Abstract
zfh-1 is a member of the zfh family of proteins, which all contain zinc finger and homeodomains. The roles and mechanisms of action of most family members are still unclear. However, we have shown previously that another member of the family, the vertebrate ZEB protein, is a transcriptional repressor that binds E box sequences and inhibits myotube formation in cell culture assays. zfh-1 is downregulated in Drosophila embryos prior to myogenesis. Embryos with zfh-1 loss-of-function mutation show alterations in the number and position of embryonic somatic muscles, suggesting that zfh-1 could have a regulatory role in myogenesis. However, nothing is known about the nature or mechanism of action of zfh-1. Here, we demonstrate that zfh-1 is a transcription factor that binds E box sequences and acts as an active transcriptional repressor. When zfh-1 expression was maintained in the embryo beyond its normal temporal pattern of downregulation, the differentiation of somatic but not visceral muscle was blocked. One potential target of zfh-1 in somatic myogenesis could be the myogenic factor mef2. mef2 is known to be regulated by the transcription factor twist, and we show here that zfh-1 binds to sites in the mef2 upstream regulatory region and inhibits twist transcriptional activation. Even though there is little sequence similarity in the repressor domains of ZEB and zfh-1, we present evidence that zfh-1 is the functional homologue of ZEB and that the role of these proteins in myogenesis is conserved from Drosophila to mammals.
Classically, myogenic differentiation in vertebrates was believed to be dependent only on the activity of the positive myogenic regulators mef2 and MRF (myogenic regulatory factors [myoD, myf-5, myogenin, and MRF-4]) proteins. Members from the two protein families synergize to promote skeletal muscle differentiation (32). However, recent evidence indicates that muscle differentiation is also under negative regulation and that a proper temporal and spatial pattern of muscle gene expression is the result of a fine balance between positive and negative factors (4, 9, 41). Previously, we and others demonstrated that a zinc finger/homeodomain protein most commonly known as ZEB (zinc finger E box binding protein [7, 14, 15, 18, 19, 40]), blocks formation of myotubes in culture by binding to E box sequences in the promoters of myogenic genes and actively repressing their transcription (36, 39). We proposed a model where ZEB would control the timing of myogenesis, although no in vivo evidence for such model is available (36).
In Drosophila, the basic helix-loop-helix protein twist is necessary and sufficient for somatic muscle development (2, 3), similar to the way in which MRF proteins serve as the switch for myogenic fate in vertebrates (43, 44). Recent studies demonstrated that twist regulates somatic muscle differentiation by direct activation of mef2 transcription through a 175-bp enhancer located 2.3 kb upstream of the mef2 gene (11). mef2 is also essential for muscle differentiation in Drosophila, embryos mutant for mef2 have muscle precursors, but they fail to differentiate and express the differentiation marker, myosin heavy chain (MHC) (5, 30).
zfh-1 is member of the zfh family, characterized by multiple zinc finger and homeodomain motifs, that is required for the normal development of myogenic and gonadal precursors (6, 13, 25, 27, 33, 47). zfh-1 is initially expressed throughout the presumptive mesoderm but later is downregulated (26, 27). Although zfh-1 diminishes in embryonic muscle precursors before they differentiate to muscle, mutant embryos with loss of function for zfh-1 showed defects in embryonic myogenesis, and although muscles still differentiate, there are subtle defects in the number and positioning of the muscles (26, 27). These results suggest that although zfh-1 is not essential for embryonic muscle differentiation to proceed, it may have a role in regulating the process. zfh-1 was originally described as a nuclear protein (26), but nothing is known about its nature, its mechanism of action, or whether it is a positive or negative regulator of such processes.
zfh-1 and ZEB are two members of the zfh family that share sequence similarity in their zinc fingers and homeodomain (13, 18). The fact that both proteins seem to be involved in myogenesis suggested that they may be functionally related. Here we examine the molecular mechanism of action of zfh-1. We show that zfh-1 is a transcriptional factor that binds E boxes. We also show that despite the lack of sequence similarity in the repressor domain, zfh-1 and ZEB have identical repressor specificity. We also found that zfh-1 is able to block myotube conversion in mammalian cell culture systems and that maintenance of zfh-1 expression beyond its normal temporal pattern blocks differentiation of somatic muscle differentiation in Drosophila embryos by disrupting the pattern of expression of the muscle differentiation factor mef2.
MATERIALS AND METHODS
Cell culture.
Schneider L2 cells were obtained from R. Cagan (Washington University, St. Louis, Mo.) and grown at 25°C in Schneider’s Drosophila medium (Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS; Life Technologies). The HT1080 fibrosarcoma and C33a cervical carcinoma cells were obtained from the American Type Culture Collection depository (Rockville, Md.) and were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) containing 5% FBS and 5% calf serum (Life Technologies). C3H10T1/2 (hereafter called 10T1/2) fibroblasts (American Type Culture Collection) were grown in DMEM containing 13% FBS.
Plasmid construction.
A zfh-1 cDNA (pBluescript P19 clone) was obtained from Z. C. Lai (University of Pennsylvania, Philadelphia). Mammalian expression vectors for zfh-1 were constructed as follows. Full-length zfh-1 cDNA cloned in the EcoRI site of the P19 clone was moved to the EcoRI site of pCI-neo (Promega Corporation, Madison, Wis.). DB-zfh-1 (containing C-terminal zinc fingers [DNA binding domains]) was obtained by PCR amplification of zfh-1 cDNA and cloned in the MluI/XbaI site of pCI-neo. A Kozak sequence, ATG codon, and Flag tag sequences were cloned in frame and upstream of the C-terminal zinc fingers in the XhoI/MluI sites of pCI-neo. Nuclear localization and stop codon signals were cloned in frame downstream of this fragment in the XbaI/NotI site of pCI-neo. DB-zfh-1–RD-ZEB was constructed by cloning the repressor domain of ZEB (containing the central region between amino acids 294 and 902) into the XbaI/NotI sites of pCI-DB-zfh-1. The expression of all these constructs was assessed by Western blotting using Flag antibody (Kodak, New Haven, Conn.) or anti-zfh-1 antibody (Z.-C. Lai) as described below.
To construct Gal4 and LexA fusion proteins, the repressor domain of zfh-1 (amino acids 416 to 972) was fused in frame with the DNA binding domain of Gal4 and LexA proteins by cloning into the EcoRI/XbaI sites of PM1 and pBXL3, respectively (10, 45). Gal4-ZEB and LexA-ZEB constructs were previously described (36, 37). To construct a frameshift L-ZEB plasmid, used as a control, ZEB cDNA was released with SmaI/XbaI from PM1-ZEB and cloned out of frame in the SmaI/XbaI site of pBXL3. Gal4 activators used in this study were previously described (45).
Drosophila expression vectors for zfh-1 and ZEB were constructed as follows. Full-length zfh-1 was released as an EcoRI fragment from the P19 clone and cloned in the EcoRI site from the actin 5 C promoter-driven ActPP construct (obtained from R. Cagan). The C-terminal zinc fingers of zfh-1 were released as an XhoI/NotI fragment from its corresponding pCI-neo version and cloned in the corresponding sites of the pBluescript KS. Then, an KpnI/NotI fragment was released from pBluescript and inserted in the KpnI/NotI site of a polylinker modified version of the pPac actin 5C-driven plasmid (from C. Thummel, University of Utah, Salt Lake City). Similarly, an XhoI/NotI fragment encoding the C-terminal zinc fingers and full-length ZEB were released from the pCI-neo version of these molecules and, after cloning in pBluescript, released as a KpnI/NotI fragment and cloned in the corresponding sites of the modified version of pPac. pPac-snail was obtained from T. Ip (University of Massachusetts, Worcester).
A 2.2-kb fragment of the rhomboid promoter cloned in pGEM7Z(−) was obtained from T. Ip (22). A BamHI fragment was released from pGEM7Z(−) and cloned in the corresponding sites of pSP73. Then a ClaI/EcoRV fragment was released from pSP73 and cloned in the same sites of pBluescript upstream of the chloramphenicol acetyltransferase (CAT) gene cloned in the PstI/BamHI sites (38).
A 3.7-kb fragment of the PL promoter of single-minded (23) was obtained as a pBluescript-derived form from S. Crews (University of North Carolina, Chapel Hill), amplified by PCR, and cloned in the XhoI/HindIII sites of pBluescript-CAT (38).
pGxSV-CAT (containing four Gal4 binding sites upstream of the simian virus 40 [SV40] enhancer driving the CAT gene), PG2 and PG5 (containing, respectively, two and five Gal4 sites upstream of the E1B TATA box and the CAT gene), and PL6G2 and PL6G5 (containing six LexA sites upstream of the Gal4 sites in PG2 and PG5) were previously described (10, 45). 76α4-E361CAT, containing a ZEB binding site (−361 bp of the α4 gene promoter) upstream of the ets-driven α4 enhancer, was previously described (37, 38). The CAT reporter plasmid pD33A3 contains six E box sequences (three copies of the tandem CACCTG/CAGGTG) and was obtained from S. Hayashi (National Institute of Genetics, Mishima, Japan) (16).
Recombinant proteins for the N-terminal zinc fingers (amino acids 201 to 468) of zfh-1 were obtained by PCR and cloned in the EcoRI/NotI site of pGEX-4T-1 (Pharmacia Fine Chemicals, Uppsala, Sweden). The C-terminal zinc fingers (amino acids 904 to 1060) of zfh-1 were obtained by PCR and cloned in the BamHI/EcoRI site of pGEX-2T (Pharmacia Fine Chemicals). A prokaryotic expression vector for N-terminal zinc fingers of ZEB was obtained from T. Kadesch (18). A glutathione S-transferase (GST) expression vector for snail was obtained from T. Ip.
Production of recombinant proteins.
Bacteria transformed with pGEX constructs encoding the N- and C- terminal zinc finger domains of zfh-1, the N-terminal domains of ZEB, and full-length snail were induced to produce recombinant proteins by incubation with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as instructed by the manufacturer (Pharmacia Fine Chemicals). After incubation with IPTG, bacteria were lysed in NETN (20 mM Tri HCl [pH 8.0], 100 mM NaCl, 0.5% NP-40, 1 mM EDTA) and sonicated, and the lysate was tested and quantified for the expression of the different GST proteins by Western blotting for GST, using an anti-GST horseradish peroxidase (HRP)-conjugated antibody (Santa Cruz Biotechnologies, Santa Cruz, Calif.).
Gel shift experiments.
As probes in the gel shift experiments, annealed oligonucleotides containing the ZEB site (E361/E399) in the α4 integrin promoter (37, 38), snail sites (Sna1 and Sna5ab) in the single-minded promoter (23), snail sites (s1 through s4) in the rhomboid promoter (22), and zfh-1 sites at bp −2782 and −8564 in the Drosophila mef2 promoter (35a) were end labeled with [γ-32P]ATP by using T4 polynucleotide kinase. Bacterial lysates containing recombinant proteins for the zinc fingers of zfh-1 and ZEB were incubated with 1 μg of bovine serum serum albumin and 0.5 μg of poly(dI-dC) in 25 μl of reaction mix containing 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 1 mM EDTA, and 10% glycerol for 10 min on ice in the presence or absence of 50-fold excess of unlabeled probe. The mixture was incubated for 10 min, 6 fmol of labeled probe was added, and the mixture was incubated for another 10 min at room temperature. Then, samples were subjected to electrophoresis as described elsewhere (37).
Transient transfections and CAT assays.
Cells were transfected by the calcium phosphate method (38). After 48 h, lysates were collected, transfection efficiency was corrected by the cotransfection of a luciferase reporter vector, and CAT assays were performed as described previously (38). DNA was brought to a total of 6 μg for 60-mm-diameter dishes or 20 μg for 100-mm-diameter dishes. CAT results are averages of duplicate assays and are all representative of at least five separate experiments with standard deviations below 15%.
Western blot assays.
C33a cells were cotransfected by the calcium phosphate method with a Flag-tagged modification of the pCI-neo expression vector (Promega) containing cDNA for snail, zfh-1, ZEB, DB-zfh-1, and DB-zfh-1–RD-ZEB. After 48 h, cells were lysed in ELB buffer (150 mM NaCl, 50 mM HEPES [pH 7.0], 5 mM EDTA, 0.1% NP-40) and sonicated briefly. The precleared lysates boiled in sample buffer with 5% of β-mercaptoethanol, the samples were loaded in a 4 to 15% polyacrylamide gradient gel (Bio-Rad Laboratories, Hercules, Calif.) and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, Mass.) by using a 10 mM CAPS {(3-[cyclohexylamino]-1-propanesulfonic acid)}–10% methanol transfer buffer. After blotting for at least 6 h, the membrane was incubated with either anti-zfh-1 mouse polyclonal or anti-Flag polyclonal (Santa Cruz Biotechnologies) antibodies; after incubation with the corresponding HRP-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, Pa.), the Western blot was develop by the enhanced chemiluminescence technique (NEN Life Sciences Products, Boston, Mass.) according to the manufacturer’s instructions.
Myogenic conversion assays and immunostaining.
For 10T1/2 cell myogenic conversion assays, 10T1/2 cells were transfected by the lipofectamine method (Life Technologies) in OPTIMEM medium (Life Technologies) as instructed by the manufacturer. After 12 h, medium was removed and replaced with 2% horse serum–DMEM (differentiation medium). After 5 to 6 days, cells were fixed in methanol and stained with antimyosin antibody MF-20 (from R. Kopan, Washington University, St. Louis, Mo.). After washing to remove unbound antibody, cultures were incubated sequentially with HRP-conjugated anti-mouse immunoglobulin G antibody (Jackson ImmunoResearch) and with diaminobenzidine (DAB) substrate (Vector Laboratories, Burlingame, Calif.). Nuclei were counterstained with hematoxylin (Zymed, South San Francisco, Calif.).
Drosophila stocks and induction of zfh-1 expression.
Oregon R flies were used as wild-type stocks. The fly stocks for heat shock-driven zfh-1 [P(hsp70-zfh-1/Cyo)] were obtained from Z.-C. Lai (26). To induce expression of zfh-1 protein, embryos were collected from agar plates during 1 h at 25°C and allowed to age for different times at 25°C. Embyros were then collected into Nytex nets, covered with 80% glycerol, and subjected to a 15-min heat shock treatment at 37°C. Following heat shock, embryos were rinsed with embryo wash solution (0.7% NaCl, 0.1% Triton X-100) and allowed to recover for different times, and analyzed for the expression of different proteins (see below).
Immunostaining and analysis of embryos.
After the appropriate time of incubation, embryos were dechorionated by incubation for 3 min in bleach, fixed in an heptane-formaldehyde solution, and extracted with methanol (12). Embryos were then blocked with 50% normal goat serum in phosphate-buffered saline, incubated with antibodies against MHC (1:10 supernatant dilution; gift from D. Kiehart, Duke University, Chapel Hill, N.C.), mef2 (rabbit serum, 1:1,000; from B. Patterson), twist (rabbit serum, 1:2,000, gift from E. Bier, University of California, San Diego, La Jolla, Calif.), and zfh-1 (polyclonal mouse serum, 1:450; gift from Z. C. Lai) for 4 h to overnight. All primary antibodies were preabsorbed twice against fixed and blocked embryos. The reaction was followed by incubation with biotinylated anti-rabbit or anti-mouse secondary antibodies (1:300 dilution) for 2 to 4 h and with ABC complex (Novocastra-Vector, Burlingame, Calif.) for 1 h. The reaction was developed with an HRP Elite kit and DAB substrate (Pierce, Rockford, Ill.). Following color development, the embryos were mounted in 80% glycerol and examined on a Zeiss Axioplan-2 microscope (Carl Zeiss, Oberkochen, Germany).
RESULTS
zfh-1 is a DNA binding protein that recognizes a subset of E box sequences.
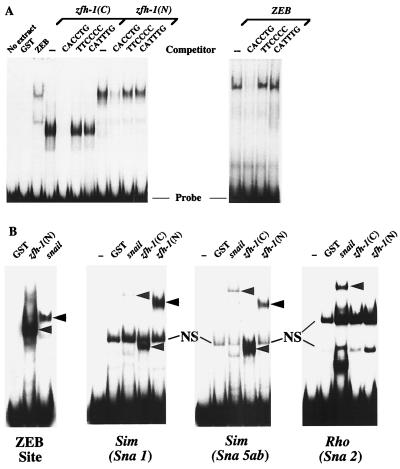
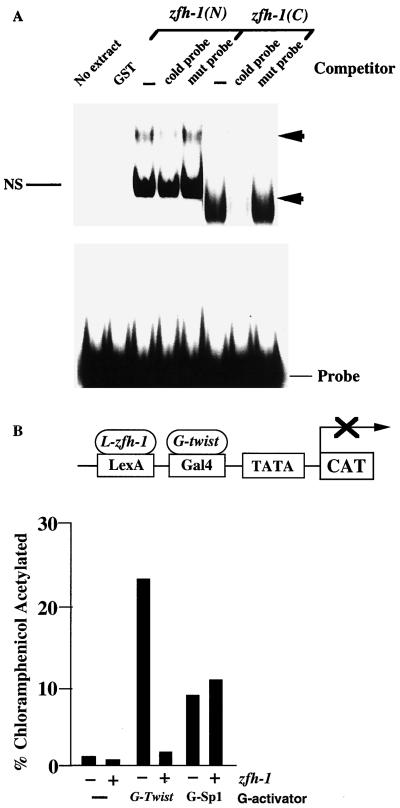
Among all zfh family members, zfh-1 and ZEB share the most sequence similarity in the zinc fingers and homeodomain (13, 18) (Table 1). The zinc fingers of ZEB bind to a subset of E boxes (and E box-like sequences), with highest affinity for the CACCTG site (18, 39). The similarity in the zinc fingers of ZEB and zfh-1 suggested that these motifs in zfh-1 might also be DNA binding domains. Therefore, we examined whether zfh-1 can bind to the CACCTG site. As shown in Fig. 1A, both the N- and C-terminal zinc fingers of recombinant zfh-1 bound to the site in gel retardation assays. This binding was abolished when the site was mutated (Fig. 1A). Furthermore, as observed for ZEB (18), zfh-1 binds to only a subset of E box sequences; it failed to bind the CATTTG E box sequence (Fig. 1A and results not shown). Interestingly, the zfh-1 binding site also matches the high-affinity site recognized by the zinc finger protein snail (17, 23, 31), and we found that zfh-1 bound quite efficiently to various snail binding sites in the single-minded gene (zfh-1 binds better than snail to Sna5ab, the highest-affinity site [23]) (Fig. 1B). These results demonstrate for the first time that zfh-1 is a DNA binding protein and that it shows DNA binding specificity similar to that of ZEB and snail.
TABLE 1.
Sequence comparison of zfh-1 and ZEB
| Region(s) | Amino acids
|
% Similarity | |
|---|---|---|---|
| ZEB | zfh-1 | ||
| N-terminal region | 1–168 | 1–252 | 8 |
| N-terminal zinc fingers | 169–294 | 253–415 | 50 |
| Central repressor domain | 295–902 | 416–972 | 12 |
| Homeodomain | 590–637 | 714–762 | 30 |
| C-terminal zinc fingers | 903–987 | 973–1056 | 80 |
| C-terminal region | 988–1124 | NA | NA |
NA, not applicable (zfh-1 has only a four-amino-acid C-terminal region [1057 to 1060]).
FIG. 1.
zfh-1 binds E box sequences. (A) Gel retardation assays using a probe containing CACCTG sites (36). Recombinant proteins encoding the C- and N-terminal zinc fingers of zfh-1 [zfh-1(C) and zfh-1(N), respectively] and N-terminal zinc fingers of ZEB were obtained from overexpression in bacteria as described in Materials and Methods. zfh-1 and ZEB binding was competed with a 50-fold excess of unlabeled probe but not with the mutant non-E box probe (TTCCCC) or an unrelated E box sequence (CATTTG). (B) Snail and zfh-1 share DNA binding specificities. A probe containing a ZEB binding site (E361/E399 in the α4 integrin promoter [36]), the highest-affinity sites for snail from the single-minded (sim) promoter (Sna1 and Sna5b as in reference 23) and from the rhomboid promoter (Sna2 as in reference 22) were used in gel shift experiments to test the binding of GST-snail and GST–N- and C-terminal zfh-1 zinc fingers [zfh-1(N) and zfh-1(C), respectively]. Equal molar amounts of GST fusion proteins (as determined by Western blotting with anti-GST antibodies [data not shown]) were used. Note that zfh-1 binds to snail sites in the single-minded promoter (even to the highest-affinity site, Sna5ab [23]) with even higher affinity than snail. However, binding of zfh-1 to the snail sites in the rhomboid promoter were weak or neglible (this figure and data not shown). Arrowheads indicate specific complex; NS denotes a nonspecific band. Free probes are indicated at the bottom.
zfh-1 is a transcriptional repressor.
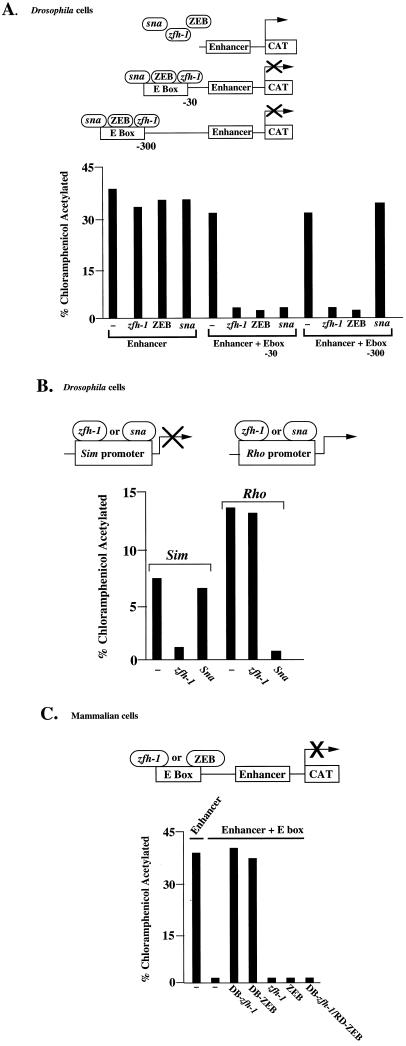
Both ZEB and snail are transcriptional repressors (20, 29, 36). Therefore, we wondered whether zfh-1 might also be a transcription factor, even though there is little evidence of sequence similarity in the central region (in between the N- and C-terminal zinc fingers) where the ZEB repressor domain is located (Table 1) (36). To determine whether zfh-1 has transcriptional activity, a reporter containing the CACCTG binding site 30 bp upstream of an enhancer was transfected in Drosophila Schneider L2 cells. These cells do not express endogenous zfh-1 or snail (data not shown), and thus the presence of the E box site had no effect on promoter activity. However, cotransfection of a zfh-1 or snail expression vector resulted in repression (Fig. 2A). A similar level of repression by zfh-1 was observed when the CACCTG sequence was moved 300 bp upstream of the enhancer (Fig. 1A), demonstrating that zfh-1 (and ZEB) can repress at long range. In contrast, snail failed to repress transcription at this long range as previously described (20) (Fig. 2A). Expression of DB-zfh-1 did not repress (data not shown), suggesting that the protein has separate DNA binding and repressor domains. These results demonstrate that zfh-1, like snail and ZEB, functions as an active transcriptional repressor when it binds to E box sequences.
FIG. 2.
zfh-1 is an active transcriptional repressor. (A) Five micrograms of a reporter containing two copies of a zfh-1 site cloned either 30 or 300 bp upstream of an enhancer element (from the α4 integrin promoter [38]) was cotransfected in Drosophila Schneider cells with 8 μg of actin 5 promoter-driven constructs for zfh-1, ZEB, or snail (sna). A reporter lacking the zfh-1 site was used as a control. (B) zfh-1 represses the single-minded promoter by binding through snail binding sites. Ten micrograms of a CAT reporter construct driven by the single-minded promoter and 2 μg of a CAT reporter driven by the rhomboid promoter were cotransfected in Drosophila Schneider cells with 8 μg of actin 5 promoter-driven constructs for snail or zfh-1. (C) zfh-1 represses transcription in mammalian cells. DB-zfh-1 and DB-ZEB are able to displace endogenous ZEB and release transcriptional repression. Full-length zfh-1, full-length ZEB, and DB-zfh-1–RD-ZEB block transcription. Three micrograms of a reporter construct containing two copies of the CACCTG site upstream of the enhancer (38) was cotransfected in human C33a cells (and HT1080 cells, with identical results) with equal molar concentrations of zfh-1, DB-zfh-1, DB-ZEB, and DB-zfh-1–RD-ZEB. CAT results are averages of duplicate assays and are all representative of at least five separate experiments with standard deviations below 15%.
Because of the overlap in DNA binding specificity, we wondered whether zfh-1 could target the same genes as snail. One such snail-regulated gene is single-minded, which is normally restricted to midline cells and a subset of somatic muscle precursor cells (23, 34). Snail functions to block ectopic expression of single-minded and other nonmesodermal genes in the mesoderm (29). We found that zfh-1 not only bound to the snail sites on the single-minded promoter (Figure 1B) but also repressed the activity of the single-minded promoter in transfection assays in Schneider cells even more efficiently than snail, consistent with the finding that sites from the single-minded promoter bind to zfh-1 more efficiently than snail (Fig. 1B and 2B).
However, snail also binds other sequences that are not shared with zfh-1 (reference 22, Fig. 1B, and data not shown). In the rhomboid promoter, snail sites are important to block the expression of rhomboid in the ventral regions during embryogenesis (22). Contrary to what we found for the snail sites in the single-minded promoter, zfh-1 showed little or no binding to the four snail sites of the rhomboid promoter (Fig. 1B and data not shown). And, accordingly, zfh-1 failed to repress the transcriptional activity of the rhomboid promoter (Fig. 2B). These results demonstrate that zfh-1 can interact with only a subset of snail sites.
It is important to point out that snail is required for zfh-1 expression and that zfh-1 persists after snail diminishes (8, 24, 26). Thus, the two proteins appear to be temporally distinguishable in the developing embryo. This suggests that the two proteins may regulate separate or perhaps partially overlapping sets of genes, albeit at distinct developmental stages or in distinct tissues.
zfh-1 contains an independent repressor domain that functions in mammalian cells.
Next, we wondered whether the repressor functions of zfh-1 and ZEB may cross species. Therefore, we cotransfected into the Schneider cells a ZEB expression vector with a reporter containing the CACCTG site upstream of an enhancer. We found that ZEB can also repress transcription in Drosophila cells (Fig. 2A).
We then investigated whether zfh-1 could repress transcription in mammalian cells. We used a reporter construct containing a CACCTG binding site upstream of an enhancer (Fig. 2C). Coexpression of DB-zfh-1 (or DB-ZEB) did not repress the activity of the enhancer (Fig. 2C). However, transfection of an expression vector for either full-length zfh-1 (or full-length ZEB) or DB-zfh-1–RD-ZEB did repress transcription through the binding site (Fig. 2C and data not shown). Together, these results suggest that zfh-1 also recognizes E box binding sites in mammalian cells and represses transcription when bound to these sites.
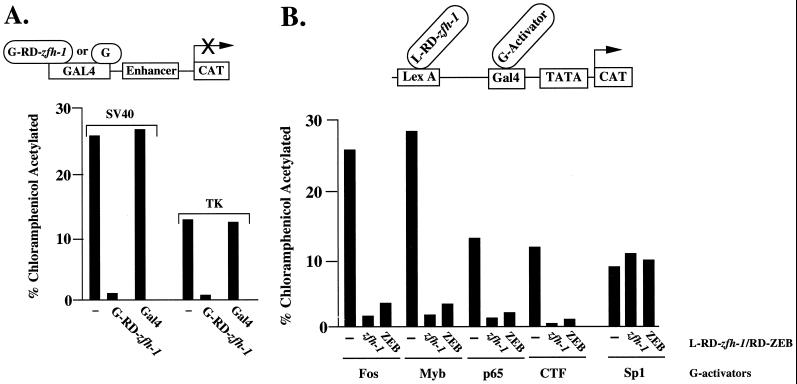
To determine whether zfh-1 contains an independent repressor domain that can function when fused to a heterologous DNA binding domain, we created a construct where the region of zfh-1 between the zinc finger domains (corresponding to the repressor domain in ZEB) was fused to the DNA binding domain of the yeast protein Gal4. Gal4–zfh-1 was tested in transfection assays with reporter plasmids containing Gal4 binding sites cloned upstream of various enhancers. Gal4–zfh-1 efficiently repressed the SV40 enhancer and thymidine kinase (TK) promoter (Fig. 3A), indicating that zfh-1 indeed contains an independent repressor domain located between the zinc finger regions.
FIG. 3.
zfh-1 is an active and selective transcriptional repressor. (A) The region between the zinc finger domains of zfh-1 acts a repressor when fused to the DNA binding domain of the yeast Gal4 protein (GRD-zfh-1). Three micrograms of a reporter construct containing five Gal4 sites upstream of the SV40 enhancer/promoter and the TK promoter were cotransfected in human HT1080 cells (or C33a cells, with identical results) with 3 μg of Gal4–RD–zfh-1. No effect was observed when the reporter was cotransfected with the molar equivalent amount of the control Gal4 expression vector. (B) zfh-1 is a selective transcriptional repressor. The region of zfh-1 between the zinc finger regions was fused to the bacterial protein LexA and tested for its ability to block the activity of a set of Gal4 activators by cotransfection with the pLG reporter construct (45) containing six LexA binding sites upstream of two or five Gal4 binding sites (results were the same with either construct); 0.8 μg of the pLG construct was cotransfected with 0.1 to 0.8 μg of different Gal4 activators and 3 μg of L–RD–zfh-1 into HT1080 cells. As a control, LexA was also cotransfected instead of LexA–zfh-1. LexA had no effect on the activity of the different Gal4 activators (data not shown). CAT results are averages of duplicate assays with standard deviations below 15%.
As shown in Table 1, the overall sequence similarity between zfh-1 and ZEB in their repressor domains is very low. Nevertheless, when we tested the ability of zfh-1 to repress the activity of a number of transcription factors, we found that zfh-1 and ZEB had similar transcription factor specificities in transfection assays (Fig. 3B and data not shown). zfh-1 is expressed in other tissues in addition to muscle (heart, gonadal cells, central nervous system [CNS], etc. [6, 26, 27, 33]), and the ability of zfh-1 to repress various transcription factors may have a role in the regulation of gene expression in these tissues.
These results indicate that zfh-1 is an active transcriptional repressor and that the repressor domains in zfh-1 and ZEB may be functionally similar.
zfh-1 blocks myogenesis in mammalian cells.
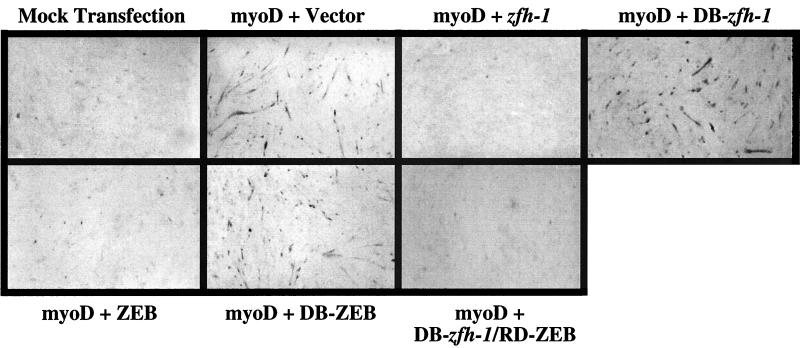
Given the similarity between ZEB and zfh-1 in DNA binding specificity and repressor activity, we examined whether zfh-1 could substitute for ZEB and block muscle differentiation in mammalian cells. Transfection of myoD is sufficient to drive cells down a myogenic pathway by inducing a cascade of transcription factors including members of the myocyte enhancer family (e.g., mef2) which collaborate with myoD to amplify the muscle differentiation program (32, 43). Previously, we and others have found that overexpression of ZEB blocks this myogenic conversion (36, 39). Here, we show that a construct encoding full-length zfh-1 also efficiently blocks myogenic differentiation (Fig. 4). As with ZEB, DB-zfh-1 alone did not affect myogenic differentiation (Fig. 4), even though DB-zfh-1 binds DNA more efficiently than the full-length protein and efficiently displaces wild-type ZEB from the promoter (Fig. 2C and results not shown). Therefore, zfh-1 and ZEB do not block myogenic differentiation simply by displacing MRF proteins from the promoter; instead, their repressor domains is required for this activity (36). Accordingly, fusion proteins containing DB-zfh-1 fused to RD-ZEB, DB-ZEB fused to RD-zfh-1, or DB-zfh-1 fused to RD-zfh-1 also blocked myotube formation (Fig. 4 and data not shown).
FIG. 4.
zfh-1 blocks myogenesis in vertebrate cells by active transcriptional repression. 10T1/2 mouse fibroblasts were transfected with 0.5 μg of a myoD expression vector (or its parent vector; mock transfection) along with equal molar concentrations of expression vectors for full-length zfh-1 and ZEB, DB-zfh-1, DB-ZEB, DB-zfh-1–RD-ZEB, or the empty vector as a control. After 5 to 6 days, cells were immunostained for MHC and developed with HRP and DAB as previously described (36). The images are representative of at least five different myogenic differentiation assays. The expression levels of ZEB, zfh-1, DB-ZEB, DB-zfh-1, and DB-zfh-1–RD-ZEB proteins were similar by Western blot assay (results not shown).
The above results indicate that the RD-zfh-1 can block myogenesis in mammalian cells, suggesting that the function of zfh-1 and ZEB may be conserved from Drosophila to mammals.
zfh-1 inhibits somatic myogenesis in vivo.
Our transfection assays in Drosophila and mammalian cells suggested that zfh-1 might play a negative role during muscle development in the Drosophila embryo. Loss of zfh-1 function did not cause drastic alterations to muscle development (27). In zfh-1 mutant embryos, somatic and visceral muscles form and differentiate, but there are subtle defects such as loss, misplacement, and disorganization of some muscles (27). These results demonstrate that zfh-1 is not required for muscle differentiation per se, but they are consistent with a regulatory role for zfh-1 in the process. From these studies, there was no indication about its mechanism of action and whether zfh-1 might act as a positive or negative regulator of myogenesis. Moreover, studies on the role of ZEB in muscle differentiation had been confined to in vitro assays (36, 39). Therefore, we decided to investigate the role of zfh-1 during myogenesis in vivo.
zfh-1 is downregulated prior to somatic muscle differentiation (reference 26 and data not shown), raising the possibility that this downregulation is essential for the onset of myogenesis. While the loss-of-function phenotype appeared mild in muscle (27), we wondered whether maintenance of zfh-1 expression beyond the time that endogenous zfh-1 diminishes might have a more drastic phenotype (e.g., blocking myogenesis as occurs in cultured cells [Fig. 4]).
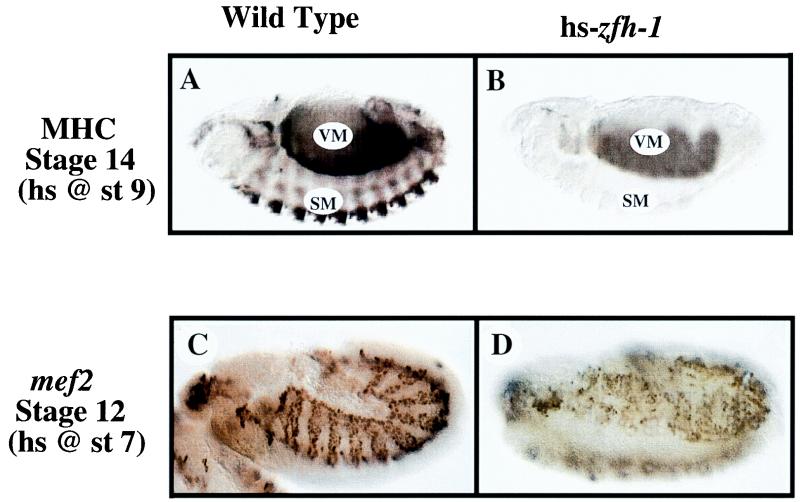
zfh-1 is initially expressed throughout the mesoderm, but after gastrulation it is downregulated in muscle precursors as well as most other mesodermal derivatives (data not shown and reference 26). We maintained expression of zfh-1 throughout embryogenesis by expressing the protein under control of the heat shock protein 70 promoter and assayed muscle development by following MHC expression (1). First, we heat shocked the embryos at stage 9–10, which corresponds to the time that zfh-1 is normally downregulated and is prior to MHC expression in muscle (reference 26 and data not shown). zfh-1 expression following heat shock was confirmed by immunohistochemistry (data not shown). At stage 14, we observed a loss of MHC expression in somatic muscles; however, surprisingly, MHC expression in visceral muscle appeared relatively normal (Fig. 5). In embryos that completed embryogenesis, we observed milder but still clear defects in MHC expression in somatic muscles (data not shown).
FIG. 5.
Overexpression of zfh-1 inhibits somatic muscle differentiation in Drosophila embryos and disrupts mef2 expression. MHC expression was analyzed as a marker of myogenic differentiation in wild-type and zfh-1-overexpressing embryos (hs-zfh-1). Embryos were analyzed with antibodies against MHC and mef2 as described in Materials and Methods. The embryos are oriented with the anterior to the left in lateral views. (A) Wild-type embryos were heat shocked (hs) for 15 min at 37°C at stage (st) 9 as described in Materials and Methods and immunostained for MHC at stage 14. VM, visceral muscle; SM, somatic muscle. (B) hs-zfh-1 embryos were heat shocked at stage 9 and immunostained for MHC at stage 14. Visceral muscle (VM) remained relatively unaffected, whereas somatic muscle (SM) showed a complete absence of MHC-positive cells. (C) Staining for mef2 in wild-type embryos (stage 12, lateral view); embryos were heat shocked at stage 7 for 15 min at 37°C as described in Materials and Methods. mef2 expression is restricted to the visceral (internal) and somatic (external) mesodermal layers and the cephalic mesoderm (most anterior part of the embryo). (D) Staining for mef2 in hs-zfh-1 embryos (stage 12, in ventrolateral view); embryos were heat shocked at stage 7. Maintained expression of zfh-1 causes inhibition of mef2 expression and severe derangement of its pattern in the mesoderm.
We also heat shocked the embryos to induce zfh-1 expression after the onset of MHC expression (stage 12–13). In this case, we observed little, if any, defect in MHC expression in somatic muscles (data not shown), indicating that once the muscles cells begin to express MHC, they are refractory to the negative effects of zfh-1 expression. Taken together, these results are consistent with a model in which extinction of zfh-1 expression in embryonic muscle precursors is necessary to allow muscle differentiation to proceed.
zfh-1 inhibits the pattern of mef-2 expression.
We noticed that maintaining zfh-1 expression resulted in a muscle differentiation phenotype similar to that seen with the loss of mef2 (where there is a block in MHC expression in somatic muscle with less effect on visceral muscle) (5). This phenotype is also similar to that observed when the transcriptional activator twist is disrupted after gastrulation via a temperature-sensitive mutant (2). twist is required for activation of the mef2 gene in somatic muscle, and these observations raised the possibility that zfh-1 may act to inhibit somatic myogenesis by blocking the expression of mef2.
The pattern of mef2 expression is complex and dynamic in the embryo, but mef2 expression increases in muscle precursors as they appear in the embryo (35). mef2 is first evident at the late cellular blastoderm stage in mesoderm primordia and continues to be expressed throughout the mesoderm during mesoderm invagination. At mid-germband extension, mef2 expression is reduced in the ventrolateral mesoderm but maintained in the dorsal region. During germband retraction, expression increases in visceral mesoderm and in somatic muscle precursors (35). This is around the time when zfh-1 is downregulated (data not shown and reference 26). mef2 expression then increases dramatically in all somatic mesoderm, and throughout germband retraction expression continues to be high in somatic muscles (35).
zfh-1 is also expressed in a dynamic fashion in the mesoderm, and it is downregulated in muscle precursors as they began to appear (26). When we double immunostained for mef2 and zfh-1, we found that the expression of zfh-1 and that of mef2 were mutually exclusive (data not shown).
Taken together, the above results suggested that zfh-1 might have some role in controlling the pattern of mef2 expression in muscle precursors. To test this possibility, we analyzed the pattern of mef2 expression in embryos where zfh-1 expression is maintained by using the heat shock construct. Wild-type embryos exhibited a normal mef2 pattern following heat shock at all stages examined (Fig. 5). However, heat-shocked-expressing zfh-1 embryos showed a range of defects. (i) In the most severe cases, mef2 was highly disrupted (data not shown). These embryos failed to complete germband retraction and appear not to have developed far past this stage. (ii) Other embryos showed a fairly normal morphology and completed embryogenesis. In these embryos, there was still clear disruption of mef2 in somatic muscle and a reduction in the number of mef2-positive cells (stage 12 [Fig. 5]). These results suggested that the downregulation of zfh-1, associated with the onset of somatic myogenesis, is required for expression of mef2.
Cripps et al. (11) have recently shown that regulation of mef2 depends on the existence of an enhancer element 2.3 kb upstream of the mef2 gene which is directly activated by twist. Examination of the mef2 promoter sequence (kindly provided by E. N. Olson and R. M. Cripps) revealed multiple zfh-1 sites throughout the sequence which bind zfh-1 in gel retardation assays (Fig. 6A).
FIG. 6.
zfh-1 binds sites from the mef2 gene promoter and blocks transcriptional activation by twist. (A) Gel retardation assays using a probe containing zfh-1 sites in the mef2 promoter sequence (35a). Sites at bp −2782 and −8564 were tested. Recombinant proteins encoding the C- and N-terminal zinc fingers of zfh-1 [zfh-1(C) and zfh-1(N), respectively] were obtained from expression in bacteria as described in Materials and Methods. zfh-1 binding was competed with a 50-fold excess of unlabeled probe (CACCTG or CACCTA) but not with the mutant non-E box probe (TTCCCC). Arrowheads indicate specific complex; NS denotes a nonspecific band. (B) zfh-1 represses twist activity. The ability of L–RD–zfh-1 (as in Fig. 3B) to block the activity of Gal4-twist was tested by cotransfection with the pLG reporter (as in Fig. 3B and reference 45); 0.8 μg of pLG reporter was cotransfected with 0.4 of Gal4-twist or 0.6 μg of Gal4-Sp1 activators and 3 μg of LexA–zfh-1. As a control, LexA was also cotransfected instead of L–RD–zfh-1. LexA had no effect on the activity of the different GAL4 activators (data not shown). L–RD–zfh-1 failed to repress the activity of Sp1 which was included as a control. CAT results are averages of duplicate assays with standard deviations below 15%.
Do the zfh-1 sites in the mef2 promoter block transcriptional activation by twist? In transfection assays, we show that zfh-1 blocks transcriptional activation by twist (Fig. 6B), and we propose that zfh-1 blocks twist-mediated activation of the mef2 gene in muscle precursors until zfh-1 expression diminishes.
DISCUSSION
Here we have examined the nature and molecular mechanism of action of zfh-1 and shown that the protein regulates myogenesis in Drosophila embryos. We demonstrate that zfh-1 is a transcription factor that binds E boxes, acts as a repressor, and functions as the homologue of the vertebrate ZEB protein. While zfh-1 and ZEB show sequence similarity in the zinc finger DNA binding domains, the transcriptional repressor domains of the two proteins shows very little sequence similarity (Table 1). However, we have found within the repressor domain of ZEB binding sites for the corepressor CtBP (37a, 38a, 42), which are also conserved in zfh-1. Nevertheless, the functional role of CtBP binding sites remains unclear. Despite the lack of extensive sequence similarity in the repressor domains, the two proteins show similar transcription factor specificities, and we provide evidence that zfh-1 can substitute for ZEB in inhibition of vertebrate myogenesis. Another possibility is that although the homology at the amino acid level is low, the tertiary structure of their repressor domains is conserved. This has been observed with the pleckstrin homology domains from several signal transduction molecules, which show almost identical crystal structures with no evident similarity at the amino acid level (28). Further characterization of the repressor domains in zfh-1 and ZEB may allow us to identify additional sequences that are important for repressor structure and thus are indicative of their repressor motif.
In this report we also provide evidence that zfh-1 and snail have some similar features. Both proteins are zinc finger transcriptional repressors, and zfh-1 can bind a subset of snail sites. zfh-1 is dependent on snail for expression (8, 21, 26) and persists after snail diminishes (24). Thus, there would be little overlapping in the protein themselves or their target genes. Rather, we suggest that the mode of action of snail and zfh-1—transcriptional repression by binding to E box (and E box-like) sequences—is an efficient regulatory mechanism for controlling differentiation that is maintained during embryogenesis and used early by snail to regulate mesoderm formation and later by zfh-1 to regulate the subsequent differentiation of certain mesodermal derivatives.
Creation of muscles with the correct orientation and placement in Drosophila embryonic hemisegments involves a highly orchestrated series of differentiation events where timing of differentiation and positioning of muscle founder cells are critical (1). Although somatic myogenesis occurs in zfh-1 mutants, these embryos show muscle defects, with some muscles disorganized and mispositioned (27). We found that a much more severe phenotype was evident when expression of zfh-1 was maintained during embryogenesis: block of somatic muscle differentiation. This phenotype is consistent with a role for zfh-1 as a negative regulator of muscle differentiation. Since zfh-1 is not normally expressed in differentiating somatic muscle precursors, classical loss-of-function analysis (27) was unable to identify the need to turn zfh-1 expression off in these cells.
The muscle phenotype that we observed when zfh-1 expression was maintained in the embryos is very similar to that found in mef2 mutant embryos: a block in differentiation and MHC expression in somatic muscle and little effect on visceral muscle (5). zfh-1 decreases in embryonic myogenic precursors before mef2 expression increases in these cells (26, 35). We also found that when zfh-1 expression was maintained during embryogenesis, the expression of mef2 was inhibited in somatic muscle. These results are consistent with the possibility that zfh-1 directly represses the mef2 gene. Expression of mef2 in embryonic muscle precursors is dependent on twist, which directly activates the mef2 gene (11). Interestingly, the mef2 promoter contains multiple zfh-1 binding sites, and we found that they bind efficiently zfh-1. Moreover, transfection assays indicate that zfh-1 is able to efficiently repress twist-mediated transcriptional activation, suggesting that interaction with the mef2 promoter could delay twist-mediated activation of the mef2 gene until zfh-1 diminishes in the embryo.
Although zfh-1 is downregulated in most tissues during embryogenesis, zfh-1 expression persists in the adult muscle precursors (references 26 and 27 and data not shown). Interestingly, these cells do not express mef2 even though they express twist. This is consistent with the idea that zfh-1 blocks twist activation of mef2 in these cells until after embryogenesis.
The finding that zfh-1 is able to repress a number of different promoters and transcription factors in transfection assays suggests that it may be capable of regulating genes in other tissues. In addition to somatic muscle, zfh-1 is expressed in the CNS and in other mesoderm-derived tissues such as gonadal and fat body precursors, heart, and gut (26, 27). Studies of zfh-1 mutant embryos and embryos overexpressing zfh-1 have suggested roles for zfh-1 in the differentiation of some of these other tissues, indicating that its role extends beyond somatic muscle. We also found that zfh-1 binds to the same sequences recognized by other zinc finger repressors such as snail and escargot (16, 17, 31). It has been shown that escargot (and snail) competes with a heterodimer containing the proneural scute-daughterless complex for DNA binding sites (16, 17, 46). In transfection assays into Schneider L2 cells, we found that zfh-1, like snail, competed for DNA binding with daughterless-scute (data not shown). zfh-1 is expressed in the CNS, and overexpression of the protein has been shown to disrupt CNS differentiation (26), implying that zfh-1 also may have a regulatory role in CNS differentiation. Therefore, competition for binding between zfh-1 and the proneural daughterless-scute complex may have a functional role in regulating zfh-1 activity in motorneurons in a fashion similar to that which we proposed for myoD family members and ZEB in mammalian muscle (36).
ACKNOWLEDGMENTS
We are extremely thankful to E. Bier, R. Cagan, S. T. Crews, T. Genetta, S. Hayashi, Y. T. Ip, D. Kiehart, R. Kopan, R. Krusnow, Z. C. Lai, B. Patterson, and C. Thummel for kindly providing antibodies, plasmids, and fly stocks. We specially thank E. N. Olson and R. Cripps for providing unpublished results on the sequence of the mef2 promoter and also E. N. Olson for critical reading of the manuscript. We also thank P. Taghert and R. Benveniste for help in managing fly stocks.
A.A.P. was supported by the Leukemia Society. This work was supported by grants to J.B.S. (from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Award [DRS-9] and from HHMI Res. Resources Program for Medical Schools Junior Faculty award 76296-538202) and to D.C.D. (from the NIH).
REFERENCES
- 1.Bate M. The mesoderm and its derivatives. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanoganster. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 1013–1090. [Google Scholar]
- 2.Baylies M K, Bate M. Twist: a myogenic switch in Drosophila. Science. 1996;272:1481–1484. doi: 10.1126/science.272.5267.1481. [DOI] [PubMed] [Google Scholar]
- 3.Baylies M K, Bate M, Gomez M R. Myogenesis: a view from Drosophila. Cell. 1998;93:921–927. doi: 10.1016/s0092-8674(00)81198-8. [DOI] [PubMed] [Google Scholar]
- 4.Benezra R, Davis R L, Lockshon D, Turner D L, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- 5.Bour B A, O’Brien M A, Lockwood W L, Goldstein E S, Bodmer R, Taghert P H, Abmayr S M, Nguyen H T. Drosophila Mef2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;15:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- 6.Broihier H, Moore L, Doren M, Newman S, Lehmann R. zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development. 1998;125:655–666. doi: 10.1242/dev.125.4.655. [DOI] [PubMed] [Google Scholar]
- 7.Cabanillas A M, Darling D S. Alternative splicing gives rise to two isoforms of Zfhep, a zinc finger/homeodomain protein that binds T3-response elements. DNA Cell Biol. 1996;15:643–651. doi: 10.1089/dna.1996.15.643. [DOI] [PubMed] [Google Scholar]
- 8.Casal J, Leptin M. Identification of novel genes in Drosophila reveals the complex regulation of early genes activity in the mesoderm. Proc Natl Acad Sci USA. 1996;93:10327–10332. doi: 10.1073/pnas.93.19.10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C M A, Kraut N, Groudine M, Weintraub H. I-mf, a novel myogenic repressor that interacts with members of the MyoD family. Cell. 1996;86:731–741. doi: 10.1016/s0092-8674(00)80148-8. [DOI] [PubMed] [Google Scholar]
- 10.Chow K N, Dean D C. Domains A and B in the Rb pocket interact to form a transcriptional repressor motif. Mol Cell Biol. 1996;16:4862–4868. doi: 10.1128/mcb.16.9.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cripps R M, Black B L, Zhao B, Lien C L, Schulz R A, Olson E N. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 1998;12:422–434. doi: 10.1101/gad.12.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiNardo S, Kuner J M, Theis J, O’Farrell P H. Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell. 1985;43:59–69. doi: 10.1016/0092-8674(85)90012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fortini M E, Lai Z C, Rubin G M. The Drosophila zfh-1 and zfh-2 genes encode novel proteins containing both zinc-finger and homeodomain motifs. Mech Dev. 1991;34:113–122. doi: 10.1016/0925-4773(91)90048-b. [DOI] [PubMed] [Google Scholar]
- 14.Franklin A J, Jetton T L, Shelton K D, Magnuson M A. BZP, a novel serum-responsive zinc finger protein that inhibits gene transcription. Mol Cell Biol. 1994;14:6773–6788. doi: 10.1128/mcb.14.10.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funahashi J, Seikido R, Murai K, Kamachi Y, Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993;119:433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- 16.Fuse N, Hirose S, Hayashi S. Diploidy of Drosophila imaginal cells is maintained by a transcriptional repressor encoded by escargot. Genes Dev. 1994;8:2270–2281. doi: 10.1101/gad.8.19.2270. [DOI] [PubMed] [Google Scholar]
- 17.Fuse N, Hirose S, Hayashi S. Determination of wing cell fate by the escargot and snail genes in Drosophila. Development. 1996;122:1059–1067. doi: 10.1242/dev.122.4.1059. [DOI] [PubMed] [Google Scholar]
- 18.Genetta T, Ruezinsky D, Kadesch T. Displacement of an E box-binding repressor by basic helix-loop-helix proteins: implications for B-cell specificity of the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1994;14:6153–6163. doi: 10.1128/mcb.14.9.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genetta T, Kadesch T. Cloning of a cDNA encoding a mouse transcriptional repressor displaying striking sequence conservation across vertebrates. Gene. 1996;169:289–290. doi: 10.1016/0378-1119(95)00824-1. [DOI] [PubMed] [Google Scholar]
- 20.Gray S, Levine M. Short-range transcriptional repressors mediate both quenching and direct repression within complex loci in Drosophila. Genes Dev. 1996;10:700–710. doi: 10.1101/gad.10.6.700. [DOI] [PubMed] [Google Scholar]
- 21.Hemavathy K, Meng X, Ip Y T. Differential regulation of gastrulation and neuroectodermal gene expression by snail in the Drosophila embryo. Development. 1997;124:3683–3691. doi: 10.1242/dev.124.19.3683. [DOI] [PubMed] [Google Scholar]
- 22.Ip Y T, Park R E, Kosman D, Bier E, Levine M. The dorsal gradient morphogen regulates stripes of rhomboid expression in the presumptive neuroectoderm of the Drosophila embryo. Genes Dev. 1992;6:1728–1739. doi: 10.1101/gad.6.9.1728. [DOI] [PubMed] [Google Scholar]
- 23.Kasai Y, Nambu J R, Lieberman P M, Crews S T. Dorsal-ventral patterning in Drosophila: DNA binding of snail protein to the single-minded gene. Proc Natl Acad Sci USA. 1992;89:3414–3418. doi: 10.1073/pnas.89.8.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosman D, Ip Y T, Levine M, Arora K. Establishment of the mesoderm-neuroectoderm boundary in the Drosophila embryo. Science. 1991;254:118–122. doi: 10.1126/science.1925551. [DOI] [PubMed] [Google Scholar]
- 25.Kostich W A, Sanes J R. Expression of zfh-4, a new member of the zinc finger-homeodomain family, in developing brain and muscle. Dev Dyn. 1995;202:145–152. doi: 10.1002/aja.1002020206. [DOI] [PubMed] [Google Scholar]
- 26.Lai Z, Fortini M E, Rubin G M. The embryonic expression patterns of zfh-1 and zfh-2, two Drosophila genes encoding novel zinc-finger homeodomain proteins. Mech Dev. 1991;34:123–134. doi: 10.1016/0925-4773(91)90049-c. [DOI] [PubMed] [Google Scholar]
- 27.Lai Z, Rushton E, Bate M, Rubin G M. Loss of function of the Drosophila zfh-1 gene results in abnormal development of mesodermally derived tissues. Proc Natl Acad Sci USA. 1993;90:4122–4126. doi: 10.1073/pnas.90.9.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemmon M A, Ferguson K M, Schlessinger J. PH domains: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 29.Leptin M. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 1991;5:1568–1576. doi: 10.1101/gad.5.9.1568. [DOI] [PubMed] [Google Scholar]
- 30.Lilly B, Zhao B, Ranganayakulu G, Paterson B M, Schulz R A, Olson E N. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 31.Mauhin V, Lutz Y, Dennefeld C, Alberga A. Definition of the DNA-binding site repertoire for the Drosophila transcription factor SNAIL. Nucleic Acids Res. 1993;21:3951–3957. doi: 10.1093/nar/21.17.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molkentin J D, Black B L, Martin J F, Olson E N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 33.Moore L, Broihier H, Doren M, Lehmann R. Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development. 1998;125:837–844. doi: 10.1242/dev.125.5.837. [DOI] [PubMed] [Google Scholar]
- 34.Nambu J R, Lewis J O, Wharton K A, Crews S T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991;67:1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen H T, Bodmer R, Abmayr S M, McDermott J C, Spoerel N A. D-Mef2: a Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc Natl Acad Sci USA. 1994;91:7520–7524. doi: 10.1073/pnas.91.16.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35a.Olson, E., and R. Cripps. Personal communication.
- 36.Postigo A A, Dean D C. ZEB, a vertebrate homologue of Drosophila zfh-1, is a negative regulator of muscle differentiation. EMBO J. 1997;16:3935–3943. doi: 10.1093/emboj/16.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postigo A A, Sheppard A M, Mucenski M L, Dean D C. c-myb and ets factors synergize to overcome transcriptional repression by ZEB. EMBO J. 1997;16:3924–3934. doi: 10.1093/emboj/16.13.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Postigo A A, Dean D C. ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci USA. 1999;96:6683–6688. doi: 10.1073/pnas.96.12.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen G D, Barks J L, Iademarco M F, Fisher R J, Dean D C. An intricate arrangement of binding sites for the Ets family of transcription factors regulates activity of the alpha 4 integrin gene promoter. J Biol Chem. 1994;269:15652–15660. [PubMed] [Google Scholar]
- 38a.Schaeper U, Boyd J M, Verma S, Uhlmann E, Subramanian T, Chinnadurai G. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc Natl Acad Sci USA. 1995;92:10467–10471. doi: 10.1073/pnas.92.23.10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekido R, Murai K, Funahashi J, Kamachi Y, Fujisawa-Sehara A, Nabeshima K, Kondoh H. The delta-crystallin enhancer-binding protein delta EF1 is a repressor of E2-box-mediated gene activation. Mol Cell Biol. 1994;14:5692–5700. doi: 10.1128/mcb.14.9.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sekido R, Takagi T, Okanami M, Moribe H, Yamamura M, Higashi Y, Kondoh H. Organization of the gene encoding transcriptional repressor delta EF1 and cross-species conservation of its domains. Gene. 1996;173:227–232. doi: 10.1016/0378-1119(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 41.Spicer D B, Rhee J, Cheung W L, Lassar A B. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH twist. Science. 1996;254:1385–1387. doi: 10.1126/science.272.5267.1476. [DOI] [PubMed] [Google Scholar]
- 42.Sundqvist A, Sollerbrant K, Svensson C. The carboxy-terminal region of adenovirus E1A activates transcription through targeting of a C-terminal binding protein-histone deacetylase complex. FEBS Lett. 1998;429:183–188. doi: 10.1016/s0014-5793(98)00588-2. [DOI] [PubMed] [Google Scholar]
- 43.Thayer M J, Weintraub H. Activation and repression of myogenesis in somatic cell hybrids: evidence for trans-negative regulation of myoD in primary fibroblasts. Cell. 1990;63:23–32. doi: 10.1016/0092-8674(90)90285-m. [DOI] [PubMed] [Google Scholar]
- 44.Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell T K, Turner D, Rupp R, Hollenberg S, Zhuang Y, Lassar A. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 45.Weintraub S J, Chow K N, Luo R X, Zhang S H, He S, Dean D C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 46.Whiteley M, Noguchi P D, Sensabaugh S M, Odenwald W F, Kassis J A. The Drosophila gene escargot encodes a zinc finger motif found in snail-related genes. Mech Dev. 1992;36:117–127. doi: 10.1016/0925-4773(92)90063-p. [DOI] [PubMed] [Google Scholar]
- 47.Yasuda H, Mizuno A, Tamaoki T, Morinaga T. ATBF1, a multiple-homeodomain zinc finger protein, selectively down-regulates AT-rich elements of the human alpha-fetoprotein gene. Mol Cell Biol. 1994;14:1395–1401. doi: 10.1128/mcb.14.2.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]