Abstract
Pyroptosis is the process of inflammatory cell death. The primary function of pyroptosis is to induce strong inflammatory responses that defend the host against microbe infection. Excessive pyroptosis, however, leads to several inflammatory diseases, including sepsis and autoimmune disorders. Pyroptosis can be canonical or noncanonical. Upon microbe infection, the canonical pathway responds to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), while the noncanonical pathway responds to intracellular lipopolysaccharides (LPS) of Gram-negative bacteria. The last step of pyroptosis requires the cleavage of gasdermin D (GsdmD) at D275 (numbering after human GSDMD) into N- and C-termini by caspase 1 in the canonical pathway and caspase 4/5/11 (caspase 4/5 in humans, caspase 11 in mice) in the noncanonical pathway. Upon cleavage, the N-terminus of GsdmD (GsdmD-N) forms a transmembrane pore that releases cytokines such as IL-1β and IL-18 and disturbs the regulation of ions and water, eventually resulting in strong inflammation and cell death. Since GsdmD is the effector of pyroptosis, promising inhibitors of GsdmD have been developed for inflammatory diseases. This review will focus on the roles of GsdmD during pyroptosis and in diseases.
KEY WORDS: Pyroptosis, Inflammasome, Caspase, Gasdermin, Sepsis, Inflammation, Pathogen-associated molecular patterns (PAMPs), Damage-associated molecular patterns (DAMPs)
Abbreviations: 7DG, 7-desacetoxy-6,7-dehydrogedunin; Ac-FLTD-CMK, acetyl-FLTD-chloromethylketone; ADRA2B, α-2B adrenergic receptor; AIM, absent in melanoma; ASC, associated speck-like protein; BMDM, bone marrow-derived macrophages; cAMP, cyclic adenosine monophosphate; CARD, caspase activation; CD, Crohn’s disease; cGAS, cyclic GMP–AMP synthase; CTM, Chinese traditional medicine; CTSG, cathepsin G; DAMP, damage-associated molecular pattern; DFNA5, deafness autosomal dominant 5; DFNB59, deafness autosomal recessive type 59; DKD, diabetic kidney disease; DMF, dimethyl fumarate; ELANE, neutrophil expressed elastase; ESCRT, endosomal sorting complexes required for transport; FADD, FAS-associated death domain; FDA, U.S. Food and Drug Administration; FIIND, function to find domain; FMF, familial Mediterranean fever; GI, gastrointestinal; GPX, glutathione peroxidase; GsdmA/B/C/D/E, gasdermin A/B/C/D/E; HAMP, homeostasis altering molecular pattern; HIN, hematopoietic expression, interferon-inducible nature, and nuclear localization; HIV, human immunodeficiency virus; HMGB1, high mobility group protein B1; IBD, inflammatory bowel disease; IFN, interferon; ITPR1, inositol 1,4,5-trisphosphate receptor type 1; LPS, lipopolysaccharide; LRR, leucine-rich repeat; MAP3K7, mitogen-activated protein kinase kinase kinase 7; MCC950, N-[[(1,2,3,5,6,7-hexahydro-s-indacen-4-yl)amino]carbonyl]-4-(1-hydroxy-1-methylethyl)-2-furansulfonamide; mtDNA, mitochondrial DNA; NAIP, NLR family apoptosis inhibitory protein; NBD, nucleotide-binding domain; NEK7, NIMA-related kinase 7; NET, neutrophil extracellular trap; NIK, NF-κB inducing kinase; NLR, NOD-like receptor; NLRP, NLR family pyrin domain containing; NSAID, non-steroidal anti-inflammatory drug; NSCLC, non-small cell lung cancer; NSP, neutrophil specific serine protease; PAMP, pathogen-associated molecular pattern; PKA, protein kinase A; PKN1/2, protein kinase1/2; PKR, protein kinase-R; PRR, pattern recognition receptors; PYD, pyrin domain; ROS, reactive oxygen species; STING, stimulator of interferon genes; TLR, Toll-like receptor; UC, ulcerative colitis
Graphical abstract
As the effector, activated GsdmD triggers pyroptosis by forming transmembrane pores and releasing inflammatory cytokines. This will lead to the resolution of cellular insults or cause inflammation-related diseases.
1. Introduction to GsdmD and pyroptosis
Gasdermin D, encoded by GSDMD on chromosome 8 (8q24.3), is a protein made of a 31 kDa N-terminus (GsdmD-N) and a 22 kDa C-terminus (GsdmD-C) connected by a peptide linker. Upon activation, the linker is cleaved to separate GsdmD-N from its autoinhibitory domain, GsdmD-C1, 2, 3. GsdmD-N forms a transmembrane pore that releases cytokines such as interleukin IL-1β4,5 and IL-186 and also disturbs the regulation of ions and water7, eventually resulting in pyroptosis. Pyroptosis is a form of inflammatory cell death via GsdmD-mediated cell lysis, which involves the participation of inflammatory caspases1. The inflammatory caspases are caspases 1, 4, and 5 in Homo sapiens, and 1 and 11 in Murinae2. Mouse caspase 11 is the counterpart to caspases 4 and 5 in humans. The most critical role of pyroptosis is to induce strong inflammatory responses that aid in host defenses against pathogen infection.
The pyroptotic pathways are important drug targets because they play critical roles in several diseases, including, but not limited to, sepsis3, Alzheimer's disease8, human immunodeficiency virus (HIV)9, and gout10. Several compounds, such as VX-765, are currently being tested for pyroptosis-associated diseases. Chemical danger signals like cytokines are released upon pyroptotic cell death, while some may even be released before cell death11. These danger signals result in the expansion of blood vessels in the localized area, leading to an increase in blood flow. This leads to other symptoms of inflammation, such as swelling and excess heat in the inflamed area. Collectively, the induction of inflammation is a double-edged sword in that it could defend against intracellular bacteria leading to resolution; however, it could also promote pathological inflammations, leading to disease development such as aberrant blood clotting and sepsis3,12,13.
The gasdermin family of proteins consists of 6 proteins: gasdermins A–E and deafness autosomal recessive type 59 (DFNB59, also called pejvakin). Except for DFNB59, the gasdermin family plays various roles in pyroptosis14. The mechanism of how pyroptosis plays a role in the innate immune system are discussed briefly in this review, and more details can be found in many excellent review papers15, 16, 17, 18.
2. Other members of gasdermin family and their roles in pyroptosis
The gasdermin family found in humans is composed of six members: gasdermins A–E, and DFNB59. Mice have no GsdmB but do have three GsdmA homologues (GsdmA1–3) and four GsdmC homologues (GsdmC1–4). Except for DFNB59, the family members have two domains, an N-terminal domain and a C-terminal domain connected by a peptide linker. These two domains of the gasdermins are similar in structure and have a sequence homology of ∼45%. The N-terminal domains are generally considered the effector domain capable of forming transmembrane pores, while the C-terminal domains of GsdmA/D/E have been shown to play autoinhibitory roles. Among the gasdermins, GsdmD and GsdmE have been better characterized for their activation and function.
2.1. GsdmA
GSDMA maps on chromosome 17 at 17q21 and has been associated with autoimmune diseases and cancers19. As mentioned above, mice have three homologues of GsdmA, and most of the mutation phenotypes in mice are mapped to GsdmA3. These mutations of GsdmA3 have been shown to cause strong skin inflammations20,21 suggesting a role in pyroptosis. When the N-terminus is expressed by itself in cells, pyroptosis is triggered22; however, the cleavage enzyme of GsdmA is still unknown. GsdmA3 has been well characterized in structure, including full length (FL) GsdmA323,24 and the pore form of the N-terminal domain24. Similar to GsdmD, its C-terminus inhibits the N-terminus, the activation of which triggers the formation of the transmembrane pore to execute pyroptosis23.
2.2. GsdmB
Like GSDMA, GSDMB maps at 17q21, and its polymorphisms are linked to autoimmune diseases such as asthma and inflammatory bowel disease (IBD)25. GsdmB enhances the activity of caspase 4 during noncanonical pyroptosis, suggesting its role in inflammation26. Paradoxically, GsdmB can be cleaved by apoptotic caspases 3/6/7, but not by inflammatory caspases, with previous evidence suggesting the cleaved N-terminal product may not contain the full N-terminal domain to form pores or directly participate in inflammation27. In a recent report, however, it was found that granzyme A from cytotoxic T cells and natural killer cells cleave and activate gasdermin B (GsdmB), triggering pyroptosis in tumor cells28. This suggests a more direct involvement in pyroptosis than what was previously understood. Intriguingly, GSDMB is overexpressed and considered an oncogene in several cancer types such as breast cancer29, gastric cancer27, and cervical cancer30. As such, further studies of GsdmB regarding its roles in pyroptosis and cancers are needed.
2.3. GsdmE
GSDME, mapping on chromosome 7 at 7p15, is also named deafness autosomal dominant 5 (DFNA5). Initially, it was associated with hereditary hearing loss, but without assigned physiological function31. Recently, GsdmE has been discovered to contribute to the regulation of both apoptosis and pyroptosis32,33. Its C-terminal domain also plays an inhibitory role in the N-terminal domain. Upon activation by caspase 3, the N-terminal domain forms lytic pores to execute apoptosis and/or pyroptosis32,33. GsdmE is also activated by killer-cell granzyme B, which is released into tumor cells by cytotoxic lymphocytes. Subsequently, granzyme B cleaves GsdmE to induce pyroptosis in tumors34. Consistently, GsdmE mediated pyroptosis contributes to the cytotoxicity of many chemotherapeutic reagents33, including a potential target for hepatocellular carcinoma patients, which uses the secondary metabolite miltirone35.
2.4. DFNB59
DFNB59 is located at 2q31 and has not been associated with either pore formation or pyroptosis. Compared with other gasdermin members, DFNB59 has a shorter C-terminal domain that does not share sequence homology with other gasdermin proteins. Mutations of DFNB59 cause a neuronal defect, leading to nonsyndromic deafness36,37. It has been suggested that DFNB59 functions as a receptor/adaptor in pexophagy, which maintains redox homeostasis of auditory hair cells to prevent noise-induced damage38.
2.5. GsdmC
GSDMC maps on chromosome 8 at 8q24.21. Limited is known about GsdmC, although artificial mutations cause cytotoxicity in cell lines23. It is highly expressed in the gastrointestinal (GI) tract and may play a role in GI cancers19,39.
3. Introduction to pyroptotic pathways
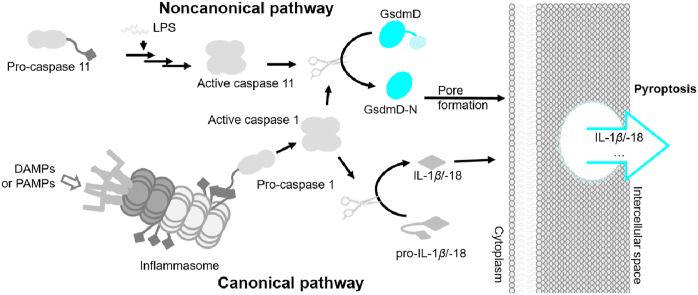
There are two major pathways for pyroptosis: canonical and noncanonical pyroptosis (Fig. 1). Both pathways use GsdmD as the downstream effector40, 41, 42. The canonical pathway detects both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) as well as cytoplasmic disturbances, recently coined as homeostasis altering molecular properties (HAMPs)43. PAMPs and DAMPs are recognized intracellularly by cytoplasmic pattern recognition receptors (PRRs, Fig. 2), the activation of which assembles the inflammasome complex to execute pyroptosis. In contrast, the recognition of HAMPs is through the detection of molecular processes that perturbs cytoplasmic homeostasis, e.g., the phosphorylation of pyrin inflammasome44,45. Generally, after the binding of a PAMP or DAMP, PRRs interact and activate the apoptosis-associated speck-like protein (ASC), which then oligomerizes and uses its caspase activation and recruitment domain (CARD) to bind to the CARD of pro-caspase 1. The binding complex of PRRs, ASC, and pro-caspase 1 is termed the inflammasome. A CARD–CARD interaction triggers the activation of pro-caspase 1 in order to cleave GsdmD and pro-IL-1β/IL-18 into their active forms, initiating pyroptosis. During pyroptosis, GsdmD-N selectively interacts with membrane lipids to form transmembrane pores, through which cellular contents, especially danger signals, are released23,46. The canonical pathway also utilizes toll-like receptors (TLRs) for priming certain PRRs to enhance immune responses. The noncanonical pathway detects intracellular lipopolysaccharides (LPS) of Gram-negative bacteria. LPS directly activates pro-caspase 11 (pro-caspase 4/5 in humans) by binding to CARD, resulting in the oligomerization and autoproteolysis of caspase 111,13. Similar as caspase 1, active caspase 11 cleaves the linker region of GsdmD to separate GsdmD-N and GsdmD-C, initiating pyroptosis. The noncanonical pathway crosstalks with the canonical pathway via NOD-like receptor family pyrin domain containing 3 (NLRP3), which activates caspase 1 to cleave pro-IL-1β/IL-18, promoting inflammation. Collectively, these events trigger downstream effects, such as cell death by membrane disruption and the release of cytokines through GsdmD-N pores, as shown in Fig. 141,47.
Figure 1.
Activation of either caspase 1 or caspase 11 can lead to pyroptosis. Pro-caspase 11 is directly activated by LPS, which leads to activated caspase 11 that cleaves GsdmD. Pro-caspase 1 is activated by the CARD recruiting domain after receptor activation and inflammasome formation. Caspase 1 can activate GsdmD and IL-1β/IL-18.
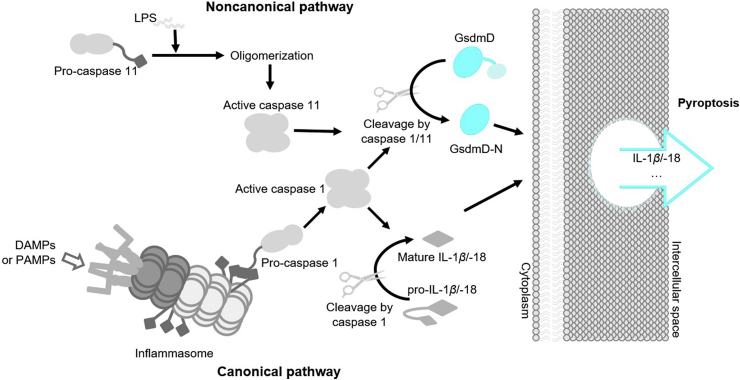
Figure 2.
Inflammasomes in canonical pyroptosis pathway. Ligands are differentially recognized by PRRs, which then assembles corresponding inflammasome complexes to activate caspase 1. Activated caspase 1 subsequently cleaves GsdmD to free its N-terminus, which forms the transmembrane pore and functions as the executor of pyroptosis. Note: the oligomerization of inflammasome proteins is not shown.
3.1. Canonical cascade
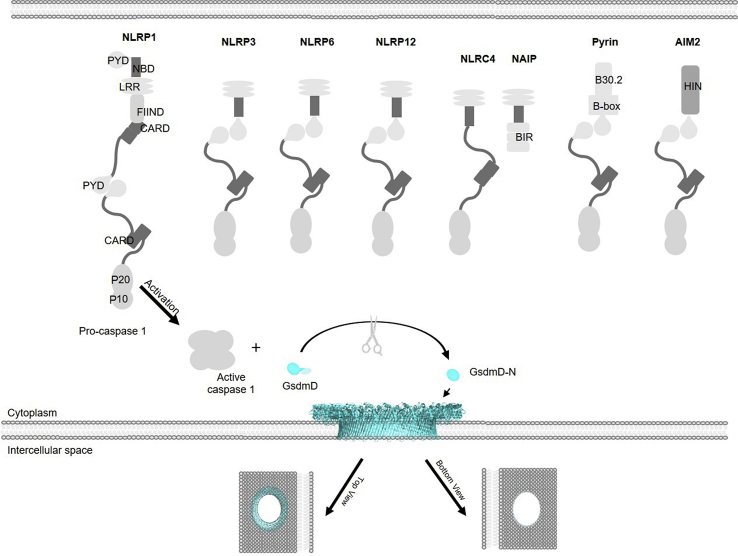
NOD-like receptors (NLRs), which are the cytoplasmic pattern-recognition receptors (PRRs), can recognize PAMPs and DAMPs to initiate the canonical inflammasome pathway. After binding with a DAMP or PAMP, NLRs are activated (Fig. 2), followed by the recruitment of the adaptor ASC complex. This results in the formation of inflammasomes, which recruit and activate pro-caspase 1. Activated caspase 1 then cleaves GsdmD to free its N-terminus, which forms the lytic pore as the pyroptotic effector48. Some well-characterized pathways are shown in Fig. 2 10,49,50. Except for NLRP1, NLRs typically contains three domains: an N-terminal adaptor domain (such as CARD or pyrin domain (PYD)), a central nucleotide-binding domain (NBD), and a C-terminal leucine-rich repeat (LRR) domain. NLRP1 contains two additional domains at its C-terminus, a CARD followed by a function to find domain (FIIND) domain. The LRR domains are used to sense bacterial components; the NBD domain is critical for oligomerization and activation; the N-terminal domain is responsible for CARD recruitment and CARD–CARD interactions as well as for activating caspase 151,52. In addition to NLRs, absent in melanoma 2 (AIM2) and pyrin, two other cytoplasmic PRRs, detect bacterial components and form inflammasomes to activate caspase 1. Individual pathways are explained in more detail in the following paragraphs.
NLRP1, which was first identified in humans, was later found in mice and was the first discovered NLR family member discovered to be involved in the formation of an inflammasome complex. NLRP1 does not require ASC and thus is able to activate pro-caspase 1, leading to IL-β secretion without ASC oligomerization53. It is coded by a single gene in humans with three homologues NLRP1a, b, and c in mice12,50,54. NLRP1b is activated by anthrax lethal toxin after it cleaves an N-terminal peptide from NLRP1b55, while human NLRP1 is not activated by the same toxin56. However, the molecular mechanism of cleavage-mediated NLRP1b inflammasome activation remains elusive. Using an in vitro system consisting of recombinant proteins, NLRP1 in humans was found to be activated by muramyl dipeptide and ATP57. In addition, inhibitors of dipeptidyl peptidases 8 and 9 (DPP8/9) can selectively activate NLRP1 and its related protein CARD8 in both mouse and human lymphocytes58. Interestingly, NLRP1b responds to metabolic stress to produce IL-1859 (known to prevent obesity and diet-induced metabolic syndrome), which paradoxically can induce inflammation. The activation of the NLRP1 inflammasome is also essential to the secretion of high mobility group protein B1 (HMGB1), which stimulates inflammatory responses by modulating both the innate and the adaptive immune responses50.
NLRP3 uses a similar overall mechanism as other NLRs such as NLRP1. However, the major difference is that NLRP3 may not directly interact with its activators; rather, it must first be primed by a cytokine receptor or another PRR60. NLRP3 can be activated by a wide range of DAMPs and PAMPs such as bacterial LPS, fungal zymosan, viral RNA extracellular ATP, reactive oxygen species, nigericin, crystallines, and amyloid-β plaque60. Lysosomal damage has also been attributed to NLRP3 activation, with lysosomal membrane damage resulting in K+ efflux and the release of lysosomal protease cathepsin B, both of which initiate NLRP3 inflammasome activation61. Despite the diverse activators, a common downstream event is the disassembly of the trans-Golgi network (TGN), which recruits NLRP3 using phosphatidylinositol-4-phosphate (PtdIns4P) and activates NLRP3 to form the inflammasome complexes62. Another crucial player during NLRP3 activation is the protein NIMA-related kinase 7 (NEK7), which participates by regulating NLRP3 inflammasome formation following potassium release and preventing inflammasome formation during mitotic division stages of the cell cycle63,64. Co-crystal structures show that NEK7 directly binds to two neighboring NLRP3 subunits as a necessity for inflammasome formation65. After activation, the NLRP3 receptor recruits the ASC by PYD–PYD interaction, assembling NLRP3 inflammasome (Fig. 2). When NLRP3 is knocked out in mice or antagonized with inhibitors, there is a significant reduction of pyroptosis, suggesting NLRP3's key involvement in the pyroptotic pathway. NLRP3 crosstalks with other innate immune pathways66,67, suggesting that the inhibition of the NLRP3 could have a broad inhibitory effect for inflammatory responses. As such, NLRP3 is an attractive drug target for patients with inflammatory diseases68. Research involving treatments for septic shock has recently shown cardamonin, a secondary herbal metabolite used in Chinese traditional medicine (CTM), showed inhibitory effects on NLRP3 activation within mouse bone marrow-derived macrophages (mBMDM) and human peripheral blood mononuclear cells (PBMC) by preventing ASC oligomerization69. It has also been shown that overactivation of the NLRP3 pathway causes gouty arthritis in mice models10. OLT1177, an inhibitor of NLRP3, is in the U.S. Food and Drug Administration (FDA) clinical trials phase II for gouty arthritis68.
NLRP6 has an N-terminal PYD, an internal NBD, and a C-terminal LRR. Similar to other NLR inflammasomes, activated NLRP6 causes ASC speck formation70 and subsequently caspase 1 activation. NLRP6 has been reported to play an inflammasome-dependent role in host defenses and inflammation and an inflammasome-independent role in intestinal homeostasis and cancer. However, the molecular mechanisms in these processes are not fully elucidated, and significant discrepancies exist71,72.
Like NLRP6, NLRP12 plays both inflammasome-dependent and -independent roles. NLRP12 inflammasome participates in the canonical pyroptotic pathway by assembling the NLRP12 pyroptosomes using a similar mechanism to NLRP6 (Fig. 2). Conversely, NLRP12 is also capable of negatively regulating inflammation by promoting the degradation of NF-κB inducing kinase (NIK)73. The reduction of NIK leads to attenuated noncanonical NF-κB signaling, which is crucial to inflammatory responses to pathogens in the innate immune system73. NLRP12, similar to NLRP6, is highly-expressed within intestinal tissues, and plays a central role in maintaining intestinal inflammation, tumorigenesis and homeostasis73,74.
Pyrin, not an NLR, has an N-terminal PYD, followed by a B-box domain, a coiled-coil domain, and a B30.2 domain at the C-terminus. Pyrin detects the inactivation of Rho GTPase, especially RhoA, which is targeted by various bacterial toxins75. Without bacterial infection, pyrin stays inactivated when phosphorylated by protein kinase1/2 (PKN1/2), and bound to protein 14-3-3. This inactive form is a monomer, which dimerizes to become active76. As a means of regulation, active RhoA promotes PKN1/2 to phosphorylate pyrin76; toxin-induced inactivation of RhoA leads to pyrin activation. Microtubules have also been shown to facilitate the dimerization of pyrin. Upon dimerization, pyrin triggers the ASC speck formation, which subsequently activates pro-caspase 175. Mutations in pyrin have been correlated with inflammatory diseases, familial Mediterranean fever (FMF)77, and hyperimmunoglobulinemia D syndrome (HIDS). AIM2, not an NLR, consists of an N-terminal PYD and a C-terminal positively charged HIN (hematopoietic expression, interferon-inducible nature, and nuclear localization) domain. AIM2 plays an important role in the innate immunity for the detection of cytoplasmic pathogenic dsDNA43. The HIN domain uses its positive charge to bind the negatively charged dsDNA. This binding is independent of DNA sequence but requires a minimum of 80 base pairs in length49. Once the HIN domain detects dsDNA in the cytoplasm, AIM2 is activated and uses its PYD to recruit ASC by PYD–PYD interaction78. The ASC speck forms and recruits pro-caspase 1 via CARD–CARD interactions, similar to NLRP3 inflammasome49. This activation pathway further facilitates immune response, ensuring escaped foreign DNA particles from phagosomes are targeted79. Like other inflammasomes, The AIM2 inflammasome is tightly controlled in cells and has been shown to be negatively regulated by interferon-inducible protein p202 within mBMDMs for the activation of pro-caspase 180.
NLRC4, composed of an N-terminal CARD, an NBD, and a C-terminal LRR, plays a major role in protection against certain Gram-negative bacteria with type III or type IV secretion systems81. NLRC4 forms complexes with NAIPs (NLR family apoptosis inhibitory proteins), and activates NLRC4 by binding to pathogenic proteins from flagellin or type III/IV secretion systems. Once the NLRC4 is activated, it oligomerizes and directly interacts with pro-caspase 1 via a CARD–CARD interaction (Fig. 2), bypassing the participation of ASC. This direct CARD–CARD interaction between the NLRC4 and pro-caspase 1 leads to its activation as well as the formation of GsdmD-N pores and interleukin release81. There are several gain-of-function mutations in NLRC4, which have been shown to result in the development of autoinflammatory diseases82.
3.2. Noncanonical cascade
The noncanonical pathway plays a critical role in cell immunological responses to intracellular Gram-negative bacteria13,41,83, where LPS is detected by caspase 4/5/11.
Intracellular LPS can be derived directly from intracellular bacteria or the extracellular space. Before encountering LPS, pro-caspase 11 has negligible catalytic activity. The CARD domain of pro-caspase 4/5/11 directly interacts with the lipid A moiety of LPS, causing a significant conformational rearrangement of pro-caspases 4/5/11. The conformational changes of these pro-caspases result in oligomerization using their CARD domains, further enabling limited activity for auto-proteolysis. It is important to note that the oligomerization of caspase 11 directly involves CARD, while the CARD of caspase 1 is used for ASC binding instead of oligomerization84,85. After oligomerization, caspase 11 auto-proteolyze after D285 with a sequence of MEAD|A to gain full activity in proteolyzing GsdmD22,41. In contrast to caspase 1, which cleaves pro-IL-1β/IL-18 and GsdmD, activated caspases 4/5/11 has been considered only recognize GsdmD and cleave at D275 of hGsdmD or D276 of mGsdmD22,41 for generating pyroptotic pores (Fig. 1). Interestingly, caspase 4/11 were shown to be activated by guanylate-binding proteins 1–4, which activate innate immunity against intracellular pathogens, on the surface of intracellular bacterium to cleavage pro-IL-1886. Additionally, the active caspase 4/5/11 also crosstalk with NLRP3, giving rise to the formation of the NLRP3 inflammasome, which can then activate caspase 1 for the production of mature cytokines, IL-1β/IL-1812. This crosstalk is suggested to involve a drastic efflux of K+ due to membrane rupturing caused by caspase 11 working as a signal to elicit NLRP3 inflammasome activation87. The noncanonical pathway has also been suggested to involve metabolic diseases due to mitochondrial dysfunction with the release of mitochondrial reactive oxygen species (ROS) and mitochondrial DNA (mtDNA). Activation of inflammasomes via both the canonical and noncanonical pathways display immune response variability enabling the host to elicit a variety of defense mechanisms dependent upon the type of pathogen infection88.
4. Mechanism of GsdmD activation in pyroptosis
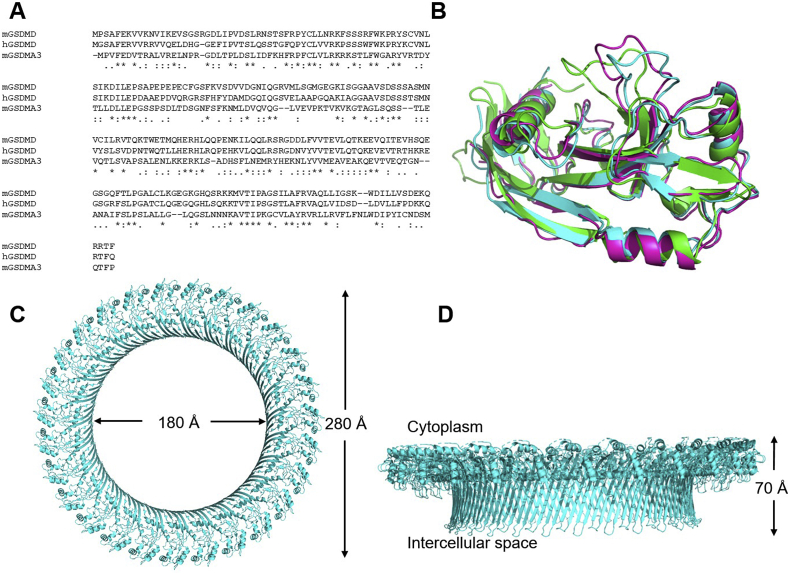
The activation mechanisms of hGsdmD and mGsdmD are considered identical, but the cutting sites on the linker-region are slightly different, with FLTD|GVP in hGsdmD and LLSD|GID in mGsdmD41. Neither hGsdmD nor mGsdmD has a high-resolution structure available in the pore form, although the structures of inactive FL proteins have been solved. The N-termini of mGsdmD and hGsdmD have a ∼60% sequence similarity with the N-terminus of mGsdmA3 (Fig. 3A). As shown in Fig. 3B, there is a significant structure overlap among the inactive N-termini of hGsdmD (PDB:6N9O), mGsdmD (PDB:6N9N), and mGsdmA3 (PDB:5B5R), with an RMSD of 1.2 Å23,24,89. Therefore, the structure of mGsdmA3 in the pore form resembles that of a GsdmD pore (Fig. 3). Using cryo-electron microscopy (Cryo-EM), the structure of mGsdmA3 pore is solved with a resolution of 3.8 Å24. Cryo-EM resolved the GsdmD pore structure with a resolution of low nanometers because of insufficient homogeneity48. mGsdmA3 pore is formed by 26–28 subunits, while GsdmD uses a similar number of subunits for pore formation. The GsdmA3 pore, which is formed by a series of β-barrels, has a ∼180 Å inner diameter and ∼280 Å outer diameter (Fig. 3C). The inner diameter is large enough to pass proteins like IL-18, IL-1β, and metabolites. Additionally, galectin-3, an effector of the NLRP3 inflammasome, could also pass through the pore90, suggesting the GsdmD pore may selectively release cellular proteins. The height of this cross-membrane β-barrel is ∼70 Å (Fig. 3D), which is the typical thickness of mammalian membranes.
Figure 3.
Similarities of the N-termini of hGsdmD, mGsdmD, and mGsdmA3, and the structure of the transmembrane pore of mGsdmA3. (A) The sequence and (B) structural alignments of the unactivated N-termini of hGsdmD, mGsdmD, and mGSDMA3 suggest the three proteins adopt similar structures after pore formation. Green: hGsdmD, PDB:6N9O; Pink: mGsdmD, PDB:6N9N; Cyan: mGsdmA3, PDB:5B5R. Note: There are ∼30 amino acids at the end of N-terminal domain that are not all structurally available for all three proteins, and these amino acids are not shown in (A) and (B). The (C) top to bottom and (D) side views show the symmetric assembly of mGsdmA3 pore.
Without proper activation, the inflammatory caspases 1/4/5/11 are in catalytic inactive pro-forms, leaving GsdmD in the inactive form40. Previous studies have shown that by mutation of the aspartic acid within the linker, GsdmD stays in its inactive form without being cleaved, and a pyroptotic pore cannot form91. Additional studies have shown that overexpression of the C-terminus suppresses the creation of pores in the membrane. This suggests that GsdmD-C plays an inhibitory role for GsdmD-N and does not require the linker region for the inhibition of GsdmD-N89,92.
Without the activation of GsdmD, pore-forming activity and pyroptosis are inhibited. Similarly, without the cleavage of pro-IL-1β or pro-IL-18 to their mature forms, inflammatory responses are limited. A full spectrum of pyroptosis includes the cleavage of pro-IL-1β, and pro-IL-18 as well as GsdmD, capable of eliciting significantly damaging effects to the local tissue and is not the best choice for immune cells to resolve minor insults. Under a mild challenge, DAMPs and PAMPs can be released independent of the activation of the inflammatory caspases and pyroptosis13,47,93, suggesting that pyroptosis, the highly inflammatory form of cell death, is under tight regulation. This tight regulation of the inflammatory caspases prevents long-term damage such as organ damage33,94,95, sepsis3, Alzheimer's disease8, atherosclerosis96, and gout10.
Compared to caspase 5, caspase 4's and 11's autocleavage is better characterized and shares a similar mechanism. Upon activation, the autocleavage of caspase 4/11 generates an enzymatically active caspase 4/11 dimers. However, it is still not fully elucidated if the dimer consists of p10+p32 or p10+p22. The p10 subunit binds to the GsdmD-C domain for substrate recognition before cleavage to separate GsdmD-N and GsdmD-C97. When GsdmD-C is replaced with mCherry with the cleavage site intact, free GsdmD-N cannot be generated as the mutant GsdmD is not recognized by the inflammatory caspases97. Consistently, in the caspase 4/GsdmD-C complex structure, the N-terminus of p10 is shown to stretch toward a hydrophobic patch of GsdmD-C, suggesting a direct interaction with GsdmD-C is required for cleavage. After aligning the GsdmD structure along with the caspase 4/GsdmD-C complex, it shows that the cleavage site, P4–P1, of GsdmD is next to the autoprocessing (P4–P1 of caspase 4) site, suggesting the binding of caspase 4 and GsdmD-C in the crystal structure is physiologically relevant. Collectively, caspase 4 binds to GsdmD-C for recognition, followed by the cleavage and release of GsdmD-N from GsdmD-C97. In addition to covalent interaction using the peptide linker, GsdmD-C interacts with GsdmD-N using a large hydrophobic patch to prevent the conformational changes necessary for pore formation. Interestingly, some GsdmD-C is secreted out of the pore during pyroptosis. This could be a strategy to eliminate the noncovalent inhibition of GsdmD-C.
5. Examples of pyroptotic inhibitors
Since the uncontrolled activation of GsdmD can cause disease in both human and mouse models, caspase 1/4/5/11 are important drug targets for inflammation-related diseases3,8,98,99. Z-VAD-FMK is a cell-permeable inhibitor that was originally developed to bind irreversibly and inhibit inflammatory caspases100. The tripeptide, VAD, is for binding, while FMK is the warhead that can form a covalent bond with the catalytic cysteines, permanently preventing catalytic activities101. However, Z-VAD-FMK does not specifically inhibit only the inflammatory caspases 1/4/5/11 as originally designed; it also inhibits caspases 3/7/8 as a suicide inhibitor, inhibiting both pyroptosis and apoptosis. The promiscuity between caspases makes it unsuitable as a drug because of the off-target effects100,101.
Acetyl-FLTD-chloromethylketone (Ac-FLTD-CMK) has also been developed to inhibit inflammatory caspases (Table 1) with Kd values of 21.7 and 29.6 μmol/L to caspases 1 and 4, respectively. Unlike pan-caspase inhibitors, it does not bind to apoptotic caspase 3. Using ATP or nigericin-treated macrophage cells, Ac-FLTD-CMK significantly reduced the secretion of IL-1β102, suggesting its inhibitory action for pyroptosis. In an in vitro caspase activity assay using a synthetic substrate, N-acetyl-Trp-Glu-His-Asp-7-amido-4-methylcoumarin (Ac-WEHD-AMC), IC50 values of Ac-FLTD-CMK differ significantly for caspases 1, 4, and 5 with values of 0.0467, 1.49, and 0.329 μmol/L, respectively. The compound is water-soluble, similar to the previously mentioned pan-caspase inhibitors, although it slightly violates Lipinski's rule of five by having a molecular weight of 569 Da103.
Table 1.
Pyroptosis inhibitors, including their names, structures, targets, and type of inhibition.
| Name of inhibitor | Structure of inhibitor | Target | Type of inhibitor |
|---|---|---|---|
| Ac-YVAD-CHO | 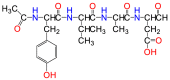 |
Caspase 1/4 | Suicide inhibitor |
| Ac-FLTD-CMK | 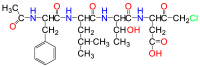 |
Caspase 1/4/5 | Suicide inhibitor |
| Z-VAD-FMK | 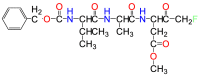 |
Pan-caspase | Suicide inhibitor |
| MCC950 | 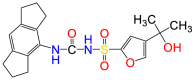 |
NLRP3 | Allosteric or competitive inhibitor |
| VX-765 | 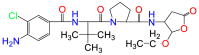 |
Caspase 1/4 | Competitive inhibitor |
| 7DG | 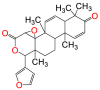 |
Protein kinase-R | Activity-independent inhibitor |
NEK7 directly interacts with NLRP3 for its activation63,104 (Table 1). The expression level of NEK7 has been shown to be closely correlated with the degree of pyroptosis. As such, NEK7 has been suggested to be a marker for pyroptosis. Even though NEK7 plays roles in multiple pathways such as cell cycle regulation, NEK7 has been suggested to be a drug target for the prevention of inflammation in pyroptosis105. Studies have suggested a linkage between IBD and NEK7. During the development of IBD, which has been directly linked to NLRP3 and pyroptosis, NEK7 is upregulated through NF-κB and modulates NLRP3 activation by direct interaction94,106
Rv3364c, a protein secreted by Mycobacterium tuberculosis, was recently reported to inhibit host serine protease cathepsin G (CTSG), enabling M. tuberculosis survival in macrophages107. Rv3364c binds tightly to CTSG on the host cell membrane, suppressing its catalytic activity and downstream activation of caspase 1. This hinders the maturation of caspase 1 and inhibits pyroptosis within infected macrophages. Consistently, the exposure of M. tuberculosis resulted in the recruitment of a large number of infected macrophages but with a lower pyroptotic rate. However, when exposed to M. tuberculosis with Rv3364c knocked out, cellular immunity by pyroptosis was restored. This decreased the number of infected macrophages, with the rate of pyroptosis increasing back to normal levels. The restoration of pyroptosis levels was then able to prevent intracellular M. tuberculosis from proliferating13,107. Currently, inhibitors mimicking Rv3364c binding patterns are under development for inhibition of inflammation107.
N-[[(1,2,3,5,6,7-Hexahydro-s-indacen-4-yl)amino]carbonyl]-4-(1-hydroxy-1-methylethyl)-2-furansulfonamide (MCC950) is a promising inhibitor targeting the NLRP3 pathway108. MCC950 directly binds to the Walker B motif of NLRP3, which prevents ATP hydrolysis and associated conformational changes of NLRP3. This locks NLRP3 in the inactive form and prevents inflammasome activation109. Subsequently, the formation of an NLRP3-induced ASC oligomerization (spike) is abrogated, preventing the downstream maturation of caspase 1. As a result, the maturation and release of cytokines and the formation of pyroptotic pores are all inhibited110,111. The IC50 for MCC950 is 7.5 nmol/L for the release of IL-1β from IMBM cells. MCC950 decreases the expression of caspase 1/11, NLRP3, and ASC in mice heart, lungs, and brain upon exposure to LPS108. Further experiments are looking at a wide range of NLRP-3 associated diseases, and it has promising inhibitory effects for NLRP3 in blood samples from patients with Muckle–Wells syndrome108,110,111.
(S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765) is a prodrug of (S)-3-({1-[(S)-1-((S)-2-{[1-(4-amino-3-chlorophenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidin-2yl]-methanoyl}-amino)-4-oxo-butyric acid (VRT-043198), a competitive inhibitor of caspase 1/4. VX-765 has been shown to be useful for inflammatory diseases, such as hepatic ischemia-reperforation112, Alzheimer's disease8,111, and bacterial infections49 (Table 1). VRT-043198, the active form of VX-765, has IC50 values of 0.2, 14.5, and 10.6 nmol/L when tested using purified caspase 1, 4 and 5 respectively in the presence of a peptide substrate113. In animal studies, VX-765 has been shown to protect LPS-challenged mice by inhibiting caspase 1, thus decreasing the production of IL-1β/IL-18. VRT-043198 selectively inhibits inflammatory caspases without inhibiting apoptotic caspases. This selectivity for inflammatory caspases makes it safer for the treatment of inflammatory diseases, in contrast to the pan-caspase inhibitors113. This drug is currently in phase II trials for psoriasis, while phase II trials for Alzheimer's disease have ceased due to the discovery of insignificant efficacy levels. In mice, VX-765 also showed a significant benefit in skin inflammation8,114.
7-Desacetoxy-6,7-dehydrogedunin (7DG) is a selective inhibitor of protein kinase-R (PKR), which plays a kinase-independent role in pyroptosis115 (Table 1). 7DG inhibits PKR by binding to its C-terminus, preventing ASC oligomerization in both the NLRP1 and NLRP3 inflammasome pathways. When macrophages are challenged with Bacillus anthracis (anthrax) lethal toxin (LT), 7DG treatment almost completely blocked pyroptotic cell death with an IC50 of 5 μmol/L112. Although this compound has shown high efficacy in blocking pyroptosis, PKR also participates in apoptosis and plays a vital role in the actin dynamics within cells116, making it an unideal drug target.
Disulfiram, an inhibitor of the enzyme acetaldehyde dehydrogenase, is an FDA-approved drug to help patients overcome alcoholism (Table 1)117. It has been suggested to be useful for cancer prevention in alcohol-dependent people, showing a significant decrease in the development of cancer118. Disulfiram was first discovered to inhibit pyroptosis when existing clinical drugs were screened in a cell-free assay, measuring Pd3+-release from liposomes in the presence of active caspase 11 and GsdmD. Subsequent studies showed that disulfiram inhibited caspase 1, caspase 11, and the ring form of GsdmD-N by covalently attaching to the catalytic cysteines of caspases 1 and 11, and to the cysteine (human/mouse Cys191/Cys192) within the channel of the GsdmD-N pore119,120. When LPS-primed macrophages were treated with disulfiram following nigericin challenge, disulfiram significantly inhibited pyroptosis in these cells with an IC50 of 7.67 μmol/L. In vivo, disulfiram increased viability in mice challenged with high doses of LPS119. Disulfiram has been shown to have inhibitory effects on a variety of proteins with differing functions. It is speculated that disulfiram's function is dependent on the expression level of the target proteins. For instance, when there is a strong pyroptotic response, it tends to target GsdmD, whose expression is upregulated. Meanwhile, it primarily targets acetaldehyde dehydrogenase during alcoholism treatment due to acetaldehyde dehydrogenase being the rate-limiting enzyme for ethanol metabolism and its high expression levels in alcohol-dependent people117,119. Because disulfiram is a safe drug in the clinic, its repurposing is promising to combat inflammation117,119.
Dimethyl fumarate (DMF) is the methyl ester of fumarate, an intermediate of the citric acid cycle. It is currently used as an orally administrated drug to treat relapsing multiple sclerosis. DMF succinates cysteine C192 of mGsdmD to S-(2-succinyl)-cysteine, which inhibits activation by inflammatory caspases. This ultimately results in the inhibition of pyroptosis. When DMF was used to treat mice models with multiple sclerosis and FMF, clinical scores of the treated mice were critically decreased, suggesting that the diseases were alleviated with reduced neuropathology. Interestingly, DMF succinates GsdmE in GsdmD knockout BMDMs, where GsdmE-dependent cell death was blocked due to its succination121.
While many of these studies have shown promise in vivo treating inflammatory diseases of mice, it is important to note that little data has been reported where clinical research has been done in human inflammatory diseases. The listed drug candidates above show promising ex vivo results. However, this can differ greatly in vivo.
6. GsdmD and membrane repair
Recent evidence has been brought to light that not all cells with an activated GsdmD protein undergo pyroptosis, suggesting an alternate repair mechanism cells have in place. Utilizing mBMDMs and HeLa cells, it has been shown that calcium influx through GsdmD pores served as a signal for membrane repair downstream of GsdmD cleavage122. The influx of calcium recruits the endosomal sorting complexes required for transport (ESCRT) to the GsdmD pores, where the GsdmD-damaged plasma membrane is repaired. ESCRT uses annexin V+ vesicles to remove the damaged membrane that contains embedded GsdmD pores. A competitive dominant-negative ESCRT mutant revealed an increase in both pyroptosis and IL-1β secretion upon exposure to LPS as compared to the control, further supporting the repairing role of ESCRT122,123. Hence, the ESCRT machinery repairs the GsdmD-damaged membrane to counteract pyroptotic cell death and cytokine release.
7. GsdmD in other pathways
Proteins of the canonical and noncanonical pyroptotic pathways have been suggested to be the drug targets for inflammatory diseases such as sepsis3,124. While GsdmD is commonly known to be activated via the caspase 1 dependent canonical pathway or via caspase 4/5/11-dependent noncanonical pathway, recent studies have suggested alternate proteases to cleave GsdmD. The alternative proteases for GsdmD have shed light on alternative drug targets for pyroptosis and inflammatory responses.
As previously mentioned, GsdmD is cleaved at D275 in humans (D276 in mice) by caspase 1 in the canonical pathway and caspases 4/5/11 in the noncanonical pathway. Recent investigations showed that an apoptotic caspase, caspase 8, also could cleave GsdmD at the same site, providing an alternative method of activation. This further alludes to caspase 8's plasticity within immune functions, including the ability to both induce apoptosis and inhibit necroptosis, programmed necrosis125. Caspase 8-dependent cleavage of GsdmD was first discovered during Yersinia pseudotuberculosis infections, where mitogen-activated protein kinase kinase kinase 7 (MAP3K7/TAK1) and IκB kinase complex (IKK) are inhibited by the Y. pseudotuberculosis outer protein J (YopJ). This prevents downstream caspase 1 cleavage, accompanied by an increase of interleukin 1 (IL-1) secretion. In this process, GsdmD cleavage is driven by a RIP1-caspase 8 signaling cascade126. Consistently, in vitro experiments utilizing macrophages with TAK1 inhibition successfully induced the cleavage of GsdmD by caspase 8 and pyroptosis, independent of caspase 1/11127. This alternate pathway has further been tested utilizing a study of IBD in a mice model during which suppression of FAS-associated death domain protein (FADD) elicited an enhanced inflammatory response within intestinal epithelial. The exaggerated inflammation results from caspase 8-mediated GsdmD cleavage and pore formation, not from ASC-caspase1-dependent cleavage128. It also suggests that FADD is a key player in suppressing pyroptosis in favor of non-inflammatory apoptosis within intestinal epithelial tissues. Consistently, recent evidence also suggests that caspase 8 is able to directly activate IL-1β in response to the activation by TLR, death receptor and dectin-1 pathways129.
While GsdmD cleavage is well established in macrophages and monocytes, recent studies suggest that neutrophil specific serine proteases (NSPs) are also capable of cleaving and activating GsdmD within neutrophils independent of caspases. Neutrophil expressed elastase (ELANE), a serine protease in cytosolic granules, has recently been found to cleave GsdmD and produce a functional N-terminal fragment capable of pore formation on plasma membranes. The absence of ELANE or GsdmD increases neutrophil's lifespan, suggesting ELANE and GsdmD activation assist in neutrophil death130. The cleavage by ELANE occurs at C268, seven residues upstream of the caspase 1 cleavage site of D275 of hGsdmD. Another NSP that cleaves GsdmD is CTSG, which cleaves at L274 of mGsdmD, two amino acids upstream from D276 of mGsdmD. The cleaved GsdmD triggers pyroptotic death and releases inflammatory cytokines, including IL-1β, from neutrophils. GsdmD cleavage by CTSG is negatively regulated by Serpinb1 and Serpinb6, suggesting a strict regulation in neutrophil death pathways and cytokine release131,132. GsdmD-induced pyroptosis in neutrophils has also been correlated with the release of neutrophil extracellular traps (NETs) where caspase 11 and GsdmD rupture the neutrophil plasma membrane to extrude NETs as the last step to execute NET associated cell death (NETosis)133.
While cleavage and activation of GsdmD via both caspase-dependent and caspase-independent mechanisms have been noted, cellular inhibitory pathways of GsdmD inflammasome activity have also been studied. Two key players within apoptotic pathways, caspases 3 and 7, have recently shown negative regulation of GsdmD in human macrophages134. These two caspases have been suggested to cleave GsdmD via an alternate pathway resulting in a truncated N-terminal domain dictating a loss of function. The cleaving by caspases 3 and 7 occurs at D87 of hGsdmD, as opposed to D275 of caspase 1/4/5 site. This loss of function within GsdmD has been proposed to execute anti-inflammatory processes by blocking pyroptosis and promoting apoptosis134. Consistent with this, a recent study shows that caspase 8-dependent GsdmD-induced cell death is limited by caspase 3 cleavage of GsdmD in mBMDM, while mutation of D88 in mGsdmD leads to an increase in pyroptosis135.
In addition to pyroptosis and cytokine release, GsdmD also restricts Type 1 interferon (IFN-1) responses to DNA in the cytosol, which is to limit the production of damaging interferon. Like AIM2, cyclic GMP-AMP synthase (cGAS) is a dsDNA sensor in innate immune cells and promotes cGAS–STING (stimulator of interferon genes) for IFN-1 production136. Recent research suggests possible crosstalks between AIM2 and cGAS pathways in that the activation of GsdmD leads to a potassium efflux through GsdmD-N pore, which inhibits cGAS sensing and IFN-1 production7. In endothelial cells, GsdmD can activate cGAS by releasing mtDNA upon LPS treatment137. Intriguingly, STING, when triggered by bacterial infection-induced DNA damage, can also activate GsdmD. In this process, STING is first phosphorylated and then interacts with inositol 1,4,5-trisphosphate receptor type 1 (ITPR1). The STING–ITPR1 complex enables calcium influx from the stressed endoplasmic reticulum (ER). This influx of calcium ions further activates caspase 1/11 for GsdmD cleavage, which releases coagulation factor 3 (F3) to trigger blood coagulation and sepsis138. These findings suggest the diversity of roles of GsdmD in both immune and non-immune cells.
8. GsdmD and diseases
Studies using non-small cell lung cancer (NSCLC) cells show that knockdown of GsdmD increases caspase 3-induced apoptosis of tumors without apoptotic stimuli, while mouse models show a decrease in tumor growth upon inhibition of GsdmD139. When the expression levels of GSDMD mRNA were analyzed from patients with lung cancers, a high level of GSDMD was shown to be associated with NSCLC. When NSCLC was subcategorized as lung adenocarcinoma and lung squamous cell carcinoma, GsdmD expression is correlated with the former for larger tumors and poor prognosis139. It has also been shown that acid reflux can cause pyroptotic cell death, which subsequently causes esophageal cancers. Similarly, overexpression of GsdmD has also been associated with cancer development in the bladder140. Paradoxically, pyroptosis is suppressed in many cancer types, with studies showing that pyroptosis has hindering effects on cancer proliferation and metastasis141, such as in ovarian cancer142, colorectal cancer143, and gastric cancers144. GsdmD is either down-regulated or inhibited within these cancers, preventing the death of the cancerous cells.
Alzheimer's and Parkinson's diseases are neurodegenerative diseases in aging populations. Chronic neuronal inflammation plays a major role in the neural degeneration seen in Parkinson's and Alzheimer's diseases8,145. A linkage of GsdmD to both Alzheimer's and Parkinson's diseases have been suggested. After treatment with VX-765, a caspase 1 inhibitor, mice with Alzheimer's disease display more activity and a higher memory score than without treatment8. On a cellular level, neuronal death is protected by VX-765. This further suggests a connection to the inflammatory death of neurons via GsdmD mediated pyroptosis8.
Vascular disease has also been shown to be related to inflammasome activation and pyroptosis pathways. Atherosclerosis is a chronic inflammatory disease that is related to endothelial dysfunction in vascular walls. This disease is characterized by the buildup of fat- and calcium-rich plaques, where cell death is triggered, and inflammatory cells are recruited. Although the full mechanism of plaque formation is not elucidated, it was found to directly activate the AIM2 pyroptotic pathway146 resulting in GSDMD activation and pyroptosis of vascular smooth muscle cells146. When AIM2 was overexpressed, ApoE–/– mice fed a high-fat diet had larger plaque lesions where pyroptosis was prominent146. Another study showed that aspirin, known for decades to have anti-inflammatory effects, inhibits NLRP3 activation in mice vascular endothelial cells. The results warrant further research for the protective effects of aspirin for cardiovascular diseases associated with NLRP3 inflammasomes147.
In 2010–2012, arthritis was found in an estimated 23% (more than 52 million) of adults in the U.S., with the most common treatment medications being non-steroidal anti-inflammatory drugs (NSAIDs)148. Gouty arthritis (gout) is a well-studied disease with respect to its relationship to GsdmD. Gout is caused by uric acid crystal formation in joints during purine degradation. This disease is exacerbated by activation of the NLRP3/GsdmD pathway in neutrophils, causing the release of activated ILs149. The prevention of IL activation in the NLRP3 pathway alleviates gout symptoms149. Calcium pyrophosphate deposition disease, commonly referred to as pseudogout, participates in pyroptosis the same way as gout, with the main difference being calcium pyrophosphate deposit instead of uric acid deposit95.
FMF, also known as autoimmune encephalomyelitis, is the most common hereditary autoinflammatory disorder. FMF is caused by mutations on the MEFV gene, encoding pyrin. The symptoms of FMF are fever, serositis, and ventral pain. Cells that have mutations within MEFV have uncontrolled pyroptotic death, which is triggered by the activation of the pyrin inflammasome. Consistently, the viability of GsdmD–/– cells, which contain MEFV mutations, are significantly higher than cells expressing GsdmD, and the viability of GsdmD–/–/caspase 1−/− cells is even greater150. Anti-inflammatory drugs are currently used to treat this chronic disease.
Diabetic kidney disease (DKD), also called diabetic nephropathy, is caused by prolonged uncontrolled diabetes. DKD is the major cause of end-stage renal disease. Pyroptotic cell death of renal tubular cells has recently been suggested to cause the progression of DKD. Upregulation of TLR4 and GsdmD is found in DKD patients and DKD animal models, which is subsequently associated with TLR4/NF-κB signaling pathway151.
Steatohepatitis is a type of nonalcoholic fatty liver disease. A pathologic hallmark is chronic inflammation of the liver and buildup of fat within liver cells. GsdmD has recently been revealed to play a critical role in steatohepatitis in that GsdmD–/– mice with nonalcoholic fatty liver disease have less hepatic fibrosis and cytokine production. When GsdmD is over-expressed, the mice have an increase of GsdmD-N, pyroptosis, and a decrease in overall activity score152. In addition to its role in inflammation, GsdmD also regulates lipogenesis and the NF-κB signaling pathway in steatohepatitis. Currently, there is no drug approved to treat steatohepatitis, and GsdmD is a potential drug target.
While GsdmD has been found to be involved in provoking liver diseases such as steatohepatitis, it has also been shown to improve hepatocyte viability in noninfectious liver injuries such as acetaminophen-induced liver damage. A recent study utilizing GsdmD–/– mice shows that GsdmD–/– mice have a significantly higher level of liver damage when exposed to toxic levels of acetaminophen153. This is correlated with an increase in caspase 8-induced apoptosis or/and necroptosis in GsdmD–/– mice, favoring apoptosis over pyroptosis153. This research further suggests that GsdmD may play a role in diseases by regulating pyroptosis, apoptosis and necroptosis.
IBD, including ulcerative colitis (UC) and Crohn's disease (CD), are driven by chronic inflammation in the intestines42,94. IBD are common chronic diseases that share symptoms of nausea, diarrhea, constipation, and severe abdominal pain. GsdmD-mediated pyroptotic cell death occurs if these diseases are left untreated, causing prolonged damage to the bowel. It has been shown that GsdmD is over-expressed in patients with IBD, triggering the death of the epithelial lining in the bowel94. Treatment with an anti-pyroptotic compound, ABX464, has reversed bowel damage of the epithelial cells in mice with CD and has been shown to be safe for patients with UC154.
HIV is the primary cause of acquired immunodeficiency syndrome (AIDS). The disease can be treated with a wide range of drug cocktails, limiting the progression of AIDS. Current medications allow for an increase in CD4+ T-cells to the point of AIDS remission. CD4+ T-cell death in HIV is caused by the NLRP3 pathway by the formation of GsdmD-N pores on the membrane9.
Both IL-1β and IL-18 play roles in antiviral infections using a caspase 1 dependent pathway; however, the roles of GsdmD in viral infections remain largely elusive. Recent work with enterovirus 71 (EV71), the virus known for causing hand-foot-and-mouth disease in children, has shown that EV71 disables GsdmD using its viral protease 3C in host cells. Viral protease 3C, known for inducing apoptosis, can cleave GsdmD at Q193, 82 residues upper to D275, the caspase 1 cleavage site. Cleavage by 3C resulted in a stunted N-terminal fragment inducing a loss of function, preventing the induction of pyroptosis within infected cells155. The result suggests that GsdmD may play a role in regulating viral infection, possibly by favoring pyroptosis over apoptosis within virally infected cells. The study shines a light on the antiviral roles of GsdmD as well as viral abilities to evade immune responses.
Sepsis occurs when a patient's body responds overwhelmingly to infection. Common clinical symptoms include high lactic acid levels in the blood, fever, hypotension, and, if left untreated, expiration. Uncontrolled pyroptosis leads to sepsis, where GsdmD-N pores release a large number of cytokines (IL-18/IL-1β), causing an overwhelming inflammatory response system-wide. Mice treated with LPS go into septic shock, which is an established sepsis model156. LPS-induced caspase 11 inflammasome has been well-established regarding involvement with sepsis. Mice studies showed that inhibition of either GsdmD or caspase 11 had increased viability when exposed to the Gram-negative endotoxins157. Upon co-treatment of LPS and a GsdmD inhibitor, disulfiram, the number of mice that expired due to sepsis is significantly lowered120. As such, proteins of the noncanonical pathway are critical targets for inhibitor screening treating sepsis.
One study has also found positive results utilizing adrenaline, a fight-or-flight hormone, to treat sepsis. Adrenaline reduces IL-1β release in human monocytic cell line THP1 and significantly decreases the septic death of LPS-treated mice158. Adrenaline plays its role by binding to its α-2B adrenergic receptor (ADRA2B), and inducing the production of cyclic adenosine monophosphate (cAMP). Subsequently, cAMP activates protein kinase A (PKA), which then phosphorylates caspase 11 to inhibit its activity for the cleavage of GsdmD158. A flavonoid, scutellarin, has also been suggested as a possible treatment for sepsis also believed to inhibit pyroptosis by affecting the cAMP/PKA signaling pathway159.
Lipid peroxidation, which activates caspase 11 for GsdmD cleavage, can induce sepsis via GsdmD-mediated pyroptosis in infections. Glutathione peroxidase 4 (GPX4), an antioxidant enzyme, protects mice from septic death by degrading hydrogen peroxide to antagonize lipid peroxidation160. Among the eight glutathione peroxidases (GPX1–8), GPX4 is the only one upregulated in peritoneal macrophages and peripheral blood mononuclear cells from mice with polymicrobial infections. Consistently, vitamin E administration showed a similar protective effect from GsdmD activation by lipid peroxidation160.
9. Conclusions
Pyroptosis induces strong inflammation to defend against intracellular pathogens, and its proper function leads to pathogen resolution. However, excessive pyroptotic activities will lead to numerous inflammatory diseases, including autoimmune disorders. Hence, pyroptotic proteins are targets for drug development for inflammatory diseases and sepsis. In addition, GsdmD is upregulated in many cancer types, suggesting that inhibitors for GsdmD may be used for cancer treatment. As vital players in pyroptotic pathways, NLRP3, GsdmD, and caspase 1/4/5/11 are actively pursued drug targets. Because the inflammatory caspases are cysteine dependent proteases, suicide inhibitors, containing a tri-/tetra-peptide and a covalent interaction warhead, are the frequently designed inhibitors for caspase 1/4/5/11. The tri-/tetra-peptides, usually selected by peptide library screening, are preferred sequences at P3–P1/P4–P1 of the cleavage site. Recently discovered GsdmD inhibitors covalently attach to C191 and inhibit its function of leaking cell contents; however, they show a lack of specificity by attaching caspase 1/11120 or GsdmE121. Future drug development should aim for specific inhibitors of caspase 4/5/11 or GsdmD. Caspase 1 activates GsdmD and inflammatory cytokines IL-18 and IL-1β for pyroptosis. However, caspase 1 inhibitors do not significantly inhibit pyroptosis in mice. Hence, inhibitors upstream of caspase 1 are considered more potent than inhibitors directly interacting with caspase 1 or GsdmD. As such, inhibitors antagonizing NLRP3 assembly have been developed and cause the failure of ASC oligomerization and subsequent caspase 1 activation.
Caspases 1 and 11 seem to use the same chemical mechanism to cleave GsdmD, which requires the binding to GsdmD-C first. However, it is puzzling why caspase 1, not caspase 11, could cleave pro-IL-1β/IL-18. It is unclear how many overlapping roles gasdermins play in inflammation and cancer, although they all participate in pyroptosis in a different context. The expression of gasdermins is differential in tissues. However, it still requires further understanding of the tissue-specific function of each gasdermin, which plays a critical role in related inflammatory diseases and cancers. Enzymes, especially proteases, are critical players in pyroptosis. Inhibitor design should include further characterizations of the kinetic mechanisms of caspase 1/11, aiming to design differential inhibitors as future drugs.
In conclusion, pyroptosis, as a form of programmed cell death, has implications in many diseases. As a topic of a robust field, more specific roles of pyroptotic proteins in inflammation and related diseases are to be elucidated. This will provide further insight into the mechanisms and treatment of inflammatory conditions and cancers.
Acknowledgments
The authors would like to thank Amie Brint and Sahana Bettadapura of UA Little Rock for critical reading and editing. This publication was made possible by the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences (P20 GM103429, USA), and grants from National Heart, Lung and Blood Institute (HL153876, USA) and National Eye Institute (EY030621, USA).
Author contributions
All authors participated in writing the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Hua Zhu, Email: hua.zhu@osumc.edu.
Shanzhi Wang, Email: sxwang2@ualr.edu.
References
- 1.de Vasconcelos N.M., van Opdenbosch N., van Gorp H., Parthoens E., Lamkanfi M. Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 2019;26:146–161. doi: 10.1038/s41418-018-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aglietti R.A., Estevez A., Gupta A., Ramirez M.G., Liu P.S., Kayagaki N. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Lu W., Zhang Y., Zhang G., Shi X., Hisada Y. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50:1401–1411. doi: 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evavold C.L., Ruan J., Tan Y., Xia S., Wu H., Kagan J.C. The pore-forming protein Gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity. 2018;48:35–44. doi: 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilig R., Dick M.S., Sborgi L., Meunier E., Hiller S., Broz P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol. 2018;48:584–592. doi: 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 6.Xiao J., Wang C., Yao J.C., Alippe Y., Xu C., Kress D. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee I., Behl B., Mendonca M., Shrivastava G., Russo A.J., Menoret A. Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity. 2018;49:413–426. doi: 10.1016/j.immuni.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores J., Noël A., Foveau B., Lynham J., Lecrux C., LeBlanc A.C. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer's disease mouse model. Nat Commun. 2018;9:3916. doi: 10.1038/s41467-018-06449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doitsh G., Galloway N.L.K., Geng X., Yang Z., Monroe K.M., Zepeda O. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szekanecz Z., Szamosi S., Kovács G.E., Kocsis E., Benkő S. The NLRP3 inflammasome—interleukin 1 pathway as a therapeutic target in gout. Arch Biochem Biophys. 2019;670:82–93. doi: 10.1016/j.abb.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Conos S.A., Lawlor K.E., Vaux D.L., Vince J.E., Lindqvist L.M. Cell death is not essential for caspase-1-mediated interleukin-1β activation and secretion. Cell Death Differ. 2016;23:1827–1838. doi: 10.1038/cdd.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broz P., Dixit V.M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 13.Rathinam V.A.K., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nat Immunol. 2019;20:527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng S., Fox D., Man S.M. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430:3068–3080. doi: 10.1016/j.jmb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink S.L., Cookson B.T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen I., Miao E. Pyroptotic cell death defends against intracellular pathogens Ine. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao E., Leaf I., Treuting P., Mao D., Dors M., Sarkar A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2011;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saeki N., Usui T., Aoyagi K., Kim D.H., Sato M., Mabuchi T. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Gene Chromosome Cancer. 2009;48:261–271. doi: 10.1002/gcc.20636. [DOI] [PubMed] [Google Scholar]
- 20.Ruge F., Glavini A., Gallimore A.M., Richards H.E., Thomas C.P., O'Donnell V.B. Delineating immune-mediated mechanisms underlying hair follicle destruction in the mouse mutant defolliculated. J Invest Dermatol. 2011;131:572–579. doi: 10.1038/jid.2010.379. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y., Jiang X., Gu P., Chen W., Zeng X., Gao X. Gsdma3 mutation causes bulge stem cell depletion and alopecia mediated by skin inflammation. Am J Pathol. 2012;180:763–774. doi: 10.1016/j.ajpath.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 22.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 23.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 24.Ruan J., Xia S., Liu X., Lieberman J., Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557:62–67. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao K.L., Kulakova L., Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci U S A. 2017;114:E1128–E1137. doi: 10.1073/pnas.1616783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q., Shi P., Wang Y., Zou D., Wu X., Wang D. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11:496–508. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komiyama H., Aoki A., Tanaka S., Maekawa H., Kato Y., Wada R. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of Gasdermin B (GSDMB) Genes Genet Syst. 2010;85:75–83. doi: 10.1266/ggs.85.75. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z., He H., Wang K., Shi X., Wang Y., Su Y. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368 doi: 10.1126/science.aaz7548. eaaz7548. [DOI] [PubMed] [Google Scholar]
- 29.Hergueta-Redondo M., Sarrio D., Molina-Crespo Á., Vicario R., Bernadó-Morales C., Martínez L. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget. 2016;7:56295–56308. doi: 10.18632/oncotarget.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q., Yang J., Xing G., Sun Q., Zhang L., He F. Expression of GSDML associates with tumor progression in uterine cervix cancer. Transl Oncol. 2008;1:73–83. doi: 10.1593/tlo.08112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Laer L., Huizing E.H., Verstreken M., Van Zuijlen D., Wauters J.G., Bossuyt P.J. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20:194–197. doi: 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- 32.Rogers C., Fernandes-Alnemri T., Mayes L., Alnemri D., Cingolani G., Alnemri E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Gao W., Shi X., Ding J., Liu W., He H. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Zhang Y., Xia S., Kong Q., Li S., Liu X. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–420. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Zhang P., An L., Sun N., Peng L., Tang W. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharm Sin B. 2020;10:1397–1413. doi: 10.1016/j.apsb.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delmaghani S., Del Castillo F.J., Michel V., Leibovici M., Aghaie A., Ron U. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat Genet. 2006;38:770–778. doi: 10.1038/ng1829. [DOI] [PubMed] [Google Scholar]
- 37.Schwander M., Sczaniecka A., Grillet N., Bailey J.S., Avenarius M., Najmabadi H. A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci. 2007;27:2163–2175. doi: 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defourny J., Aghaie A., Perfettini I., Avan P., Delmaghani S., Petit C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc Natl Acad Sci U S A. 2019;116:8010–8017. doi: 10.1073/pnas.1821844116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miguchi M., Hinoi T., Shimomura M., Adachi T., Saito Y., Niitsu H. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0166422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayagaki N., Stowe I.B., Lee B.L., O'Rourke K., Anderson K., Warming S. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 42.Thornberry N. Interleukin-1β converting enzyme. Methods Enzymol. 1994;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- 43.Liston A., Masters S.L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat Rev Immunol. 2017;17:208–214. doi: 10.1038/nri.2016.151. [DOI] [PubMed] [Google Scholar]
- 44.Park Y.H., Wood G., Kastner D.L., Chae J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao W., Yang J., Liu W., Wang Y., Shao F. Site-specific phosphorylation and microtubule dynamics control Pyrin inflammasome activation. Proc Natl Acad Sci U S A. 2016;113:E4857–E4866. doi: 10.1073/pnas.1601700113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X., Zhang Z., Ruan J., Pan Y., Magupalli V.G., Wu H. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanoni I., Tan Y., Di Gioia M., Broggi A., Ruan J., Shi J. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sborgi L., Rühl S., Mulvihill E., Pipercevic J., Heilig R., Stahlberg H. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Man S.M., Karki R., Kanneganti T.D. AIM2 inflammasome in infection, cancer, and autoimmunity: role in DNA sensing, inflammation, and innate immunity. Eur J Immunol. 2016;46:269–280. doi: 10.1002/eji.201545839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chavarría-Smith J., Vance R.E. The NLRP1 inflammasomes. Immunol Rev. 2015;265:22–34. doi: 10.1111/imr.12283. [DOI] [PubMed] [Google Scholar]
- 51.Shaw M., Reimer T., Kim Y.G., Nuñez G. NOD-like receptors (NLRs): Bona fide intracellular microbial sensors. Curr Opin Immunol. 2008;20:377–382. doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franchi L., Warner N., Viani K., Nuñez G. Function of NOD-like receptors in microbial recognition and host defense. Immunol Rev. 2010;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 54.van Opdenbosch N., Gurung P., Vande Walle L., Fossoul A., Kanneganti T.D., Lamkanfi M. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat Commun. 2014;5:3209. doi: 10.1038/ncomms4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levinsohn J.L., Newman Z.L., Hellmich K.A., Fattah R., Getz M.A., Liu S. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavarría-Smith J., Mitchell P.S., Ho A.M., Daugherty M.D., Vance R.E. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faustin B., Lartigue L., Bruey J.M., Luciano F., Sergienko E., Bailly-Maitre B. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Johnson D.C., Okondo M.C., Orth E.L., Rao S.D., Huang H.C., Ball D.P. DPP8/9 inhibitors activate the CARD8 inflammasome in resting lymphocytes. Cell Death Dis. 2020;11:628. doi: 10.1038/s41419-020-02865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy A.J., Kraakman M.J., Kammoun H.L., Dragoljevic D., Lee M.K.S., Lawlor K.E. IL-18 production from the NLRP1 inflammasome prevents obesity and metabolic syndrome. Cell Metabol. 2016;23:155–164. doi: 10.1016/j.cmet.2015.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alu A., Han X., Ma X., Wu M., Wei Y., Wei X. The role of lysosome in regulated necrosis. Acta Pharm Sin B. 2020;10:1880–1903. doi: 10.1016/j.apsb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J., Chen Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Y., Zeng M., Yang D., Motro B., Núñez G. Nek7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi H., Wang Y., Li X., Zhan X., Tang M., Fina M. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat Immunol. 2016;17:250–258. doi: 10.1038/ni.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharif H., Wang L., Wang W.L., Magupalli V.G., Andreeva L., Qiao Q. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rathinam V.A.K., Vanaja S.K., Waggoner L., Sokolovska A., Becker C., Stuart L.M. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Venegas C., Heneka M.T. Inflammasome-mediated innate immunity in Alzheimer's disease. FASEB J. 2019;33:13075–13084. doi: 10.1096/fj.201900439. [DOI] [PubMed] [Google Scholar]
- 68.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z., Xu G., Gao Y., Zhan X., Qin N., Fu S. Cardamonin from a medicinal herb protects against LPS-induced septic shock by suppressing NLRP3 inflammasome. Acta Pharm Sin B. 2019;9:734–744. doi: 10.1016/j.apsb.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen C., Lu A., Xie W.J., Ruan J., Negro R., Egelman E.H. Molecular mechanism for NLRP6 inflammasome assembly and activation. Proc Natl Acad Sci U S A. 2019;116:2052–2057. doi: 10.1073/pnas.1817221116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghimire L., Paudel S., Jin L., Jeyaseelan S. The NLRP6 inflammasome in health and disease. Mucosal Immunol. 2020;13:388–398. doi: 10.1038/s41385-020-0256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li R., Zhu S. NLRP6 inflammasome. Mol Aspect Med. 2020;76:100859. doi: 10.1016/j.mam.2020.100859. [DOI] [PubMed] [Google Scholar]
- 73.Chen G.Y. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur J Immunol. 2014;44:321–327. doi: 10.1002/eji.201344135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuladhar S., Kanneganti T.D. NLRP12 in innate immunity and inflammation. Mol Aspect Med. 2020;76:100887. doi: 10.1016/j.mam.2020.100887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnappauf O., Chae J.J., Kastner D.L., Aksentijevich I. The pyrin inflammasome in health and disease. Front Immunol. 2019;10:1745. doi: 10.3389/fimmu.2019.01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heilig R., Broz P. Function and mechanism of the pyrin inflammasome. Eur J Immunol. 2018;48:230–238. doi: 10.1002/eji.201746947. [DOI] [PubMed] [Google Scholar]
- 77.Xue Y., Enosi Tuipulotu D., Tan W.H., Kay C., Man S.M. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40:1035–1052. doi: 10.1016/j.it.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandes-Alnemri T., Yu J.W., Datta P., Wu J., Alnemri E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts T., Idris A., Dunn J., Kelly G., Burnton C., Hodgson S. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 81.Duncan J., Canna S. The NLRC4 inflammasome. Immunol Rev. 2018;281:115–123. doi: 10.1111/imr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romberg N., Vogel T.P., Canna S.W. NLRC4 inflammasomopathies. Curr Opin Allergy Clin Immunol. 2017;17:398–404. doi: 10.1097/ACI.0000000000000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Broz P., Ruby T., Belhocine K., Bouley D.M., Kayagaki N., Dixit V.M. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ross C., Chan A.H., von Pein J., Boucher D., Schroder K. Dimerization and auto-processing induce caspase-11 protease activation within the non-canonical inflammasome. Life Sci Alliance. 2018;1:1–10. doi: 10.26508/lsa.201800237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Winkler S., Rösen-Wolff A. Caspase-1: an integral regulator of innate immunity. Semin Immunopathol. 2015;37:419–427. doi: 10.1007/s00281-015-0494-4. [DOI] [PubMed] [Google Scholar]
- 86.Wandel M.P., Kim B.H., Park E.S., Boyle K.B., Nayak K., Lagrange B. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat Immunol. 2020;21:880–891. doi: 10.1038/s41590-020-0697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rühl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur J Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 88.Aachoui Y., Sagulenko V., Miao E.A., Stacey K.J. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Z., Wang C., Rathway J., Yang J., Zhou B., Yang R. Crystal structures of the full-length murine and human Gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity. 2019;51:43–49. doi: 10.1016/j.immuni.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y., Wang H., Shen J., Deng R., Yao X., Guo Q. Gasdermin D drives the nonexosomal secretion of galectin-3, an insulin signal antagonist. J Immunol. 2019;203:2712–2723. doi: 10.4049/jimmunol.1900212. [DOI] [PubMed] [Google Scholar]
- 91.Anton L., Sborgi L., Hiller S., Broz P., Maier T. Insights into Gasdermin D activation from the crystal structure of its C-terminal domain. bioRxiv. 2017 doi: 10.1101/187211. Available from: [DOI] [Google Scholar]
- 92.Kuang S., Zheng J., Yang H., Li S., Duan S., Shen Y. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci U S A. 2017;114:10642–10647. doi: 10.1073/pnas.1708194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsu L.C., Ali S.R., McGillivray S., Tseng P.H., Mariathasan S., Humke E.W. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuan Y.Y., Xie K.X., Wang S.L., Yuan L.W. Inflammatory caspase-related pyroptosis: mechanism, regulation and therapeutic potential for inflammatory bowel disease. Gastroenterol Rep. 2018;6:167–176. doi: 10.1093/gastro/goy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alippe Y., Mbalaviele G. Omnipresence of inflammasome activities in inflammatory bone diseases. Semin Immunopathol. 2019;41:607–618. doi: 10.1007/s00281-019-00753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wen H., Ting J.P.Y., O'Neill L.A.J. A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation?. Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang K., Sun Q., Zhong X., Zeng M., Zeng H., Shi X. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang X., Cheng X., Tang Y., Qiu X., Wang Y., Kang H. Bacterial endotoxin activates the coagulation cascade through Gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51:983–996. doi: 10.1016/j.immuni.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Slee E.A., Zhu H., Chow S.C., MacFarlane M., Nicholson D.W., Cohen G.M. Benzyloxycarbonyl-Val-Ala-Asp (OMe) fluoromethylketone (Z-VAD.FMK) inhibits apoptosis by blocking the processing of CPP32. Biochem J. 1996;315:21–24. doi: 10.1042/bj3150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ekert P.G., Silke J., Vaux D.L. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 102.Yang J., Liu Z., Wang C., Yang R., Rathkey J.K., Pinkard O.W. Mechanism of gasdermin D recognition by inflammatory caspases and their inhibition by a gasdermin D-derived peptide inhibitor. Proc Natl Acad Sci U S A. 2018;115:6792–6797. doi: 10.1073/pnas.1800562115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lipinski C., Lombardo F., Dominy B., Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 104.Chen X., Liu G., Yuan Y., Wu G., Wang S., Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019;10:1–12. doi: 10.1038/s41419-019-2157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saloura V., Cho H.S., Kiyotani K., Alachkar H., Zuo Z., Nakakido M. WHSC1 promotes oncogenesis through regulation of NIMA-related kinase-7 in squamous cell carcinoma of the head and neck. Mol Cancer Res. 2015;13:293–304. doi: 10.1158/1541-7786.MCR-14-0292-T. [DOI] [PubMed] [Google Scholar]
- 106.Liu N., Su H., Zhang Y., Liu Z., Kong J. Cholecalciterol cholesterol emulsion attenuates experimental autoimmune myocarditis in mice via inhibition of the pyroptosis signaling pathway. Biochem Biophys Res Commun. 2017;493:422–428. doi: 10.1016/j.bbrc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 107.Danelishvili L., Everman J.L., McNamara M.J., Bermudez L.E. Inhibition of the plasma-membrane-associated serine protease cathepsin G by Mycobacterium tuberculosis Rv3364c suppresses caspase-1 and pyroptosis in macrophages. Front Microbiol. 2012;2:281. doi: 10.3389/fmicb.2011.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dolunay A., Senol S.P., Temiz-Resitoglu M., Guden D.S., Sari A.N., Sahan-Firat S. Inhibition of NLRP3 inflammasome prevents LPS-induced inflammatory hyperalgesia in mice: contribution of NF-κB, caspase-1/11, ASC, NOX, and NOS isoforms. Inflammation. 2017;40:366–386. doi: 10.1007/s10753-016-0483-3. [DOI] [PubMed] [Google Scholar]
- 109.Coll R.C., Hill J.R., Day C.J., Zamoshnikova A., Boucher D., Massey N.L. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15:556–559. doi: 10.1038/s41589-019-0277-7. [DOI] [PubMed] [Google Scholar]
- 110.Salla M., Butler M.S., Pelingon R., Kaeslin G., Croker D.E., Reid J.C. Identification, synthesis, and biological evaluation of the major human metabolite of NLRP3 inflammasome inhibitor MCC950. ACS Med Chem Lett. 2016;7:1034–1038. doi: 10.1021/acsmedchemlett.6b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coll R.C., Robertson A.A.B., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–257. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J., Zhao J., Xu M., Li M., Wang B., Qu X. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia–reperfusion injury. Cell Death Dis. 2020;11:244. doi: 10.1038/s41419-020-2437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Boxer M., Quinn A., Shen M., Jadhav A., Leister W., Simeonov A. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5:730–739. doi: 10.1002/cmdc.200900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wannamaker W., Davies R., Namchuk M., Pollard J., Ford P., Ku G. (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther. 2007;321:509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 115.Hett E., Slater L., Mark K., Kawate T., Monks B.G., Stutz A. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat Chem Biol. 2013;9:398–405. doi: 10.1038/nchembio.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Irving A.T., Wang D., Vasilevski O., Latchoumanin O., Kozer N., Clayton A.H.A. Regulation of actin dynamics by protein kinase R control of gelsolin enforces basal innate immune defense. Immunity. 2012;36:795–806. doi: 10.1016/j.immuni.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 117.Wright C., Moore R. Disulfiram treatment of alcoholism. Am J Med. 1990;88:647–655. doi: 10.1016/0002-9343(90)90534-k. [DOI] [PubMed] [Google Scholar]
- 118.Skrott Z., Mistrik M., Andersen K.K., Friis S., Majera D., Gursky J. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature. 2017;552:194–199. doi: 10.1038/nature25016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu J., Liu X., Zhao J., Xia S., Ruan J., Luo X. Identification of pyroptosis inhibitors that target a reactive cysteine in gasdermin D. bioRxiv. 2018 doi: 10.1101/365908. Available from: [DOI] [Google Scholar]
- 120.Hu J.J., Liu X., Xia S., Zhang Z., Zhang Y., Zhao J. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Humphries F., Shmuel-Galia L., Ketelut-Carneiro N., Li S., Wang B., Nemmara V.V. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rühl S., Shkarina K., Demarco B., Heilig R., Santos J.C., Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–960. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 123.Fomby P., Cherlin A. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014;24:734–742. doi: 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mariathasan S., Hewton K., Monack D.M., Vucic D., French D.M., Lee W.P. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 125.Newton K., Wickliffe K.E., Maltzman A., Dugger D.L., Reja R., Zhang Y. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–682. doi: 10.1038/s41586-019-1752-8. [DOI] [PubMed] [Google Scholar]
- 126.Sarhan J., Liu B.C., Muendlein H.I., Li P., Nilson R., Tang A.Y. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888–E10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Orning P., Weng D., Starheim K., Ratner D., Best Z., Lee B. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwarzer R., Jiao H., Wachsmuth L., Tresch A., Pasparakis M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity. 2020;52:978–993. doi: 10.1016/j.immuni.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 129.Vince J.E., Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73:2349–2367. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kambara H., Liu F., Zhang X., Liu P., Bajrami B., Teng Y. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018;22:2924–2936. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burgener S.S., Leborgne N.G.F., Snipas S.J., Salvesen G.S., Bird P.I., Benarafa C. Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep. 2019;27:3646–3656. doi: 10.1016/j.celrep.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi Y.J., Kim S., Choi Y., Nielsen T.B., Yan J., Ruan J. SERPINB1-mediated checkpoint of inflammatory caspase activation. Nat Immunol. 2019;20:276–287. doi: 10.1038/s41590-018-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen K.W., Monteleone M., Boucher D., Sollberger G., Ramnath D., Condon N.D. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aar6676. eaar6676. [DOI] [PubMed] [Google Scholar]
- 134.Taabazuing C.Y., Okondo M.C., Bachovchin D.A. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen K.W., Demarco B., Heilig R., Shkarina K., Boettcher A., Farady C.J. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP 3 inflammasome assembly. EMBO J. 2019;38 doi: 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Feltham R., Vince J.E. Ion man: GSDMD punches pores to knock out cGAS. Immunity. 2018;49:379–381. doi: 10.1016/j.immuni.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 137.Huang L.S., Hong Z., Wu W., Xiong S., Zhong M., Gao X. mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity. 2020;52:475–486. doi: 10.1016/j.immuni.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H., Zeng L., Xie M., Liu J., Zhou B., Wu R. TMEM173 drives lethal coagulation in sepsis. Cell Host Microbe. 2020;27:556–570. doi: 10.1016/j.chom.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gao J., Qiu X., Xi G., Liu H., Zhang F., Lv T. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol Rep. 2018;40:1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peng J., Jiang H., Guo J., Huang J., Yuan Q., Xie J. CD147 expression is associated with tumor proliferation in bladder cancer via GSDMD. BioMed Res Int. 2020;2020:7638975. doi: 10.1155/2020/7638975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fang Y., Tian S., Pan Y., Li W., Wang Q., Tang Y. Pyroptosis: a new frontier in cancer. Biomed Pharmacother. 2020;121:109595. doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 142.Qiao L., Wu X., Zhang J., Liu L., Sui X., Zhang R. α-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB J. 2019;33:12760–12767. doi: 10.1096/fj.201900483RR. [DOI] [PubMed] [Google Scholar]
- 143.Ma Y., Chen Y., Lin C., Hu G. Biological functions and clinical significance of the newly identified long non-coding RNA RP1-85F18.6 in colorectal cancer. Oncol Rep. 2018;40:2648–2658. doi: 10.3892/or.2018.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang W., Chen D., Jiang M., Xu B., Li X., Chu L. Downregulation of GSDMD promotes gastric cancer proliferation by regulating cell cycle-related proteins. J Dig Dis. 2018;19:74–83. doi: 10.1111/1751-2980.12576. [DOI] [PubMed] [Google Scholar]
- 145.Orr C.F., Rowe D.B., Halliday G.M. An inflammatory review of Parkinson's disease. Prog Neurobiol. 2002;68:325–340. doi: 10.1016/s0301-0082(02)00127-2. [DOI] [PubMed] [Google Scholar]
- 146.Pan J., Han L., Guo J., Wang X., Liu D., Tian J. AIM2 accelerates the atherosclerotic plaque progressions in ApoE−/− mice. Biochem Biophys Res Commun. 2018;498:487–494. doi: 10.1016/j.bbrc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 147.Zhou X., Wu Y., Ye L., Wang Y., Zhang K., Wang L. Aspirin alleviates endothelial gap junction dysfunction through inhibition of NLRP3 inflammasome activation in LPS-induced vascular injury. Acta Pharm Sin B. 2019;9:711–723. doi: 10.1016/j.apsb.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hootman J.M., Helmick C.G., Barbour K.E., Theis K.A., Boring M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015‒2040. Arthritis Rheum. 2016;68:1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goldberg E.L., Asher J.L., Molony R.D., Shaw A.C., Zeiss C.J., Wang C. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017;18:2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Magnotti F., Lefeuvre L., Benezech S., Malsot T., Waeckel L., Martin A. Pyrin dephosphorylation is sufficient to trigger inflammasome activation in familial Mediterranean fever patients. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201910547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y., Zhu X., Yuan S., Wen S., Liu X., Wang C. TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front Endocrinol (Lausanne) 2019;10:603. doi: 10.3389/fendo.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu B., Jiang M., Chu Y., Wang W., Chen D., Li X. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 153.Yang C., Sun P., Deng M., Loughran P., Li W., Yi Z. Gasdermin D protects against noninfectious liver injury by regulating apoptosis and necroptosis. Cell Death Dis. 2019;10:481. doi: 10.1038/s41419-019-1719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vermeire S., Hebuterne X., Napora P., Wisniewska-Jarosinska M., Kiss G., Bourreille A. ABX464 is safe and efficacious in proof of concept study in ulcerative colitis patients. Gastroenterology. 2019;156 S-218. [Google Scholar]
- 155.Lei X., Zhang Z., Xiao X., Qi J., He B., Wang J. Enterovirus 71 inhibits pyroptosis through cleavage of Gasdermin D. J Virol. 2017;91 doi: 10.1128/JVI.01069-17. e01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee B.L., Stowe I.B., Gupta A., Kornfeld O.S., Roose-Girma M., Anderson K. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med. 2018;215:2279–2288. doi: 10.1084/jem.20180589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kayagaki N., Warming S., Lamkanfi M., Walle L.V., Louie S., Dong J. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 158.Chen R., Zeng L., Zhu S., Liu J., Zeh H.J., Kroemer G. CAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci Adv. 2019;5 doi: 10.1126/sciadv.aav5562. eaav5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ye J., Zeng B., Zhong M., Li H., Xu L., Shu J. Scutellarin inhibits caspase-11 activation and pyroptosis in macrophages via regulating PKA signaling. Acta Pharm Sin B. 2021;11:112–126. doi: 10.1016/j.apsb.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kang R., Zeng L., Zhu S., Xie Y., Liu J., Wen Q. Lipid peroxidation drives Gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]