Abstract
Aquaculture is a growing sector, providing several products for human consumption, and it is therefore important to guarantee its quality and safety. This study aimed to contribute to the knowledge of bacterial composition of Crassostrea gigas, Mytilus spp. and Ruditapes decussatus, and the antibiotic resistances/resistance genes present in aquaculture environments. Two hundred and twenty-two bacterial strains were recovered from all bivalve mollusks samples belonging to the Aeromonadaceae, Bacillaceae, Comamonadaceae, Enterobacteriaceae, Enterococcaceae, Micrococcaceae, Moraxellaceae, Morganellaceae, Pseudomonadaceae, Shewanellaceae, Staphylococcaceae, Streptococcaceae, Vibrionaceae, and Yersiniaceae families. Decreased susceptibility to oxytetracycline prevails in all bivalve species, aquaculture farms and seasons. Decreased susceptibilities to amoxicillin, amoxicillin/clavulanic acid, cefotaxime, cefoxitin, ceftazidime, chloramphenicol, florfenicol, colistin, ciprofloxacin, flumequine, nalidixic acid and trimethoprim/sulfamethoxazole were also found. This study detected six qnrA genes among Shewanella algae, ten qnrB genes among Citrobacter spp. and Escherichia coli, three oqxAB genes from Raoultella ornithinolytica and blaTEM-1 in eight E. coli strains harboring a qnrB19 gene. Our results suggest that the bacteria and antibiotic resistances/resistance genes present in bivalve mollusks depend on several factors, such as host species and respective life stage, bacterial family, farm’s location and season, and that is important to study each aquaculture farm individually to implement the most suitable measures to prevent outbreaks.
Keywords: bivalve mollusks, aquaculture, antibiotic resistance, oxytetracycline, PMQR
1. Introduction
Aquaculture is an ancient activity, practiced since the Roman Empire (140 B.C.) in Europe. It has developed over the centuries, but it was in the last three decades that it experienced its greatest growth, pressured by increased demand [1,2].
Bivalve mollusks are known to be rich in proteins, vitamin D, long-chain omega-3 fatty acids, iodine and selenium, contributing to a healthy diet [3]. These organisms represent the main aquaculture production in Portugal and, in 2012, 95.2% of the active establishments were for bivalve mollusks’ production [4]. In 2015, the main species produced were Ruditapes decussatus, with 2300 t, Mytilus spp., with 1200 t, and Crassostrea gigas and Ostrea edulis, with 650 t. Other important species are Cerastoderma edule (264 t). The central and southern regions of Portugal (regions B and A, respectively) are the most relevant in the national production of bivalve mollusks [4,5].
C. gigas (common name: Japanese oyster) is the mollusk most commonly consumed worldwide. Along with Mytilus spp. (common name: mussels), they have a global geographical distribution, facilitated by features such as high fertility, rapid growth, and resistance to environmental variations (salinity, temperature, etc.). These are euryhaline species, whose natural habitat is in the lower limit of the intertidal zone until the subtidal (about 15 m) in estuaries and coastal lagoons for C. gigas, and in the high intertidal to subtidal regions in estuarine areas to oceanic seawaters for Mytilus spp. [1,6]. On the other hand, R. decussatus (common name: clams) are mostly cultivated in Portugal, Spain, the Atlantic coast of France and in the Mediterranean basin. This species is usually found in shallow waters, burrowed in sand and silty mud. These bivalve species feed by filtration of phytoplankton and organic matter (detritus) from the surrounding water [1,7,8]. This type of feeding allows an accumulation of numerous contaminants in these animals, such as toxins, antibiotic residues, bacteria, viruses, and protozoa. Therefore, bivalve mollusks can suffer from numerous infectious diseases, especially if cultured in high densities, that cause high mortality rates and have a significant commercial impact [9]. Among the most frequent diseases are those caused by bacteria, which leads to an increase in antibiotic consumption to treat and prevent the spread of these diseases. Moreover, the accumulation of antibiotic residues can submit commensal and pathogenic bacteria of these organisms and bacteria from the aquatic environment to a selective pressure, contributing to the rise of antibiotic resistance [7]. Among the most frequently found bacteria in bivalve mollusks are those belonging to the Proteobacteria phylum [10,11]. In this phylum, we can find normal commensal bacteria (e.g., Bacillus spp., Vibrio spp. and Aeromonas spp.) and non-commensal bacteria (e.g., Shewanella algae). Bacteria from both groups can become pathogenic to these organisms [9,12]. Previous studies detected antibiotic resistance, namely to amoxicillin and quinolones, in bivalve mollusks [13,14]. Other studies estimate 700,000 deaths per year around the world due to antibiotic-resistant bacteria [15]. Antibiotic resistance is a growing and global threat, reaching not only human, but also veterinary medicine, since there are studies that indicate the transfer of antibiotic resistance genes between these two reservoirs [16,17]. Bacteria present in bivalve mollusks (e.g., Vibrio spp. and Photobacterium damselae) can be responsible for infections in humans through the consumption (e.g., gastroenteritis) or handling of these organisms (e.g., wound infections that can evolve to necrotizing fasciitis with multiple organ failure and septicemia) [18,19,20].
Given this scenario, we designed a study to understand the diversity of antibiotic-resistant bacterial species present in the three mainly produced bivalve mollusks in two locally distant regions of Portugal (R. decussatus, Mytilus spp. and C. gigas), and the molecular mechanisms of antibiotic resistance that are circulating in these aquaculture environments.
2. Results
Overall, after the initial screening with selective media containing antibiotics (amoxicillin, cefotaxime, chloramphenicol, colistin, nalidixic acid and/or oxytetracycline), two hundred and twenty-two bacterial strains were recovered from the bivalve mollusks’ samples included in this study. One hundred and ninety-two were Gram-negative bacteria, whereas only thirty were Gram-positive bacteria. Gram-negative bacteria prevail in all three species of bivalve mollusks, when compared with Gram-positive bacteria. All bacterial families and respective species found in this study are listed in Table 1.
Table 1.
Bacterial families and respective species found in our study.
| Bacterial Family | Bacterial Species | Bivalve Species |
|---|---|---|
| Aeromonadaceae | Aeromonas punctata | R. decussatus |
| Aeromonas sp. | Mytilus spp. | |
| Bacillaceae | Bacillus sp. | R. decussatus |
| Bacillus cereus group | C. gigas, Mytilus spp. and R. decussatus | |
| Comamonadaceae | Comamonas aquatica | R. decussatus |
| Enterobacteriaceae | Citrobacter werkmanii | |
| Pseudocitrobacter faecalis | ||
| Enterobacter cancerogenus | Mytilus spp. | |
| Escherichia fergusonii | C. gigas | |
| Raoultella ornithinolytica | Mytilus spp. and R. decussatus | |
| Citrobacter braakii | C. gigas and R. decussatus | |
| Klebsiella aerogenes | ||
| Enterobacter spp. (E. hormaechei, E. kobei) | C. gigas and Mytilus spp. | |
| Klebsiella spp. (K. pneumoniae, K. oxytoca) | ||
| Citrobacter freundii | C. gigas, Mytilus spp. and R. decussatus | |
| Enterobacter cloacae | ||
| Escherichia coli | ||
| Enterococcaceae | Enterococcus spp. (E. faecalis, E. hirae) | |
| Enterococcus faecium | C. gigas | |
| Vagococcus fluvialis | Mytilus spp. | |
| Micrococcaceae | Micrococcus luteus | |
| Moraxellaceae | Acinetobacter spp. (A. beijerinckii, A. junii, A. pittii, A. ursingii) | C. gigas |
| Moraxella osloensis | ||
| Morganellaceae | Morganella morganii | C. gigas and R. decussatus |
| Proteus hauseri | Mytilus spp. | |
| Proteus vulgaris | C. gigas, Mytilus spp. and R. decussatus | |
| Providencia spp. (P. rettgeri, P. stuartii) | ||
| Pseudomonadaceae | Pseudomonas mendocina | Mytilus spp. and R. decussatus |
| Pseudomonas putida | C. gigas and Mytilus spp. | |
| Shewanellaceae | Shewanella algae | |
| Staphylococcaceae | Staphylococcus pasteuri | Mytilus spp. and R. decussatus |
| Staphylococcus warneri | Mytilus spp. | |
| Staphylococcus xylosus | R. decussatus | |
| Streptococcaceae | Lactococcus garvieae | |
| Vibrionaceae | Photobacterium damselae | C. gigas and Mytilus spp. |
| Vibrio alginolyticus | C. gigas, Mytilus spp. and R. decussatus | |
| Vibrio fluvialis | Mytilus spp. | |
| Vibrio spp. (V. furnissii, V. vulnificus) | R. decussatus | |
| Yersiniaceae | Serratia marcescens | C. gigas |
2.1. Bacterial Diversity in Clams Samples
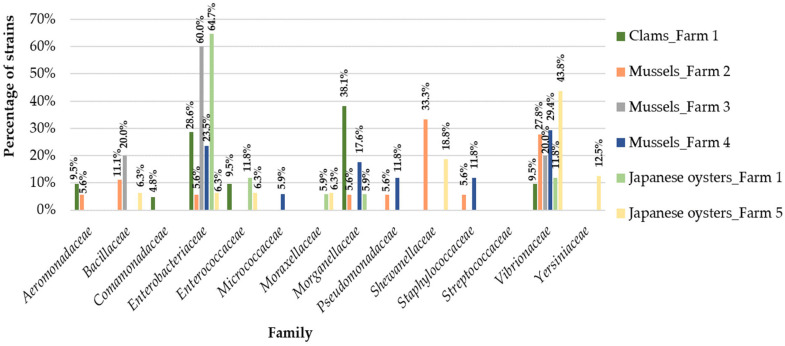
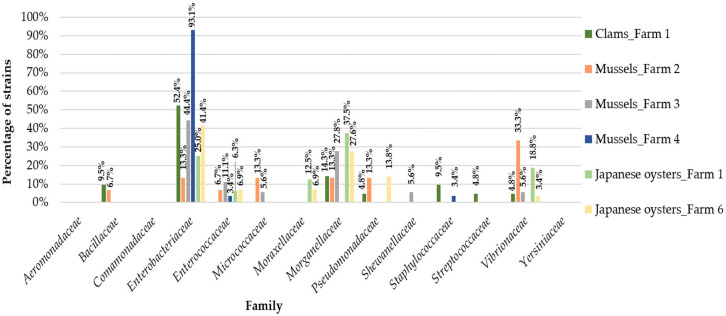
In clams samples we identified ten different families of bacteria (Figure 1 and Figure 2 and Table S1): Aeromonadaceae, Comamonadaceae, Enterococcaceae (only in summer), Bacillaceae, Staphylococcaceae, Streptococcaceae (only in autumn), Enterobacteriaceae, Morganellaceae, Pseudomonadaceae and Vibrionaceae (in both seasons). In summer, Morganellaceae was the most frequent bacterial family (38.1%). In fact, Morganellaceae appears more in this season in clams than in other bivalves studied (p = 0.02; Table 2). However, in autumn the results differ, with Enterobacteriaceae representing the most frequently isolated bacterial family (52.4%).
Figure 1.
Distribution of the bacterial families among the five aquaculture farms in the summer.
Figure 2.
Distribution of the bacterial families among the five aquaculture farms in the autumn.
Table 2.
Odds ratio (OR) and 95% confidence intervals (CI) (p ≤ 0.05) from the analyses of positive and negative associations between bivalve species and each bacterial family, bivalve species/bacterial family and season, C. gigas/bacterial family and location, bivalve species/bacterial family and nonsusceptibility to different antibiotic’s class (using the results from the initial screening in selective media).
| Bivalve’s Common Name | Bivalve Species | Bacterial Family | Season | Collection Site | Antibiotic | OR 1 | 95% CI | p Value |
|---|---|---|---|---|---|---|---|---|
| Clams | R. decussatus/All | Morganellaceae | Summer | All | NA | 6.933 | 1.02–54.16 | 0.02 |
| Mussels | Mytilus spp./All | Enterobacteriaceae | Summer | All | NA | 0.1908 (P) | 0.05897–0.597 | ≤0.01 |
| Mytilus spp./All | Enterobacteriaceae | Autumn | All | NA | 5.242 | 1.675–16.96 | ≤0.01 | |
| Mytilus spp. | Enterobacteriaceae | Summer | All | NA | 0.1689 (P) | 0.0584–0.4595 | ≤0.01 | |
| Mytilus spp. | Enterobacteriaceae | Autumn | All | NA | 5.92 | 2.176–17.12 | ≤0.01 | |
| Mytilus spp. | Morganellaceae | All | All | NA | 0.437 (P) | 0.1842–0.983 | 0.02 | |
| Mytilus spp. | Shewanellaceae | Summer | All | NA | 10.76 | 1.202–503 | ≤0.01 | |
| Mytilus spp. | Vibrionaceae | Summer | All | NA | 3.54 | 1.058–12.75 | 0.02 | |
| Japanese oysters | C. gigas/All | Enterobacteriaceae | Summer | All | NA | 9.429 | 2.263–45.46 | ≤0.01 |
| C. gigas | Morganellaceae | Summer | All | NA | 0.0692 (P) | 0.001588–0.5202 | ≤0.01 | |
| C. gigas | Morganellaceae | Autumn | All | NA | 14.45 | 1.922–629.7 | ≤0.01 | |
| C. gigas | All | All | All | Oxytetracycline | 0.4167 (P) | 0.2293–0.7527 | ≤0.01 | |
| C. gigas | All | Summer | All | Oxytetracycline | 2.786 | 1.061–7.35 | 0.02 |
Only significant associations are presented: p values ≤ 0.05 and confidence limits excluding null values (0, 1, or [n]). 1 Odds Ratio. (P) indicates an OR value for a protective or negative association; otherwise, values should be interpreted as a positive association. CI: Confidence intervals. NA: not applicable.
2.2. Bacterial Diversity in Mussels samples
Ten different families of bacteria were found among mussels samples: Aeromonadaceae (only in summer), Enterococcaceae (only in autumn), Bacillaceae, Enterobacteriaceae, Micrococcaceae, Morganellaceae, Pseudomonadaceae, Shewanellaceae, Staphylococcaceae and Vibrionaceae (in both seasons). In aquaculture farm 2, the most frequently found bacterial family in summer was Shewanellaceae (33.3%), whereas in autumn the most frequently found was Vibrionaceae (33.3%). In aquaculture farm 3, Enterobacteriaceae predominated in both seasons (with 60.0% in summer and 44.4% in autumn). In aquaculture farm 4, the most frequently found bacterial family in summer was Vibrionaceae (29.4%) and in the autumn it was Enterobacteriaceae (93.1%) (Figure 1 and Figure 2 and Table S1). Overall, we verified that Enterobacteriaceae appears more in autumn in mussels than in other bivalves (p ≤ 0.01) and was most frequent in autumn than in summer among mussels samples (p ≤ 0.01). Our statistical analyses showed that Morganellaceae were not usually associated with mussels samples (protective association; p = 0.02). In this bivalve species, Shewanellaceae and Vibrionaceae were most frequently found in summer than in autumn (p ≤ 0.01 and p = 0.02, respectively) (Table 2).
2.3. Bacterial Diversity in Japanese Oysters Samples
In Japanese oysters samples we identified nine different bacterial families: Bacillaceae, Shewanellaceae, Yersiniaceae (only in summer), Pseudomonadaceae (only in autumn), Enterobacteriaceae, Enterococcaceae, Moraxellaceae, Morganellaceae and Vibrionaceae (in both seasons). In this group of samples, in aquaculture farm 1 Enterobacteriaceae was the most frequently found bacterial family in summer (64.7%; Figure 1 and Figure 2 and Table S1). Indeed, this family appears more in summer in Japanese oysters than in the other bivalves studied (p ≤ 0.01; Table 2). In autumn, Morganellaceae was the most common family (37.5%) in aquaculture farm 1. In fact, Morganellaceae is more frequently found in autumn than in summer on all the studied aquaculture farms (p ≤ 0.01). In farm 5 (only samples collected in summer), Vibrionaceae was the most frequently found bacterial family (43.8%), while in farm 6 (only samples collected in autumn), Enterobacteriaceae predominated (41.4%).
2.4. Initial Evaluation of Decreased Susceptibilities
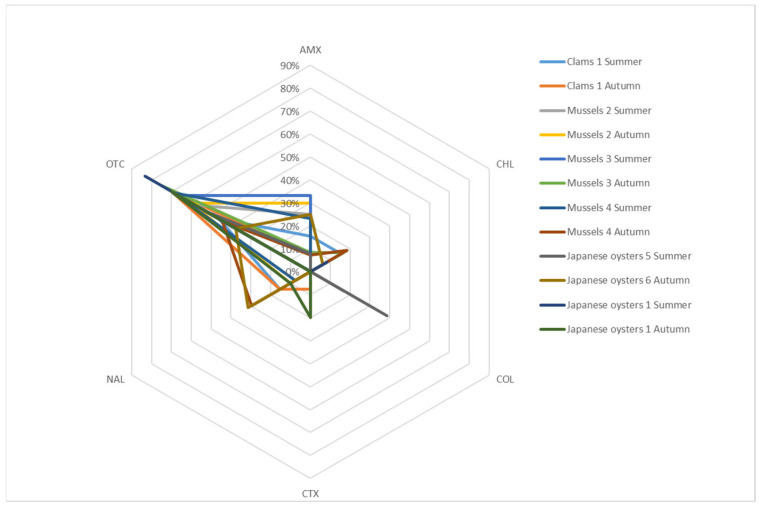
Initial screening with selective media containing antibiotics allowed the identification of decreased susceptibilities. Figure 3 (Figure S1 and Table S2) presents the results of this screening (eliminating known intrinsic resistances for the analysis). Decreased susceptibility to oxytetracycline prevails in all bivalve species, aquaculture farms and seasons.
Figure 3.
Decreased susceptibilities found in bivalves samples. These results were obtained through the initial screening with selective media containing antibiotics and do not include known intrinsic resistances. AMX: amoxicillin; CHL: chloramphenicol; COL: colistin; CTX: cefotaxime; NAL: nalidixic acid; OTC: oxytetracycline.
In clams, no decreased susceptibility to colistin was found and decreased susceptibility to chloramphenicol was only identified in samples collected in summer (15.4%).
In mussels, no reduced susceptibility to cefotaxime was found. In contrast, amoxicillin and oxytetracycline reduced susceptibility were present in all farms and seasons. Autumn was the only season where reduced susceptibility to chloramphenicol was found (16.7% in farm 3 and 18.5% in farm 4). However, reduced susceptibility to colistin was only found in a sample from farm 2, collected in summer (16.7%). Reduced susceptibility to nalidixic acid was not found in farm 3.
Although, in Japanese oysters, only decreased susceptibility to oxytetracycline was found in all farms and seasons, this decreased susceptibility was not so frequent in these bivalve species (protective association) when compared to other species of bivalve analyzed in this study (p ≤ 0.01; Table 2). Furthermore, this decreased susceptibility appears more associated with summer than autumn in these bivalves (p = 0.02; Table 2). Decreased susceptibility to amoxicillin and colistin were only found in samples from region B (farms 5 and 6), whereas decreased susceptibility to cefotaxime was only recovered in samples from region A (farm 1). Decreased susceptibility to nalidixic acid was only observed in samples collected in autumn (31.3% in farm 6 and 10.0% in farm 1).
2.5. Antibiotic Susceptibility of β-Lactamase- and Plasmid-Mediated Quinolone Resistance (PMQR)-Producing Strains
Investigation of resistance genes by PCR revealed six qnrA genes among S. algae, ten qnrB genes among C. braakii, C. freundii and E. coli, and three oqxAB genes from R. ornithinolytica strains (Table 3). qnrA genes were found in region A and B aquaculture farms, in both seasons, although they predominated in summer. However, qnrB and oqxAB genes were only found in region A aquaculture farms in autumn. Three PMQR-producing strains (C. braakii, C. freundii and S. algae) revealed a susceptibility profile to all quinolones tested, with zone diameters ranging from 31 to 33 mm (disk diffusion) and concentrations of <0.015 to 0.125 mg/L (MIC) for ciprofloxacin, concentrations of 0.5 to 2 mg/L for flumequine, and a zone diameter of 27 mm for levofloxacin. The remaining sixteen PMQR-producing strains revealed a decreased susceptibility to at least one quinolone tested, with concentrations of 0.5 to >16 mg/L for ciprofloxacin and 2 to 64 mg/L for flumequine. Non-susceptibilities to amoxicillin, colistin and oxytetracycline (MIC concentrations of 8 to 64 mg/L for the last one) were also found among S. algae strains. All R. ornithinolytica harboring an oqxAB gene were also resistant to oxytetracycline, with concentrations ranging from 16 to 64 mg/L. Decreased susceptibility to β-lactam antibiotics among Citrobacter spp. was mostly intrinsic. This genus also revealed decreased susceptibility to phenicols and oxytetracycline, with a concentration of 32 mg/L for chloramphenicol (C. braakii), 8 to 16 mg/L for florfenicol and 8 mg/L for oxytetracycline. E. coli harboring qnrB19 and blaTEM-1 genes revealed non-susceptibility to β-lactam antibiotics as well, in addition to non-susceptibilities to chloramphenicol, florfenicol, ciprofloxacin, flumequine, oxytetracycline and trimethoprim/sulfamethoxazole. Our study detected 11 multidrug resistance strains: one C. braakii recovered from clams, one C. freundii recovered from Japanese oysters, and eight E. coli and one S. algae recovered from mussels.
Table 3.
Phenotype and genotype profile of the nineteen β-lactamase- and PMQR-producing strains.
| Bacterial Species | Farm (No. of Strains) | Bivalve Mollusk Species | Season | Decreased Susceptibility Profile |
AR Genes |
|---|---|---|---|---|---|
| C. braakii | 1 (n = 1) | R. decussatus | A | AMX, AMC, FOX, CHL, FLO, OTC | qnrB-type 1 |
| C. freundii | 1 (n = 1) | C. gigas | A | AMC, CAZ, FOX, FLO, OTC | qnrB44 |
| E. coli | 4 (n = 8) | Mytilus spp. | A | (AMX), AMC, (CAZ), (CIP), CHL, FLO, (FMQ), OTC, SXT | qnrB19, bla TEM-1 |
| R. ornithinolytica | 4 (n = 3) | Mytilus spp. | A | (CIP), FMQ, NAL, OTC | oqxAB |
| S. algae | 2 (n = 3) | Mytilus spp. | S | (AMX), (FMQ), OTC | qnrA3 |
| 2 (n = 1) | Mytilus spp. | S | CIP, FMQ, OTC | qnrA11 | |
| 5 (n = 1) | C. gigas | S | COL | qnrA12 | |
| 3 (n = 1) | Mytilus spp. | A | FMQ, OTC | qnrA2 |
1 This qnrB sequence contains a premature stop codon due to naturally occurring deletion (suggesting a non-functional protein), so it was not possible to assign an allele number (accession number GenBank MW183827). AMX: amoxicillin; AMC: amoxicillin/clavulanic acid; FOX: cefoxitin; CAZ: ceftazidime; CIP: ciprofloxacin; CHL: chloramphenicol; COL: colistin; FLO: florfenicol; FMQ: flumequine; NAL: nalidixic acid; OTC: oxytetracycline; SXT: trimethoprim/sulfamethoxazole. AR: antibiotic resistance. A: Autumn. S: summer. Variable presence of nonsusceptibility phenotype is indicated by parentheses.
3. Discussion
As we observed in our study, bivalve mollusks usually concentrate a great diversity of bacterial species/families, which makes them susceptible to various diseases and may represent a risk to human health, since some of these bivalves are eaten raw (e.g., oysters). Indeed, statistically significant differences in bacterial composition between bivalve species from the same aquaculture farm and season (clams and Japanese oysters from farm 1, region A) were detected. Within the same bivalve species, we also observed variations between farms from the same region (mussels in region A) and different regions (Japanese oysters in region A and B), although these variations were not statistically significant. Fernández et al. detected variations in bacterial composition of post larvae and adult stages of Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea at different cultivation sites [13]. They concluded that the most frequent phyla were Proteobacteria, Bacteroidetes, Actinobacteria and Firmicutes (in that order), using a high-throughput sequencing approach (pyrosequencing). In a different study, Pierce and Ward evaluated the gut microbiome from Crassostrea virginica and Mytilus edulis and confirmed that these species had similar (but not identical) gut microbiomes that vary with the seasons [11]. The most abundant phyla were Proteobacteria, Tenericutes, Verrucomicrobia, Bacteroidetes, Cyanobacteria, Planctomycetes, Actinobacteria, Firmicutes, and Fusobacteria. In our study, the most frequently identified phylum was also Proteobacteria, which comprised the following families: Aeromonadaceae, Comamonadaceae, Enterobacteriaceae, Moraxellaceae, Pseudomonadaceae, Shewanellaceae, Vibrionaceae, and Yersiniaceae. These findings agree with the hypothesis previously proposed that bacterial composition in bivalve mollusks is influenced by host species and respective life stage, diet, rearing conditions, bacterial composition of the aquatic habitat, salinity, and temperature [9,21,22].
The bacteria found in this study belonging to the genera Acinetobacter, Aeromonas, Bacillus, Micrococcus, Photobacterium, Pseudomonas, and Vibrio are ubiquitous in the water environments and commensal microbiota of bivalves [9]. Some of these bacteria, especially those belonging to Proteobacteria phylum, are important for bivalve mollusks’ metabolism, since they are able to fix nitrogen in the gastrointestinal tract of these organisms and degrade cellulose and agar (the main elements of the food ingested by bivalve mollusks) [21]. Species belonging to the genera Aeromonas, Pseudomonas and Vibrio are also responsible for diseases in bivalve larvae [9]. Information about the frequency and pathogenicity of other genera found in this study in bivalve mollusks is scarce. However, there are several studies reporting human infections caused by all seven genera described above, namely wound infections, foodborne diseases (by ingestion of raw seafood), myonecrosis, septicemia, necrotizing fasciitis, empyema, bacteremia, endocarditis, severe respiratory, urinary and biliary tract infections, meningitis, and keratitis [9,18,19,20,22,23,24,25,26,27].
In addition to commensal microbiota of bivalve mollusks, we also found non-commensal bacteria, some already reported in bivalve mollusks, others with no information about their presence in these organisms. The genera Enterobacter, Enterococcus, Klebsiella, Proteus, Providencia, and Staphylococcus, as well as the species from Citrobacter freundii complex, Escherichia coli, Morganella morganii, Shewanella algae and Vagococcus fluvialis, found in this study, were already reported in bivalve mollusks (especially clams, mussels and oysters) [12,13,28,29,30,31,32,33,34,35,36]. These groups of bacteria, some of which are fish pathogens, are commonly found in aquatic environments [12,25,34,35,37,38]. All these bacteria were already associated with human infections, such as ear and eye infections, osteomyelitis, infective arthritis, endocarditis, bacteremia, meningitis, intestinal and urinary tract infections, brain abscess, peritonitis, enteritis and septicemia, and some are recognized as important agents in nosocomial infections [12,13,34,37,39,40,41,42,43,44,45,46,47,48,49].
To our knowledge, this is the first report associating Comamonas aquatica, Escherichia fergusonii, Lactococcus garvieae, Moraxella osloensis, Pseudocitrobacter faecalis, Raoultella ornithinolytica, and Serratia marcescens with bivalve mollusks from aquaculture. These bacteria were already recovered from a wide range of environments, such as water, soil, plants, fish, insects, milk, cheese, sugar cane, mango, and the feces of warm-blooded animals, among others [45,50,51,52,53,54,55,56,57]. Furthermore, they are responsible for bacteremia, septic shock, biliary, gastrointestinal, urinary tract and wound infections, meningitis, infective endocarditis, lumbar osteomyelitis, and hepatic abscess in humans [52,55,56,57,58].
Non-commensal bacteria of bivalve mollusks such as Enterobacter spp., Enterococcus faecalis, Enterococcus faecium, E. coli, Klebsiella aerogenes, K. pneumoniae, M. osloensis, M. morganii, and Providencia rettgeri could be indicators of fecal contamination, since these bacteria are widely found in the gastrointestinal tract of humans and several other animals [13,25,32,34,35,40,46,48,51]. These and other bacteria can enter aquaculture farms through runoff from land (especially during periods of high precipitation), sewage, maritime traffic and birds or marine mammals. Fecal material from land and sewage can concentrate a high bacterial diversity, as well as several heavy metals, antibiotics, and organic substances, promoting a selective pressure on bacteria normally present in an aquatic environment [13].
The initial screening with selective media containing antibiotics and, subsequently, the MIC results revealed the prevalence of decreased susceptibility to oxytetracycline in all bivalve species, aquaculture farms and seasons. This prevalence could be explained by the high frequency of prescription of this antibiotic in aquaculture, due to broad spectrum of activity, low cost, and potency [7]. With one exception, we could not establish an association between an antibiotic and a specific bivalve species, location, or season. The exception was the decreased susceptibility to oxytetracycline that was statistically associated (p = 0.02) with summer in Japanese oysters.
Decreased susceptibility to β-lactams was also found in the present study. This antibiotic class is frequently used in aquaculture in several countries [25] and high resistance rates are usually observed in bivalve mollusks (especially regarding amoxicillin), often associated with blaTEM and blaCTX-M [13]. In our study, we also detected the blaTEM-1 gene in eight multidrug resistant E. coli strains with decreased susceptibility to amoxicillin, amoxicillin/clavulanic acid and/or ceftazidime. Noteworthy, strains with non-susceptibilities to β-lactams might have other resistance mechanisms not studied here (e.g., efflux pumps).
The other decreased susceptibility detected in this study was to chloramphenicol, although this antibiotic has already been banned for use in food-producing animals in Europe since the 1990s. This decreased susceptibility can persist in the environment due to coselection with other antibiotics (especially florfenicol, which is widely used in aquaculture, and decreased susceptibility to this antibiotic was also found in this study) and/or heavy metals. Moreover, there are soil bacteria that are capable of producing this substance [25]. Other studies also confirmed the presence of this antibiotic resistance in bivalve mollusks [13,59].
Worryingly, our study detected decreased susceptibility to colistin, an antibiotic of last resort against multidrug resistant Gram-negative bacteria in human medicine. This antibiotic is also used in veterinary medicine, including aquaculture, the latter hypothesized by some authors as the source of certain colistin resistance genes [60,61]. In our study, fourteen strains revealed decreased susceptibility to this antibiotic. Of these, seven, identified as Shewanella algae and Photobacterium damselae, had non-intrinsic resistance and were isolated from mussels collected in aquaculture farm 2 and Japanese oysters collected in aquaculture farm 5 (in the region A and B, respectively). These results may reflect a selective pressure in these regions that facilitates the dissemination of strains with decreased susceptibility to colistin. No plasmid-mediated colistin resistance-encoding genes were detected, which suggested that other resistance mechanisms not studied here are responsible for the decreased susceptibility to this antibiotic (other mcr-variant genes; efflux pumps; or pmrC, pmrE, mgrB genes, among others genes and operons that play a role in lipopolysaccharide modification and consequent decreased susceptibility to colistin) [62]. Previous studies had already detected decreased susceptibility to colistin and mcr-1 gene in clams and mussels, respectively [61,63].
Our study revealed a low prevalence of decreased susceptibility to nalidixic acid (13%) in all bivalve samples analyzed. This antibiotic belongs to a class commonly used in aquaculture, quinolones [25]. Former studies in bivalve mollusks, also detected low levels of decreased susceptibility to quinolones [13,14]. Of the twenty strains with decreased susceptibility to nalidixic acid, only three strains of R. ornithinolytica revealed a positive result for the oqxAB gene. These three strains also revealed decreased susceptibility to other quinolones, such as flumequine and ciprofloxacin. Investigation of quinolones resistance genes by PCR in all Gram-negative strains, regardless their phenotype, revealed the presence of six qnrA-type genes among S. algae and ten qnrB-type genes among C. braakii, C. freundii and E. coli. Interestingly, not all strains harboring a qnr gene revealed a resistance phenotype to quinolones (one C. braakii with a qnrB-type, one C. freundii with a qnrB44 and one S. algae with a qnrA12). This may be caused by non-functional proteins, such as in the case of C. braakii with a deletion in qnrB gene that originated a premature stop codon, or a low expression of these genes, difficult to detect by phenotypic methods [64]. These results highlight the importance of using both phenotypic and genotypic methods in research of antibiotic resistances/resistance genes, since there is not always phenotypic and/or genotypic expression. Although qnrA, qnrB and oqxAB genes are frequently found in aquaculture environments [25,65,66], little information is known about their frequency in bivalve mollusks, thus this study can contribute to better knowledge in this field.
All E. coli strains that harbor a qnrB19 gene had a decreased susceptibility to quinolones (ciprofloxacin and/or flumequine) and also presented resistance to the combination trimethoprim/sulfamethoxazole (also used in aquaculture [25]). This resistance was previously reported in bivalve mollusks [67] and could be associated with the acquisition of genes sulI and sulII (for sulfamethoxazole resistance) and dhfrI and dhfrII (for trimethoprim resistance), causing the alteration of the antibiotic target [68].
In this study, we observed low resistance rates/few resistance genes to the antibiotics tested (except for oxytetracycline). However, it is important to implement surveillance plans in aquaculture farms, since this environment can be a reservoir of antibiotic resistance and/or antibiotic resistance genes. The implementation of measures that help to prevent outbreaks is also crucial, because fighting an outbreak is more difficult and expensive. Examples of such measures are limiting stock movements, avoiding exposure to elevated temperatures and high or low salinity, strict hygiene measures, and decreasing stock densities, among others. In the presence of an outbreak, it is important to identify the pathogen responsible and, if possible, to test its susceptibility to antibiotics, so that veterinarians can use a narrow-spectrum antibiotic at the correct concentration. Whenever possible, antibiotic administration by bath or feed should be avoided, giving preference to more individual methods to prevent the exposure of healthy individuals and the aquatic environment to a selective pressure. Investment in alternatives to antibiotics should be made, such as antimicrobial peptides (produced by several species of bivalve mollusks), bacteriophages, probiotics, and vaccines, always considering animal welfare and the product’s safety for human consumption [9,69].
4. Materials and Methods
4.1. Sample Characterization
The Portuguese Institute for the Sea and Atmosphere (IPMA) provided the bivalve samples used in this study. In summer of 2019, were collected one sample of clams (R. decussatus) and one sample of Japanese oyster (C. gigas) in aquaculture farm 1 from region A (south of Portugal); three samples of mussels (Mytilus spp.) from aquaculture farms 2, 3 and 4, also in region A; and one sample of Japanese oyster collected from aquaculture farm 5 in region B (central region of Portugal). The aquaculture farms from region A present in this study are distributed along its coastline.
In autumn of 2019, the sampling previously described was repeated for both regions, except for the sample of Japanese oyster in region B, which was collected in a different aquaculture (farm 6).
All samples were frozen and transported on ice to the National Institute of Health Dr. Ricardo Jorge, where they were analyzed. In this study, one sample corresponds to 3 to 10 individuals, depending on the species (minimum 50 g, for each sample).
4.2. Bacterial Isolation and Identification
Fifty grams of each sample were homogenized in peptone water (Stomacher 80 Biomaster®, Seward, UK), making a 1:10 dilution, and incubated for 12 to 18 h at 37 °C. Each dilution was plated in selective media, containing specific concentrations of different antibiotics (allowing an initial screening of decreased susceptibilities), and incubated for 18 to 20 h at 37 °C. Aeromonas agar, MacConkey agar and Thiosulfate Citrate Bile Salts Sucrose Agar contained the following standard antibiotic concentrations to select antibiotic resistant strains: 100 mg/L of amoxicillin, 2 mg/L of cefotaxime, 20 mg/L of chloramphenicol, 0.5 mg/L of colistin, 50 mg/L of nalidixic acid and 8 mg/L of oxytetracycline.
Mannitol salt agar and UriSelect™4 chromogenic agar contained 8 mg/L of oxytetracycline. Plates with and without antibiotic were used as controls. Colonies with different morphology (to avoid duplications) were selected and DNA extracted, according to manufacturer’s instructions (MagNA Pure 96 Instrument, Roche, Manheim, Germany). Strains were identified by MALDI-TOF and amplification of the 16S rRNA gene, as previously described [70].
4.3. Statistical Analyses of Results
Statistical analyses were performed to detect positive or negative associations between bivalve species and each bacterial family, bivalve species/bacterial family and season, C. gigas/bacterial family and location, bivalve species/bacterial family and nonsusceptibility to different classes of antibiotics (using the results from the initial screening in selective media). Only factors identified as statistically significant are shown. Fisher’s exact test was used to assess differences in bacterial families/season/location/nonsusceptibility to different classes of antibiotics between bivalve species and one-tailed p values of ≤0.05 were considered to be statistically significant. Associations were established by calculation of odds ratios with 95% confidence intervals. The null hypothesis was rejected for p values of ≤0.05. All statistical analyses were calculated using OpenEpi software, version 3.01 [71].
4.4. Molecular Detection of Resistance Genes
All Gram negative strains were investigated for the presence of blaOXA-48, blaVIM, blaIMP-1-type, blaNDM, blaKPC, blaGES, blaSME (β-lactams resistance genes), qnrA, qnrB, qnrC, qnrD, qnrS, aac(6′)-Ib, qepA (quinolones resistance genes), mcr-1, mcr-2, mcr-3, mcr-4 mcr-5 and mcr-9 genes (colistin resistance genes) through Polymerase Chain Reaction (PCR), using primers already reported [25], with the exception of mcr-9 primers. Primers and conditions for the search of mcr-9 gene are here described for the first time (mcr9-F, 5′-TTCCCTTTGTTCTGGTTG-3′, and mcr9-R, 5′–GGATTATAGACGCTGGTG-3′; initial denaturation at 94 °C for 7 min, followed by 30 cycles of 94 °C for 45 s, 55.6 °C for 45 s and 72 °C for 1 min and 45 s with a final extension at 72 °C for 10 min). For 5 strains recovered from MacConkey agar with cefotaxime, we investigated the presence of blaCTX-M, blaTEM, blaSHV, and blaOXA-1-type (β-lactams resistance genes) [25]. Furthermore, the presence of oqxAB gene (a quinolones resistance gene) was investigated for 20 strains recovered from Aeromonas/MacConkey agar with nalidixic acid [25]. Four more strains were searched for blaTEM, blaSHV, and blaOXA-1-type genes according to the antibiotic susceptibility testing (see next subtitle): two with an intermediate phenotype to ceftazidime and the other two with positive results in disc combination test (DCT).
All Staphylococcus spp. were tested for the presence of mecA, mecC, vanA, vanB and vanD genes [25], whereas all Enterococcus spp. were studied for the presence of vanA, vanB and vanD genes.
4.5. Antibiotic Susceptibility Testing of Strains with Resistance Genes
Antibiotic susceptibility was studied by disk diffusion (Bio-Rad, Marnes-la-Coquette, France) and minimum inhibitory concentration (MIC) by in-house broth microdilution for nineteen strains that revealed the presence of resistance genes. Antibiotics tested and respective concentrations and breakpoints are listed in Table 4. The antibiogram was completed with disc combination test (DCT), double disc synergy test (DDST), faropenem (10 µg) and temocillin (30 µg) to search for extended-spectrum β-lactamase (ESBL), metallo-β-lactamase (MBL), AmpC cephalosporinases and carbapenemases, as already reported [25]. The strains were considered multidrug resistant if they presented resistance to three or more structurally unrelated antibiotics. EUCAST species-specific intrinsic resistances were considered (https://www.eucast.org/expert_rules_and_intrinsic_resistance/) (accessed on 13 April 2021).
Table 4.
Antibiotics and respective concentrations and breakpoints used, by bacterial family.
| Bacterial Family | Method | Antibiotics Tested (Concentration) | Breakpoints |
|---|---|---|---|
| Enterobacteriaceae | Disk diffusion | AMC (20 + 10 µg), AZT (30 µg), FEP (30 µg), CTX (5 µg), FOX (30 µg), CAZ (10 µg), ERT (10 µg), IMP (10 µg), MEM (10 µg), PTZ (36 µg), CIP (5 µg), SXT (25 µg), GEN (10 µg) | EUCAST 1 |
| MIC | CHL, FLO, OTC FMQ CIP |
CLSI VET08 2 CASFM VET 2019 3 EUCAST |
|
| Shewanellaceae | Disk diffusion | AZT (30 µg), FEP (30 µg), CAZ (10 µg), IMP (10 µg), MEM (10 µg), PTZ (36 µg), CIP (5 µg), LEV (5 µg), AN (30 µg), GEN (10 µg), NET (10 µg), TMN (10 µg) | EUCAST 4 |
| MIC | CHL, FLO, OTC, CIP, FMQ | CLSI M100 4,5 |
AMC: amoxicillin/clavulanic acid; AN: amikacin; AZT: aztreonam; CAZ: ceftazidime; CHL: chloramphenicol; CTX: cefotaxime; CIP: ciprofloxacin; ERT: ertapenem; FEP: cefepime; FLO: florfenicol; FMQ: flumequine; FOX: cefoxitin; GEN: gentamicin; IPM: imipenem; LEV: levofloxacin; MEM: meropenem; NET: netilmicin; OTC: oxytetracycline; PTZ: piperacillin/tazobactam; SXT: trimethoprim/sulfamethoxazole; TMN: tobramycin; EUCAST: European Committee on Antimicrobial Susceptibility Testing. CLSI: Clinical and Laboratory Standards Institute; CASFM VET: Comité de l’antibiogramme de la Société Française de Microbiologie Recommandations Vétérinaires. 1 https://www.eucast.org/clinical_breakpoints/ (accessed on 13 April 2021). 2 https://clsi.org/ (accessed on 13 April 2021). 3 https://www.sfm-microbiologie.org/2019/07/09/casfm-veterinaire-2019/ (accessed on 13 April 2021). 4 Breakpoints for Shewanella spp. were not available, therefore breakpoints from EUCAST and CLSI M100 for Pseudomonas spp. were used, as reported elsewhere [72]. 5 Breakpoints for CHL were used for FLO as well; breakpoints for CIP were used for FMQ; and breakpoints for tetracycline were used for OTC.
5. Conclusions
In recent years, there has been an increase in studies on microbiota and antibiotic resistances/resistance genes present in aquaculture, mainly on fish. This study presents an important contribution to fill the gaps in the knowledge of bacterial diversity and antibiotic resistance mechanisms in bivalve mollusks. We could observe a great variety of bacterial species and antibiotic resistances among clams, mussels and Japanese oysters, seasons, and locations. This fact highlights the need to study and adapt the surveillance plans and measures to prevent the spread of antibiotic resistance to each specific location and animal species. Therefore, bivalve mollusks can play an important role in monitoring these aquaculture environments, since their filter feeding habits make them excellent indicators of environmental pollution [13].
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10091135/s1, Figure S1: Decreased susceptibilities found in bivalves samples. These results were obtained through the initial screening with selective media containing antibiotics and do not include known intrinsic resistances. AMX: amoxicillin; CHL: chloramphenicol; COL: colistin; CTX: cefotaxime; NAL: nalidixic acid; OTC: oxytetracycline. Table S1: Distribution of bacterial families among the six aquaculture farms in summer and autumn. Table S2: Percentage of strains with decreased susceptibility to antibiotics used in the initial screening.
Author Contributions
Conceptualization, M.C.; methodology, V.S., V.M., M.C.; data curation, V.M. and V.S.; formal analysis, V.S., V.M., M.C.; investigation, V.S., L.R., E.F., M.J.B., V.M., M.C.; validation, M.C.; writing—original draft preparation, V.S.; visualization, V.M. and M.C.; writing—review and editing, V.M., M.C.; supervision, resources, project administration, and funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
V.S. has a Ph.D. fellowship granted by the FCT (Fundação para a Ciência e a Tecnologia) with the reference SFRH/BD/133100/2017 cofinanced by European Social Fund and the Operational Program for Human Capital (POCH), Portugal. The work was supported by UIDB/00211/2020 with funding from FCT/MCTES through national funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gaspar P., Pires I., Magalhães A., Lda C. Boas Práticas em Cultivo de Ostra—Algarve. APA/ARH do Algarve; Amadora, Portugal: 2017. [Google Scholar]
- 2.Ottinger M., Clauss K., Kuenzer C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016;119:244–266. doi: 10.1016/j.ocecoaman.2015.10.015. [DOI] [Google Scholar]
- 3.Elbashir S., Parveen S., Schwarz J., Rippen T., Jahncke M., DePaola A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018;70:85–93. doi: 10.1016/j.fm.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Direção-Geral de Recursos Naturais Segurança e Serviços Marítimos . Plano Estratégico Para a Aquicultura Portuguesa 2014–2020. Direção-Geral de Recursos Naturais Segurança e Serviços Marítimos; Lisbon, Portugal: 2013. [Google Scholar]
- 5.Araújo J., Soares F., Pousão-Ferreira P. Offshore Production of Mediterranean Mussels in Southern Portugal. [(accessed on 25 October 2020)];World Aquac. 2018 49:55–57. Available online: https://www.was.org/Magazine/Vol/49/2#.X5VTYIj7RPY. [Google Scholar]
- 6.Goulletquer P. Cultured Aquatic Species Information Programme. Mytilus edulis. [(accessed on 13 August 2020)]. Available online: http://www.fao.org/fishery/culturedspecies/Mytilus_edulis/en.
- 7.Chiesa L.M., Nobile M., Malandra R., Panseri S., Arioli F. Occurrence of antibiotics in mussels and clams from various FAO areas. Food Chem. 2018;240:16–23. doi: 10.1016/j.foodchem.2017.07.072. [DOI] [PubMed] [Google Scholar]
- 8.Figueras A. Cultured Aquatic Species Information Programme. Ruditapes decussatus. [(accessed on 13 August 2020)]. Available online: http://www.fao.org/fishery/culturedspecies/Ruditapes_decussatus/en.
- 9.Zannella C., Mosca F., Mariani F., Franci G., Folliero V., Galdiero M., Tiscar P.G., Galdiero M. Microbial Diseases of Bivalve Mollusks: Infections, Immunology and Antimicrobial Defense. Mar. Drugs. 2017;15:182. doi: 10.3390/md15060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández N.T., Mazón-Suástegui J.M., Vázquez-Juárez R., Ascencio-Valle F., Romero J. Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during commercial production. FEMS Microbiol. Ecol. 2014;88:69–83. doi: 10.1111/1574-6941.12270. [DOI] [PubMed] [Google Scholar]
- 11.Pierce M.L., Ward J.E. Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) and the Blue Mussel (Mytilus edulis): Temporal Variation and the Influence of Marine Aggregate-Associated Microbial Communities. mSphere. 2019;4:e00730-19. doi: 10.1128/mSphere.00730-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng S.Y., Liu P.Y., Lee Y.H., Wu Z.Y., Huang C.C., Cheng C.C., Tung K.C. The pathogenicity of Shewanella algae and ability to tolerate a wide range of temperatures and salinities. Can. J. Infect. Dis. Med. Microbiol. 2018;2018:6976897. doi: 10.1155/2018/6976897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grevskott D.H., Svanevik C.S., Sunde M., Wester A.L., Lunestad B.T. Marine bivalve mollusks as possible indicators of multidrug-resistant Escherichia coli and other species of the Enterobacteriaceae family. Front. Microbiol. 2017;8:1–10. doi: 10.3389/fmicb.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopatek M., Wieczorek K., Osek J. Prevalence and antimicrobial resistance of Vibrio parahaemolyticus isolated from raw shellfish in Poland. J. Food Prot. 2015;78:1029–1033. doi: 10.4315/0362-028X.JFP-14-437. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. London, UK: 2014. [(accessed on 12 April 2021)]. Review on Antimicrobial Resistance. Available online: https://amr-review.org/Publications.html. [Google Scholar]
- 16.Furushita M., Shiba T., Maeda T., Yahata M., Kaneoka A., Takahashi Y., Torii K., Hasegawa T., Ohta M. Similarity of tetracycline resistance genes isolated from fish farm bacteria to those from clinical isolates. Appl. Environ. Microbiol. 2003;69:5336–5342. doi: 10.1128/AEM.69.9.5336-5342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akinbowale O.L., Peng H., Barton M.D. Diversity of tetracycline resistance genes in bacteria from aquaculture sources in Australia. J. Appl. Microbiol. 2007;103:2016–2025. doi: 10.1111/j.1365-2672.2007.03445.x. [DOI] [PubMed] [Google Scholar]
- 18.Rivas A.J., Lemos M.L., Osorio C.R. Photobacterium damselae subsp. Damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 2013;4:1–6. doi: 10.3389/fmicb.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froelich B.A., Noble R.T. Vibrio bacteria in raw oysters: Managing risks to human health. Philos. Trans. R. Soc. B Biol. Sci. 2016;371:20150209. doi: 10.1098/rstb.2015.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tewari A., Abdullah S. Bacillus cereus food poisoning: International and Indian perspective. J. Food Sci. Technol. 2015;52:2500–2511. doi: 10.1007/s13197-014-1344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasa A., Mira A., Camelo-Castillo A., Belda-Ferre P., Romalde J.L. Characterization of the microbiota associated to Pecten maximus gonads using 454-pyrosequencing. Int. Microbiol. 2016;19:93–99. doi: 10.2436/20.1501.01.267. [DOI] [PubMed] [Google Scholar]
- 22.Kacar A. Some microbial characteristics of mussels (Mytilus galloprovincialis) in coastal city area. Environ. Sci. Pollut. Res. 2011;18:1384–1389. doi: 10.1007/s11356-011-0487-3. [DOI] [PubMed] [Google Scholar]
- 23.Radó J., Kaszab E., Benedek T., Kriszt B., Szoboszlay S. First isolation of carbapenem-resistant Acinetobacter beijerinckii from an environmental sample. Acta Microbiol. Immunol. Hung. 2019;66:113–130. doi: 10.1556/030.66.2019.004. [DOI] [PubMed] [Google Scholar]
- 24.Richards G.P., Watson M.A., Crane E.J., Burt I.G., Bushek D. Shewanella and Photobacterium spp. in oysters and seawater from the Delaware bay. Appl. Environ. Microbiol. 2008;74:3323–3327. doi: 10.1128/AEM.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgueiro V., Manageiro V., Bandarra N.M., Reis L., Caniça M. Bacterial Diversity and Antibiotic Susceptibility of Sparus aurata from Aquaculture. Microorganisms. 2020;8:1343. doi: 10.3390/microorganisms8091343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernández-Robles M.F., Álvarez-Contreras A.K., Juárez-García P., Natividad-Bonifacio I., Curiel-Quesada E., Vázquez-Salinas C., Quiñones-Ramírez E.I. Virulence factors and antimicrobial resistance in environmental strains of Vibrio alginolyticus. Int. Microbiol. 2016;19:191–198. doi: 10.2436/20.1501.01.277. [DOI] [PubMed] [Google Scholar]
- 27.Khan A., Aung T.T., Chaudhuri D. The First Case of Native Mitral Valve Endocarditis due to Micrococcus luteus and Review of the Literature. Case Rep. Cardiol. 2019;2019:1–3. doi: 10.1155/2019/5907319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.W., Hong Y.J., Jo J.I., Ha S.D., Kim S.H., Lee H.J., Rhee M.S. Raw ready-to-eat seafood safety: Microbiological quality of the various seafood species available in fishery, hyper and online markets. Lett. Appl. Microbiol. 2017;64:27–34. doi: 10.1111/lam.12688. [DOI] [PubMed] [Google Scholar]
- 29.Bozcal E., Dagdeviren M. Bacterial metagenome analysis of Mytilus galloprovincialis collected from Istanbul and Izmir coastal stations of Turkey. Environ. Monit. Assess. 2020;192:186. doi: 10.1007/s10661-020-8129-1. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Delgado M., Contreras M., García-Amado M.A., Gueneau P., Suárez P. Occurrence of Proteus mirabilis associated with two species of Venezuelan oysters. Rev. Inst. Med. Trop. Sao Paulo. 2007;49:355–359. doi: 10.1590/S0036-46652007000600004. [DOI] [PubMed] [Google Scholar]
- 31.Sacramento A.G., Fernandes M.R., Sellera F.P., Dolabella S.S., Zanella R.C., Cerdeira L., Lincopan N. VanA-type vancomycin-resistant Enterococcus faecium ST1336 isolated from mussels in an anthropogenically impacted ecosystem. Mar. Pollut. Bull. 2019;142:533–536. doi: 10.1016/j.marpolbul.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Roslev P., Iversen L., Sønderbo H.L., Iversen N., Bastholm S. Uptake and persistence of human associated Enterococcus in the mussel Mytilus edulis: Relevance for faecal pollution source tracking. J. Appl. Microbiol. 2009;107:944–953. doi: 10.1111/j.1365-2672.2009.04272.x. [DOI] [PubMed] [Google Scholar]
- 33.Chapman C. Ph.D. Thesis. University of Tasmania; Hobart, Australia: 2012. Investigation into the Microbiological Causes of Epizootics of Pacific Oyster Larvae (Crassostrea gigas) in Commercial Production. [Google Scholar]
- 34.Drzewiecka D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb. Ecol. 2016;72:741–758. doi: 10.1007/s00248-015-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Sousa O.V., Dos Fernandes Vieira R.H.S., De Menezes F.G.R., Dos Reis C.M.F., Hofer E. Detection of Vibrio parahaemolyticus and Vibrio cholerae in oyster, Crassostrea rhizophorae, collected from a natural nursery in the Cocó river estuary, Fortaleza, Ceará, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2004;46:59–62. doi: 10.1590/S0036-46652004000200001. [DOI] [PubMed] [Google Scholar]
- 36.Baldi F., Gallo M., Marchetto D., Faleri C., Maida I., Fani R. Manila clams from Hg polluted sediments of Marano and Grado lagoons (Italy) harbor detoxifying Hg resistant bacteria in soft tissues. Environ. Res. 2013;125:188–196. doi: 10.1016/j.envres.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Santoiemma P.P., Kalainov D.M., Mehta M.P., Bolon M.K. An unusual case of Staphylococcus pasteuri osteomyelitis. Infect. Dis. Rep. 2020;12:32–34. doi: 10.4081/idr.2020.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadhav K.P., Pai P.G. A rare infective endocarditis caused by Vagococcus fluvialis. J. Cardiol. Cases. 2019;20:129–131. doi: 10.1016/j.jccase.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuo T., Mori N., Kawai F., Sakurai A., Toyoda M., Mikami Y., Uehara Y., Furukawa K. Vagococcus fluvialis as a causative pathogen of bloodstream and decubitus ulcer infection: Case report and systematic review of the literature. J. Infect. Chemother. 2020;6 doi: 10.1016/j.jiac.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Liu H., Zhu J., Hu Q., Rao X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016;50:10–17. doi: 10.1016/j.ijid.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Sharma D., Sharma P., Soni P. First case report of Providencia Rettgeri neonatal sepsis. BMC Res. Notes. 2017;10:17–19. doi: 10.1186/s13104-017-2866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanuparthy A., Challa T., Meegada S., Siddamreddy S., Muppidi V. Staphylococcus warneri: Skin Commensal and a Rare Cause of Urinary Tract Infection. Cureus. 2020;12:e8337. doi: 10.7759/cureus.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y.H., Xu C.G., Yang Y.B., Xing X.X., Liu X., Qu Q.W., Ding W.Y., Bello-Onaghise G., Li Y.H. Histidine metabolism and IGPD play a key role in cefquinome inhibiting biofilm formation of Staphylococcus xylosus. Front. Microbiol. 2018;9:665. doi: 10.3389/fmicb.2018.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walczak N., Puk K., Guz L. Bacterial flora associated with diseased freshwater ornamental fish. J. Vet. Res. 2017;61:445–449. doi: 10.1515/jvetres-2017-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Kharousi Z.S., Guizani N., Al-Sadi A.M., Al-Bulushi I.M., Shaharoona B. Hiding in Fresh Fruits and Vegetables: Opportunistic Pathogens May Cross Geographical Barriers. Int. J. Microbiol. 2016;2016 doi: 10.1155/2016/4292417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herridge W.P., Shibu P., O’Shea J., Brook T.C., Hoyles L. Bacteriophages of Klebsiella spp., their diversity and potential therapeutic uses. J. Med. Microbiol. 2020;69:176–194. doi: 10.1099/jmm.0.001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-Vazquez M., Oteo-Iglesias J., Sola-Campoy P.J., Carrizo-Manzoni H., Bautista V., Lara N., Aracil B., Alhambra A., Martínez-Martínez L., Campos J., et al. Characterization of Carbapenemase-Producing Klebsiella oxytoca in Spain, 2016–2017. Antimicrob. Agents Chemother. 2019;63:e02529-18. doi: 10.1128/AAC.02529-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Z., Li H., Feng J., Li Y., Chen X., Guo X., Chen W., Wang L., Lin L., Yang H., et al. NDM-1 encoded by a pNDM-BJ01-like plasmid p3SP-NDM in clinical Enterobacter aerogenes. Front. Microbiol. 2015;6:294. doi: 10.3389/fmicb.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abriouel H., Ben Omar N., Molinos A.C., López R.L., Grande M.J., Martínez-viedma P., Ortega E., Cañamero M.M., Galvez A. Comparative analysis of genetic diversity and incidence of virulence factors and antibiotic resistance among enterococcal populations from raw fruit and vegetable foods, water and soil, and clinical samples. Int. J. Food Microbiol. 2008;123:38–49. doi: 10.1016/j.ijfoodmicro.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 50.Kubota H., Mitani A., Niwano Y., Takeuchi K., Tanaka A., Yamaguchi N., Kawamura Y., Hitomi J. Moraxella species are primarily responsible for generating malodor in laundry. Appl. Environ. Microbiol. 2012;78:3317–3324. doi: 10.1128/AEM.07816-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkhatib N.J., Younis M.H., Alobaidi A.S., Shaath N.M. An unusual osteomyelitis caused by Moraxella osloensis: A case report. Int. J. Surg. Case Rep. 2017;41:146–149. doi: 10.1016/j.ijscr.2017.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaeuffer C., Schramm F., Meyer A., Hansmann Y., Guffroy A., Argemi X. First case of Comamonas aquatica bacteremia complicated by septic shock. Med. Mal. Infect. 2018;48:540–542. doi: 10.1016/j.medmal.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Parthasarathy A., Gan H.M., Wong N.H., Savka M.A., Steiner K.K., Henry K.R., Hudson A.O. Isolation and genomic characterization of six endophytic bacteria isolated from Saccharum sp (sugarcane): Insights into antibiotic, secondary metabolite and quorum sensing metabolism. J. Genom. 2018;6:117–121. doi: 10.7150/jgen.28335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaastra W., Kusters J.G., van Duijkeren E., Lipman L.J.A. Escherichia fergusonii . Vet. Microbiol. 2014;172:7–12. doi: 10.1016/j.vetmic.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 55.Hajjar R., Ambaraghassi G., Sebajang H., Schwenter F., Su S.H. Raoultella ornithinolytica: Emergence and resistance. Infect. Drug Resist. 2020;13:1091–1104. doi: 10.2147/IDR.S191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rösch R.M., Buschmann K., Brendel L., Schwanz T., Vahl C.-F. Lactococcus garvieae Endocarditis in a Prosthetic Aortic Valve: A Case Report and Literature Review. J. Investig. Med. High. Impact Case Rep. 2019;7:1–7. doi: 10.1177/2324709619832052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iguchi A., Nagaya Y., Pradel E., Ooka T., Ogura Y., Katsura K., Kurokawa K., Oshima K., Hattori M., Parkhill J., et al. Genome evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol. Evol. 2014;6:2096–2110. doi: 10.1093/gbe/evu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baek S.D., Chun C., Hong K.S. Hemolytic uremic syndrome caused by Escherichia fergusonii infection. Kidney Res. Clin. Pract. 2019;38:253–255. doi: 10.23876/j.krcp.19.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R.X., Wang A., Wang J.Y. Antibiotic resistance monitoring in heterotrophic bacteria from anthropogenic-polluted seawater and the intestines of oyster Crassostrea hongkongensis. Ecotoxicol. Environ. Saf. 2014;109:27–31. doi: 10.1016/j.ecoenv.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 60.Catry B., Cavaleri M., Baptiste K., Grave K., Grein K., Holm A., Jukes H., Liebana E., Navas A.L., Mackay D., et al. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. Int. J. Antimicrob. Agents. 2015;46:297–306. doi: 10.1016/j.ijantimicag.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Shen Y., Zhang R., Schwarz S., Wu C., Shen J., Walsh T.R., Wang Y. Farm animals and aquaculture: Significant reservoirs of mobile colistin resistance genes. Environ. Microbiol. 2020;22:2469–2484. doi: 10.1111/1462-2920.14961. [DOI] [PubMed] [Google Scholar]
- 62.Aghapour Z., Gholizadeh P., Ganbarov K., Bialvaei A.Z., Mahmood S.S., Tanomand A., Yousefi M., Asgharzadeh M., Yousefi B., Kafil H.S. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect. Drug Resist. 2019;12:965–975. doi: 10.2147/IDR.S199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahanayake P.S., Hossain S., Wickramanayake M.V.K.S., Wimalasena S.H.M.P., Heo G.-J. Manila clam (Ruditapes philippinarum) marketed in Korea as a source of vibrios harbouring virulence and β-lactam resistance genes. Lett. Appl. Microbiol. 2020;71:46–53. doi: 10.1111/lam.13229. [DOI] [PubMed] [Google Scholar]
- 64.Jones-dias D., Manageiro V., Francisco A.P., Martins A.P., Domingues G., Louro D., Ferreira E., Caniça M. Assessing the molecular basis of transferable quinolone resistance in Escherichia coli and Salmonella spp. from food-producing animals and food products. Vet. Microbiol. 2013;167:523–531. doi: 10.1016/j.vetmic.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Su H., Hu X., Wang L., Xu W., Xu Y., Wen G., Li Z., Cao Y. Contamination of antibiotic resistance genes (ARGs) in a typical marine aquaculture farm: Source tracking of ARGs in reared aquatic organisms. J. Environ. Sci. Heal. Part. B. 2020;55:220–229. doi: 10.1080/03601234.2019.1684747. [DOI] [PubMed] [Google Scholar]
- 66.Antunes P., Campos J., Mourão J., Pereira J., Novais C., Peixe L. Inflow water is a major source of trout farming contamination with Salmonella and multidrug resistant bacteria. Sci. Total Environ. 2018;642:1163–1171. doi: 10.1016/j.scitotenv.2018.06.143. [DOI] [PubMed] [Google Scholar]
- 67.De Silva B.C.J., Hossain S., Dahanayake P.S., Heo G.-J. Aeromonas spp. from marketed Yesso scallop (Patinopecten yessoensis): Molecular characterization, phylogenetic analysis, virulence properties and antimicrobial susceptibility. J. Appl. Microbiol. 2019;126:288–299. doi: 10.1111/jam.14106. [DOI] [PubMed] [Google Scholar]
- 68.Giedraitiene A., Vitkauskiene A., Naginiene R., Pavilonis A. Antibiotic resistance mechanisms of clinically important bacteria. Medicina (B. Aires) 2011;47:137–146. doi: 10.3390/medicina47030019. [DOI] [PubMed] [Google Scholar]
- 69.Cabello F.C., Godfrey H.P., Buschmann A.H., Dölz H.J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016;16:e127–e133. doi: 10.1016/S1473-3099(16)00100-6. [DOI] [PubMed] [Google Scholar]
- 70.Jones-Dias D., Manageiro V., Caniça M. Influence of agricultural practice on mobile bla genes: IncI1-bearing CTX-M, SHV, CMY and TEM in Escherichia coli from intensive farming soils. Environ. Microbiol. 2016;18:260–272. doi: 10.1111/1462-2920.13021. [DOI] [PubMed] [Google Scholar]
- 71.Dean A.G., Sullivan K.M., Soe M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. [(accessed on 29 July 2020)]. Available online: www.OpenEpi.com.
- 72.McAuliffe G.N., Hennessy J., Baird R.W. Relative frequency, characteristics, and antimicrobial susceptibility patterns of Vibrio spp., Aeromonas spp., Chromobacterium violaceum, and Shewanella spp. in the Northern Territory of Australia, 2000–2013. Am. J. Trop. Med. Hyg. 2015;92:605–610. doi: 10.4269/ajtmh.14-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.