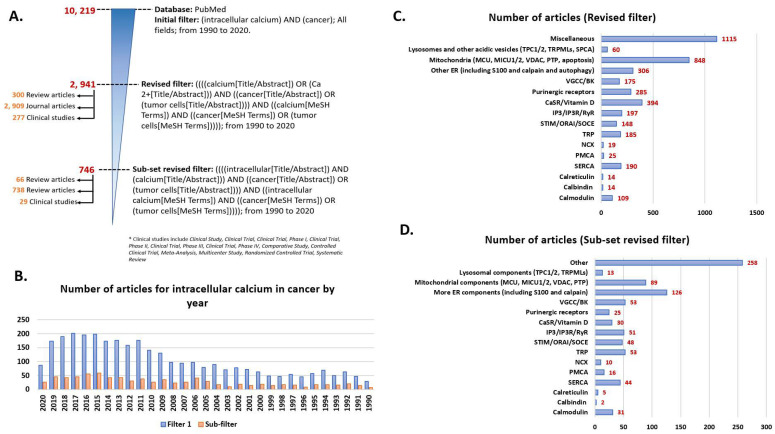
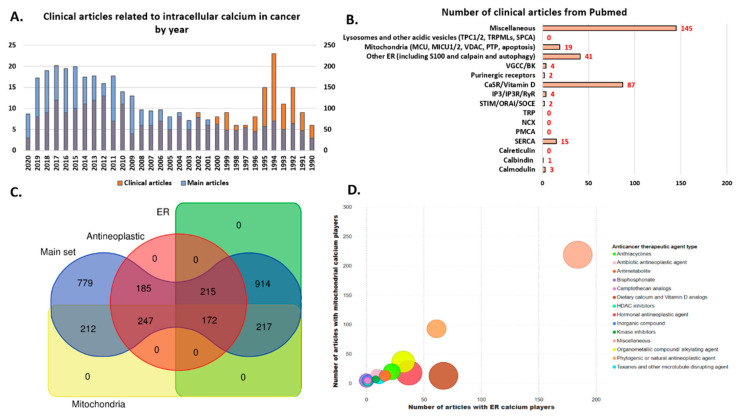
Abstract
Intracellular Ca2+ distribution is a tightly regulated process. Numerous Ca2+ chelating, storage, and transport mechanisms are required to maintain normal cellular physiology. Ca2+-binding proteins, mainly calmodulin and calbindins, sequester free intracellular Ca2+ ions and apportion or transport them to signaling hubs needing the cations. Ca2+ channels, ATP-driven pumps, and exchangers assist the binding proteins in transferring the ions to and from appropriate cellular compartments. Some, such as the endoplasmic reticulum, mitochondria, and lysosomes, act as Ca2+ repositories. Cellular Ca2+ homeostasis is inefficient without the active contribution of these organelles. Moreover, certain key cellular processes also rely on inter-organellar Ca2+ signaling. This review attempts to encapsulate the structure, function, and regulation of major intracellular Ca2+ buffers, sensors, channels, and signaling molecules before highlighting how cancer cells manipulate them to survive and thrive. The spotlight is then shifted to the slow pace of translating such research findings into anticancer therapeutics. We use the PubMed database to highlight current clinical studies that target intracellular Ca2+ signaling. Drug repurposing and improving the delivery of small molecule therapeutics are further discussed as promising strategies for speeding therapeutic development in this area.
Keywords: SOCE, ORAI, STIM, STIMATE, SERCA, PNCA, calmodulin, TRP, IP3R, MCU, VGCC
1. Introduction
Ca2+ is the quintessential ion central to numerous cellular homeostasis and physiological functions. Low hydration energy, high polarizability, relative flexibility of coordination sites and bond length, and large concentration gradient across cellular membranes (100 nM intracellular to 2 mM extracellular) due to low intracellular levels make it the ion of choice at the core of cellular signaling in prokaryotes and eukaryotes alike [1,2]. The mechanisms adopted by cells for intracellular Ca2+ buffering involve sequestration by special proteins [3,4] (Figure 1). Some of these proteins exist in the soluble or non-membranous parts of the cytoplasm within or outside organelles that serve as repositories for Ca2+ ions [3]. Such proteins sequester cytosolic Ca2+ upon sensing an increase in its levels and participate in relaying the associated cellular messages. Other proteins that work as intracellular Ca2+ buffers exist in the lipid bilayers, plasma membrane, or organelle membranes, like pumps or transporters. Apart from these proteins, intracellular Ca2+ is regulated by inter-organellar transport and the influx of Ca2+ ions from extracellular space [5]. In this review, we provide an overview of key components and the associated major mechanisms of intracellular Ca2+ regulation under physiological conditions. It is followed by delineating how these proteins and pathways are manipulated by cancerous cells during tumorigenesis and progression.
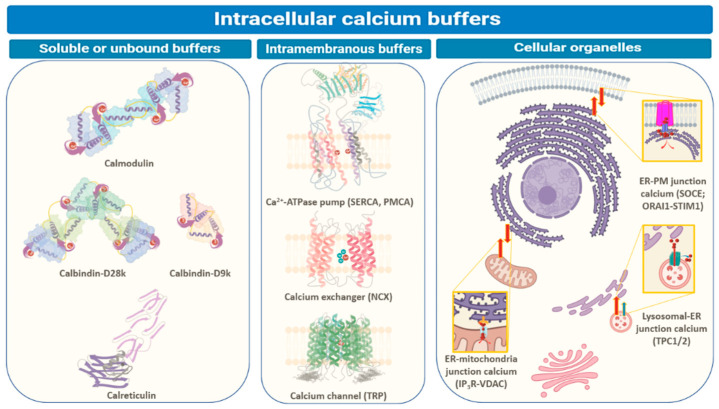
Figure 1.
Types of intracellular Ca2+ buffers.
Intracellular Ca2+ levels are managed through binding to special proteins or sequestration within different cellular compartments. The three main ways by which intracellular Ca2+ is buffered are depicted above—soluble or unbound proteins that are found in the non-membranous parts of a cell (cytosol or inside organelles), membrane-bound or intramembranous proteins (generally Ca2+ channels (like TRP), ATP-driven pumps (SERCA or PMCA), and ion exchangers (NCX)), and organellar compartments such as endoplasmic reticulum (ER), mitochondria, acidic vesicles (mainly lysosomes and Golgi bodies) or organellar junctions (endoplasmic reticulum-plasma membrane (ER-PM), endoplasmic reticulum-mitochondria, or endoplasmic reticulum-lysosomes) [3,4,5]. The major players regulating inter-organellar Ca2+ are mentioned in parenthesis within the cellular organelles section. IP3R, inositol-3,4,5-triphosphate; NCX, sodium-Ca2+ exchanger; ORAI1 (or CRACM1), Ca2+ release activated modulator 1; PMCA, plasma-membrane Ca2+ ATPase; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; STIM1, stromal interaction molecule 1; SOCE (store-operated Ca2+ entry); TPC1/2, two-pore channel; TRP, transient receptor potential; VDAC, voltage-dependent anion channel.
2. Intracellular Ca2+ Buffers in Normal Cells
2.1. Soluble and Unbound Intracellular Proteins: Calmodulin, Calbindin, and Calretinin
The non-membranous proteins inside a cell can act as both Ca2+ sensors and buffers [2,3]. Most of these proteins have EF-hand motif(s) that allows Ca2+ ions to bind and trigger changes in protein folding, thereby influencing downstream or linked cellular pathways [3,4]. Calmodulin (CaM) is one of the best-studied and ubiquitously expressed Ca2+-sensing proteins known to play a key role in intracellular Ca2+ homeostasis [3]. As the prototype for intracellular Ca2+ sensors, its 148 amino acid structure is comprised of two Ca2+-binding sites, each with two EF-hand motifs: N- and C-termini alpha-helices with a Ca2+ coordination loop in between providing affinity for Ca2+ ion docking and sequestration [6]. The ability of CaM to transmit a change in free intracellular Ca2+ levels into modulation of a cellular response comes through its Ca2+-dependent structural flexibility. CaM can exist in a Ca2+-free closed conformational state (Apo-CaM), a semi-open (Ca2-CaM), or an open state (Holo-CaM or Ca4-CaM) after Ca2+-binding [6,7,8] (Figure 2). The latter two conformational states expose hydrophobic residues of this protein, thus allowing it to bind to target or effector molecules and acting as a fast-acting intermediary between change in intracellular Ca2+ and cellular processes. Differential Ca2+-binding on the two lobes of CaM makes fast buffering of a wide range of free intracellular Ca2+ possible for this protein. Analysis of CaM kinetics by Faas et al. has revealed that the N-lobe of CaM acts as the first site for Ca2+-binding during a massive increase in intracellular Ca2+ levels (>100 mM) in a nanodomain [9], whereas the C-lobe, having a higher affinity for Ca2+ than the N-lobe, captures Ca2+ in its EF-hand motifs when the Ca2+ concentration in the pool of intracellular fluid is 1–10 mM and both the motifs have Ca2+-bound to them. The presence of methionine residues in its lobes and plasticity of the central linker in its structure also provides CaM with properties to function as an adaptor protein in intracellular Ca2+ signaling [10]. CaM can bind to several targets or effector molecules over a variable distance and in multiple orientations to mediate change in intracellular Ca2+ signaling. Some major effector proteins that are regulated by CaM binding and are relevant for Ca2+ homeostasis include ORAI, EGFR, PI3K, IQGAP, and connexins [1].
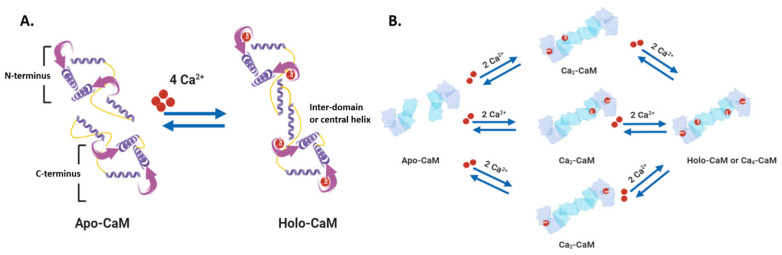
Figure 2.
Overview of calmodulin structure and Ca2+ binding conformations. (A) Calmodulin (CaM) structure comprises four EF-hand structures, two each on N- and C-termini that are connected via an inter-domain helix [6]. CaM exists either as Apo-CaM (no Ca2+ bound to EF-hands) or Holo-CaM (Ca4-CaM or Ca2+ ions bound to all EF-hands); (B) Different intermediate calmodulin conformations (Ca2-CaM) between Apo-CaM and Holo-CaM with Ca2+ ions binding to two EF-hands during each step [8].
Calbindin D-28k is another Ca2+-binding protein with six EF-hand motifs that buffers and transports free cytosolic Ca2+ but, unlike calmodulin, does not act as a linker or adaptor protein in shaping intracellular Ca2+ signaling [11,12]. Additionally, its expression is limited to a few cell types such as mammalian kidney ductal cells, intestinal absorptive epithelia, and neurons. Calretinin or calbindin D-29k, with 58% homology to calbindin D-28k, acts both as a nonlinear Ca2+ buffer and sensor predominantly in the neurons [13,14]. Expressed in the kidney and duodenum epithelial cells, calbindin D-9k or S100G is a monomer comprised of two EF motifs [15]. With no known binding partners, it is only considered a Ca2+ buffer. ER (endoplasmic reticulum) molecular chaperones, calreticulin (in the lumen), and calnexin (on the membrane) are also known to be Ca2+ buffers [5,16].
Physiological relevance: CaM is required for spatial and temporal regulation of [Ca2+]i as evident by its role in modulation (activation or inactivation) of Ca2+ pumps (such as PMCA and SERCA) and Ca2+ channels (such as CaV1.3, TRPV5 and 6, ORAI) [17,18,19]. CaM also acts via serine/threonine kinases known as Calmodulin-activated Kinases (CaMKs) to influence cellular processes like proliferation (for example, centrosome duplication at G1/S or anaphase to metaphase transition via CaMKII) [20]. Calbindin D-28k acts as a Ca2+ buffer proximal to Ca2+ channels like TRPV5 and maintains a steep gradient for ion entry [21].
2.2. Intramembranous Molecular Buffers: SERCA, PMCA, NCX, and TRP
Intra-membrane Ca2+ buffers primarily translocate free Ca2+ between domains and organelles. These mainly comprise ion exchangers, channels, and ATP-driven pumps [22]. SERCA or Sarcoendoplasmic Reticulum Ca2+ ATPase is an ATP-dependent ion pump known to significantly maintain free cytosolic Ca2+ concentration via actively pumping the ion into the endoplasmic reticulum (or sarcoplasmic reticulum in muscle cell). Among the eleven of these P-type ATPase pump isoforms (and variants) recognized so far, SERCA1a and SERCA1b are mainly expressed in adult and neonatal skeletal muscle cells, respectively. SERCA2a is found in cardiomyocytes, while SERCA2b and 2c are expressed ubiquitously. SERCA3 (all the six splice variants) are largely co-expressed with SERCA2b in hematopoietic, endothelial, and epithelial cells. With 85 percent and 75 percent overlap of SERCA2 and SERCA3 primary sequences to that of SERCA1, these isoforms exhibit differential affinity for Ca2+; SERCA3 demonstrates a fivefold lower propensity to bind Ca2+ than other isoforms [23,24,25]. Regardless, the isoforms share a general structure that includes 10-pass transmembrane helices and three cytoplasmic domain lobes (Figure 3A) [26,27]. Two closely spaced Ca2+-binding sites are present on the cytoplasmic side of transmembrane domains. These sites act cooperatively with each other such that the binding of Ca2+ ions to site I increases the binding affinity for site II [27]. Once the two Ca2+-sites are occupied, the cytoplasmic lobes—the nucleotide-binding (N)-domain followed by phosphorylation (P), and actuator (A)-domains—undergo conformational shifts and translocate Ca2+ ions [25,26] (Figure 3B).
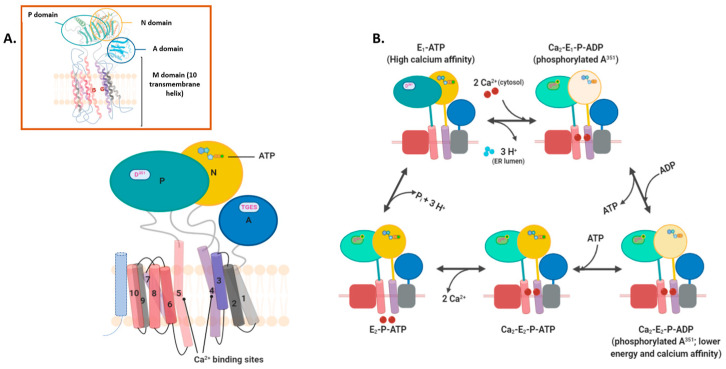
Figure 3.
Sarcoplasmic endoplasmic Ca2+-ATPase structure and mechanism of Ca2+ ion transport. (A) SERCA pump structure is comprised of Actuator or A domain (for dephosphorylation), transmembrane or M domain (10 helices and two Ca2+ ion binding sites between helices 4 and 5), Nucleotide or N domain (source of phosphorylation), and Phosphorylation or P domain (consists of the site of phosphorylation; aspartate 351 residue) [26]. Picture in the inset is a simplified representation of SERCA crystal structure; (B) Numerous steps are involved in the transport of Ca2+ via SERCA across the endoplasmic reticulum membrane [28,29,30,31]. As per the above model, the pump cycle begins with SERCA having a high affinity to Ca2+ in its physiologically predominant E1-ATP state. After cytosolic Ca2+ ions bind to SERCA, the pump transitions into Ca2-E1-P-ADP state utilizing the energy from the transfer of ATP γ-phosphate in the N domain to D351 amino acid residue in P domain. Transport of Ca2+ ions to the ER lumen parallels the shift of pump from its high-energy intermediate conformation to the low energy E2-ATP state (with less affinity to Ca2+; not shown) triggered by replacement of ADP with ATP. Ca2+ ions are then exchanged in the lumen with 2–3 protons compensating for the loss of positive charge. Followed by dephosphorylation at D351. In a Ca2+-free resting stage, the γ-phosphate of ATP in N-domain is masked from phosphorylation site (P domain Asp351) as well as the dephosphorylation site (A-domain TGES sequence) [25,32]. When two cytosolic Ca2+ ions bind to SERCA pump in its higher Ca2+ affinity state (known as E1-ATP), it results in rotational changes within the transmembrane and cytoplasmic domains, thus bringing N-domain ATP closer to Asp351 for transfer of γ-phosphate. From this high-energy intermediate state (Ca2-E1-P-ADP) the pump prepares to transition into a lower energy state, Ca2-E2-P-ADP by completely transferring the γ-phosphate to P-domain and coordinating Mg2+ ions to all the phosphate groups present. In a two-step process again, first ADP is exchanged for ATP in the ER causing appropriate conformational changes to create a channel and exposing the Ca2+-binding residues in its lumen (E2P-ATP). Ca2+ ions are then released in exchange for protons and the pump restores its resting state. ADP, adenosine diphosphate; ATP, adenosine triphosphate; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase.
Although phospholamban (PLN) has been shown to be a stronger inhibitor of the pump, some studies indicate sarcolipin (SLN) to inhibit SERCA at high Ca2+ concentrations [24,28]. Both PLN and SLN are type I transmembrane micropeptides that bind as dephosphorylated monomers (active form) to a groove surrounded by TM2, TM4, TM8, and TM9 of SERCA. SERCA2b being the more widely expressed isoform in non-muscle cells is modulated by other means than PLN and SLN. The inhibitors include another-regulin (ALN; a ubiquitously expressed inhibitor with PLN/SLN key SERCA2b-interacting amino acids), an additional transmembrane helix (TM11), and a cytoplasmic end luminal extension of SERC2b [28] (Table 1).
Table 1.
Sarcoplasmic or endoplasmic Ca2+-ATPase isoforms.
| SERCA Isoform | Tissue Localization | Inhibitor(s) |
|---|---|---|
| SERCA1 | Adult and neonatal skeletal muscles | Myoregulin (MLN), PLN, and SLN |
| SERCA2a | Cardiac muscles | PLN, SLN, miRNA-25 |
| SERCA2b | Non-muscle tissues | ALN, TM11 and its luminal extension |
| SERCA3 | Co-expressed with SERCA2b in endothelial, epithelial, and hematopoietic cells. | Endoregulin (ELN) |
SERCA has three isoforms and multiple splice variants. The table summarizes the localization of major isoforms and splice variants along with a mention of their endogenous inhibitors [29].
P-type Ca2+-ATPases also exist within the plasma membrane and maintain cytosolic Ca2+ levels by transferring them into the extracellular space. The Plasma Membrane Ca2+ ATPases (PMCAs) were earlier known only as housekeeping proteins required for intracellular Ca2+ homeostasis, but some isoforms and splice variants are now known to have a more active role [33,34]. PMCAs transport one Ca2+ ion per ATP molecule which differs from two Ca2+ ions per ATP molecule stoichiometry of SERCA [35]. Four PMCA isoforms are known in mammals, each one with many splice variants [34]. PMCA1 (especially PMCA1b) has a ubiquitous expression with its presence being essential even during embryonic development. PMCA2 and PMCA3 are expressed more selectively in tissues such as the brain, pancreatic β cells, inner ear cells, mammary glands, and the heart. The pattern of PMCA4 tissue expression of overlaps with that of PMCA1, however, its absence does not cause embryonic lethality. The isoforms and variants also differ in the activation/inactivation rates which makes it possible for PMCAs to manage intracellular Ca2+ in the form of fast-acting spikes or slowly released puffs [34,36]. The general structure of such Ca2+ transporters comprises 10 transmembrane segments with large cytosolic loops TM 1–2 and TM 3–4, a cytosolic N- and C-termini tails [34,36,37] (Figure 4A). The first cytosolic loop consists of splice site A (regulates membrane-targeting) and phospholipid-binding sites, the second loop has an aspartyl-containing phosphorylation site, and the C-terminus is strewn with the calmodulin-binding site, splice site C and PDZ domain. The activity of all PMCA isoforms (and variants) is heavily regulated. Short-term regulation of catalytic activity of most of the “b” or splice site 2 variants is mainly calmodulin-dependent [37,38,39,40]. The binding of CaM reverses auto-inhibition of the pump due to conformational shifts which displace C-tail from cytosolic loops. Other means of autoinhibition reversal include phosphorylation of C-tail (Ser/Thr residues) by protein kinase A or C, proteolytic cleavage of C-tail, or dimerization via C-terminus. Change in the localization via interaction with PDZ proteins like MAGUK (membrane-associated guanylate kinase) at the C-tail or transcriptional and translational modulation influences the long-term activity of PMCAs.
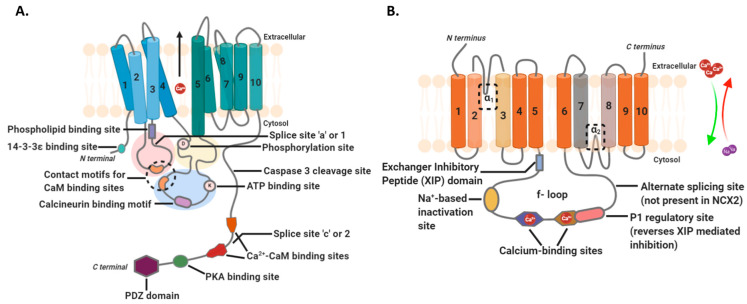
Figure 4.
Structures of intracellular Ca2+ efflux machinery. (A) Plasma membrane Ca2+-ATPase (PMCA) is comprised of cytosolic N- and C-termini, two Ca2+-binding sites (akin to SERCA; not shown here), and 10 transmembrane (TM) segments. Between the N- and C-tails lie three loop structures named like the domains in SERCA—A domain (red circle between TM 2–3), P domain (blue circle between TM 4–5), and N domain (yellow circle between TM 4–5) [34,36,37,38,39,40]. Various binding motifs and regulatory or interaction sites exist on each terminus and cytosolic domain with variations observed in their presence between PMCA isoforms and splice variants. Ca2+-bound calmodulin or Ca2+-CaM is a major regulator for all PMCA family members. The proximity of Ca2+-CaM binding sites on the C-tail to their corresponding motifs in A- and N-domains determine the open or closed states of the pump; (B) Sodium–Ca2+ exchanger (NCX) also has a ten transmembrane topology with N- and C-tails facing the extracellular space [42,43,44,45,49]. The cytosolic side consists of an f-loop (between TM 5–6) that has two Ca2+ ion binding sites, an alternate splicing site, a regulatory site, a sodium ion-dependent inactivation site, and an inhibitory domain. The structure also has two conserved alpha-helical repeats (α1 and α2) that influence ion binding and transport. ATP, adenosine triphosphate; CaM, calmodulin; NCX2, sodium-Ca2+ exchanger 2; PKA, protein kinase A; PDZ, a structural domain; XIP, exchanger inhibitory peptide.
PMCAs also partner with sodium-Ca2+ exchanger (NCX) in some cells to remove Ca2+ from the cytosol [41]. PMCA has high Ca2+ ion affinity and low capacity when compared to NCX that has low Ca2+ ion affinity but a high capacity for ion efflux. This means that PMCA maintains basal cytosolic levels or small bursts of Ca2+ ion entry while NCX is responsible for regulating large but transient increase in intracellular Ca2+. NCX or SLC8 belongs to a superfamily of the Ca2+ ion/cation antiporter gene family. Within the SLC8 family, NCX1, 2, and 3 are the identified functional members encoded by separate genes in mammals [42]. NCX1 is expressed ubiquitously, NCX2 is found in the brain and skeletal muscles, and NCX3 in neurons. Topological analysis of this family based on NCX1 predicts a structure with ten transmembrane alpha-helices. The first five helices form the N-terminus which is separated by a cytosolic loop from the remaining helices forming the C-terminus. The cytosolic loop (500 a.a.) is a regulatory site—a beta repeat region with two Ca2+ ion binding regions, CBD1 and CBD2. CBD1 is the primary site for detecting small changes in cytosolic Ca2+ ion concentration resulting in greater structural changes that activate the exchanger. CBD2 only responds to moderate change in cytosolic Ca2+ ion levels [42,43,44,45] (Figure 4B).
Physiological relevance: Blocking the function of SERCA isoforms can lead to disproportionate Ca2+ levels in the cell cytosol, thus activating apoptosis signals in select cell types. For instance, reduced SERCA2b activity in hepatocytes results in ER stress followed by cell death due to accumulation of excessive cytosolic Ca2+ [46]. PMCA can help maintain the local intracellular Ca2+ ion [Ca2+]i gradient required for cellular motility. Migrating endothelial cells have higher expression of PMCA at their leading edges to maintain low basal [Ca2+]i levels thereby, preventing continued activation of Myosin Light Chain Kinase (MLCK) and extended contraction of the cell membrane at the migration frontier [47]. On the other hand, NCX1 inhibition and thus impairment of [Ca2+]i extrusion allows the proliferation of pancreatic beta cells [48].
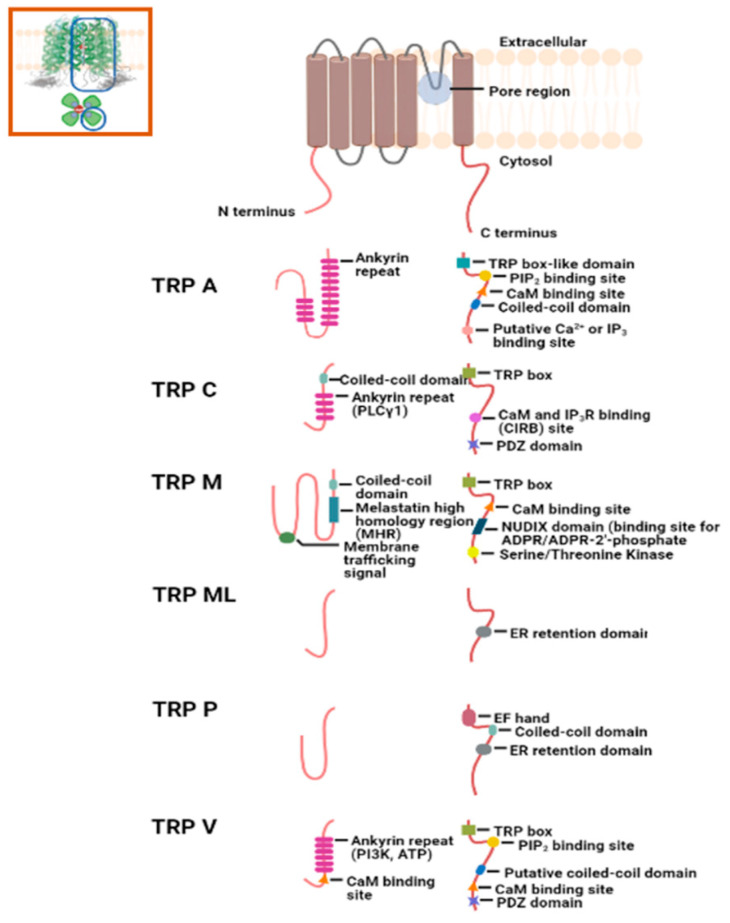
Initially ascribed to Ca2+ homeostasis in sensory neurons, Transient Receptor Potential (TRP) channels have lately been appreciated for a similar function in epithelial and immune cells [50]. Mammalian TRP channel superfamily is composed of 28 family members belonging to six subfamilies—TRPC (Canonical), TRPA (Ankyrin), TRPM (Melastatin), TRPV (Vanilloid), TRPP (Polycystin), and TRPML (Mucopilin)—that differ in their sensitivity to various sensory stimulations and affinity for cations (including Ca2+ ions) sequestration [51]. Commonly, TRP family members share a structure with six transmembrane domains, intracellular N- and C-termini, and a pore-forming TM 5–6 loop [52] Figure 5. Cation selectivity of a TRP channel is determined via an extracellular portion of the pore-forming TM 5–6 helices [51,53]. To have a functional channel for Ca2+ transport, TRP members form homo- or hetero-tetramers within and across the subfamily members [54]. The N-terminus of each tetramer subunit of a TRP channel along with corresponding transmembrane helices are associated with channel assembly and pore regulation [55]. The N-terminus within and across each TRP subfamily varies in the presence and number of ankyrin repeats; having such repeats in general at the amino end provides a site for protein-protein interaction or ligand binding [51,56,57]. The intracellular C-terminus of each subunit is a site for protein interaction and post-translational modification. It also brings structural and functional diversity between subfamilies [52,53,58]. For example, TRPC, TRPV, and TRPM subfamilies share a C-terminus TRP box motif—a short cytosolic hydrophobic stretch at the end of the transmembrane domain putatively holding the channel in a closed conformation [53,59]. Moreover, the C-tail of these channels can have PDZ protein binding domains (TRPV and C), sites for interaction with G-proteins (Gq/11)/calmodulin/PLCβ, ADP-ribose binding (NUDIX; TRPM2), or PLC-interacting kinase (PLIK; TRPM6 and 7) domains [59] (Table 2).
Figure 5.
Structure of TRP channel isoforms. The general topology of a TRP channel includes a homo- or hetero-tetrameric formation (see inset). The basic structure of each subunit consists of cytosolic N- and C-termini, and six transmembrane domains with the pore region between TM 5–6. A variable number of ankyrin repeats on the N-terminus are found in TRP A, TRP C, and TRP V. No specific interaction site or functional domain has been identified so far on the N-terminus of TRP ML and TRP P. The most common domains found on the C-terminus across TRP isoforms are TRP box, coiled-coiled domain, and calmodulin (CaM) binding site [59,60,61,62]. ADPR, adenosine diphosphate ribose; CIRB, calmodulin and IP3R binding site; NUDIX, nucleoside diphosphate-linked moiety X; PI3K, phosphoinositide 3-kinase; PIP2, Phosphatidylinositol 4,5-bisphosphate; PLCγ, phospholipase C gamma.
Table 2.
Protein interactions of TRP channel isoforms.
| TRP Isoform | Protein Interactors |
|---|---|
| TRP A | PIP2, AKAP 79/150, Ca2+-CaM, TRPV1, Sig-1R |
| TRP C | CaBP1, CaM, Caveolin1, Homer, Immunophilins, IP3Rs, Junctate, Junctophilin, NCX1, PLCγ1 RhoA, Stathmin, VAMP, ZO-1, actin cytoskeleton (myosin, ERM proteins, NHERF, etc.), focal adhesion kinase, contactin, Src, STIM, ORAI, RyR, TRPV4, TRPP1, TRPM4 |
| TRP M | 14-3-3γ, 5HT1B, AKAP5/150, CaM, PKCα, PTPL1, Rac1, S100A10, Sig1R, TRPC3, RACK1, ENAC, Synaptotagmin1, α-actin, Myosin heavy chain, Annexin 1, Gαq |
| TRP ML | PDCD6, Cdc42, HSP40, HSP90, Rho1, Rac1, Rac2, RhoG, TPC1, TPC2, TRPV5 |
| TRP P | EGFR, eIF-2α, Filamin-A, HDAC6, IP3R1, IP3R3, PERK, α-actinin, PKD1, PKD1L1, RACK1, PLCγ, Troponin I, Tropomyosin, TRPC (1–4), TRPV4, RyR2, |
| TRP V | AKAP5/150, Calbindin D-28k, CaM, Caveolin1, Cyclophilin B, CAMKII, E-cadherin, EGFR, e-NOS, GABARAP, Fyn, F-actin, IP3R3, Klotho, Lck, Lyn, Myosin, α-integrin, α-tubulin, PKC, PPARα, S100A10, Src, TMEM16A, TRPA1, TRPC1, TRPML3, TRPP1 |
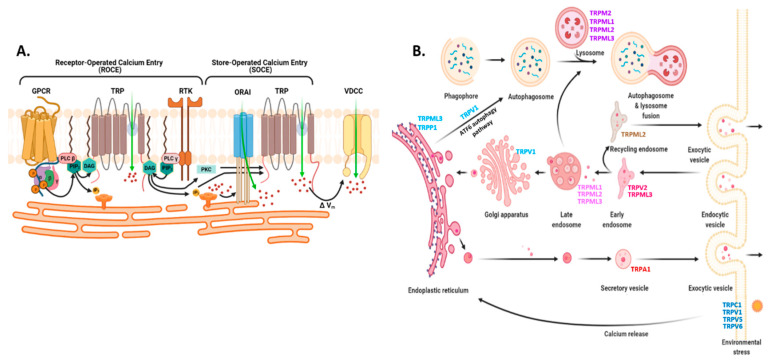
Physiological relevance: On a high level, TRP channels act as activators, integrators, as well as downstream effectors of Ca2+ signaling at the plasma membrane and in intracellular compartments [54,55,69] (Figure 6). Almost all TRP channels permeable to Ca2+ ions (other than TRPM4 and 5), can directly activate intracellular Ca2+ signaling [51]. Taking TRPC as an example, many members of this subfamily are activated by DAG (Diacylglycerol) which is produced by PLC β- or γ-mediated cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) after the ligand binding at GPCRs or RTKs [59]. Being a family of non-selective cation channels reacting to numerous stimuli, TRP channels can also indirectly influence the activation of receptor-operated bulk entry of Ca2+ from the extracellular space [58]. TRPP1/2, TRPA1, TRPM8, and TRPV1-4 are all expressed on the ER membrane [54,70]. At this site, PLC-independent activation of the TRP channels (such as TRPV1) is suggested to induce ER Ca2+ release via inositol triphosphate receptor (IP3R) which further triggers bulk entry of extracellular Ca2+ into the cell (discussed further in a later section) [71,72,73,74]. On the flip side, cytosolic Ca2+ regulates the activity of TRP channels in response to physiological stimuli. This regulatory effect is usually through CaM binding (inhibition of TRPV5, TRPV6, and sensitization of TRPV3) and indirectly through CaM-binding kinase II (CaMKII) [69]. Among the extra-neuronal TRP channels, TRPA1, TRPC1, TRPM8, TRPV1, and TRPV4 have been recognized for their role in epithelial and immune cell Ca2+ homeostasis [50,75]. By regulating intracellular Ca2+, some of these channels like TRPV1 and TRPM8 which are expressed in human bronchial and lung epithelium, respectively, aid the release of chemoattractants and promote immune cell–epithelial cell interaction.
Figure 6.
Many roles of TRP channels in intracellular Ca2+ homeostasis. (A) TRP channels drive Ca2+ influx either through Receptor-Operated Ca2+ Entry or Store-Operated Ca2+ Entry. ROCE requires activated GPCRs or Receptor-tyrosine kinases to initiate phospholipase C beta (PLC β) or PLC γ mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol 1,4,5-triphosphate (yellow circles) after which DAG binds to a TRP channel to stimulate the inflow of Ca2+ ions. IP3 generated via PIP2 hydrolysis triggers a reduction in Ca2+ ion levels in the endoplasmic reticulum by simultaneously promoting the release of the cations through IP3 receptors (IP3Rs) [54,63,69,71,72,76,77,78]. This results in the formation of SOC complex (ORAI-STIM or ORAI-TRP-STIM) for transporting Ca2+ ions from extracellular space into the cytosol. Some TRP isoforms can also be activated by direct binding of protein kinase C that triggers Ca2+ influx (or sodium influx) followed by membrane depolarization leading to more Ca2+ influx via the opening of voltage-dependent Ca2+ channels [54]. Other TRP isoforms (especially expressed in muscle cells) can directly induce Ca2+ release from IP3Rs on the ER membrane (Ca2+-induced Ca2+ release or CICR; not shown); (B) TRP channels are expressed on intracellular membranes as well where they play an active role in the regulation of Ca2+ ion concentration during processes such as autophagy, endocytosis, and exocytosis (58,61,75). DAG, Diacylglycerol; GPCRs, G-protein coupled receptors; IP3, inositol 1,4,5-triphosphate; ORAI (Ca2+ release activated Ca2+ modulator; RTKs, Receptor-tyrosine kinases; ROCE, Receptor-Operated Ca2+ Entry; SOC, store-operated Ca2+; SOCE, Store-Operated Ca2+ Entry; STIM, stromal interaction molecule; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C; TRP, transient receptor potential; VDCCs, voltage-dependent Ca2+ channels.
2.3. Cellular Organelles
2.3.1. Endoplasmic Reticulum: STIM, ORAI, IP3Rs, and TRPC1 in SOCE and SOCIC Ca2+ Entry Models
The ER serves as the largest and most dynamic organelle reservoir for intracellular Ca2+ and is therefore central to an array of cell signaling processes for protein synthesis, folding, and post-translational modifications [79,80,81,82]. In contrast to the cytosol, ER Ca2+ ion levels can range from 100 uM to 1 mM based on the cell type [72,80]. ER and other intracellular organelles buffer excessive cytosolic Ca2+ by both housing Ca2+-binding proteins (example: calreticulin in ER) and via active transport (example: SERCA pumps in ER) [5,79,80]. Depletion of Ca2+ from the ER lumen actuates an indirect mode of Ca2+ entry into the organelle which is termed Store-Operated Ca2+ Entry (SOCE) or Ca2+ Release Activated Ca2+ (CRAC) entry; it is activated when plasma membrane receptors like PLC-coupled GPCRs (but not voltage-gated channels) trigger Ca2+ ion release from the organelle [83,84]. Exhaustion of the intraluminal ER Ca2+ ion store following such prolonged release is then sensed by STIM (Stromal Interaction Molecule) tethered to the ER membrane and subsequently relayed to the CRAC channels on the plasma membrane.
Physiological relevance: SOCE and its key players participate in multiple normal cellular processes that go awry during cancer progression. For example, siRNA mediated downregulation of STIM1 and ORAI1 in keratinocytes impairs cellular differentiation [85], whereas SOCE is found to be inactivated during mitosis [86]. By contrast, stimulation of IP3Rs (and RyRs) promotes cell cycle progression of stem cells, pancreatic beta cells, renal cells, and more.
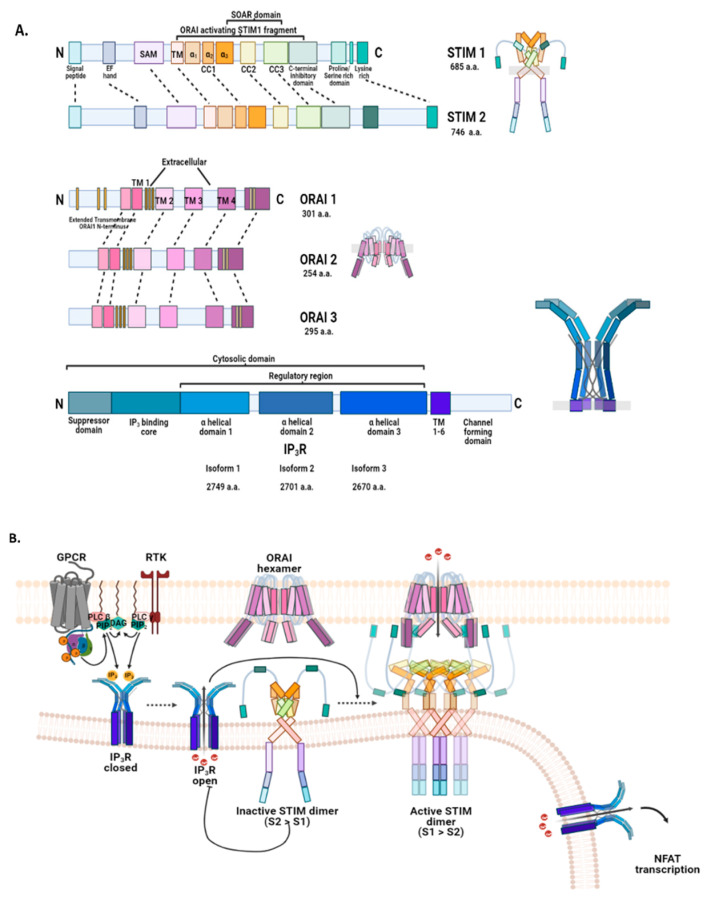
STIM, a type I transmembrane protein, was originally identified during a search for transmembrane and secretory proteins in stromal and pre-B lymphocytes [84,87]. The role of STIM in [Ca2+]i signaling was later confirmed by independent work of two groups using high-throughput RNAi screens to identify inhibitors of thapsigargin evoked CRAC current (ICRAC) [88,89,90]. Both the isoforms, STIM1 and STIM2 have a luminal N-terminus and a cytoplasmic C-terminus [91]. Starting at the N-terminus, the basic STIM structure is comprised of EF-hand motifs (canonical and non-canonical) and a sterile α-motif (SAM), all of which help in intraluminal Ca2+ ion sequestration [83,88]; Figure 7A. The C-terminus has various coiled-coiled domains (CC1-3) that include CRAC activating domain (CAD; also known as STIM-ORAI activating region (SOAR)), and a polybasic site (PBS). The cytoplasm-facing C-terminus of STIM is essential for localization to ER-PM junctions and for interaction with CRAC channels. Even with greater than 74 percent sequence similarity, the STIM isoforms perform differently as ER Ca2+ sensors—the Ca2+ affinity of EF-SAM domains being greater for STIM1 (200 mM; 400 mM for STIM2) while the time to form oligomers for plasma membrane CRAC channel association is nearly 70 times longer for STIM2 [84,91,92,93]. This makes STIM2 more suitable for the regulation of basal ER Ca2+, although only a small contribution to SOCE was recorded in some studies [54,76]. In fact, STIM2 has been shown to inhibit STIM1-mediated SOCE [94]. With the expression of STIM1 on both the plasma membrane and the ER membrane, the difference between the cellular localization of these isoforms also highlights their functional disparity [90].
Figure 7.
Major molecules in store-operated Ca2+ entry. (A) STIM, ORAI, and IP3Rs are central to the influx of Ca2+ ions from extracellular space into cytosol re-quired for replenishing the depleted intracellular stores. In humans, STIM has two isoforms. Both, STIM1 and STIM2 have a type I transmembrane structure comprised of a signal peptide at the N-terminus for localization to the ER, an EF-hand motif, and a sterile alpha motif for binding of luminal Ca2+ ions. SAM is also a site for protein–protein interactions), and a short transmembrane segment followed by three coiled-coiled domains (CC1, CC2, and CC3, that form ORAI-activating STIM fragment or OASF). CC2-CC3 region (or SOAR) is adequate as well for STIM–ORAI interactions [106,107,108,109]. The ORAI interacting sites on the domain are hidden in inactive STIM dimers. A Ca2+-dependent inhibitory domain, a proline/serine-rich region (polybasic; PBS), and a lysine-rich tail segment are found at the C-terminus. ORAI channels have three isoforms in humans. All the ORAI isoforms have an N-terminal ETON segment (cytosolic), four transmembrane domains, and a cytosolic C-terminus. The structure between TM 1–2 and TM 3–4 is exposed to the extracellular space. The yellow vertical bars depict regions of amino acid residues (positive, negative, hydrophobic, or special) that influence ORAI channel pore selectivity. ORAI channel exists as a hexamer that is formed by oligomerization of 3 dimer subunits. Inositol 1,4,5-triphosphate receptor subunits have most of their structure (5 domains) exposed to the cytosol. Each IP3R is formed of four subunits [110,111,112,113]. The transmembrane segments and the C-terminus are involved in pore-forming, gate-keeping, and tetramerization; (B) Subsequent to the activation of IP3Rs on the ER membrane by ligand IP3 and cytosolic Ca2+, STIM dimers are activated once the luminal Ca2+ concentration drops below basal levels [114,115,116,117,118,119]. The coiled-coiled domains experience a shift in their conformation, thus exposing the SOAR/OASF region for interaction with ORAI channels on the plasma membrane [120]. STIM-ORAI functional coupling for SOCE pore formation requires 3 STIM dimers interacting with one ORAI hexamer [87]. STIM dimer composition and function vary under different cellular states. Under resting or physiologically inactive state (or with low stimuli), STIM dimers are largely composed of STIM2 (S2) which has an inhibitory effect on IP3R activation. As the activation stimuli become stronger and intracellular store gets depleted of Ca2+, STIM dimers composed mostly of STIM1 takeover to participate in SOCE. IP3Rs are also expressed on parts of ER membrane away from the ER-PM microdomain involved in SOCE. These receptors provide intracellular Ca2+ ions for downstream Ca2+ signaling including NFAT-mediated transcription. Red semi-circle in the ER represents high luminal Ca2+ levels, pink semi-circle is for moderately low Ca2+ ion concentration, and pale semi-circle indicates extremely low Ca2+ concentration. CC, coiled-coil; NFAT, nuclear factor of activated T-cells; SAM, sterile alpha motif; SOAR, STIM1 Orai1-activating region; TM, transmembrane.
A year after STIM molecules were recognized as ER Ca2+ ion sensors, ORAI1 was confirmed as Ca2+ Release-Activated Ca2+ Modulator 1 (CRACM1) or simply, the CRAC channel [84,95]. Genome-wide RNAi screening, linkage analysis, and positional cloning isolated and identified ORAI1 as the mutated gene responsible for severe combined immunodeficiency in humans (with low ICRAC in T lymphocytes). Three isoforms of ORAI proteins exist in mammals—ORAI1, ORAI2, and ORAI3 [95,96]. Topologically, an ORAI channel-forming monomer has four transmembrane domains forming two intracellular (I-II, III-IV) and one extracellular peptide loop (II-III) [97,98] (Figure 7A). Both N- and C-termini are cytoplasmic with each having a STIM1-binding site or CRAC activation domain (CAD; 73–91 a.a. on amino side and 268–291 a.a. on the carboxyl side of ORAI1). A Ca2+-binding site is present at E106 that resides in the conserved TM1 segment. The three isoforms have high sequence homology in the transmembrane domains (92%) but only 62 percent overall due to some differences in the C-terminus (coiled-coiled domain) and III-IV loop. This results in variations between the isoforms in terms of activation time, Ca2+ ion-dependent inactivation kinetics, activation, or inhibition by 2-aminoethoxydiphenyl borate, affinity for STIM1, redox sensitivity, and activation by STIM2 [96,99,100].
The mechanistic model of SOCE has evolved over the last two decades. Going by the widely accepted “interaction model” or “diffusion trap model”, STIM1 exists as a dimer in resting (inactive) state where the Ca2+-bound canonical EF-hand, the non-canonical EF-hand, and SAM domain on each monomer together impart structural stability [95,99,100] (Figure 7B). It is complemented by the conformation of the cytosolic segment that keeps the ORAI-binding CAD domain, and the plasma membrane interacting polybasic C-tail hidden away. Depletion of unbound ER Ca2+ then triggers the freely diffusing STIM1 dimers on the ER surface to lose Ca2+ ions from cEF; this causes a conformational change on the C-termini exposing STIM CAD domains and extending the polybasic tails to bind ORAI hexamers and PIP2, respectively, at the ER-PM junction. Some studies have recognized STIM1 expressed on PM along with ORAI1 as key players for a store-independent, arachidonic acid-regulated Ca2+ current in association with ORAI3 [101,102]. Nonetheless, the function of plasma membrane STIM1 is debatable to date.
The release of intraluminal ER Ca2+ that evokes an influx of extracellular Ca2+ via ORAI1-STIM1complex at the ER-PM puncta is best known to be mediated by activated IP3R [103,104]. Certain types of ligand-stimulated GPCRs and RTKs generate IP3 and DAG as secondary effectors; the IP3 molecules then bind to its receptors expressed on the ER surface. Sampieri and Vaca et al. discovered IP3Rs to be spatially and functionally associated with STIM1 localized at the puncta [105]. In their study, activated IP3Rs immediately localized to STIM1 to form a complex that allowed the cEF domain of the latter to effectively sense nearby intraluminal Ca2+ depletion. Furthermore, overexpression of IP3Rs was shown to enhance the SOCE Ca2+ influx. Contrastingly, IP3Rs remain inactive during ER Ca2+ leak due to SERCA pump inhibition. Consequently, Ca2+ ion depletion around STIM1 cEF is slow and ineffectivewhich leads to smaller ORAI1 currents.
Mikoshiba et al. were the first to identify and characterize IP3Rs in Purkinje cells of cerebellar mutated mice as binding sites for the second messenger, IP3 [121]. Three members of this receptor have been identified in mammals all of which share a 2700 amino acid structure composed of six transmembrane segments and five distinct domains—IP3-binding suppressor/coupling domain at N-terminus, IP3-binding domain, internal modulatory/coupling domain, Ca2+ pore-forming transmembrane domain, gate-keeper domain at the C-terminal [122] (Figure 7A). Four IP3R subunits, each with the five domains, come together to form a functional IP3R in a homomeric or heteromeric fashion. Other than the pore-forming domain, the majority of the IP3R structure is extracellular (Figure 7A). The affinity of the IP3-binding domain for IP3 is similar between the isoforms. However, the binding affinity is modified by the N-terminal coupling domain [123]. It has been now revealed that the 225 a.a. segment in the N-terminus of IP3R1 decreases the binding of IP3 to the receptor-binding site by directly interacting with it. Different studies agree that IP3R2 is the most sensitive and IP3R3 is the least sensitive for IP3 docking [122]. The N-terminal domain also allows for receptor regulation via proteins like HOMER, CaM, Ca2+ Binding Protein 1 (CaBP1), Ankyrin, and IRBIT (IP3R binding protein released with inositol 1,4,5-trisphosphate) [124,125,126,127,128,129]. IP3 and Ca2+ ions are the main regulators of IP3R channel activity [123]. Each subunit of the functional IP3R tetramer has a binding site for IP3 and the predominant observation is that channel opening requires occupancy of more than one but not all four IP3-binding sites. N-terminal residues 1–223 and internal modulatory domain residues 651–1130 have been shown to be essential for coupling IP3 binding to channel opening [130]. Studies have found IP3 docking to also be necessary for IP3 receptor cluster formation in the ER membrane for localized Ca2+ release and SOCE activation [131,132]. Regulation by cytosolic Ca2+ ions is biphasic; a minor increase in its concentration accelerates channel activity in response to IP3 while higher levels inhibit channel opening [72,122,133]. Purportedly, intraluminal Ca2+ influences IP3 binding to the receptor as well with high levels sensitizing the ligand–receptor interaction and vice versa [133]. IP3R-mediated Ca2+ release has another positive regulator, OAG (1-oleoyl-2-acyl-sn-glycerol) that indirectly amplifies the channel activity by increasing IP3 production through the PLC pathway [134].
Physiologically, intracellular Ca2+ release by IP3R and store refilling by SOCE occur less in phasic format and overlap to quite an extent [88]. This means that in between the IP3-dependent Ca2+ ion leaks, closure of IP3Rs, and initiation of Ca2+ influx by SOCE, there is a time window when some IP3Rs are active and releasing Ca2+ while ion influx by STIM-ORAI complex and SERCA pump-mediated ER store repletion are occurring. The active IP3Rs in that duration are, however, not clustered the ER-PM junction and thereby contribute to differential activation of SOCE downstream effectors situated near puncta but away from the Ca2+ influx nanodomain [88,98]. Apart from IP3R, the translocon, which is a complex of proteins that help newly formed polypeptides having signaling sequence to be transported from cytosol to ER, acts as another Ca2+-leak channel [135]. When bound to a ribosome, a translocon complex forms a leak pore of 4–5 nm in size that is permeable to intraluminal Ca2+ during the resting state of ER.
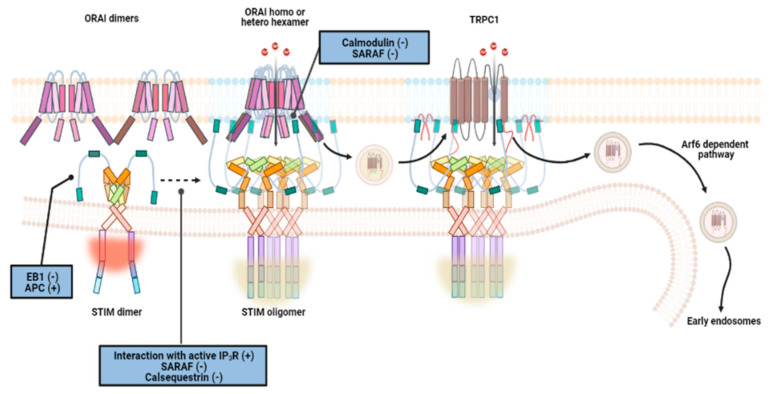
Newer studies have provided evidence for more proteins besides STIM, ORAI, and IP3R to be participants in store-operated Ca2+ entry. Luis Vaca was the first to describe a protein complex termed Store-Operated Ca2+ Influx Complex or SOCIC that involves many key proteins such as TRPC1, SERCA, and microtubule end-tracking protein, EB1 [136]. The quintessential CRAC channels required for SOCE have a voltage-independent, highly Ca2+ ion-selective, inwardly rectifying (reversal potential near 40 mV), low amplitude (6 fA) current at physiological negative membrane potential which is blocked by low concentration of lanthanides and a high dose of 2-APB (2-aminoethoxydiphenyl borate) [137]. On the other hand, the delayed global Ca2+ influx mediated by SOCIC complex that simultaneously utilizes TRPC as its ion channel generates a current amplitude up to 1–2 pA [84]. TRPCs were originally speculated to be CRAC channels [138]. Typically, electrophysiological properties of TRPC homomers include an inwardly rectifying (reversal potential about 15 mV) Ca2+ ion-selective current that is activated by store depletion (via thapsigargin or IP3 inclusion in micropipette during whole-cell patch clamp) and inhibited by lanthanides as well as 2APB [139]. Experimental evidence has also demonstrated a decline in SOCE response corresponding to downregulation or no expression of endogenous TRPCs in vitro and in vivo, respectively. Nonetheless, certain dissimilarities in their current properties with that of ICRAC made the role of these channels in SOCE debatable. Presently, TRP channels are known as the key components of receptor-operated Ca2+ entry (ROC) where these channels open for Ca2+ entry in response to increased DAG levels from PIP2 hydrolysis upon Gq-coupled receptor activation [139]. However, with the proposed SOCIC model and some recent studies, the contribution of TRPC channels to ISOC currents is beginning to unravel [136,139].
The SOCIC model is based on the findings that TRPC-mediated Ca2+ influx is dependent on several interactors [138]. STIM1 is a major interactor and regulator of TRPC expressed within the ER-PM junctions. Vesicular TRPC1 is positioned near ER–PM junctions in unstimulated cells with the help of Rab4-dependent fast-recycling endosomes [140]. Store depletion and subsequent Ca2+ entry by ORAI1-STIM1 complexes translocate vesicular TRPC1s into the proximity of ORAI1-STIM1 clusters present on the cell surface. At this point, critical interaction between TRPC1s and caveolin-1 allows insertion of these channel proteins into cholesterol-rich lipid rafts in the junctions, thereby placing them proximal to ORAI1 for subsequent activation by Ca2+ influx. To be activated and participating as a store-operated Ca2+ channel, TRPC1 in the lipid rafts dissociates from caveolin-1 before engaging STIM1. STIM1 gates TRPC1 through the interaction of lysine residues at 684–685 a.a. in its polybasic tail with the aspartate residues at 639–640 a.a in the tetrameric channel protein. Other regulatory interactions between these proteins involve STIM1 ERM and SOAR domains, and TRPC1 coiled-coiled domains. Following a sustained and global Ca2+ release inside the cells, TRPC1 dissociates from STIM1 to re-associate with caveolin-1 for internalization into Rab5-linked early endosomes (via Arf6-dependent pathway). Due to the experimental proof of direct interaction between ORAI1-TRPC1-STIM1 shown by coimmunoprecipitation, Luis Vaca et al. considered TRPC1 to be the pore-forming component and the surrounding ORAI1 to be the regulator in their proposed SOCIC model [136]. Lately, studies have diverged from this model with mounting evidence supporting the formation of distinct but interacting ORA1-STIM1 and TRPC1-STIM1 Ca2+ ion conducting pores within the lipid rafts [139,141]. Using the new model, ORAI1-STIM1 based Ca2+ entry explains the “oscillatory” changes in intracellular levels of the ion whereas, the larger scale intracellular Ca2+ modulations are attributed to TRPC1-STIM1 associated influx. Physiologically, ICRAC activates proteins like calcineurin that promote translocation of NFAT and subsequent gene expression that is distinct from activation of NFκB based transcription triggered by SOCIC complex (ORAI-STIM1 + TRPC1-STIM1)-mediated ISOC.
Knockdown and mutation analyses reveal that the TRPC1-mediated store-operated Ca2+ currents are very much dependent on the expression and normal function of both STIM1 and ORAI1 [141]. Scaffolding proteins (example: Homer1), junction stabilizing proteins (example: Junctate, junctophilins (JP), and extended synaptotagmins (E-Syts)), vesicle-membrane fusion protein (example: synaptosome-associated protein (SNAP-25)), and STIM1 inhibitor protein called SARAF (store-operated Ca2+ entry (SOCE)-associated regulatory factor) also regulate TRPC1-mediated store-operated Ca2+ entry [142,143]. Some SOCIC modulators directly impact ORAI1 and STIM1 function too. For instance, Homer1 (cytoplasmic) and junctate proteins coimmunoprecipitate and apparently interact with both ORAI and STIM1, thereby promoting the SOCE complex formation [139]. Similarly, CLCA2 or human chloride channel accessory protein 2, a putative tumor suppressor expressed on the cell surface known to enhance SOCE response, colocalizes and coimmunoprecipitates with ORAI1 and STIM1 [144]. STIMATE (STIM-Activating Enhancer) or TMEM110, an ER-resident protein that colocalizes with STIM1 positively impacts SOCE in a two-prong manner. It modulates STIM1-ORAI1 mediated Ca2+ signaling by promoting STIM1 translocation to ER-PM junctions and via stabilizing the puncta [145,146]. By contrast, proteins such as CaM (cytoplasmic) and SARAF (ER membrane) directly interact with the ORAI1-STIM1 complex that causes suppression of SOCE response by ORAI1 inactivation (both) and deoligomerization of STIM1 (SARAF only) [141]. The revised SOCIC-based current model for SOCE is thus, visualized, to begin with, ER store depletion that results in the release of STIM1 dimers from the impact of regulators like SARAF and calsequestrin [141,147,148] (Figure 8). As the “free” STIM1 dimers oligomerize, ORAI1 molecules cluster into homo or hetero hexamers in plasma membrane domains that are low in PIP2 and cholesterol. ORAI1 clustering triggers the recruitment of TRPC1 tetramers from proximal vesicles into the lipid raft domains. In coordination with the ER-PM junctions coming closer and being stabilized by the tethering proteins (such as junctates, junctophilins (JPH3, JPH4), and E-Syt1), the STIM1 oligomers form independent complexes with the ORAI1 and TRPC1 clusters. APC (adenomatous polyposis coli) facilitates this localization of STIM1 into ER-PM puncta by dissociating the ER Ca2+ sensor from microtubule end-tracking protein, EB1. Subsequently, the ORAI1-STIM1 and TRPC1-STIM1 complexes move into cholesterol and PIP2 rich domains to activate ICRAC and ISOC for replenishing Ca2+ stores.
Figure 8.
Store-operated Ca2+ entry with TRPC1. Per the store-operated Ca2+ influx complex (SOCIC) model, Ca2+ influx mediated via STIM-ORAI complex has oscillatory patterns whereas bulk cellular entry of Ca2+ ions is mediated via TRPC1-STIM1 complex [122,124,146]. As described in the model, depletion of Ca2+ ions from the luminal space of the endoplasmic reticulum promotes oligomerization of STIM dimers on ER membrane [105,142,147,148]. Various STIM interacting proteins regulate this process including SARAF, calsequestrin, and activated IP3R. The symbol opposite each of these proteins in the figure depicts the outcome of their interaction with STIM on its oligomerization. As regulators of STIM insertion in the ER membrane, EB1 and APC indirectly influence STIM-ORAI puncta formation as well [136]. ORAI subunits also translocate from low lipid regions (yellow) on the plasma membrane to cluster into hexamers within cholesterol-rich lipid rafts (green). The ORAI-STIM puncta formation allows Ca2+ entry while simultaneously triggering detachment of TRPC1 from caveolae in vesicles and insertion into lipid-rich rafts on the plasma membrane [139,141]. From thereon, functional coupling between TRPC1 and STIM brings a large intracellular flow of Ca2+ ions. Activated ORAI hexamer also is purported to promote TRPC1 insertion into lipid rafts. After SOCIC-mediated Ca2+ ion influx, Rab4 coated vesicles transport TRPC1 via the Arf6 pathway to early endosomes for recycling [140,141]. APC, adenomatous polyposis coli; Arf6, ADP ribosylation factor 6; EB1, microtubule plus end-binding protein; SARAF, SOCE-associated regulatory factor.
2.3.2. Mitochondria and Acidic Vesicles (Mainly Lysosomes)
Mitochondria, known to be the “powerhouse of the cell”, also play a critical role in maintaining Ca2+ ion levels in the cytosol and endoplasmic reticulum [83,149,150]. These sphero-cylindrical organelles that are found mostly aggregated around the nucleus store similar levels of intracellular Ca2+ as the cytosol (0.1 μM) [140]. Electrochemical proton gradient or membrane potential (~Ψmt = −150 to −180 mV) and close apposition to ER are the two key factors responsible for Ca2+ uptake in mitochondria [151]. The free movement of small molecules (less than 5 kDa) from the outer mitochondrial membrane (OMM) into the inner mitochondrial space and their impermeability across the latter generates a high electrochemical proton gradient for ATP synthesis [152]. This gradient simultaneously draws Ca2+ ions from the cytosol.
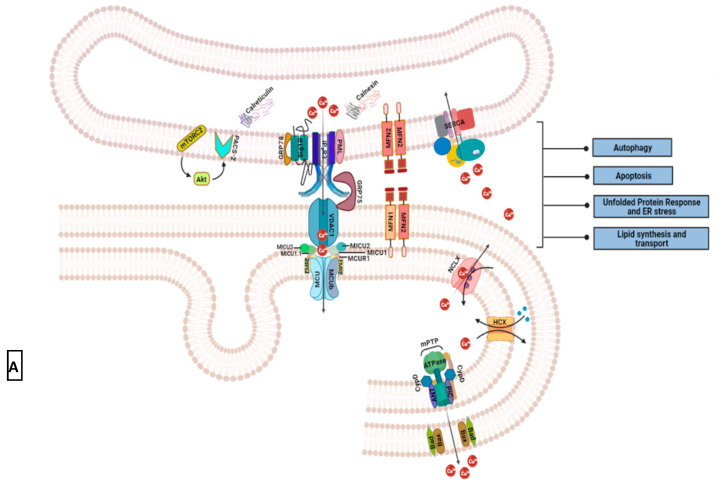
Transfer of Ca2+ ions from ER to mitochondria occurs at specialized microdomains or contact sites known as Mitochondrial Associated Membranes (MAMs). These are characterized by the ER and OMM apposed at 10–25 nm from each other and are strewn with a cluster of channels, transporters, exchangers, and tethering proteins for facilitating Ca2+ ion transfer [151,152,153,154]. IP3Rs localized at the ER side of the MAMs release Ca2+ ions that gate voltage-dependent anion channels (VDACs) located on the OMM [149,153]; Figure 9A. VDACs (1, 2, and 3) are 30 kDa polypeptides having a 19-strand beta-barrel structure that regulates the flux of metabolites (polyvalent anions like ADP and ATP) across the outer mitochondria membranes [155]. These channels transport cations including Ca2+ more readily than anions like chloride. Due to voltage-dependent electrostatic gating, the ion selectivity and flux across VDACs change between open and closed states. For instance, the movement of alpha-helix positive charge to the channel outer walls in the closed state increases Ca2+ ion flux by 10 times relative to the open state. The importance of proximity between IP3Rs and VDACs in MAMs became clearer when it was realized that the channels on the inner mitochondrial membrane (IMM) transporting Ca2+ ions into the matrix have a low affinity (Km ~5–10 mM, KD ~10–50 mM) to these cations [151,156]. These channels, known as Mitochondrial Ca2+ Uniporters (MCUs), are highly selective for Ca2+ ions, and their opening demonstrates sigmoidal dependence on the cation concentration partly due to lowering of Ψmt that subsequently diminishes drive for cation flux. MCU (40 kDa protein) oligomers form a functional multi-protein complex with their regulators—the mitochondria Ca2+ uptake proteins (MICU1, 2, 3) and the essential MCU regulators (EMRE). MICU1 or CBARA1 and MICU2 form obligate heterodimers together in IMM to regulate MCU. These proteins have EF-hands that sense Ca2+ ions concentration in the IMS and accordingly inhibit or promote MCU activity. MICU1 is known to stimulate the rapid agonist-mediated Ca2+ ions uptake while MICU2 acts as a gatekeeper for MCU during low Ca2+ ion concentrations.
Figure 9.
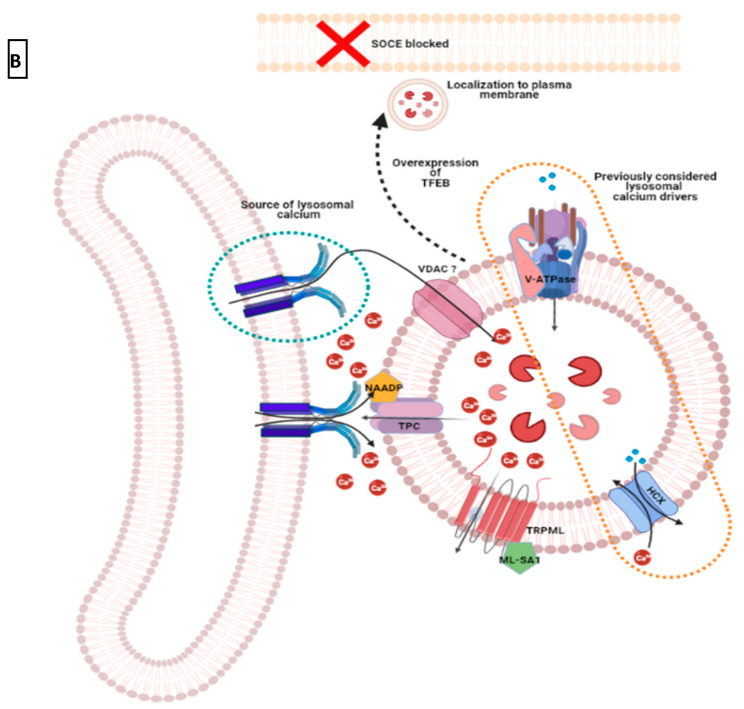
Mitochondrial and lysosomal impact on intracellular Ca2+ signal. (A) Primary components of Ca2+ signaling at the mitochondrial associated membranes (MAMs) include IP3R3 on the endoplasmic reticulum, VDAC1 on the outer mitochondrial membrane, and MCU complex on the inner mitochondrial membrane [151,154,156,161]. Transport of Ca2+ ions from ER to mitochondria plays a crucial role in cellular metabolism (autophagy), cell survival (during unfolded protein response and impinging cell death signals), lipid production, and distribution [162,163,164,165,166]. For these cellular processes to proceed normally, the integrity of MAMs is essential. Various tethering proteins regulate MAM structural integrity. Such secondary MAM components include GRP75, PML, GRP78 (or BiP), mitofusins (MFN1 and 2), and phosphofurin acidic cluster sorting protein (PACS2) [158,159,160,164,167,168,169]. Localized to the ER side of MAMs, growth factor-activated mTORC2 utilizes phosphorylated PACS2 (via Akt) to help avoid structural disruption of this sub-compartment [170]. It also phosphorylates IP3R3 to promote Ca2+ release at MAM sites. Other key MAM localized proteins maintain Ca2+ homeostasis in the region. These are calreticulin and calmodulin in the ER lumen, and SERCA on the ER membrane (184). Excessive Ca2+ ions in the mitochondrial matrix are extruded mainly by a permeability transition pore (mPTP). This Ca2+ efflux pore is situated on the inner mitochondrial membrane and is comprised of F1F0 ATPase with cyclophilin D (CyPD), adenine nucleotide translocase (ANT), and mitochondrial phosphate carrier (PiC) as its regulators [171,172]. Proapoptotic molecules Bax and Bak influence mPTP opening by controlling outer mitochondrial membrane permeability [173]. Ca2+ exchangers such as NCLX and HCX are less understood efflux mechanisms that may prevent overload of these cations in the organelle [151,174]; (B) Lysosomal Ca2+ promotes IP3R3 mediated Ca2+ release from the endoplasmic reticulum [175,176]. Activated by NAADP, two-pore channels (TPCs) and TRPMLs are the main mode of Ca2+ extrusion from lysosomes. Multi-subunit V-ATPases actively transport hydrogen ions into the lysosomal lumen to maintain acidic pH that has been previously considered as the driving force for Ca2+ entry into lysosomes via HCX. Although any direct association between acidic lumen and Ca2+ store maintenance is now considered controversial, any indirect impact on lysosomal Ca2+ stores has not been ruled out. Parallel to this, the dependence of lysosomal Ca2+ uptake on ER Ca2+ stores is speculated [177]. Lysosomes can also play a more active role in intracellular Ca2+ homeostasis as indicated by their inhibition of SOCE [178]. Akt, a serine-threonine kinase; BiP, binding immunoglobulin protein; CyPD, cyclophilin D or peptidyl-prolyl cis-trans isomerase D; EMRE, essential mitochondria regulator; GRP75, glucose-regulated protein 75; HCX, hydrogen-Ca2+ exchanger; MCU; mitochondrial Ca2+ uniporter; MCUR1, mitochondrial Ca2+ uniporter regulator 1; MICU1/2, mitochondrial Ca2+ uptake 1 or 2; MFN1/2, mitofusin 1 or 2; mPTP, mitochondrial permeability transition pore; ML-SA1, mucolipin synthetic agonist 1; mTORC2, mTOR complex 2; NAADP, nicotinic acid adenine dinucleotide phosphate; NCLX, mitochondrial sodium Ca2+ exchanger; PML, promyeloctic leukemia protein; PACS2, phosphofurin acidic cluster sorting protein 2; SERCA, sarcoendoplasmic reticulum Ca2+ ATPase; Sig1R, sigma 1 receptor; TFEB, transcription factor EB; TPC, two-pore channel; TRPML, transient receptor potential mucolipin channel; V-ATPase, vacuolar ATPase; VDAC, voltage-dependent anion channel.
MAMs are stabilized by several chaperones and tethering proteins for optimal functioning of key Ca2+ ion transporting components, IP3R-VDAC-MCU/MICU1/MICU2 [157,158] (Figure 9A). Mitofusin 1 and 2 (MFN1/2) promote and regulate ER-mitochondria connectivity at MAMs by maintaining ER shape along with the interaction between adjacent mitochondria. Out of the OMM-linked GTPases, MFN2 is directly involved in MAM formation and tends to cluster more in the microdomain via either homotypic or heterotypic (with mitostatin or THCP) interactions, whereas MFN1 has a dominant role in mitochondrial fusion [159]. GRP75, a member of the heat shock protein 70 family (glucose-regulated protein 75 or HSPA9 or mortalin), is an essential cytosolic tethering protein that stabilizes the interaction between IP3R N-terminus and VDAC by acting as a bridge [160]. PML (or pro-myelocytic leukemia), a tumor-suppressor protein enriched on the ER side of MAM microdomains, modulates Ca2+ ion release from IP3R by forming a multi-protein complex with the receptor, AKT (or protein kinase B), and protein phosphatase A, thereby modifying the ER-mitochondria Ca2+ ion transfer [158]. MAMs are also enriched with ER chaperone proteins like Sigma 1 Receptor (Sig1R) and BiP (immunoglobulin heavy chain binding protein or GRP78) that interact with each other under normal cytosolic Ca2+ ion levels. However, with the release of ER Ca2+ ions, SigR1 dissociates from BiP to bind and prevent IP3R degradation, thus enhancing Ca2+ transfer to mitochondria.
Physiological relevance: Mitochondrial-associated membranes are not just linkage points for ER and mitochondria. These sites finely regulate the movement of ions, metabolites (including Reactive Oxygen Species and lipids), and signaling molecules between the two organelles and thus, are central to normal ER function and mitochondrial biogenesis [164]. Note that triggers for vital processes such as autophagy and apoptosis hinge on the functioning of these dynamic protein bridges that maintain Ca2+ ion flow between the two organelles. For example, it is evident from certain studies that post-metabolic stress, autophagy-inducing proteins (like Beclin 1) and the ones involved in autophagosome formation (Atg 14L and Atg5) localize at MAMs. Moreover, the knockdown of MAM complex proteins such as mitofusins impairs autophagosome formation.
Ca2+ extrusion is as important as uptake for homeostasis in mitochondria. Two Ca2+ exchangers that localize to IMM, a sodium-Ca2+-lithium exchanger (NCLX) and a hydrogen-Ca2+ exchanger (mCHE) [151,174]. NCLX or SLC24A6 (solute carrier family 24 member 6) is an isoform of plasma membrane sodium-Ca2+ exchanger and is mainly expressed in excitable cells [179,180]. It is assumed to exchange three Na+ inside the matrix for one Ca2+ ion and its electrogenic activity (Ψmt dependent) is inhibited by a selective inhibitor (CGP-37157). Much less is known about the hydrogen-Ca2+ exchanger that extrudes one Ca2+ ion per 2-3 hydrogen ions [159]. Mitochondrial permeability transition pore (PTP; 3 nm pore diameter), a high conductance non-selective ion channel also has a considerable role in mitochondrial Ca2+ ion efflux [181,182]. The remarkable difference in Vmax (maximum rate of reaction) of MCU and the combined efflux rate of Ca2+ exchangers creates a huge kinetic imbalance that predisposes mitochondria toward Ca2+ ion overload [181]. Opening of PTP prevents this overload via fast Ca2+ ion efflux leading to depolarization and subsequent increase in permeability of the inner mitochondrial membrane. The open state of PTP is favored by the presence of Ca2+ ions, reactive oxygen species (ROS), mitochondrial matrix pH (around 7.4), and cyclophilin D (CypD) [183]. Inhibitors of PTP include divalent ions like Mg2+, Sr2+, Mn2+, cyclosporin (directly inhibits cyclophilin D), nucleotides, and matrix acidification [181]. The quest to molecularly identify PTP led to the discovery of it being formed by F0F1 ATPase dimers, though the exact location of the pore within the dimer remains to be identified [184]. PTP is active under normal physiological conditions as well where it functions in a transient low conductance mode to maintain cytosolic Ca2+ level without irreversibly changing Ψmt [171]. The positioning of these sphero-cylindrical organelles within the cell, too impacts cytosolic and mitochondrial Ca2+ buffering. For instance, the rise in cytosolic Ca2+ is limited to the apical or secretory side of pancreatic acinar cells by a “belt” of mitochondria unless the organellar Ca2+ buffering capacity is superseded [185].
While IP3 acts as the dominant Ca2+-mobilizing messenger, cADPR (cyclic ADP-ribose) and NAADP (nicotinic acid adenine dinucleotide phosphate) are also known to modulate intracellular Ca2+ stores [186]. cADPR evokes Ca2+ ion release from ER by acting on ryanodine receptors (RyR; counterpart of IP3R in myocytes and co-expressed in some other cell types). NAADP releases Ca2+ from acidic and/or secretory vesicles such as lysosomes and endosomes [187]. Although the exact stimulus for intracellular NAADP synthesis has not been established, some studies link activation of certain GPCRs and tyrosine kinase receptors to the formation of this Ca2+ messenger.
In most mammalian cells, lysosomes comprise ~5 percent of the cell volume and store similar levels of intracellular Ca2+ (0.5 mM) as the ER [175,188] Due to relatively smaller size than ER, lysosomes release nearly undetectable amounts of intracellular Ca2+ in response to NAADP trigger [175]. It is hypothesized that this weak Ca2+ ion signal is subsequently amplified by ER Ca2+ release. Such a model of anterograde Ca2+ signal coupling can work only when ER and lysosomes are adjacent. Two-pore channels (TPCs; TPC1 in both lysosomes and endosomes; TPC2 only in endosomes) and TRPML are the prime modes for Ca2+ ion release (others include P2X4, VGCCs, TRPA1, and TRPM2) while V-type H+ ATPase and H+/Ca2+ exchanger may concertedly transport Ca2+ ions into lysosomes [175,176] (Figure 9B). Some studies support that anterograde ER-lysosomal coupling happens at specialized membrane contact sites (MCS) and have experimentally demonstrated how the removal of TPCs from such sites disrupts the NAADP-based inter-organelle signaling [189,190]. Nonetheless, without a clear understanding of the MCS structure, its functional significance is difficult to verify in anterograde ER-lysosomal Ca2+ coupling. Absent the anterograde Ca2+ release, it is suggested that lysosomes can participate in refining the ER Ca2+ release signal by sequestering Ca2+ ions in the MCS microdomain and inactivating background IP3R activity [170,175]. Few cell types are predisposed to retrograde or reverse Ca2+ ion signal coupling between ER and lysosomes where IP3 triggers ER Ca2+ release that eventually stimulates NAADP synthesis and lysosomal Ca2+ ion release via TPCs [191] (Figure 9B). Research is still underway to determine why some cell types have this form of ER-lysosomal signaling and what its physiological relevance is.
3. Redistribution of Intracellular Ca2+ and Hijack of Its Regulatory Machinery in Cancer Cells
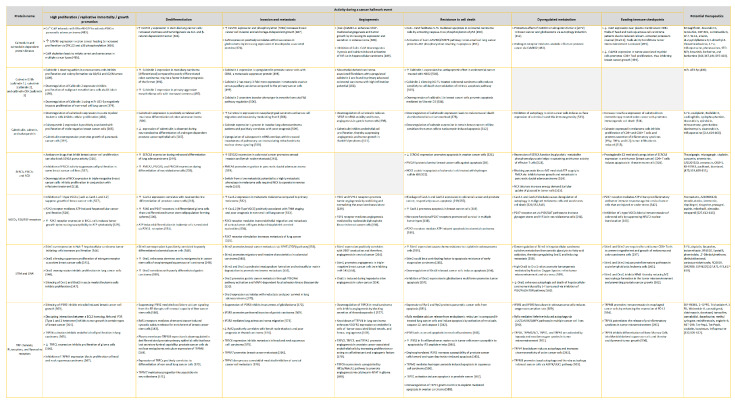
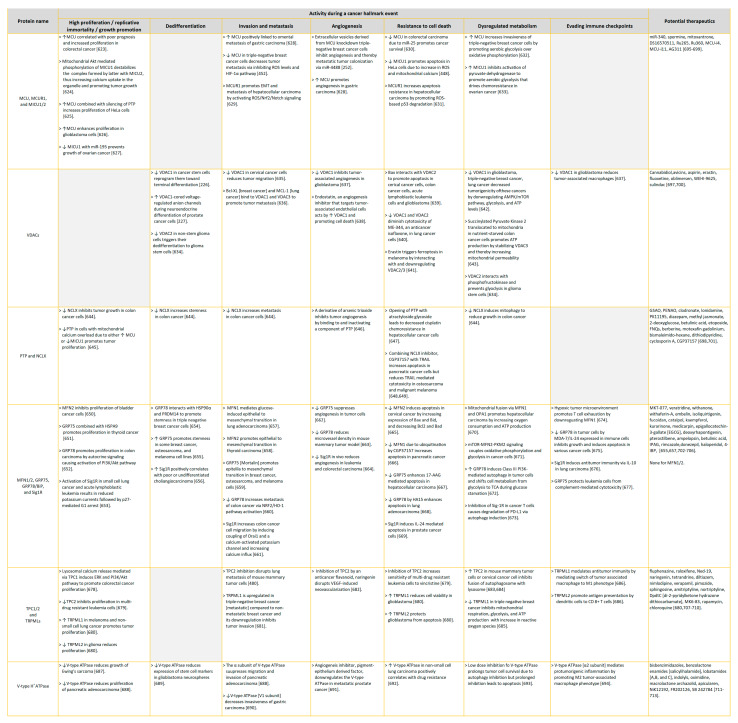
The relationship between Ca2+ and carcinogenesis goes back to the 1946 pioneer study by Carruthers et al. where [Ca2+]i in mouse and human squamous cell carcinoma was measured to be 57 percent and 47 percent lower, respectively, than respective normal epidermises [192]. Following research further highlighted reduced cellular Ca2+ dependency with an increasing degree of neoplastic transformation [193,194,195,196,197,198,199]. Separately, some studies consistently showed how a reduction in extracellular Ca2+ beyond a certain point accelerated cellular transformation [193,200,201,202]. Moreover, reduction in cell adhesiveness, enhancement in cell motility, and increased leakage of proteolytic enzymes in cancer cells have been attributed to the loss of cellular Ca2+ [203,204,205]. To complement the in vitro findings, various epidemiological studies have yielded evidence for the inverse correlation between intake of dietary or supplementary Ca2+ and cancers of colon, rectum, breast, gastric tract, endometrium, renal system, and ovaries [206]. Although the above-mentioned evidence on the differential levels of Ca2+ ion between normal and tumor cells would suggest this cation to have a tumor-inhibitory effect, newer studies indicate a rather complex dynamic [207,208,209]. In this section, we will bring to light multiple ways in which cancer cells manipulate intracellular [Ca2+]i levels and the associated molecular machinery during different stages of tumor progression. Each subsection will provide an overview of studies supporting distortion of signaling in a specific intracellular Ca2+ pool, thereby explaining the mechanisms underlying various cancer hallmarks—excessive proliferation, inhibition of growth suppressors, activation of invasion and metastasis, replicative immortality, induction of angiogenesis, resistance to cell death, dysregulated cellular metabolism, and immune surveillance evasion [210]. Additionally, the tables summarize the role of individual Ca2+ signaling components described earlier during each of the hallmark cellular processes involved in tumorigenesis and cancer progression. They also provide a corresponding list of potential therapeutic drugs targeting Ca2+ signaling proteins under preclinical conditions.
3.1. Intracellular Ca2+ Pool in the Endoplasmic Reticulum and at the ER-PM Junction
Because of the substantial observed association of aberrant expression or dysfunction of ion channels with tumor initiation and progression, cancer is at times termed as “oncochannelopathy” [211]. Multiple Ca2+ channels expressed on the plasma membrane are functionally altered in cancer cells [210,211,212,213] including voltage-gated Ca2+ channels (VGCCs), ligand-gated channels, TRP channels, and ORAI channels. Bioinformatic analysis has revealed abundant expression of genes encoding most VGCCs (L-, R-, N-, P/Q-, and T-type) in cancer tissues [214,215,216,217,218]. Accordingly, CACNA1G encoding the pore-forming alpha subunit of T-type VGCC, Cav 3.1, is expressed in lung adenocarcinoma (A549), colon cancer (HCT116), breast cancer (MCF-7, MDA-MB-231), ovarian cancer (A2780), and melanoma [208,215,217,218,219,220,221,222]. Similarly, Cav 1.3 (CACNA1D encoded L-type VGCC) is shown to be upregulated in breast, endometrial, prostate, neuroblastoma, and colorectal cancer biopsies [217,223]. Consequently, blocking VGCC activity either chemically (BK10040 and KYSO5090; T-type channel blockers or Verapamil; L-type blocker) or by gene silencing is reported to inhibit growth, induce death, or reduce migration of tumor cells by causing a drop in intracellular Ca2+ [217,223,224,225,226,227].
The somewhat controvertible, but the generally accepted, role of ligand-gated Ca2+ channels in cancer cells is anti-inflammatory/protumorigenic for P1 receptors and proinflammatory/anti-tumorigenic for P2 [228,229,230]. Due to higher levels of ATP in tumors than normal tissues, purinergic receptors, P2X and P2Y, are the most extensively studied ligand-gated Ca2+ channels in tumors [231]. ATP binding to P2X receptors results in increased Ca2+ influx through them while P2Y receptor (G-protein coupled) activation by the nucleotide enhances ER Ca2+ release or cAMP production [231,232]. The receptor expression levels and related Ca2+ signaling are both altered during growth factor-induced epithelial–mesenchymal transition (EMT) [232,233]. For instance, EGF-mediated EMT in MDA-MB-468 breast cancer cells is reported to reduce the sensitivity of P2Rs to ATP binding with simultaneous downregulation of P2Y13 and upregulation of P2X5 and P2Y6 transcript levels. ATP-mediated increase in [Ca2+]i can exert anti-migratory and antiangiogenic effects on tumor-derived endothelial cells. Two separate studies have shown that treatment of Breast Tumor-derived Endothelial cells (BTEC) and Renal Tumor-derived Endothelial Cells (RTEC) with 100 mM ATP leads to a biphasic increase in [Ca2+]i—an initial transient release from ER followed by SOCE and then a prolonged release rise due to Ca2+ influx via P2X7R and P2Y11R [234,235]. This raised [Ca2+]i then activates adenylate cyclase10/cAMP/EPAC-1 pathway to disrupt actin cytoskeleton and thus, inhibit migration of tumor-derived endothelial cells.
The modification of Ca2+ ion efflux machinery at the ER-PM junction during tumor formation or progression also requires attention. PMCA and Na+/Ca2+ Exchanger (NCX) lead a concerted effort on a normal cell surface to expel excessive free cytosolic Ca2+ ions and thereby prevent cytotoxicity [236]. Functionally, it is logical to consider deactivation or negligible expression of the efflux machinery in cancer cells so that high intracellular Ca2+ is available to drive tumor proliferation and metabolism [41,237,238]. Such has been observed in colon cancer where PMCA4 mRNA levels were found to be significantly lower in high-grade colon adenocarcinoma, lymph node metastasis, and benign tumors relative to healthy tissue [36,239,240]. Using the HT29 colon cancer cell line, Aung et al. showed that PMCA4 overexpression indeed minimizes cell proliferation in colon cancer [239]. Moreover, PMCA4 expression is observed to be induced during differentiation of colon cancer (HT29), neuroblastoma (IMR-32), and breast cancer (MCF7) [36,241,242,243]. By contrast, pancreatic cancer cells that exhibit high glycolytic over mitochondrial metabolism need PMCA for cell survival [244]. PMCA4 function in these PDAC (pancreatic duct adenocarcinoma) cell lines relies on ATP derived from glycolysis. Therefore, inhibiting ATP production by disrupting glycolysis stops PMCA driven Ca2+ ion efflux resulting in excessive Ca2+ filling and cell death [245]. Expression of PMCA4 mRNA is muted in breast cancer cell lines too. However, expression of its splice variant PMCA4b is complex due to distinctive regulation by histone deacetylase inhibitors and ERα [243,245,246]. As an example, PMCA4b exhibits low expression with high sensitivity to HDAC inhibitors in MCF-7 (ER-positive cell line), but high expression and low sensitivity to 17β estradiol or HDAC inhibitors in MDA-MB-231 cells (triple-negative breast cancer) [245]. Kenealey et al. have also shown that inhibition of PMCA activity (likely PMCA1 and 4) by resveratrol induces programmed cell death in MDA-MB-231 cells [241].
The Ca2+ antiporter NCX1 is the most widely distributed and well-studied isoform within its family. Its mRNA and protein expression levels are dramatically reduced in renal cancer cells and nephroblastoma, conferring several advantages [247]. Knockdown of NCX1 in MDCK cells induces EMT via the Ca2+-dependent ERK signaling activation. However, the ability of NCX1-knockdown kidney cells to grow in an anchorage-independent manner and their increased junctional permeability is independent of the Ca2+ transport function of the exchanger [248]. In penile tumors, knockdown of NCX1 lifts the brakes on proliferation and reduces apoptosis [247,249]. For therapy-resistant medulloblastoma and ovarian cancer, on the other hand, knockdown of NCX1 results in sensitization of these cancers to ionizing radiation and cisplatin [250,251]. Similarly, OSW-1, a natural saponin, and potential anticancer treatment blocks NCX activity in acute leukemia cell line (HL-60) and induces cytotoxicity via accumulation of excess Ca2+ ion in the cytosol [252]. In cancer cells exposed to hypoxia, the reverse mode NCX functioning is coupled to carbonic anhydrase IX (CA1X) and sodium-hydrogen exchanger (NHE1) for converting the intracellular proton load (occurring due to metabolic changes during hypoxia) into interstitial acidosis [253,254]. This allows the breakdown of the extracellular matrix and consequently, promotes tumor cell migration and invasion [253,255].
SOCE is central to intracellular Ca2+ signaling as it forms the major Ca2+ ion influx route in non-excitable cells [256]. Conceivably, the expression and activity of SOCE components are remodeled during each stage of cancer progression. However, the pattern of SOCE alteration for advancing cancer hallmarks is complex because it is based on the cancer cell type, progression stage, and isoforms of participating components [257,258,259]. As discussed earlier, STIM1 and ORAI1 are the “classical SOC” channel components with STIM2, ORAI2, and ORAI3 taking the center stage in selective cases for SOCE-mediated Ca2+ influx [257,260]. Evidence from pharmacological and molecular inhibition studies underscores the role of SOCE in cell cycle progression [257,261,262,263,264,265]. Store-operated Ca2+ entry is elevated during G1/S transition but decreased during entry into the G2/M phase [266]. Diminishing SOCE via STIM1 inhibited the proliferation of cervical cancer, glioblastoma (U251), osteosarcoma (143B and U2OS), lung carcinoma (A549 and SK-MES-1), and breast cancer (MCF7) [265,266,267,268,269,270,271,272]. Knockdown of STIM1 in cervical cancer (SiHa and HeLa) caused inhibition of CDK2 phosphorylation, increase in cyclin inhibitors p21 and p27, and accumulation of Cyclin E [272]. In hepatoma cell lines (Huh-7 and HepG2), simultaneous knockdown of STIM1 and ORAI1 dropped protein levels of cyclin D1, thus causing G0/G1 cell cycle arrest [272]. Various studies have also shown that ORAI3-actuated SOCE in MCF7 upregulates c-myc/NFAT/p-ERK axis to increase in CDK2/4, cyclins D1 and E, and promote G1/S transition [266,273,274]. Interestingly, STIM2 upregulation in melanoma cells has been known to contribute to antiproliferative but invasive phenotype [275]. Modulation of SOCE components and the associated Ca2+ uptake by cancer cells also influences programmed cell death. Based on the cell type and stimuli, SOCE can either aid apoptosis or provide apoptotic resistance in cancer cells [276,277,278,279,280]. The pivotal proapoptotic role of STIM1/ORAI1 was first noted in pancreatic cancer where downregulation of ORAI1 or ectopic expression of its dominant-negative mutant reduced the susceptibility of tumor cells to apoptotic stimuli [280]. On the other hand, overexpression of ORAI1 in androgen-independent prostate cancer cells can reinstate the normal level of apoptosis. The proapoptotic role of STIM1/ORAI1-based SOCE is reversed in non-small cell lung cancer (A549), ovary carcinoma (A2780), pancreatic adenocarcinoma (HC67, Panc1, Capan1, ASPC1, and MiaPaca2), multiple myeloma (immortalized human cell lines and patient-derived tumor cells), and melanoma (B16BL6-8) with the general accompaniment of elevated Akt pathway activity [277,281,282,283,284,285]. ORAI1-based Ca2+ entry also slows down the rate of CD95 mediated apoptosis in leukemic T cells [286]. Moreover, STIM2, found abundantly in colon cancer cells, can elicit an anti-apoptotic effect on such tumor cells [287]. Likewise, ORAI3 overexpression in ER-positive T47D cell line confers apoptotic resistance to cisplatin, paclitaxel, or other chemotherapeutics [288].
Unlike apoptosis, all STIM and ORAI isoforms exert a uniform positive effect on tumor migration and invasiveness [276]. Studies on human cervical cancer have revealed that patients with STIM1 upregulated primary tumors have poorer clinical outcomes due to excessive tumors and lymph node metastasis [257,289]. Similarly, STIM1/ORAI1 overexpression in multiple myeloma or upregulation of ORAI1 in esophageal squamous cell carcinoma is associated with poorer progression-free survival [257,269,284]. One of the pioneer studies demonstrating the proinvasive role of STIM1/ORAI1 in breast cancer cells determined that SOCE enhanced turnover of Rac- and Ras-based focal adhesions to increase cancer cell migration [257,290]. A similar role for STIM1/ORAI mediated SOCE has been observed in cervical cancer, hepatocellular carcinoma, renal cell carcinoma, nasopharyngeal cancer, and glioblastoma where this Ca2+ influx regulates focal adhesion turnover, cytoskeletal reorganization, and actomyosin-based mechanotransduction [291,292]. Except for melanoma, STIM2 overexpression in cancer cells and their invasiveness are inversely correlated [284,293]. Understandably, therefore, a high STIM1/STIM2 ratio in breast cancer cells combined with increased SOCE correlates with poorer prognosis in patients [276,284].
Increased angiogenesis is another quintessential feature of cancer [210]. Therefore, manipulation of SOCE has been carefully studied in cancer-associated stromal cells, especially endothelial cells [294]. Silencing of STIM1 or ORAI1 in vascular endothelial cells (HUVECs) attenuates cell proliferation and VEGF-triggered Ca2+ influx [276,295]. On the other hand, STIM1 in vivo overexpression in cancer cells positively correlates with increased VEGF secretion, endothelial cell proliferation, and thus angiogenesis [296]. Studies have also shown ORAI1 to stimulate in vitro tubulogenesis and in vivo angiogenesis [269]. Immune cells are another integral and active part of the tumor microenvironment. Since SOCE components were first discovered and studied in T cells, it is presumable that their function in cancer-promoting or -killing immune cells has been extensively explored [297,298]. Surprisingly, distortion of the role of SOCE components and thereby, the intracellular Ca2+ flux in cancer-related T cells is not well investigated. One of the early studies in this area delineated how downregulation or absence of STIM1 and STIM2 in cytotoxic T lymphocytes (CTLs; a subset of anti-tumor immune cells) prevents the production of cytolytic factors (interferon-γ and TNF-α), thus releasing control on tumor cell engraftment and in vivo tumor growth [299]. Contradictorily, partial inhibition of ORAI1 in a separate study resulted in the killing of CTLs by tumor cells; more research is, therefore, warranted [300].
IP3Rs, as the principal Ca2+ ion release channels for endoplasmic reticulum, are found localized at various sites on the ER membrane: near the SOCE nanodomain, the ER-PM junction outside of the nanodomain, and at the mitochondrial-associated membranes (MAMs) [301,302,303,304]. The functionality of these receptors is determined by their localization and isoform type. Receptors expressed in the ER-PM junction participate in the initiation and propagation of SOCE, whereas the receptors expressed at MAMs regulate ER-mitochondrial Ca2+ ion movement, thereby influencing cellular bioenergetics and apoptosis [165]. As our focus here is on the ER-PM junction, the role of IP3Rs at MAMs will be discussed in the latter part of this article.
IP3Rs (and the smooth muscle counterparts, RyRs) control cellular functions like contractility, cellular motility, and migration [305]. Although the research on the role of IP3Rs in cell migration is still new, studies, in general, support a promigratory effect [305,306]. A migrating cell has polarized front and rear ends that differ in the cytoskeletal arrangement and Ca2+ ion gradient. The repetitive attachment-detachment of leading and trailing edges during cell migration are accompanied by Ca2+ ion oscillations. As a result, migrating tumor cells should have a strong dependency on Ca2+ channels [307,308,309]. This hypothesis is validated by Baljinnyam et al. via their study demonstrating that the activation of PLCε/IP3/IP3R in melanoma cell lines (Mel-2 and SK-Mel-24) increases cell migration; the resulting Ca2+ ion flux promotes interaction between S100A4 (Ca2+-activated invasion protein) and myosin light chain kinase II which rearranges the actin cytoskeleton via EPAC/cAMP-induced Ca2+ elevation [305,310]. IP3R3 as well has been found to cause peritoneal dissemination of gastric cancer [305,311]. Ano1, or TMEM16A, a Ca2+-activated chloride channel (CaCC) at the ER-PM junction, is highly upregulated in several cancer types—gastrointestinal tumors, head and neck squamous carcinoma, glioblastoma, pancreatic, breast, and colorectal cancer— as a pro-proliferative and pro-migratory signaling molecule [312,313,314,315,316,317]. IP3Rs are now known to tether Ano1 to ER-PM junctions, provide localized Ca2+ ion for their activation, and link the opening of CaCCs to SOCE augmentation along with GPCR stimulation [318,319].
By maintaining intracellular Ca2+ homeostasis, voltage-independent Ca2+ channel family known as Transient Receptor Potential or TRP channels influence various Ca2+ ion-sensitive downstream effectors of cellular processes like proliferation, motility, and apoptosis [320,321]. TRP channels having a pro-proliferative effect in cancer cells are mostly derived from TRPC (5, 6), TRPM (2, 4, 5, 7, 8), and TRPV (1, 2, 6) subfamilies [322,323]. TRPC5 downregulation reduces growth in adriamycin-resistant breast cancer cells due to concomitant decline in drug resistance imparting P-glycoproteins [324]. TRPV1, TRPV2, and TRPM2 have been observed to stimulate the proliferation of prostate cancer [325]. Moreover, overexpression of TRPV2 or upregulation of TRPV6 positively correlates with poor prognosis of esophageal squamous cancer and malignant transformation in leukemia or other tumors, respectively [322,326,327,328]. Interestingly, TRPV channels trigger cancer cell proliferation based on cellular subtypes. For example, TRPV1 is expressed in several breast cancer cell lines, peculiarly is found to inhibit cellular proliferation in triple-negative breast cancer [329]. Proapoptotic members of TRP channels largely belong to TRPC, TRPM, and TRPV subfamilies [322]. TRPC1 in conjunction with STIM1 has been recently shown to induce cisplatin cytotoxicity in non-small cell lung cancer cells via reactive oxygen species and DNA damage response [280,330]. In addition, TRPC1 and TRPC4 are postulated to sensitize triple-negative breast cancer to various chemotherapeutics [331]. Among the TRPM members, TRPM2 activation with subsequent Ca2+ ion elevation causes oxidative stress and apoptotic death in MCF7 and CaCo-2 cell lines treated with 5-fluorouracil and leucovorin [332]. Conversely, TRPM8 protects pancreatic cancer cell lines Panc-1 and BxPC3 from gemcitabine by maintaining the expression of multi-drug resistance proteins like P-glycoprotein [333]. TRPV1 and downstream Ca2+ ion signaling get activated in breast cancer MCF7 cells and glioblastoma treated with TRPV1 positive allosteric modulator MRS1477 or capsaicin, respectively, to trigger cell death [334,335]. Members of the TRP family are also involved in mechanotransduction that allows the cancer cell to invade and metastasize [336]. TRPM7 is the best-studied example in this context with its expression in nasopharyngeal and pancreatic cancer related to poor prognosis [337,338]. This non-selective Ca2+ ion-permeable channel forms a mechanosensory complex in breast cancer cells [336,339]. Thus, silencing TRPM7 in ER-positive MCF7 and triple-negative breast cancer MDA-MB-231 redistributes filamentous actin in cell cortices, raises the number of focal adhesions due to rearrangement of local Ca2+ ion levels, and phosphorylates myosin light chain and paxillin [339]. Amongst the TRPV subfamily, TRPV4 is significantly upregulated during breast cancer metastasis, and in the clinical samples with poor overall survival has been observed [340,341].
By dint of its central role in ER Ca2+ replenishment, SERCA pumps are essential for proper protein folding and maturation and other functions required for cancer cell proliferation and survival [342,343]. Therefore, various cancer types such as colon, lung, and prostate carcinomas are rife with mutations and changes in SERCA expression levels (expression datasets of human cancer cell lines) [343,344,345,346,347,348,349]. On the other hand, SERCA3 expression is induced during differentiation but negatively correlated to colon cancer progression (DLD-1, COLO-205, and Caco-2) [345,349]. Following suit, high expression of SERCA3 is observed in differentiated forms of gastric cancer and myeloid leukemias [350,351]. SERCA pumps are also recognized as regulators of Notch1 receptors that are common drivers of tumor proliferation, especially for leukemia [352]. As a result, SERCA inhibition blocks the activity of mutated Notch1 receptors (by impairing protein folding) which leads to G0/G1 arrest in leukemia cells. SERCA isoforms are required even for the survival of cancer stem cells (CSCs) against metabolic stress [353]. In a study utilizing metabolic stress-resistant breast cancer CSCs derived from MCF-7 and MDA-MB-231 cell lines, SERCA expression was induced in a calmodulin kinase 2α (CaMK2α)/NFκB-dependent manner to prevent stress elicited by 2-deoxy-glucose treatment. Treatment of such CSCs with a combination of 2-deoxy-glucose and thapsigargin, however, sensitized the cells to apoptosis via cytosolic Ca2+ ion overload. To prevent unnecessary cytotoxicity induced by thapsigargin in normal cells, its soluble prodrug, G-202 or mipsagargin, was designed and tested in a Phase II study against sorafenib-resistant hepatocellular carcinoma (HCC) [354]. In the study, mipsagargin was shown to stabilize tumor progression by specifically targeting PSMA (prostate-specific membrane antigen) expressing endothelial cells that form the HCC-associated vasculature. It has also been tested against glioblastoma, prostate cancer, and renal cell carcinoma; however, results of those trials have not yet been published.
The anomalous signaling, localization, and mobilization of Ca2+ ions seen in tumor cells are partly attributed to peculiar expression levels of calmodulin (CaM) and its target proteins [355]. It is a common observation for tumor cells to express higher levels of CaM which complements the raised levels of cytosolic Ca2+ ions relative to benign tissues [356,357,358,359,360]. Although it was contended that this observation was cell type-, culture condition-, or transformation agent-specific, Wang et al. (1992) confirmed a significant increase in CaM levels at the individual cell level between transformed and normal cells during G1 to S phase transition [361]. It was further validated that the surge in CaM levels was specific to cellular transformation and not due to a rise in total intracellular protein during cell cycle progression. Inhibition of CaM by the naphthalensulfonamide-derived selective CaM antagonists W-7 and W-13 caused p21cip1-dependent growth arrest and apoptosis of multiple myeloma tumors in a mouse xenograft model [362]. Some of the protumorigenic effects of calmodulin are facilitated through its binding to the Ca2+/calmodulin-stimulated protein kinase (CaMK) family that modulate cell cycle progression by interacting with phase-specific cyclins and cyclin-dependent kinases [363]. For instance, CaMKI allows G1/S transition via phosphorylation and activation of cdk4 while CaMKII triggers metaphase to anaphase progression by stimulating cdc2 [364,365,366]. Therefore, pharmacological (STO-609, KN-62, KN-93, berbamine, etc.) and siRNA inhibition of CaMKs have demonstrated anti-proliferative effects in numerous cancer cell lines [367]. Direct interaction between CaM and nuclear hormone receptors is another exploitable mechanism for limiting tumor proliferation. This is exemplified in studies where the growth of estrogen receptor- and androgen receptor-positive cancer cell lines, MCF7 and LnCaP, respectively, is inhibited by CaM antagonists. In such cell lines, CaM antagonists disrupt stabilization and activation of estrogen receptor and androgen receptor, both of which are dependent upon direct interaction with CaM [368,369,370]. The functional interdependencies of calmodulin and numerous [Ca2+]i transporters too can be leveraged for killing cancer cells. It is well known that calmodulin regulates the slow inactivation of CRAC currents by direct interaction with the STIM1 SOAR domain, thereby disrupting the STIM1-ORAI1 complex [371]. It has also been established that calmodulin-binding is required for IP3R activation [372]. On the other hand, CaMKII potentiates SOCE by promoting STIM1-ORAI1 complex formation [373]. CaM and CaMKs are not only regulators of SOCE, but also form integral components of its downstream signaling cascade. This is well evident by their activation of Akt, ERK1/2, and Raf/Pyk2 (via cytosolic Ca2+ elevation) for tumor survival, growth, migration, and invasion [374,375,376,377,378].
Calreticulin (CRT) and calbindin are other major EF-hand containing Ca2+-binding proteins that are altered by cancer cells [379,380,381,382]. Cancer cells gain many advantages over normal tissue by dysregulating calreticulin since it is a critical modulator of Ca2+ ion-dependent processes including proliferation, differentiation, cell adhesion, migration, intercellular interactions, immune response, and apoptosis [383,384,385,386,387,388,389,390]. Accordingly, upregulation of CRT expression is recorded in various tumors such as invasive breast carcinoma, high microsatellite instability colorectal carcinoma, squamous cell carcinoma, leukemia, and many more [391,392,393,394,395]. CRT expression also positively correlates with tumor size, grade, and development stage in breast and lung cancers [396,397]. Even more, its high expression levels are commensurate with poor survival in prostate cancer and neuroblastoma patients [398,399]. The effect of CRT on tumor proliferation is cell type-dependent with most cancer cells responding to upregulation of this protein with rapid growth [400,401]. For instance, overexpression of CRT in pancreatic and gastric cancer cells causes remarkable growth of these tumor types while stable knockdown of the same in oral squamous cell carcinoma led to significant G0/G1 arrest with a negative impact on anchorage-independent growth and colony formation. In certain cancers like gastric carcinoma, CRT overexpression is reported to promote VEGF expression as well, thus leading to enhanced angiogenesis, tumor invasiveness, and migration [401]. Under normal conditions, calreticulin is essential for cellular adhesion via integrin-associated Ca2+ signaling with the Wnt pathway as the potential downstream effector [402,403]. In contrast, CRT N-terminus expressed on the cancer cell surface is found to be essential for thrombospondin (TSP) mediated invasion and metastasis [404]. Studies have elucidated that the N-terminus of TSP binds to the CRT and low-density lipoprotein receptor co-complex to induce disassembly of focal adhesion kinase, thus reducing cellular adhesion. CRT as a promoter of cancer cell invasion and metastasis has been verified through overexpression (MDCK cells, gastric cancer cell line AGS) and knockdown (HL60 leukemia cell line and J82 bladder cancer cells) studies as well [405,406,407,408]. Strikingly, CRT expressed on the cancer cell surface can engage in immunomodulatory activities and pose a threat to tumor survival. Work from de Bruyn and Bremer et al. revealed that TRAIL-induced apoptosis relocates CRT from ER lumen to cell surface where the Ca2+-binding protein is shown to attract dendritic cells and macrophages for phagocytosis [409]. In glioma, an increase in CRT expression likewise correlates with higher radiosensitivity and, thus, a greater rate of apoptosis [410]. In pancreatic adenocarcinoma, cancer cell survival and chemoresistance decrease significantly post CRT knockdown [411]. Very limited research has been conducted on modifications of calbindin activities in cancer cells. This Ca2+ ion buffer which has dominant expression in neurons has so far only been found to protect osteosarcoma cells from apoptotic stimuli [412].
3.2. Intracellular Ca2+ Pool at ER-Mitochondrial Junction
Aberrant cellular metabolism, a hallmark of a wide variety of tumors, stems from the dysfunction of mitochondrial pathways for ATP production [210]. The shift from oxidative phosphorylation to glycolysis for ATP generation in a subset of cancers is induced by the cumulative effect of mutations in the participating mitochondrial enzymes and Ca2+ signaling [413,414,415,416]. Specifically, activities of TCA cycle enzymes, alpha-ketoglutarate, isocitrate dehydrogenase, and pyruvate dehydrogenase rely on proper Ca2+ ion uptake by mitochondria. Thus, plummeting Ca2+ ion transport from ER to mitochondria in transformed cells portends tumor progression.
ER-mitochondria junctions or Mitochondrial-Associated Membranes (MAMs) are hubs for proteins that are integral to vital processes such as phospholipid synthesis and translocation, mitochondrial Ca2+ ion transport, ER stress, ER Ca2+ release, mitochondrial morphology regulation, cellular bioenergetics, apoptosis and survival, autophagy, and ROS generation [165]. IP3R, as discussed earlier in this review, is one of the MAM proteins involved in ER to mitochondria Ca2+ ion release, and thus crucial for autophagy and apoptosis induction [417,418,419]. Simplistically, inhibition of IP3R (mainly IP3R3) mediated Ca2+ ionrelease into mitochondria creates an imbalance in ATP/ADP ratio that derepresses AMPK/mTOR/ULK-1 axis and initiates autophagy flux (based on nutrient starvation) [418]. Notably, abrogation of IP3R activity outside of MAMs perturbs autophagy induction independent of mTOR suppression—no binding of IP3R to Beclin1, a protein required for autophagosome formation, is observed [420]. Conversely, IP3Rs located away from MAM regions of the ER can promote autophagy induction by acting as Ca2+ ion leak channels as less Ca2+ storage in the ER manifests into poor ion supply for ATP production [418]. Depending on the tumor stage and type, autophagy flux can either cause a cancer cell to thrive or perish [421,422]. By and large, autophagy activation supports tumor suppression during the early stages of cancer development whereas, more advanced tumors utilize it for drug resistance and sustained growth. Autophagy induction due to inadequate ATP production in normal cells halts G1/S cell cycle progression because of an increase in p53/p21 expression and activity [423,424,425]. In cancer cells with mutant p53, however, aberrant proliferation continues post autophagy induction that results in mitotic catastrophe and eventually cell death due to deprivation of mitochondrial metabolites [423,424,425,426,427]. Removal of dysfunctional proteins and mitochondria during autophagy can also prevent tumorigenesis [428]. In highly developed tumors and related stem cells, autophagy induction contradictorily promotes survival from chemotherapeutic (such as 5-FU or bortezomib) assault; competition between a plethora of tumor suppressors and promoters or oncoproteins to interact with IP3R is consistent with the above-stated observation [429,430,431]. Within this context, Bax inhibitor-1 (BI-1) and Bcl2, both located on the ER membrane, stymie IP3R mediated Ca2+ ion release into the mitochondria, ergo inducing autophagy [432,433,434]. Inversely, tumor suppressors such as Beclin-1 prevent inhibition of IP3R-mediated Ca2+ ion release into the mitochondria partly by interacting with Bcl2 which then dissociates from IP3R, thereby restoring the ER-mitochondrial Ca2+ ion transfer [420]. Chemotherapeutics like Arsenic trioxide also increase this Ca2+ ion transport by upregulating the expression of tumor suppressor PML (promyelocytic leukemia) that blocks phosphorylation and inactivation of IP3R by p-Akt [418,435,436].
The ER-mitochondria Ca2+ ion transport has emerged as an integral component of the crosstalk between autophagy and apoptosis [437]. Inhibition of autophagy or excessive mitochondrial Ca2+ ion uptake via MAMs triggers intrinsic apoptosis pathways. Thus, tumor suppressors and proapoptotic molecules like BRCA1 or PTEN bind to IP3R to boost Ca2+ ion uptake at MAMs while tumor promoters and anti-apoptotic molecules such as Bcl2 or Bcl-xL repress mitochondrial Ca2+ ion overload by binding to and inhibiting the channel activity [438,439]. VDAC1 and GRP75, members of a trio comprising IP3R and forming a Ca2+ ion transporting complex at MAMs are also exploited by cancer cells [440,441]. It is noteworthy that the impact of VDAC1 manipulation on cancer cell bioenergetics and survival stretches beyond its role in the trio [442,443]. Protumorigenic proteins, hexokinase I and II (HKI and HKII) partner with VDAC1 on the outer mitochondrial membrane to utilize its nucleotide shuttling property (ATP/ADP) for generating high energy storage forms like glucose-6-phosphate and fuel rapid cell proliferation via glycolysis [442,444]. Aside from this, Bcl2, Bcl-xL, or hexokinase bind to the N-terminus of VDAC1 and prevent the release of cytochrome C through a VDAC1 multimer pore that assembles in response to apoptotic stimuli [445,446,447]. At the inner mitochondrial membrane, the Ca2+ uptake channel, MCU with its regulators MICU1/2 act as the key mediators of Ca2+-based mitochondrial functions [448,449]. Because MICU1/2 limits Ca2+ ion uptake via MCU and its absence causes cell death via mitochondrial overload, tumor cells are widely observed to have elevated levels of these proteins, although expression of MCU is subtype-dependent [172,450]. For example, in invasive breast carcinoma such as Triple-Negative Breast Cancer, upregulated MCU expression is critical for xenograft size, lymph node metastasis, and lung infiltration [451,452]. On the other hand, downregulation of MCU can negatively impact breast cancer cell size and motility [452].
3.3. Intracellular Ca2+ Pool at ER-Lysosome Junction
Ca2+ signaling at lysosomal membranes and surfaces of acidic vesicles provides a functional scaffolding for endocytic traffic and autophagy, thereby directly influencing cellular health [453,454].
Alteration of such signaling in cancer contributes to cancer hallmarks such as uninhibited growth, angiogenesis, and metastasis [455,456,457]. TPC1 and TPC2 are considered the drivers of lysosomal Ca2+ signaling [458]. Initial research in a mouse model of B16 melanoma cells and xenografts established the role of these Ca2+ ion release channels in tumor invasiveness [459]. The study found that VEGF treatment caused NAADP-mediated, sustained Ca2+ ion signals via TPCs on lysosomal membranes, and this promoted G0/G1 cell cycle transition, tumor vascularization, focal adhesion kinase (FAK) formation, and migration. Both in vitro and in vivo silencing or pharmacological inhibition of TPC1/2 have also been shown to block invasion and migration of urinary bladder carcinoma (T24), hepatoma (HUH7), and mammary carcinoma [189,460]. Nguyen et. al used a mouse mammary carcinoma model and silenced TPC expression to demonstrate reduction in lung metastasis. The delineated mechanism involved a downstream failure in the trafficking of β1-integrin to plasma membrane that further prevented phosphorylation of FAK, Src, and vinculin, and thus lamellipodia formation [460]. This complements the previously established role of lysosomes in enhancing tumor migration via cathepsin-based disintegration of extracellular matrix [189,461].
TRPML1 is another Ca2+ ion release channel expressed on lysosomal membranes and is known to finely control autophagy [458,462]. Presently, there is low evidence of its involvement in cancer progression, but the expression of its downstream Ca2+ activated transcription factor, TFEB is noticeably correlated with cellular malignancy [189,463,464,465,466]. Nutrient starvation and ROS production can activate TRPML1 mediated Ca2+ ion release followed by calcineurin activation that subsequently dephosphorylates TFEB and releases it from 14-3-B-B; free TFEB is then translocated to the nucleus to evoke transcription of autophagy-related genes [467]. In short, cancer cells benefit by mai17ntaining dephosphorylated TFEB levels. Supporting this hypothesis, non-small cell lung carcinoma patients with the poor outcome often have TFEB (dephosphorylated) overexpression with simultaneous upregulation of other lysosomal markers like LAMP2a and cathepsin D [468]. TFEB upregulation is also linked to higher invasiveness in colorectal cancer cells [469]. Although a less known function of TFEB, DNA repair triggered by this protein is exploited by Triple-Negative Breast Cancer cells for chemoresistance against doxorubicin [470].
4. Conclusions and Discussion
Intracellular Ca2+ signaling involves a smorgasbord of proteins that chelate or transport Ca2+ ions across various cellular compartments and thereby assist in signal induction, relay, or integration. With Ca2+ signaling being pivotal for vital cellular processes, distortion of this machinery can be highly advantageous to cancer cells. Although there has been a steady upward trend in the number of published studies that delineate the functional exploitation of various intracellular Ca2+ signaling components in tumors, limited headway has occurred in translating those research outcomes into clinical applications. To demonstrate this, we first utilized the PubMed database to get a list of all the publications within the realm of intracellular Ca2+ in cancer. Using the advanced search feature, we selected “intracellular Ca2+” and “cancer” as the keywords to look for in all fields (author, title, abstract, MeSH words, journal name, etc.) of PubMed articles. We got more than 11,000 studies (not shown). As the published articles between 1960 and 1990 only accounted for less than 0.1 percent of the total, we set our year range for subsequent searches as 1990–2020. To minimize the occurrence of non-relevant articles in our dataset (that is, studies only focused on one of the two keywords and not both), we used stricter filtration criteria [Figure 10A]. Based on the distribution pattern of articles by the year and the overall categorization per major intracellular Ca2+ molecules, Revised Filter 2 was determined to be a sub-set of Revised Filter 1 [Figure 10B–D]. A notable difference between the two filters in terms of sorting of articles by the intracellular Ca2+ signaling molecules in focus was that Revised Filter 2 had a lower percentage of articles on “CaSR or Vitamin D” amongst all the ones with main Ca2+ signaling molecules from the endoplasmic reticulum or its interorganellar junctions [Figure 10C,D; from “Calmodulin” up to “Other ER” categories] in Revised Filter 2 (6% (30/454)) than Revised Filter 1 (19% (394/2061)). Therefore, it seemed that the search criteria used in Revised Filter 1 captured most studies relevant to our search without being too stringent; we chose to use the list of articles generated from Revised Filter 1 for subsequent analysis.
Figure 10.
Trends observed in published articles on the role of intracellular Ca2+ in cancer. (A) Schematic of finding articles published from 1990 to 2020 on intracellular Ca2+ in cancer. The number of articles published based on each advanced search filter combination is indicated in red. All the articles found with revised filter and subset-revised filter are further broken down into review articles, journal articles, and clinical studies. (B) Year-based distribution of the total number of published articles on intracellular Ca2+ in cancer differentiated per the search filters. The yearly distribution of the number of published articles identified using subset-revised filter is uniformly correlated with that of the revised filter; (C) Categorization of the number of published articles found using the revised filter per the main intracellular Ca2+ signaling molecule(s) in each study. (D) Categorization of the number of published articles found using the sub-set revised filter per the main intracellular Ca2+ signaling molecule(s) in each study. The articles were sorted by using the categories listed on the y-axis in panels (C,D) as keywords to be identified in the list of articles (title, abstract, and MeSH words) extracted with Revised Filter 1 by creating a dynamic search feature in Excel.
Clinical studies that were extracted from the list of articles in Revised Filter 1 were labeled in PubMed with one or more of these terms— Clinical Study, Clinical Trial, Clinical Trial Phase I, Clinical Trial Phase II, Clinical Trial Phase III, Clinical Trial Phase IV, Comparative Study, Controlled Clinical Trial, Meta-Analysis, Multi-Center Study, Randomized Controlled Trial, Systematic Review. Two-hundred-and-seventy-seven clinical studies were identified with most of them concentrated between year ranges 1991 to 1996 and 2010 to 2017 (with approximately 10 or more studies published each year) [Figure 11A]. With only 0.1% (277 out of 2941) of the clinical studies for over 30 years, it is interesting to observe that most of such research has explored the role of dietary Ca2+ and Vitamin D on cancer (31%) [Figure 11B]. Translational research on any other key Ca2+ signaling player in the endoplasmic reticulum or its interorganellar junctions accounted for less than 10 percent of all the published clinical articles. We further evaluated from the main set of published articles the number of studies that used antineoplastic agents (mainly chemotherapeutics) by searching for appropriate keywords. There are 819 articles in the main set that had utilized antineoplastic agent(s) to address a hypothesis related to the role of a major Ca2+ signaling molecule in cancer [Figure 11C]. Out of those studies, there are 215 ER-related and 247 that have mitochondrial Ca2+ signaling molecules as major components of research. Studies with bisphosphonates and not chemotherapeutics were predominant in the antineoplastic group of articles followed by the ones that utilized dietary Ca2+ and/or Vitamin D [Figure 11D]. Among the remaining articles, hormonal, phytogenic or natural, and organometallic agents were the most popular anticancer chemotherapeutics to be used. The above conclusions have been derived with the caveat that PubMed is not an exhaustive database of biomedical research. It is nevertheless surprising to note the lack of clinical development of anticancer therapeutics targeting Ca2+ signaling.
Figure 11.
The focus of published clinical studies in the area of intracellular Ca2+ in cancer. (A) Year-wise distribution of clinical articles (same as clinical studies in the previous figure) extracted from the published articles obtained using the revised filter. The left y-axis corresponds to clinical articles while the right y-axis is for depicting the number of main articles. (B) The number of extracted clinical articles categorized by the major intracellular Ca2+ signaling molecule(s) each study is focused on. (C) The published articles (referred to as the main set) found using the revised filter are broken down into subsets of studies that have utilized antineoplastic agents or highlighted intracellular Ca2+ signaling molecules either from the endoplasmic reticulum and its inter-organellar junctions or mitochondria. Venn diagram shows the overlap between the number of studies in each subset and the main set. (D) Bubble chart indicates the major groups of antineoplastic agents used in the clinical articles with the main intracellular signaling molecules from the endoplasmic reticulum and its inter-organellar junctions or mitochondria. The size of bubbles is proportional to the number of clinical studies documented in PubMed per anticancer therapeutic agent category.
Research using small molecule drugs targeting numerous Ca2+ signaling proteins is currently in the early stages. Some of the examples include RP4010 (ORAI1 inhibitor; Phase I/IB clinical study terminated), Synta66 (SOCE inhibitor; preclinical), CAD204520 (SERCA inhibitor; preclinical), and SKF96365 (TRP channel and SOCE inhibition; preclinical) [471,472,473,474,475,476]. The time-intensive process (median time 7.3 years) of drug development that simultaneously demands heavy intellectual and monetary investments (roughly $648 million per drug in R&D) could be a possible reason for this gap [477]. Toxicity associated with targeting ubiquitously expressed proteins, the heterogeneity within populations of cancer cells, and the development of multidrug resistance are other critical barriers to be overcome [478,479,480,481]. The effectiveness of small molecule inhibitors of Ca2+ signaling could be increased by leveraging the advancements made in the nanocarrier-based targeted drug delivery systems. For example, taking from the design of Antp-LP4 related peptides (VDAC1 inhibitors), Venetoclax (a Bcl2 inhibitor) can be encapsulated in inorganic (such as gold nanoparticle or quantum dots) or organic nanocarriers (such as liposomes) coated with transferrin to specifically target cancer cells that demonstrate significant surface expression of transferrin receptors [445,482,483]. However, some studies have also highlighted the independent effect of nanoparticles themselves on the regulation of Ca2+ signaling [479]. Drug repurposing is another way to find effective therapeutic modalities targeting Ca2+ signaling in cancer. Several FDA-approved anticancer agents (such as 5-fluorouracil, cisplatin, tamoxifen, paclitaxel, and doxorubicin) and drugs against other disease conditions (such as leflunomide, tolvaptan, and teriflunomide) can impact Ca2+ signaling machinery [484,485]. Some of these drugs, formulated as prodrugs or nanocarrier loads, are under investigation as antagonists of cancer-promoting Ca2+ signaling See Figure 12 [46,226,227,252,440,441,486,487,488,489,490,491,492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551,552,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590,591,592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608,609,610,611,612,613,614,615,616,617,618,619,620,621,622,623,624,625,626,627,628,629,630,631,632,633,634,635,636,637,638,639,640,641,642,643,644,645,646,647,648,649,650,651,652,653,654,655,656,657,658,659,660,661,662,663,664,665,666,667,668,669,670,671,672,673,674,675,676,677,678,679,680,681,682,683,684,685,686,687,688,689,690,691,692,693,694,695,696,697,698,699,700,701,702,703,704,705,706,707,708,709,710,711,712,713].
Figure 12.
Roles of intracellular calcium regulators in cancer and potential therapeutics.
Even after decades of research on intracellular Ca2+ in tumor cells, the vast clinical potential of targeting key players in this area remains underappreciated. Whether it is through redesigning existing therapeutics or developing novel treatments for directly modulating the intracellular Ca2+ levels, greater efforts are needed to find ways to reverse the hijack of intracellular Ca2+ signaling in cancer.
Author Contributions
Conceptualization, R.C.E. and A.S.; analysis, A.S.; writing-review and editing, A.S., G.T.R. and R.C.E.; visualization, A.S.; supervision, R.C.E. All authors have read and agreed to the published version of the manuscript.
Funding
RCE was funded by an Excellence in Academic Medicine award from the State of Illinois.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell A.K. Intracellular Calcium. John Wiley & Sons; Hoboken, NJ, USA: 2014. [DOI] [Google Scholar]
- 2.Carafoli E., Krebs J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagur R., Hajnóczky G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017;66:780–788. doi: 10.1016/j.molcel.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwaller B. Cytosolic Ca2+ Buffers. Cold Spring Harb. Perspect. Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prins D., Michalak M. Organellar Calcium Buffers. Cold Spring Harb. Perspect. Biol. 2011;3:a004069. doi: 10.1101/cshperspect.a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin D., Means A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/S0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 7.Nelson M.R., Chazin W.J. An interaction-based analysis of calcium-induced conformational changes in Ca2+ sensor proteins. Protein Sci. 1998;7:270–282. doi: 10.1002/pro.5560070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Day D.H., Eshak K., Myre M.A. Calmodulin Binding Proteins and Alzheimer’s Disease. J. Alzheimer’s Dis. 2015;46:553–569. doi: 10.3233/JAD-142772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faas C., Raghavchri S., Lisman J.E., Mody I. Calmodulin as a Direct Detector of Ca2+ Signals. Nat. Neurosci. 2011;14:301–304. doi: 10.1038/nn.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalobo A., Hiriaki I., Vogel H.J., Berchtold M.W. Calmodulin as a protein linker and a regulator of adaptor/scaffold proteins. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018;1865:507–521. doi: 10.1016/j.bbamcr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt H. Three functional facets of calbindin D-28k. Front. Mol. Neurosci. 2012;5:25. doi: 10.3389/fnmol.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawasaki H., Kretsinger R.H. Structural and functional diversity of EF-hand proteins: Evolutionary perspectives. Protein Sci. 2017;26:1898–1920. doi: 10.1002/pro.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers J.H. Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 1987;105:1343–1353. doi: 10.1083/jcb.105.3.1343. Erratum in 1990, 110, 1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugli A., Forster Y., Haas P., Nocito A., Bucher C., Bissig H., Mirlacher M., Storz M., Mihatsch M.J., Sauter G. Calretinin expression in human normal and neoplastic tissues: A tissue microarray analysis on 5233 tissue samples. Hum. Pathol. 2003;34:994–1000. doi: 10.1053/S0046-8177(03)00339-3. [DOI] [PubMed] [Google Scholar]
- 15.Marenholz I., Heizmann C.W., Fritz G. S100 proteins in mouse and man: From evolution to function and pathology (Including an update of the nomenclature) Biochem. Biophys. Res. Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 16.Dudek E., Michalak M. Calnexin and calreticulin. In: Kretsinger R.H., Uversky V.N., Permyakov E.A., editors. Encyclopedia of Metalloproteins. Springer; Berlin/Heidelberg, Germany: 2013. pp. 555–562. [DOI] [Google Scholar]
- 17.Ben Johny M., Yang P.S., Bazzazi H., Yue D.T. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat. Commun. 2013;4:1717. doi: 10.1038/ncomms2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambers T.T., Weidema A.F., Nilius B., Hoenderop J.G.J., Bindels R.J.M. Regulation of the mouse epithelial Ca2+ channel trpv6 by the Ca2+-sensor calmodulin. J. Biol. Chem. 2004;279:28855–28861. doi: 10.1074/jbc.M313637200. [DOI] [PubMed] [Google Scholar]
- 19.Nitsche J., Josts I., Heidemann J., Mertens H.D., Maric S., Moulin M., Haertlein M., Busch S., Forsyth V.T., Svergun D.I., et al. Structural basis for activation of plasma-membrane Ca2+-ATPase by calmodulin. Commun. Biol. 2018;1:206. doi: 10.1038/s42003-018-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahl C.R., Means A.R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 21.Lambers T.T., Mahieu F., Oancea E., Hoofd L., de Lange F., Mensenkamp A.R., Voets T., Nilius B., Clapham D.E., Hoenderop J.G., et al. Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J. 2006;25:2978–2988. doi: 10.1038/sj.emboj.7601186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlin J.R., Bassani J.W., Bers D.M. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys. J. 1994;67:1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrashekera P.C., Kargacin M.E., Deans J.P., Lytton J. Determination of apparent calcium affinity for endogenously expressed human sarco(endo)plasmic reticulum calcium-ATPase isoform SERCA3. Am. J. Physiol. Cell Physiol. 2009;296:C1105–C1114. doi: 10.1152/ajpcell.00650.2008. [DOI] [PubMed] [Google Scholar]
- 24.Gorski P.A., Ceholski D.K., Young H.S. Structure-function relationship of the serca pump and its regulation by phospholamban and sarcolipin. In: Krebs J., editor. Membrane Dynamics and Calcium Signaling. Springer; Berlin/Heidelberg, Germany: 2017. pp. 77–119. [DOI] [PubMed] [Google Scholar]
- 25.Vandecaetsbeek I., Trekels M., De Maeyer M., Ceulemans H., Lescrinier E., Raeymaekers L., Wuytack F., Vangheluwe P. Structural basis for the high affinity of the ubiquitous SERCA2b Ca2+ pump. Proc. Natl. Acad. Sci. USA. 2009;106:18533–18538. doi: 10.1073/pnas.0906797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toyoshima C., Nakasako M., Nomura H., Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z., Lewis D., Strock C., Inesi G., Nakasako M., Nomura H., Toyoshima C. Detailed Characterization of the Cooperative Mechanism of Ca2+ Binding and Catalytic Activation in the Ca2+ Transport (SERCA) ATPase. Biochemistry. 2000;39:8758–8767. doi: 10.1021/bi000185m. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M., Skuta N., Watanabe S., Zhang Y., Yoshikaie K., Tanaka Y., Ushioda R., Kato Y., Takagi J., Tsukazaki T., et al. Structural Basis of Sarco/Endoplasmic Reticulum Ca2+-ATPase 2b Regulation via Transmembrane Helix Interplay. Cell Rep. 2019;27:1221–1230. doi: 10.1016/j.celrep.2019.03.106. [DOI] [PubMed] [Google Scholar]
- 29.Anderson D.M., Makarewich C.A., Anderson K.M., Shelton J.M., Bezprozvannaya S., Bassel-Duby R., Olson E.N. Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci. Signal. 2016;9:ra119. doi: 10.1126/scisignal.aaj1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Inoue M., Tsutsumi A., Watanabe S., Nishizawa T., Nagata K., Kikkawa M., Inaba K. Cryo-EM structures of SERCA2b reveal the mechanism of regulation by the luminal extension tail. Sci. Adv. 2020;6:eabb0147. doi: 10.1126/sciadv.abb0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dencher N.A., Choli T., Dresselhaus D., Fimmel F., Grzesiek S., Papadopoulos G., Wittmann-Liebold B., Büldt G. Structure-function relationship of the light-driven proton pump bacteriorhodopsin. J. Protein Chem. 1989;8:340–343. doi: 10.1007/BF01674270. [DOI] [PubMed] [Google Scholar]
- 32.A buffering serca pump in models of calcium dynamics. Biophys. J. 2006;91:151–163. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinoza-Fonseca L.M. Probing the effects of nonannular lipid binding on the stability of the calcium pump SERCA. Sci. Rep. 2019;9:3349. doi: 10.1038/s41598-019-40004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strehler E.E., Caride A.J., Filoteo A.G., Xiong Y., Penniston J.T., Enyedi A. Plasma membrane Ca2+-ATPases as dynamic regulators of cellular calcium handling. Ann. N. Y. Acad. Sci. 2007;1099 doi: 10.1196/annals.1387.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stafford N., Wilson C., Oceandy D., Neyses L., Cartwright E.J. The plasma membrane calcium atp ases and their role as major new players in human disease. Physiol. Rev. 2017;97:1089–1125. doi: 10.1152/physrev.00028.2016. [DOI] [PubMed] [Google Scholar]
- 36.Padányi R., Pászty K., Hegedűs L., Varga K., Papp B., Penniston J.T., Enyedi A. Multifaceted plasma membrane Ca2+ pumps: From structure to intracellular Ca2+ handling and cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016;1863:1351–1363. doi: 10.1016/j.bbamcr.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Lopreiato R., Giacomello M., Carafoli E. The plasma membrane calcium pump: New ways to look at an old enzyme. J. Biol. Chem. 2014;289:10261–10268. doi: 10.1074/jbc.O114.555565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Gordones M.C., Ramírez-Iglesias J.R., Cervino V., Uzcanga G.L., Benaim G., Mendoza M. Evidence of the presence of a calmodulin-sensitive plasma membrane Ca2+-ATPase in Trypanosoma equiperdum. Mol. Biochem. Parasitol. 2017;213:1–11. doi: 10.1016/j.molbiopara.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Bruce J.I.E. Metabolic regulation of the PMCA: Role in cell death and survival. Cell Calcium. 2018;69:28–36. doi: 10.1016/j.ceca.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strehler E.E. Plasma membrane calcium atpases as novel candidates for therapeutic agent development. J. Pharm. Pharm. Sci. 2013;16:190. doi: 10.18433/J3Z011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brini M., Carafoli E. The Plasma Membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium. Cold Spring Harb Perspect Biol. 2011;3:a004168. doi: 10.1101/cshperspect.a004168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hilge M. Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J. Biol. Chem. 2012;287:31641–31649. doi: 10.1074/jbc.R112.353573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giladi M., Lee S.Y., Ariely Y., Teldan Y., Granit R., Strulovich R., Haitin Y., Chung K.Y., Khananshvili D. Structure-based dynamic arrays in regulatory domains of sodium-calcium exchanger (Ncx) isoforms. Sci. Rep. 2017;7:993. doi: 10.1038/s41598-017-01102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emery L., Whelan S., Hirschi K.D., Pittman J.K. Protein phylogenetic analysis of Ca2+/cation antiporters and insights into their evolution in plants. Front. Plant. Sci. 2012;3:1. doi: 10.3389/fpls.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annunziato L., Pignataro G., Di Renzo G.F. Pharmacology of brain Na+/Ca2+ exchanger: From molecular biology to therapeutic perspectives. Pharmacol. Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- 46.Chemaly E.R., Troncone L., Lebeche D. SERCA control of cell death and survival. Cell Calcium. 2018;69:46–61. doi: 10.1016/j.ceca.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsai F.-C., Seki A., Yang H.W., Hayer A., Carrasco S., Malmersjö S., Meyer T. A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat. Cell Biol. 2014;16:133–144. doi: 10.1038/ncb2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papin J., Zummo F.P., Pachera N., Guay C., Regazzi R., Cardozo A.K., Herchuelz A. Na+/Ca2+ Exchanger a Druggable Target to Promote β-Cell Proliferation and Function. J. Endocr. Soc. 2018;2:631–645. doi: 10.1210/js.2017-00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molinaro P., Pannaccione A., Sisalli M.J., Secondo A., Cuomo O., Sirabella R., Cantile M., Ciccone R., Scorziello A., Di Renzo G., et al. A New Cell-penetrating Peptide That Blocks the Autoinhibitory XIP Domain of NCX1 and Enhances Antiporter Activity. Mol. Ther. 2015;23:465–476. doi: 10.1038/mt.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalil M., Alliger K., Weidinger C., Yerinde C., Wirtz S., Becker C., Engel M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018;9:174. doi: 10.3389/fimmu.2018.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnitzler M.M., Wäring J., Gudermann T., Chubanov V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 2008;22:1540–1551. doi: 10.1096/fj.07-9694com. [DOI] [PubMed] [Google Scholar]
- 52.Voolstra O., Huber A. Post-translational modifications of trp channels. Cells. 2014;3:258–287. doi: 10.3390/cells3020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Goor M.K.C., Hoenderop J.G.J., van der Wijst J. TRP channels in calcium homeostasis: From hormonal control to structure-function relationship of TRPV5 and TRPV6. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017;1864:883–893. doi: 10.1016/j.bbamcr.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Gees M., Colsoul B., Nilius B. The Role of Transient Receptor Potential Cation Channels in Ca2+ Signaling. Cold Spring Harb. Perspect. Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaudet R. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. CRC Press; Boca Raton, FL, USA: Taylor & Francis; Abingdon, UK: 2007. Structural Insights into the Function of TRP Channels (Chapter 25) [Google Scholar]
- 56.Cohen M., Moiseenkova-Bell V.Y. Structure of Thermally Activated TRP Channels. Curr. Top. Membr. 2014;74:181–211. doi: 10.1016/B978-0-12-800181-3.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol. Biosyst. 2008;4:372–379. doi: 10.1039/b801481g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Earley S., Brayden J.E. Transient receptor potential channels in the vasculature. Physiol. Rev. 2015;95:645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 60.Muller C., Morales P., Reggio P.H. Cannabinoid ligands targeting trp channels. Front. Mol. Neurosci. 2019;11:487. doi: 10.3389/fnmol.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J., Hackos D.H. TRPA1 as a drug target—Promise and challenges. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015;388:451–463. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li M., Yu Y., Yang J. Structural biology of trp channels. Adv. Exp. Med. Biol. 2011;704:1–23. doi: 10.1007/978-94-007-0265-3_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimova L., Barvikova K., Macikova L., Vyklicka L., Sinica V., Barvik I., Vlachova V. Proximal c-terminus serves as a signaling hub for trpa1 channel regulation via its interacting molecules and supramolecular complexes. Front. Physiol. 2020;11:189. doi: 10.3389/fphys.2020.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eder P., Schindl R., Romanin C., Groschner K. Protein–protein interactions in trpc channel complexes. In: Liedtke W.B., Heller S., editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. CRC Press; Boca Raton, FL, USA: Taylor & Francis; Abingdon, UK: 2007. [Google Scholar]
- 65.Noyer L., Lemonnier L., Mariot P., Gkika D. Partners in crime: Towards new ways of targeting calcium channels. Int. J. Mol. Sci. 2019;20:6344. doi: 10.3390/ijms20246344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shin Y.-C., Shin S.-Y., Chun J.N., Cho H.S., Lim J.M., Kim H.-G., So I., Kwon D., Jeon J.-H. Trip database 2.0: A manually curated information hub for accessing trp channel interaction network. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0047165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin Y.-C., Shin S.-Y., So I., Kwon D., Jeon J.-H. TRIP Database: A manually curated database of protein–protein interactions for mammalian TRP channels. Nucleic Acids Res. 2011;39((Suppl. 1)):D356–D361. doi: 10.1093/nar/gkq814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chun J.N., Lim J.M., Kang Y., Kim E.H., Shin Y.-C., Kim H.-G., Jang D., Kwon D., Shin S.-Y., So I., et al. A network perspective on unraveling the role of TRP channels in biology and disease. Pflügers Arch. Eur. J. Physiol. 2014;466:173–182. doi: 10.1007/s00424-013-1292-2. [DOI] [PubMed] [Google Scholar]
- 69.Vangeel L., Voets T. Transient receptor potential channels and calcium signaling. Cold Spring Harb. Perspect. Biol. 2019;11 doi: 10.1101/cshperspect.a035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong X., Wang X., Xu H. Trp channels of intracellular membranes. J. Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pecze L., Blum W., Henzi T., Schwaller B. Endogenous TRPV1 stimulation leads to the activation of the inositol phospholipid pathway necessary for sustained Ca2+ oscillations. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016;1863:2905–2915. doi: 10.1016/j.bbamcr.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Thillaiappan N.B., Chakraborty P., Hasan G., Taylor C.W. IP3 receptors and Ca2+ entry. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019;1866:1092–1100. doi: 10.1016/j.bbamcr.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Cullen P.J., Steinberg F. To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2018;19:679–696. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- 74.Peinelt C., Beck A., Monteilh-Zoller M.K., Penner R., Fleig A. IP3 receptor subtype-dependent activation of store-operated calcium entry through ICRAC. Cell Calcium. 2009;45:326–330. doi: 10.1016/j.ceca.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao J.N., Platoshyn O., Golovina V.A., Liu L., Zou T., Marasa B.S., Turner D.J., Yuan J.X.-J., Wang J.-Y. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am. J. Physiol. Liver Physiol. 2006;290:G782–G792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- 76.Haustrate A., Prevarskaya N., Lehen’kyi V. Role of the trpv channels in the endoplasmic reticulum calcium homeostasis. Cells. 2020;9:317. doi: 10.3390/cells9020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Firth A.L., Yuan J.X.-J. Ion channels and transporters in the pulmonary vasculature: A focus on smooth muscle. In: Yuan J.X.-J., Garcia J.G.N., West J.B., Hales C.A., Rich S., Archer S.L., editors. Textbook of Pulmonary Vascular Disease. Springer; Jersey, NJ, USA: 2011. pp. 223–244. [DOI] [Google Scholar]
- 78.Sukumaran P., Schaar A., Sun Y., Singh B.B. Functional role of TRP channels in modulating ER stress and Autophagy. Cell Calcium. 2016;60:123–132. doi: 10.1016/j.ceca.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lam A.K., Galione A. The endoplasmic reticulum and junctional membrane communication during calcium signaling. Biochim. Biophys. Acta (BBA)—Bioenerg. 2013;1833:2542–2559. doi: 10.1016/j.bbamcr.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Michalak M., Robert Parker J.M., Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/S0143416002001884. [DOI] [PubMed] [Google Scholar]
- 81.Berridge M.J. The endoplasmic reticulum: A multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/S0143416002001823. [DOI] [PubMed] [Google Scholar]
- 82.Burdakova D., Petersenb O.H., Verkhratskya A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Raffaello A., Mammucari C., Gherardi G., Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prakriya M., Lewis R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015;85:757–810. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Numaga-Tomita T., Putney J.W. Role of STIM1- and Orai1-mediated Ca2+ entry in Ca2+-induced epidermal keratinocyte differentiation. J. Cell Sci. 2013;126:605–612. doi: 10.1242/jcs.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodeify R., Yu F., Courjaret R., Nader N., Dib M., Sun L., Adap E., Hubrack S., Machaca K. Regulation and role of store-operated Ca2+ entry in cellular proliferation. In: Kozak J.A., Putney J.W., editors. Calcium Entry Channels in Non-Excitable Cells. CRC Press; Boca Raton, FL, USA: Taylor & Francis; Abingdon, UK: 2018. [DOI] [PubMed] [Google Scholar]
- 87.Oritani K., Kincade P.W. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roos J., DiGregorio P.J., Yeromin A.Y., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J.A., Wagner S.L., Cahalan M.D., et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liou J., Kim M.L., Heo W.D., Jones J.T., Myers J.W., Ferrell J.E., Jr., Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–2141. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hewavitharana T., Deng X., Soboloff J., Gill D.L. Role of Stim and Orai proteins in calcium signaling pathways. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 91.Zheng L., Stathopulos P.B., Schindl R., Li G.-Y., Romanin C., Ikura M. Auto-inhibitory role of the EF-SAM domain of STIM proteins in store-operated calcium entry. Proc. Natl. Acad. Sci. USA. 2011;108:1337–1342. doi: 10.1073/pnas.1015125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stathopulos P.B., Zheng L., Ikura M. Stromal Interaction Molecule (STIM) 1 and STIM2 Calcium Sensing Regions Exhibit Distinct Unfolding and Oligomerization Kinetics. J. Biol. Chem. 2009;284:728–732. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 93.Brandman O., Liou J., Park W.S., Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soboloff J., Spassova M.A., Hewavitharana T., He L.-P., Xu W., Johnstone L.S., Dziadek M.A., Gill D.L. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr. Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 95.Vig M., Peinelt C., Beck A., Koomoa D.L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., et al. Cracm1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A., Penner R. CRACM1, CRACM2, and CRACM3 Are Store-Operated Ca2+ Channels with Distinct Functional Properties. Curr. Biol. 2007;27:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prakriya M. Store-Operated Orai Channels: Structure and Function. Curr. Top. Membr. 2013;71:1–32. doi: 10.1016/B978-0-12-407870-3.00001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hogan P.G., Rao A. Store-operated calcium entry: Mechanisms and modulation. Biochem. Biophys. Res. Commun. 2015;460:40–49. doi: 10.1016/j.bbrc.2015.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoover P.J., Lewis R.S. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (Crac) channels by stromal interaction molecule 1 (Stim1) Proc. Natl. Acad. Sci. USA. 2011;108:13299–13304. doi: 10.1073/pnas.1101664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu M.M., Covington E.D., Lewis R.S. Single-molecule analysis of diffusion and trapping of STIM1 and Orai1 at endoplasmic reticulum—Plasma membrane junctions. Mol. Biol. Cell. 2014;25:3672–3685. doi: 10.1091/mbc.e14-06-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thompson J.L., Shuttleworth T.J. Molecular basis of activation of the arachidonate-regulated Ca2+ (Arc) channel, a store-independent Orai channel, by plasma membrane STIM1. J. Physiol. 2013;591:3507–3523. doi: 10.1113/jphysiol.2013.256784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Facilitation of Orai3 targeting and store-operated function by Orai1. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015;1853:1541–1550. doi: 10.1016/j.bbamcr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 103.Putney J.W., McKay R.R. Capacitative calcium entry channels. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 104.Mikoshiba K., Hattori M. Ip3 receptor-operated calcium entry. Sci. Signal. 2000;2000:pe1. doi: 10.1126/stke.2000.51.pe1. [DOI] [PubMed] [Google Scholar]
- 105.Sampieri A., Santoyo K., Asanov A., Vaca L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018;8:13252. doi: 10.1038/s41598-018-31621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Derler I., Jardin I., Romanin C. Molecular mechanisms of STIM/Orai communication. Am. J. Physiol. Physiol. 2016;310:C643–C662. doi: 10.1152/ajpcell.00007.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tiffner A., Derler I. Molecular Choreography and Structure of Ca2+ Release-Activated Ca2+ (CRAC) and KCa2+ Channels and Their Relevance in Disease with Special Focus on Cancer. Membranes. 2020;10:425. doi: 10.3390/membranes10120425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Derler I., Madl J., Schütz G., Romanin C. Structure, regulation and biophysics of icrac, stim/orai1. In: Md. Islam S., editor. Calcium Signaling. Springer; Dordrecht, The Netherlands: 2012. pp. 383–410. [DOI] [Google Scholar]
- 109.Chung S., Zhang M., Stathopulos P. The 2β Splice Variation Alters the Structure and Function of the Stromal Interaction Molecule Coiled-Coil Domains. Int. J. Mol. Sci. 2018;19:3316. doi: 10.3390/ijms19113316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamada K., Miyatake H., Terauchi A., Mikoshiba K. IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc. Natl. Acad. Sci. USA. 2017;114:4661–4666. doi: 10.1073/pnas.1701420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narayanan D., Adebiyi A., Jaggar J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol. Circ. Physiol. 2012;302:H2190–H2210. doi: 10.1152/ajpheart.01146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor C.W., da Fonseca P., Morris E. IP3 receptors: The search for structure. Trends Biochem. Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 113.Serysheva I.I. Toward a high-resolution structure of IP3R channel. Cell Calcium. 2014;56:125–132. doi: 10.1016/j.ceca.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou Y., Nwokonko R.M., Baraniak J.H., Jr., Trebak M., Lee K.P.K., Gill D.L. The remote allosteric control of Orai channel gating. PLoS Biol. 2019;17:e3000413. doi: 10.1371/journal.pbio.3000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cai X., Zhou Y., Nwokonko R., Loktionova N.A., Wang X., Xin P., Trebak M., Wang Y., Gill D.L. The Orai1 Store-operated Calcium Channel Functions as a Hexamer. J. Biol. Chem. 2016;291:25764–25775. doi: 10.1074/jbc.M116.758813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan Z., Ma J., Zui P., Jianjie M. Open Sesame: Treasure in store-operated calcium entry pathway for cancer therapy. Sci. China Life Sci. 2014;58:48–53. doi: 10.1007/s11427-014-4774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhai X., Sterea A.M., El Hiani Y. Lessons from the Endoplasmic Reticulum Ca2+ Transporters—A Cancer Connection. Cells. 2020;9:1536. doi: 10.3390/cells9061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emrich S.M., Yoast R.E., Xin P., Arige V., Wagner L.E., Hempel N., Gill D.L., Sneyd J., Yule D.I., Trebak M. Omnitem-poral choreographies of IP3R and all five STIM/Orai underlie the complexity of mammalian Ca2+ signaling. BioRxiv. 2020 doi: 10.1101/2020.10.04.325480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Korzeniowski M.K., Baird B., Holowka D. STIM1 activation is regulated by a 14 amino acid sequence adjacent to the CRAC activation domain. AIMS Biophys. 2016;3:99–118. doi: 10.3934/biophy.2016.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lopez J.J., Jardin I., Sanchez-Collado J., Salido G.M., Smani T., Rosado J.A. TRPC Channels in the SOCE Scenario. Cells. 2020;9:126. doi: 10.3390/cells9010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nat. Cell Biol. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- 122.Taylor C.W., Tovey S.C. IP3 Receptors: Toward Understanding Their Activation. Cold Spring Harb. Perspect. Biol. 2010;2:a004010. doi: 10.1101/cshperspect.a004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hisatsune C., Mikoshiba K. IP3receptor mutations and brain diseases in human and rodents. J. Neurochem. 2017;141:790–807. doi: 10.1111/jnc.13991. [DOI] [PubMed] [Google Scholar]
- 124.Prole D.L., Taylor C. Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J. Physiol. 2016;594:2849–2866. doi: 10.1113/JP271139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tu J.C., Xiao B., Yuan J.P., A Lanahan A., Leoffert K., Li M., Linden D.J., Worley P.F. Homer Binds a Novel Proline-Rich Motif and Links Group 1 Metabotropic Glutamate Receptors with IP3 Receptors. Neuron. 1998;21:717–726. doi: 10.1016/S0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 126.Li C., Enomoto M., Rossi A.M., Seo M.-D., Rahman T., Stathopulos P., Taylor C., Ikura M., Ames J.B. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc. Natl. Acad. Sci. 2013;110:8507–8512. doi: 10.1073/pnas.1220847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hirota J., Michikawa T., Natsume T., Furuichi T., Mikoshiba K. Calmodulin inhibits inositol 1,4,5-trisphosphate-induced calcium release through the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett. 1999;456:322–326. doi: 10.1016/S0014-5793(99)00973-4. [DOI] [PubMed] [Google Scholar]
- 128.Bourguignon L.Y., Jin H., Iida N., Brandt N.R., Zhang S.H. The involvement of ankyrin in the regulation of inositol 1,4,5-trisphosphate receptor-mediated internal Ca2+ release from Ca2+ storage vesicles in mouse T-lymphoma cells. J. Biol. Chem. 1993;268:7290–7297. doi: 10.1016/S0021-9258(18)53175-6. [DOI] [PubMed] [Google Scholar]
- 129.Ando H., Mizutani A., Matsu-Ura T., Mikoshiba K. IRBIT, a Novel Inositol 1,4,5-Trisphosphate (IP3) Receptor-binding Protein, Is Released from the IP3 Receptor upon IP3 Binding to the Receptor. J. Biol. Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 130.Uchida K., Miyauchi H., Furuichi T., Michikawa T., Mikoshiba K. Critical Regions for Activation Gating of the Inositol 1,4,5-Trisphosphate Receptor. J. Biol. Chem. 2003;278:16551–16560. doi: 10.1074/jbc.M300646200. [DOI] [PubMed] [Google Scholar]
- 131.Rahman T. Dynamic clustering of IP3 receptors by IP3. Biochem. Soc. Trans. 2012;40:325–330. doi: 10.1042/BST20110772. [DOI] [PubMed] [Google Scholar]
- 132.Taylor C.W., Konieczny V. IP3receptors: Take four IP3to open. Sci. Signal. 2016;9:pe1. doi: 10.1126/scisignal.aaf6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bezprozvanny L., Watras J., Ehrlich B. Bell-shaped calcium-response curves of lns(l,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nat. Cell Biol. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 134.Hisatsune C., Nakamura K., Kuroda Y., Nakamura T., Mikoshiba K. Amplification of Ca2+ Signaling by Diacylglycerol-mediated Inositol 1,4,5-Trisphosphate Production. J. Biol. Chem. 2005;280:11723–11730. doi: 10.1074/jbc.M409535200. [DOI] [PubMed] [Google Scholar]
- 135.Van Coppenolle F., Vanden Abeele F., Slomianny C., Flourakis M., Hesketh J., Dewailly E., Prevarskaya N. Ribosome-translocon complex mediates calcium leakage from endoplasmic reticulum stores. J. Cell Sci. 2004;117:4135–4142. doi: 10.1242/jcs.01274. [DOI] [PubMed] [Google Scholar]
- 136.Vaca L. SOCIC: The store-operated calcium influx complex. Cell Calcium. 2010;47:199–209. doi: 10.1016/j.ceca.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 137.Gudlur A., Hogan P.G. The STIM-Orai Pathway: Orai, the Pore-Forming Subunit of the CRAC Channel. Adv. Exp. Med. Biol. 2017;993:39–57. doi: 10.1007/978-3-319-57732-6_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeHaven W.I., Jones B.F., Petranka J.G., Smyth J.T., Tomita T., Bird G.S., Putney J.W. TRPC channels function independently of STIM1 and Orai1. J. Physiol. 2009;587:2275–2298. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng K.T., Ong H.L., Liu X., Ambudkar I.S. Contribution and Regulation of TRPC Channels in Store-Operated Ca2+ Entry. Curr. Top. Membr. 2013;71:149–179. doi: 10.1016/b978-0-12-407870-3.00007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Souza L.B., Ong H.L., Liu X., Ambudkar I.S. Fast endocytic recycling determines TRPC1–STIM1 clustering in ER–PM junctions and plasma membrane function of the channel. Biochim. Biophys. Acta (BBA)—Bioenerg. 2015;1853:2709–2721. doi: 10.1016/j.bbamcr.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 141.Ambudkar I.S., de Souza L.B., Ong H.L. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium. 2017;63:33–39. doi: 10.1016/j.ceca.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pacheco J., Vaca L. Stim-trp pathways and microdomain organization: Auxiliary proteins of the stim/orai complex. In: Groschner K., Graier W.F., Romanin C., editors. Store-Operated Ca2+ Entry (SOCE) Pathways: Emerging Signaling Concepts in Human (Patho)physiology. Springer; Berlin/Heidelberg, Germany: 2017. pp. 189–210. [DOI] [Google Scholar]
- 143.Redondo P.C., Harper A.G.S., Salido G.M., Pariente J.A., Sage S.O., Rosado J.A. A role for SNAP-25 but not VAMPs in store-mediated Ca2+entry in human platelets. J. Physiol. 2004;558:99–109. doi: 10.1113/jphysiol.2004.064899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma A., Ramena G., Yin Y., Premkumar L., Elble R.C. CLCA2 is a positive regulator of store-operated calcium entry and TMEM16A. PLoS ONE. 2018;13:e0196512. doi: 10.1371/journal.pone.0196512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lopez J.J., Albarran L., Gómez L.J., Smani T., Salido G.M., Rosado J.A. Molecular modulators of store-operated calcium entry. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016;1863:2037–2043. doi: 10.1016/j.bbamcr.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 146.Quintana A., Rajanikanth V., Farber-Katz S., Gudlur A., Zhang C., Jing J., Zhou Y., Rao A., Hogan P.G. TMEM110 regulates the maintenance and remodeling of mammalian ER–plasma membrane junctions competent for STIM–ORAI signaling. Proc. Natl. Acad. Sci. USA. 2015;112:E7083–E7092. doi: 10.1073/pnas.1521924112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang L., Wang L., Li S., Xue J., Luo D. Calsequestrin-1 Regulates Store-Operated Ca2+ Entry by Inhibiting STIM1 Aggregation. Cell. Physiol. Biochem. 2016;38:2183–2193. doi: 10.1159/000445574. [DOI] [PubMed] [Google Scholar]
- 148.Albarran L., Lopez J.J., Ben Amor N., Cano F.E.M., Erro A.B., Smani T., Salido G.M., Rosado J.A. Dynamic interaction of SARAF with STIM1 and Orai1 to modulate store-operated calcium entry. Sci. Rep. 2016;6:24452. doi: 10.1038/srep24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malli R., Frieden M., Trenker M., Graier W.F. The Role of Mitochondria for Ca2+ Refilling of the Endoplasmic Reticulum. J. Biol. Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- 150.DeLuca H.F., Engstrom G.W. Calcium uptake by rat kidney mitochondria. Proc. Natl. Acad. Sci. USA. 1961;47:1744–1750. doi: 10.1073/pnas.47.11.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finkel T., Menazza S., Holmström K., Parks R.J., Liu J., Sun J., Liu J., Pan X., Murphy E. The Ins and Outs of Mitochondrial Calcium. Circ. Res. 2015;116:1810–1819. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Patergnani S., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Marchi S., Missiroli S., Poletti F., et al. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Alberts B., editor. Molecular Biology of the Cell. 4th ed. Garland Science; New York, NY, USA: 2002. [Google Scholar]
- 154.Hajnóczky G., Csordás G., Yi M. Old players in a new role: Mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium. 2002;32:363–377. doi: 10.1016/S0143416002001872. [DOI] [PubMed] [Google Scholar]
- 155.Bononi A., Missiroli S., Poletti F., Suski J.M., Agnoletto C., Bonora M., De Marchi E., Giorgi C., Marchi S., Patergnani S., et al. Mitochondria-associated membranes (Mams) as hotspot Ca2+ signaling units. In: Islam M.S., editor. Calcium Signaling. Vol. 740. Springer; Dordrecht, The Netherlands: 2012. pp. 411–437. [DOI] [PubMed] [Google Scholar]
- 156.Colombini M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta (BBA) Biomembr. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mishra J., Jhun B.S., Hurst S., O-Uchi J., Csordás G., Sheu S.-S. The mitochondrial Ca2+ uniporter: Structure, function, and pharmacology. In: Singh H., Sheu S.-S., editors. Pharmacology of Mitochondria. Vol. 240. Springer; Berlin/Heidelberg, Germany: 2017. pp. 129–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Paillard M., Csordás G., Szanda G., Golenár T., Debattisti V., Bartok A., Wang N., Moffat C., Seifert E.L., Spät A., et al. Tissue-specific mitochondrial decoding of cytoplasmic Ca2+ signals is controlled by the stoichiometry of micu1/2 and mcu. Cell Rep. 2017;18:2291–2300. doi: 10.1016/j.celrep.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sassano M.L., van Vliet A.R., Agostinis P. Mitochondria-associated membranes as networking platforms and regulators of cancer cell fate. Front. Oncol. 2017;7:174. doi: 10.3389/fonc.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schrepfer E., Scorrano L. Mitofusins, from Mitochondria to Metabolism. Mol. Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 161.Vallese F., Barazzuol L., Maso L., Brini M., Calì T. Er-mitochondria calcium transfer, organelle contacts and neurodegen-erative diseases. In: Islam M.S., editor. Calcium Signaling. Springer; Berlin/Heidelberg, Germany: 2020. pp. 719–746. [DOI] [PubMed] [Google Scholar]
- 162.Morgan A.J., Davis L.C., Wagner S.K.T.Y., Lewis A.M., Parrington J., Churchill G.C., Galione A. Bidirectional Ca2+ signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao P., Yan Z., Zhu Z. Mitochondria-associated endoplasmic reticulum membranes in cardiovascular diseases. Front. Cell Dev. Biol. 2020;8:1309. doi: 10.3389/fcell.2020.604240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van Vliet A.R., Agostinis P. Mitochondria-associated membranes and er stress. In: Wiseman R.L., Haynes C.M., editors. Coordinating Organismal Physiology through the Unfolded Protein Response. Springer; Berlin/Heidelberg, Germany: 2018. pp. 73–102. [DOI] [PubMed] [Google Scholar]
- 165.Perrone M., Caroccia N., Genovese I., Missiroli S., Modesti L., Pedriali G., Vezzani B., Vitto V.A.M., Antenori M., Lebiedzinska-Arciszewska M., et al. The role of mitochondria-associated membranes in cellular homeostasis and diseases. Int. Rev. Cell Mol. Biol. 2020;350:119–196. doi: 10.1016/bs.ircmb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 166.Betz C., Stracka D., Prescianotto-Baschong C., Frieden M., Demaurex N., Hall M.N. Mtor complex 2-akt signaling at mitochondria-associated endoplasmic reticulum membranes (Mam) regulates mitochondrial physiology. Proc. Natl. Acad. Sci. USA. 2013;110:12526–12534. doi: 10.1073/pnas.1302455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ouyang Y.-B., Giffard R.G. Er-mitochondria crosstalk during cerebral ischemia: Molecular chaperones and er-mitochondrial calcium transfer. Int. J. Cell Biol. 2012 doi: 10.1155/2012/493934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Matsuzaki H., Fujimoto T., Tanaka M., Shirasawa S. Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem. Biophys. Res. Commun. 2013;433:322–326. doi: 10.1016/j.bbrc.2013.02.099. [DOI] [PubMed] [Google Scholar]
- 169.Lee S., Min K.-T. The interface between er and mitochondria: Molecular compositions and functions. Mol. Cells. 2018;41:1000–1007. doi: 10.14348/molcells.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kilpatrick B.S., Eden E.R., Hockey L.N., Yates E., Futter C.E., Patel S. An endosomal naadp-sensitive two-pore Ca2+ channel regulates er-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bernardi P., Di Lisa F., Fogolari F., Lippe G. From ATP to PTP and back: A dual function for the mitochondrial ATP synthase. Circ. Res. 2015;116:1850–1862. doi: 10.1161/CIRCRESAHA.115.306557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vultur A., Gibhardt C.S., Stanisz H., Bogeski I. The role of the mitochondrial calcium uniporter (Mcu) complex in cancer. Pflügers Arch. Eur. J. Physiol. 2018;470:1149–1163. doi: 10.1007/s00424-018-2162-8. [DOI] [PubMed] [Google Scholar]
- 173.Halestrap A.P. The c ring of the f1fo atp synthase forms the mitochondrial permeability transition pore: A critical appraisal. Front. Oncol. 2014;4:234. doi: 10.3389/fonc.2014.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gomez-Suaga P., Paillusson S., Stoica R., Noble W., Hanger D.P., Miller C.C.J. The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy. Curr. Biol. 2017;27:371–385. doi: 10.1016/j.cub.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lively Lysosomes. [(accessed on 20 September 2020)]. Available online: https://www.asbmb.org/asbmb-today/science/050116/lively-lysosomes.
- 176.Yang J., Zhao Z., Gu M., Feng X., Xu H. Release and uptake mechanisms of vesicular Ca2+ stores. Protein Cell. 2019;10:8–19. doi: 10.1007/s13238-018-0523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Karch J., Molkentin J.D. Identifying the components of the elusive mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA. 2014;111:10396–10397. doi: 10.1073/pnas.1410104111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Garrity A.G., Wang W., Collier C.M., Levey S.A., Gao Q., Xu H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife. 2016;5:e15887. doi: 10.7554/eLife.15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Natarajan G.K., Glait L., Mishra J., Stowe D.F., Camara A.K.S., Kwok W.-M. Total Matrix Ca2+ Modulates Ca2+ Efflux via the Ca2+/H+ Exchanger in Cardiac Mitochondria. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.510600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pathak T., Trebak M. Mitochondrial Ca2+ signaling. Pharmacol. Ther. 2018;192:112–123. doi: 10.1016/j.pharmthera.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bernardi P., Von Stockum S. The permeability transition pore as a Ca2+ release channel: New answers to an old question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Massari S., Azzone G.F. The equivalent pore radius of intact and damaged mitochondria and the mechanism of active shrinkage. Biochim. Biophys. Acta (BBA)—Bioenerg. 1972;283:23–29. doi: 10.1016/0005-2728(72)90094-1. [DOI] [PubMed] [Google Scholar]
- 184.Di Lisa F., Carpi A., Giorgio V., Bernardi P. The mitochondrial permeability transition pore and cyclophilin D in cardioprotection. Biochim. Biophys. Acta (BBA)—Bioenerg. 2011;1813:1316–1322. doi: 10.1016/j.bbamcr.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 185.Ichas F., Mazat J.-P. From calcium signaling to cell death: Two conformations for the mitochondrial permeability transition pore. Switching from low- to high-conductance state. Biochim. Biophys. Acta (BBA)—Bioenerg. 1998;1366:33–50. doi: 10.1016/S0005-2728(98)00119-4. [DOI] [PubMed] [Google Scholar]
- 186.Tinel H., Cancela J.M., Mogami H., Gerasimenko J., Gerasimenko O., Tepikin A., Petersen O. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 1999;18:4999–5008. doi: 10.1093/emboj/18.18.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gerasimenko J., Sherwood M., Tepikin A., Petersen O., Gerasimenko O. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J. Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- 188.Patel S., Ramakrishnan L., Rahman T., Hamdoun A., Marchant J., Taylor C., Brailoiu E. The endo-lysosomal system as an NAADP-sensitive acidic Ca2+ store: Role for the two-pore channels. Cell Calcium. 2011;50:157–167. doi: 10.1016/j.ceca.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Faris P., Shekha M., Montagna D., Guerra G., Moccia F. Endolysosomal Ca2+ signalling and cancer hallmarks: Two-pore channels on the move, trpml1 lags behind! Cancers. 2019;11:27. doi: 10.3390/cancers11010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Phillips M.J., Voeltz G.K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.López-Sanjurjo C.I., Tovey S.C., Prole D.L., Taylor C.W. Lysosomes shape Ins(1,4,5) P 3 -evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013;126:289–300. doi: 10.1242/jcs.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sbano L., Bonora M., Marchi S., Baldassari F., Medina D.L., Ballabio A., Giorgi C., Pinton P. TFEB-mediated increase in peripheral lysosomes regulates store-operated calcium entry. Sci. Rep. 2017;7:40797. doi: 10.1038/srep40797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Carruthers C., Suntzeff V. Calcium, Copper, and Zinc in the Epidermal Carcinogenesis of Mouse and Man. Cancer Res. 1946;6:296. [PubMed] [Google Scholar]
- 194.Borowiec A.S., Bidaux G., Pigat N., Goffin V., Bernichtein S., Capiod T. Calcium Channels, External Calcium Concentration and Cell Proliferation. Eur. J. Pharmacol. 2014;739:19–25. doi: 10.1016/j.ejphar.2013.10.072. [DOI] [PubMed] [Google Scholar]
- 195.Whitfield J.F. Calcium signals and cancer. Crit. Rev. Oncog. 1992;3:55–90. [PubMed] [Google Scholar]
- 196.Cook S.J., Lockyer P.J. Recent advances in Ca2+-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 197.Boynton A.L., Whitfield J.F., Isaacs R.J. The different roles of serum and calcium in the control of proliferation of BALB/c 3T3 mouse cells. In Vitro-Plant. 1976;12:120–123. doi: 10.1007/BF02796358. [DOI] [PubMed] [Google Scholar]
- 198.Hazelton B., Mitchell B., Tupper J. Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J. Cell Biol. 1979;83:487–498. doi: 10.1083/jcb.83.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Prevarskaya N., Ouadid-Ahidouch H., Skryma R., Shuba Y. Remodelling of Ca2+ transport in cancer: How it contributes to cancer hallmarks? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130097. doi: 10.1098/rstb.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Humeau J., Bravo-San Pedro J.M., Vitale I., Nuñez L., Villalobos C., Kroemer G., Senovill L. Calcium signaling and cell cycle: Progression or death. Cell Calcium. 2018;70:3–15. doi: 10.1016/j.ceca.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 201.Capiod T., Shuba Y., Skryma R., Prevarskaya N. Calcium signalling and cancer cell growth. Subcell Biochem. 2007;45:405–427. doi: 10.1007/978-1-4020-6191-2_15. [DOI] [PubMed] [Google Scholar]
- 202.Clowes G., Frisbie W. No. 32. On the relationship between the rate of growth, age, and potassium and calcium content of mouse tumors (adeno-carcinoma, jensen) Am. J. Physiol. Leg. Content. 1905;14:173–192. doi: 10.1152/ajplegacy.1905.14.3.173. [DOI] [Google Scholar]
- 203.Carruthers C., Suntzeff V. The role of calcium in carcinogenesis summary. Science. 1944;99:245–247. doi: 10.1126/science.99.2569.245-a. [DOI] [PubMed] [Google Scholar]
- 204.Miller K. Calcium and cancer. Med. Hypotheses. 1977;3:263–264. doi: 10.1016/0306-9877(77)90034-2. [DOI] [PubMed] [Google Scholar]
- 205.Kadio B., Yaya S., Basak A., Djè K., Gomes J., Mesenge C. Calcium role in human carcinogenesis: A comprehensive analysis and critical review of literature. Cancer Metastasis Rev. 2016;35:391–411. doi: 10.1007/s10555-016-9634-0. [DOI] [PubMed] [Google Scholar]
- 206.Peterlik M., Grant W.B., Cross H.S. Calcium, Vitamin D and Cancer. Anticancer Res. 2009;29:3687–3698. [PubMed] [Google Scholar]
- 207.Pottle J., Sun C., Gray L., Li M. Exploiting MCF-7 Cells’ calcium dependence with interlaced therapy. J. Cancer Ther. 2013;4:32–40. doi: 10.4236/jct.2013.47A006. [DOI] [Google Scholar]
- 208.Taylor J.M., Simpson R.U. Inhibition of Cancer Cell Growth by Calcium Channel Antagonists in the Athymic Mouse. Cancer Res. 1992;52:2413–2418. [PubMed] [Google Scholar]
- 209.Xu M.M., Seas A., Kiyani M., Ji K.S.Y., Bell H.N. A temporal examination of calcium signaling in cancer- from tumorigenesis, to immune evasion, and metastasis. Cell Biosci. 2018;8:25. doi: 10.1186/s13578-018-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 211.Prevarskaya N., Skryma R., Shuba Y. Ion Channels in cancer: Are cancer hallmarks oncochannelopathies? Physiol. Rev. 2018;98:559–621. doi: 10.1152/physrev.00044.2016. [DOI] [PubMed] [Google Scholar]
- 212.Monteith G.R., Prevarskaya N., Roberts-Thomson S.J. The calcium–cancer signalling nexus. Nat. Rev. Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 213.Roberts-Thomson S.J., Chalmers S.B., Monteith G.R. The Calcium-Signaling Toolkit in Cancer: Remodeling and Targeting. Cold Spring Harb. Perspect. Biol. 2019;11:a035204. doi: 10.1101/cshperspect.a035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Roderick H.W., Cook S.J. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat. Rev. Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 215.Phan N.N., Wang C.Y., Chen C.F., Sun Z., Lai M.D., Lin Y.L. Voltage-gated calcium channels: Novel targets for cancer therapy. Oncol. Lett. 2017;14:2059–2074. doi: 10.3892/ol.2017.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang C.Y., Lai M.D., Phan N.N., Sun Z., Lin Y.C. Meta-analysis of public microarray datasets reveals voltage-gated calcium gene signatures in clinical cancer patients. PLoS ONE. 2015;10:e0125766. doi: 10.1371/journal.pone.0125766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wang X.T., Nagaba Y., Cross H.S., Wrba F., Zhang L., Guggino S.E. The mRNA of L-type calcium channel elevated in colon cancer: Protein distribution in normal and cancerous colon. Am. J. Pathol. 2000;157:1549–1562. doi: 10.1016/S0002-9440(10)64792-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Buchanan P.J., McCloskey K.D. CaV channels and cancer: Canonical functions indicate benefits of repurposed drugs as cancer therapeutics. Eur. Biophys. J. 2016;45:621–633. doi: 10.1007/s00249-016-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Taylor J.T., Huang L., Pottle J.E., Liu K., Yang Y., Zeng X., Keyser B.M., Agrawal K.C., Hansen J.B., Li M. Selective blockade of T-type Ca2+ channels suppresses human breast cancer cell proliferation. Cancer Lett. 2008;267:116–124. doi: 10.1016/j.canlet.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 220.Azimi I., Roberts-Thomson S.J., Monteith G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014;171:945–960. doi: 10.1111/bph.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Antal L., Martin-Caraballo M. T-Type Calcium Channels in Cancer. Cancers. 2019;11:134. doi: 10.3390/cancers11020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Barceló C., Sisó P., Maiques O., de la Rosa I., Martí R.M., Macià A. T-Type Calcium Channels in Cnacer: A Potential Target in Melanoma. Cancers. 2020;12:391. doi: 10.3390/cancers12020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Choi D.L., Jang S.J., Cho S., Choi H.E., Rim H.K., Lee K.T., Lee J.Y. Inhibition of Cellular Proliferation and Induction of Apoptosis in Human Lung Adenocarcinoma A549 Cells by T-type Calcium Channel Antagonist. Bioorg. Med. Chem. Lett. 2014;24:1565–1570. doi: 10.1016/j.bmcl.2014.01.071. [DOI] [PubMed] [Google Scholar]
- 224.Rim H.K., Cho S., Shin D.H., Chung K.S., Cho Y.W., Choi J.H., Lee J.Y., Lee K.T. T-type Ca2+ channel blocker, KYS05090 induces autophagy and apoptosis in A549 cells through inhibiting glucose uptake. Molecules. 2014;19:9864–9875. doi: 10.3390/molecules19079864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rim H.K., Lee H.W., Choi I.S., Park J.Y., Choi H.W., Choi J.H., Cho Y.W., Lee J.Y., Lee K.T. T-type Ca2+ channel blocker, KYS05047 induces G1 phase cell cycle arrest by decreasing intracellular Ca2+ levels in human lung adenocarcinoma A549 cells. Bioorg. Med. Chem. Lett. 2012;22:7123–7126. doi: 10.1016/j.bmcl.2012.09.076. [DOI] [PubMed] [Google Scholar]
- 226.Arif T., Amsalem Z., Shoshan-Barmatz V. Metabolic Reprograming Via Silencing of Mitochondrial VDAC1 Expression Encourages Differentiation of Cancer Cells. Mol. Ther.—Nucleic Acids. 2019;17:24–37. doi: 10.1016/j.omtn.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thinnes F.P. Neuroendocrine differentiation of LNCaP cells suggests: VDAC in the cell membrane is involved in the extrinsic apoptotic pathway. Mol. Genet. Metab. 2009;97:241–243. doi: 10.1016/j.ymgme.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 228.Fourbon Y., Guéguinou M., Félix R., Constantin B., Uguen A., Fromont G., Lajoie L., Magaud C., LeComte T., Chamorey E., et al. Ca2+ protein alpha 1D of CaV1.3 regulates intracellular calcium concentration and migration of colon cancer cells through a non-canonical activity. Sci. Rep. 2017;7:14199. doi: 10.1038/s41598-017-14230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scarpellino G., Genova T., Avanzato D., Bernardini M., Bianco S., Petrillo S., Tolosano E., de Almeida Vieira J.R., Bussolati B., Fiorio Pla A., et al. Purinergic Calcium Signals in Tumor-Derived Endothelium. Cancers. 2019;11:766. doi: 10.3390/cancers11060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chong J.-H., Zheng G.-G., Zhu X.-F., Guo Y., Wang L., Ma C.-H., Liu S.-Y., Xu L.-L., Lin Y.-M., Wu K.-F. Abnormal expression of P2X family receptors in Chinese pediatric acute leukemias. Biochem. Biophys. Res. Commun. 2010;391:498–504. doi: 10.1016/j.bbrc.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 231.Vijayan D., Smyth M.J., Teng M.W.L. Purinergic Receptors: Novel Targets for Cancer Immunotherapy. In: Zitvogel L., Kroemer G., editors. Oncoimmunology. Springer; Cham, Switzerland: 2018. [DOI] [Google Scholar]
- 232.Maehara Y., Kusumoto H., Anai H., Kusumoto T., Sugimachi K. Human tumor tissues have higher ATP contents than normal tissues. Clin. Chim. Acta. 1987;169:341–343. doi: 10.1016/0009-8981(87)90337-8. [DOI] [PubMed] [Google Scholar]
- 233.Azimi I., Beilby H., Davis F.M., Marcial D.L., Kenny P.A., Thompson E.W., Roberts-Thomson S., Monteith G.R. Altered purinergic receptor-Ca2+ signaling associated with hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Mol. Oncol. 2016;10:166–178. doi: 10.1016/j.molonc.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Davis F.M., Kenny P.A., Soo E.T.L., van Denderen B.J.W., Thompson E.W., Cabot P.J., Parat M.O., Roberts-Thomson S.J., Monteith G.R. Remodeling of Purinergic Receptor-Mediated Ca2+ Signaling as a Consequence of EGF-induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. PLoS ONE. 2011;6:e23464. doi: 10.1371/journal.pone.0023464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Avanzato D., Genova T., Fiorio A., Bernardini M., Bianco S., Bussolati B., Mancardi D., Giraudo E., Maione F., Cassoni P., et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016;6:32602. doi: 10.1038/srep32602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Feng W., Yang X., Wang L., Wang R., Yang F., Wang H., Liu X., Ren Q., Zhang Y., Zhu X., et al. P2X7 promotes the progression of MLL-AF9 induced acute myeloid leukemia by upregulation of Pbx3. Haematol. 2020;106:1278–1289. doi: 10.3324/haematol.2019.243360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reisner P.D., Brandt P.C., Vanaman T.C. Analysis of plasma membrane Ca2+-ATPase expression in control and SV40-transformed human fibroblasts. Cell Calcium. 1997;21:53–62. doi: 10.1016/S0143-4160(97)90096-8. [DOI] [PubMed] [Google Scholar]
- 238.Usachev Y.M., Toutenhoofd S.L., Goellner G.M., Strehler E.E., Thayer S.A. Differentiation induces up-regulation of plasma membrane Ca2+-ATPase and concomitant increase in Ca2+ efflux in human neuroblastoma cell line IMR-32. J. Neurochem. 2001;76:1756–1765. doi: 10.1046/j.1471-4159.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 239.Roberts-Thomson S.J., Curry M.C., Monteith G.R. Plasma membrane calcium pumps and their emerging roles in cancer. World J. Biol. Chem. 2010;1:248–253. doi: 10.4331/wjbc.v1.i8.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Aung C.S., Ye W., Plowman G., Peters A.A., Monteith G., Roberts-Thomson S.J. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis. 2009;30:1962–1969. doi: 10.1093/carcin/bgp223. [DOI] [PubMed] [Google Scholar]
- 241.Peterson J.A., Oblad R.V., Mecham J.C., Kenealey J.D. Resveratrol inhibits plasma membrane Ca2+-ATPase inducing an increase in cytoplasmic calcium. Biochem. Biophys. Rep. 2016;7:253–258. doi: 10.1016/j.bbrep.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rüschoff J.H., Brandenburger T., Strehler E.E., Filoteo A.G., Heinmöller E., Aumüller G., Wilhelm B. Plasma Membrane Calcium ATPase Expression in Human Colon Multistep Carcinogenesis. Cancer Investig. 2012;30:251–257. doi: 10.3109/07357907.2012.657817. [DOI] [PubMed] [Google Scholar]
- 243.Ribiczey P., Papp B., Homolya L., Enyedi Á., Kovács T. Selective upregulation of the expression of plasma membrane calcium ATPase isoforms upon differentiation and 1,25(OH)2D3-vitamin treatment of colon cancer cells. Biochem. Biophys. Res. Commun. 2015;464:189–194. doi: 10.1016/j.bbrc.2015.06.113. [DOI] [PubMed] [Google Scholar]
- 244.Varga K., Pászty K., Padányi R., Hegedűs L., Brouland J.P., Papp B., Enyedi A. Histone deacetylase inhibitor- and PMA-induced upregulation of PMCA4b enhances Ca2+ clearance from MCF-7 breast cancer cells. Cell Calcium. 2014;55:78–92. doi: 10.1016/j.ceca.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 245.James A.D., Patel W., Butt Z., Adiamah M., Dakhel R., Latif A., Uggenti C., Swanton E., Imamura H., Siriwardena A.K., et al. The Plasma Membrane Calcium Pump in Pancreatic Cancer Cells Exhibiting the Warburg Effect Relies on Glycolytic ATP. J. Biol. Chem. 2015;290:24760–24771. doi: 10.1074/jbc.M115.668707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Varga K., Hollósi A., Pászty K., Hegedűs L., Szakács G., Tímár J., Papp B., Enyedi A., Padányi A. Expression of Calcium Pumps Is Differentially Regulated by Histone Deacetylase Inhibitors and Estrogen Receptor Alpha in Breast Cancer Cells. BMC Cancer. 2018;18:1029. doi: 10.1186/s12885-018-4945-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Peters A.A., Milevskiy M.J.G., Lee W.C., Curry M.C., Smart C.E., Saunus J.M., Reid L., da Silva L., Marcial D.L., Dray E., et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci. Rep. 2016;6:25505. doi: 10.1038/srep25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Balasubramaniam S.L., Gopalakrishnapillai A., Petrelli N.J., Barwe S.P. Knockdown of Sodium-Calcium Exchanger 1 Induces Epithelial to Mesenchymal Transition in Kidney Epithelial Cells. J. Biol. Chem. 2017;292:11388–11399. doi: 10.1074/jbc.M116.752352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Mahdi S.H., Cheng H., Li J., Feng R. The effect of TGF-beta induced epithelial-mesenchymal transition on the expression of intracellular calcium-handling proteins in T47D and MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 2015;42:1240–1251. doi: 10.1016/j.abb.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 250.Munoz J.J., Drigo S.A., Barros-Filho M.C., Marchi F.A., Scapulatempo-Neto C., Pessoa G.S., Guimaraes G.C., Trindade Filho J.C., Lopes A., Arruda M.A., et al. Down-Regulation of SLC8A1 as a Putative Apoptosis Evasion Mechanism by Modulation of Calcium Levels in Penile Carcinoma. J. Urol. 2015;194:245–251. doi: 10.1016/j.juro.2014.11.097. [DOI] [PubMed] [Google Scholar]
- 251.Pelzl L., Hosseinzadeh Z., al-Maghout T., Singh Y., Sahu I., Bissinger R., Schmidt S., Alkahtani S., Stournaras C., Toulany M., et al. Role of Na+/Ca2+ Exchangers in Therapy Resistance of Medulloblastoma Cells. Cell Physiol. Biochem. 2017;42:1240–1251. doi: 10.1159/000478953. [DOI] [PubMed] [Google Scholar]
- 252.Zheng X., Lu S., He Z., Huang H., Yao Z., Miao Y., Cai C., Zou F. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene. 2020;39:6975–6989. doi: 10.1038/s41388-020-01514-6. [DOI] [PubMed] [Google Scholar]
- 253.Kucukkaya B., Basoglu H., Erdag D., Akbas F., Susgun S., Yalcintepe L. Calcium homeostasis in cisplatin resistant epithelial ovarian cancer. Gen. Physiol. Biophys. 2019;38:353–363. doi: 10.4149/gpb_2019013. [DOI] [PubMed] [Google Scholar]
- 254.Liskova V., Hudecova S., Lencesova L., Iuliano F., Sirova M., Ondrias K., Pastorekova S., Krizanova O. Type 1 Sodium Calcium Exchanger Forms a Complex with Carbonic Anhydrase IX and Via Reverse Mode Activity Contributes to pH Control in Hypoxic Tumors. Cancers. 2019;11:1139. doi: 10.3390/cancers11081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Svastova E., Witarski W., Csaderova L., Kosik I., Skvarkova L., Hulikova A., Zatovicova M., Barathova M., Kopacek J., Pastorek J., et al. Carbonic Anhydrase IX Interacts with Bicarbonate Transporters in Lamellipodia and Increases Cell Migration via Its Catalytic Domain. J. Biol. Chem. 2012;287:3392–3402. doi: 10.1074/jbc.M111.286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sennoune S.R., Santos J.M., Hussain F., Martínez-Zaguilán R. Sodium calcium ex-changer operates in the reverse mode in metastatic human melanoma cells. Cell. Mol. Biol. 2015;61:40–49. [PubMed] [Google Scholar]
- 257.Tojyo Y., Morita T., Nezu A., Tanimura A. Key Components of Store-Operated Ca2+ Entry in Non-Excitable Cells. J. Pharmacol. Sci. 2014;125:340–346. doi: 10.1254/jphs.14R06CP. [DOI] [PubMed] [Google Scholar]
- 258.Chen Y.F., Lin P.C., Yeh Y.M., Chen L.H., Shen M.R. Store-Operated Ca2+ Entry in Tumor Progression: From Molecular Mechanisms to Clinical Implications. Cancers. 2019;11:899. doi: 10.3390/cancers11070899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Stewart T.A., Yapa K.T.D.S., Monteith G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 260.Pierro C., Sneyers F., Bultynck G., Roderick H.W. ER Ca2+ Release and Store-Operated Ca2+ Entry—Partners in Crime or Independent Actors in Oncogenic Transformation? Cell Calcium. 2019;82:102061. doi: 10.1016/j.ceca.2019.102061. [DOI] [PubMed] [Google Scholar]
- 261.Hoth M., Niemeyer B.A. The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr. 2013;71:237–271. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 262.Azimi I., Bong A.H., Poo G.X.H., Armitage K., Lok D., Roberts-Thomson S.J., Monteith G.R. Pharmacological Inhibition of Store-Operated Calcium Entry in MDA-MB-468 Basal A Breast Cancer Cells: Consequences on Calcium Signalling, Cell Migration and Proliferation. Cell Mol. Life Sci. 2018;75:4525–4537. doi: 10.1007/s00018-018-2904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chen Y.W., Chen Y.F., Chen Y.T., Chiu W.T., Shen M.R. The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition. Sci. Rep. 2016;6:22142. doi: 10.1038/srep22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jardin I., Lopez J.J., Salido G.M., Rosado J.M. Store-Operated Ca2+ Entry in Breast Cancer Cells: Remodeling and Functional Role. Int. J. Mol. Sci. 2018;19:4053. doi: 10.3390/ijms19124053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yang S., Zhang J.J., Huang X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 266.El Boustany C., Bidaux G., Enfissi A., Delcourt P., Prevarskaya N., Capiod T. Capacitative Calcium Entry and Transient Receptor Potential Canonical 6 Expression Control Human Hepatoma Cell Proliferation. Hepatology. 2008;47:2068–2077. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 267.Feng M., Grice D.M., Faddy H.M., Nguyen N., Leitch S., Wang Y., Muend S., Kenny P.A., Sukumar S., Roberts-Thomson S.J., et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Li G., Zhang Z., Wang R., Ma W., Yang Y., Wei J., Wei Y. Suppression of STIM1 inhibits human glioblastoma cell proliferation and induces G0/G1 phase arrest. J. Exp. Clin. Cancer Res. 2013;32:20. doi: 10.1186/1756-9966-32-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen Y.F., Chiu W.T., Chen Y.T., Lin P.Y., Huang H.J., Chou C.Y., Chang H.C., Tang M.J., Shen M.R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA. 2011;31:203–211. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zang J., Zuo D., Shogren K.L., Gustafson C.T., Zhou Z., Thompson M.A., Guo R., Prakash Y.S., Lu L., Guo W., et al. STIM1 expression is associated with osteosarcoma cell survival. Chin J Cancer Res. 2019;31:203–211. doi: 10.21147/j.issn.1000-9604.2019.01.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cheng H., Wang S.-Q., Feng R. STIM1 plays an important role in TGF-β-induced suppression of breast cancer cell proliferation. Oncotarget. 2016;7:16866–16878. doi: 10.18632/oncotarget.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ge C., Zeng B., Li R., Li Z., Fu Q., Wang W., Wang Z., Dong S., Lai Z., Wang Y., et al. Knockdown of STIM1 expression inhibits non-small-cell lung cancer cell proliferation in vitro and in nude mouse xenografts. Bioengineered. 2019;10:425–436. doi: 10.1080/21655979.2019.1669518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Motiani R.K., Zhang X.X., Harmon K.E., Keller R.S., Matrougui K., Bennett J.A., Trebak M. Orai3 is an estrogen receptor α-regulated Ca 2+ channel that promotes tumorigenesis. FASEB J. 2013;27:63–75. doi: 10.1096/fj.12-213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Benzerdjeb N., Sevestre H., Ahidouch A., Ouadid-Ahidouch H. Orai3 is a predictive marker of metastasis and survival in resectable lung adenocarcinoma. Oncotarget. 2016;7:81588–81597. doi: 10.18632/oncotarget.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Dubois C., Abeele F.V., Lehen’Kyi V., Gkika D., Guarmit B., Lepage G., Slomianny C., Borowiec A.S., Bidaux G., Benahmed M., et al. Remodeling of Channel-Forming ORAI Proteins Determines an Oncogenic Switch in Prostate Cancer. Cancer Cell. 2014;26:19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 276.Stanisz H., Saul S., Müller C.S.L., Kappl R., Niemeyer B.A., Vogt T., Hoth M., Roesch A., Bogeski I. Inverse regulation of melanoma growth and migration by Orai1/STIM2-dependent calcium entry. Pigment. Cell Melanoma Res. 2014;27:442–453. doi: 10.1111/pcmr.12222. [DOI] [PubMed] [Google Scholar]
- 277.Fiorio Pla A., Kondratska K., Prevarskaya N. STIM and ORAI proteins: Crucial roles in hallmarks of cancer. Am. J. Physiol. Cell Physiol. 2016;310:C509–C519. doi: 10.1152/ajpcell.00364.2015. [DOI] [PubMed] [Google Scholar]
- 278.Kondratska K., Kondratskyi A., Yassine M., Lemonnier L., Lepage G., Morabito A., Skryma R., Prevarskaya N. Orai1 and STIM1 mediate SOCE and contribute to apoptotic resistance of pancreatic adenocarcinoma. Biochim. Biophys. Acta (BBA)—Bioenerg. 2014;1843:2263–2269. doi: 10.1016/j.bbamcr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 279.Schmidt S., Liu G., Liu G., Yang W., Honisch S., Pantelakos S., Stournaras C., Hönig A., Lang F. Enhanced Orai1 and STIM1 expression as well as store operated Ca2+ entry in therapy resistant ovary carcinoma cells. Oncotarget. 2014;5:4799–4810. doi: 10.18632/oncotarget.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Gualdani R., De Clippele M., Ratbi I., Gailly P., Tajeddine N. Store-Operated Calcium Entry Contributes to Cisplatin-Induced Cell Death in Non-Small Cell Lung Carcinoma. Cancers. 2019;11:430. doi: 10.3390/cancers11030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Flourakis M., Lehen’Kyi V., Beck B., Raphael M., Vandenberghe M., Van Denabeele F., Roudbaraki M., Lepage G., Mauroy B., Romanin C., et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Li Q., Wang J., He Y.-Q., Feng C., Zhang X.-J., Sheng J.-Q., Li P.-F. MicroRNA-185 regulates chemotherapeutic sensitivity in gastric cancer by targeting apoptosis repressor with caspase recruitment domain. Cell Death Dis. 2014;5:e1197. doi: 10.1038/cddis.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Fedida-Metula S., Feldman B., Koshelev V., Levin-Gromiko U., Voronov E., Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012;33:740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 284.Mimura N., Hideshima T., Shimomura T., Suzuki R., Ohguchi H., Rizq O., Kikuchi S., Yoshida Y., Cottini F., Jakubikova J., et al. Selective and potent Akt inhibition triggers anti-myeloma activities and enhances fatal endoplasmic reticulum stress induced by proteasome inhibition. Oncotarget. 2014;74:4458–4469. doi: 10.1158/0008-5472.CAN-13-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wang W., Ren Y., Wang L., Zhao W., Dong X., Pan J., Gao X., Tian Y. Orai1 and Stim1 Mediate the Majority of Store-Operated Calcium Entry in Multiple Myeloma and Have Strong Implications for Adverse Prognosis. Cell. Physiol. Biochem. 2018;48:2273–2285. doi: 10.1159/000492645. [DOI] [PubMed] [Google Scholar]
- 286.Zhan Z.Y., Zhong L.X., Feng M., Wang J.F., Liu D.B., Xiong J.P. Over-expression of Orai1 mediates cell proliferation and associates with poor prognosis in human non-small cell lung carcinoma. Int. J. Clin. Exp. Pathol. 2015;8:5080–5088. [PMC free article] [PubMed] [Google Scholar]
- 287.Khadra N., Bresson-Bepoldin L., Penna A., Chaigne-Delalande B., Ségui B., Levade T., Vacher A.M., Reiffers J., Ducret T., Moreau J.F., et al. CD95 triggers Orai1-mediated localized Ca2+ entry, regulates recruitment of protein kinase C (PKC) β2, and prevents death-inducing signaling complex formation. Proc. Natl. Acad. Sci. USA. 2011;108:19072–19077. doi: 10.1073/pnas.1116946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Sobradillo D., Hernández-Morales M., Ubierna D., Moyer M.P., Núñez L., Villalobos C. A Reciprocal Shift in Transient Receptor Potential Channel 1 (TRPC1) and Stromal Interaction Molecule 2 (STIM2) Contributes to Ca2+ Remodeling and Cancer Hallmarks in Colorectal Carcinoma Cells. J. Biol. Chem. 2014;289:28765–28782. doi: 10.1074/jbc.M114.581678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hasna J., Hague F., Rodat-Despoix L., Geerts D., Leroy C., Tulasne D., Ouadid-Ahidouch H., Kischel P. Orai3 calcium channel and resistance to chemotherapy in breast cancer cells: The p53 connection. Cell Death Differ. 2018;25:691–705. doi: 10.1038/s41418-017-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Zhu H., Zhang H., Jin F., Fang M., Huang M., Yang C.S., Chen T., Fu L., Pan Z. Elevated Orai1 Expression Mediates Tumor-Promoting Intracellular Ca2+ Oscillations in Human Esophageal Squamous Cell Carcinoma. Oncotarget. 2014;5:3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Didiasova M., Zakrzewicz D., Magdolen V., Nagaraj C., Bálint Z., Rohde M., Preissner K.T., Wygrecka M. STIM1/ORAI1-mediated Ca2+ Influx Regulates Enolase-1 Exteriorization. J. Biol. Chem. 2015;290:11983–11999. doi: 10.1074/jbc.M114.598425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Chen Y.F., Hsu K.F., Shen M.R. The Store-Operated Ca2+ Entry-Mediated Signaling Is Important for Cancer Spread. Biochim. Biophys. Acta. 2016;1863:1427–1435. doi: 10.1016/j.bbamcr.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 293.Mo P., Yang S. The Store-Operated Calcium Channels in Cancer Metastasis: From Cell Migration, Invasion to Metastatic Colonization. Front. Biosci. 2018;23:1241–1256. doi: 10.2741/4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.McAndrew D., Grice D.M., Peters A.A., Davis F.M., Stewart T., Rice M., Smart C.E., Brown M.A., Kenny P.A., Roberts-Thomson S.J., et al. ORAI1-mediated Calcium Influx in Lactation and in Breast Cancer. Mol. Cancer Ther. 2011;10:448–460. doi: 10.1158/1535-7163.MCT-10-0923. [DOI] [PubMed] [Google Scholar]
- 295.Moccia F. Endothelial Ca2+ Signaling and the Resistance to Anticancer Treatments: Partners in Crime. Int. J. Mol. Sci. 2018;19:217. doi: 10.3390/ijms19010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Li J., Cubbon R.M., Wilson L.A., Amer M.S., McKeown L., Hou B., Majeed Y., Tumova S., Seymour V.A.L., Taylor H., et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2entry and endothelial tube formation. Circ. Res. 2011;108:1190–1198. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Li J., Bruns A.F., Hou B., Rode B., Webster P.J., Bailey M.A., Appleby H.L., Moss N.K., Ritchie J.E., Yuldasheva N.Y., et al. Orai3 Surface Accumulation and Calcium Entry Evoked by Vascular Endothelial Growth Factor. Arterioscler. Thromb. Vasc. Biol. 2015;35:1987–1994. doi: 10.1161/ATVBAHA.115.305969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 299.Zweifach A., Lewis R.S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Weidinger C., Shaw P.J., Feske S. STIM1 and STIM2-mediated Ca2+ influx regulates antitumour immunity by CD8+ T cells. EMBO Mol. Med. 2013;5:1311–1321. doi: 10.1002/emmm.201302989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhou X., Friedmann K.S., Lyrmann H., Zhou Y., Schoppmeyer R., Knörck A., Mang S., Hoxha C., Angenendt A., Backes C.S., et al. A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity. J. Physiol. 2018;596:2681–2698. doi: 10.1113/JP274964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Endo M. Calcium Release from the Sarcoplasmic Reticulum. Physiol. Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 303.Courjaret R., Dib M., Machaca K. Spatially restricted subcellular Ca2+ signaling downstream of store-operated calcium entry encoded by a cortical tunneling mechanism. Sci. Rep. 2018;8:11214. doi: 10.1038/s41598-018-29562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Thillaiappan N.B., Chavda A.P., Tovey S.C., Prole D.L., Taylor C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017;8:1505. doi: 10.1038/s41467-017-01644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 306.Mandeville J.T.H., Ghosh R.N., Maxfield F.R. Intracellular calcium levels correlate with speed and persistent forward motion in migrating neutrophils. Biophys. J. 1995;68:1207–1217. doi: 10.1016/S0006-3495(95)80336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Ritaine A., Shapovalov G., Prevarskaya N. Metabolic Disorders and Cancer: Store-Operated Ca2+ Entry in Cancer: Focus on IP 3 R-Mediated Ca2+ Release from Intracellular Stores and Its Role in Migration and Invasion. Adv. Exp. Med. Biol. 2017;993:623–637. doi: 10.1007/978-3-319-57732-6_31. [DOI] [PubMed] [Google Scholar]
- 308.Wei C., Wang X., Chen M., Ouyang K., Song L.S., Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Brundage R.A., Fogarty K.E., Tuft R.A., Fay F.A. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science. 1991;254:703–706. doi: 10.1126/science.1948048. [DOI] [PubMed] [Google Scholar]
- 310.Okeke E., Parker T., Dingsdale H., Concannon M., Awais M., Voronina S., Molgó J., Begg M., Metcalf D., Knight A.E., et al. Epithelial–mesenchymal transition, IP3 receptors and ER–PM junctions: Translocation of Ca2+ signalling complexes and regulation of migration. Biochem. J. 2016;473:757–767. doi: 10.1042/BJ20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Baljinnyam E., De Lorenzo M.S., Xie L.H., Iwatsubo M., Chen S., Goydos J.S., Nowycky M.C., Iwatsubo K. Exchange Protein Directly Activated by Cyclic AMP Increases Melanoma Cell Migration by a Ca2+-dependent Mechanism. Cancer Res. 2010;70:5607–5617. doi: 10.1158/0008-5472.CAN-10-0056. [DOI] [PubMed] [Google Scholar]
- 312.Jin X., Shah S., Liu Y., Zhang H., Lees M., Fu Z., Lippiat J.D., Beech D.J., Sivaprasadarao A., Baldwin S.A., et al. Activation of the Cl- Channel ANO1 by Localized Calcium Signals in Nociceptive Sensory Neurons Requires Coupling With the IP3 Receptor. Sci. Signal. 2013;6:ra73. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ruiz C., Martins J.R., Rudin F., Schneider S., Dietsche T., Fischer C.A., Tornillo L., Terracciano L.M., Schreiber R., Bubendorf L., et al. Enhanced Expression of ANO1 in Head and Neck Squamous Cell Carcinoma Causes Cell Migration and Correlates with Poor Prognosis. PLoS ONE. 2012;7:e43265. doi: 10.1371/journal.pone.0043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Zeng X., Pan D., Wu H., Chen H., Yuan W., Zhou J., Shen Z., Chen S. Transcriptional Activation of ANO1 Promotes Gastric Cancer Progression. Biochem. Biophys. Res. Commun. 2019;512:131–136. doi: 10.1016/j.bbrc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 315.Lee Y.S., Lee J.K., Bae Y., Lee B.S., Kim E., Cho C.H., Ryoo K., Yoo J., Kim C.H., Yi G.S., et al. Suppression of 14-3-3γ-mediated surface expression of ANO1 inhibits cancer progression of glioblastoma cells. Sci. Rep. 2016;6:26413. doi: 10.1038/srep26413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Crottès D., Lin Y.H.T., Peters C.J., Gilchrist J.M., Wiita A.P., Jan Y.N., Jan L.Y. TMEM16A Controls EGF-induced Calcium Signaling Implicated in Pancreatic Cancer Prognosis. Proc. Natl. Acad. Sci. USA. 2019;116:13026–13035. doi: 10.1073/pnas.1900703116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Britschgi A., Bill A., Brinkhaus H., Rothwell C., Clay I., Duss S., Rebhan M., Raman P., Guy C.T., Wetzel K., et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc. Natl. Acad. Sci. USA. 2013;110:E1026–E1034. doi: 10.1073/pnas.1217072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sakakura C., Hagiwara A., Fukuda K., Shimomura K., Takagi T., Kin S., Nakase Y., Fujiyama J., Mikoshiba K., Okazaki Y., et al. Possible involvement of inositol 1,4,5-trisphosphate receptor type 3 (IP3R3) in the peritoneal dissemination of gastric cancers. Anticancer Res. 2003;23:3691–3697. [PubMed] [Google Scholar]
- 319.Courjaret R., Machaca K. Mid-range Ca2+ Signalling Mediated by Functional Coupling Between Store-Operated Ca2+ Entry and IP3-dependent Ca2+ Release. Nat. Commun. 2014;5:3916. doi: 10.1038/ncomms4916. [DOI] [PubMed] [Google Scholar]
- 320.Sui Y., Sun M., Wu F., Yang L., Di W., Zhang G., Zhong L., Ma Z., Zheng J., Fang X., et al. Inhibition of TMEM16A Expression Suppresses Growth and Invasion in Human Colorectal Cancer Cells. PLoS ONE. 2014;9:e115443. doi: 10.1371/journal.pone.0115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Prevarskaya Na Zhang L., Barritt G. TRP channels in cancer. Biochim. Biophys. Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 322.Pla A.F., Gkika D. Emerging role of TRP channels in cell migration: From tumor vascularization to metastasis. Front. Physiol. 2013;4:311. doi: 10.3389/fphys.2013.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Shapovalov G., Ritaine A., Skryma R., Prevarskaya N. Role of TRP Ion Channels in Cancer and Tumorigenesis. Semin. Immunopathol. 2016;38:357–369. doi: 10.1007/s00281-015-0525-1. [DOI] [PubMed] [Google Scholar]
- 324.Ma X., Cai Y., He D., Zou C., Zhang P., Lo C.Y., Xu Z., Chan F.L., Yu S., Chen Y., et al. Transient receptor potential channel TRPC5 is essential for P-glycoprotein induction in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA. 2012;109:16282–16287. doi: 10.1073/pnas.1202989109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yang D. Emerging role of transient receptor potential (TRP) channels in cancer progression. BMB Rep. 2020;53:125–132. doi: 10.5483/BMBRep.2020.53.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhou K., Zhang S.-S., Yan Y., Zhao S. Overexpression of transient receptor potential vanilloid 2 is associated with poor prognosis in patients with esophageal squamous cell carcinoma. Med. Oncol. 2014;31:17. doi: 10.1007/s12032-014-0017-5. [DOI] [PubMed] [Google Scholar]
- 327.Semenova S.B., Vassilieva I., Fomina A.F., Runov A.L., Negulyaev Y. Endogenous expression of TRPV5 and TRPV6 calcium channels in human leukemia K562 cells. Am. J. Physiol. Physiol. 2009;296:C1098–C1104. doi: 10.1152/ajpcell.00435.2008. [DOI] [PubMed] [Google Scholar]
- 328.Bödding M., Wissenbach U., Flockerzi V. The Recombinant Human TRPV6 Channel Functions as Ca2+Sensor in Human Embryonic Kidney and Rat Basophilic Leukemia Cells. J. Biol. Chem. 2002;277:36656–36664. doi: 10.1074/jbc.M202822200. [DOI] [PubMed] [Google Scholar]
- 329.Weber L.V., Al-Refae K., Wölk G., Bonatz G., Altmüller J., Becker C., Gisselmann G., Hatt H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer. 2016;8:243–252. doi: 10.2147/BCTT.S121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Elzamzamy O.M., Penner R., Hazlehurst L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells. 2020;9:388. doi: 10.3390/cells9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Grant C.V., Carver C.M., Hastings S.D., Ramachandran K., Muniswamy M., Risinger A.L., Beutler J.A., Mooberry S.L. Triple-negative breast cancer cell line sensitivity to englerin A identifies a new, targetable subtype. Breast Cancer Res. Treat. 2019;177:345–355. doi: 10.1007/s10549-019-05324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Santoni G., Maggi F., Morelli M.B., Santoni M., Marinelli O. Transient Receptor Potential Cation Channels in Cancer Therapy. Med. Sci. 2019;7:108. doi: 10.3390/medsci7120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liu J., Hu G., Gong Y., Yu Q., He B., Li W., He Z., Hao W., He Z., Liu Y. Silencing of TRPM8 inhibits aggressive tumor phenotypes and enhances gemcitabine sensitivity in pancreatic cancer. Pancreatology. 2018;18:935–944. doi: 10.1016/j.pan.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 334.Nazıroğlu M., Çiğ B., Blum W., Vizler C., Buhala A., Marton A., Katona R., Jósvay K., Schwaller B., Oláh Z., et al. Targeting breast cancer cells by MRS1477, a positive allosteric modulator of TRPV1 channels. PLoS ONE. 2017;12:e0179950. doi: 10.1371/journal.pone.0179950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Amantini C., Mosca M., Nabissi M., Lucciarini R., Caprodossi S., Arcella A., Giangaspero F., Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 336.Canales J., Morales D., Blanco C., Rivas J., Díaz N., Angelopoulos I., Cerda O. A TR(i)P to Cell Migration: New Roles of TRP Channels in Mechanotransduction and Cancer. Front. Physiol. 2019;10:757. doi: 10.3389/fphys.2019.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chen J.-P., Wang J., Luan Y., Wang C.-X., Li W.-H., Zhang J.-B., Sha D., Shen R., Cui Y.-G., Zhang Z., et al. TRPM7 promotes the metastatic process in human nasopharyngeal carcinoma. Cancer Lett. 2015;356:483–490. doi: 10.1016/j.canlet.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 338.Rybarczyk P., Gautier M., Hague F., Dhennin-Duthille I., Chatelain D., Kerr-Conte J., Pattou F., Regimbeau J.-M., Sevestre H., Ouadid-Ahidouch H. Transient receptor potential melastatin-related 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J. Cancer. 2012;131:E851–E861. doi: 10.1002/ijc.27487. [DOI] [PubMed] [Google Scholar]
- 339.Middelbeek J., Kuipers A.J., Henneman L., Visser D., Eidhof I., Van Horssen R., Wieringa B., Canisius S.V., Zwart W., Wessels L.F., et al. TRPM7 Is Required for Breast Tumor Cell Metastasis. Cancer Res. 2012;72:4250–4261. doi: 10.1158/0008-5472.CAN-11-3863. [DOI] [PubMed] [Google Scholar]
- 340.Lee W.H., Choong L.Y., Jin T.H., Mon N.N., Chong S., Liew C.S., Putti T., Lu S.Y., Harteneck C., Lim Y.P. TRPV4 plays a role in breast cancer cell migration via Ca2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncog. 2017;6:e338. doi: 10.1038/oncsis.2017.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Lee W.H., Choong L.Y., Mon N.N., Lu S., Lin Q., Pang B., Yan B., Krishna V.S.R., Singh H., Tan T.Z., et al. TRPV4 Regulates Breast Cancer Cell Extravasation, Stiffness and Actin Cortex. Sci. Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lodish H., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J. Biol. Chem. 1992;267:12753–12760. doi: 10.1016/S0021-9258(18)42340-X. [DOI] [PubMed] [Google Scholar]
- 343.Dang D., Rao R. Calcium-ATPases: Gene disorders and dysregulation in cancer. Biochim. Biophys. Acta (BBA)—Bioenerg. 2016;1863:1344–1350. doi: 10.1016/j.bbamcr.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chung F.-Y., Lin S.-R., Lu C.-Y., Yeh C.-S., Chen F.-M., Hsieh J.-S., Huang T.-J., Wang J.-Y. Sarco/Endoplasmic Reticulum Calcium-ATPase 2 Expression as a Tumor Marker in Colorectal Cancer. Am. J. Surg. Pathol. 2006;30:969–974. doi: 10.1097/00000478-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 345.Gou W.-F., Niu Z.-F., Zhao S., Takano Y., Zheng H.-C. Aberrant SERCA3 expression during the colorectal adenoma-adenocarcinoma sequence. Oncol. Rep. 2014;31:232–240. doi: 10.3892/or.2013.2837. [DOI] [PubMed] [Google Scholar]
- 346.Bergner A., Kellner J., Tufman A., Huber R.M. Endoplasmic reticulum Ca2+-homeostasis is altered in small and non-small cell lung cancer cell lines. J. Exp. Clin. Cancer Res. 2009;28:25. doi: 10.1186/1756-9966-28-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Arbabian A., Brouland J.P., Apáti Á., Pászty K., Hegedűs L., Enyedi Á., Chomienne C., Papp B. Modulation of endoplasmic reticulum calcium pump expression during lung cancer cell differentiation. FEBS J. 2013;280:5408–5418. doi: 10.1111/febs.12064. [DOI] [PubMed] [Google Scholar]
- 348.O’Neill J.P., Velalar C.N., Lee D.I., Zhang B., Nakanishi T., Tang Y., Selaru F., Ross D., Meltzer S.J., Hussain A. Thapsigargin resistance in human prostate cancer cells. Cancer. 2006;107:649–659. doi: 10.1002/cncr.22027. [DOI] [PubMed] [Google Scholar]
- 349.Brouland J.P., Gélébart P., Kovàcs T., Enouf J., Grossmann J., Papp B. The Loss of Sarco/Endoplasmic Reticulum Calcium Transport ATPase 3 Expression Is an Early Event during the Multistep Process of Colon Carcinogenesis. Am. J. Pathol. 2005;167:233–242. doi: 10.1016/S0002-9440(10)62968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Launay S., Giannì M., Kovàcs T., Bredoux R., Bruel A., Gélébart P., Zassadowski F., Chomienne C., Enouf J., Papp B. Lineage-specific Modulation of Calcium Pump Expression During Myeloid Differentiation. Blood. 1999;93:4395–4405. doi: 10.1182/blood.V93.12.4395. [DOI] [PubMed] [Google Scholar]
- 351.Gélébart P., Kovács T., Brouland J.P., van Gorp R., Grossmann J., Rivard N., Panis Y., Martin V., Bredoux R., Enouf J., et al. Expression of Endomembrane Calcium Pumps in Colon and Gastric Cancer Cells. J. Biol. Chem. 2002;277:26310–26320. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- 352.Roti G., Carlton A., Ross K.N., Markstein M., Pajcini K., Su A.H., Perrimon N., Pear W.S., Kung A., Blacklow S.C., et al. Complementary Genomic Screens Identify SERCA as a Therapeutic Target in NOTCH1 Mutated Cancer. Cancer Cell. 2013;23:390–405. doi: 10.1016/j.ccr.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Park K.C., Kim S.W., Jeon J.Y., Jo A.R., Choi H.J., Kim J., Lee H.G., Kim Y., Mills G., Noh S.H., et al. Survival of Cancer Stem-Like Cells Under Metabolic Stress via CaMK2α-mediated Upregulation of Sarco/Endoplasmic Reticulum Calcium ATPase Expression. Clin. Cancer Res. 2017;24:1677–1690. doi: 10.1158/1078-0432.CCR-17-2219. [DOI] [PubMed] [Google Scholar]
- 354.Mahalingam D., Peguero J., Cen P., Arora S.P., Sarantopoulos J., Rowe J., Allgood V., Tubb B., Campos L. A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers. 2019;11:833. doi: 10.3390/cancers11060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Berchtold M.W., Villalobo A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim. Biophys. Acta (BBA)—Bioenerg. 2014;1843:398–435. doi: 10.1016/j.bbamcr.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 356.Colomer J., Agell N., Engel P., Bachs O. Expression of calmodulin and calmodulin binding proteins in lymphoblastoid cells. J. Cell. Physiol. 1994;159:542–550. doi: 10.1002/jcp.1041590318. [DOI] [PubMed] [Google Scholar]
- 357.Ye Q., Wei Y., Fischer R., Borner C., Berchtold M.W. Expression of calmodulin and calmodulin binding proteins in rat fibroblasts stably transfected with protein kinase C and oncogenes. Biochim. Biophys. Acta (BBA)—Bioenerg. 1997;1359:89–96. doi: 10.1016/S0167-4889(97)00086-4. [DOI] [PubMed] [Google Scholar]
- 358.Krishnaraju K., Murugesan K., Vij U., Kapur B., Farooq A. Calmodulin levels in oestrogen receptor positive and negative human breast tumours. Br. J. Cancer. 1991;63:346–347. doi: 10.1038/bjc.1991.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wei J.W., Morris H.P., A Hickie R. Positive correlation between calmodulin content and hepatoma growth rates. Cancer Res. 1982;42:2571–2574. [PubMed] [Google Scholar]
- 360.Veigl M.L., Vanaman T.C., E Branch M., Sedwick W.D. Differences in calmodulin levels of normal and transformed cells as determined by culture conditions. Cancer Res. 1984;44:3184–3189. [PubMed] [Google Scholar]
- 361.Wang R., Zhang H., Li S., Xue S. Intracellular levels of calmodulin are increased in transformed cells. Cell Res. 1992;2:119–127. doi: 10.1038/cr.1992.12. [DOI] [Google Scholar]
- 362.Yokokura S., Yurimoto S., Matsuoka A., Imataki O., Dobashi H., Bandoh S., Matsunaga T. Calmodulin antagonists induce cell cycle arrest and apoptosis in vitro and inhibit tumor growth in vivo in human multiple myeloma. BMC Cancer. 2014;14:1–11. doi: 10.1186/1471-2407-14-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Skelding K.A., Rostas J.A.P., Verrills N.M. Controlling the cell cycle: The role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle. 2011;10:631–639. doi: 10.4161/cc.10.4.14798. [DOI] [PubMed] [Google Scholar]
- 364.Kahl C.R., Means A.R. Regulation of Cyclin D1/Cdk4 Complexes by Calcium/Calmodulin-dependent Protein Kinase I. J. Biol. Chem. 2004;279:15411–15419. doi: 10.1074/jbc.M312543200. [DOI] [PubMed] [Google Scholar]
- 365.Patel R., Holt M., Philipova R., Moss S., Schulman H., Hidaka H., Whitaker M. Calcium/Calmodulin-dependent Phosphorylation and Activation of Human Cdc25-C at the G2/M Phase Transition in HeLa Cells. J. Biol. Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- 366.Wang Y., Zhao R., Zhe H. The emerging role of CaMKII in cancer. Oncotarget. 2015;6:11725–11734. doi: 10.18632/oncotarget.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Brzozowski J.S., Skelding K.A. The Multi-Functional Calcium/Calmodulin Stimulated Protein Kinase (CaMK) Family: Emerging Targets for Anti-Cancer Therapeutic Intervention. Pharmaceuticals. 2019;12:8. doi: 10.3390/ph12010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Li Z., Joyal J.L., Sacks D. Calmodulin Enhances the Stability of the Estrogen Receptor. J. Biol. Chem. 2001;276:17354–17360. doi: 10.1074/jbc.M010238200. [DOI] [PubMed] [Google Scholar]
- 369.Li Z., Zhang Y., Hedman A.C., Ames J.B., Sacks D.B. Calmodulin Lobes Facilitate Dimerization and Activation of Estrogen Receptor-α. J. Biol. Chem. 2017;292:4614–4622. doi: 10.1074/jbc.M116.754804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Cifuentes E., Mataraza J.M., Yoshida B.A., Menon M., Sacks D., Barrack E.R., Reddy G.P.-V. Physical and functional interaction of androgen receptor with calmodulin in prostate cancer cells. Proc. Natl. Acad. Sci. USA. 2004;101:464–469. doi: 10.1073/pnas.0307161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Li X., Wu G., Yang Y., Fu S., Liu X., Kang H., Yang X., Su X.-C., Shen Y. Calmodulin dissociates the STIM1-Orai1 complex and STIM1 oligomers. Nat. Commun. 2017;8:1–14. doi: 10.1038/s41467-017-01135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Sun Y., Rossi A.M., Rahman T., Taylor C. Activation of IP3 receptors requires an endogenous 1-8-14 calmodulin-binding motif. Biochem. J. 2012;449:39–49. doi: 10.1042/BJ20121034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Li S., Xue J., Sun Z., Liu T., Zhang L., Wang L., You H., Fan Z., Zheng Y., Luo D. CaMKII Potentiates Store-Operated Ca2+ Entry Through Enhancing STIM1 Aggregation and Interaction with Orai1. Cell. Physiol. Biochem. 2018;46:1042–1054. doi: 10.1159/000488835. [DOI] [PubMed] [Google Scholar]
- 374.Nussinov R., Wang G., Tsai C.-J., Jang H., Lu S., Banerjee A., Zhang J., Gaponenko V. Calmodulin and PI3K Signaling in KRAS Cancers. Trends Cancer. 2017;3:214–224. doi: 10.1016/j.trecan.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Umemura M., Baljinnyam E., Feske S., De Lorenzo M.S., Xie L.-H., Feng X., Oda K., Makino A., Fujita T., Yokoyama U., et al. Store-Operated Ca2+ Entry (SOCE) Regulates Melanoma Proliferation and Cell Migration. PLoS ONE. 2014;9:e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Villalobo A., Berchtold M.W. The Role of Calmodulin in Tumor Cell Migration, Invasiveness, and Metastasis. Int. J. Mol. Sci. 2020;21:765. doi: 10.3390/ijms21030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Li T., Yi L., Hai L., Ma H., Tao Z., Zhang C., Abeysekera I., Zhao K., Yang Y., Wang W., et al. The interactome and spatial redistribution feature of Ca2+ receptor protein calmodulin reveals a novel role in invadopodia-mediated invasion. Cell Death Dis. 2018;9:1–17. doi: 10.1038/s41419-017-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Choi H.S., Kim D.-A., Chung H., Park I.H., Kim B.H., Oh E.-S., Kang D.-H. Screening of breast cancer stem cell inhibitors using a protein kinase inhibitor library. Cancer Cell Int. 2017;17:1–12. doi: 10.1186/s12935-017-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Kaur A., Raghavan M. A Calreticulin Tail: C-terminal Mutants of Calreticulin Allow Cancer Cells to Evade Phagocytosis. Mol. Cell. 2020;77:683–685. doi: 10.1016/j.molcel.2020.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Venkateswaran K., Verma A., Bhatt A.N., Shrivastava A., Manda K., Raj H.G., Prasad A., Len C., Parmar V.S., Dwarakanath B.S. Emerging Roles of Calreticulin in Cancer: Implications for Therapy. Curr. Protein Pept. Sci. 2018;19:344–357. doi: 10.2174/1389203718666170111123253. [DOI] [PubMed] [Google Scholar]
- 381.Han A., Li C., Zahed T., Wong M., Smith I., Hoedel K., Green D., Boiko A.D. Calreticulin is a Critical Cell Survival Factor in Malignant Neoplasms. PLoS Biol. 2019;17:e3000402. doi: 10.1371/journal.pbio.3000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Jin C., Lin T., Shan L. Downregulation of Calbindin 1 by miR-454-3p Suppresses Cell Proliferation in Nonsmall Cell Lung Cancer In Vitro. Cancer Biother. Radiopharm. 2019;34:119–127. doi: 10.1089/cbr.2018.2598. [DOI] [PubMed] [Google Scholar]
- 383.Pfyffer G.E., Humbel B., Sträuli P., Mohrmann I., Murer H., Heizmann C.W. Calcium-binding proteins in carcinoma, neuroblastoma and glioma cell lines. Virchows Archiv. 1987;412:135–144. doi: 10.1007/BF00716185. [DOI] [PubMed] [Google Scholar]
- 384.Lu Y.-C., Weng W.-C., Lee H. Functional Roles of Calreticulin in Cancer Biology. BioMed Res. Int. 2015;2015:1–9. doi: 10.1155/2015/526524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sun J., Mu H., Dai K., Yi L. Calreticulin: A potential anti-cancer therapeutic target. Die Pharm. 2017;72:503–510. doi: 10.1691/ph.2017.7031. [DOI] [PubMed] [Google Scholar]
- 386.Villagomez M., Szabo E., Podcheko A., Feng T., Papp S., Opas M. Calreticulin and focal-contact-dependent adhesion. Biochem. Cell Biol. 2009;87:545–556. doi: 10.1139/O09-016. [DOI] [PubMed] [Google Scholar]
- 387.Huang G., Sun Z., Wu J., Shui S., Han X., Guo D., Li T. Calreticulin Promotes Proliferation and Migration but Inhibits Apoptosis in Schwann Cells. Med. Sci. Monit. 2016;22:4516–4522. doi: 10.12659/MSM.900956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Clark R.A., Li S.-L., Pearson D.W., Leidal K.G., Clark J.R., Denning G.M., Reddick R., Krause K.-H., Valente A.J. Regulation of Calreticulin Expression during Induction of Differentiation in Human Myeloid Cells. J. Biol. Chem. 2002;277:32369–32378. doi: 10.1074/jbc.M205269200. [DOI] [PubMed] [Google Scholar]
- 389.Martins I., Kepp O., Galluzzi L., Senovilla L., Schlemmer F., Adjemian S., Menger L., Michaud M., Zitvogel L., Kroemer G. Surface-exposed calreticulin in the interaction between dying cells and phagocytes. Ann. N. Y. Acad. Sci. 2010;1209:77–82. doi: 10.1111/j.1749-6632.2010.05740.x. [DOI] [PubMed] [Google Scholar]
- 390.Raghavan M., Wijeyesakere S.J., Peters L.R., Del Cid N. Calreticulin in the immune system: Ins and outs. Trends Immunol. 2013;34:13–21. doi: 10.1016/j.it.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zamanian M., Hamadneh L.A.Q., Veerakumarasivam A., Rahman S.A., Shohaimi S., Rosli R. Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell Int. 2016;16:56. doi: 10.1186/s12935-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Banerjea A., Ahmed S., E Hands R., Huang F., Han X., Shaw P.M., Feakins R., A Bustin S., Dorudi S. Colorectal cancers with microsatellite instability display mRNA expression signatures characteristic of increased immunogenicity. Mol. Cancer. 2004;3:21. doi: 10.1186/1476-4598-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Harada K., Takenawa T., Ferdous T., Kuramitsu Y., Ueyama Y. Calreticulin is a novel independent prognostic factor for oral squamous cell carcinoma. Oncol. Lett. 2017;13:4857–4862. doi: 10.3892/ol.2017.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Chiang W.-F., Hwang T.-Z., Hour T.-C., Wang L.-H., Chiu C.-C., Chen H.-R., Wu Y.-J., Wang C.-C., Wang L.-F., Chien C.-Y., et al. Calreticulin, an endoplasmic reticulum-resident protein, is highly expressed and essential for cell proliferation and migration in oral squamous cell carcinoma. Oral Oncol. 2013;49:534–541. doi: 10.1016/j.oraloncology.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 395.Hacken E., Gounari M., Back J.W., Shimanovskaya E., Scarfò L., Kim E., Burks J., Ponzoni M., Ramirez G.A., Wierda W.G., et al. Calreticulin as a novel B-cell receptor antigen in chronic lymphocytic leukemia. Haematologica. 2017;102:e394–e396. doi: 10.3324/haematol.2017.169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Lwin Z.-M., Guo C., Salim A., Yip G.W.-C., Chew F.-T., Nan J., Thike A.A., Tan P.-H., Bay B.-H. Clinicopathological significance of calreticulin in breast invasive ductal carcinoma. Mod. Pathol. 2010;23:1559–1566. doi: 10.1038/modpathol.2010.173. [DOI] [PubMed] [Google Scholar]
- 397.Liu R., Gong J., Chen J., Li Q., Song C., Zhang J., Li Y., Liu Z., Dong Y., Chen L., et al. Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer Immunol. Immunother. 2011;61:855–864. doi: 10.1007/s00262-011-1146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Alur M., Nguyen M.M., Eggener S.E., Jiang F., Dadras S.S., Stern J., Kimm S., Roehl K., Kozlowski J., Pins M., et al. Suppressive Roles of Calreticulin in Prostate Cancer Growth and Metastasis. Am. J. Pathol. 2009;175:882–890. doi: 10.2353/ajpath.2009.080417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Hsu W.-M., Hsieh F.J., Jeng Y.-M., Kuo M.L., Chen C.-N., Lai D.M., Wang B.T., Tsao P.-N., Lee H., Lin M.T., et al. Calreticulin expression in neuroblastoma—A novel independent prognostic factor. Ann. Oncol. 2005;16:314–321. doi: 10.1093/annonc/mdi062. [DOI] [PubMed] [Google Scholar]
- 400.Sheng W., Chen C., Dong M., Zhou J., Liu Q., Dong Q., Li F. Overexpression of Calreticulin Contributes to the Development and Progression of Pancreatic Cancer. J. Cell. Physiol. 2014;229:887–897. doi: 10.1002/jcp.24519. [DOI] [PubMed] [Google Scholar]
- 401.Lee P.-C., Chiang J.-C., Chen C.-Y., Chien Y.-C., Chen W.-M., Huang C.-W., Weng W.-C., Chen C.-I., Lee P.-H., Chen C.-N., et al. Calreticulin regulates vascular endothelial growth factor-A mRNA stability in gastric cancer cells. PLoS ONE. 2019;14:e0225107. doi: 10.1371/journal.pone.0225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Fadel M.P., Szewczenko-Pawlikowski M., Leclerc P., Dziak E., Symonds J.M., Blaschuk O., Michalak M., Opas M. Calreticulin Affects β-Catenin-associated Pathways. J. Biol. Chem. 2001;276:27083–27089. doi: 10.1074/jbc.M101676200. [DOI] [PubMed] [Google Scholar]
- 403.Coppolino M.G., Woodside M.J., Demaurex N., Grinstein S., St-Arnaud R., Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nat. Cell Biol. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 404.Goicoechea S., Pallero M.A., Eggleton P., Michalak M., Murphy-Ullrich J.E. The Anti-adhesive Activity of Thrombospondin Is Mediated by the N-terminal Domain of Cell Surface Calreticulin. J. Biol. Chem. 2002;277:37219–37228. doi: 10.1074/jbc.M202200200. [DOI] [PubMed] [Google Scholar]
- 405.Hayashida Y., Urata Y., Muroi E., Kono T., Miyata Y., Nomata K., Kanetake H., Kondo T., Ihara Y. Calreticulin Represses E-cadherin Gene Expression in Madin-Darby Canine Kidney Cells via Slug. J. Biol. Chem. 2006;281:32469–32484. doi: 10.1074/jbc.M607240200. [DOI] [PubMed] [Google Scholar]
- 406.Chen C., Su T., Lu Y., Lee H. Calreticulin regulates cell proliferation and migration in gastric cancer cell line AGS. FASEB J. 2007;21:A1318. doi: 10.1096/fasebj.21.6.a1318-b. [DOI] [Google Scholar]
- 407.Yi L., Shan J., Chen X., Li G., Li L., Tan H., Su Q. Involvement of calreticulin in cell proliferation, invasion and differentiation in diallyl disulfide-treated HL-60 cells. Oncol. Lett. 2016;12:1861–1867. doi: 10.3892/ol.2016.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Lu Y.-C., Chen C.-N., Wang B., Hsu W.-M., Chen S.-T., Chang K.-J., Chang C.-C., Lee H. Changes in Tumor Growth and Metastatic Capacities of J82 Human Bladder Cancer Cells Suppressed by Down-Regulation of Calreticulin Expression. Am. J. Pathol. 2011;179:1425–1433. doi: 10.1016/j.ajpath.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.de Bruyn M., Wiersma V.R., Helfrich W., Eggleton P., Bremer E. The Ever-Expanding Immunomodulatory Role of Calreticulin in Cancer Immunity. Front. Oncol. 2015;5:35. doi: 10.3389/fonc.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Okunaga T., Urata Y., Goto S., Matsuo T., Mizota S., Tsutsumi K., Nagata I., Kondo T., Ihara Y., Kendall H.E., et al. Calreticulin, a Molecular Chaperone in the Endoplasmic Reticulum, Modulates Radiosensitivity of Human Glioblastoma U251MG Cells. Cancer Res. 2006;66:8662–8671. doi: 10.1158/0008-5472.CAN-05-4256. [DOI] [PubMed] [Google Scholar]
- 411.Matsukuma S., Yoshimura K., Ueno T., Oga A., Inoue M., Watanabe Y., Kuramasu A., Fuse M., Tsunedomi R., Nagaoka S., et al. Calreticulin is highly expressed in pancreatic cancer stem-like cells. Cancer Sci. 2016;107:1599–1609. doi: 10.1111/cas.13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Huang Z., Fan G., Wang D. Downregulation of calbindin 1, a calcium-binding protein, reduces the proliferation of osteosarcoma cells. Oncol. Lett. 2017;13:3727–3733. doi: 10.3892/ol.2017.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Favier J., Brière J.-J., Burnichon N., Rivière J., Vescovo L., Benit P., Giscos-Douriez I., De Reyniès A., Bertherat J., Badoual C., et al. The Warburg Effect Is Genetically Determined in Inherited Pheochromocytomas. PLoS ONE. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Seyfried T.N., Shelton L.M. Cancer as a metabolic disease. Nutr. Metab. 2010;7:1–9. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Hirschey M.D., DeBerardinis R.J., Diehl A.M.E., Drew J.E., Frezza C., Green M., Jones L.W., Ko Y.H., Le A., Lea M.A., et al. Dysregulated metabolism contributes to oncogenesis. Semin. Cancer Biol. 2015;35:S129–S150. doi: 10.1016/j.semcancer.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Amuthan G., Biswas G., Ananadatheerthavarada H.K., Vijayasarathy C., Shephard H.M., Avadhani N.G. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- 417.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 418.Kania E., Roest G., Vervliet T., Parys J.B., Bultynck G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017;7:140. doi: 10.3389/fonc.2017.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Parys J.B., Decuypere J.-P., Bultynck G. Role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy. Cell Commun. Signal. 2012;10:17. doi: 10.1186/1478-811X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Vicencio J.M., Ortiz C., Criollo A., E Jones A.W., Kepp O., Galluzzi L., Joza N., Vitale I., Morselli E., Tailler M., et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 421.Choi K.S. Autophagy and cancer. Exp. Mol. Med. 2012;44:109–120. doi: 10.3858/emm.2012.44.2.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zheng K., He Z., Kitazato K., Wang Y. Selective Autophagy Regulates Cell Cycle in Cancer Therapy. Theranostics. 2019;9:104–125. doi: 10.7150/thno.30308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Jones R.G., Plas D.R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M., Thompson C.B. AMP-Activated Protein Kinase Induces a p53-Dependent Metabolic Checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 425.Mathiassen S.G., De Zio D., Cecconi F. Autophagy and the Cell Cycle: A Complex Landscape. Front. Oncol. 2017;7:51. doi: 10.3389/fonc.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Sorokina I.V., Denisenko T.V., Imreh G., Tyurin-Kuzmin P.A., Kaminskyy V., Gogvadze V., Zhivotovsky B. Involvement of autophagy in the outcome of mitotic catastrophe. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-14901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Blandino G., Di Agostino S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J. Exp. Clin. Cancer Res. 2018;37:1–13. doi: 10.1186/s13046-018-0705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Chen H.-Y., White E. Role of Autophagy in Cancer Prevention. Cancer Prev. Res. 2011;4:973–983. doi: 10.1158/1940-6207.CAPR-10-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Li X., Zhou Y., Li Y., Yang L., Ma Y., Peng X., Yang S., Liu J., Li H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019;119:109415. doi: 10.1016/j.biopha.2019.109415. [DOI] [PubMed] [Google Scholar]
- 430.Smith A., MacLeod K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019;247:708–718. doi: 10.1002/path.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sui X., Chen R., Wang Z., Huang Z., Kong N., Zhang M., Han W., Lou F., Yang J., Zhang Q., et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013;4:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Liu E.Y., Ryan K.M. Autophagy and cancer—Issues we need to digest. J. Cell Sci. 2012;125:2349–2358. doi: 10.1242/jcs.093708. [DOI] [PubMed] [Google Scholar]
- 433.Patergnani S., Missiroli S., Marchi S., Giorgi C. Mitochondria-associated endoplasmic reticulum membranes microenvironment: Targeting autophagic and apoptotic pathways in cancer therapy. Front. Oncol. 2015;5:173. doi: 10.3389/fonc.2015.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Pihán P., Carreras-Sureda A., Hetz C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Lallemand V., Zhu J., Puvion F., Koken M., Honoré N., Doubeikovsky A., Duprez E., Pandolfi P.P., Puvion E., Freemont P., et al. Role of Promyelocytic Leukemia (Pml) Sumolation in Nuclear Body Formation, 11s Proteasome Recruitment, and as2O3-Induced Pml or Pml/Retinoic Acid Receptor α Degradation. J. Exp. Med. 2001;193:1361–1372. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Missiroli S., Bonora M., Patergnani S., Poletti F., Perrone M., Gafà R., Magri E., Raimondi A., Lanza G., Tacchetti C., et al. PML at Mitochondria-Associated Membranes Is Critical for the Repression of Autophagy and Cancer Development. Cell Rep. 2016;16:2415–2427. doi: 10.1016/j.celrep.2016.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Endoplasmic Reticulum-Mitochondria Ca 2+ Crosstalk in the Control of the Tumor Cell Fate. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:858–864. doi: 10.1016/j.bbamcr.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 438.Bultynck G., Campanella M. Tumor suppressive Ca2+ signaling is driven by IP3 receptor fitness. Cell Stress. 2017;1:73–78. doi: 10.15698/cst2017.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Hedgepeth S.C., Garcia M.I., Wagner L.E., II, Rodriguez A.M., Chintapalli S.V., Snyder R.R., Hankins G.D.V., Henderson B.R., Brodie K.M., Yule D.I., et al. The BRCA1 Tumor Suppressor Binds to Inositol 1,4,5-Trisphosphate Receptors to Stimulate Apoptotic Calcium Release. J. Biol. Chem. 2015;290:7304–7313. doi: 10.1074/jbc.M114.611186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Nussinov R., Muratcioglu S., Tsai C.-J., Jang H., Gursoy A., Keskin O. K-Ras4B/calmodulin/PI3Kα: A promising new adenocarcinoma-specific drug target? Expert Opin. Ther. Targets. 2016;20:831–842. doi: 10.1517/14728222.2016.1135131. [DOI] [PubMed] [Google Scholar]
- 441.Chen W., An P., Quan X.-J., Zhang J., Zhou Z.-Y., Zou L.-P., Luo H.-S. Ca2+/calmodulin-dependent protein kinase II regulates colon cancer proliferation and migration via ERK1/2 and p38 pathways. World J. Gastroenterol. 2017;23:6111–6118. doi: 10.3748/wjg.v23.i33.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Rizzuto R., De Stefani D., Raffaello A. Cristina Mammucari Mitochondria as sensors and regulators of calcium signaling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 444.Shoshan-Barmatz V., Mizrachi D., Mizrachi D. VDAC1: From structure to cancer therapy. Front. Oncol. 2012;2:164. doi: 10.3389/fonc.2012.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A., Arif T. Voltage-Dependent Anion Channel 1 As an Emerging Drug Target for Novel Anti-Cancer Therapeutics. Front. Oncol. 2017;7:154. doi: 10.3389/fonc.2017.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: Cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Abu-Hamad S., Arbel N., Calo D., Arzoine L., Israelson A., Keinan N., Ben-Romano R., Friedman O., Shoshan-Barmatz V. The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J. Cell Sci. 2009;122:1906–1916. doi: 10.1242/jcs.040188. [DOI] [PubMed] [Google Scholar]
- 448.Mallilankaraman K., Doonan P., Cárdenas C., Chandramoorthy H.C., Müller M., Miller R., Hoffman N.E., Gandhirajan R.K., Molgó J., Birnbaum M.J., et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Plovanich M., Bogorad R.L., Sancak Y., Kamer K.J., Strittmatter L., Li A.A., Girgis H.S., Kuchimanchi S., De Groot J., Speciner L., et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Graier W.F., Malli R. Mitochondrial calcium: A crucial hub for cancer cell metabolism? Transl. Cancer Res. 2017;6:S1124–S1127. doi: 10.21037/tcr.2017.08.28. [DOI] [Google Scholar]
- 451.Curry M.C., Peters A.A., Kenny P.A., Roberts-Thomson S.J., Monteith G.R. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2013;434:695–700. doi: 10.1016/j.bbrc.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 452.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T., Berecz T., Duchen M., Rosato A., Bogeski I., Szabadkai G., et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF -1α. EMBO Mol. Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Lawrence R.E., Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019;21:133–142. doi: 10.1038/s41556-018-0244-7. [DOI] [PubMed] [Google Scholar]
- 454.Galione A. A primer of NAADP-mediated Ca2+ signalling: From sea urchin eggs to mammalian cells. Cell Calcium. 2015;58:27–47. doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 455.Piao S., Amaravadi R.K. Targeting the lysosome in cancer. Ann. N. Y. Acad. Sci. 2016;1371:45–54. doi: 10.1111/nyas.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Yang S., Wang X., Contino G., Liesa M., Sahin E., Ying H., Bause A., Li Y., Stommel J.M., Dell’Antonio G., et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–729. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Hämälistö S., Jäättelä M. Lysosomes in cancer—living on the edge (of the cell) Curr. Opin. Cell Biol. 2016;39:69–76. doi: 10.1016/j.ceb.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yamaguchi S., Jha A., Li Q., Soyombo A., Dickinson G., Churamani D., Brailoiu E., Patel S., Muallem S. Transient Receptor Potential Mucolipin 1 (TRPML1) and Two-pore Channels Are Functionally Independent Organellar Ion Channels. J. Biol. Chem. 2011;286:22934–22942. doi: 10.1074/jbc.M110.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Favia A., Pafumi I., Desideri M., Padula F., Montesano C., Passeri D., Nicoletti C., Orlandi A., Del Bufalo D., Sergi M., et al. NAADP-Dependent Ca2+ Signaling Controls Melanoma Progression, Metastatic Dissemination and Neoangiogenesis. Sci. Rep. 2016;6:18925. doi: 10.1038/srep18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Nguyen O.N.P., Grimm C., Schneider L.S., Chao Y.-K., Atzberger C., Bartel K., Watermann A., Ulrich M., Mayr D., Wahl-Schott C., et al. Two-Pore Channel Function Is Crucial for the Migration of Invasive Cancer Cells. Cancer Res. 2017;77:1427–1438. doi: 10.1158/0008-5472.CAN-16-0852. [DOI] [PubMed] [Google Scholar]
- 461.Kern U., Wischnewski V., Biniossek M.L., Schilling O., Reinheckel T. Lysosomal protein turnover contributes to the acquisition of TGFβ-1 induced invasive properties of mammary cancer cells. Mol. Cancer. 2015;14:39. doi: 10.1186/s12943-015-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Zhang X., Cheng X., Yu L., Yang J., Calvo R., Patnaik S., Hu X., Gao Q., Yang M., Lawas M., et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016;7:12109. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Settembre C., Di Malta C., Polito V.A., Arencibia M.G., Vetrini F., Serkan E., Erdin S.U., Huynh T., Medina D., Colella P., et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Durinck S., Stawiski E.W., Pavía-Jiménez A., Modrusan Z., Kapur P., Jaiswal B.S., Zhang N., Toffessi-Tcheuyap V., Nguyen T., Pahuja K.B., et al. Spectrum of diverse genomic alterations define non–clear cell renal carcinoma subtypes. Nat. Genet. 2015;47:13–21. doi: 10.1038/ng.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Calcagni A., Kors L., Verschuren E., De Cegli R., Zampelli N., Nusco E., Confalonieri S., Bertalot G., Pece S., Settembre C., et al. Modelling TFE renal cell carcinoma in mice re-veals a critical role of WNT signaling. eLife. 2016;5:e17047. doi: 10.7554/eLife.17047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Perera R.M., Stoykova S., Nicolay B.N., Ross K.N., Fitamant J., Boukhali M., Lengrand J., Deshpande V., Selig M.K., Ferrone C.R., et al. Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nat. Cell Biol. 2015;524:361–365. doi: 10.1038/nature14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Medina D.L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Rosato A.S., Prezioso C., Forrester A., et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Giatromanolaki A., Kalamida D., Sivridis E., Karagounis I.V., Gatter K.C., Harris A.L., Koukourakis M.I. Increased expression of transcription factor EB (TFEB) is associated with autophagy, migratory phenotype and poor prognosis in non-small cell lung cancer. Lung Cancer. 2015;90:98–105. doi: 10.1016/j.lungcan.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 469.Liang J., Jia X., Wang K., Zhao N. High expression of TFEB is associated with aggressive clinical features in colorectal cancer. OncoTargets Ther. 2018;11:8089–8098. doi: 10.2147/OTT.S180112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Slade L., Biswas D., Ihionu F., El Hiani Y., Kienesberger P.C., Pulinilkunnil T. A Lysosome Independent Role for TFEB in Activating DNA Repair and Inhibiting Apoptosis in Breast Cancer Cells. Biochem. J. 2020;477:137–160. doi: 10.1042/BCJ20190596. [DOI] [PubMed] [Google Scholar]
- 471.Cui C., Chang Y., Zhang X., Choi S., Tran H., Penmetsa K.V., Viswanadha S., Fu L., Pan Z. Targeting Orai1-mediated store-operated calcium entry by RP4010 for anti-tumor activity in esophagus squamous cell carcinoma. Cancer Lett. 2018;432:169–179. doi: 10.1016/j.canlet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Serafini M., Cordero-Sanchez C., Di Paola R., Bhela I.P., Aprile S., Purghè B., Fusco R., Cuzzocrea S., Genazzani A.A., Riva B., et al. Store-Operated Calcium Entry as a Therapeutic Target in Acute Pancreatitis: Discovery and Development of Drug-Like SOCE Inhibitors. J. Med. Chem. 2020;63:14761–14779. doi: 10.1021/acs.jmedchem.0c01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Waldherr L., Tiffner A., Mishra D., Sallinger M., Schober R., Frischauf I., Schmidt T., Handl V., Sagmeister P., Köckinger M., et al. Blockage of Store-Operated Ca2+ Influx by Synta66 is Mediated by Direct Inhibition of the Ca2+ Selective Orai1 Pore. Cancers. 2020;12:2876. doi: 10.3390/cancers12102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Marchesini M., Gherli A., Montanaro A., Patrizi L., Sorrentino C., Pagliaro L., Rompietti C., Kitara S., Heit S., Olesen C.E., et al. Blockade of Oncogenic NOTCH1 with the SERCA Inhibitor CAD204520 in T Cell Acute Lymphoblastic Leukemia. Cell Chem. Biol. 2020;27:678–697.e13. doi: 10.1016/j.chembiol.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Pagliaro L., Marchesini M., Roti G. Targeting oncogenic Notch signaling with SERCA inhibitors. J. Hematol. Oncol. 2021;14:1–17. doi: 10.1186/s13045-020-01015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Patergnani S., Danese A., Bouhamida E., Aguiari G., Previati M., Pinton P., Giorgi C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020;21:8323. doi: 10.3390/ijms21218323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Prasad V., Mailankody S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Internal Med. 2017;177:1569–1575. doi: 10.1001/jamainternmed.2017.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018;3:7. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Lee D., Hong J.H. Physiological application of nanoparticles in calcium-related proteins and channels. Nanomedicine. 2019;14:2479–2486. doi: 10.2217/nnm-2019-0004. [DOI] [PubMed] [Google Scholar]
- 480.Colone M., Calcabrini A., Stringaro A. Drug Delivery Systems of Natural Products in Oncology. Molecules. 2020;25:4560. doi: 10.3390/molecules25194560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Bong A.H., Monteith G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta (BBA)—Bioenerg. 2018;1865:1786–1794. doi: 10.1016/j.bbamcr.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 482.Shen Y., Li X., Dong D., Zhang B., Xue Y., Shang P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018;8:916–931. [PMC free article] [PubMed] [Google Scholar]
- 483.Choudhary D., Goykar H., Karanwad T., Kannaujia S., Gadekar V., Misra M. An understanding of mitochondria and its role in targeting nanocarriers for diagnosis and treatment of cancer. Asian J. Pharm. Sci. 2020 doi: 10.1016/j.ajps.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Rahman S., Rahman T. Unveiling some FDA-approved drugs as inhibitors of the store-operated Ca2+ entry pathway. Sci. Rep. 2017;7:12881. doi: 10.1038/s41598-017-13343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Frandsen S.K., Vissing M., Gehl J. A Comprehensive Review of Calcium Electroporation—A Novel Cancer Treatment Modality. Cancers. 2020;12:290. doi: 10.3390/cancers12020290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Munk M., Alcalde J., Lorentzen L., Villalobo A., Berchtold M.W., Panina S. The impact of calmodulin on the cell cycle analyzed in a novel human cellular genetic system. Cell Calcium. 2020;88:102207. doi: 10.1016/j.ceca.2020.102207. [DOI] [PubMed] [Google Scholar]
- 487.Chai S., Xu X., Wang Y., Zhou Y., Zhang C., Yang Y., Xu H., Xu R., Wang K., Yang Y. Ca2+/calmodulin-dependent protein kinase IIγ enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget. 2015;6:16069–16083. doi: 10.18632/oncotarget.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Chi M., Evans H., Gilchrist J., Mayhew J., Hoffman A., Pearsall E.A., Jankowski H., Brzozowski J., Skelding K.A. Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci. Rep. 2016;6:33132. doi: 10.1038/srep33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Daft P.G., Yang Y., Napierala D., Zayzafoon M. The Growth and Aggressive Behavior of Human Osteosarcoma Is Regulated by a CaMKII-Controlled Autocrine VEGF Signaling Mechanism. PLoS ONE. 2015;10:e0121568. doi: 10.1371/journal.pone.0121568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Jung H.J., Kim J.H., Shim J.S., Kwon H.J. A Novel Ca2+/Calmodulin Antagonist HBC Inhibits Angiogenesis and Down-regulates Hypoxia-inducible Factor. J. Biol. Chem. 2010;285:25867–25874. doi: 10.1074/jbc.M110.135632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Can G., Akpinar B., Baran Y., Zhivotovsky B., Olsson M. 5-Fluorouracil signaling through a calcium–calmodulin-dependent pathway is required for p53 activation and apoptosis in colon carcinoma cells. Oncogene. 2013;32:4529–4538. doi: 10.1038/onc.2012.467. [DOI] [PubMed] [Google Scholar]
- 492.Ren T., Tang Y., Wang M., Wang H., Liu Y., Qian X., Chang C., Chen M. Triptolide induces apoptosis through the calcium/calmodulin-dependent protein kinase kinaseβ/AMP-activated protein kinase signaling pathway in non-small cell lung cancer cells. Oncol. Rep. 2020;44:2288–2296. doi: 10.3892/or.2020.7763. [DOI] [PubMed] [Google Scholar]
- 493.Karacosta L.G., Foster B.A., Azabdaftari G., Feliciano D., Edelman A.M. A Regulatory Feedback Loop Between Ca2+/Calmodulin-dependent Protein Kinase Kinase 2 (CaMKK2) and the Androgen Receptor in Prostate Cancer Progression. J. Biol. Chem. 2012;287:24832–24843. doi: 10.1074/jbc.M112.370783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Chimote A.A., Gawali V., Newton H.S., Wise-Draper T.M., Conforti L. A Compartmentalized Reduction in Membrane-Proximal Calmodulin Reduces the Immune Surveillance Capabilities of CD8+ T Cells in Head and Neck Cancer. Front. Pharmacol. 2020;11:143. doi: 10.3389/fphar.2020.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Racioppi L., Nelson E.R., Huang W., Mukherjee D., Lawrence S.A., Lento W., Masci A.M., Jiao Y., Park S., York B., et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer. Nat. Commun. 2019;10:1–16. doi: 10.1038/s41467-019-10424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Blum W., Schwaller B. Calretinin is essential for mesothelioma cell growth/survival in vitro: A potential new target for malignant mesothelioma therapy? Int. J. Cancer. 2013;133:2077–2088. doi: 10.1002/ijc.28218. [DOI] [PubMed] [Google Scholar]
- 497.Winn B., Tavares R., Fanion J., Noble L., Gao J., Sabo E., Resnick M.B. Differentiating the undifferentiated: Immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum. Pathol. 2009;40:398–404. doi: 10.1016/j.humpath.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Wörthmüller J., Oberson A., Salicio V., Blum W., Schwaller B. Calretinin Functions in Malignant Mesothelioma Cells Cannot Be Replaced by the Closely Related Ca2+-Binding Proteins Calbindin-D28k and Parvalbumin. Int. J. Mol. Sci. 2018;19:4015. doi: 10.3390/ijms19124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Dodla P., Bhoopalan V., Khoo S.K., Miranti C., Sridhar S. Gene expression analysis of human prostate cell lines with and without tumor metastasis suppressor CD82. BMC Cancer. 2020;20:1–17. doi: 10.1186/s12885-020-07675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Bignotti E., Tassi R.A., Calza S., Ravaggi A., Bandiera E., Rossi E., Donzelli C., Pasinetti B., Pecorelli S., Santin A.D. Gene expression profile of ovarian serous papillary carcinomas: Identification of metastasis-associated genes. Am. J. Obstet. Gynecol. 2007;196:245.e1–245.e11. doi: 10.1016/j.ajog.2006.10.874. [DOI] [PubMed] [Google Scholar]
- 501.Wörthmüller J., Blum W., Pecze L., Salicio V., Schwaller B. Calretinin promotes invasiveness and EMT in malignant mesothelioma cells involving the activation of the FAK signaling pathway. Oncotarget. 2018;9:36256–36272. doi: 10.18632/oncotarget.26332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Gordillo C.H., Sandoval P., Muñoz-Hernández P., Pascual-Antón L., López-Cabrera M., Jiménez-Heffernan J.A. Mesothelial-to-Mesenchymal Transition Contributes to the Generation of Carcinoma-Associated Fibroblasts in Locally Advanced Primary Colorectal Carcinomas. Cancers. 2020;12:499. doi: 10.3390/cancers12020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Jung E.M., Choi K.C., Jeung E.B. Expression of calbindin-D28k is inversely correlated with proapototic gene expression in hydrogen peroxide-induced cell death in endometrial cancer cells. Int. J. Oncol. 2011;38:1059–1066. doi: 10.3892/ijo.2011.916. [DOI] [PubMed] [Google Scholar]
- 504.Stevenson L., Allen W.L., Proutski I., Stewart G., Johnston L., McCloskey K., Wilson P.M., Longley D.B., Johnston P.G. Calbindin 2 (CALB2) Regulates 5-Fluorouracil Sensitivity in Colorectal Cancer by Modulating the Intrinsic Apoptotic Pathway. PLoS ONE. 2011;6:e20276. doi: 10.1371/journal.pone.0020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Sergeev I.N. Vitamin D and cellular Ca2+ signaling in breast cancer. Anticancer. Res. 2012;32:299–302. [PubMed] [Google Scholar]
- 506.Kim J.H., sil Hong B., Heo W., Han J.M., Han W., Noh D.-Y., Moon H.-G. Abstract 33: Calsequestrin 2 regulates prolif-eration, migration, and invasion in triple-negative breast cancer cells. Cancer Res. 2018;78((Suppl. 13)):33. doi: 10.1158/1538-7445.am2018-33. [DOI] [Google Scholar]
- 507.Toquet C., Jarry A., Bou-Hanna C., Bach K., Denis M.G., Mosnier J.F., Laboisse C.L. Altered Calreticulin expression in human colon cancer: Maintenance of Calreticulin expression is associated with mucinous differentiation. Oncol. Rep. 2007;17:1101–1107. doi: 10.3892/or.17.5.1101. [DOI] [PubMed] [Google Scholar]
- 508.Vanoverberghe K., Abeele F.V., Mariot P., Lepage G., Roudbaraki M., Bonnal J.L., Mauroy B., Shuba Y., Skryma R., Prevarskaya N. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2003;11:321–330. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- 509.Han Y., Liao Q., Wang H., Rao S., Yi P., Tang L., Tian Y., Oyang L., Wang H., Shi Y., et al. High expression of calreticulin indicates poor prognosis and modulates cell migration and invasion via activating Stat3 in nasopharyngeal carcinoma. J. Cancer. 2019;10:5460–5468. doi: 10.7150/jca.35362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Pouliquen D.L., Boissard A., Coqueret O., Guette C. Biomarkers of tumor invasiveness in proteomics (Review) Int. J. Oncol. 2020;57:409–432. doi: 10.3892/ijo.2020.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Amuthan G., Biswas G., Zhang S., Klein-Szanto A., Vijayasarathy C., Avadhani N.G. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Pike S.E., Yao L., Setsuda J., Jones K.D., Cherney B., Appella E., Sakaguchi K., Nakhasi H., Atreya C.D., Teruya-Feldstein J., et al. Calreticulin and calreticulin fragments are endothelial cell inhibitors that suppress tumor growth. Blood. 1999;94:2461–2468. doi: 10.1182/blood.V94.7.2461.419a26_2461_2468. [DOI] [PubMed] [Google Scholar]
- 513.Delom F., Emadali A., Cocolakis E., Lebrun J.-J., Nantel A., Chevet E. Calnexin-dependent regulation of tunicamycin-induced apoptosis in breast carcinoma MCF-7 cells. Cell Death Differ. 2006;14:586–596. doi: 10.1038/sj.cdd.4402012. [DOI] [PubMed] [Google Scholar]
- 514.Li D.-D., Xie B., Wu X.-J., Li J.-J., Ding Y., Wen X.-Z., Zhang X., Zhu S.-G., Liu W., Zhang X.-S., et al. Late-stage inhibition of autophagy enhances calreticulin surface exposure. Oncotarget. 2016;7:80842–80854. doi: 10.18632/oncotarget.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Obeid M., Tesniere A., Ghiringhelli F., Fimia G.M., Apetoh L., Perfettini J.-L., Castedo M., Mignot G., Panaretakis T., Casares N., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 516.Chen Y., Ma D., Wang X., Fang J., Liu X., Song J., Li X., Ren X., Li Q., Li Q., et al. Calnexin Impairs the Antitumor Immunity of CD4+ and CD8+ T Cells. Cancer Immunol. Res. 2018;7:123–135. doi: 10.1158/2326-6066.CIR-18-0124. [DOI] [PubMed] [Google Scholar]
- 517.Loulousis M., Krager S.L., Darcy Y.L., Tischkau S.A., Copello J.A. Drugs that inhibit the sarcoplasmic reticulum Ca2+ atpase (Serca) and prevention of breast cancer cell proliferation. FASEB J. 2016;30:768.4. doi: 10.1096/fasebj.30.1_supplement.768.4. [DOI] [Google Scholar]
- 518.Lee W.J., Robinson J.A., Holman N.A., McCall M.N., Roberts-Thomson S., Monteith G. Antisense-mediated Inhibition of the Plasma Membrane Calcium-ATPase Suppresses Proliferation of MCF-7 Cells. J. Biol. Chem. 2005;280:27076–27084. doi: 10.1074/jbc.M414142200. [DOI] [PubMed] [Google Scholar]
- 519.Chovancova B., Liskova V., Babula P., Krizanova O. Role of Sodium/Calcium Exchangers in Tumors. Biomolecules. 2020;10:1257. doi: 10.3390/biom10091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Sritangos P., Alarcon E.P., James A., Sultan A., Richardson D.A., Bruce J.I.E. Plasma Membrane Ca2+ ATPase Isoform 4 (PMCA4) Has an Important Role in Numerous Hallmarks of Pancreatic Cancer. Cancers. 2020;12:218. doi: 10.3390/cancers12010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Fondello C., Agnetti L., Glikin G.C., Finocchiaro L.M. Mechanisms Enhancing the Cytotoxic Effects of Bleomycin plus Suicide or Interferon-β Gene Lipofection in Metastatic Human Melanoma Cells. Anti-Cancer Agents Med. Chem. 2019;18:1338–1348. doi: 10.2174/1871520618666180604084849. [DOI] [PubMed] [Google Scholar]
- 522.Szadvari I., Hudecova S., Chovancova B., Matuskova M., Cholujova D., Lencesova L., Valerian D., Ondrias K., Babula P., Krizanova O. Sodium/calcium exchanger is involved in apoptosis induced by H2S in tumor cells through decreased levels of intracellular pH. Nitric Oxide. 2019;87:1–9. doi: 10.1016/j.niox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 523.Ho P.-C., Bihuniak J.D., Macintyre A., Staron M., Liu X., Amezquita R., Tsui Y.-C., Cui G., Micevic G., Perales J.C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162:1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.James A., Richardson D.A., Oh I.-W., Sritangos P., Attard T., Barrett L., Bruce J.I.E. Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: Role of pyruvate kinase-M2 (PKM2) Br. J. Cancer. 2020;122:266–278. doi: 10.1038/s41416-019-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Márián T., Szabó-Péli J., Németh E., Trón L., Friedländer E., Szabó A., Balkay L., Veress G., Krasznai Z. Na+/Ca2+ exchanger inhibitors modify the accumulation of tumor-diagnostic PET tracers in cancer cells. Eur. J. Pharm. Sci. 2007;30:56–63. doi: 10.1016/j.ejps.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 526.Ghosh S., Adhikary A., Chakraborty S., Nandi P., Mohanty S., Chakraborty S., Bhattacharjee P., Mukherjee S., Putatunda S., Chakraborty S., et al. Nifetepimine, a Dihydropyrimidone, Ensures CD4+ T Cell Survival in a Tumor Microenvironment by Maneuvering Sarco(endo)plasmic Reticulum Ca2+ ATPase (SERCA) J. Biol. Chem. 2012;287:32881–32896. doi: 10.1074/jbc.M112.357889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Bhargava A., Saha S. T-Type voltage gated calcium channels: A target in breast cancer? Breast Cancer Res. Treat. 2019;173:11–21. doi: 10.1007/s10549-018-4970-0. [DOI] [PubMed] [Google Scholar]
- 528.Xie R., Xu J., Wen G., Jin H., Liu X., Yang Y., Ji B., Jiang Y., Song P., Dong H., et al. The P2Y2 Nucleotide Receptor Mediates the Proliferation and Migration of Human Hepatocellular Carcinoma Cells Induced by ATP. J. Biol. Chem. 2014;289:19137–19149. doi: 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Adinolfi E., Melchiorri L., Falzoni S., Chiozzi P., Morelli A., Tieghi A., Cuneo A., Castoldi G., Di Virgilio F., Baricordi O.R. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood. 2002;99:706–708. doi: 10.1182/blood.V99.2.706. [DOI] [PubMed] [Google Scholar]
- 530.Ledur P.F., Villodre E.S., Paulus R., Cruz L.A., Flores D.G., Lenz G. Extracellular ATP reduces tumor sphere growth and cancer stem cell population in glioblastoma cells. Purinergic Signal. 2011;8:39–48. doi: 10.1007/s11302-011-9252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Conigrave A.D., van der Weyden L., Holt L., Jiang L., Wilson P., I Christopherson R., Morris M.B. Extracellular ATP-dependent suppression of proliferation and induction of differentiation of human HL-60 leukemia cells by distinct mechanisms. Biochem. Pharmacol. 2000;60:1585–1591. doi: 10.1016/S0006-2952(00)00465-2. [DOI] [PubMed] [Google Scholar]
- 532.Maiques O., Macià A., Moreno S., Barceló C., Santacana M., Vea A., Herreros J., Gatius S., Ortega E., Valls J., et al. Immunohistochemical analysis of T-type calcium channels in acquired melanocytic naevi and melanoma. Br. J. Dermatol. 2016;176:1247–1258. doi: 10.1111/bjd.15121. [DOI] [PubMed] [Google Scholar]
- 533.Zhou X., Wang W., Zhang S., Wang X., Tang Z., Gu J., Li J., Huang J. CACNA1B (Cav2.2) Overexpression and Its Association with Clinicopathologic Characteristics and Unfavorable Prognosis in Non-Small Cell Lung Cancer. Dis. Markers. 2017;2017:1–8. doi: 10.1155/2017/6136401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Schumacher D., Strilic B., Sivaraj K.K., Wettschureck N., Offermanns S. Platelet-Derived Nucleotides Promote Tumor-Cell Transendothelial Migration and Metastasis via P2Y2 Receptor. Cancer Cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 535.Takai E., Tsukimoto M., Harada H., Kojima S. Autocrine signaling via release of ATP and activation of P2X7 receptor influences motile activity of human lung cancer cells. Purinergic Signal. 2014;10:487–497. doi: 10.1007/s11302-014-9411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Rumjahn S.M., A Javed M., Wong N., E Law W., O Buxton I.L. Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. Br. J. Cancer. 2007;97:1372–1380. doi: 10.1038/sj.bjc.6604019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Dziegielewska B., Gray L.S., Dziegielewski J. T-type calcium channels blockers as new tools in cancer therapies. Pflügers Archiv—Eur. J. Physiol. 2014;466:801–810. doi: 10.1007/s00424-014-1444-z. [DOI] [PubMed] [Google Scholar]
- 538.Gilbert S.M., Oliphant C.J., Hassan S., Peille A.L., Bronsert P., Falzoni S., Di Virgilio F., McNulty S., Lara R. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene. 2019;38:194–208. doi: 10.1038/s41388-018-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Höpfner M., Maaser K., Barthel B., Von Lampe B., Hanski C., Riecken E.-O., Zeitz M., Scherübl H. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: Involvement of intracellular calcium and cyclic adenosine monophosphate. Int. J. Color. Dis. 2001;16:154–166. doi: 10.1007/s003840100302. [DOI] [PubMed] [Google Scholar]
- 540.Sallán M.C., Visa A., Shaikh S., Nàger M., Herreros J., Cantí C. T-type Ca2+ Channels: T for Targetable. Cancer Res. 2018;78:603–609. doi: 10.1158/0008-5472.CAN-17-3061. [DOI] [PubMed] [Google Scholar]
- 541.Amoroso F.S., Capece M., Rotondo A., Cangelosi D., Ferracin M., Franceschini A., Raffaghello L., Pistoia V., Varesio L., Adinolfi E. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: Evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 542.Pfaffenzeller M.S., Franciosi M.L.M., Cardoso A.M. Purinergic signaling and tumor microenvironment in cervical Cancer. Purinergic Signal. 2020;16:123–135. doi: 10.1007/s11302-020-09693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Wu L., Lin W., Liao Q., Wang H., Lin C., Tang L., Lian W., Chen Z., Li K., Xu L., et al. Calcium Channel Blocker Nifedipine Suppresses Colorectal Cancer Progression and Immune Escape by Preventing NFAT2 Nuclear Translocation. Cell Rep. 2020;33:108327. doi: 10.1016/j.celrep.2020.108327. [DOI] [PubMed] [Google Scholar]
- 544.Karacicek B., Erac Y., Tosun M. Functional consequences of enhanced expression of STIM1 and Orai1 in Huh-7 hepatocellular carcinoma tumor-initiating cells. BMC Cancer. 2019;19:1–10. doi: 10.1186/s12885-019-5947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Faouzi M., Kischel P., Hague F., Ahidouch A., Benzerdjeb N., Sevestre H., Penner R., Ouadid-Ahidouch H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim. Biophys. Acta. 2013;1833:752–760. doi: 10.1016/j.bbamcr.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 546.Hou M.-F., Kuo H.-C., Li J.-H., Wang Y.-S., Chang C.-C., Chen K.-C., Chen W.-C., Chiu C.-C., Yang S., Chang W.-C. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2011;1810:1278–1284. doi: 10.1016/j.bbagen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 547.Diez-Bello R., Jardin I., Salido G.M., Rosado J. Orai1 and Orai2 mediate store-operated calcium entry that regulates HL60 cell migration and FAK phosphorylation. Biochim. Biophys. Acta (BBA)—Bioenerg. 2017;1864:1064–1070. doi: 10.1016/j.bbamcr.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 548.Zhang Z., Liu X., Feng B., Liu N., Wu Q., Han Y., Nie Y., Wu K., Shi Y., Fan D. STIM1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in colorectal cancer. Oncogene. 2015;34:4808–4820. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Lee S.H., Rigas N.K., Lee C.-R., Bang A., Srikanth S., Gwack Y., Kang M.K., Kim R.H., Park N.-H., Shin K.-H. Orai1 promotes tumor progression by enhancing cancer stemness via NFAT signaling in oral/oropharyngeal squamous cell carcinoma. Oncotarget. 2016;7:43239–43255. doi: 10.18632/oncotarget.9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Wu S., Chen M., Huang J., Zhang F., Lv Z., Jia Y., Cui Y.-Z., Sun L.-Z., Wang Y., Tang Y., et al. ORAI2 Promotes Gastric Cancer Tumorigenicity and Metastasis through PI3K/Akt Signaling and MAPK-Dependent Focal Adhesion Disassembly. Cancer Res. 2021;81:986–1000. doi: 10.1158/0008-5472.CAN-20-0049. [DOI] [PubMed] [Google Scholar]
- 551.Miao Y., Shen Q., Zhang S., Huang H., Meng X., Zheng X., Yao Z., He Z., Lu S., Cai C., et al. Calcium-sensing stromal interaction molecule 2 upregulates nuclear factor of activated T cells 1 and transforming growth factor-β signaling to promote breast cancer metastasis. Breast Cancer Res. 2019;21:1–12. doi: 10.1186/s13058-019-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Sun J., Lu F., He H., Shen J., Messina J., Mathew R., Wang D., Sarnaik A.A., Chang W.-C., Kim M., et al. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J. Cell Biol. 2014;207:535–548. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Pan S., Zhao X., Shao C., Fu B., Huang Y., Zhang N., Dou X., Zhang Z., Qiu Y., Wang R., et al. STIM1 promotes angiogenesis by reducing exosomal miR-145 in breast cancer MDA-MB-231 cells. Cell Death Dis. 2021;12:1–15. doi: 10.1038/s41419-020-03304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Liu X., Wan X., Kan H., Wang Y., Yu F., Feng L., Jin J., Zhang P., Ma X. Hypoxia-induced upregulation of Orai1 drives colon cancer invasiveness and angiogenesis. Eur. J. Pharmacol. 2018;832:1–10. doi: 10.1016/j.ejphar.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 555.Sun X., Wei Q., Cheng J., Bian Y., Tian C., Hu Y., Li H. Enhanced Stim1 expression is associated with acquired chemo-resistance of cisplatin in osteosarcoma cells. Hum. Cell. 2017;30:216–225. doi: 10.1007/s13577-017-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Faouzi M., Hague F., Potier M., Ahidouch A., Sevestre H., Ouadid-Ahidouch H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J. Cell. Physiol. 2011;226:542–551. doi: 10.1002/jcp.22363. [DOI] [PubMed] [Google Scholar]
- 557.Liu H., Hughes J.D., Rollins S., Chen B., Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp. Mol. Pathol. 2011;91:753–760. doi: 10.1016/j.yexmp.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 558.Zhao H., Yan G., Zheng L., Zhou Y., Sheng H., Wu L., Zhang Q., Lei J., Zhang J., Xin R., et al. STIM1 is a metabolic checkpoint regulating the invasion and metastasis of hepatocellular carcinoma. Theranostics. 2020;10:6483–6499. doi: 10.7150/thno.44025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Frisch J., Angenendt A., Hoth M., Prates Roma L., Lis A. STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment. Cancers. 2019;11:457. doi: 10.3390/cancers11040457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Tang B.-D., Xia X., Lv X.-F., Yu B.-X., Yuan J.-N., Mai X.-Y., Shang J.-Y., Zhou J.-G., Liang S.-J., Pang R.-P. Inhibition of Orai1-mediated Ca2+entry enhances chemosensitivity of HepG2 hepatocarcinoma cells to 5-fluorouracil. J. Cell. Mol. Med. 2016;21:904–915. doi: 10.1111/jcmm.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Fleur-Lominy S.S., Maus M., Vaeth M., Lange I., Zee I., Suh D., Liu C., Wu X., Tikhonova A., Aifantis I., et al. STIM1 and STIM2 Mediate Cancer-Induced Inflammation in T Cell Acute Lymphoblastic Leukemia. Cell Rep. 2018;24:3045–3060.e5. doi: 10.1016/j.celrep.2018.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Xu Y., Zhang S., Niu H., Ye Y., Hu F., Chen S., Li X., Luo X., Jiang S., Liu Y., et al. STIM1 accelerates cell senescence in a remodeled microenvironment but enhances the epithelial-to-mesenchymal transition in prostate cancer. Sci. Rep. 2015;5:11754. doi: 10.1038/srep11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Szatkowski C., Parys J.B., Ouadid-Ahidouch H., Matifat F. Inositol 1,4,5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol. Cancer. 2010;9:156. doi: 10.1186/1476-4598-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Nougarède A., Popgeorgiev N., Kassem L., Omarjee S., Borel S., Mikaelian I., Lopez J., Gadet R., Marcillat O., Treilleux I., et al. Breast Cancer Targeting through Inhibition of the Endoplasmic Reticulum-Based Apoptosis Regulator Nrh/BCL2L10. Cancer Res. 2018;78:1404–1417. doi: 10.1158/0008-5472.CAN-17-0846. [DOI] [PubMed] [Google Scholar]
- 565.Thoppil R.J., Adapala R.K., Cappelli H.C., Kondeti V., Dudley A.C., Gary Meszaros J., Paruchuri S., Thodeti C.K. TRPV4 channel activation selectively inhibits tumor endothelial cell proliferation. Sci. Rep. 2015;5:14257. doi: 10.1038/srep14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Bomben V.C., Sontheimer H. Disruption of transient receptor potential canonical channel 1 causes incomplete cytokinesis and slows the growth of human malignant gliomas. Glia. 2010;58:1145–1156. doi: 10.1002/glia.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Jiang J., Li M.-H., Inoue K., Chu X.-P., Seeds J., Xiong Z.-G. Transient Receptor Potential Melastatin 7–like Current in Human Head and Neck Carcinoma Cells: Role in Cell Proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Sun C., Shui B., Zhao W., Liu H., Li W., Lee J.C., Doran R., Lee F.K., Sun T., Shen Q.S., et al. Central role of IP3R2-mediated Ca2+ oscillation in self-renewal of liver cancer stem cells elucidated by high-signal ER sensor. Cell Death Dis. 2019;10:1–13. doi: 10.1038/s41419-019-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Bidaux G., Flourakis M., Thébault S., Zholos A., Beck B., Gkika D., Roudbaraki M., Bonnal J.-L., Mauroy B., Shuba Y., et al. Prostate cell differentiation status determines transient receptor potential melastatin member 8 channel subcellular localization and function. J. Clin. Investig. 2007;117:1647–1657. doi: 10.1172/JCI30168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Jiang H.-N., Zeng B., Zhang Y., Daskoulidou N., Fan H., Qu J.-M., Xu S.-Z. Involvement of TRPC Channels in Lung Cancer Cell Differentiation and the Correlation Analysis in Human Non-Small Cell Lung Cancer. PLoS ONE. 2013;8:e67637. doi: 10.1371/journal.pone.0067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Middelbeek J., Visser D., Henneman L., Kamermans A., Kuipers A.J., Hoogerbrugge P.M., Jalink K., Van Leeuwen F.N. TRPM7 maintains progenitor-like features of neuroblastoma cells: Implications for metastasis formation. Oncotarget. 2015;6:8760–8776. doi: 10.18632/oncotarget.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Kang S.S., Han K.-S., Ku B.M., Lee Y.K., Hong J., Shin H.Y., Almonte A., Woo D.H., Brat D.J., Hwang E.M., et al. Caffeine-Mediated Inhibition of Calcium Release Channel Inositol 1,4,5-Trisphosphate Receptor Subtype 3 Blocks Glioblastoma Invasion and Extends Survival. Cancer Res. 2010;70:1173–1183. doi: 10.1158/0008-5472.CAN-09-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Huang X., Jin M., Chen Y.-X., Wang J., Chang Y., Yuan Q., Yao K.-T., Ji G. ERP44 inhibits human lung cancer cell migration mainly via IP3R2. Aging. 2016;8:1276–1286. doi: 10.18632/aging.100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Xu N., Zhang D., Chen J., He G., Gao L. Low expression of ryanodine receptor 2 is associated with poor prognosis in thyroid carcinoma. Oncol. Lett. 2019;18:3605–3612. doi: 10.3892/ol.2019.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.De Quirós S.B., Merlo A., Secades P., Zambrano I., de Santa María I.S., Ugidos N., Jantus-Lewintre E., Sirera R., Suarez C., Chiara M.-D. Identification of TRPC6 as a possible candidate target gene within an amplicon at 11q21-q22.2 for migratory capacity in head and neck squamous cell carcinomas. BMC Cancer. 2013;13:116. doi: 10.1186/1471-2407-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Ramer R., Merkord J., Rohde H., Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010;79:955–966. doi: 10.1016/j.bcp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 577.Gaunt H.J., Vasudev N.S., Beech D.J. Transient receptor potential canonical 4 and 5 proteins as targets in cancer therapeutics. Eur. Biophys. J. 2016;45:611–620. doi: 10.1007/s00249-016-1142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Kanugula A.K., Adapala R.K., Midha P., Cappelli H.C., Meszaros J.G., Paruchuri S., Chilian W.M., Thodeti C.K. Novel noncanonical regulation of soluble VEGF/VEGFR2 signaling by mechanosensitive ion channel TRPV4. FASEB J. 2019;33:195–203. doi: 10.1096/fj.201800509R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Bernardini M., Brossa A., Chinigo G., Grolez G.P., Trimaglio G., Allart L., Hulot A., Marot G., Genova T., Joshi A., et al. Transient Receptor Potential Channel Expression Signatures in Tumor-Derived Endothelial Cells: Functional Roles in Prostate Cancer Angiogenesis. Cancers. 2019;11:956. doi: 10.3390/cancers11070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Santoni G., Morelli M., Santoni M., Nabissi M. New deals on the transcriptional and post-transcriptional regulation of TRP channel target genes during the angiogenesis of glioma. J. Exp. Integr. Med. 2011;1:221–234. doi: 10.5455/jeim.290711.ir.006. [DOI] [Google Scholar]
- 581.Mariot P., Prevarskaya N., Roudbaraki M.M., Le Bourhis X., Van Coppenolle F., Vanoverberghe K., Skryma R. Evidence of functional ryanodine receptor involved in apoptosis of prostate cancer (Lncap) cells. Prostate. 2000;43:205–214. doi: 10.1002/(SICI)1097-0045(20000515)43:3<205::AID-PROS6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 582.Shin D.-H., Leem D.-G., Shin J.-S., Kim J.-I., Kim K.-T., Choi S.Y., Lee M.-H., Choi J.-H., Lee K.-T. Compound K induced apoptosis via endoplasmic reticulum Ca2+ release through ryanodine receptor in human lung cancer cells. J. Ginseng Res. 2018;42:165–174. doi: 10.1016/j.jgr.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Rezuchova I., Hudecova S., Soltysova A., Matuskova M., Durinikova E., Chovancova B., Zuzcak M., Cihova M., Burikova M., Penesova A., et al. Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis. 2019;10:186. doi: 10.1038/s41419-019-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Akl H., Monaco G., La Rovere R., Welkenhuyzen K., Kiviluoto S., Vervliet T., Molgó J., Distelhorst C.W., Missiaen L., Mikoshiba K., et al. IP3R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP3R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Boutin B., Tajeddine N., Monaco G., Molgo J., Vertommen D., Rider M., Parys J., Bultynck G., Gailly P. Endoplasmic reticulum Ca2+ content decrease by PKA-dependent hyperphosphorylation of type 1 IP3 receptor contributes to prostate cancer cell resistance to androgen deprivation. Cell Calcium. 2015;57:312–320. doi: 10.1016/j.ceca.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 586.Kiss F., Pohóczky K., Szállási A., Helyes Z. Transient Receptor Potential (TRP) Channels in Head-and-Neck Squamous Cell Carcinomas: Diagnostic, Prognostic, and Therapeutic Potentials. Int. J. Mol. Sci. 2020;21:6374. doi: 10.3390/ijms21176374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Monet M., Gkika D., Lehen’Kyi V., Pourtier A., Abeele F.V., Bidaux G., Juvin V., Rassendren F., Humez S., Prevarsakaya N. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim. Biophys. Acta (BBA)—Bioenerg. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 588.Liu X., Zou J., Su J., Lu Y., Zhang J., Li L., Yin F. Downregulation of transient receptor potential cation channel, subfamily C, member 1 contributes to drug resistance and high histological grade in ovarian cancer. Int. J. Oncol. 2015;48:243–252. doi: 10.3892/ijo.2015.3254. [DOI] [PubMed] [Google Scholar]
- 589.Cardenas C., Lovy A., Silva-Pavez E., Urra F., Mizzoni C., Ahumada-Castro U., Bustos G., Jaňa F., Cruz P., Farias P., et al. Cancer cells with defective oxidative phosphorylation require endoplasmic reticulum–to–mitochondria Ca2+transfer for survival. Sci. Signal. 2020;13:eaay1212. doi: 10.1126/scisignal.aay1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.Law B.Y.K., Michelangeli F., Qu Y.Q., Xu S.-W., Han Y., Mok S.W.F., de Seabra Rodrigues Dias I.R., Javed M.-H., Chan W.-K., Xue W.-W., et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca2+-dependent mechanism. Sci. Rep. 2019;9:20034. doi: 10.1038/s41598-019-56675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Fels B., Bulk E., Pethő Z., Schwab A. The Role of TRP Channels in the Metastatic Cascade. Pharmaceuticals. 2018;11:48. doi: 10.3390/ph11020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Liu X., Zhang P., Xie C., Sham K.W.-Y., Ng S.S.M., Chen Y., Cheng C.H.K. Activation of PTEN by inhibition of TRPV4 suppresses colon cancer development. Cell Death Dis. 2019;10:1–14. doi: 10.1038/s41419-019-1700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Huang Y., Li S., Jia Z., Zhao W., Zhou C., Zhang R., Ali D.W., Michalak M., Chen X.-Z., Tang J. Transient Receptor Potential Melastatin 8 (TRPM8) Channel Regulates Proliferation and Migration of Breast Cancer Cells by Activating the AMPK-ULK1 Pathway to Enhance Basal Autophagy. Front. Oncol. 2020;10:2645. doi: 10.3389/fonc.2020.573127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Lan X., Zhao J., Song C., Yuan Q., Liu X. TRPM8 facilitates proliferation and immune evasion of esophageal cancer cells. Biosci. Rep. 2019;39:BSR20191878. doi: 10.1042/BSR20191878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Yu S., Huang S., Ding Y., Wang W., Wang A., Lu Y. Transient receptor potential ion-channel subfamily V member 4: A potential target for cancer treatment. Cell Death Dis. 2019;10:1–17. doi: 10.1038/s41419-019-1708-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Yamamoto M., Horie I., Isohama Y., Tsukimoto M. Activation of TRPV4 Channel Regulates Differentiation to and Function of Myeloid-Derived Suppressor Cells. BPB Rep. 2020;3:70–75. doi: 10.1248/bpbreports.3.2_70. [DOI] [Google Scholar]
- 597.Eliaa S.G., Al-Karmalawy A.A., Saleh R.M., ElShal M.F. Empagliflozin and Doxorubicin Synergistically Inhibit the Survival of Triple-Negative Breast Cancer Cells via Interfering with the mTOR Pathway and Inhibition of Calmodulin: In Vitro and Molecular Docking Studies. ACS Pharmacol. Transl. Sci. 2020;3:1330–1338. doi: 10.1021/acsptsci.0c00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Pawar P., Ma L., Byon C.H., Liu H., Ahn E.-Y., Jhala N., Arnoletti J.P., McDonald J.M., Chen Y. Molecular Mechanisms of Tamoxifen Therapy for Cholangiocarcinoma: Role of Calmodulin. Clin. Cancer Res. 2009;15:1288–1296. doi: 10.1158/1078-0432.CCR-08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Mine N., Yamamoto S., Saito N., Yamazaki S., Suda C., Ishigaki M., Kufe D.W., Von Hoff D.D., Kawabe T. CBP501-Calmodulin Binding Contributes to Sensitizing Tumor Cells to Cisplatin and Bleomycin. Mol. Cancer Ther. 2011;10:1929–1938. doi: 10.1158/1535-7163.MCT-10-1139. [DOI] [PubMed] [Google Scholar]
- 600.Chen P., Wu Q., Feng J., Yan L., Sun Y., Liu S., Xiang Y., Zhang M., Pan T., Chen X., et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Target. Ther. 2020;5:1–11. doi: 10.1038/s41392-020-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Merlot A.M., Shafie N.H., Yu Y., Richardson V., Jansson P.J., Sahni S., Lane D., Kovacevic Z., Kalinowski D.S., Richardson D. Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase. Biochem. Pharmacol. 2016;109:27–47. doi: 10.1016/j.bcp.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 602.Rewcastle G.W., Baguley B.C., Atwell G.J., Denny W.A. Potential antitumor agents. 52. Carbamate analogs of amsacrine with in vivo activity against multidrug-resistant P388 leukemia. J. Med. Chem. 1987;30:1576–1581. doi: 10.1021/jm00392a009. [DOI] [PubMed] [Google Scholar]
- 603.Mayur Y.C., Jagadeesh S., Thimmaiah K.N. Targeting calmodulin in reversing multi drug resistance in cancer cells. Mini-Reviews Med. Chem. 2006;6:1383–1389. doi: 10.2174/138955706778993021. [DOI] [PubMed] [Google Scholar]
- 604.Kim C., Kim B. Anti-Cancer Natural Products and Their Bioactive Compounds Inducing ER Stress-Mediated Apoptosis: A Review. Nutrients. 2018;10:1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Koido S., Kan S., Yoshida K., Yoshizaki S., Takakura K., Namiki Y., Tsukinaga S., Odahara S., Kajihara M., Okamoto M., et al. Immunogenic modulation of cholangiocarcinoma cells by chemoimmunotherapy. Anticancer. Res. 2014;34:6353–6361. [PubMed] [Google Scholar]
- 606.Humeau J., Sauvat A., Cerrato G., Xie W., Loos F., Iannantuoni F., Bezu L., Lévesque S., Paillet J., Pol J., et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol. Med. 2020;12:e11622. doi: 10.15252/emmm.201911622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Charlier H.A., Jr., Olson R.D., Thornock C.M., Mercer W.K., Olson D.R., Broyles T.S., Muhlestein D.J., Larson C.L., Cusack B.J., Shadle S.E. Investigations of Calsequestrin as a Target for Anthracyclines: Comparison of Functional Effects of Daunorubicin, Daunorubicinol, and Trifluoperazine. Mol. Pharmacol. 2005;67:1505–1512. doi: 10.1124/mol.104.005728. [DOI] [PubMed] [Google Scholar]
- 608.Gorini S., De Angelis A., Berrino L., Malara N., Rosano G., Ferraro E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxidative Med. Cell. Longev. 2018;2018:1–15. doi: 10.1155/2018/7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Tadini-Buoninsegni F., Smeazzetto S., Gualdani R., Moncelli M.R. Drug Interactions With the Ca2+-ATPase From Sarco(Endo)Plasmic Reticulum (SERCA) Front. Mol. Biosci. 2018;5 doi: 10.3389/fmolb.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Yiallouris A., Patrikios I., Johnson E.O., Sereti E., Dimas K., De Ford C., Fedosova N.U., Graier W.F., Sokratous K., Kyriakou K., et al. Annonacin promotes selective cancer cell death via NKA-dependent and SERCA-dependent pathways. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-018-0772-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 611.Varghese E., Samuel S.M., Sadiq Z., Kubatka P., Liskova A., Benacka J., Pazinka P., Kruzliak P., Büsselberg D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019;20:3017. doi: 10.3390/ijms20123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Stenius U., Miraglia E., Högberg J. Statins exhibit anticancer effects through modifications of the pAkt signaling pathway. Int. J. Oncol. 2011;40:867–875. doi: 10.3892/ijo.2011.1223. [DOI] [PubMed] [Google Scholar]
- 613.Stokes L., Bidula S., Bibič L., Allum E. To Inhibit or Enhance? Is There a Benefit to Positive Allosteric Modulation of P2X Receptors? Front. Pharmacol. 2020;11:627. doi: 10.3389/fphar.2020.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Mitrugno A., Sylman J.L., Rigg R.A., Yunga S.T., Shatzel J.J., Williams C.D., Mccarty O.J. Carpe low-dose aspirin: The new anti-cancer face of an old anti-platelet drug. Platelets. 2017;29:773–778. doi: 10.1080/09537104.2017.1416076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Ballerini P., Dovizio M., Bruno A., Tacconelli S., Patrignani P. P2Y12 Receptors in Tumorigenesis and Metastasis. Front. Pharmacol. 2018;9:66. doi: 10.3389/fphar.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Bentham Science Publisher Bentham Science Publisher Pharmacological Inhibition of Voltage-gated Ca2+ Channels for Chronic Pain Relief. Curr. Neuropharmacol. 2013;11:606–620. doi: 10.2174/1570159X11311060005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Vashisht A., Trebak M., Motiani R.K. STIM and Orai proteins as novel targets for cancer therapy. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am. J. Physiol. Physiol. 2015;309:C457–C469. doi: 10.1152/ajpcell.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Echakraborty S., Eghosh S., Ebanerjee B., Santra A., Eadhikary A., Misra A.K., Sen P.C. Phemindole, a Synthetic Di-indole Derivative Maneuvers the Store Operated Calcium Entry (SOCE) to Induce Potent Anti-Carcinogenic Activity in Human Triple Negative Breast Cancer Cells. Front. Pharmacol. 2016;7:114. doi: 10.3389/fphar.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Ali S., Doan D., Ojong T., Solomon H., Corpuz E., Huynh T., Haziq M., Shahid M., Siddiqui M.R., Newaz M., et al. Metformin Attenuates High Glucose-Induced Coronary Vascular Endothelial Hyper Permeability Via Inhibition of Orai-1 Mediated Store-Operated Calcium Entry. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.06270. [DOI] [Google Scholar]
- 620.Song M., Chen D., Yu S.P. The TRPC channel blocker SKF 96365 inhibits glioblastoma cell growth by enhancing reverse mode of the Na+/Ca2+exchanger and increasing intracellular Ca2+ Br. J. Pharmacol. 2014;171:3432–3447. doi: 10.1111/bph.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Rodrigues T., Sieglitz F., Bernardes G. Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem. Soc. Rev. 2016;45:6130–6137. doi: 10.1039/C5CS00916B. [DOI] [PubMed] [Google Scholar]
- 622.Distelhorst C.W., Bootman M. Creating a New Cancer Therapeutic Agent by Targeting the Interaction between Bcl-2 and IP3Receptors. Cold Spring Harb. Perspect. Biol. 2019;11:a035196. doi: 10.1101/cshperspect.a035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Liu Y., Jin M., Wang Y., Zhu J., Tan R., Zhao J., Ji X., Jin C., Jia Y., Ren T., et al. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct. Target. Ther. 2020;5:1–13. doi: 10.1038/s41392-020-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Marchi S., Corricelli M., Branchini A., Vitto V.A.M., Missiroli S., Morciano G., Perrone M., Ferrarese M., Giorgi C., Pinotti M., et al. Akt-mediated phosphorylation of MICU 1 regulates mitochondrial Ca 2+ levels and tumor growth. EMBO J. 2019;38:e99435. doi: 10.15252/embj.201899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Marchi S., Vitto V.A.M., Danese A., Wieckowski M., Giorgi C., Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Li X., Spelat R., Bartolini A., Cesselli D., Ius T., Skrap M., Caponnetto F., Manini I., Yang Y., Torre V. Mechanisms of malignancy in glioblastoma cells are linked to MCU upregulation and higher intracellular calcium level. J. Cell Sci. 2020;133:237503. doi: 10.1242/jcs.237503. [DOI] [PubMed] [Google Scholar]
- 627.Rao G., Dwivedi S.K.D., Zhang Y., Dey A., Shameer K., Karthik R., Srikantan S., Hossen N., Wren J.D., Madesh M., et al. Micro RNA -195 controls MICU 1 expression and tumor growth in ovarian cancer. EMBO Rep. 2020;21:48483. doi: 10.15252/embr.201948483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Wang X., Song X., Cheng G., Zhang J., Dong L., Bai J., Luo D., Xiong Y., Li S., Liu F., et al. The Regulatory Mechanism and Biological Significance of Mitochondrial Calcium Uniporter in the Migration, Invasion, Angiogenesis and Growth of Gastric Cancer. OncoTargets Ther. 2020;13:11781–11794. doi: 10.2147/OTT.S262049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Jin M., Wang J., Ji X., Cao H., Zhu J., Chen Y., Yang J., Zhao Z., Ren T., Xing J. MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2019;38:1–13. doi: 10.1186/s13046-019-1135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Marchi S., Lupini L., Patergnani S., Rimessi A., Missiroli S., Bonora M., Bononi A., Corrà F., Giorgi C., De Marchi E., et al. Downregulation of the Mitochondrial Calcium Uniporter by Cancer-Related miR-25. Curr. Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Ren T., Wang J., Zhang H., Yuan P., Zhu J., Wu Y., Huang Q., Guo X., Zhang J., Jiaojiao W., et al. MCUR1-Mediated Mitochondrial Calcium Signaling Facilitates Cell Survival of Hepatocellular Carcinoma via Reactive Oxygen Species-Dependent P53 Degradation. Antioxidants Redox Signal. 2018;28:1120–1136. doi: 10.1089/ars.2017.6990. [DOI] [PubMed] [Google Scholar]
- 632.Yu C., Wang Y., Peng J., Shen Q., Chen M., Tang W., Li X., Cai C., Wang B., Cai S., et al. Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget. 2017;8:83831–83844. doi: 10.18632/oncotarget.19747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Chakraborty P.K., Mustafi S.B., Xiong X., Dwivedi S.K.D., Nesin V., Saha S., Zhang M., Dhanasekaran D., Jayaraman M., Mannel R., et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat. Commun. 2017;8:14634. doi: 10.1038/ncomms14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Zhou K., Yao Y.-L., He Z.-C., Chen C., Zhang X.-N., Yang K.-D., Liu Y.-Q., Liu Q., Fu W.-J., Chen Y.-P., et al. VDAC2 interacts with PFKP to regulate glucose metabolism and phenotypic reprogramming of glioma stem cells. Cell Death Dis. 2018;9:1–14. doi: 10.1038/s41419-018-1015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Wu C.-H., Lin Y.-W., Wu T.-F., Ko J.-L., Wang P.-H. Clinical implication of voltage-dependent anion channel 1 in uterine cervical cancer and its action on cervical cancer cells. Oncotarget. 2016;7:4210–4225. doi: 10.18632/oncotarget.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 636.White C. The Regulation of Tumor Cell Invasion and Metastasis by Endoplasmic Reticulum-to-Mitochondrial Ca2+ Transfer. Front. Oncol. 2017;7:171. doi: 10.3389/fonc.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.Arif T., Krelin Y., Nakdimon I., Benharroch D., Paul A., Dadon-Klein D., Shoshan-Barmatz V. VDAC1 is a molecular target in glioblastoma, with its depletion leading to reprogrammed metabolism and reversed oncogenic properties. Neuro-Oncol. 2017;19:951–964. doi: 10.1093/neuonc/now297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Yuan S., Fu Y., Wang X., Shi H., Huang Y., Song X., Li L., Song N., Luo Y. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 2008;22:2809–2820. doi: 10.1096/fj.08-107417. [DOI] [PubMed] [Google Scholar]
- 639.Chin H.S., Li M.X., Tan I.K.L., Ninnis R.L., Reljic B., Scicluna K., Dagley L.F., Sandow J.J., Kelly G.L., Samson A.L., et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat. Commun. 2018;9:4976. doi: 10.1038/s41467-018-07309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Zhang L., Townsend D.M., Morris M., Maldonado E.N., Jiang Y.-L., Broome A.-M., Bethard J.R., Ball L.E., Tew K.D. Voltage-Dependent Anion Channels Influence Cytotoxicity of ME-344, a Therapeutic Isoflavone. J. Pharmacol. Exp. Ther. 2020;374:308–318. doi: 10.1124/jpet.120.000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Yang Y., Luo M., Zhang K., Zhang J., Gao T., Connell D.O., Yao F., Mu C., Cai B., Shang Y., et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-14324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Arif T., Paul A., Krelin Y., Shteinfer-Kuzmine A., Shoshan-Barmatz V. Mitochondrial VDAC1 Silencing Leads to Metabolic Rewiring and the Reprogramming of Tumour Cells into Advanced Differentiated States. Cancers. 2018;10:499. doi: 10.3390/cancers10120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.Qi H., Ning X., Yu C., Ji X., Jin Y., McNutt M.A., Yin Y. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 2019;10:1–16. doi: 10.1038/s41419-018-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Pathak T., Gueguinou M., Walter V., Delierneux C., Johnson M.T., Zhang X., Xin P., Yoast R., Emrich S.M., Yochum G.S., et al. Dichotomous role of the human mitochondrial Na+/Ca2+/Li+ exchanger NCLX in colorectal cancer growth and metastasis. eLife. 2020;9 doi: 10.7554/eLife.59686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Marchi S., Vitto V.A.M., Patergnani S., Pinton P. High mitochondrial Ca2+ content increases cancer cell proliferation upon inhibition of mitochondrial permeability transition pore (mPTP) Cell Cycle. 2019;18:914–916. doi: 10.1080/15384101.2019.1598729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Don A., Kisker O., Dilda P., Donoghue N., Zhao X., Decollogne S., Creighton B., Flynn E., Folkman J., Hogg P.J. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell. 2003;3:497–509. doi: 10.1016/S1535-6108(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 647.Ling X., Zhou Y., Li S.-W., Yan B., Wen L. Modulation of mitochondrial permeability transition pore affects multidrug resistance in human hepatocellular carcinoma cells. Int. J. Biol. Sci. 2010;6:773–783. doi: 10.7150/ijbs.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Kaddour-Djebbar I., Lakshmikanthan V., Shirley R.B., Ismail K.-D., Lewis R.W., Kumar M.V. Therapeutic advantage of combining calcium channel blockers and TRAIL in prostate cancer. Mol. Cancer Ther. 2006;5:1958–1966. doi: 10.1158/1535-7163.MCT-06-0011. [DOI] [PubMed] [Google Scholar]
- 649.Takata N., Ohshima Y., Suzuki-Karasaki M., Yoshida Y., Tokuhashi Y. Mitochondrial Ca2+ removal amplifies TRAIL cytotoxicity toward apoptosis-resistant tumor cells via promotion of multiple cell death modalities. Int. J. Oncol. 2017;51:193–203. doi: 10.3892/ijo.2017.4020. [DOI] [PubMed] [Google Scholar]
- 650.Pang G., Xie Q., Yao J. Mitofusin 2 inhibits bladder cancer cell proliferation and invasion via the Wnt/β-catenin pathway. Oncol. Lett. 2019;18:2434–2442. doi: 10.3892/ol.2019.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Starenki D., Sosonkina N., Hong S.-K., Lloyd R.V., Park J.-I. Mortalin (GRP75/HSPA9) Promotes Survival and Proliferation of Thyroid Carcinoma Cells. Int. J. Mol. Sci. 2019;20:2069. doi: 10.3390/ijms20092069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Fu R., Yang P., Wu H.-L., Li Z.-W., Li Z.-Y. GRP78 secreted by colon cancer cells facilitates cell proliferation via PI3K/Akt signaling. Asian Pac. J. Cancer Prev. 2014;15:7245–7249. doi: 10.7314/APJCP.2014.15.17.7245. [DOI] [PubMed] [Google Scholar]
- 653.Crottès D., Guizouarn H., Martin P., Borgese F., Soriani O. The sigma-1 receptor: A regulator of cancer cell electrical plasticity? Front. Physiol. 2013;4:175. doi: 10.3389/fphys.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Moriya C., Taniguchi H., Nagatoishi S., Igarashi H., Tsumoto K., Imai K. PRDM14 directly interacts with heat shock proteins HSP90α and glucose-regulated protein 78. Cancer Sci. 2017;109:373–383. doi: 10.1111/cas.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Kabakov A., Yakimova A., Matchuk O. Molecular Chaperones in Cancer Stem Cells: Determinants of Stemness and Potential Targets for Antitumor Therapy. Cells. 2020;9:892. doi: 10.3390/cells9040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Xu D., Yi W., Chen Y., Ma L., Wang J., Yu G. Overexpression of Sig1R is closely associated with tumor progression and poor outcome in patients with hilar cholangiocarcinoma. Med Oncol. 2014;31:261. doi: 10.1007/s12032-014-0261-8. [DOI] [PubMed] [Google Scholar]
- 657.Liu X., Feng C., Wei G., Kong W., Meng H., Du Y., Li J. Mitofusin1 Is a Major Mediator in Glucose-Induced Epithelial-to-Mesenchymal Transition in Lung Adenocarcinoma Cells. OncoTargets Ther. 2020;13:3511–3523. doi: 10.2147/OTT.S238714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658.You M.-H., Jeon M.J., Kim S.R., Lee W.K., Cheng S.-Y., Jang G., Kim T.Y., Kim W.B., Shong Y.K. Mitofusin-2 modulates the epithelial to mesenchymal transition in thyroid cancer progression. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-81469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Na Y., Kaul S., Ryu J., Lee J.-S., Ahn H.M., Kaul Z., Kalra R.S., Li L., Widodo N., Yun C.-O., et al. Stress Chaperone Mortalin Contributes to Epithelial-to-Mesenchymal Transition and Cancer Metastasis. Cancer Res. 2016;76:2754–2765. doi: 10.1158/0008-5472.CAN-15-2704. [DOI] [PubMed] [Google Scholar]
- 660.Chang Y.-J., Chen W.-Y., Huang C.-Y., Liu H.-H., Wei P.-L. Glucose-regulated protein 78 (GRP78) regulates colon cancer metastasis through EMT biomarkers and the NRF-2/HO-1 pathway. Tumor Biol. 2014;36:1859–1869. doi: 10.1007/s13277-014-2788-x. [DOI] [PubMed] [Google Scholar]
- 661.Gueguinou M., Crottès D., Chantôme A., Rapetti-Mauss R., Potier-Cartereau M., Clarysse L., Girault A., Fourbon Y., Jézéquel P., Guérin-Charbonnel C., et al. The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca2+ homeostasis. Oncogene. 2017;36:3640–3647. doi: 10.1038/onc.2016.501. [DOI] [PubMed] [Google Scholar]
- 662.Yoo J.Y., Ryu J., Gao R., Yaguchi T., Kaul S.C., Wadhwa R., Yun C.-O. Tumor suppression by apoptotic and anti-angiogenic effects of mortalin-targeting adeno-oncolytic virus. J. Gene Med. 2010;12:586–595. doi: 10.1002/jgm.1471. [DOI] [PubMed] [Google Scholar]
- 663.Dong D., Stapleton C., Luo B., Xiong S., Ye W., Zhang Y., Jhaveri N., Zhu G., Ye R., Liu Z., et al. A Critical Role for GRP78/BiP in the Tumor Microenvironment for Neovascularization during Tumor Growth and Metastasis. Cancer Res. 2011;71:2848–2857. doi: 10.1158/0008-5472.CAN-10-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Crottès D., Rapetti-Mauss R., Pérez F.A., Tichet M., Gariano G., Martial S., Guizouarn H., Pellissier B., Loubat A., Popa A.M., et al. SIGMAR1 Regulates Membrane Electrical Activity in Response to Extracellular Matrix Stimulation to Drive Cancer Cell Invasiveness. Cancer Res. 2016;76:607–618. doi: 10.1158/0008-5472.CAN-15-1465. [DOI] [PubMed] [Google Scholar]
- 665.Wang W., Liu X., Guo X., Quan H. Mitofusin-2 Triggers Cervical Carcinoma Cell Hela Apoptosis via Mitochondrial Pathway in Mouse Model. Cell. Physiol. Biochem. 2018;46:69–81. doi: 10.1159/000488410. [DOI] [PubMed] [Google Scholar]
- 666.Choudhary V., Kaddour-Djebbar I., Alaisami R., Kumar M.V., Bollag W.B. Mitofusin 1 degradation is induced by a disruptor of mitochondrial calcium homeostasis, CGP37157: A role in apoptosis in prostate cancer cells. Int. J. Oncol. 2014;44:1767–1773. doi: 10.3892/ijo.2014.2343. [DOI] [PubMed] [Google Scholar]
- 667.Guo W., Yan L., Yang L., Liu X., E Q., Gao P., Ye X., Liu W., Zuo J. Targeting GRP75 Improves HSP90 Inhibitor Efficacy by Enhancing p53-Mediated Apoptosis in Hepatocellular Carcinoma. PLoS ONE. 2014;9:e85766. doi: 10.1371/journal.pone.0085766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668.Wu J., Wu Y., Lian X. Targeted inhibition of GRP78 by HA15 promotes apoptosis of lung cancer cells accompanied by ER stress and autophagy. Biol. Open. 2020;9:bio053298. doi: 10.1242/bio.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Do W., Herrera C., Mighty J., Shumskaya M., Redenti S.M., Sauane M. Sigma 1 Receptor plays a prominent role in IL-24-induced cancer-specific apoptosis. Biochem. Biophys. Res. Commun. 2013;439:215–220. doi: 10.1016/j.bbrc.2013.08.057. [DOI] [PubMed] [Google Scholar]
- 670.Li M., Wang L., Wang Y., Zhang S., Zhou G., Lieshout R., Ma B., Liu J., Qu C., Verstegen M.M.A., et al. Mitochondrial Fusion Via OPA1 and MFN1 Supports Liver Tumor Cell Metabolism and Growth. Cells. 2020;9:121. doi: 10.3390/cells9010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Li T., Han J., Jia L., Hu X., Chen L., Wang Y. PKM2 coordinates glycolysis with mitochondrial fusion and oxidative phosphorylation. Protein Cell. 2019;10:583–594. doi: 10.1007/s13238-019-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672.Li Z., Wang Y., Newton I.P., Zhang L., Ji P., Li Z. GRP78 is implicated in the modulation of tumor aerobic glycolysis by promoting autophagic degradation of IKKβ. Cell. Signal. 2015;27:1237–1245. doi: 10.1016/j.cellsig.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 673.Maher C.M., Thomas J.D., Haas D., Longen C.G., Oyer H.M., Tong J., Kim F.J. Small-Molecule Sigma1 Modulator Induces Autophagic Degradation of PD-L1. Mol. Cancer Res. 2017;16:243–255. doi: 10.1158/1541-7786.MCR-17-0166. [DOI] [PubMed] [Google Scholar]
- 674.Liu Y.-N., Yang J.-F., Huang D.-J., Ni H.-H., Zhang C.-X., Zhang L., He J., Gu J.-M., Chen H.-X., Mai H.-Q., et al. Hypoxia Induces Mitochondrial Defect That Promotes T Cell Exhaustion in Tumor Microenvironment Through MYC-Regulated Pathways. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675.Lee A.S. GRP78 Induction in Cancer: Therapeutic and Prognostic Implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 676.Zhu L.X., Sharma S., Gardner B., Escuadro B., Atianzar K., Tashkin D.P., Dubinett S.M. IL-10 Mediates Sigma1 Receptor-Dependent Suppression of Antitumor Immunity. J. Immunol. 2003;170:3585–3591. doi: 10.4049/jimmunol.170.7.3585. [DOI] [PubMed] [Google Scholar]
- 677.Pilzer D., Saar M., Koya K., Fishelson Z. Mortalin inhibitors sensitize K562 leukemia cells to complement-dependent cytotoxicity. Int. J. Cancer. 2009;126:1428–1435. doi: 10.1002/ijc.24888. [DOI] [PubMed] [Google Scholar]
- 678.Faris P., Pellavio G., Ferulli F., Di Nezza F., Shekha M., Lim D., Maestri M., Guerra G., Ambrosone L., Pedrazzoli P., et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) induces intracellular Ca2+ release through the two-pore channel TPC1 in metastatic colorectal cancer cells. Cancers. 2019;11:542. doi: 10.3390/cancers11040542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Müller M., Geisslinger F., Gerndt S., Schütz R., Chao Y.-K., Bracher F., Grimm C., Vollmar A., Bartel K. Blocking Lysosomal Two-Pore Channel 2 Function Inhibits Proliferation of Multidrug Resistant Leukemia Cells and Sensitizes Them to Vincristine Treatment. Blood. 2019;134((Suppl. 1)):2081. doi: 10.1182/blood-2019-128195. [DOI] [Google Scholar]
- 680.Santoni G., Santoni M., Maggi F., Marinelli O., Morelli M.B. Emerging Role of Mucolipins TRPML Channels in Cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Xu M., Almasi S., Yang Y., Yan C., Sterea A.M., Syeda A.K.R., Shen B., Derek C.R., Huang P., Gujar S., et al. The lysosomal TRPML1 channel regulates triple negative breast cancer development by promoting mTORC1 and purinergic signaling pathways. Cell Calcium. 2019;79:80–88. doi: 10.1016/j.ceca.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Pafumi I., Festa M., Papacci F., Lagostena L., Giunta C., Gutla V., Cornara L., Favia A., Palombi F., Gambale F., et al. Naringenin Impairs Two-Pore Channel 2 Activity And Inhibits VEGF-Induced Angiogenesis. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-04974-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 683.Tang T., Yang Z.-Y., Wang D., Yang X.-Y., Wang J., Li L., Wen Q., Gao L., Bian X.-W., Yu S.-C. The role of lysosomes in cancer development and progression. Cell Biosci. 2020;10:1–18. doi: 10.1186/s13578-020-00489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Sun W., Yue J. TPC2 mediates autophagy progression and extracellular vesicle secretion in cancer cells. Exp. Cell Res. 2018;370:478–489. doi: 10.1016/j.yexcr.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 685.Almasi S., Kennedy B.E., Yoast R.E., Emrich S.M., Trebak M., Hiani Y.E. The lysosomal TRPML1 channel promotes breast cancer survival by supporting mitochondrial function and cellular metabolism. BioRxiv. 2020 doi: 10.1101/2020.09.04.283242. [DOI] [Google Scholar]
- 686.Xu M., Dong X.-P. Endolysosomal TRPMLs in Cancer. Biomol. 2021;11:65. doi: 10.3390/biom11010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Avnet S., Di Pompo G., Lemma S., Salerno M., Perut F., Bonuccelli G., Granchi D., Zini N., Baldini N. V-ATPase is a candidate therapeutic target for Ewing sarcoma. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013;1832:1105–1116. doi: 10.1016/j.bbadis.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 688.Flinck M., Hagelund S., Gorbatenko A., Severin M., Pedraz-Cuesta E., Novak I., Stock C., Pedersen S.F. The Vacuolar H+ ATPase α3 Subunit Negatively Regulates Migration and Invasion of Human Pancreatic Ductal Adenocarcinoma Cells. Cells. 2020;9:465. doi: 10.3390/cells9020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689.Di Cristofori A., Ferrero S., Bertolini I., Gaudioso G., Russo M.V., Berno V., Vanini M., Locatelli M., Zavanone M., Rampini P., et al. The vacuolar H+ ATPase is a novel therapeutic target for glioblastoma. Oncotarget. 2015;6:17514–17531. doi: 10.18632/oncotarget.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 690.Liu P., Chen H., Han L., Zou X., Shen W. Expression and role of V1A subunit of V-ATPases in gastric cancer cells. Int. J. Clin. Oncol. 2015;20:725–735. doi: 10.1007/s10147-015-0782-y. [DOI] [PubMed] [Google Scholar]
- 691.Sennoune S.R., E Bermudez L., Lees J.C., Hirsch J., Filleur S., Martínez-Zaguilán R. Vacuolar H+-ATPase is down-regulated by the angiogenesis-inhibitory pigment epithelium-derived factor in metastatic prostate cancer cells. Cell. Mol. Boil. 2014;60:45–52. [PubMed] [Google Scholar]
- 692.Lu Q., Lu S., Huang L., Wang T., Wan Y., Zhou C.X., Zhang C., Zhang Z., Li X. The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn. Pathol. 2013;8:145. doi: 10.1186/1746-1596-8-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Stransky L., Cotter K., Forgac M. The Function of V-ATPases in Cancer. Physiol. Rev. 2016;96:1071–1091. doi: 10.1152/physrev.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Katara G.K., Jaiswal M.K., Kulshrestha A., Kolli B., Gilman-Sachs A., Beaman K.D. Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene. 2013;33:5649–5654. doi: 10.1038/onc.2013.532. [DOI] [PubMed] [Google Scholar]
- 695.Cui C., Yang J., Fu L., Wang M., Wang X. Progress in understanding mitochondrial calcium uniporter complex-mediated calcium signalling: A potential target for cancer treatment. Br. J. Pharmacol. 2019;176:1190–1205. doi: 10.1111/bph.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Szabo I., Zoratti M., Biasutto L. Targeting mitochondrial ion channels for cancer therapy. Redox Biol. 2021;42:101846. doi: 10.1016/j.redox.2020.101846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Parrasia S., Mattarei A., Furlan A., Zoratti M., Biasutto L. Small-Molecule Modulators of Mitochondrial Channels as Chemotherapeutic Agents. Cell. Physiol. Biochem. 2019;53:11–43. doi: 10.33594/000000192. [DOI] [PubMed] [Google Scholar]
- 698.Esuh D.H., Ekim M.-K., Ekim H.S., Echung H.H., Esong Y.S. Mitochondrial permeability transition pore as a selective target for anti-cancer therapy. Front. Oncol. 2013;3:41. doi: 10.3389/fonc.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Via L., Argaez A.N.G., Martínez-Vázquez M., Grancara S., Martinis P., Toninello A. Mitochondrial Permeability Transition as Target of Anticancer Drugs. Curr. Pharm. Des. 2014;20:223–244. doi: 10.2174/13816128113199990033. [DOI] [PubMed] [Google Scholar]
- 700.Olivas-Aguirre M., Torres-López L., Valle-Reyes J.S., Hernández-Cruz A., Pottosin I., Dobrovinskaya O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019;10:779. doi: 10.1038/s41419-019-2024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Ruiz A., Alberdi E., Matute C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis. 2014;5:e1156. doi: 10.1038/cddis.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 702.Mashayekhi M., Zhou D.H. The advance of anti-cancer flexible heteroarotinoids compounds. J. Biochem. Mol. Biol. Res. 2020;5:230–241. doi: 10.17554/j.issn.2313-7177.2020.05.47. [DOI] [Google Scholar]
- 703.Yun C.W., Kim H.J., Lim J.H., Lee S.H. Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy. Cells. 2019;9:60. doi: 10.3390/cells9010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 704.Bailly C., Waring M.J. Pharmacological effectors of GRP78 chaperone in cancers. Biochem. Pharmacol. 2019;163:269–278. doi: 10.1016/j.bcp.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 705.Oyer H.M., Sanders C.M., Kim F.J. Small-Molecule Modulators of Sigma1 and Sigma2/TMEM97 in the Context of Cancer: Foundational Concepts and Emerging Themes. Front. Pharmacol. 2019;10:1141. doi: 10.3389/fphar.2019.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 706.Georgiadis M.-O., Karoutzou O., Foscolos A.-S., Papanastasiou I. Sigma Receptor (σR) Ligands with Antiproliferative and Anticancer Activity. Mol. 2017;22:1408. doi: 10.3390/molecules22091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 707.Alharbi A.F., Parrington J. Endolysosomal Ca2+ Signaling in Cancer: The Role of TPC2, From Tumorigenesis to Metastasis. Front. Cell Dev. Biol. 2019;7:302. doi: 10.3389/fcell.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Jin X., Zhang Y., Alharbi A., Hanbashi A., Alhoshani A., Parrington J., Alharbi A.F. Targeting Two-Pore Channels: Current Progress and Future Challenges. Trends Pharmacol. Sci. 2020;41:582–594. doi: 10.1016/j.tips.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 709.Huang T., Sun Y., Li Y., Wang T., Fu Y., Li C., Li C. Growth Inhibition of a Novel Iron Chelator, DpdtC, against Hepatoma Carcinoma Cell Lines Partly Attributed to Ferritinophagy-Mediated Lysosomal ROS Generation. Oxidative Med. Cell. Longev. 2018;2018:1–13. doi: 10.1155/2018/4928703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Zhang X., Chen W., Gao Q., Yang J., Yan X., Zhao H., Su L., Yang M., Gao C., Yao Y., et al. Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. PLoS Biol. 2019;17:e3000252. doi: 10.1371/journal.pbio.3000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 711.Chen D., Xie J., Fiskesund R., Dong W., Liang X., Lv J., Jin X., Liu J., Mo S., Zhang T., et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018;9:873. doi: 10.1038/s41467-018-03225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Pérez-Sayáns M., Martín J.M.S., Barros-Angueira F., Rey J.M.G., García-García A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat. Rev. 2009;35:707–713. doi: 10.1016/j.ctrv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 713.Patil R., Powrozek O., Kumar B., Seibel W., Beaman K., Waris G., Sharma-Walia N., Patil S. Bisbenzimidazoles: Anticancer vacuolar (H+)-atpase inhibitors. Chem. Appl. Benzimidazole Its Deriv. 2019 doi: 10.5772/intechopen.8523. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.