Abstract
Insulin derivatives such as insulin detemir and insulin degludec are U.S. Food and Drug Administration (FDA)-approved long-acting insulin currently used by millions of people with diabetes. These derivatives are modified in C-terminal B29 lysine to retain insulin bioactivity. New and efficient methods for facile synthesis of insulin derivatives may lead to new discovery of therapeutic insulin. Herein, we report a new method using sortase A (SrtA)-mediated ligation for the synthesis of insulin derivatives with high efficiency and functional group tolerance in the C-terminal B chain. This new insulin molecule (Ins-SA) with an SrtA-recognizing motif can be conjugated to diverse groups with N-terminal oligoglycines to generate new insulin derivatives. We further demonstrated that a new insulin derivative synthesized by this SrtA-mediated ligation shows strong cellular and in vivo bioactivity. This enzymatic method can therefore be used for future insulin design and development.
KEY WORDS: Diabetes mellitus, Insulin synthesis, Sortase A (SrtA) ligation, Albumin-binding peptide SA21, Long-acting insulin
Abbreviations: Alb, albumin; Boc, tert-butyloxycarbonyl; DCM, dichloromethane; DIEA, N,N-diisopropylethylamine; DMEM, Dulbecco's Modified Eagle Medium; DMF, dimethylformamide; DMSO, dimethyl sulfoxide; DOI, desoctapeptide (B23−30) insulin; EDT, 1,2-ethanedithiol; FBS, fetal bovine serum; Fmoc, 9-fluorenylmethoxycarbonyl; HATU, 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate; HBTU, O-(benxontriazol-1-yl)-1,1,3,3-tetramethyluronium; HPLC, high performance liquid chromatography; HTRF, homogeneous time resolved fluorescence; i.p., intraperitoneal; IR-B, human insulin receptor isoform B; ITT, insulin tolerance test; LC‒MS, liquid chromatography mass spectrometry; Mtt, 4-methyltrityl; NBD-X, 6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoic acid; pAkt, phosphorylated protein kinase B; SrtA, sortase A; STZ, streptozotocin; t-Bu, tert-butyl; THF, triflouroacetic acid; TIS, triisoproylsilane
Graphical abstract
A new method of synthesis of insulin derivatives through sortase A (SrtA) ligation was reported. The insulin substrate (Ins-SA) can be site-selectively modified using sortase A in high efficiency.
1. Introduction
Diabetes mellitus is a chronic degenerative metabolic disease characterized by loss of pancreatic β-cell function and marked by high glucose levels1. Individuals with diabetes require insulin therapy and must monitor their blood glucose levels to maintain normoglycemia and prevent hypoglycemic shock which could lead to premature death2. For people with diabetes relying on exogenous insulin, fast-acting insulin and long-acting insulin are available clinically to provide better glycemic control3,4. Among the long-acting insulins, insulin detemir and degludec are derivatives with hydrophobic modifications on B29 lysine on the C-terminal B chain4,5. The hydrophobic groups promote insulin multimerizations and serum albumin association to increase the in vivo half-life of insulin to 20–48 h5,6. Furthermore, C-terminal B chain modified insulin derivatives have been used to demonstrate sugar dependent properties7, 8, 9, 10 and reduced insulin aggregation11. Therefore, modifications on insulin C-terminal B chain may provide new potential therapeutic candidates.
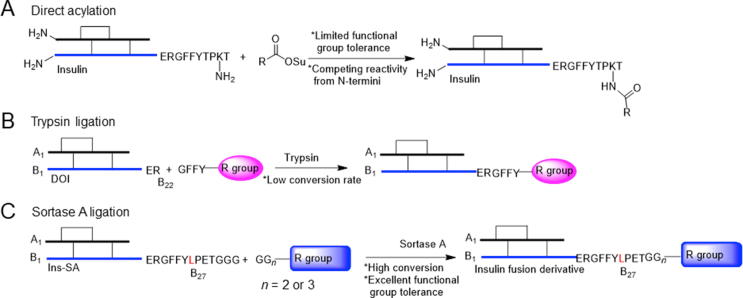
Correspondingly, there are two available methods for synthesis of insulin: direct acylation on B29 lysine or trypsin-mediated ligation using desoctapeptide (B23‒30) insulin (DOI). However, these methods suffer from limited chemical functionality tolerance or low conversion (Scheme 1)12,13. Total chemical synthesis of insulin derivatives is another approach10,11 but currently it is far from large-scale synthesis. Therefore, a new modification method that provides high functional group tolerance and conjugation efficiency will be extremely useful in creating new insulin derivatives. To develop this method, we focused our efforts on sortase A (SrtA); a member of a class of thiol-containing transpeptidases that anchor proteins to the bacterial cell wall14. SrtA reactions have been used extensively to modify various peptides, proteins, and small molecules due to its high conversion, selectivity, chemical functionality, and simplistic working conditions15, 16, 17.
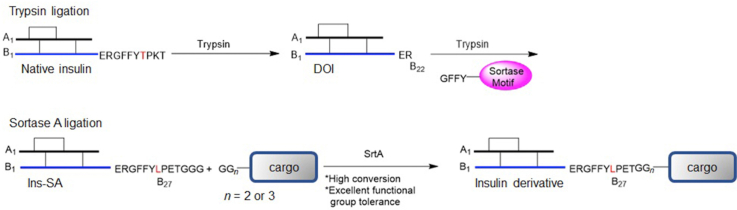
Scheme 1.
Synthesis of insulin derivatives via (A) direct acylation, (B) trypsin ligation, and (C) sortase A ligation.
SrtA recognizes an LPXTG sequence and cleaves the threonine–glycine bond to form a thioacyl-linked intermediate. This intermediate is primed to react with the N-terminal amino group of an oligoglycine motif via nucleophilic attack (Scheme 1). This transpeptidation reaction has been used extensively to attach virtually any water-soluble molecule to a protein with LPXTG sequence on the C-terminus18,19.
2. Results and discussion
2.1. Design of Ins-SA
Des B27‒30 insulin was reported to have full activity, which suggests that this segment of insulin is not involved in its bioactivity20,21. The insulin B-chain sequence ends with TPKT (B27‒30), which shares a similarity with the SrtA-recognizing sequence (LPXTG). Therefore, we hypothesized that a new insulin analogue with a B-chain sequence ending with the sorting motif, LPETGGG, with two simple substitutions (T27L and K29E) on the C-terminus will also be active toward the insulin signaling pathway. To test this hypothesis, we synthesized Ins-SA (human insulin with B27‒33: LPETGGG) and confirmed this new insulin analogue has preserved potency as native insulin in activating the insulin signaling pathway. Using Ins-SA and SrtA-mediated synthesis, we have synthesized a series of insulin fusion derivatives that maintain the bioactivity in high yields (70%–80%). These findings provide a new approach to synthesize new insulin derivatives for the treatment of diabetes and the potential to develop novel tools to study this incurable life-long disease.
2.2. Synthesis of Ins-SA
We used the trypsin cleavage/ligation method (Scheme 1B) to synthesize Ins-SA (Scheme 2). Noted, Ins-SA can also be recombinantly expressed using traditional insulin expression system in a large scale. In this case, DOI was first obtained with excellent purity and high yield (65%–75%) after the trypsin cleavage of native insulin as confirmed by LC‒MS and HPLC (Supporting Information Fig. S1). We then synthesized GFFYLPETGGG using a standard Fmoc/t-Bu solid-phase peptide synthesis protocol. The trypsin-mediated ligation of the peptide GFFYLPETGGG to DOI led to Ins-SA with 20%–30% yield after purification, which is consistent with previous reports using this method22.
Scheme 2.
Synthesis of Ins-SA via trypsin mediated ligation and cleavage. Cleavage of insulin to DOI was completed by dissolving trypsin in pH 7.5 buffer. This mixture was added to native insulin dissolved in 1:1 (v/v) DMSO:1,4-butanediol, 3 mL total. Ligation of octapeptide to DOI was completed by dissolving 1 mg/μmol trypsin in pH 7.6 buffer. This mixture was added to native insulin dissolved in 1:1 (v/v) DMSO:1,4-butanediol, 348 μL total.
2.3. Bioactivity of Ins-SA
To compare the potency of Ins-SA with native insulin, we measured pAkt Ser473 levels as an indication of signal activation in a mouse fibroblast cell line, NIH 3T3, overexpressing human insulin receptor isoform B (IR-B). pAkt levels in the cell lysates were measured after treatment23 with Ins-SA and native insulin was used as a positive control. As shown, Ins-SA has preserved bioactivity compared to native insulin (Fig. 1). This further suggests that Ins-SA is a good substrate for modifications.
Figure 1.
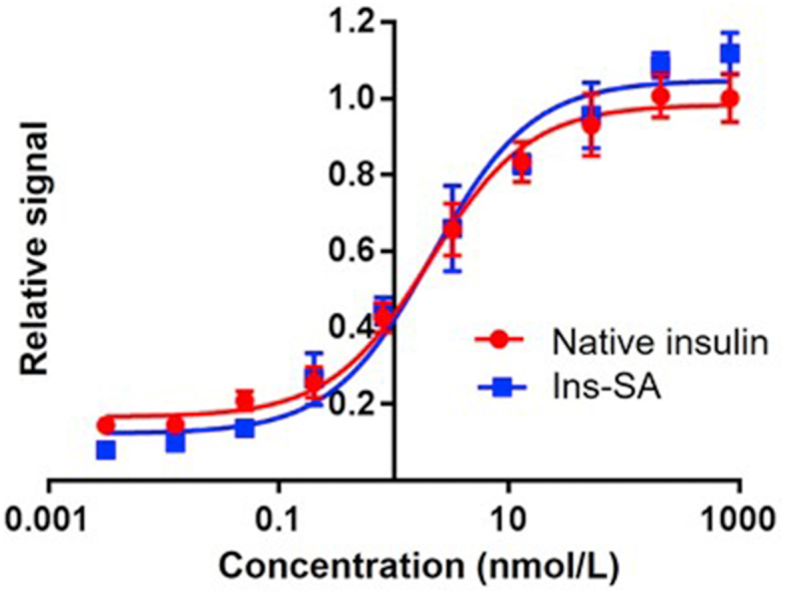
In vitro pAkt assay using NIH 3T3 cells overexpressing insulin receptors to compare the bioactivity of native insulin and Ins-SA (native insulin EC50 = 2.126 nmol/L, Ins-SA EC50 = 2.196 nmol/L). Data were represented as mean ± SD, n = 4.
2.4. Ligation test and bioactivity of Ins-SA-NBDX
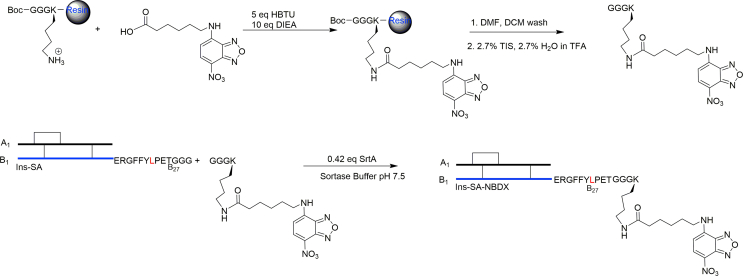
To test the utility of using SrtA-mediated ligations with Ins-SA, we introduced a fluorophore, NBD-X, to Ins-SA. In this case, NBD-X was coupled to GGGK via the ε-amino group of Lys to generate the oligoglycine motif needed for SrtA ligation. First, GGGK was synthesized on resin via standard Fmoc/t-Bu protocol and orthogonally protected using Boc and Mtt protecting groups on the N-terminus and lysine side chain, respectively. The Mtt group on Lys was removed after SPPS and NBD-X were coupled on lysine using standard coupling reagents. The peptide was then cleaved from resin using a standard cleavage cocktail. This peptide was then coupled to Ins-SA via SrtA (Scheme 3). Ins-SA-NBDX was then characterized and purified via HPLC and LC‒MS (Supporting Information Fig. S4). The yield for this reaction was 75%–80%. These results suggest that SrtA mediated ligation is a facile method to generate new insulin analogues with higher conjugation efficiency and improved functional group tolerance. Unlike direct acylation of lysine B29, this SrtA-mediated conjugation can achieve site-selective insulin modifications without competitions from the N-termini24,25.
Scheme 3.
Synthesis of Ins-SA-NBDX. NBD-X was coupled to the ε-amino group of the lysine on GGGK and the product was coupled to Ins-SA via SrtA-mediated ligation (10.5 μmol/L SrtA) using pH 7.5 sortase buffer.
Next, to compare the potency of Ins-SA-NBDX with native insulin, pAkt Ser473 levels were measured as previously described. As shown, Ins-SA-NBDX has a ∼5.5-fold reduced bioactivity vitro assays26. These results demonstrate insulin activity was retained after SrtA-mediated insulin modifications (Fig. 2).
Figure 2.
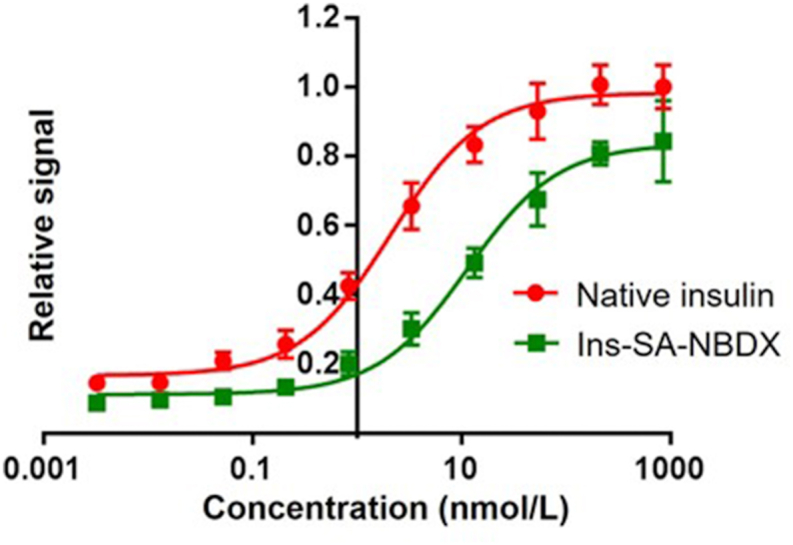
In vitro pAkt assay using NIH 3T3 cells overexpressing insulin receptors to compare the bioactivity of native insulin and Ins-SA-NBDX (native insulin EC50 = 2.126 nmol/L, In-SA-NBDX EC50 = 11.49 nmol/L). Data were represented as mean ± SD, n = 4.
2.5. Synthesis of Ins-SA-Alb by SrtA ligation
To determine the feasibility of using SrtA for the fusion of large peptides, we introduced an albumin binding peptide (SA21) to Ins-SA. SA21 was discovered through a phage display screen as a potent peptide binder of serum albumin. Due to its strong interaction with serum album, it has a long half-life of 2.3 h when injected by intravenous bolus into rabbits27. Therefore, addition of SA21 to native insulin may lead to a long-acting insulin derivative for diabetes treatment. While fatty acid such as myristic acid has been used to modify insulin through B29 lysine acylation, the synthesis of insulin–peptide conjugate through the C-terminal of B chain is challenging. First, direct acylation on B29 lysine would not work due to the competition from the side chain functional groups on SA21. Furthermore, trypsin ligation between DOI and long peptide would lead to low conversion. We hypothesize that the SrtA-mediated ligaiton may provide a superior route for insulin−SA21 conjugate synthesis. In this case, SA21 was first synthesized via standard Fmoc/t-Bu protocol and we added two Gly on the N-terminus to perform SrtA-mediated ligation. This peptide was then conjugated to Ins-SA, which led to the product Ins-SA-Alb with a 50%–65% yield (Scheme 4). Ins-SA-Alb was then characterized and purified via HPLC and LC‒MS (Supporting Information Fig. S5).
Scheme 4.
Synthesis of Ins-SA-Alb via SrtA-mediated ligation using SA21 (GGRLIEDICLPRWGCLWEDD). This one-pot reaction was completed using 60 μmol/L SrtA and pH 7.5 sortase buffer and DMSO: 1,4-butanediol 1:1 (v/v), 167 μL total. Organic solvents were used to help stabilize SA21 in aqueous solution.
2.6. Bioactivity and insulin tolerance test (ITT) of Ins-SA-Alb
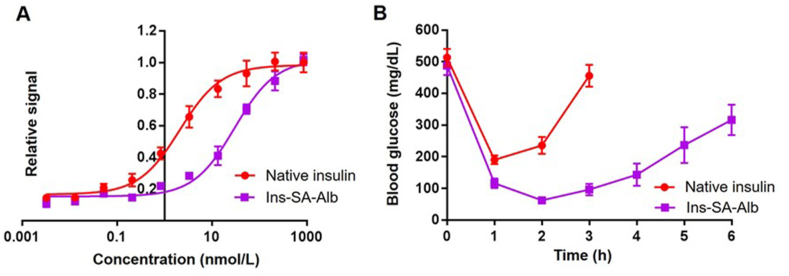
To compare the potency of Ins-SA-Alb with native insulin we measured pAkt Ser473 levels as previously described. As shown, Ins-SA-Alb is ∼15-fold weaker than native insulin in activating insulin signaling which we hypothesize may be due to the large substituent appended or the short glycine linker between Ins-SA and SA21 (Fig. 3A). The short spacer may reduce the flexibility of B-chain insulin, which may result in lower binding affinity to the insulin receptor. Notably, studies indicate that commercially available long-acting insulin analogues exhibit reduced in vitro bioactivity: insulin detemir exhibits a consistent 2–10 fold lower potency, insulin peglispro has a 10-fold lower potency, and insulin degludec exhibits a 6–8 fold lower potency when compared to native insulin26,28, 29, 30, 31. Next, to evaluate the in vivo effects of Ins-SA-Alb, an insulin tolerance test was performed by injecting streptozotocin (STZ) treated mice via subcutaneous injection with Ins-SA-Alb or native insulin (both at 0.15 μg/kg per mouse). Native insulin was used as a positive control in this study and the mice blood glucose levels were measured every hour to measure the duration of lowering blood glucose effects. As shown, even with reduced bioactivity compared to native insulin, Ins-SA-Alb has a longer blood glucose lowering effect in STZ-treated mice compared to an equal dose of native insulin which suggests a longer half-life for Ins-SA-Alb (Fig. 3B).
Figure 3.
(A) In vitro pAkt assay comparing the bioactivity of native insulin and Ins-SA-Alb (native insulin EC50 = 2.126 nmol/L, Ins-SA-Alb EC50 = 30.32 nmol/L). (B) In vivo effects of Ins-SA-Alb and native insulin in STZ treated mice. Data were represented as mean ± SD, n = 4.
3. Conclusions
In conclusion, this work describes a rapid and facile chemical strategy for the synthesis of new insulin analogues and derivatives. We developed a new methodology that uses SrtA to modify the C-terminal of the insulin B chain. The new insulin precursor, Ins-SA, has a similar bioactivity as native insulin. Using Ins-SA, we have synthesized a series of insulin fusion derivatives that maintain bioactivity in high yields (70%–80%). As a proof of concept, we utilized this method to conjugate a fluorophore to Ins-SA and observed similar bioactivity as Ins-SA. This study has laid the foundation for the use of SrtA-mediated ligations to produce insulin imaging agents and insulin delivery systems. Additionally, we used this method to synthesize an insulin fusion derivative Ins-SA-Alb. Although Ins-SA-Alb is ∼15-fold weaker than native insulin in activating insulin signaling, in STZ-treated diabetic mice, Ins-SA-Alb has a longer half-life than native insulin indicating SrtA ligations are a viable method to produce new insulin fusion derivatives. Notably, because only a couple of mutations are needed in the C-terminal B chain, novel insulin skeletons with therapeutic potentials32,33 can also be modified using this method. We are now applying our method to synthesize other insulin fusion derivatives with therapeutic potentials.
4. Experimental
4.1. Materials
Fmoc protected amino acids were obtained from Protein Technologies Inc. N-α-Fmoc-N-ε-4-methyltrityle-l-lysine [Fmoc-Lys (Mtt)-OH] and 6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)hexanoic acid (NBD-X) was obtained from AnaSpec Egt Group. O-(Benxontriazol-1-yl)-1,1,3,3,-tetramethyluronium (HBTU) and 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo [4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU) were purchased from ChemPep. Rink Amide MBHA resin HL was obtained from Novabiochem and H-Rink Amide ChemMatrix was provided by Biotage. Dimethylformamide (DMF), triflouroacetic acid (TFA), acetonitrile and ethyl ether were purchased from Fisher Scientific. Piperidine, triisoproylsilane (TIS), 1,2-ethanedithiol (EDT), N,N-diisopropylethylamine (DIEA), dichloromethane (DCM), methyl sulfoxide (DMSO), 1,4-butanediol, Boc-Gly-OH, and trypsin from bovine pancreas was obtained from Sigma–Aldrich. Native insulin was obtained from Invitrogen Life Technologies.
4.2. Liquid chromatography mass spectrometry (LC‒MS) analysis
Characterization of crude peptides was performed by LC‒MS on an Xbridge C18 5-μm (50 mm × 2.1 mm) column at 0.4 mL/min with a water/acetonitrile gradient in 0.1% formic acid on an Agilent 6120 Quadrupole LC‒MS system. Fractions collected from HPLC runs were also analyzed by LC‒MS and fractions containing the targeted product were collected and lyophilized using a Labconcon Freeze Dryer.
4.3. High performance liquid chromatography (HPLC)
Preparative reverse-phase HPLC of crude peptides was performed on Luna 5u C8 100 Å (250 mm × 10 mm) column at 3 mL/min with a water/acetonitrile gradient in 0.1% TFA on an Agilent 1260 HPLC system.
4.4. Synthesis of desoctapeptide insulin (DOI)
Native insulin was cleaved at Arg B22 via trypsin-mediated cleavage. 50 mg of native insulin and 8.75 mg of trypsin was dissolved in 3 mL 1:1 (v/v) solvent mixture of DMSO, 1,4-butanediol, and 0.25 mol/L pH 7.5 Tris-base buffer with 0.2 mol/L CaCl2. This mixture was incubated at room temperature and mixed for 8–10 h. DOI was then characterized via LC‒MS and the mixture was diluted with double distilled water (ddwater) in a 1:5 ratio. Upon dilution the mixture was centrifuged and filtered with a GHP Acrodisc 25 mm Syringe filter with 0.45 μm GHP membrane obtained from Pall Life Sciences prior to collection of targeted fractions which were then lyophilized.
4.5. Peptide synthesis
Peptides (insulin analogue modified sequence: GFFYLPETGGG and SA21: GGRLIEDDICLPRWGCLWEDD) were synthesized via Fmoc solid phase peptide synthesis on a commercial peptide synthesizer (Alstra; Biotage, Inc.). Automated peptide synthesis was carried in a 10 mL reactor vial with the following protocols for 0.1 mmol scale. For Fmoc deprotection: (i) 4.5 mL of 20% piperidine in DMF; (ii) mix 2 × 3 min (new solvent delivered for each mix cycle). For amino acid coupling: (i) 1.25 mL of 0.4 mol/L Fmoc-protected amino acid in DMF; (ii) 1.225 mL of 0.4 mol/L HBTU or HATU (HBTU and Rink Amide MBHA resin HL was used for GFFYLPETGGG; HATU and H-Rink Amide ChemMatrix were used for SA21) in DMF; and (iii) mixed for 5 min at 75 °C (cysteine coupling: mix 10 min at 50 °C). DMF washing was completed between the deprotection and coupling steps: (i) 4.5 mL of DMF; (ii) mix 45 s. Upon completion of the peptide chain resins were washed with DCM and dried under vacuum for 20 min. The peptide was then cleaved from resin via cleavage for 2.5 h using 12.5 mL TFA, 330 μL water, 330 μL TIS and 330 μL EDT (for cysteine containing peptides). The peptide was precipitated with ethyl ether at 4 °C and lyophilized using a Labconco Freeze Dryer.
4.6. Synthesis of NBD-X bound GGGK
4.6.1. Synthesis of resin bound (Boc)GGGK
The peptide was synthesized via Fmoc solid phase peptide synthesis on a commercial peptide synthesizer (Alstra; Biotage, Inc.). Automated peptide synthesis was carried in a 10 mL reactor vial with the following protocols for 0.1 mmol scale. For Fmoc deprotection: (i) 4.5 mL of 20% piperidine in DMF; (ii) mix 2 × 3 min (new solvent delivered for each mix cycle). For amino acid coupling: (i) 1.25 mL of 0.4 mol/L Fmoc-protected amino acid in DMF; (ii) 1.225 mL of 0.4 mol/L HBTU (Rink Amide MBHA resin HL was used) in DMF; and (iii) mixed for 5 min at 75 °C. For DMF washing, completed between the deprotection and coupling steps: (i) 4.5 mL of DMF; (ii) mix 45 s). The N-terminal glycine was Boc protected and the group was not removed until cleavage of resin. To remove the Mtt protecting group on Lys, upon completion of the peptide chain, (i) 1% TFA and 5% TIS in DCM was added to the resin and the reaction was incubated and mixed for 30 min, (ii) the resin was washed using 1% TFA and DCM, and steps (i) and (ii) were repeated until the yellow color was completely removed. Next, the resin was washed in the following order i) DCM ii) DMF iii) DIEA in DMF (1 mol/L), and iv) DMF. The resin was then washed with DCM and dried under vacuum for 30 min and the peptide was left on resin to allow coupling with NBD-X.
4.6.2. Coupling and cleavage of NBD-X bound GGGK
N-terminal Boc protected and Mtt deprotected GGGK on resin was coupled to 2 equivalents NBD-X using 5 equivalents HBTU, 10 equivalents DIEA, in DMF (0.16 mol/L by Boc-GGGK). The reaction was incubated on rotator for 1.5 h. This was followed by 3 times DMF wash and 3 times DCM wash. Standard cleavage protocol using 2.7% TIS and 2.7% water in TFA was used to remove peptide from resin and remove the Boc protecting group from N-terminal Gly.
4.7. Synthesis of Ins-SA
Upon purification and lyophilization of DOI and synthesis of GFFYLPETGGG (GFFYLPETGGG did not need further purification after synthesis) 1.6 μmol DOI, 10 equivalents GFFYLPETGGG, and 1 mg/μmol of trypsin were dissolved in 348 μL 1:1 (v/v) solvent mixture of DMSO, 1,4-butanediol, and 0.25 mol/L Tris-base buffer pH 7.6 with 0.2 mol/L CaCl2. The reaction was incubated at rt under mild shaking for 12 h. Upon characterization of Ins-SA formation by LC‒MS, the reaction mixture was diluted with ddwater in a 1:2.7 ratio. For this particular synthesis the HPLC method was critical to collect targeted fractions. The method used was as Table 1.
Table 1.
Conditions of chromatography for purifications.
| Time (min) | 0.1% TFA in ddwater (%) | 0.1% TFA in acetonitrile (%) | Flow (mL/min) |
|---|---|---|---|
| 0 | 70 | 30 | 3.0 |
| 6 | 70 | 30 | 3.0 |
| 25 | 45.3 | 54.7 | 3.0 |
| 26 | 5 | 95 | 3.0 |
| 28 | 5 | 95 | 3.0 |
| 29 | 70 | 30 | 3.0 |
Fractions were collected then lyophilized.
4.8. Synthesis of Ins-SA-NBDX
0.25 μmol Ins-SA, 10 equivalents GGGK-NBDX, and 0.42 equivalents (10.5 μmol/L) SrtA were mixed in 2.5 mL of sortase buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 150 mmol/L NaCl, pH 7.5). This mixture was incubated at rt and allowed to react for 1.5 h. Ins-SA-NBDX formation was confirmed via LC‒MS and reaction was quenched using 0.2% TFA in water. The targeted fraction was collected by HPLC and the product collected was lyophilized.
4.9. Synthesis of Ins-SA-Alb
To Ins-SA 5 equivalents of SA21 and 0.6 equivalents (60 μmol/L) SrtA of total reactants were mixed in 100 μL 1:1 (v/v) solvent mixture of DMSO, 1,4-butanediol, and sortase buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 150 mmol/L NaCl, pH 7.5). This mixture was incubated at rt and allowed to react 1.5 h. Ins-SA-Alb formation was confirmed via LC‒MS and reaction was quenched using 0.2% TFA in water. The targeted fraction was collected by HPLC and the product collected was lyophilized.
4.10. In vitro studies using insulin signaling activation assay
To determine the extent of insulin signaling induced by analogues and derivatives, pAkt Ser 473 levels were measured in a mouse fibroblast cell line (provided by Prof. Terri Wood, Rutgers New Jersey Medical School, USA), NIH 3T3, overexpressing human insulin receptor isoform B (IR-B). The cell line was cultured in Dulbecco's Modified Eagle Medium (DMEM, Thermofisher Scientific, MA, USA) with 10% fetal bovine serum (FBS), 100 U/mL penicillin-streptomycin (Thermofisher Scientific) and 2 mg/mL puromycin (Thermofisher Scientific). For each assay, 60,000 cells per well and 100 μL per well, were plated in a 96-well plate with culture media containing 1% FBS. 24 h later, 50 μL of insulin analogue or derivative was pipetted into each well after the removal of the original media. After a 30-min treatment, the insulin solution was removed and the HTRF pAkt Ser473 kit (Cisbio, MA, USA) was used to measure the intracellular level of pAkt Ser473. Briefly, the cells were first treated with cell lysis buffer (50 μL per well) for 1 h under mild shaking. 16 μL of cell lysate was then added to 4 μL of detecting reagent in a white 384-well plate. After 4-h incubation, the plate was read in a Synergy Neo plate reader (BioTek, VT, USA) and the data processed according to the manufacturer's protocol.
4.11. In vivo studies using a rodent animal model
For this study C57BL/6 mice were obtained from The Jackson Laboratory. Mice were treated with streptozotocin (STZ) to produce a type 1 diabetes mouse model. All procedures were performed in accordance with the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Utah. In brief, mice with a body weight of 20–25 g were labeled on their tails prior to intraperitoneal (i.p.) injection with 20 mg/mL STZ in 0.1 mol/L citrate buffer pH 4.6. The injection volume for each mouse was calculated based on their body weight. Citrate buffer was prepared using 0.1 mol/L citric acid and 0.1 mol/L sodium citrate. The buffer was filter-sterilized and stored at 4 °C. To test the synthesized insulin fusion derivatives, mice (n = 6) were fasted over a 2-h period. After fasting, baseline blood glucose levels were measured using a glucometer and the mice were subsequently subcutaneously injected with 7.5 μg/mL of native insulin or Ins-SA-Alb. Blood glucose levels were measured every hour for 6 h after insulin injection.
Acknowledgments
We thank Prof. David R. Liu's Lab from Harvard University for providing the SrtA plasmid34. This work is supported by NIGMS (GM125001, USA) and NIDDK (DK121336, USA).
Author contributions
Maria M. Disotuar, Conceptualization, Methodology, Investigation, Writing—original draft; Jake A. Smith, Investigation, Resources. Jinze Li, Iinvestigation, Resources. Steve Alam, Resources. Nai-Pin Lin, Resources, Writing—review & editing. Danny Hung-Chieh Chou, Supervision, Funding acquisition.
Conflicts of interest
The authors declare no competing interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Appendix A. Supporting information
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2020.11.011.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Multimedia component 1
References
- 1.Cnop M., Welsh N., Jonas J.C., Jorns A., Lenzen S., Eizirik D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Department of Health and Human Services; U.S: 2014. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014.https://stacks.cdc.gov/view/cdc/23442/cdc_23442_DS1.pdf Available from: [Google Scholar]
- 3.Nathan D.M. Diabetes: advances in diagnosis and treatment. J Am Med Assoc. 2015;314:1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 4.Webber M.J., Anderson D.G. Smart approaches to glucose-responsive drug delivery. J Drug Target. 2015;23:651–655. doi: 10.3109/1061186X.2015.1055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens D.R. New horizons—alternative routes for insulin therapy. Nat Rev Drug Discov. 2002;1:529–540. doi: 10.1038/nrd836. [DOI] [PubMed] [Google Scholar]
- 6.Havelund S., Plum A., Ribel U., Jonassen I., Vølund A., Markussen J. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res (N Y) 2004;21:1498–1504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- 7.Hoeg-Jensen T., Havelund S., Nielsen P.K., Markussen J. Reversible insulin self-assembly under carbohydrate control. J Am Chem Soc. 2005;127:6158–6159. doi: 10.1021/ja051038k. [DOI] [PubMed] [Google Scholar]
- 8.Chou D.H., Webber M.J., Tang B.C., Lin A.B., Thapa L.S., Deng D. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci U S A. 2015;112:2401–2406. doi: 10.1073/pnas.1424684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disotuar M.M., Chen D., Lin N.P., Chou D.H. Glucose-responsive insulin through bioconjugation approaches. J Diabetes Sci Technol. 2020;14:198–203. doi: 10.1177/1932296819854105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu Y.B., Agrawal R., Chen D., Zheng N., Durupt G., Kim J.H. Long-lasting designer insulin with glucose-dependent solubility markedly reduces risk of hypoglycemia. Adv Ther. 2019;2:1900128. [Google Scholar]
- 11.Guan X., Chaffey P.K., Wei X., Gulbranson D.R., Ruan Y., Wang X. Chemically precise glycoengineering improves human insulin. ACS Chem Biol. 2018;13:73–81. doi: 10.1021/acschembio.7b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose K., De Pury H., Offord R.E. Rapid preparation of human insulin and insulin analogues in high yield by enzyme-assisted semi-synthesis. Biochem J. 1983;211:671–676. doi: 10.1042/bj2110671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermeier R., Geiger R. A new semisynthesis of human insulin. Hoppe Seylers Z Physiol Chem. 1976;357:759–767. doi: 10.1515/bchm2.1976.357.1.759. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickx A.P.A., Budzik J.M., Oh S.Y., Schneewind O. Architects at the bacterial surface—sortases and the assembly of pili with isopeptide bonds. Nat Rev Microbiol. 2011;9:166–176. doi: 10.1038/nrmicro2520. [DOI] [PubMed] [Google Scholar]
- 15.Theile C.S., Witte M.D., Blom A.E.M., Kundrat L., Ploegh H.L., Guimaraes C.P. Site-specific N-terminal labeling of proteins using sortase-mediated reactions. Nat Protoc. 2013;8:1800–1807. doi: 10.1038/nprot.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao H., Hart S.A., Schink A., Pollok B.A. Sortase-mediated protein ligation: a new method for protein engineering. J Am Chem Soc. 2004;126:2670–2671. doi: 10.1021/ja039915e. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z., Guo Z. Sortase-mediated transpeptidation for site-specific modification of peptides, glycopeptides, and proteins. J Carbohydr Chem. 2012;31:48–66. doi: 10.1080/07328303.2011.635251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F., Luo E.Y., Flora D.B., Mezo A.R. Irreversible sortase A-mediated ligation driven by diketopiperazine formation. J Org Chem. 2014;79:487–492. doi: 10.1021/jo4024914. [DOI] [PubMed] [Google Scholar]
- 19.Ton-That H., Mazmanian S.K., Faull K.F., Schneewind O. Anchoring of surface proteins to the cell wall of staphylococcus aureus: sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH2-Gly3 substrates. J Biol Chem. 2000;275:9876–9881. doi: 10.1074/jbc.275.13.9876. [DOI] [PubMed] [Google Scholar]
- 20.Cosmatos A., Ferderigos N., Katsoyannis P.G. Chemical synthesis of [des(tetrapeptide B27−30), Tyr(NH2)26-B] and [des(pentapeptide B26−30), Phe(NH2)25-B] bovine insulins. Int J Pept Protein Res. 1979;14:457–471. doi: 10.1111/j.1399-3011.1979.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 21.Fischer W.H., Saunders D., Brandenburg D., Wollmer A., Zahn H. A shortened insulin with full in vitro potency. Biol Chem Hoppe Seyler. 1985;366:521–525. doi: 10.1515/bchm3.1985.366.1.521. [DOI] [PubMed] [Google Scholar]
- 22.Inouye K., Watanabe K., Morihara K., Tochino Y., Kanaya T., Emura J. Enzyme-assisted semisynthesis of human insulin. J Am Chem Soc. 1979;101:751–752. [Google Scholar]
- 23.Saltiel A.R., Kahn C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 24.Baker D.P., Lin E.Y., Lin K., Pellegrini M., Petter R.C., Chen L.L. N-rerminally PEGylated human interferon-β-1a with improved pharmacokinetic properties and in vivo efficacy in a melanoma angiogenesis model. Bioconjugate Chem. 2006;17:179–188. doi: 10.1021/bc050237q. [DOI] [PubMed] [Google Scholar]
- 25.Gilmore J.M., Scheck R.A., Esser-Kahn A.P., Joshi N.S., Francis M.B. N-terminal protein modification through a biomimetic transamination reaction. Angew Chem Int Ed Engl. 2006;45:5307–5311. doi: 10.1002/anie.200600368. [DOI] [PubMed] [Google Scholar]
- 26.Werner H., Chantelau E.A. Differences in bioactivity between human insulin and insulin analogues approved for therapeutic use—compilation of reports from the past 20 years. Diabetol Metab Syndrome. 2011;3:13. doi: 10.1186/1758-5996-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis M.S., Zhang M., Meng Y.G., Kadkhodayan M., Kirchhofer D., Combs D. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277:35035–35043. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 28.Varewijck A.J., Janssen J.A.M.J.L. Insulin and its analogues and their affinities for the IGF1 receptor. Endocr Relat Cancer. 2012;19:F63–F75. doi: 10.1530/ERC-12-0026. [DOI] [PubMed] [Google Scholar]
- 29.Tennagels N., Werner U. The metabolic and mitogenic properties of basal insulin analogues. Arch Physiol Biochem. 2013;119:1–14. doi: 10.3109/13813455.2012.754474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasrallah S.N., Reynolds L.R. Insulin degludec, the new generation basal insulin or just another basal insulin?. Clin Med Insights Endocrinol Diabetes. 2012;5:31–37. doi: 10.4137/CMED.S9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owens R.A., Hansen R.J., Kahl S.D., Zhang C., Ruan X., Koester A. In vivo and in vitro characterization of basal insulin peglispro: a novel insulin analog. J Pharmacol Exp Therapeut. 2016;357:459–465. doi: 10.1124/jpet.115.231035. [DOI] [PubMed] [Google Scholar]
- 32.Xiong X., Blakely A., Karra P., VandenBerg M.A., Ghabash G., Whitby F. Novel four-disulfide insulin analog with high aggregation stability and potency. Chem Sci. 2020;11:195–200. doi: 10.1039/c9sc04555d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng N., Karra P., VandenBerg M.A., Kim J.H., Webber M.J., Holland W.L. Synthesis and characterization of an A6-A11 methylene thioacetal human insulin analogue with enhanced stability. J Med Chem. 2019;62:11437–11443. doi: 10.1021/acs.jmedchem.9b01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen I., Dorr B.M., Liu D.R. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc Natl Acad Sci U S A. 2011;108:11399–11404. doi: 10.1073/pnas.1101046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1