical thick ascending limb of rat kidney. Am J Physiol Renal Physiol 248: F821–F829, 1985. doi: 10.1152/ajprenal.1985.248.6.F821. [DOI] [PubMed] [Google Scholar]
- 69.Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Physiol Renal Physiol 265: F736–F742, 1993. doi: 10.1152/ajprenal.1993.265.5.F736. [DOI] [PubMed] [Google Scholar]
- 70.Packer RK, Desai SS, Hornbuckle K, Knepper MA. Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J Am Soc Nephrol 2: 77–83, 1991. doi: 10.1681/ASN.V2177. [DOI] [PubMed] [Google Scholar]
- 71.Bookstein C, Musch MW, DePaoli A, Xie Y, Villereal M, Rao MC, Chang EB. A unique sodium-hydrogen exchange isoform (NHE-4) of the inner medulla of the rat kidney is induced by hyperosmolarity. J Biol Chem 269: 29704–29709, 1994. doi: 10.1016/S0021-9258(18)43937-3. [DOI] [PubMed] [Google Scholar]
- 72.Numata M, Orlowski J. Molecular cloning and characterization of a novel (Na+,K+)/H+ exchanger localized to the trans-Golgi network. J Biol Chem 276: 17387–17394, 2001. doi: 10.1074/jbc.M101319200. [DOI] [PubMed] [Google Scholar]
- 73.Knepper MA, Agre P. Structural biology. The atomic architecture of a gas channel. Science 305: 1573–1574, 2004. doi: 10.1126/science.1103191. [DOI] [PubMed] [Google Scholar]
- 74.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH4/NH+4 transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002. doi: 10.1097/01.asn.0000025280.02386.9d. [DOI] [PubMed] [Google Scholar]
- 75.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins RhBG and RhCG in mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003. doi: 10.1152/ajprenal.00050.2002. [DOI] [PubMed] [Google Scholar]
- 76.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003. doi: 10.1097/01.asn.0000050413.43662.55. [DOI] [PubMed] [Google Scholar]
- 77.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011. doi: 10.1152/ajprenal.00554.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Good DW, Burg MB. Ammonia production by individual segments of the rat nephron. J Clin Invest 73: 602–610, 1984. doi: 10.1172/JCI111250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA 98: 4221–4226, 2001. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 81.Karniski LP, Lötscher M, Fucentese M, Hilfiker H, Biber J, Murer H. Immunolocalization of sat-1 sulfate/oxalate/bicarbonate anion exchanger in the rat kidney. Am J Physiol Renal Physiol 275: F79–F87, 1998. doi: 10.1152/ajprenal.1998.275.1.F79. [DOI] [PubMed] [Google Scholar]
- 82.Kuo SM, Aronson PS. Oxalate transport via the sulfate/HCO3 exchanger in rabbit renal basolateral membrane vesicles. J Biol Chem 263: 9710–9717, 1988. doi: 10.1016/S0021-9258(19)81576-4. [DOI] [PubMed] [Google Scholar]
- 83.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA 98: 9425–9430, 2001. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu J, Worrell RT, Li HC, Barone SL, Petrovic S, Amlal H, Soleimani M. Chloride/bicarbonate exchanger SLC26A7 is localized in endosomes in medullary collecting duct cells and is targeted to the basolateral membrane in hypertonicity and potassium depletion. J Am Soc Nephrol 17: 956–967, 2006. doi: 10.1681/ASN.2005111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bazúa-Valenti S, Castañeda-Bueno M, Gamba G. Physiological role of SLC12 family members in the kidney. Am J Physiol Renal Physiol 311: F131–F144, 2016[Erratum inAm J Physiol Renal Physiol311: F830, 2016]. doi: 10.1152/ajprenal.00071.2016. [DOI] [PubMed] [Google Scholar]
- 86.César-Razquin A, Snijder B, Frappier-Brinton T, Isserlin R, Gyimesi G, Bai X, Reithmeier RA, Hepworth D, Hediger MA, Edwards AM, Superti-Furga G. A call for systematic research on solute carriers. Cell 162: 478–487, 2015. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 87.Dimke H, Hoenderop JG, Bindels RJ. Molecular basis of epithelial Ca2+ and Mg2+ transport: insights from the TRP channel family. J Physiol 589: 1535–1542, 2011. doi: 10.1113/jphysiol.2010.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gagnon KB, Delpire E. Sodium transporters in human health and disease. Front Physiol 11: 588664, 2021. doi: 10.3389/fphys.2020.588664. [DOI] [PMC free article] [PubMed] [Google Scholar]