Abstract
Objective
To summarize the level of knowledge regarding the effects of microcurrent therapy (MCT) on musculoskeletal pain in adults.
Data Sources
The PubMed, Physiotherapy Evidence Database, Cumulative Index to Nursing Allied Health Literature, Cochrane Central Register of Controlled Trials, and Igaku Chuo Zasshi database were searched from the time of their inception to December 2020.
Study Selection
Randomized controlled trials (RCTs) investigating the effects of MCT on musculoskeletal pain were included. Additionally, non-RCTs were included to assess the adverse events.
Data Extraction
The primary outcomes were pain and adverse events related to MCT. To assess the reproducibility of MCT, we evaluated the completeness of treatment description using the Template for Intervention Description and Replication (TIDieR) checklist. We also assessed the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
Data Synthesis
A comprehensive assessment of 4 RCTs and 5 non-RCTs that met the inclusion criteria revealed that MCT significantly improved shoulder pain (1 study, 40 patients) and knee pain (1 study, 52 patients) compared with sham MCT without any severe adverse events. MCT has clinically significant benefits for knee pain. This study also revealed a clinically significant placebo response in treating knee pain. This evidence highlights the substantial effect of placebo response in clinical care. These treatment effects on knee pain are further supported by the high quality of evidence in GRADE with high reproducibility in TIDieR.
Conclusions
The findings of this meta-analysis highlight the effect of placebo response in treating knee pain. MCT is a potential, core nonpharmacologic treatment option in clinical care with minimal adverse events and should be further investigated. This study proposes a framework for the future investigation of the effect of MCT on musculoskeletal pain to enhance the study quality and reproducibility.
KEYWORDS: Electric stimulation therapy, Meta-analysis, Musculoskeletal pain, Placebo effect, Rehabilitation
List of abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; LBP, low back pain; MCID, minimum clinically important difference; MCT, microcurrent therapy; NSAIDs, nonsteroidal anti-inflammatory drugs; RCTs, randomized controlled trials; TIDieR, Template for Intervention Description and Replication
Musculoskeletal pain is the most common cause of disability worldwide.1 Although treatment options in primary care comprise both nonpharmacologic and pharmacologic treatments, the latter, which includes nonsteroidal anti-inflammatory drugs (NSAIDs), have significant gastrointestinal adverse effects.2 This led physicians to consider nonpharmacologic treatments such as self-management and exercise as the first-line treatment option.3 However, the effects of the currently available nonpharmacologic treatment are far from adequate.4 Hence, there is an urgent need to improve the quality of care for musculoskeletal pain.4
Microcurrent therapy (MCT) is a new Food and Drug Administration–approved electrotherapy for treating musculoskeletal pain, with a 510K certificate. MCT delivers small electric currents of <1 mA across the skin without activation of muscle contractions and noticeable sensations, and its potential mechanism is different from that of conventional transcutaneous electrical nerve stimulation.5 To our knowledge, to date, no systematic review has evaluated the risks and benefits of MCT in treating musculoskeletal pain; therefore, summarizing a comprehensive picture of MCT is a necessary step toward establishing effective musculoskeletal pain management in primary care.
In previous clinical trials, MCT was only considered effective when its effect was superior to the sham treatment-induced placebo response. Nevertheless, in patient care, treatment is unavoidably delivered with placebo response. Given that the beneficial effects of the placebo effect are often clinically significant, especially in chronic pain,6 assessing the overall treatment effect that includes placebo response is important in optimizing patient care.
With this in mind, the goal of this study was to summarize the level of knowledge regarding the effects of MCT in patients with musculoskeletal pain. Specifically, this systematic review summarized the knowledge from randomized controlled trials (RCTs) regarding synthesized placebo and the true therapeutic effects of MCT on musculoskeletal pain. We then calculated the overall treatment effect as a summation of the placebo response and true therapeutic effect. Lastly, to evaluate the risk-benefit balance, we summarized the adverse events associated with MCT from both RCTs and non-RCTs.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement,7 Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols,8 Meta-analysis of Observational Studies in Epidemiology checklist,9 and Cochrane Handbook for Systematic Reviews of Interventions10 (supplemental appendix S1 and S2, available online only at http://www.archives-pmr.org/).
Terminology
We separated the placebo responses from placebo effects, as suggested by Kirsch.11 Placebo response is defined as improvement observed with a sham intervention, whereas placebo effect is defined as the difference in responses between the no treatment and sham treatment groups. In this review, musculoskeletal pain was divided into 3 categories based on the duration of pain experience: acute (pain that persisted for less than 3 months), subacute (a subset of acute pain that has been present for at least 6 weeks but less than 3 months), and chronic (pain that has persisted for more than 3 months).12
Eligibility criteria
This review included original articles that investigated the effects of MCT interventions on musculoskeletal pain. Articles meeting the following criteria were included: (1) published as an original article in a peer-reviewed journal; (2) written in English or Japanese; (3) included adults with musculoskeletal pain; (4) had a treatment strategy that included MCT; and (5) reported pain and/or discomfort level as outcomes. Articles meeting the following criteria were excluded: (1) included patients with a history of surgery and (2) included treatment arms involving other interventions (eg, exercise) in addition to MCT. There were no restrictions on the study period and follow-up duration. In this review, safety outcomes were summarized by reviewing both RCTs and non-RCTs; however, therapeutic effects were summarized by reviewing only RCTs to report the true treatment effects of MCT compared with those of sham MCT. RCTs including sham MCT as the control arm were included.
Literature search and study selection
The PubMed, Physiotherapy Evidence Database, Cumulative Index to Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, and Igaku Chuo Zasshi databases were searched. PubMed searches used combined key terms, including “Microcurrent,” “Microamperage,” “Low-intensity direct current,” and “Pain” by combining Medical Subject Headings and non–Medical Subject Headings terms. For each electronic database, we searched articles from the time of database inception to December 2020. Google Scholar was also used as a complementary search engine. The reference lists of relevant systematic reviews13 were manually searched. Furthermore, a citation search of the original articles included in this review was performed using the Web of Science.
A single reviewer (H.I.), who is a content and statistical expert, assessed the eligibility of the studies in accordance with the Cochrane Handbook.10 The reviewer first screened the titles and abstracts and then the full text of each article that met the eligibility criteria. If multiple studies described the same trial, all studies were considered.
Data extraction
The same reviewer collected data, including information on author names, years, country, study design, study population, demographic characteristics, MCT conditions (administrator, device name, electrode placement, pulse frequency, pulse duration, current intensity, treatment time), frequency and duration of MCT, follow-up duration, and outcome measurements using standardized data forms. When an article reported outcomes using multiple pain scales, we used only the scales with the highest rank on the pain outcome hierarchy. Among the scales, the global knee pain score (visual analog scale or Likert scale score) was the highest hierarchy. To standardize the pain outcomes measured across the studies, all pain scales were converted to a scale of 0-100 points (higher values indicated severe pain), as in a previous meta-analysis.14 This had 2 advantages: an intuitive and easily interpretable mean difference across different studies and the consideration of the concept of minimum clinically important difference (MCID) in the global knee pain score. We contacted the authors when there were missing or unclear data. If there was no response from the authors, missing data were calculated from the available data (eg, figures), if possible. For studies with multiple evaluation points, the outcome data from the last available evaluation point were used.15
Synthesis of results
Mean differences (postsham MCT vs post MCT and presham MCT vs postsham MCT) and 95% confidence intervals for each outcome were calculated using Review Manager version 5.3.16,a The prediction interval and study heterogeneity for each outcome variable were not calculated because of the small sample size; P values <.05 were considered statistically significant. To interpret the effect size in a clinical context, the mean difference for each outcome was compared with the MCID after administration of NSAIDs (ie, 19.9-mm improvement in the global knee pain score, 0-100mm).17 The placebo response (presham MCT vs postsham MCT) and true treatment effect (postsham MCT vs post MCT) were calculated separately. The overall treatment effect was calculated by the summation of the placebo response and true treatment effect. The proportion attributable to placebo response was calculated using the ratio of the mean difference between the placebo response and overall treatment effect. These calculations are based on the assumption that the overall treatment effects can be divided into placebo response and true therapeutic effect, as done in previous systematic reviews.18,19
Adverse events in patients treated with MCT were evaluated in each study. Adverse events were considered “serious” if the MCT resulted in any of the following outcomes: death, life-threatening complications, hospitalization, or substantial disruption of normal life function.
Overall quality of evidence
The Cochrane Risk of Bias Tool version 2.0 was used to assess the risk of bias.20 It includes bias assessment in the following 5 domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported result. The grade for each domain was determined as “low risk of bias,” “some concerns,” or “high risk of bias.” Each study was then given an overall grade from the above grades based on the Cochrane guideline documents.20 A single reviewer (H.I.) assessed the risk of bias for RCTs only.
To evaluate the quality of evidence, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.21 The same reviewer graded the quality of evidence for each outcome based on the 5 domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Evidence quality was downgraded if (1) the overall risk of bias was “some concerns” or “high risk of bias” (risk of bias domain) or (2) the sample size was inadequate, defined as optimal information size and wide 95% confidence interval that included 0 (imprecision domain). Optimal information size was calculated using Power and Sample Size program version 3.1.22,b The domain of indirectness was not downgraded because all studies included adults with musculoskeletal pain. In addition, this systematic review did not consider the domains of inconsistency and publication bias because there were limited studies (ie, 1 study in each outcome for meta-analysis).
To assess the reproducibility of MCT, we evaluated the completeness of the treatment description using the Template for Intervention Description and Replication (TIDieR) checklist,23 which was slightly modified (item 8: when and how much) in accordance with that used in the study by Bartholdy et al.24 This scoring system included the 12 items, as shown in table 1. All items were scored “yes” when they fulfilled the criterion (complete) and “no” when they did not (noncomplete). The same reviewer assessed all items. Only published information, including supplemental materials and reference lists, was used for scoring.
Table 1.
Brief description of the TIDieR items that were used to assess intervention reporting (adapted from Bartholdy et al24)
| Item No. | Item Name | Item Description |
|---|---|---|
| 1 | Brief name | Provides the name or a phrase that describes the intervention |
| 2 | Why | Describes any rationale, theory, or goal of the elements essential to the intervention |
| 3 | What: materials | Describes any physical or informational materials provided to participants or used in the intervention delivery or in training of intervention providers Provide information on where the materials can be accessed (for example, online appendix, URL) |
| 4 | What: procedures | Describes each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities |
| 5 | Provider | Describes the intervention provider and their expertise, background, and any specific training given |
| 6 | How | Describe the modes of delivery (such as face to face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group |
| 7 | Where | Describes the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features |
| 8 | When and how much | Describes the dose/scheduling of the intervention including the following 4 subitems. This item is only complete if all 4 subitems are complete reported. |
| a. Intensity | The intensity of the intervention | |
| b. Frequency | The frequency of the intervention sessions | |
| c. Session time | The duration of each individual intervention session | |
| d. Overall duration | The overall duration of the intervention | |
| 9 | Tailoring | Describes the what, why, when, and how of intervention titration, personalization, or progression |
| 10 | Modifications | Describes any modifications to the intervention during the course of the study |
| 11 | How well: planned | Describes strategies used to maintain or improve fidelity (how and by whom) |
| 12 | How well: actual | Describes the extent to which the intervention was delivered as planned (if adherence or fidelity as assessed) |
Results
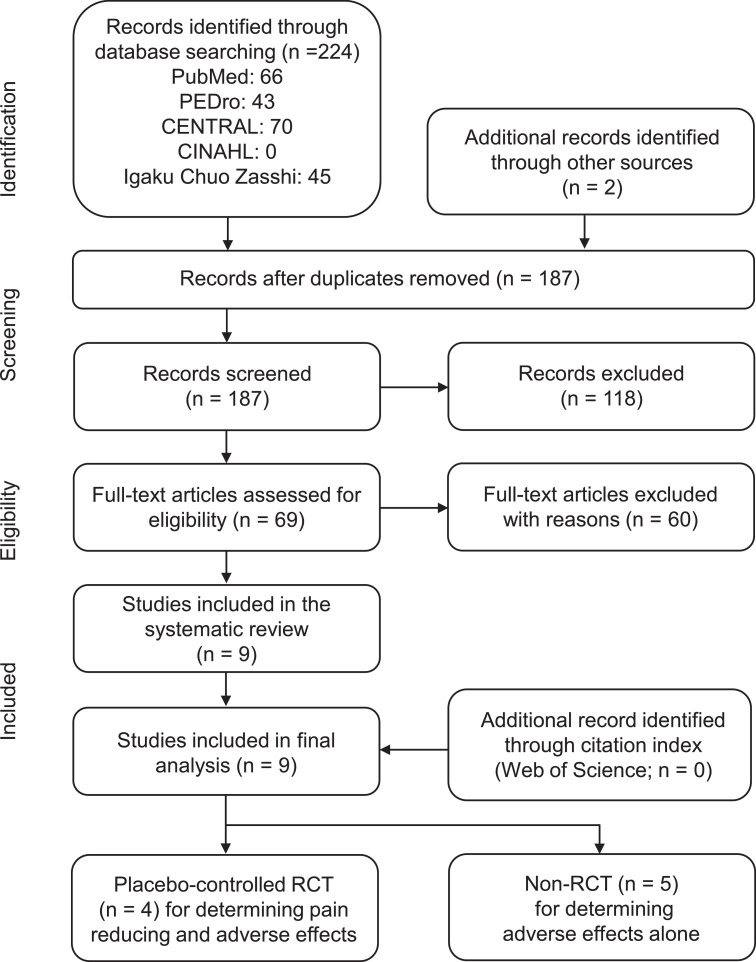
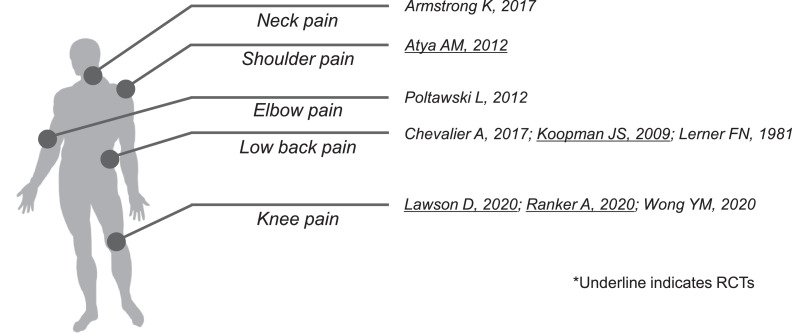
The electronic databases identified a total of 226 articles, of which 9 articles met the inclusion criteria for this systematic review25, 26, 27, 28, 29, 30, 31, 32, 33 (fig 1). These studies included patients with chronic neck pain, chronic low back pain (LBP), knee pain, tennis elbow, and subacromial impingement-induced shoulder pain (fig 2). Of the 9 studies, 4 (40.0%) were RCTs in which MCT was used to treat adults with subacromial impingement-induced shoulder pain (n=1), chronic LBP (n=1), and knee pain (n=2) (see fig 2). Notably, only 1 study used MCT as a self-management technique.29 The trial duration was 6 weeks for shoulder pain, 10 weeks for LBP, and 3-4 weeks for knee pain. The placebo response in 1 RCT treating knee pain was not calculated because of a lack of numerical data on the global knee pain scale score.32 Table 2 (RCTs) and table 3 (non-RCTs) summarize the participants’ characteristics. Supplemental appendix S3 (available online only at http://www.archives-pmr.org/) provides detailed information about the study characteristics.
Fig 1.
Flow diagram of the review. Abbreviations: CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index to Nursing and Allied Health; PEDro, Physiotherapy Evidence Database.
Fig 2.
Graphic summary of microcurrent therapy targeted pain identified in 4 randomized controlled trials and 5 nonrandomized controlled trials.
Table 2.
Study characteristics of randomized controlled trials
| Author (Country) | Participants | Sham Microcurrent Therapy |
Microcurrent Therapy |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Age (y) | BMI | %Women | N | Age (y) | BMI | %Women | ||
| Atya26 (Egypt) | With subacromial impingement | 21 | 49.1 | 30.5 | 57.1 | 19 | 48.8 | 33.3 | 47.4 |
| Koopman et al28 (Netherlands) | With LBP | 5 | 52.0 | 27.6 | 40.0 | 5 | 49.0 | 28.0 | 80.0 |
| Lawson et al29 (US) | With knee pain | 26 | 40.4 | 26.3 | 67.3* | 26 | 44.3 | 30.7 | 67.3* |
| Ranker et al32 (Germany) | With KOA | 12 | 69.9 | - | 91.7 | 14† 13‡ |
70.1† 74.1‡ |
- | 64.3† 61.5‡ |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); KOA, knee osteoarthritis; US, United States.
Averaged value of all participants.
High-intensity microcurrent.
Low-intensity microcurrent.
Table 3.
Study characteristics of nonrandomized controlled trials
| Author (Country) | Participants | Condition | Intervention | N | Age (y) | BMI | %Women |
|---|---|---|---|---|---|---|---|
| Armstrong et al25 (UK) | With LBP | E | MCT | 34 | 46.0 | - | 70.6 |
| Chevalier et al27 (UK) | With LBP | E | MPS | 68 | 47.0 | - | 73.5 |
| Lerner & Kirsch30 (US) | With LBP | C E |
Sham MCT MCT |
40* | 38.3* | - | 50.0* |
| Poltawski et al31 (UK) | With tennis elbow | E1† E2† E3† E4† |
MCT MCT MCT MCT |
61* | 53.0* | - | 47.5* |
| Wong et al33 (Hong Kong) | With chondromalacia patella | E | MPS | 1 | 35.0 | 23.0 | 0 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); C, control; E, experimental; MPS, microcurrent point stimulation; UK, United Kingdom; US, United States.
Averaged value of all participants.
Different microcurrent device (Elexoma [E1 and E2], WeWo [E3], Tendonworks [E4]) and different current intensity (E1 and E2) were used.
Sham MCT induces clinically significant placebo responses in adults with subacute to chronic knee pain
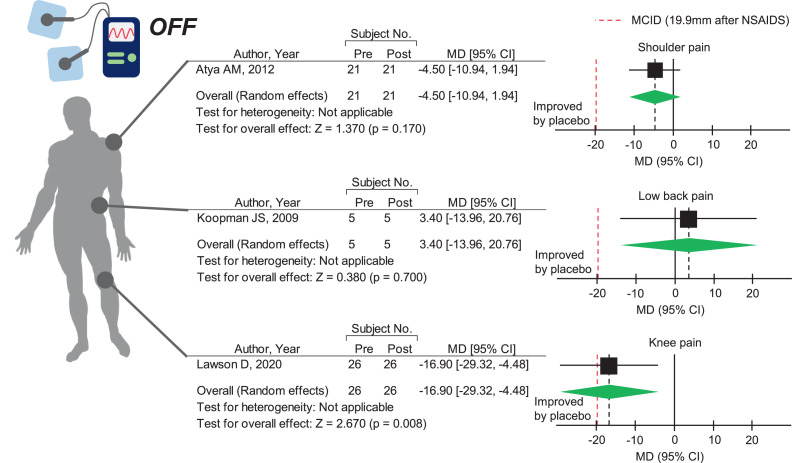
First, we summarized the currently available evidence on placebo response that has not been assessed in previous clinical trials. Sham MCT did not significantly improve chronic LBP28 and subacromial impingement-induced shoulder pain26 (fig 3), but it significantly improved subacute to chronic knee pain29 (see fig 3). Notably, the pain relief effect on knee pain was close to an MCID of 19.9 mm, indicating clinically significant improvement in the global knee pain score.17 These findings suggest that placebo response may be joint- or disease-dependent and that sham MCT may elicit a clinically beneficial response in subacute to chronic knee pain.
Fig 3.
Placebo response after sham microcurrent therapy in shoulder pain, low back pain, and knee pain. The green diamonds represent the pooled effect sizes. The vertical solid line at 0 represents no difference. The black vertical dashed line represents the average effect size. The red vertical dashed line represents the minimum clinically important difference after administration of nonsteroidal anti-inflammatory drugs (ie, 19.9-mm improvement in the global knee pain score, 0-100mm). Abbreviations: CI, confidence interval; MD, mean difference.
MCT adds clinically significant benefits in sham MCT-induced subacute to chronic knee pain relief
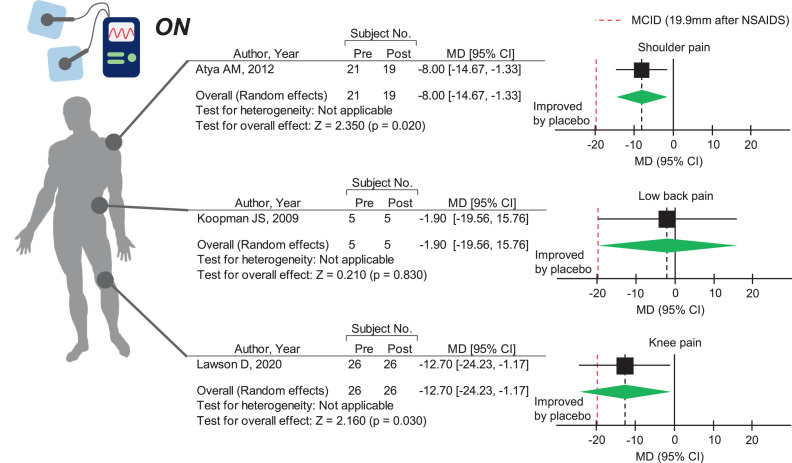
We sought to determine whether MCT has a higher pain-reducing effect than the sham treatment. Meta-analysis revealed that MCT did not improve chronic LBP28 (fig 4). However, MCT significantly improved subacromial impingement-induced shoulder pain26 and subacute to chronic knee pain29 compared with sham MCT (see fig 4). Most notably, the pain-reducing effect of MCT on knee pain reached the MCID. The beneficial effect of MCT on knee pain is further supported by another RCT showing that MCT significantly improved evening pain in patients with symptomatic mild to moderate knee osteoarthritis, regardless of the current intensity.32
Fig 4.
Pain-reducing effect of microcurrent therapy on shoulder pain, low back pain, and knee pain. The green diamonds represent the pooled effect sizes. The vertical solid line at 0 represents no difference. The black vertical dashed line represents the average effect size. The red vertical dashed line represents the minimum clinically important difference after administration of nonsteroidal anti-inflammatory drugs (ie, 19.9-mm improvement in the global knee pain score, 0-100mm). Abbreviations: CI, confidence interval; MD, mean difference.
To account for placebo response in MCT treatment effects, we calculated the overall treatment effects (placebo response plus true treatment effect) for each treatment. As expected, the mean MCT treatment effect for subacute to chronic knee pain was 29.6 mm, which was above the MCID. However, the overall treatment effects of MCT for shoulder pain (12.5mm) and chronic LBP (1.9mm) did not reach the MCID. Notably, placebo response accounted for 57% of the overall treatment effect on knee pain, which was higher than that for shoulder pain (36%) and LBP (0%).
MCT for subacute to chronic knee pain has high quality of evidence with high reproducibility
To evaluate the quality of evidence for the treatment effects, we used the GRADE assessment.34 The quality of evidence for shoulder pain and LBP was downgraded because (1) the potential risk of bias was judged to be “some concerns” (supplemental appendix S4, available online only at http://www.archives-pmr.org/) and/or (2) the sample size was lower than the optimal information size. However, no downgrading was done for subacute to chronic knee pain, and the level of evidence was judged to be high.
Treatment needs to have high reproducibility for better translation from clinical trials to bedside care. Therefore, we further evaluated the reproducibility of MCT using the TIDieR checklist23 (supplemental appendix S5, available online only at http://www.archives-pmr.org/). Notably, studies on MCT for knee pain have almost completely described the essential information needed for reproducible treatment. Thus, there is high quality of evidence with high reproducibility for MCT for knee pain (table 4).
Table 4.
Summary of findings comparing the effects of microcurrent therapy and sham microcurrent therapy
| Outcome | Sample Size (Studies) | Treatment Effect (95% CI) | Level of Evidence (GRADE) |
|---|---|---|---|
| Shoulder pain Follow-up: 6 wk | n=40 (1 × RCT) | −8.0 (−14.7 to −1.33) mm | Moderate* |
| Low back pain Follow-up: 10 wk | n=10 (1 × RCT) | −1.9 (−19.6 to 15.8) mm | Low*,† |
| Knee pain Follow-up: 4 wk | n=52 (1 × RCT) | −12.7 (−24.2 to −1.2) mm | High |
Abbreviation: CI, confidence interval.
Downgraded for risk of bias (overall grade of “some concerns”).
Downgraded for imprecision (sample size is smaller than the optimal information size; n=890).
MCT has no severe adverse effect
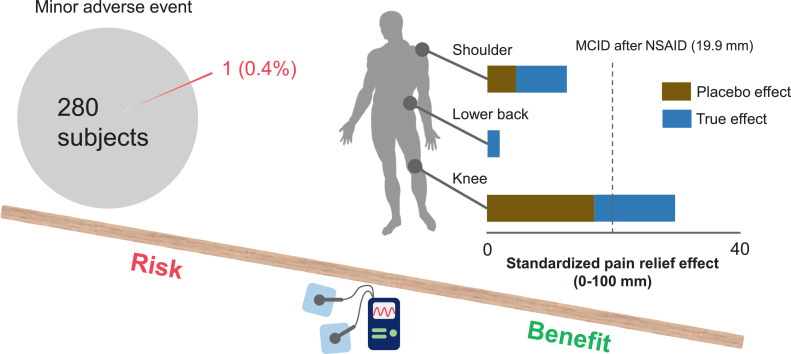
To provide a comprehensive picture of MCT-related adverse effects, we summarized adverse events from 9 RCTs and non-RCTs (281 patients in total). Only 1 patient (0.4%) in a non-RCT dropped out because of tingling in the feet the night after the first MCT treatment session.31 No serious adverse events requiring medical treatment have been reported to date.
Discussion
This meta-analysis summarizes the current evidence on the effect of MCT on musculoskeletal pain. A comprehensive assessment of 4 RCTs and 5 non-RCTs revealed that MCT significantly improved shoulder pain (1 study, 40 patients, “moderate” quality of evidence) and knee pain (1 study, 52 patients, “high” quality of evidence) compared with sham MCT, without any severe adverse events (fig 5). By assessing the placebo response in addition to the true treatment effects, this study also revealed that patients with subacute to chronic knee pain displayed the largest and a clinically significant placebo response. This evidence highlights the substantial effect of placebo response in treating patients with musculoskeletal pain. These treatment effects on knee pain are further supported by the high reproducibility of the treatment. Although small studies with varying levels of treatment approaches had limitations in providing a strong conclusion, this systematic review serves as a framework for future high-quality clinical trials.
Fig 5.
Graphic abstract showing the risk-benefit balance of microcurrent therapy on musculoskeletal pain. Of the 281 participants, only 1 patient (0.4%) reported a minor adverse event. When we consider the placebo response, MCT significantly improved subacute to chronic knee pain over the minimum clinically important difference after administration of nonsteroidal anti-inflammatory drugs, as indicated by the black vertical dashed line. The placebo response accounts for 57% of the overall treatment effects for subacute to chronic knee pain, suggesting a substantial effect of placebo response in patient care. Thus, these findings indicate that the clinical benefits of MCT may outweigh its harmful effects in people with subacute to chronic knee pain.
MCT as a potential and promising self-management option for subacute to chronic knee pain
The benefit of the treatment results based on the true treatment effect and placebo response depends in the context in which the treatment is delivered.35 These placebo effects are often clinically significant, especially in chronic painful conditions, including musculoskeletal pain. Although overall treatment effects including placebo response are important in patient care, placebo response has not been assessed in previous clinical trials. Here, we have summarized the current evidence on the effect of MCT on musculoskeletal pain while considering placebo response. Placebo response accounted for 57% of the overall treatment effect on knee pain, which generally supports the findings of previous studies that showed that placebo response accounted for 60% of the overall treatment effects in other diseases.36 Furthermore, placebo response further enhanced the effect of MCT on knee pain, with a clinically significant difference. This evidence further reinforces the importance of considering placebo response when interpreting clinical trial data and translating it to patient care.
High quality of evidence and reproducibility are essential factors in incorporating evidence from clinical trials to patient care. We found that MCT treatment for knee pain has a “high” quality of evidence according to the GRADE approach with high reproducibility. These results indicate that the treatment effect for knee pain has less bias, and clinicians can easily reproduce MCT treatment. Because MCT for knee pain was used as a self-management technique,29 the findings of this study support an effective and promising self-management program for subacute to chronic knee pain at home. Because there are only few adverse events associated with MCT, our findings suggest that MCT may be a core treatment option, especially for patients who cannot use NSAIDs because of the associated gastrointestinal problems.2
Implications for future research
This meta-analysis provides an opportunity for further improvement in the methodological rigor and serves as a foundation for additional clinical trials. Additional high-quality clinical trials for shoulder pain and LBP are needed, given that these are common chronic conditions leading to disability, but the quality of evidence and sample size are far from adequate. Evaluating the treatment effects on pain from multiple sites is particularly important in the context of primary care because the general population and individuals accessing primary care often display musculoskeletal pain that coexists in multiple regions of the body.37,38
An enhanced understanding of the mechanism of MCT is also imperative for establishing MCT as a promising treatment option. Currently, MCT is known to enhance adenosine triphosphate synthesis, amino acid transportation, and protein synthesis and inhibit the inflammatory response in an experimental skin model.5,39,40 This knowledge indicates that MCT may also be beneficial for the treatment of acute pain because of its anti-inflammatory effects. Nevertheless, the clinical trials included in this meta-analysis focused on subacute to chronic pain conditions. This knowledge gap should be considered in future studies.
Study limitations
The review processes were performed by a single reviewer, possibly yielding more errors than the preferred method of independent review by 2 reviewers.10 However, to overcome this issue, the reviewer performed full-text screening twice and additionally searched the references of the original articles. In addition, this review did not include studies comparing MCT with other treatment approaches. Therefore, this study cannot address whether MCT is more effective than other nonpharmacologic therapeutics. Finally, the small number of included studies limited our analyses. The placebo response and true treatment effect were calculated from 1 study for each outcome. We cannot address generalizability, and the MCT treatment effect may be specific to the patients included in each study.
This study has several strengths: (1) only RCTs with sham MCT control were included for effectiveness analysis; (2) observational studies were also included for risk analysis; and (3) replicability was examined using the TIDieR checklist.
Conclusions
This systematic review demonstrated that MCT significantly improved shoulder and knee pain compared with sham MCT, without any severe adverse events. Considering the placebo response, MCT has clinically significant benefits in the treatment of knee pain with self-management, but further investigation is needed to explore its other possible applications, such as for the treatment of shoulder pain and LBP. This study proposes a framework for future investigations on the effects of MCT on musculoskeletal pain. Continuous efforts considering placebo response in high-quality clinical trials could help enhance the translation of MCT from clinical trials to patient care.
Suppliers
-
a.
Review Manager, version 5.3; Nordic Cochrane Center, Cochrane Collaboration.
-
b.
Power and Sample Size program, version 3.1.2; Vanderbilt University Medical Center.
Acknowledgment
We thank Editage (www.editage.jp) for English language editing.
Footnotes
Supported in part by funding from Omron Healthcare Co, Ltd. The funding source had no role in the study design, data collection, data analyses, interpretation, or writing of the report.
Disclosures: none
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arrct.2021.100145.
Appendix. Supplementary materials
References
- 1.Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang W, Moskowitz RW, Nuki G. OARSI recommendations for the management of hip and knee osteoarthritis, part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Babatunde OO, Jordan JL, Van der Windt DA, Hill JC, Foster NE, Protheroe J. Effective treatment options for musculoskeletal pain in primary care: a systematic overview of current evidence. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin I, Wiles L, Waller R. What does best practice care for musculoskeletal pain look like? Eleven consistent recommendations from high-quality clinical practice guidelines: systematic review. Br J Sports Med. 2020;54:79–86. doi: 10.1136/bjsports-2018-099878. [DOI] [PubMed] [Google Scholar]
- 5.Mercola JM, Kirsch DL. The basis for microcurrent electrical therapy in conventional medical practice. J Adv Med. 1995;8:107–120. [Google Scholar]
- 6.Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2008;67:1716–1723. doi: 10.1136/ard.2008.092015. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 8.Shamseer L, Moher D, Clarke M. Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 9.Stroup DF, Berlin JA, Morton SC. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Green S. John Wiley & Sons; Hoboken, NJ: 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 11.Kirsch I. The placebo effect revisited: lessons learned to date. Complement Ther Med. 2013;21:102–104. doi: 10.1016/j.ctim.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 12.King W. In: Encyclopedia of pain. Gebhart GF, Schmidt RF, editors. Springer Berlin Heidelberg; Berlin, Heidelberg: 2013. Acute pain, subacute pain, and chronic pain; pp. 60–63. editors. [Google Scholar]
- 13.Poltawski L, Watson T. Bioelectricity and microcurrent therapy for tissue healing–a narrative review. Phys Ther Rev. 2009;14:104–114. [Google Scholar]
- 14.Wandel S, Juni P, Tendal B. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15. doi: 10.1038/s41536-018-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks JJ, Higgins JP. Statistical algorithms in Review Manager 5. Statistical Methods Group of The Cochrane Collaboration. 2010:1–11. [Google Scholar]
- 17.Tubach F, Ravaud P, Baron G. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou K, Wong J, Abdullah N. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2016;75:1964–1970. doi: 10.1136/annrheumdis-2015-208387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AT, Shrestha S, Collins JE, Sullivan JK, Losina E, Katz JN. Estimating contextual effect in nonpharmacological therapies for pain in knee osteoarthritis: a systematic analytic review. Osteoarthritis Cartilage. 2020;28:1154–1169. doi: 10.1016/j.joca.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Savović J, Page MJ. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Balshem H, Helfand M, Schunemann HJ. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Dupont WD, Plummer WD., Jr. Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann TC, Glasziou PP, Boutron I. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 24.Bartholdy C, Nielsen SM, Warming S, Hunter DJ, Christensen R, Henriksen M. Poor replicability of recommended exercise interventions for knee osteoarthritis: a descriptive analysis of evidence informing current guidelines and recommendations. Osteoarthritis Cartilage. 2019;27:3–22. doi: 10.1016/j.joca.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong K, Gokal R, Chevalier A, Todorsky W, Lim M. Microcurrent point stimulation applied to lower back acupuncture points for the treatment of nonspecific neck pain. J Altern Complement Med. 2017;23:295–299. doi: 10.1089/acm.2016.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atya AM. Efficacy of microcurrent electrical stimulation on pain, proprioception accuracy and functional disability in subacromial impingement: RCT. Indian J Physiother Occup Ther. 2012;6:15–18. [Google Scholar]
- 27.Chevalier A, Armstrong K, Gokal R. Microcurrent point stimulation applied to acupuncture points for the treatment of non-specific lower back pain. J Altern Complement Integr Med. 2016;2:016. doi: 10.1089/acm.2016.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koopman JS, Vrinten DH, van Wijck AJ. Efficacy of microcurrent therapy in the treatment of chronic nonspecific back pain: a pilot study. Clin J Pain. 2009;25:495–499. doi: 10.1097/AJP.0b013e31819a6f3e. [DOI] [PubMed] [Google Scholar]
- 29.Lawson D, Lee KH, Kang HB, Yang N, Llewellyn T, Takamatsu S. Efficacy of microcurrent therapy for treatment of acute knee pain: a randomized double-blinded controlled clinical trial. Clin Rehabil. 2021;35:390–398. doi: 10.1177/0269215520965320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lerner FN, Kirsch DL. A double blind comparative study of microstimulation and placebo effect in short term treatment of the chronic back pain patient. J Am Chiropr Assoc. 1981;15:101–106. [Google Scholar]
- 31.Poltawski L, Johnson M, Watson T. Microcurrent therapy in the management of chronic tennis elbow: pilot studies to optimize parameters. Physiother Res Int. 2012;17:157–166. doi: 10.1002/pri.526. [DOI] [PubMed] [Google Scholar]
- 32.Ranker A, Husemeyer O, Cabeza-Boeddinghaus N, Mayer-Wagner S, Crispin A, Weigl MB. Microcurrent therapy in the treatment of knee osteoarthritis: could it be more than a placebo effect? A randomized controlled trial. Eur J Phys Rehabil Med. 2020;56:459–468. doi: 10.23736/S1973-9087.20.05921-3. [DOI] [PubMed] [Google Scholar]
- 33.Wong YM, Suzuki S, Odagiri K. The micro-current stimulation of knee acupoints in management of chondromalacia patella: a case report. J Phys Ther Sci. 2020;32:772–774. doi: 10.1589/jpts.32.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyatt GH, Oxman AD, Vist GE. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wampold BE, Minami T, Tierney SC, Baskin TW, Bhati KS. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. J Clin Psychol. 2005;61:835–854. doi: 10.1002/jclp.20129. [DOI] [PubMed] [Google Scholar]
- 36.Walach H, Sadaghiani C, Dehm C, Bierman D. The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials–a secondary analysis. BMC Med Res Methodol. 2005;5:26. doi: 10.1186/1471-2288-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartvigsen J, Davidsen M, Hestbaek L, Sogaard K, Roos EM. Patterns of musculoskeletal pain in the population: a latent class analysis using a nationally representative interviewer-based survey of 4817 Danes. Eur J Pain. 2013;17:452–460. doi: 10.1002/j.1532-2149.2012.00225.x. [DOI] [PubMed] [Google Scholar]
- 38.Kamaleri Y, Natvig B, Ihlebaek CM, Bruusgaard D. Localized or widespread musculoskeletal pain: does it matter? Pain. 2008;138:41–46. doi: 10.1016/j.pain.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Cheng N, Van Hoof H, Bockx E. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin Orthop Relat Res. 1982:264–272. [PubMed] [Google Scholar]
- 40.Kaur S, Lyte P, Garay M. Galvanic zinc-copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin. Arch Dermatol Res. 2011;303:551–562. doi: 10.1007/s00403-011-1145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.