Abstract
Yeast strains lacking the yeast nuclear cap-binding complex (yCBC) are viable, although impaired in growth. We have taken advantage of this observation to carry out a genetic screen for components that show synthetic lethality (SL) with a cbp20-Δ cbp80-Δ double mutation. One set of SL interactions was due to mutations that were complemented by components of U1 small nuclear RNP (snRNP) and the yeast splicing commitment complex. These interactions confirm the role of yCBC in commitment complex formation. Physical interaction of yCBC with the commitment complex components Mud10p and Mud2p, which may directly mediate yCBC function, was demonstrated. Unexpectedly, we identified multiple SL mutations that were complemented by Cbf5p and Nop58p. These are components of the two major classes of yeast small nucleolar RNPs, which function in the maturation of rRNA precursors. Mutants lacking yCBC were found to be defective in rRNA processing. Analysis of the yCBC deletion phenotype suggests that this is likely to be due to a defect in the splicing of a subset of ribosomal protein mRNA precursors.
Most eukaryotic organisms have a complement of three specialized RNA polymerases (pol I, pol II, and pol III) responsible mainly for rRNA, mRNA, and tRNA synthesis, respectively. pol II transcripts have characteristic 5′ ends consisting of a 7-methylguanosine cap structure attached by a 5′-5′ phosphotriester linkage to the first encoded nucleotide of the transcript (66). Aside from providing protection against 5′-to-3′ exonuclease activities, the cap structure plays important roles at multiple steps in the function of pol II transcripts. In vertebrates, the cap has been shown to stimulate pre-mRNA splicing (30, 37, 38, 57), pre-mRNA 3′ end formation by cleavage and polyadenylation (11, 15, 19, 23), export from the nucleus of U small nuclear RNAs (snRNAs) (22, 32), and the initiation of translation (67).
The effect of the cap in mRNA translation is mediated by eukaryotic initiation factor 4F (eIF-4F), a multicomponent complex whose cap-binding subunit is eIF-4E (67). In contrast, the nuclear functions of the cap are all thought to be mediated by CBC, the nuclear cap-binding complex. CBC consists of a heterodimer of two proteins, CBP80 and CBP20 (30, 31, 33, 34), and evidence from in vivo and in vitro experiments supports its role in both pre-mRNA splicing and U snRNA export (30, 31, 44). CBC associates with the cap structures of pre-mRNA and nuclear mRNA in vivo and accompanies mRNA through nuclear pore complexes to the cytoplasm (78). There is, however, no evidence that CBC plays an important role in the nuclear export of mRNA, in contrast to its function in the export of U snRNAs (31, 32).
While the in vitro evidence for the function of CBC in cleavage and polyadenylation is direct (15), the current in vivo data on this topic are less definitive. It has, however, been observed that in cells whose largest pol II subunit is truncated, transcripts are not efficiently capped (9, 48), and the majority of the RNAs produced thus lack a high-affinity CBC binding site. Such uncapped transcripts are neither spliced nor cleaved and polyadenylated efficiently (49). Although the latter effect may reflect direct interaction between the carboxy-terminal domain of the pol II subunit and the cleavage and polyadenylation machinery (24, 49), the lack of CBC binding to the transcripts may also contribute to the inefficiency of their 3′ end formation.
CBC has been identified in yeast (10, 20, 43). Yeast CBP80 (yCBP80) is encoded by the GCR3 gene (75), and yCBP20 is encoded by MUD13 (10, 20). In contrast to the data for multicellular eukaryotes discussed above, the data reported on CBC function in yeast relate only to pre-mRNA splicing. mud13 and gcr3 strains exhibit reduced splicing of a reporter gene that carries a nonconsensus 5′ splice site (10) or a nonconsensus sequence in the branchpoint region (15a). In vitro splicing is also decreased in extracts that were biochemically depleted of CBC (43) or extracts from a mud13 strain (10). Yeast strains that lack RNA capping activity do not show obvious defects in mRNA cleavage or polyadenylation (16, 63), and extracts from yeast cells that lack CBC do not exhibit defects in 3′ end formation in vitro (15a). It is unclear whether yeast U snRNAs resemble their vertebrate counterparts in being transported out of the nucleus during maturation, and yCBC function in U snRNA export has therefore not yet been tested.
In pre-mRNA splicing, yCBC and human CBC (hCBC) play analogous roles. They increase the efficiency with which U1 snRNP binds to the cap-proximal 5′ splice site and thus increase the rate of recognition and splicing of the cap-proximal intron (10, 43, 44). In biochemical terms, this manifests itself as an increase in commitment complex formation (64) in the presence of CBC (10, 43). Although the functions in pre-mRNA splicing of yCBC and hCBC are therefore likely to be mechanistically related, the detailed mechanism by which CBC exerts its role is not known. Attempts to demonstrate direct interaction between hCBC and human U1 snRNP were unsuccessful (44), resulting in the hypothesis that one or more unknown factors mediate the CBC-dependent increase in U1 snRNP-5′ splice site interaction.
To identify this intermediary and, more generally, to obtain additional insight into CBC function, a genetic analysis of CBC in Saccharomyces cerevisiae has been undertaken. Twelve distinct genes whose mutation leads to lethality in the absence of yCBC are identified. Complementation of these defects reveals that the great majority of these genetic interactions can be explained on the basis of yCBC function in the commitment complex assembly step of pre-mRNA splicing. Further, evidence of physical interaction between yCBC and two commitment complex components, Mud2p and Mud10p, is presented.
MATERIALS AND METHODS
DNA constructs.
To construct pHT80, the GCR3 gene was PCR amplified from genomic DNA from position −800 before the ATG to position 290 after the stop codon of yCBP80. The amplified DNA was digested with SmaI and SalI and cloned into the same sites of the polylinker of pHT4467, a single-copy plasmid with ADE2 and URA markers. The functionality of the gene was checked by its ability to restore growth and splicing efficiency to wild-type levels in the cbp80-Δ strain.
pSEY8-yCBP20, a full-length yCBP20 clone from a yeast genomic library (20), was digested with HindIII and repaired with Klenow enzyme to obtain a 1-kbp fragment that was cloned using the SmaI site of pHT4467 or pHT80 to generate pHT20 and pHT8020, respectively. The expression of yCBP20 and yCBP80 in these pHT4467-derived plasmids was assayed by growth restoration of the cbp disrupted strains and by Western blotting using extracts isolated from cbp20-Δ, cbp80-Δ, and cbp20/80-Δ strains transformed with pHT20, pHT80, and pHT8020 respectively.
To construct YEp20, the open reading frame (ORF) of MUD13 was amplified from pSEY8-yCBP20 (20). The fragment was digested with BamHI and HindIII and cloned in the same sites under the GAL10 promoter of YEp51, a multicopy plasmid with a LEU marker (62). Similarly, to construct YEp80, the ORF of yCBP80 was amplified with oligonucleotides that avoid intronic sequences. The PCR fragment was end repaired and cloned in the SmaI site of pBluescript SK+ (Stratagene) to generate pBS-GCR3. This plasmid was then digested with EagI and BglI; the ends were repaired with Klenow enzyme and digested with SalI. The fragment containing GCR3 was cloned under the control of the GAL10 promoter in YEp51. The expression of yCBP80 and yCBP20 in YEp51-derived plasmids was analyzed by growth rate restoration and by Western blotting.
Deletion of yCBC genes.
The techniques used for growing yeast are described elsewhere (68). Yeast cells were transformed with DNA by the lithium acetate method (29). Strains used for the synthetic lethal (SL) screen were derived from YJV159 (MATa ade2 ade3 his3 leu2-3,112 trp1 ura3) (76a).
The cbp20-Δ strain (MATa ade2 ade3 his3 leu2-3,112 trp1 ura3 ycbp20/mud13::HIS3) was obtained by transfection of YJV159 with a linear DNA in which the HIS3 gene from plasmid Ydp-H (5) had been inserted between the BclI and SnaBI sites of MUD13. Transformed cells were grown on SD-His medium, and the MUD13 deletion was confirmed by PCR amplification of genomic DNA and Southern blotting.
To obtain the cbp80-Δ strain (MATa ade2 ade3 his3 leu2-3,112 rp1 ura3 ycbp80/gcr3::TRP1) and the double-knockout strain (MATa ade2 ade3 his3 leu2-3,112 trp1 ura3 ycbp80/gcr3::TRP1 ycbp20/mud13::HIS3), YJV159 and cbp20-Δ cells were transfected with a linear DNA carrying a disrupted copy of the GCR3 gene. The sequence from 20 nucleotides before the ATG to position 2700, just after the last ATG in frame, were replaced by the TRP1 gene from plasmid Ydp-W (5). Transformed cells where grown on SD-Trp medium, and the GCR3 deletion was confirmed by PCR amplification of genomic DNA and Southern blotting. The doubling times of these strains were measured, and the expression of yCBP80 and yCBP20 was analyzed by Western blotting. A disruption of GCR3 was also generated in a MATα strain. A GCR3 gene fragment from the SnaBI site, 200 nucleotides upstream of the ATG, to the BglII site was replaced by the HIS3 gene in strain D209 (MATα ade2 leu2 ura3 his3 rp1). PCR amplification of genomic DNA and Southern blotting were used to verify the genotype. This strain was crossed with YJV159, the diploid was sporulated and tetrads were dissected. Strain cbp80-Δ α (MATα ade2 ade3 leu2 trp1 ura3 ycbp80/gcr3::HIS3) was identified among the haploid progeny by screening for the desired phenotypes. The doubling time of this strain was 230 min, similar to that of a cbp80-Δ strain (Fig. 1).
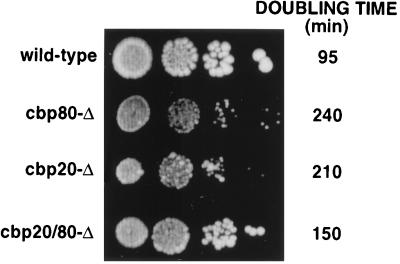
FIG. 1.
yCBC affects vegetative growth rate. Strain YJV159 (wild type) was disrupted for GCR3 (cbp80-Δ), for MUD13 (cbp20-Δ), or for both (cbp20/80-Δ). These strains were grown to mid-log phase, and four dilutions of each were plated to compare the growth rates. The doubling time of each strain was also assayed in liquid culture and is indicated on the right.
The ssd1-Δ allele was constructed by one-step PCR (4) using the HIS3 selective marker with integration targeting sequences that precisely delete the entire ORF.
Doubling time and viability tests.
Yeast control strain or strains disrupted for GCR3, MUD13, or both were grown in liquid medium to mid-log phase (optical density at 600 nm of approximately 0.8). Cells were then diluted 20 and 40 times, and optical density was monitored. Doubling time was calculated during logarithmic growth. Dilutions of these strains were also plated on YPD and allowed to grow for 48 h at 30°C (Fig. 1). Similarly, temperature-sensitive LUC mutants (see below for description) were checked for viability. Cells grown to mid-log phase were diluted and shifted to 37°C, and growth was monitored.
Mutagenesis and the SL screen.
An SL screen was performed as described elsewhere (39). The cbp20/80-Δ strain was transformed with plasmid pHT8020, which encodes yCBP80, yCBP20, Ura3p, and Ade3p. The resulting cells are red due to the ADE3-ade2 combination. When cells were grown on 4% glucose–YPD medium, a red to white sectoring phenotype was observed, indicating that the strain could lose plasmid pHT8020. Cells plated on 4% glucose–YPD were exposed to 254-nm UV light (Desaga) for 30 s to allow 10% survival; 2.8 × 105 colonies were then screened, and 560 colonies that did not sector were isolated. Of these, 155 did not grow on 5-fluoro-orotic acid (FOA) plates, indicating that they needed pHT8020 to survive; 42 clones showed a red color and FOA sensitivity at different temperatures. To eliminate false positives, these strains were transformed with plasmid YEp80, YEp20, or both. Thirty-two strains were then able to survive on FOA plates, indicating that the phenotype was indeed yCBC related. Most of the strains needed both yCBP80 and yCBP20 to survive on FOA plates, but some showed weak growth with only yCBP20 or yCBP80.
A genetic characterization was then carried out. The mutants were crossed to the cbp80-Δ α strain, and in all cases red to white sectoring was observed, indicating that all the mutations were recessive. Diploids were sporulated, and 10 to 12 tetrads were dissected and analyzed phenotypically. In all cases where four spores were recovered, the sectoring phenotype and the FOA lethality segregated 2:2, indicating that the synthetic lethality was probably caused by mutation at a single locus. As some of the mutants showed temperature sensitivity, these crosses also allowed determination of whether temperature sensitivity was linked to synthetic lethality. Finally, the mutant strains were crossed pairwise and plated on FOA plates. Combinations that could not grow were assigned to a complementation group. The 32 strains were sorted into 21 complementation groups named LUC, as they are lethal unless CBC is produced.
Cloning of genes that complement LUC1 to LUC14.
Mutants were transformed with a low-copy-number plasmid library (6), using conditions that predict that five times the whole yeast genome was being transformed and a probability of recovery higher than 95%. Transformants were selected on minimal plates. The mutant strains that showed temperature sensitivity linked to the synthetic lethality were grown at 37°C after transformation. The other strains were grown at 30°C, and the sectoring phenotype was allowed to develop after replica plating to YPD–4% glucose, using nitrocellulose membranes (Protran BA 85/20; 0.45-mm pore size; Schleicher & Schuell). Plates were screened, and sectoring colonies were plated on FOA plates. Plasmids containing complementing genomic DNA fragments were recovered from the positives and amplified in Escherichia coli XL1-Blue. Retransformation into the mutant strains and rechecking of the sectoring/FOA or temperature resistance phenotype was performed. Insert DNA boundaries were sequenced and compared to the MIPS (Munich Information Centre for Protein Sequences) yeast database (50–52) to define the complementing region. As the average insert size of the library was 10 kb, several genes were usually present in the inserts. When several positives were isolated from a single mutant strain, the overlapping region of the inserts helped to define the complementing ORF. When two or more genes were still partially or totally included in the overlap, they were cloned independently in pRS315, a single-copy plasmid with a LEU marker (69) and retransformed in the mutant strain. Plasmids expressing Mud1p, and Mud2p, and SmD3p were kindly provided by M. Rosbash and B. Séraphin. Sectoring phenotype and FOA (or temperature) resistance were used to define the complementing ORF.
yCBC column preparation and binding assays.
The yCBC column was prepared as described previously (20, 43). After preparation, 10 μl of the column was boiled in sodium dodecyl sulfate (SDS) sample buffer without reducing agents, and the proteins bound to it were separated by electrophoresis in an SDS–12% polyacrylamide gel and stained with Coomassie blue dye. Only two proteins were detected. Western blotting analysis identified them as yCBP80 and yCBP20. An unrelated antibody column was incubated with yeast extracts under conditions similar to those used with a negative control.
The U1 snRNP proteins isolated in the screen and Mud2p were labeled with [35S]methionine in an in vitro T7 coupled transcription-translation reaction (TNT-T7 kit; Promega). The T7 promoter-containing template was obtained as a PCR amplification product. The 5′ oligonucleotides used contained the T7 promoter sequence followed by 20 nucleotides around the ATG region of the ORF. In the case of MUD1, the 5′ oligonucleotide was longer and included the sequence from the ATG to the sixth nucleotide after the intron. The 3′ end oligonucleotides used contained the sequence complementary to the last 20 nucleotides of the ORF. The PCR was done with standard conditions for cloned Pfu polymerase (Stratagene), 1 μg of plasmid DNA, and 20 to 25 cycles. The PCR product was purified by using a Qiaquick PCR purification kit (Qiagen); 0.5 μg of the amplified product was incubated in a 50-μl reaction mixture with the reticulocyte lysate TNT-T7 mix (Promega) that couples transcription and translation. The labeled proteins were then diluted to 500 μl with phosphate-buffered saline-8.5% glycerol and centrifuged through a Nanosep 30K filter (Pall Filtron) at 10,000 rpm (Biofuge A; Heraeus) at 4°C until a 10-fold concentration was achieved. This step was repeated twice to eliminate the unincorporated [35S]methionine.
The control and yCBC columns were washed in binding buffer (50 mM Tris [pH 7.5], 150 mM NaCl, and 10% glycerol in complete protease inhibitor cocktail from Boehringer Mannheim); 15 μl of control or yCBC column beads (corresponding to 1.5 μg of yCBC) was mixed with 3 μl of labeled proteins in a final volume of 200 μl of binding buffer. The mixture was rotated for 2 h at 4°C. The beads were pelleted, and the supernatant was recovered. The beads were washed three times with 1 ml of binding buffer. Supernatant and pellet fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and visualized by fluorography.
Northern hybridization.
RNA was extracted as described previously (71), separated by electrophoresis, and transferred to a filter. For hybridization to snoRNAs, the oligonucleotides anti-U3 (5′-CUAUAGAAAUGAUCCU), anti-U14 (5′-TCACTCAGACATCCTAGG), anti-snR10 (5′-CUIUUAAAUUUICIUU), snR3 (TCGATCTTCGTACTGTCT), and anti-snR30 (ATGTCTGCAGTATGGTTTTAC) were used. Oligonucleotide anti-U3 is largely composed of 2′-O-methyl RNA.
For hybridization to mRNAs, the oligonucleotides used were anti-ACT1 (5′-TCTTGGTCTACCGACGATAGATGGGAAGACAGCA), anti-RPS9A (5′-GTTGTATACTTTTGTATTTCT), anti-RPS9B (5′-TGTTGCTTAGTCTTAGTTG), anti-RPS11A (5′-CTTGCTGGTTGCTTAATTT), anti-RPS11B (5′-TCCCTGGCTTGATACGTT), anti-RPS3 (5′-GACACCGTCAGCGACTAG), anti-RPS10A (5′-GCTTGGTTGAAATCCTTC), anti-RPL16A (5′-CTCGATTTGTTCTTCACCTTC), anti-RPL16B (5′-CCAACCAACCAACAATAATAC), anti-RPL22A (5′-CTTAATCTGTTGTTTTGGTGG), anti-RPL22B (5′-GTGGTTGATATTTGTGAAACG), anti-RPL10 (5′-CTGTAACATCTAGCTGGTC), anti-RPL30 (5′-GGTTGATAGAATCTTGGGAT), anti-RPL28 (5′-GTGCTTTCTGTGCTTACCGATACGACCTTTACCG), and anti-RPL25 (TTTCTTAGCGGCAGTAGCC).
For pre-rRNA hybridization, oligonucleotides depicted in Fig. 4A were used: 001 (5′-CCAGTTACGAAAATTCTTG), 002 (5′-GCTCTTTGCTCTTGCC), 003 (5′-TGTTACCTCTGGGCCC), 007 (5′-CTCCGCTTATTGATATGC), 008 (5′-CATGGCTTAATCTTTGAGAC), and 013 (5′-GGCCAGCAATTTCAAGTTA).
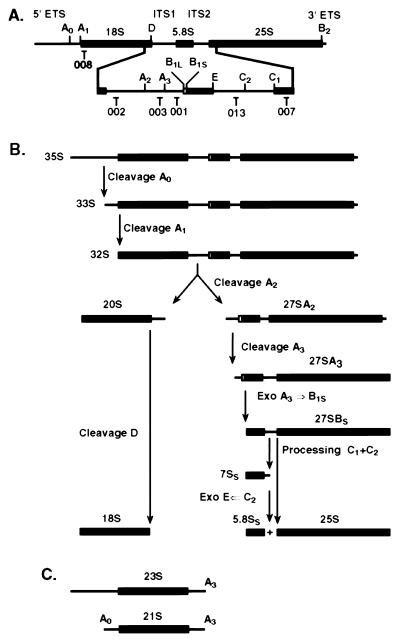
FIG. 4.
The yeast pre-rRNA processing pathway. (A) Structure of the pre-rRNA with positions of oligonucleotides used for hybridization. In the 35S pre-rRNA, the mature 18S, 5.8S, and 25S rRNA sequences are flanked by the 5′ and 3′ external transcribed spacers (5′ ETS and 3′ ETS) and separated by internal transcribed spacers 1 and 2 (ITS1 and ITS2). (B) Major pre-rRNA processing pathway in yeast. Note that a minor alternative pathway in ITS1 generates an alternative form of 5.8S rRNA (5.8SL) that is extended 5′ to site BIL (not shown). (C) Structures of the aberrant 23S and 21S RNAs.
RESULTS
It was previously determined that GCR3, which encodes yCBP80, and MUD13, which encodes yCBP20, are not essential genes in S. cerevisiae (10, 75), strongly suggesting that yCBC is not necessary for yeast vegetative growth. To further investigate this, the growth of yeast strains lacking GCR3 (cbp80-Δ), MUD13 (cbp20-Δ) or both genes (cbp20/80-Δ) was analyzed. As shown in Fig. 1, strains that lack either CBP80 or CBP20 individually grow slowly at 30°C on rich medium either on plates or in liquid culture. The growth defects are similar at higher (37°C) or lower (23°C) temperatures (data not shown). Analysis of extracts made from the strains by Western blotting showed that while the cbp20-Δ strain accumulated amounts of yCBP80 similar to those accumulated by the wild-type strain, the cbp80-Δ strain accumulated fourfold less yCBP20 than the wild type, suggesting that yCBP20 is unstable in the absence of yCBP80 (data not shown). Since CBP80 and CBP20 need to heterodimerize to bind to capped RNA (30, 31) it was not surprising that the two single-deletion strains showed similar growth defects. It was unexpected, however, that a strain lacking both yCBP80 and yCBP20 (cbp20/80-Δ) grew better than strains with either single deletion (Fig. 1). This suggested that the production of either CBP80 or CBP20 alone had a dominant negative effect on growth.
SL interactions.
Since a yeast strain lacking both yCBP80 and yCBP20 could grow reasonably well, the double-deletion background served as the basis for a search for genes whose mutation would prove lethal in the absence of CBC (see Materials and Methods for details). In this way, 21 complementation groups that were lethal unless CBC was produced (LUC) were characterized (see Materials and Methods). Fourteen of these complementation groups were rescued by transformation with low-copy-number plasmids containing yeast genomic DNA inserts and named LUC1 to LUC14. In 12 of the 14 cases, the gene responsible for complementation was identified by further analysis, usually through transformation with subfragments of the original DNA insert of the complementing plasmid (Table 1). Many of these complementation groups were represented only once in the collection of SL strains, showing that the screen is unlikely to be saturated.
TABLE 1.
Summary of SL mutationsa
| Mutant | nb | Gene(s) characterized | Characteristic(s)c |
|---|---|---|---|
| LUC1 | 2 | MUD1 | U1 snRNP |
| LUC2 | 2 | MUD2 | U2AF65 homologue |
| LUC3 | 1 | NAM8 | U1 snRNP |
| (MRE2/MUD15) | |||
| LUC4 | 3 | SNU56 | U1 snRNP |
| (MUD10) | |||
| LUC5 | 1 | SNU71 | U1 snRNP |
| LUC6 | 2 | SMD3 | U snRNP core protein |
| LUC7 | 1 | YDL087c | Metazoan homologues with SR domains |
| LUC8 | 2 | CBF5 | snoRNP (H+ACA) |
| LUC9 | 5 | NOP58 | snoRNP (C+D) |
| LUC10 | 1 | SSD1 | Predicted exonuclease; Ts mutation |
| LUC11 | 1 | GCR1 | Transcription factor; Ts mutation |
| LUC12 | 1 | SRV2 | Binds actin and adenylate cyclase |
| LUC13 | 1 | RPO31 | Subunit of pol III |
| YTA1 | 26S proteasome component | ||
| LUC14 | 1 | YNL206c | Similar to HMG/SSRP proteins |
| SPS18 | Unknown function | ||
| SPS19 | Peroxisomal 2,4-dienoyl-CoA reductase |
Twenty-one complementation groups were isolated in an SL screen with yCBC and named LUC mutants. The strains were transformed with a library of yeast genomic DNA inserts in a low-copy-number plasmid, and plasmids able to suppress the SL phenotype were isolated for 14 complementation groups (LUC1 to LUC14). In 12 of these cases, plasmid analysis allowed the definition of a single open reading frame whose expression reverts the SL phenotype (LUC1 to LUC12). The genes are indicated in each case, and a brief summary of their characteristics is shown on the right. Single genes have not yet been defined for LUC13 and LUC14, but they are contained between nucleotides 5357982 and 547020 of chromosome XV (LUC13) and nucleotides 256819 and 260289 of chromosome XIV (LUC14).
Number of isolates from the same complementation group.
Ts, temperature sensitive; CoA, coenzyme A.
The LUC genes can be divided into four main groups: (i) those that encode splicing factors that are components of yeast commitment complexes (LUC1 to LUC6); (ii) components of yeast small nucleolar RNPs (snoRNPs) (LUC8 and LUC9); (iii) genes with a function in RNA metabolism that seems unconnected to known CBC functions (LUC10 and LUC13); and (iv) genes with no obvious direct connection to RNA metabolism (LUC11, LUC12, and LUC14).
Genetic interactions between yCBC and splicing factors.
MUD13, which encodes yCBP20, was characterized on the basis of a mutant allele that caused synthetic lethality when present in combination with an otherwise viable mutant form of U1 snRNA (10). This finding, together with biochemical data (10, 43), showed that yCBC functioned in the commitment complex assembly step of yeast pre-mRNA splicing. Commitment complexes form on intron-containing pre-mRNAs in the absence of ATP hydrolysis. There are two commitment complexes, CC1 and CC2, both of which depend on U1 snRNP-5′ splice site interaction (64, 65). In addition, CC2 requires interaction between Mud2p and branch point binding protein (BBP), which bind at and near the branchpoint region of the intron, and U1 snRNP bound at the 5′ splice site (1, 2, 7, 65). The identities of genes complementing LUC1 to LUC6 are consistent with the function of yCBC in commitment complex assembly.
LUC1, LUC2, and LUC6 were initially assigned to this category. LUC1/MUD1 encodes the yeast U1A homologue and also causes synthetic lethality with the truncated U1 snRNA used to identify MUD13 (46). U1A is a conserved component of U1 snRNP. LUC2/MUD2 was also found in the truncated U1 snRNA SL screen and encodes the yeast homologue of U2AF65 (1). Both U2AF65 and Mud2p are involved in very early steps of intron recognition (1, 2, 7, 61, 83). LUC6 is complemented by SMD3, which encodes one of the core components of the spliceosomal snRNPs (60; see also reference 47). Although not specific for U1 snRNP, in fact an SMD3 allele that causes synthetic lethality together with a mutant U2 snRNA has previously been isolated (81); mutation of SmD3p could be expected to affect U1 snRNP function at early stages of splicing.
The product of NAM8, which complements LUC3, was originally proposed to have a role in mitochondrial RNA splicing (13) and later implicated in meiosis-specific nuclear pre-mRNA splicing events (54). Recently, however, it was identified, along with the products of SNU56/MUD10, which complements LUC4, and SNU71, which complements LUC5, as a novel component of the yeast U1 snRNP (21). These three proteins are all stably associated with yeast U1 snRNA but are not present in vertebrate U1 snRNP (14, 21). Since yCBC and U1 snRNP are both commitment complex components, these findings provide a reasonable explanation for the synthetic lethality that results when these three genes are mutated on a yCBC null background.
YDL087c, which complements LUC7, is not functionally characterized. We have found putative vertebrate homologues by examination of the DNA databases. These homologues have SR domains, characteristic of a large family of metazoan splicing factors (59, 82), consistent with the possibility that the LUC7 SL phenotype is also due to mutation of a protein involved in pre-mRNA splicing. Further characterization of this complementation group is in progress.
Physical interaction between CBC and yeast splicing factors.
As described in the introduction, hCBC was shown to stimulate U1 snRNP binding to the cap-proximal 5′ splice site but not to interact directly with U1 snRNP (44). This suggested that one or more mediators of hCBC-U1 snRNP interaction must exist. The analogous role in splicing of yCBC, and the presence of several additional proteins in yeast U1 snRNP compared to its human counterpart (14, 21), suggested that one or more of these proteins might form a direct interaction with yCBC.
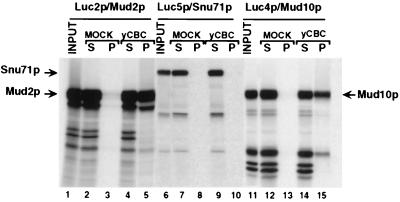
To examine this possibility, yCBC was purified from yeast extracts by immune-affinity chromatography using an antibody directed against the N terminus of yCBP80 (20, 43). The column was washed extensively with buffer containing 1 M NaCl; upon subsequent SDS elution, only yCBP80 and yCBP20 were detected by Coomassie blue staining. Mud2p, Snu71p, and Mud10p were synthesized and labeled with [35S]methionine by in vitro transcription and translation and passed over the column. Mud2p and Mud10p were clearly retained on the column (Fig. 2, lanes 1 to 5 and 11 to 15), while Snu71p (lanes 6 to 10), Nam8p and Luc7p (data not shown) were not retained. Since yCBC could not be prepared in recombinant form but had to be purified from yeast extract, and since the Mud2p and Mud10p proteins were produced in reticulocyte lysate, we cannot be certain that the interactions observed are direct rather than mediated by a factor in the lysate, nor is it certain whether posttranslational modifications are required for the interactions. Indeed, Mud10p produced in E. coli lysate did not bind to the CBC column (data not shown), suggesting a possible role for modification of this protein in CBC interaction. Despite these caveats, the interactions observed make Mud2p and Mud10p strong candidates for mediating the interactions that allow yCBC to stimulate commitment complex formation. Additional support for this possibility comes from the observation that yCBC and Mud10p were found to interact by the two-hybrid method in yeast (16a).
FIG. 2.
yCBC interacts with Mud2p and with Mud10p. [35S]methionine-labeled Mud2p/Luc2p (lanes 1 to 5), Snu71p/Luc5p (lanes 6 to 10), and Mud10p/Luc4p (lanes 11 to 15) were incubated with a control column (MOCK) or with a yCBC column as indicated. Samples were fractionated into nonbound supernatant (S) and bound pellet (P) fractions and analyzed by SDS-polyacrylamide gel electrophoresis. In lanes 1, 6, and 11, 25% of the input protein was loaded.
yCBC deletion strains exhibit defects in rRNA processing.
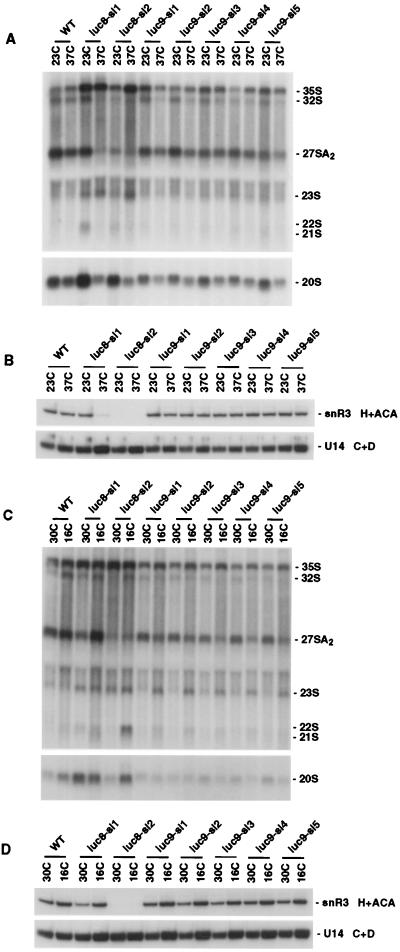
LUC8 (two strains) and LUC9 (five strains) were complemented by the CBF5 and NOP58 genes, respectively. Cbf5p and Nop58p are both components of snoRNP complexes. The large number of snoRNA species present in eukaryotic cells can be divided into two families on the basis of conserved sequence elements (reviewed in reference 41). Nop58p associates with the box C+D family of snoRNAs (18, 80), most of which function as guides to direct ribose methylation on pre-rRNA (35, 72). Cbf5p is likely to be the rRNA pseudouridine synthase which is guided by the box H+ACA family of snoRNAs to sites of pseudouridine formation on pre-rRNA (17, 40, 55, 72). In addition to their roles in pre-rRNA modification, both classes of snoRNA include members that are critical for pre-rRNA processing at three early cleavage sites designated A0, A1, and A2 (see Fig. 4).
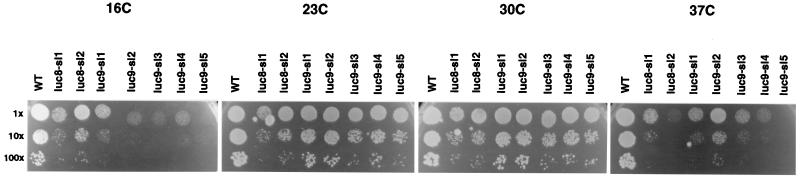
In the presence of functional CBC, the two LUC8 strains and five LUC9 strains were temperature sensitive for growth at 37°C and the LUC9 strains were additionally strongly cold sensitive for growth at 16°C (Fig. 3). Following transfer from 25 to 37°C, the luc8-sl1 strain showed an inhibition of pre-rRNA processing (Fig. 4 and 5A), while the luc8-sl2 strain showed a largely nonconditional processing inhibition (Fig. 5A and C). The processing defects resemble those seen in strain depleted of Cbf5p; the 35S pre-rRNA accumulated, while the 32S, 27SA2, and 20S pre-rRNAs were depleted (Fig. 4). Aberrant processing intermediates (the 21S, 22S, and 23S RNAs) were also detected (Fig. 5A and data not shown). These phenotypes are indicative of the inhibition of processing at sites A0, A1, and A2. The LUC9 strains showed a mild pre-rRNA processing defect at 37°C (Fig. 5A) and stronger inhibition of processing following transfer from 30 to 16°C (Fig. 5C). Again, the phenotype was indicative of the inhibition of processing at sites A0, A1, and A2. Similar inhibition is seen in strains genetically depleted of Nop58p (80).
FIG. 3.
Growth of the LUC8 and LUC9 strains carrying functional CBC. Dilutions (1- to 102-fold) of luc8 and luc9 strains along with the wild-type isogenic (WT) control strain were spotted on minimal plates at 16, 23, 30, and 37°C and incubated for 3 days.
FIG. 5.
Northern analysis of pre-rRNA (A and C) and snoRNA (B and D) levels in LUC8 and LUC9 strains. RNA was extracted following growth at 23°C and 18 h after transfer to 37°C (A and B) or following growth at 30°C and 12 h after transfer to 16°C (C and D). The oligonucleotide probes used in panels A and C were 003 (top) and 002 (bottom). WT, wild type.
The SL strains LUC8 and LUC9, expressing yCBP80 and yCBP20, have both rRNA processing and snoRNA stability defects that are consistent with mutations in CBF5 and NOP58, respectively (Fig. 5). Nop58p is required for the stability of the box C+D class of snoRNAs, while Cbf5p is required for stability of box H+ACA snoRNAs (18, 41a). The luc8-sl1 strain was found to result in conditional depletion of the box H+ACA snoRNA snR3 at 37°C, while luc8-sl2 resulted in nonconditional depletion of snR3 (Fig. 5B and D). Depletion of the essential box H+ACA snoRNA, snR30, was substantially less marked at 23 or 30°C (data not shown). None of the LUC9 strains resulted in clear depletion of the box C+D snoRNA, U14 (Fig. 5B and D).
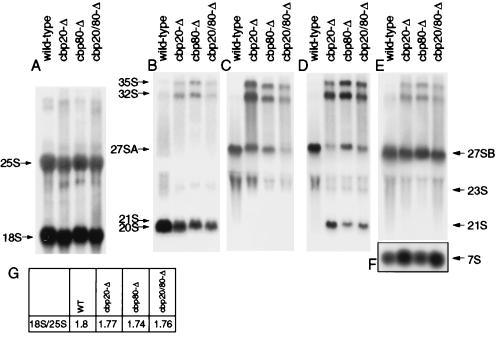
The genetic interaction of CBC with components of both major classes of snoRNP suggested that deletion of CBC might affect pre-rRNA processing. This possibility was tested by Northern hybridization using probes specific for either mature rRNAs (Fig. 6A) or pre-rRNAs (Fig. 6B to F) in strains lacking yCBP80 and/or yCBP20.
FIG. 6.
yCBC is required for normal pre-rRNA processing. For Northern blot analysis of mature and precursor rRNAs, RNA was extracted from wild-type (WT) and cbp strains as indicated. (A) Hybridization with a probe complementary to the mature 18S and 25S RNAs; (B) hybridization with a probe complementary to the 5′ region of ITS1 (oligonucleotide 002); (C) hybridization with a probe complementary to the 3′ region of ITS1, downstream of site A3 (oligonucleotide 001); (D) hybridization with a probe complementary to the central region of ITS1, between sites A2 and A3 (oligonucleotide 003); (E and F) hybridization with a probe complementary to the 5′ region of ITS2 (oligonucleotide 013). (G) Ratios of steady-state levels of mature 18S and 25S rRNAs. The positions of mature and precursor rRNA species are indicated; 21S and 20S pre-rRNAs are not well resolved; the identity of the 32S intermediate was verified by hybridizing a riboprobe complementary to the region between sites A0 and A1. Positions of the oligonucleotide probes are depicted in Fig. 4.
Several pre-rRNA species accumulated to abnormally high levels in all three deletion strains; the 35S primary transcript, the 32S pre-rRNA, and an aberrant 21S rRNA (see also Fig. 4). In contrast, the level of the 27SA2 pre-rRNA was strongly reduced. The 21S intermediate extends from site A0 to A3, and results from cleavage of the 32S pre-rRNA at site A3 in the absence of cleavage at site A2. We conclude that A2 cleavage is particularly inhibited in the mutants. The overall pattern of defects, however, suggests that not only A2, but also the A0 and A1 cleavage events are slowed. Levels of 27SB and 7S pre-rRNAs were not altered (Fig. 6E and F), indicating that the pathway of 5.8S/25S rRNA synthesis is not affected by yCBC deletion (Fig. 4). We conclude that the absence of Cbp20p or Cbp80p inhibits processing at sites A0, A1, and A2, with the greatest effects on A2. Processing at later steps on the pathway of 5.8S/25S synthesis does not appear to be affected. No clear reduction in the levels of mature 25S or 18S rRNAs was observed (Fig. 6A and G), so the inhibition of mature rRNA synthesis is unlikely to be directly responsible for the slow growth of the cbp deletion strains.
Four snoRNA species are required for pre-rRNA processing at sites A0, A1, and A2: U3 and U14, which are associated with Nop58p (27, 41a, 45, 80), and snR30 and snR10, which are associated with Cbf5p (40, 53, 70). Among these, the rRNA processing phenotype observed in the cbp deletions strains is most similar to this observed upon deletion of the SNR10 gene (70). Depletion of Nop58p or Cbf5p leads to loss of the snoRNAs with which they are associated; moreover, several snoRNAs, including U18 and U24, are encoded within pre-mRNA introns, and their synthesis could be inhibited by splicing defects. We therefore examined the steady-state levels of snoRNAs in the CBC deletion strains. No change in the steady-state levels of any of these snoRNAs was observed (Fig. 7 and data not shown).
FIG. 7.
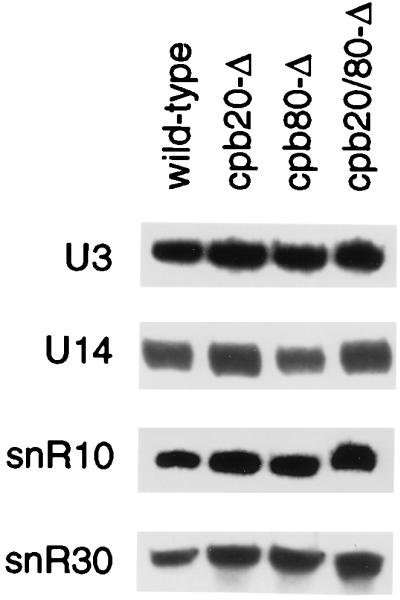
yCBC does not affect accumulation of various snoRNAs. For Northern blot analysis of low-molecular-weight RNAs, RNA was extracted from wild-type and cbp strains as indicated. The probes used for hybridization are described in Materials and Methods.
Many snoRNAs, including U3, snR10, and snR30 snoRNAs, carry hypermethylated 5′ cap structures (26, 53, 79). The cap structures on the U3 and U8 snoRNAs have been reported to be required for nucleolar localization (28; but see reference 42) and therefore, presumably, for function. The efficiency of cap hypermethylation in the cbp strains was assessed by immunoprecipitation using a m2,2,7G cap-specific serum (R1131) and an monoclonal antibody that reacts with both m2,2,7G and m7G cap structures (H20; kindly provided by R. Lührmann). No difference in immunoprecipitation was observed between RNAs extracted from the wild-type and cbp20-Δ strains, suggesting that the cbp strains were not deficient in snoRNA cap hypermethylation (data not shown).
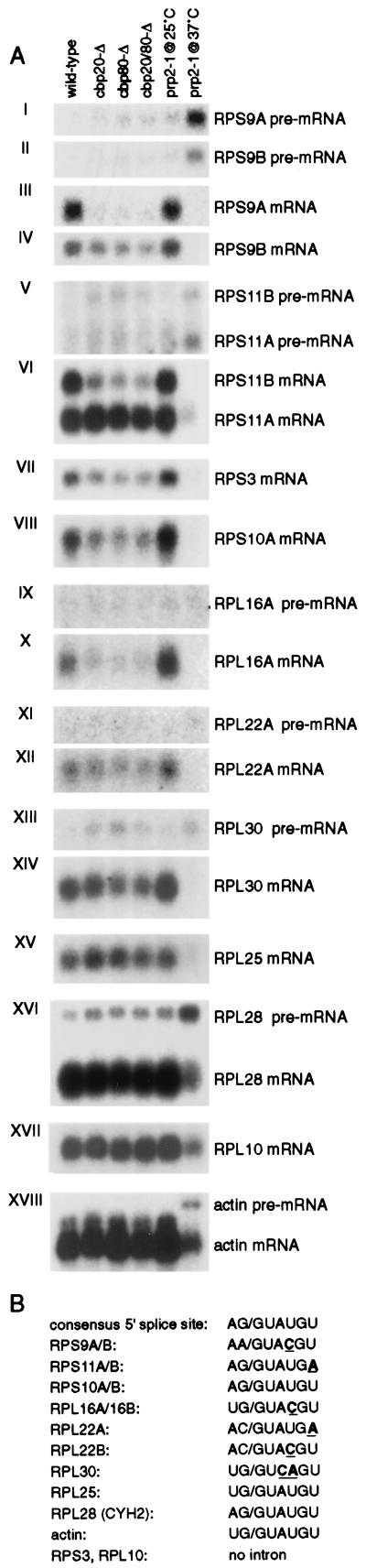
Defects in ribosome assembly caused by inefficient splicing of the pre-mRNAs encoding ribosomal proteins can inhibit pre-rRNA processing in yeast (references 8 and 58 and references therein). Since processing defects were detected mainly in the small ribosomal subunit rRNA, we first investigated the splicing of small subunit ribosomal protein (RPS) pre-mRNAs in the CBC deletion strains. As a control, we utilized the temperature sensitive prp2-1 strain, which exhibits a strong splicing block at the nonpermissive temperature (37°C) and consequent accumulation of pre-mRNAs (reference 58 and Fig. 8). Since CBC plays roles in the U1 snRNP-5′ splice site interaction and commitment complex assembly (10, 43, 44), we initially analyzed pre-mRNAs with nonconsenus 5′ splice sites (73). RPS9A and RSP9B contain GUACGU instead of GUAUGU; while RPS11A and RPS11B contain GUAUGA instead of GUAUGU (Fig. 8B).
FIG. 8.
yCBC affects steady-state levels of mRNAs of ribosomal proteins. RNA was extracted from either wild-type or cbp strains as indicated. Additionally, control RNA was extracted from a temperature-sensitive-lethal splicing-deficient prp2-1 strain grown either at the permissive temperature (25°C) or after shift to the nonpermissive temperature (37°C) for 60 min. (A) Analysis of RPS and RPL mRNAs and pre-mRNAs. The positions of mature and precursor mRNAs are indicated. Probes used for hybridization are described in Materials and Methods. (B) Sequences at the 5′ splice sites of pre-mRNAs analyzed in this study. Nonconsensus residues are underlined. All pre-mRNAs tested contain one intron, and in all cases it is located close to the 5′ end of the pre-mRNA.
Analysis of the steady-state levels of these mRNAs shows that splicing of the pre-mRNAs is inhibited. A similar degree of splicing inhibition was observed in the single and double cbp deletion strains (Fig. 8A, I to VI), while the different pre-mRNAs showed various degrees of inhibition. The level of RPS11A mRNA was not significantly altered, and there was no detectable accumulation of nonspliced pre-mRNA. By contrast, the mature RPS9A, RSP9B, and RPS11B mRNAs were depleted in the deletion strains and pre-mRNAs accumulated (Fig. 8A, I to VI; Table 2). The accumulation of unspliced precursors indicated that splicing of these pre-mRNAs is indeed defective. The defects in the splicing of RPS9A and RPS11B were particularly strong. These differences may be explained by examination of the pre-mRNA sequences. In addition to nonconsensus 5′ splice sites, RPS9A and RPS11B also lack optimal polypyrimidine tract and branchpoint region sequences (CACUAAC and GACUAAU, respectively, instead of UACUAAC). Since reporter introns with either 5′ splice site or branchpoint mutations are very poorly spliced in strains lacking yCBC (references 10 and 15a), this may contribute to their inefficient splicing in the absence of CBC. The splicing of actin pre-mRNA (Fig. 8A, XVIII) was not altered in the yCBC deletion strains. RPS3 encodes a small subunit ribosomal protein but does not contain an intron (Fig. 8A VII), while the introns in RPS10A and RPS10B (data shown only for RPS10A [Fig. 8A, VIII]) have a consensus 5′ splice site. For each of these genes there was a clear decrease in mRNA, although this was not accompanied by an increase in the RPS10A or RPS10B pre-mRNAs (data not shown).
TABLE 2.
PhosphorImager (Molecular Dynamics) analysis of the accumulation of RPS9A, RPS11B, RPL30, and RPL28 pre-mRNAs and mRNAsa
| Strain |
RPS9A
|
RPS11B
|
RPL30
|
RPL28
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-mRNA | mRNA | Pre-mRNA/mRNA | Pre-mRNA | mRNA | Pre-mRNA/RNA | Pre-mRNA | mRNA | Pre-mRNA/mRNA | Pre-mRNA | mRNA | Pre-mRNA/ mRNA | ||
| Wild type | 1 | 65.05 | 0.015 | 1 | 10.27 | 0.1 | 1 | 14.3 | 0.07 | 1 | 13.7 | 0.073 | |
| cbc20-Δ | 5.16 | 6.3 | 0.82 | 4.08 | 2.1 | 1.94 | 1.58 | 10.4 | 0.15 | 1.34 | 12.6 | 0.11 | |
| cbc80-Δ | 9.76 | 9 | 1.08 | 4.11 | 2.25 | 1.83 | 1.89 | 7.33 | 0.26 | 1.35 | 10.45 | 0.13 | |
| cbc20/80-Δ | 7.65 | 9.69 | 0.77 | 3.43 | 2.18 | 1.57 | 1.66 | 7.8 | 0.2 | 1.31 | 11.27 | 0.12 | |
| prp2-1 | |||||||||||||
| 25°C | 10.22 | 66.08 | 0.15 | 3.79 | 10.33 | 0.37 | 1.37 | 18.4 | 0.074 | 1.38 | 14.2 | 0.097 | |
| 37°C | 50.7 | 7.05 | 7.2 | 2.1 | 0.14 | 15 | 1.98 | 1.29 | 1.53 | 3.9 | 2.26 | 1.73 | |
Values relative to those of wild-type pre-mRNA are shown.
Since the levels of many pre-mRNAs and mRNAs encoding small subunit ribosomal proteins were affected in cbc strains, we examined whether this would also be the case for large ribosomal subunit (RPL) pre-mRNAs. The 5′ splice sites of these pre-mRNAs are GUACGU for RPL16A and RPL16B, GUAUGA for RPL22A, GUACGU for RPL22B, and GUCAGU for RPL30, compared to the consensus GUAUGU (Fig. 8B). The steady-state levels of these mRNAs were also decreased in all cbc strains, particularly for RPL16A and RPL16B (Fig. 8A, IX-XIV; RPL16B and RPL22B were very similar to RPL16A and RPL22A [data not shown]). The decrease in the level of mRNA was clearly accompanied by accumulation of pre-mRNA only in the case of RPL30 (Table 2). Note that the 5′ splice site of RPL30 pre-mRNA has two nonconsensus residues. RPL25 and RPL28 (CYH2) pre-mRNAs have consensus 5′ splice sites, whereas RPL10 pre-mRNA has no intron. There was no significant reduction in the levels of these three mRNAs in the cbc strain. We conclude that the splicing of the pre-mRNAs with suboptimal splice sites is strongly inhibited in strains lacking CBC. The degree of inhibition varies between different pre-mRNAs with weak 5′ splice sites (Fig. 8A; Table 2), reflecting the differing relative levels of importance of CBC function in the splicing of those pre-mRNAs. Note that the reduction in some ribosomal protein mRNAs without concomitant increase in pre-mRNA, and the reduction in mRNAs from genes without introns, could well be a consequence of the impaired growth of the strains and consequent reduction in ribosome synthesis.
Other SL complementing genes.
LUC11 (Table 1) is complemented by GCR1, a transcriptional activator required for expression of multiple genes involved in glucose metabolism (3, 25). Since the gene encoding yCBP80, GCR3, was first identified in a search for additional mutants that affected growth on glucose (75), the finding of gcr1 mutants in our screen was not a surprise. As would be expected, the alleles of GCR1 recovered from the synthetic lethal screen did not cause lethality when the strains were plated on nonfermentable carbon sources (data not shown). Three additional complementation groups among the seven for which no complementing plasmid was recovered also failed to produce lethality when grown on nonfermentable carbon sources, suggesting that additional genes involved in glucose metabolism are likely to be involved in producing the SL phenotype. A possible explanation for both the earlier and present findings with GCR1 is the report that the GCR1 gene includes an intron with a nonconsensus 5′ splice site (GUAUGA instead of GUAUGU [73]).
There is no obvious reason why SRV2 (LUC12) or any of the genes on the LUC14 complementing plasmid (Table 1) should, when mutated, generate a lethal phenotype in the absence of CBC. Similarly, although it is possible to speculate on possible functional connections between pol III transcripts (e.g., U6 snRNA) and CBC, the identity of any of the genes that complement LUC13 is not readily explicable. Given the role of CBC in U snRNA transport in vertebrates (31) and the existence of an abundant complex in yeast between CBC and yeast importin α (Srp1p), a mediator of nuclear protein import (20), it was of interest that the temperature-sensitive allele of LUC10/SSD1 recovered in this screen accumulates poly(A)-containing RNA in the nucleus at nonpermissive temperature (data not shown). LUC10/SSD1 was the only strain isolated in the screen showing this phenotype. However, an ssd1-Δ allele with a precise deletion of the entire ORF did not exhibit nuclear poly(A) accumulation (21a). Sequence analysis suggests that SSD1 likely encodes an exonuclease of the RNase II family (76), consistent with a role in RNA metabolism, but no change in mRNA stability was detected in either the cbp deletion strains or luc10-1 strain (15a).
DISCUSSION
An extensive genetic analysis has been carried out with yCBC. Although there is strong evidence that vertebrate CBC is multifunctional (see the introduction), all of the genetic interactions with yCBC that are readily explicable are consistent with the function of yCBC at the commitment complex assembly stage of yeast pre-mRNA splicing (10, 43). Two strong candidates for direct physical interaction with yCBC in the commitment complex were identified: Mud10p, which does not yet have an identified vertebrate homologue; and Mud2p, the yeast U2AF65 homologue (1). Examination of interaction between human CBC and U2AF65, using techniques similar to those used here, indicate that these proteins also interact in vitro (15a). Surprisingly, however, the SR repeat-containing domain of U2AF65 (83) which is not conserved in yeast Mud2p (1) is the region required for this interaction. Further investigation of this interaction is in progress.
The approach used here, that of screening for genetic interactions that produce synthetic lethality, has previously been used successfully to identify several components of the yeast commitment complex (1, 2, 10, 46), and our analysis provides a strong confirmation of the usefulness of the approach in this case. Like the nuclear pore complex (12) or the Srb complex that forms part of the basal pol II transcription machinery (36), the commitment complex consists of a large number of components held together by multiple individual interactions, many of which may be relatively weak. Such complexes appear to represent particularly sensitive, and therefore productive, targets for this form of genetic analysis. This is presumably because a mutation that affects an individual interaction is often insufficient to destabilize the whole complex, whereas disruption of multiple combinations of two interactions will cause destabilization.
An interesting aspect of our data concerns the role of CBC in commitment complex formation and function. Based on previous data on vertebrate splicing, CBC has been viewed functionally as a cofactor that increases the interaction between U1 snRNP and the cap-proximal 5′ splice site (44). The yeast equivalent of this function might be fulfilled by the CBC-Mud10p interaction. Other results presented here suggest the yCBC function may be more complicated. First, both genetic and physical interaction between CBC and Mud2p were observed. Mud2p is not required for the interaction of U1 snRNP with pre-mRNA during formation of the initial commitment complex, CC1 (1); rather, it is needed for CC2 formation. Since Mud2p binds to the pyrimidine tract of the intron, i.e., 3′ of the BBP-branchpoint region complex (2, 7), this might suggest that yCBC’s role extends beyond U1 snRNP function. Further evidence for this hypothesis comes from comparing the efficiencies of splicing between RSP9A and RSP9B pre-mRNAs and between RSP11A and RSP11B pre-mRNAs. These two pairs of pre-mRNAs exhibit markedly different splicing efficiencies in the absence of CBC. These differences do not correlate with differences at the 5′ splice site, where U1 snRNP interaction occurs, but with differences in the pyrimidine tract regions of the introns. These results might suggest that yCBC stabilizes both U1 snRNP-5′ splice site and Mud2p-3′ splice site interactions.
CBC and pre-rRNA processing.
An unexpected result from these analyses was the identification of five SL strains that could be complemented by NOP58 and two that were complemented by CBF5. These genes encode essential nucleolar proteins that are core components of the box C+D and box H+ACA families of snoRNPs, respectively (reviewed in reference 41). Nop58p and Cbf5p are both required for the early pre-rRNA processing steps at sites A0, A1, and A2 on the pathway of 18S rRNA synthesis. The SL strains were each found to have defects in pre-rRNA processing at these steps, in the presence of functional CBC. Similarly, strains lacking CBC were found to be defective in the cleavage of sites A0, A1, and A2, with the greatest effect on site A2. Synergistic inhibition of rRNA synthesis is therefore likely to underlie the observed SL interactions. A large number of genes encode pre-rRNA processing factors, and it is unclear why only two complementation groups were isolated in multiple strains. One possibility is that snoRNAs with which Nop58p and Cbf5p associate also play roles in the modification of spliceosomal snRNAs and therefore participate, indirectly, in pre-mRNA splicing. Box C+D snoRNAs guide 2′-O-methylation of several positions in the U6 snRNA in vertebrates (74); however, equivalent guide RNAs have not been identified in yeast.
Strains lacking CBC were found to be defective in the splicing of pre-mRNAs that encode ribosomal proteins, particularly those in which the sequences at both the 5′ and 3′ ends of the intron were nonconsensus. The pre-rRNA processing defect in the cbc mutants may therefore be a consequence of reduced, or imbalanced, ribosomal protein synthesis. In the cbc strain, the steady-state level of the mature rRNAs was not clearly altered, suggesting that reduced rRNA synthesis is not the direct cause of the growth defect. This phenotype resembles that seen in strains lacking the snoRNA, snR10 (70). Like Cbp20p and Cbp80p, snR10 is not essential, but its absence impairs cell growth. Pre-rRNA processing is inhibited at sites A0, A1, and A2, with the greatest effect on A2. Accumulation of mature rRNA is, however, not prevented, and the impaired growth is probably due to a defect in ribosome assembly (77). We speculate that an alteration in the stoichiometry of the ribosomal proteins interferes with normal ribosome assembly, leading to the synthesis of partially defective ribosomal subunits.
ACKNOWLEDGMENTS
We thank B. Séraphin for the plasmid expressing SmD3p and the single-copy plasmid genomic library, M. Rosbash for plasmids expressing Mud1p and Mud2p, R. Lührmann for yeast extracts and anticap antibodies, D. Görlich for anti-yCBP80 antibody and the pSEY8-yCBP20 plasmid, H. Tekotte for plasmid pHT4467, B. Dichtl and J. Venema for advice on the SL screen, and E. Hartmann for discussions early in the project and for providing a different yCBP20 deletion strain. Bertrand Séraphin, Mutsuhito Ohno, Juan Valcárcel, Alexandra Segref, and Gert-Jan Arts provided useful criticisms of the manuscript.
P.F. was a recipient of a fellowship from the EU TMR program, and J.K. received a fellowship from EMBO.
REFERENCES
- 1.Abovich N, Liao X C, Rosbash M. The yeast MUD2 protein: an interaction with PRP11 defines a bridge between commitment complexes and U2 snRNP addition. Genes Dev. 1994;8:843–854. doi: 10.1101/gad.8.7.843. [DOI] [PubMed] [Google Scholar]
- 2.Abovich N, Rosbash M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell. 1997;89:403–412. doi: 10.1016/s0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 3.Baker H V. GCR1 of Saccharomyces cerevisiae encodes a DNA binding protein whose binding is abolished by mutations in the CTTCC sequence motif. Proc Natl Acad Sci USA. 1991;88:9443–9447. doi: 10.1073/pnas.88.21.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudin-Baillieu A, Guillemet E, Cullinand C, Lacroute F. Construction of a yeast strain deleted for the TRP1 promoter and coding region that enhances the efficiency of the polymerase chain reaction-disruption method. Yeast. 1997;13:353–356. doi: 10.1002/(SICI)1097-0061(19970330)13:4<353::AID-YEA86>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 5.Berben G, Dumont J, Gilliquet V, Bolle P A, Hilger F. The Ydp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast. 1991;7:475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- 6.Berges T, Petfalski E, Tollervey D, Hurt E C. Synthetic lethality with fibrillarin identifies Nop77p, a nucleolar protein required for pre-rRNA processing and modification. EMBO J. 1994;13:3136–3148. doi: 10.1002/j.1460-2075.1994.tb06612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund J A, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/s0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 8.Bromley S, Hereford L, Rosbash M. Further evidence that the rna2 mutation of Saccharomyces cerevisiae affects mRNA processing. Mol Cell Biol. 1982;2:1205–1211. doi: 10.1128/mcb.2.10.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho E J, Takagi T, Moore C R, Buratowski S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 1997;11:3319–3326. doi: 10.1101/gad.11.24.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colot H V, Stutz F, Rosbash M. The yeast splicing factor Mud13p is a commitment complex component and corresponds to CBP20, the small subunit of the nuclear cap-binding complex. Genes Dev. 1996;10:1699–1708. doi: 10.1101/gad.10.13.1699. [DOI] [PubMed] [Google Scholar]
- 11.Cooke C, Alwine J C. The cap and the 3′ splice site similarly affect polyadenylation efficiency. Mol Cell Biol. 1996;16:2579–2584. doi: 10.1128/mcb.16.6.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doye V, Hurt E. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 13.Ekwall K, Kermorgant M, Dujardin G, Groudinsky O, Slonimski P P. The NAM8 gene in Saccharomyces cerevisiae encodes a protein with putative RNA binding motifs and acts as a suppressor of mitochondrial splicing deficiencies when overexpressed. Mol Gen Genet. 1992;233:136–144. doi: 10.1007/BF00587571. [DOI] [PubMed] [Google Scholar]
- 14.Fabrizio P, Esser S, Kastner B, Lührmann R. Isolation of S. cerevisiae snRNPs: comparison of U1 and U4/U6.U5 to their human counterparts. Science. 1994;264:261–265. doi: 10.1126/science.8146658. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty S M, Fortes P, Izaurralde E, Mattaj I W, Gilmartin G M. Participation of the nuclear cap binding complex in pre-mRNA 3′ processing. Proc Natl Acad Sci USA. 1997;94:11893–11898. doi: 10.1073/pnas.94.22.11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Fortes, P., and I. W. Mattaj. Unpublished data.
- 16.Fresco L D, Buratowski S. Conditional mutants of the yeast mRNA capping enzyme show that the cap enhances, but is not required for, mRNA splicing. RNA. 1996;2:584–596. [PMC free article] [PubMed] [Google Scholar]
- 16a.Fromont-Racine, M., and P. Legrain. Personal communication.
- 17.Ganot P, Caizergues-Ferrer M, Kiss T. The family of box ACA small nucleolar RNAs is defined by an evolutionarily defined secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev. 1997;11:941–956. doi: 10.1101/gad.11.7.941. [DOI] [PubMed] [Google Scholar]
- 18.Gautier T, Berges T, Tollervey D, Hurt E. Nucleolar KKE/D repeat proteins Nop56p and Nop58p interact with Nop1p and are required for ribosome biogenesis. Mol Cell Biol. 1997;17:7088–7098. doi: 10.1128/mcb.17.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilmartin G M, McDevitt M A, Nevins J R. Multiple factors are required for specific RNA cleavage at a poly(A) addition site. Genes Dev. 1988;2:578–587. doi: 10.1101/gad.2.5.578. [DOI] [PubMed] [Google Scholar]
- 20.Görlich D, Kraft R, Kostka S, Vogel F, Hartmann E, Laskey R A, Mattaj I W, Izaurralde E. Importin provides a link between nuclear protein import and U snRNA export. Cell. 1996;87:21–32. doi: 10.1016/s0092-8674(00)81319-7. [DOI] [PubMed] [Google Scholar]
- 21.Gottschalk A, Tang J, Puig O, Salgado J, Neubauer G, Colot H V, Mann M, Séraphin B, Rosbash M, Lührmann R, Fabrizio P A. Comprehensive biochemical and genetic analysis of the yeast U1 snRNP reveals five novel proteins. RNA. 1998;4:374–393. [PMC free article] [PubMed] [Google Scholar]
- 21a.Grosshans, H., J. Kufel, and D. Tollervey. Unpublished data.
- 22.Hamm J, Mattaj I W. Monomethylated cap structures facilitate RNA export from the nucleus. Cell. 1990;63:109–118. doi: 10.1016/0092-8674(90)90292-m. [DOI] [PubMed] [Google Scholar]
- 23.Hart R P, McDevitt M A, Nevins J R. Poly(A) site cleavage in a HeLa nuclear extract is dependent on downstream sequences. Cell. 1985;43:677–683. doi: 10.1016/0092-8674(85)90240-5. [DOI] [PubMed] [Google Scholar]
- 24.Hirose Y, Manley J L. RNA polymerase II is an essential mRNA polyadenylation factor. Nature. 1998;395:93–96. doi: 10.1038/25786. [DOI] [PubMed] [Google Scholar]
- 25.Holland M J, Yokoi T, Holland J P, Myambo K, Innis M A. The GCR1 gene encodes a positive transcriptional regulator of the enolase and glyceraldehyde-3-phosphate dehydrogenase gene families in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:813–820. doi: 10.1128/mcb.7.2.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes J M X, Konings D A M, Cesareni G. The yeast homologue of U3 snRNA. EMBO J. 1987;6:2145–2155. doi: 10.1002/j.1460-2075.1987.tb02482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes J M, Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991;10:4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobson M R, Pederson T. A 7-methylguanosine cap commits U3 and U8 small nuclear RNAs to the nucleolar localization pathway. Nucleic Acids Res. 1998;26:756–760. doi: 10.1093/nar/26.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izaurralde E, Lewis J, McGuigan C, Jankowska M, Darzynkiewicz E, Mattaj I W. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell. 1994;78:657–668. doi: 10.1016/0092-8674(94)90530-4. [DOI] [PubMed] [Google Scholar]
- 31.Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj I W. A cap-binding protein complex mediating U snRNA export. Nature. 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- 32.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataoka N, Ohno M, Kangawa K, Tokoro Y, Shimura Y. Cloning of a complementary DNA encoding an 80 kilodalton nuclear cap binding protein. Nucleic Acids Res. 1994;22:3861–3865. doi: 10.1093/nar/22.19.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kataoka N, Ohno M, Moda I, Shimura Y. Identification of the factors that interact with NCBP, an 80 kDa nuclear cap binding protein. Nucleic Acids Res. 1995;23:3638–3641. doi: 10.1093/nar/23.18.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiss-László Z, Henry Y, Bachellerie J-P, Caizergues-Ferrer M, Kiss T. Site-specific ribose methylation of preribosomal RNA: a novel function for small nucleolar RNAs. Cell. 1996;85:1077–1088. doi: 10.1016/s0092-8674(00)81308-2. [DOI] [PubMed] [Google Scholar]
- 36.Koleske A J, Young R A. The RNA polymerase II holoenzyme and its implications for gene regulation. Trends Biochem Sci. 1995;20:113–116. doi: 10.1016/s0968-0004(00)88977-x. [DOI] [PubMed] [Google Scholar]
- 37.Konarska M M, Padgett R A, Sharp P A. Recognition of cap structure in splicing in vitro of mRNA precursors. Cell. 1984;38:731–736. doi: 10.1016/0092-8674(84)90268-x. [DOI] [PubMed] [Google Scholar]
- 38.Krainer A R, Maniatis T, Ruskin B, Green M R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984;36:993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- 39.Kranz J E, Holm C. Cloning by function: an alternative approach for identifying yeast homologues of genes from other organisms. Proc Natl Acad Sci USA. 1990;87:6629–6633. doi: 10.1073/pnas.87.17.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafontaine D L J, Bousquet-Antonelli C, Henry Y, Caizergues-Ferrer M, Tollervey D. The box H+ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafontaine D L J, Tollervey D. Birth of the snoRNPs: the evolution of the modification guide snoRNAs. Trends Biochem Sci. 1998;23:383–388. doi: 10.1016/s0968-0004(98)01260-2. [DOI] [PubMed] [Google Scholar]
- 41a.Lafontaine, D. L. J., and D. Tollervey. Unpublished data.
- 42.Lange T S, Borovjagin A V, Gerbi S A. Nucleolar localization elements in U8 snoRNA differ from sequences required for rRNA processing. RNA. 1998;4:789–800. doi: 10.1017/s1355838298980438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis J D, Görlich D, Mattaj I W. A yeast cap binding protein complex (yCBC) acts at an early step in pre-mRNA splicing. Nucleic Acids Res. 1996;24:3332–3336. doi: 10.1093/nar/24.17.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis J D, Izaurralde E, Jarmolowski A, McGuigan C, Mattaj I W. A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5′ splice site. Genes Dev. 1996;10:1683–1698. doi: 10.1101/gad.10.13.1683. [DOI] [PubMed] [Google Scholar]
- 45.Li H D, Zagorski J, Fournier M J. Depletion of U14 small nuclear RNA (snR128) disrupts production of 18S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:1145–1152. doi: 10.1128/mcb.10.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao X C, Tang J, Rosbash M. An enhancer screen identifies a gene that encodes the yeast U1 snRNP A protein: implications for snRNP protein function in pre-mRNA splicing. Genes Dev. 1993;7:419–428. doi: 10.1101/gad.7.3.419. [DOI] [PubMed] [Google Scholar]
- 47.Lührmann R, Kastner B, Bach M. Structure of spliceosomal snRNPs and their role in pre-mRNA splicing. Biochim Biophys Acta. 1990;1087:265–292. doi: 10.1016/0167-4781(90)90001-i. [DOI] [PubMed] [Google Scholar]
- 48.McCracken S, Fong N, Rosonina E, Yankulov K, Brothers G, Siderovski D, Hessel A, Foster S, Shuman S, Bentley D L. 5′-capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 1997;11:3306–3318. doi: 10.1101/gad.11.24.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson S D, Wickens M, Bentley D L. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- 50.Mewes H W, Albermann K, Heumann K, Liebl S, Pfeiffer F. MIPS: a database for protein sequences, homology data and yeast genome information. Nucleic Acids Res. 1997;25:28–30. doi: 10.1093/nar/25.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mewes H W, Albermann K, Bähr M, Frishman D, Gleissner A, Hani J, Heumann K, Kleine K, Maierl A, Oliver S G, Pfeiffer F, Zollner A. Overview of the yeast genome. Nature. 1997;387:7–65. doi: 10.1038/42755. [DOI] [PubMed] [Google Scholar]
- 52.Mewes H W, Hani J, Pfeiffer F, Frishman D. MIPS: a database for protein sequences and complete genomes. Nucleic Acids Res. 1998;26:33–37. doi: 10.1093/nar/26.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrissey J P, Tollervey D. Yeast snR30 is a small nucleolar RNA required for 18S rRNA synthesis. Mol Cell Biol. 1993;13:2469–2477. doi: 10.1128/mcb.13.4.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa T, Ogawa H. Involvement of the MRE2 gene of yeast in formation of meiosis-specific double-strand breaks and crossover recombination through RNA splicing. Genes Cells. 1997;2:65–79. doi: 10.1046/j.1365-2443.1997.d01-283.x. [DOI] [PubMed] [Google Scholar]
- 55.Ni J, Tien A L, Fournier M J. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell. 1997;89:565–573. doi: 10.1016/s0092-8674(00)80238-x. [DOI] [PubMed] [Google Scholar]
- 56.Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohno M, Sakamoto H, Shimura Y. Preferential excision of the 5′ proximal intron from mRNA precursors with two introns as mediated by the cap structure. Proc Natl Acad Sci USA. 1987;84:5187–5191. doi: 10.1073/pnas.84.15.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosbash M, Harris P K, Woolford J L, Jr, Teem J L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981;24:679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- 59.Roth M B, Zahler A M, Stolk J A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991;115:587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy J, Zheng B, Rymond B C, Woolford J L., Jr Structurally related but functionally distinct yeast SmD core small nuclear ribonucleoprotein particle proteins. Mol Cell Biol. 1995;15:445–455. doi: 10.1128/mcb.15.1.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruskin B, Zamore P D, Green M R. A factor, U2AF is required for U2 snRNP binding and splicing complex assembly. Cell. 1988;52:207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- 62.Sander P, Grunewald S, Bach M, Haase W, Reilander H, Michel H. Heterologous expression of the human D2S dopamine receptor in protease-deficient Saccharomyces cerevisiae strains. Eur J Biochem. 1994;226:697–705. doi: 10.1111/j.1432-1033.1994.tb20098.x. [DOI] [PubMed] [Google Scholar]
- 63.Schwer B, Mao X, Shuman S. Accelerated mRNA decay in conditional mutants of yeast mRNA capping enzyme. Nucleic Acids Res. 1998;26:2050–2057. doi: 10.1093/nar/26.9.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Séraphin B, Rosbash M. Identification of functional U1 snRNA-pre-mRNA complexes committed to spliceosome assembly and splicing. Cell. 1989;59:349–358. doi: 10.1016/0092-8674(89)90296-1. [DOI] [PubMed] [Google Scholar]
- 65.Séraphin B, Rosbash M. The yeast branchpoint sequence is not required for the formation of a stable U1 snRNA–pre-mRNA complex and is recognized in the absence of U2 snRNA. EMBO J. 1991;10:1209–1216. doi: 10.1002/j.1460-2075.1991.tb08062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shatkin A J. Capping of eucaryotic mRNAs. Cell. 1976;9:645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- 67.Shatkin A J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985;40:223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- 68.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 69.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tollervey D. A yeast small nuclear RNA is required for normal processing of pre-ribosomal RNA. EMBO J. 1987;20:4169–4175. doi: 10.1002/j.1460-2075.1987.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tollervey D, Mattaj I W. Fungal small nuclear ribonucleoproteins share properties with plant and vertebrate U snRNPs. EMBO J. 1987;6:469–476. doi: 10.1002/j.1460-2075.1987.tb04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tollervey D, Kiss T. Function and synthesis of small nucleolar RNAs. Curr Opin Cell Biol. 1997;9:337–342. doi: 10.1016/s0955-0674(97)80005-1. [DOI] [PubMed] [Google Scholar]
- 73.Tornow J, Santangelo G. The GCR1 gene of Saccharomyces cerevisiae is a split gene with an unusually long intron. Genetics. 1994;138:973–974. doi: 10.1093/genetics/138.3.973. . (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tycowski K T, You Z-H, Graham P J, Steitz J A. Modification of U6 Spliceosomal RNA is guided by other small RNAs. Mol Cell. 1998;2:629–638. doi: 10.1016/s1097-2765(00)80161-6. [DOI] [PubMed] [Google Scholar]
- 75.Uemura H, Jigami Y. GCR3 encodes an acidic protein that is required for expression of glycolytic genes in Saccharomyces cerevisiae. J Bacteriol. 1992;174:5526–5532. doi: 10.1128/jb.174.17.5526-5532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uesono Y, Toh-e A, Kikuchi Y. Ssd1p of Saccharomyces cerevisiae associates with RNA. J Biol Chem. 1997;272:16103–16109. doi: 10.1074/jbc.272.26.16103. [DOI] [PubMed] [Google Scholar]
- 76a.Venema, J. Personal communication.
- 77.Venema J, Bousquet-Antonelli C, Gelugne J-P, Caizergues-Ferrer M, Tollervey D. Rok1p is a putative RNA helicase required for pre-rRNA processing. Mol Cell Biol. 1997;17:337–342. doi: 10.1128/mcb.17.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visa N, Izaurralde E, Ferreira J, Daneholt B, Mattaj I W. A nuclear cap-binding complex binds Balbiani Ring pre-mRNA cotranscriptionally and accompanies the ribonucleoprotein particle during nuclear export. J Cell Biol. 1996;133:5–14. doi: 10.1083/jcb.133.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wise J A, Tollervey D, Maloney D, Swerdlow H, Dunn E J, Guthrie C. Yeast contains small nuclear RNAs encoded by single copy genes. Cell. 1983;35:743–751. doi: 10.1016/0092-8674(83)90107-1. [DOI] [PubMed] [Google Scholar]
- 80.Wu P, Brockenbrough J S, Metcalfe A C, Chenand S, Aris J P. Nop5p is a small nucleolar ribonucleoprotein component required for pre-18S rRNA processing in yeast. J Biol Chem. 1998;273:16453–16463. doi: 10.1074/jbc.273.26.16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu D M, Field D J, Tang S J, Moris A, Bobechko B P, Friesen J D. Synthetic lethality of yeast slt mutations with U2 small nuclear RNA mutations suggests functional interactions between U2 and U5 snRNPs that are important for both steps of pre-mRNA splicing. Mol Cell Biol. 1998;18:2055–2066. doi: 10.1128/mcb.18.4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zahler A M, Lane W S, Stolk J A, Roth M B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992;6:837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- 83.Zamore P D, Patton J G, Green M R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992;355:609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]