Abstract
Significant sex differences exist across cellular, tissue organization, and body system scales to serve the distinct sex-specific functions required for reproduction. They are present in all animals that reproduce sexually and have widespread impacts on normal development, aging, and disease. Observed from the moment of fertilization, sex differences are patterned by sexual differentiation, a lifelong process that involves mechanisms related to sex chromosome complement and the epigenetic and acute activational effects of sex hormones. In this mini-review, we examine evidence for sex differences in cellular responses to DNA damage, their underlying mechanisms, and how they might relate to sex differences in cancer incidence and response to DNA-damaging treatments.
Keywords: sex differences, DNA repair, sexual differentiation, senescence, radiation
It is well-established that more males get cancer and die from cancer than females do. Sex differences in cancer incidence are widespread and include hematological malignancies, as well as cancers of the bladder, colon, skin, liver, and brain (1,2), with incidence ratios on the order of 1.26:1 to 4.86:1 (male:female) (3). In addition to developing cancer more frequently, cancer-related mortality is also higher in males than in females (4-6), and clinically important sex differences are evident in metastasis, expression of prognostic biomarkers, and treatment response in multiple cancers (7-9). Despite the overwhelming evidence of their importance, sex differences are not adequately considered in basic cancer research or in the design and execution of clinical trials (10). In part, this is a consequence of the general lack of familiarity that scientists, clinicians, and the public have with the mechanisms of sexual differentiation—the process through which sex differences are established. This, together with the overattribution of sex differences to the actions of circulating sex hormones alone, impedes the incorporation of the biology of sex differences in lab- and clinic-based cancer research. Furthermore, the majority of bioinformatics tools, which are increasingly applied in genomic and transcriptomic data analysis, do not account for sex as a biological variable (11). This confounds current data analyses and has the potential for propagating conclusions that are not fully accurate for males or females. In this mini-review, we will focus on sex differences in DNA damage response, with a concise review of epidemiological, clinical, and lab-based research as an illustration of the significance of sex differences research and the investigative path from population data to molecular and developmental mechanisms.
Sex Differences in Cancer Risk and Survival Following DNA Damage
While sex differences undoubtedly impact most aspects of cancer cell physiology, it is worthwhile to consider the cellular response to DNA damage as a potential common mechanism underlying sex differences in cancer incidence and response to treatment. Both the genesis of cancer and effective treatment for most cancers are dependent upon cellular responses to DNA damage. Cancer occurs because of the persistence and expansion of cells that carry mutations affecting the cancer hallmark pathways (12). Protective mechanisms that are normally triggered by the DNA damage itself, or by the resultant gain/loss of gene function, must be bypassed for cancers to arise. If we assume similar rates of inherited and somatically acquired mutations in males and females, we might hypothesize that the greater rates of cancer in males are related to less vigorous protective responses to early mutational events in male compared to female incipient cancer cells. To address this hypothesis, it is important to ask whether it is supported by population-level data, such as the patterns of cancer in individuals exposed to mutagenic doses of radiation (eg, survivors of atomic weapon attacks) or those with prior cancer treatment.
Survivors of the atomic bombings of Hiroshima and Nagasaki have been carefully studied for decades. The most recent report on solid tumor rates in survivors clearly demonstrates a significant sex effect. When sex-specific cancers are excluded, the solid cancer rate for males is 93.7 and 86.9 per 104 person-years in Hiroshima and Nagasaki, respectively. For female survivors, these rates are 63.7 and 48.8 (13), respectively. Similar rigor is characteristic of the SEER data collection and analysis of secondary cancer risk after primary cancer treatment. In aggregate, the lifetime excess absolute risk (EAR; cancer risk/10 000 person-years) is slightly higher in females. However, a substantial component of the secondary cancers in females are breast cancers, and overall, rates in male primary cancer survivors are consistently greater in those whose initial diagnosis was at less than 60 years of age (14). Together, these epidemiological studies indicate that significant sex differences in cancer risk are evident after exposure to mutagens, with much of that risk biased toward males. In addition, male mice and rats are more susceptible to cancer development in multiple models that utilize mutagens to induce DNA damage and promote carcinogenesis (15-18).
In a complimentary fashion, it may be important to consider evidence for sex differences in cancer in individuals with cancer predisposition syndromes from germline DNA repair defects. Lynch syndrome is the most common inherited DNA repair defect. It arises most frequently from mutations in the DNA mismatch repair genes MLH1 or MSH2 and substantially increases the risk for colorectal cancer and, to a lesser extent, several other cancers. The risk for colorectal and urinary tract cancer is significantly less for females with Lynch syndrome than for males across the lifespan (19,20). Li-Fraumeni syndrome (LFS) involves germline mutation in TP53. p53 is essential for coordinating cellular responses to DNA damage and predisposes affected individuals to multiple cancers in an age-, location-, and sex-biased manner. In young children, adrenocortical carcinoma is more common in females, while choroid plexus carcinoma and sarcomas are more common in males (21). In adults, breast cancer in females dominates the LFS phenotype, while brain tumors, a common diagnosis in both males and females with LFS, have an overall increased risk in males. Together, these additional epidemiological studies of cancer risk in the setting of increased DNA mutation demonstrate strong sex effects that vary according to age and cancer type but which, overall, suggest that males carry a higher risk for those cancers that occur in both sexes. Thus, it is reasonable to conclude that male and female responses to DNA damage are significantly different in a clinically relevant manner.
Sex differences in DNA repair are also critical to consider when looking into the basis for sex differences in cancer survival. As the mainstay of cancer treatment is the induction of cancer cell senescence or death through radiation- and chemotherapy-mediated DNA damage, the mechanisms underlying sex differences in survival are likely to involve sex differences in cellular responses to DNA damage. We directly measured response to radiation and chemotherapy in a cohort of glioblastoma patients through specialized analysis of standard magnetic resonance imaging. They found that female patients exhibited a significantly better response to radiation and temozolomide treatment than male patients did (22).
Sex Differences in Cellular Response to DNA Damage
The population level considerations previously presented make it reasonable to investigate cellular responses to DNA damage at greater depth. Following DNA damage, a cell fate decision must be made between transient cell cycle arrest and attempt at DNA repair, terminal exit from the cell cycle and senescence, or programmed cell death (23,24). Reentry into the cell cycle without adequate DNA repair results in the persistence of potentially cancer-promoting mutations within cells. Male cells accumulate more somatic mutations than female cells, which appears to be due to earlier accumulation of mutations in males, rather than sex differences in the actual mutation rate (25). This may suggest decreased DNA damage repair or impaired elimination of damaged cells in males compared to females.
Three major players in cell fate decisions following DNA damage are p53, p21, and Rb. In particular, level and duration of p53 expression is thought to be the critical determinant in whether a cell survives or dies (23,24). Sex differences in p53 function have been observed in both development (26,27) and aging (28). A study of male and female vascular smooth muscle cells exposed to UVB radiation found sex differences in both p53 localization and cell fate, with male cells exhibiting higher rates of apoptosis, while female cells had higher rates of senescence (29).
A critical target of p53 is p21, which inhibits the cyclin dependent kinase Cdk2 and thus prevents Rb hyperphosphorylation and inactivation. Rb is an important tumor suppressor that acts as a gatekeeper of the cell cycle; it can both transiently inhibit cell cycle progression and also induce permanent cell cycle arrest in the form of senescence (30,31). Following loss of function of the Nf1 and p53 tumor suppressors, female astrocytes were shown to be less likely to undergo malignant transformation than male astrocytes due to sex differences in Rb regulation (32), which was traced to differences in the expression of cyclin dependent kinase inhibitors, including p21. Female astrocytes with p53 and Nf1 loss were more likely to upregulate p21 following treatment with the chemotherapy etoposide, even though levels of DNA damage were equivalent in both sexes (33). Sex differences in p21 expression have also been reported in healthy tissues. In a study of sex differences in gene expression across species (ie, human, macaque, mouse, rat, and dog), p21 had conserved higher expression levels in multiple tissues in females compared to males (34).
The X-chromosome has been linked to regulation of both the p53 and Rb pathways through the lysine demethylase Kdm6a, a tumor suppressor that escapes X-inactivation. In Kdm6a knockout mice, p53 tumor suppressor pathway was 1 of the top 3 most affected canonical pathways, and Kdm6a had been shown to regulate transcription of canonical p53 targets, including p21 (35). Kdm6a also regulates a network of Rb pathway genes, and depletion of Kdm6a led to increased cell proliferation and S-phase entry (36). Thus, both p53 and Rb pathways could potentially be upregulated in female cells via an X-chromosome dependent mechanism.
Together these results suggest that female cells have greater upregulation of pathways that promote senescence in response to cellular stress and thus may have a lower threshold for senescence induction. Senescence plays an important biological role as a tumor suppressor mechanism (30,37) and is also induced by cancer therapies, including radiation and chemotherapy (38,39). Senescence could potentially contribute to lower rates of cancer and better survival in females.
Cellular Response to DNA Damage Is Patterned by Sexual Differentiation
Mechanisms of Sexual Differentiation
Biological sex, which is rooted in the evolutionary differences between males and females that arose as a product of sexual reproduction, results in fundamental differences in males and females at the genetic, epigenetic, and hormonal levels, with profound consequences for disease mechanisms and treatment responses (40). All sex differences in normal and pathophysiological development, aging, and disease are traceable to the mechanisms of sexual differentiation—the biological process by which males and females diverge physiologically from the undifferentiated zygote. Sexual differentiation begins at fertilization and continues throughout the lifespan. It is initiated by the unique complement of sex chromosomes in male (XY) and female (XX) cells. Since female cells lack a Y chromosome, all Y-linked genes are necessarily specific to male embryos. In contrast, females have 2 X chromosomes, resulting in higher expression levels of X-linked genes in female embryos prior to X-inactivation, as well as continued higher levels of those genes that escape from X-inactivation (41,42).
Differences in X and Y gene expression can exert broad downstream effects, regulating genome-wide transcriptional differences in males and females (43-45). This is demonstrated in preimplantation embryos, which exhibit sex differences in the expression levels of hundreds to thousands of genes, many of which are located on autosomes (45-47). Since this is prior to gonadal differentiation, these expression differences cannot be explained by sex hormone exposure but instead must be driven by the sex chromosomes. Sex differences in early embryo developmental rates and metabolism have both been linked to sex chromosomes (48,49), demonstrating important phenotypic consequences of sex chromosome complement.
In eutherian mammals, the expression of the testis-determining factor gene, SRY, from the Y chromosome exerts a profound effect on sexual differentiation. SRY is a transcription factor that initiates testes differentiation in XY embryos (50). In the absence of SRY expression, the gonads develop into the ovaries. The establishment of differentiated testes or ovaries is known as “sexual determination,” but it is gonadal hormones that drive sexual differentiation and pattern the sexual phenotype of the rest of the body (51). The fetal testes secrete testosterone beginning around gestational week 9 in humans, with the maximal sex difference in serum testosterone levels occurring between weeks 12 and 18 (52). Multiple studies have demonstrated that gonadal steroid exposure during a critical period of development is essential for normal sexual differentiation, and the embryonic period is now understood to be a time of “organization,” during which cells and tissues are permanently patterned by the effects of gonadal hormone exposure (53,54). Sex hormone-dependent epigenetic programming appears to be a mechanism underlying the long-lasting effects of the organizational period (3,55,56).
From the onset of puberty to menopause, gonadal hormones circulate at high levels, and their effects on secondary sexual characteristics and physiology are referred to as the “activational” period of sexual differentiation (54). During this period, circulating testosterone in males and estradiol and progesterone in females act on the sexually differentiated substrate established by the genetic and organizational effects of hormones during development. In contrast to the long-lasting, stable effects of in utero hormone exposure, activational effects of hormones are classically seen as acute and reversible (54).
Methods for Identifying Involved Mechanisms of Sexual Differentiation
The role that activational effects of sex hormones play in a biological sex difference can be readily interrogated through manipulations such as gonadectomy, with and without hormone replacement and before or after sexual maturity, as well as genetic and pharmacological inhibition or activation of hormone production or hormone response. It is more complicated to distinguish sex chromosome effects and the epigenetic effects of gonadal hormone exposure in utero. This is because testes and ovary development, and the resultant sex hormone secretion, is dependent upon the presence or absence of SRY, a Y-chromosome gene. Patient-derived specimens and induced pluripotent stem cells from individuals with disorders of sexual development can provide an experimental platform for investigating these mechanisms in humans, but this is not an approach that has been widely utilized. Alternatively, the four core genotypes (FCG) transgenic mouse model is a readily available experimental platform for parsing chromosomal and gonadal sex effects on biology (57).
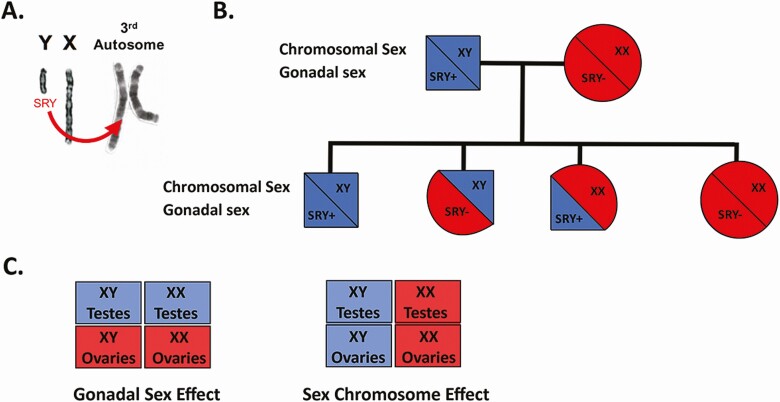
In the FCG model, Sry has been deleted from the Y chromosome and inserted in multiple copies as a transgene onto an autosome (SryA) (58). Thus, inheritance of Sry and the Y chromosome are independent, which enables separation of gonadal and chromosomal sex effects. Crossing XY−/SryA FCG males with normal XX females leads to offspring of 4 genotypes: XY/Sry+ (XY with testes; normal male), XY/Sry− (XY with ovaries), XX/Sry+ (XX with testes), and XX/Sry− (XX with ovaries; normal female). Mice that inherit Sry, regardless of whether they are XY or XX, develop testes and are exposed to masculinizing levels of gonadal hormones during development, while mice that lack Sry develop ovaries and display behaviors associated with a feminized brain (57). This model can be used to investigate whether a sex difference is driven primarily by sex chromosomes or gonadal sex (Fig. 1).
Figure 1.
The four core genotypes model. To separate chromosomal and gonadal sex effects on biology, Sry, the testis-determining gene, was deleted from the Y-chromosome and cloned onto chromosome 3 (A). The presence of Sry on an autosome (SryA), and not the Y-chromosome, results in 4 differing genotypes in pups from FCG male (XY;SryA) and wildtype female breeding (B). Comparisons between mice based on either gonads or sex chromosomes supports attribution to gonadal vs chromosomal sex effects for a phenotype (C).
Sex Chromosome Effects on Cellular Responses to DNA Damage
Astrocytes expressing a dominant negative form of p53 retain the ability to upregulate the p53 target gene Cdkn1a (p21) in response to DNA damage. The mechanisms for this upregulation remain unknown. It may be through the actions of other p53 family members, such as p63 and p73 (59), through some retained wildtype p53 function or through alternative p53-independent mechanisms. Regardless of the exact mechanism, upregulation of p21 in response to etoposide-induced DNA damage has been observed to be greater in female DNp53-expressing astrocytes with co-occurring deletion of Nf1 (Nf1−/−;DNp53) than in their male counterparts (33). The mechanism(s) underlying this sex difference may include X chromosome effects such as those mediated by the lysine demethylase Kdm6a, which escapes X-inactivation and regulates p53 canonical targets, including Cdkn1a (p21) (35). Through this mechanism, Kdm6a was shown to possess a bladder cancer protective effect. Tumor suppressive interactions between the X chromosomes and the p53 pathway have also been described by Haupt et al, who identified a network of X-linked genes associated with p53 function (60), supporting the importance of sex chromosomes in modifying p53 target gene expression.
In Utero Sex Hormone Effects on Cellular Responses to DNA Damage
Cellular senescence is a therapeutic response to DNA damaging cancer treatments. Senescence is primarily regulated by 2 central pathways: p53/p21WAF1/Cip1 and p16INK4A/Rb (30,37,61). Sex differences in the regulation of p21, p16, and Rb in a glioblastoma model have been described and indicate that upregulation of these pathways occurs with a female bias following cellular stress (32,33). Broestl et al examined the relationship between p21 expression and cellular senescence following DNA damage (62). In this study, wildtype, Nf1−/−;DNp53, and Nf1−/−;p53−/− astrocytes were irradiated, and p21 expression and cellular senescence were measured and correlated. Both wildtype and mutant female astrocytes exhibited significantly greater p21-dependent senescence in response to irradiation compared to males. Using the FCG model, it was determined that this difference was patterned by in utero gonadal sex; the presence of ovaries, but not testes, during development was associated with significant p21-dependent senescence (62). Since these studies were performed on isolated astrocytes in vitro, it supports a role for gonadal hormone-mediated epigenetic changes in regulating p21-induced senescence following DNA damage.
Activational Sex Hormone Effects on Cellular Responses to DNA Damage
Sex effects on DNA damage response are not solely patterned by in utero sexual differentiation. In sex-specific cancers such as prostate and breast cancers, activational effects of androgens, estrogens, and progestins impact on the kinetics and efficiency of DNA repair and exhibit complex relationships with genomic stability (63). Direct effects of androgens on the expression of multiple components of the DNA damage response pathway contribute to radio resistance in prostate cancer (64,65). Similarly, ligand activation of the estrogen receptor results in complex formation with mediator of DNA damage checkpoint 1, which promotes DNA repair through nonhomologous end joining or homologous recombination (66). Estrogen receptor (ER) has additional complex effects on DNA repair that are mediated through activation of protein kinase B and inhibition of ataxia telangiectasia mutated (ATM) and ATM and RAD3 related (ATR) (67). Similar to androgen receptor activation, estrogen receptor activation contributes to radio resistance in breast cancer.
Summary and Future Directions
Sex differences in normal development, aging, and disease are widespread. As briefly reviewed here, this includes fundamental aspects of cellular physiology such as response to DNA damage. The profound impact these differences can have on cancer incidence and survival cannot be overstated. Rigorous evaluation of the mechanisms underlying these differences is essential and includes not only studying male vs female cell biology but also discovery of which mechanisms of sexual differentiation are operative in patterning specific sex differences. These parallel lines of investigation are sure to advance our understanding of both normal biology and disease, and speed the development of more effective treatments for all patients.
Database Search Parameters
PubMed and Google searches were performed using terms specific to the subject, such as sex differences, DNA repair, DNA mutation, sex hormones, sex chromosomes, atomic bomb survivors, secondary cancer risks, Lynch or Li-Fraumeni syndromes, and sexual differentiation.
Acknowledgments
Work in the Rubin Lab related to this manuscript was supported by NIH RO1 CA174737-06 (J.B.R.), NIH R21 NS098210 (J.B.R.), and Joshua’s Great Things (J.B.R.).
Additional Information
Disclosure Summary : The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1. Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex disparities in cancer mortality and survival. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1629-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. [DOI] [PubMed] [Google Scholar]
- 3. Rubin JB, Lagas JS, Broestl L, et al. . Sex differences in cancer mechanisms. Biol Sex Differ. 2020;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conforti F, Pala L, Bagnardi V, et al. . Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19(6):737-746. [DOI] [PubMed] [Google Scholar]
- 5. Rampen FH. Malignant melanoma: sex differences in response to chemotherapy? Eur J Cancer Clin Oncol. 1982;18(1):107-110. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 7. Mervic L. Time course and pattern of metastasis of cutaneous melanoma differ between men and women. Plos One. 2012;7(3):e32955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28(26):4086-4093. [DOI] [PubMed] [Google Scholar]
- 9. Rampen F. Malignant melanoma: sex differences in survival after evidence of distant metastasis. Br J Cancer. 1980;42(1):52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geller SE, Koch AR, Roesch P, Filut A, Hallgren E, Carnes M. The more things change, the more they stay the same: a study to evaluate compliance with inclusion and assessment of women and minorities in randomized controlled trials. Acad Med. 2018;93(4):630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bond KM, McCarthy MM, Rubin JB, Swanson KR. Molecular omics resources should require sex annotation: a call for action. Nat Methods. 2021;18(6):585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 13. Grant EJ, Brenner A, Sugiyama H, et al. . Solid cancer incidence among the life span study of atomic bomb survivors: 1958-2009. Radiat Res. 2017;187(5):513-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curtis RE, Freedman DM, Ron E, et al. . New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute; 2006. [Google Scholar]
- 15. Bertram JS, Craig AW. Specific induction of bladder cancer in mice by butyl-(4-hydroxybutyl)-nitrosamine and the effects of hormonal modifications on the sex difference in response. Eur J Cancer. 1972;8(6):587-594. [DOI] [PubMed] [Google Scholar]
- 16. Thomas-Ahner JM, Wulff BC, Tober KL, Kusewitt DF, Riggenbach JA, Oberyszyn TM. Gender differences in UVB-induced skin carcinogenesis, inflammation, and DNA damage. Cancer Res. 2007;67(7):3468-3474. [DOI] [PubMed] [Google Scholar]
- 17. Lee SM, Kim N, Son HJ, et al. . The effect of sex on the azoxymethane/dextran sulfate sodium-treated mice model of colon cancer. J Cancer Prev. 2016;21(4):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kadekar S, Peddada S, Silins I, French JE, Högberg J, Stenius U. Gender differences in chemical carcinogenesis in National Toxicology Program 2-year bioassays. Toxicol Pathol. 2012;40(8):1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wischhusen JW, Ukaegbu C, Dhingra TG, et al. . Clinical factors associated with urinary tract cancer in individuals with lynch syndrome. Cancer Epidemiol Biomarkers Prev. 2020;29(1):193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bucksch K, Zachariae S, Aretz S, et al. ; German Consortium for Familial Intestinal Cancer . Cancer risks in Lynch syndrome, Lynch-like syndrome, and familial colorectal cancer type X: a prospective cohort study. BMC Cancer. 2020;20(1):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amadou A, Achatz MIW, Hainaut P. Revisiting tumor patterns and penetrance in germline TP53 mutation carriers: temporal phases of Li-Fraumeni syndrome. Curr Opin Oncol. 2018;30(1):23-29. [DOI] [PubMed] [Google Scholar]
- 22. Yang W, Warrington NM, Taylor SJ, et al. . Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. 2019;11(473):eaao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16(1):20-33. [DOI] [PubMed] [Google Scholar]
- 24. Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15(11):1139-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Podolskiy DI, Lobanov AV, Kryukov GV, Gladyshev VN. Analysis of cancer genomes reveals basic features of human aging and its role in cancer development. Nat Commun. 2016;7:12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen X, Watkins R, Delot E, et al. . Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68(2):265-273. [DOI] [PubMed] [Google Scholar]
- 27. Delbridge ARD, Kueh AJ, Ke F, et al. . Loss of p53 causes stochastic aberrant X-chromosome inactivation and female-specific neural tube defects. Cell Rep. 2019;27(2):442-454.e5. [DOI] [PubMed] [Google Scholar]
- 28. Waskar M, Landis GN, Shen J, et al. . Drosophila melanogaster p53 has developmental stage-specific and sex-specific effects on adult life span indicative of sexual antagonistic pleiotropy. Aging (Albany NY). 2009;1(11):903-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malorni W, Straface E, Matarrese P, et al. . Redox state and gender differences in vascular smooth muscle cells. FEBS Lett. 2008;582(5):635-642. [DOI] [PubMed] [Google Scholar]
- 30. Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482-496. [DOI] [PubMed] [Google Scholar]
- 31. Harrington EA, Bruce JL, Harlow E, Dyson N. pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc Natl Acad Sci U S A. 1998;95(20):11945-11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun T, Warrington NM, Luo J, et al. . Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest. 2014;124(9):4123-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kfoury N, Sun T, Yu K, et al. . Cooperative p16 and p21 action protects female astrocytes from transformation. Acta Neuropathol Commun. 2018;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365(6450):eaaw7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaneko S, Li X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci Adv. 2018;4(6):eaar5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang JK, Tsai MC, Poulin G, et al. . The histone demethylase UTX enables RB-dependent cell fate control. Genes Dev. 2010;24(4):327-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. te Poele RH, Okorokov AL, Jardine L, Cummings J, Joel SP. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res. 2002;62(6):1876-1883. [PubMed] [Google Scholar]
- 39. Jeon HY, Kim JK, Ham SW, et al. . Irradiation induces glioblastoma cell senescence and senescence-associated secretory phenotype. Tumour Biol. 2016;37(5):5857-5867. [DOI] [PubMed] [Google Scholar]
- 40. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, et al. . Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396(10250):565-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am J Physiol Cell Physiol. 2014;306(1):C3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moreira de Mello JC, Fernandes GR, Vibranovski MD, Pereira LV. Early X chromosome inactivation during human preimplantation development revealed by single-cell RNA-sequencing. Sci Rep. 2017;7(1):10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wijchers PJ, Yandim C, Panousopoulou E, et al. . Sexual dimorphism in mammalian autosomal gene regulation is determined not only by Sry but by sex chromosome complement as well. Dev Cell. 2010;19(3):477-484. [DOI] [PubMed] [Google Scholar]
- 44. Bramble MS, Roach L, Lipson A, et al. . Sex-specific effects of testosterone on the sexually dimorphic transcriptome and epigenome of embryonic neural stem/progenitor cells. Sci Rep. 2016;6:36916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Werner RJ, Schultz BM, Huhn JM, Jelinek J, Madzo J, Engel N. Sex chromosomes drive gene expression and regulatory dimorphisms in mouse embryonic stem cells. Biol Sex Differ. 2017;8(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci U S A. 2010;107(8):3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lowe R, Gemma C, Rakyan VK, Holland ML. Sexually dimorphic gene expression emerges with embryonic genome activation and is dynamic throughout development. BMC Genomics. 2015;16:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350(1333):253-260; discussion 260. [DOI] [PubMed] [Google Scholar]
- 49. Gutiérrez-Adán A, Oter M, Martínez-Madrid B, Pintado B, De La Fuente J. Differential expression of two genes located on the X chromosome between male and female in vitro-produced bovine embryos at the blastocyst stage. Mol Reprod Dev. 2000;55(2):146-151. [DOI] [PubMed] [Google Scholar]
- 50. Eggers S, Sinclair A. Mammalian sex determination—insights from humans and mice. Chromosome Res. 2012;20(1):215-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arnold AP. A general theory of sexual differentiation. J Neurosci Res. 2017;95(1-2):291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christine Knickmeyer R, Baron-Cohen S. Fetal testosterone and sex differences. Early Hum Dev. 2006;82(12):755-760. [DOI] [PubMed] [Google Scholar]
- 53. Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369-382. [DOI] [PubMed] [Google Scholar]
- 54. Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55(5):570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology. 2011;93(3):150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McCarthy MM, Nugent BM. At the frontier of epigenetics of brain sex differences. Front Behav Neurosci. 2015;9:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Itoh Y, Mackie R, Kampf K, et al. . Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dötsch V, Bernassola F, Coutandin D, Candi E, Melino G. p63 and p73, the ancestors of p53. Cold Spring Harb Perspect Biol. 2010;2(9):a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haupt S, Caramia F, Herschtal A, et al. . Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat Commun. 2019;10(1):5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729-740. [DOI] [PubMed] [Google Scholar]
- 62. Broestl L, Grandison L, Shenoy S, et al. . Gonadal sex patterns p21-induced cellular senescence in mouse and human glioblastoma. BioRxiv. Posted June 2, 2021. doi: 10.1101/2021.06.02.446756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ganguly S, Naik D, Muskara A, Mian OY. The nexus of endocrine signaling and cancer: how steroid hormones influence genomic stability. Endocrinology. 2021;162(1):endocr/bqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goodwin JF, Schiewer MJ, Dean JL, et al. . A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Polkinghorn WR, Parker JS, Lee MX, et al. . Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zou R, Zhong X, Wang C, et al. . MDC1 enhances estrogen receptor-mediated transactivation and contributes to breast cancer suppression. Int J Biol Sci. 2015;11(9):992-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pedram A, Razandi M, Evinger AJ, Lee E, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009;20(14):3374-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.