Abstract
We have identified two Gcn5-dependent histone acetyltransferase (HAT) complexes from Saccharomyces cerevisiae, the 0.8-MDa ADA complex and the 1.8-MDa SAGA complex. The SAGA (Spt-Ada-Gcn5-acetyltransferase) complex contains several subunits which also function as part of other protein complexes, including a subset of TATA box binding protein-associated factors (TAFIIs) and Tra1. These observations raise the question of whether the 0.8-MDa ADA complex is a subcomplex of SAGA or whether it is a distinct HAT complex that also shares subunits with SAGA. To address this issue, we sought to determine if the ADA complex contained subunits that are not present in the SAGA complex. In this study, we report the purification of the ADA complex over 10 chromatographic steps. By a combination of mass spectrometry analysis and immunoblotting, we demonstrate that the adapter proteins Ada2, Ada3, and Gcn5 are indeed integral components of ADA. Furthermore, we identify the product of the S. cerevisiae gene YOR023C as a novel subunit of the ADA complex and name it Ahc1 for ADA HAT complex component 1. Biochemical functions of YOR023C have not been reported. However, AHC1 in high copy numbers suppresses the cold sensitivity caused by particular mutations in HTA1 (I. Pinto and F. Winston, personal communication), which encodes histone H2A (J. N. Hirschhorn et al., Mol. Cell. Biol. 15:1999–2009, 1995). Deletion of AHC1 disrupted the integrity of the ADA complex but did not affect SAGA or give rise to classic Ada− phenotypes. These results indicate that Gcn5, Ada2, and Ada3 function as part of a unique HAT complex (ADA) and represent shared subunits between this complex and SAGA.
Posttranslational modifications of nucleosomal histones have been correlated with the modulation of the structure and function of chromatin (7). One of the most extensively studied modifications is the acetylation of the highly conserved amino-terminal histone tails. The steady-state level of acetylation of histone proteins is accomplished by the action of histone acetyltransferases (HATs) and histone deacetylases (HDACs) (37). Acetylation affects higher-order folding of chromatin fibers (16) and the interaction of nonhistone proteins with histones (14). It also plays an important role in histone deposition and nucleosome assembly during S phase (48) and can increase the affinity of transcription factors for nucleosomal DNA (35, 61). Correlations between transcription and histone acetylation are strengthened by reports showing that active chromosomal domains are hyperacetylated (6, 14, 32), while heterochromatic domains are hypoacetylated (10, 31).
A large number of recent studies have provided a direct molecular link between histone acetylation and transcriptional activation (24, 63). In these reports, it has been shown that several previously identified coactivators-adapters of transcription possess intrinsic HAT activity. Among these coactivators are yeast Gcn5 (11), human Gcn5 (65, 69), p300/Creb-binding protein (CBP)-associated factor (P/CAF) (71), TATA box binding protein (TBP)-associated factor 250 (TAFII250) (41), p300/CBP (2, 43), ACTR (12), and steroid receptor coactivator 1 (SRC-1( (55). Conversely, several transcriptional repressors and/or corepressors have been shown to be associated with HDACs, including Rpd3 (59), Sin3 (27, 33, 34, 73), and N-CoR/SMRT (1, 28). Moreover, human and Xenopus complexes containing both HDAC activity and ATP-dependent nucleosome remodeling activity have been isolated (62, 70, 74).
Many of these chromatin-modifying activities have been found within large multisubunit protein complexes that also contain several components with homology or identity to known transcriptional regulators (25, 58). Indeed, the coactivator-adapter protein Gcn5 is part of large multisubunit complexes in Saccharomyces cerevisiae, which enhances its ability to acetylate nucleosomal histones (20, 30, 46, 50, 51). In yeast, Gcn5 is involved in the regulation of a variety of genes (9, 18, 39, 51, 66). The largest of the Gcn5-dependent HAT complexes is the 1.8-MDa SAGA complex. SAGA comprises at least four distinct classes of gene products (22, 23). First, there are the Ada proteins Ada1, Ada2, Ada3, Gcn5 (Ada4), and Ada5 (Spt20), which have been isolated as proteins interacting functionally with the yeast activator Gcn4 and the herpes simplex virus activation domain VP16 (3, 4). The second group comprises Spt3, Spt7, Spt8, and Spt 20 (Ada5). These proteins are all members of the TBP-related set of Spt proteins, initially identified as suppressors of transcription initiation defects caused by promoter insertions of the transposable element Ty (68). The third group of proteins found to be part of SAGA are a subset of TAFIIs, including TAFII20/17, TAFII25/23, TAFII60, TAFII68/61, and TAFII90 (22). Finally, the product of the essential gene TRA1 has been shown to be a component of SAGA (23, 52). Apparent counterparts of the SAGA complex have been isolated from mammalian cells (40, 42, 67).
The second Gcn5-dependent HAT complex is the 0.8-MDa ADA complex, which differs from SAGA in many aspects. While the ADA complex is also dependent on and cofractionates with Ada2, it is not dependent on Ada1, Ada5 (Spt20), or the other Spt proteins found in SAGA (20, 57). Both the ADA and SAGA complexes can stimulate in vitro transcription from nucleosome templates in an acetyl coenzyme A-dependent reaction (56). However, the SAGA complex has been shown to physically interact with the acidic activators Gcn4 and VP16, whereas ADA failed to do so (60). In addition, we recently demonstrated that the ADA and SAGA HAT complexes generate overlapping, yet distinct, patterns of lysine acetylation on histone H3. While ADA can acetylate lysine residues 14 and 18 in histone H3, SAGA acetylates to some extent all four lysines in H3 (21).
Despite these differences between the two Gcn5-dependent HAT complexes, it remained unclear whether the smaller ADA is a subcomplex of the larger SAGA or functions as a distinct HAT complex in yeast. Fourteen subunits contained in the SAGA complex have been identified so far (22, 23). On the other hand, the proteins contained in ADA, other than the three adapter proteins (i.e., Ada2, Ada3, and Gcn5), were unknown. The best way to address whether ADA is distinct from SAGA is through the identification of ADA complex-specific components. We therefore purified the native ADA HAT complex from yeast. Mass spectrometry and immunoblotting analysis of the purified complex demonstrated that the yeast adapter proteins Ada2, Ada3, and Gcn5 are indeed components of the ADA complex. Importantly, we demonstrate by several criteria that the gene product of the open reading frame YOR023C is a novel component of ADA and is not present in SAGA. YOR023C in high copy numbers suppresses the cold sensitivity caused by particular mutations in HTA1 (45a), which encodes histone H2A (29). We named this protein Ahc1 for ADA HAT complex component 1. The presence of Ahc1 in the ADA complex indicates that it is a unique HAT complex in yeast that shares a subset of Ada proteins (Ada2, Ada3, and Gcn5) with the SAGA complex.
MATERIALS AND METHODS
Yeast strains.
ADA was purified from yeast strain CY396 (swi2Δ::HIS3, HO-lacZ, SWI2-HA-6HIS::URA3) and was described previously (44). Construction of a complete disruption of the YOR023C gene was carried out applying the one-step gene disruption (49) method with LEU2 as the disrupting marker. Transformation into yeast strain YJW 100 (MATa ade2-1 his3-11 leu2-3,112 trp1-1 ura3-1 can1-100) created YJW 103 (MATa ade2-1 his3-11 ahc1Δ::LEU2 trp1-1 ura3-1 can1-100). We verified the correct integration by PCR with two different primer pairs. For the complementation experiment (see Fig. 6) we transformed YJW 103 with plasmid pJW 100 (pRS314-AHC1:HA3-TRP1/CEN), thereby creating yeast strain YJW 104 (MATa ade2-1 his3-11 ahc1Δ::LEU2 trp1-1 ura3-1 can1-100; pAHC1:HA3 TRP/CEN). For the Ada− phenotype and in vivo transcription experiments, the strains used were BY4741 (MATa his3Δ1 leu2Δ met15Δ ura3Δ; wild type) and two mutants thereof, which had the mutations ahc1Δ and ada2Δ. The former mutant, with a deletion of open reading frame YOR023C, was purchased from Research Genetics (Huntsville, Ala.). The latter strain, SB608, was prepared by transforming BY4741 with an ada2Δ::hisG-URA3 fragment from plasmid pyADA2-KO (4); Ura+ transformants were then plated on fluoro-orotic acid media to select for loss of the URA3 marker (5). Yeast cells were transformed with DNA by the lithium acetate method (19). Yeast strains were grown at 30°C in YPD broth (1% yeast extract, 2% peptone, 2% glucose) or in minimal medium (0.67% yeast nitrogen base without amino acids, 2% glucose) and supplemented as required.
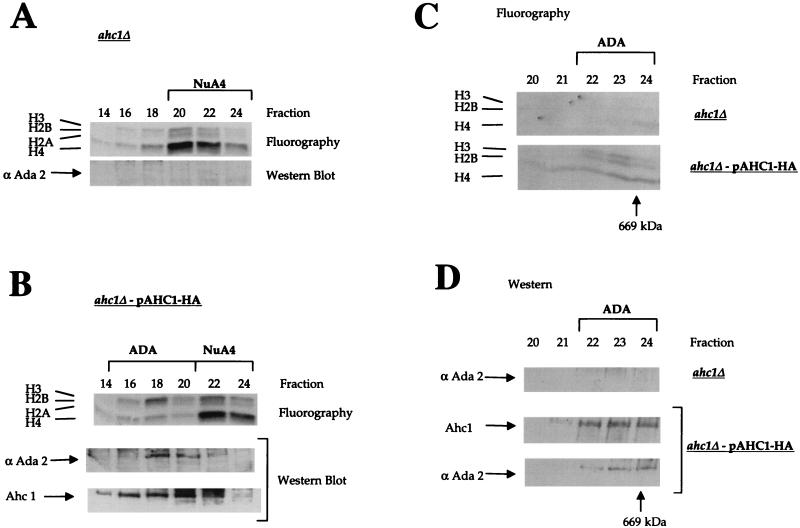
FIG. 6.
The ADA HAT complex can be rescued by plasmid expression of AHC1. (A and B) Mono Q fractionation of partially purified whole-cell extracts prepared from YJW 103 (ahc1Δ) and YJW 104 (ahc1Δ-pAHC1:HA3). HAT assay fluorograms and Western blots of fractions containing ADA and NuA4 (fractions 14 to 24) are shown. Immunodetection of Ahc1 was accomplished with an anti-HA antibody. The ADA HAT complex was specifically restored in YJW 104 (B) and was absent in the AHC1 deletion (A). HAT assays (C) and Western blots (D) from Superose 6 size exclusion chromatography of Mono Q fractions (A) are shown. Fractions 14 to 20 from a Mono Q column were pooled, concentrated, and fractionated on a Superose 6 column. Ahc1 was immunodetected by monoclonal anti-HA antibody. ADA is present only in YJW 104 bearing pAHC1-HA3 and was eluted at a molecular mass of ∼800 kDa.
Cloning and epitope tagging of AHC1.
The AHC1 coding sequence, including an upstream sequence of 1 kb spanning the endogenous promoter, was amplified by PCR from yeast genomic DNA. The fragment was subsequently digested with ApaI-SalI and cloned with these sites into pRS314 (54) bearing the triple hemagglutinin (HA) and a CYC1 termination sequence at the C-terminal end. Further details about the cloning process will be provided upon request.
Purification of the ADA complex.
For purification of the ADA complex, we began with 90 liters of the yeast strain CY396 grown to mid-log phase. Elution of ADA from each column was monitored by a combination of immunoblotting and HAT assays. Whole-cell extract was prepared according to a previously published procedure (13, 20). Extracts were incubated on a rotating wheel with 90 ml of Ni2+ nitrilotriacetic acid (NTA) agarose (Qiagen) overnight at 4°C. The resin was then sequentially washed in a column with extraction buffer (40 mM HEPES [pH 7.5], 350 mM NaCl, 10% glycerol, 0.1% Tween 20, 2 μg of leupeptin per ml, 2 μg of pepstatin A per ml, 5 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride [PMSF]) and 20 mM imidazole buffer (100 mM NaCl, 10% glycerol, 0.1% Tween 20, 2 μg of leupeptin per ml, 2 μg of pepstatin A per ml, 5 μg of aprotinin per ml, 1 mM PMSF [pH 7.5]), followed by elution of proteins with 300 mM imidazole buffer. The eluted material from the Ni2+ NTA agarose was directly loaded onto a 20-ml Mono Q HR16/10 (Pharmacia) column equilibrated with 100 mM NaCl in buffer A (50 mM Tris-HCl [pH 8.0], 10% glycerol, 0.1% Tween 20, 2 μg of leupeptin per ml, 2 μg of pepstatin A per ml, 5 μg of aprotinin per ml, 1 mM PMSF). Bound proteins were eluted by applying a 500-ml linear salt gradient from 100 to 500 mM NaCl in buffer A. Peak ADA fractions were pooled, diluted to 100 mM NaCl, and loaded onto a Mono Q HR5/5 column (Pharmacia); the elution from this column was done with a 25-ml linear gradient from 100 to 500 mM NaCl in buffer A. ADA fractions from the 1-ml Mono Q column (typically fractions 18 to 20) were brought to 100 mM NaCl in buffer B (50 mM HEPES [pH 7.8], 10% glycerol, 0.1% Tween 20, 2 μg of leupeptin per ml, 2 μg of pepstatin A per ml, 5 μg of aprotinin per ml, 1 mM PMSF) and applied to a Mono S HR5/5 column (Pharmacia). Proteins were step eluted with 200 mM NaCl and 500 mM NaCl in buffer B. ADA, which was eluted in the 500 mM NaCl wash, was then concentrated to 0.5 ml in Biomax-30 (Millipore) and subsequently loaded onto a Superose 6 HR10/30 size exclusion column (Pharmacia) equilibrated with 250 mM NaCl in buffer B. Peak fractions of ADA from the Superose 6 column were pooled, diluted to 100 mM NaCl, and loaded onto a 1-ml native DNA cellulose column (Pharmacia). Bound proteins were eluted with a 12-ml linear gradient from 100 mM to 1 M NaCl. ADA peak fractions were immediately dialyzed against 100 mM NaCl in buffer B and applied onto a 1-ml histone agarose column (Sigma). Elution from histone agarose was accomplished with a 12-ml linear salt gradient from 100 mM to 1 M NaCl. Fractions containing ADA were pooled, concentrated to 0.5 ml with Biomax-30, and loaded onto a Superose 6 HR10/30 column. Peak Superose 6 fractions were diluted to 100 mM NaCl, immediately concentrated to 0.5 ml, and directly loaded onto a Mini Q PC 3.2/3 column (Pharmacia). Bound proteins were eluted with a 2-ml linear salt gradient from 100 to 500 mM NaCl. For the isolation of ADA from yeast YJW 100, YJW 103, and YJW 104, we used 6 liters of cells. The strains were grown to an absorbance at 600 nm of 1.0 at 30°C. Whole-cell extracts were prepared as described and subsequently purified with 5 ml of Ni2+ NTA agarose, Mono Q HR5/5 fractionation, and Superose 6 chromatography as described above.
HAT assays, Western blotting, antibodies, and immunoprecipitation.
HAT assays were performed as previously described (13). After each chromatography, equivalent amounts of fractionated samples were subjected to electrophoresis with sodium dodecyl sulfate (SDS)–10% polyacrylamide gels, transferred to nitrocellulose membranes, and processed for immunoblotting. Anti-Ahc1 antibodies were raised in rabbits against a synthetic peptide spanning the amino-terminal 16 amino acids of Yor023C (MMSPAQDKLQHQHHNP) by Research Genetics. A monoclonal anti-HA antibody was purchased from Boehringer Mannheim (Indianapolis, Ind.). Immunodetection was performed with an enhanced chemiluminescence kit from Amersham according to the manufacturer’s protocol. For immunoprecipitation experiments, equivalent titers of anti-Ada2 or anti-Ahc1 antibodies were incubated with 20 μl of preequilibrated protein A-Sepharose resin (Pharmacia) for 1 h at room temperature. Beads were then washed with immunoprecipitation (IP) buffer (50 mM HEPES [pH 7.8], 150 mM NaCl, 10% glycerol, 0.1% Tween 20, 2 μg of leupeptin per ml, 2 μg of pepstatin A per ml, 1 mM PMSF), and purified ADA and SAGA fractions were added and incubated in IP buffer for 4 to 16 h at 4°C on a rotating wheel. After incubation supernatants were collected, the beads were washed five times with IP buffer. Input material, supernatants, and beads were directly assayed for HAT activity with free-core histones as a substrate. Protein concentrations were determined according to the method described by Bradford (8).
Mass spectrometry analysis.
ADA peak fractions after the final Mini Q column were concentrated in Microconcentrator-30 apparatus (Amicon), loaded onto a SDS–10% polyacrylamide gel, and stained with Coomassie blue. After destaining, the bands were excised and digested in gel with trypsin, according to the method of Shevchenko et al. (53). Identification of proteins was accomplished by microcolumn high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry and database searching. A 100- by 365-μm fused silica capillary (Polymetrics Inc., Phoenix, Ariz.) (17) was packed to a length of ∼10 cm with 10-mm POROS 10 R2 reverse-phase material (Perseptives Biosystems, Framingham, Mass.). The sample was directly loaded onto the microcolumn by helium pressurization of the sample in a stainless-steel bomb (72). Liquid chromatography was performed with a dual-syringe pump (Applied Biosystems, Foster City, Calif.). The mobile phase consisted of 0.5% acetic acid (solvent A) and 80:20 acetonitrile-water containing 5% acetic acid (solvent B). A precolumn split was used to deliver a flow rate of 250 to 400 nl/min through the column. The high-performance liquid chromatography pump was programmed to ramp solvent B from 2 to 60% in 30 min. Electrospray ionization was done at a voltage of 1.8 kV. Tandem mass spectra were automatically acquired during the entire gradient run (36). Tandem mass spectra were searched against the Saccharomyces genome database obtained from Stanford University with the SEQUEST program (15). Every sequence with high scores that matched a tandem mass spectrum was manually verified. To facilitate the identification of potential contaminants, sequences for human keratin and bovine trypsin were included.
β-Galactosidase assays and overexpression of Gal4-VP16.
For plate growth experiments, wild-type, ahc1Δ, and ada2Δ yeast strains were transformed with high-copy-number Gal4-VP16 plasmid (4) or empty vector (pDB20L-BglII) bearing the LEU2 selective marker and plated directly onto synthetic dextrose minimal medium. Plates were grown for 3 days at 30°C; Gal4-VP16 plates were incubated for an additional day at room temperature. For in vivo transcription assays, wild-type, ahc1Δ, and ada2Δ double transformants, containing pLGSD5 reporter plasmid (26) and low-copy-number Gal4-VP16 (4) or Gal4-VP16FA plasmid or empty vector (pRS315) (54), were grown to an optical density of 0.8 at 600 nm in selective synthetic complete medium. Extracts, prepared by breaking cells with glass beads, were tested for β-galactosidase activity and protein concentration as described previously (47). Reported values are the averages of the results from two to four independent transformants for each strain-plasmid combination.
RESULTS
Purification of the ADA HAT complex.
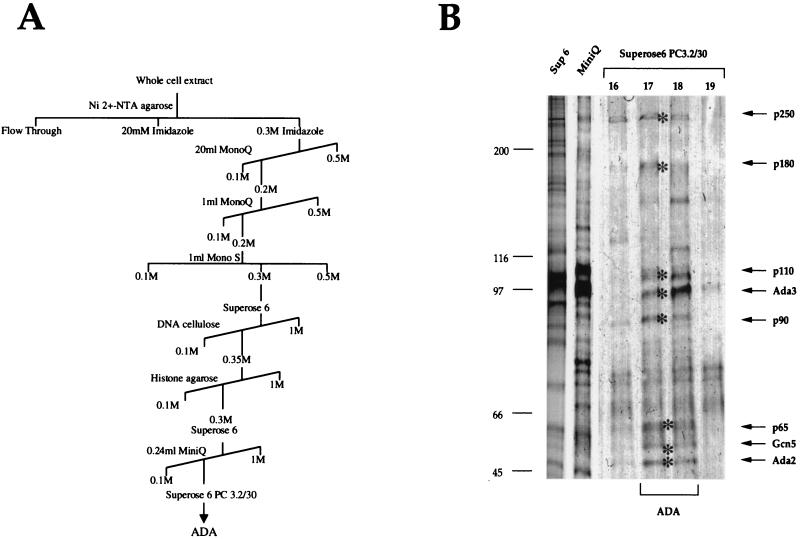
To gain a better insight into the protein composition of the ADA complex and to investigate its relationship to SAGA, we purified the ADA complex from S. cerevisiae whole-cell extract prepared from 90 liters of yeast cell culture. The purification strategy is outlined in Fig. 1A. After each chromatographic separation, we monitored for the ADA complex by two criteria. Column fractions were tested for their HAT activity, including monitoring the substrate specificity, since the ADA complex preferentially acetylates nucleosomal histones H3 and H2B (20). We also examined column fractions by Western blot analysis with antibodies to Ada2 and Gcn5. Only the peak fractions containing ADA complex activity from each column were used for subsequent chromatographic steps. The results of the purification are presented in Table 1. Aliquots of fractions from the 10th column, a Superose 6 PC 3.2/30 size exclusion column, were run on an SDS–7.5% polyacrylamide gel and analyzed by silver staining (Fig. 1B). Eight proteins with approximate molecular masses of 50, 55, 65, 90, 97, 110, 180, and 250 kDa coeluted with the ADA HAT activity (Fig. 1B, lanes 17 and 18). These protein bands were excised and analyzed by mass spectrometry.
FIG. 1.
Purification of ADA. (A) Schematic representation of the chromatographic steps applied for purification of the ADA complex. ADA was followed by HAT assay and immunoblotting. (B) Silver staining of purified ADA. Aliquots of ADA peak fractions which were eluted from the last three chromatographic steps (Superose 6, Mini Q, and Superose 6 PC 3.2/30) were separated by SDS-polyacrylamide gel electrophoresis and stained with silver. For the final Superose 6 column, side fractions are also shown. Arrows and asterisks indicate proteins which were coeluted with the purified ADA fraction in amounts apparently stoichiometric with one another.
TABLE 1.
Purification of the ADA HAT complex
| Purification stepa | Total protein (μg) | Total units (μU)b | Sp Act (μU/mg) | Purification (fold) |
|---|---|---|---|---|
| NiAg eluate | 580,000 | 3,000 | 5.2 | 1 |
| 20 ml Mono Q | 19,500 | 1,620 | 83 | 16 |
| 1 ml Mono Q | 4,000 | 4,800 | 1,200 | 214 |
| Mono | 186 | 4,350 | 23,387 | 4,498 |
| Superose 6 | 52.5 | 4,125 | 78,572 | 14,030 |
| DNA cellulose | 16.8 | 3,090 | 184,000 | 34,783 |
| Histone agarose | 7.9 | 1,680 | 212,658 | 40,580 |
| Superose 6 | 2.52 | 1,305 | 517,875 | 98,550 |
| Mini Q | 0.82 | 620 | 755,854 | 147,826 |
| Superose 6 PC 3.2/30 | 0.34 | 525 | 1.54 × 106 | 298,550 |
Purification was done with whole-cell extract prepared from 90 liters of yeast cell culture.
One microunit is defined as the activity required to transfer 1 fmol of acetyl residues to histones under standard histone acetyltransferase assay conditions (see Materials and Methods).
The proteins migrating with molecular masses of 50, 55, and 97 kDa were identified as the adapter proteins Ada2, Gcn5, and Ada3, respectively (Fig. 2). Sixteen peptides from p97 identified this protein as Ada3. Ada2 and Gcn5 were identified by nine and five peptides, respectively. These findings confirmed previous genetic and biochemical studies with less-purified material (20). Mass spectrometry also tentatively identified the remaining subunits of the ADA complex. However, to confirm that each putative subunit is not a contaminant in the final fraction, it is necessary to generate antibodies against peptides from the protein. This allows an examination of the copurification and immunoprecipitation of the putative subunit with the ADA complex. Moreover, by generating a yeast strain where the corresponding open reading frame is deleted, its importance for the activity and integrity of the ADA complex can be examined. Using these criteria, we have thus far confirmed the presence of one novel subunit of the ADA complex (see below).
FIG. 2.
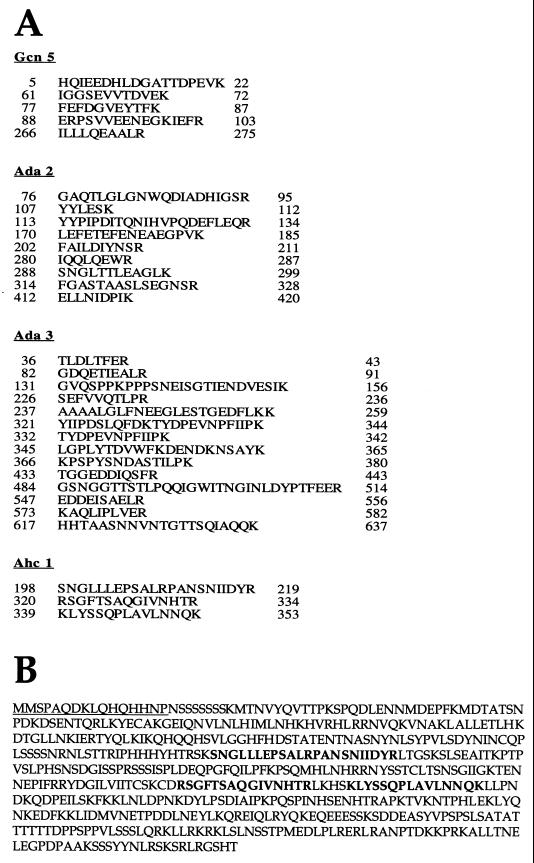
Mass spectrometry analysis. Mass spectrometry identified the four ADA subunits as Ada2, Ada3, Gcn5, and Ahc1. (A) Peptide sequences obtained by mass spectrometry for Ada2, Ada3, Gcn5, and Ahc1. Numbers at the left and the right of each sequence indicate the first and the last amino acids identified, respectively. For all four proteins, numerous hits were obtained. (B) Complete amino acid sequence for Ahc1. The underlined 16 N-terminal amino acid residues were used to generate the Ahc1 antiserum. Residues shown in boldface represent amino acids identified by mass spectrometry.
Identification of a novel component of the ADA complex.
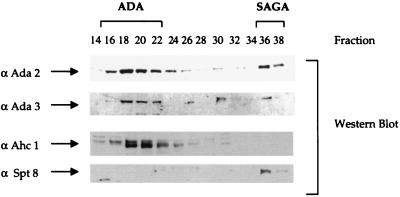
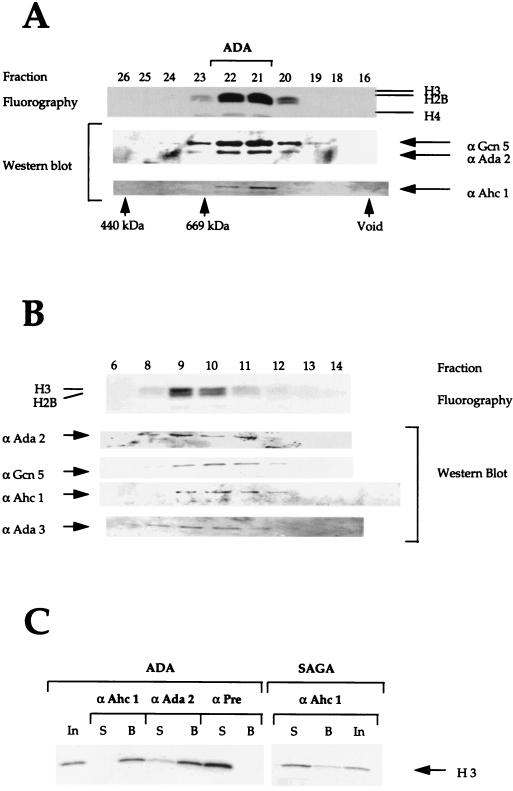
Mass spectrometry analysis of the p65 band revealed the presence of three peptides from the same open reading frame, YOR023C (Fig. 2). This previously uncharacterized open reading frame has been renamed AHC1 for ADA HAT complex component 1. The amino acid sequence of the entire protein is shown in Fig. 2B. We generated antibodies against the peptide spanning the first 16 N-terminal amino acid residues (Fig. 2B) and used this antiserum to follow this protein during the course of purification. Fractions from the initial Mono Q column were tested with the Ahc1 antiserum, since this column separates the ADA, NuA4, NuA3, and SAGA complexes (13). The Western blot results in Fig. 3 demonstrate that Ada2 and Ada3 cofractionated, as expected, with the ADA and SAGA complexes. Spt8 cofractionated only with SAGA and was not detected in fractions containing ADA. By contrast, Ahc1 cofractionated only with the ADA complex and was not found in the fractions containing the SAGA complex. Thus, while Ada2, Ada3, and Gcn5 are contained in both complexes, each also appears to have unique subunits not found in the other, i.e., Spt8 in SAGA and Ahc1 in ADA.
FIG. 3.
Western blot analysis of Mono Q chromatography. Ten microliters of indicated fractions was separated after Mono Q chromatography by SDS–10% polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. Membranes were incubated with antibodies (α) against Ahc1, Ada2, Ada3, and Spt8.
To confirm the copurification of Ahc1 with the ADA complex, we performed Western blotting on highly purified ADA fractions from further chromatographic steps. Figure 4 shows Western blots with antibodies against ADA subunits and Ahc1 on fractions from columns, that were used very late in the purification process, Superose 6 and Mini Q. As shown in Fig. 4A, Gcn5, Ada2, and Ahc1 coeluted with ADA HAT activity on the Superose 6 column that represented the eighth chromatographic step. Similarly, Ada2, Ada3, Gcn5, and Ahc1 coeluted with ADA complex HAT activity on the Mini Q column, the ninth column. Thus, Ahc1 copurifies with the other subunits and the HAT activity of the ADA complex through multiple chromatographic steps, suggesting that it is a bona fide subunit of the complex.
FIG. 4.
Ahc1 is coeluted with purified ADA. (A) Fractions from the seventh column, Superose 6 size exclusion chromatography, were tested in a nucleosomal HAT assay and Western blotting with the indicated antibodies. The upper panel shows the fluorogram from the HAT assay depicting the specificity of ADA. Histones H3 and H2B were acetylated by ADA. (B) Fluorogram after nucleosomal HAT assay and Western blotting with the indicated antibodies (α) of purified fractions from the Mini Q column. (C) Immunoprecipitation with purified ADA and SAGA complex. ADA and SAGA were incubated with preimmune serum, anti-Ada2 antiserum, or anti-Ahc1 antiserum immobilized on protein A-Sepharose beads. The fluorogram of HAT reaction products obtained with free histones is shown.
To confirm the physical association of Ahc1 with the ADA complex, we tested whether the anti-Ahc1 antisera were able to immunoprecipitate the HAT activity of the complex. As shown in Fig. 4C, anti-Ahc1 antibodies immunoprecipitated the ADA complex as efficiently as antibodies against Ada2. Both antibodies were able to deplete ADA HAT activity from the supernatant, in contrast to the preimmune serum. In addition, the ADA HAT activity in each case was detected on the beads (Fig. 4C). By contrast, anti-Ahc1 antisera failed to immunoprecipitate the SAGA complex, confirming that Ahc1 is a component of ADA but not SAGA.
The ADA complex is dependent on the AHC1 gene.
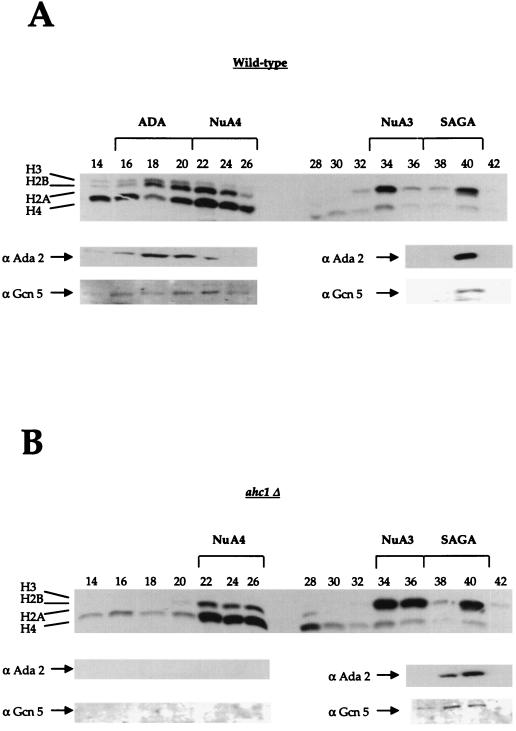
From the biochemical data presented above, we concluded that the protein Ahc1 is a distinct component of the ADA HAT complex. To examine the importance of Ahc1, we generated an AHC1 deletion strain and investigated the effects of this deletion on the ADA complex. To do this, we prepared whole-cell extracts from a wild-type strain and the mutant strain bearing the complete deletion of AHC1. Extracts from both strains were partially purified with Ni2+ NTA agarose and Mono Q anion exchange chromatography. Fractions eluting from the Mono Q column were then subjected to HAT assays with both nucleosomes and core histones as substrates. As demonstrated in Fig. 5, partially purified ADA prepared from the wild-type strain was eluted in fractions 18 to 22 (identified by H3-H2B HAT activity and Ada2 and Gcn5 Western blotting). In addition, the other previously identified HATs, NuA4, NuA3, and SAGA, were eluted as predicted (13). Figure 5B shows the fractionation of complexes from extract prepared from the strain bearing a disruption in AHC1. In this instance, we found that extracts from ahc1Δ cells specifically lacked the ADA complex (fractions 18 to 22), while the SAGA, NuA4, and NuA3 complexes were unaffected. There was neither detectable nucleosomal ADA HAT activity (Fig. 5) nor free-core histone HAT activity for ADA (results not shown). Furthermore, immunoblotting analysis with antibodies against Ada2 and Gcn5 indicated that, in addition to the loss of activity, the ADA complex itself was lost in the ahc1Δ preparation. Importantly, SAGA was unaffected (fractions 36 to 38). Therefore, Ahc1 behaved like Ada2 and Ada3 (20) in that it was required for the overall structural integrity of the ADA complex (19a).
FIG. 5.
An AHC1 mutant specifically affects the ADA HAT complex. Whole-cell extracts from a wild-type strain and a yeast strain bearing a mutation in AHC1 were partially purified with Ni2+ NTA agarose and Mono Q chromatography. (A) In the top panel, a typical fluorogram from a nucleosomal HAT assay with fractions from a Mono Q column prepared from a wild-type strain is presented. The four HAT complexes are indicated at the top. The lower panels show results from Western blotting with antibodies (α) raised against Ada2 and Gcn5. (B) Fluorogram from a nucleosomal HAT assay of Mono Q fractions prepared from the ahc1Δ yeast strain. NuA4, NuA3, and SAGA were eluted in the same fractions as in the wild type. ADA was absent in the AHC1 mutant. Western blots (lower panels) demonstrate that anti-Ada2 and anti-Gcn5 antibodies showed immunoreactivity only for SAGA (fractions 38 to 40).
To further substantiate the importance of Ahc1 for ADA integrity, we wished to address the possibility that the complex would be restored when AHC1 is expressed on a low-copy-number plasmid in ahc1Δ cells. To this end, we cloned AHC1 bearing a triple HA epitope tag at the C terminus into the ARS-CEN vector pRS314 (54) and expressed it under its endogenous promoter in the AHC1 deletion strain. Whole-cell extracts were prepared from yeast strains YJW 103 (ahc1Δ) and YJW 104 (ahc1Δ pAHC1:HA3), and ADA was fractionated as described (see Materials and Methods). While ADA was missing in YJW 103 (Fig. 6A), we found that ADA HAT activity was present in Mono Q fractions 16 to 20 in YJW 104 (Fig. 6B). Immunodetection with antibodies against Ada2 and a monoclonal anti-HA antibody to detect epitope-tagged Ahc1 confirmed the presence of ADA in this preparation. The ADA complex was apparently fully restored in YJW 104 cells as demonstrated by size exclusion chromatography (Fig. 6C and D). The complex eluted in fractions 22 to 24 from a Superose 6 column, giving it a size of about 800 kDa. Again, no ADA complex was detectable in ahc1Δ cells; however, the ADA complex was present in ahc1-pAHC1-HA as detected by its H3 HAT activity (Fig. 6C) and by Western blotting (Fig. 6D). Note that the slight histone H4 activity in fraction 24 on Superose 6 is from a slightly smaller contaminating HAT complex which peaks in Mono Q fractions 14 and 15 (Fig. 5) and is unrelated to ADA (12a).
An AHC1 deletion does not display an Ada phenotype.
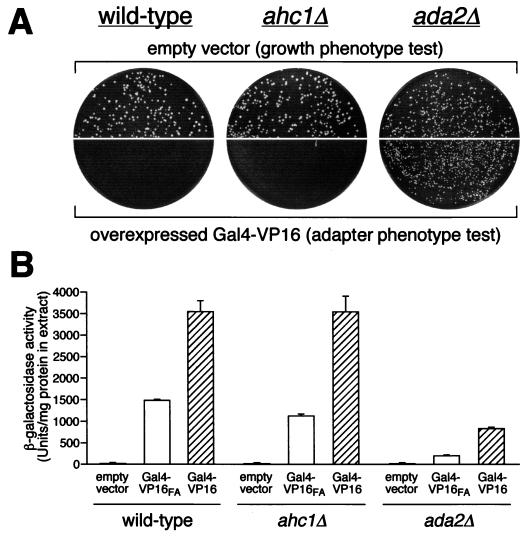
Mutations in ADA2, ADA3, and GCN5 (ADA4) were isolated in a selection for mutants that confer resistance to toxicity from overexpressed Gal4-VP16. In addition, mutations in any of these genes reduced transcriptional activation by the acidic activators VP16 and GCN4 (4, 38, 45). Therefore, we wished to ask whether a mutation in AHC1 is resistant to overexpression of Gal4-VP16 and shows reduced Gal-VP16-mediated transcription levels. Overexpression of Gal4-VP16 and in vivo transcription assays were performed as described in Materials and Methods. As demonstrated in Fig. 7, AHC1 deletion did not exert the typical adapter (Ada−) phenotypes as described for deletions of ADA2, ADA3, or GCN5 (4, 38, 45). First, we found that the ahc1Δ strain did not have a growth defect on minimal medium (Fig. 7A, upper panels). The wild type and the AHC1 deletion strain showed similar growth on minimal media while the adapter ada2Δ mutant grew more poorly and resulted in smaller colonies. Second, the AHC1 deletion was unable to relieve the toxicity of overexpressed Gal4-VP16 (Fig. 7A, lower panels). No significant growth was seen for either the wild type or the ahc1Δ strain, whereas ada2Δ cells were able to grow. Third, Gal4-VP16-mediated transcription levels were similar in the wild type and the mutant (Fig. 7B). Thus, results from these three assays measuring Ada− phenotypes indicate that a strain lacking Ahc1 does not exhibit these defects.
FIG. 7.
An AHC1 deletion does not display a classic Ada phenotype. (A) Transformants of wild-type, ahc1Δ, and ada2Δ (adapter control) cells containing high-copy-number empty vector were plated on minimal medium to assess overall growth phenotype (upper panels). To test for adapter phenotype (Ada−; relief of toxicity of overexpressed chimeric activator Gal4-VP16), cells were transformed with high-copy-number activator plasmid and plated on minimal medium (lower panels). (B) Quantitation of acidic-activator-mediated in vivo transcription is presented. Wild-type, ahc1Δ, and ada2Δ cells were transformed with pLGSD5 reporter plasmid and low-copy-number empty vector, Gal4-VP16, or Gal4-VP16FA plasmids, and extracts were assayed for β-galactosidase activity.
DISCUSSION
The discovery that several transcriptional coactivator proteins are HATs provided a direct molecular link between histone acetylation and activation of transcription. One of these coactivators possessing HAT activity, the yeast protein Gcn5, functions as the catalytic subunit in several native high-molecular-weight complexes (20, 46, 50, 51). The largest of these Gcn5-dependent native HAT complexes, the 1.8-MDa SAGA complex, has recently been purified and characterized (22, 23). SAGA contains Tra1, several Ada proteins, the TBP class of Spt proteins, and a subset of TAFII proteins. Members from each of these classes of proteins have been demonstrated to be essential for structural integrity (20, 57), transcriptional stimulation (64), or nucleosomal histone acetylation (22) by SAGA.
While many subunits of the SAGA complex have been identified, proteins other than Ada2, Ada3, and Gcn5 comprising the 0.8-MDa ADA complex were not known. A long-standing question to address was whether ADA functions as a distinct complex in S. cerevisiae or is a subcomplex of the larger SAGA complex. There are several lines of evidence which suggested that the ADA complex may be distinct from SAGA. First, ADA is capable of acetylating nucleosomal histones although lacking TAFII68. A depletion of TAFII68 from SAGA resulted in the loss of both nucleosomal acetylation and transcriptional stimulation for SAGA (22). Second, ADA and SAGA show different lysine specificities within histone H3 (21). One can speculate that there are distinct proteins within either complex that are required for this specificity. Third, in contrast to SAGA, ADA fails to interact with the acidic activators Gcn4 and VP16 in vitro (60), even though it contains Ada2, which has been shown to physically interact with these activation domains (3). Fourth, a recent study has demonstrated that a deletion of the bromodomain within Gcn5 significantly reduced nucleosomal HAT activity of SAGA, while the ability of ADA to acetylate nucleosomes was unchanged (57). All of these observations suggest distinct activities of the ADA complex relative to the SAGA complex. However, these differences could arise from the fact that ADA lacks many subunits found in SAGA and/or that the ADA complex may contain distinct subunits not found in the SAGA complex.
In this study, we have purified the ADA HAT complex and have identified a novel subunit of this complex. Indeed, we find that a protein of previously unknown function, Yor023C, herein named Ahc1, is a unique subunit of the ADA complex. Several lines of evidence demonstrate that Ahc1 is an integral component of the ADA complex. First, Ahc1 was identified by mass spectrometry analysis as a protein in the highly purified ADA complex (Fig. 2). Second, Western blot experiments using an anti-Ahc1 antiserum confirmed the copurification of Ahc1 with the HAT activity of ADA. Third, anti-Ahc1 antibodies immunoprecipitated the ADA complex, demonstrating that Ahc1 is a stably interacting component of the purified complex (Fig. 4A and B). Fourth, an AHC1 deletion strain specifically lacked the ADA complex, while the larger SAGA complex was unaffected by this deletion. Thus, the structural integrity of the ADA complex was dependent on the presence of the AHC1 gene product. Fifth, reintroducing Ahc1 on a plasmid restored the ADA complex, as shown in Fig. 6.
The finding that the ADA complex contains unique subunits not found in the SAGA complex illustrates that it is a distinct complex and not merely a subcomplex of SAGA. While the functions of the ADA complex remain under investigation, it is clear that it is not responsible for the classic Ada− phenotypes (e.g., the lower Gal4-VP16 function in vivo) (4). While the deletion of AHC1 disrupted the ADA complex, it did not result in an Ada− phenotype (Fig. 7). It is therefore likely that ADA is not involved in transcriptional activation mediated by acidic activators in the same manner as SAGA is. Consistent with this is the observation that SAGA, but not ADA, interacts with the acidic activators VP16 and Gcn4 (60). Moreover, deletion of the SAGA components Ada1, Spt20, and Spt7, which are not in the ADA complex, disrupts the SAGA complex and also results in Ada− phenotypes (57). Thus, the functions of Ada2, Ada3, and Gcn5 which give rise to the Ada− phenotypes are most likely attributable to the functions of the SAGA complex. However, a genetic link between the ADA complex and histone function is suggested by the fact that AHC1 in high copy numbers suppresses the cold sensitivity mediated by particular mutations in histone H2A (29, 45a). The presence of the adapter proteins Ada2, Ada3, and Gcn5 in two unique complexes indicates that these proteins may perform important roles in complexes with distinct functions. In this regard, the Ada proteins are similar to other proteins that are involved in transcriptional regulation (e.g., several TAFIIs and Tra1 [22, 23]).
ACKNOWLEDGMENTS
We thank members of the Workman lab for many helpful discussions. A.E. also thanks David Steger, Sam John, and Patrick Grant for their continuous encouragement during this work. We also thank LeAnn Howe for her help during the initial cloning steps.
This work was supported by the National Center for Research Resources, National Institutes of Health, and by grants from the National Institute of General Medical Sciences awarded to J.L.W., National Institutes of Health grant 11823-02 and NSF Science and Technology Center grant BIR9214821AM awarded to J.R.Y., and National Institutes of Health and NSF grants to S.L.B. A.E. was a recipient of a postdoctoral fellowship from the Austrian Science Foundation (Fonds zur Förderung der wissenschaftlichen Forschung, J1571-GEN) and was a postdoctoral associate of the Howard Hughes Medical Institute. D.E.S. was supported by an NIH postdoctoral fellowship. J.L.W. is an associate investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Alland L, Muhle R, Hou H J, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP coactivator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Barlev N A, Candau R, Wang L, Darpino P, Silverman N, Berger S L. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. doi: 10.1074/jbc.270.33.19337. [DOI] [PubMed] [Google Scholar]
- 4.Berger S L, Pina B, Silverman N, Marcus G A, Agapite J, Reigier J L, Triezenberg S J, Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 5.Boeke J D, LaCroute F, Fink G R. A positive selection for mutants lacking orotidine-5-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 6.Bone J R, Lavender J, Richman R, Palmer M J, Turner B M, Kuroda M I. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 1994;8:96–104. doi: 10.1101/gad.8.1.96. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury E M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992;14:9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Brandl C J, Martens J A, Margaliot A, Stenning D, Furlanetto A M, Saleh A, Hamilton K S, Genereaux J. Structure/functional properties of the yeast dual regulator protein NGG1 that are required for glucose repression. J Biol Chem. 1996;271:9298–9306. doi: 10.1074/jbc.271.16.9298. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein M, Sobel R E, Allis C D, Turner B M, Broach J R. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 12a.Eberharter, A., and P. Grant. Unpublished data.
- 13.Eberharter A, John S, Grant P A, Utley R T, Workman J L. Identification and analysis of yeast nucleosomal histone acetyltransferase complexes. Methods. 1998;14:315–321. doi: 10.1006/meth.1998.0635. [DOI] [PubMed] [Google Scholar]
- 14.Edmondson D G, Smith M M, Roth S Y. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996;10:1247–1259. doi: 10.1101/gad.10.10.1247. [DOI] [PubMed] [Google Scholar]
- 15.Eng J K, McCormack A L, Yates J R., III An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher T M, Hansen J C. The nucleosome array: structure/function relationships. Crit Rev Eukaryot Gene Expr. 1996;6:149–188. doi: 10.1615/critreveukargeneexpr.v6.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 17.Gatlin C L, Kleemann G R, Hays L G, Link A J, Yates J R., III Protein identification at the low femtomole level from silver-stained gels using a new fritless electrospray interface for liquid chromatography-microspray and nanospray mass spectrometry. Anal Biochem. 1998;263:93–101. doi: 10.1006/abio.1998.2809. [DOI] [PubMed] [Google Scholar]
- 18.Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992;11:4145–4152. doi: 10.1002/j.1460-2075.1992.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz D A, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Grant, P. A. Unpublished data.
- 20.Grant P A, Duggan L, Côté J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, Berger S L, Workman J L. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 21.Grant P A, Eberharter A, John S, Cook R G, Turner B M, Workman J L. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274:5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 22.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcription stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 23.Grant P A, Schieltz D, Pray-Grant M G, Yates J R, Workman J L. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol Cell. 1998;2:863–867. doi: 10.1016/s1097-2765(00)80300-7. [DOI] [PubMed] [Google Scholar]
- 24.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 25.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 26.Guarente L, Yocum R R, Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci USA. 1982;79:7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassig C A, Fleischer T C, Billin A N, Schreiber S L, Ayer D E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 28.Heinzel T, Lavinsky R M, Mullen T-M, Söderström M, Laherty C D, Torchia J, Yang W-M, Brard G, Ngo S, Davie J R, Seto E, Eisenman R N, Rose D W, Glass C K, Rosenfeld M G. A complex containing N-CoR, mSin3 and histone deacetylates mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 29.Hirschhorn J N, Bortvin A L, Ricupero-Hovasse S L, Winston F. A new class of histone H2A mutations in Saccharomyces cerevisiae causes specific transcriptional defects in vivo. Mol Cell Biol. 1995;15:1999–2009. doi: 10.1128/mcb.15.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horiuchi J, Silverman N, Piña B, Marcus G A, Guarente L. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol Cell Biol. 1997;17:3220–3228. doi: 10.1128/mcb.17.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeppesen P, Turner B M. The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenic marker for gene expression. Cell. 1993;74:281–289. doi: 10.1016/0092-8674(93)90419-q. [DOI] [PubMed] [Google Scholar]
- 32.Johnson C A, O’Neill L P, Mitchell A, Turner B M. Distinctive patterns of histone H4 acetylation are associated with defined sequence elements within both heterochromatic and euchromatic regions of the human genome. Nucleic Acids Res. 1998;26:994–1001. doi: 10.1093/nar/26.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 34.Laherty C D, Yang W-M, Sun J-M, Davie J R, Seto E, Eisenman R N. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee D Y, Hayes J J, Pruss D, Wolffe A P. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993;72:73–84. doi: 10.1016/0092-8674(93)90051-q. [DOI] [PubMed] [Google Scholar]
- 36.Link A J, Hays L G, Carmack E B, Yates J R., III Identifying the major proteome components of Haemophilus influenzae type-strain NCTC 8143. Electrophoresis. 1997;18:1314–1334. doi: 10.1002/elps.1150180808. [DOI] [PubMed] [Google Scholar]
- 37.Loidl P. Histone acetylation: facts and questions. Chromosoma. 1994;103:441–449. doi: 10.1007/BF00337382. [DOI] [PubMed] [Google Scholar]
- 38.Marcus G A, Silverman N, Berger S L, Horiuchi J, Guarente L. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 1994;13:4807–4815. doi: 10.1002/j.1460-2075.1994.tb06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martens J A, Genereaux J, Saleh A, Brandl C J. Transcription activation by PDR1p is inhibited by its association with NGG1p/ADA3p. J Biol Chem. 1996;271:15884–15890. doi: 10.1074/jbc.271.27.15884. [DOI] [PubMed] [Google Scholar]
- 40.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 41.Mizzen C A, Yang X-J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J L, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 42.Ogryzko V V, Kotani T, Zhang X, Schiltz R L, Howard T, Yang X-J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 43.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 44.Peterson C L, Dingwall A, Scott M P. Five SWI/SNF gene products are components of a large multisubunit complex required for transcriptional enhancement. Proc Natl Acad Sci USA. 1994;91:2905–2908. doi: 10.1073/pnas.91.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piña B, Berger S, Marcus G A, Silverman N, Agapite J, Guarente L. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol Cell Biol. 1993;13:5981–5989. doi: 10.1128/mcb.13.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Pinto, I., and F. Winston. Personal communication.
- 46.Pollard K J, Peterson C L. Role for ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol Cell Biol. 1997;17:6212–6222. doi: 10.1128/mcb.17.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 48.Roth S Y, Allis C D. Histone acetylation and chromatin assembly: a single escort, multiple dances? Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- 49.Rothstein R. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Garcia A B, Sendra R, Pamblanco M, Tordera V. Gcn5p is involved in the acetylation of histone H3 in nucleosomes. FEBS Lett. 1997;403:186–190. doi: 10.1016/s0014-5793(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 51.Saleh A, Lang V, Cook R, Brandl C J. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272:5571–5578. doi: 10.1074/jbc.272.9.5571. [DOI] [PubMed] [Google Scholar]
- 52.Saleh A, Schieltz D, Ting N, McMahon S B, Litchfield D W, Yates J R, Lees-Miller S P, Cole M D, Brandl C J. Tra1p is a component of the yeast Ada·Spt transcriptional regulatory complexes. J Biol Chem. 1998;273:26559–26565. doi: 10.1074/jbc.273.41.26559. [DOI] [PubMed] [Google Scholar]
- 53.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 54.Sikorski R, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M-J, O’Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 56.Steger D J, Eberharter A, John S, Grant P A, Workman J L. Purified histone acetyltransferases stimulate HIV-1 transcription from preassembled nucleosomal arrays. Proc Natl Acad Sci USA. 1998;95:12924–12929. doi: 10.1073/pnas.95.22.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Struhl K, Moqtaderi Z. The TAFs in the HAT. Cell. 1998;94:1–4. doi: 10.1016/s0092-8674(00)81213-1. [DOI] [PubMed] [Google Scholar]
- 59.Taunton J, Hassig C A, Schreiber S L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 60.Utley R T, Ikeda K, Grant P A, Côté J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators target histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 61.Vettese-Dadey M, Grant P A, Hebbes T R, Crane-Robinson C, Allis C D, Workman J L. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 1996;15:2508–2518. [PMC free article] [PubMed] [Google Scholar]
- 62.Wade P A, Jones P L, Vermaak D, Wolffe A P. A multiple subunit Mi-2 histone deacetylase from Xenopus laevis cofractionates with an associated Snf2 superfamily ATPase. Curr Biol. 1998;8:843–846. doi: 10.1016/s0960-9822(98)70328-8. [DOI] [PubMed] [Google Scholar]
- 63.Wade P A, Pruss D, Wolffe A P. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Liu L, Berger S L. Critical residues for histone acetylation by Gcn5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 1998;12:640–653. doi: 10.1101/gad.12.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis C D, Berger S L. Histone acetyltransferase activity is conserved between yeast and human GCN5 and is required for complementation of growth and transcriptional activation. Mol Cell Biol. 1997;17:519–527. doi: 10.1128/mcb.17.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welihinda A A, Tirasophon W, Green S R, Kaufman R J. Gene induction in response to unfolded protein in the endoplasmic reticulum is mediated through Ire1p kinase interaction with a transcriptional coactivator complex containing Ada5p. Proc Natl Acad Sci USA. 1997;94:4289–4294. doi: 10.1073/pnas.94.9.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 68.Winston F, Carlson M. Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet. 1992;8:387–391. doi: 10.1016/0168-9525(92)90300-s. [DOI] [PubMed] [Google Scholar]
- 69.Xu W, Edmondson D G, Roth S Y. Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates. Mol Cell Biol. 1998;18:5659–5669. doi: 10.1128/mcb.18.10.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xue Y, Wong J, Moreno T G, Young M K, Côté J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- 71.Yang X-J, Ogryzko V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 72.Yates J R, III, McCormack A L, Hatden J B, Davey M P. Peptide sequence analysis on quadrupole mass spectrometers. In: Celis J E, editor. Cell biology, a laboratory handbook. San Diego, Calif: Academic Press; 1994. pp. 380–389. [Google Scholar]
- 73.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylase and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/s0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y, LeRoy G, Seelig H P, Lane W S, Reinberg D. The dermatomyositis-specific autoantigen Mi2 is a component of a complex containing histone deacetylase and nucleosome remodeling activities. Cell. 1998;95:279–289. doi: 10.1016/s0092-8674(00)81758-4. [DOI] [PubMed] [Google Scholar]