Abstract
Genetic recombination and the repair of double-strand DNA breaks in Saccharomyces cerevisiae require Rad51, a homologue of the Escherichia coli RecA protein. In vitro, Rad51 binds DNA to form an extended nucleoprotein filament and catalyzes the ATP-dependent exchange of DNA between molecules with homologous sequences. Vertebrate Rad51 is essential for cell proliferation. Using site-directed mutagenesis of highly conserved residues of human Rad51 (hRad51) and gene targeting of the RAD51 locus in chicken DT40 cells, we examined the importance of Rad51’s highly conserved ATP-binding domain. Mutant hRad51 incapable of ATP hydrolysis (hRad51K-133R) binds DNA less efficiently than the wild type but catalyzes strand exchange between homologous DNAs. hRad51 does not need to hydrolyze ATP to allow vertebrate cell proliferation, form nuclear foci, or repair radiation-induced DNA damage. However, cells expressing hRad51K-133R show greatly reduced targeted integration frequencies. These findings show that ATP hydrolysis is involved in DNA binding by hRad51 and suggest that the extent of DNA complexed with hRad51 in nucleoprotein influences the efficiency of recombination.
The Escherichia coli RecA protein polymerizes on DNA to form helical nucleoprotein structures and catalyzes the formation of heteroduplex DNA molecules between homologous DNAs in an ATP-dependent manner (reviewed in references 19 and 33). Following the identification of Saccharomyces cerevisiae Rad51 (ScRad51) as a structural homologue of RecA (1, 2, 37), mammalian versions of Rad51 were cloned (36). Purification and in vitro analysis of both ScRad51 and human Rad51 (hRad51) demonstrated ATP-dependent filament formation and strand exchange activity on DNA substrates, confirming their being functionally homologous to RecA (4, 7, 14, 31, 44, 45), although there are significant biochemical differences between RecA and Rad51 in terms of reaction kinetics and substrate preference (recently reviewed in reference 5).
Genetic analysis of RAD51 in yeast has placed it in the RAD52 epistasis group; rad51 mutation leads to meiotic and mitotic recombination defects, along with hypersensitivity to ionizing radiation. However, homozygous null mutation of murine RAD51 results in very early embryonic lethality (22, 50). Similarly, Rad51−/− chicken DT40 cells expressing a repressible RAD51 transgene accumulate chromosomal abnormalities and die rapidly upon repression of RAD51 (39). One model to explain this lethality invokes Rad51 acting to repair spontaneous DNA lesions occurring during replication (40). Such DNA breaks occur during replication in E. coli (28), and replication-associated recombination has been described for both E. coli and S. cerevisiae (34, 53). Furthermore, the observation of chromatid-type breaks in Rad51-deficient cells suggests the occurrence of DNA lesions during S phase (39). That none of the Rad51 homologues described to date (Rad51B, Rad51C, Rad51D, Xrcc2, and Xrcc3 [summarized in reference 23]) can substitute for Rad51 in cell survival emphasizes the key role the protein plays in vertebrate cells.
Comparison of the sequences of RecA and Rad51 shows that several residues have been entirely conserved throughout evolution (10), notably those in regions assigned to ATP binding or hydrolysis from the RecA crystal structure (41–43). In the case of RecA, ATP binding alone can promote DNA strand exchange (32), but ATP hydrolysis is required for the final resolution of heteroduplex products (6, 20, 25); E. coli cells with mutations in the A-site of the NTP-binding consensus (G/AXXXXGKT/S [51]) of the RecA ATP-binding site show greatly impaired recombination ability (24). Mutations affecting the corresponding site in S. cerevisiae Rad51 also impair recombination (11, 13, 37): abrogation of ATP binding in the Rad51 K-191A mutant leads to severe recombination and repair defects (13, 37), while a weak mutant (K-191R), which supports ATP binding but not hydrolysis, mediates DNA strand exchange and can restore cellular resistance to DNA damage to normal levels when highly expressed (37, 46).
We now report site-directed mutagenesis of a number of conserved residues in the hRad51 ATP-binding pocket and analysis of vertebrate cell survival and hRad51 function in vivo and in vitro with the mutants. Our findings reveal that hRad51 need not hydrolyze ATP for the recombinational repair necessary for vertebrate cell proliferation.
MATERIALS AND METHODS
Plasmids.
Site-directed mutagenesis of pHsRAD51, a pBluescript KS(+) construct containing hRAD51 cDNA (36), was performed with the QuikChange kit as specified by the manufacturer (Stratagene, La Jolla, Calif.). hRad51 mutations were generated as follows (5′ to 3′ from the hRAD51 start site; mutant bases are underlined): G-132A, ACT GGG→ACT GCT; K-133A, GGG AAG→GGC GCC; K-133R, GGG AAG→GGG CGT; D-161A, ATT GAC→ATC GCG; E-163A, ACT GAG→ACT GCG; V-221A, ATT GTA→ATT GCT; D-222A, GAC AGT→GCT AGC; and S-223A, GAC AGT→GAT GCC.
Following confirmatory restriction digestion and DNA sequencing, a BamHI-SalI fragment of each pBluescript-Rad51 plasmid was cloned into the EcoRI site of pApuroII (21) for expression in DT40 cells. For expression of Rad51K-133R in E. coli, an NcoI-partial XhoI fragment was cloned into the corresponding sites of pET15b (Novagen, Madison, Wis.). The prokaryotic expression vector for wild-type hRad51 consisted of the coding sequence inserted into the multicloning site of pET8c (Novagen). The targeting constructs used for the chicken RAD51 locus have been described (39). The OVALBUMIN targeting vector was generated by the insertion of a histidinol resistance cassette (47) into the HindIII site of an 8-kb PmaCI-PshAI genomic fragment in pSP72 (40). Targeting vectors for the chicken RAD54B and XRCC2 loci will be published elsewhere.
Purification of recombinant Rad51 proteins.
Wild-type hRad51 and hRad51K-133R proteins were purified by selective spermidine precipitation (3). The proteins were further purified by hydroxyapatite (Bio-Rad, Hercules, Calif.), Mono-Q (Pharmacia, Uppsala, Sweden), and Mono-S column chromatography. The Rad51K-133R protein behaved very similarly to wild-type protein during purification. The concentrations of the proteins were determined by the Bradford dye-binding assay with bovine serum albumin (BSA) as a standard. The Rad51K-133A mutant protein was precipitated less well than wild-type protein and could not be recovered efficiently from the columns during further purification.
Measurement of Rad51 ATPase activity.
ATP hydrolysis was measured in the presence of 2 μM poly(dT) (average length, 200 nucleotides; Pharmacia) by using [α-32P]ATP as a substrate as described previously (38). The concentration of ATP was 200 μM. The products were analyzed on polyethyleneimine paper and analyzed with a BAS 2000 phosphorimager (Fuji Film, Kanagawa, Japan).
DNA binding analysis. (i) Gel shift analysis.
Closed circular φX174 DNA (3 mM, containing open circular DNA) was incubated with various concentrations of Rad51 protein in a buffer containing 20 mM Tris-HCl (pH 7.5), 1 mM dithiothreitol (DTT), 5 mM MgCl2, and 0.05 mg of BSA per ml for 10 min at 37°C. After the addition of dye solution (0.05% bromophenol blue, 20% glycerol), the products were analyzed by electrophoresis through a 0.9% agarose gel. The DNA was visualized by staining the gel with SYBR-Green II (Molecular Probes, Eugene, Oreg.).
(ii) Etheno-DNA binding assay.
Etheno-DNA was prepared by using heat-denatured calf thymus DNA as a substrate as described previously (27). Etheno-DNA (1 mM) was incubated with various protein concentrations in buffer (20 mM Tris-HCl [pH 7.5], 1 mM DTT, 1 mM ATP, 5 mM MgCl2) at 25°C. Fluorescence of etheno-DNA was measured at 400 nm after excitation at 300 nm on a fluorescence spectrophotometer (RF-5000; Shimazu, Columbia, Md.).
Strand exchange assay.
The strand exchange reaction was carried out as follows. A 20 μM concentration of the 60-mer oligonucleotide A+ (ATT CGA CCT ATC CTT GCG CAG CTC GAG AAG CTC TTA CTT TGC CAC CTT TCG CCA TCA ACT), 5′ end labelled with 32P, was preincubated with 1.7 or 3.3 μM Rad51 in buffer (20 mM Tris-HCl [pH 7.5], 1 mM DTT, 1 mM MgCl2, 0.05 mg of BSA per ml, 2 mM ATP) for 5 min at 37°C. After the incubation at 37°C, the Mg2+ concentration was increased to 30 mM, followed by the addition of double-stranded DNA (dsDNA) end labelled with 32P (oligonucleotide A+ annealed with the 60-mer oligonucleotide A− [AGT TGA TGG CGA AAG GTC GCA AAG TAA GAG CTT CTC GAG CTG CGC AAG GAT AGG TCG AAT]). The final concentration of dsDNA was 20 μM. At various time points, aliquots were withdrawn and deproteinized in the presence of 0.1% sodium dodecyl sulfate and 0.5 mg of proteinase K per ml for 10 min, and products were resolved by 12.5% polyacrylamide gel electrophoresis. Following electrophoresis, the gel was dried and visualized by autoradiography and bands were quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Reverse transcriptase PCR (RT-PCR).
cDNA was reverse transcribed by using oligo(dT) primers from 1 μg of Trizol reagent-isolated total RNA (Gibco BRL, Grand Island, N.Y.) and the SuperScript II kit (Gibco), as prescribed by the supplier. A total of 1/20 of each reverse transcription reaction mixture was PCR amplified by 30 cycles of 45 s at 94°C, 45 s at 55°C, and 60 s at 68°C, followed by 5 min at 72°C, with 0.4 U of Ex Taq polymerase (Takara, Shiga, Japan) per reaction. Primers were used at 250 nM; the sequences were GCC GCC ATG GAG GCT GTT GCC TAT GCG CCA and GGC GGT GGC ACT GTC AGC AAT AAG CAG TGC.
Conditions of DT40 cell culture, transfection, selection, and cytotoxicity analysis.
Cell culture and DNA transfections were performed as described previously, as was suppression of the tet-controlled hRAD51 gene in the RAD51−/− tet-RAD51+ clone 110 (39). The efficiency of transfection into clone 110 cells was monitored by placing half the transfected cells under selection with 0.5 μg of puromycin per ml and counting the surviving colonies after 1 week. Selection conditions for histidinol, blasticidin, and neomycin were as previously described, as was Southern analysis for targeting at the RAD51 and OVALBUMIN loci (39, 48); conditions for Southern analysis of targeting at the chicken RAD54B and XRCC2 loci will be published elsewhere. Colony formation following gamma irradiation from a 137Cs source was assessed as in previous work by members of our group (48).
Immunofluorescence microscopy and immunoblotting for Rad51.
Confocal microscopy (with an MRC-1024 microscope; Bio-Rad) of Rad51 foci visualized with anti-Rad51 antibody in untreated cells or cells gamma irradiated with 8 Gy, 8 h after treatment, was performed as previously described (52). Western blot analysis with the same antibody was done as previously described (39).
RESULTS
We used site-directed mutagenesis to generate a series of hRad51 mutants in which highly conserved and putatively functionally important residues were altered (Table 1). All mutants were verified by sequencing and mutation-specific restriction digestion. In order to assess whether these mutant constructs were capable of rescuing the lethality incurred in the absence of Rad51 and of permitting cell proliferation, expression vectors containing them under the control of the chicken β-actin promoter were transfected into conditionally Rad51-null chicken cells (39). Expression of wild-type hRad51 in these cells allows survival and confers a normal level of resistance to gamma irradiation (40). The ability of the mutant expressed to support proliferation upon repression of the Rad51 gene was assessed by the number of colonies formed (Table 2). We used the puromycin resistance cassette incorporated into the expression vectors to ensure comparable transfection efficiencies between samples. These findings show that while various mutants of hRad51 are capable of rescuing the lethality incurred in the absence of Rad51, mutations of the ATP binding and hydrolysis A-site residue K-133 or of the B-site residue D-222 greatly impede this ability. As a control, Western blot analysis confirmed that the K-133A, K-133R, and D-222A expression constructs were indeed capable of producing hRad51 protein (data not shown). A low background level of survivors was obtained with the vector alone, and a wild-type signal occasionally accompanied the mutant after RT-PCR and mutation-specific restriction digestion, especially with the stronger mutations (data not shown), perhaps suggesting some interference with the tet repression system. Despite these concerns, this assay gave reproducible levels of rescue by the various transgenes and no revertants or survivors were observed without DNA transfection. Therefore, we believe that it gives a useful, though qualitative, assessment of the essential residues in the hRad51 protein.
TABLE 1.
Highly conserved (10) hRad51 residues chosen for mutagenesis
| Residue
|
Putative function(s)a | ||
|---|---|---|---|
| hRad51 | ScRad51 | RecA | |
| G-132 | G-190 | G-71 | ATP binding and hydrolysis (A-site) |
| K-133 | K-191 | K-72 | ATP binding and hydrolysis (A-site) |
| D-161 | D-219 | D-94 | Monomer-monomer interaction; stabilizes E-96 loop |
| E-163 | E-221 | E-96 | Monomer-monomer interaction; H2O activation |
| V-221 | V-279 | V-143 | ATP binding and hydrolysis (B-site) |
| D-222 | D-280 | D-144 | ATP binding and hydrolysis (B-site) |
| S-223 | S-281 | S-145 | ATP binding and hydrolysis (B-site); E-96 interaction |
RecA functions were assigned from crystal structure (41); not all roles suggested have been validated experimentally.
TABLE 2.
Ability of hRad51 mutant constructs to complement Rad51 deficiency in cell survival
| Construct transfected | Location of mutationa | Survival following hRad51 suppressionb |
|---|---|---|
| Wild type hRad51 | + | |
| hRad51 with mutation | ||
| G-132A | ATP-binding A-site | + |
| K-133A | ATP-binding A-site | − |
| K-133R | ATP-binding A-site | +/− |
| D-161A | Monomer-monomer interface | + |
| E-163A | Monomer-monomer interface | + |
| V-221A | ATP-binding B-site | + |
| D-222A | ATP-binding B-site | − |
| S-223A | ATP-binding B-site | + |
| Empty vector | − |
Based on RecA locations assigned from the crystal structure (41).
Results are the averages of at least two experiments and represent the percentages of colonies obtained after transfection with each mutant relative to the number obtained by transfection with wild-type hRad51, as follows: −, <10% of the wild type; +/−, 10 to 20% of the wild type; and +, 60 to >100% of the wild type. Transfection efficiencies were generally consistent, as measured by the number of puromycin-resistant colonies obtained in parallel experiments.
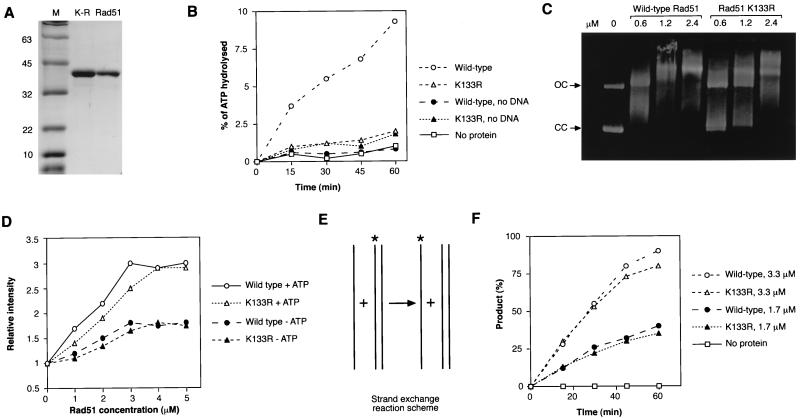
One of the best-studied motifs in the Rad51 structure is the A-site for ATP binding. We examined the role this site plays in vertebrate Rad51 function more carefully, in particular because our findings with tet-repressible Rad51 showed that it has a key role (Table 2). After purification of the K-133R mutant protein (Fig. 1A), we compared its ability to hydrolyze ATP in the presence of single-stranded DNA (ssDNA) with that of the wild type. We were unable to purify the K-133A protein, thereby precluding a direct comparison between these two mutants. While wild-type hRad51 had a very low ATPase activity, the ATPase activity of hRad51K-133R was even lower, at background levels (Fig. 1B). While there was some residual ATP hydrolysis associated with our K-133R preparation, this was not affected by the addition of DNA, so we conclude that the DNA-dependent ATPase activity of hRad51 is abrogated by the K-133R mutation. To characterize the mutant’s ability to bind DNA, we incubated purified protein with dsDNA and monitored the formation of protein-DNA complexes by gel shift analysis. Figure 1C shows that hRad51K-133R protein binds dsDNA with lower efficiency than the wild-type protein, with the formation of larger nucleoprotein complexes requiring higher concentrations of the protein. Our experiments examining ssDNA binding using etheno-DNA (Fig. 1D) confirmed this lower DNA-binding efficiency but also showed that nucleotide cofactor binding is necessary for the proficient formation of the hRad51K-133R–ssDNA complex. From these data, we conclude that hRad51K-133R binds, but does not hydrolyze, ATP during complexation with DNA in a nucleoprotein filament and that this complexation is slower than that which the wild-type protein undergoes. We next examined whether hRad51K-133R could mediate homologous pairing and strand exchange between short oligonucleotide DNA molecules in vitro. The reaction diagrammed in Fig. 1E revealed that the hRad51K-133R protein is indeed capable of catalyzing efficient homologous pairing and strand exchange (Fig. 1F).
FIG. 1.
Biochemical characterization of hRad51. (A) Purification of Rad51 protein from E. coli. A total of 2 μg of hRad51K-133R (K-R) and 1.5 μg of the wild-type hRad (Rad51) protein were loaded in the lanes indicated. The sizes of the molecular mass markers (M) are shown at the left, in kilodaltons. (B) ATP hydrolysis mediated by hRad51 and hRad51K-133R proteins. hRad51 was used at 1 μM and ATP was used at 200 μM in the presence or absence of 20 μM poly(dT) as indicated. Results are the averages of five experiments. (C) Binding of φX174 DNA by wild-type hRad51 and hRad51K-133R in the presence of ATP. As shown in the left lane, no DNA is bound in the absence of protein. The arrows at the left indicate open circular (OC) and closed circular (CC) forms of the DNA. A total of 130 ng of DNA was incubated with various concentrations of the protein in the presence of ATP for 5 min and then complexes were analyzed by 0.9% agarose gel electrophoresis. Longer times (up to 30 min) of incubation of DNA with protein yielded the same results. (D) Binding of etheno-DNA by wild-type hRad51 and hRad51K-133R in the presence or absence of 1 mM ATP as indicated. Fluorescence was measured after 5 min of incubation, when the excitation values reached a plateau. (E) Diagram of the strand exchange reaction. The assay monitors the appearance of labelled ssDNA (indicated by asterisks), showing the exchange of the labelled and unlabelled strands. (F) DNA strand exchange mediated by wild-type hRad51 or hRad51K-133R. Different concentrations of protein were incubated first with unlabelled single-stranded oligonucleotide and then with labelled homologous dsDNA, and the exchange of labelled and unlabelled ssDNA over time was monitored by 12% polyacrylamide gel electrophoresis. Data are plotted as percentages of products over total input label as calculated with a phosphorimager.
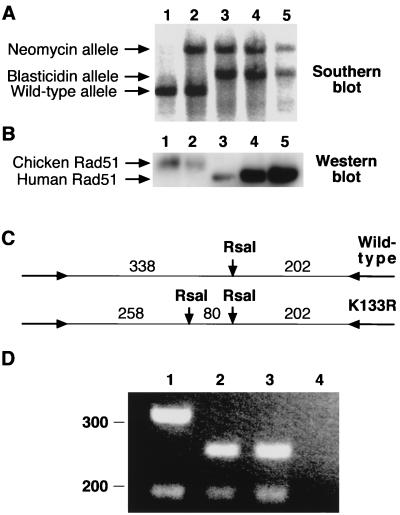
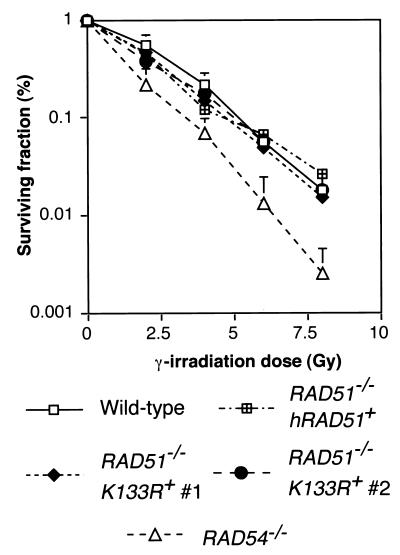
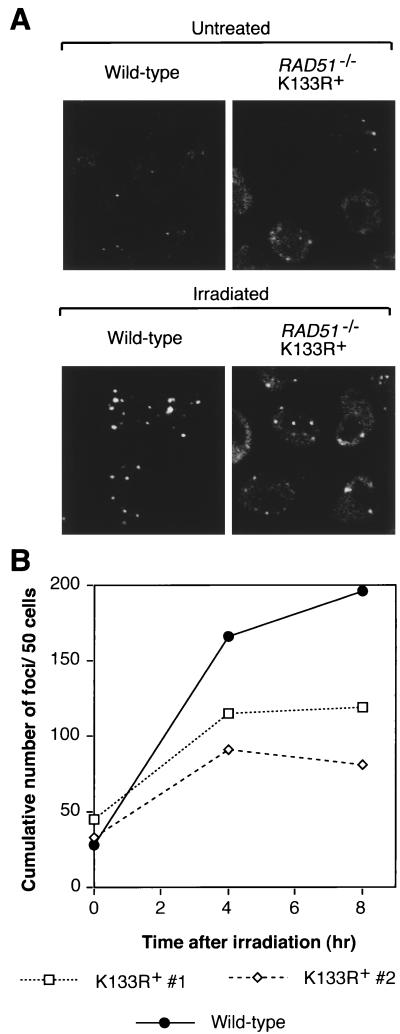
We next asked whether this hRad51 mutant could function in vivo. To rule out possible concerns with the tet-repressible system, we chose to generate Rad51-null cells expressing only the transgene of interest. Since high expression levels of ScRad51K-133R are necessary to complement rad51Δ, we reasoned that high expression levels of the corresponding hRad51 mutant might be necessary to permit vertebrate cell viability (37, 46). Consistent with this idea, the few hRAD51K-133R-transfected clones surviving tet repression of the wild-type hRAD51 transgene showed very high expression levels of mutant protein. Therefore, we generated RAD51+/− cells expressing the transgene of interest and then targeted the second RAD51 allele. This approach worked well for K-133R, but not RAD51+/− cells expressing the K-133A transgene at a level equal to or higher than that of the endogenous protein were obtained. A possible explanation for this is the dominant-negative effect in S. cerevisiae described for the equivalent K-191A mutation (13). Figure 2 shows that these cells were indeed RAD51−/− clones expressing only hRad51K-133R. Southern analysis confirmed the disruption of the endogenous RAD51 locus (Fig. 2A), and we used the slight difference in electrophoretic mobility between chicken and human Rad51 to show by Western blotting that only hRad51 is expressed (Fig. 2B). Finally, RT-PCR followed by diagnostic restriction digestion, as diagrammed in Fig. 2C, confirmed that only the K-133R mutant hRAD51 gene was expressed (Fig. 2D). Thus, the K-133R mutant is indeed capable of rescuing Rad51 deficiency. Perhaps surprisingly, these cells were no more sensitive to double-strand breaks induced in their DNA by gamma irradiation than wild-type DT40 cells (Fig. 3). Similar overexpression of the wild-type protein also confers normal levels of radioresistance (Fig. 3). RAD51−/− hRAD51K-133R+ cells were also capable of forming foci of Rad51, putative sites of active recombinational repair, in response to such treatment (Fig. 4), although the extent of this induction was slightly lower than that found in wild-type cells (Fig. 4B). We attribute the elevated background levels of immunofluorescence in mutant cells to the very high hRad51 expression levels.
FIG. 2.
Generation of RAD51−/− hRAD51K-133R+ DT40 cells. (A) Southern blot analysis of targeting of the chicken RAD51 locus (39). DNA from wild-type (lane 1), RAD51+/− (lane 2), RAD51−/− tet-hRAD51+ clone 110 (lane 3), RAD51−/− hRAD51K-133R+ clone 1 (lane 4), and RAD51−/− hRAD51K-133R+ clone 2 (lane 5) DT40 cells was used. (B) Western blot analysis. Total protein (10 μg/lane) was loaded from wild-type (lane 1), RAD51+/−(lane 2), RAD51−/− tet-hRAD51+ clone 110 (lane 3), RAD51−/− hRAD51K-133R+ clone 1 (lane 4), and RAD51−/− hRAD51K-133R+ clone 2 (lane 5) DT40 cells and immunoblotted with anti-hRad51 antiserum. (C) Scheme of diagnostic restriction digestion for the K-133R mutation. A second RsaI site in the PCR fragment shown was introduced as indicated into the mutant hRad51 gene. Expected sizes are indicated, in base pairs. (D) RT-PCR analysis of K-133R transgene expression. After reverse transcription and amplication of 1 μg of total RNA, 20 μl of each 100-μl reaction mixture was digested with RsaI and run on a 2.5% agarose gel. Shown are digests from RAD51−/− tet-hRAD51+ clone 110 (lane 1), RAD51−/− hRAD51K-133R+ clone 1 (lane 2), and RAD51−/− hRAD51K-133R+ clone 2 (lane 3) DT40 cells and a no reverse transcriptase control amplification (lane 4). Sizes are shown at the left, in base pairs.
FIG. 3.
Gamma ray sensitivity of RAD51−/− hRAD51K-133R+ DT40 cells. Survival 1 week after 137Cs irradiation with the indicated dose was quantified by colony formation in methylcellulose-containing medium relative to that of nonirradiated cells. Hypersensitive RAD54−/− cells (8) and a RAD51−/− cell line expressing comparably high levels of hRad51 (40) were included as controls. Plating efficiency was ≈100% for RAD51−/− hRAD51K-133R+ cells, and the data are the means ± the standard deviations of three experiments.
FIG. 4.
Formation of Rad51 foci by Rad51K-133R mutant proteins. (A) Immunofluorescent visualization of Rad51 foci in RAD51−/− hRAD51K-133R+ DT40 cells. Cells were fixed either directly or 8 h after gamma irradiation with 8 Gy of 137Cs. The high background levels in mutant cells are attributed to the very high hRad51 expression levels. This experiment was carried out at least twice for all genotypes shown. Images were processed with Adobe Photoshop, version 4.0J. (B) Kinetics of focus formation. Following microscopy and image processing with Adobe Photoshop, version 4.0J, color-inverted images were printed and distinct foci were counted. At least 80 cells from three randomly chosen frames were counted per time point.
The analysis of gene targeting is probably the most sensitive tool for examining deficiencies in recombination (40, 52). We next analyzed targeting frequencies at the RAD54B, XRCC2, and OVALBUMIN loci in wild-type and RAD51−/− hRAD51K-133R+ DT40 cells. Although cells expressing hRad51K-133R were capable of gene targeting (Table 3), this occurred at a markedly lower frequency than in wild-type cells. This is unlikely to be an artifact of mutant hRad51 overexpression, since a clone overexpressing the wild-type protein demonstrated normal levels of targeted integration frequency in similar experiments (40).
TABLE 3.
Gene targeting efficiency in Rad51−/− hRad51K-133R+ DT40 cells
| Locus targeted | No. (%) of clones targeting locus/no. of clones examined
|
||
|---|---|---|---|
| RAD51−/− K-133R+ clone 1 | RAD51−/− K-133R+ clone 2 | Wild type | |
| RAD54B | 1/28 (4) | NDa | 8/33 (24) |
| XRCC2 | 22/63 (35) | ND | 41/52 (79) |
| OVALBUMIN | 1/34 (3) | 4/36 (11) | 23/35 (66) |
ND, not determined.
DISCUSSION
Our findings implicate the hRad51 ATP binding consensus in vertebrate cell survival, although changes at certain residues in the A- and B-sites of ATP binding and hydrolysis have a relatively minor effect. To analyze hRad51 residues essential for vertebrate cell survival, we concentrated on those believed to be involved in the catalytically important binding and hydrolysis of ATP. Mutation to A of the highly conserved NTP-binding A-site G-132 residue, the B-site V-221 and S-223 residues, and the putative H2O-activating D-161 and E-163 residues allowed efficient rescue of Rad51-deficient lethality. Yeast with mutations at two of the corresponding sites, rad51G190D and rad51E221K, show recombinational activity reduced to that observed in rad51 mutants (11), although our experiments changing G-132 and E-163 to A involved more conservative mutations than those. On the other hand, a G-71V mutation in E. coli RecA results in a recA mutant phenotype (18). Nonconservative changes at the putative H2O activation site residues, D-161 and E-163 (41, 42), were very effective at complementing Rad51 deficiency, suggesting that these residues are not essential for the vital (recombination) activities of hRad51. Mutations which had a strong effect on DT40 cell survival were those altering K-133 to A or R and D-222 to A. The equivalent ScRad51 K-191A, K-191R (13, 37, 46), and D-280G (11) mutations also have strongly disruptive effects on Rad51 activity, genetic recombination, and repair of DNA damage, although overexpression of ScRad51K-191R appears to confer normal Rad51 activity (46).
Appropriate nucleotide cofactor binding is necessary for Rad51 to form an extended nucleoprotein filament on DNA and to mediate strand exchange in vitro (4, 7, 31, 44, 45). While the prototypic RecA protein can mediate strand exchange between homologous DNAs without the hydrolysis of ATP, as shown by the use of nonhydrolyzable ATP analogues (26) or K-72R mutant RecA (32), it appears that this exchange differs from that permitted by the ATP-hydrolyzing reaction, so the biochemical role of this hydrolysis is still a topic of active debate (6, 12, 20, 25, 35). Perhaps significantly, expression of RecAK-72R does not complement RecA deficiency (18). Although the inability to bind ATP results in a catalytically inactive ScRad51 protein, the loss of ScRad51’s ability to hydrolyze ATP is compatible with biochemical and biological activity, as nonhydrolyzable ATP analogues can support ScRad51-mediated strand exchange and ScRAD51K-191R can rescue rad51Δ, if expressed at high levels (37, 46). It should be noted that the normal ATPase activity of hRad51 is very low, so the reduction of this to background levels is not a dramatic change. However, the corresponding mutation of K-191 in ScRad51 or K-72 in RecA to R results in a protein clearly capable of binding, but not hydrolyzing, ATP, so we believe that the hRad51K-133R protein has no ATPase activity. Although it is possible that some minor contaminating fraction of another ATP-hydrolyzing protein was present in the Rad51 preparations used in our experiments, any residual ATP hydrolysis activity was not DNA dependent, so we conclude that the K-133R mutation removes the ATPase activity of hRad51.
To find out what effects such a mutation has on the activities of hRad51, we expressed hRad51K-133R in E. coli and examined the ability of the purified protein to bind DNA and to mediate pairing and strand exchange between homologous DNA molecules. We found by gel shift and etheno-DNA binding analyses that the mutant protein binds both ssDNA and dsDNA less efficiently than the wild type (Fig. 1C and D), albeit in an ATP-dependent manner. Like the corresponding yeast mutant protein, hRad51K-133R mediates homologous pairing and strand exchange between two homologous DNA molecules (Fig. 1F). While nonhydrolyzable ATPγS is not as effective a cofactor for strand exchange by the hRad51 protein as ATP (4, 14), it does not abrogate the reaction, so our findings are consistent with previous work on the involvement of ATP hydrolysis in Rad51 function.
DT40 cells engineered to express only the K-133R mutant protein are viable, confirming our findings with the tet-repressible Rad51 assay (Fig. 2). Our inability to generate RAD51+/− lines expressing high levels of hRad51K-133A in parallel experiments may suggest some dominant-negative effects of these proteins on wild-type Rad51, as has been described in yeast (11, 29). RAD51−/− hRAD51K-133R+ cells carry out normal levels of recombinational repair, as measured by their ability to withstand gamma irradiation (Fig. 3). In addition, this mutant Rad51 protein can form subnuclear aggregates, believed to represent recombination structures required to repair induced or replication-associated DNA damage (9, 16, 17, 40, 49), both spontaneously and in response to ionizing radiation (Fig. 4). However, the induction of nuclear foci of Rad51K-133R is slightly retarded. That the mutant Rad51 can polymerize on damaged DNA is consistent with the normal levels of radiation sensitivity found in cells expressing hRad51K-133R. Nevertheless, the inability to hydrolyze ATP impedes this protein’s ability to support homologous recombination, as measured by gene targeting (Table 3). This is not a feature of overexpression of the human transgene, despite previous findings on the species specificity of the interactions of the RAD52 group proteins (13), because high expression of wild-type Rad51 restores gene targeting to at least wild-type DT40 levels (40).
Why does Rad51 hydrolyze ATP? Rad51 requires ATP to bind DNA (4, 7, 44, 45) and dissociates upon its hydrolysis (30), suggesting that turnover of the nucleoprotein filament might require hydrolysis. This idea has been advanced for RecA (20). An alternative notion, that efficient strand exchange requires ATP hydrolysis, is supported by the poor activity of ATPγS in acting as a cofactor for strand exchange by hRad51 (4, 14) but not by the efficient strand exchange mediated by ScRad51 in the absence of ATP hydrolysis (46). Our findings with hRad51K-133R indicate that strand exchange is not abrogated by the K-133R mutation, despite the accompanying inability to hydrolyze ATP. Rescue of lethality and the continued occurrence of gene targeting at detectable levels further indicate that hRad51 need not hydrolyze ATP to function.
While the high expression levels in our RAD51−/− hRAD51K-133R+ cells might argue that low turnover necessitates overexpression to cope with spontaneous mitotic DNA lesions, this idea seems insufficient to explain the reduced gene targeting efficiency, given the large amount of hRad51 available. However, the altered DNA binding by the mutant protein may explain the reduction in targeting efficiency. If Rad51 assembly on a recombination substrate occurs slowly, it will delay recombination. The limited time available in which targeted integration may occur before transfected constructs are lost may explain the reduced efficiency. The reduced induction of Rad51K-133R foci after irradiation (Fig. 4) may reflect this polymerization defect. Since a minimum polymer size is likely necessary for immunofluorescent visualization of a nucleoprotein structure as a focus, sufficient Rad51 for double-strand break repair may be assembled at double-strand breaks (Fig. 3) without, perhaps, giving rise to detectable foci. The extent to which hRad51 initiates and mediates extensive heteroduplex DNA formation in a chromosomal environment is still under debate (15), but the ability to do so may be dependent on the extent of the DNA sequence bound by Rad51. If that is the case, one possible model explaining the restoration of viability along with the reduced targeting frequency in RAD51−/− hRAD51K-133R+ cells is that ATP hydrolysis permits more rapid and extensive nucleoprotein filament formation and homology searching by Rad51. The essential recombinational repair of sister chromatids, which lie adjacent to one another, is therefore less disrupted by ATPase deficiency than the complex search for homology throughout the nuclear volume required by gene targeting. The diminution of the Rad51 ATPase activity in higher organisms may reflect the diminishing reliance on, and indeed the general avoidance of, recombination between homologous chromosomes or repeat sequences during evolution of complex organisms. The pressure retaining this activity may be primarily during occasions when such recombination is both necessary and useful, e.g., meiosis.
ACKNOWLEDGMENTS
We thank Y. Sato, M. Hashishin, O. Koga, and M. Hirao for their excellent technical assistance. We are also grateful to H. Kurumizaka, T. Shibata (both of RIKEN), and H. Ogawa (Iwate College of Nursing) for their comments on the manuscript.
C.M. is the recipient of a JSPS Postdoctoral Fellowship. The Bayer-Chair Department of Molecular Immunology and Allergology is supported by Bayer Yakuhin, Kyoto, Japan. This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science and Culture of Japan, CREST, JST, and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research.
REFERENCES
- 1.Aboussekhra A, Chanet R, Adjiri A, Fabre F. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol Cell Biol. 1992;12:3224–3234. doi: 10.1128/mcb.12.7.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basile G, Aker M, Mortimer R K. Nucleotide sequence and transcriptional regulation of the yeast recombinational repair gene RAD51. Mol Cell Biol. 1992;12:3235–3246. doi: 10.1128/mcb.12.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann P, Benson F E, Hajibagheri N, West S C. Purification of human Rad51 protein by selective spermidine precipitation. Mutat Res. 1997;384:65–72. doi: 10.1016/s0921-8777(97)00028-1. [DOI] [PubMed] [Google Scholar]
- 4.Baumann P, Benson F E, West S C. Human Rad51 protein promotes ATP-dependent homologous pairing and strand transfer reactions in vitro. Cell. 1996;87:757–766. doi: 10.1016/s0092-8674(00)81394-x. [DOI] [PubMed] [Google Scholar]
- 5.Baumann P, West S C. Role of the human Rad51 protein in homologous recombination and double-stranded break repair. Trends Biochem Sci. 1998;23:247–251. doi: 10.1016/s0968-0004(98)01232-8. [DOI] [PubMed] [Google Scholar]
- 6.Bedale W A, Cox M M. Evidence for the coupling of ATP hydrolysis to the final (extension) phase of recA protein-mediated DNA strand exchange. J Biol Chem. 1996;271:5725–5732. doi: 10.1074/jbc.271.10.5725. [DOI] [PubMed] [Google Scholar]
- 7.Benson F E, Stasiak A, West S C. Purification and characterization of the human Rad51 protein, an analogue of E. coli recA. EMBO J. 1994;13:5764–5771. doi: 10.1002/j.1460-2075.1994.tb06914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezzubova O, Silbergleit A, Yamaguchi-Iwai Y, Takeda S, Buerstedde J-M. Reduced X-ray resistance and homologous recombination frequencies in a RAD54−/− mutant of the chicken DT40 cell line. Cell. 1997;89:185–193. doi: 10.1016/s0092-8674(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 9.Bishop D K, Ear U, Bhattacharyya A, Calderone C, Beckett M, Weichselbaum R R, Shinohara A. Xrcc3 is required for assembly of Rad51 complexes in vivo. J Biol Chem. 1998;273:21482–21488. doi: 10.1074/jbc.273.34.21482. [DOI] [PubMed] [Google Scholar]
- 10.Brendel V, Brocchieri L, Sandler S J, Clark A J, Karlin S. Evolutionary comparisons of RecA-like proteins across all major kingdoms of living organisms. J Mol Evol. 1997;44:528–541. doi: 10.1007/pl00006177. [DOI] [PubMed] [Google Scholar]
- 11.Chanet R, Heude M, Adjiri A, Maloisel L, Fabre F. Semidominant mutations in the yeast Rad51 protein and their relationships with the Srs2 helicase. Mol Cell Biol. 1996;16:4782–4789. doi: 10.1128/mcb.16.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox M M. Why does RecA protein hydrolyse ATP? Trends Biochem Sci. 1994;19:217–222. doi: 10.1016/0968-0004(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 13.Donovan J W, Milne G T, Weaver D T. Homotypic and heterotypic protein associations control Rad51 function in double-strand break repair. Genes Dev. 1994;8:2552–2562. doi: 10.1101/gad.8.21.2552. [DOI] [PubMed] [Google Scholar]
- 14.Gupta R C, Bazemore L R, Golub E I, Radding C M. Activities of human recombination protein Rad51. Proc Natl Acad Sci USA. 1997;94:463–468. doi: 10.1073/pnas.94.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R C, Folta-Stogniew E, Radding C M. Human Rad51 protein can form homologous joints in the absence of net strand exchange. J Biol Chem. 1999;274:1248–1256. doi: 10.1074/jbc.274.3.1248. [DOI] [PubMed] [Google Scholar]
- 16.Haaf T, Golub E I, Reddy G, Radding C M, Ward D C. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci USA. 1995;92:2298–2302. doi: 10.1073/pnas.92.6.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haaf T, Raderschall E, Reddy G, Ward D C, Radding C M, Golub E I. Sequestration of mammalian Rad51-recombination protein into micronuclei. J Cell Biol. 1999;144:11–20. doi: 10.1083/jcb.144.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konola J T, Logan K M, Knight K L. Functional characterization of residues in the P-loop motif of the RecA protein ATP-binding site. J Mol Biol. 1994;237:20–34. doi: 10.1006/jmbi.1994.1206. [DOI] [PubMed] [Google Scholar]
- 19.Kowalczykowski S C, Eggleston A K. Homologous pairing and DNA strand-exchange proteins. Annu Rev Biochem. 1994;63:991–1043. doi: 10.1146/annurev.bi.63.070194.005015. [DOI] [PubMed] [Google Scholar]
- 20.Kowalczykowski S C, Krupp R A. DNA strand exchange promoted by RecA protein in the absence of ATP: implications for the mechanism of energy transfer in protein-promoted nucleic acid transactions. Proc Natl Acad Sci USA. 1995;92:3478–3482. doi: 10.1073/pnas.92.8.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B-cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim D-S, Hasty P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol Cell Biol. 1996;16:7133–7143. doi: 10.1128/mcb.16.12.7133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N, Lamerdin J E, Tebbs R S, Schild D, Tucker J D, Shen M R, Brookman K W, Siciliano M J, Walter C A, Fan W, Narayana L S, Zhou Z Q, Adamson A W, Sorensen K J, Chen D J, Jones N J, Thompson L H. Xrcc2 and Xrcc3, new human Rad51-family members, promote chromosome stability and protect against DNA cross-links and other damages. Mol Cell. 1998;1:783–793. doi: 10.1016/s1097-2765(00)80078-7. [DOI] [PubMed] [Google Scholar]
- 24.Logan K M, Knight K L. Mutagenesis of the P-loop motif in the ATP-binding site of the RecA protein from Escherichia coli. J Mol Biol. 1993;232:1048–1059. doi: 10.1006/jmbi.1993.1459. [DOI] [PubMed] [Google Scholar]
- 25.MacFarland K J, Shan Q, Inman R B, Cox M M. RecA as a motor protein. J Biol Chem. 1997;272:17675–17685. doi: 10.1074/jbc.272.28.17675. [DOI] [PubMed] [Google Scholar]
- 26.Menetski J P, Bear D G, Kowalczykowski S C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli recA protein in the absence of ATP hydrolysis. Proc Natl Acad Sci USA. 1990;87:21–25. doi: 10.1073/pnas.87.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menetski J P, Kowalczykowski S C. Interaction of recA protein with single-stranded DNA. Quantitative aspects of binding affinity modulation by nucleotide cofactors. J Mol Biol. 1985;181:281–295. doi: 10.1016/0022-2836(85)90092-0. [DOI] [PubMed] [Google Scholar]
- 28.Michel B, Ehrlich S D, Uzest M. DNA double-strand breaks caused by replication arrest. EMBO J. 1997;16:430–438. doi: 10.1093/emboj/16.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milne G T, Weaver D T. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 1993;7:1755–1765. doi: 10.1101/gad.7.9.1755. [DOI] [PubMed] [Google Scholar]
- 30.Namsaraev E A, Berg P. Binding of Rad51p to DNA. J Biol Chem. 1998;273:6177–6182. doi: 10.1074/jbc.273.11.6177. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa T, Yu X, Shinohara A, Egelman E H. Similarity of the yeast Rad51 filament to the bacterial recA filament. Science. 1993;259:1896–1899. doi: 10.1126/science.8456314. [DOI] [PubMed] [Google Scholar]
- 32.Rehrauer W M, Kowalczykowski S C. Alteration of the nucleoside triphosphate (NTP) catalytic domain within Escherichia coli RecA protein attenuates NTP hydrolysis but not joint molecule formation. J Biol Chem. 1993;268:1292–1297. [PubMed] [Google Scholar]
- 33.Roca A I, Cox M M. The RecA protein: structure and function. Crit Rev Biochem Mol Biol. 1990;25:415–456. doi: 10.3109/10409239009090617. [DOI] [PubMed] [Google Scholar]
- 34.Seigneur M, Bidnenko V, Ehrlich S D, Michel B. RuvAB acts at arrested replication forks. Cell. 1998;95:419–430. doi: 10.1016/s0092-8674(00)81772-9. [DOI] [PubMed] [Google Scholar]
- 35.Shan Q, Cox M M, Inman R B. DNA strand exchange promoted by RecA K72R. J Biol Chem. 1996;271:5712–5724. doi: 10.1074/jbc.271.10.5712. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara A, Ogawa H, Matsuda Y, Ushio N, Ikeo K, Ogawa T. Cloning of human, mouse and fission yeast recombination genes homologous to RAD51 and recA. Nat Genet. 1993;4:239–243. doi: 10.1038/ng0793-239. [DOI] [PubMed] [Google Scholar]
- 37.Shinohara A, Ogawa H, Ogawa T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell. 1992;69:457–470. doi: 10.1016/0092-8674(92)90447-k. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara A, Ogawa T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature. 1998;391:404–407. doi: 10.1038/34943. [DOI] [PubMed] [Google Scholar]
- 39.Sonoda E, Sasaki M S, Buerstedde J-M, Bezzubova O, Shinohara A, Ogawa H, Takata M, Yamaguchi-Iwai Y, Takeda S. Rad51-deficient vertebrate cells accumulate chromosomal breaks prior to cell death. EMBO J. 1998;17:598–608. doi: 10.1093/emboj/17.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonoda E, Sasaki M S, Morrison C, Yamaguchi-Iwai Y, Takata M, Takeda S. Sister chromatid exchanges are mediated by homologous recombination in vertebrate cells. Mol Cell Biol. 1999;19:5166–5169. doi: 10.1128/mcb.19.7.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Story R M, Bishop D K, Kleckner N, Steitz T A. Structural relationship of bacterial recA proteins to recombination proteins from bacteriophage T4 and yeast. Science. 1993;259:1892–1896. doi: 10.1126/science.8456313. [DOI] [PubMed] [Google Scholar]
- 42.Story R M, Steitz T A. Structure of the recA-protein-ADP complex. Nature. 1992;355:374–376. doi: 10.1038/355374a0. [DOI] [PubMed] [Google Scholar]
- 43.Story R M, Weber I T, Steitz T A. The structure of the E. coli recA protein monomer and polymer. Nature. 1992;355:318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- 44.Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast Rad51 protein. Science. 1994;265:1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- 45.Sung P, Robberson D L. DNA strand exchange mediated by a Rad51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell. 1995;82:453–461. doi: 10.1016/0092-8674(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 46.Sung P, Stratton S A. Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J Biol Chem. 1996;271:27983–27986. doi: 10.1074/jbc.271.45.27983. [DOI] [PubMed] [Google Scholar]
- 47.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takata M, Sasaki M S, Sonoda E, Morrison C, Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A, Takeda S. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 1998;17:5497–5508. doi: 10.1093/emboj/17.18.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tashiro S, Kotomura N, Shinohara A, Tanaka K, Ueda K, Kamada N. S phase specific formation of the human Rad51 protein nuclear foci in lymphocytes. Oncogene. 1996;12:2165–2170. [PubMed] [Google Scholar]
- 50.Tsuzuki T, Fujii Y, Sakumi K, Tominaga Y, Nakao K, Sekiguchi M, Matsushiro A, Yoshimura Y, Morita T. Targeted disruption of the Rad51 gene leads to lethality in embryonic mice. Proc Natl Acad Sci USA. 1996;93:6236–6240. doi: 10.1073/pnas.93.13.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaguchi-Iwai Y, Sonoda E, Buerstedde J-M, Bezzubova O, Morrison C, Takata M, Shinohara A, Takeda S. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol Cell Biol. 1998;18:6430–6435. doi: 10.1128/mcb.18.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou H, Rothstein R. Holliday junctions accumulate in replication mutants via a RecA homolog-independent mechanism. Cell. 1997;90:87–96. doi: 10.1016/s0092-8674(00)80316-5. [DOI] [PubMed] [Google Scholar]