Abstract
Biliary tract cancer, comprising gallbladder cancer, cholangiocarcinoma and ampullary cancer, represents a more uncommon entity outside high-endemic areas, though global incidence is rising. The majority of patients present at a late stage, and 5-year survival remains poor. Advanced stage disease is incurable, and though palliative chemotherapy has been shown to improve survival, further diagnostic and therapeutic options are required in order to improve patient outcomes. Although certain subtypes of biliary tract cancer are relatively rich in targetable mutations, attaining tumour tissue for histological diagnosis and treatment monitoring is challenging due to locoregional anatomical constraints and patient fitness. Liquid biopsies offer a safe and convenient alternative to invasive procedures and have great potential as diagnostic, predictive and prognostic biomarkers. In this review, the current standard of care for patients with biliary tract cancer, future treatment horizons and the possible utility of liquid biopsies within a variety of contexts will be discussed. Circulating tumour DNA, circulating microRNA and circulating tumour cells are discussed with an overview of their potential applications in management of biliary tract cancer. A summary is also provided of currently recruiting clinical trials incorporating liquid biopsies within biliary tract cancer research.
Keywords: Biliary tract cancer, Liquid biopsy, Circulating tumour DNA, Cell free DNA, Circulating tumour cells, Biomarkers
Core Tip: Liquid biopsies represent an enticing prospect in biliary tract cancer. In this review, we discuss the rationale, methods and utility of liquid biopsies for predictive and prognostic purposes, including circulating tumour DNA, circulating tumour cells and circulating microRNA. A summary is provided of current trials utilising liquid biopsies in biliary tract cancer.
INTRODUCTION
Biliary tract cancers (BTC) are an uncommon group of malignancies including gallbladder carcinoma (GBC), ampullary cancer and cholangiocarcinoma (CCA), the latter anatomically subdivided into intrahepatic CCA (iCCA), perihilar and distal subtypes. Incidence varies significantly by geographical region, with highest rates in East Asia, India and Chile and lower rates in Europe and North America, though incidence is increasing, particularly of iCCA[1].
Management of early-stage BTC revolves around surgical resection, though only 20%-40% of patients have surgically resectable disease at presentation[2], and a significant proportion of patients undergoing surgical resection will subsequently experience disease relapse[3]. Adjuvant chemotherapy with capecitabine has been demonstrated to improve survival[4]. Advanced disease is considered incurable, and although palliative chemotherapy with cisplatin and gemcitabine improves outcomes in eligible patients, median survival remains around 1 year. Second-line chemotherapy with 5-fluorouracil/oxaliplatin plus active symptom control has been demonstrated to improve overall survival (OS)[5], though there remains an unmet need for further systemic therapy options.
In future, additional potential therapeutic options may be available in clinical practice in the advanced setting, such as isocitrate dehydrogenase 1 (IDH1) inhibitors, immunotherapy and widespread use of fibroblast growth factor receptor (FGFR) inhibitors. Trials of molecularly targeted therapies and immune checkpoint inhibitors in unselected patients with BTC have suggested that these treatments should be used on a ‘precision medicine’ basis rather than empirically[6].
Molecularly targeted therapies have shown significant promise in iCCA, where tumours harbour an IDH1 mutation in 10%-20% of cases[7]. The phase 3 ClarIDHy trial evaluated the oral IDH1 inhibitor ivosidenib in patients with previously-treated CCA and histologically-proven IDH1 mutations. Although the statistically significant increase in progression free survival (PFS) seen with ivosidenib was meagre [2.7 mo vs 1.4 mo with placebo, hazard ratio (HR) 0.37 (95%CI: 0.25-0.54)], 22% of patients remained progression-free at 12 mo[8], and crossover-adjusted OS was recently reported as significantly prolonged (10.3 mo vs 5.1 mo with placebo)[9]. Alterations in FGFR have been observed in a variety of cancers including CCA, where the majority of aberrations are fusions in the FGFR2 gene[10]. Phase 2 trials of the oral selective FGFR tyrosine kinase inhibitors pemigatinib[11] and infigratinib[12] have shown promise, with objective response rates of 35.5% and 31% respectively in previously-treated patients with CCA and FGFR2 fusions/rearrangements. Based on data from the FIGHT-202 study[11], the United States Food and Drug Administration (FDA) granted accelerated approval in April 2020 for pemigatinib in patients with previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusion or rearrangement[13]; approval is expected imminently in Europe[14]. Phase 3 trials of first-line pemigatinib (FIGHT-302, NCT03656536[15]) and infigratinib (PROOF, NCT03773302[16]) are currently in progress.
Immune checkpoint inhibition, in monotherapy, has been less successful in the treatment of patients with BTC to date; pembrolizumab produced an overall response rate (ORR) of just 5.8% in patients with advanced BTC in the multi-tumour KEYNOTE-158 study[17], which may be related to the low incidence of microsatellite instability high/mismatch repair deficient tumours in BTC[18]. A subsequent analysis of patients (non-colorectal) with micro-satellite instability ‘high’ disease, including 22 patients with cholangiocarcinoma, reported an ORR of 34.3%[19]. Immunotherapy is an active area of investigation in biliary tract cancer, particularly in combination with other systemic or locoregional therapies.
The neurotropic tyrosine kinase receptor (NTRK), implicated in cellular proliferation, via the mitogen activated protein kinase pathway[20], has been targeted in patients with a variety of solid cancers. Larotrectinib, an oral TRK inhibitor with activity against a range of solid tumours harbouring NTRK gene fusions[21], was granted tumour-agnostic approval by the FDA and European Medicines Agency in 2018 and 2019, respectively. NTRK gene fusions are rarely observed in BTC, with one study identifying NTRK fusion in 1 of 28 patients[22]. Finally, other targets including the human epidermal growth factor receptor, the Wnt pathway and BRAF have been explored in patients with BTC. The ROAR study recently reported an ORR of 51% to dabrafenib and trametinib in 43 patients with previously-treated BRAF V600E-mutated advanced biliary cancer[23]. These targets are found in a minority of patients, however, and require tissue immunohistochemistry confirmation.
Despite the availability of standard and potentially promising investigational agents for the treatment of patients with BTC, identification of such targetable alterations usually requires adequate tumour tissue for molecular profiling. This review discusses the potential utility of ‘liquid biopsies’ in the management of patients with BTC, which may become part of future diagnostic, therapeutic or prognostic approaches and may replace the need for invasive tumour biopsies.
A review of the literature was undertaken following a PubMed literature search for {[(circulating tumour DNA) OR (circulating tumor DNA) OR (ctDNA) OR (cell free dna) OR (cell-free dna) OR (cfDNA) OR (circulating tumo* cell) OR (liquid biopsy)] AND (cancer) AND [(biliary) OR (gallbladder) OR (cholangiocarcinoma)]} NOT (colorectal).
CHALLENGES FOR TISSUE ACQUISITION IN BILIARY TRACT CANCER
The anatomical location of the biliary tract and its intimate relations with other key structures present significant challenges in the investigation, diagnosis and management of patients with BTC. Although fine needle biopsy via endoscopic ultrasound (EUS) represents the ‘gold standard’ for pathological confirmation of suspected cases, less sensitive investigations such as biliary brush cytology from endoscopic retrograde cholangiopancreatography (ERCP) or fine needle aspiration are often necessary[24]. Percutaneous biopsy, for example of metastatic lesions, may also be used. Some patients may be medically unfit for or reluctant to undergo more invasive procedures, and their limited diagnostic yield (molecular testing failed in 26.8% of 149 samples in one study[25]) may preclude assessment of pathological features such as mutational and molecular testing. Furthermore, BTCs exhibit significant intra- and inter-tumoural heterogeneity, posing the risk that an isolated biopsy from one part of a tumour may not be representative of the biology of the overall disease process in that patient[26]. Moreover, the mutational landscape across multiple metastatic sites may evolve during the course of a patient’s treatment. An alternative to tumour biopsies would be liquid biopsies.
Existing blood biomarkers utilised in the management of patients with biliary tract cancer
The only serum biomarkers currently recommended in guidelines for the management of patients with BTC are limited to a small number of assays including carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), routine liver function tests and lactate dehydrogenase[24]. CA19-9, a protein ordinarily expressed throughout the biliary and upper gastrointestinal tract, has been shown to have both predictive and prognostic applications in BTC. In patients with inoperable BTC, CA19-9 levels prior to systemic therapy were demonstrated to be prognostic for OS, with a HR for death of 2.92 for CA19-9 > 300 units/mL[27]. Although it has been studied as a biomarker to detect cholangiocarcinoma in patients with primary sclerosing cholangitis[28], the diagnostic utility of CA19-9 is limited by its relative lack of sensitivity and specificity. Furthermore, CA19-9 is not expressed in Lewis antigen-negative individuals (approximately 10% of a Caucasian population). CEA, more commonly associated with colorectal cancer, has limited application in BTC[29,30].
Why liquid biopsies?
The term ‘liquid biopsy’, referring to assessment of diagnostic, predictive or prognostic biomarkers usually via peripheral blood, has seen a rapid ascendency in research interest in the last five years, with a PubMed search for ‘liquid biopsy’ yielding 61 results from the year 2014 and 1342 from 2020. They present an attractive option for avoiding invasive procedures and potentially assessing a spectrum of DNA mutations from a variety of metastatic sites[31].
LIQUID BIOPSY IN THE MANAGEMENT OF PATIENTS WITH BTC: HORIZON OVERVIEW
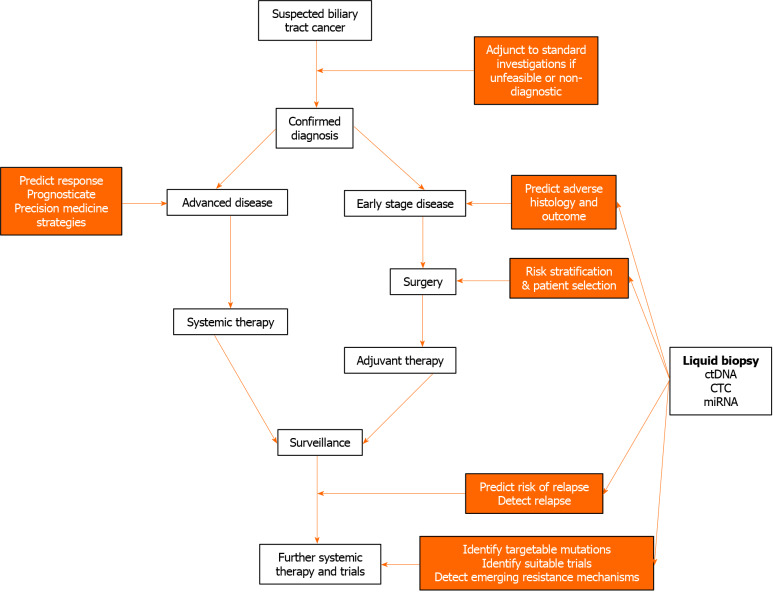
The potential applications of liquid biopsies in the management of patients with BTC are assessment of circulating tumour DNA (ctDNA), circulating microRNA (miRNA), and circulating tumour cells (CTCs). Here, the potential utility of these investigations according to diagnostic, predictive and prognostic applications will be discussed. These applications are summarised in Figure 1.
Figure 1.
Potential utilities of liquid biopsy in biliary tract cancer. ctDNA: Circulating tumour DNA; CTC: Circulating tumour cell; miRNA: Circulating micro-ribonucleic acid.
Potential liquid biopsy sources in patients with BTC
The principal attraction of liquid biopsies, namely their relative ease of collection from patients, has unsurprisingly led to the majority of studies being undertaken on samples obtained from peripheral blood. These will be discussed in more detail below. Prompted however by the relatively low yield of peripheral blood for CTCs, Catenacci et al[32] investigated paired portal venous and peripheral blood samples in 18 patients with clinicoradiologically-suspected pancreaticobiliary cancer[32]. While peripheral CTCs were identified in only 4 patients, portal venous CTCs were isolated in all 18 patients. Though this process requires invasive EUS, nullifying the benefit of a peripheral blood sample, it may have relevance for further study of CTCs and prognostication in patients with BTC.
Similarly, bile has been studied as a potential source of ctDNA in patients with BTC. A series of 10 patients with GBC or CCA underwent paired bile and tumour sampling via percutaneous transhepatic or surgical route, with targeted deep sequencing for 150 tumour genes showing a sensitivity of 94.7% and specificity of 99.9% vs tumour sampling[33]. A larger study of 30 patients with confirmed GBC examined paired bile and tumour samples, identifying bile ctDNA mutations in 87.5% of the 57.1% samples with a tumour mutation[34]. The majority of this review will now focus on liquid biopsies obtained from peripheral blood.
ctDNA
Background: Although the terms ‘ctDNA’ and ‘cfDNA’ are used inconsistently in the literature, ctDNA is here used to refer to the proportion of cfDNA which has specifically originated from tumour tissue, as opposed to physiological tissue. Genomic alterations in ctDNA can be identified via polymerase chain reaction (PCR) or next generation sequencing (NGS)[35]. While digital PCR permits highly sensitive analysis of point mutations of interest[36], NGS allows analysis of a large number of genes in parallel, with improved sensitivities achieved via novel methods such as capture-based NGS[37].
Analysis of ctDNA in the context of BTC has repeatedly been shown to be feasible and reliable in recent years. An early study by Zill et al[38] comparing NGS via cell free DNA and genomic DNA from tumour biopsies demonstrated cell free DNA in 16/26 patients with advanced BTC, with 92.3% sensitivity and 100% specificity for five genes[38]. A 2017 study measuring cfDNA via quantitative PCR in 34 patients with GBC successfully distinguished patients with GBC from those with cholecystitis and healthy controls[39]. Larger, more recent studies have achieved similarly successful results, with Mody et al[35] identifying clinically relevant mutations in 55% of 138 (unpaired) ctDNA samples from patients with BTC[35].
Diagnostic application of ctDNA in patients with biliary tract cancer: The standard of care cytological investigations for suspected BTC are ERCP or EUS[24]. These procedures may be technically difficult or precluded by comorbidity, and so a non-invasive diagnostic test is an attractive prospect. A study of ctDNA obtained by PCR from 34 patients with GBC and 39 controls (including 22 with cholecystitis) demonstrated significantly higher ctDNA levels in the malignant group, with levels also correlating with tumour/node/metastasis status[39]. Differential ctDNA gene methylation has been explored as an adjunctive tool in differentiating malignant from benign biliary tract disease. One study quantified methylation of opioid-binding protein/cell adhesion molecule (OPCML), homeobox A9 (HOXA9) and HOXD9 in serum ctDNA in 40 patients with CCA vs 40 with benign disease, including cholecystitis, cholangitis and papillary adenoma, among others[40]. The methylation level of OPCML and HOXD9 was significantly higher in the malignant group than the benign group, with area under curve (AUC) 0.850 and 0.789 respectively, while HOXA9 showed no significant difference.
Predictive application of ctDNA in patients with biliary tract cancer: Given the limited existing cytotoxic treatment options for advanced BTC and the relatively high rates of targetable mutations, particularly in intrahepatic cholangiocarcinoma, liquid biopsies present a significant opportunity for improving patient outcomes as predictive biomarkers for PFS, OS, response and toxicity.
While the ClarIDHy trial required inclusion of patients with histological confirmation of IDH1 mutations, a recent post-hoc analysis demonstrated that IDH1 mutation detection via plasma ctDNA PCR was feasible, with a concordance of 92% with tumour tissue[7]. Longitudinal plasma clearance of IDH1 mutation, defined as variant allele frequency (VAF) below the assay’s sensitivity, was observed in a subset of patients who responded to ivosidenib. Another recent study demonstrated concordance in 6/6 paired tissue and ctDNA samples for patients with IDH1 mutation and FGFR2 alteration[25]. Liquid biopsies have also been employed to confirm resistance to FGFR inhibitor therapy in patients with advanced, FGFR-fusion positive CCA via FGFR2 multiple recurrent point mutations detected on cfDNA, and confirmed on tumour biopsy[41]. These mutations were subsequently used to predict response to a novel FGFR inhibitor molecule, futibatinib (TAS-120)[42].
Given the high risk of relapse after BTC resection, a biomarker to aid selection of patients for adjuvant therapies is an enticing prospect. In patients with resected colorectal cancer, ctDNA has been used to identify patients with minimal residual disease and therefore could aid selection for adjuvant therapy, and also to detect subsequent relapse[43]. A study of 11 patients with resected pancreaticobiliary malignancies (8 biliary) was able to demonstrate feasibility of ctDNA detection via the Foundation Medicine Liquid platform, detecting ctDNA in 3 of the 8 patients with BTC[44]. A trend towards increased relapse risk was identified in patients with detectable ctDNA after surgery.
Finally, when BTC has relapsed after initial systemic therapy, ctDNA may in the future show utility in matching to an optimal clinical trial via molecular profiling via a similar process to Okamura et al[45] and the TARGET study[46].
Prognostic application of ctDNA in patients with biliary tract cancer: In matched ctDNA and tissue samples from patients with locally advanced or metastatic CCA prior to and during first line chemotherapy, Ettrich and colleagues reported that VAF correlated with tumour load, and with worse PFS in patients with iCCA[47].
Circulating miRNA
Background: Circulating miRNA refers to short, non-coding strands of approximately 20 nucleotides, existing freely in plasma, protein-bound or within extracellular vesicles[48]. In addition to the role of miRNA in gene expression regulation, miRNA has been shown to be differentially expressed in patients with CCA vs healthy controls[49].
Diagnostic application of miRNA in patients with biliary tract cancer: Studies involving miRNA investigating its potential ability to diagnose BTC have yielded promising results, most notably with miRNA-21, miRNA-26, miRNA-122 and miRNA-150[49]. A study of miRNA-21 derived via PCR from plasma samples in 94 patients who had undergone curative or non-curative resection for BTC and 50 healthy controls, demonstrated that miRNA-21 could distinguish malignant disease with an AUC of 0.93 (sensitivity 84%, specificity 98%)[50]. When comparing patients with BTC and 23 with benign biliary disease, an AUC to detect cancer of 0.83 was demonstrated. Similar studies report high sensitivity for BTC vs healthy controls with miRNA-21 (sensitivity 87.8%, specificity 90.5%[51]), miRNA-26a (sensitivity 84.8%, specificity 81.8%[52] and miRNA-150 (sensitivity 93.3%, specificity 53.3%[53]. Cheng et al[54] observed significant upregulation of miRNA-21 in patients with CCA vs healthy controls, but conversely, miRNA-106a was significantly downregulated in CCA vs benign biliary disease or healthy controls (AUC 0.79 CCA vs benign biliary disease; AUC 0.89 CCA vs healthy controls)[54].
In a related study, Lapitz et al[55] isolated RNA from extracellular vesicles derived from serum and urine in 12 patients with CCA, using nanoparticle tracking analysis, transmission electron microscopy and immunoblotting[55]. When compared with healthy individuals and those with primary sclerosing cholangitis or ulcerative colitis, patients with CCA exhibited a differential RNA profile, suggesting a possible diagnostic role in distinguishing patients with CCA from those with benign biliary disease or healthy individuals.
Predictive and prognostic applications of miRNA in patients with biliary tract cancer: A number of studies have reported association between tissue or serum miRNA dysregulation and unfavourable prognosis in patients with BTC[49]. In addition to being a promising diagnostic biomarker, miRNA-106a has shown promise in predictive (for tumour biology, PFS and OS) and prognostic roles. Compared to CA19-9, tumour differentiation, neural invasion, p53 and MUC1 expression, decreased pre-operative serum miRNA-106a expression was the only variable independently associated with lymph node metastases in 103 patients who underwent curative-intent resection for CCA[54]. Moreover, low miRNA-106a expression (taken as level < 1.00) was associated with a significantly shorter OS of 11.4 mo, vs 45.0 mo in patients with miRNA-106a level > 1.00.
In another study of 66 patients undergoing curative or palliative resection for CCA (29 stage I/II, 37 stage III/IV) vs 66 healthy controls, serum miRNA-26a upregulation was significantly associated with advanced stage, lymphatic invasion, tumour differentiation and metastasis status[52]. On multivariable analysis, upregulated serum miRNA-26a was also significantly associated with adverse PFS (HR 4.226, 95%CI: 1.415-10.321) and OS (HR 3.461, 95%CI: 1.331-5.364).
CTCs
Background: First recognised in 1869, CTCs are rapidly becoming a valuable but elusive tool in the oncological armamentarium in several solid cancers[56], with utility both in enumeration and characterisation. Conventional epithelial CTC (eCTC) detection relies on positive identification typically via the epithelial cell adhesion molecule (EpCAM), and exclusion of CD45 positive leukocytes. The ‘CellSearch’ system remains the only FDA-approved platform for eCTC detection via EpCAM. Given the relative scarcity of eCTCs, however, with cells detectable in just 17%-46% of patients with BTC, novel techniques have been developed to expand the pool of available cells to include non-conventional CTCs (ncCTC)[57]. Inclusion of this cell population, lacking EpCAM or leukocyte markers, but identified via copy number alterations, detected eCTCs or ncCTCs in 83% of 41 samples, vs eCTC positivity alone in only 19%. Although only eCTCs were associated with disease-specific survival, ncCTCs were associated with response to therapy. This novel technique offers promise in expanding yields of detectable CTCs in BTC.
Diagnostic application of CTCs in the management of patients with biliary tract cancer: Awasthi et al[58] isolated EpCAM-positive, CD45-negative CTCs in 25 of 27 treatment-naïve patients with confirmed GBC (5 stage I/II, 22 stage III/IV), with a sensitivity and specificity of 92.6% and 91.7% respectively[58]. Higher cut-off points for CTCs were able to distinguish between disease stages.
Predictive and prognostic application of CTCs in the management of patients with biliary tract cancer: Liquid biopsies have been extensively investigated for utility in monitoring treatment response and prognosticating. The phase 2 ABC-03 study randomised 124 treatment-naïve patients with advanced BTC to 8 cycles of cisplatin and gemcitabine with either cediranib or placebo[59]. The presence and increasing levels of eCTCs (detected by the CellSearch platform) were strongly associated with adverse prognosis; OS in patients with CTC 0/7.5 mL was 18.1 mo, compared to 10.3 mo (CTC 1/7.5 mL) and 8.7 mo (CTC ≥ 2/7.5 mL). However, a subsequent subgroup analysis of 43 patients demonstrated that change in eCTC level during treatment was not predictive of outcome[60]. A larger, prospective study of 88 patients with advanced CCA similarly demonstrated strong associations between higher CellSearch-detected CTC level during first line therapy and adverse outcome[61]. Median OS with CTC < 5/7.5 mL was 20 mo, vs 5 mo in patients with CTC ≥ 5/7.5 mL.
Patients with advanced BTC refractory to chemotherapy present a significant challenge in the clinic. Identification of these patients prior to commencing systemic therapy may avoid exposure to potentially harmful treatment, and aid selection for clinical trials. In small cell lung cancer, copy number alterations in CTCs successfully classified 83% of cases as either chemorefractory or chemosensitive[62]; a similar approach in BTC may predict which patients may be refractory to conventional treatment and prompt an alternative therapy strategy.
GENE ALTERATION DETECTION IN BILIARY TRACT CANCER USING LIQUID BIOPSIES
Several other mutations have been targeted in small numbers of patients with BTC, including alterations in ERBB2/HER2, TP53, KRAS, PIK3CA and BRAF, though data for liquid biopsies in these small populations are limited. The ERBB2/HER2 pathway is established as an important therapeutic target in a variety of solid cancers. Conventional ERBB2 quantification in tumour tissue is via immunochemistry or fluorescence in situ hybridisation, though an assay via ctDNA copy number has been validated in patients with HER2-amplified colorectal cancer[63]. HER2 is overexpressed in a minority of patients with cholangiocarcinoma, more so in extrahepatic CCA (8.5%, vs 0.9% in intrahepatic CCA)[64] and most commonly (16%) in GBC[65]. There are limited data to support ERBB2/HER2 determination via ctDNA in BTC, though Yarlagadda and colleagues reported a case of chemo-refractory CCA with 3+ HER2 amplification (assayed via ctDNA and confirmed histologically), who maintained a partial response to trastuzumab/pertuzumab therapy for over 12 mo[66].
A prospective study of the genomic landscape in BTC assessed ctDNA or tissue DNA mutations in 121 patients with BTC[45]. ctDNA was available from 71 patients (67 patients with advanced stage disease). Seventy five percent of patients were considered to have at least one theoretically targetable mutation, on or off-label. The most prevalent mutations were in TP53, KRAS and PIK3CA, identified in 38%, 28% and 14% of patients respectively. Of the 40 patients with matched ctDNA and tissue DNA samples, concordance was 68%, 80% and 90% respectively. Eighty patients commenced systemic therapy following molecular profiling, of whom 43% received a ‘matched’ therapy to an identified mutation (as first-line therapy in 67%). Although the majority of these patients were treated with gemcitabine/platinum (patients with BRCA-associated alteration) or anti-FGFR or -IDH therapies, 2 patients were treated based on ctDNA PIK3CA mutations (carboplatin and everolimus (first-line) and everolimus and lenvatinib (following prior gemcitabine/cisplatin), both achieving stable disease). Additionally, one patient was found to harbour a BRAF mutation in tissue and ctDNA.
SOME CURRENT LISTED LIQUID BIOPSY TRIALS IN PATIENTS WITH BTC
Although liquid biopsies in patients with BTC have shown promise in identifying targetable mutations, further study is required before these tests can be integrated into routine clinical practice. Some current listed BTC trials in which liquid biopsies are incorporated are summarised in Table 1.
Table 1.
Biliary tract cancer trial list
|
NCT
|
Setting
|
Recruitment status
|
Expected enrolment number
|
Relevant intervention
|
Phase
|
Relevant liquid biopsy outcomes
|
| NCT04561453 | Resected CCA/GBC | Recruiting | 20 | ctDNA monitoring (interval not stated) | NA | Success rate in obtaining ctDNA. Predictive value of ctDNA for recurrence and response to medical therapy |
| NCT03377179 | Unresectable CCA, 1st or 2nd line | Recruiting | 105 | ABC294640 + hydroxychloroquine | 2 | Serial ctDNA monitoring during/after treatment |
| NCT04484636 | Advanced HCC, CCA, GBC, pancreatic, gastric or oesophageal cancer in 1st line therapy | Recruiting | 200 | FoundationOneCDx. FoundationOneLiquid | NA | Frequency of targetable mutations. Heterogeneity of targetable alterations in paraffin embedded specimen vs cfDNA. Number of patients receiving therapies in accordance to their genomic profiles |
| NCT04400357 | Operable eCCA, ampullary, duodenal or pancreatic cancer | Recruiting | 244 | Robotic versus open pancreaticoduoedenectomy | NA | Baseline ctDNA. Effect of operative approach on ctDNA at post-operative day 1-30 |
| NCT03278106 | Stage 3/4 GBC | Active, not recruiting | 28 | Trifluridine/tipiracil following at least 1 line of systemic therapy | 2 | Baseline ctDNA or CTC. Change in ctDNA or CTC during and after treatment |
| NCT04072445 | Advanced GBC | Recruiting | 28 | Trifluridine/tipiracil + irinotecan following at least 1 line of systemic therapy | 2 | Baseline ctDNA or CTC. Change in ctDNA or CTC during and after treatment (frequency not stated) |
| NCT04445532 | BTC (any stage), HCC, healthy controls. Ampullary eligibility not stated | Recruiting | 450 | ctDNA monitoring during standard surgical or systemic therapy | NA | Biomarkers of DFS/OS and treatment efficacy |
| NCT03718897 | BTC newly diagnosed by ERCP | Recruiting | 100 | Baseline ctDNA and tissue whole genome sequencing | NA | OS |
| NCT04005339 | Advanced BTC (excluding ampullary) | Recruiting | 44 | 1st or 2nd line fluorouracil, leucovorin, liposomal irinotecan | 2 | ctDNA as surrogate for disease burden. Change in ctDNA compared to CA19-9 (ctDNA frequency not stated) |
ClinicalTrials.gov last accessed 20 January 2021. BTC: Biliary tract cancer; CA19-9: Carbohydrate antigen 19-9; CCA: Cholangiocarcinoma; cfDNA: Cell-free DNA; CTC: Circulating tumour cell; ctDNA: Circulating tumour DNA; DFS: Disease-free survival; eCCA: Extrahepatic cholangiocarcinoma; ERCP: Endoscopic retrograde cholangiopancreatography; GBC: Gallbladder cancer; HCC: Hepatocellular carcinoma; OS: Overall survival.
CONCLUSION
Although several novel therapies are likely to emerge in the near future, BTC poses significant diagnostic and therapeutic challenges to oncologists, and prognosis remains poor. Liquid biopsies offer hope of improved diagnostic pathways and easier identification of molecular alterations, thus potentially allowing access to molecularly targeted therapies without the need for invasive biopsies. Further unanswered questions regarding the validity of liquid biopsies for diagnosis and monitoring treatment require research attention. In particular, individual liquid biopsy platforms require independent validation in coordinated, collaborative studies in these rare cancers. Financial and logistic implications of incorporating these novel techniques will also require careful consideration. Liquid biopsies have been shown to have utility in other tumour sites, potentially paving the way for further study in BTC. Patients with chemorefractory advanced disease present a particular challenge, and identification of this cohort prior to initiation of systemic therapy may aid trial enrolment or alternative treatment modalities.
Footnotes
Conflict-of-interest statement: RS: Received travel and meeting funding from Servier; AL: Received travel and educational support from Ipsen, Pfizer, Bayer, AAA, Sirtex, Novartis, Mylan and Delcath. Speaker honoraria from Merck, Pfizer, Ipsen, and Incyte. Advisory honoraria from EISAI, Nutricia Ipsen, QED, and Roche. Member of the Knowledge Network and NETConnect Initiatives funded by Ipsen; JWV: Consulting or advisory role for Agios, AstraZeneca, Delcath Systems, Keocyt, Genoscience Pharma, Incyte, Ipsen, Merck, Mundipharma EDO, Novartis, PCI Biotech, Pfizer, Pieris Pharmaceuticals, QED, and Wren Laboratories; Speakers’ Bureau for Imaging Equipment Limited, Ipsen, Novartis, Nucana; and received Travel Grants from Celgene and Nucana; MMN: Received research grant support from Servier, Ipsen, and NuCana. She has received travel and accommodation support from Bayer and Ipsen and speaker honoraria from Pfizer, Ipsen, NuCana, and Mylan. She has served on advisory boards for Incyte, Celgene, Ipsen, Sirtex, and Baxalta.
Manuscript source: Invited manuscript
Peer-review started: February 16, 2021
First decision: May 3, 2021
Article in press: August 11, 2021
Specialty type: Oncology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee HJ, Taglieri E S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
Contributor Information
Rohan Shotton, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom.
Angela Lamarca, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom; Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom.
Juan Valle, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom; Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom.
Mairéad G McNamara, Department of Medical Oncology, The Christie NHS Foundation Trust, Manchester M20 4BX, United Kingdom; Division of Cancer Sciences, School of Medical Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, United Kingdom. mairead.mcnamara@nhs.net.
References
- 1.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60:1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 3.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663–673. doi: 10.1016/S1470-2045(18)30915-X. [DOI] [PubMed] [Google Scholar]
- 5.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, Anthoney A, Maraveyas A, Iveson T, Waters JS, Hobbs C, Barber S, Ryder WD, Ramage J, Davies LM, Bridgewater JA, Valle JW Advanced Biliary Cancer Working Group. Second-line FOLFOX chemotherapy vs active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Kamgar M, Mahipal A. Targeted Therapies in Advanced Biliary Tract Cancer: An Evolving Paradigm. Cancers (Basel) 2020;12 doi: 10.3390/cancers12082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguado E, Abou-Alfa GK, Zhu AX, Macarulla T, Fan B, Nejad P, Choe S, Jiang L, Gliser C, Pandya SS, Wu B. IDH1 mutation detection in plasma circulating tumor DNA (ctDNA) and association with clinical response in patients with advanced intrahepatic cholangiocarcinoma (IHC) from the phase III ClarIDHy study. J Clin Oncol. 2020;38 (15_suppl):4576–4576. [Google Scholar]
- 8.Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DVT, Borad MJ, Bridgewater JA, Harris WP, Murphy AG, Oh D-Y, Whisenant JR, Wu B, Jiang L, Gliser C, Pandya SS, Valle JW, Abou-Alfa GK. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) vs placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 ( IDH1 ) mutation. J Clin Oncol. 2021;39 (3_suppl):266–266. [Google Scholar]
- 10.Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res. 2016;22:259–267. doi: 10.1158/1078-0432.CCR-14-3212. [DOI] [PubMed] [Google Scholar]
- 11.Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, Paulson AS, Borad MJ, Gallinson D, Murphy AG, Oh DY, Dotan E, Catenacci DV, Van Cutsem E, Ji T, Lihou CF, Zhen H, Féliz L, Vogel A. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Javle M, Kelley RK, Roychowdhury S, Weiss KH, Abou-Alfa GK, Macarulla T, Sadeghi S, Waldschmidt D, Zhu AX, Goyal L, Borad M, Yong WP, Borbath I, El-Khoueiry A, Philip P, Moran S, Ye Y, Ising M, Lewis N, Bekaii-Saab T. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions. Ann Oncol . 2018;29:viii720. [Google Scholar]
- 13.US Food and Drug Administration. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion|FDA (Internet). fda.gov. 2020. [cited 21 January 2021]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion .
- 14.Incyte Announces Positive CHMP Opinion for Pemigatinib for the Treatment of Adults With Previously Treated. Unresectable Locally Advanced or Metastatic Cholangiocarcinoma With a Fibroblast Growth Factor Receptor 2 (FGFR2) Fusion or Rearrangement|Busines (Internet). 2021. [cited 7 February 2021]. Available from: https://www.businesswire.com/news/home/20210129005533/en/Incyte-Announces-Positive-CHMP-Opinion-for-Pemigatinib-for-the-Treatment-of-Adults-With-Previously-Treated-Unresectable-Locally-Advanced-or-Metastatic-Cholangiocarcinoma-With-a-Fibroblast-Growth-Fac .
- 15.Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, Melisi D, Macarulla T, Bridgewater JA, Wasan HS, Borad MJ, Lihou CF, Zhen H, Féliz L, Asatiani E, Jiang P, Vogel A. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) vs gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J Clin Oncol. 2020;38 (4_suppl):TPS592–TPS592. doi: 10.2217/fon-2020-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javle MM, Borbath I, Clarke SJ, Hitre E, Louvet C, Mercade TM, Oh D-Y, Spratlin JL, Valle JW, Weiss KH, Berman C, Howland M, Ye Y, Cho T, Moran S, Abou-Alfa GK. Infigratinib vs gemcitabine plus cisplatin multicenter, open-label, randomized, phase 3 study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: The PROOF trial. J Clin Oncol. 2019;37 (15_suppl):TPS4155–TPS4155. [Google Scholar]
- 17.Bang YJ, Ueno M, Malka D, Chung HC, Nagrial A, Kelley RK, Piha-Paul SA, Ros W, Italiano A, Nakagawa K, Rugo HS, De Braud FG, Varga AI, Hansen AR, Gao C, Krishnan S, Norwood K, Doi T. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: Results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. J Clin Oncol. 2019;37 (15_suppl):4079. [Google Scholar]
- 18.Rashid A, Ueki T, Gao YT, Houlihan PS, Wallace C, Wang BS, Shen MC, Deng J, Hsing AW. K-ras mutation, p53 overexpression, and microsatellite instability in biliary tract cancers: a population-based study in China. Clin Cancer Res. 2002;8:3156–3163. [PubMed] [Google Scholar]
- 19.Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr, Italiano A, Kao S, Piha-Paul SA, Delord JP, McWilliams RR, Fabrizio DA, Aurora-Garg D, Xu L, Jin F, Norwood K, Bang YJ. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 20.Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023. doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross JS, Wang K, Gay L, Al-Rohil R, Rand JV, Jones DM, Lee HJ, Sheehan CE, Otto GA, Palmer G, Yelensky R, Lipson D, Morosini D, Hawryluk M, Catenacci DV, Miller VA, Churi C, Ali S, Stephens PJ. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist. 2014;19:235–242. doi: 10.1634/theoncologist.2013-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Subbiah V, Lassen U, Élez E, Italiano A, Curigliano G, Javle M, de Braud F, Prager GW, Greil R, Stein A, Fasolo A, Schellens JHM, Wen PY, Viele K, Boran AD, Gasal E, Burgess P, Ilankumaran P, Wainberg ZA. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 24.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 25.Lamarca A, Kapacee Z, Breeze M, Bell C, Belcher D, Staiger H, Taylor C, McNamara MG, Hubner RA, Valle JW. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J Clin Med. 2020;9 doi: 10.3390/jcm9092854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bragazzi MC, Ridola L, Safarikia S, Matteo SD, Costantini D, Nevi L, Cardinale V. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann Gastroenterol. 2018;31:42–55. doi: 10.20524/aog.2017.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harder J, Kummer O, Olschewski M, Otto F, Blum HE, Opitz O. Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2097–2100. doi: 10.1158/1055-9965.EPI-07-0155. [DOI] [PubMed] [Google Scholar]
- 28.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 29.Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, Jiang XQ, Peng ZH. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085–4092. doi: 10.3748/wjg.v20.i14.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunnet M, Mau-Sørensen M. Serum tumor markers in bile duct cancer--a review. Biomarkers. 2014;19:437–443. doi: 10.3109/1354750X.2014.923048. [DOI] [PubMed] [Google Scholar]
- 31.Rizvi S, Eaton J, Yang JD, Chandrasekhara V, Gores GJ. Emerging Technologies for the Diagnosis of Perihilar Cholangiocarcinoma. Semin Liver Dis. 2018;38:160–169. doi: 10.1055/s-0038-1655775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catenacci DV, Chapman CG, Xu P, Koons A, Konda VJ, Siddiqui UD, Waxman I. Acquisition of Portal Venous Circulating Tumor Cells From Patients With Pancreaticobiliary Cancers by Endoscopic Ultrasound. Gastroenterology. 2015;149:1794–1803.e4. doi: 10.1053/j.gastro.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen N, Zhang D, Yin L, Qiu Y, Liu J, Yu W, Fu X, Zhu B, Xu X, Duan A, Chen Z, Wang X, Cao X, Zhao T, Zhou Z, Yu L, Qin H, Fang Z, Li JY, Liu Y, Xiong L, Yuan B, Li F, Zhang Y. Bile cellfree DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol Rep. 2019;42:549–560. doi: 10.3892/or.2019.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinugasa H, Nouso K, Ako S, Dohi C, Matsushita H, Matsumoto K, Kato H, Okada H. Liquid biopsy of bile for the molecular diagnosis of gallbladder cancer. Cancer Biol Ther. 2018;19:934–938. doi: 10.1080/15384047.2018.1456604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mody K, Kasi PM, Yang J, Surapaneni PK, Bekaii-Saab T, Ahn DH, Mahipal A, Sonbol MB, Starr JS, Roberts A, Nagy R, Lanman R, Borad MJ. Circulating Tumor DNA Profiling of Advanced Biliary Tract Cancers. JCO Precis Oncol . 2019:1–9. doi: 10.1200/PO.18.00324. [DOI] [PubMed] [Google Scholar]
- 36.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, Antonarakis ES, Azad NS, Bardelli A, Brem H, Cameron JL, Lee CC, Fecher LA, Gallia GL, Gibbs P, Le D, Giuntoli RL, Goggins M, Hogarty MD, Holdhoff M, Hong SM, Jiao Y, Juhl HH, Kim JJ, Siravegna G, Laheru DA, Lauricella C, Lim M, Lipson EJ, Marie SK, Netto GJ, Oliner KS, Olivi A, Olsson L, Riggins GJ, Sartore-Bianchi A, Schmidt K, Shih lM, Oba-Shinjo SM, Siena S, Theodorescu D, Tie J, Harkins TT, Veronese S, Wang TL, Weingart JD, Wolfgang CL, Wood LD, Xing D, Hruban RH, Wu J, Allen PJ, Schmidt CM, Choti MA, Velculescu VE, Kinzler KW, Vogelstein B, Papadopoulos N, Diaz LA Jr. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, Shrager JB, Loo BW Jr, Alizadeh AA, Diehn M. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zill OA, Greene C, Sebisanovic D, Siew LM, Leng J, Vu M, Hendifar AE, Wang Z, Atreya CE, Kelley RK, Van Loon K, Ko AH, Tempero MA, Bivona TG, Munster PN, Talasaz A, Collisson EA. Cell-Free DNA Next-Generation Sequencing in Pancreatobiliary Carcinomas. Cancer Discov. 2015;5:1040–1048. doi: 10.1158/2159-8290.CD-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari S, Tewari S, Husain N, Agarwal A, Pandey A, Singhal A, Lohani M. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol Oncol Res. 2017;23:91–97. doi: 10.1007/s12253-016-0087-0. [DOI] [PubMed] [Google Scholar]
- 40.Wasenang W, Chaiyarit P, Proungvitaya S, Limpaiboon T. Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin Epigenetics. 2019;11:39. doi: 10.1186/s13148-019-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, Lennerz JK, Vu P, Deshpande V, Kambadakone A, Mussolin B, Reyes S, Henderson L, Sun JE, Van Seventer EE, Gurski JM Jr, Baltschukat S, Schacher-Engstler B, Barys L, Stamm C, Furet P, Ryan DP, Stone JR, Iafrate AJ, Getz G, Porta DG, Tiedt R, Bardelli A, Juric D, Corcoran RB, Bardeesy N, Zhu AX. Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyal L, Shi L, Liu LY, Fece de la Cruz F, Lennerz JK, Raghavan S, Leschiner I, Elagina L, Siravegna G, Ng RWS, Vu P, Patra KC, Saha SK, Uppot RN, Arellano R, Reyes S, Sagara T, Otsuki S, Nadres B, Shahzade HA, Dey-Guha I, Fetter IJ, Baiev I, Van Seventer EE, Murphy JE, Ferrone CR, Tanabe KK, Deshpande V, Harding JJ, Yaeger R, Kelley RK, Bardelli A, Iafrate AJ, Hahn WC, Benes CH, Ting DT, Hirai H, Getz G, Juric D, Zhu AX, Corcoran RB, Bardeesy N. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion-Positive Intrahepatic Cholangiocarcinoma. Cancer Discov. 2019;9:1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriksen T V, Tarazona N, Reinert T, Carbonell-Asins JA, Renner D, Sharma S, Roda D, Huerta M, Roselló S, Iversen LH, Gotschalck KA, Madsen AH, Andersen PV, Thorlacius-Ussing O, Løve US, Sethi H, Aleshin A, Cervantes A, Andersen CL. Circulating tumor DNA analysis for assessment of recurrence risk, benefit of adjuvant therapy, and early relapse detection after treatment in colorectal cancer patients. J Clin Oncol. 2021;39 (3_suppl):11. [Google Scholar]
- 44.Lamarca A, McNamara MG, Hubner R, Valle JW. Role of ctDNA to predict risk of recurrence following potentially curative resection of biliary tract and pancreatic malignancies. J Clin Oncol . 2021;39 (3_suppl):336. [Google Scholar]
- 45.Okamura R, Kurzrock R, Mallory RJ, Fanta PT, Burgoyne AM, Clary BM, Kato S, Sicklick JK. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int J Cancer. 2021;148:702–712. doi: 10.1002/ijc.33230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothwell DG, Ayub M, Cook N, Thistlethwaite F, Carter L, Dean E, Smith N, Villa S, Dransfield J, Clipson A, White D, Nessa K, Ferdous S, Howell M, Gupta A, Kilerci B, Mohan S, Frese K, Gulati S, Miller C, Jordan A, Eaton H, Hickson N, O'Brien C, Graham D, Kelly C, Aruketty S, Metcalf R, Chiramel J, Tinsley N, Vickers AJ, Kurup R, Frost H, Stevenson J, Southam S, Landers D, Wallace A, Marais R, Hughes AM, Brady G, Dive C, Krebs MG. Utility of ctDNA to support patient selection for early phase clinical trials: the TARGET study. Nat Med. 2019;25:738–743. doi: 10.1038/s41591-019-0380-z. [DOI] [PubMed] [Google Scholar]
- 47.Ettrich TJ, Schwerdel D, Dolnik A, Beuter F, Blätte TJ, Schmidt SA, Stanescu-Siegmund N, Steinacker J, Marienfeld R, Kleger A, Bullinger L, Seufferlein T, Berger AW. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep. 2019;9:13261. doi: 10.1038/s41598-019-49860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Letelier P, Riquelme I, Hernández AH, Guzmán N, Farías JG, Roa JC. Circulating MicroRNAs as Biomarkers in Biliary Tract Cancers. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puik JR, Meijer LL, Le Large TY, Prado MM, Frampton AE, Kazemier G, Giovannetti E. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18:1343–1358. doi: 10.2217/pgs-2017-0010. [DOI] [PubMed] [Google Scholar]
- 50.Kishimoto T, Eguchi H, Nagano H, Kobayashi S, Akita H, Hama N, Wada H, Kawamoto K, Tomokuni A, Tomimaru Y, Umeshita K, Doki Y, Mori M. Plasma miR-21 is a novel diagnostic biomarker for biliary tract cancer. Cancer Sci. 2013;104:1626–1631. doi: 10.1111/cas.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma JL, Wu L, Wang H, Han SX, Zhu Q. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015;6:5932–5946. doi: 10.18632/oncotarget.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang LJ, Zhang KL, Zhang N, Ma XW, Yan SW, Cao DH, Shi SJ. Serum miR-26a as a diagnostic and prognostic biomarker in cholangiocarcinoma. Oncotarget. 2015;6:18631–18640. doi: 10.18632/oncotarget.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Yin J, Li T, Yuan L, Wang D, He J, Du X, Lu J. Upregulated circulating miR-150 is associated with the risk of intrahepatic cholangiocarcinoma. Oncol Rep. 2015;33:819–825. doi: 10.3892/or.2014.3641. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Q, Feng F, Zhu L, Zheng Y, Luo X, Liu C, Yi B, Jiang X. Circulating miR-106a is a Novel Prognostic and Lymph Node Metastasis Indicator for Cholangiocarcinoma. Sci Rep. 2015;5:16103. doi: 10.1038/srep16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lapitz A, Arbelaiz A, O'Rourke CJ, Lavin JL, Casta A, Ibarra C, Jimeno JP, Santos-Laso A, Izquierdo-Sanchez L, Krawczyk M, Perugorria MJ, Jimenez-Aguero R, Sanchez-Campos A, Riaño I, Gónzalez E, Lammert F, Marzioni M, Macias RIR, Marin JJG, Karlsen TH, Bujanda L, Falcón-Pérez JM, Andersen JB, Aransay AM, Rodrigues PM, Banales JM. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells. 2020;9 doi: 10.3390/cells9030721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol. 2017;22:421–430. doi: 10.1007/s10147-017-1105-2. [DOI] [PubMed] [Google Scholar]
- 57.Reduzzi C, Vismara M, Silvestri M, Celio L, Niger M, Peverelli G, De Braud F, Daidone MG, Cappelletti V. A novel circulating tumor cell subpopulation for treatment monitoring and molecular characterization in biliary tract cancer. Int J Cancer. 2020;146:3495–3503. doi: 10.1002/ijc.32822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awasthi NP, Kumari S, Neyaz A, Gupta S, Agarwal A, Singhal A, Husain N. EpCAM-based Flow Cytometric Detection of Circulating Tumor Cells in Gallbladder Carcinoma Cases. Asian Pac J Cancer Prev. 2017;18:3429–3437. doi: 10.22034/APJCP.2017.18.12.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valle JW, Wasan H, Lopes A, Backen AC, Palmer DH, Morris K, Duggan M, Cunningham D, Anthoney DA, Corrie P, Madhusudan S, Maraveyas A, Ross PJ, Waters JS, Steward WP, Rees C, Beare S, Dive C, Bridgewater JA. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16:967–978. doi: 10.1016/S1470-2045(15)00139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Backen AC, Lopes A, Wasan H, Palmer DH, Duggan M, Cunningham D, Anthoney A, Corrie PG, Madhusudan S, Maraveyas A, Ross PJ, Waters JS, Steward WP, Rees C, McNamara MG, Beare S, Bridgewater JA, Dive C, Valle JW. Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial. Br J Cancer. 2018;119:27–35. doi: 10.1038/s41416-018-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang JD, Campion MB, Liu MC, Chaiteerakij R, Giama NH, Ahmed Mohammed H, Zhang X, Hu C, Campion VL, Jen J, Venkatesh SK, Halling KC, Kipp BR, Roberts LR. Circulating tumor cells are associated with poor overall survival in patients with cholangiocarcinoma. Hepatology. 2016;63:148–158. doi: 10.1002/hep.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carter L, Rothwell DG, Mesquita B, Smowton C, Leong HS, Fernandez-Gutierrez F, Li Y, Burt DJ, Antonello J, Morrow CJ, Hodgkinson CL, Morris K, Priest L, Carter M, Miller C, Hughes A, Blackhall F, Dive C, Brady G. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med. 2017;23:114–119. doi: 10.1038/nm.4239. [DOI] [PubMed] [Google Scholar]
- 63.Siravegna G, Sartore-Bianchi A, Nagy RJ, Raghav K, Odegaard JI, Lanman RB, Trusolino L, Marsoni S, Siena S, Bardelli A. Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-targeted Therapy in Metastatic Colorectal Cancer. Clin Cancer Res. 2019;25:3046–3053. doi: 10.1158/1078-0432.CCR-18-3389. [DOI] [PubMed] [Google Scholar]
- 64.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross JS, Wang K, Javle MM, Catenacci DVT, Shroff RT, Ali SM, Elvin JA, Chmielecki J, Yelensky R, Lipson D, Miller VA, Stephens PJ, Meric-Bernstam F. Comprehensive genomic profiling of biliary tract cancers to reveal tumor-specific differences and frequency of clinically relevant genomic alterations. J Clin Oncol. 2015;33 (15_suppl):4009. [Google Scholar]
- 66.Yarlagadda B, Kamatham V, Ritter A, Shahjehan F, Kasi PM. Trastuzumab and pertuzumab in circulating tumor DNA ERBB2-amplified HER2-positive refractory cholangiocarcinoma. NPJ Precis Oncol. 2019;3:19. doi: 10.1038/s41698-019-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]