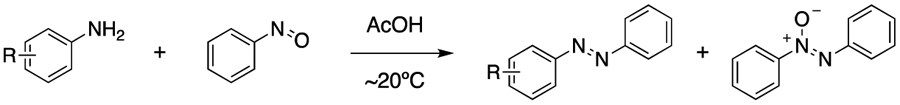
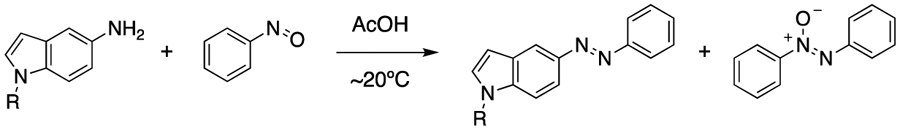
Abstract
Azobenzenes are widely used as dyes and photochromic compounds, with the Baeyer-Mills reaction serving as the most common method for their preparation. This transformation is often plagued by low yields due to the formation of undesired azoxybenzene. Here, we explore electronic effects dictating the formation of the azoxybenzene side-product. Using calculated oxidation potentials, we were able to predict reaction outcomes and improve reaction efficiency simply by modulating the oxidation potential of the aryl amine component.
Keywords: azobenzene, azoxybenzene, Baeyer-Mills reaction, Mills reaction, photoswitch, photochromic ligand
Graphical Abstract
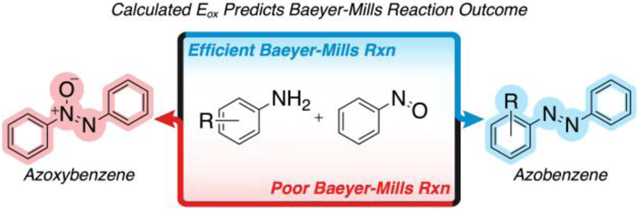
The Baeyer-Mills reaction is widely utilized to synthesize azobenzenes, but often produces azoxybenzene as an undesired byproduct. Here, we use a computational approach to predict the outcomes of Baeyer-Mills reactions.
INTRODUCTION
The Baeyer-Mills reaction—a condensation between an aryl amine and a nitrosoarene—is the most common reaction employed for constructing the diazene functional group of azobenzenes.1–3 Azobenzenes have classically been utilized as organic dyes,4 and many of these compounds exhibit photochromism, making them extremely useful in materials science5 and biology.6 Despite being discovered in the late 19th century, the Baeyer-Mills reaction remains highly substrate dependent, often requiring extensive trial and error to optimize. Three primary outcomes are typical of a Baeyer-Mills reaction—formation of the desired azobenzene product, production of the undesired azoxybenzene side-product, or lack of starting material reactivity. As the utility of azobenzenes has grown, so has their synthetic complexity, making predictive insight into potential reaction success increasingly valuable.
Typically, the nitrosoarene component of a Baeyer-Mills reaction comes from either oxidation of an aniline or reduction of a nitrobenzene. Given the instabilities of many nitrosoarenes, these compounds are often used in the subsequent condensation reaction without purification. Thus, incomplete aniline oxidation or partial nitro reduction can produce hydroxylamines capable of condensing with the nitrosoarene to yield undesired azoxybenzene. However, the use of purified nitrosoarenes in Baeyer-Mills reactions also often results in azoxybenzene formation (Figure 1A), indicating that hydroxylamine impurities resulting from nitrosoarene preparation cannot entirely explain the production of azoxybenzene.
Figure 1.
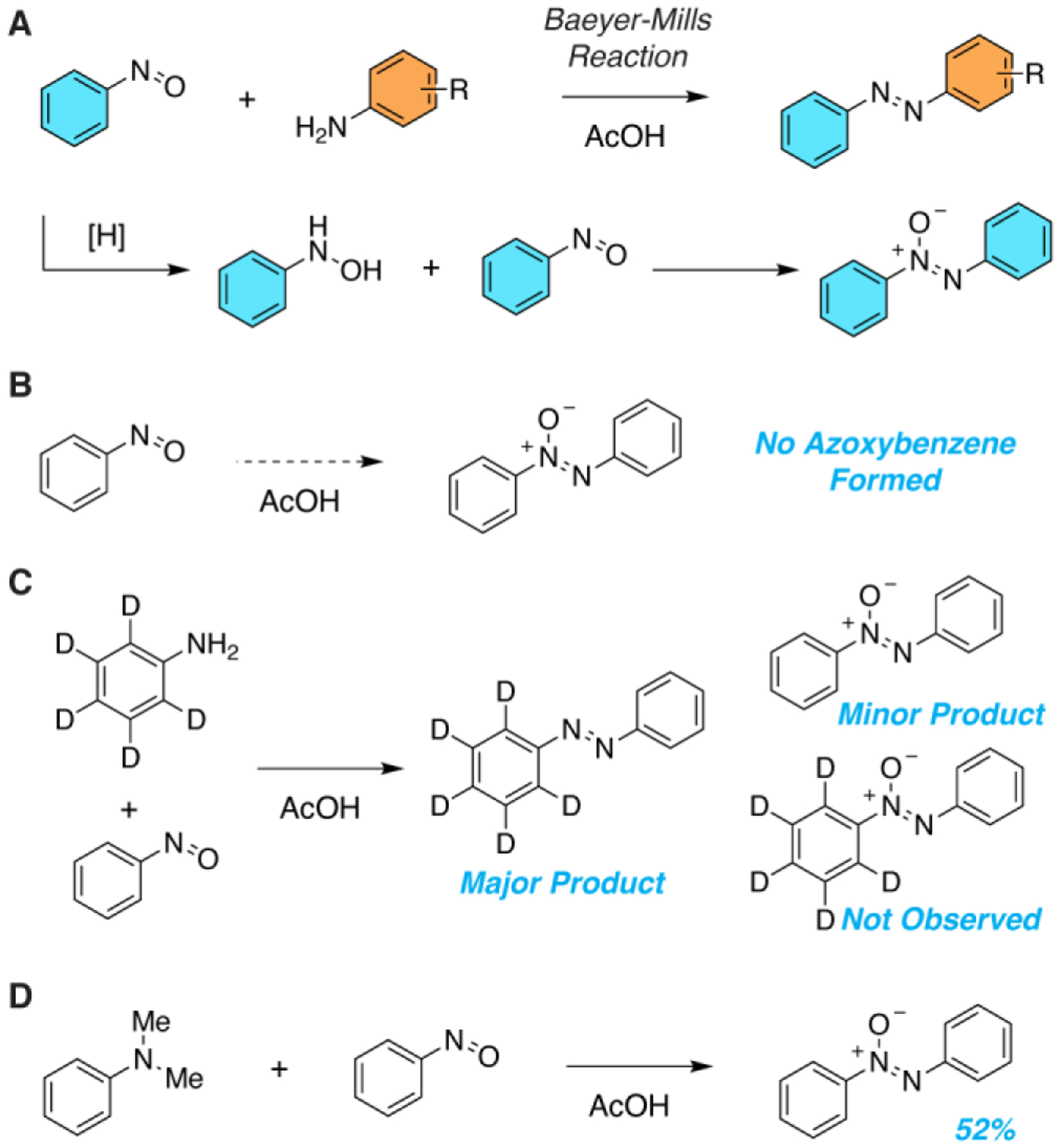
Mechanistic Studies of the Baeyer-Mills Reaction (A) Baeyer-Mills reactions to generate azobenzenes are complicated by the production of azoxybenzene via a proposed nitrosobenzene reduction pathway. (B) Purified nitrosobenzene does not form azoxybenzene through disproportionation. (C) The aniline component of a Baeyer-Mills reaction is not incorporated into the azoxybenzene side product. (D) An electron-rich aniline facilitates the formation of azoxybenzene from nitrosobenzene.
Disproportionation of nitrosoarenes has the potential to produce aryl hydroxylamines leading to azoxybenzenes. However, subjecting purified nitrosobenzene to standard Baeyer-Mills reaction conditions (i.e. AcOH, room temperature) did not produce any azoxybenzene (Figure 1B), suggesting that the interplay of the aniline and nitroso reaction components is essential. We hypothesized that the nitroso compound might be oxidizing the aniline to produce hydroxylamines originating from each component, which could ultimately condense with nitrosobenzene to produce azoxybenzene. However, a Baeyer-Mills reaction between aniline-d5 and nitrosobenzene did not yield any deuterated azoxybenzene (Figure 1C). When reacting nitrosobenzene with substituted anilines, any observed azoxybenzene byproduct is exclusively unsubstituted, indicating that both aryl groups of azoxybenzene come from nitrosobenzene. Combining nitrosobenzene with N,N-dimethylaniline—a compound incapable of participating in a Baeyer-Mills reaction—yielded a significant amount of azoxybenzene (Figure 1D). Electron-rich aryl amines like N,N-dimethylaniline are easily oxidized,7 and can readily reduce the nitrosoarene leading to a hydroxylamine product. The coupling of phenylhydroxylamine and nitrosobenzene to produce azoxybenzene has been studied previously and is quite favorable under the standard acidic conditions of a Baeyer-Mills reaction.8 Taken together, our results suggest that the reduction of the nitrosoarene by the arylamine is likely a competing pathway in Baeyer-Mills reactions leading to the formation of undesired azoxybenzene and reducing the yield of the desired azobenzene.
Given the importance of the aryl amine in the formation of azoxybenzene under standard Baeyer-Mills reaction conditions, it seems likely that the aryl amine reduces the nitrosoarene to an aryl hydroxylamine. This hydroxylamine then couples with another equivalent of nitrosoarene to form azoxybenzene. Thus, for every one equivalent of azoxybenzene formed, two equivalents of nitrosoarene starting material are consumed, drastically lowering the efficiency of the Baeyer-Mills reaction. Accordingly, we set out to understand how the electronic properties of substituted anilines impacted the formation of azobenzene and azoxybenzene.
Results and Discussion
We performed a series of Baeyer-Mills reactions between substituted anilines and nitrosobenzene at ambient temperature in acetic acid, which are typical reaction conditions for this transformation.3,9,10 Yields were measured via 1H NMR using an internal standard to avoid issues associated with E→Z isomerization of azobenzenes during chromatography (full synthetic details and isolated yields are provided in the supplementary information). These data are presented in Table 1.
Table 1.
Reaction of substituted anilines with nitrosobenzene. Reagents and conditions: appropriate aniline (1.0 eq.), nitrosobenzene (1.0 eq.), AcOH (0.5 M), ambient temperature, 24 h.
| Entry | R | Calculated Eoxa | σp+ | Azobenzene % Yieldb | Azoxybenzene % Yieldb |
|---|---|---|---|---|---|
| 1 | p-NMe2 | 0.18 | −1.70 | 34 | 45 |
| 2 | p-NH2 | 0.38 | −1.30 | 28 | 43 |
| 3 | o-NH2 | 0.73 | N/A | 8 | 91 |
| 4 | p-OMe | 0.77 | −0.78 | ≥95 | 5 |
| 5 | 2,6-dimethoxy | 0.77 | N/A | 46 | 35 |
| 6 | p-NHCO2tBu | 0.80 | N/A | 87 | 6 |
| 7 | p-OH | 0.83 | −0.92 | 34 | 69 |
| 8 | o-OMe | 0.93 | N/A | 82 | 13 |
| 9 | p-Me | 0.97 | −0.31 | 95 | ≤5 |
| 10 | o-NHCO2tBu | 1.04 | N/A | 85 | 12 |
| 11 | o-Et | 1.08 | N/A | 77 | 20 |
| 12 | p-I | 1.16 | 0.14 | ≥95 | ≤5 |
| 13 | H | 1.20 | 0.00 | ≥95 | ≤5 |
| 14 | o-Br | 1.46 | N/A | 49 | 8 |
| 15 | p-CO2Me | 1.49 | 0.49 | 82 | 6 |
| 16 | 2,6-difluoro | 1.55 | N/A | 12 | ≤5 |
| 17 | p-CN | 1.63 | 0.66 | 64 | 7 |
| 18 | p-CF3 | 1.67 | 0.61 | ≥95 | ≤5 |
| 19 | o-NO2 | 1.88 | N/A | ≤5 | ≤5 |
| 20 | p-NO2 | 1.90 | 0.79 | 19 | ≤5 |
Volts vs. SHE.
Yield determined by 1H NMR using dibromomethane as an internal standard.
N/A = not available.
The donor/acceptor strength of the aniline substituents, characterized by their associated Hammett sigma constants (σp+),11 was able to effectively predict the efficiency of the Baeyer-Mills reactions (Figure 2B). Electron-rich anilines (σp+ < −0.8) perform poorly in Baeyer-Mills reactions because they have a high propensity to reduce the nitrosoarene, and this is reflected in higher yields of the azoxybenzene side products (Figure 2C). Anilines with moderately withdrawing and donating substituents (σp+ = −0.8–0.6) produce excellent yields of the desired azobenzenes with minimal formation of azoxybenzene. Azobenzene yields drop sharply when aryl amines containing highly electron-withdrawing substituents (σp+ > 0.6) are utilized, presumably due to reduced nucleophilicity of the anilines. Azoxybenzene yields are also low when electron-deficient anilines are employed, due to their inability to reduce nitrosobenzene.
Figure 2.
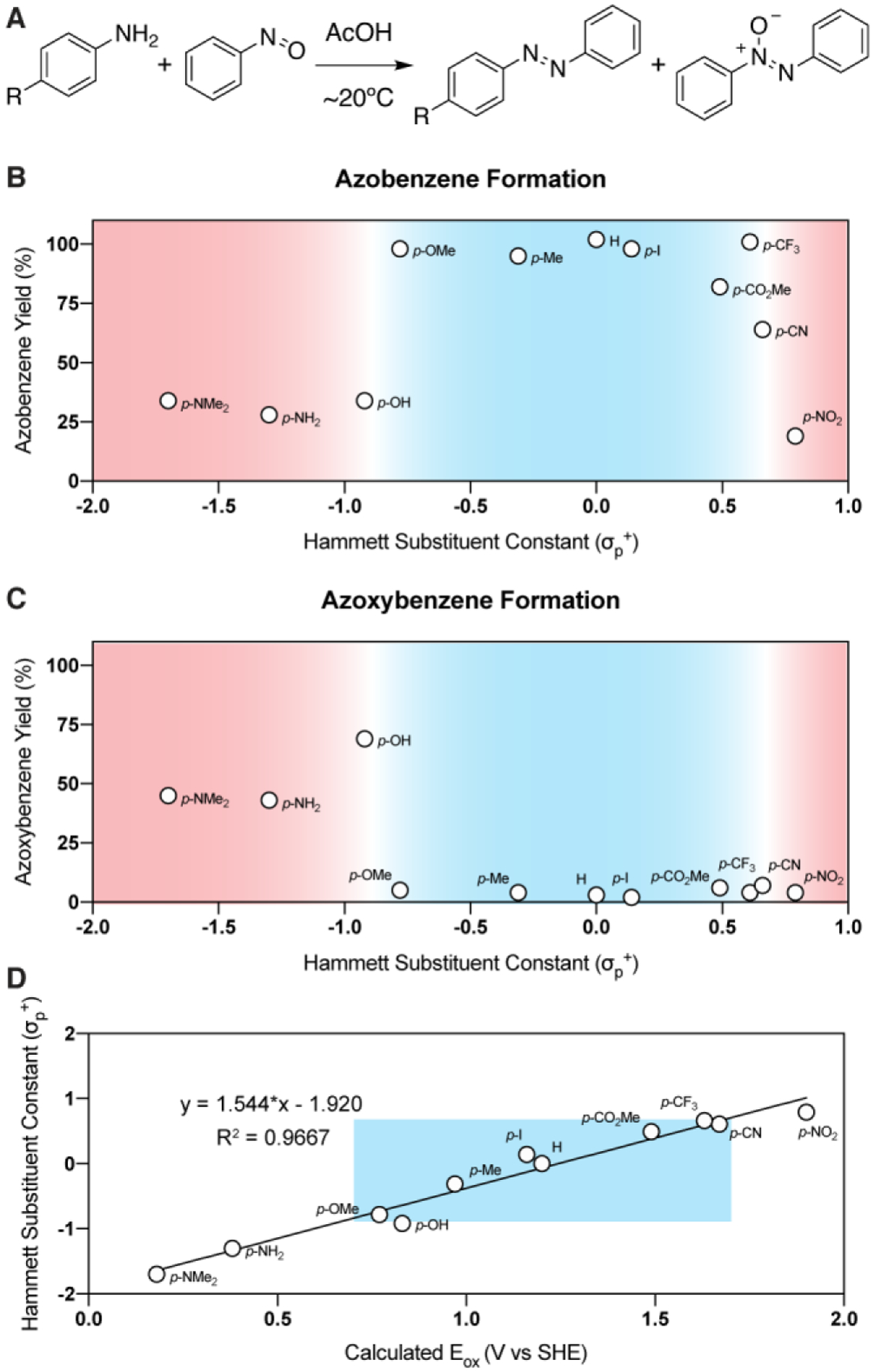
(A) General reaction scheme. (B) Azobenzene yield is high when arylamine sigma constants exist within an optimal range (blue, −0.8 < σp+ <0.6). (C) Azoxybenzene yield is high when σp+ < −0.8. (D) Calculated Eox values correlate well with known sigma constants (σp+). Suboptimal arylamine sigma values are shown in red, while sigma values associated with high Baeyer-Mills reaction efficiencies are highlighted in blue. Yields determined by 1H NMR using dibromomethane as an internal standard.
As a surrogate for oxidation potentials, σp+ values can be extremely useful for predicting the efficiency of Baeyer-Mills reactions. However, these values are not available for many substituted anilines or more complex arylamines. Thus, we were interested in using computationally determined oxidation potentials to predict the efficiency of Baeyer-Mills reactions. Voltages necessary to achieve single electron oxidation (Eox) were calculated for a variety of arylamines using Gaussian 16 A.03 at the B3LYP/6–31+g(d,p) level of theory.12 This combination of a classic functional with a Pople basis set has been used by others,13 though we opted to utilize a reduced basis set to improve computational speed for trend analysis. The implicit Solvation Model based on Density (SMD) developed by Marinech et al. was used to account for acetic acid during modeling through the typical Born-Haber cycle approach.14 Not only is this method for calculating oxidation potentials low cost and highly accessible, but it also produces values that correlate strongly with known σp+ values (Figure 2D). A similar correlation between σp+ and oxidation potentials has been reported elsewhere.15
Using calculated Eox values, we replotted our initial data set and included nine additional arylamines that lack known σp+ values, including various ortho-substituted anilines and p-Boc phenylenediamine. As with σp+ values, there seems to be an optimal range of calculated Eox values for achieving efficient Baeyer-Mills reactions (Figure 3B). Electron-rich anilines with low calculated Eox values (i.e., Eox < 0.7 V) produce large quantities of azoxybenzene (Figure 3C), anilines with moderate Eox values yield high amounts of desired azobenzene, and electron-deficient anilines with exceptionally high calculated Eox values exhibit low reactivity. Notably, Boc protection of o- and p-phenylenediamine shifted the calculated oxidation potentials of the parent compounds into the desired range, resulting in diminished formation of azoxybenzene side products and drastically improved yields of the desired azobenzenes.
Figure 3.
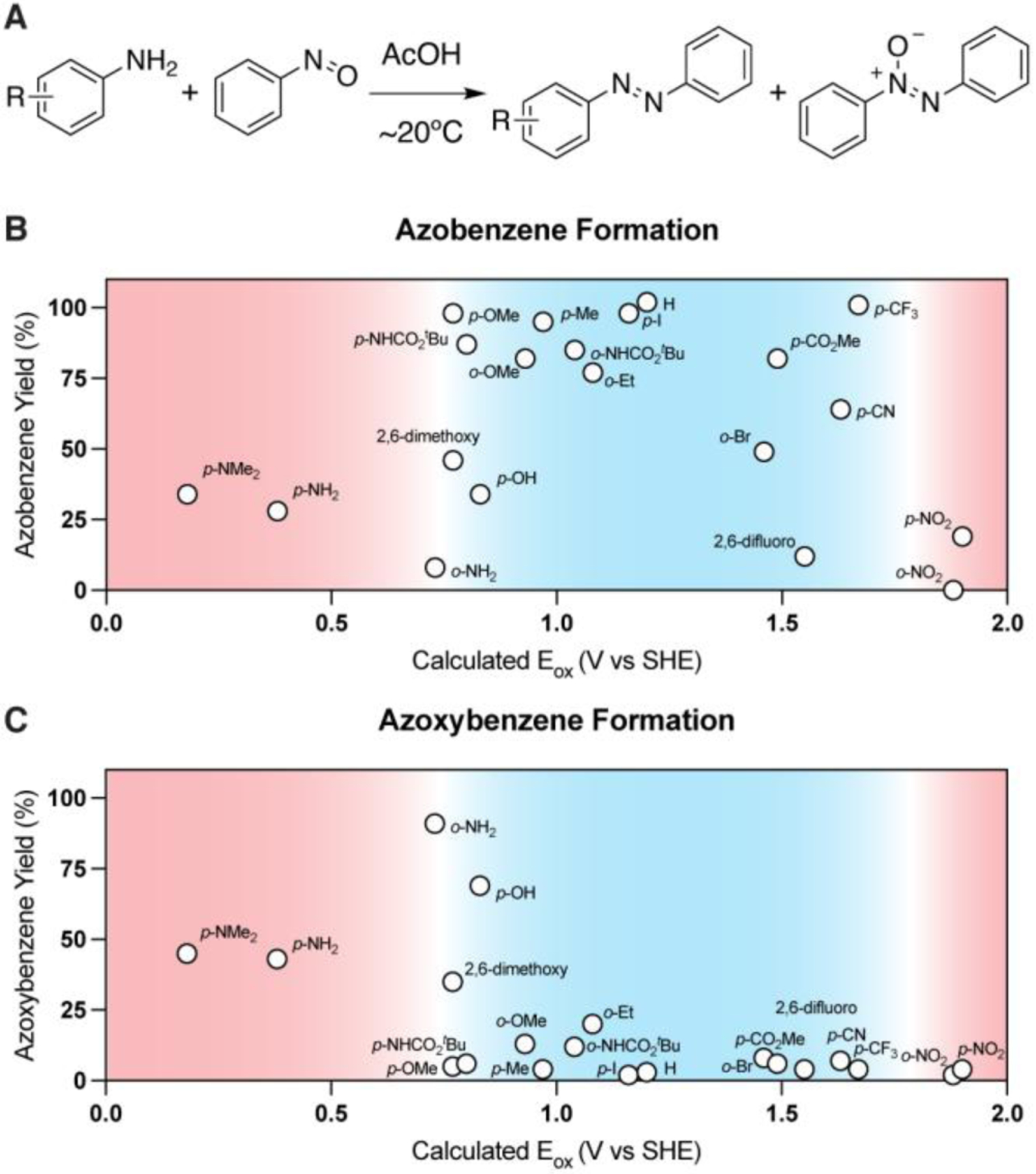
(A) General reaction scheme. (B) Azobenzene yield is high when arylamine oxidation potentials exist within an optimal range (blue, 0.7 V < Eox < 1.7 V). (C) Azoxybenzene yield is high when Eox < 0.7 V. Suboptimal calculated Eox values are shown in red, while calculated Eox values associated with high Baeyer-Mills reaction efficiencies are highlighted in blue. Yields determined by 1H NMR using dibromomethane as an internal standard.
We conducted a screen of various typical Baeyer-Mills reactions with p-nitroaniline, aniline, and p-phenylenediamine (Table S1) and found that while some conditions using either DCM or EtOH with stoichiometric amounts of AcOH increased reaction efficiency, the trends of our calculations remain intact across all conditions, as aniline outperforms p-phenylenediamine, which outperforms p-nitroaniline.
Overall, this method for predicting Baeyer-Mills reaction efficiency works quite well for arylamines with most calculated Eox values. However, the method is less reliable for arylamines with calculated Eox values ~0.7 V. For these substrates, it is important to consider how likely it is that an aryl amine will be oxidized, and in turn reduce nitrosobenzene during the reaction. For example, p-methoxyaniline, p-N-Boc-phenylenediamine, p-hydroxyaniline, and o- phenylenediamine all possess calculated Eox values of approximately 0.7 V, yet only the latter two compounds produce low yields of the desired azobenzenes. Both p-hydroxyaniline and o-phenylenediamine lack substitution protecting them from oxidation to the corresponding quinone and quinonediimine species, thus, it is reasonable to suspect that they would produce high levels of undesired azoxybenzene side product (Figure 3C).
With an understanding that very electron-rich aryl amines tend to perform poorly in Baeyer-Mills reactions due to competing reduction of nitrosobenzene, we decided to use calculated oxidation potentials to improve the synthesis of a target azoheteroarene. Azoheteroarenes have shown immense promise as azo scaffolds with many advantages over traditional azobenzenes including optimized photophysical properties.16–19 However, the electronic properties of the arylamines required to synthesize these scaffolds can pose significant challenges for Baeyer-Mills reactions, as is the case with the highly electron-rich 5-aminoindole. Reaction of 5-aminoindole with nitrosobenzene produces little to no desired azoheteroarene, and instead yields azoxybenzene in >40% yield (Table 2, Figure 4). To solve this issue, we attempted to modulate the oxidation potential of the indole through N-substitution. Our computational modeling revealed that Boc, Ac, and Ts protected indoles have Eox values suitable for efficient Baeyer-Mills reactions. As expected, all of these starting materials produced the desired azoheteroarene products in good yields, and the formation of azoxybenzene was most efficiently suppressed as the calculated Eox values increased (Table 2, Figure 4).
Table 2.
Reaction of N-substituted 5-aminoindoles with nitrosobenzene. Reagents and conditions: appropriate aniline (1.0 eq.), nitrosobenzene (1.0 eq.), AcOH (0.5 M), ambient temperature, 24 h.
| Entry | R | Calculated Eoxa | σp+ | Azobenzene % Yieldb | Azoxybenzene % Yieldb |
|---|---|---|---|---|---|
| 1 | H | 0.70 | N/A | 6 | 42 |
| 2 | Boc | 0.89 | N/A | 58 | 18 |
| 3 | Ac | 0.99 | N/A | 72 | 17 |
| 4 | Ts | 1.01 | N/A | 63 | 10 |
Volts vs. SHE.
Yield determined by 1H NMR using dibromomethane as an internal standard.
N/A = not available
Figure 4.
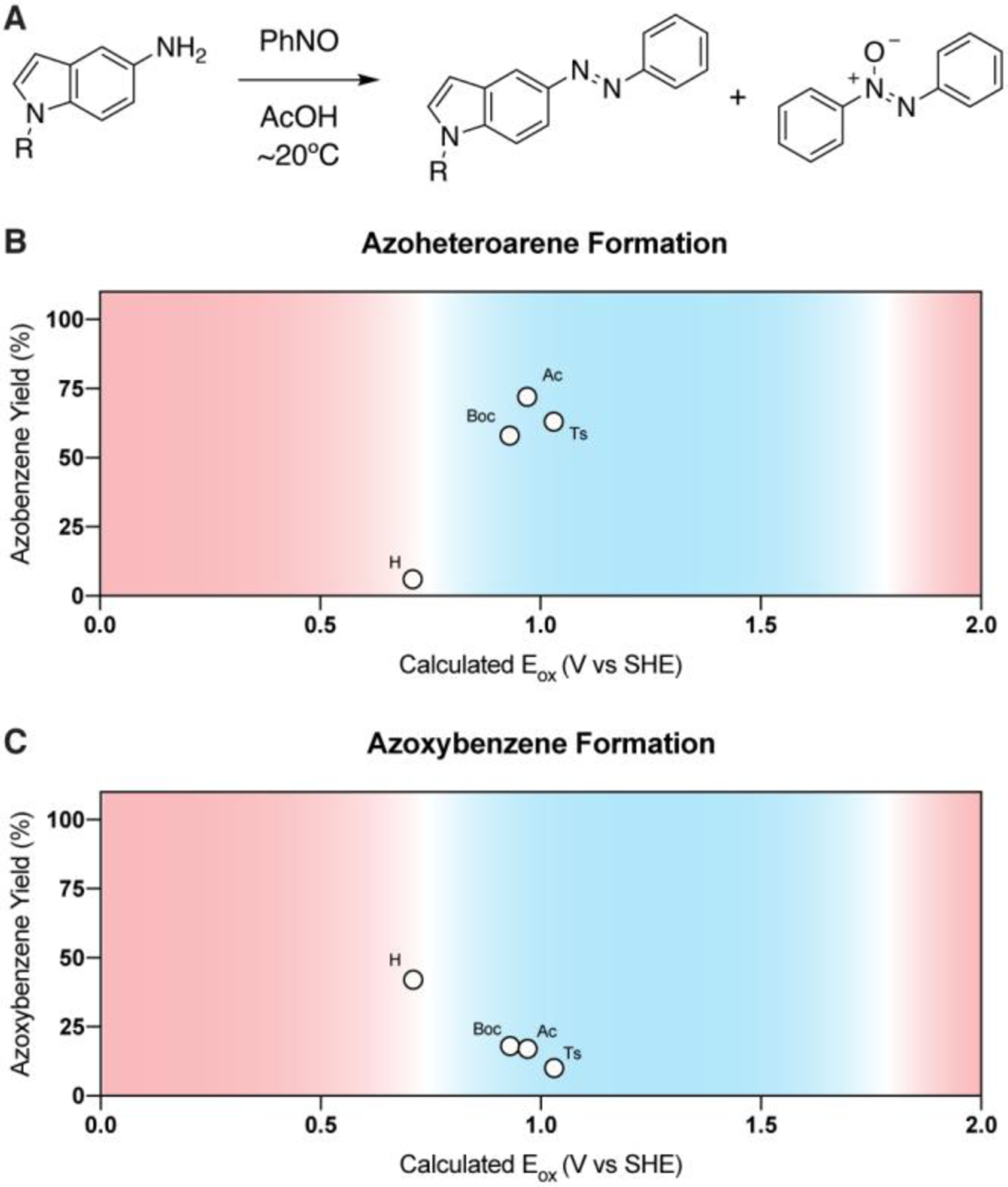
(A) General reaction scheme. (B) Azoheteroarene yield is high when the oxidation potentials for N1-substituted 5-aminoindoles exist within an optimal range (blue, 0.7 V < Eox < 1.7 V). Suboptimal oxidation potentials (red) result in lower reaction efficiency. (C) Azoxybenzene yield is high when Eox < 0.7 V. Suboptimal calculated Eox values are shown in red, while calculated Eox values associated with high Baeyer-Mills reaction efficiencies are highlighted in blue. Yields determined by 1H NMR using dibromomethane as an internal standard.
In conclusion, we have used a combination of mechanistic experiments and computational chemistry to investigate the electronic effects governing the outcome of condensation reactions between aryl amines and nitrosobenzene. Importantly, we have demonstrated that calculated oxidation potentials can be utilized to predict the formation of both desired azobenzene and undesired azoxybenzene products in the Baeyer-Mills reaction. Electron-rich aryl amines with low calculated Eox values drive the formation of undesired azoxybenzene, presumably through reduction of nitrosobenzene. In addition, highly electron-deficient aryl amines lack the nucleophilicity required to effectively condense with nitrosobenzene. Thus, we identified an intermediate oxidation potential range that is ideal for generating desired azobenzene products in high yields. We also demonstrated that protecting group strategies can be used to modulate the oxidation potential of the arylamine to improve Baeyer-Mills reaction efficiency. Taken together, these results represent a significant step toward predicting Baeyer-Mills reaction efficiencies and will prove valuable in the construction of new azobenzene dyes and photochromic compounds.
Supplementary Material
Acknowledgements
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to D.E.O.; T32GM113770 to R.J.T.), a Provost’s Undergraduate Fellowship (N.Y.), an R. Bryan Miller Graduate Fellowship (R.J.T.), a Francesca Miller Undergraduate Research Award (N.Y.), and the AggieLight Program (D.E.O.). Funding for NMR spectrometers was provided by the National Science Foundation (Grants CHE9808183 and CHE0443516). Analysis for this project was performed in the UC Davis Campus Mass Spectrometry Facilities, with instrument funding provided by the NIH (Grant 1S10OD025271). The authors thank C.R. Carr and L.A. Berben (UC Davis) for helpful discussions.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Baeyer A, Chem. Ber, 1874, 7, 1638–1640. [Google Scholar]
- 2.Mills C, J. Chem. Soc, 1895, 67, 925–933. [Google Scholar]
- 3.Merino E, Chem. Soc. Rev, 2011, 40, 3835–3853. [DOI] [PubMed] [Google Scholar]
- 4.Benkhaya S, M’rabet S, and El Harfi A, Heliyon, 2020, 6, e03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For references related to photoswitchable materials, see:; (a) Russew M-M and Hecht S, Adv. Mater, 2010, 22, 3348–3360. [DOI] [PubMed] [Google Scholar]; (b) Pianowski ZL, Chem. Eur. J, 2019, 25, 5128–5144. [DOI] [PubMed] [Google Scholar]; (c) ckmann M, Doltsinis NL, and Marx D, Phys. Rev. E, 2008, 78, 036101. [DOI] [PubMed] [Google Scholar]
- 6.For references related to biologically relevant photoswitches, see:; (a) Hüll K, Morstein J, and Trauner D, Chem. Rev, 2018, 118, 10710–10747. [DOI] [PubMed] [Google Scholar]; (b) Beharry AA and Woolley GA, Chem. Soc. Rev, 2011, 40, 4422–4437. [DOI] [PubMed] [Google Scholar]; (c) Velema WA, Szymanski W, and Feringa BL, J. Am. Chem. Soc, 2014, 136, 2178–2191. [DOI] [PubMed] [Google Scholar]; (d) Fehrentz T, Schönberger M, and Trauner D, Angew. Chem., Int. Ed, 2011, 50, 12156–12182. [DOI] [PubMed] [Google Scholar]; (e) Kramer RH, Mourot A, and Adesnik H, Nat. Neurosci, 2013, 16, 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Szymanński W, Beierle JM, Kistemaker HA, Velema WA, and Feringa BL, Chem. Rev, 2013, 113, 6114–6178. [DOI] [PubMed] [Google Scholar]; (g) Broichhagen J, Frank JA, and Trauner D, Acc. Chem. Res, 2015, 48, 1947–1960. [DOI] [PubMed] [Google Scholar]
- 7.Pavitt AS, Bylaska EJ, and Tratnyek PG, Environ. Sci. Process. Impacts, 2017, 19, 339–349. [DOI] [PubMed] [Google Scholar]
- 8.Pizzolatti MG and Yunes RA, J. Chem. Soc. Perkin Trans 2, 1990, 759–764. [Google Scholar]
- 9.Davey MH, Lee VY, Miller RD, and Marks TJ, J. Org. Chem, 1999, 64, 4976–4979. [DOI] [PubMed] [Google Scholar]
- 10.Priewisch B and Rück-Braun K, J. Org. Chem, 2005, 70, 2350–2352. [DOI] [PubMed] [Google Scholar]
- 11.Hansch C, Leo A, and Taft RW, Chem. Rev, 1991, 91, 165–195. [Google Scholar]
- 12.Gaussian 16, Revision C.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, and Fox DJ, Gaussian, Inc., Wallingford CT, 2016. [Google Scholar]
- 13.For references related to B3LYP, see:; (a) Becke AD, J. Chem. Phys, 1993, 98, 1372–1377. [Google Scholar]; (b) Becke AD, J. Chem. Phys, 1993, 98, 5648–5652. [Google Scholar]; (c) Lee C, Yang W, and Parr RG, Phys. Rev. B, 1988, 37, 785–789. [DOI] [PubMed] [Google Scholar]; (d) Stephens PJ, Devlin FJ, Chabalowski CF, and Frisch MJ, J. Phys. Chem, 1994, 98, 11623–11627. [Google Scholar]; (e) Tirado-Rives J and Jorgensen WL, J. Chem. Theory Comput, 2008, 4, 297–306. [DOI] [PubMed] [Google Scholar]
- 14.Marenich AV, Cramer CJ, and Truhlar DG, J. Phys. Chem. B, 2009, 113, 6378–6396. [DOI] [PubMed] [Google Scholar]
- 15.Jovanovic SV, Tosic M, and Simic MG, J. Phys. Chem, 1991, 95, 10824–10827. [Google Scholar]
- 16.Stefano C, Nadja AS, and König B, Nat. Rev. Chem, 2019, 3, 133–146. [Google Scholar]
- 17.Weston CE, Richardson RD, Haycock PR, White AJP, and Fuchter MJ, J. Am. Chem. Soc, 2014, 136, 11878–11881. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Gao C, Andréasson J, and Grøtli M, Org. Lett, 2018, 20, 4875–4879. [DOI] [PubMed] [Google Scholar]
- 19.Calbo J, Weston CE, White AJP, Rzepa HS, Contreras-García J, and Fuchter MJ, J. Am. Chem. Soc, 2017, 139, 1261–1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.