Abstract
Objectives
To identify potential predictors of COVID-19 vaccine hesitancy (C19-VH) in adults with immune-mediated inflammatory diseases (IMID).
Methods
A total of 1000 IMID patients were enrolled in this web-based cross-sectional study. A standardised and self-administered survey was designed by members of the Brazilian Society of Rheumatology Steering Committee for Infectious and Endemic diseases and distributed to IMID patients spread across Brazil.
Results
Of the 908 (90.8%) respondents eligible for analysis, 744 (81.9%) were willing to get vaccinated against COVID-19. In our multivariable logistic regression model, concurrent malignancy, fibromyalgia, hydroxychloroquine use, and recent corticosteroid pulse therapy were independently associated with higher odds of C19-VH. The short duration of COVID-19 vaccine clinical trials was the main reason for C19-VH.
Conclusion
We identified novel characteristics potentially associated with C19-VH among adults with IMID. Greater awareness on the safety and efficacy of COVID-19 vaccines is needed for both IMID patients and attending physicians.
Keywords: COVID-19, Vaccine hesitancy, Vaccine acceptance, Immune-mediated inflammatory diseases
Abbreviations: COVID-19, coronavirus disease 2019; IMID, immune-mediated chronic inflammatory diseases; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; DMARD, disease-modifying antirheumatic drugs; OR, odds ratio; CI, confidence interval; RMD, rheumatic and musculoskeletal diseases
1. Introduction
Coronavirus disease 2019 (COVID-19) has affected over 220 million people globally since it was declared a pandemic by the World Health Organization on March 11, 2020 [1]. To overcome this still-expanding pandemic and its devastating economic, social, and health consequences, it is fundamental to have far-reaching COVID-19 vaccination coverage. However, the achievement of this goal may be hampered, among other factors, by vaccine hesitancy, a complex and multifactorial phenomenon defined as a delay in acceptance or refusal of vaccination despite the availability of vaccination services [2]. To date, limited information is available on COVID-19 vaccine hesitancy in patients with immune-mediated chronic inflammatory diseases (IMID) [3], [4], [5], [6], who might be at higher risk of severe COVID-19, especially those under intense immunosuppression.
To address this knowledge gap, members of the Brazilian Society of Rheumatology Steering Committee for Infectious and Endemic diseases designed an online survey aiming to explore IMID patients’ perceptions and behaviours towards COVID-19 vaccine. As pre-planned, we herein present an analysis of the first 1000 respondents, focusing on the identification of correlates of COVID-19 vaccine hesitancy and/or acceptance, and we describe the potential concerns related to the vaccination of IMID patients against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
2. Methods
2.1. Study design and population
In this cross-sectional study, the committee members sent an invitation to participate in the survey to adult patients diagnosed with an IMID who were followed up in their private offices, rheumatology outpatient clinics in referral hospitals, or in infusion centres (list of participating hospitals/infusion centres available as supplementary material). In doing so, we expected to recruit a large number of patients within a short period of time and, at the same time, ensure a reliable diagnosis. The study protocol was approved by the Research Ethics Committee at Universidade Federal Fluminense (CAAE: 42336321.5.0000.5243), State of Rio de Janeiro, Brazil.
2.2. Online questionnaire
In this self-administered survey, which was sent by the committee members to IMID patients via email or WhatsApp, the participants answered questions related to the underlying IMID and health in general, sociodemographic aspects, personal history of SARS-CoV-2 infection, in addition to their expectations and perceptions related to COVID-19 vaccination.
The following comorbidities were assessed in the questionnaire: cardiovascular disease (includes systemic arterial hypertension, coronary artery disease, cardiopathy, heart failure, stroke, and arrhythmia); diabetes mellitus; chronic lung disease (includes asthma, bronchitis, emphysema, chronic obstructive pulmonary disease, and fibrosis); chronic liver disease (includes hepatitis B, hepatitis C, cirrhosis, and non-alcoholic fatty liver disease); chronic kidney disease (includes haemodialysis and peritoneal dialysis); human immunodeficiency virus infection; cancer (includes solid and haematopoietic malignancies); obesity; depressive & anxiety disorder; and fibromyalgia.
According to the study protocol, access to the survey questions was only allowed for those who confirmed not to be vaccinated against COVID-19; aged 18 years or older; and who agreed with the electronic consent form.
2.3. Statistical analyses
We used Mann-Whitney test, Chi-square or Fisher’s exact test to study differences in characteristics between IMID patients willing to get vaccinated against COVID-19 versus IMID patients hesitant (i.e., uncertain or unwilling) to receive a COVID-19 vaccine (two-sided, significance level < 0.05). Variables with a p value < 0.05 in the univariable analysis were entered into a multivariable logistic regression model. The strength of association between age and willingness to accept COVID-19 vaccination was measured by calculating the point-biserial correlation coefficient (rpb). IBM SPSS (version 22.0) was used for the statistical analyses.
3. Results
3.1. Patient characteristics
One thousand IMID patients responded to the online survey between 30 January 2021 and 8 February 2021. Of these, 92 (9.2%) were excluded from analysis due to the following: refusal to participate (8), age < 18 years (32), or prior receipt of a COVID-19 vaccine (52). The characteristics of the included participants (9 0 8) are shown in Table 1, Table 2 . Patients were mostly middle aged, female, white, married, of lower socioeconomic status (classes C, D, or E, as defined by the Brazilian Institute of Geography and Statistics), and had completed higher education. Just over half of them lived in the Southeast region (54.4%), which is the most populous macro-region in Brazil, followed by the Northeast (18.3%), South (13.7%), Central-West (9.6%), and North (4.1%) regions. The proportion of individuals who reported a previous diagnosis of SARS-CoV-2 infection was approximately 16%, and a much smaller proportion claimed to be active smoker.
Table 1.
General characteristics of participants with immune-mediated inflammatory diseases.
| Variables | All participants N = 908 | COVID-19 vaccine acceptance group n = 744 | COVID-19 vaccine hesitant group n = 164 | P value† |
|---|---|---|---|---|
| Female | 714 (78.6) | 568 (76.3) | 146 (89.0) | <0.05 |
| Age, years, median (range) | 47 (18–92) | 47 (18–92) | 46 (21–79) | 0.52 |
| Self-reported skin colour/ethnicity | 0.25 | |||
| White | 638 (70.3) | 533 (71.6) | 105 (64.0) | 0.06 |
| Brown | 213 (23.5) | 166 (22.3) | 47 (28.6) | 0.08 |
| Black | 37 (4.1) | 30 (4.0) | 7 (4.3) | 0.82 |
| Indigenous or Yellow | 20 (2.2) | 15 (2.0) | 5 (3.0) | 0.38 |
| Marital status | 0.48 | |||
| Married/civil partnership | 555 (61.1) | 448 (60.2) | 107 (65.2) | 0.25 |
| Single | 229 (25.2) | 192 (25.8) | 37 (22.6) | 0.42 |
| Divorced, separated, or widowed | 124 (13.7) | 104 (14.0) | 20 (12.2) | 0.61 |
| Socioeconomic class by monthly household income (minimum wages), N = 773* | <0.05 | |||
| A or B (≥10) | 261 (33.8) | 232/649 (35.7) | 29/124 (23.4) | <0.05 |
| C (4–10) | 227 (29.4) | 189/649 (29.1) | 38/124 (30.6) | 0.74 |
| D or E (<4) | 285 (36.9) | 228/649 (35.1) | 57/124 (46.0) | <0.05 |
| Education level | <0.05 | |||
| Bachelor’s or postgraduate degree | 582 (64.1) | 492 (66.1) | 90 (54.9) | <0.05 |
| High school | 236 (26) | 177 (23.8) | 59 (36.0) | <0.05 |
| Less than high school | 56 (6.2) | 45 (6.0) | 11 (6.7) | 0.72 |
| Other | 34 (3.7) | 30 (4.0) | 4 (2.4) | 0.49 |
| Currently working | 493 (54.3) | 414 (55.6) | 79 (48.2) | 0.08 |
| Prior COVID-19 infection | 148 (16.3) | 117 (15.7) | 31 (18.9) | 0.34 |
| Years since disease diagnosis, N = 892 | 0.57 | |||
| <5 years | 177 (19.8) | 150/732 (20.5) | 27/160 (16.9) | 0.32 |
| 5–10 years | 262 (29.4) | 214/732 (29.2) | 48/160 (30) | 0.84 |
| >10 years | 453 (50.8) | 368/732 (50.3) | 85/160 (53.1) | 0.54 |
| Current use of non-biologic DMARD | 594 (65.4) | 480 (64.5) | 114 (69.5) | 0.23 |
| Current use of biologic DMARD | 404 (44.5) | 327 (44) | 77 (46.9) | 0.48 |
| Current use of oral corticosteroid | 515 (56.7) | 412 (55.4) | 103 (62.8) | 0.09 |
| Corticosteroid pulse therapy in the last 30 days, N = 850 | 39 (4.6) | 26/701 (3.7) | 13/149 (8.7) | <0.05 |
| ≥1 comorbidity | 491 (54.1) | 398 (53.5) | 93 (56.7) | 0.48 |
| Body mass index, kg/m2, median (range), N = 884 | 26 (15.2–55.1) | 26 (15.2–55.1) | 25.5 (17.1–45.2) | 0.37 |
| Active smoker | 44 (4.8) | 38 (5.1) | 6 (3.6) | 0.54 |
Unless otherwise stated, data are shown as number of participants (%).
Abbreviations: DMARD, disease-modifying antirheumatic drug
†Proportions were compared with Chi-square or Fisher’s exact test, and continuous values with Mann-Whitney test.
According to the Brazilian Institute of Geography and Statistics (IBGE).
Table 2.
Immune-mediated inflammatory diseases, comorbidities, and concomitant therapies stratified by willingness for COVID-19 vaccination.
| COVID-19 vaccine acceptance group n = 744 | COVID-19 vaccine hesitant group n = 164 | P value | |
|---|---|---|---|
| Immune-mediated inflammatory diseases | |||
| RA | 248 (33.3) | 49 (29.9) | 0.40 |
| SLE | 143 (19.2) | 45 (27.4) | <0.05 |
| AS | 111 (14.9) | 21 (12.8) | 0.54 |
| PsA/Pso | 60 (8.1) | 15 (9.1) | 0.63 |
| SS | 24 (3.2) | 4 (2.4) | 0.80 |
| SSc | 19 (2.5) | 7 (4.3) | 0.29 |
| IBD | 16 (2.1) | 0 | 0.09 |
| PM/DM | 10 (1.3) | 2 (1.2) | >0.99 |
| TAK | 7 (0.9) | 0 | 0.36 |
| Behçet | 7 (0.9) | 1 (0.6) | >0.99 |
| GPA | 5 (0.7) | 1 (0.6) | >0.99 |
| GCA | 2 (0.3) | 2 (1.2) | 0.15 |
| Other/overlap syndromes | 92 (12.4) | 17 (10.4) | 0.59 |
| Non-biologic DMARDs (not mutually exclusive) | |||
| Azathioprine | 91 (12.2) | 26 (15.8) | 0.24 |
| Cyclophosphamide | 6 (0.8) | 1 (0.6) | >0.99 |
| Antimalarial agent | 184 (24.7) | 65 (39.6) | <0.05 |
| Leflunomide | 54 (7.2) | 17 (10.4) | 0.19 |
| Methotrexate | 172 (23.1) | 27 (16.5) | 0.07 |
| Mycophenolate | 35 (4.7) | 11 (6.7) | 0.32 |
| Sulfasalazine | 37 (5.0) | 4 (2.4) | 0.21 |
| Tacrolimus or cyclosporine | 11 (1.5) | 2 (1.2) | >0.99 |
| Tofacitinib, baricitinib or upadacitinib | 32 (4.3) | 3 (1.8) | 0.17 |
| Biologic DMARDs (mutually exclusive) | |||
| Adalimumab | 60 (8.1) | 14 (8.5) | 0.87 |
| Etanercept | 18 (2.4) | 8 (4.9) | 0.11 |
| Golimumab | 28 (3.8) | 5 (3.0) | 0.81 |
| Infliximab | 74 (9.9) | 12 (7.3) | 0.37 |
| Certolizumab | 18 (2.4) | 3 (1.8) | >0.99 |
| Secukinumab | 17 (2.3) | 6 (3.6) | 0.28 |
| Ixekizumab | 4 (0.5) | 0 | >0.99 |
| Abatacept | 20 (2.7) | 5 (3.0) | 0.79 |
| Belimumab | 7 (0.9) | 5 (3.0) | <0.05 |
| Rituximab | 36 (4.8) | 11 (6.7) | 0.33 |
| Tocilizumab | 35 (4.7) | 7 (4.3) | >0.99 |
| Ustekinumab | 9 (1.2) | 1 (0.6) | >0.99 |
| Vedolizumab | 1 (0.1) | 0 | >0.99 |
| Comorbidities (not mutually exclusive) | |||
| Cardiovascular disease* | 196 (26.3) | 35 (21.3) | 0.19 |
| Diabetes mellitus | 66 (8.9) | 14 (8.5) | >0.99 |
| Chronic lung disease § | 69 (9.3) | 17 (10.4) | 0.65 |
| Chronic liver disease † | 21 (2.8) | 8 (4.9) | 0.21 |
| Chronic kidney disease ‡ | 12 (1.6) | 9 (5.5) | <0.05 |
| HIV infection | 4 (0.5) | 1 (0.6) | >0.99 |
| Obesity | 39 (5.2) | 12 (7.3) | 0.34 |
| Cancer ¶ | 7 (0.9) | 5 (3) | <0.05 |
| Depressive/anxiety disorder | 171 (23) | 34 (20.7) | 0.60 |
| Fibromyalgia | 88 (11.8) | 33 (20.1) | <0.05 |
Abbreviations: AS, ankylosing spondylitis; DMARD, disease-modifying antirheumatic drug; PM/DM, polymyositis or dermatomyositis; GCA, giant cell arteritis; GPA, granulomatosis with polyangiitis; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; IL-17, interleukin-17; PsA/Pso, psoriatic arthritis or psoriasis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren syndrome; SSc, systemic sclerosis; TAK, Takayasu’s arteritis; TNF, tumour necrosis factor.
Proportions were compared with chi-square or Fisher’s exact test.
* Includes systemic arterial hypertension, coronary artery disease, cardiopathy, heart failure, stroke, and arrhythmia.
§ Includes asthma, bronchitis, emphysema, chronic obstructive pulmonary disease, and fibrosis.
† Includes hepatitis B, hepatitis C, cirrhosis, and non-alcoholic fatty liver disease.
‡ Includes haemodialysis and peritoneal dialysis.
¶ Includes haematopoietic malignancies.
Rheumatoid arthritis was the most prevalent IMID in our survey (32.7%), followed by systemic lupus erythematosus (20.7%), ankylosing spondylitis (14.5%), psoriatic arthritis or psoriasis (8.3%), Sjogren’s syndrome (3.1%), systemic sclerosis (2.9%), Crohn’s disease or ulcerative colitis (1.8%), polymyositis or dermatomyositis (1.3%), Behçet’s disease (0.9%), Takayasu’s arteritis (0.8%), granulomatosis with polyangiitis (0.7%), and giant cell arteritis (0.4%). Miscellaneous causes and overlapping IMIDs accounted for 109 cases. Overall, disease duration was relatively long (>10 years for 50.8% of participants). At study enrolment, most patients were being treated with oral corticosteroid and non-biologic disease-modifying antirheumatic drugs (DMARD). Use of biological DMARD was reported by 44.5% of the participants, whereas 54% of them had at least one comorbidity.
3.2. Predictors of COVID-19 vaccine hesitancy and/or acceptance
Among the 908 participants who completed the survey, 164 (18.1%) reported being hesitant about receiving a COVID-19 vaccine when it becomes available. In the unadjusted analysis (Table 1, Table 2), the hesitant patients were predominantly female, of lower socioeconomic status, and less educated than non-hesitant patients. Vaccine hesitancy was also more common among patients with systemic lupus erythematosus; those treated with hydroxychloroquine, belimumab, and pulse corticosteroid therapy; and those with comorbidities such as chronic kidney disease, cancer, and fibromyalgia (all p < 0.05 in univariable analysis). In a multivariable model (odds ratio [OR], 95% confidence interval [CI]), current use of hydroxychloroquine (OR: 2.38, 95% CI: 1.39–4.08), recent corticosteroid pulse therapy (OR: 2.73, 95% CI: 1.24–6.0), concomitant malignancy (OR: 5.02, 95% CI: 1.31–19.19), and fibromyalgia (OR: 1.88, 1.08–3.25) remained independently associated with higher odds of COVID-19 vaccine hesitancy (all p < 0.05) (supplementary material). Importantly, 178/908 cases (19.6%) were excluded from the final model due to missing data on household income (answer not obligatory) and/or receipt of corticosteroid pulse therapy in the last 30 days (answers: yes, no, or unsure). No significant correlation was observed between age and COVID-19 vaccine acceptance (rpb = 0.023, p = 0.49).
3.3. Reasons for COVID-19 vaccine hesitancy
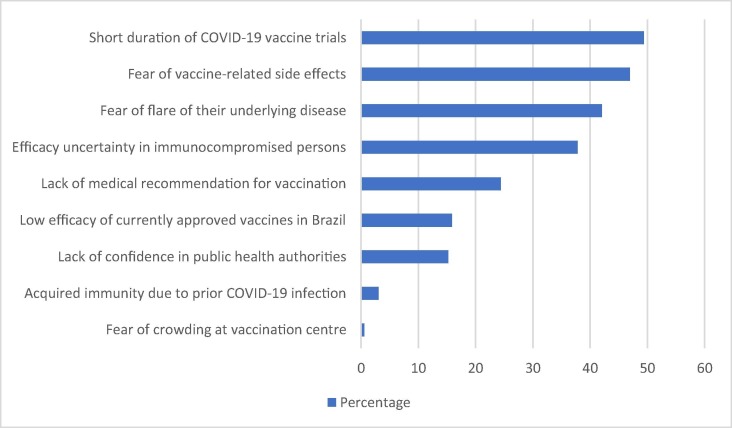
Fig. 1 illustrates the reasons provided by the participants who were doubtful or unwilling to get vaccinated against COVID-19. The most common cause of concern was the relatively short duration of pre-licensure COVID-19 vaccine clinical trials (49.4%), followed by fear of vaccine-related adverse events (47%), fear of IMID aggravation (42.1%), uncertainties due to the lack of efficacy data on immunocompromised individuals at that time (37.8%), and absence of medical recommendation for COVID-19 vaccination (24.4%).
Fig. 1.
Reasons for COVID-19 vaccine hesitancy among 164 adults with immune-mediated inflammatory diseases.
4. Discussion
To the best of our knowledge, the potential acceptance rate of COVID-19 vaccine in this study (81.9%) was the highest of all published surveys among patients with rheumatic and musculoskeletal diseases (RMDs) (range: 54.2–80.2%) [3], [4], [5], [6]. Vaccination intent, however, was not homogeneous across the Brazilian macro-regions, with COVID-19 vaccine acceptance being proportionally higher in the South (87.9%) and lower in Central-West Brazil (70.1%) (data not shown). Of interest, the overall potential acceptance rate in our study was greater than that of a global survey with 13,426 adults (71.5%) [7], and also among healthcare personnel (51.1%) [8], who have been regarded as a high-risk group for SARS-CoV-2 infection. Despite the apparently good result observed in our large sample of IMID patients, one must consider the need for mass vaccination in order to minimise the global burden of the COVID-19 pandemic [9].
According to available data in the literature, the potential acceptance of COVID-19 vaccination amongst RMD patients has been positively associated with higher education level, older age, male gender, and prior receipt of other vaccines [3], [4], [5], [6]. In our univariable analysis, acceptance of the COVID-19 vaccine was also greater among men and those with higher education level; however, these associations did not retain significance after adjusting for the covariates. Contrary to prior studies [4], [5], [6], we found no correlation between age and potential COVID-19 vaccination. As a limitation of our research, data on immunisation coverage were not collected. However, a recent study on systemic lupus erythematosus had already shown greater odds of vaccination among patients who had previously received other vaccines [10].
As the major finding of our study, potential acceptance of COVID-19 vaccination was negatively influenced by the presence of certain comorbidities, such as malignancy and fibromyalgia, as well as exposure to hydroxychloroquine and corticosteroid pulse therapy. Hence, these data might be regarded as somewhat paradoxical given that moderate-to-high glucocorticoid dosages (prednisone-equivalent doses ≥ 10 mg/day [11] and methylprednisolone pulse therapy [12], respectively) have been associated with unfavourable outcomes of COVID-19 in patients with rheumatic diseases. Moreover, COVID-19 vaccines, which do not contain live virus, have been proven to be safe and effective, and should therefore be recommended for IMID patients [13], [14], [15], especially those severely immunocompromised. Regarding the relationship between hydroxychloroquine usage and COVID-19 vaccine hesitancy, we hypothesised that this association might be due to a false sense of protection against COVID-19 considering the purported benefits conferred by the drug on SARS-CoV-2 clearance [16]. Of note, the types of comorbidities linked with COVID-19 vaccine hesitancy in our study are distinct from those associated with hospitalisation from SARS-CoV-2 infection (hypertension/cardiovascular disease, lung disease, chronic renal insufficiency/end-stage renal disease, and diabetes), as shown by the COVID-19 Global Rheumatology Alliance physician-reported registry [11].
Our data are in line with the literature with regard to the most common reasons for refusing or doubting COVID-19 vaccination [3], [4], [5], [6]. Of concern, almost 25% of the hesitant participants linked their attitude to a lack of medical recommendation for COVID-19 vaccination, therefore suggesting that attending physicians should be more engaged in spreading a message in favour of vaccination against COVID-19. Interestingly, even among our IMID patients willing to get vaccinated against COVID-19, 41.5% were fearful of worsening their underlying disease, while 20.8% believed they were more likely to have side effects from the COVID-19 vaccine than the general population (data not shown).
As strength of our study, we included a large number of IMID patients from across the country who were followed up in the public (16.9%), private (62.1%), or both (21%) healthcare systems. As limitations of our research, whose protocol did not allow the collection of any sort of data that could identify the participant, it is impossible to rule out that some participants may have submitted their answers more than once. In addition, we did not recruit random patients. Participants in this study were probably cared for by academic physicians, which may have led to inclusion of patients who are more health conscious and perhaps more confident about vaccination in general.
In conclusion, we have identified novel characteristics potentially associated with COVID-19 vaccine hesitancy among adults with IMID, in addition to the new data provided on the reasons for refusing or doubting COVID-19 vaccination. Attending physicians should spend more time with IMID patients who bear risk characteristics for COVID-19 vaccination, reinforcing the overall safety and efficacy of COVID-19 vaccines, including among immunocompromised individuals [14], [15], so that we can achieve global mass vaccination.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank Dr. Bruna Costa da Mata Moura and Dr. Valéria Valim for their contributions in recruiting patients for this work at Reuma–Reumatologia Avançada (private infusion centre) and at Universidade Federal do Espírito Santo University Hospital, respectively.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.
Ethics statement
The Research Ethics Committee at Universidade Federal Fluminense approved the study protocol (CAAE: 42336321.5.0000.5243).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.09.057.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Johns Hopkins University Coronavirus Resource Center [Internet]. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University; 2020. [Cited 05 September 2021]. Available from: https://coronavirus.jhu.edu/map.html.
- 2.MacDonald NE, SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine 2015;33:4161–4. https://doi.org/ /10.1016/j.vaccine.2015.04.036. [DOI] [PubMed]
- 3.Campochiaro C., Trignani G., Tomelleri A., Cascinu S., Dagna L. COVID-19 Vaccine Study Group. Potential acceptance of COVID-19 vaccine in rheumatological patients: a monocentric comparative survey. Ann Rheum Dis. 2021;80(6):816–817. doi: 10.1136/annrheumdis-2020-219811. [DOI] [PubMed] [Google Scholar]
- 4.Priori R., Pellegrino G., Colafrancesco S., Alessandri C., Ceccarelli F., Di Franco M., et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. 2021;80(7):953–954. doi: 10.1136/annrheumdis-2021-220059. [DOI] [PubMed] [Google Scholar]
- 5.Felten R., Dubois M., Ugarte-Gil M.F., Chaudier A., Kawka L., Bergier H., et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021;3(4):e243–e245. doi: 10.1016/S2665-9913(21)00039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boekel L., Hooijberg F., van Kempen Z.L.E., Vogelzang E.H., Tas S.W., Killestein J., et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3(4):e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maltezou H.C., Pavli A., Dedoukou X., Georgakopoulou T., Raftopoulos V., Drositis I., et al. Determinants of intention to get vaccinated against COVID-19 among healthcare personnel in hospitals in Greece. Infect Dis Health. 2021;26(3):189–197. doi: 10.1016/j.idh.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R.M., Vegvari C., Truscott J., Collyer B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614–1616. doi: 10.1016/S0140-6736(20)32318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieira de Rezende R.P., Mattos G.A., de Mello Leal Augusto R., Machado Gayer C.R., Mendes Klumb E. Predictors for seasonal influenza vaccination and reasons for inadequate vaccination coverage against a broad spectrum of vaccine-preventable diseases: a cross-sectional study among a Brazilian cohort of adult patients with systemic lupus erythematosus. Lupus. 2019;28(6):794–796. doi: 10.1177/0961203319846383. [DOI] [PubMed] [Google Scholar]
- 11.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila M, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. https://http://dx.doi.org/10.1136/annrheumdis-2020-217871 [DOI] [PMC free article] [PubMed]
- 12.Marques CDL, Kakehasi AM, Pinheiro MM, Mota LMH, Albuquerque CP, Silva CR, et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalized patients with rheumatic diseases and COVID-19: first results of ReumaCoV Brazil registry. RMD Open 2021;7:e001461. https://doi.org/10.1136/rmdopen-2020-001461 [DOI] [PMC free article] [PubMed]
- 13.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases - Version 1. Arthritis Rheumatol. 2021;73(7):1093–1107. doi: 10.1002/art.v73.710.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med (2021). https://doi.org/10.1038/s41591-021-01469-5. [DOI] [PubMed]
- 15.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021 Jun 14:annrheumdis-2021-220647. doi: 10.1136/annrheumdis-2021-220647. Epub ahead of print. [DOI] [PubMed]
- 16.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.