Abstract
Problem:
The aim of this study was to investigate the possible relationship between vaginal/rectal microbiome disbalances and miRNA expression with infertility.
Method of study:
Observational, exploratory, preliminary study. A total of 287 multiple IVF failure infertile patients were recruited. Twenty fertile women, not IVF failure, were recruited as the control group. Swab samples were collected from the vagina and rectum. Microbial composition by NGS and miRNA expression by real-time PCR of vaginal and rectal samples was measured. Immunometabolic markers from blood (insulin, vitamin D, LDL-cholesterol, ANA, TPO, Tg, and ASCA antibodies) and saliva (sIgA) were analyzed.
Result(s):
Infertile patients showed a lower bacterial richness and increased Firmicutes/Bacteroidetes ratio at rectal level and an increased Lactobacillus brevis/Lactobacillus iners ratio in vaginal samples regarding the fertile group. In the same rectal swab samples, we found that miR-21–5p, which is associated with tight junction disruption and yeast overgrowth, is upregulated and that miR-155–5p, which is associated with inflammation, is overexpressed in the unexplained infertile group (*p < .05). These deregulated miRNAs were also upregulated in the vaginal samples from the same patients (*p < .05).
Conclusion:
miRNAs could be potential biomarkers of the inflammatory impact of microbiome disbalances in unexplained infertile women.
Keywords: autoantibodies, infertility, intestinal permeability, microbiota, miRNAs
1 |. INTRODUCTION
Infertility is an important public health problem, with a global prevalence of 8–12% among couples at reproductive age1 that has serious adverse effects on society, economy, and the mental health of the couple involved.2 The main causes of female infertility are (1) ovulation disorders, (2) uterine or cervical issues, (3) tubal alterations, (4) endometriosis, (5) immune factors, and/or (6) pelvic infections that are mainly associated with Chlamydia trachomatis and Neisseria gonorrhoeae.1 However, approximately 30% of cases cannot be explained, defined as “unexplained infertility” (UI).3
Currently, there is growing evidence demonstrating the impact of human microbiota as a factor of health and disease.4,5 The microbiota is a group of microorganisms found in mucosal tissues such as the gut, reproductive tract and the skin, which are beneficial for the normal physiology of the host. The human microbiota plays a critical role in multiple biological processes such as nutrient and drug metabolism, maintenance of the structural integrity of the mucosal barrier, immunomodulation, and protection against pathogens.6 Disruption of the microbiota composition, which results from a decrease in the ratio of beneficial/harmful bacteria, is defined as “dysbiosis.”7 Dysbiosis can be categorized into three different types: loss of beneficial organisms, excessive growth of potentially harmful organisms, and loss of overall microbial diversity. Moreover, these three types are not mutually exclusive and can occur simultaneously.7–10
The microbiota of the female reproductive tract is receiving increasing attention in human reproduction because it may not only impact the chances of achieving a pregnancy, but also the health status of the mother and the child before and after delivery. The vaginal microbiota is most often dominated by Lactobacillus species. However, in some women it lacks Lactobacillus spp. and is composed of a wide array of strict and facultative anaerobes, a state that broadly correlates with increased risk for infection, disease, and poor reproductive and obstetric outcomes. Interestingly, the level of protection against infection can also vary by species and strains of Lactobacillus, and some species that dominate vagina microbiome are not always optimal.11 Recent studies have demonstrated that the relative abundance of L. iners, L. crispatus, and L. gasseri in the vagina can distinguish idiopathic infertile women from fertile women.12–14
In addition, a normal gut microbiota is essential for the function of the immune system, and dysbiosis can have a major impact on its normal function resulting in deviation of normal immune responses.7 Moreover, there is growing evidence about the impact of gut microbiota dysbiosis and intestinal inflammation in inflammatory conditions that affect male and female fertility.15–19 However, the mechanisms associated with this regulation are still poorly understood. Microbiome integrity is associated with a number of beneficial effects including promoting the integrity of the gastrointestinal barrier.20–22 The permeability of the intestinal epithelium depends on the regulation of the mucosal immune system and the intercellular tight junctions (TJs). The pathophysiological regulation of TJs is influenced by many factors, including secretory immunoglobulin A (IgA), lectins, yeast, aerobic and anaerobic bacteria, and microRNAs (miRNAs).23,24
Increased intestinal permeability has been found to play a key role in the development of various inflammatory and autoimmune disorders.25–29 Immune disorders are also implicated in reproductive failure, and the seroprevalence of certain auto-antibodies such us anti-nuclear antibodies, anti-TPO, antithyroglobulin antibodies and anti-phospholipids in unexplained infertile women was reported to be higher than in fertile women.30–32 Since the microbiome composition affects the repertoire of immunological cells in the mucosa, and dysbiosis is associated with inflammatory diseases,33–37 we hypothesize that the pathogenesis of infertility might be associated with abnormal immunological responses due to alterations in the microbiota. In this sense, it is plausible that microbiota can play a role in the development of infertility by affecting the epigenetic, immunologic, and/or biochemical functions of the host.
The miRNAs are a class of small noncoding RNA molecules that control gene expression at the post-transcriptional level regulating mRNA through its degradation and adjusting protein levels.38 In recent years, extraordinary progress has been made in terms of identifying the origin and exact functions of miRNA, focusing on their potential use in both the research and the clinical field. There is promising evidence that in spite of the lack of standardized protocols regarding the use of miRNAs in current clinical practice, they could be a reliable tool for future use in diagnosis. These molecules meet most of the required criteria for being an ideal biomarker, such as accessibility, high specificity, and sensitivity.39 Several miRNAs have been described to be associated with dysbiosis and with an immune disbalance of the two main immune cell populations of the mucosae: macrophages (Ms) and dendritic cells (DCs), suggesting that tissue infiltration and inflammation remediation could be regulated by this small molecules.40,41 It is of our interest to focus on miRNAs known to be related with intestinal permeability, microbiome disbalance, and immune regulation. A thorough analysis of the literature consulting resources available in online databases such as NCBI, PubMed, Medline, ScienceDirect, and UpToDate was performed and four out of thousand miRNAs has been selected for this study. miR-21 which is associated with tight junction disruption in the gut,42–46 immune disorders linked to autoimmune diseases,47,48 macrophage polarization toward M2 phenotype,41 fungal overgrowth and missing bacterial species49; miR-155 which is associated with inflammatory diseases,50 macrophage activation toward M1 phenotype,51,52 endometriosis,53 and bacterial overgrowth after Gram-negative bacterial exposure54; miR-193b which is associated with bacterial vaginosis,55 and anti-inflammatory function in asthma56; and miR-141 which is related to intestinal cell proliferation and immune system regulation57,58; and microbial fluctuations along with the gut.59 Potential influence of these miRNAs in gut microbiome and its association with infertility is unknown.
The objective of this study was to compare the rectal and vaginal microbiota between fertile and unexplained infertile women and its correlation with the expression levels of vaginal and rectal miR-21 miR-155, miR-141, and miR193b. In this sense, as current techniques evolve, we anticipate that miRNAs could become a potential biomarker in the development of personalized patient microbiome profiles, thus permitting more specific therapeutic interventions in the future.
2 |. MATERIAL AND METHODS
2.1 |. Study groups
Women were recruited from March 2018 to December 2019. Participation in this preliminary study was voluntary, and written informed consent was obtained from the subjects. The study was approved by the Ethics Review Committee of Halitus Medical Institute.
2.1.1 |. Infertile population
Patients were defined as unexplained infertility (UI) when they met all of the following criteria: (1) Body Mass Index (BMI) under 25; (2) normal ovarian function was required by cycle day 3 (±2 days) FSH ≤ 12 IU/L within 1 year before study initiation; (3) normal tubal and peritoneal anatomy as determined by hysterosalpingography and/or laparoscopy; (4) midluteal serum progesterone >10 ng/ml; (5) no evidence of male infertility; (6) number 2 and 3 not apply if they are in an ovodonation (OD) program because of ovarian failure diagnose; and (7) history of at least 2 IVF-ET or 1 OD unsuccessful procedures.
In the UI group, the following criteria were considered to exclude patients: the presence of hydrosalpinx, severe endometriosis, antibiotic treatments, and hormonal untreated disorders like high prolactin level, insulin insensitivity, hypo, and hyperthyroidism.
2.1.2 |. Fertile group
Women recruited for the control fertile group met the following criteria: aged between 21 and 39 years; BMI under 25; at least one healthy baby born conceived without assisted reproductive technologies (ART) and younger than 2 years; a body mass index equal to or lower than 25. In addition, exclusion criteria were considered for this group: being pregnant and/or breastfeeding, being under hormonal treatment, taking antibiotics, using an intrauterine device use (IUD), having a personal history of endocrine, autoimmune disease, infertility, or recurrent miscarriages.
2.2 |. Blood sample analysis
Quantification of anti-thyroid peroxidase (TPO), thyroid antithyroglobulin antibody (TgAb), anti-Saccharomyces cerevisiae antibodies (ASCA), lupus anticoagulant, and anti-nuclear antibodies (ANA) was determined together with thyroid-stimulating hormone (TSH), hemoglobin, vitamins D and B12, insulin and blood glucose levels, following standard protocols in certified clinical laboratories.
2.3 |. Vaginal fluid and rectal sample preparation
Two vaginal and rectal samples were obtained from each patient using a sterile Dacron swab. Regarding the vaginal samples, patients opened the folds of skin at the vaginal opening, inserted the swab 3 to 5 cm into the vagina, moved the swab in several full circles along the vaginal walls for 20 s, and immediately inserted the swab into the collection tube. Regarding the rectal samples, patients inserted the swab 1 to 2 cm into the anal hole, moved the swab in several full circles for 20 s, and immediately inserted the swab into the collection tube. These swabs were suspended in 1 ml of RNA later solution to stabilize the microbial DNA and RNA and stored at −80°C in individual tubes until processing.
2.4 |. Microbiological studies
In the present investigation, we used conventional agar-based culture methods for the vaginal and rectal samples and Giemsa and Gram staining. The agar culture lasted for 72 h, in order to evaluate possible infections.
2.5 |. Sample processing and DNA extraction
Metagenomic DNA extraction was carried out from 200 μl of the suspension using the QIAamp DNA Mini kit (Qiagen), following the manufacturer’s instructions. The final working elution volume for NGS was optimized to 50 μl. All DNA samples were stored at −20°C prior to sequencing.
2.6 |. 16S rRNA library preparation and sequencing
Metagenomic DNA samples were quantified using Quant-iT PicoGreen dsDNA assay kit (Invitrogen Corporation) and further processed using the Illumina 16S Sample Preparation Guide, with some modifications. The DNA concentration of samples was normalized to 5 ng/μl, and then, 12.5 ng of DNA was used to amplify the 16S rRNA V4 hypervariable region using polymerase chain reaction (PCR) (20 cycles) and the following primers (overhang adapter sequence are underlined): 515F, 5-TCGTCGGCAGCGTCAGATGTG TATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3 and 806RB, 5-T CTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACNVGG GTWTCTAAT-3. Amplicons were purified using AMPure XP beads (Beckman Coulter Life Sciences), and a second amplification round was performed using 5 μl of DNA and the Nextera XT Index Primers (N7xx and S5XX). After a final purification using AMPure XP beads and after quantification, each DNA library was pooled, quantified, denatured, and loaded into a NextSeq500 platform using the NextSeq System Denature and Dilute Libraries Guide (Illumina Inc.). The libraries were sequenced using 2 × 150 cycles.
2.7 |. RNA isolation
Total RNA (including miRNAs) was isolated from each sample using the mirVana miRNA isolation kit (Life Technologies), according to the manufacturer’s instructions. The purity (Absorbance 260/280) and quantity of the extracted RNA were measured using a Nanodrop One spectrophotometer (Thermo Scientific).
2.8 |. cDNA synthesis
cDNA was synthesized using specific predesigned TaqMan Reverse Transcription (RT) and the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems), according to the manufacturer’s instructions. Reverse transcription reactions were performed in a final volume of 15 μl, and each reaction contained 4 ng of total RNA from the vaginal samples and 10 ng of total RNA from the rectal samples. The reactions were incubated at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min, with a final hold at 4°C. Reverse transcription reactions without an RNA template were used as the RT negative control (for potential contamination with genomic DNA).
2.9 |. qRT-PCR analysis
The final reaction volume was 20 μl, which contained 1.33 μl of the RT reaction product. Real-time PCR cycling was conducted on a Thermal Cycler C1000 Touch CFX96 Real-Time System (Bio-Rad) using the following parameters: 95°C for 10 min, followed by 40–45 cycles of 95°C for 15 s, and 60°C for 1 min to identify the miRNAs. The threshold cycle (Ct) values were automatically calculated using Bio-Rad CFX Maestro software, and the fold changes in expression were calculated using the 2-ΔΔCt method using RNU48 (vaginal samples) and RNU6B (rectal samples) as endogenous controls for miRNA expression.60 All sample-assay combinations were detected in duplicates for individual samples, and negative controls were included in each plate.
2.10 |. Statistical and bioinformatics analyses
The expression levels of the four selected miRNAs (miR-21–5p, miR-155–5p, miR-193b-3p, miR-141–3p) were normalized to the endogenous RNU48/RNU6B levels (Table 1). The relative miRNA quantity in the tested samples from control women vs. infertile women was calculated separately by using the comparative ΔCt method. ΔCt was calculated by subtracting the Ct values of the endogenous control by those of the miRNA of interest: ΔCt (CtmiR of interest − CtRNU48/RNU6B). The fold-change cutoff for miRNAs was calculated using the following expression: 2-ΔΔCt.61
TABLE 1.
miRNA probe details and sequence information
| Probe | Mature miRNA sequence | Accession number |
|---|---|---|
| RNU48 | GATGACCCCAGGTAACTCTGAGTGTGT CGCTGATGCCATCACCGCAGCGCTCTGACC |
NR_002745 |
| RNU6B | CGCAAGGATGACACGCAAATTCGTG AAGCGTTCCATATTTTT |
NR_002752 |
| miR-21–5p | UAGCUUAUCAGACUGAUGUUGA | MI0000077 |
| miR-155–5p | UUAAUGCUAAUCGUGAUAGGGGUU | MI0000681 |
| miR-193b-3p | AACUGGCCCUCAAAGUCCCGCU | MIMAT0002819 |
| miR-141–3p | UAACACUGUCUGGUAAAGAUGG | MIMAT0000432 |
The ΔCt distribution was compared with the control reference values by using Mann–Whitney U-test or unpaired t-test, according to the variance evaluation (p values <.05 were considered statistically significant). Means and ranges of ΔCt values were established for each miRNA. The presence of outliers was evaluated using the Grubbs test. The t-test was used, and the area under the receiver operating characteristic (ROC) curve (AUC) values was analyzed for each miRNA to assess its suitability as a single biomarker. These results were computed using GraphPad Prism 8.0 software (GraphPad Software). Differences were significant at p values <.05, and an AUC value close to 1 indicated a high diagnostic value.
The power curve for two-sample t-test was performed for each of the miRNAs, calculating the power for Mean 1 = Mean 2 + difference α = 0.05. The power was calculated for each miRNA, in order to determine whether it was over 80%.
3 |. RESULTS
3.1 |. Clinical characterization of the study groups
3.1.1 |. Infertile population
A total of 287 UI patients were enrolled in this observational study. Average subject age was 40 years (Range: 27–52); 26% of patients had had a positive pregnancy test resulted in 22 chemical pregnancies and 53 spontaneous miscarriages before week 10 of pregnancy; average Gravity/Parity = 1.2/0; average failed IVF cycles = 4.2; and average time trying to conceive = 10 years. 120/287 patients had already failed at least 1 ET with donated oocytes.
We found that 15% of the 287 UI with recurrent IVF-ET failures women included in the study showed anemia; 73.2% had hypovitaminosis D and/or B12; 65.5% were positive for one of the autoantibodies tested; 30.7% were positive for ASCA testing, among others, as shown in Table 2. Moreover, most of the patients referred gastrointestinal symptoms (63% of women with increased microRNA levels and dysbiosis and 60% of women with normal miRNA levels), such as gastritis, diarrhea, and abdominal pain, which together with anemia, hypovitaminosis, and gastrointestinal autoantibodies, are linked to a “leaky gut” condition characterized by an increased intestinal permeability.
TABLE 2.
Clinical characterization of systemic biomarkers. Data is presented as the number of patients (n) and percentage of total studied patients (%). Total UI women = 287. Total fertile women = 20
| Clinical characterization | UI women | Fertile women | ||
|---|---|---|---|---|
| n | % | n | % | |
| Anemia | 43 | 15.0 | 1 | 5.0 |
| Hypovitaminosis B and/or D | 210 | 73.2 | 2 | 10.0 |
| Hypothyroidism | 147 | 51.2 | 2 | 10.0 |
| Metabolic syndrome | 161 | 56.1 | 0 | 0 |
| Polycystic ovary syndrome | 53 | 18.5 | 0 | 0 |
| Endometriosis | 78 | 27.2 | 0 | 0 |
| Autoimmunity | 188 | 65.5 | 0 | 0 |
| TPO + | 57 | 19.9 | 0 | 0 |
| TgAb + | 53 | 18.5 | 0 | 0 |
| ANA + | 59 | 20.6 | 0 | 0 |
| ASCA (IgA, IgG) | 88 | 30.7 | 1 | 5.0 |
Abbreviations: Anemia: hemoglobin <12 g/dl; Hypovitaminosis B: Vitamin B12 <200 pg/ml; Hypovitaminosis D: Vitamin D <30 ng/ml; Hypothyroidism: TSH >4 UI/ml; Metabolic syndrome: altered oral glucose tolerance test (OGTT), glycemia >100 mg/dl, insulin >24 mU/L and/or Homeostatic Model Assessment (HOMA) >3; Polycystic ovary syndrome: ultrasound diagnosis and/or inositol-metformin intake; endometriosis: laparoscopic diagnosis and/or CA125 > 35 UI/ml; Autoimmunity: diagnosis of celiac disease, Hashimoto’s disease, Crohn’s disease, autoimmune diabetes, lupus, Graves, rheumatoid arthritis, scleroderma, myasthenia gravis, and/or Sjogren; TPO, Anti-Thyroid Peroxidase; TgAb, Thyroid Antithyroglobulin Antibody; ANA, Anti-nuclear Antibody; ASCA, Anti-Saccharomyces cerevisiae antibodies; IgA, Immunoglobulin A; IgG, Immunoglobulin G.
3.1.2 |. Fertile group
Twenty fertile women not seeking for pregnancy were recruited as the control group for all the new biomarkers tested. They were 35 years old on average with an age range of 29 to 38 years old; 1.7 kids on average by natural conception (Range: 1–4) and they all met the inclusion and exclusion criteria. None of them showed infection in vaginal and rectal samples according to previously described microbiological conventional analysis.9 We calculated if the sample size was sufficient to obtain a power of 80%, and considering the difference between means and the standard deviation we confirmed that 18 women per group was sufficient to obtain the expected power (data not shown).
3.2 |. Differences in bacterial communities using 16S rRNA sequencing
A beta diversity analysis based on the phylogenetic distances between OTUs was performed using UniFrac weighted. This analysis was visualized with principal coordinate analysis (PCoA). This analysis revealed differences in the composition of the communities between the different sampling sites, vaginal and anal, showing as expected a separation into two groups (data not shown).
The relative abundance was analyzed at the level of order and family, for each sample type. First, we examined the general phylogenetic composition at the rectal and vaginal samples from fertile and UI women. Next, we determined the taxonomic levels at the genera and species level in the vaginal and rectal samples. Using the described primer set (see M&M) and miSeq platform, an average of 49.100 reads were obtained for each sequencing reaction. Our analysis showed significant differences in bacterial populations between the two groups.
In the rectal swabs, we found a lower richness at the genera level of UI women. On average, 69 genera were observed in UI patients, compared to 85 in fertile women (*p < .05; Figure 1A). Moreover, in the rectal samples of UI patients, there was a significantly increased ratio of Firmicutes/Bacteroidetes (*p < .05, Figure 1B) compared to fertile women. However, 30.4% of UI patients showed the same Firmicutes/Bacteroidetes ratio as the control fertile group.
FIGURE 1.
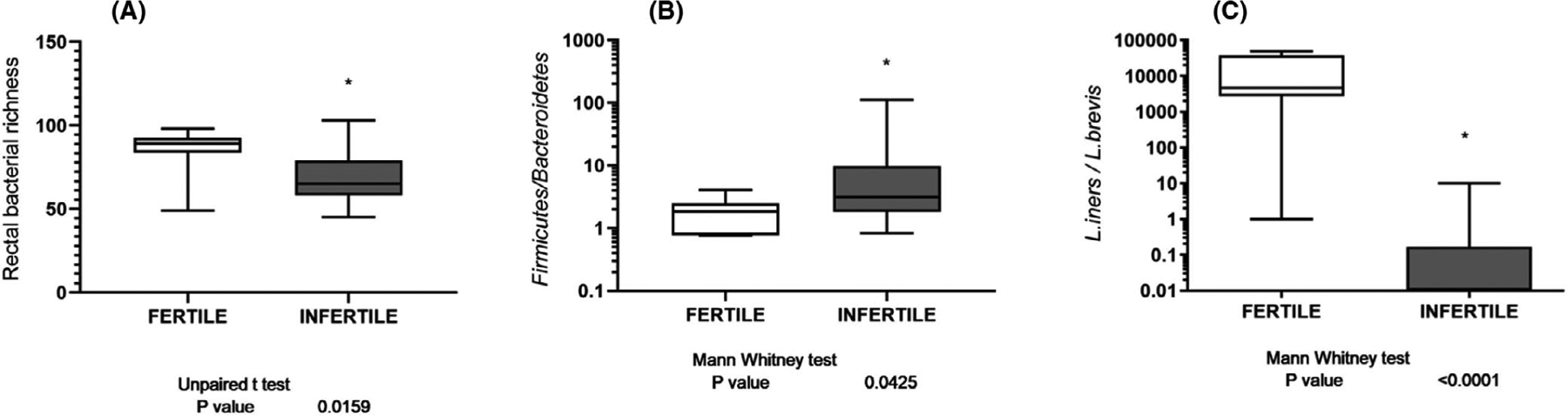
Differences in bacterial communities using 16S rRNA sequencing of vaginal and rectal swabs. Bacterial gene count using 16S rRNA sequencing of rectal swabs (A). The relative proportion of microorganisms in rectal (B) and vaginal (C) swabs. Data are presented as total gene counts [median (middle line), interquartile range (top and bottom lines)], and statistical significance (Unpaired t-test) was defined as p < .05
On the other hand, analyzing the abundance at the different taxonomic levels in the vaginal swab samples, no significant difference was observed with respect to the ratio Lactobacillus spp/anaerobic bacteria (Gardnerella spp, Mobiluncus spp), suggesting that there is no a common dysbiosis, product of a bacterial vaginosis, in our patients. In fact, the difference was in the specific Lactobacillus species that colonize the vagina of the UI patients. Commonly, L. iners is one of the predominant species in a healthy vagina, and when we analyzed the Lactobacillus species in our UI patients, we observed that the vagina of UI women was colonized by L. brevis, which is not one of the common five Lactobacillus communities’ subclasses.62 In this sense, the ratio of Lactobacillus iners/Lactobacillus brevis was significantly higher in the vaginal swab of fertile women compared to the UI group (***p < 0001, Figure 1C). However, 22.7% of UI patients showed the same Lactobacillus iners/Lactobacillus brevis ratio as the control fertile group.
3.3 |. Total miRNA expression
Our next objective was to evaluate the expression levels of miRNAs by qPCR in the vaginal and rectal swabs of our population of fertile and UI women. Only 2 out of the 4 studied miRNAs showed significant difference between UI and fertile women. No difference was observed in vaginal and/or rectal miR-193b and miR-141 levels between fertile and UI women (Data not shown, nsp > .05). In the vaginal samples, we found an overexpression of miR-21 and miR-155 in the UI group compared to the fertile group (*p < .05, Figure 2A,B). Similar differences were found in rectal samples obtained from IU and fertile patients (*p < .05, Figure 2C,D). Moreover, we found a positive correlation between vaginal and rectal values of miR21 and miR155 (Data not shown, *p = .05). Considering that UI patients showed differences in the rectal and vaginal microbiome composition regarding the control group, we study the correlation between this result and miRNAs expression. A significant increased expression of rectum Lactobacillus spp was observed in UI patients with miR21 overexpression in vaginal swabs (Data not shown, *p = .042), whereas there was not a significant difference in Lactobacillus spp expression in association with vaginal miR155 results (Data not shown, nsp = .075). In this sense, miRNA differential expression could be associated with a microbiota disbalance in UI women.
FIGURE 2.
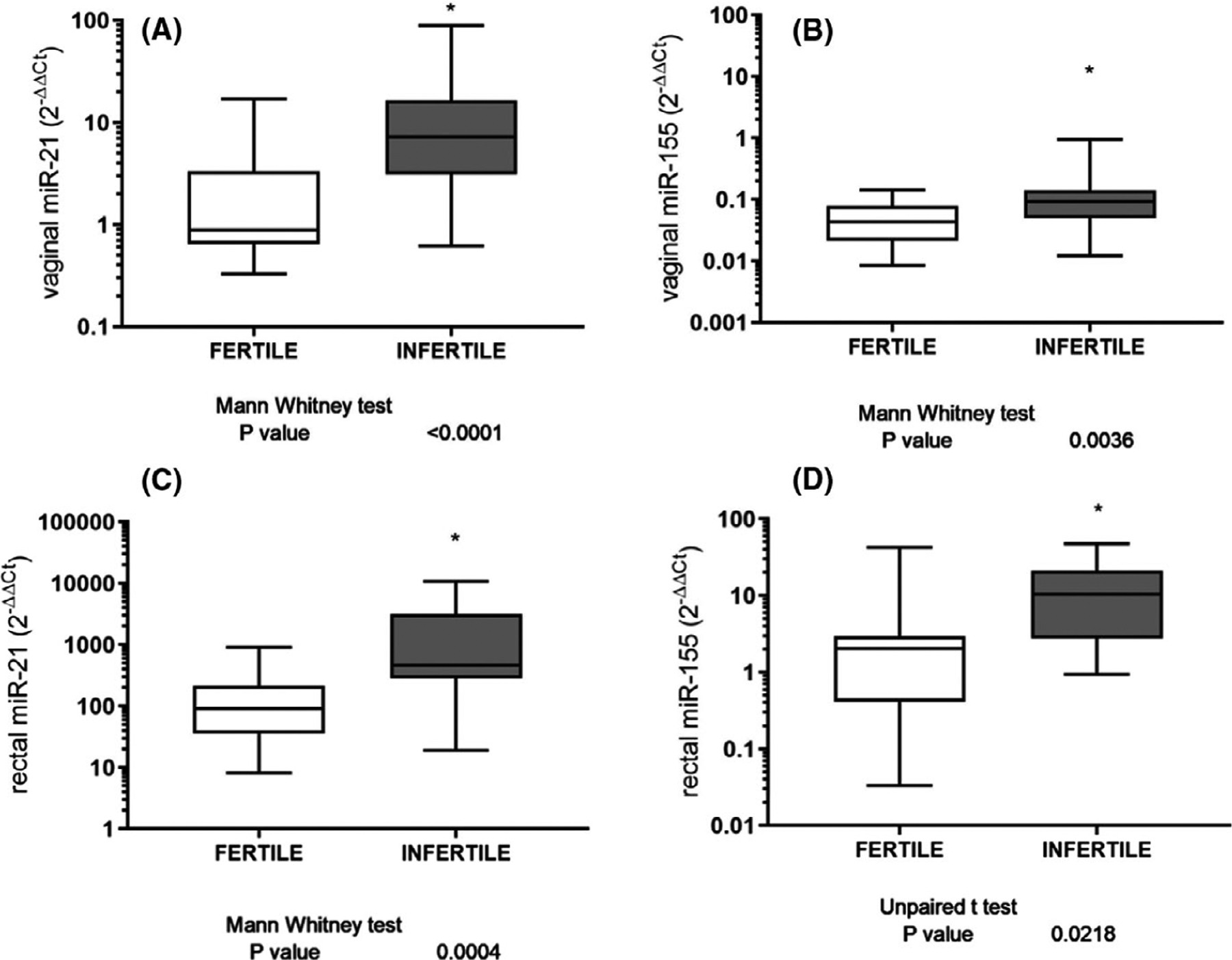
Expression levels of dysregulated miRNAs identified in the selection cohort. Expression profiles of significantly altered miRNAs identified in the vaginal and anal swabs from infertile women. Data are presented as the relative expression values normalized to RNU48/RNU6B [median (middle line), interquartile range (top and bottom lines)], statistical significance (Mann-Whitney U test or Unpaired t-test) was defined as p < .05. *** designates p ≤ .001; ** designates p < .01; * designates p < .05. (A) For vaginal miR-21 (B) and miR-155 (C); for rectal miR-21 (D) and miR-155
3.4 |. Evaluation of miR-21–5p and miR-155–5p as biomarkers for female infertility
To investigate whether these two miRNAs could differentiate between fertile and infertile women, ROC curves were constructed using the data from UI patients, compared to 20 control women. ROC curve analysis allowed us to obtain AUC values that enabled the classification of the predictive power of miRNAs in the measurable categories.
Considering that targeted miRNAs are associated with different functions: miR-21 is associated with tight junction disruption in the gut, fungal overgrowth, and missing bacterial species; and miR-155 is associated with inflammatory disorders and bacterial overgrowth, and the ROC curve analysis was performed for each marker individually (Figure 3A–D) showing that both miRNAs (vaginal and rectal) have significant discriminating ability to differentiate UI patients from fertile controls, as AUCs are all significantly above 0.5 (Table 3). miR21 and miR155 in both sample types, vaginal and rectal, have greater than 80% sensitivity for selected cutoff values.
FIGURE 3.
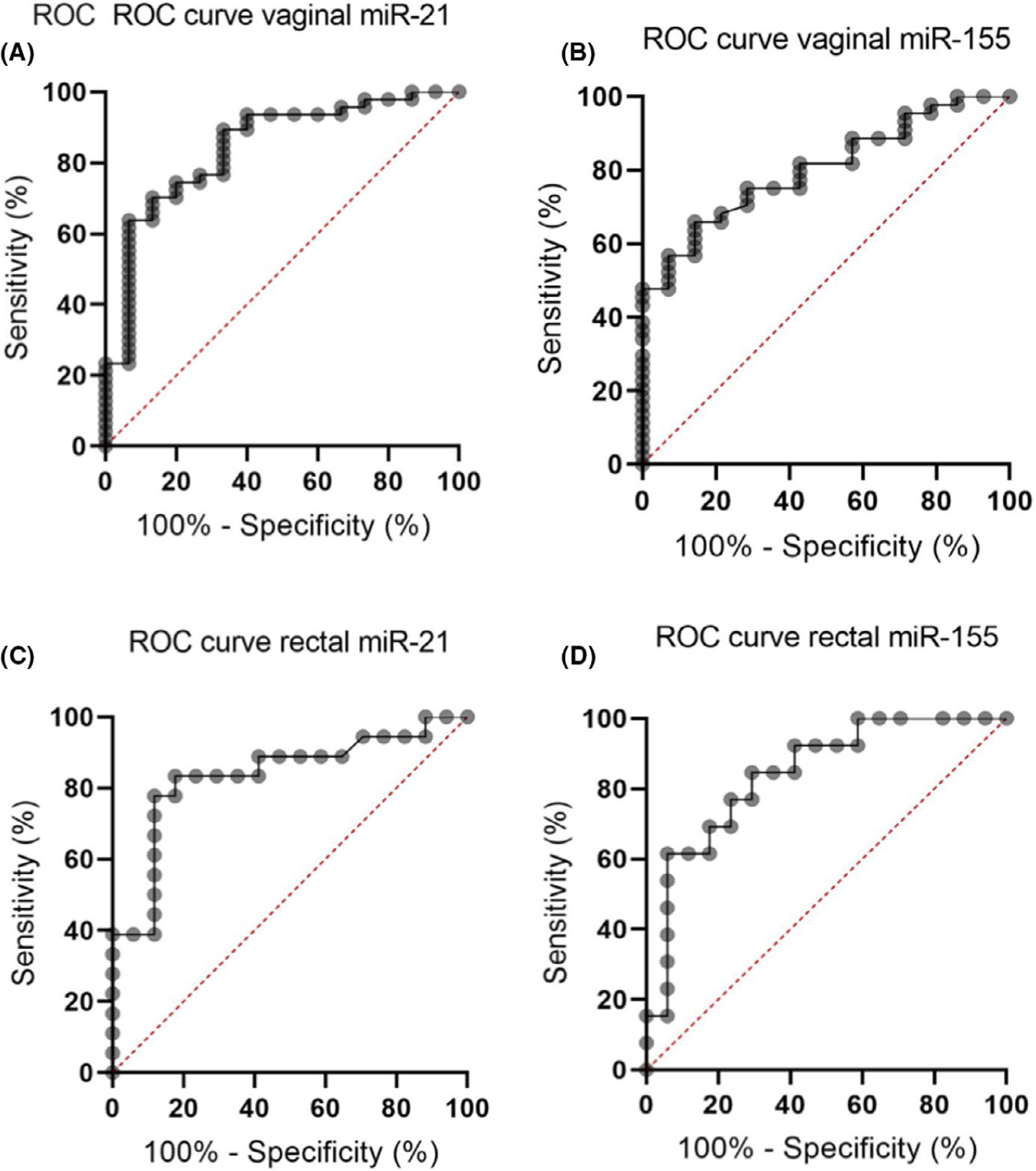
Diagnostic estimates of miRNAs identified as dysregulated in the selection cohort. ROC curve analysis was performed for each of the miRNAs identified as being dysregulated in the selection cohort and the associated AUC and diagnostic sensitivities and specificities for individual miRNAs are presented in Table 1. (A) For vaginal miR-21 (B) and miR-155. (C) For rectal miR-21 (D) and miR-155
TABLE 3.
Analytical validation of miRNAs. The accuracy of miRNAs was evaluated using ROC curve analysis. Once the cutoff value was selected for each miRNA, according to the sensitivity and specificity, positive and negative predictive values and power were calculated to analyze the performance of our biomarkers
| Vaginal miR-21 | Vaginal miR-155 | Anal miR-21 | Anal miR-155 | |
|---|---|---|---|---|
| AUC | 0.8426 | 0.8028 | 0.8350 | 0.8416 |
| p | <.0001 | .0007 | .0007 | .0016 |
| Sensitivity (%) | 89.36 | 84.09 | 83.33 | 84.62 |
| Specificity (%) | 66.67 | 75.00 | 82.35 | 70.59 |
| Positive predictive value | 87.50 | 90.20 | 75.00 | 61.00 |
| Negative predictive value | 70.6 | 63.16 | 76.00 | 75.00 |
| Power | 80.63 | 80.87 | 82.26 | 80.35 |
Abbreviation: AUC, Area Under Curve.
4 |. DISCUSSION
Unknown infertility is the category that includes all couples without an explanation for their inability to achieve a successful pregnancy. Looking for the correct biomarkers that discriminate and identify the following diagnosis is something that science owes to these couples.
In addition, chronic inflammatory conditions have been associated with poor reproductive outcomes, whereas it has been hypothesized that subfertility can be effectively treated by controlling inflammatory and autoimmune processes. Moreover, gastrointestinal disorders had been implicated in infertility and recurrent pregnancy loss, whereas a growing evidence connecting inflammatory and autoimmune disorders with intestinal conditions has been reported.16,63,64
We consider that it is important to identify whether the peripheral blood markers of immunometabolic pathways tested in this study are connected to intestinal dysfunction because of a specific microbiome signature, which is related to increased intestinal permeability.
We tested serum vitamin B12 levels because one of the most common causes of chronic anemia is vitamin B12 deficiency, which is synthesized by intestinal bacteria, and it is associated with autoimmune or dystrophic gastritis.65,66 We tested insulin and LDL levels because it has been demonstrated that intestinal dysbiosis, by altering microbiome metabolism and consequently host metabolism, not only affects inflammatory responses but also contributes to metabolic disorders.67 We compared autoantibody levels because in addition to a genetic predisposition and exposure to triggering non-self-antigens, the loss of the protective function of the normal microbiome and the effect of dysbiosis on the function of mucosal barriers that interact with the underlying immune cells is related to the development of autoantibodies.68 Among them, it has been reported that individuals with intestinal barrier dysfunction express higher levels of ASCA antibodies than healthy individuals and the expression of this antibody was correlated with yeast overexpression and TJ dysfunction. Lipopolysaccharides (LPS) from gut bacteria have been shown to play a role in systemic inflammation, leading to the opening of the gut and blood barrier.69
Changes in gut microbiota perturb homeostatic interaction between microbiota and the intestine and might contribute to metabolic disorders. Individuals with lower bacterial richness in the gut are characterized by more marked overall adiposity, insulin resistance, dyslipidemia, and a more pronounced inflammatory phenotype when compared with high bacterial richness individuals.70–72 Although the composition of intestinal microbiota is highly diverse in healthy individuals, those exhibiting overall adiposity, insulin resistance, and dyslipidemia are characterized by low bacterial richness. Moreover, composition of gut microbiota in obesity individuals differs from that in lean individuals. Bacteroidetes prevalence is lower in obese people and a proportional increase in members of the Firmicutes phylum, revealing an association with a higher presence of enzymes for complex carbohydrate degradation and fermentation,73 which are related to elevated levels of energy harvesting from the diet.74 The altered ratio of Firmicutes/Bacteroidetes has been associated with obesity and with abnormal intestinal homeostasis.75–77 UI women with increased expression of miR-21 and miR-155 showed lower bacterial richness with respect to fertile women and a lower prevalence of Bacteroidetes together with higher levels of Firmicutes leading to an increased ratio Firmicutes/Bacteroidetes. The main difference between both miRNAs is that only the UI women with overexpression of miR-21 showed increased levels of total Lactobacillus species in the rectal swab with regard to the rest of Lactic Acid Bacteria (*p < .05).
In addition, it is known that the vaginal microbiota is most often dominated by Lactobacillus species. However, in some women the level of protection against infection can also vary by species and strains of Lactobacillus, and some species although dominant are not always optimal.11 In fact, UI women with overexpression of both miRNAs show normal proportion of Lactobacillus spp/anaerobes and the difference is in the specific Lactobacillus species that colonize the vagina. Commonly, L. iners is one of the predominant species in a healthy vagina, as we observed in our fertile control group, but when we analyzed the Lactobacillus species in our UI patients, we observed that they are mainly colonized by L. brevis, which is not one of the common five Lactobacillus communities’ subclasses.56,62,70,78 The ratio of Lactobacillus iners/Lactobacillus brevis is significantly lower in the vaginal swab of UI women with miRNAs overexpression.
We report that the identification of specific miRNAs biomarkers that correlates with the presence of a specific microbiome signature could be considered in the study of unexplained infertility. We demonstrate that the group of UI patients had a microbiome disbalance in the rectum and vagina, when compared to NGS patterns of the fertile control group and that this pattern was associated with higher levels of miR-21 and miR-155. This result also confirms that the link with microbiome disbalance is specific to those miRNAs since no association has been shown with miR-193b and miR-141 expression. Moreover, the association between the bacterial composition and immunometabolic disorders has been proposed. Thus, when we studied miRNAs that communicate the microbiome with the immune system and that are linked to TJ disruption, we observed that miR-21 and miR-155 were overexpressed at the rectal and vaginal levels in these women. miR-21 is associated with tight junction disruption in the gut,42–46,79 immune disorders,47,48,80 fungal overgrowth, and missing bacterial species,49 and miR-155 is associated with inflammatory diseases,50 macrophage activation toward the M1 phenotype,51,53 and bacterial overgrowth.81
Considering the clinical background of our patients and the results obtained during the study, we consider that a microorganism disbalance at the intestinal level is associated with a disrupted intestinal barrier through an opened TJ, resulting in the entry of foreign immunogenic antigens and in the activation of the mucosal immune system. The disrupted inflamed epithelial barrier is linked to the overexpression of miR-21 and miR-155. These miRNAs could travel via blood circulation and target the reproductive system, in which we also observed a dysbiosis, an inflamed mucosa, and a disrupted epithelial barrier.
5 |. CONCLUSION
In conclusion, this exploratory study hypothesizes that we could analyze a swab to hunt for the microRNA signature. This result together with specific blood and saliva markers could be a potential tool to identify a microbiome imbalance which is affecting different immune pathways in UI patients. Further studies, including a placebo control age-matched group, should be conducted in order to evaluate the efficacy of the test and the following treatment on reproductive outcomes.
ACKNOWLEDGEMENTS
The authors thank all the participants for their contribution. The study was funded by World Bank, EMPRETECNO 2016, and PAEBT 016/16.
Funding information
World Bank; EMPRETECNO, Grant/Award Number: 2016; PAEBT, Grant/Award Number: 016/16
Footnotes
CONFLICT OF INTEREST
US 63/076690 has been filled and assigned to Microgenesis Corporation. Authors stated that there is no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. [DOI] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2014;21:411–426. [DOI] [PubMed] [Google Scholar]
- 3.Sadegui MR. Unexplained infertility, the controversial matter in management of infertile couples – PubMed. J Reprod Infertil. 2015;16:1–2. 10.1093/humupd/dmv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kåhrström CT, Pariente N, Weiss U. Intestinal microbiota in health and disease. Nature. 2016;535:47. [DOI] [PubMed] [Google Scholar]
- 5.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am. 2017;46:77–89. [DOI] [PubMed] [Google Scholar]
- 6.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. [DOI] [PubMed] [Google Scholar]
- 7.Fallucca F, Porrata C, Fallucca S, Pianesi M. Influence of diet on gut microbiota, inflammation and type 2 diabetes mellitus. First experience with macrobiotic Ma-Pi 2 diet. Diabetes Metab Res Rev. 2014;30:48–54. [DOI] [PubMed] [Google Scholar]
- 8.Aagaard K, Riehle K, Ma J, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:e36466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boers SA, Jansen R, Hays JP. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur J Clin Microbiol Infect Dis. 2019;38:1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016;22:1137–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril. 2018;110:327–336. [DOI] [PubMed] [Google Scholar]
- 12.Campisciano G, Florian F, D’Eustacchio A, et al. Subclinical alteration of the cervical–vaginal microbiome in women with idiopathic infertility. J Cell Physiol. 2017;232:1681–1688. [DOI] [PubMed] [Google Scholar]
- 13.Borgdorff H, Armstrong SD, Tytgat HLP, et al. Unique insights in the cervicovaginal Lactobacillus iners and L. crispatus proteomes and their associations with microbiota dysbiosis. PLoS One. 2016;11:e0150767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu M, Zhang X, Liang Y, Lin S, Qian W, Fan S. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio. 2020;11:e03242–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol. 2017;17:469–482. [DOI] [PubMed] [Google Scholar]
- 16.He FF, Li YM. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res. 2020;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komiya S, Naito Y, Okada H, et al. Characterizing the gut microbiota in females with infertility and preliminary results of a water-soluble dietary fiber intervention study. J Clin Biochem Nutr. 2020;67:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Peñarrubia P, Ruiz-Alcaraz AJ, Martínez-Esparza M, Marín P, Machado-Linde F. Hypothetical roadmap towards endometriosis: prenatal endocrine-disrupting chemical pollutant exposure, ano-genital distance, gut-genital microbiota and subclinical infections. Hum Reprod Update. 2020;26:214–246. [DOI] [PubMed] [Google Scholar]
- 19.Lundy SD, Sangwan N, Parekh NV, et al. Functional and taxonomic dysbiosis of the gut, urine, and semen microbiomes in male infertility. Eur Urol. 2021. 10.1016/j.eururo.2021.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Snelson M, de Pasquale C, Ekinci EI, Coughlan MT. Gut microbiome, prebiotics, intestinal permeability and diabetes complications. Best Pract Res Clin Endocrinol Metab. 2021;101507. 10.1016/j.beem.2021.101507 [DOI] [PubMed] [Google Scholar]
- 21.Chakaroun RM, Massier L, Kovacs P. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients. 2020;12:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camara-Lemarroy CR, Metz LM, Yong VW. Focus on the gut-brain axis: multiple sclerosis, the intestinal barrier and the microbiome. World J Gastroenterol. 2018;24:4217–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon MK. Intestinal barrier: molecular pathways and modifiers. World J Gastrointest Pathophysiol. 2013;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catanzaro JR, Strauss JD, Bielecka A, et al. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci Rep. 2019;9:13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vojdani A For the assessment of intestinal permeability, size matters. Altern Ther Health Med. 2013;19:12–24. [PubMed] [Google Scholar]
- 26.Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. [DOI] [PubMed] [Google Scholar]
- 27.Arvonen M, Berntson L, Pokka T, Karttunen TJ, Vähäsalo P, Stoll ML. Gut microbiota-host interactions and juvenile idiopathic arthritis. Pediatr Rheumatol. 2016;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahlén A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304:15–22. [DOI] [PubMed] [Google Scholar]
- 29.Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleicher N, El-Roeiy A. The reproductive autoimmune failure syndrome. Am J Obstet Gynecol. 1988;159:223–227. [DOI] [PubMed] [Google Scholar]
- 31.Cubillos J, Lucena A, Lucena C, et al. Incidence of autoantibodies in the infertile population. Early Pregnancy. 1997;3:119–124. [PubMed] [Google Scholar]
- 32.Deroux A, Dumestre-Perard C, Dunand-Faure C, Bouillet L, Hoffmann P. Female infertility and serum auto-antibodies: a systematic review. Clin Rev Allergy Immunol. 2017;53:78–86. [DOI] [PubMed] [Google Scholar]
- 33.De Luca F, Shoenfeld Y. The microbiome in autoimmune diseases. Clin Exp Immunol. 2019;195:74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu LCH. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flores R, Shi J, Fuhrman B, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng B, Lai Z, Sun L, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019;170:43–52. [DOI] [PubMed] [Google Scholar]
- 37.Yurtdaş G, Akdevelioğlu Y. A new approach to polycystic ovary syndrome: the gut microbiota. J Am Coll Nutr. 2020;39:371–382. [DOI] [PubMed] [Google Scholar]
- 38.Gulyaeva LF, Kushlinskiy NE. Regulatory mechanisms of microRNA expression. J Transl Med. 2016;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condrat CE, Thompson DC, Barbu MG, et al. miRNAs as biomarkers in disease: latest findings regarding their role in diagnosis and prognosis. Cells. 2020;9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mortha A, Chudnovskiy A, Hashimoto D, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtale G, Rubino M, Locati M. MicroRNAs as molecular switches in macrophage activation. Front Immunol. 2019;10:799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Shen J, Cheng J, Fan X. MicroRNA-21 regulates intestinal epithelial tight junction permeability. Cell Biochem Funct. 2015;33:235–240. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Zhang F, He DK, Fan XM, Shen J. MicroRNA-21 is upregulated during intestinal barrier dysfunction induced by ischemia reperfusion. Kaohsiung J Med Sci. 2018;34:556–563. [DOI] [PubMed] [Google Scholar]
- 44.Nakata K, Sugi Y, Narabayashi H, et al. Commensal Microbiota-induced microRNA modulates intestinal epithelial permeability through the small GTPase ARF4. J Biol Chem. 2017;292:15426–15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi C, Yang Y, Xia Y, et al. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65:1470–1481. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Li X. Molecular analysis of inflammatory bowel disease: clinically useful tools for diagnosis, response prediction, and monitoring of targeted therapy. Mol Diagnosis Ther. 2015;19:141–158. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Brandt S, Medeiros A, et al. MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin e2 - mediated M2 generation. PLoS One. 2015;10:e0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu WD, Pan HF, Li JH, Ye DQ. MicroRNA-21 with therapeutic potential in autoimmune diseases. Expert Opin Ther Targets. 2013;17:659–665. [DOI] [PubMed] [Google Scholar]
- 49.Croston TL, Lemons AR, Beezhold DH, Green BJ. MicroRNA regulation of host immune responses following fungal exposure. Front Immunol. 2018;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdul-Maksoud RS, Sediq AM, Kattaia A, et al. Serum miR-210 and miR-155 expression levels as novel biomarkers for rheumatoid arthritis diagnosis. Br J Biomed Sci. 2017;74:209–213. [DOI] [PubMed] [Google Scholar]
- 51.Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64:1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287:21816–21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saare M, Rekker K, Laisk-Podar T, et al. Challenges in endometriosis miRNA studies — from tissue heterogeneity to disease specific miRNAs. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2282–2292. [DOI] [PubMed] [Google Scholar]
- 54.Maudet C, Mano M, Eulalio A. MicroRNAs in the interaction between host and bacterial pathogens. FEBS Lett. 2014;588:4140–4147. [DOI] [PubMed] [Google Scholar]
- 55.Smith S, Ravel J. Identification and characterization of regulatory miRNAs and mRNAs in the longitudinal human host response to vaginal microbiota. 2017.
- 56.Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L727–L734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One. 2011;6:e17745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong H, Weng C, Bai R, et al. The regulatory network of miR-141 in the inhibition of angiogenesis. Angiogenesis. 2019;22:251–262. [DOI] [PubMed] [Google Scholar]
- 59.Moloney GM, Viola MF, Hoban AE, Dinan TG, Cryan JF. Faecal microRNAs: indicators of imbalance at the host-microbe interface? Benef Microbes. 2018;9:175–183. [DOI] [PubMed] [Google Scholar]
- 60.Rotelli MT, Di Lena M, Cavallini A, et al. Fecal microRNA profile in patients with colorectal carcinoma before and after curative surgery. Int J Colorectal Dis. 2015;30:891–898. [DOI] [PubMed] [Google Scholar]
- 61.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 62.Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. 2017;129:643–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bradley RJ, Rosen MP. Subfertility and gastrointestinal disease: ‘unexplained’ is often undiagnosed. Obstet Gynecol Surv. 2004;59:108–117. [DOI] [PubMed] [Google Scholar]
- 64.Swartwout B, Luo XM. Implications of probiotics on the maternal-neonatal interface: gut microbiota, immunomodulation, and auto-immunity. Front Immunol. 2018;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulnigg-Dabsch S Autoimmune gastritis. Wien Med Wochenschr. 2016;166:424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez-Castro KI, Franceschi M, Noto A, et al. Clinical manifestations of chronic atrophic gastritis. Acta Biomed. 2018;89:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belizário JE, Faintuch J, Garay-Malpartida M. New frontiers for treatment of metabolic diseases. Mediators Inflamm. 2018;66(6):696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalinkovich A, Gabdulina G, Livshits G. Autoimmunity, inflammation, and dysbiosis mutually govern the transition from the preclinical to the clinical stage of rheumatoid arthritis. Immunol Res. 2018;66:696–709. [DOI] [PubMed] [Google Scholar]
- 69.Vojdani A, Vojdani E, Herbert M, Kharrazian D. Correlation between antibodies to bacterial lipopolysaccharides and barrier proteins in sera positive for ASCA and ANCA. Int J Mol Sci. 2020;21:1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. [DOI] [PubMed] [Google Scholar]
- 71.Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98:16–24. [DOI] [PubMed] [Google Scholar]
- 72.Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018;9:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. [DOI] [PubMed] [Google Scholar]
- 74.Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magne F, Gotteland M, Gauthier L, et al. The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74:1251–1262. [DOI] [PubMed] [Google Scholar]
- 77.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. 2020;8:1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi C, Liang Y, Yang J, et al. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One. 2013;8:e66814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang S, Wan X, Ruan Q. The microRNA-21 in autoimmune diseases. Int J Mol Sci. 2016;17:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nisenblat V, Sharkey DJ, Wang Z, et al. Plasma miRNAs display limited potential as diagnostic tools for endometriosis. J Clin Endocrinol Metab. 2019;104:1999–2022. [DOI] [PubMed] [Google Scholar]


