Abstract
Changes in cell growth and metabolism are affected by the surrounding environmental factors to adapt to the cell’s most appropriate growth model. However, abnormal cell metabolism is correlated with the occurrence of many diseases and is accompanied by changes in galectin (Gal) performance. Gals were found to be some of the master regulators of cell–cell interactions that reconstruct the microenvironment, and disordered expression of Gals is associated with multiple human metabolic-related diseases including cancer development. Cancer cells can interact with surrounding cells through Gals to create more suitable conditions that promote cancer cell aggressiveness. In this review, we organize the current understanding of Gals in a systematic way to dissect Gals’ effect on human disease, including how Gals’ dysregulated expression affects the tumor microenvironment’s metabolism and elucidating the mechanisms involved in Gal-mediated diseases. This information may shed light on a more precise understanding of how Gals regulate cell biology and facilitate the development of more effective therapeutic strategies for cancer treatment by targeting the Gal family.
Keywords: galectin, metabolism, tumor microenvironment, cancer
1. Introduction
In 1977, Halina Den and coworkers performed β-D-galectinoside-specific lectin isolation from chickens [1], introducing the roles of Gal in cell biology. Until now, the versatility and controversial roles of galectins have remained diverse according to an increasing number of studies [2]. How specific galectins connect and remodel their ordinary function to disease development remains an elusive question. In this review, we organize the current understanding of galectins, from their fundamental roles to disease development, and their druggable potential for readers to appreciate their importance.
2. Classification and Distribution of Galectins
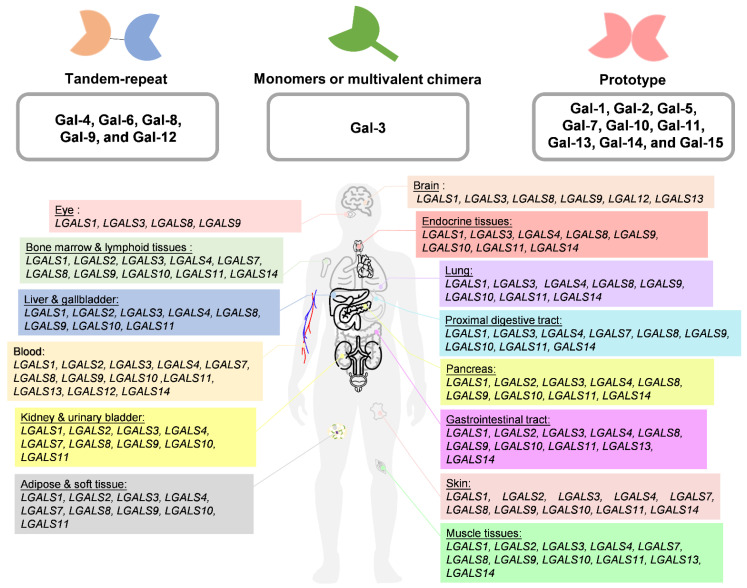
Gal is one of the carbohydrate-binding proteins that belongs to the animal lectin family. Gals are ubiquitous in the cytosol, nucleus, plasma membrane, and extracellular regions of cells through binding to glycans, which contain lactose or N-acetyllactosamine (LacNAc; Galβ1-4GlcNAc), via van der Waals interactions [3]. Gals are transcribed and translated into proteins by LGALS genes. Their main structure is divided into the N-terminal domain (NTD), which is composed of 12 amino acids containing serine phosphorylation [4]. The middle comprises a proline–glycine-rich domain, and the β-sheet consists of approximately 130 amino acids that form a highly conserved, small, and soluble structure composed of the functional carbohydrate recognition domain (CRD), which has an affinity for binding to the β-galactoside and carbohydrates that further classify Gal family proteins through CRDs [4,5]. Currently, Gals are divided into three groups according to their different structures. (1) Dimeric Gals are composed of the same subunit as the single CRD component, and the prototype includes Gal-1, Gal-2, Gal-5, Gal-7, Gal-10, Gal-11, Gal-13, Gal-14, and Gal-15. (2) Tandem-repeat Gals include Gal-4, Gal-6, Gal-8, Gal-9, and Gal-12 and are composed of at least two carbohydrate CRDs that covalently interact. (3) Finally, Gals can present as monomers or multivalent chimera types based on NTD self-oligomerization (Gal-3) (Figure 1A). In addition, Gal can undergo polymerization through non-covalent bonds [6], which results in different carbohydrate associations and allows presentation in different subcellular components and tissues [7]. The conformational change in the subunits may affect Gal’s interactions with different partners through either carbohydrate-dependent binding or carbohydrate-independent binding to perform diverse functions [8,9]. Furthermore, posttranscriptional and posttranslational modifications affect the multiple isoforms of Gal that may decide subcellular distribution and protein stability. For example, Haddad et al. identified that LGALS3 has an alternative reading frame called the GALIG gene, which can translate into mitogaligin and cytogaligin [10]. Apart from LGALS5, LGALS6, LGALS11, LGALS14, and LGALS15 are expressed in other species, such as sheep and goats. There are currently approximately eleven LGALS whose subcellular location and expression in the human body have been characterized, as depicted in Figure 1B (Table 1).
Figure 1.
The groups of Gal’s family structures. The distribution of galectin (LGALS) is dependent on the RNA level in the human body. All profiles were collected from the human protein atlas website (https://www.proteinatlas.org/, accessed on 15 September 2020) (see Appendix A).
Table 1.
Relative RNA expression of galectin (LGALS) in organs.
| LGALS Expression | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All Organs | LGALS1 | LGALS2 | LGALS3 | LGALS4 | LGALS7 | LGALS8 | LGALS9 | LGALS10 | LGALS12 | LGALS13 | LGALS14 |
| Brain | ● | ● | ● | ● | ● | ● | |||||
| Eye | ● | ● | ● | ● | |||||||
| Endocrine tissues | ● | ● | ● | ● | ● | ● | ● | ||||
| Lung | ● | ● | ● | ● | ● | ● | ● | ||||
| Proximal digestive tract | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Gastrointestinal tract | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Liver and gallbladder | ● | ● | ● | ● | ● | ● | ● | ||||
| Pancreas | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Kidney and urinary bladder | ● | ● | ● | ● | ● | ● | ● | ● | |||
| Muscle tissues | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | |
| Adipose and soft tissue | ● | ● | ● | ● | ● | ● | ● | ||||
| Skin | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Bone marrow and lymphoid tissues | ● | ● | ● | ● | ● | ● | ● | ● | ● | ||
| Blood | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● |
● Means positive expressed. All profiles were collected from the human protein atlas website (https://www.proteinatlas.org/, accessed on 15 September 2020).
3. Gal Functions in Cell Biology
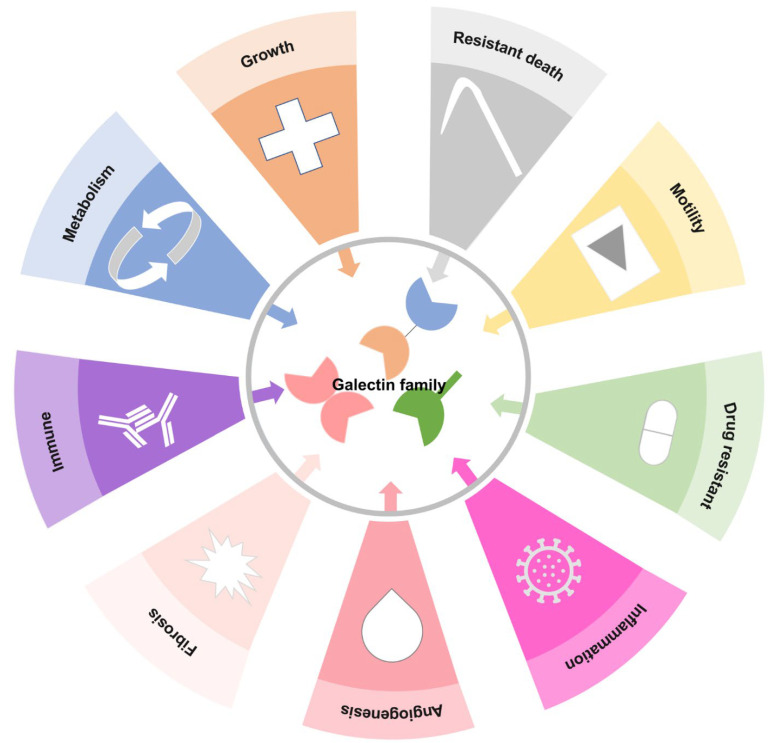
To date, these Gals have been found to regulate different cellular functions, including the interaction of galactoside ligands with different proteins in embryonic development, inflammation, immune response, metabolic disease, premRNA splicing, cell cycle, motility, survival, organ fibrosis, and cancer development (Figure 2) [9,10,11,12,13,14,15].
Figure 2.
Pluripotency of the galectin family in hallmarks of cell biology. The schematic diagram illustrates previous Gal functions; Gals are involved in cell proliferation, migration, anti-apoptosis, carbohydrates, proteins, lipid metabolism or nucleic acid synthesis, resistance to drug treatment, immune system or inflammation response, vessel generation, organ fibrogenesis, and resistance to death.
3.1. Embryonic Development
Gal families are involved in chicken lens development during embryogenesis, i.e., Gal-1, Gal-3, and Gal-8 [16]. Increased expression of LGALS1 may regulate the differentiation of human embryonic stem cells into pancreatic β cells [17]. Motohashi et al. reported that Gal-1 was highly expressed and enhanced neural crest generation in mouse embryonic stem cells [18]. Tang and others have shown that Gal-1 regulates trophoblast stem cell differentiation [11,19]. Gal-3 has been observed in myeloid cells, which can differentiate into macrophages in the developing lung and kidney [20,21].
3.2. Immunol Responses
During inflammation, Gal-1 induces neutrophil and T cell apoptosis [22,23]. Inflammatory bowel disease patients produce high serum levels of Gal-1 and Gal-3 [24]. Moreover, recombinant Gal-1 attenuates anti-ovalbumin glucose immunoglobulin E and interleukin to alleviate allergic airway inflammation [12]. Recombinant Gal-9 reverses lipopolysaccharide (LPS)-induced preeclampsia by promoting M2 macrophage polarization [25]. Interestingly, apart from Gal-1, Gal-3, a promoter during autoimmune cholangitis, is induced by activation of IL-1β and the NLRP3 inflammasome [26]. However, Gal-13 promotes neutrophil function by inducing reactive oxygen species (ROS), hepatocyte growth factor (HGF), matrix metalloproteinase 9 (MMP9), and programmed death-ligand 1 (PD-L1) during pregnancy [27].
3.3. Metabolic Processes
In addition, Gal expression is related to metabolic disorders. Glucocorticoid treatment can reverse diabetic retinopathy-induced Gal-1 expression in hypoxia [13]. During pregnancy, Gal-2 was observed in a metabolic disorder that caused gestational diabetes mellitus [28]. Increased serum Gal-3 and Gal-4 expression was observed in diabetes patients [29,30], and levels of Gal-3 were correlated with cardiovascular events in type 2 diabetes mellitus patients [31,32,33]. In a type 1 diabetes model, targeting Gal-3 decreased pro-inflammatory cytokine production [34]. Damage to pancreatic β cells induced by Gal-3 was present in both type 1 and 2 diabetes [34,35]. Sun et al. found that Gal-3 mediates high glucose-induced cardiomyocyte injury by regulating NADPH oxidase [36]. Moreover, diabetes mellitus is accompanied by organ fibrosis, such as in cardiac and lung tissue [15,37]. Hernández-Romero et al. observed that Gal-3 is involved in diabetes-induced atrial fibrillation [38]. In agreement with other reports, Wu et al. showed that diabetes mellitus-induced atrial fibrillation was accompanied by increased Gal-3 expression [39]. Similar observations were described by Al-Obaidi et al., who reported increased Gal-1 expression in both type 1 and 2 diabetes that regulates hyperglycemia-induced renal fibrosis [40]. Altogether, current observations suggest that Gal participates in diverse cellular pathways; therefore, it is crucial to understand and elucidate Gal diversity.
4. Abnormal Regulation of Galectin in Cancer Progression
Based on the expression of Gals and their different functions in cells as described above, it is predictable that Gal is involved in tumor progression. The correlation between Gal and survival outcomes from The Cancer Genome Atlas (TCGA) is shown in Table 2. In pan-cancer, we found that different Gals will have coordinated and redundant effects. Studies have also shown that Gal has a multifunctional effect in certain cancers (Table 3). For example, Califice et al. demonstrated that cytoplasmic Gal-3 promotes prostate cancer motility, proliferation, and angiogenesis. However, it inhibits prostate cancer in the nucleus [14]. Similarly, distinct roles were observed in Gal-9 isoforms, including the C-terminus and N-terminus of the Gal-9 CRD [41] in which the different CRDs of Gal-9 mediated opposing functions in a tube formation assay. The C-terminus of Gal-9 suppresses endothelial sprouting; conversely, the N-terminus promotes endothelial sprouting. In agreement with other reports, Rao et al. showed that treating cells with Gal-4 antibody increased cell proliferation and treating cells with recombinant Gal-4 increased cell cycle arrest, causing apoptosis through p27 induction and suppressing cyclin D1 and c-Myc expression in colorectal cancer [42]. These diverse findings indicate the need to better understand the functionality of different Gals in different cell types. Several studies have demonstrated that Gals regulate the growth of cancer cells. Gal-1 has been reported to participate in immune surveillance escape to regulate colorectal cancer growth [43]. Another study from Liang and coworkers found that Gal-3 enhances tumor initiation in hepatocellular carcinoma [44]. Furthermore, Liebscher et al. observed that Gal-1 regulates neuroblastoma cell growth, and knockdown of Gal-1 induced cell apoptosis [45]. Gal-9 promotes cell proliferation and was negatively regulated by lncMX1–215-mediated H3K27 acetylation on the promoter, and treating cells with histone deacetylase inhibitors increased metastasis in head and neck squamous cell carcinoma [46]. Similar observations were described by Enninga et al. who found that Gal-9 induces tumor growth by regulating CD206 macrophages in melanoma [47]. Furthermore, many studies have confirmed that the expression of Gals is associated with cancer stemness and resistance to drug treatment. Gal-1 increases invasion by stabilizing Ras to control the ERK pathway and promotes castration-resistant prostate cancer progression [48]. Cristiani et al. showed that the co-expression of PD-L1 and galectin-9 increases lung cancer sphere formation [49]. Mechanistically, Gal-9 promotes a stemness phenotype by modulating CCR7–CCL19 axes. This effect is in agreement with another cancer type wherein Gal-3-mediated immunosuppressive was required for prostate cancer stemness and metastasis [50]. Interestingly, Gal-3 is also reportedly increased in lung cancer, and monocytic MDSC with Gal-3 may induce cellular resistance to chemotherapy treatment [51], confirming that posttranslational modification of Gal affects subcellular location and functionality.
Table 2.
The prognostic role of galectin family members of pan-cancer in TCGA cohorts.
| Prototype | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Type | Endpoint | Case | Cohort | LGALS1 | HR | Risk | LGALS2 | HR | Risk | LGALS7 | HR | Risk | LGALS10 | HR | Risk | LGALS13 | HR | Risk | LGALS14 | HR | Risk |
| ACC | Overall survival | 76 | TCGA | 0.7800 | 1.1 | high | 0.2300 | 0.7 | low | N.S. | 0.7600 | 0.9 | low | N.S. | N.S. | ||||||
| BLCA | Overall survival | 402 | TCGA | 0.0450 | 1.4 | high | 0.3800 | 0.9 | low | 0.1100 | 1.3 | high | 0.9200 | 1.0 | low | N.S. | N.S. | ||||
| BRCA | Overall survival | 1070 | TCGA | 0.1600 | 1.3 | high | 0.3300 | 0.8 | low | 0.5400 | 1.1 | low | N.S. | N.S. | N.S. | ||||||
| CESC | Overall survival | 292 | TCGA | 0.3100 | 1.3 | high | 0.1400 | 0.6 | low | 0.0510 | 0.6 | low | 0.0089 | 0.4 | low | N.S. | N.S. | ||||
| CHOL | Overall survival | 36 | TCGA | 0.0980 | 2.2 | high | 0.1700 | 0.5 | low | 0.5500 | 1.3 | high | N.S. | N.S. | N.S. | ||||||
| COAD | Overall survival | 270 | TCGA | 0.2700 | 1.3 | high | 0.8400 | 1.0 | low | 0.9100 | 1.0 | high | 0.1500 | 0.7 | low | N.S. | N.S. | ||||
| DLBC | Overall survival | 46 | TCGA | 0.6200 | 0.7 | low | 0.1200 | 0.4 | low | N.S. | 0.5300 | 0.7 | low | N.S. | N.S. | ||||||
| ESCA | Overall survival | 182 | TCGA | 0.6300 | 1.1 | high | 0.1300 | 0.7 | low | 0.8900 | 1.0 | low | 0.9900 | 1.0 | high | N.S. | N.S. | ||||
| GBM | Overall survival | 161 | TCGA | 0.4500 | 1.2 | high | 0.3300 | 1.2 | high | 0.3800 | 1.2 | high | 1.0000 | 1.0 | low | N.S. | N.S. | ||||
| HNSC | Overall survival | 518 | TCGA | 0.0420 | 1.4 | high | 0.9900 | 1.0 | high | 0.1700 | 1.3 | high | 0.1700 | 0.8 | low | N.S. | N.S. | ||||
| KICH | Overall survival | 64 | TCGA | 0.0250 | 5.0 | high | 0.9400 | 1.1 | high | N.S. | N.S. | N.S. | N.S. | ||||||||
| KIRC | Overall survival | 516 | TCGA | 0.0023 | 1.8 | high | 0.0003 | 0.5 | low | 0.6400 | 0.9 | low | 0.0058 | 0.6 | low | N.S. | N.S. | ||||
| KIRP | Overall survival | 282 | TCGA | 0.3100 | 1.3 | high | 0.1800 | 1.5 | high | 0.6100 | 0.9 | low | N.S. | N.S. | N.S. | ||||||
| LAML | Overall survival | 106 | TCGA | 1.0000 | 1.0 | N.S. | 1.0000 | 1.0 | N.S. | N.S. | 1.0000 | 1.0 | N.S. | N.S. | N.S. | ||||||
| LGG | Overall survival | 514 | TCGA | 0.0061 | 1.5 | high | 0.0062 | 0.7 | low | N.S. | N.S. | N.S. | N.S. | ||||||||
| LIHC | Overall survival | 364 | TCGA | 0.4800 | 1.1 | high | 0.0230 | 0.7 | low | N.S. | N.S. | N.S. | N.S. | ||||||||
| LUAD | Overall survival | 478 | TCGA | 0.4500 | 1.1 | high | 0.0230 | 0.7 | low | 0.5600 | 1.1 | high | 0.0370 | 0.7 | low | N.S. | N.S. | ||||
| LUSC | Overall survival | 482 | TCGA | 0.0470 | 1.4 | high | 0.4600 | 1.1 | high | 0.5600 | 0.9 | low | 0.9600 | 1.0 | low | N.S. | N.S. | ||||
| MESO | Overall survival | 82 | TCGA | 0.2200 | 1.4 | high | 0.6200 | 0.9 | low | N.S. | N.S. | N.S. | N.S. | ||||||||
| OV | Overall survival | 424 | TCGA | 0.1500 | 1.2 | high | 0.1500 | 0.8 | low | 0.7800 | 1.0 | N.S. | 0.3300 | 0.9 | low | N.S. | 0.9900 | 1.0 | high | ||
| PAAD | Overall survival | 178 | TCGA | 0.0680 | 1.5 | high | 0.3600 | 0.8 | low | 0.4500 | 0.9 | low | 0.0059 | 1.9 | high | N.S. | N.S. | ||||
| PCPG | Overall survival | 182 | TCGA | 0.7400 | 1.2 | low | 0.3800 | 0.7 | low | N.S. | N.S. | N.S. | N.S. | ||||||||
| PRAD | Overall survival | 492 | TCGA | 0.7900 | 1.1 | high | 0.2900 | 1.3 | high | 0.0780 | 0.7 | low | N.S. | N.S. | N.S. | ||||||
| READ | Overall survival | 92 | TCGA | 0.1900 | 1.8 | high | 0.6500 | 1.2 | high | 0.2900 | 1.8 | high | 0.3100 | 0.6 | low | N.S. | N.S. | ||||
| SARC | Overall survival | 262 | TCGA | 0.2500 | 1.2 | high | 0.1000 | 0.7 | low | N.S. | N.S. | N.S. | N.S. | ||||||||
| SKCM | Overall survival | 584 | TCGA | 0.2100 | 1.2 | high | 0.1900 | 0.9 | low | 0.0270 | 1.3 | high | N.S. | N.S. | N.S. | ||||||
| STAD | Overall survival | 384 | TCGA | 0.4500 | 1.2 | low | 0.2500 | 0.8 | low | 0.0077 | 1.7 | high | 0.2200 | 1.3 | high | N.S. | N.S. | ||||
| TGCT | Overall survival | 136 | TCGA | 0.0970 | 1.9 | high | 0.2900 | 1.5 | high | 0.8900 | 1.1 | high | 0.5800 | 1.2 | high | N.S. | N.S. | ||||
| THCA | Overall survival | 450 | TCGA | 0.7900 | 0.9 | N.S. | 0.2300 | 1.4 | high | 0.7000 | 1.1 | high | N.S. | N.S. | N.S. | ||||||
| THYM | Overall survival | 118 | TCGA | 0.0800 | 0.4 | low | 0.7700 | 1.1 | high | 0.6100 | 1.3 | low | 0.0310 | 3.2 | high | N.S. | N.S. | ||||
| UCEC | Overall survival | 172 | TCGA | 0.2200 | 0.7 | low | 0.1300 | 0.6 | low | 0.7300 | 0.9 | low | N.S. | N.S. | N.S. | ||||||
| UCS | Overall survival | 56 | TCGA | 0.6800 | 0.9 | low | 0.4900 | 0.8 | low | 0.0960 | 0.6 | low | 0.0140 | 0.4 | low | N.S. | N.S. | ||||
| UVM | Overall survival | 78 | TCGA | 0.0007 | 5.6 | high | 0.1500 | 2.0 | high | N.S. | N.S. | N.S. | N.S. | ||||||||
| Tandem | Chimeric | ||||||||||||||||||||
| Cancer Type | Endpoint | Case | Cohort | LGALS4 | HR | Risk | LGALS8 | HR | Risk | LGALS9 | HR | Risk | LGALS12 | HR | Risk | LGALS3 | HR | Risk | |||
| ACC | Overall survival | 76 | TCGA | 0.0002 | 3.6 | high | 0.0001 | 3.8 | high | 0.7600 | 1.1 | low | 0.4700 | 0.7 | low | 0.0059 | 3.0 | high | |||
| BLCA | Overall survival | 402 | TCGA | 0.8900 | 1.0 | low | 0.0480 | 1.4 | high | 0.0260 | 0.7 | low | 0.1900 | 1.2 | high | 0.0830 | 1.3 | high | |||
| BRCA | Overall survival | 1070 | TCGA | 0.2300 | 0.8 | low | 0.2500 | 0.8 | low | 0.2700 | 0.8 | low | 0.2100 | 0.8 | low | 0.7000 | 1.1 | high | |||
| CESC | Overall survival | 292 | TCGA | 0.1200 | 1.6 | high | 0.1100 | 1.6 | high | 0.0250 | 0.6 | low | 0.1500 | 1.5 | high | 0.7300 | 0.9 | low | |||
| CHOL | Overall survival | 36 | TCGA | 0.4000 | 1.5 | high | 0.0830 | 0.5 | low | 0.0320 | 0.6 | low | 0.0140 | 0.3 | low | 0.5400 | 0.7 | low | |||
| COAD | Overall survival | 270 | TCGA | 0.0570 | 0.6 | low | 0.3400 | 1.3 | high | 0.6100 | 0.9 | low | 0.3000 | 0.8 | low | 0.0790 | 0.7 | low | |||
| DLBC | Overall survival | 46 | TCGA | 0.8800 | 1.1 | high | 0.3300 | 1.8 | high | 0.0320 | 7.2 | high | 0.5600 | 0.6 | low | 0.8300 | 1.2 | low | |||
| ESCA | Overall survival | 182 | TCGA | 0.8200 | 1.0 | high | 0.4500 | 1.3 | low | 0.7900 | 0.9 | low | 0.6400 | 0.9 | low | 0.7100 | 1.1 | high | |||
| GBM | Overall survival | 161 | TCGA | 0.2800 | 2.8 | low | 0.0320 | 1.6 | high | 0.2700 | 1.2 | high | 0.1400 | 1.3 | high | 0.0560 | 1.4 | high | |||
| HNSC | Overall survival | 518 | TCGA | 0.6000 | 1.0 | high | 0.0390 | 1.4 | high | 0.0120 | 0.7 | low | 0.6800 | 0.9 | high | 0.2600 | 0.9 | low | |||
| KICH | Overall survival | 64 | TCGA | 0.5400 | 1.5 | high | 0.4200 | 1.7 | high | 0.1200 | 0.3 | low | 0.7000 | 0.8 | low | 0.1400 | 3.1 | high | |||
| KIRC | Overall survival | 516 | TCGA | 0.4000 | 0.9 | low | 0.1000 | 0.7 | low | 0.9700 | 1.0 | low | 0.0140 | 1.5 | high | 0.9500 | 1.0 | low | |||
| KIRP | Overall survival | 282 | TCGA | 0.0031 | 2.4 | high | 0.2100 | 1.4 | high | 0.5500 | 0.8 | low | 0.5800 | 1.2 | low | 0.7500 | 1.1 | high | |||
| LAML | Overall survival | 106 | TCGA | 1.0000 | 1.0 | N.S. | 1.0000 | 1.0 | N.S. | 0.0550 | 1.7 | high | 0.6800 | 1.1 | high | 0.8900 | 1.0 | low | |||
| LGG | Overall survival | 514 | TCGA | 0.1600 | 1.3 | high | 0.0028 | 1.6 | high | 0.0004 | 1.9 | high | 0.0760 | 1.4 | high | 0.0005 | 1.9 | high | |||
| LIHC | Overall survival | 364 | TCGA | 0.4800 | 1.1 | high | 0.5800 | 0.9 | low | 0.1100 | 1.3 | high | 0.3700 | 1.2 | high | 0.0037 | 1.7 | high | |||
| LUAD | Overall survival | 478 | TCGA | 0.0690 | 0.8 | low | 0.3000 | 1.2 | low | 0.2500 | 0.8 | low | 0.0680 | 0.8 | low | 0.0580 | 1.3 | high | |||
| LUSC | Overall survival | 482 | TCGA | 0.5700 | 0.9 | low | 0.4600 | 0.9 | low | 0.5100 | 1.1 | high | 0.6100 | 1.1 | high | 0.3600 | 0.9 | low | |||
| MESO | Overall survival | 82 | TCGA | 0.6700 | 1.1 | low | 0.5200 | 0.8 | low | 0.0700 | 0.6 | low | 0.4700 | 1.2 | high | 0.9400 | 1.0 | low | |||
| OV | Overall survival | 424 | TCGA | 0.8000 | 1.0 | low | 0.5800 | 1.1 | high | 0.5200 | 1.1 | low | 0.9400 | 1.0 | high | 0.6000 | 0.9 | low | |||
| PAAD | Overall survival | 178 | TCGA | 0.2500 | 1.3 | high | 0.4100 | 1.2 | high | 0.0770 | 1.4 | high | 0.2400 | 0.8 | low | 0.0430 | 1.5 | high | |||
| PCPG | Overall survival | 182 | TCGA | 0.8400 | 1.1 | high | 0.0060 | 2.6 | high | 0.0680 | 0.2 | low | 0.6100 | 0.7 | low | 0.3500 | 2.2 | high | |||
| PRAD | Overall survival | 492 | TCGA | 0.0081 | 1.8 | high | 0.7800 | 0.9 | low | 0.2800 | 0.5 | low | 0.6100 | 1.4 | high | 0.7300 | 1.3 | high | |||
| READ | Overall survival | 92 | TCGA | 0.8800 | 1.1 | low | 0.3800 | 1.5 | low | 0.5200 | 0.7 | low | 0.3600 | 0.7 | low | 0.2500 | 0.6 | low | |||
| SARC | Overall survival | 262 | TCGA | 0.5700 | 1.1 | high | 0.3900 | 1.2 | high | 0.0085 | 0.6 | low | 0.9100 | 1.0 | high | 0.3100 | 0.8 | low | |||
| SKCM | Overall survival | 584 | TCGA | 0.9500 | 1.0 | high | 0.3100 | 0.9 | low | 0.0004 | 0.6 | low | 0.2900 | 0.9 | low | 0.8000 | 1.0 | high | |||
| STAD | Overall survival | 384 | TCGA | 0.1900 | 0.8 | low | 0.3300 | 1.2 | high | 0.2900 | 0.9 | low | 0.0047 | 1.6 | high | 0.3700 | 0.9 | low | |||
| TGCT | Overall survival | 136 | TCGA | 0.8700 | 0.9 | low | 0.3800 | 0.7 | low | 0.2000 | 4.2 | high | 0.7300 | 0.7 | low | 0.0230 | 900,000,000.0 | high | |||
| THCA | Overall survival | 450 | TCGA | 0.9900 | 1.0 | high | 0.5800 | 1.2 | high | 0.8700 | 0.9 | high | 0.7900 | 0.9 | low | 0.2300 | 0.5 | low | |||
| THYM | Overall survival | 118 | TCGA | 0.7400 | 0.9 | low | 0.4400 | 0.7 | low | 0.9600 | 1.0 | low | 0.6300 | 0.7 | high | 0.5800 | 1.5 | high | |||
| UCEC | Overall survival | 172 | TCGA | 0.8200 | 0.9 | high | 0.8000 | 0.9 | low | 0.7700 | 0.9 | low | 0.2400 | 0.7 | low | 0.9600 | 1.0 | low | |||
| UCS | Overall survival | 56 | TCGA | 0.0360 | 0.5 | low | 0.3700 | 1.4 | high | 0.5100 | 0.8 | low | 0.3700 | 1.4 | high | 0.6400 | 0.9 | low | |||
| UVM | Overall survival | 78 | TCGA | 0.5300 | 1.3 | high | 0.1300 | 2.0 | high | 0.0210 | 2.9 | high | 0.4300 | 0.7 | low | 0.0410 | 2.5 | high | |||
Abbreviations of cancer type as: Adrenocortical Cancer (ACC), Bladder Cancer (BLCA), Breast Cancer (BRCA), Cervical Cancer (CESC), Bile Duct Cancer (CHOL), Colon Cancer (COAD), Colon and Rectal Cancer (COADREAD), Large B Cell Lymphoma (DLBC), Mesothelioma (MESO), Esophageal Cancer (ESCA), Glioblastoma (GBM), Kidney Chromophobe (KICH), Kidney Clear Cell Carcinoma (KIRC), Kidney Papillary Cell Carcinoma (KIRP), Acute Myeloid Leukemia (LAML), Lower Grade Glioma (LGG), Liver Cancer (LIHC), Lung Adenocarcinoma (LUAD), Lung Squamous Cell Carcinoma (LUSC), Head and Neck Cancer (HNSC), Ovarian Cancer (OV), Pancreatic Cancer (PAAD), Pheochromocytoma and Paraganglioma (PCPG), Prostate Cancer (PRAD), Rectal Cancer (READ), Melanoma (SKCM), Stomach Cancer (STAD), Testicular Cancer (TGCT), Thyroid Cancer (THCA), Thymoma (THYM), Endometrioid Cancer (UCEC), Uterine Carcinosarcoma (UCS), Ocular Melanoma (UVM). All data collected and manipulated from UCSC Xena website (https://xenabrowser.net/, accessed on 15 September 2020).
Table 3.
Functions of LGALS in cancer.
| Cancer Type | LGALS Expression | Biological Relevance | Year | Author | Reference |
|---|---|---|---|---|---|
| Bladder | Gal-1 | Regulation of proliferation and invasion | 2018 | Li, C.F. | [66] |
| Gal-3 | Tumor Growth | 2008 | Fang, T. | [67] | |
| Gal-9 | Contribution to tumor invasion and immune surveillance | 2019 | Qi, Y. | [68] | |
| Blood | Gal-12 | Regulation of lipid raft formation | 2016 | Xue, H. | [69] |
| Bone marrow | Gal-1 | Regulation of M2 macrophage activation | 2017 | Andersen, M.N. | [70] |
| Gal-1 | Required for tumor development | 2019 | Muller, J. | [71] | |
| Breast | Gal-1 | Contributes to tumor progression and drug resistant | 2017 | Nam, K. | [72] |
| Gal-1 | Tumor metastasis and immune evasion | 2019 | Patrick, M.E. | [73] | |
| Gal-1 | Associated with chemoresistance | 2016 | Upreti, M. | [74] | |
| Gal-3 | Associated with metastasis | 2019 | Pereira, J.X. | [75] | |
| Gal-3 | Involved in osteoclastogenesis | 2016 | Nakajima, K. | [76] | |
| Gal-3 | Regulates tumor growth and metastasis | 2003 | John, C.M. | [77] | |
| Cervical | Gal-1 | promotes the invasive | 2020 | Wu, H. | [63] |
| Gal-7 | Negative regulation of tumor progression | 2016 | Higareda-Almaraz, J. C. | [78] | |
| Colon | Gal-1 | Promotes invasion | 2017 | Park, G. | [79] |
| Gal-3 | Promotes cancer metastasis | 2013 | Dovizio, M. | [80] | |
| Gal-12 | Inhibits glutamine uptake | 2019 | Katzenmaier, E.M. | [81] | |
| Colorectal | Gal-1 | Associated to immunosuppressive | 2021 | Cagnoni, A.J. | [43] |
| Gal-1 | Associated to tumor progression | 2019 | Sandberg, T.P. | [82] | |
| Gal-3 | Promotes metastasis | 2017 | Wu, K.L. | [56] | |
| Gal-4 | causing apoptosis | 2017 | Rao, U. | [42] | |
| Gal-4 | Growth inhibition | 2019 | Michalak, M. | [83] | |
| Gal-9 | Increases immune surveillance | 2019 | Sakhnevych, S.S. | [84] | |
| Esophageal | Gal-9 | Apoptosis inductor | 2019 | Chiyo, T. | [85] |
| Gastric | Gal-1 | Involved in metastasis | 2016 | Chong, Y. | [86] |
| Head and neck | Gal-1 | Regulates vessel normalization | 2017 | Koonce, N.A. | [87] |
| Liver | Gal-3 | Regulates cell proliferation | 2021 | Liang, Z. | [44] |
| Gal-9 | Promotes tumor apoptosis | 2017 | Tadokoro, T. | [88] | |
| Lung | Gal-1 | Correlated to metabolism and poor prognosis | 2019 | Zheng, H. | [89] |
| Gal-1 | Immune suppression | 2016 | Hsu, Y. L. | [90] | |
| Gal-3 | Associated with drug-resistance | 2019 | He, F. | [91] | |
| Gal-3 | Immune surveillance escape | 2019 | Vuong, L. | [92] | |
| Gal-9 | Associated with chemoresistance | 2019 | Limagne, E. | [51] | |
| Ovarian | Gal-3 | Chemotherapy sensitivity | 2019 | Wang, D. | [93] |
| Gal-3 | Cell apoptosis | 2016 | El-Kott, A.F. | [94] | |
| Gal-3 | Cell motility and sphere formation | 2019 | Hossein, G. | [95] | |
| Pancreatic | Gal-1 | Promotes cancer progression | 2018 | Tang, D. | [96] |
| Gal-1 | Crosstalk with stromal cells | 2018 | Orozco, C. A. | [97] | |
| Prostate | Gal-1 | Associated to invasion ability | 2018 | Shih, T.C. | [48] |
| Gal-1 | Regulates cells proliferation and apoptosis | 2018 | Corapi, E. | [98] | |
| Gal-3 (cytoplasmic) | Promotes tumor progression | 2004 | Califice, S. | [14] | |
| Gal-3 (nucleus) | Inhibits tumor progression | 2004 | Califice, S. | [14] | |
| Gal-3 | Immunosuppressive and Metastasis | 2020 | Caputo, S. | [50] | |
| Gal-3 | Regulates osteoclastogenesis | 2016 | Nakajima, K. | [76] | |
| Skin | Gal-1 | Involved in immune surveillance escape and cause drug resistance | 2020 | Gorniak, P. | [99] |
| Gal-3 | Lung metastasis | 2005 | Krishnan, V. | [100] |
All the biological relevancies of Gals in cancer are collected and listed based on current research and referred to in this article.
Gal also participates in other processes during cancer progression, including angiogenesis and motility. Gal-1 is reportedly induced by communicating glycosylated receptors to regulate cell angiogenesis [52]. In colon and breast cancer, Gal-3 can interact with glycoprotein VI, and activation of Gal-3 from cancer cells promotes extravasation by stimulating activation and degranulation in platelets [53]. Similar observations were described for in vivo experiments; endogenous Gal-8 can be secreted by MCF-7 cells, which increases microvascular permeability by binding to integrin-β1 and VEGFR2 to activate AKT-eNOS-FAK signaling that promotes angiogenesis and metastasis [54]. Conversely, in multiple myeloma patients, knockdown of Gal-1 upregulates MMP9, CCL2, SEMA3A, and CXCL10 to promote angiogenesis [55]. Additionally, Wu et al. and coworkers showed the interaction of Gal-3 and secreted carcinoembryonic antigen in colorectal cancer cells; knockdown of Gal-3 blocked carcinoembryonic antigen-mediated cell migration and metastasis [56]. Taken together, these findings indicate that molecules associate with Gal to affect the functions of Gal. The functions of these molecules are also affected by the presence or absence of Gal. As described above, several factors influence Gal functions, including posttranslational modification of mRNA or protein, subcellular location, and interacting partners. According to current studies, the mechanism by which Gal is secreted into the extracellular environment remains unknown because of its lack of a secretion signal peptide or transmembrane domain [57]. For example, posttranslational modification or interaction partners may shift Gal’s location. Gong et al. showed that phosphorylation of Gal-3 on the N-terminal domain alters its subcellular location, particularly residues 89-96 [7,58]. Phosphorylation of Gal-3 at tyrosine by calpain 4 has been found to increase extracellular secretion [59]. Interestingly, artificial plus acylation sequences accelerate Gal secretion in cos-7 cells [60]. Sato and coworkers found that Gal can regulate baby hamster kidney cell attachment and spreading, and the secretion of Gal is affected by methylamine, serum starvation, heat shock, and calcium ionophores [61]. Mutation of arginine to alanine at residue 224 was critical for nuclear localization of Gal-3 and protein stability [62]. Furthermore, inhibition of miR-1275 by RACK1 induced Gal-1 expression and secretion in cervical cancer [63]. Gal-3-binding proteins, such as synexin and importin, were found to be associated with intracellular Gal-3 and translocation into the nucleus [62,64,65], revealing that identifying Gal-binding proteins may unveil mechanisms of Gal translocation. In addition to these molecules, scientists have also found various potential interaction partners with the Gal family. Thus, these binding partners of Gal have been collected on the website through prediction or experimental proof (Table 4).
Table 4.
Interaction partners of galectin.
| LGALS Type | Protein Partner | Dataset |
| LGALS1 | AAR2, ACO, AGR2, ALCAM, ALDOA, ANXA2, ANXA22, APEX, APOA1, ARF4, AFP, ATP6AP2, C110RF87, CD2, CD4, CD7, CD28, CD44, CD68, CDC42, CDHR2, CDHR5, CHL1, CHORDC1, CLNS1A, CRIP1, CYLD, DBN1, DCPS, DDX19B, DYRK1A, EFNB3, EFTUD2, EGFR, EREG, ESR2, F5, FGA, FGG, FAM24B, FLNA, FN1, FUBP1, FZD10, GEMIN4, GOLT1B, GTF2I, HEPACAM2, HIST1H2BO, HNRNPL, HRAS, HSPA5, HSPB2, ICAM2, ICOSLG, IGBP1, IL6, ITGA4, LAMC1, LAMP1, LAMTOR3, LASP1, LGALS3BP, LGALS3, LIMA1, LINGO2, LMAN1, LRFN4, MB21D1, MCM2, MCM5, MUC16, MYC, MYH9, NLGN3, NPM1, NTRK3, PCBD2, PCBP1, PCBP2, PECAM1, PHB2, PIGR, PIH1D1, PLIN3, POLE4, PRKCZ, PSG1, PSMG1, PTEN, PTGER3, PTPRA, PTPRC, PTPRZ1, RAB5C, RAB10, RAC1, RAE1, RNF4, SIPR2, SEMA4C, SERPINH1, SIGLEC7, SLAMF1, SLAMF7, SMN1, SNRPB, SOD1, SOX2, SPANXC, SPN, STUB1, SUSD2, TALDO1, TIMP1, TNFRSF10C, TNF, TYW3, U2AF2, UBE2N, UQCRFS1, USP4, VASN, VCAM1, VIPR2, WWP2, ZNF131 | Abbott KL(2008), Agrawal P(2010), Amith SR (2017), Byron A(2012), Caron P(2019), Chen R(2013), Cho Y(2018), Chung LY (2012), Drissi R (2015), Elliott PR (2016), Ewing RM (2007), Fang X (2011), Foerster S (2013), Giurato G (2018), Grose JH (2015), Guo HB (2009), Havugimana PC (2012), Heidelberger JB (2018), Hein MY (2015), Hou C (2018), Humphries JD (2009), Hutchins JR (2010), Huttlin EL (2014/pre-pub), Huttlin EL (2015), Huttlin EL (2017), Kristensen AR (2012), Kumar R (2017), Kupka S (2016), Lin TW (2015), Lum KK (2018), Malinova A (2017), Pace KE (1999), Park JW (2001), Paz A (2001), Roewenstrunk J (2019), Seelenmeyer C (2003), Shen C (2015), Tiemann K (2018), Tinari N (2001), Varier RA (2016), Verrastro I (2015), Voss PG (2008), Walzel H (2000), Wan C (2015), Wang J (2008), Whisenant TC (2015), Yamauchi T (2018), Zhao B (2012) |
| LGALS2 | ALOX5AP, APP, GCSAM, IGSF23, IKBKG, LTA, NAT8, NR1H4, NXPE1, PAICS, PSMA6, SDCBP2, SDCBP, SDPR, TRIM16, TUBA1B, TUBB, WDYHV1 | Chauhan S (2016), Fenner BJ (2010), Luck K (2020), Ozaki K (2004), Rolland T (2014) |
| LGALS3 | ORF3A, ORF7B, ABCB1, ABCB1, ABCC4, ABCC4, ACAA2, ACOT1, ACP2, ACTA1, ACTA2, ADCY3, ADCY3, ADCY6, ADCY6, ADCY9, ADCY9, AGPS, AGR2, AHCY, AK3, ALCAM, ALCAM, APLNR, APP, ATG9A, ATG9A, ATP1A1, ATP2B4, ATP2B4, ATP5C1, ATP13A3, ATP13A3, BARD1, BARD1, BARD1, BARD1, BCL2L14, BRCA1, BSG, C1GALT1C1, C1ORF85, C11ORF87, CACNG1, CACNG5, CADM1, CAPN1, CAPN1, CBFB, CCT3, CD58, CD58, CD63, CD63, CD68, CD109, CD109, CDC5L, CDK15, CFTR, CKAP4, CLCN3, CLCN3, CLCN5, CLEC7A, CLNS1A, COASY, COASY, COLEC12, COLEC12, COX17, CRIP1, CRX, CRYZ, CSPG4, CSPG4, CTNNB1, CYLD, DBI, DDOST, DSTN, DUT, ECE1, ECE1, EGFR, EGFR, EGFR, ELMOD1, ELN, ELN, EMB, EMB, EMP3, ENO1, ENPP4, ENPP4, ESR1, ESR2, FAHD2A, FBXL4, FBXO6, FCF1, FCGR2A, FKBP2, FLT4, FLT4, FN1, FN1, FOXA1, GEMIN4, GLB1, GOLGA2, GPR35, GPR35, GPR52, GPR52, GPR55, GPR55, GPR84, GRPR, GSTP1, GTF2I, GTF2I, GTF2I, HEBP1, HEG1, HEG1, HRAS, HRNR, HSP90AB1, HTR2C, ICOSLG, IDH2, IGBP1, IL31RA, IMPA2, INCA1, IPO5, IPPK, ISOC2, ITGA1, ITGA1, ITGA2, ITGA2, ITGB1, KCTD12, KIAA0319L, KIAA0319L, KIAA1549, KIAA1549, KIAA1549, KIF16B, KPNB1, KRAS, LAMA1, LAMA1, LAMA4, LAMA4, LAMB1, LAMB1, LAMC1, LAMP1, LAMP1, LAMP2, LGALS1, LGALS3BP, LGALS3BP, LGALS3BP, LGALS3BP, LGALS3BP, LGALS3BP, LGALS3, LGALS9C, LGALS9C, LGALS9, LGALS9, LNPEP, LNPEP, LPAR1, LPAR1, LPAR2, LRP1, MAP1LC3A, MAP1LC3A, MAPK3, MCCC1, MCCC2, MCPH1, MEFV, MFAP3, MFAP3, MFSD4, MPZL1, MPZL1, MRC2, MRC2, MSRB2, MUC, MYH10, MYL6, MYL12B, MYO1E, MYOC, NAP1L1, NCSTN, NHLRC2, NID2, NID2, NPTN, NRAS, OPALIN, OPTN, OSTM1, OSTM1, P2RY6, P2RY12, PARK7, PARP1, PAXIP1, PAXIP1, PCBP2, PCCA, PDCD1LG2, PDHX, PEBP1, PKM, PLIN3, PODXL, PODXL, PPARG, PPIA, PPIG, PPP2R1A, PRDX5, PRPF8, PRPF19, PRR13, PTGFRN, PTGFRN, PTPN11, PTPN11, PTPRH, PTPRJ, PTPRJ, PTPRK, PTPRK, PTPRO, PTPRZ1, PTPRZ1, PYHIN1, PYHIN1, RAB7A, RAB11B, RNF167, RPL7A, RPL12, RPL35, RPS20, RRAGB, RRAGB, RRAGC, RRAGC, RTN4RL2, RTN4RL2, RUNX1, SCARA3, SCARA3, SDK1, SDK1, SDK2, SDK2, SERINC1, SERINC2, SERPINH1, SGOL2, SH3BGRL, SLC1A3, SLC1A5, SLC1A5, SLC2A14, SLC3A2, SLC4A2, SLC4A2, SLC4A7, SLC4A7, SLC5A5, SLC5A8, SLC6A1, SLC7A2, SLC7A2, SLC9A6, SLC9A6, SLC12A2, SLC12A2, SLC12A2, SLC12A4, SLC12A4, SLC12A6, SLC12A6, SLC12A7, SLC12A7, SLC14A1, SLC15A4, SLC15A4, SLC17A5, SLC25A5, SLC26A2, SLC26A2, SLC30A1, SLC30A1, SLC38A9, SLC38A9, SLC46A3, SNCA, SOD1, SOD2, SPR, SQSTM1, SS18L1, SUFU, SYPL1, TACR1, TBK1, TEX35, TEX35, TKT, TMEM9, TMEM63A, TMEM63A, TMEM63C, TMEM179B, TMEM182, TMPO, TMSB10, TPCN2, TRIM5, TRIM5, TRIM6, TRIM16, TRIM16, TRIM17, TRIM22, TRIM23, TRIM49, TRIM49, TSPAN2, TSPAN31, TXN, VIM, VSTM1, YWHAZ | Bussi C (2018), Carvalho RS (2014), Chauhan S (2016), Chen X (2014), Cortegano I (2000), Elliott PR (2016), Fautsch MP (2006), Foerster S (2013), Giurato G (2018), Guo HB (2009), Havugimana PC (2012), Hein MY (2015), Hubel P (2019), Huttlin EL (2014/pre-pub), Huttlin EL (2015), Huttlin EL (2017), Jozwik KM (2016), Ju T (2008), Koths K (1993), Kumar P (2017), Li CG (2019), Li X (2016), Lin TW (2015), Liu X (2017), Luck K (2020), Malinova A (2017), Merlin J (2011), Mohammed H (2013), Moutaoufik MT (2019), Ochieng J (1999), Olah J (2011), Park JW (2001), Rolland T (2014), Rosenberg I (1991), Rosenbluh J (2016), Stukalov A (2020), Tiemann K (2018), Tinari N (2001), Ulmer TA (2006), Voss PG (2008), Wan C (2015), Wang X (2006), Woods NT (2012), Yeung ATY (2019), Zhong Y (2017) |
| LGALS4 | ARPC3, BANP, CEP55, CHD7, CFTR, ELAVL1, ELAVL2, ELAVL3, ELAVL4, EYA2, GOLGA6L9, HOMEZ, HOXA1, HSF2BP, KRTAP11-1, MLH1, NOTUM, NCKIPSD, PAICS, RFX6, PHGDH, RRAS2, SHKBP1, SPANXA1, TENM4, TOX2 | Wang X (2006), Luck K (2020), Brieger A (2010), Stelzl U (2005) |
| LGALS7 | ADSS, AEBP2, AHSG, APAF1, CBWD1, CDK2, CHD3, CHD4, CHST6, COPS5, CYLD, DDX19B, DOCK7, DPYSL2, DPYSL4, DPYSL5, E2F6, ESR1, EZH2, FGFR1, GAB1, HECW2, HIV2GP4, HNRNPA1, HRAS, HSPA6, IFI16, INSIG2, KLHL8, LGALS7B, LSM2, LUCAT1, LZTR1, MCM2, METTL3, MKS1, MTPAP, MYC, NAMPT, PALD1, PCGF5, PIK3C3, PKN2, POLA2, POLE2, PPARG, PRPF8, RHEB, ST3GAL1, STG3GAL2, ST3GAL4, ST6GALNAC2, ST6GALNAC3, ST6GALNAC4, ST8SIA6, SSBP1, SUZ12, TAB1, TCF3, TGM5, TUBA1B, TUBA3C, TUBA4A, TUBB3, TUBBB, TUBG1, USP1, USP4, USP15, USP38, VPS13B, VWA9, WIPI1, XPO1, YAF2 | Hauri S (2016), Adhikari H (2018), Behrends C (2010), Bennett EJ (2010), Cao Q (2014), Diner BA (2015), Drissi R (2015), Elliott PR (2016), Fogeron ML (2013), Guardia-Laguarta C (2019), Hauri S (2016), Heidelberger JB (2018), Hoffmeister H (2017), Huttlin EL (2014/pre-pub), Huttlin EL (2017), Ikeda Y (2009), Kirli K (2015), Landsberg CD (2019), Li CG (2019), Lu L (2013), Luck K (2020), Malinova A (2017), Neganova I (2011), Pladevall-Morera D (2019), Roy R (2014), Sowa ME (2009), Tarallo R (2011), Teachenor R (2012), Zhou Q (2019) |
| LGALS8 | ABCC1, ABCC4, ACP2, ADCY6, ADCY9, ALCAM, ANO6, APEH, ATG9A, ATP2B2, ATP2B3, ATP2B4, ATP6VOA1, ATP6VOA2, ATP13A3, BARD1, C10RF85, C10RF159, C4A, CALCOCO2, CD47, CD58, CD63, CD276, CLCN3, CLCN5, CLCN7, COLEC12, CSAD, CSPG4, CUL1, DAG1, ECE1, EGFR, ELTD1, EMB, ENPP4, ESR2, FGFR1, FLE4, FN1, HEG1, ITGA1, ITGA2, ITGA3, ITGA5, ITGA6, ITGA7, ITGB1, KIAA0319L, KIAA1549, LAMA4, LNPEP, LRRC4B, LRRC8A, LRRC8C, LRRC8E, LRRK2, LRSAM1, MCAM, MEFV, MFAP3, MID2, MRC2, NACC1, NCR3LG1, NDP, NPC1, NPTN, NR1D2, OPTN, OSTM1, PAN2, PDPN, PHACTR1, PODXL, PROCR, PTGFRN, PTPRA, PTPRG, PTPRJ, PTPRK, PVRL1, PVRL3, RNF13, RRAGB, RRAGC, SCARB2, SDCBP, SDK1, SEZ6L2, SLC1A1, SLC4A2, SLC4A7, SLC9A6, SLC12A2, SLC12A4, SLC12A6, SLC12A7, SLC12A9, SLC17A5, SLC22A23, SLC26A2, SLC30A1, SLC31A1, SLC35A5, SLC36A1, SLC38A9, SORL1, SPPL2A, SUSD5, SV2A, TAX1BP1, TMEM63A, TMEM63B, TMEM237, TMEM242, TRIM5, TRIM6, TRIM17, TRIM22, TRIM23, TRIM49, TRPM4, WBP2 | Beilina A (2014), Bennett EJ (2010), Bett JS (2013), Chauhan S (2016), Chen S (2018), Foerster S (2013), Giurato G (2018), Hadari YR (2000), Huttlin EL (2014/pre-pub), Huttlin EL (2015), Kim BW (2013), Li S (2013), Luck K (2020), Rolland T (2014), Rual JF (2005), Thurston TL (2012), Tomkins JE (2018), Verlhac P (2015), Woods NT (2012) |
| LGALS9 | ACP2, ALCAM, ATG9A, ATG16L1, ATP2B4, ATP7A, ATP11C, ATP13A3, C2CD5, CD44, CD47, CD58, CD63, CD86, CD109, CD274, CD276, CLCN3, COLEC12, CSPG4, CTLA4, CTNNB1, DAG1, DAZAP2, ECE1, ENPP4, ENPP4, FBLN1, FN1, FOXP3, HAVCR2, IGF2R, IGSF3, ITGA4, ITGB1, JUP, KIAA0319L, KIAA15459, KRTAP6-3, LAG3, LAGALS3, LGALS9B, LGALS9C, LAMA1, LAMA4, LAMB1, LGALS3, LGALS9B, LGALS9C, LNPEP, LRP1, LUM, MAN2B1, MB21D1, MET, MFAP3, MPZL1, MRC2, NAGLU, NCR3LG1, NICD1, NICD2, NR2C2, OSTM1, P4HB, PBK, PCDH9, PDIA3, PDIA6, PLXNA1, PODXL, PTGFRN, PTPN11, PTPRJ, PTPRK, RNF13, RRAGB, RRAGC, SLC1A5, SLC4A7, SLCSLC6A6, SLC9A3R2, SLC9A6, SLC12A2, SLC12A4, SLC12A6, SLC12A7, SLC12A9, SLC29A1, SLC38A9, SORL1, SUSD5, TSPAN3, USP39, ZER1 | Arbogast F (2019), Bi S (2011), Ewing RM (2007), Huttlin EL (2014/pre-pub), Huttlin EL (2015), Luck K (2020), Lum KK (2018), Wan C (2015), Yu L (2018) |
| LGALS10 | ADH1A, CHD7, CLC, EPX, GALE, ISYNA1, LGALS3, LGALS12, PAICS, RNASE2, RNASE3 | Rolland T (2014), Luck K (2020) |
| LGALS12 | CLC, EMB, HAPLN1, LGALS13, LIMD1, RYR3, SLC7A2, VPS12C, VPS13C | Huttlin EL (2015), Huttlin EL (2017) |
| LGALS13 | ADAM12, ANXA2, BTBD1, CENPV, CREB5, DNPEP, ENG, ENDOU, HOXA1, LGALS3, LGALS12, NUTF2, NUFIP2, OTX1, PACSIN3, PAPPA, PGF, PHLDA1, POLR1A, POU4F2, PPEF1, PWP1, UBB | Huttlin EL (2017), Lambert B (2012), Luck K (2020), Rolland T (2014), Yu H (2011) |
| LGALS14 | ADAMTSL4, AJUBA, ALS2CR12, BANP, BLZF1, C1QTNF2, COG6, CRLF3, DDIT4L, DOK6, ENTHD2, FCHO1, FLNA, GFNA, GFAP, IKZF3, IL16, ITLN2, JRK, KRT19, KRT35, LNX1, LNX2, MEI4, MID2, NFKBID, PCBID, PCBD2, PICK1, POF1B, PPM1J, REL, RIMBP3C, SDCBP2, SH3GLB2, SPAG5, STAT5B, TARSL2, TBC1D25, TCF4, TEKT1, TNR, TRIM23, TRIM27, VIM, ZBTB8A, ZNF71, ZNF248, ZNF438, ZNF558, ZNF655 | Luck K (2020), Rolland T (2014), Yachie N (2016) |
The relative interaction proteins of Gals were collected based on the BioGRID website (https://thebiogrid.org/, accessed on 15 September 2020).
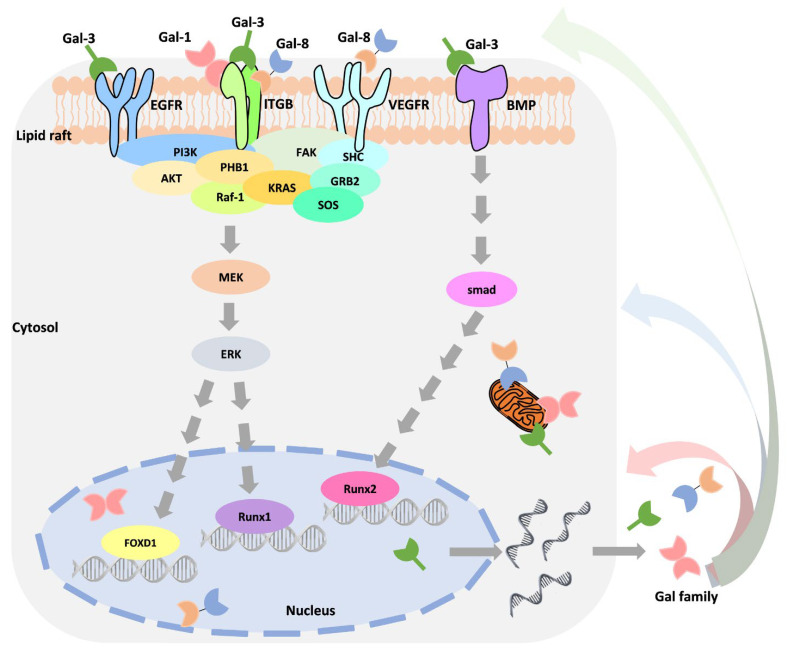
Moreover, many studies have confirmed that Gals interact with different partners or have different functions in different locations. Thus, distinguishing among Gal locations (extracellular and intracellular) may unveil these roles. Table 5 is organized based on the current observations. However, it is worth noting that Gal’s expression in subcellular localization is regulated in response to different types of stimuli. Using the widely discussed Gal-3 as an example, its expression is stimulated by growth factors, cytokines, environmental changes, death signals, and in response to drug treatment [101,102,103,104]. Through stimulus-induced activation of intracellular signaling, such as Ras/MAPK/ERK, Smad signaling increases the binding of transcription factors to the Gal-3 promoter [101,105]. Park et al. demonstrated that Toll-like receptor 4 activates Gal-1 expression by stimulating lipopolysaccharide in colon cancer cells [79]. Interestingly, as an autocrine molecule, Gal can regulate its own expression by associating with extracellular receptors, such as integrin, EGFR, VEGFR2, and BMPR (Figure 3) [101,105]. For example, Gal-1 regulates triple-negative breast cancer progression and drug resistance by interacting with integrin-β1 to activate the integrin-β1/FAK/c-Src/ERK/STAT3/survivin pathway [72]. Recently, Oysnsdel et al. showed that Gal-8 interacts with integrin α5β1 to induce epithelial transformation into a mesenchymal-like phenotype [106]. The interaction of Gal-3 and EGFR may partially mediate MUC1 to promote cancer progression [107]. Importantly, this mechanism may increase the crosstalk between different signaling pathways and enable cells to respond to different stimuli. Seguin demonstrated that the interaction of Gal-3 and integrin-β3 bypasses the inhibition of EGFR inhibitors, promoting drug resistance and stemness [108]. Conversely, Gal-3 is a negative regulator of melanoma that acts by regulating integrin-β3 expression [109]. EGFR inhibitor treatment causes increased integrin αvβ3 expression, resulting in drug resistance by activating Gal-3/KRAS/RalB/TBK1/NF-κB signaling in non-small cell lung cancer [91]. However, how Gal is transactivated by unique mechanisms in response to drug treatment is still unknown.
Table 5.
Subcellular distribution of galectin.
| LGALS Type | Nucleus | Endoplasmic Reticulum | Cytosol | Plasma Membrane | Extracellular | Cytoskeleton | Mitochondrion | Peroxisome | Endosome | Lysosome | Golgi Apparatus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LGALS1 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS2 | + | + | + | + | + | + | + | ||||
| LGALS3 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS4 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS7 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS8 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS9 | + | + | + | + | + | + | + | + | + | + | |
| LGALS10 | + | + | + | + | + | + | + | + | + | + | + |
| LGALS12 | + | + | + | + | + | + | + | + | + | ||
| LGALS13 | + | + | + | + | + | + | + | + | + | ||
| LGALS14 | + | + | + | + | + | + | + |
+ Means positive expressed. Listed are the distributions of Gals in cell subcellular components, which were collected from the GeneCards website (https://www.genecards.org/, accessed on 15 September 2020).
Figure 3.
Crosstalk of various signaling molecules that regulate galectin expression. The schematic diagram illustrates the complex regulation involved in Gal expression and interaction components.
5. The Roles of Galectins in Cancer Metabolism Reprogramming
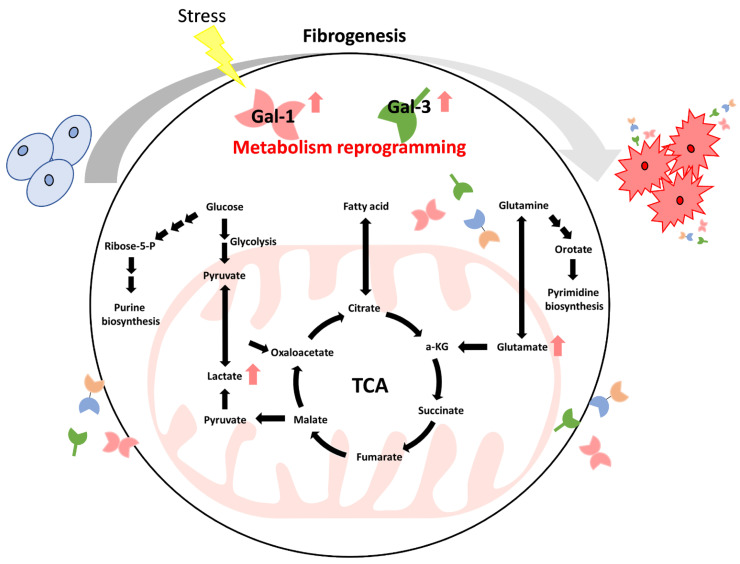
Common metabolic abnormalities involve sugars, lipids, proteins, and nucleotides. Abnormal metabolism leads to long-term inflammation in cells and promotion of tumorigenesis [110]. Cancer cells can also improve cell survival by changing their metabolism and adapting the way nutrients are taken up [111]. Interestingly, cancer cells express increased glucose transporter (GLUT) expression levels to obtain increased nutrients [112]. Understanding tumorigenesis caused by metabolic abnormalities and how cancer cells grow in response to metabolic abnormalities has become the subject of multiple studies [110,111,112]. Moreover, metabolic disorders are usually accompanied by organ fibrosis, such as diabetes mellitus-mediated aortic stenosis or lung fibrosis [15,37]. Evidence shows that patients with pulmonary fibrosis may be more prone to lung cancer development [113,114,115,116]. Tang et al. revealed that Gal-1 was overexpressed in pancreatic stellate cells that participated in chronic pancreatitis and pancreatic cancer progression [96]. However, cancer patients also exhibit fibrosis and increased Gal-1 production during progression or chemotherapy [96,117]. Radiation-induced pulmonary fibrosis was observed to increase Gal-1 expression in a murine model [118]. Thus, these findings indicate that both the abnormal expression of Gal and cancer therapy result in fibrosis (Figure 4). Additionally, metabolic reprogramming is considered one of the hallmarks of fibrosis [119]. Gals have abnormal expression patterns in metabolic abnormalities, and changes in Gal have been identified in the progression of many cancers [120].
Figure 4.
Metabolic reprogramming-associated fibrogenesis induction of galectin expression. The schematic diagram illustrates that metabolic reprogramming accompanies organ fibrogenesis and dysregulated Gal expression during stress stimulation.
5.1. Carbohydrate Metabolism
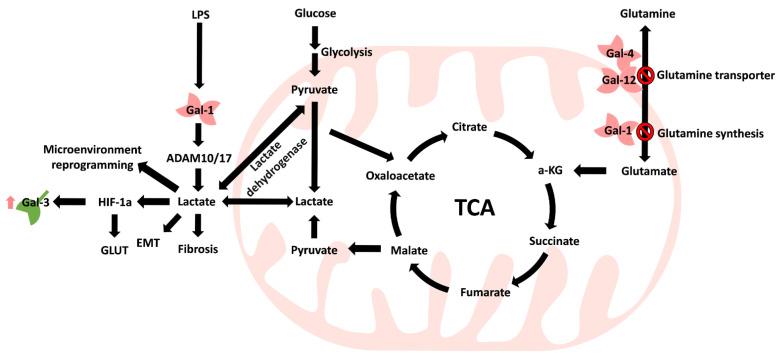
Zheng et al. reported that Gal-1 expression was correlated with tumor volume and glycolysis-related markers (GLUT-1 and hexokinase II), which may serve as an independent prognostic marker in lung adenocarcinoma [89]. Park et al. showed that Toll-like receptor 4 (TLR4) increases Gal-1-mediated ADAM metallopeptidase domain 10 (ADAM10) and ADAM17, which promotes lactate (2-hydroxypropanoic acid) production [79]. Lactate is the primary metabolite of glucose through anaerobic glycolysis in normal cells [121]. Lactate was once considered a waste product in the metabolic process [121]. In some tissues, such as the liver, brain, heart, and skeletal muscle, lactate can serve as a source of energy or as a carbon source for gluconeogenesis through the Cori cycle in the liver [122]. In addition, cancer cells can generate energy through the Warburg effect to accelerate ATP production [123]. However, anaerobic glycolysis produces approximately 85% lactic acid that is transported to the extracellular environment and acidifies the microenvironment [124]. Furthermore, Apicella et al. showed that tumor-associated stromal cells, such as cancer-associated fibroblasts, could take up lactate to stimulate production and to increase tumor cell resistance to therapy [125], indicating that Gal promotes lactate production and may create a more acidic microenvironment for tumor cells. In addition, cancer cell-mediated lactate production triggers hypoxia-inducible factor 1α (HIF-1α) expression under hypoxic conditions [126] and the expression of glucose transporters but also the regulated expression of Gal-1 [89], which may form a regulatory loop between tumor cells and tumor-associated cells. Gal-1 is highly expressed in lymphoma, and its concentration was correlated with lactate dehydrogenase (LDH) expression [127]. Moreover, more aggressive metastatic cells exhibit high Gal-1 expression and LDH B expression in melanoma [128].
5.2. Amino Acid Metabolism
In contrast, glutamine, which is an energy source, can be incorporated into the tricarboxylic acid (TCA) cycle, and while glutamine is a primary energy resource, tumor cells can provide more lactate [129]. However, Li et al. confirmed that glutamine synthesis and uptake can be negatively regulated by Gal expression, and showed that upregulation of Gal-1 was significantly associated with reduced glutamine synthetase expression in urinary bladder urothelial carcinoma [66]. Similar results can be observed when recombinant Gal-4 treatment inhibits tumor growth by decreasing the phosphorylation of SLC1A5 (a glutamine transporter) in colon cancer cells [83], and Kazenmaier et al. identified that Gal-12 can bind to SLC1A5 to reduce glutamine uptake in colon cancer cells [81].
5.3. Lipid Metabolism
Fatty acids are an essential energy source for cancer metabolism as well. In healthy cells, obesity caused by an abnormal diet can reduce weight gain by targeting Gal-1 [130]. In tumor cells, Mukherjee et al. observed that Gal-12 regulates lipid draft formation to inhibit human promyelocytic leukemia lipogenesis [69].
Energy generation and metabolism primarily come from the mitochondria. Consequently, these findings show that overall mitochondrial metabolism is also altered during the process of carcinogenesis (Figure 5) in which the TCA cycle is central for the conversion of different metabolites to produce lactate for reprogramming the tumor microenvironment.
Figure 5.
Galectin family involvement in mitochondrial metabolism reprogramming. Gal expression accompanies both metabolism and microenvironment reprogramming, which can be recognized in several diseases, including tumor progression. ↑ Means increase.
5.4. Disorder of Mitochondria
Mitochondria also regulate cell survival through the intrinsic pathway, and abnormal mitochondrial function was observed in the absence of Gal-3 [131]. Yu et al. showed that Gal-3 regulates cytochrome c release to prevent mitochondrial damage in human breast epithelial cells [64]. Wang et al. found that cisplatin-induced mitochondrial dysfunction is inhibited by Gal-3 in ovarian carcinoma [93]. Similarly, targeting of Gal-3 decreased bcl-2 protein levels in ovarian carcinoma [94]. Tadokoro et al. reported that treating cells with Gal-9 induced mitochondrial release of apoptosis-related molecules, such as cytochrome c, Smac/Diablo, and HtrA2, inhibiting liver cancer proliferation [88]. In agreement with other reports, Chiyo and coworkers demonstrated that Gal-9 induces apoptosis in esophageal squamous cell carcinoma through the mitochondria [85]. Sakhnevych et al. found that Gal-9 and its receptor Tim-3 form a complex and accumulate in the mitochondria in response to a Bcl-XL antagonist in colorectal cancer cells [84]. Extracellular Gal-7 can reenter cells and translocate into the nucleus to interact with bcl-2 as an anti-apoptosis function to promote breast cancer chemoresistance [132]. Altogether, these findings indicate that Gal participates in metabolic reprogramming and mitochondrial dysfunction during cancer progression. Dysregulated metabolism-mediated Gal expression contributes further to microenvironment alterations.
6. Galectin in the Microenvironment
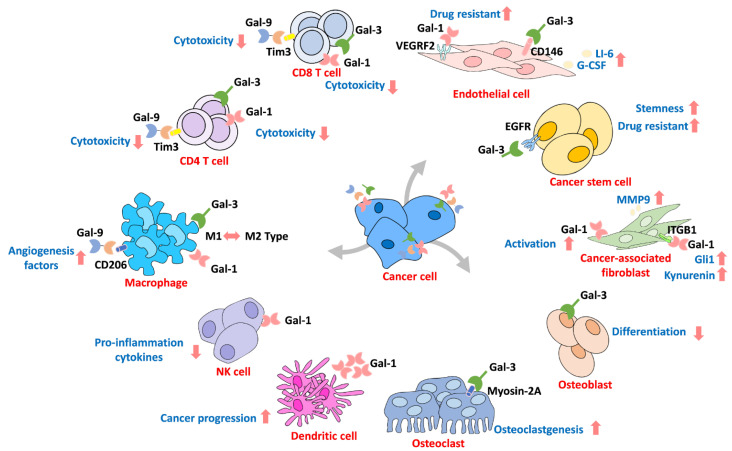
The interactions in the tumor microenvironment are considered another critical mechanism for cancer progression that assimilate peripheral cells through secretion of various molecules [133,134]. Furthermore, there are various cells surrounding the microenvironment, such as stromal cells and immune cells, which are reportedly regulated by Gals [82,135,136]. Additionally, immune evasion is one of the most important mechanisms for cancer survival [137]. The interactions of extracellular Gal not only activate downstream cell signaling pathways but also contribute to environmental reprogramming (Figure 6). For example, increased Gal-3 levels are often detected in cancer patients’ blood, indicating that circulating Gal contributes to microenvironment reprogramming during cancer progression [75,138]. Circulating Gal-3 interacts with endothelial cells, which induce cytokine production that promotes cancer progression [139,140]. Colomb et al. showed that Gal-3 interacts with CD146 on endothelial cells through affinity purification assays, which leads to AKT signaling activation and IL-6 and G-CSF secretion to promote cancer progression [141]. Croci and coworkers demonstrated that tumors develop resistance to anti-VEGF therapy by secreting Gal-1 to interact with VEGFR2 in endothelial cells [142]. Tumor-mediated Gal-1 and Gal-3 have also been identified as inhibiting T cell cytotoxicity by interacting with the T cell receptor or lymphocyte activation gene 3 (LAG-3) and inducing T cell apoptosis [143,144]. In addition, Gal-3 functions as a switch for macrophage polarization and regulates CD8+ T cell infiltration into lung adenocarcinoma cells [92]. Treatment with Gal-3 antagonists promotes T cell infiltration by recognizing cancer-mediated interferon-gamma in vivo [145]. It has been reported that the expression of Gal-1 is a marker of lymphocyte infiltrates in cutaneous head and neck cancers [146] and triple-negative breast cancer patients [73]. Evidence also shows that prostate cancer-mediated Gal-1 downregulates lymphocyte proliferation and apoptosis [98]. Tesone et al. showed that tumor-associated macrophages express Gal-9 to promote cancer progression [68]. Overexpression of Gal-1 activates hepatocellular carcinoma and promotes cancer cell immune surveillance escape by inducing T cell apoptosis [147]. Andersen et al. revealed that circulating Gal-1 in serum might promote M2 macrophage activation in multiple myeloma patients [70]. Suppression of Gal-1 in glioma cells sensitized them to natural killer cells (NK cells), which was caused by cancer cells producing more pro-inflammatory cytokines for recruitment of monocytic myeloid-derived suppressor cells to differentiate into dendritic cells, leading to further recruitment of NK cells [148,149]. Moreover, treating melanoma patients with a BRAP/MEK inhibitor increased Gal-1 expression by an unknown mechanism that may lead to immune surveillance escape and cause drug resistance [99]. Similarly, Gal-9 has been found to be associated with immune tolerance during pregnancy [150]. In contrast, tumor-associated macrophages expressing Gal-9 are associated with invasive bladder tumor stage and decreased immune surveillance [68]. In addition, evidence suggests that Gal-9 binds to CD206 on M2 macrophages to induce the secretion of angiogenesis factors to promote tumor growth [47]. Additionally, evidence has revealed that Gal-9 interacts with its receptor Tim3 on T cells, prompting immunosuppression of the tumor microenvironment [151,152]. However, Luo et al. demonstrated that treating T cells with Gal-7 suppresses TGF-β signaling to activate polarization toward CD4 T cells [153]. In contrast, Higareda-Almaraz and coworkers showed that Gal-7 is a negative regulator of cervical cancer that acts through reprogramming the tumor microenvironment [78]. Interestingly, fibroblasts were shown to be activated by cancer cells known as cancer-associated fibroblasts, and the expression of Gals was associated with fibroblast activation and promoted growth [135,136]. Toti et al. found that knockdown of Gal-1 in tumor stromal cells, such as human pancreatic stellate cells, decreased pancreatic ductal adenocarcinoma growth in vivo [97]. In addition, cancer-associated fibroblasts mediate Gal-1 regulation of cancer cell motility through macrovesicle release, and knockdown of Gal-1 prevents cancer-associated fibroblast-mediated prostate and pancreatic cancer migration [136]. This finding is in agreement with previously described cancer-associated fibroblasts expressing Gal-1 to promote melanoma cell migration [154]. The possible mechanism might be due to Gal-1’s interaction with integrin-β1 on fibroblasts to activate Gli1 expression, resulting in increased metastasis of gastric cancer [86]. In addition, Gal-1 secreted by cancer cells can induce cancer-associated fibroblasts to activate TDO2-ATK signaling and produce the tryptophan metabolite kynurenine [90], which reportedly can induce T cell apoptosis [155,156]. Furthermore, bone remodeling is an essential mechanism for cancer cell interactions with bone marrow stromal cells in the microenvironment. Gals have been reported to participate in cancer-mediated bone remodeling [71,76,157]. Muller and coworkers found that Gal-1 expression was important for multiple myeloma development and bone mass [71]. Evidence shows that Gal-3 cleavage is observed in metastatic bone cancers and plays a different role than Gal-3 during osteoclastogenesis, and cancer-mediated Gal-3 regulates osteoclastogenesis by binding to myosin-2A in breast and prostate cancer [76]. A murine model revealed that depletion of Gal-3 increases bone metastasis in breast cancer through the CXCR4/CXCL12 axis [157].
Figure 6.
The galectin family contributes to tumor microenvironment reprogramming. Gals can act as a reprogramming messenger to connect tumor cells and other cells in the microenvironment. ↑, ↓ Means increase or decrease.
7. Available Inhibitors for Targeting Galectins
Based on the reported Gal functions in disease progression above, investigators have attempted to design specific inhibitors to block Gals’ function as therapeutic reagents for related diseases. There are various methods that have been used to block Gal functions, such as neutralizing antibodies, synthetic compounds, carbohydrate derivatives, and binding peptides. Several inhibitors used in clinical trials for testing in multiple human diseases in different phases are listed in Table 6.
Table 6.
Galectin-related ongoing clinical trials.
| Drug | Target | Phase | Status | Disease | NCT Number |
|---|---|---|---|---|---|
| GCS-100 | Gal-3 | I | Completed | Chronic Kidney Disease | NCT01717248 |
| II | Completed | Chronic Kidney Disease | NCT01843790 | ||
| II | Withdrawn | Diffuse Large B-cell Lymphoma | NCT00776802 | ||
| GM-CT-01 | Gal-3 | II | Terminated | Metastatic Melanoma | NCT01723813 |
| II | Withdrawn | Colorectal Cancer | NCT00388700 | ||
| II | Withdrawn | Cancer of the Bile Duct, Gallbladder Cancer | NCT00386516 | ||
| II | Terminated | Colorectal Cancer | NCT00110721 | ||
| GR-MD-02 | Gal-3 | I | Completed | Metastatic Melanoma | NCT02117362 |
| I | Recruiting | Melanoma, Non-Small Cell Lung Cancer, Squamous Cell Carcinoma of the Head and Neck | NCT02575404 | ||
| I | Completed | Non-Alcoholic Steatohepatitis (NASH) | NCT01899859 | ||
| II | Completed | Hypertension, Portal | NCT02462967 | ||
| II | Recruiting | Prevention of Esophageal Varices, NASH - Nonalcoholic Steatohepatitis, Cirrhosis | NCT04365868 | ||
| II | Completed | Nonalcoholic Steatohepatitis | NCT02421094 | ||
| II | Completed | Psoriasis | NCT02407041 | ||
| OTX008 | Gal-3 | I | Unknown | Solid Tumors | NCT01724320 |
| PectaSol-C Modified Citrus Pectin (MCP) | Gal-3 | II | Completed | Prostatic Neoplasms | NCT01681823 |
| TD139 | Gal-3 | II | Recruiting | Idiopathic Pulmonary Fibrosis (IPF) | NCT03832946 |
| II | Completed | Idiopathic Pulmonary Fibrosis | NCT02257177 | ||
| II | Recruiting | COVID-19 | NCT04473053 |
All data were collected and manipulated from the NIH ClinicalTrials website (https://clinicaltrials.gov/, accessed on 15 September 2020).
7.1. Neutralizing Antibodies
Neutralizing antibodies are commonly used to block protein functions. Stasenko et al. showed that invasion and proliferation were suppressed in high-grade serous ovarian cancer, and showed improved overall survival, in response to treatment with an anti-Gal-3 antibody [158]. Evidence shows that neutralizing antibodies may partially suppress the inhibition of lactose treatment [159]. In addition, an in vivo model showed that ischemia-induced angiogenesis was decreased by treatment with neutralizing anti-Gal-3 [160]. Saez et al. demonstrated that abnormal angiogenesis can be blocked by a specific Gal-1 neutralizing antibody [52]. Using a neutralizing antibody to block the expression of Gal-9 in colon adenocarcinoma resulted in increased T cell cytotoxicity and immunosuppression [161]. Metastatic melanoma adhesion to endothelial cells can be blocked by response to Gal-1 antibody [162]. Moreover, β4-integrin/PI3K activation to epidermoid carcinoma cells can be blocked by treating cells with a Gal-3 antibody [104]. In vivo, targeting Gal-3 with antibodies inhibited B16 melanoma and UV-2237 fibrosarcoma metastasis to the lung [163]. Similarly, Nakamura et al. found that an anti-Gal-3 antibody inhibited adhesion and liver metastasis of adenocarcinoma [164]. Interestingly, drug delivery can be conducted using Gal as a target. Ma and coworkers found that anti-Gal-3-based nanoparticles could control drug delivery and increase the concentration of doxorubicin in thyroid cancer in vivo [165]. In contrast, blocking Gal-4 using a neutralizing antibody on the cell surface induced cell proliferation [42]. Blocking Gal-3 also boosted cytokine INF-gamma secretion of CD8(+) tumor-infiltrating lymphocytes [143,166]. Dovizio et al. found that anti-Gal-3 antibodies prevented cox-2 expression during platelet adhesion in colon carcinoma [80]. Gal-3 neutralizing antibody also inhibited Gal-3-mediated ERK signaling as well as neuroblastoma-mediated IL-6 expression in bone marrow stromal cells [167]. Targeting Gal-3 with an antibody blocked tumor adhesion to endothelial cells, inhibiting cancer metastasis [168]. Furthermore, targeting Gal interaction partners can also block Gal functions. For example, blocking CD166, a Gal-8 binding partner, suppresses Gal-8-mediated migration and tube formation of endothelial cells [169].
7.2. Carbohydrate Derivatives
Gal interacts with its partners by recognizing N-glycans, such as N-acetyl-D-lactosamine, by the carbohydrate recognition domain; therefore, there are various ways to block this interaction. Pretreating cells with lactose blocks Gal-3-mediated cell adhesion [100]. Lactose blocks the association between Tim-3 and Gal-9, inducing immune molecule expression of T cell immunoreceptors with Ig and ITIM domains [170]. Pan et al. showed that Gal-3-mediated neutrophil infiltration could be blocked by lactose treatment to reverse acute pancreatitis [171]. Moreover, Gal antagonists, such as LacNAc (N-acetyl-D-lactosamine) and TetraLacNAc (tetra-N-acetyl-D-lactosamine), reduce tumor growth by inducing IFN-gamma and chemokine production to induce CD8(+) T cell infiltration [145]. Lactose treatment blocks exosomes derived from HIV-infected dendritic cells [172]. In addition, evidence shows that synthetic inhibitors, such as synthetic lactulose amines, have been reported to suppress tumor progression by binding to Gal-1 and Gal-3 [159].
7.3. Galectin-Binding Peptides
The truncated Gal-3 carbohydrate recognition domain (Gal-3C), a Gal-3-binding peptide designed based on Gal-3′s structure, was found to inhibit cancer progression, which was generated by matrilysin-1 [173,174]. John et al. showed that Gal-3C suppresses breast cancer growth and metastasis in vivo [77]. This outcome is in agreement with previously described Gal-3C inhibiting multiple myeloma development and synergistically boosting the effect of bortezomib [175]. Evidence also shows that recombinant Gal-3C regulates the integrin/FAK/SRC/NDRG1 axis to suppress hepatocellular carcinoma progression [176]. Identifying peptides that can interfere with the carbohydrate recognition domain of specific Gals, such as Thomsen–Friedenreich antigen-specific peptide P-30, may disrupt Gal functions [177,178]. Lian and coworkers identified the Gal-binding peptide G3-C12-HPMA-KLA, which has dual effects on cancer cells and subcellular mitochondria [179]. In addition, the Gal-1-binding peptide “anginex” was designed to induce apoptosis of endothelial cells and to decrease angiogenesis [180]. Anginex-conjugated arsenic–cisplatin combined liposomes enhanced therapeutic efficiency [74]; evidence suggests that anginex may also have dual activity by suppressing H-Ras translocation [181].
7.4. Synthetic Compounds
Non-peptide compounds, such as OTX008, have been designed to target Gal-1 based on the effect of anginex [87], which has been found to serve as a therapeutic reagent for cancer and diabetic patients [40,87,182,183,184]. Treatment with BH3I-1 blocks the interaction between Tim-3 and Gal-9, which increases immune surveillance in colorectal cancer [84]. In addition, plant-derived galactomannans (known as DAVANAT) have been found to bind both Gal-1 and Gal-3, leading to CD8(+) tumor-infiltrating lymphocyte cytotoxicity [166,185,186]. The same effect can be observed from another Gal-3 inhibitor, “GR-MD-02” (also known as belapectin), which has been identified to reduce liver fibrosis [187,188]. Saccharide derivatives have been found to inhibit Gal functions and include related inhibitors, such as GCS-100, a modified form of citrus pectin (MCP), RN1, and disaccharide thiodigalactoside (TDG) [189]. Chauhan et al. showed that GCS-100 activates NF-KB signaling to induce apoptosis in lymphoma [189]. In agreement with other reports, the authors also showed that GCS-100, an antagonist of Gal-3, induced cancer cell death [190,191,192], boosted tumor-infiltrating T lymphocyte secretion of IFN-gamma [143], and increased prostate cancer sensitivity to cisplatin [193]. Similarly, an effect was observed in response to modified citrus pectin (MCP) on cancer [67,95,194,195]; however, MCP can also be used for other Gal-related disease treatments, such as decreasing Gal-3 levels in type 2 diabetes [196], decreasing doxorubicin-induced cardiovascular diseases [197], ameliorating cardiac dysfunction [198], and improving ischemic heart failure [199]. In addition, the calixarene derivative compound “OTX-008” was designed as a Gal-1 inhibitor [200]. Evidence shows that OTX-008 suppresses cancer progression by targeting Gal-1 [87,182,184,200]. Moreover, OTX-008 treatment blocks Gal-1-mediated retinal neovascularization, renal fibrosis in diabetes, and proliferative diabetic retinopathy [40,183,201]. The Gal-3 inhibitor “GB1107” also increased PD-L1-mediated immune surveillance in lung adenocarcinoma [92].
7.5. Other Derivatives
Other polysaccharide derivatives include “RN1” and “HH1-1.” Zhang and coworkers demonstrated that RN1 binds to Gal-3 and blocks Gal-3-mediated downstream signaling, which suppresses transcription factors, such as RUNX binding to the Gal-3 promoter in pancreatic ductal adenocarcinoma [105]. Yao et al. showed that HH1-1 blocks the interaction between Gal-3 and EGFR, which decreases EGFR/AKT/FOXO3 signaling to halt the progression of pancreatic cancer [202]. The same effect can be observed with another Gal-1 inhibitor, thiodigalactoside (TDG) [203]. Lai et al. found that sorafenib-resistant breast cancer-induced Gal-1 was suppressed by TDG treatment [204]. In agreement with other reports [205,206], the authors also showed that TDG increased both CD4 (+) and CD8 (+) T cells by blocking Gal-1 in murine breast, colon, and lung cancer models. TDG halted Gal-1-induced cisplatin in hepatocellular carcinoma [207]. Moreover, targeting Gal-1 through TDG treatment suppressed diet-induced obesity [208]. Similarly, this effect can be observed in “TD139,” a specific Gal-3 inhibitor derived from thiodigalactoside, which blocks TGF-β-induced lung fibrosis [209]. In addition, several Gal-1-related inhibitors, such as lactobionic acid (LBA), inhibit diet-mediated obesity [210], and act as a third-generation photosensitizer (PS) that increases the cytotoxicity of irradiation in bladder cancer [211]. Moreover, LLS2 treatment increases paclitaxel-induced cytotoxicity in ovarian cancer [212].
8. Conclusions
Homeostasis of the metabolic process is vital for cell growth and stability. Abnormal metabolism promotes a long-term inflammatory response, which also contributes to fibrosis and accompanying tumorigenesis. The Gal family is also involved in these processes. Gal exists in different tissues and cells. According to current research on Gal, the functions of Gal are very complicated, which may be due to its posttranscriptional modifications, subcellular localization, cell type, and interacting partners. In particular, several studies have indicated that the functions of extracellular Gal are different from those of intracellular Gal [132,213]. This may be due to the extracellular form of Gal being modified by other mediators or interacting with different molecules that promote distinct functions. Gal has also been found to interact with different molecules through non-carbohydrate binding to regulate different cellular functions [8,9]. Therefore, it is imperative to analyze and to identify molecules and functions inherent to Gal. However, the current understanding shows that Gal interactions and distribution are not thoroughly understood. It is essential to distinguish regulators of Gal expression in response to environmental pressures. For example, when cells receive chemotherapy drugs long term, Gal may be induced by drug resistance, resulting in more drug resistance, and hindering clinical treatment. Therefore, extensive studies are needed to fully understand Gal. According to Table 5, different Gals can exist in the same subcellular compartments; however, whether they function synergistically or regulate one another is still uncertain. In particular, extracellular Gal has also been found to be a mediator that affects cell–cell interactions. Based on current evidence, we know that when abnormal cell metabolism or tumorigenesis occurs, certain Gals are upregulated to promote organ fibrosis. However, dysregulation of metabolic disease-induced fibrosis promotes cancer progression by inducing Gal in the tumor microenvironment, but precisely how this occurs is still unclear. Therefore, systematically investigating how these interactions are regulated by Gal in the microenvironment and the complex molecular mechanisms involved may further enhance the effects and reduce the toxicity caused by non-specificity in the design of specific inhibitory drug treatments. Whether inhibition will achieve therapeutic effects needs further research and discussion. Furthermore, designing inhibitors of partners that associate with Gal and combinations with current therapeutic drugs may achieve a synergistic response in Gal-related disease.
Acknowledgments
This research was supported by Academia Sinica (AS-SUMMIT-108), (AS-SUMMIT-109) and (ASKPQ-109-BioMed); Ministry of Science and Technology, Taiwan (MOST-107-2320-B-001-016-MY3), (MOST-108-3114-Y-001-002).
Abbreviations
| ADAM | ADAM metallopeptidase domain |
| CRD | Carbohydrate recognition domain |
| Gal | Galectin |
| Gal-3C | Gal-3 carbohydrate recognition domain |
| GLUT | Glucose transporter |
| HGF | Hepatocyte growth factor |
| HIF-1α | Hypoxia-inducible factor 1α |
| Lactate | 2-hydroxypropanoic acid |
| LacNAc | N-acetyllactosamine |
| LBA | Lactobionic acid |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MCP | Modified citrus pectin |
| MMP9 | Matrix metalloproteinase 9 |
| NK cell | Natural killer cell |
| NTD | N-terminal domain |
| PD-L1 | Programmed death-ligand 1 |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| TCA | Tricarboxylic acid |
| TCGA | The Cancer Genome Atlas |
| TDG | Thiodigalactoside |
| TetraLacNAc | Tetra-N-acetyl-D-lactosamine |
| TGF-β | Transforming growth factor-beta |
| TLR4 | Toll-like receptor 4 |
Appendix A
https://www.proteinatlas.org/20200915 (accessed on 30 July 2021).
https://xenabrowser.net/20200915 (accessed on 30 July 2021).
https://thebiogrid.org/20200915 (accessed on 30 July 2021).
https://www.genecards.org/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02117362?term=galectin&phase=0&draw=2&rank=1/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02575404?term=galectin&phase=0&draw=2&rank=3/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01724320?term=galectin&phase=0&draw=2&rank=4/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01899859?term=galectin&phase=0&draw=2&rank=6/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01717248?term=galectin&phase=0&draw=2&rank=8/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT03832946?term=galectin&phase=1&draw=2&rank=1/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01723813?term=galectin&phase=1&draw=2&rank=2/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02257177?term=galectin&phase=1&draw=2&rank=3/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02462967?term=galectin&phase=1&draw=2&rank=4/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT04365868?term=galectin&phase=1&draw=2&rank=5/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01843790?term=galectin&phase=1&draw=2&rank=6/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02556450?term=galectin&phase=1&draw=2&rank=7/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02421094?term=galectin&phase=1&draw=2&rank=8/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT02407041?term=galectin&phase=1&draw=2&rank=9/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT00388700?term=galectin&phase=1&draw=2&rank=10/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT00386516?term=galectin&phase=1&draw=2&rank=11/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT00776802?term=galectin&phase=1&draw=2&rank=13/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT01681823?term=galectin&phase=1&draw=2&rank=15/20200915 (accessed on 30 July 2021).
https://clinicaltrials.gov/ct2/show/NCT04473053?term=galectin&phase=1&draw=2&rank=18/20200915 (accessed on 30 July 2021).
Author Contributions
Conceptualization, C.-H.L., Y.-C.C.; methodology, C.-H.L., Y.-C.C.; software, C.-H.L., Y.-C.C.; resources, C.-H.L., M.-H.C.; data curation, C.-H.L., M.-H.C. and Y.-F.Y.; writing—original draft preparation, C.-H.L., Y.-C.C.; writing—review and editing, all authors; visualization, C.-H.L., M.-H.C. and Y.-F.Y.; supervision, S.-M.L. and M.H.; project administration, M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Related available websites for datasets generated in this article were noted.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Den H., Malinzak D.A. Isolation and properties of beta-D-galactoside-specific lectin from chick embryo thigh muscle. J. Biol. Chem. 1977;252:5444–5448. doi: 10.1016/S0021-9258(19)63370-3. [DOI] [PubMed] [Google Scholar]
- 2.Thijssen V.L., Heusschen R., Caers J., Griffioen A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta-Rev. Cancer. 2015;1855:235–247. doi: 10.1016/j.bbcan.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Vasta G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012;946:21–36. doi: 10.1007/978-1-4614-0106-3_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seyrek K., Richter M., Lavrik I.N. Decoding the sweet regulation of apoptosis: The role of glycosylation and galectins in apoptotic signaling pathways. Cell Death Differ. 2019;26:981–993. doi: 10.1038/s41418-019-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rustiguel J.K., Soares R.O., Meisburger S.P., Davis K.M., Malzbender K.L., Ando N., Dias-Baruffi M., Nonato M.C. Full-length model of the human galectin-4 and insights into dynamics of inter-domain communication. Sci. Rep. 2016;6:33633. doi: 10.1038/srep33633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue H., Liu L., Zhao Z., Zhang Z., Guan Y., Cheng H., Zhou Y., Tai G. The N-terminal tail coordinates with carbohydrate recognition domain to mediate galectin-3 induced apoptosis in T cells. Oncotarget. 2017;8:49824–49838. doi: 10.18632/oncotarget.17760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menon R.P., Hughes R.C. Determinants in the N-terminal domains of galectin-3 for secretion by a novel pathway circumventing the endoplasmic reticulum-Golgi complex. Eur. J. Biochem. 1999;264:569–576. doi: 10.1046/j.1432-1327.1999.00671.x. [DOI] [PubMed] [Google Scholar]
- 8.Grigorian A., Demetriou M. Manipulating cell surface glycoproteins by targeting N-glycan-galectin interactions. Methods Enzymol. 2010;480:245–266. doi: 10.1016/S0076-6879(10)80012-6. [DOI] [PubMed] [Google Scholar]
- 9.MacKinnon A.C., Farnworth S.L., Hodkinson P.S., Henderson N.C., Atkinson K.M., Leffler H., Nilsson U.J., Haslett C., Forbes S.J., Sethi T. Regulation of alternative macrophage activation by galectin-3. J. Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 10.El Haddad S., Serrano A., Normand T., Robin C., Dubois M., Brule-Morabito F., Mollet L., Charpentier S., Legrand A. Interaction of Alpha-synuclein with Cytogaligin, a protein encoded by the proapoptotic gene GALIG. Biochem. Biophys. Res. Commun. 2018;495:787–792. doi: 10.1016/j.bbrc.2017.11.078. [DOI] [PubMed] [Google Scholar]
- 11.You J.L., Wang W., Tang M.Y., Ye Y.H., Liu A.X., Zhu Y.M. A potential role of galectin-1 in promoting mouse trophoblast stem cell differentiation. Mol. Cell. Endocrinol. 2018;470:228–239. doi: 10.1016/j.mce.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Lv Y., Dai M., Wang M., Chen F., Liu R. Anti-inflammatory Property of Galectin-1 in a Murine Model of Allergic Airway Inflammation. J. Immunol. Res. 2019;2019:9705327. doi: 10.1155/2019/9705327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanda A., Hirose I., Noda K., Murata M., Ishida S. Glucocorticoid-transactivated TSC22D3 attenuates hypoxia- and diabetes-induced Muller glial galectin-1 expression via HIF-1alpha destabilization. J. Cell. Mol. Med. 2020;24:4589–4599. doi: 10.1111/jcmm.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Califice S., Castronovo V., Bracke M., van den Brule F. Dual activities of galectin-3 in human prostate cancer: Tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527–7536. doi: 10.1038/sj.onc.1207997. [DOI] [PubMed] [Google Scholar]
- 15.Talakatta G., Sarikhani M., Muhamed J., Dhanya K., Somashekar B.S., Mahesh P.A., Sundaresan N., Ravindra P.V. Diabetes induces fibrotic changes in the lung through the activation of TGF-beta signaling pathways. Sci. Rep. 2018;8:11920. doi: 10.1038/s41598-018-30449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia Caballero G., Schmidt S., Manning J.C., Michalak M., Schlotzer-Schrehardt U., Ludwig A.K., Kaltner H., Sinowatz F., Schnolzer M., Kopitz J., et al. Chicken lens development: Complete signature of expression of galectins during embryogenesis and evidence for their complex formation with alpha-, beta-, delta-, and tau-crystallins, N-CAM, and N-cadherin obtained by affinity chromatography. Cell Tissue Res. 2020;379:13–35. doi: 10.1007/s00441-019-03129-0. [DOI] [PubMed] [Google Scholar]
- 17.Russ H.A., Landsman L., Moss C.L., Higdon R., Greer R.L., Kaihara K., Salamon R., Kolker E., Hebrok M. Dynamic Proteomic Analysis of Pancreatic Mesenchyme Reveals Novel Factors That Enhance Human Embryonic Stem Cell to Pancreatic Cell Differentiation. Stem Cells Int. 2016;2016:6183562. doi: 10.1155/2016/6183562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motohashi T., Nishioka M., Kitagawa D., Kawamura N., Watanabe N., Wakaoka T., Kadoya T., Kunisada T. Galectin-1 enhances the generation of neural crest cells. Int. J. Dev. Biol. 2017;61:407–413. doi: 10.1387/ijdb.160380tm. [DOI] [PubMed] [Google Scholar]
- 19.Tang M., You J., Wang W., Lu Y., Hu X., Wang C., Liu A., Zhu Y. Impact of Galectin-1 on Trophoblast Stem Cell Differentiation and Invasion in In Vitro Implantation Model. Reprod. Sci. 2018;25:700–711. doi: 10.1177/1933719117725816. [DOI] [PubMed] [Google Scholar]
- 20.Munro D.A.D., Wineberg Y., Tarnick J., Vink C.S., Li Z., Pridans C., Dzierzak E., Kalisky T., Hohenstein P., Davies J.A. Macrophages restrict the nephrogenic field and promote endothelial connections during kidney development. Elife. 2019;8 doi: 10.7554/eLife.43271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan S.Y., Krasnow M.A. Developmental origin of lung macrophage diversity. Development. 2016;143:1318–1327. doi: 10.1242/dev.129122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earl L.A., Bi S., Baum L.G. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem. 2010;285:2232–2244. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Law H.L., Wright R.D., Iqbal A.J., Norling L.V., Cooper D. A Pro-resolving Role for Galectin-1 in Acute Inflammation. Front. Pharmacol. 2020;11:274. doi: 10.3389/fphar.2020.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu T.B., Dodd S., Yu L.G., Subramanian S. Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS ONE. 2020;15:e0227306. doi: 10.1371/journal.pone.0227306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z.H., Wang L.L., Liu H., Muyayalo K.P., Huang X.B., Mor G., Liao A.H. Galectin-9 Alleviates LPS-Induced Preeclampsia-Like Impairment in Rats via Switching Decidual Macrophage Polarization to M2 Subtype. Front. Immunol. 2018;9:3142. doi: 10.3389/fimmu.2018.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arsenijevic A., Milovanovic J., Stojanovic B., Djordjevic D., Stanojevic I., Jankovic N., Vojvodic D., Arsenijevic N., Lukic M.L., Milovanovic M. Gal-3 Deficiency Suppresses Novosphyngobium aromaticivorans Inflammasome Activation and IL-17 Driven Autoimmune Cholangitis in Mice. Front. Immunol. 2019;10:1309. doi: 10.3389/fimmu.2019.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vokalova L., Balogh A., Toth E., Van Breda S.V., Schafer G., Hoesli I., Lapaire O., Hahn S., Than N.G., Rossi S.W. Placental Protein 13 (Galectin-13) Polarizes Neutrophils Toward an Immune Regulatory Phenotype. Front. Immunol. 2020;11:145. doi: 10.3389/fimmu.2020.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hepp P., Unverdorben L., Hutter S., Kuhn C., Ditsch N., Gross E., Mahner S., Jeschke U., Knabl J., Heidegger H.H. Placental Galectin-2 Expression in Gestational Diabetes: A Systematic, Histological Analysis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atalar M.N., Abusoglu S., Unlu A., Tok O., Ipekci S.H., Baldane S., Kebapcilar L. Assessment of serum galectin-3, methylated arginine and Hs-CRP levels in type 2 diabetes and prediabetes. Life Sci. 2019;231:116577. doi: 10.1016/j.lfs.2019.116577. [DOI] [PubMed] [Google Scholar]
- 30.Tromp J., Voors A.A., Sharma A., Ferreira J.P., Ouwerkerk W., Hillege H.L., Gomez K.A., Dickstein K., Anker S.D., Metra M., et al. Distinct Pathological Pathways in Patients With Heart Failure and Diabetes. JACC Heart Fail. 2020;8:234–242. doi: 10.1016/j.jchf.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo-Almoros A., Pello A., Acena A., Martinez-Milla J., Gonzalez-Lorenzo O., Tarin N., Cristobal C., Blanco-Colio L.M., Martin-Ventura J.L., Huelmos A., et al. Galectin-3 Is Associated with Cardiovascular Events in Post-Acute Coronary Syndrome Patients with Type-2 Diabetes. J. Clin. Med. 2020;9 doi: 10.3390/jcm9041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vora A., de Lemos J.A., Ayers C., Grodin J.L., Lingvay I. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019;104:4449–4458. doi: 10.1210/jc.2019-00398. [DOI] [PubMed] [Google Scholar]
- 33.Tan K.C.B., Cheung C.L., Lee A.C.H., Lam J.K.Y., Wong Y., Shiu S.W.M. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia. 2018;61:1212–1219. doi: 10.1007/s00125-018-4552-z. [DOI] [PubMed] [Google Scholar]
- 34.Mensah-Brown E.P., Al Rabesi Z., Shahin A., Al Shamsi M., Arsenijevic N., Hsu D.K., Liu F.T., Lukic M.L. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009;130:83–88. doi: 10.1016/j.clim.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Petrovic I., Pejnovic N., Ljujic B., Pavlovic S., Miletic Kovacevic M., Jeftic I., Djukic A., Draginic N., Andjic M., Arsenijevic N., et al. Overexpression of Galectin 3 in Pancreatic beta Cells Amplifies beta-Cell Apoptosis and Islet Inflammation in Type-2 Diabetes in Mice. Front. Endocrinol. 2020;11:30. doi: 10.3389/fendo.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J., Zhang L., Fang J., Yang S., Chen L. Galectin-3 mediates high-glucose-induced cardiomyocyte injury by NADPH oxidase/reactive oxygen species pathway. Can. J. Physiol. Pharmacol. 2020 doi: 10.1139/cjpp-2019-0708. [DOI] [PubMed] [Google Scholar]
- 37.Giritharan S., Cagampang F., Torrens C., Salhiyyah K., Duggan S., Ohri S. Aortic Stenosis Prognostication in Patients With Type 2 Diabetes: Protocol for Testing and Validation of a Biomarker-Derived Scoring System. JMIR Res. Protoc. 2019;8:e13186. doi: 10.2196/13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Romero D., Vilchez J.A., Lahoz A., Romero-Aniorte A.I., Jover E., Garcia-Alberola A., Jara-Rubio R., Martinez C.M., Valdes M., Marin F. Galectin-3 as a marker of interstitial atrial remodelling involved in atrial fibrillation. Sci. Rep. 2017;7:40378. doi: 10.1038/srep40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X., Liu Y., Tu D., Liu X., Niu S., Suo Y., Liu T., Li G., Liu C. Role of NLRP3-Inflammasome/Caspase-1/Galectin-3 Pathway on Atrial Remodeling in Diabetic Rabbits. J. Cardiovasc. Transl. Res. 2020 doi: 10.1007/s12265-020-09965-8. [DOI] [PubMed] [Google Scholar]
- 40.Al-Obaidi N., Mohan S., Liang S., Zhao Z., Nayak B.K., Li B., Sriramarao P., Habib S.L. Galectin-1 is a new fibrosis protein in type 1 and type 2 diabetes. FASEB J. 2019;33:373–387. doi: 10.1096/fj.201800555RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aanhane E., Schulkens I.A., Heusschen R., Castricum K., Leffler H., Griffioen A.W., Thijssen V.L. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis. 2018;21:545–555. doi: 10.1007/s10456-018-9607-8. [DOI] [PubMed] [Google Scholar]
- 42.Rao U.S., Rao P.S. Surface-bound galectin-4 regulates gene transcription and secretion of chemokines in human colorectal cancer cell lines. Tumour Biol. 2017;39:1010428317691687. doi: 10.1177/1010428317691687. [DOI] [PubMed] [Google Scholar]
- 43.Cagnoni A.J., Giribaldi M.L., Blidner A.G., Cutine A.M., Gatto S.G., Morales R.M., Salatino M., Abba M.C., Croci D.O., Marino K.V., et al. Galectin-1 fosters an immunosuppressive microenvironment in colorectal cancer by reprogramming CD8(+) regulatory T cells. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2102950118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang Z., Wu B., Ji Z., Liu W., Shi D., Chen X., Wei Y., Jiang J. The binding of LDN193189 to CD133 C-terminus suppresses the tumorigenesis and immune escape of liver tumor-initiating cells. Cancer Lett. 2021;513:90–100. doi: 10.1016/j.canlet.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Liebscher L., Weissenborn C., Langwisch S., Gohlke B.O., Preissner R., Rabinovich G.A., Christiansen N., Christiansen H., Zenclussen A.C., Fest S. A minigene DNA vaccine encoding peptide epitopes derived from Galectin-1 has protective antitumoral effects in a model of neuroblastoma. Cancer Lett. 2021;509:105–114. doi: 10.1016/j.canlet.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Ma H., Chang H., Yang W., Lu Y., Hu J., Jin S. A novel IFNalpha-induced long noncoding RNA negatively regulates immunosuppression by interrupting H3K27 acetylation in head and neck squamous cell carcinoma. Mol. Cancer. 2020;19:4. doi: 10.1186/s12943-019-1123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enninga E.A.L., Chatzopoulos K., Butterfield J.T., Sutor S.L., Leontovich A.A., Nevala W.K., Flotte T.J., Markovic S.N. CD206-positive myeloid cells bind galectin-9 and promote a tumor-supportive microenvironment. J. Pathol. 2018;245:468–477. doi: 10.1002/path.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shih T.C., Liu R., Wu C.T., Li X., Xiao W., Deng X., Kiss S., Wang T., Chen X.J., Carney R., et al. Targeting Galectin-1 Impairs Castration-Resistant Prostate Cancer Progression and Invasion. Clin. Cancer Res. 2018;24:4319–4331. doi: 10.1158/1078-0432.CCR-18-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cristiani C.M., Turdo A., Ventura V., Apuzzo T., Capone M., Madonna G., Mallardo D., Garofalo C., Giovannone E.D., Grimaldi A.M., et al. Accumulation of Circulating CCR7(+) Natural Killer Cells Marks Melanoma Evolution and Reveals a CCL19-Dependent Metastatic Pathway. Cancer Immunol. Res. 2019;7:841–852. doi: 10.1158/2326-6066.CIR-18-0651. [DOI] [PubMed] [Google Scholar]
- 50.Caputo S., Grioni M., Brambillasca C.S., Monno A., Brevi A., Freschi M., Piras I.S., Elia A.R., Pieri V., Baccega T., et al. Galectin-3 in Prostate Cancer Stem-Like Cells Is Immunosuppressive and Drives Early Metastasis. Front. Immunol. 2020;11:1820. doi: 10.3389/fimmu.2020.01820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Limagne E., Richard C., Thibaudin M., Fumet J.D., Truntzer C., Lagrange A., Favier L., Coudert B., Ghiringhelli F. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. Oncoimmunology. 2019;8:e1564505. doi: 10.1080/2162402X.2018.1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez Saez J.M., Hockl P.F., Cagnoni A.J., Mendez Huergo S.P., Garcia P.A., Gatto S.G., Cerliani J.P., Croci D.O., Rabinovich G.A. Characterization of a neutralizing anti-human galectin-1 monoclonal antibody with angioregulatory and immunomodulatory activities. Angiogenesis. 2021;24:1–5. doi: 10.1007/s10456-020-09749-3. [DOI] [PubMed] [Google Scholar]
- 53.Mammadova-Bach E., Gil-Pulido J., Sarukhanyan E., Burkard P., Shityakov S., Schonhart C., Stegner D., Remer K., Nurden P., Nurden A.T., et al. Platelet glycoprotein VI promotes metastasis through interaction with cancer cell-derived galectin-3. Blood. 2020;135:1146–1160. doi: 10.1182/blood.2019002649. [DOI] [PubMed] [Google Scholar]
- 54.Zamorano P., Koning T., Oyanadel C., Mardones G.A., Ehrenfeld P., Boric M.P., Gonzalez A., Soza A., Sanchez F.A. Galectin-8 induces endothelial hyperpermeability through the eNOS pathway involving S-nitrosylation-mediated adherens junction disassembly. Carcinogenesis. 2019;40:313–323. doi: 10.1093/carcin/bgz002. [DOI] [PubMed] [Google Scholar]
- 55.Storti P., Marchica V., Airoldi I., Donofrio G., Fiorini E., Ferri V., Guasco D., Todoerti K., Silbermann R., Anderson J.L., et al. Galectin-1 suppression delineates a new strategy to inhibit myeloma-induced angiogenesis and tumoral growth in vivo. Leukemia. 2016;30:2351–2363. doi: 10.1038/leu.2016.137. [DOI] [PubMed] [Google Scholar]
- 56.Wu K.L., Huang E.Y., Yeh W.L., Hsiao C.C., Kuo C.M. Synergistic interaction between galectin-3 and carcinoembryonic antigen promotes colorectal cancer metastasis. Oncotarget. 2017;8:61935–61943. doi: 10.18632/oncotarget.18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutzner T.J., Higuero A.M., Sussmair M., Kopitz J., Hingar M., Diez-Revuelta N., Caballero G.G., Kaltner H., Lindner I., Abad-Rodriguez J., et al. How presence of a signal peptide affects human galectins-1 and -4: Clues to explain common absence of a leader sequence among adhesion/growth-regulatory galectins. Biochim. Biophys. Acta Gen. Subj. 2020;1864:129449. doi: 10.1016/j.bbagen.2019.129449. [DOI] [PubMed] [Google Scholar]
- 58.Gong H.C., Honjo Y., Nangia-Makker P., Hogan V., Mazurak N., Bresalier R.S., Raz A. The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999;59:6239–6245. [PubMed] [Google Scholar]
- 59.Menon S., Kang C.M., Beningo K.A. Galectin-3 secretion and tyrosine phosphorylation is dependent on the calpain small subunit, Calpain 4. Biochem. Biophys. Res. Commun. 2011;410:91–96. doi: 10.1016/j.bbrc.2011.05.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mehul B., Hughes R.C. Plasma membrane targetting, vesicular budding and release of galectin 3 from the cytoplasm of mammalian cells during secretion. J. Cell Sci. 1997;110 (Pt 10):1169–1178. doi: 10.1242/jcs.110.10.1169. [DOI] [PubMed] [Google Scholar]
- 61.Sato S., Burdett I., Hughes R.C. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: A pathway independent of the endoplasmic reticulum-Golgi complex. Exp. Cell Res. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- 62.Nakahara S., Hogan V., Inohara H., Raz A. Importin-mediated nuclear translocation of galectin-3. J. Biol. Chem. 2006;281:39649–39659. doi: 10.1074/jbc.M608069200. [DOI] [PubMed] [Google Scholar]
- 63.Wu H., Song S., Yan A., Guo X., Chang L., Xu L., Hu L., Kuang M., Liu B., He D., et al. RACK1 promotes the invasive activities and lymph node metastasis of cervical cancer via galectin-1. Cancer Lett. 2020;469:287–300. doi: 10.1016/j.canlet.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Yu F., Finley R.L., Jr., Raz A., Kim H.R. Galectin-3 translocates to the perinuclear membranes and inhibits cytochrome c release from the mitochondria. A role for synexin in galectin-3 translocation. J. Biol. Chem. 2002;277:15819–15827. doi: 10.1074/jbc.M200154200. [DOI] [PubMed] [Google Scholar]
- 65.Funasaka T., Raz A., Nangia-Makker P. Nuclear transport of galectin-3 and its therapeutic implications. Semin. Cancer Biol. 2014;27:30–38. doi: 10.1016/j.semcancer.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li C.F., Shen K.H., Chien L.H., Huang C.H., Wu T.F., He H.L. Proteomic Identification of the Galectin-1-Involved Molecular Pathways in Urinary Bladder Urothelial Carcinoma. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang T., Liu D.D., Ning H.M., Dan L., Sun J.Y., Huang X.J., Dong Y., Geng M.Y., Yun S.F., Yan J., et al. Modified citrus pectin inhibited bladder tumor growth through downregulation of galectin-3. Acta Pharmacol. Sin. 2018;39:1885–1893. doi: 10.1038/s41401-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi Y., Chang Y., Wang Z., Chen L., Kong Y., Zhang P., Liu Z., Zhou Q., Chen Y., Wang J., et al. Tumor-associated macrophages expressing galectin-9 identify immunoevasive subtype muscle-invasive bladder cancer with poor prognosis but favorable adjuvant chemotherapeutic response. Cancer Immunol. Immunother. 2019;68:2067–2080. doi: 10.1007/s00262-019-02429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xue H., Yang R.Y., Tai G., Liu F.T. Galectin-12 inhibits granulocytic differentiation of human NB4 promyelocytic leukemia cells while promoting lipogenesis. J. Leukoc. Biol. 2016;100:657–664. doi: 10.1189/jlb.1HI0316-134R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen M.N., Ludvigsen M., Abildgaard N., Petruskevicius I., Hjortebjerg R., Bjerre M., Honore B., Moller H.J., Andersen N.F. Serum galectin-1 in patients with multiple myeloma: Associations with survival, angiogenesis, and biomarkers of macrophage activation. Onco Targets Ther. 2017;10:1977–1982. doi: 10.2147/OTT.S124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muller J., Duray E., Lejeune M., Dubois S., Plougonven E., Leonard A., Storti P., Giuliani N., Cohen-Solal M., Hempel U., et al. Loss of Stromal Galectin-1 Enhances Multiple Myeloma Development: Emphasis on a Role in Osteoclasts. Cancers. 2019;11 doi: 10.3390/cancers11020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nam K., Son S.H., Oh S., Jeon D., Kim H., Noh D.Y., Kim S., Shin I. Binding of galectin-1 to integrin beta1 potentiates drug resistance by promoting survivin expression in breast cancer cells. Oncotarget. 2017;8:35804–35823. doi: 10.18632/oncotarget.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patrick M.E., Egland K.A. SUSD2 Proteolytic Cleavage Requires the GDPH Sequence and Inter-Fragment Disulfide Bonds for Surface Presentation of Galectin-1 on Breast Cancer Cells. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20153814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Upreti M., Jyoti A., Johnson S.E., Swindell E.P., Napier D., Sethi P., Chan R., Feddock J.M., Weiss H.L., O’Halloran T.V., et al. Radiation-enhanced therapeutic targeting of galectin-1 enriched malignant stroma in triple negative breast cancer. Oncotarget. 2016;7:41559–41574. doi: 10.18632/oncotarget.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pereira J.X., Dos Santos S.N., Pereira T.C., Cabanel M., Chammas R., de Oliveira F.L., Bernardes E.S., El-Cheikh M.C. Galectin-3 Regulates the Expression of Tumor Glycosaminoglycans and Increases the Metastatic Potential of Breast Cancer. J. Oncol. 2019;2019:9827147. doi: 10.1155/2019/9827147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakajima K., Kho D.H., Yanagawa T., Harazono Y., Hogan V., Chen W., Ali-Fehmi R., Mehra R., Raz A. Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis. Cancer Res. 2016;76:1391–1402. doi: 10.1158/0008-5472.CAN-15-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.John C.M., Leffler H., Kahl-Knutsson B., Svensson I., Jarvis G.A. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin. Cancer Res. 2003;9:2374–2383. [PubMed] [Google Scholar]
- 78.Higareda-Almaraz J.C., Ruiz-Moreno J.S., Klimentova J., Barbieri D., Salvador-Gallego R., Ly R., Valtierra-Gutierrez I.A., Dinsart C., Rabinovich G.A., Stulik J., et al. Systems-level effects of ectopic galectin-7 reconstitution in cervical cancer and its microenvironment. BMC Cancer. 2016;16:680. doi: 10.1186/s12885-016-2700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park G.B., Kim D. TLR4-mediated galectin-1 production triggers epithelial-mesenchymal transition in colon cancer cells through ADAM10- and ADAM17-associated lactate production. Mol. Cell. Biochem. 2017;425:191–202. doi: 10.1007/s11010-016-2873-0. [DOI] [PubMed] [Google Scholar]
- 80.Dovizio M., Maier T.J., Alberti S., Di Francesco L., Marcantoni E., Munch G., John C.M., Suess B., Sgambato A., Steinhilber D., et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol. Pharmacol. 2013;84:25–40. doi: 10.1124/mol.113.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katzenmaier E.M., Fuchs V., Warnken U., Schnolzer M., Gebert J., Kopitz J. Deciphering the galectin-12 protein interactome reveals a major impact of galectin-12 on glutamine anaplerosis in colon cancer cells. Exp. Cell Res. 2019;379:129–139. doi: 10.1016/j.yexcr.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 82.Sandberg T.P., Oosting J., van Pelt G.W., Mesker W.E., Tollenaar R., Morreau H. Erratum: Molecular profiling of colorectal tumors stratified by the histological tumor-stroma ratio—Increased expression of galectin-1 in tumors with high stromal content. Oncotarget. 2019;10:2416. doi: 10.18632/oncotarget.26820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michalak M., Warnken U., Schnolzer M., Gabius H.J., Kopitz J. Detection of malignancy-associated phosphoproteome changes in human colorectal cancer induced by cell surface binding of growth-inhibitory galectin-4. IUBMB Life. 2019;71:364–375. doi: 10.1002/iub.1987. [DOI] [PubMed] [Google Scholar]
- 84.Sakhnevych S.S., Yasinska I.M., Fasler-Kan E., Sumbayev V.V. Mitochondrial Defunctionalization Supresses Tim-3-Galectin-9 Secretory Pathway in Human Colorectal Cancer Cells and Thus Can Possibly Affect Tumor Immune Escape. Front. Pharmacol. 2019;10:342. doi: 10.3389/fphar.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiyo T., Fujita K., Iwama H., Fujihara S., Tadokoro T., Ohura K., Matsui T., Goda Y., Kobayashi N., Nishiyama N., et al. Galectin-9 Induces Mitochondria-Mediated Apoptosis of Esophageal Cancer In Vitro and In Vivo in a Xenograft Mouse Model. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chong Y., Tang D., Xiong Q., Jiang X., Xu C., Huang Y., Wang J., Zhou H., Shi Y., Wu X., et al. Galectin-1 from cancer-associated fibroblasts induces epithelial-mesenchymal transition through beta1 integrin-mediated upregulation of Gli1 in gastric cancer. J. Exp. Clin. Cancer Res. 2016;35:175. doi: 10.1186/s13046-016-0449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koonce N.A., Griffin R.J., Dings R.P.M. Galectin-1 Inhibitor OTX008 Induces Tumor Vessel Normalization and Tumor Growth Inhibition in Human Head and Neck Squamous Cell Carcinoma Models. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18122671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tadokoro T., Fujihara S., Chiyo T., Oura K., Samukawa E., Yamana Y., Fujita K., Mimura S., Sakamoto T., Nomura T., et al. Induction of apoptosis by Galectin-9 in liver metastatic cancer cells: In vitro study. Int. J. Oncol. 2017;51:607–614. doi: 10.3892/ijo.2017.4053. [DOI] [PubMed] [Google Scholar]
- 89.Zheng H., Cui Y., Li X., Du B., Li Y. Prognostic Significance of (18)F-FDG PET/CT Metabolic Parameters and Tumor Galectin-1 Expression in Patients With Surgically Resected Lung Adenocarcinoma. Clin. Lung Cancer. 2019;20:420–428. doi: 10.1016/j.cllc.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 90.Hsu Y.L., Hung J.Y., Chiang S.Y., Jian S.F., Wu C.Y., Lin Y.S., Tsai Y.M., Chou S.H., Tsai M.J., Kuo P.L. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7:27584–27598. doi: 10.18632/oncotarget.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He F., Wang Y., Cai W., Li M., Dong L. Reversal of EGFR inhibitors’ resistance by co-delivering EGFR and integrin alphavbeta3 inhibitors with nanoparticles in non-small cell lung cancer. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vuong L., Kouverianou E., Rooney C.M., McHugh B.J., Howie S.E.M., Gregory C.D., Forbes S.J., Henderson N.C., Zetterberg F.R., Nilsson U.J., et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to PD-L1 Blockade. Cancer Res. 2019;79:1480–1492. doi: 10.1158/0008-5472.CAN-18-2244. [DOI] [PubMed] [Google Scholar]
- 93.Wang D., You D., Li L. Galectin-3 regulates chemotherapy sensitivity in epithelial ovarian carcinoma via regulating mitochondrial function. J. Toxicol. Sci. 2019;44:47–56. doi: 10.2131/jts.44.47. [DOI] [PubMed] [Google Scholar]
- 94.El-Kott A.F., Shati A.A., Ali Al-Kahtani M., Alharbi S.A. The apoptotic effect of resveratrol in ovarian cancer cells is associated with downregulation of galectin-3 and stimulating miR-424-3p transcription. J. Food Biochem. 2019;43:e13072. doi: 10.1111/jfbc.13072. [DOI] [PubMed] [Google Scholar]
- 95.Hossein G., Halvaei S., Heidarian Y., Dehghani-Ghobadi Z., Hassani M., Hosseini H., Naderi N., Sheikh Hassani S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019;8:4315–4329. doi: 10.1002/cam4.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang D., Wu Q., Zhang J., Zhang H., Yuan Z., Xu J., Chong Y., Huang Y., Xiong Q., Wang S., et al. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-beta1/Smad pathway. Oncol. Rep. 2018;39:1347–1355. doi: 10.3892/or.2018.6202. [DOI] [PubMed] [Google Scholar]
- 97.Orozco C.A., Martinez-Bosch N., Guerrero P.E., Vinaixa J., Dalotto-Moreno T., Iglesias M., Moreno M., Djurec M., Poirier F., Gabius H.J., et al. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc. Natl. Acad. Sci. USA. 2018;115:E3769–E3778. doi: 10.1073/pnas.1722434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corapi E., Carrizo G., Compagno D., Laderach D. Endogenous Galectin-1 in T Lymphocytes Regulates Anti-prostate Cancer Immunity. Front. Immunol. 2018;9:2190. doi: 10.3389/fimmu.2018.02190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gorniak P., Wasylecka-Juszczynska M., Lugowska I., Rutkowski P., Polak A., Szydlowski M., Juszczynski P. BRAF inhibition curtails IFN-gamma-inducible PD-L1 expression and upregulates the immunoregulatory protein galectin-1 in melanoma cells. Mol. Oncol. 2020 doi: 10.1002/1878-0261.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krishnan V., Bane S.M., Kawle P.D., Naresh K.N., Kalraiya R.D. Altered melanoma cell surface glycosylation mediates organ specific adhesion and metastasis via lectin receptors on the lung vascular endothelium. Clin. Exp. Metastasis. 2005;22:11–24. doi: 10.1007/s10585-005-2036-2. [DOI] [PubMed] [Google Scholar]
- 101.Li C.H., Chang Y.C., Hsiao M., Liang S.M. FOXD1 and Gal-3 Form a Positive Regulatory Loop to Regulate Lung Cancer Aggressiveness. Cancers. 2019;11 doi: 10.3390/cancers11121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee S.H., Khwaja Rehman F., Tyler K.C., Yu B., Zhang Z., Osuka S., Zerrouqi A., Kaluzova M., Hadjipanayis C.G., Cummings R.D., et al. A Chimeric Signal Peptide-Galectin-3 Conjugate Induces Glycosylation-Dependent Cancer Cell-Specific Apoptosis. Clin. Cancer Res. 2020;26:2711–2724. doi: 10.1158/1078-0432.CCR-18-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang P., Sun Y., Peng R., Chen W., Fu X., Zhang L., Peng H., Zhang Z. Long non-coding RNA Rpph1 promotes inflammation and proliferation of mesangial cells in diabetic nephropathy via an interaction with Gal-3. Cell Death Dis. 2019;10:526. doi: 10.1038/s41419-019-1765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kariya Y., Oyama M., Hashimoto Y., Gu J., Kariya Y. beta4-Integrin/PI3K Signaling Promotes Tumor Progression through the Galectin-3-N-Glycan Complex. Mol. Cancer Res. 2018;16:1024–1034. doi: 10.1158/1541-7786.MCR-17-0365. [DOI] [PubMed] [Google Scholar]
- 105.Zhang L., Wang P., Qin Y., Cong Q., Shao C., Du Z., Ni X., Li P., Ding K. RN1, a novel galectin-3 inhibitor, inhibits pancreatic cancer cell growth in vitro and in vivo via blocking galectin-3 associated signaling pathways. Oncogene. 2017;36:1297–1308. doi: 10.1038/onc.2016.306. [DOI] [PubMed] [Google Scholar]
- 106.Oyanadel C., Holmes C., Pardo E., Retamal C., Shaughnessy R., Smith P., Cortes P., Bravo-Zehnder M., Metz C., Feuerhake T., et al. Galectin-8 induces partial epithelial-mesenchymal transition with invasive tumorigenic capabilities involving a FAK/EGFR/proteasome pathway in Madin-Darby canine kidney cells. Mol. Biol. Cell. 2018;29:557–574. doi: 10.1091/mbc.E16-05-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Piyush T., Chacko A.R., Sindrewicz P., Hilkens J., Rhodes J.M., Yu L.G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017;24:1937–1947. doi: 10.1038/cdd.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seguin L., Kato S., Franovic A., Camargo M.F., Lesperance J., Elliott K.C., Yebra M., Mielgo A., Lowy A.M., Husain H., et al. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayashi Y., Jia W., Kidoya H., Muramatsu F., Tsukada Y., Takakura N. Galectin-3 Inhibits Cancer Metastasis by Negatively Regulating Integrin beta3 Expression. Am. J. Pathol. 2019;189:900–910. doi: 10.1016/j.ajpath.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 110.He F., Antonucci L., Karin M. NRF2 as a regulator of cell metabolism and inflammation in cancer. Carcinogenesis. 2020;41:405–416. doi: 10.1093/carcin/bgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puschel F., Favaro F., Redondo-Pedraza J., Lucendo E., Iurlaro R., Marchetti S., Majem B., Eldering E., Nadal E., Ricci J.E., et al. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci. USA. 2020;117:9932–9941. doi: 10.1073/pnas.1913707117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weyandt J.D., Thompson C.B., Giaccia A.J., Rathmell W.K. Metabolic Alterations in Cancer and Their Potential as Therapeutic Targets. Am. Soc. Clin. Oncol. Educ. Book. 2017;37:825–832. doi: 10.1200/EDBK_175561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jang H.J., Park M.S., Kim Y.S., Chang J., Lee J.H., Lee C.T., Lee S.H., Yoon H.I. The relationship between the severity of pulmonary fibrosis and the lung cancer stage. J. Cancer. 2021;12:2807–2814. doi: 10.7150/jca.51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim H.C., Lee S., Song J.W. Impact of idiopathic pulmonary fibrosis on clinical outcomes of lung cancer patients. Sci. Rep. 2021;11:8312. doi: 10.1038/s41598-021-87747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Q., Liu P., Zhou H., Kong H., Xie W. An increased risk of lung cancer in combined pulmonary fibrosis and emphysema patients with usual interstitial pneumonia compared with patients with idiopathic pulmonary fibrosis alone: A systematic review and meta-analysis. Ther. Adv. Respir. Dis. 2021;15:17534666211017050. doi: 10.1177/17534666211017050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yoneshima Y., Iwama E., Matsumoto S., Matsubara T., Tagawa T., Ota K., Tanaka K., Takenoyama M., Okamoto T., Goto K., et al. Paired analysis of tumor mutation burden for lung adenocarcinoma and associated idiopathic pulmonary fibrosis. Sci. Rep. 2021;11:12732. doi: 10.1038/s41598-021-92098-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dolgormaa G., Harimoto N., Ishii N., Yamanaka T., Hagiwara K., Tsukagoshi M., Igarashi T., Watanabe A., Kubo N., Araki K., et al. Mac-2-binding protein glycan isomer enhances the aggressiveness of hepatocellular carcinoma by activating mTOR signaling. Br. J. Cancer. 2020;123:1145–1153. doi: 10.1038/s41416-020-0971-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Im H.S., Kim H.D., Song J.Y., Han Y., Lee D.Y., Kim C.W., Yun Y.S. Overexpression of alpha1-protease inhibitor and galectin-1 in radiation-induced early phase of pulmonary fibrosis. Cancer. Res. Treat. 2006;38:92–98. doi: 10.4143/crt.2006.38.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao H., Dennery P.A., Yao H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314:L544–L554. doi: 10.1152/ajplung.00521.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y.S., Li X.T., Yu L.G., Wang L., Shi Z.Y., Guo X.L. Roles of galectin-3 in metabolic disorders and tumor cell metabolism. Int. J. Biol. Macromol. 2020;142:463–473. doi: 10.1016/j.ijbiomac.2019.09.118. [DOI] [PubMed] [Google Scholar]
- 121.Brooks G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kumar R.M.M., Narayanan N.K., Raghunath K.J., Rajagopalan S. Composite Pheochromocytoma Presenting as Severe Lactic Acidosis and Back Pain: A Case Report. Indian J. Nephrol. 2019;29:353–356. doi: 10.4103/ijn.IJN_67_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun X., Peng Y., Zhao J., Xie Z., Lei X., Tang G. Discovery and development of tumor glycolysis rate-limiting enzyme inhibitors. Bioorg. Chem. 2021;112:104891. doi: 10.1016/j.bioorg.2021.104891. [DOI] [PubMed] [Google Scholar]
- 124.Hu X., Chao M., Wu H. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal Transduct. Target. Ther. 2017;2:16047. doi: 10.1038/sigtrans.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Apicella M., Giannoni E., Fiore S., Ferrari K.J., Fernandez-Perez D., Isella C., Granchi C., Minutolo F., Sottile A., Comoglio P.M., et al. Increased Lactate Secretion by Cancer Cells Sustains Non-cell-autonomous Adaptive Resistance to MET and EGFR Targeted Therapies. Cell Metab. 2018;28:848–865 e846. doi: 10.1016/j.cmet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 126.Singh D., Arora R., Kaur P., Singh B., Mannan R., Arora S. Overexpression of hypoxia-inducible factor and metabolic pathways: Possible targets of cancer. Cell Biosci. 2017;7:62. doi: 10.1186/s13578-017-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holst J.M., Ludvigsen M., Hamilton-Dutoit S.J., Bendix K., Plesner T.L., Norgaard P., Moller M.B., Steiniche T., Rabinovich G.A., d’Amore F., et al. High intratumoural galectin-1 expression predicts adverse outcome in ALK(-) ALCL and CD30(+) PTCL-NOS. Hematol. Oncol. 2020;38:59–66. doi: 10.1002/hon.2702. [DOI] [PubMed] [Google Scholar]
- 128.Rondepierre F., Bouchon B., Bonnet M., Moins N., Chezal J.M., D’Incan M., Degoul F. B16 melanoma secretomes and in vitro invasiveness: Syntenin as an invasion modulator. Melanoma Res. 2010;20:77–84. doi: 10.1097/CMR.0b013e32833279f2. [DOI] [PubMed] [Google Scholar]
- 129.Damiani C., Colombo R., Gaglio D., Mastroianni F., Pescini D., Westerhoff H.V., Mauri G., Vanoni M., Alberghina L. A metabolic core model elucidates how enhanced utilization of glucose and glutamine, with enhanced glutamine-dependent lactate production, promotes cancer cell growth: The WarburQ effect. PLoS Comput. Biol. 2017;13:e1005758. doi: 10.1371/journal.pcbi.1005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baek J.H., Kim D.H., Lee J., Kim S.J., Chun K.H. Galectin-1 accelerates high-fat diet-induced obesity by activation of peroxisome proliferator-activated receptor gamma (PPARgamma) in mice. Cell Death Dis. 2021;12:66. doi: 10.1038/s41419-020-03367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coppin L., Jannin A., Ait Yahya E., Thuillier C., Villenet C., Tardivel M., Bongiovanni A., Gaston C., de Beco S., Barois N., et al. Galectin-3 modulates epithelial cell adaptation to stress at the ER-mitochondria interface. Cell Death Dis. 2020;11:360. doi: 10.1038/s41419-020-2556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bibens-Laulan N., St-Pierre Y. Intracellular galectin-7 expression in cancer cells results from an autocrine transcriptional mechanism and endocytosis of extracellular galectin-7. PLoS ONE. 2017;12:e0187194. doi: 10.1371/journal.pone.0187194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhong S.W., Jeong J.H., Chen Z.K., Chen Z.H., Luo J.L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020;13:57–69. doi: 10.1016/j.tranon.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jin M.Z., Jin W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.AbuSamra D.B., Mauris J., Argueso P. Galectin-3 initiates epithelial-stromal paracrine signaling to shape the proteolytic microenvironment during corneal repair. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aaw7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toti A., Santi A., Pardella E., Nesi I., Tomasini R., Mello T., Paoli P., Caselli A., Cirri P. Activated fibroblasts enhance cancer cell migration by microvesicles-mediated transfer of Galectin-1. J. Cell Commun. Signal. 2021;15:405–419. doi: 10.1007/s12079-021-00624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Horn L.A., Fousek K., Palena C. Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer. 2020;6:432–441. doi: 10.1016/j.trecan.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sturgill E.R., Rolig A.S., Linch S.N., Mick C., Kasiewicz M.J., Sun Z., Traber P.G., Shlevin H., Redmond W.L. Galectin-3 inhibition with belapectin combined with anti-OX40 therapy reprograms the tumor microenvironment to favor anti-tumor immunity. Oncoimmunology. 2021;10:1892265. doi: 10.1080/2162402X.2021.1892265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Balakrishnan B., Subramanian S., Mallia M.B., Repaka K., Kaur S., Chandan R., Bhardwaj P., Dash A., Banerjee R. Multifunctional Core-Shell Glyconanoparticles for Galectin-3-Targeted, Trigger-Responsive Combination Chemotherapy. Biomacromolecules. 2020;21:2645–2660. doi: 10.1021/acs.biomac.0c00358. [DOI] [PubMed] [Google Scholar]
- 140.Dos Santos S.N., Sheldon H., Pereira J.X., Paluch C., Bridges E.M., El-Cheikh M.C., Harris A.L., Bernardes E.S. Galectin-3 acts as an angiogenic switch to induce tumor angiogenesis via Jagged-1/Notch activation. Oncotarget. 2017;8:49484–49501. doi: 10.18632/oncotarget.17718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colomb F., Wang W., Simpson D., Zafar M., Beynon R., Rhodes J.M., Yu L.G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017;292:8381–8389. doi: 10.1074/jbc.M117.783431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Croci D.O., Cerliani J.P., Dalotto-Moreno T., Mendez-Huergo S.P., Mascanfroni I.D., Dergan-Dylon S., Toscano M.A., Caramelo J.J., Garcia-Vallejo J.J., Ouyang J., et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 143.Demotte N., Wieers G., Van Der Smissen P., Moser M., Schmidt C., Thielemans K., Squifflet J.L., Weynand B., Carrasco J., Lurquin C., et al. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–7488. doi: 10.1158/0008-5472.CAN-10-0761. [DOI] [PubMed] [Google Scholar]
- 144.Kouo T., Huang L., Pucsek A.B., Cao M., Solt S., Armstrong T., Jaffee E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015;3:412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gordon-Alonso M., Hirsch T., Wildmann C., van der Bruggen P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017;8:793. doi: 10.1038/s41467-017-00925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nambiar D.K., Aguilera T., Cao H., Kwok S., Kong C., Bloomstein J., Wang Z., Rangan V.S., Jiang D., von Eyben R., et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Investig. 2019;129:5553–5567. doi: 10.1172/JCI129025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.You Y., Tan J.X., Dai H.S., Chen H.W., Xu X.J., Yang A.G., Zhang Y.J., Bai L.H., Bie P. MiRNA-22 inhibits oncogene galectin-1 in hepatocellular carcinoma. Oncotarget. 2016;7:57099–57116. doi: 10.18632/oncotarget.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Baker G.J., Chockley P., Zamler D., Castro M.G., Lowenstein P.R. Natural killer cells require monocytic Gr-1(+)/CD11b(+) myeloid cells to eradicate orthotopically engrafted glioma cells. Oncoimmunology. 2016;5:e1163461. doi: 10.1080/2162402X.2016.1163461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Baker G.J., Chockley P., Yadav V.N., Doherty R., Ritt M., Sivaramakrishnan S., Castro M.G., Lowenstein P.R. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res. 2014;74:5079–5090. doi: 10.1158/0008-5472.CAN-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Enninga E.A.L., Harrington S.M., Creedon D.J., Ruano R., Markovic S.N., Dong H., Dronca R.S. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2018;79 doi: 10.1111/aji.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li X., Chen Y., Liu X., Zhang J., He X., Teng G., Yu D. Tim3/Gal9 interactions between T cells and monocytes result in an immunosuppressive feedback loop that inhibits Th1 responses in osteosarcoma patients. Int. Immunopharmacol. 2017;44:153–159. doi: 10.1016/j.intimp.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 152.Chen T.C., Chen C.H., Wang C.P., Lin P.H., Yang T.L., Lou P.J., Ko J.Y., Wu C.T., Chang Y.L. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci. Rep. 2017;7:10349. doi: 10.1038/s41598-017-10386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo Z., Ji Y., Tian D., Zhang Y., Chang S., Yang C., Zhou H., Chen Z.K. Galectin-7 promotes proliferation and Th1/2 cells polarization toward Th1 in activated CD4+ T cells by inhibiting The TGFbeta/Smad3 pathway. Mol. Immunol. 2018;101:80–85. doi: 10.1016/j.molimm.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 154.Benedicto A., Hernandez-Unzueta I., Sanz E., Marquez J. Ocoxin Increases the Antitumor Effect of BRAF Inhibition and Reduces Cancer Associated Fibroblast-Mediated Chemoresistance and Protumoral Activity in Metastatic Melanoma. Nutrients. 2021;13 doi: 10.3390/nu13020686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Giesbrecht K., Former S., Sahr A., Heeg K., Hildebrand D. Streptococcal Pyrogenic Exotoxin A-Stimulated Monocytes Mediate Regulatory T-Cell Accumulation through PD-L1 and Kynurenine. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20163933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y., Gong S., Pan W., Chen Y., Liu B., Li N., Tang B. A tumor acidity activatable and Ca(2+)-assisted immuno-nanoagent enhances breast cancer therapy and suppresses cancer recurrence. Chem. Sci. 2020;11:7429–7437. doi: 10.1039/D0SC00293C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pereira J.X., Azeredo M.C., Martins F.S., Chammas R., Oliveira F.L., Santos S.N., Bernardes E.S., El-Cheikh M.C. The deficiency of galectin-3 in stromal cells leads to enhanced tumor growth and bone marrow metastasis. BMC Cancer. 2016;16:636. doi: 10.1186/s12885-016-2679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stasenko M., Smith E., Yeku O., Park K.J., Laster I., Lee K., Walderich S., Spriggs E., Rueda B., Weigelt B., et al. Targeting galectin-3 with a high-affinity antibody for inhibition of high-grade serous ovarian cancer and other MUC16/CA-125-expressing malignancies. Sci. Rep. 2021;11:3718. doi: 10.1038/s41598-021-82686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rabinovich G.A., Cumashi A., Bianco G.A., Ciavardelli D., Iurisci I., D’Egidio M., Piccolo E., Tinari N., Nifantiev N., Iacobelli S. Synthetic lactulose amines: Novel class of anticancer agents that induce tumor-cell apoptosis and inhibit galectin-mediated homotypic cell aggregation and endothelial cell morphogenesis. Glycobiology. 2006;16:210–220. doi: 10.1093/glycob/cwj056. [DOI] [PubMed] [Google Scholar]
- 160.Yan Y.P., Lang B.T., Vemuganti R., Dempsey R.J. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009;1288:116–124. doi: 10.1016/j.brainres.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 161.Yang R., Sun L., Li C.F., Wang Y.H., Yao J., Li H., Yan M., Chang W.C., Hsu J.M., Cha J.H., et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat. Commun. 2021;12:832. doi: 10.1038/s41467-021-21099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu X., Li J., Connolly E.M., Liao X., Ouyang J., Giobbie-Hurder A., Lawrence D., McDermott D., Murphy G., Zhou J., et al. Combined Anti-VEGF and Anti-CTLA-4 Therapy Elicits Humoral Immunity to Galectin-1 Which Is Associated with Favorable Clinical Outcomes. Cancer Immunol. Res. 2017;5:446–454. doi: 10.1158/2326-6066.CIR-16-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Meromsky L., Lotan R., Raz A. Implications of endogenous tumor cell surface lectins as mediators of cellular interactions and lung colonization. Cancer Res. 1986;46:5270–5275. [PubMed] [Google Scholar]
- 164.Inufusa H., Nakamura M., Adachi T., Aga M., Kurimoto M., Nakatani Y., Wakano T., Miyake M., Okuno K., Shiozaki H., et al. Role of galectin-3 in adenocarcinoma liver metastasis. Int. J. Oncol. 2001;19:913–919. doi: 10.3892/ijo.19.5.913. [DOI] [PubMed] [Google Scholar]
- 165.Ma X., Li X., Shi J., Yao M., Zhang X., Hou R., Shao N., Luo Q., Gao Y., Du S., et al. Host-Guest Polypyrrole Nanocomplex for Three-Stimuli-Responsive Drug Delivery and Imaging-Guided Chemo-Photothermal Synergetic Therapy of Refractory Thyroid Cancer. Adv. Healthc. Mater. 2019;8:e1900661. doi: 10.1002/adhm.201900661. [DOI] [PubMed] [Google Scholar]
- 166.Demotte N., Bigirimana R., Wieers G., Stroobant V., Squifflet J.L., Carrasco J., Thielemans K., Baurain J.F., Van Der Smissen P., Courtoy P.J., et al. A short treatment with galactomannan GM-CT-01 corrects the functions of freshly isolated human tumor-infiltrating lymphocytes. Clin. Cancer Res. 2014;20:1823–1833. doi: 10.1158/1078-0432.CCR-13-2459. [DOI] [PubMed] [Google Scholar]
- 167.Fukaya Y., Shimada H., Wang L.C., Zandi E., DeClerck Y.A. Identification of galectin-3-binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J. Biol. Chem. 2008;283:18573–18581. doi: 10.1074/jbc.M803115200. [DOI] [PubMed] [Google Scholar]
- 168.Glinskii O.V., Huxley V.H., Glinsky G.V., Pienta K.J., Raz A., Glinsky V.V. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005;7:522–527. doi: 10.1593/neo.04646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Delgado V.M., Nugnes L.G., Colombo L.L., Troncoso M.F., Fernandez M.M., Malchiodi E.L., Frahm I., Croci D.O., Compagno D., Rabinovich G.A., et al. Modulation of endothelial cell migration and angiogenesis: A novel function for the "tandem-repeat" lectin galectin-8. FASEB J. 2011;25:242–254. doi: 10.1096/fj.09-144907. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y., Jiang N., Zhang T., Chen R., Feng Y., Sang X., Yang N., Chen Q. Tim-3 signaling blockade with alpha-lactose induces compensatory TIGIT expression in Plasmodium berghei ANKA-infected mice. Parasit. Vectors. 2019;12:534. doi: 10.1186/s13071-019-3788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan L.L., Deng Y.Y., Wang R., Wu C., Li J., Niu W., Yang Q., Bhatia M., Gudmundsson G.H., Agerberth B., et al. Lactose Induces Phenotypic and Functional Changes of Neutrophils and Macrophages to Alleviate Acute Pancreatitis in Mice. Front. Immunol. 2018;9:751. doi: 10.3389/fimmu.2018.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kulkarni R., Prasad A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017;7:14787. doi: 10.1038/s41598-017-14817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Puthenedam M., Wu F., Shetye A., Michaels A., Rhee K.J., Kwon J.H. Matrilysin-1 (MMP7) cleaves galectin-3 and inhibits wound healing in intestinal epithelial cells. Inflamm. Bowel Dis. 2011;17:260–267. doi: 10.1002/ibd.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ochieng J., Green B., Evans S., James O., Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim. Biophys. Acta. 1998;1379:97–106. doi: 10.1016/S0304-4165(97)00086-X. [DOI] [PubMed] [Google Scholar]
- 175.Mirandola L., Yu Y., Chui K., Jenkins M.R., Cobos E., John C.M., Chiriva-Internati M. Galectin-3C inhibits tumor growth and increases the anticancer activity of bortezomib in a murine model of human multiple myeloma. PLoS ONE. 2011;6:e21811. doi: 10.1371/journal.pone.0021811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang M., Tian F., Ying W., Qian X. Quantitative proteomics reveal the anti-tumour mechanism of the carbohydrate recognition domain of Galectin-3 in Hepatocellular carcinoma. Sci. Rep. 2017;7:5189. doi: 10.1038/s41598-017-05419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hoffmann M., Hayes M.R., Pietruszka J., Elling L. Synthesis of the Thomsen-Friedenreich-antigen (TF-antigen) and binding of Galectin-3 to TF-antigen presenting neo-glycoproteins. Glycoconj. J. 2020 doi: 10.1007/s10719-020-09926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Santarsia S., Grosso A.S., Trovao F., Jimenez-Barbero J., Carvalho A.L., Nativi C., Marcelo F. Molecular Recognition of a Thomsen-Friedenreich Antigen Mimetic Targeting Human Galectin-3. ChemMedChem. 2018;13:2030–2036. doi: 10.1002/cmdc.201800525. [DOI] [PubMed] [Google Scholar]
- 179.Sun W., Li L., Li L.J., Yang Q.Q., Zhang Z.R., Huang Y. Two birds, one stone: Dual targeting of the cancer cell surface and subcellular mitochondria by the galectin-3-binding peptide G3-C12. Acta Pharmacol. Sin. 2017;38:806–822. doi: 10.1038/aps.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Griffioen A.W., van der Schaft D.W., Barendsz-Janson A.F., Cox A., Struijker Boudier H.A., Hillen H.F., Mayo K.H. Anginex, a designed peptide that inhibits angiogenesis. Biochem. J. 2001;354:233–242. doi: 10.1042/bj3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thijssen V.L., Barkan B., Shoji H., Aries I.M., Mathieu V., Deltour L., Hackeng T.M., Kiss R., Kloog Y., Poirier F., et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 182.Leung Z., Ko F.C.F., Tey S.K., Kwong E.M.L., Mao X., Liu B.H.M., Ma A.P.Y., Fung Y.M.E., Che C.M., Wong D.K.H., et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J. Exp. Clin. Cancer Res. 2019;38:423. doi: 10.1186/s13046-019-1402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abu El-Asrar A.M., Ahmad A., Allegaert E., Siddiquei M.M., Alam K., Gikandi P.W., De Hertogh G., Opdenakker G. Galectin-1 studies in proliferative diabetic retinopathy. Acta Ophthalmol. 2020;98:e1–e12. doi: 10.1111/aos.14191. [DOI] [PubMed] [Google Scholar]
- 184.Michael J.V., Wurtzel J.G., Goldfinger L.E. Inhibition of Galectin-1 Sensitizes HRAS-driven Tumor Growth to Rapamycin Treatment. Anticancer Res. 2016;36:5053–5061. doi: 10.21873/anticanres.11074. [DOI] [PubMed] [Google Scholar]
- 185.Traber P.G., Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS ONE. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ahmad N., Gabius H.J., Andre S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 187.Harrison S.A., Marri S.R., Chalasani N., Kohli R., Aronstein W., Thompson G.A., Irish W., Miles M.V., Xanthakos S.A., Lawitz E., et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment. Pharmacol. Ther. 2016;44:1183–1198. doi: 10.1111/apt.13816. [DOI] [PubMed] [Google Scholar]
- 188.Harrison S.A., Dennis A., Fiore M.M., Kelly M.D., Kelly C.J., Paredes A.H., Whitehead J.M., Neubauer S., Traber P.G., Banerjee R. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS ONE. 2018;13:e0203054. doi: 10.1371/journal.pone.0203054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chauhan D., Li G., Podar K., Hideshima T., Neri P., He D., Mitsiades N., Richardson P., Chang Y., Schindler J., et al. A novel carbohydrate-based therapeutic GCS-100 overcomes bortezomib resistance and enhances dexamethasone-induced apoptosis in multiple myeloma cells. Cancer Res. 2005;65:8350–8358. doi: 10.1158/0008-5472.CAN-05-0163. [DOI] [PubMed] [Google Scholar]
- 190.Streetly M.J., Maharaj L., Joel S., Schey S.A., Gribben J.G., Cotter F.E. GCS-100, a novel galectin-3 antagonist, modulates MCL-1, NOXA, and cell cycle to induce myeloma cell death. Blood. 2010;115:3939–3948. doi: 10.1182/blood-2009-10-251660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Clark M.C., Pang M., Hsu D.K., Liu F.T., de Vos S., Gascoyne R.D., Said J., Baum L.G. Galectin-3 binds to CD45 on diffuse large B-cell lymphoma cells to regulate susceptibility to cell death. Blood. 2012;120:4635–4644. doi: 10.1182/blood-2012-06-438234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ruvolo P.P., Ruvolo V.R., Benton C.B., AlRawi A., Burks J.K., Schober W., Rolke J., Tidmarsh G., Hail N., Jr., Davis R.E., et al. Combination of galectin inhibitor GCS-100 and BH3 mimetics eliminates both p53 wild type and p53 null AML cells. Biochim. Biophys. Acta. 2016;1863:562–571. doi: 10.1016/j.bbamcr.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang Y., Nangia-Makker P., Balan V., Hogan V., Raz A. Calpain activation through galectin-3 inhibition sensitizes prostate cancer cells to cisplatin treatment. Cell Death Dis. 2010;1:e101. doi: 10.1038/cddis.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Conti S., Vexler A., Hagoel L., Kalich-Philosoph L., Corn B.W., Honig N., Shtraus N., Meir Y., Ron I., Eliaz I., et al. Modified Citrus Pectin as a Potential Sensitizer for Radiotherapy in Prostate Cancer. Integr. Cancer Ther. 2018;17:1225–1234. doi: 10.1177/1534735418790382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Xue H., Zhao Z., Lin Z., Geng J., Guan Y., Song C., Zhou Y., Tai G. Selective effects of ginseng pectins on galectin-3-mediated T cell activation and apoptosis. Carbohydr. Polym. 2019;219:121–129. doi: 10.1016/j.carbpol.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 196.Ma S., Li S., Lv R., Hou X., Nie S., Yin Q. Prevalence of mild cognitive impairment in type 2 diabetes mellitus is associated with serum galectin-3 level. J. Diabetes Investig. 2020 doi: 10.1111/jdi.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tian Y., Lv W., Lu C., Jiang Y., Yang X., Song M. Galectin-3 inhibition attenuates doxorubicin-induced cardiac dysfunction by upregulating the expression of peroxiredoxin-4. Can. J. Physiol. Pharmacol. 2020 doi: 10.1139/cjpp-2019-0700. [DOI] [PubMed] [Google Scholar]
- 198.Xu G.R., Zhang C., Yang H.X., Sun J.H., Zhang Y., Yao T.T., Li Y., Ruan L., An R., Li A.Y. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-kappaB signaling pathway. Biomed. Pharmacother. 2020;126:110071. doi: 10.1016/j.biopha.2020.110071. [DOI] [PubMed] [Google Scholar]
- 199.Li S., Li S., Hao X., Zhang Y., Deng W. Perindopril and a Galectin-3 Inhibitor Improve Ischemic Heart Failure in Rabbits by Reducing Gal-3 Expression and Myocardial Fibrosis. Front. Physiol. 2019;10:267. doi: 10.3389/fphys.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Astorgues-Xerri L., Riveiro M.E., Tijeras-Raballand A., Serova M., Rabinovich G.A., Bieche I., Vidaud M., de Gramont A., Martinet M., Cvitkovic E., et al. OTX008, a selective small-molecule inhibitor of galectin-1, downregulates cancer cell proliferation, invasion and tumour angiogenesis. Eur. J. Cancer. 2014;50:2463–2477. doi: 10.1016/j.ejca.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 201.Yang N., Zhang W., He T., Xing Y. Suppression of Retinal Neovascularization by Inhibition of Galectin-1 in a Murine Model of Oxygen-Induced Retinopathy. J. Ophthalmol. 2017;2017:5053035. doi: 10.1155/2017/5053035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yao Y., Zhou L., Liao W., Chen H., Du Z., Shao C., Wang P., Ding K. HH1-1, a novel Galectin-3 inhibitor, exerts anti-pancreatic cancer activity by blocking Galectin-3/EGFR/AKT/FOXO3 signaling pathway. Carbohydr. Polym. 2019;204:111–123. doi: 10.1016/j.carbpol.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 203.Espelt M.V., Croci D.O., Bacigalupo M.L., Carabias P., Manzi M., Elola M.T., Munoz M.C., Dominici F.P., Wolfenstein-Todel C., Rabinovich G.A., et al. Novel roles of galectin-1 in hepatocellular carcinoma cell adhesion, polarization, and in vivo tumor growth. Hepatology. 2011;53:2097–2106. doi: 10.1002/hep.24294. [DOI] [PubMed] [Google Scholar]
- 204.Lai J., Lu D., Zhang C., Zhu H., Gao L., Wang Y., Bao R., Zhao Y., Jia B., Wang F., et al. Noninvasive small-animal imaging of galectin-1 upregulation for predicting tumor resistance to radiotherapy. Biomaterials. 2018;158:1–9. doi: 10.1016/j.biomaterials.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 205.Ito K., Ralph S.J. Inhibiting galectin-1 reduces murine lung metastasis with increased CD4(+) and CD8 (+) T cells and reduced cancer cell adherence. Clin. Exp. Metastasis. 2012;29:561–572. doi: 10.1007/s10585-012-9471-7. [DOI] [PubMed] [Google Scholar]
- 206.Kuo P., Bratman S.V., Shultz D.B., von Eyben R., Chan C., Wang Z., Say C., Gupta A., Loo B.W., Jr., Giaccia A.J., et al. Galectin-1 mediates radiation-related lymphopenia and attenuates NSCLC radiation response. Clin. Cancer Res. 2014;20:5558–5569. doi: 10.1158/1078-0432.CCR-14-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Su Y.C., Davuluri G.V., Chen C.H., Shiau D.C., Chen C.C., Chen C.L., Lin Y.S., Chang C.P. Galectin-1-Induced Autophagy Facilitates Cisplatin Resistance of Hepatocellular Carcinoma. PLoS ONE. 2016;11:e0148408. doi: 10.1371/journal.pone.0148408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Parray H.A., Yun J.W. Combined inhibition of autophagy protein 5 and galectin-1 by thiodigalactoside reduces diet-induced obesity through induction of white fat browning. IUBMB Life. 2017;69:510–521. doi: 10.1002/iub.1634. [DOI] [PubMed] [Google Scholar]
- 209.Mackinnon A.C., Gibbons M.A., Farnworth S.L., Leffler H., Nilsson U.J., Delaine T., Simpson A.J., Forbes S.J., Hirani N., Gauldie J., et al. Regulation of transforming growth factor-beta1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mukherjee R., Yun J.W. Lactobionic acid reduces body weight gain in diet-induced obese rats by targeted inhibition of galectin-1. Biochem. Biophys. Res. Commun. 2015;463:1311–1316. doi: 10.1016/j.bbrc.2015.06.114. [DOI] [PubMed] [Google Scholar]
- 211.Pereira P.M., Silva S., Bispo M., Zuzarte M., Gomes C., Girao H., Cavaleiro J.A., Ribeiro C.A., Tome J.P., Fernandes R. Mitochondria-Targeted Photodynamic Therapy with a Galactodendritic Chlorin to Enhance Cell Death in Resistant Bladder Cancer Cells. Bioconjug. Chem. 2016;27:2762–2769. doi: 10.1021/acs.bioconjchem.6b00519. [DOI] [PubMed] [Google Scholar]
- 212.Shih T.C., Liu R., Fung G., Bhardwaj G., Ghosh P.M., Lam K.S. A Novel Galectin-1 Inhibitor Discovered through One-Bead Two-Compound Library Potentiates the Antitumor Effects of Paclitaxel in vivo. Mol. Cancer Ther. 2017;16:1212–1223. doi: 10.1158/1535-7163.MCT-16-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fukumori T., Takenaka Y., Yoshii T., Kim H.R., Hogan V., Inohara H., Kagawa S., Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–8311. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Related available websites for datasets generated in this article were noted.